Abstract
Background and objectives
Atherosclerotic renal artery stenosis may cause kidney function loss, but effects of stenting on eGFR and clinical events associated with CKD are uncertain. Our study objectives were to determine effects of stenting on eGFR and predictors of clinical events.
Design, setting, participants, & measurements
Participants (n=931) in the Cardiovascular Outcomes in Renal Artery Stenosis Trial (from May of 2005 to September of 2012) had >60% atherosclerotic renal artery stenosis and systolic hypertension on two or more antihypertensive drugs and/or stage ≥3 CKD. The intervention was stenting versus no stenting on a background of risk factor management: renin-angiotensin system inhibition, statin, antiplatelet therapy, and smoking cessation education. The effect of stenting on eGFR by the serum creatinine-cystatin C Chronic Kidney Disease Epidemiology Collaboration equation was the prespecified analysis of kidney function. Predictors of eGFR and CKD outcomes (≥30% eGFR loss, ESRD, and death) and cardiovascular disease outcomes (stroke, myocardial infarction, heart failure, and death) controlling for eGFR and albuminuria were also determined.
Results
eGFR was 59±24 ml/min per 1.73 m2 (mean±SD) at baseline. Over 3 years, eGFR change, assessed by generalized estimating equations, was −1.5±7.0 ml/min per 1.73 m2 per year in the stent group versus −2.3±6.3 ml/min per 1.73 m2 per year in the medical therapy only group (P=0.18). eGFR predictors (multiple variable generalized estimating equations) were age, albuminuria, systolic BP, and diabetes (inverse associations) as well as men, total cholesterol, and HDL cholesterol (positive associations). CKD outcomes events occurred in 19% (175 of 931), and predictors (Cox proportional hazards models) included albuminuria (positive association), systolic BP (positive association), and HDL cholesterol (inverse association). Cardiovascular disease outcomes events occurred in 22% (207 of 931), and predictors included age, albuminuria, total cholesterol, prior cardiovascular disease, and bilateral atherosclerotic renal artery stenosis (positive associations).
Conclusions
Stenting did not influence eGFR in participants with atherosclerotic renal artery stenosis receiving renin-angiotensin system inhibition–based therapy. Predictors of clinical events were traditional risk factors for CKD and cardiovascular disease.
Keywords: chronic kidney disease, cardiovascular disease, glomerular filtration rate, renin angiotensin system, albuminuria, blood pressure, hypertension, Renal Artery Obstruction, Stents
Introduction
Atherosclerotic renal artery stenosis (ARAS) may cause kidney function loss, leading to CKD, cardiovascular disease (CVD), and death (1–4). The effect of renal artery revascularization by stenting on kidney function is unclear, even after recent randomized trials, because of highly selected study populations, crossovers from medical therapy to stenting, and lack of power or ascertainment (5–9). Moreover, predictors of kidney function over time have not been previously determined in patients with ARAS.
In the Cardiovascular Outcomes in Renal Artery Stenosis (CORAL) Clinical Trial, patients with ARAS were randomized to medical therapy plus stenting or medical therapy only (10). Medical therapy was designed to achieve optimal targets for CKD and CVD risk factors, including hypertension, increased LDL cholesterol, hyperglycemia, platelet aggregation, and smoking. Risk factor management was target driven with add-on therapies on the basis of clinical practice guidelines current at the time of the study (11–14). Revascularization by stenting did not reduce the primary outcome, a composite of clinical events, including death, myocardial infarction, stroke, hospitalization for congestive heart failure, ESRD, and ≥30% reduction in eGFR.
The CORAL Trial had a large international sample of participants with ARAS, which provided a unique opportunity to determine effects of stenting on eGFR and predictors of eGFR over time. Additional study objectives were to ascertain predictors of clinical events associated with CKD.
Materials and Methods
Study Participants and Enrollment
The CORAL Trial enrollment began on May 16, 2005 and concluded on January 30, 2012, with follow-up until September 28, 2012 (ClinicalTrials.gov; NCT00081731) (10). The sample size was calculated for 90% power to detect a 25% reduction in incidence of the primary outcome. The trial included multiple sites with international locations (North America, South America, Europe, Australia/New Zealand, and Africa). The study protocol adhered to the principles of the Declaration of Helsinki and was approved by institutional review boards or ethics committees at each participating site. All participants signed written informed consent to participate. They had ARAS with >60% stenosis and systolic hypertension on two or more antihypertensive drugs and/or CKD stage ≥3. ARAS was identified by renal angiography, duplex ultrasonography, magnetic resonance angiography, or computed tomographic angiography. Exclusion criteria were fibromuscular dysplasia, CKD attributable to other causes, serum creatinine >4 mg/dl, kidney length <7 cm, or stenoses that could not be treated with a single stent. Renal angiograms were analyzed by an angiographic core laboratory (10). Global ischemia was defined as >60% bilateral stenoses or >60% stenosis to a single functioning kidney (10).
Randomization and Interventions
Participants with ARAS were randomized in a 1:1 ratio to medical therapy plus stenting or medical therapy only by means of an interactive voice randomization system with a permuted block design (the Consolidated Standards of Reporting Trials diagram) (Supplemental Figure 1) (10). Participants, investigators, and study coordinators were not masked to group assignment. Both groups received antihypertensive therapy with a stepwise approach to achieve the BP target starting with a renin-angiotensin system (RAS) inhibitor and either an angiotensin receptor blocker or an angiotensin–converting enzyme inhibitor (10). They also received a statin adjusted to achieve the LDL cholesterol target, aspirin at 81–325 mg daily, and clopidogrel at 75 mg daily for those receiving stents (according to local standard of care). Goals for risk factors included BP<130/80 mmHg with CKD or diabetes and <140/90 mmHg otherwise, LDL cholesterol <70 mg/dl, hemoglobin A1c of approximately 7% (diabetes subgroup), and smoking cessation by an education program (11–14). Candesartan (16–32 mg) with or without hydrochlorothiazide (12.5–50 mg; Astra Zeneca) and atorvastatin-amlodipine (10–80/2.5–10 mg; Pfizer Inc.) were provided to participants through use of a voucher system at local pharmacies.
For participants randomized to stenting, each renal artery with >60% stenosis was stented (Palmaz–Genesis Stent; Cordis Corporation) (10). An Angioguard embolic protection device was required until August of 2006, when embolic protection was subsequently used at operator discretion (10). Crossovers from the medical therapy only group to stenting were allowed only on approval by the CORAL Trial crossover committee after occurrence of a primary outcome event.
Study Outcomes
The effect of stenting on eGFR was a prespecified secondary analysis in the CORAL Trial protocol. Post hoc analyses were formulated for assessing predictors of eGFR and clinical event outcomes associated with CKD. For the rate of change in eGFR, the post hoc power was estimated at 40%. eGFR was calculated by the serum creatinine-cystatin C Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation, because this method is considered the most accurate and precise, even at eGFR levels >60 ml/min per 1.73 m2, on the basis of clinically available filtration markers, serum creatinine, and cystatin C (15). eGFR was also calculated by other equations: serum creatinine CKD-EPI, serum creatinine Modification of Diet in Renal Disease (MDRD), and serum cystatin C CKD-EPI. CKD events (≥30% eGFR loss for ≥60 days, ESRD, and death caused by kidney disease) and CVD events (stroke, myocardial infarction, congestive heart failure hospitalizations, and death caused by CVD) were prespecified in the study protocol (10). The definition of the eGFR end point to ≥30% loss was adjusted before unmasking or awareness of event rates with the approval of the US Food and Drug Administration because of changes in clinical outcome definitions during the trial. Death caused by kidney disease was defined as death attributable to uremia, a dialysis procedure, acute renal failure, renal transplant, hyperkalemia in association with renal insufficiency, and other complications of renal failure in the study protocol. An independent clinical events committee, whose members were unaware of randomization assignment, adjudicated all study events.
Statistical Analyses
Analyses were on the basis of the intent to treat principle. Differences in baseline characteristics, laboratory values, risk factors, and lesion-based measures were assessed by unpaired t tests and chi-squared tests for continuous and categorical variables, respectively. eGFR over time was evaluated by univariate and multiple variable generalized estimating equations (GEEs). Predictors of CKD and CVD events were assessed by Cox proportional hazards models. Multiple variable models were treatment adjusted (stent versus no stent) with age and sex forced into the models. Other covariates were derived from baseline characteristics with stepwise selection for multiple variable analyses with entry and stay criteria of P<0.10. Kaplan–Meier survival plots were constructed and evaluated with the log rank test for analyses of CKD or CVD events. Statistical significance was set at α with P<0.05. All analyses were performed using SAS, version 9.1.3 (SAS Institute Inc., Cary, NC).
Results
Study Participants
From the parent study, 931 CORAL Trial participants (stent plus medical therapy group, n=459; medical therapy only group, n=472) had data for inclusion in the analyses of eGFR and clinical event outcomes. The CORAL Trial participants were 69±9 (mean±SD) years of age (Table 1). Women (466 of 931) and men (465 of 931) were equally represented. Baseline BP was 150±23/79±13 mmHg on 2.1±1.6 antihypertensive agents (Table 2). One third (310 of 929) had diabetes, and 59% (526 of 890) of participants had CKD stage ≥3. CKD stage 4 was present in 8% (69 of 890). Bilateral ARAS was present in 20% (155 of 760). In the group assigned to receive stenting, arterial dissection occurred in 11 participants, and one required dialysis between 30 and 90 days after the procedure. One participant assigned to the medical therapy only group had a stroke on the day of randomization (10).
Table 1.
Demographics and baseline kidney function measures for the Cardiovascular Outcomes in Renal Artery Stenosis Trial participants by randomization group
| Measures | Stent Plus Medical Therapy, n=459 | Medical Therapy Only, n=472 | Difference [95% CI] | P Value |
|---|---|---|---|---|
| Age, yr | ||||
| Mean±SD (N) | 69±9 (459) | 69±9 (472) | 0.3 [−0.9 to 1.5] | 0.60 |
| No. of men | 51% (234/459) | 49% (231/472) | 2 [−4 to 8] | 0.56 |
| Hispanic or Latino | 5.2% (24/459) | 6.6% (31/472) | −1.3 [−4.4 to 1.7] | 0.41 |
| Race | 0.96 | |||
| American Indian or Alaska Native | 0.2% (1/459) | 0.2% (1/472) | 0.0 [−0.6 to 0.6] | |
| Asian | 1.1% (5/459) | 1.5% (7/472) | −0.4 [−1.8 to 1.1] | |
| Black | 7.0% (32/459) | 7.0% (33/472) | −0.0 [−3.3 to 3.3] | |
| Native Hawaiian or Pacific Islander | 0.2% (1/459) | 0.4% (2/472) | −0.2 [−0.9 to 0.5] | |
| White | 91.5% (420/459) | 90.9% (429/472) | 0.6 [−3.0 to 4.3] | |
| Serum creatinine, mg/dl | ||||
| Mean±SD (N) | 1.26±0.46 (436) | 1.25±0.47 (454) | 0.01 [−0.05 to 0.07] | 0.78 |
| Creatinine-cystatin C CKD-EPI eGFR (ml/min per 1.73 m2) | ||||
| Mean±SD (N) | 59±24 (436) | 59±23 (454) | 0.1 [−3.0 to 3.1] | 0.96 |
| Urine albumin-to-creatinine ratio, mg/g | ||||
| Median (IQR) | 24 (10–98.0) | 21 (9–75) | 2 [−4 to 8] | 0.29 |
| CKD stage ≥3 | 60% (261/436) | 58% (265/454) | −3 [−10 to 3] | 0.47 |
P values for comparisons are generated with two–sample t tests for continuous measurements and chi-squared tests for binary measurements. 95% CI, 95% confidence interval; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; IQR, interquartile range.
Table 2.
Risk factors, medications, and renal artery stenosis measures for the Cardiovascular Outcomes in Renal Artery Stenosis Trial participants by randomization group
| Measures | Stent Plus Medical Therapy, n=459 | Medical Therapy Only, n=472 | Difference [95% CI] | P Value |
|---|---|---|---|---|
| Systolic BP, mmHg | ||||
| Mean±SD (N) | 150±23 (457) | 150±23 (467) | −0.5 [−3.5 to 2.4] | 0.50 |
| Diastolic BP, mmHg | ||||
| Mean±SD (N) | 79±13 (457) | 79±13 (467) | 0.3 [−1.5 to 2.0] | 0.23 |
| Antihypertensive counts, mean±SD (N) | 2.2±1.6 (455) | 2.1±1.6 (467) | 0.1 [−0.1 to 0.3] | 0.95 |
| Antihypertensive classes, mean±SD (N) | 2.0±1.5 (455) | 2.0±1.4 (467) | 0.1 [−0.1 to 0.2] | 0.58 |
| ACE inhibitor or ARB | 83% (380/459) | 84% (397/472) | −1.3 [6.1 to 3.5] | 0.60 |
| Diuretic | 65% (298/459) | 71% (337/472) | −6.5 [−12.4 to 0.5] | 0.04 |
| Calcium channel blocker | 62% (284/459) | 65% (307/472) | −3.2 [−9.4 to 3.0] | 0.34 |
| β-Blocker | 78% (358/450) | 77% (362/472) | 1.3 [−4.1 to 6.7] | 0.64 |
| Diabetes mellitus | 32% (148/457) | 34% (162/472) | 0.1 [−0.1 to 0.3] | 0.58 |
| HbA1c, % | ||||
| Mean±SD (N) | 7.0±1.4 (132) | 6.8±1.2 (150) | 0.1 [−0.2 to 0.4] | 0.39 |
| Total cholesterol, mg/dl | ||||
| Mean±SD (N) | 164±43 (434) | 162±42 (450) | 2 [−4 to 8] | 0.50 |
| LDL cholesterol, mg/dl | ||||
| Mean±SD (N) | 92±34 (419) | 89±33 (445) | 3 [−1 to 8] | 0.15 |
| HDL cholesterol, mg/dl | ||||
| Mean±SD (N) | 43±14 (432) | 44±13 (451) | −1 [−3 to 1] | 0.23 |
| Statin use | 83% (382/459) | 87% (410/472) | −4% [−8% to 1%] | 0.12 |
| Body mass index | ||||
| Mean±SD (N) | 28.2±5.3 (458) | 28.7±5.7 (468) | −0.4 [−1.1 to 0.3] | 0.24 |
| Global ischemia | 20% (89/445) | 16% (51/315) | 4% [−2% to 9%] | 0.19 |
| Stenosis, % (core laboratory), mean±SD (N) | 67±11 (557) | 67±12 (377) | 0.96 |
P values for comparisons are generated with two–sample t tests for continuous measurements and chi-squared tests for binary measurements. HbA1c analyses include only study participants with diabetes. 95% CI, 95% confidence interval; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; HbA1c, hemoglobin A1c.
Kidney Function
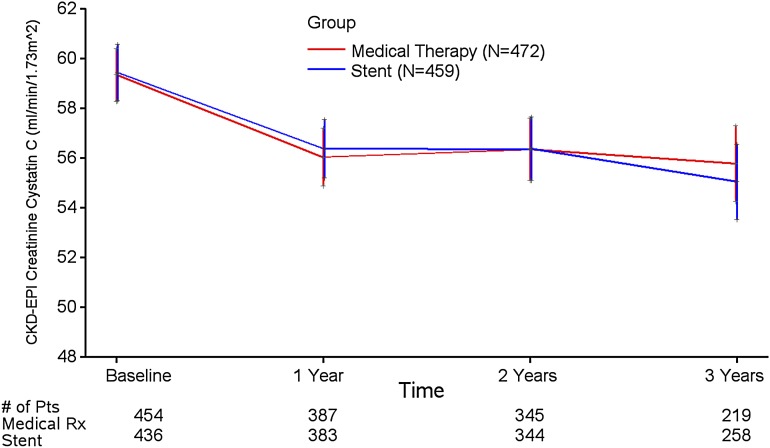
The CORAL Trial participants had a serum creatinine-cystatin C CKD-EPI eGFR of 59±24 ml/min per 1.73 m2 at baseline and 55±23 ml/min per 1.73 m2 (mean±SD) after 3 years (Figure 1). The rate of change in eGFR was −1.5±7.0 ml/min per 1.73 m2 per year in the group randomized to stent plus medical therapy versus −2.3±6.3 ml/min per 1.73 m2 per year in the group who received medical therapy only (P=0.18) during 3 years of follow-up. In addition to the prespecified outcome of eGFR by the serum creatinine-cystatin C CKD-EPI equation, analyses by the CKD-EPI equation on the basis of serum cystatin C alone or creatinine–based MDRD or CKD-EPI equations yielded similar results (Supplemental Table 1, Supplemental Figure 2A). Serum cystatin C measurements were made at baseline and yearly, whereas creatinine was measured at baseline and 3 and 6 months followed by annual measurements. Despite lower creatinine–based eGFR in the medical therapy only group at 3 and 6 months, no difference in creatinine-based eGFR was detected between groups after 1–3 years of follow-up (Supplemental Figure 2).
Figure 1.
Creatinine-cystatin C Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) eGFR (milliliters per minute per 1.73 m2) in randomized treatment groups of the Cardiovascular Outcomes in Renal Artery Stenosis Trial. Data are displayed as means±SDs. Pts, patients.
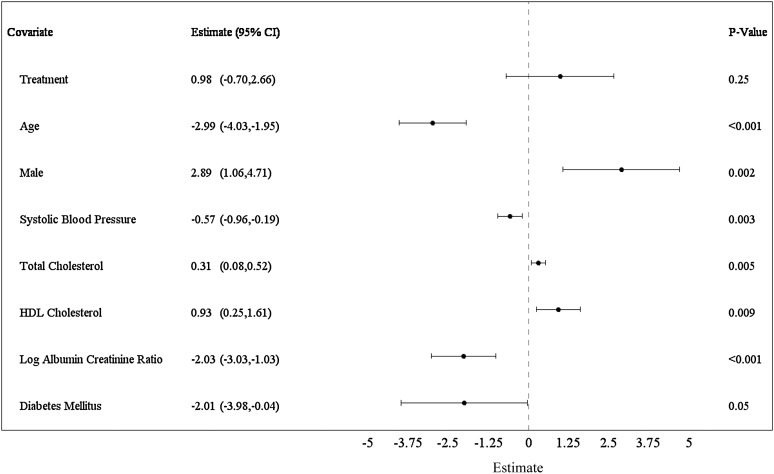
Predictors of eGFR (serum creatinine-cystatin C CKD-EPI) adjusted for age, sex, and randomization group (stent plus medical therapy versus medical therapy only) and covariates selected stepwise in the models were determined by GEE (Figure 2). Covariates retained in the eGFR model are shown as estimates with 95% confidence intervals (95% CIs) for change over mean follow-up of 1.84 years: age (per decade, −2.99; 95% CI, −4.03 to −1.95; P=0.001), men (2.89; 95% CI, 1.06 to 4.71; P=0.002), systolic BP (per 10 mmHg, −0.57; 95% CI, −0.96 to −0.19; P=0.003), total cholesterol (per 10 mg/dl, 0.31; 95% CI, 0.08 to 0.52; P<0.01), HDL cholesterol (per 10 mg/dl, 0.93; 95% CI, 0.25 to 1.61; P<0.01), log urine albumin-to-creatinine ratio (UACR; per SD, −2.03; 95% CI, −3.03 to −1.03; P<0.001), and diabetes (−2.01; 95% CI, −3.98 to −0.04; P=0.05). Stent treatment, bilateral ARAS, and baseline eGFR were not retained in the GEE models. Moreover, no significant interactions were detected to indicate differences in predictors of eGFR by subgroups, including CKD stage, albuminuria status, systolic BP level, use of antihypertensive agents, degree of stenosis, or prior CVD events (Supplemental Figure 3).
Figure 2.
Forest plot displaying a multivariate model for baseline covariates of eGFR (Chronic Kidney Disease Epidemiology Collaboration creatinine-cystatin C). All models were treatment and visit adjusted. Age, sex, and treatment were forced into the multiple variable model. Covariates were added to the model sequentially and kept in the model if the significance level was P<0.10 (n=713). Age is displayed per 10 years. Systolic BP is displayed per 10 mmHg. Total cholesterol and HDL cholesterol are displayed per 10-mg/dl units. Log of urine albumin-to-creatinine ratio is displayed per SD. Estimates are per mean follow-up of 1.84 years. 95% CI, 95% confidence interval.
CKD Events
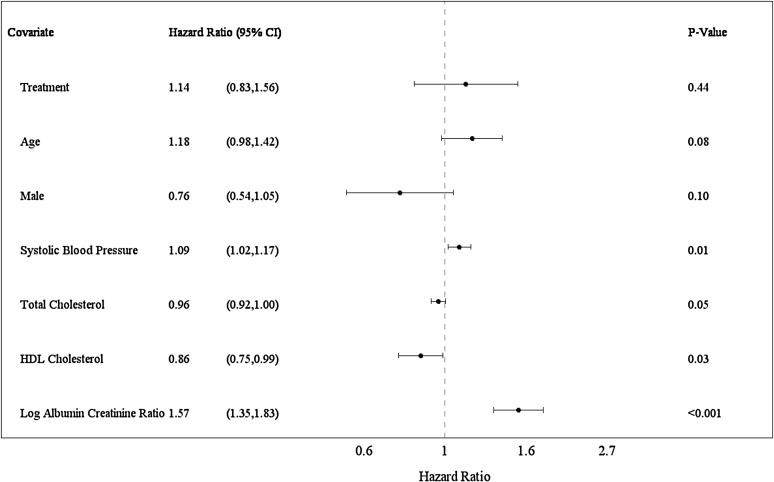
The CKD event outcomes (ESRD, ≥30% eGFR loss, and death caused by kidney disease) occurred in 19% (175 of 931) of the CORAL Trial participants. CKD events occurred among 18% (84 of 459) in the stent group and 19% (91 of 472) in the medical therapy only group (P=0.61; Kaplan–Meier survival plot) (Supplemental Figure 4). Numbers of individual events in stent and medical therapy only groups have been previously reported (10). To assess predictors of events, Cox proportional hazards models were applied with multiple variable analyses adjusted for age, sex, and randomization group with stepwise selection of covariates (Figure 3). Baseline covariates independently predicting CKD events are reported as hazard ratios (HRs) with 95% CIs over mean follow-up of 2.96 years: systolic BP (per 10 mmHg; HR, 1.09; 95% CI, 1.02 to 1.17; P=0.01), log UACR (per SD; HR, 1.57; 95% CI, 1.35 to 1.83; P<0.001), and HDL cholesterol (per 10 mg/dl; HR, 0.86; 95% CI, 0.75 to 0.99; P=0.03).
Figure 3.
Forest plot displaying a multiple variable Cox proportional hazards model of baseline covariates for CKD events. CKD events were a composite of ≥30% eGFR loss for >60 days, ESRD, and death caused by kidney disease. All models were treatment adjusted. A stepwise model selection method was used with a significance level of P<0.10 for covariates to enter and stay in the model (n=811). Age, sex, and treatment were forced into the multiple variable model. Age is displayed per 10 years. Systolic BP is displayed per 10 mmHg. Total cholesterol and HDL cholesterol are displayed per 10-mg/dl units. Log of urine albumin-to-creatinine ratio is displayed per SD. Estimates are per mean follow-up of 2.96 years. 95% CI, 95% confidence interval.
CVD Events
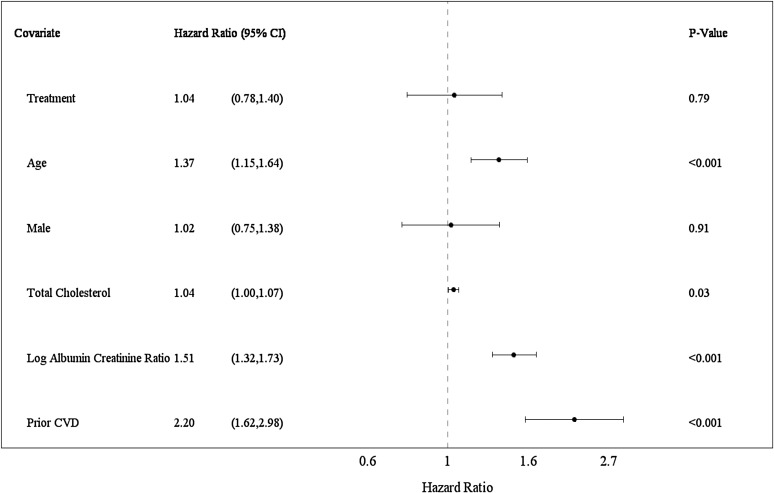
The CVD event outcomes (stroke, myocardial infarction, hospitalization for congestive heart failure, and death) occurred in 22% (207 of 931) of the CORAL Trial participants. CVD events occurred among 22% (103 of 459) in the stent group and 22% (104 of 472) in the medical therapy only group (P=0.90; Kaplan–Meier survival plot) (Supplemental Figure 5). Numbers of individual events in stent and medical therapy only groups have been previously reported (10). To assess predictors of events, Cox proportional hazards models were applied with multiple variable analyses adjusted for age, sex, and randomization group with stepwise selection of covariates (Figure 3). Baseline covariates independently predicting CVD events are reported as HRs with 95% CIs over mean follow-up of 3.12 years: age (per decade; HR, 1.37; 95% CI, 1.15 to 1.64; P<0.001), baseline log UACR (per SD; HR, 1.51; 95% CI, 1.32 to 1.73; P<0.001), history of a prior CVD event (HR, 2.20; 95% CI, 1.62 to 2.98; P<0.001), and baseline total cholesterol (per 10 mg/dl; HR, 1.04; 95% CI, 1.00 to 1.07; P=0.03). Bilateral ARAS associated with CVD events in univariate analyses (HR, 1.58; 95% CI, 1.12 to 2.22; P<0.01). Data on bilateral ARAS had missing records, such that inclusion of this variable in the Cox models substantially lowered the sample size (from n=817 to n=664). Therefore, two stepwise regressions, one with bilateral ARAS and one without it, were conducted in sensitivity analyses. When included in the modeling, bilateral ARAS was retained as a predictor (HR, 1.47; 95% CI, 1.02 to 2.10; P=0.04). Otherwise, the same set of predictor variables resulted regardless of whether bilateral ARAS was included (Figure 4, Supplemental Table 1).
Figure 4.
Forest plot displaying a multiple variable Cox proportional hazards model of baseline covariates for cardiovascular disease (CVD) events. CVD events were a composite of stroke, myocardial infarction, hospitalization for congestive heart failure, and death caused by CVD. Prior CVD was defined as CVD events that occurred before entry into the Cardiovascular Outcomes in Renal Artery Stenosis (CORAL) Trial. All models were treatment adjusted. A stepwise model selection method was used with a significance level P<0.10 for covariates to enter and stay in the model (n=817). Age, sex, and treatment were forced into the multiple variable model. Age is displayed per 10 years. Total cholesterol is displayed per 10-mg/dl units. Log of urine albumin-to-creatinine ratio is displayed per SD. Estimates are per mean follow-up of 3.12 years. Two stepwise regressions, one with bilateral atherosclerotic renal artery stenosis (ARAS) and one without ARAS, were conducted. Because bilateral ARAS had missing records, inclusion of this variable in the multivariate model substantially lowered the sample size (n=664). Therefore, the model without ARAS is presented. The same set of predictors was obtained regardless of whether bilateral ARAS was included in the model by sensitivity analysis. 95% CI, 95% confidence interval.
Discussion
In the CORAL Trial participants with ARAS, mean eGFR was slightly low at baseline and remained so after 3 years of follow-up. Stent treatment, when added to optimal medical therapy, neither mitigated nor worsened eGFR. Although more than one half of the participants had at least CKD stage 3 at study enrollment, eGFR over time was not predicted by baseline eGFR. Instead, eGFR predictors included other CKD risk factors, such as age, diabetes, sex, systolic BP, and albuminuria. Notably, more severe ARAS did not predict eGFR. Stent treatment did not modify eGFR or risks of clinical events associated with CKD.
The clinical effects of renal artery revascularization by stenting have been controversial, despite recent clinical trials (5,6,10). The CORAL Trial participants with ARAS had traditional risk profiles for CKD. Importantly, RAS inhibition and target-driven control of multiple risk factors were feasible, were safe, and produced outcomes for eGFR and clinical events associated with CKD that were not bested by addition of stenting. An earlier collection of reports suggested that renal artery stenting may benefit hypertension, kidney function, and various other clinical outcomes (16–25). Most of these data were derived from observational cohorts (often retrospective), single-center experiences, and uncontrolled or underpowered clinical trials. Frequent crossovers to stenting also limited interpretation of the results. These studies did not assure optimal risk factor management by contemporary standards, and none mandated, although many avoided, RAS inhibitor use. The primary outcomes were typically surrogates, most commonly measures of BP. Indeed, the CORAL Trial showed a small reduction in systolic BP by stenting, but it did not translate into higher eGFR or lower risk of either CKD or CVD events (10).
Effects of revascularization by stenting on longitudinal eGFR have not been previously reported. The CORAL Trial group receiving only medical therapy had transiently lower serum creatinine–based eGFR than the stented group at 3 and 6 months. Calculations of eGFR with cystatin C were only available from baseline and annual assessments. Nevertheless, from 1 year onward and in the overall analysis, no between-group difference in eGFR was detected by any estimating method with serum creatinine and/or cystatin C. This response is reminiscent of early GFR decline followed by stabilization after reducing BP by RAS inhibition in patients with CKD (26,27). With BP management including RAS inhibition in the CORAL Trial, a similar phenomenon likely occurred. The CORAL Trial risk prediction models for eGFR, CKD events, and CVD events uniquely controlled for severity of kidney disease at baseline (eGFR and albuminuria). Predictors of eGFR in the CORAL Trial were traditional CKD risk factors. In particular, the inverse relationship between systolic BP and eGFR reflects an association of lower BP with higher eGFR over time. Baseline levels of HDL and total cholesterol also favorably associated with eGFR and CKD events. Dyslipidemia exacerbates kidney injury early in the course of experimental ARAS (28). These data suggest that a more favorable lipid profile may benefit kidney function in patients with ARAS.
Observational and uncontrolled studies suggest that high-risk patients, such as those with more severe ARAS or global ischemia, low eGFR or rapid eGFR decline, uncontrolled hypertension, congestive heart failure, or flash pulmonary edema, may benefit from stenting (29–31). However, assessments of such cohorts have inherent bias for treatment selection. Much of these data were collected during an earlier era, when optimal medical management was not well defined and event rates for CKD, CVD, and death were considerably higher (30). For example, in the CORAL Trial, bilateral ARAS predicted CVD outcomes, but stenting did not reduce risk of these events. Bilateral ARAS is an indicator of more severe vascular disease and thereby, may associate with higher CVD risk. From this randomized clinical trial testing optimal medical therapy alone versus with stenting, these analyses do not reveal variation in response to treatment by high-risk features. Therefore, the CORAL Trial data support the effectiveness of risk factor treatment and BP control with RAS inhibition as standard of care in patients with ARAS (32).
This study has both limitations and strengths. High-risk patients may have been less likely to be referred to the CORAL Trial. Few participants with a history of recent hospitalization for congestive heart failure or advanced CKD were enrolled. Patients with rapid decline in eGFR may also have been less likely to be referred to the CORAL Trial or other clinical trials of randomized treatment. Nevertheless, findings from the CORAL Trial provide a reasonable guide to ARAS management among patients commonly seen in clinical practice. It is unlikely that a randomized clinical trial of stenting could be conducted in high-risk patients, who are relatively infrequent and at greater risk of procedural complications, such as AKI and/or atheroemboli. Thus, the risk-benefit tradeoff of stenting among patients with high-risk ARAS is uncertain. Questions about reproducibility and accuracy of GFR estimated from measures of serum creatinine and the validity of a 20% decline in eGFR as an end point for studies in ARAS have been raised (33). A recent report of using both serum cystatin C and serum creatinine found substantially improved accuracy and precision for eGFR calculated by the CKD-EPI equation across a wide range of eGFR levels (15). Therefore, these analyses incorporated the newer eGFR method and a definition of this end point as ≥30% eGFR decline to align with the evolution of clinical outcome definitions since embarking on the CORAL Trial.
In conclusion, the largest prospective, randomized clinical trial on ARAS to date, the CORAL Trial, did not show that stent treatment influenced eGFR over 3 years in participants receiving a foundation of RAS inhibition–based therapy with target-driven control of BP, LDL cholesterol, and hyperglycemia. Predictors of kidney function and clinical events for CKD and CVD were traditional risk factors for kidney disease and atherosclerosis.
Disclosures
K.R.T. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses. K.R.T. was responsible for study design and conduct, data analyses and reporting, and writing the manuscript. K.R.T. has received grants from the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Diabetes, Digestive, and Kidney Diseases. K.R.T. has received consulting fees from Eli Lilly (Indianapolis, IN); Amgen, Inc. (Thousand Oaks, CA); and Noxxon Pharma as well as research support from Eli Lilly. L.D.D. was involved in the design of the study, collection and analysis of data, and the writing of the manuscript. L.D.D. received grants from the National Institutes of Health (NIH) and research support from Pfizer Inc., Astra Zeneca, and Johnson & Johnson. W.H. provided input on data inclusion and analyses. W.H. reports no disclosures. B.A.G. contributed to the design of the analyses, review of study data, and writing of the manuscript. B.A.G. has received grants from the NIH. M.S. supervised generation of data from the research laboratory and contributed to writing the manuscript. M.S. has received grants from the NIH. S.T. contributed to the design of the analyses and review of study data. S.T. has received personal fees from AbbVie. J.I.S. contributed to writing and editing the manuscript. J.I.S. has received grants from the NIH, the BrickStreet Insurance Endowment, and the Huntington Foundation Endowment. K.J. contributed to the design of the analyses, review of study data, and writing of the manuscript. K.J. has received grants from the Medical College of Toledo. A.L. was responsible for and conducted the data analyses. K.P. was responsible for and conducted the data analyses. K.P. has received grants from the NHLBI. J.M.M. was responsible for and conducted the data analyses. J.M.M. has received personal fees from the Harvard Clinical Research Institute and grants from the NHLBI. R.B.D. was responsible for and conducted the data analyses. R.B.D. contributed considerable input and review as the senior statistician for the Cardiovascular Outcomes in Renal Artery Stenosis Clinical Trial. R.B.D. has received grants from the NHLBI. D.E.C. was responsible for and conducted the data analyses. D.E.C. also made substantial contribution to study design and data acquisition along with critical review of the manuscript for intellectual content as well as final approval of the manuscript. D.E.C. agrees to be accountable for all aspects of the work. D.E.C. has received grants from the NHLBI and research support from Medtronic, Boston Scientific, and Abbott Vascular. T.P.M. provided input into writing the manuscript and edited final revisions. T.P.M. reports no disclosures. C.J.C. was responsible for study design and analysis and the writing of the manuscript. C.J.C. has received research funding from Cordis Corporation, study drug from Astra Zeneca, and study drug and research funding from Pfizer Inc.
Supplementary Material
Acknowledgments
The authors appreciate the support and encouragement provided by Diane M. Reid (Medical Officer, Division of Cardiovascular Sciences, National Heart, Lung and Blood Institute [NHLBI], Bethesda, MD).
The Cardiovascular Outcomes in Renal Artery Stenosis Clinical Trial was supported by NHLBI of the National Institutes of Health grants U01HL071556, U01HL072734, U01HL072735, U01HL072736, and U01HL072737. Study drugs were provided by Astra Zeneca and Pfizer Inc. Study devices were provided by Cordis Corporation, and supplemental financial support was granted by both Cordis Corporation and Pfizer Inc.
None of the sponsors had roles in design and conduct of the study; collection, management, analysis, and interpretation of the data; or review or approval of the manuscript. The content of this paper is solely the responsibility of the authors.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Does Renal Artery Stenting Prevent Clinical Events?,” on pages 1125–1127.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10491015/-/DCSupplemental.
References
- 1.Chábová V, Schirger A, Stanson AW, McKusick MA, Textor SC: Outcomes of atherosclerotic renal artery stenosis managed without revascularization. Mayo Clin Proc 75: 437–444, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Conlon PJ, Athirakul K, Kovalik E, Schwab SJ, Crowley J, Stack R, McCants CB Jr., Mark DB, Bashore TM, Albers F: Survival in renal vascular disease. J Am Soc Nephrol 9: 252–256, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Edwards MS, Craven TE, Burke GL, Dean RH, Hansen KJ: Renovascular disease and the risk of adverse coronary events in the elderly: A prospective, population-based study. Arch Intern Med 165: 207–213, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Zalunardo N, Tuttle KR: Atherosclerotic renal artery stenosis: Current status and future directions. Curr Opin Nephrol Hypertens 13: 613–621, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Bax L, Woittiez AJ, Kouwenberg HJ, Mali WP, Buskens E, Beek FJ, Braam B, Huysmans FT, Schultze Kool LJ, Rutten MJ, Doorenbos CJ, Aarts JC, Rabelink TJ, Plouin PF, Raynaud A, van Montfrans GA, Reekers JA, van den Meiracker AH, Pattynama PM, van de Ven PJ, Vroegindeweij D, Kroon AA, de Haan MW, Postma CT, Beutler JJ: Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: A randomized trial. Ann Intern Med 150: 840–848, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Wheatley K, Ives N, Gray R, Kalra PA, Moss JG, Baigent C, Carr S, Chalmers N, Eadington D, Hamilton G, Lipkin G, Nicholson A, Scoble J; ASTRAL Investigators : Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 361: 1953–1962, 2009 [DOI] [PubMed] [Google Scholar]
- 7.van Jaarsveld BC, Krijnen P, Pieterman H, Derkx FH, Deinum J, Postma CT, Dees A, Woittiez AJ, Bartelink AK, Man in ’t Veld AJ, Schalekamp MA; Dutch Renal Artery Stenosis Intervention Cooperative Study Group : The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. N Engl J Med 342: 1007–1014, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Plouin PF, Chatellier G, Darné B, Raynaud A: Blood pressure outcome of angioplasty in atherosclerotic renal artery stenosis: A randomized trial. Essai Multicentrique Medicaments vs Angioplastie (EMMA) Study Group. Hypertension 31: 823–829, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Webster J, Marshall F, Abdalla M, Dominiczak A, Edwards R, Isles CG, Loose H, Main J, Padfield P, Russell IT, Walker B, Watson M, Wilkinson R; Scottish and Newcastle Renal Artery Stenosis Collaborative Group : Randomised comparison of percutaneous angioplasty vs continued medical therapy for hypertensive patients with atheromatous renal artery stenosis. J Hum Hypertens 12: 329–335, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, Cohen DJ, Matsumoto AH, Steffes M, Jaff MR, Prince MR, Lewis EF, Tuttle KR, Shapiro JI, Rundback JH, Massaro JM, D’Agostino RB Sr., Dworkin LD; CORAL Investigators : Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 370: 13–22, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr., Jones DW, Materson BJ, Oparil S, Wright JT Jr., Roccella EJ; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee : The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA 289: 2560–2572, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults : Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 285: 2486–2497, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Kidney Disease Outcomes Quality Initiative (K/DOQI) : K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 43[Suppl 1]: S1–S290, 2004 [PubMed] [Google Scholar]
- 14.American Diabetes Association : Standards of medical care in diabetes—2006. Diabetes Care 29[Suppl 1]: S4–S42, 2006 [PubMed] [Google Scholar]
- 15.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beutler JJ, Van Ampting JM, Van De Ven PJ, Koomans HA, Beek FJ, Woittiez AJ, Mali WP: Long-term effects of arterial stenting on kidney function for patients with ostial atherosclerotic renal artery stenosis and renal insufficiency. J Am Soc Nephrol 12: 1475–1481, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Blum U, Krumme B, Flügel P, Gabelmann A, Lehnert T, Buitrago-Tellez C, Schollmeyer P, Langer M: Treatment of ostial renal-artery stenoses with vascular endoprostheses after unsuccessful balloon angioplasty. N Engl J Med 336: 459–465, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Burket MW, Cooper CJ, Kennedy DJ, Brewster PS, Ansel GM, Moore JA, Venkatesan J, Henrich WL: Renal artery angioplasty and stent placement: Predictors of a favorable outcome. Am Heart J 139: 64–71, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Dejani H, Eisen TD, Finkelstein FO: Revascularization of renal artery stenosis in patients with renal insufficiency. Am J Kidney Dis 36: 752–758, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Harden PN, MacLeod MJ, Rodger RS, Baxter GM, Connell JM, Dominiczak AF, Junor BJ, Briggs JD, Moss JG: Effect of renal-artery stenting on progression of renovascular renal failure. Lancet 349: 1133–1136, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Muray S, Martín M, Amoedo ML, García C, Jornet AR, Vera M, Oliveras A, Gómez X, Craver L, Real MI, García L, Botey A, Montanyà X, Fernández E: Rapid decline in renal function reflects reversibility and predicts the outcome after angioplasty in renal artery stenosis. Am J Kidney Dis 39: 60–66, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Nordmann AJ, Woo K, Parkes R, Logan AG: Balloon angioplasty or medical therapy for hypertensive patients with atherosclerotic renal artery stenosis? A meta-analysis of randomized controlled trials. Am J Med 114: 44–50, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Paulsen D, Kløw NE, Rogstad B, Leivestad T, Lien B, Vatne K, Fauchald P: Preservation of renal function by percutaneous transluminal angioplasty in ischaemic renal disease. Nephrol Dial Transplant 14: 1454–1461, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Tuttle KR, Chouinard RF, Webber JT, Dahlstrom LR, Short RA, Henneberry KJ, Dunham LA, Raabe RD: Treatment of atherosclerotic ostial renal artery stenosis with the intravascular stent. Am J Kidney Dis 32: 611–622, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Watson PS, Hadjipetrou P, Cox SV, Piemonte TC, Eisenhauer AC: Effect of renal artery stenting on renal function and size in patients with atherosclerotic renovascular disease. Circulation 102: 1671–1677, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, Striker G; Modification of Diet in Renal Disease Study Group : The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med 330: 877–884, 1994 [DOI] [PubMed] [Google Scholar]
- 27.Levey AS, Beck GJ, Bosch JP, Caggiula AW, Greene T, Hunsiker LG; Modification of Diet in Renal Disease Study Group : Short-term effects of protein intake, blood pressure, and antihypertensive therapy on glomerular filtration rate in the Modification of Diet in Renal Disease Study. J Am Soc Nephrol 7: 2097–2109, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Porcel M, Krier JD, Lerman A, Sheedy PF 2nd, Romero JC, Napoli C, Lerman LO: Combination of hypercholesterolemia and hypertension augments renal function abnormalities. Hypertension 37[2 Pt 2]: 774–780, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Herrmann SMS, Saad A, Textor SC: Management of atherosclerotic renovascular disease after Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL). Nephrol Dial Transplant 30: 366–375, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ritchie J, Green D, Chrysochou C, Chalmers N, Foley RN, Kalra PA: High-risk clinical presentations in atherosclerotic renovascular disease: Prognosis and response to renal artery revascularization. Am J Kidney Dis 63: 186–197, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Jaff MR, Bates M, Sullivan T, Popma J, Gao X, Zaugg M, Verta P; HERCULES Investigators : Significant reduction in systolic blood pressure following renal artery stenting in patients with uncontrolled hypertension: Results from the HERCULES trial. Catheter Cardiovasc Interv 80: 343–350, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Greco BA, Freda BJ: What is the optimal treatment for patients with atherosclerotic renal artery stenosis? Am J Kidney Dis 64: 174–177, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Madder RD, Hickman L, Crimmins GM, Puri M, Marinescu V, McCullough PA, Safian RD: Validity of estimated glomerular filtration rates for assessment of baseline and serial renal function in patients with atherosclerotic renal artery stenosis: Implications for clinical trials of renal revascularization. Circ Cardiovasc Interv 4: 219–225, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.