Abstract
Mammalian or mechanistic target of rapamycin (mTOR) senses nutrient, energy, and hormone signals to regulate metabolism and energy homeostasis. mTOR activity in the hypothalamus, which is associated with changes in energy status, plays a critical role in the regulation of food intake and body weight. mTOR integrates signals from a variety of “energy balancing” hormones such as leptin, insulin, and ghrelin, although its action varies in response to these distinct hormonal stimuli as well as across different neuronal populations. In this review, we summarize and highlight recent findings regarding the functional roles of mTOR complex 1 (mTORC1) in the hypothalamus specifically in its regulation of body weight, energy expenditure, and glucose/lipid homeostasis. Understanding the role and underlying mechanisms behind mTOR-related signaling in the brain will undoubtedly pave new avenues for future therapeutics and interventions that can combat obesity, insulin resistance, and diabetes.
Keywords: mTOR, hypothalamus, hormones, nutrients, energy homeostasis
balanced food intake and energy expenditure are key to maintaining energy homeostasis, whereas impaired regulation of this balance leads to excessive weight gain and obesity. Food intake and energy expenditure are primarily controlled by the central nervous system (CNS), especially the hypothalamus, which acts as a key signaling hub linking CNS action to peripheral organ metabolism. Along with other nutrient sensors in different brain areas, the hypothalamus senses and integrates changes in circulating hormone and nutrient levels by relaying these peripheral signals to neuroendocrine cells, which signal behavioral and metabolic effectors to regulate whole body energy homeostasis (6, 53, 57, 82).
Extensive studies have been performed to explore the unique roles and distinct actions of several anatomically well-defined hypothalamic areas in the regulation of energy balance, including the arcuate nucleus (ARC) and ventromedial (VMH), dorsomedial (DMH), paraventricular (PVH), and lateral (LH) hypothalamus (74, 99). In the ARC, hormone and nutrient signals from the periphery induce activity changes of two subpopulations of neurons: an orexigenic population coexpressing the neurotransmitters neuropeptide Y (NPY) and agouti-related peptide (AgRP) and an anorexigenic population coexpressing proopiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART). Numerous studies have demonstrated that POMC/CART and AgRP/NPY neurons have direct synaptic connections with some neuronal populations located in the VMH, DMH, PVH, and LH, all of which have profound effects on feeding behavior, energy expenditure, and glucose homeostasis (82).
Leptin and insulin are two important hormonal regulators of peripheral energy homeostasis, which play critical roles in mediating CNS-controlled glucose, lipid, and energy metabolism by acting on the hypothalamus. Both the insulin and leptin receptors are widely expressed in the ARC, VMH, DMH, LH, and PVH, and together or independently these two pathways can mediate their respective signals to dynamically control whole body energy homeostasis (75). Within the hypothalamus, POMC and AgRP neurons have been identified as major targets of leptin and insulin action (75). As an energy sensor and a downstream target of the insulin-stimulated phosphatidylinositol 3-kinase (PI3K) pathway, mTOR has emerged as a newly important player of CNS-regulated energy homeostasis. The coordinated action of the mammalian or mechanistic target of rapamycin (mTOR) in peripheral insulin target tissues and in the CNS, particularly within neuronal networks responsible for food intake and body weight, plays a key role in regulating glucose and lipid metabolism as well as energy balance. In this review, we highlight the latest findings on the central roles of hypothalamic mTOR in regulating energy balance and glucose and lipid homeostasis. We also discuss potential new targets of mTOR action and the underlying mechanisms that mediate the central regulation of energy homeostasis.
mTOR Signaling and Energy Homeostasis
The Ser/Thr protein kinase mTOR integrates various internal and external signals to regulate many cellular activities, including mRNA translation, protein synthesis, cell proliferation and growth, autophagy, lipogenesis, and thermogenesis (8, 79, 89). mTOR exerts its biological activities in mammalian cells by forming two distinct complexes: mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2), which differ in their components, regulation, function, and sensitivity to rapamycin.
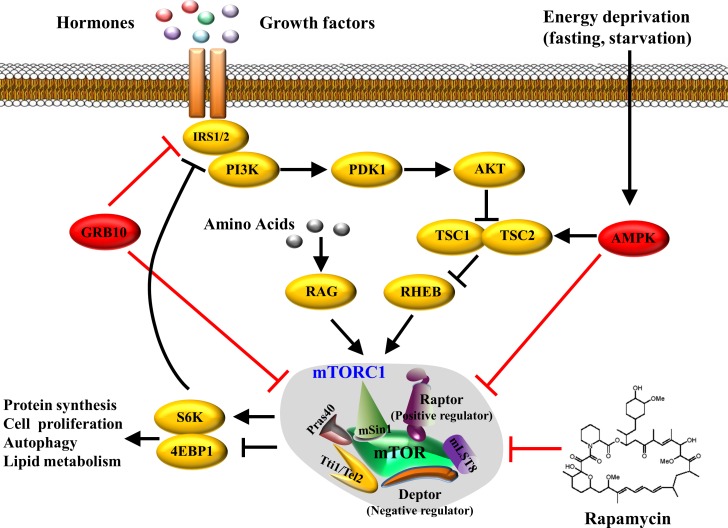
The rapamycin-sensitive mTORC1 contains mTOR, mLST8 (mammalian lethal with SEC13 protein-8, also known as GβL), and Deptor (DEP domain-containing mTOR-interacting protein), which are also the components of mTORC2. However, mTORC1 has its unique accessory proteins including Raptor (regulatory associated protein of mTOR) and PRAS40 (40-kDa Pro-rich Akt substrate, also known as AKT1S1). The activity of mTORC1 is stimulated by growth factors, nutrients, hormones, hypoxia, and many other factors. Growth factors and insulin promote PI3K/Akt-mediated phosphorylation of tuberous sclerosis 1/2 (TSC1/2), which leads to activation of a small GTPase Rheb (Ras homolog enriched in brain) and subsequent activation of mTORC1 and its downstream signaling cascade (4). The mTORC1 signaling pathway could also be regulated by nutrients such as glucose and amino acids through interaction with Rag proteins, another set of small GTPases (64). Branched-chain amino acids (BCAA) such as leucine and arginine are also potent activators of mTORC1, although the underlying mechanisms are not well understood (27). Energy limitation, such as glucose deprivation and starvation, strongly inhibits mTORC1 activation by stimulating AMP-activated protein kinase (AMPK), which is another critical cellular energy sensor that suppresses mTORC1 activity by phosphorylating TSC2 or Raptor (19) (Fig. 1).
Fig. 1.
Mammalian or mechanistic target of rapamycin complex 1 (mTORC1) signaling pathway. The mTORC1 activate can be induced by hormones and growth factors (e.g., insulin, IGF-I, etc.) through a PI3K/Akt-mediated pathway, and by amino acids (mainly leucine) through Rag GTPases. On the other hand, activation of mTORC1 can be suppressed by rapamycin, mTORC1 downstream substrate Grb10, and energy deprivation-induced AMPK activation. Activated mTORC1 subsequently activates downstream S6K or inhibits 4E-BP1, resulting in protein translation, cellular proliferation, autophagy, or lipid metabolism.
Activation of mTORC1 stimulates or inhibits its downstream substrates, including 4E-binding proteins (4EBPs), 40S ribosomal protein S6 kinases (S6Ks), the autophagy inducer ULK1, PRAS40, and the growth factor receptor-binding protein-10 (Grb10) (23, 31, 53, 84). Recently, we have identified Grb10 as a key negative feedback regulator of mTORC1 signaling (36). On the other hand, activated mTOR/p70S6K has a negative feedback regulation on insulin signaling by phosphorylating and inactivating insulin receptor substrate (IRS) (71). Thus, prolonged activation or overactivation of mTORC1 may result in desensitization of the insulin signaling cascade, thereby contributing to insulin resistance and obesity.
The second mTOR complex, mTORC2, contains Rictor (Raptor-independent companion of mTOR), mSin1 (mammalian stress-activated protein kinase-interacting protein), and PROTOR1/2 (protein observed with Rictor-1 and -2), which distinguishes this complex from mTORC1 (32). Unlike mTORC1, mTORC2 activity is less sensitive to acute rapamycin treatment, although chronic drug administration may suppress its activity in some cell types and tissues (39). The mTORC2 signaling pathway was thought to mainly regulate cellular survival, lipid metabolism, and cytoskeleton organization by phosphorylation-activating protein kinase Cα (PKCα), Akt, or serum- and glucocorticoid-induced protein kinase-1 (SGK1) (40). However, there is some evidence suggesting that mTORC2 is essential for the regulation of neuronal morphology and synaptic activity (69). A recent study shows that neuron-specific disruption of Rictor, a key regulatory subunit of the mTORC2 complex, leads to a profound increase in fat composition and adiposity, impaired glucose tolerance, and insulin sensitivity, indicating that Rictor plays an important role in hypothalamic regulation of energy balance (34). In particular, ablation of Rictor in POMC neurons leads to hyperphagia, obesity, and glucose intolerance, whereas Rictor disruption in AgRP neurons shows no significant changes on energy homeostasis in mice (34). Currently, the exact mechanisms underlying these effects of mTORC2 are still largely unknown and require greater investigation. For the remainder of our review, we will mainly be discussing the roles of mTORC1 within various neuronal populations and across different regions in the hypothalamus.
mTORC1 Signaling in the Hypothalamus
mTORC1 and its well-established downstream target S6K are widely expressed in the brain. However, the activity of mTORC1 is found rather restrictively in the hypothalamus, particularly in the ARC, PVH, VMH, and SCN (suprachiasmatic nucleus) (13, 26, 39, 58). Within the ARC, mTORC1 activity can be detected in the vast majority (more than 90%) of AgRP neurons and in a portion (∼45%) of POMC neurons in rats with free access to food (13, 26), providing an anatomical basis for the regulatory action on these neurons.
Similar to its action in the peripheral tissues, mTORC1 acts as a sensor of energy status in the hypothalamus (13, 58, 77). Fasting and refeeding reduces and increases, respectively, phosphorylation of mTORC1, S6K1, and S6 in the medial-basal hypothalamus (MBH), including the ARC and the VMH in rats (13), suggesting that hypothalamic mTORC1 activity is closely associated with the energy status of the animals. Nutrient-induced hypothalamic mTORC1 activity mediates central administration of leucine-decreased food intake and body weight in rats by decreasing the expression levels of Agrp and NPY while increasing POMC expression within the ARC (13, 45). On the other hand, overnutrition impairs mTORC1 activity and decreases mTORC1 signaling in the hypothalamus, which is implicated to contribute to the development of hyperphagia, weight gain, and leptin resistance in high-fat-diet (HFD)-induced obesity (12). Of note, nutrient-activated hypothalamic mTORC1 signaling is neuron- or area-specific. S6K phosphorylation is increased in the ARC, particularly in the AgRP neurons, but is suppressed in the VMH under fasting or in leptin-deficient ob/ob mice (58, 77). In addition, refeeding markedly decreases mTORC1 activity in VMH and LH in both lean and obese rats (25), suggesting that hypothalmic mTORC1 activation is neuron type and context dependent. In contrast to what was observed in the hypothalamus (12), the activity of mTOR signaling is increased by overnutrition in some peripheral tissues such as liver and muscle (30), and in the hippocampus of rats (12), indicating that differential regulation of mTORC1 may exist outside the hypothalamus. However, the underlying mechanisms and related pathways require further investigation.
Manipulation of hypothalamic mTORC1 activity has a profound impact on food intake, body weight, and insulin sensitivity in rodents (2, 12, 13, 20). Pharmacological inhibition of mTORC1 in the hypothalamus using rapamycin inhibits leucine-decreased food intake and body weight (13). Similarly, modulation of the downstream target of the mTORC1, S6K, also leads to changes in feeding behavior and body weight (5, 12, 13). Adenovirus-mediated acute overexpression of a constitutively active S6K1 in the MBH suppresses food intake, lowers weight gain, and improves insulin sensitivity in rats, while dominant-negative S6K1 increases food intake and weight gain, suggesting a bidirectional regulation of energy homeostasis by S6K1 (5). However, several other studies show opposite results. Adenovirus-mediated overactivation or suppression of hypothalamic S6K mimics or blocks, respectively, the inhibitory effects of short-term HFD feeding on central insulin-induced suppression of hepatic gluconeogenesis and insulin sensitivity (52). Very recently, Caron et al. (10) found that mice overexpressing DEPTOR, a known negative regulator of mTORC1, in the MBH prevents HFD-induced obesity, improves glucose metabolism and protects against hepatic steatosis, along with a reduction in food intake and feeding efficiency. The discrepancy in these studies might be due to differences between acute and long-term activation of the mTORC1 signaling and energy availability of the animals. Interestingly, while specific deletion of S6K1 in either POMC or AgRP neurons has no effects on feeding behavior and bodyweight, mTOR-S6K1 signaling in POMC neurons probably regulates hepatic glucose production and peripheral lipid metabolism, but controls skeletal muscle insulin sensitivity and glucose level in AgRP neurons (66). These findings suggest that mTOR-S6K signaling has distinct roles in POMC and AgRP neurons. However, how mTORC1 signaling in POMC and AgRP neurons regulate distinct metabolic events is unclear. Therefore, future genetic models manipulating mTORC1 activity in distinct neuronal populations will greatly enhance our understanding of the role of mTORC1 signaling in nutrient sensing and feeding behavior.
It is well established that prolonged activation of mTORC1 signaling is closely related to aging and aging-related disease, including obesity, diabetes, and cardiovascular and neurodegenerative diseases (24, 89). Consistently, overactivation of mTORC1 in hypothalamic neurons induces aging-associated feeding behavior and obesity (44, 83). Mori et al. (44) found that POMC neuron-specific deletion of the Tsc1 gene, a major negative regulator of mTORC1, leads to long-term elevation of mTORC1 signaling associated not only with cellular hypertrophy but also with reduced axonal projections to the hypothalamic PVN, which are linked with hyperphagic obesity. These findings are confirmed by Yang et al. (83), who found that Tsc deletion-induced activation of mTORC1 in POMC neurons leads to elevation of KATP channel activity associated with age-related silencing of POMC neurons, which may contribute to hyperphagia and obesity. These studies imply that hypothalamic mTORC1 signaling may control food intake and energy homeostasis by modulating neuronal plasticity and synaptic activity, which lead to functional changes of neuronal circuits.
mTORC1 in the Hypothalamus Mediating the Effects of Hormone Regulators on Energy Homeostasis
mTORC1 and leptin.
It is well established that leptin plays a major role in the regulation of feeding and energy expenditure and is thus is critical for maintaining energy homeostasis and body weight control. Through its receptors (LepRs), leptin acts on POMC and AgRP neurons to suppress food intake and promote energy expenditure, respectively (49).
A growing body of evidence has uncovered a very close link between mTORC1 and leptin signaling in the CNS. Intracerebroventricular (icv) administration of leptin activates mTORC1 in rat hypothalamus (12), suggesting that leptin is an activator of mTORC1 signaling in the hypothalamus. In response to changes in nutrient availability and energy status, hypothalamic mTORC1 has been shown to mediate the effect of leptin to regulate food intake (13). Inhibition of mTORC1 by rapamycin disrupts the action of leptin on feeding, suggesting that the mTORC1 signaling pathway in the hypothalamus is required for the appetite-suppressing effects of this hormone (13). In addition, activation of mTORC1-S6K enhances the phosphorylation-dependent inhibition of AMPK by leptin (13). Interestingly, mTORC1 has also been found to mediate leptin action on lipid metabolism and inflammation in peripheral tissues (41, 57). Taken together, these findings highlight the importance of the mTORC1 pathway in mediating the effects of leptin. Consistent with these results, Blouet et al. (5) found that targeted deletion of MBH S6K activity in rats significantly alters hypothalamic orexigenic neuropeptide expression and leptin sensitivity, leading to changes in food intake and body weight as well as metabolic and feeding responses to fasting. In addition, mice with S6K deletion do not respond to leptin administration (12). These data support the notion that inhibition of the mTORC1-S6K signaling pathway in the hypothalamus may contribute to the onset of leptin resistance. Another possibility by which hypothalamic mTORC1 mediates leptin's satiating effects may be through a positive feedback loop involving POMC neurons and adipose tissue. In effector T cells, for example, leptin activates mTORC1 via a PI3K-Akt-dependent pathway, which in turn controls leptin production and signaling and leads to more sensitive cellular responses (57). Whether this positive feedback regulatory loop between leptin and mTORC1 exists in the hypothalamus to control feeding behavior is unknown and requires further investigation.
AMPK is an important nutrient sensor that has broad and mostly opposing effects on the metabolic functions of mTORC1. AMPK activity is inhibited by leptin in ARC and PVH, in addition to insulin, high levels of glucose, and refeeding (43). The effects of leptin on food intake and body weight require the suppression of hypothalamic AMPK activity (43). S6K-dependent phosphorylation of AMPK at Ser485/491 is required for leptin to silence AMPK activity in the hypothalamus (14). These results imply a negative feedback regulation of AMPK by mTORC1 signaling and vice versa. The feedback regulation between mTORC1 and AMPK has also been described in the muscle, where deletion of S6K1 leads to overactivation of AMPK (1).
The endoplasmic reticulum (ER) is a cellular organelle responsible for protein translation, folding, posttranslational modification, and transportation. A chronic failure of ER function causes the accumulation of misfolded proteins, resulting in ER stress. Overnutrition-induced hypothalamus ER stress has been implicated to play a central role in insulin resistance and obesity (54, 87) and development of leptin resistance (58, 87). An increasing body of evidence suggests a bidirectional functional cross-talk between mTOR signaling and ER stress in the regulation of apoptosis, autophagy, hepatic lipid synthesis, and insulin resistance (3, 29). Recently, Zhang et al. (85) reported that manipulation of activating transcription factor 4 (ATF4), an important downstream responsive element of ER stress, in the hypothalamus negatively affects insulin sensitivity in the liver and is in part mediated by the mTORC1-S6K pathway. An interesting question that remains to be answered is whether cross-talk between mTORC1 and ER stress in the hypothalamus involves the action of leptin. In addition, it is unknown whether mTORC1 signaling and ER stress regulate body weight and energy metabolism via common or distinct mechanisms.
mTORC1 and insulin.
Similarly to leptin, insulin in the brain has also been recognized as an important regulator of energy homeostasis. Insulin receptor (IR) and insulin receptor substrate-1 (IRS-1) distribute throughout the brain regions with the higher expression levels in the hypothalamus (16, 74). Insulin in the brain not only regulates neuronal function and synaptogenesis, but also modulates appetite, body weight, white fat mass, and hepatic glucose output as well as whole body energy metabolism (7, 33). Since the pioneering work by Woods et al. (77a) showed that icv infusion of insulin markedly decreased food intake and body weight gain in baboons, a growing body of evidence demonstrates a critical role of hypothalamic insulin in the regulation of body weight and energy homeostasis (88). Insulin administration into the third ventricle decreases food intake accompanied by decreased expression of NPY and AgRP and increased expression of POMC and CART in the ARC, which may contribute to the increased activity of α-melanocyte-stimulating hormone (α-MSH) in the PVH neurons (33, 65). Furthermore, an increase in NPY and AgRP along with a decrease in POMC and CART has been reported in the hypothalamus of streptozotocin (STZ)-induced insulin-deficient mice (33, 65). Brain-specific deletion of IR also leads to increased food intake and mild obesity in mice (7). However, POMC- or AgRP-restricted IR knockout mice do not exhibit changes of energy homeostasis, suggesting that insulin action in POMC and AgRP cells maybe unnecessary for the steady-state control of feeding behavior and body weight (35).
It is well established that the mTORC1 signaling pathway is activated by insulin, and, when activated, mTORC1-S6K functions as a negative feedback regulator of insulin signaling by serine phosphorylation of IRS-1/2 (38, 73). In line with these findings, icv administration of insulin also activates the mTORC1 pathway in mouse ARC via a PI3K/Akt-dependent mechanism (48). On the other hand, rapamycin-induced inhibition of hypothalamic mTORC1 reverses the effects of insulin on food intake and body weight (48). Hypothalamic insulin signaling is known to be an important regulator of hepatic glucose production (HGP) in rodents (17, 51). Overexpression of S6K in the hypothalamus abolishes, whereas overexpression of dominant-negative S6K or dominant-negative Raptor in the MBH restores the ability of insulin to suppress HGP after HFD feeding (52). Since the MBH mediates HFD-induced insulin resistance in the liver, these results indicate that the effects of the hypothalamic mTORC1-S6K pathway on diet-induced hepatic insulin resistance may act through the suppression of insulin signaling in the MBH. Consistent with this finding, mice with MBH overexpression of DEPTOR, the negative regulator of mTORC1, show increased hypothalamic insulin signaling and reduced HFD-induced obesity and hepatic steatosis as well as improved glucose metabolism (10). Taken together, current data demonstrate the importance of hypothalamic mTORC1 pathway in mediating the action of insulin to regulate energy homeostasis.
mTORC1 and ghrelin.
Ghrelin, a peptide hormone secreted by specialized cells in the stomach and duodenum, is the well-known gastric hormone acting in the brain to increase food intake and body weight (50, 72). Unlike anorexigenic actions of leptin and insulin, fasting-elevated ghrelin is a feeding motivator and potently increases food intake and adiposity via actions in the hypothalamus (72). The stimulatory actions of ghrelin on appetite and body weight are mediated through the growth factor secretagogue receptor (GHSR) in the ARC and VMH (68). In the ARC, ghrelin rapidly increases NPY/AgRP neuronal firing and meanwhile increases the NPY and AgRP mRNA concentrations and peptide release from nerve terminals in the PVH while inhibiting POMC neuron activity (28). Furthermore, ghrelin promotes eating by stimulating GABA inhibitory postsynaptic currents and synaptic contacts on the POMC neurons (6, 20, 70).
Ghrelin-activated NPY/AgRP neurons have been suggested to be controlled by hypothalamic Sirtuin 1 (Sirt1)/p53 and AMPK signaling pathways, leading to inhibition of de novo lipogenesis and increased fatty acid oxidation (37, 50). In rats, the stimulatory effect of hypothalamic ghrelin on AgRP and NPY gene expression is controlled by the homeobox domain transcription factor BSX, which involves two other transcription factors, pCREB (phosphorylated cAMP response element-binding protein) and FoxO1 (forkhead box O1) (75). Recent studies suggest that hypothalamic mTORC1 signaling may mediate the orexigenic action of ghrelin. Ghrelin administration through icv injection induces mTORC1 activity in the ARC accompanied by an increase in AgRP, NPY, pCREB, and FOXO1 and whose effects are blocked by central rapamycin treatment (45). Thus, mTORC1 activity might be necessary for ghrelin-induced AgRP and NPY expression, probably through the modulation of pCREB and FOXO1 (45). Similarly, chronic peripheral administration of ghrelin promotes weight gain and fat accumulation in wild-type but not in S6K1-deleted mice (67).
Since ghrelin and leptin exert opposite regulatory effects on POMC and AgRP neurons in the ARC and the actions of both hormones on feeding behavior are mediated by mTORC1 activity (13, 40), it is conceivable that mTORC1 may serve as a switch to mediate the distinct role of these two hormones in the regulation of food intake, depending upon the nutrition status and activated neuronal populations (20). Indeed, the regulation of mTORC1 to nutritional and hormonal perturbations in the MBH depends on cell and stimulus types rather than in a uniform manner (77).
In addition to mediating the actions of leptin, insulin, and ghrelin on feeding behavior and body weight, hypothalamic mTORC1 is also involved in controlling the actions of orexigenic thyroid hormones (TH) in ARC and anorectic GLP-1 in VMH and LH (25, 76). Although it is still unclear how mTORC1 signaling in a distinct neuron population mediates various hormone actions to control glucose/lipid metabolism and energy homeostasis, the ability of mTORC1 to sense various nutrient and hormone signals, control gene transcription, and regulate neuronal development and plasticity suggests that mTORC1 serves as a key signaling hub that allows hypothalamic neurons to produce coordinated responses that maintain whole body energy homeostasis.
Roles of Hypothalamic mTORC1 Signaling in the Regulation of Beige Fat Development and Thermogenesis
Thermogenesis dissipates energy in the form of heat in response to environmental changes of temperature, stress, diet, and other unfavorable conditions. The brown adipose tissue (BAT) is a specialized fat tissue responsible for adaptive thermogenesis, as it contains a large number of mitochondria that generate heat through uncoupling protein-1 (UCP1)(9). Although classical brown adipocytes, such as those that reside in the interscapular region in rodents, originate from Myf5-positive cells, brown-like adipocytes can also be induced in white adipose tissue (WAT) through a process termed “browning” or “beigeing” (22). These recruitable brown-like adipocytes, known as beige or brite adipocytes, increase energy expenditure in mice and correlate with leanness in humans; thus, pharmacological expansion of beige fat represents a potential strategy for combating obesity and metabolic diseases (22, 61, 78).
It is well established that CNS networks govern sympathetic nerve activity (SNA) to regulate BAT thermogenesis and energy expenditure, thereby contributing to overall energy homeostasis (22, 42, 47, 86). In both rodents and humans, the sympathetic nervous system (SNS) is known to regulate BAT mass and activity. The activation and expansion of BAT requires the release of norepinephrine (NE) from postganglionic sympathetic nerve terminals, which in turn stimulate lipid oxidation through the β3-adrenoreceptor (88). In addition, the browning/beigeing of white adipocytes also involves increased NE release from the SNS; all together, the neural networks in the CNS determine sympathetic neural outflow and regulate SNA of BAT and WAT (46, 47).
The peptides and neuronal populations in the hypothalamic areas, including the PVH, LH, DMH, ARC, and preoptic area (POA), are also important regulators of BAT thermogenesis and WAT browning (47, 86). For example, activated POMC neurons promote, whereas AgRP neurons suppress, browning of white fat (15, 63). The effects of hypothalamic leptin and insulin on body weight and energy utilization are believed to act, in part, through their actions on thermogenesis in BAT (60, 62). Both hormones increase the activity of the SNS (59, 60) and stimulate the expression of UCP1 (11, 18). Leptin is also important for beige fat development by activating SNA to WAT via stimulating PI3K signaling in the CNS (55). A very recent study by Dodd et al. (15) demonstrates that insulin and leptin may act together on POMC neurons to promote WAT browning and weight loss. However, as an essential mediator of both leptin and insulin, whether and how hypothalamic mTORC1 is involved in the actions of leptin and insulin on the regulation of thermogenesis and WAT browning are largely unknown and deserve comprehensive investigation.
In addition to its role in the CNS, the mTOR signaling pathway has also been shown to play a direct role in regulating thermogenesis in both BAT and beige fat. Constitutive activation of mTORC1 signaling by knocking out the Tsc1 gene leads to decreased expression of UCP1 and PPARγ coactivator 1α (PGC-1α) in BAT (81). On the other hand, fat-specific knockout of Raptor, a positive regulator of the mTORC1 signaling pathway, increases UCP1 expression and the beigeing of WAT (56). Recently, we have found that increasing mTORC1 signaling by fat-specific knocking out of Grb10, a negative regulator of mTORC1 signaling, suppresses UCP1 expression, decreases core body temperature and cold tolerance, and increases adiposity in mice (36).
Much less is known about the role of hypothalamic mTORC1 signaling in the regulation of beige fat development, BAT function, and thermogenesis. In response to cold exposure, adenoviral-mediated constitutive activation of S6K1 in the MBH of rats leads to higher core temperature and elevated UCP1 levels in the BAT, whereas overexpression of dominant-negative S6K mutant reduces cold tolerance and body temperature compared with controls (5, 52). These results suggest that central manipulation of mTORC1 signaling may promote BAT function and thermogenesis, but the underlying mechanisms are still largely unknown.
Recent studies show that mTORC1 is involved in the action of SNS. Both icv injection of rapamycin and adenovirus-mediated overexpression of dominant-negative S6K substantially reduced leptin-induced renal SNA and arterial pressure in rats (21). Melanocortin-3/4 receptors (MC3/4Rs) are known downstream mediators of the sympathetic and cardiovascular actions of leptin. Interestingly, mice pretreated with rapamycin have no response to icv injection of MTII, an activator of the MC3/4Rs that increases renal SNA or arterial pressure, indicating that mTORC1 may mediate the sympathetic and cardiovascular effects of leptin on the melanocortin system (21). Similarly, adenoviral-mediated hypothalamic constitutive activation of S6K1 decreases leucine deprivation-stimulated energy expenditure, and the effect is mediated by modulation of corticotropin-releasing hormone (CRH) expression in a MC4R-dependent manner (80). Very recently, Muta et al. (48) demonstrated that hypothalamic mTORC1 is also involved in insulin action on the SNS. Inhibition of hypothalamic mTORC1 with rapamycin not only reverses the suppressing central effects of insulin on food intake and body weight gain, but also abolishes insulin-stimulated lumbar SNA (48). Because the SNS mediates the central leptin or insulin action on BAT thermogenesis, it is reasonable to postulate that hypothalamic mTORC1 may mediate the action of anorexigenic hormones on BAT function and WAT browning by regulating the SNS. Further studies will be needed to verify this possibility.
Concluding Remarks and Future Directions
As an essential energy sensor, the mTORC1 signaling pathway plays a critical role, not only in peripheral tissues but also in the CNS, in the regulation of whole body energy balance. Current data suggest that the activity of hypothalamic mTORC1 changes in response to various energy and hormone stimuli, and manipulation of this signaling pathway, has profound impacts on feeding behavior and energy expenditure as well as insulin sensitivity and glucose/lipid metabolism in rodents (Fig. 2). Importantly, although hypothalamic mTORC1 signaling is a critical mediator of hormone and nutrient actions on whole body energy metabolism, a number of key questions remain to be answered. First, it remains unclear how mTORC1 signaling in distinct neuron population is regulated by hormonal and nutrient factors. It is also unclear how mTORC1 regulates distinct neuronal activity and function and thus the different neuronal circuits controlling energy homeostasis. In addition, it is unknown whether and how mTORC1 cross-talks to other energy sensors, such as AMPK, in the hypothalamus to control energy balance. Furthermore, ER and mitochondrial function are critical for insulin sensitivity and energy balance and are closely linked to mTORC1 signaling in the peripheral tissues. However, it remains to be determined whether mTORC1 plays a role in ER and/or mitochondrial function in the hypothalamus to regulate whole body energy metabolism. Finally, whether and how hypothalamic mTORC1 regulates the SNS outflow to BAT and WAT to regulate thermogenesis and energy expenditure are still largely unknown. In the future, animal models with nucleus-specific manipulation of the different proteins constituting mTORC1 signaling pathways, along with the use of noninvasive neuroimaging, optogenetics, and electrophysiology, will help shed light on the bona fide roles and specific cellular and molecular mechanisms of mTORC1 in the hypothalamus. Undoubtedly, fully understanding the role and the underlying mechanisms of mTOR signaling in the brain will pave new avenues for future therapeutics and medical interventions for obesity, insulin resistance, diabetes, and related metabolic disorders.
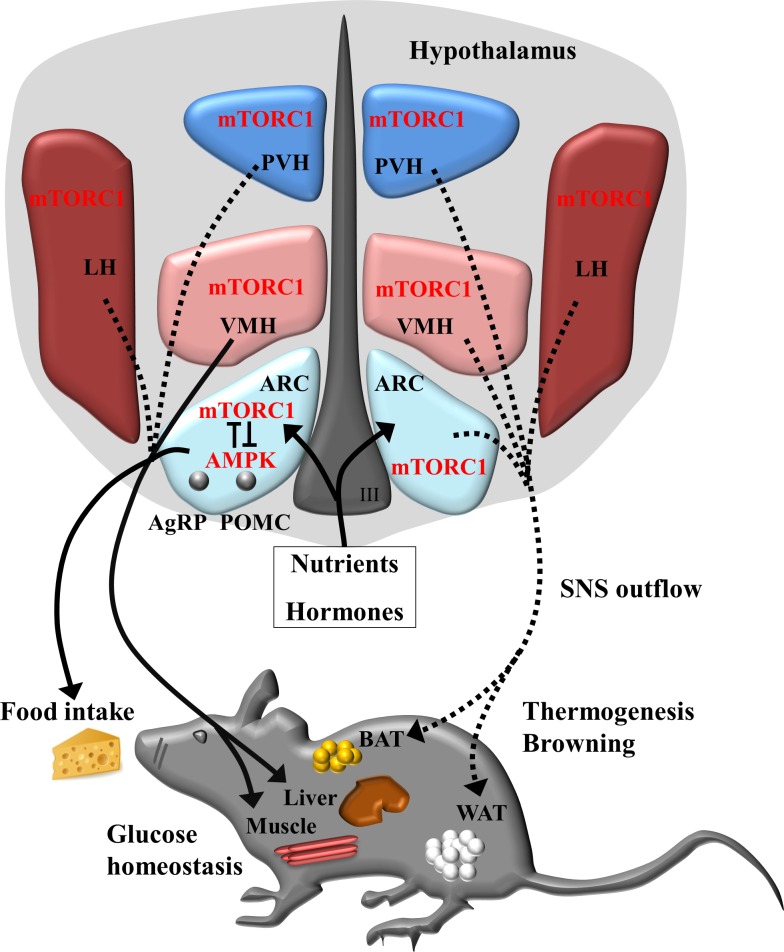
Fig. 2.
Schematic model for hypothalamic mTORC1 in the regulation of whole body energy homeostasis. Current evidence shows that, in response to various hormone and nutrient signals, mTORC1 activities can be detected in the neurons of the arcuate nucleus (ARC) and ventromedial (VMH), lateral (LH), and paraventricular (PVH) hypothalamus. In the proopiomelanocortin (POMC) and agouti-reated peptide (AgRP) neurons of hypothalamic ARC, the bidirectional negative regulation between mTORC1and AMPK may regulate activity of neurons that project to and act on distinct downstream neuron populations to suppress or increase food intake as well as insulin sensitivity and glucose homeostasis in the liver and muscle. mTORC1 in hypothalamic neurons may also integrate energy signals to regulate energy expenditure by modulating sympathetic nervous system (SNS) firing to the brown adipose tissue (BAT) and white adipose tissue (WAT). Solid lines and arrows indicate the known pathways; dashed lines and arrows indicate the unknown pathways.
GRANTS
This work was supported by Grants 2014CB910500 and 2012CB524900 from the National Program on Key Basic Research Project, Grants 31471131 and 81130015 from the National Natural Science Foundation of China, and Grants RO1 DK-100697, RO1 DK-093587, and RO1 DK-101379 from the National Institute of Diabetes and Digestive and Kidney Diseases.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
F.H. prepared figures; F.H. drafted manuscript; F.H., Y.X., and F.L. edited and revised manuscript; F.H. and F.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Guangdi Li for assistance with the graphic presentations and Christopher Cervantes for language editing.
REFERENCES
- 1.Aguilar V, Alliouachene S, Sotiropoulos A, Sobering A, Athea Y, Djouadi F, Miraux S, Thiaudiere E, Foretz M, Viollet B, Diolez P, Bastin J, Benit P, Rustin P, Carling D, Sandri M, Ventura-Clapier R, Pende M. S6 kinase deletion suppresses muscle growth adaptations to nutrient availability by activating AMP kinase. Cell Metab 5: 476–487, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Andre C, Cota D. Coupling nutrient sensing to metabolic homoeostasis: the role of the mammalian target of rapamycin complex 1 pathway. Proc Nutr Soc 71: 502–510, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Appenzeller-Herzog C, Hall MN. Bidirectional crosstalk between endoplasmic reticulum stress and mTOR signaling. Trends Cell Biol 22: 274–282, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Astrinidis A, Henske EP. Tuberous sclerosis complex: linking growth and energy signaling pathways with human disease. Oncogene 24: 7475–7481, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Blouet C, Ono H, Schwartz GJ. Mediobasal hypothalamic p70 S6 kinase 1 modulates the control of energy homeostasis. Cell Metab 8: 459–467, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briggs DI, Andrews ZB. Metabolic status regulates ghrelin function on energy homeostasis. Neuroendocrinology 93: 48–57, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science 289: 2122–2125, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Cai H, Dong LQ, Liu F. Recent advances in adipose mTOR signaling and function: therapeutic prospects. Trends Pharmacol Sci 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Caron A, Labbe SM, Lanfray D, Blanchard PG, Villot R, Roy C, Sabatini DM, Richard D, Laplante M. Mediobasal hypothalamic overexpression of DEPTOR protects against high-fat diet-induced obesity. Mol Metab 5: 102–112, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Commins SP, Watson PM, Levin N, Beiler RJ, Gettys TW. Central leptin regulates the UCP1 and ob genes in brown and white adipose tissue via different beta-adrenoceptor subtypes. J Biol Chem 275: 33059–33067, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Cota D, Matter EK, Woods SC, Seeley RJ. The role of hypothalamic mammalian target of rapamycin complex 1 signaling in diet-induced obesity. J Neurosci 28: 7202–7208, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science 312: 927–930, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Dagon Y, Hur E, Zheng B, Wellenstein K, Cantley LC, Kahn BB. p70S6 kinase phosphorylates AMPK on serine 491 to mediate leptin's effect on food intake. Cell Metab 16: 104–112, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dodd GT, Decherf S, Loh K, Simonds SE, Wiede F, Balland E, Merry TL, Munzberg H, Zhang ZY, Kahn BB, Neel BG, Bence KK, Andrews ZB, Cowley MA, Tiganis T. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell 160: 88–104, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folli F, Bonfanti L, Renard E, Kahn CR, Merighi A. Insulin receptor substrate-1 (IRS-1) distribution in the rat central nervous system. J Neurosci 14: 6412–6422, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelling RW, Morton GJ, Morrison CD, Niswender KD, Myers MG Jr, Rhodes CJ, Schwartz MW. Insulin action in the brain contributes to glucose lowering during insulin treatment of diabetes. Cell Metab 3: 67–73, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Geloen A, Trayhurn P. Regulation of the level of uncoupling protein in brown adipose tissue by insulin requires the mediation of the sympathetic nervous system. FEBS Lett 267: 265–267, 1990. [DOI] [PubMed] [Google Scholar]
- 19.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30: 214–226, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haissaguerre M, Saucisse N, Cota D. Influence of mTOR in energy and metabolic homeostasis. Mol Cell Endocrinol 397: 67–77, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Harlan SM, Guo DF, Morgan DA, Fernandes-Santos C, Rahmouni K. Hypothalamic mTORC1 signaling controls sympathetic nerve activity and arterial pressure and mediates leptin effects. Cell Metab 17: 599–606, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med 19: 1252–1263, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev 18: 1926–1945, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Hu F, Liu F. Targeting tissue-specific metabolic signaling pathways in aging: the promise and limitations. Protein Cell 5: 21–35, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hurtado-Carneiro V, Sanz C, Roncero I, Vazquez P, Blazquez E, Alvarez E. Glucagon-like peptide 1 (GLP-1) can reverse AMP-activated protein kinase (AMPK) and S6 kinase (P70S6K) activities induced by fluctuations in glucose levels in hypothalamic areas involved in feeding behaviour. Mol Neurobiol 45: 348–361, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Inhoff T, Stengel A, Peter L, Goebel M, Tache Y, Bannert N, Wiedenmann B, Klapp BF, Monnikes H, Kobelt P. Novel insight in distribution of nesfatin-1 and phospho-mTOR in the arcuate nucleus of the hypothalamus of rats. Peptides 31: 257–262, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jewell JL, Guan KL. Nutrient signaling to mTOR and cell growth. Trends Biochem Sci 38: 233–242, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Central effect of ghrelin, an endogenous growth hormone secretagogue, on hypothalamic peptide gene expression. Endocrinology 141: 4797–4800, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferre P, Foufelle F. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest 119: 1201–1215, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology 146: 1473–1481, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13: 132–141, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JG, Horvath TL. mTOR signaling fades POMC neurons during aging. Neuron 75: 356–357, 2012. [DOI] [PubMed] [Google Scholar]
- 33.Kleinridders A, Ferris HA, Cai W, Kahn CR. Insulin action in brain regulates systemic metabolism and brain function. Diabetes 63: 2232–2243, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kocalis HE, Hagan SL, George L, Turney MK, Siuta MA, Laryea GN, Morris LC, Muglia LJ, Printz RL, Stanwood GD, Niswender KD. Rictor/mTORC2 facilitates central regulation of energy and glucose homeostasis. Mol Metab 3: 394–407, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Bruning JC. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab 5: 438–449, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Liu M, Bai J, He S, Villarreal R, Hu D, Zhang C, Yang X, Liang H, Slaga TJ, Yu Y, Zhou Z, Blenis J, Scherer PE, Dong LQ, Liu F. Grb10 promotes lipolysis and thermogenesis by phosphorylation-dependent feedback inhibition of mTORC1. Cell Metab 19: 967–980, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez M, Lage R, Saha AK, Perez-Tilve D, Vazquez MJ, Varela L, Sangiao-Alvarellos S, Tovar S, Raghay K, Rodriguez-Cuenca S, Deoliveira RM, Castaneda T, Datta R, Dong JZ, Culler M, Sleeman MW, Alvarez CV, Gallego R, Lelliott CJ, Carling D, Tschop MH, Dieguez C, Vidal-Puig A. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab 7: 389–399, 2008. [DOI] [PubMed] [Google Scholar]
- 38.Manning BD. Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J Cell Biol 167: 399–403, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez de Morentin PB, Martinez-Sanchez N, Roa J, Ferno J, Nogueiras R, Tena-Sempere M, Dieguez C, Lopez M. Hypothalamic mTOR: the rookie energy sensor. Curr Mol Med 14: 3–21, 2014. [DOI] [PubMed] [Google Scholar]
- 40.Martins L, Fernandez-Mallo D, Novelle MG, Vazquez MJ, Tena-Sempere M, Nogueiras R, Lopez M, Dieguez C. Hypothalamic mTOR signaling mediates the orexigenic action of ghrelin. PLos One 7: e46923, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maya-Monteiro CM, Bozza PT. Leptin and mTOR: partners in metabolism and inflammation. Cell Cycle 7: 1713–1717, 2008. [DOI] [PubMed] [Google Scholar]
- 42.McGlashon JM, Gorecki MC, Kozlowski AE, Thirnbeck CK, Markan KR, Leslie KL, Kotas ME, Potthoff MJ, Richerson GB, Gillum MP. Central serotonergic neurons activate and recruit thermogenic brown and beige fat and regulate glucose and lipid homeostasis. Cell Metab 21: 692–705, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428: 569–574, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Mori H, Inoki K, Munzberg H, Opland D, Faouzi M, Villanueva EC, Ikenoue T, Kwiatkowski D, MacDougald OA, Myers MG Jr, Guan KL. Critical role for hypothalamic mTOR activity in energy balance. Cell Metab 9: 362–374, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morrison CD, Xi X, White CL, Ye J, Martin RJ. Amino acids inhibit Agrp gene expression via an mTOR-dependent mechanism. Am J Physiol Endocrinol Metab 293: E165–E171, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morrison SF, Madden CJ, Tupone D. Central control of brown adipose tissue thermogenesis. Front Endocrinol 3, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morrison SF, Madden CJ, Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab 19: 741–756, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muta K, Morgan DA, Rahmouni K. The role of hypothalamic mTORC1 signaling in insulin regulation of food intake, body weight, and sympathetic nerve activity in male mice. Endocrinology 156: 1398–1407, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol 70: 537–556, 2008. [DOI] [PubMed] [Google Scholar]
- 50.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature 409: 194–198, 2001. [DOI] [PubMed] [Google Scholar]
- 51.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 8: 1376–1382, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Ono H, Pocai A, Wang Y, Sakoda H, Asano T, Backer JM, Schwartz GJ, Rossetti L. Activation of hypothalamic S6 kinase mediates diet-induced hepatic insulin resistance in rats. J Clin Invest 118: 2959–2968, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oshiro N, Takahashi R, Yoshino K, Tanimura K, Nakashima A, Eguchi S, Miyamoto T, Hara K, Takehana K, Avruch J, Kikkawa U, Yonezawa K. The proline-rich Akt substrate of 40 kDa (PRAS40) is a physiological substrate of mammalian target of rapamycin complex 1. J Biol Chem 282: 20329–20339, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306: 457–461, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Plum L, Rother E, Munzberg H, Wunderlich FT, Morgan DA, Hampel B, Shanabrough M, Janoschek R, Konner AC, Alber J, Suzuki A, Krone W, Horvath TL, Rahmouni K, Bruning JC. Enhanced leptin-stimulated Pi3k activation in the CNS promotes white adipose tissue transdifferentiation. Cell Metab 6: 431–445, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Polak P, Cybulski N, Feige JN, Auwerx J, Ruegg MA, Hall MN. Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab 8: 399–410, 2008. [DOI] [PubMed] [Google Scholar]
- 57.Procaccini C, De Rosa V, Galgani M, Carbone F, Cassano S, Greco D, Qian K, Auvinen P, Cali G, Stallone G, Formisano L, La Cava A, Matarese G. Leptin-induced mTOR activation defines a specific molecular and transcriptional signature controlling CD4+ effector T cell responses. J Immunol 189: 2941–2953, 2012. [DOI] [PubMed] [Google Scholar]
- 58.Proulx K, Cota D, Woods SC, Seeley RJ. Fatty acid synthase inhibitors modulate energy balance via mammalian target of rapamycin complex 1 signaling in the central nervous system. Diabetes 57: 3231–3238, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rahmouni K, Morgan DA, Morgan GM, Liu X, Sigmund CD, Mark AL, Haynes WG. Hypothalamic PI3K and MAPK differentially mediate regional sympathetic activation to insulin. J Clin Invest 114: 652–658, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rezai-Zadeh K, Munzberg H. Integration of sensory information via central thermoregulatory leptin targets. Physiol Behav 121: 49–55, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell 156: 20–44, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rothwell NJ, Stock MJ. Insulin and thermogenesis. Int J Obes 12: 93–102, 1988. [PubMed] [Google Scholar]
- 63.Ruan HB, Dietrich MO, Liu ZW, Zimmer MR, Li MD, Singh JP, Zhang K, Yin R, Wu J, Horvath TL, Yang X. O-GlcNAc transferase enables AgRP neurons to suppress browning of white fat. Cell 159: 306–317, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320: 1496–1501, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 404: 661–671, 2000. [DOI] [PubMed] [Google Scholar]
- 66.Smith MA, Katsouri L, Irvine EE, Hankir MK, Pedroni SM, Voshol PJ, Gordon MW, Choudhury AI, Woods A, Vidal-Puig A, Carling D, Withers DJ. Ribosomal S6K1 in POMC and AgRP Neurons Regulates Glucose Homeostasis but Not Feeding Behavior in Mice. Cell Reports 11: 335–343, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stevanovic D, Trajkovic V, Muller-Luhlhoff S, Brandt E, Abplanalp W, Bumke-Vogt C, Liehl B, Wiedmer P, Janjetovic K, Starcevic V, Pfeiffer AF, Al-Hasani H, Tschop MH, Castaneda TR. Ghrelin-induced food intake and adiposity depend on central mTORC1/S6K1 signaling. Mol Cell Endocrinol 381: 280–290, 2013. [DOI] [PubMed] [Google Scholar]
- 68.Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci USA 101: 4679–4684, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thomanetz V, Angliker N, Cloetta D, Lustenberger RM, Schweighauser M, Oliveri F, Suzuki N, Ruegg MA. Ablation of the mTORC2 component rictor in brain or Purkinje cells affects size and neuron morphology. J Cell Biol 201: 293–308, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci 11: 998–1000, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tremblay F, Marette A. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J Biol Chem 276: 38052–38060, 2001. [DOI] [PubMed] [Google Scholar]
- 72.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature 407: 908–913, 2000. [DOI] [PubMed] [Google Scholar]
- 73.Um SH, D'Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab 3: 393–402, 2006. [DOI] [PubMed] [Google Scholar]
- 74.Unger J, McNeill TH, Moxley RT 3rd, White M, Moss A, Livingston JN. Distribution of insulin receptor-like immunoreactivity in the rat forebrain. Neuroscience 31: 143–157, 1989. [DOI] [PubMed] [Google Scholar]
- 75.Varela L, Horvath TL. Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. EMBO Rep 13: 1079–1086, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Varela L, Martinez-Sanchez N, Gallego R, Vazquez MJ, Roa J, Gandara M, Schoenmakers E, Nogueiras R, Chatterjee K, Tena-Sempere M, Dieguez C, Lopez M. Hypothalamic mTOR pathway mediates thyroid hormone-induced hyperphagia in hyperthyroidism. J Pathol 227: 209–222, 2012. [DOI] [PubMed] [Google Scholar]
- 77.Villanueva EC, Munzberg H, Cota D, Leshan RL, Kopp K, Ishida-Takahashi R, Jones JC, Fingar DC, Seeley RJ, Myers MG Jr. Complex regulation of mammalian target of rapamycin complex 1 in the basomedial hypothalamus by leptin and nutritional status. Endocrinology 150: 4541–4551, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77a.Woods SC, Lotter EC, McKay LD, Porte D Jr. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature 282: 503–505, 1979. [DOI] [PubMed] [Google Scholar]
- 78.Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev 27: 234–250, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 124: 471–484, 2006. [DOI] [PubMed] [Google Scholar]
- 80.Xia T, Cheng Y, Zhang Q, Xiao F, Liu B, Chen S, Guo F. S6K1 in the central nervous system regulates energy expenditure via MC4R/CRH pathways in response to deprivation of an essential amino acid. Diabetes 61: 2461–2471, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xiang X, Lan H, Tang H, Yuan F, Xu Y, Zhao J, Li Y, Zhang W. Tuberous sclerosis complex 1-mechanistic target of rapamycin complex 1 signaling determines brown-to-white adipocyte phenotypic switch. Diabetes 64: 519–528, 2015. [DOI] [PubMed] [Google Scholar]
- 82.Xu Y, Elmquist JK, Fukuda M. Central nervous control of energy and glucose balance: focus on the central melanocortin system. Ann NY Acad Sci 1243: 1–14, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang SB, Tien AC, Boddupalli G, Xu AW, Jan YN, Jan LY. Rapamycin ameliorates age-dependent obesity associated with increased mTOR signaling in hypothalamic POMC neurons. Neuron 75: 425–436, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu Y, Yoon SO, Poulogiannis G, Yang Q, Ma XM, Villen J, Kubica N, Hoffman GR, Cantley LC, Gygi SP, Blenis J. Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science 332: 1322–1326, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang Q, Yu J, Liu B, Lv Z, Xia T, Xiao F, Chen S, Guo F. Central activating transcription factor 4 (ATF4) regulates hepatic insulin resistance in mice via S6K1 signaling and the vagus nerve. Diabetes 62: 2230–2239, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang W, Bi S. Hypothalamic regulation of brown adipose tissue thermogenesis and energy homeostasis. Front Endocrinol 6: 136, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 135: 61–73, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao J, Unelius L, Bengtsson T, Cannon B, Nedergaard J. Coexisting beta-adrenoceptor subtypes: significance for thermogenic process in brown fat cells. Am J Physiol Cell Physiol 267: C969–C979, 1994. [DOI] [PubMed] [Google Scholar]
- 89.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12: 21–35, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]