Abstract
Assessment of total vitamin D intake from foods and dietary supplements (DSs) may be incomplete if 25-hydroxyvitamin D [25(OH)D] intake is not included. However, 25(OH)D data for such intake assessments are lacking, no food or DS reference materials (RMs) are available, and comparison of laboratory performance has been needed. The primary goal of this study was to evaluate whether vitamin D3 and 25(OH)D3 concentrations in food and DS materials could be measured with acceptable reproducibility. Five experienced laboratories from the U.S. and other countries participated, all using liquid chromatography tandem-mass spectrometry but no common analytical protocol; however, various methods were used for determining vitamin D3 in the DS. Five animal-based materials (including three commercially available RMs) and one DS were analyzed. Reproducibility results for the materials were acceptable. Thus it is possible to obtain consistent results among experienced laboratories for vitamin D3 and 25(OH)D3 in foods and a DS.
Keywords: reference material, analytical, food, dietary supplement, SRM 1577c Bovine Liver, SRM 1546a Meat Homogenate, SRM 1845a Whole Egg Powder, Vitamin D3 (cholecalciferol), Vitamin D2 (ergocalciferol), 25-Hydroxyvitamin D3 (25-hydroxycholcalciferol), 25-Hydroxyvitamin D2 (25-hydroxyergocalciferol)
TOC Image
INTRODUCTION
Vitamin D deficiency is a worldwide concern because of its major health consequences, including rickets and the postulated increased risk of other diseases.1–3 Numerous epidemiological evaluations of vitamin D intake and vitamin D status have been reported, as have studies on the relationship of these factors with occurrence of various diseases.4–8 Humans and animals obtain vitamin D3 (cholecalciferol) from dietary sources and from synthesis when 7-dehydrocholesterol in the skin is exposed to UV light.9 Vitamin D3 is the primary form in the diet, although vitamin D2 (ergocalciferol) can be present in some foods and supplements.5–7, 10–13 These “parent” compounds of vitamin D are metabolized by humans and animals to 25(OH)D and other metabolites before they are used by the body. Therefore, animal tissue—and, in turn, animal-derived human foods—contain 25-hydroxyvitamin D [25(OH)D] as well as the parent vitamin D, and the 25(OH)D can contribute to the overall vitamin D value of the food. In fact, evidence suggests that the content of 25(OH)D may be two to five times more potent than the content of parent vitamin D.10–12 Therefore, accurate estimates of dietary vitamin D intake require the inclusion of the 25(OH)D content of foods.13
Current estimates of vitamin D intake suggest that many people in the United States consume considerably less than the established dietary requirement.14 The serum 25(OH)D concentrations of the U.S. population, an indicator of vitamin D status, are higher than expected based on estimated vitamin D intakes alone.15, 16 Although sun exposure is often cited a major factor in this discrepancy, another possibility is the failure to account for intakes of 25(OH)D in foods. This potentially important omission could result in significant underestimation of vitamin D intake because the reliability of calculated vitamin D intake rests heavily on the trueness and availability of food composition data,13 which do not typically include 25(OH)D assessments. For example, information on vitamin D in the major source of food composition data in the United States, the U.S. Department of Agriculture’s (USDA’s) National Nutrient Database for Standard Reference,17 is currently being updated,18 but SR does not provide 25(OH)D values. Some limited data on the 25(OH)D content of foods are available in other databases outside the U.S.19, 20, but the lack of data in the U.S. on 25(OH)D3 in foods and the extent to which dietary 25(OH)D3 contributes to vitamin D status merits further exploration.
In addition to limited 25(OH)D measurements in food composition data, the lack of reliable food composition data on vitamin D is also due to uncertainty about the accuracy (trueness and precision) of the methods used to generate such data. Furthermore, obtaining reliable estimates of average concentrations in foods and sources of variability in the food supply, as well as in dietary supplements (DSs), requires representative sampling, validated analytical methods, expert analysts, and accredited, documented quality-control measures.
In the 1990s and early 2000s, the methodology for quantification of vitamin D3 and 25(OH)D3 used high-performance liquid chromatography (HPLC) with UV detection, which necessitated time-consuming clean-up steps to obtain accurate results.21, 22 Recent progress has been made in the development of methodology for quantitation of vitamin D in foods using liquid chromatography-tandem mass spectrometry (LC-MS/MS) for measurements of vitamin D3 and 25(OH)D3.23–27
When published values from different studies are compared, variability due to real differences in food composition versus those due to differences in analytical methodology and skill in assay performance cannot be discerned in the absence of common control or reference samples. In a 2008 study28 that quantified vitamin D3 in the same five food matrices at six experienced laboratories, each using their standard in-house methodology, the results showed varying degrees of difference among the foods and laboratories. Whether this variability was due to differences in the methods used and/or individual skills in laboratory procedures is an open question. Differences in matrix and analyte levels can affect assay performance even when the same basic methodology is used. Therefore, control and reference materials representing different food matrices are needed to ensure the trueness and precision of quantitative results across a wide spectrum of foods.
The first specific aim of this study was to determine whether vitamin D3 and 25(OH)D3 concentrations in a range of animal-based food matrix materials and a DS could be measured with acceptable reproducibility by laboratories that are experienced in vitamin D analysis, without a common analytical protocol. The secondary aim was to assess the potential for the use of the materials as control or reference materials in future research.
MATERIALS AND METHODS
Overview
Through an interagency agreement between the Nutrient Data Laboratory (NDL) (Beltsville, MD) of the USDA Agricultural Research Service and the Office of Dietary Supplements of the National Institutes of Health (Bethesda, MD), NDL designed and conducted a pilot study that measured vitamin D and 25(OH)D levels in foods and a DS. A “roundtable” group of five experts identified and critically evaluated scientific issues related to sample selection, preparation, distribution, and tracking as well as laboratory participation and evaluation of results. The roundtable members were representatives from Health Canada (Longueuil, Quebec), U.S. Food and Drug Administration (College Park, MD), National Institute of Standards and Technology (NIST) (Gaithersburg, MD), and USDA’s Food Composition and Method Development Laboratory (Beltsville, MD). In addition, scientists from the Office of Dietary Supplements participated in the roundtable discussions. Five animal-based foods and one DS were selected by NDL in consultation with the roundtable group. Samples were prepared at a central location and distributed to each laboratory on two occasions for analysis of vitamin D and 25(OH)D content.
Identification of participating laboratories
NDL identified analytical laboratories with expertise in measuring vitamin D and 25(OH)D in various matrices. These laboratories had either published their vitamin D methodology or had previously provided NDL with vitamin D data that met data quality evaluation standards.29 The participating laboratories were Covance Laboratories (Madison, Wisconsin), Health Canada (Longueuil, Quebec), Heartland Laboratories (Ames, Iowa), NIST (Gaithersburg, Maryland), and the Technical University of Denmark (Søborg, Denmark). All of the laboratories were using LC-MS/MS for separation, detection, and quantitation but varied analytical protocols to analyze vitamin D and 25(OH)D content. Two of the laboratories also had experience analyzing DSs for vitamin D content. The five laboratories are identified in this report using randomly assigned letters A through E.
Selection of food samples
Standard animal-based food reference materials from NIST that could contain 25(OH)D3 were initially considered for the study, including NIST Standard Reference Material (SRM) 1577c Bovine Liver; NIST SRM 1546a Meat Homogenate; and NIST SRM 1845a Whole Egg Powder. Foods included in the initial screening were cooked chicken liver, cooked beef liver, rotisserie chicken with skin, a cooked pork and egg yolk composite, and cooked ground beef. The materials chosen for screening represented a variety of food matrices with potential for measurable levels of 25(OH)D.
NDL collaborated with scientific partners at Virginia Tech (Blacksburg, Virginia) to screen these materials and samples of other foods for vitamin D3 and 25(OH)D3 content. A homogeneous composite of locally procured (Blacksburg, Virginia) retail samples of chicken liver, beef liver, and rotisserie chicken was prepared for each of these three foods using validated protocols.28, 30 The ground beef and the pork and egg yolk samples had been previously prepared using the same protocols for use as control materials in the USDA National Food and Nutrient Analysis Program.30 Subsamples of all composites were maintained in glass jars, sealed under nitrogen at −60°C and protected from light. The frozen samples were shipped on dry ice via express overnight delivery to Covance Laboratories, where they were analyzed for vitamin D3 and 25(OH)D3 content.
The screening results were evaluated by the roundtable experts who selected the following materials to represent a range of expected vitamin D3 and 25(OH)D3 concentrations and diverse matrices in this study: NIST SRM 1577c Bovine Liver; NIST SRM 1546a Meat Homogenate; NIST SRM 1845a Whole Egg Powder; cooked chicken liver; and cooked ground beef. The roundtable experts selected these materials because they were sufficiently diverse to represent variability in method performance based on specific matrix characteristics that might affect extraction and quantitation (e.g., fat content, concentration of vitamin D3 and 25(OH)D3, and animal tissue type [e.g., muscle, liver, or egg]).
Selection of the DS
The DS product for this study was selected with the aim of developing a DS in-house control material. Scientists on the Dietary Supplement Ingredient Database team at NDL studied current vitamin D3 supplements on the market, evaluating claimed composition and manufacturing information for both animal and non-animal based vitamin D3 supplements (Supplemental Table 1). Their goal was to purchase supplements that represented a diversity of vitamin D source materials, production methods, matrices, and supplement strength (vitamin D3 labeled content), and test them for 25(OH)D3 content. Based on these criteria, six products sold as capsules, tablets, softgels, and powder labeled as having vitamin D3 content and one product labeled as having 25(OH)D3 content were chosen for preliminary analyses. These products had a wide range of labeled vitamin D content per serving (0 IU to 12 500 IU). A subsample of 90 units of each product was placed in amber Nalgene bottles with desiccant packs, sealed under ambient conditions, and shipped via next-day delivery to Covance Laboratories for screening analysis of vitamin D3 and 25(OH)D3 content.
The screening results (presented in Supplemental Table 2) also indicated that all six DS products sold with vitamin D3 content claims contained 25(OH)D3 at levels similar to those in the foods tested for inclusion in the study. However, their 25(OH)D3 levels were minute compared to the amount of vitamin D3 in the products. The label of the product ultimately chosen for the study claimed that it contained 2000 IU vitamin D3/serving, a level commonly taken by the U.S. population. This product also contained a measurable amount of 25(OH)D3. It was a private-brand softgel capsule sold in warehouse stores and was produced using a common manufacturing process of lanolin recrystallization.31 Thus, purchasing this product by the case provided a large amount of serving units of the same lot, enabling its use as an in-house control material in future studies.
Sample preparation and shipment
Separate sample sets of the selected materials were shipped to each laboratory on two occasions (hereafter referred to as “Trial 1” and “Trial 2”) separated by approximately two months. The NIST materials were shipped in their original packaging. The DS for the study was stored at −30 °C in its original containers, and individual bottles were opened only as needed. Each shipment included a total of 40 g to 100 g of material per food and 90 DS softgel capsules. Sample bottle labels identified the material type. The samples were shipped on dry ice in an insulated cooler via express overnight delivery to each laboratory. General estimates of the expected concentrations of vitamin D3 and 25(OH)D3 in each sample were given to the laboratories to assist in determining the assay parameters.
Sample analysis
The laboratories were asked to use their usual methodology to analyze vitamin D in duplicate or triplicate in each sample. Although results for vitamin D2 were not requested, some laboratories also included vitamin D2 in their assays. All laboratories spectroscopically characterized their standards before use; the only materials supplied to the laboratories were the samples to be analyzed. Laboratories were asked to describe their methodology in detail and to answer a set of questions on the analyses that they performed. For the DS, the laboratories were advised to combine 30 units (i.e., capsules, tablets, softgels) for each assay. (U.S. Pharmacopeia provides guidelines on the amounts of capsules or tablets to be combined for analysis that range from at least 10 to at least 30 for products with vitamin D only or with vitamin D in multivitamin matrices).32–34 Guidelines for subsampling the DS were provided. The guidelines indicated that each laboratory had the option of analyzing for each replicate assay the contents (fill only) of the combined 30 softgels or to dissolve 30 whole softgels before subsampling and analysis. Laboratories that conducted their analyses after dissolving the whole softgels calculated results for the amount of fill based on the weights of the whole capsules and their fill. Thus, for this report, DS concentrations from all laboratories were reported as μg/100 g of fill contents.
Data review
Each laboratory’s Trial 1 data report was reviewed by an internal NDL group to ensure that all required information was received and to detect any potential problems with analyses, such as deviations from the study protocol or major discrepancies in results. NDL contacted laboratories that had report deviations or discrepancies to alert them of any non-representative analytical errors before these laboratories analyzed the second set of samples. Trial 2 datasets were also reviewed by an internal NDL group.
Statistical methods
Results for each material included the replicate values for both trials from all laboratories. Data analysis was performed using PROLAB Plus™ software (QuoData Quality & Statistics, Dresden, Germany). Analyses included calculations of reproducibility relative standard deviations (RSDs) and repeatability RSDs for each material according to ISO 5725-2.35 The reproducibility RSD expresses the precision of the average of the results from different laboratories and was calculated using the SD between the averages of replicate measurements by each laboratory in each trial. Based on Probability of Detection (POD) model, predicted reproducibility RSD is calculated as 2×C−0.15, where C is a mass fraction of analyte, and estimated RSD is expressed as %. Predicted repeatability RSD is considered to be half of this value. As the concentration decreases, the POD decreases and predicted RSD increases, as illustrated by the Horwitz curve.36,37
The repeatability RSD for each material expresses the variability between independent test results obtained within each laboratory. In this study, the repeatability RSD was calculated using the SD between the replicate measurements of each laboratory within each trial (the square root of the sum of the squares of the within-laboratory SD divided by 2, followed by multiplication by the number of laboratory/trials) as an estimate of overall within-laboratory variability for each analyte in each sample.
The Horwitz ratio (HorRat) was used to evaluate the reproducibility RSDs from data provided by the different laboratories for the materials.37, 38 The HorRat indicates the acceptability of the reproducibility of interlaboratory measurements, taking into consideration the analyte concentration. This ratio is the RSD of the mean divided by a predicted reproducibility RSD based on the concentration of the analyte. An acceptable HorRat is ≤2.0.37
Outliers were identified using the Cochran test (analysis of variance) and Grubbs test (analysis of mean value deviations) according to ISO 5725-2.35 Because the purpose of this study was to evaluate both inter- and intra-laboratory variability for each material, the outliers identified from these tests were not excluded from calculation of overall mean and SD. One exception, however, was a value identified as an outlier by the Grubbs test that had already been manually excluded as a result of the analyst’s data evaluation (Laboratory D’s vitamin D3 measurement of the meat homogenate in Trial 1). This outlier is explained in the results section of this report.
RESULTS
Analytical methods used for foods
After receipt of Trial 1 data, it was evident that results from Laboratory E differed markedly from those of the other laboratories. Laboratory E was not given details on the results from the other laboratories. However, because this laboratory had experience primarily in determining vitamin D and 25(OH)D levels in serum, the laboratory optimized its method to measure these analytes in food matrices before analyzing additional study samples. A new set of samples was then sent to Laboratory E, which reported values for the meat homogenate, ground beef, and liver samples. Laboratories A, B, C, and E analyzed each food material in triplicate (three subsamples from the same mixture on the same day), and Laboratory D submitted duplicate data.
Table 1a summarizes the methodology used by each laboratory. All laboratories used LC-MS/MS to quantify vitamin D3 and 25(OH)D3 levels without using a common sample preparation protocol. The laboratories labeled their internal standards in varied ways as well, using, for example, deuterium, tritium, or carbon-13. All laboratories calibrated their standards before use, which is important for obtaining accurate results. They also optimized their methodologies to measure the low concentrations of 25(OH)D in the foods and DS and of vitamin D in the foods. Laboratories A, B, D, and E derivatized the samples prior to LC-MS/MS analysis, but Laboratory C did not. Another significant difference was that Laboratory E did not saponify the samples but used an enzymatic digestion with lipase prior to LC-MS/MS analysis. Laboratory E indicated that its method had not been sufficiently validated for eggs and supplements, so it did not report results for those materials. In addition, Laboratory D’s analyst indicated concern about the validity of the vitamin D3 value for the meat homogenate when reporting its Trial 1 results. Therefore, Laboratory D’s data were excluded from these study results.
Table 1a.
Methodology Used by Participating Laboratories to Analyze Vitamin D Content in Foods and 25(OH)D Content in Foods and a Dietary Supplement
| Laboratory A | Laboratory B | Laboratory C | Laboratory D | Laboratory E | |
|---|---|---|---|---|---|
| Method reference | Huang, M.; LaLuzerne, P.; Winters, D.; Sullivan, D., Measurement of vitamin D in foods and nutritional supplements by liquid chromatography/tandem mass spectrometry. J AOAC Int 2009, 92, 1327–1335. | Not available | Not available | Burild, A.; Frandsen, H. L.; Poulsen, M.; Jakobsen, J., Quantification of physiological levels of vitamin D(3) and 25-hydroxyvitamin D(3) in porcine fat and liver in subgram sample sizes. J. Sep. Sci. 2014, 37, 2659–2663. | Not available |
| Sample size | 3 g to 10 g depending on moisture content | 0.8 g to 3 g depending on moisture content | 0.6 g to 2 g | 1 g | 2 g to 3 g |
| Internal standard | (2H3)vitamin D3 and (2H3)25(OH)D3 (IsoSciences, King of Prussia, PA) | (2H6)vitamin D3 and (2H6)25(OH)D3 (Chemaphor, Ottawa, Canada) | (3H)vitamin D3 (in-house) and (2H3)25(OH)D3 (Sigma-Aldrich) | (2H6)vitamin D3 and (2H6)25(OH)D3 (Chemaphor, Ottawa, Canada) | (13C5)vitamin D3 and (13C5)25(OH)D3 (IsoSciences, King of Prussia, PA) |
| Initial treatment | reagent-grade alcohol with 2 % pyrogallic acid, 50 % KOH added | 10 mL pyrogallol 1% in EtOH and 8 mL KOH 60 % | methanoic KOH | sodium ascorbate, KOH, and ethanol | lipase and ethanol |
| Lipid treatment | saponification under N2 overnight | saponification under N2 at room temperature overnight | saponification for 2 hours at 60°C | saponification under N2 at room temperature for 16–18 hours | enzymatic digestion for 2 hours at 37° C |
| Extraction step | solution of 30 mL 20 % ether and 80 % hexane | 3× petroleum ether/diethyl ether (80/20) | 3× hexane/ethyl acetate (85/15) | ethyl acetate/n-heptane (20/80) | 2× hexane extracted, pooled, with addition of ~1g MgSO4 to remove extra water; centrifuged; and dried |
| Clean-up step | dried and redissolved in 1 mL 70 % acetonitrile–H2O [not done for 25(OH)D] | organic phase washed with water, taken to dryness, and dissolved in 800 μL 1-chlorobutane | dried and redissolved in 1 mL hexane/methylene chloride | dried and redissolved in 2-propanol in n-heptane | |
| Additional clean-up | purified using HPLC normal phase (silica column) and fractions collection | loaded into an SPE cartridge, eluted with methylene chloride/isopropanol, two cleanings through HPLC columns, final solution methylene chloride and isopropanol | loaded onto a silica SPE cartridge, rinsed with 0.5 % 2-propanol in n-heptane, eluted with 6% and 10 % 2-propanol in n-heptane | ||
| Derivatization | evaporated to dryness and 2 mg/mL PTAD in 100 % ACN added. | fractions evaporated to dryness, PTAD reagent in ethyl acetate added, evaporated to dryness, reconstituted in 0.2 mL of methanol | not derivatized | dried under N2 and derivatized with PTAD in anhydrous acetonitrile, solvent evaporated, sample reconstituted in 60% methanol | derivatized with PTAD in acetonitrile, dried, reconstituted in 500 uL 70:30 methanol:water. |
| HPLC column | Thermo, UHPLC column, Hypersil GOLD aQ (1.9μm) 100 mm × 2.1 mm | Phenomenex LUNA-C18 (3.0 um) 150 mm × 2.1 mm | Supelco-Sil LC-18 ODS column (5μm) 3.3 cm × 4.6 mm | Ascentis Express C18 (2.1 mm × 10 cm, 2.7 μm particles) column and C18 (2.1 × 5 mm, 2.7 μm particles) guard column | YMC-Pack Pro C18 RS (5μm,8nm) 150 mm × 4.6 mm |
| HPLC separation | mobile phase: (1) 0.1% formic acid, 20% MeOH in ultrapure water; (2) 0.1 % formic acid in methanol | mobile phase: (1) 45 % ACN/55 % H2O; (2) methanol, both with 2 mM ammonium acetate and 0.04 % formic acid | mobile phase: methanol/acetonitrile/water (48.5/48.5/3) | mobile phase: (1) Milli-Q water, methylamine (5 mM), and formic acid (0.1 %); (2) methanol, methylamine (5 mM), and formic acid 0.1 % | mobile phase: (1) water, (2) methanol |
| Quantification | 4000 LC-MS/MS System (Sciex): triple quadrupole tandem mass spectrometer | LC-Agilent 1100 with binary pump coupled to a WATERS Quattro Premier XE MSMS system (ESi mode) | Agilent 1290 UHPLC system coupled to an Agilent 6460 triple quadrupole LC/MS/MS ESI operated in positive MRM mode | Agilent 1200 series HPLC coupled to an Agilent 6460 series triple quadrupole mass spectrometer operated in positive MRM mode | Agilent 6410B MS/MS system, APCI, positive ionization mode |
| Ion Massesa | D3 - 560 (383, 298, 280) 25(OH)D3 558 (298, 280, 247) D2 572 (298) 25(OH)D2 N/A |
D3 - 560 (298, 280) 25(OH)D3 593 (298, 280) D2 572 (298, 280) 25(OH)D2 605 (298, 280) |
D3 - 385 (367, 259) 25(OH)D3 401 (383, 365) D2 397 (379, 107) 25(OH)D2 413 (395, 355) |
D3 - 591 (298) 25(OH)D3 607 (298) D2 603 (298) 25(OH)D2 619 (298) |
D3 - 560 (298) 25(OH)D3 558 (298) D2 572 (298) 25(OH)D2 570 (298) |
| Limit of quantification | Vitamin D3/D2: 0.05/0.20 μg/100g; 25(OH)D3: 0.012 μg/100g | high moisture: 0.08 ng/g low moisture: 0.2 ng/g | 0.5 ng/g | <0.1 ng/g | vitamin D3/D2: 0.4/0.3 ng/g 25(OH)D3/D2: 0.2/0.7 ng/g |
Masses in parenthesis are the product ions.
Abbreviations: HPLC, high-performance liquid chromatography; SPE, solid-phase extraction; PTAD, 4-phenyl-1,2,4-triazole-3,5-dione; ACN, acetonitrile; UHPLC, ultra-high-performance liquid chromatography; MS/MS, tandem mass spectrometry; ESi, electrospray ionization; MRM, multiple reaction monitoring
Analytical methods used for the DS
The DS was analyzed by Laboratories A, B, and C (Tables 1a and 1b). Laboratory A analyzed the fill contents, whereas Laboratories B and C analyzed whole softgels. Laboratories A and C analyzed the DS in triplicate. Laboratory B submitted one value for Trial 1 and values from duplicate analysis for Trial 2. Laboratory C conducted both of its trials for 25(OH)D3 on the same day, rather than two months apart. Because Laboratory C used a separate set of material and assay batches for each sample set, the data are reported here for two separate trials, even though the assays were not separated in time as was the case at the other laboratories. Because the 25(OH)D3 content of the DS was similar to that of the foods, the laboratories measured it as described in Table 1a. The DS’s vitamin D3 content was orders of magnitude higher than its 25(OH)D3 content and the vitamin D3 in foods. As a result the laboratories optimized their methodologies to measure the high levels of vitamin D3 in the DS (Table 1b). Laboratory B used LC-MS/MS to measure vitamin D3 but did not derivatize the sample (since it is not necessary for high levels), whereas Laboratories A and C used high-performance liquid chromatography-UV (a less sensitive detection method).
Table 1b.
Methodology for Analysis of Vitamin D3 Content in the Dietary Supplement
| Laboratory A | Laboratory B | Laboratory C | |
|---|---|---|---|
| Subsampling | fill from 30 softgels | 30 softgels dissolved in 20 mL of water at 50 °C | 10 softgels dissolved |
| Internal standard | none | (2H6)vitamin D3 (Chemaphor, Ottawa, Canada) | (3H)vitamin D3 (in-house) |
| Initial treatment | none | 20 mL water at 50 °C; 70 mL 1 % pyrogallol in ethanol added, followed by 50 mL KOH 60 % (w/v) | methanolic KOH |
| Saponification | none | at room temperature overnight under N2 | 2 hours at 60 °C |
| Extraction step | weighed and dissolved in hexane | further extraction of an aliquot of the solution using 3× petroleum ether/diethyl ether (80/20) | 3× hexane/ethyl acetate (85/15) |
| Clean-up step | organic phase is washed with water before evaporation to dryness, dissolution of residue in 2.0 mL 1-chlorobutane | dried and redissolved in 1 mL hexane/methylene chloride | |
| Additional clean-up | purification using HPLC normal phase (silica column) and fractions collection, evaporation of fraction evaporated reconstitution in MeOH | loaded into an SPE cartridge, eluted with methylene chloride/isopropanol, two clean-ups through HPLC columns | |
| HPLC column | Partisil (250 mm × 4.6 mm), 5 micron | ACE-C18 3.0um, 150 × 3.0 mm | Supelco-Sil LC-18 ODS column |
| HPLC separation | mobile phase: (1)1.5 % isopropanol/hexane; (2) 5 % isopropanol/hexane | mobile phase: (1) 50 % methanol/H2O + 0.1% acetic acid; (2) methanol 95 % + isopropanol 5 % + 0.1 % acetic acid | mobile phase: methanol/acetonitrile/water (48.5/48.5/3) |
| Quantification | HPLC-UV detection at 265 nm | LC-MS/MS APCi Agilent 1100 LC coupled to a Thermo Quantum Ultra triple quad MS/MS | HPLC-UV with liquid scintillation counting for internal standard |
| Limit of quantification | 0.05 μg/100g | 700 μg/100 g vitamin D3 | 5 ng by ratio of weight to calculated concentration |
Abbreviations: HPLC, high-performance liquid chromatography; SPE, solid-phase extraction; UV, ultraviolet; LC-MS/MS, liquid chromatography-tandem mass spectrometry; APCi, atmospheric pressure chemical ionization
Vitamin D3 and 25(OH)D3 results for the food materials
Figure 1 and Table 2a summarize the results for vitamin D3 and 25(OH)D3 levels in the foods analyzed. The mean assayed vitamin D3 concentrations ranged from 0.043 μg/100 g (dried bovine liver) to 4.49 μg/100 g (whole egg powder). The mean vitamin D3 levels in the meat homogenate, whole egg powder, and chicken liver were higher than the corresponding 25(OH)D3 levels in these foods, and the 25(OH)D3 levels were higher than the vitamin D3 levels in the dried bovine liver and ground beef.
Figure 1.
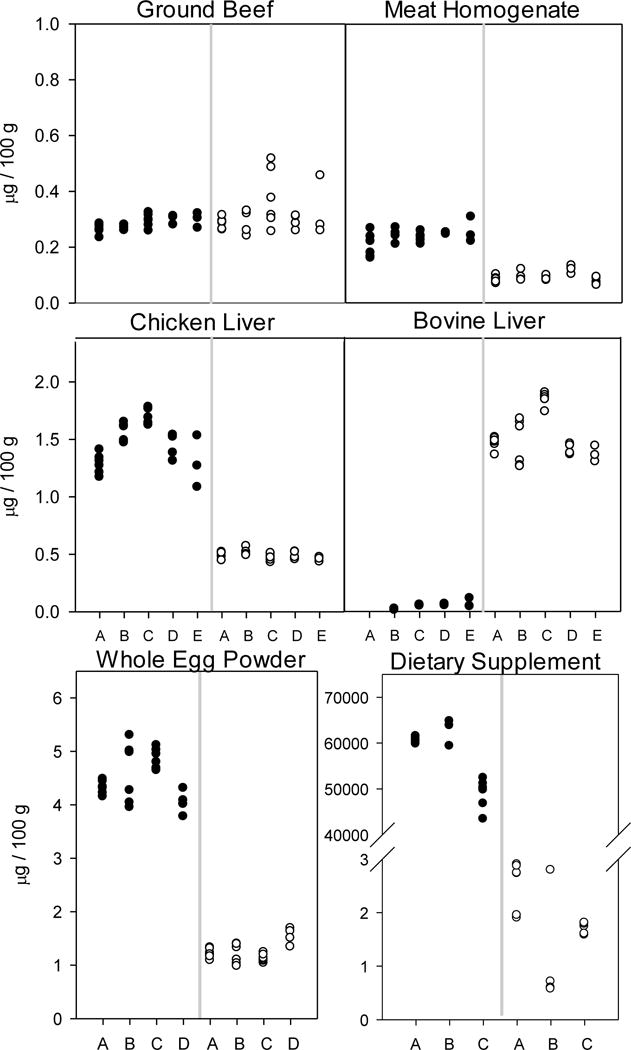
Results for analyses of vitamin D3 and 25(OH)D3 (μg/100 g) levels in six matrices in two trials from five laboratories (A,B,C,D,E). Vitamin D3=black circles; 25(OH)D3=open circles
Table 2a.
|
|
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mean vitamin D3 (μg/100 g)
|
mean 25(OH) D3 (μg/100 g)
|
|||||||||||
| chicken liver | dried bovine liver (NIST 1577c) | whole egg powder (NIST 1845a) | ground beef | meat homogenate (NIST 1546a) | dietary supplement (fill only) | chicken liver | dried bovine liver (NIST 1577c) | whole egg powder (NIST 1845a) | ground beef | meat homogenate (NIST 1546a) | dietary supplement (fill only) | |
| Trial 1 | ||||||||||||
|
| ||||||||||||
| Laboratory A | 1.35 | <0.05c | 4.42 | 0.28 | 0.24 | 61 200 | 0.48 | 1.42 | 1.29 | 0.28 | 0.09 | 2.79 |
| Laboratory B | 1.63 | 0.016 | 5.10 | 0.27 | 0.257 | 59 357 | 0.57 | 1.62 | 1.37 | 0.33 | 0.12 | 1.75 |
| Laboratory C | 1.65 | 0.05 | 5.03 | 0.31 | 0.23 | 51 256 | 0.50 | 1.81 | 1.12 | 0.29 | 0.09 | 1.78d |
| Laboratory D | 1.46 | 0.06 | 4.20 | 0.31 | e | 0.46 | 1.39 | 1.66 | 0.27 | 0.11 | ||
|
| ||||||||||||
| Trial 2 | ||||||||||||
|
| ||||||||||||
| Laboratory A | 1.22 | <0.05c | 4.23 | 0.25 | 0.17 | 60 200 | 0.52 | 1.46 | 1.15 | 0.29 | 0.08 | 2.24 |
| Laboratory B | 1.48 | 0.014 | 4.09 | 0.28 | 0.23 | 64 280 | 0.50 | 1.26 | 1.04 | 0.25 | 0.09 | 0.58 |
| Laboratory C | 1.72 | 0.05 | 4.71 | 0.28 | 0.24 | 46 653 | 0.45 | 1.83 | 1.14 | 0.46 | 0.08 | 1.66e |
| Laboratory D | 1.42 | 0.05 | 3.90 | 0.29 | 0.25 | 0.52 | 1.39 | 1.46 | 0.31 | 0.13 | ||
| Laboratory E | 1.29 | 0.07 | 0.30 | 0.26 | 0.46 | 1.34 | 0.33 | 0.08 | ||||
|
| ||||||||||||
| combined | ||||||||||||
|
| ||||||||||||
| mean | 1.47 | 0.043 | 4.49 | 0.284 | 0.233 | 56 390 | 0.495 | 1.51 | 1.25 | 0.315 | 0.093 | 1.82 |
| SD | 0.20 | 0.025 | 0.45 | 0.023 | 0.034 | 7160 | 0.041 | 0.21 | 0.20 | 0.074 | 0.019 | 0.82 |
| reproducibility% RSD | 13.3 | 58.0 | 10.0 | 8.0 | 14.8 | 12.7 | 8.3 | 14.1 | 16.0 | 23.5 | 20.7 | 45.1 |
| Horwitz ratio | 0.4 | 1.1 | 0.4 | 0.2 | 0.4 | 2.1 | 0.2 | 0.5 | 0.5 | 0.6 | 0.5 | 1.6 |
| repeatability % RSD | 6.9 | 38.7 | 2.8 | 6.2 | 9.7 | 2.9 | 3.4 | 3.6 | 5.4 | 15.6 | 10.6 | 14.8 |
Shaded cells indicate no data reported.
Values for foods in this table are means from triplicate analyses within each trial reported by Laboratories A, B, C and E and duplicate analyses from Laboratory D.
Laboratory A reported a vitamin D3 level of <0.05 for dried bovine liver (NIST 1577c) in Trial 1 and Trial 2, which is lower than the laboratory’s limit of quantification. Therefore, these values were omitted from mean and SD calculations.
Laboratory C conducted both of its trials on the same day, not two months apart as the other laboratories did.
Laboratory D indicated uncertainty about the quality of its data on meat homogenate (NIST 1546a). Therefore, this value was considered an outlier and excluded from the results reported here.
Abbreviations: NIST, National Institute of Standards and Technology (Gaithersburg, MD); SD, standard deviation; RSD, relative standard deviation
The mean assayed 25(OH)D3 concentrations ranged from 0.093 μg/100 g (meat homogenate) to 1.51 μg/100 g (dried bovine liver). Dried bovine liver had the lowest vitamin D3 content of all materials analyzed (0.043 μg/100 g), near the limit of quantitation for all of the laboratories. However, dried bovine liver had the highest 25(OH)D3 content (1.51 μg/100 g) of the food materials.
The reproducibility for vitamin D3 ranged from 8% (ground beef) to 58% RSD (dried bovine liver; predicted reproducibility RSD for this very low analyte concentration is 51.28%). The corresponding HorRat values were all acceptable, ranging from 0.2 to 1.1. Repeatability for vitamin D3 ranged from 2.8% RSD (whole egg powder) to 38.7% RSD (dried bovine liver; predicted repeatability RSD for this low analyte concentration is 25.64%, Table 2a).
The reproducibility for 25(OH)D3 ranged from 8.3 % RSD (chicken liver) to 23.5 % RSD (ground beef). The HorRat values ranged from 0.2 (chicken liver) to 0.6 (ground beef). Repeatability for 25(OH)D3 ranged from 3.4 % RSD (chicken liver) to 15.6 % RSD (ground beef; Table 2a).
Vitamin D3 and 25(OH)D3 results for the DS
The mean assayed concentration of vitamin D3 in the DS was 56,400 μg/100 g. The mean 25(OH)D3 value was 1.82 μg/100 g, which was comparable to the levels found in the foods. The mean vitamin D3 amount for the DS in this study was 14.8% above the labeled level (% difference for screened supplements ranged from 10.8 to 57.5% (see Supplemental Table 2). Reproducibility was 12.7% RSD for vitamin D3 and 45.1 % RSD for 25(OH)D3. Repeatability was 2.9 % RSD for vitamin D3 and 14.8 % RSD for 25(OH)D3. For comparison, predicted reproducibility and repeatability RSDs for 25(OH)D3 were 40.93 % and 20.46%, respectively. However, the three laboratories handled the DS differently. Two laboratories dissolved the capsules and provided the data in μg per 100 g (whole capsule). The other laboratory cut the capsules open, emptied the contents, and provided the data in μg per 100 g of fill as well as the total weights of the fill and capsule shell. The weight data were applied to the first laboratory’s results to calculate the results in μg per 100 g of fill for direct comparison. For the DS results reported based on fill weight, the HorRat value was 2.1 for vitamin D3 and 1.6 for 25(OH)D3 (see Table 2a). The HorRat for the DS results based on the weight of the whole capsule were 1.8 and 1.3 respectively (see Supplemental Table 3).
Vitamin D2 and 25(OH)D2 results for the foods and DS
Only three laboratories reported results for vitamin D2 and 25(OH)D2 levels, and only Laboratory B reported values for vitamin D2 and 25(OH)D2 in all materials. Therefore, results for vitamin D2 and 25(OH)D2 are provided for descriptive purposes only, and only values above the limit of quantification are reported (Table 2b). The vitamin D2 level reported by Laboratory B was below the limit of quantitation in all materials except the ground beef, and 25(OH)D2 was measurable only in the ground beef and dried bovine liver. The vitamin D2 and 25(OH)D2 levels reported by Laboratory B were considerably lower than those of vitamin D3 and 25(OH)D3 (<0.1 μg/100 g).
Table 2b.
Vitamin D2 and 25(OH)D2 in Dried Bovine Liver and Ground Beef Measured by Three Participating Laboratoriesa,b
|
|
|
|||
|---|---|---|---|---|
| mean Vitamin D2 (μg/100g) |
mean 25(OH)D2 (μg/100g) |
|||
| dried bovine liver (NIST 1577c) |
ground beef | dried bovine liver (NIST 1577c) |
ground beef | |
| Trial 1 | ||||
|
| ||||
| Laboratory B | <0.01 | 0.071 | 0.08 | 0.072 |
| Laboratory D | 0.067 | 0.053 | 0.0435 | |
|
| ||||
| Trial 2 | ||||
|
| ||||
| Laboratory B | <0.03 | 0.078 | 0.07 | 0.073 |
| Laboratory D | 0.055 | 0.040 | 0.044 | |
| Laboratory E | 0.033 | 0.184 | 0.063 | 0.090 |
|
| ||||
| combined | ||||
|
| ||||
| mean | 0.099 | 0.064 | 0.067 | |
| SD | 0.058 | 0.015 | 0.024 | |
| reproducibility% RSD | 59.0 | 24.2 | 35.2 | |
| Horwitz ratio | 1.3 | 0.5 | 0.7 | |
| repeatability % RSD | 27.6 | 22.3 | 13.4 | 22.7 |
Shaded cells indicate no data reported or not applicable.
Values for foods in this table represent means from triplicate analyses within trials reported by Laboratories B and E and duplicate analyses from Laboratory D.
‐ Abbreviations: NIST, National Institute of Standards and Technology (Gaithersburg, MD); <, value below laboratory’s limit of quantitation; SD, standard deviation; RSD, relative standard deviation
DISCUSSION
For the foods in this study, the HorRat values for vitamin D3 and 25(OH)D3 (ranging from 0.2 to 1.1) demonstrated acceptable between-laboratory reproducibility at the analyte concentration present for all of the matrices (ground beef, chicken liver, whole egg powder, meat homogenate, and dried bovine liver). In our study, the low repeatability RSDs for vitamin D3 and for 25(OH)D3, seen in all the matrices except the vitamin D3 in the bovine liver, suggest that the laboratories had consistent within-laboratory results and, thus, acceptable within-laboratory precision for all study materials. Therefore, our study shows that it is possible to obtain results with acceptable overall reproducibility among experienced laboratories using LC-MS/MS to measure levels of vitamin D3 and 25(OH)D3 for a variety of food matrices having naturally occurring vitamin D3 and 25(OH)D3.
For the DS, the three laboratories demonstrated that they were able to measure vitamin D3 at high levels as well as very low levels of 25(OH)D3. The HorRat values of 2.1 and 1.8 for vitamin D3 and 1.6 and 1.3 for 25(OH)D3 indicate acceptable or close to acceptable reproducibility relative to the analyte concentration. The elevated RSDs obtained experimentally are close to theoretically predicted RSDs at such low analyte concentrations, and this is reflected in acceptable HorRat ratios. For example, the % RSD for the very low level 25(OH)D3 in bovine liver seems very high. However, it is an acceptable RSD because as the concentration decreases, the predicted RSD increases, as illustrated by the Horwitz curve.37
The amount of 25(OH)D3 in the vitamin D supplement was very low but was consistently present in all DS analyzed in the screening and in the study. Vitamin D levels above label claims (overages) were seen in the DS selected for this study and in the products screened for inclusion in this study. Manufacturers may add ingredient overages to provide the minimum content stated on the label at the end of the product shelf life.39
Vitamin D2 and 25(OH)D2 were found in small quantities in the ground beef and bovine liver, with acceptable HorRat ratios, although our study’s focus was on measuring vitamin D3 and 25(OH)D3. Future studies need to identify whether different samples of beef in the food supply have variable vitamin D2 content and identify sources of vitamin D2 in food samples such as components of animals’ diets.
The source of 25(OH)D3 in meat, poultry, and eggs could be due to the animal’s exposure to UV light, resulting in in vivo production of vitamin D3, or derived from the animal’s feed containing vitamin D3 or 25(OH)D3. Thus, variation in animal diet and environment can affect vitamin D3 and 25(OH)D3 levels in animal-based foods.21, 40–48 Unfortunately, data on the 25(OH)D3 content of foods are limited and existing data can vary greatly.21,49, 50
Comparison of studies of 25(OH)D3 in foods is hindered by the lack of relevant certified reference materials,51 which makes it difficult to ascertain whether the differences between studies are due to methodological or physiological factors. Matrix-matched reference or control samples make it possible to quantify the contribution of analytical differences that are separate from differences in food composition. Developing reference materials for use in analyses, for foods and for DSs, is relevant to ensuring the trueness of vitamin D3 and 25(OH)D3 estimates. The data from this study will be used to help assign reference values for vitamin D3 and 25(OH)D3 in several NIST SRMs—Bovine Liver (SRM 1577c), Meat Homogenate (SRM 1546a), and Whole Egg Powder (SRM 1845a).52 As for reference values for DSs, this study provided data on a vitamin D product that could be used as in-house control material in future studies.
Our study had several limitations. Because it included only experienced analysts, validated methods, and appropriate equipment to obtain results, we cannot draw conclusions about the likely performance of other laboratories in analyzing the same materials. We also did not seek to quantify any bias between laboratories. Although the overall reproducibility was acceptable for all materials, some systematic bias between laboratories could have affected the comparison of nutrient content results for foods analyzed at different laboratories.
Continued efforts to determine amounts of vitamin D and 25(OH)D in foods and supplements using validated methodologies and well-characterized control and reference materials will help answer questions about discrepancies between circulating amounts of 25(OH)D in the body and vitamin D intakes calculated from composition data for foods and DSs. These endeavors can also help ensure that public health policy decisions about vitamin D are based on accurate estimates of intake.
Supplementary Material
Acknowledgments
The authors thank Covance, Heartland, Health Canada, Technical University of Denmark, and Virginia Tech laboratory personnel for their participation and significant contributions to this study. We also acknowledge the analysts for their expertise, including Carolyn Burdette from NIST. We thank Will Guthrie from NIST for his statistical advice on the intra- and interlaboratory results and Debby Berlyne for her professional editorial contributions.
FUNDING SOURCE
Partial funding for this work was provided under Agreement 60 1235 3012 from ODS.
ABBREVIATIONS USED
- 25(OH)D
25-hydroxyvitamin D
- DS
dietary supplement
- USDA
U.S. Department of Agriculture
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- NDL
Nutrient Data Laboratory
- NIST
National Institute of Standards and Technology
- SRM
standard reference material
- RSD
relative standard deviation
- HorRat
Horwitz ratio
Footnotes
SUPPORTING INFORMATION AVAILABLE
Descriptions and analytical results of seven dietary supplements screened for vitamin D3 and 25(OH)D3, and per-capsule analytical results from three labs for the dietary supplement in the study are available free of charge via the Internet at http://pubs.acs.org.
Trade names are included for the reader’s information only and are not meant to imply endorsement or preferential treatment by the US Government.
References
- 1.Grober U, Spitz J, Reichrath J, Kisters K, Holick MF. Vitamin D: Update 2013: From rickets prophylaxis to general preventive healthcare. Dermatoendocrinol. 2013;5:331–347. doi: 10.4161/derm.26738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wacker M, Holick MF. Sunlight Vitamin D: A global perspective for health. Dermatoendocrinol. 2013;5:51–108. doi: 10.4161/derm.24494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 4.Au LE, Rogers GT, Harris SS, Dwyer JT, Jacques PF, Sacheck JM. Associations of vitamin D intake with 25-hydroxyvitamin D in overweight racially/ethnically diverse US children. J Acad Nutr Diet. 2013;113:1511–1516. doi: 10.1016/j.jand.2013.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobs ET, Alberts DS, Benuzillo J, Hollis BW, Thompson PA, Martinez ME. Serum 25(OH)D levels dietary intake of vitamin D colorectal adenoma recurrence. J Steroid Biochem Mol Biol. 2007;103:752–756. doi: 10.1016/j.jsbmb.2006.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Messenger W, Nielson CM, Li H, Beer T, Barrett-Connor E, Stone K, Shannon J. Serum dietary vitamin D cardiovascular disease risk in elderly men: a prospective cohort study. Nutr Metab Cardiovasc Dis. 2012;22:856–863. doi: 10.1016/j.numecd.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motsinger S, Lazovich D, MacLehose RF, Torkelson CJ, Robien K. Vitamin D intake mental health-related quality of life in older women: the Iowa Women’s Health Study. Maturitas. 2012;71:267–273. doi: 10.1016/j.maturitas.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pludowski P, Holick MF, Pilz S, Wagner CL, Hollis BW, Grant WB, Shoenfeld Y, Lerchbaum E, Llewellyn DJ, Kienreich K, Soni M. Vitamin D effects on musculoskeletal health immunity autoimmunity cardiovascular disease cancer fertility pregnancy dementia and mortality-a review of recent evidence. Autoimmun Rev. 2013;12:976–989. doi: 10.1016/j.autrev.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Holick MF. Vitamin D photobiology, metabolism, and clinical applications. In: DeGroot LJ, editor. Endocrinology. 3rd. Vol. 2. WB Saunders; Philadelphia, PA: 1995. pp. 990–1013. [Google Scholar]
- 10.Tanaka Y, Frank H, DeLuca HF. Biological activity of 1,25-dihydroxyvitamin D3 in the rat. Endocrinology. 1973;92:417–422. doi: 10.1210/endo-92-2-417. [DOI] [PubMed] [Google Scholar]
- 11.Cashman KD, Seamans KM, Lucey AJ, Stocklin E, Weber P, Kiely M, Hill TR. Relative effectiveness of oral 25-hydroxyvitamin D3 vitamin D3 in raising wintertime serum 25-hydroxyvitamin D in older adults. Am J Clin Nutr. 2012;95:1350–1356. doi: 10.3945/ajcn.111.031427. [DOI] [PubMed] [Google Scholar]
- 12.Jetter A, Egli A, Dawson-Hughes B, Staehelin HB, Stocklin E, Gossl R, Henschkowski J, Bischoff-Ferrari HA. Pharmacokinetics of oral vitamin D3 calcifediol. Bone. 2014;59:14–19. [PubMed] [Google Scholar]
- 13.Ovesen L, Brot C, Jakobsen J. Food contents biological activity of 25-hydroxyvitamin D: a vitamin D metabolite to be reckoned with? Ann Nutr Metab. 2003;47:107–113. doi: 10.1159/000070031. [DOI] [PubMed] [Google Scholar]
- 14.Institute of Medicine. Dietary reference intakes for calcium and vitamin D. National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- 15.Taylor CL, Carriquiry AL, Bailey RL, Sempos CT, Yetley EA. Appropriateness of the probability approach with a nutrient status biomarker to assess population inadequacy: a study using vitamin D. Am J Clin Nutr. 2013;97:72–78. doi: 10.3945/ajcn.112.046094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonnell SL, French CB, Heaney RP. Quantifying the food sources of basal vitamin d input. J Steroid Biochem Mol Biol. 2014;144(Pt A):149–151. doi: 10.1016/j.jsbmb.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 17.US Department of Agriculture National Nutrient Database for Standard Reference (accessed July 23, 2015) http://ndb.nal.usda.gov.
- 18.Holden JM, Lemar LE, Exler J. Vitamin D in foods: development of the US Department of Agriculture database. Am J Clin Nutr. 2008;87:1092S–1096S. doi: 10.1093/ajcn/87.4.1092S. [DOI] [PubMed] [Google Scholar]
- 19.Saxholt E, Christensen AT, Møller A, Hartkopp HB, Hess Ygil K, Hels OH. Danish Food Composition Databank, revision 7 (accessed August 19, 2015) http://www.foodcomp.dk.
- 20.Public Health England McCance and Widdowson’s ’composition of foods integrated dataset’ on the nutrient content of the UK food supply (accessed August 19, 2015) https://www.gov.uk/government/publications/composition-of-foods-integrated-dataset-cofid/.
- 21.Mattila PH, Valkonen E, Valaja J. Effect of different vitamin D supplementations in poultry feed on vitamin D content of eggs chicken meat. J Agric Food Chem. 2011;59:8298–8303. doi: 10.1021/jf2012634. [DOI] [PubMed] [Google Scholar]
- 22.Jakobsen J, Clausen I, Leth T, Ovesen L. A new method for the determination of vitamin D3 25-hydroxyvitamin D3 in meat. Journal of Food Composition and Analysis. 2004;17:777–787. [Google Scholar]
- 23.Huang M, LaLuzerne P, Winters D, Sullivan D. Measurement of vitamin D in foods nutritional supplements by liquid chromatography/tandem mass spectrometry. J AOAC Int. 2009;92:1327–1335. [PubMed] [Google Scholar]
- 24.Burild A, Frandsen HL, Poulsen M, Jakobsen J. Quantification of physiological levels of vitamin D(3) 25-hydroxyvitamin D(3) in porcine fat liver in subgram sample sizes. J Sep Sci. 2014;37:2659–2663. doi: 10.1002/jssc.201400548. [DOI] [PubMed] [Google Scholar]
- 25.Bilodeau L, Dufresne G, Deeks J, Clément G, Bertrand J, Turcotte S, Robichaud A, Beraldin F, Fouquet A. Determination of vitamin D3 25-hydroxyvitamin D3 in foodstuffs by HPLC UV-DAD LC–MS/MS. J Food Comp Anal. 2011;24:441–448. [Google Scholar]
- 26.Strobel N, Buddhadasa S, Adorno P, Stockham K, Greenfield H. Vitamin D 25-hydroxyvitamin D determination in meats by LC-IT-MS. Food Chem. 2013;138:1042–1047. doi: 10.1016/j.foodchem.2012.08.041. [DOI] [PubMed] [Google Scholar]
- 27.Byrdwell WC, Horst RL, Phillips KM, Holden JM, Patterson KY, Harnly JM, Exler J. Vitamin D levels in fish shellfish determined by liquid chromatography with ultraviolet detection mass spectrometry. J Food Comp Anal. 2013;30:109–119. [Google Scholar]
- 28.Phillips KM, Craig Byrdwell W, Exler J, Harnly JM, Holden JM, Holick MF, Hollis BW, Horst RL, Lemar LE, Patterson KY, Tarrago-Trani MT, Wolf WR. Development validation of control materials for the measurement of vitamin D3 in selected US foods. J Food Comp Anal. 2008;21:527–534. [Google Scholar]
- 29.Haytowitz DB, Pehrsson PR, Holden JM. The National Food Nutrient Analysis Program: A decade of progress. J Food Compost Anal. 2008;21:S94–S102. doi: 10.1016/j.jfca.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips KM, Patterson KY, Rasor AR, Exler J, Haytowitz DM, Holden JM, Pehrsson PR. The role of quality control reference materials in the National Food Nutrient Analysis Program. Anal Bioanal Chem. 2006;384:1341–1355. doi: 10.1007/s00216-005-0294-0. [DOI] [PubMed] [Google Scholar]
- 31.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19:73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.U.S. Pharmacopeia. Dietary Supplements Official Monographs. Vol. 1. U.S Pharmacopeia; Rockville, MD: 2012. Oil-soluble vitamins capsules, USP36; pp. 1622–1628. [Google Scholar]
- 33.U.S. Pharmacopeia. Dietary Supplements Official Monographs. Vol. 1. U.S Pharmacopeia; Rockville, MD: 2012. Oil-soluble vitamins with minerals tablets, USP36; pp. 1654–1664. [Google Scholar]
- 34.US Pharmacopeia 38(4) in-process revision: cholecalciferol capsules [NEW] (USP36-NF31 2S) (accessed August 21, 2015) http://www.usppf.com/pf/pub/index.html.
- 35.International Organization for Standardization. 5725-2:1994. Accuracy (trueness and precision) of measurement methods and results – Part 2: Basic method for the determination of repeatability and reproducibility of a standard measurement method. ISO copyright office; Geneva, Switzerland: 1994. [Google Scholar]
- 36.AOAC International Guidelines and References: Appendix F: Guidelines for Standard Method Performance Requirements. 2012 (accessed February 4, 2014). http://www.eoma.aoac.org/app_f.pdf.
- 37.Horwitz W, Albert R. The Horwitz ratio (HorRat): A useful index of method performance with respect to precision. J AOAC Int. 2006;89:1095–1109. [PubMed] [Google Scholar]
- 38.Massart DL, Smeyers-Verbeke J, Vander Heyden Y. Benchmarking for analytical methods: the Horwitz curve. LCGC Europe. 2005;18:528–531. [Google Scholar]
- 39.LeDoux MA, Appelhans KR, Braun LA, Dziedziczak D, Jennings S, Liu L, Osiecki H, Wyszumiala E, Griffiths J. A quality dietary supplement: before you start and after it’s marketed–a conference report. Eur J Nutr. 2015;54(Suppl 1):S1–8. doi: 10.1007/s00394-014-0827-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuhn J, Schutkowski A, Kluge H, Hirche F, Stangl GI. Free-range farming: a natural alternative to produce vitamin D-enriched eggs. Nutrition. 2014;30:481–484. doi: 10.1016/j.nut.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Casas E, Lippolis JD, Kuehn LA, Reinhardt TA. Seasonal variation in vitamin D status of beef cattle reared in the central United States. Domest Anim Endocrinol. 2015;52:71–74. doi: 10.1016/j.domaniend.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 42.Sunita Rao D, Raghuramulu N. Food chain as origin of vitamin D in fish. Comp Biochem Physiol A Physiol. 1996;114:15–19. [Google Scholar]
- 43.Cherian G. Eggs and health: nutrient sources and supplement carriers. Eggs and health: nutrient sources and supplement carriers. In: Watson RR, editor. Complementary and Alternative Therapies and the Aging Population. Academic Press; New York, NY: 2009. pp. 333–346. [Google Scholar]
- 44.Lu Z, Chen TC, Zhang A, Persons KS, Kohn N, Berkowitz R, Martinello S, Holick MF. An evaluation of the vitamin D3 content in fish: Is the vitamin D content adequate to satisfy the dietary requirement for vitamin D? J Steroid Biochem Mol Biol. 2007;103:642–644. doi: 10.1016/j.jsbmb.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kassis N, Drake SR, Beamer SK, Matak KE, Jaczynski J. Development of nutraceutical egg products with omega-3-rich oils. LWT Food Sci Technol. 2010;43:777–783. [Google Scholar]
- 46.Schiavone A, Barroeta AC. Egg enrichment with vitamins and trace minerals. In: Van Immerseel F, Nys Y, Bain M, editors. Improving the Safety and Quality of Eggs and Egg Products: Egg Safety and Nutritional Quality. Woodhead Publishing Limited; Cambridge, United Kingdom: 2011. pp. 289–320. [Google Scholar]
- 47.Jakobsen J, Saxholt E. Vitamin D metabolites in bovine milk butter. Journal of Food Composition and Analysis. 2009;22:472–478. [Google Scholar]
- 48.Jakobsen J, Maribo H, Bysted A, Sommer HM, Hels O. 25-hydroxyvitamin D3 affects vitamin D status similar to vitamin D3 in pigs–but the meat produced has a lower content of vitamin D. The British journal of nutrition. 2007;98:908–913. doi: 10.1017/S0007114507756933. [DOI] [PubMed] [Google Scholar]
- 49.Exler J, Phillips KM, Patterson KY, Holden JM. Cholesterol vitamin D content of eggs in the U.S. retail market. J Food Comp Anal. 2013;29:110–116. [Google Scholar]
- 50.Clausen I, Jakobsen J, Leth T, Ovesen L. Vitamin D3 25-hydroxyvitamin D3 in raw cooked pork cuts. Journal of Food Composition and Analysis. 2003;16:575–585. [Google Scholar]
- 51.Schmid A, Walther B. Natural vitamin D content in animal products. Adv Nutr. 2013;4:453–462. doi: 10.3945/an.113.003780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.National Institute of Standards and Technology Standard Reference Materials (accessed May 22, 2015) http://www.nist.gov/srm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


