Abstract
Mucositis is a major oncological problem. The entire gastrointestinal and genitourinary tract and also other mucosal surfaces can be affected in recipients of radiotherapy, and/or chemotherapy. Major progress has been made in recent years in understanding the mechanisms of oral and small intestinal mucositis, which appears to be more prominent than colonic damage. This progress is largely due to the development of representative laboratory animal models of mucositis. This review focuses on the development and establishment of the Dark Agouti rat mammary adenocarcinoma model by the Mucositis Research Group of the University of Adelaide over the past 20 years to characterize the mechanisms underlying methotrexate-, 5-fluorouracil-, and irinotecan-induced mucositis. It also aims to summarize the results from studies using different animal model systems to identify new molecular and cellular markers of mucositis.
Keywords: Rat, mucositis, methotrexate, 5-fluorouracil, irinotecan, chemotherapy
Introduction
The major problem with anticancer therapies (radiation and chemotherapy) is the unwanted toxicity to normal tissues. Chemotoxic drugs are known to act by inducing apoptosis or cell cycle arrest in cancer cells but unfortunately also in normal cells.1,2 Granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), and platelet-derived growth factor can ameliorate common bone marrow side effects such as anaemia, thrombocytopenia and neutropenia, and since their development, the doses of chemotherapeutic drugs have been able to be increased, leading to toxicity of other organ systems, particularly the gastrointestinal (GI) tract, the genitourinary system, the nervous system, and the heart.
Mucositis is a clinical term used to describe damage to mucous membranes after anticancer therapies.3 It occurs throughout the entire gastrointestinal tract (GT) (including the mouth) and genitourinary tract, and to a lesser extent in other mucosal surfaces. Its severity and duration varies with the dose and the type of drug used. The importance of mucositis is that it limits the dose of chemotherapy. Cancer therapy-associated mucosal injury of the alimentary tract (mouth to anus) (AT) is referred to as alimentary mucositis.4 The GI crypt epithelium is particularly vulnerable to chemotherapeutic toxicity, with symptoms including nausea and vomiting, abdominal pain, distension, and diarrhoea due to direct effects of the cytotoxics on the mucosa. Abdominal pain is caused by the extensive damage occurring in the abdominal region. Diarrhoea and constipation are thought to be caused by the alteration in absorptive functions of cells, mucin distribution and composition, and microbial interactions with these cells and metabolites of the drugs themselves.5,6 Direct effects of the cytotoxics result in massive apoptosis at the crypt base,7 whereas indirect effects are due to lack of enteral intake, attenuation in secretion of GI hormones secondary to the bowel injury, and acute and chronic infections,8,9 the latter due to the bacterial translocation (BT) and the systemic spread of microbiota from the gut to systemic organs. BT is a significant cause of sepsis in critically ill patients and is linked to microbial overgrowth, immunosuppression and loss of physical barrier.10 Both microbiota and endotoxins cause systemic infection and multi-organ failure.
The exact mechanisms of oral and GI mucositis are not fully understood, yet a lot of progress has been made over the last several years. This is largely due to the development of representative preclinical animal models of mucositis (mouse, hamster, rat). Since AT damage is most visibly expressed in the mouth of patients, researchers developed models for mucosal injury focusing on oral damage following irradiation and chemotherapy and to which they applied a visual scoring system of the oral damage. The models include the mouse lip (irradiation),11,12 the mouse ventral tongue mucosa (irradiation),13 and the hamster cheek pouch (5-fluorouracil (5FU)),14 and are now widely used to investigate various aspects of oral mucositis including the pathobiology and treatment as well as the mucotoxicity of the drugs used. GI mucositis models using histological evaluation of the small intestine and/or colon as the gold standard for the detection of GI inflammation and damage have also been developed, using mice and rats. Mouse GI mucositis models have been developed for melphalan,15,16 cytosine arabinoside, vincristine, doxorubicin,17,18 cyclophosphamide,19 5FU,20,21 and methotrexate (MTX).22 Rats have also become popular experimental animals in the establishment of GI mucositis models and are now used widely in mucositis studies. This review focuses on three rat models of cytotoxic drug-induced GI mucositis that have been developed and optimized by the Mucositis Research Group of Professor Dorothy Keefe over the past two decades. These models are based on work that has been done with Dark Agouti rats and the cytotoxic drugs MTX, 5FU, and irinotecan. The group also developed the rat model of lapatinib-induced diarrhoea which simulates the patients’ diarrhoea following treatment with epidermal growth factor receptor tyrosine kinase inhibitors. However, since the establishment of this model has recently been reviewed, we decided to exclude it from this overview.23 Moreover, the group is working on the development of a rat model of radiotherapy-induced GI mucositis. The models have allowed to unravel at least in part the complexity of the pathobiology of mucositis and its underlying mechanisms, and to develop preventive strategies and/or therapeutic drugs to treat mucositis without reducing the efficacy of chemotherapy.
Dark Agouti rat mammary adenocarcinoma model
In 2002, Keefe and co-workers established the Dark Agouti rat mammary adenocarcinoma model (DAMA) model for the simultaneous assessment of the effects of chemotherapy and chemoprotectants on both the entire AT and the tumor24 and it is now one of the most extensively used models to investigate chemotherapy-induced mucositis in rats.2,25–28 This model has the benefit that the rats are bearing breast cancer tumors and thus allows us to evaluate the additional effect of tumor burden on the overall chemotherapy-induced gut toxicity,29 reflecting more accurately the patient setting of chemotherapy-induced mucositis.
The model is based on the injection of breast cancer cells subcutaneously into both flanks of the female rats, which are grown over approximately two weeks to represent up to 15% of bodyweight (BW). Although the model has previously been used for studies of malnutrition following chemotherapy30,31 and for studies of neuroprotection by glutamate,32 it had not been applied to assess GI injury nor protection after chemotherapy. Initially, Keefe and co-workers developed the model to assess small intestinal toxicity; however, it is now routinely used to investigate toxicities arising at different sites along the AT (oral mucosa, stomach, and intestines).
General treatment protocol
All studies start with the approval by the Animal Ethics Committees of The Institute of Medical and Veterinary Sciences (now SA Pathology) and of The University of Adelaide and comply with the latest National Health and Medical Research Council (Australia) Code of Practice for Animal Care in Research and Training. All animals are monitored four times daily for clinical signs of stress; these scores are tallied, and if any animal exceeds the maximum acceptable total score (as defined by the Animal Ethics Committee) they are euthanized. These criteria include a dull ruffled coat with accompanying dull and sunken eyes, coolness to touch with no spontaneous movement, hunched appearance, diarrhoea, reduced food and water intake, and tumour burden >10% of body weight.
In each experiment, rats are randomly assigned to either a control or experimental group according to a specific time point. All rats in the experimental groups receive a single intramuscular dose in case of MTX, or a single intraperitoneal dose in the case of 5FU or irinotecan. Rats in the control groups receive treatment with the solvent vehicle (saline for MTX, dimethylsulphoxide for 5FU, sorbitol/lactic acid buffer for irinotecan). In the case of irinotecan administration, animals receive a subcutaneous injection of atropine immediately prior to irinotecan injection to prevent a severe cholinergic reaction. Subsequent to administration of the chemotherapy drugs, end points such as mortality, diarrhoea, and general clinical condition are assessed four times per 24 h period. Rats are killed by exsanguination and cervical dislocation usually at 6, 72, and 120 h following administration of the test drug. A section of the AT extending from the pyloric sphincter to the rectum is dissected out and flushed with chilled isotonic saline (0.9% w/v) to remove contents. Samples (1 cm in length) of the small intestine (taken at 25% of the length of the small intestine from the pylorus) and the colon (taken at midcolon position) are dissected and removed for further analysis. In addition, samples of oral mucosa (tongue and buccal mucosa) are taken. All tissue samples are immediately fixed in 10% neutral buffered formalin before processing and embedding in paraffin wax.
The DAMA model of MTX-induced mucositis
MTX is a chemotherapeutic agent that is structurally similar to folic acid which is essential for the de novo synthesis of all purine bases. MTX is a potent inhibitor of dihydrofolate reductase33 which catalyses the conversion of dihydrofolate to the active tetrahydrofolate. DNA synthesis is inhibited if tetrahydrofolate is not available for the production of a coenzyme required for thymidylate formation. Essentially, all purine synthesis and, therefore, also the synthesis of DNA, RNA, thymidylates, and proteins, will be blocked by MTX. As MTX acts specifically during DNA and RNA synthesis, it is cytotoxic during the S-phase of the cell cycle. Moreover, the anticancer effect of MTX has been linked to a decrease in tissue polyamine levels, since tumors are highly dependent on polyamines (putrescine, spermidine, and spermine) for growth.34 MTX is effective for the treatment of a number of cancers including breast, head and neck, leukemia, lymphoma, lung, osteosarcoma, bladder, and trophoblastic neoplasms.35 The most common adverse effects linked to MTX include ulcerative mucositis, low white blood cell count and thus predisposition to infection, nausea, abdominal pain, fatigue, fever, dizziness, acute pneumonitis, and rarely, pulmonary fibrosis. MTX is metabolized by the intestinal microbiota to the inactive metabolite 4-amino-4-deoxy-N-methylpteroic acid; however, this accounts for less than 5% loss of the oral dose.36
Development and characteristics of the model
In the first set of studies, the optimal dose of MTX was calculated to administer intramuscularly to the DA rats in order to produce significant, but non-lethal, small intestinal mucositis.37 Since initial tests with doses <1.5 mg MTX/kg BW given at two consecutive days showed only mild symptoms of mucositis (diarrhoea), the following studies were performed using higher doses. An intramuscular injection of 1.5 or 2 mg MTX/kg BW for two consecutive days reliably caused severe, non-lethal mucositis, clinically manifest by abdominal pain, bloating, and diarrhoea.2,28 No clinical signs of health deterioration or diarrhoea were observed with a single injection of 1.5 mg MTX/kg BW although histology revealed significant signs of GI injury.38 Moreover, no clinical signs of oral mucositis could be observed, indicating that oral mucositis is not a good indicator of changes in the GI of rats.
Histopathological changes subsequent to MTX administration were observed at different sites along the AT and at different time points. Oral thickness decreased between 90 min and 6 h post-MTX treatment, although no sign of typical ulcerations could be observed at the level of the tongue, buccal mucosa, or gingiva. An initial increase in colon crypt length occured between 6 and 48 h, which coincided with a decrease in jejunal crypt length and was followed by a decrease in colon crypt length starting from 48 h post-treatment. Other signs of mucosal damage in the jejunum included blunting and fusion of the villi and obliteration of the crypts.38
Epithelium tissue levels of nuclear factor-kappaB (NF-kappaB) showed a significant elevation in the jejunum (peaking at 6 h) and colon (peaking at 12 h) but not within the oral mucosa. Significantly elevated levels of tumor necrosis factor-alpha (TNF-alpha) were observed in the oral mucosa, jejunum, and colon peaking between 2 and 6 h in the oral mucosa, at 6 h in the jejunum, whilst in the colon the elevation of TNF-alpha peaked at 24 h.38 Conversely, significantly elevated levels of interleukin-1 beta (IL-1beta) were observed in the oral mucosa, jejunum, and colon. Within each of the three AT sites, two peaks of IL-1beta occurred at 6 and 48 h in the oral mucosa and at 2 and 24 h in the jejunum and colon. MTX further showed a systemic effect, as serum levels of NF-kappaB, TNF-alpha, and IL-6 were elevated, with peaks at 2 h, 90 min and 2 h, and 2 h, respectively, and decreased IL-1beta levels at 6 h, all preceding histological changes in tissues.39
Application of the model
The model has been extensively used by Keefe’s group to characterize the mechanisms underlying MTX toxicities recorded in different locations along the AT, and to assess cytoprotective agents. However, in this review we will focus on the group’s effort to better unravel the pathobiology of MTX-induced mucositis and discuss it in light of what other groups have found using similar animal models.
The Keefe group found that the small intestine seemed to be the predominant site of damage after high dose MTX treatment of tumor-bearing rats and that MTX-induced mucositis occurs through p53-dependent apoptosis.2,28,37A time-course study showed that MTX increased apoptosis by 28-fold in the crypts of the small intestine and by 3-fold in the tumor, both peaking at 6 h after the second dose of 1.5 mg MTX/kg BW. At this dose, the major tissue damage was found at the level of the jejunum and coincided with apoptosis and elevated p53 levels. Upregulation of p53 can either induce apoptosis or block cell proliferation. It inhibits progression of the cell cycle through p21, a cyclin-dependent kinase inhibitor necessary to induce cell cycle arrest that is p53-dependent in response to DNA damage following chemotherapy.40 However, p21 levels seem to remain constant following high doses of MTX, suggesting a pro-apoptotic role of p53 following MTX.37 Unlike the small intestine, apoptosis in the colon was recorded to be 10-fold lower, and did not induce overt damage.37 Others have shown that Paneth cells and Peyers’ patches (PP) seem to be spared from MTX-induced damage and that also the function of goblet and Paneth cells remains intact during MTX treatment.39,41
Other groups have used similar animal models (although often in absence of tumors) to identify important mediators and signalling pathways that are involved during MTX-induced toxicity, and can be summarized as follows (Table 1).
– The maintenance of intestinal barrier function is highly dependent on epithelial cell-to-cell adhesion, which is indispensable for intestinal architecture. Tight-junctions (TJs) are intercellular junctional complexes that act as a primary barrier to the diffusion of molecules. They are located at the most apical part of the lateral membranes of epithelial cells and comprise components such as transmembrane protein claudins, occludin, and peripheral membrane proteins zonula occludens (ZOs). ZOs bind to the cytoplasmic tail of claudins, which determine the properties of the cell barrier43 and regulate the localization of the claudins in the TJs ensuring their maintenance. A variety of inflammatory mediators, such as reactive oxygen species (ROS), TNF-alpha and interferon(INF)-gamma, disrupt the TJ barrier and alter the localization and phosphorylation status of ZO-1, and therefore alterations of ZO-1 may be associated with disruption of the TJ barrier, leading to enhanced intestinal permeability. MTX-mediated alterations of ZO-1 were confirmed by Hamada et al. showing that the phosphorylation status and expression of ZO-1 was significantly decreased along the apical side of the villi after MTX administration and coincided with increased intestinal permeability.42 The group further showed that other components of the TJs are altered following MTX.58 Claudin-2 levels were significantly increased in the crypts and villus tips of the small intestine in MTX-treated rats, while claudin-4 levels were decreased in the villus tips. Furthermore, ZO-1/claudin-4 binding was inhibited by MTX, indicating that MTX impairs cell–cell adhesion and hence normal epithelial intestinal barrier function. Hamada’s data further suggested that intestinal barrier dysfunction following MTX administration occurs via a pathway in which MTX-induced ROS elevates macrophage inflammatory protein-2 (MIP-2), TNF-alpha, and/or IL-1beta levels through a signaling cascade downstream of toll-like receptor (TLR4).58 Paralleling the expression pattern of TLR4,53 it is not surprising that MTX-induced mucositis occurs more readily in the small intestine than in the colon.37
– According to the Sonis model of GI mucositis, ROS generation during chemotherapy is the first step leading to intestinal inflammation, further activating NF-kappaB and upregulating the production of inflammatory cytokines. Different studies have reported that MTX administration in rats indeed enhances the production of ROS in small intestinal mucosa.45,46,59 Phagocytes, such as macrophages and neutrophils, infiltrate into the inflamed region, and are thought to be responsible for the formation of ROS,45 which can subsequently alter the localization of TJ components such as ZO-1 and occluding.47,60
– ROS probably act together with reactive nitrogen species (RNS) in damaging small intestinal cells, causing nitrosative stress. The role of nitrosative stress in MTX-induced intestinal damage was first studied by Kolli et al.61 Following treatment with MTX, they found increased staining of nitrotyrosine in the intestinal samples, which was accompanied by neutrophil infiltration and tissue degeneration. RNS are derived from nitrogen oxide (NO) and superoxide produced by inducible nitrogen oxide synthase (iNOS) and nicotinamide adenine dinucleotide phosphate oxidase, respectively. Cytokines are able to induce nitrosative stress through the expression of iNOS with consequent production of NO.44 Leitão et al. evaluated the specific role of the iNOS in mice and showed that MTX injections induced intestinal epithelial damage in wild-type mice but not in iNOS −/− mice. The intestinal damage could be prevented by NOS inhibitors, suggesting a critical role of NO, via the inducible iNOS, in MTX-induced intestinal mucositis.62
– The endocannabinoid system has been shown to be active in the GI system playing important roles in the control of both gastric and intestinal motility and secretion.63 Evidence exists that an overactive endocannabinoid system in the gut might intervene to reduce intestinal inflammation.48 In 2007, D’Argenio et al. showed elevated endocannabinoid levels peaking with atrophy in the jejunum of rats injected with MTX.49 In the muscle/serosa samples, a peak of anandamide, 2-arachidonoyl-glycerol (2-AG), and palmitoylethanolamide (PEA) levels was found three days after treatment, while in the mucosa samples, 2-AG and PEA were significantly elevated after three days in correspondence with the strongest damage.49 The authors speculated that putative protective effects of endocannabinoids are exerted at both the muscle level and the epithelial level to combat acute inflammation.
– Glucagon-like peptide-2 (GLP-2), a peptide member of a family of proglucagon-derived peptides, is secreted from L-type enteroendocrine cells of the distal small intestine and colon, stimulates crypt cell proliferation, and decreases apoptosis in small intestinal epithelium. Plasma GLP-2 levels appear to vary with intestinal mucosal condition and injury. Hirotani et al. demonstrated a relationship between plasma GLP-2 levels and changes in proliferative markers (mucosal weight, DNA and protein content in duodenum, jejunum, and ileum) resulting from MTX-induced intestinal mucositis.64 Therefore, circulating levels of GLP-2 may be a potential marker for MTX-induced intestinal mucositis.
– BT has been defined as the passage of viable microbiota from the gut lumen to extra-intestinal organs.65 When the mucosa is injured and the intestinal barrier is compromised, translocation of intestinal microorganisms can occur.50 MTX may cause intestinal BT as confirmed by Song et al. by the use of a green fluorescent protein-labelled Escherichia coli strain fed to rats.66 Mesenteric lymph nodes, spleen, liver, spleen, and kidney were all positive for the presence of E. coli after rats were treated with MTX. They further showed that G-CSF was effective in prevention of BT. Before microbiota can translocate to other tissues, they have to adhere to the intestinal mucosa. Sandovsky-Losica and Segal were the first to report that adherence of Candida albicans was significantly enhanced after total body irradiation of mice and after treatment with MTX and 5FU. The maximal increase was observed at 3–4 days post-treatment, concurrently with the maximal decrease in white blood cells and spleen weight of the treated animals.51 Unfortunately, no mechanistic work or follow-up studies have been reported.
– It is well known that intestinal differentiation is compromised during MTX treatment. De Koning et al. suggested that this may be explained by the fact that intestine-specific transcription factors caudal type homeobox 2 (Cdx2), GATA binding protein 4 (GATA-4), and hepatocyte-nuclear factor-alpha (HNF-1alpha), important for intestinal development, differentiation, and gene expression, are downregulated during the phase of intestinal damage caused by MTX.67 In combination, Cdx2, GATA-4, and HNF-1alpha have been shown to act as promoting factors of the sucrose-isomaltase (SI) gene68 as well as several other enterocyte-specific markers.69 SI is a brush border enzyme with an important function in degradation of disaccharides70 and is specifically expressed by enterocytes in a differentiation-specific pattern and therefore is a widely used marker for intestinal differentiation. Its expression is characterized by a strong abundance at the crypt-villus junction and mid-villus, and a decreased intensity towards the tips of the villi52 and is affected by MTX.39,71 De Koning et al. showed that intestinal damage in mice following MTX was associated with decreased expression of HNF-1alpha, Cdx2, and GATA-4, which correlated with decreased expression of SI, and seemed inversely correlated with enhanced proliferation of epithelial crypt cells.67 The clinical consequence of SI downregulation is dysfunction of degradation and uptake of sugars by the intestine during villus atrophy. In the rat, other enterocyte markers like lactase, fatty acid binding proteins (I-FABP and L-FABP), SGLT1, and the fructose transporter GLUT5 were also downregulated after MTX treatment similarly to SI.71
– Evidence from in vitro and in vivo experiments suggests that trefoil peptides may play a key role in protecting the GI mucosa from various insults,72–74 probably by preserving the integrity of the epithelial barrier and promoting the formation of a continuous gel with mucin glycoproteins on the mucosal surface.75 In mammals, trefoil peptides are produced predominantly in the GI tract and concentrated within the mucus layer.76 Two studies have shown that the expression of trefoil factor 3 (TFF3) which is predominantly synthesized by the goblet cells in the small and large intestine55,77 is altered after MTX administration. The number of TFF3-positive cells was decreased along the crypt-villous axis during the increasing villous atrophy and was followed by a depletion of TFF3-positive goblet cells in the lower villous region and an accumulation of TFF3-positive cells in the villous tip region.41,71 In contrast, Xian et al. reported only significant changes in TFF3 protein expression in the jejunum of MTX-treated rats during damage, which might be due to a different treatment regime.78
– In relation to markers associated with intestinal repair following MTX, it is important to mention the hepatocyte growth factor (HGF). HGF has been shown to be a potent mitogen for a variety of epithelial cells,79 and stimulates mucosal regeneration and functional recovery after massive small bowel resection.80 It plays a role in gastric ulcer repair, intestinal cell proliferation and restitution.54,81,82 Xian et al. demonstrated upregulation of both HGF and c-met during the repair of the small intestinal mucosa following MTX-induced damage in the rat. HGF and c-met upregulation coincided with the timing of hyperproliferation during the repair of the intestine, suggesting an involvement of HGF and c-met in the stimulation of crypt cell proliferation initiating mucosal regeneration.83 This hyperproliferation in the recovery phase has already been described by Taminiau et al. (1980).57 Gut epithelial renewal was altered after MTX exposure showing a proliferative phase with defects in enterocyte maturation and villi of enterocytes that exhibited an enzyme profile similar to that of crypt cells (decreased disaccharidase, alkaline phosphatase, and Na+/K+-ATPase activities and increased thymidine kinase activity.
Table 1.
Biomarkers for MTX-induced AT toxicity and injury
| Animal | Protocol | Observations | Ref |
|---|---|---|---|
| Rats | 15 mg MTX/kg BW; orally; daily for 3--5 days | Increased claudin-2 and decreased claudin-4 immunostaining. Deminished occludin mRNA levels; compromised interaction between ZO-1 and claudin-4. Increased mRNA levels of TNF-alpha, IL-1beta, MIP-2, and TLR4 in the small intestine | 42 |
| Rats | 15 mg MTX/kg BW; orally; daily for 3--5 days | Increased tyrosine dephosphorylation of ZO-1 and a reduction of ZO-1 immunostaining along the apical membrane of intestinal villi | 43 |
| Mice | Three subcutaneous MTX injections (2.5 mg/kg BW) | No significant inflammation in MTX-treated iNOS −/− mice | 44 |
| Rats | 20 mg MTX/kg BW; iv; single dose | ROS production preceeds the increase of paracellular permeability in MTX-treated rats. Treatment with NAC prevents MTX-induced ROS production and paracellular permeability | 45 |
| Rats | 20 mg MTX/kg BW; iv; single dose | ROS production preceeds an increase of myeloperoxidase activity, suggestive of neutrophil infiltration in MTX-treated rats. Both treatments of NAC and tungsten prevent MTX-induced ROS production and neutrophil infiltration | 46 |
| Rats | 7 mg MTX/kg BW; ip; daily for 3 days | Nitrotyrosine in all the parts of the small intestine (duodenum > ileum > jejunum) of MTX-treated rats | 47 |
| Rats | 30 mg MTX/kg BW; iv; single dose | Coinciding with inflammatory features in the jejunum, in muscle/serosa and mucosa layers, the levels of anandamide, 2-AG, and PEA peak 3 days after MTX-treatment and return to basal levels at remission (7 days after treatment) | 48 |
| Rats | 1.25, 2.5, and 5.0 mg MTX/kg BW per day; orally; daily for 6 days | Higher plasma GLP-2 levels in 2.5 mg/kg/day and 5.0 mg/kg/day group. Plasma GLP-2 levels seem to affect the degree of intestinal injury | 49 |
| Rats | 3.5 mg MTX/kg BW; 3 days followed by gavage with Escherichia coli for 2 days and 10 ug/kg G-CSF for 4 days to prevent intestinal barrier dysfunction and BT | BT in MTX-treated rats. G-CSF significantly increases mucosal thickness and villous height of the ileum and decreases intestinal permeability and BT | 50 |
| Mice | 60 and 120 mg MTX/kg BW; iv; daily for 2 days | Intestinal damage is associated with decreased expression of HNF-1alpha, Cdx2 and GATA-4. This correlates with decreased expression of SI, and seems inversely correlated with enhanced proliferation of epithelial crypt cells | 51 |
| Rats | 20 mg MTX/kg BW; iv; single dose followed by a second injection of 10 mg/kg/BW after 1 day | mRNA expression of enterocyte and goblet cell markers significantly decreased during damage: SI (−62%) and CPS (−82%) are correlated; also correlations between lactase (−76%) and SGLT1 (−77%) and between I-FABP (−52%) and L-FABP (−45%). Decreases in GLUT5 (−53%), MUC2 (−43%), and TFF3 (−54%) mRNAs occurring independently. Increased lysozyme mRNA present in Paneth cells (+76%). During regeneration, expression of each marker returns to control levels | 52 |
| Rats | 15 mg MTX/kg BW; orally; daily for 5 days | Increased contents of DNA, RNA, proteins and polyamines (spermine and spermidine) in the jejunal mucosa of MTX-treated rats | 53 |
| Rats | 2.5 mg MTX/kg BW; sc; daily for 3 days | Upregulation of HGF and c-met coincides with crypt hyperproliferation and mucosal recovery, suggesting a role for HGF in intestinal repair following acute injury | 54 |
| Rats | 2.5 mg MTX/kg BW; sc; daily for 3 days | Increased TFF3 mRNA but depleted protein levels during histological damage. Cell population expressing TFF3 mRNA expand from usual goblet cells to some non-goblet epithelial cells before goblet cell repopulation | 55 |
| Mice | 3 mg MTX/kg BW; ip; single dose | An increase in the adherence of C. albicans to murine GI mucosa in MTX-treated mice, concurrently with the maximal decrease in the total number of white blood cells | 56 |
| Rats | 30 mg/kg BW; iv; single dose | Four days after MTX exposure, the gut enters a proliferative phase with defects in enterocyte maturation (villi of enterocytes show the enzyme profile of crypt cells) namely decreased disaccharidase, alkaline phosphatase, and Na+/K+-ATPase activities and increased thymidine kinase activity | 57 |
The DAMA model of 5FU-induced mucositis
5FU is a potent agent against solid tumors which was introduced in 1957 for the treatment of colorectal cancer, pancreatic cancer, and inflammatory breast cancer. The primary mechanism of action is to inhibit thymidylate synthase,84 which affects pyrimidine synthesis and leads to depletion of intracellular thymidine triphosphate pools.85 5FU has also been proposed to interfere with the activity of ribosomal RNA binding protein (RRBP), at the level of pre-ribosomal RNA processing.86 5FU treatment leads to an accumulation of cancer cells in the S-phase.87–89 Side effects include myelosuppression, mucositis, dermatitis, and diarrhoea.
Development and characteristics of the model
Based on the successful development and application of the DAMA model of MTX-induced mucositis, the Keefe group adapted the model for the study of GI toxicities associated with 5FU. The model is based on studies with large groups of rats that were treated intraperitoneally with a single dose of 150 mg 5FU/kg BW and showed clear clinical and histological signs of GI mucositis.38
Morphological changes were observed at different sites along the AT and at different time points. Oral epithelial thickness decreased between 2 and 12 h post-5FU treatment. In parallel with MTX, no sign of ulcerations were observed at the level of the tongue, buccal mucosa or gingiva.38 In the jejunum, 5FU caused blunting and fusion of the villi as well as enterocyte hyperplasia. A reduction in jejunal crypt length occurred from 12 h extending over the remainder of the 72-h time period.38 Clear signs of apoptotis were observed at 12 h. Histological examination revealed minimal changes in the colon with increased numbers of apoptotic bodies within the deep aspects of the crypts. Crypt length was increased at 90 min, followed by a brief reduction at 6 h and a further reduction occurred by 72 h.38
In contrast to MTX, 5FU administration did not cause elevated tissue staining for NF-kappaB within the oral mucosa, jejunum, or the colon. However, TNF-alpha levels were significantly elevated in the oral mucosa, jejunum, and colon and peaked earlier in the oral mucosa and jejunum than in the colon. With respect to IL-1beta levels, only the oral mucosa demonstrated a significant peak at 6 h. With respect to the blood serum levels of NF-kappaB and pro-inflammatory cytokines following 5FU, NF-kappaB, TNF-alpha, IL-1beta, and IL-6 all peaked before histological evidence of tissue damage, namely at 12 h, 90 min, 60 min and 6 h, and 30 and 60 min, respectively.38 NF-kappaB is the key molecule involved in 5FU-induced mucositis, as demonstrated by Chang et al. using transgenic mice carrying the luciferase gene driven by a promoter with NF-kappaB responsive elements.90
Applications of the model
The model was used by the Keefe group to show that the GI microbiota and mucins play a role in the development of 5FU-induced alimentary mucositis.91 The microflora found within the duodenum and jejunum mainly consists of anaerobes (Peptostreptococcus spp., Fusobacterium spp., and Bacteroides spp.), Streptococcus spp., Lactobacillus spp., Veillonellae, Actinomyces spp., and a variety of fungi.92 Following 5FU, a decrease in Clostridium spp., Lactobacillus spp., and Streptococcus spp., and an increase in Escherichia spp. could be observed in the jejunum of rats.91 In contrast to the small intestine, the colonic microflora is more diverse, consisting of over 400 different species92 and includes large numbers of anaerobes (Bifidobacterium spp., Bacteroides spp., Eubacterium spp., Peptotrecoccus spp., and Clostridium spp.), enterococci and Enterobacteriaeceae.92,93 In the colon, 5FU caused decreases in Enterococcus spp., Lactobacillus spp., and Streptococcus spp. Faecal samples showed decreasing trends in Lactobacillus spp. and Bacteroides spp., an increasing trend in E. coli, and significant increases in Clostridium spp. and Staphylococcus spp. at 24 h.91
Other groups have used similar animal models (although often in absence of tumors) to identify important mediators and signaling pathways that are involved during 5FU-induced toxicity, and can be summarized as follows (Table 2).
– In response to DNA damage, cells can either undergo apoptosis or G1 cell cycle arrest. Early studies109–111 have shown that 5FU induces both apoptosis and cell cycle arrest in murine intestinal epithelial cells and tends to target cells at specific positions within the crypt. Later, it has been shown that 5FU induces apoptosis via a p53-dependent mechanism in murine intestinal crypt epithelium.103,107,112,113 Also, cell cycle progression was significantly suppressed following 5FU administration and correlated with a prolonged, p53-dependent expression of p21waf-1/cip1.112 Also, p53 −/− mice showed significant reduction in apoptosis and inhibition of cell cycle progression following 5FU compared to wild-type mice.113 Inomata et al. detected p21WAF/Cip1 positivity in the nuclei of the first appearing apoptotic cells and proposed that cells in the stem cell zone respond to 5FU in inducing p53-dependent apoptosis or cell cycle arrest.114 Apoptotic cells are eliminated while the p21WAF/Cip1-positive arrested cells appear to move up to the apex of the crypt.
– The mismatch repair (MMR) proteins have been shown to be essential for the normal response to a wide spectrum of agents including oxidative damage, ionizing radiation, cisplatin, UV, and 5FU damage.102 They signal apoptosis either directly or through cycles of repair.115 The thymine DNA glycosylase methyl-CpG binding domain protein 4 (MBD4) which interacts with MMR protein MutL homolog 1 has been shown to play a role in mediating the apoptotic response within the small intestine. MBD4 −/− mice showed significantly reduced apoptotic responses following treatment with irradiation, cisplatin, temozolomide, and 5FU.105 This means that a significant proportion of the apoptotic response to 5FU and other chemotherapeutics are dependent on functional MBD4.
– The Bcl-2 gene family of proteins control apoptosis in response to a wide variety of stimuli, including chemotherapy.104 They contain both pro- and anti-apoptotic members, which are mainly involved in the intrinsic apoptotic pathway through regulation of mitochondrial membrane permeability and caspase activation. Interestingly, the bcl-2 expression level is maximal in the colonic crypt and this seems to play a role in hindering apoptosis following 5FU. In the small intestine, however, bcl-2 did not appear to play a significant role in the control of apoptosis. Overall, bcl-2 −/− mice display higher levels of spontaneous apoptosis following 5FU.98,107 Experiments with bax −/− mice suggest that bax has only minor roles in the apoptotic response to gamma-radiation or 5FU in both the small intestine and the colon,56,98 whereas bcl-w −/− mice exhibited more apoptosis after 5FU treatment than their wild-type counterparts in both colon and small intestine. Therefore, it seems likely that the final outcome is dependent on the regional and/or temporal balance between all members of the Bcl-2 family of regulator proteins.
– Similar to MTX, 5FU causes significant increase in the frequencies of BT in liver, spleen, mesenteric lymph nodes (MLNs), and portal blood which is counteracted by GM-CSF. Microbial isolates are mostly Gram-negative microbiota.101 An increase in the adherence of C. albicans to murine GI mucosa was found following 5FU treatment.116
– A balance in expression between destructive matrix metalloproteinase (MMPs) and their inhibitors, tissue inhibitor of metalloproteinase (TIMPs), is essential to ensure that tissue homeostasis as has been shown in the hamster model for oral mucositis, where 5FU induced an increase in MMP-2 and -9 with concurrent decreases in TIMP-1 and -2 in the damaged cheek pouches.117
– Gut-associated lymphoid tissue (GALT) in the intestine has been considered a centre of mucosal immunity99 and reduction of GALT mass and function may impair mucosal defense.106 Nagayoshi et al. showed that even a small dose of 5FU reduced lymphocyte numbers at GALT effector sites, associated with a decrease in intestinal and respiratory tract immunoglobulin A (IgA) levels.108 Therefore, GI symptoms and the systemic inflammatory response accompanying 5FU treatment may be associated at least in part with these GALT changes.
– 5FU administration to rats causes epithelial barrier dysfunction of the small intestine, resulting in the increase of small intestinal permeation.100,103,118 For example, absorption of d-glucose which is actively transported across the cell membrane by Na+-dependent glucose cotransporter (SGLT1) on brush-border membrane of the small intestine119 is increased following repeated administration of 5FU to rats.120 Surprisingly, the SGLT1 protein expression was decreased, meaning that increased d-glucose absorption is probably related to the activation of the Na+/K+-ATPase activity which is a driving force of SGLT1-mediated transport. Interesting to note in this context, is that Sadoff reported that the administration of 5FU to patients with diabetes enhanced the glucose concentration in plasma and caused severe diarrhoea and blood poisoning.94 Hence, the enhancement of glucose absorption in the small intestine by the 5FU treatment would worsen the condition of diabetics.
– IL-4 is a critical mediator of intestinal inflammation and functions as either an anti- or pro-inflammatory molecule depending on the model of intestinal inflammation.121 Soares et al. observed that 5FU-induced intestinal mucositis with a concomitant increase in IL-4 concentration in wild-type mice compared to untreated wild-type mice.97 Knockouts of IL-4 did not show the pathological alterations of 5FU-induced mucositis, hence demonstrating the pro-inflammatory role of this cytokine.
– IL-6 is recognized as an important mediator of gut dysfunction in inflammatory bowel disease.122 The role of IL-6 in 5FU-induced small intestinal damage was investigated by Jin et al. who showed that IL-6 −/− mice exhibited increased caspase-3-dependent apoptosis in the ileum and colon after 5FU, relative to wild-type controls.123 Therefore, endogenous IL-6 most likely plays an important role in limiting intestinal injury and cell death following 5FU treatment.
– Platelet-activating factor (PAF) is produced endogenously during gut inflammation.124 It is a potent mediator of many inflammatory processes, important for prostaglandin and eicosanoid synthesis, the induction of apoptosis, and NF-kappaB activation.96,125,126 Soares et al. demonstrated that PAFR −/− mice were protected against intestinal damage caused by 5FU treatment. They further demonstrated that PAFR −/− mice had significantly less pro-inflammatory cytokine production in duodenal tissue, suggesting that PAF is involved in the activation of the cytokine cascade that leads to the intestinal damage.127 PAF has indeed been shown to increase the proteolytic processing of NF-kappaB and expression of pro-inflammatory cytokines, thereby mediating acute bowel injury in mice.128
– There is some evidence to suggest that developmental pathways play a role in adult tissue regenerative processes.129 The Hedgehog (Hh) pathway is known to be essential for GI development. Hh signaling occurs from the epithelial cell layer to stimulate mesenchymal growth in the developing digestive tract95 and is vital for patterning the intestinal crypt-villus axis in mice.130 Recent data have shown that Hh ligand expression displays a biphasic expression pattern of downregulation during injury phase and upregulation during the repair phase of the gut mucosa damage following 5FU.131 This finding is consistent with the results of a previous report which shows that Hh signaling is involved in gastric ulcer repair.132
Table 2.
Biomarkers for 5FU-induced AT toxicity and injury
| Animal | Protocol | Observations | Ref |
|---|---|---|---|
| mice | 450 mg 4FU/kg BW; ip; single dose | Significant damage, MPO activity and elevated levels of pro-inflammatory cytokines (IL-4, TNF-alpha, IL-1beta and CXCL-8) in the intestinal epithelium of 5FU-treated IL-4 wild-type mice but not in −/− mice | 94 |
| mice | 600 mg 5FU/kg BW; ip; single dose | Hg signaling consistently downregulated following 5FU during the injury phase followed by upregulation during the repair phase. Hg signaling inhibition augments apoptosis and suppresses mitotic activity in intestinal crypts | 95 |
| mice | 100 mg 5FU/kg BW; ip; single dose | Network analysis of 5FU-affected genes by transcriptomic tool shows that NF-kappaB is key molecule. 5-ASA inhibits 5FU-induced NF-kappaB activation and proinflammatory cytokine production | 88 |
| mice | 450 mg 5FU/kg BW; ip; single dose | In PAF-R −/− mice and PAF-R antagonist-treated mice, 5FU-induced intestinal damage is less than in wild-type mice | 96 |
| mice | 40 mg 5FU/kg BW; ip; single dose | IL-6 −/− mice exhibit increased apoptosis after 5FU relative to wild-type controls | 97 |
| rats | 50 mg 5FU/kg BW; iv; daily for 6 days | Increased plasma endotoxin,TNF-alpha and IL-6 levels in the 5FU animals. BT at liver, spleen, mesenteric lymph node, and portal blood | 98 |
| mice | 10 mg 5FU/kg BW; continuous iv infusion for 5 days | Reduced CD4/CD8 ratio in the lamina propria, GALT cell number and mucosal IgA levels after 5FU-exposure | 99 |
| rats | 30 or 60 mg 5FU/kg BW; orally; daily for 4 days | Higher Na+/K+-ATPase activity and D-glucose absorption in 5FU-treated rats | 100 |
| hamsters | 60 mg 5FU/kg BW; ip; on days 0, 5, and 10 | Decreases in cheek pouch and jejunum epithelium proliferation rates, increases in MMP-2 and -9, and plasmin, and decreases in TIMP-1 and -2 following 5FU | 101 |
| mice | 400 mg 5FU/kg BW; ip; 6 h between 2 injections | Reduced apoptosis in MBD4 −/− mice following treatment with gamma-irradiation, cisplatin, temozolomide and 5FU | 102 |
| mice | [14C] 5FU for 1 h at a flow rate of 20 ml/kg BW | 5FU induces apoptosis and/or cell cycle arrest: p21(WAF/Cip1)-positive nuclei migrate up the crypt, while bax-positive cytoplasm are observed throughout the crypt epithelial cells | 103 |
| mice | 40 mg 5FU/kg BW; ip; single dose | bcl-w −/− mice exhibit more apoptosis in the small intestine than the wild-type mice after 5FU-exposure | 104 |
| mice | 40 mg 5FU/kg BW; ip; single dose | Elevated apoptosis at base of crypts in colonic epithelium of 5FU-treated bcl-2 −/− mice. Bcl-2 seems to play a key role in determining the sensitivity of colonic stem cells to 5FU-damage | 105 |
| rats | 15 or 30 mg 5FU/kg BW, orally; daily for 3-4 days | BW loss and epithelial barrier dysfunction of the small intestine in 5FU-treated rats as shown by increased permeation of fluorescein isothiocyanate-labelled dextran | 106 |
| mice | 40 and 400 mg 5FU/kg; ip; single dose | Less 5FU-induced apoptosis and cell cycle inhibition in p53 −/− mice. | 107 |
| rats | 300 mg 5FU/kg BW; intragastrically; single dose | Reduced levels of glucose, phosphate transporter, sucrase and SGLT1 after 5FU-exposure | 108 |
| mice | 200 mg 5FU/kg BW; iv; single dose | Increase in adherence of C. albicans to murine GI mucosa after 5FU | 56 |
Irinotecan-induced DAMA model of mucositis
Irinotecan is a relatively new cytotoxic agent used to treat a variety of solid tumors. The primary mechanism of action is to inhibit DNA topoisomerase I.5,6 DNA damage is induced by trapping topoisomerase I during its normal action in regulating DNA structure.133 During DNA replication, topoisomerase I produces reversible single-strand breaks by cutting and reattaching double chain DNA. These breaks relieve the torsional strain generated by the advancing replication forks.133
Irinotecan causes severe diarrhoea in 60–80% of patients.134 Cholinergic, secretory diarrhoea occurs early, and can be prevented by the administration of atropine prior irinotecan. A delayed diarrhoea also occurs, compounded by the action of β-glucuronidase making irinotecan toxicity worse.6,135 Leukopenia is another dose-limiting side effect of irinotecan, compromising the patient and resulting in opportunistic infection.5,6 Irinotecan is converted by hepatic and GI carboxylesterases to its active metabolite, 7-ethyl-10-hydroxycamptothecin (SN-38) which is responsible for irreversible DNA damage.6,133 SN-38 has a stronger anti-carcinogenic activity but is further processed by glucuronyltransferase to become SN-38 glucuronide, a less toxic form of SN-38.6 However, the glucuronide is transported to the intestine during bile excretion and is able to be hydrolyzed by glucuronidase to return to the toxic form. Both bind the topoisomerase I-DNA complex, preventing relegation of the DNA strand. This leads to double-strand DNA breakage and cell death. A number of intestinal microbiota have β-glucoronidase activity and may be responsible in part for the intestinal cytotoxicity of irinotecan.6 Microbial β-glucoronidase is produced primarily by Enterobacteriaeceae (E.coli, Salmonella spp., Shigella spp., Yersina spp., Citrobacter spp., Hafnia spp., and Edwardia spp.) and has been reported to be produced by Flavobacterium spp., and Bacteriodes spp.136
Development and characterisation of the model
A single-dose irinotecan model of GI mucositis was established in 2003 using different doses of 100, 150, and 200 mg irinotecan/kg BW in the rat with implanted breast cancer tumors.25 Animals receiving 100 and 150 mg irinotecan/kg BW showed mild to moderate diarrhoea by 96 h; however, 50% of the animals receiving 150 mg irinotecan/kg BW had died at that time point. At 200 mg irinotecan/kg BW, all animals had severe diarrhoea and a 100% mortality rate at 96 h.
Histopathology showed that irinotecan caused increased apoptosis, crypt hypoplasia, crypt dilation, and mucus secretion in the colon. In the small intestine, the jejunum showed increased apoptosis, villous atrophy, and crypt hypoplasia. Moreover, irinotecan decreased the liver weight significantly.25 Irinotecan caused a peak of jejunal apoptosis at 6 h (100 mg/kg BW and 200 mg/kg BW) which decreased at later time intervals, but remained elevated. A similar peak of apoptosis occurred in the colon at 6 h (100 mg/kg BW and 200 mg/kg BW) which decreased more rapidly than in the small intestine. In contrast to MTX and 5FU, goblet cell numbers and mucus secretion in the small intestine did not alter after irinotecan treatment, whereas goblet cells in the colon showed mucus hypersecretion and at 96 h, goblet cell number had significantly decreased as previously reported by other studies.5,137
A more detailed analysis of the mucosal changes in the AT following a single intraperitoneal dose of 200 mg irinotecan/kg BW in non-tumor bearing rats showed that rats began to demonstrate diarrhoea and symptoms of GI mucositis already after 2 h which was more prevalent at 24 h.138 Tissue damage was most pronounced in the jejunum, where cell death was already obvious after 6 h following treatment. In general, the stomach showed the lowest severity of tissue damage, followed by the colon where cell death was significantly increased at 24 h. In the oral mucosa, changes were noted in the thickness of the epithelium over a 72 h time period, however, no obvious areas of ulceration could be identified. In the jejunum, changes included the presence of degenerative enterocytes within the crypts at 6 h followed by villus blunting, epithelial atrophy, and increased infiltration of inflammatory cells. A decrease in villus and crypt length could be observed already between 30 and 60 min and was followed by a resolution at 2 h and another significant reduction at 12 h before returning to lengths comparable to controls by 24 h. In the colon, initial histological changes included the presence of individual degenerative enterocytes within the crypts, followed by complete ablation of the crypts at later time points. Crypt length in the colon increased at 30 and 60 min, returned to normal levels at 2 h, and then increased again at 24 h.
Irinotecan administration did cause significantly elevated tissue staining for NF-kappaB within the oral mucosa, jejunum and the colon.138 Levels peaked in the oral mucosa at 2 h following irinotecan administration and were consistently higher throughout studies. In the jejunum, tissue NF-kappaB levels peaked at 12 h before subsiding and then slowly increased over the later time points. In the colon, NF-kappaB levels were significantly elevated at all time points. Oral mucosa and jejunal TNF-alpha levels peaked at 6 h, while significantly elevated levels were observed in the colon peaking early at 2 h. With regards to IL-1beta, an elevated (non-significant) trend was observed in the oral mucosa at the later time points, whilst in the jejunum, significantly elevated levels peaked at 6 h. In the colon, the tissue levels of IL-1beta peaked at 12 h. As for IL-6, tissue levels in the oral mucosa were significantly elevated at the later time points, whereas a (non-significant) peak could be observed in the jejunum and colon at 6 h.138 A peak in serum concentration of NF-kappaB was detected at 6 h following irinotecan, remaining significantly elevated at 12 h. Two peaks in serum levels of TNF-alpha at 60 min and 24 h were observed. Serum IL-1beta levels were decreased at 60 min and were then increased at 2 h following irinotecan administration. IL-6 demonstrated a steady increase in concentration peaking at 12 h and then rapidly reduced to baseline levels.139
Application of the model
The model has been used to investigate irinotecan-induced alterations in extracellular matrix (ECM) components.140 A dose of 200 mg/kg BW resulted in a substantial increase in the total collagen deposits around crypts from 24 h in both the jejunum and colon, although collagen IV expression decreased significantly in the crypt region in a delayed fashion. Likewise, fibronectin expression decreased significantly in jejunum and colon from 6 to 24 h following treatment. These changes in the ECM seemed to correlate with the altered epithelial cell kinetics in both the jejunum and colon as cellular proliferation was halved in the jejunum and colon at 48 and 24 h, respectively, while apoptosis peaked at 6 h.141 This peak in apoptosis was further confirmed by gene array analysis experiments showing that genes involved in apoptosis, mitogen-activated protein kinase (MAPK) signaling, and inflammation were upregulated in the small intestine following irinotecan treatment.26
The mechanism behind irinotecan-induced mucosal injury was further explored using tumor-bearing rats that underwent irinotecan treatment with and without the p53 inhibitor, pifithrin-alpha.142 Although pifithrin-alpha reduced severity and duration of intestinal apoptosis, it did not significantly affect p53 expression, intestinal cell death, or mucositis following irinotecan, suggesting that irinotecan may act through upregulation of pro-apoptotic proteins or the activation of caspases to induce cell death. This was shown in tumor-bearing rats treated with two consecutive doses of 150 mg irinotecan/kg BW, where an increase in caspase-1 and -3 expression and downregulated levels of bcl-xL, bcl-2 like 1 and cyclin D2 and G were noticed in the small intestine.27 A more detailed kinetic study using microarray and RT-PCR analysis of intestinal tissues was done with non-tumor-bearing rats treated with a single dose of 200 mg irinotecan/kg BW. At 1 h, many of the genes induced were known p53 targets, some of which are also negative regulators of the MAPK signaling pathway. These early response genes are probably increased in response to cellular stress, particularly DNA damage, and help control apoptosis and the cell cycle. These results suggest that irinotecan-induced damage signaling is p53-dependent to some degree and that early interference with p53 and MAPK activation could affect downstream development of tissue damage. At 6 h, the predominant inhibitory gene changes were related to the cell cycle, including cyclins and cyclin dependent kinases and their regulators which play a major role in mediating the antiproliferative effects of irinotecan. A number of genes involved in the response to DNA double-strand breaks were also identified. Genes that were upregulated at 6 h included transcription factors and cytokine receptors (like TNF and INF receptors) that are known to be activated by inflammatory signals and act through MAPK signaling to induce apoptotic and inflammatory responses in tissue injury. At 24 h, apart from the genes that reflect an anti-proliferative and metabolically inactive environment, also the ones involved in the carbohydrate metabolism were altered. At 72 h, genes involved in regulation of cell proliferation, wound healing, and blood vessel formation were upregulated, reflecting the repair phase following tissue insult.141
Also, tissue levels of MMPs and TIMPs have been shown to be significantly altered following treatment with irinotecan.143 MMPs are endopeptidases with a predominant role in ECM though regulation of its components. Recently, they have been shown to act as mediators of damage in mucositis development,143 which is not surprising given the fact that MMPs are known to play a key role in tissue injury and inflammation in many GI disorders.144,145 A dose of 200 mg irinotecan/kg BW resulted in an increase in MMP-2, -3, -9, and -12 levels at various time points depending on the tissue. These changes were associated with inflammatory infiltrate and maximum tissue damage. In contrast, MMP-1 expression correlated with tissue restitution. TIMP-1 and -2 levels were only significantly altered in the jejunum. Also, the expression of the tissue-type plasminogen activators uPA and tPA, as well as plasminogen activator inhibitor-1 (PAI-1), known to play a role in MMP activation, increased significantly in the jejunum starting from 6 h following treatment. There was no significant change in tPA or uPA and a decrease in PAI-1 was noticed in the colon.143 Given the expression profiles of MMPs correlate with the histopathological damage, these results suggest varying roles for MMPs at different stages of mucositis development within the AT.
Irinotecan-induced mucositis manifests typically as early diarrhoea observed in the rats from 2 to 6 h, and a late onset diarrhoea apparent at 72 h after treatment. The latter corresponds with changes in the luminal environment. As was reported for 5FU,90 goblet cell numbers, composition, and distribution were also altered upon irinotecan treatment. Total goblet cell number in the jejunum decreased significantly in the crypts after 48–72 h and in the villus after 24 h. In the colon, the most noticeable decrease was noted after 48–72 h. Mucin staining in the jejunum decreased in both the crypts and villi from 90 min onwards and became more sulphated in treated rats. In the colon, no major changes could be noticed, although a shift towards the sulphated form was seen. Muc2 and Muc4 expression is significantly lowered in the villi and crypts of the jejunum at 12 h and 24–48 h, respectively, and in the colon between 24 and 72 h.146
With regards to the alimentary microbiome, changes could be observed in the stomach, jejunum, colon, and faeces of rats. The majority of the changes were seen at 6, 12, and 24 h after treatment and persisted up to 72 h. Bacteroides spp., Lactobacillus spp., and Bifidobacterium spp. were shown to decrease after treatment, whereas Staphylococcus spp., Clostridium spp., and E. coli increased. These data suggest the involvement of the alimentary microbiome in the severity of irinotecan-induced mucositis.147
Other groups have used similar animal models (although often in absence of tumors) to identify important mediators and signaling pathways that are involved during irinotecan-induced toxicity, and can be summarized as follows (Table 3).
– It was reported that irinotecan causes BT in mice.149 Nakao et al. investigated the relationship between disruption of TJs and BT after irinotecan-treatment of rats.148 Microbiota were detected in 80% of irinotecan-treated rats either in the MLNs or the spleen, while no BT occurred in the control rats. Irinotecan-treated rats also showed decreased claudin-1 and occludin expression in both the small and large intestine, therefore indicating that TJ might be disturbed after irinotecan treatment permitting microbiota and toxins present in the intestinal lumen getting into the deep tissues.148
– The disruption of basal GLP-2 R signaling seems to play a role in irinotecan-induced intestinal injury and bacteremia. It was shown that exogenous activation of the GLP-2 R ameliorates experimental small and large bowel gut injury.150 Lee et al. studied the importance of the GLP-2 R for gut growth and the response to mucosal injury, and host-microbiota interactions in GLP-2 R +/+ and GLP-2 R −/− mice. GLP-2 R −/− mice exhibited significantly enhanced mucosal injury in response to irinotecan and microbial colonization of the small bowel was significantly increased.151 The authors speculate that the latter may be due to the reduced expression of antimicrobial products derived from Paneth cells in GLP-2 R −/− mice. These data reveal a role for GLP-2 in the control of gut microbial communities.
Table 3.
Biomarkers for irinotecan-induced AT toxicity and injury
| Animal | Protocol | Observations | Ref |
|---|---|---|---|
| Rats | 250 mg irinotecan/kg BW; ip; daily for 2 days | Bacteria detected in the mesenteric lymph node or spleen. Large intestinal resistance of the rats is decreased, while small intestinal resistance is increased. Decreased claudin-1 expression in small and large intestine, decreased occludin expression in small and large intestine (tendency) in IR-treated rats | 147 |
| Mice | 100 mg irinotecan/kg BW; ip; daily for 3 days (3 times) | Increased morbidity, mortality and BT in GLP-2 R −/− mice in response to IR | 148 |
| Mice | 60, 80, 100, and 150 mg irinotecan/kg BW; ip; for 4 days | Germ-free mice are more resistant to IR than the holoxenic group | 146 |
Conclusion
The data obtained from the three rat models for chemotherapy-induced mucositis generally suggest that there are differences in the mucositis pathobiology depending on the type of chemotherapeutic drug used. Although this seems logical as MTX, 5FU, and irinotecan are known to act via various molecular pathways (Figure 1), this is in contrast to what has been assumed so far. However, this may have implications for the management of mucositis, particularly with respect to the development of treatment regimens for mucositis. Further investigations are required to determine the exact pathways that lead to damage caused by the different drugs. Recent data have shown that the Dark Agouti rat model is suitable to assess the safety and efficacy of chemoprotective agents. Any agents that show response in this model without promoting tumor growth can then be considered for human clinical trials, either singly or in combination.
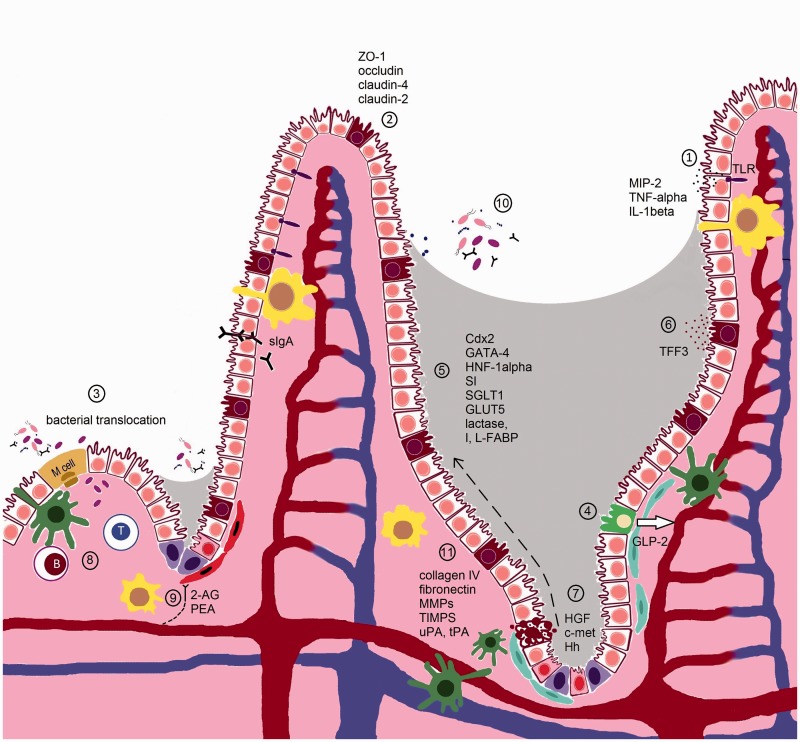
Figure 1.
Cellular and molecular targets of MTX, 5FU and irinotecan in the small intestine of the AT. In the small intestine, the barrier provided by the small IECs (white) ensures a segregation between luminal microbiota and the mucosal immune system. In the crypts, the intestinal epithelial stem cells (pink) share their niche with stromal (blue), dentritic cells (green) and macrophages (yellow), and ensure the continuous renewal of the epithelial cell layer. After differentiation, IECs migrate up the crypt–villus axis (dashed) arrow. Goblet (brown) and Paneth (purple) cells secrete mucus and antimicrobial proteins to exclude microbiota. The release of sIgA further contributes to this barrier function. M cells and goblet cells mediate transport of luminal antigens and live microbiota across the epithelial barrier to dendritic cells (green), and macrophages sample the lumen through transepithelial dendrites. Pathogen recognition receptors such as TLRs recognize conserved microbial-associated molecular motifs and pathogen-specific virulence properties. TLRs induce cytokines on ligation to signal molecules via NF-kappaB activation and the MAPK pathway. Cytotoxics MTX, 5FU, and irinotecan impair different normal epithelial intestinal functions via different mechanisms:
(1) MTX enhances the production of ROS and RNS, probably formed by phagocytes in the inflammatory regions of the small intestine, which trigger the activation of TLR4, and downstream NF-kappaB and MAPK pathways leading to elevated levels of MIP-2, TNF-alpha, and IL-1beta.
(2) MTX impairs cell–cell adhesion by destabilizing the interaction between ZO-1 and claudin-4 and altering the normal expression levels of claudin-2 (increased), claudin-4 (decreased) and occludin. Irinotecan has been shown to decrease claudin-1 and occludin expression in both the small and large intestine. This might be due to the increased expression of TNF-alpha, IL-1beta, MIP-2, and TLR4. MTX also causes the formation of RNS that probably act together with ROS in damaging small intestinal cells, either in the MLNs or the spleen.
(3) MTX increases the risk in Escherichia coli translocation and both MTX and 5FU have been shown to promote adherence of Candida albicans. Microbiota is also detected in irinotecan-treated rats.
(4) MTX stimulates the levels of GLP-2, a peptide secreted from L-type entero-endocrine cells (green) of the distal small intestine and colon, in blood and might therefore be a potential marker for MTX-induced intestinal mucositis. The disruption of basal GLP-2 R signaling seems to play a role in irinotecan-induced intestinal injury as exogenous activation of the GLP-2 receptor ameliorates gut injury.
(5) MTX downregulates the transcription factors Cdx2, GATA-4, and HNF-1alpha, which are important for intestinal development, differentiation, and gene expression. This is correlated with a decreased expression of the SI. Also enterocyte markers lactase, I-FABP, L-FABP, SGLT1, and GLUT5 are downregulated by MTX. In contrast, 5FU stimulates the activity of the Na+-dependent SGLT1 while the SGLT1 protein expression is decreased, resulting in a higher absorption of d-glucose.
(6) MTX alters the expression of TFF3, which are predominantly synthesized by the goblet cells. TFFs play an important role in preserving the integrity of the epithelial barrier.
(7) MTX, 5FU, and irinotecan induce p53-dependent apoptosis and cell cycle arrest in the epithelial stems cells of the deep crypts of the small intestine in the initial stage of mucositis. The degree of apoptosis seems to depend on the expression levels of members of the Bcl-2 family. Irinotecan, MTX, and 5FU also reduce the number of goblet cell numbers. In the mucosal regeneration phase, an upregulation of HGF and c-met can be noticed following MTX treatment while the Hh signaling seems to be important during the repair phase of the gut mucosa damage following 5FU.
(8) GALT in the intestine has been considered a centre of mucosal immunity. 5FU has been shown to reduce the number of lymphocytes at the GALT effector sites and the levels of intestinal and respiratory tract IgA levels.
(9) Endocannabinoids receptors are localized throughout the gut and predominantly located in the enteric nervous system expressed in cholinergic neurons. Receptor activation leads to relaxation in muscle cells (red) of the gut. MTX results in an overactive endocannabinoid system in the gut which might function to reduce intestinal inflammation.
(10) 5FU and irinotecan alter the microbial composition of the gut. A decrease in Clostridium spp., Lactobacillus spp., and Streptococcus spp., and an increase in Escherichia spp. could be observed in the jejunum of 5FU-treated rats. In irinotecan-treated rats, a decrease in Bacteroides spp., Lactobacillus spp., and Bifidobacterium spp can be noticed, whereas Staphylococcus spp., Clostridium spp., and E. coli are increased.
(11) Irinotecan and 5FU can cause dramatic changes in the ECM. Irinotecan results in the increase of total collagen deposits around crypts, but a decrease in collagen IV and fibronectin expression in the crypt region. Irinotecan and 5FU further alter the levels of MMP-2, -3, -9, and TIMP-1 and -2 in the jejunum. Also, the expression of the serine proteases uPA and tPA as well as PAI-1, known to play a role in MMP activation, is increased following irinotecan.
ACKNOWLEDGEMENTS
Barbara Vanhoecke’s research leading to these results has received funding from the Seventh Framework Programme (FP7/2011) under grant agreement no. 299169 (Mucositis Platform). Eline Vanlancker is a doctoral research fellow supported by the Bijzonder Onderzoeksfonds (Ghent University; B/13807/01–BOF13/DOC/280). Bronwen Mayo is funded by a University of South Australia Postgraduate Award. Andrea Stringer is financially supported by a NHMRC Postdoctoral Training Fellowship.
Author contributions
Barbara Vanhoecke was the single writer of the manuscript and developed the tables. Eline Vanlancker helped with listing the references and the final layout of the manuscript. Emma Bateman and Bronwen Mayo helped with the design of the figure. Andrea Stringer and Daniel Thorpe critically reviewed the manuscript and provided constructive feedback. Dorothy Keefe provided useful material that has not been previously published for this review.
REFERENCES
- 1.Keefe DMK, Brealey J, Goland GJ, Cummins AG. Chemotherapy for cancer causes apoptosis that precedes hypoplasia in crypts of the small intestine in humans. Gut 2000; 47: 632–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gibson RJ, Keefe DMK, Thompson FM, Clarke JM, Goland GJ, Cummins AG. Effect of interleukin-11 on ameliorating intestinal damage after methotrexate treatment of breast cancer in rats. Dig Dis Sci 2002; 47: 2751–7. [DOI] [PubMed] [Google Scholar]
- 3.Sonis ST. Complications of cancer and their treatment: oral complications. In: Holland JF, Frei E, Bast RC, Kufe DW, Morton DL, Weichselbaum RR. (eds). Cancer medicine, 3 ed London: Lea & Febiger, 1993, pp. 2381–8. [Google Scholar]
- 4.Keefe DM, Schubert MM, Elting LS, Sonis ST, Epstein JB, Raber-Durlacher JE, Migliorati CA, McGuire DB, Hutchins RD, Peterson DE. Mucositis Study Section M. Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 2007; 109: 820–31. [DOI] [PubMed] [Google Scholar]
- 5.Ikuno N, Soda H, Watanabe M, Oka M. Irinotecan (CPT-11) and characteristic mucosal changes in the mouse ileum and cecum. J Natl Cancer Inst 1995; 87: 1876–83. [DOI] [PubMed] [Google Scholar]
- 6.Takasuna K, Hagiwara T, Hirohashi M, Kato M, Nomura M, Nagai E, Yokoi T, Kamataki T. Involvement of beta-glucuronidase in intestinal microflora in the intestinal toxicity of the antitumor camptothecin derivative irinotecan hydrochloride (CPT-11) in rats. Cancer Res 1996; 56: 3752–7. [PubMed] [Google Scholar]
- 7.Potten CS, Wilson JW, Booth C. Regulation and significance of apoptosis in the stem cells of the gastrointestinal epithelium. Stem Cells 1997; 15: 82–93. [DOI] [PubMed] [Google Scholar]
- 8.Pearson ADJ, Craft AW, Pledger JV, Eastham EJ, Laker MF, Pearson GL. Small bowel function in acute lymphoblastic leukemia. Arch Dis Child 1984; 59: 460–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craft AW, Rankin A, Aherne W. Methotrexate absorption in children with acute lymphoblastic leukemia. Cancer Treatment Rep 1981; 65: 77–81. [PubMed] [Google Scholar]
- 10.Deitch EA. Bacterial translocation: the influence of dietary variables. Gut 1994; 35: S23–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parkins CS, Fowler JF, Yu S. A murine model of lip epidermal/mucosal reactions to X-irradiation. Radiother Oncol 1983; 1: 159–65. [DOI] [PubMed] [Google Scholar]
- 12.Xu F-X, van der Schueren E, Kian Ang K. Acute reactions of the lip mucosa of mice to fractionated irradiations. Radiother Oncol 1984; 1: 369–74. [DOI] [PubMed] [Google Scholar]
- 13.Moses R, Kummermehr J. Radiation response of the mouse tongue epithelium. Br J Cancer 1986; 53: 12–15. [PMC free article] [PubMed] [Google Scholar]
- 14.Sonis ST, Tracey C, Shklar G, Jenson J, Florine D. An animal model for mucositis induced by cancer chemotherapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1990; 69: 437–43. [DOI] [PubMed] [Google Scholar]
- 15.Robinson BA, Clutterbuck RD, Millar JL, McElwain TJ. Epidermal growth factor (hEGF) has no effect on murine intestine epithelial damage and regeneration after melphalan. Br J Cancer 1985; 52: 733–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castellino S, Elion GB, Griffith OW, Dewhirst M, Kurtzberg J, Cattley RC, Scott P, Bigner DD, Friedman HS. Development of a model of melphalan-induced gastrointestinal toxicity in mice. Cancer Chemother Pharmacol 1993; 31: 376–80. [DOI] [PubMed] [Google Scholar]
- 17.Thakkar NS, Potten CS. Abrogation of adriamycin toxicity in vivo by cycloheximide. Biochem Pharmacol 1992; 43: 1683–91. [DOI] [PubMed] [Google Scholar]
- 18.Anilkumar TV, Sarraf CE, Hunt T, Alison MR. The nature of cytotoxic drug-induced cell death in murine intestinal crypts. Br J Cancer 1992; 65: 552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woo PCY, Ng WF, Leung HCH, Tsoi HW, Yuen KY. Clarithromycin attenuates cyclophosphamide-induced mucositis in mice. Pharmacol Res 2000; 41: 527–32. [DOI] [PubMed] [Google Scholar]
- 20.Huang FS, Kemp CJ, Williams JL, Erwin CR, Warner BW. Role of epidermal growth factor and its receptor in chemotherapy-induced intestinal injury. Am J Physiol-Gastrointest Liver Physiol 2002; 282: G432–42. [DOI] [PubMed] [Google Scholar]
- 21.Carneiro-Filho BA, Oria RB, Rea KW, Brito GAC, Fujii J, Obrig T, Lima AAM, Guerrant RL. Alanyl-glutambie hastens morphologic recovery from 5-fluorouracil-induced mucositis in mice. Nutrition 2004; 20: 934–41. [DOI] [PubMed] [Google Scholar]
- 22.de Koning BAE, van Dieren JM, Lindenbergh-Kortleve DJ, van der Sluis M, Matsumoto T, Yamaguchi K, Einerhand AW, Samsom JN, Pieters R, Nieuwenhuis EES. Contributions of mucosal immune cells to methotrexate-induced mucositis. Int Immunol 2006; 18: 941–9. [DOI] [PubMed] [Google Scholar]
- 23.Bowen JM. Development of the rat model of lapatinib-induced diarrhoea. Scientifica 2014; 2014: 194185–194185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keefe DMK. Gastrointestinal mucositis: a new biological model. Support Care Cancer 2004; 12: 6–9. [DOI] [PubMed] [Google Scholar]
- 25.Gibson RJ, Bowen JM, Inglis MRB, Cummins AG, Keefe DMK. Irinotecan causes severe small intestinal damage, as well as colonic damage, in the rat with implanted breast cancer. J Gastroenterol Hepatol 2003; 18: 1095–100. [DOI] [PubMed] [Google Scholar]
- 26.Bowen JM, Gibson RJ, Cummins AG, Tyskin A, Keefe DMK. Irinotecan changes gene expression in the small intestine of the rat with breast cancer. Cancer Chemother Pharmacol 2007; 59: 337–48. [DOI] [PubMed] [Google Scholar]
- 27.Bowen JM, Gibson RJ, Keefe DM, Cummins AG. Cytotoxic chemotherapy upregulates pro-apoptotic Bax and Bak in the small intestine of rats and humans. Pathology 2005; 37: 56–62. [DOI] [PubMed] [Google Scholar]
- 28.Gibson RJ, Keefe DMK, Clarke JM, Regester GO, Thompson FM, Goland GJ, Edwards BG, Cummins AG. The effect of keratinocyte growth factor on tumour growth and small intestinal mucositis after chemotherapy in the rat with breast cancer. Cancer Chemother Pharmacol 2002; 50: 53–8. [DOI] [PubMed] [Google Scholar]
- 29.Gibson RJ, Bowen JM, Alvarez E, Finnie J, Keefe DMK. Establishment of a single-dose irinotecan model of gastrointestinal mucositis. Chemotherapy 2007; 53: 360–9. [DOI] [PubMed] [Google Scholar]
- 30.Rofe AM, Bourgeois CS, Washington JM, Philcox JC, Coyle P. Metabolic consequences of methotrexate therapy in tumor-bearing rats. Immunol Cell Biol 1994; 72: 43–8. [DOI] [PubMed] [Google Scholar]
- 31.Tomas FM, Chandler CS, Coyle P, Bourgeois CS, Burgoyne JL, Rofe AM. Effects of insulin and insulin-like growth factors on protein and energy metabolism in tumour-bearing rats. Biochem J 1994; 301: 769–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyle FM, Wheeler HR, Shenfield GM. Glutamate ameliorates experimental vincristine neuropathy. J Pharmacol Exp Ther 1996; 279: 410–15. [PubMed] [Google Scholar]
- 33.Rajagopalan PTR, Zhang ZQ, McCourt L, Dwyer M, Benkovic SJ, Hammes GG. Interaction of dihydrofolate reductase with methotrexate: Ensemble and single-molecule kinetics. Proc Natl Acad Sci U S A 2002; 99: 13481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russell DH. Effects of methotrexate and cytosine-arabinoside on polyamine metabolism in a mouse L1210 leukemia. Cancer Res 1972; 32: 2459–62. [PubMed] [Google Scholar]
- 35.Farber S, Diamond LK, Mercer RD, Sylvester RF, Jr, Wolff JA. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid (aminopterin). New Engl J Med 1948; 238: 787–93. [DOI] [PubMed] [Google Scholar]
- 36.Valerino DM, Johns D, Zaharko D, Oliverio V. Studies of the metabolism of methotrexate by intestinal flora—I: Identification and study of biological properties of the metabolite 4-amino-4-deoxy-N10-methylpteroic acid. Biochem Pharmacol 1972; 21: 821–31. [DOI] [PubMed] [Google Scholar]
- 37.Gibson RJ, Bowen JM, Cummins AG, Keefe DMK. Relationship between dose of methotrexate, apoptosis, p53/p21 expression and intestinal crypt proliferation in the rat. Clin Exper Med 2005; 4: 188–95. [DOI] [PubMed] [Google Scholar]
- 38.Logan R, Stringer A, Bowen J, Gibson R, Sonis S, Keefe D. Is the pathobiology of chemotherapy-induced alimentary tract mucositis influenced by the type of mucotoxic drug administered? Cancer Chemother Pharmacol 2009; 63: 239–51. [DOI] [PubMed] [Google Scholar]
- 39.Renes IB, Verburg M, Bulsing NP, Ferdinandusse S, Buller HA, Dekker J, Einerhand AWC. Protection of the Peyer's patch-associated crypt and villus epithelium against methotrexate-induced damage is based on its distinct regulation of proliferation. J Pathol 2002; 198: 60–8. [DOI] [PubMed] [Google Scholar]
- 40.Bilim V, Kawasaki T, Takahashi K, Tomita Y. Adriamycin induced G2/M cell cycle arrest in transitional cell cancer cells with wt p53 and p21(WAF1/CIP1) genes. J Exp Clin Cancer Res 2000; 19: 483–8. [PubMed] [Google Scholar]
- 41.Verburg M, Renes IB, Meijer HP, Taminiau J, Buller HA, Einerhand AWC, Dekker J. Selective sparing of goblet cells and Paneth cells in the intestine of methotrexate-treated rats. Am J Physiol-Gastrointest Liver Physiol 2000; 279: G1037–47. [DOI] [PubMed] [Google Scholar]
- 42.Hamada K, Shitara Y, Sekine S, Horie T. Zonula Occludens-1 alterations and enhanced intestinal permeability in methotrexate-treated rats. Cancer Chemother Pharmacol 2010; 66: 1031–8. [DOI] [PubMed] [Google Scholar]
- 43.Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Ann Rev Physiol 2006; 68: 403–29. [DOI] [PubMed] [Google Scholar]
- 44.Gow AJ, Duran D, Malcolm S, Ischiropoulos H. Effects of peroxynitrite-induced protein modifications on tyrosine phosphorylation and degradation. FEBS Lett 1996; 385: 63–6. [DOI] [PubMed] [Google Scholar]
- 45.Miyazono Y, Gao F, Horie T. Oxidative stress contributes to methotrexate-induced small intestinal toxicity in rats. Scand J Gastroenterol 2004; 39: 1119–27. [DOI] [PubMed] [Google Scholar]
- 46.Gao F, Horie T. A synthetic analog of prostaglandin E-1 prevents the production of reactive oxygen species in the intestinal mucosa of methotrexate-treated rats. Life Sci 2002; 71: 1091–9. [DOI] [PubMed] [Google Scholar]
- 47.Rao RK, Basuroy S, Rao VU, Karnaky KJ, Gupta A. Tyrosine phosphorylation and dissociation of occludin-Z0-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J 2002; 368: 471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Marzo V, Izzo AA. Endocannabinoid overactivity and intestinal inflammation. Gut 2006; 55: 1373–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.D'Argenio G, Petrosino S, Gianfrani C, Valenti M, Scaglione G, Grandone I, Nigam S, Sorrentini I, Mazzarella G, Di Marzo V. Overactivity of the intestinal endocannabinoid system in celiac disease and in methotrexate-treated rats. J Mol Med 2007; 85: 523–30. [DOI] [PubMed] [Google Scholar]
- 50.Berg RD. Bacterial translocation from the gastrointestinal tract. In: Paul PS, Francis DH. (eds). Mechanisms in the pathogenesis of enteric diseases 2. Advances in experimental medicine and biology, New York: Kluwer Academic/Plenum Publications, 1999, pp. 11–30.. [DOI] [PubMed] [Google Scholar]
- 51.Sandovsky-Losica H, Segal E. Interaction of Candida albicans with murine gastrointestinal mucosa: effect of irradiation on adherence in vitro. Med Mycol 1989; 27: 345–52. [PubMed] [Google Scholar]
- 52.Rings E, Krasinski SD, Vanbeers EH, Moorman AFM, Dekker J, Montgomery RK, Grand RJ, Buller HA. Restriction of lactase gene expression along the proximal-to-distal axis of rat small intestine occurs during postnatal development. Gastroenterology 1994; 106: 1223–32. [DOI] [PubMed] [Google Scholar]
- 53.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol 2010; 10: 131–43. [DOI] [PubMed] [Google Scholar]
- 54.Nishimura S, Takahashi M, Ota S, Hirano M, Hiraishi H. Hepatocyte growth factor accelerates restitution of intestinal epithelial cells. J Gastroenterol 1998; 33: 172–8. [DOI] [PubMed] [Google Scholar]
- 55.Taupin DR, Pang KC, Green SP, Giraud AS. The trefoil peptides spasmolytic polypeptide and intestinal trefoil factor are major secretory products of the rat gut. Peptides 1995; 16: 1001–5. [DOI] [PubMed] [Google Scholar]
- 56.Pritchard DM, Print C, O'Reilly L, Adams JM, Potten CS, Hickman JA. Bcl-w is an important determinant of damage-induced apoptosis in epithelia of small and large intestine. Oncogene 2000; 19: 3955–9. [DOI] [PubMed] [Google Scholar]
- 57.Taminiau J, Gall DG, Hamilton JR. Response of the rat small-intestine epithelium to methotrexate. Gut 1980; 21: 486–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hamada K, Kakigawa N, Sekine S, Shitara Y, Horie T. Disruption of ZO-1/claudin-4 interaction in relation to inflammatory responses in methotrexate-induced intestinal mucositis. Cancer Chemother Pharmacol 2013; 72: 757–65. [DOI] [PubMed] [Google Scholar]
- 59.Maeda T, Miyazono Y, Ito K, Hamada K, Sekine S, Horie T. Oxidative stress and enhanced paracellular permeability in the small intestine of methotrexate-treated rats. Cancer Chemother Pharmacol 2010; 65: 1117–23. [DOI] [PubMed] [Google Scholar]
- 60.Sheth P, Basuroy S, Li CY, Naren AP, Rao RK. Role of phosphatidylinositol 3-kinase in oxidative stress-induced disruption of tight junctions. J Biol Chem 2003; 278: 49239–45. [DOI] [PubMed] [Google Scholar]
- 61.Kolli VK, Abraham P, Rabi S. Methotrexate-induced nitrosative stress may play a critical role in small intestinal damage in the rat. Arch Toxicol 2008; 82: 763–70. [DOI] [PubMed] [Google Scholar]
- 62.Leitão RF, Brito GA, Oriá RB, Braga-Neto MB, Bellaguarda EA, Silva JV, Gomes AS, Lima-Júnior RC, Siqueira FJ, Freire RS. Role of inducible nitric oxide synthase pathway on methotrexate-induced intestinal mucositis in rodents. BMC Gastroenterol 2011; 11: 90–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hornby PJ, Prouty SM. Involvement of cannabinoid receptors in gut motility and visceral perception. Br J Pharmacol 2004; 141: 1335–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hirotani Y, Yamamoto K, Ikeda K, Arakawa Y, Li J, Kitamura K, Kurokawa N, Tanakae K. Correlation between plasma glucagon-like peptide 2 levels and proliferative makers in small intestinal injury in rats induced by methotrexate administration. Biol Pharm Bull 2006; 29: 2327–30. [DOI] [PubMed] [Google Scholar]
- 65.Berg RD, Garlington AW. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun 1979; 23: 403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Song DS, Shi B, Xue H, Li YS, Yu BJ, Xu Z, Liu FK, Li JS. Green fluorescent protein labeling Escherichia coli TG1 confirms intestinal bacterial translocation in a rat model of chemotherapy. Curr Microbiol 2006; 52: 69–73. [DOI] [PubMed] [Google Scholar]
- 67.de Koning BAE, Lindenbergh-Kortleve DJ, Pieters R, Rings E, Buller HA, Renes IB, Einerhand AWC. The effect of cytostatic drug treatment on intestine-specific transcription factors Cdx2, GATA-4 and HNF-1 alpha in mice. Cancer Chemother Pharmacol 2006; 57: 801–10. [DOI] [PubMed] [Google Scholar]
- 68.Boudreau F, Rings E, van Wering HM, Kim RK, Swain GP, Krasinski SD, Moffett J, Grand RJ, Suh ER, Traber PG. Hepatocyte nuclear factor-1 alpha, GATA-4, and caudal related homeodomain protein Cdx2 interact functionally to modulate intestinal gene transcription – Implication for the developmental regulation of the sucrose-isomaltase gene. J Biol Chem 2002; 277: 31909–17. [DOI] [PubMed] [Google Scholar]
- 69.Clatworthy JP, Subramanian V. Stem cells and the regulation of proliferation, differentiation and patterning in the intestinal epithelium: emerging insights from gene expression patterns, transgenic and gene ablation studies. Mech Dev 2001; 101: 3–9. [DOI] [PubMed] [Google Scholar]
- 70.Vanbeers EH, Buller HA, Grand RJ, Einerhand AWC, Dekker J. Intestinal brush border glycohydrolases: structur, function, and development. Crit Rev Biochem Mol Biol 1995; 30: 197–262. [DOI] [PubMed] [Google Scholar]
- 71.Verburg M, Renes IB, Van Nispen D, Ferdinandusse S, Jorritsma M, Buller HA, Einerhand AWC, Dekker J. Specific responses in rat small intestinal epithelial mRNA expression and protein levels during chemotherapeutic damage and regeneration. J Histochem Cytochem 2002; 50: 1525–36. [DOI] [PubMed] [Google Scholar]
- 72.Babyatsky MW, deBeaumont M, Thim L, Podolsky DK. Oral trefoil peptides protect against ethanol- and indomethacin-induced gastric injury in rats. Gastroenterology 1996; 110: 489–97. [DOI] [PubMed] [Google Scholar]
- 73.Chinery R, Playford RJ. Combined intestinal trefoil factor and epidermal growth factor is prophylactic against indomethacin-induced gastric damage in the rat. Clin Sci 1995; 88: 401–3. [DOI] [PubMed] [Google Scholar]
- 74.Cook GA, Thim L, Yeomans ND, Giraud AS. Oral human spasmolytic polypeptide protects against aspirin-induced gastric injury in rats. J Gastroenterol Hepatol 1998; 13: 363–70. [DOI] [PubMed] [Google Scholar]
- 75.Kindon H, Pothoulakis C, Thim L, Lynchdevaney G, Podolsky DK. Trefoil peptide protection of intestinal epithelial barrier function: cooperative interaction with mucin glycoprotein. Gastroenterology 1995; 109: 516–23. [DOI] [PubMed] [Google Scholar]
- 76.Jeffrey GP, Oates PS, Wang TC, Babyatsky MW, Brand SJ. Spasmolytic polypetide - a trefoil peptide secreted by rat gastric mucous cells. Gastroenterology 1994; 106: 336–45. [DOI] [PubMed] [Google Scholar]
- 77.Suemori S, Lynchdevaney K, Podolsky DK. Identification and characterization of rat intestinal trefoil factor: tissue- and cell-specific member of the trefoil protein family. Proc Natl Acad Sci U S A 1991; 88: 11017–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xian CJ, Howarth GS, Mardell CE, Cool JC, Familari M, Read LC, Giraud AS. Temporal changes in TFF3 expression and jejunal morphology during methotrexate-induced damage and repair. Am J Physiol-Gastrointest Liver Physiol 1999; 277: G785–95. [DOI] [PubMed] [Google Scholar]
- 79.Schmassmann A, Hirschi C, Stettler C, Poulsom R, Halter F. Recent work with hepatocyte growth scatter factor. In: Halter F, Winton D, Wright NA (eds). The gut as a model in cell and molecular biology. Dordrecht: Springer, 1997, pp.180–93.
- 80.Kato Y, Yu DH, Schwartz MZ. Enhancement of intestinal adaptation by hepatocyte growth factor. J Pediatr Surg 1998; 33: 235–9. [DOI] [PubMed] [Google Scholar]
- 81.Kinoshita Y, Kishi K, Asahara M, Matsushima Y, Wang HY, Miyazawa K, Kitamura N, Chiba T. Production and activation of hepatocyte growth factor during the healing of rat gastric ulcers. Digestion 1997; 58: 225–31. [DOI] [PubMed] [Google Scholar]
- 82.Goke M, Kanai M, Podolsky DK. Intestinal fibroblasts regulate intestinal epithelial cell proliferation via hepatocyte growth factor. Am J Physiol-Gastrointest Liver Physiol 1998; 274: G809–18. [DOI] [PubMed] [Google Scholar]
- 83.Xian CJ, Couper R, Howarth GS, Read LC, Kallincos NC. Increased expression of HGF and c-met in rat small intestine during recovery from methotrexate-induced mucositis. Br J Cancer 2000; 82: 945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peters GJ, Backus HHJ, Freemantle S, van Triest B, Codacci-Pisanelli G, van der Wilt CL, Smid K, Lunec J, Calvert AH, Marsh S, McLeod HL, Bloemena E, Meijer S, Jansen G, van Groeningen CJ, Pinedo HM. Induction of thymidylate synthase as a 5-fluorouracil resistance mechanism. Biochim Biophys Acta-Mol Basis Dis 2002; 1587: 194–205. [DOI] [PubMed] [Google Scholar]
- 85.Elstein KH, Mole ML, Setzer RW, Zucker RM, Kavlock RJ, Rogers JM, Lau C. Nucleoside-mediated mitigation of 5-fluorouracil-induced toxicity in synchronized murine erythroleukemic cells. Toxicol Appl Pharmacol 1997; 146: 29–39. [DOI] [PubMed] [Google Scholar]
- 86.Ghoshal K, Jacob ST. An alternative molecular mechanism of action of 5-fluorouracil, a potent anticancer drug. Biochem Pharmacol 1997; 53: 1569–75. [DOI] [PubMed] [Google Scholar]
- 87.Wadler S, Horowitz R, Zhang HY, Schwartz EL. Effects of perturbations of pools of deoxyribonucleoside triphosphates on expression of ribonucleotide reductase, a G(1)/S transition state enzyme, in p53-mutated cells. Biochem Pharmacol 1998; 55: 1353–60. [DOI] [PubMed] [Google Scholar]
- 88.Takeda H, Haisa M, Naomoto Y, Kawashima R, Satomoto K, Yamatuji T, Tanaka N. Effect of 5-fluorouracil on cell cycle regulatory proteins in human colon cancer cell line. Jpn J Cancer Res 1999; 90: 677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Inada T, Ichikawa A, Kubota T, Ogata Y, Moossa A, Hoffman RM. 5-FU-induced apoptosis correlates with efficacy against human gastric and colon cancer xenografts in nude mice. Anticancer Res 1997; 17: 1965–71. [PubMed] [Google Scholar]
- 90.Chang CT, Ho TY, Lin H, Liang JA, Huang HC, Li CC, Lo HY, Wu SL, Huang YF, Hsiang CY. 5-Fluorouracil induced intestinal mucositis via nuclear factor-kappa B activation by transcriptomic analysis and in vivo bioluminescence imaging. PLoS One 2012; 7: 8–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stringer AM, Gibson RJ, Logan RM, Bowen JM, Yeoh ASJ, Hamilton J, Keefe DMK. Gastrointestinal microflora and mucins may play a critical role in the development of 5-fluorouracil-induced gastrointestinal mucositis. Exp Biol Med 2009; 234: 430–41. [DOI] [PubMed] [Google Scholar]
- 92.Simon GL, Gorbach SL. Intestinal flora in health and disease. Gastroenterology 1984; 86: 174–93. [PubMed] [Google Scholar]
- 93.Simon GL, Gorbach SL. The human intestinal microflora. Dig Dis Sci 1986; 31: S147–62. [DOI] [PubMed] [Google Scholar]
- 94.Sadoff L. Overwhelming 5-fluorouracil toxicity in patients whose diabetes is poorly controlled. Am J Clin Oncol-Cancer Clin Trial 1998; 21: 605–7. [DOI] [PubMed] [Google Scholar]
- 95.Mao J, Kim BM, Rajurkar M, Shivdasani RA, McMahon AP. Hedgehog signaling controls mesenchymal growth in the developing mammalian digestive tract. Development 2010; 137: 1721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chao W, Olson MS. Platelet-activating factor: receptors and signal transduction. Biochem J 1993; 292: 617–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Soares PMG, Mota J, Souza EP, Justino PFC, Franco AX, Cunha FQ, Ribeiro RA, Souza M. Inflammatory intestinal damage induced by 5-fluorouracil requires IL-4. Cytokine 2013; 61: 46–9. [DOI] [PubMed] [Google Scholar]
- 98.Pritchard DM, Potten CS, Korsmeyer SJ, Roberts S, Hickman JA. Damage-induced apoptosis in intestinal epithelia from bcl-2-null and bax-null mice: investigations of the mechanistic determinants of epithelial apoptosis in vivo. Oncogene 1999; 18: 7287–93. [DOI] [PubMed] [Google Scholar]
- 99.Kelsall B, Strober W. Gut-associated lymphoid tissue: antigen handling and T-lymphocyte responses. In: Orgra PL, Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR (eds). Mucosal immunology. 2nd edn. San Diego, CA: Academic Press, 1999, pp. 293–317.
- 100.Tanaka H, Miyamoto K, Morita K, Haga H, Segawa H, Shiraga T, Fujioka A, Kouda T, Taketani Y, Hisano S, Fukui Y, Kitagawa K, Takeda E. Regulation of the PepT1 peptide transporter in the rat small intestine in response to 5-fluorouracil-induced injury. Gastroenterology 1998; 114: 714–23. [DOI] [PubMed] [Google Scholar]
- 101.Kucuk C, Ozkan M, Akgun H, Muhtaroglu S, Sozuer E. The effect of granulocyte macrophage-colony stimulating factor on bacterial translocation after administration of 5-fluorouracil in rats. J Surg Res 2005; 128: 15–20. [DOI] [PubMed] [Google Scholar]
- 102.Buermeyer AB, Deschenes SM, Baker SM, Liskay RM. Mammalian DNA mismatch repair. Annu Rev Genet 1999; 33: 533–64. [DOI] [PubMed] [Google Scholar]
- 103.Pritchard DM, Potten CS, Hickman JA. The relationships between p53-dependent apoptosis, inhibition of proliferation, and 5-fluorouracil-induced histopathology in murine intestinal epithelia. Cancer Res 1998; 58: 5453–65. [PubMed] [Google Scholar]
- 104.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev 1999; 13: 1899–911. [DOI] [PubMed] [Google Scholar]
- 105.Sansom OJ, Zabkiewicz J, Bishop SM, Guy J, Bird A, Clarke AR. MBD4 deficiency reduces the apoptotic response to DNA-damaging agents in the murine small intestine. Oncogene 2003; 22: 7130–6. [DOI] [PubMed] [Google Scholar]
- 106.Li J, Kudsk KA, Gocinski B, Dent D, Glezer J, Langkamphenken B. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma-Injury Inf Crit Care 1995; 39: 44–52. [DOI] [PubMed] [Google Scholar]
- 107.Merritt AJ, Potten CS, Kemp CJ, Hickman JA, Balmain A, Lane DP, Hall PA. The role of P53 in spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and P53-deficient mice. Cancer Res 1994; 54: 614–17. [PubMed] [Google Scholar]
- 108.Nagayoshi H, Fukatsu K, Ueno C, Hara E, Maeshima Y, Omata J, Hiraide H, Mochizuki H. 5-fluorouracil infusion reduces gut-associated lymphoid tissue cell number and mucosal immunoglobulin A levels. J Parenter Enter Nutr 2005; 29: 395–400. [DOI] [PubMed] [Google Scholar]
- 109.Ijiri K, Potten C. Response of intestinal cells of differing topographical and hierarchical status to ten cytotoxic drugs and five sources of radiation. Br J Cancer 1983; 47: 175–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ijiri K, Potten C. Further studies on the response of intestinal crypt cells of different hierarchical status to eighteen different cytotoxic agents. Br J Cancer 1987; 55: 113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Potten CS. The significance of spontaneous and induced apoptosis in the gastrointestinal tract of mice. Cancer Metastasis Rev 1992; 11: 179–95. [DOI] [PubMed] [Google Scholar]
- 112.Pritchard DM, Jackman A, Potten CS, Hickman JA. Chemically-induced apoptosis: p21 and p53 as determinants of enterotoxin activity. Toxicol Lett 1998; 103: 19–27. [DOI] [PubMed] [Google Scholar]
- 113.Pritchard DM, Watson AJ, Potten CS, Jackman AL, Hickman JA. Inhibition by uridine but not thymidine of p53-dependent intestinal apoptosis initiated by 5-fluorouracil: evidence for the involvement of RNA perturbation. Proc Natl Acad Sci 1997; 94: 1795–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Inomata A, Horii I, Suzuki K. 5-Fluorouracil-induced intestinal toxicity: what determines the severity of damage to murine intestinal crypt epithelia? Toxicol Lett 2002; 133: 231–40. [DOI] [PubMed] [Google Scholar]
- 115.Fishel R. Signaling mismatch repair in cancer. Nat Med 1999; 5: 1239–41. [DOI] [PubMed] [Google Scholar]
- 116.Sandovskylosica H, Segal E. Interaction of Candida albicans with murine gastrointestinal mucosa from methotrexate and 5-fluorouracil treated animals: in vitro adhesion and prevention. J Med Vet Mycol 1990; 28: 279–87. [PubMed] [Google Scholar]
- 117.Morvan FO, Baroukh B, Ledoux D, Caruelle JP, Barritault D, Godeau G, Saffar JL. An engineered biopolymer prevents mucositis induced by 5-fluorouracil in hamsters. Am J Pathol 2004; 164: 739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hirata K, Horie T. Changes in intestinal absorption of 5-fluorouracil-treated rats. Pharmacol Toxicol 1999; 85: 33–6. [DOI] [PubMed] [Google Scholar]
- 119.Lee WS, Kanai Y, Wells RG, Hediger MA. The high-affinity Na+/glucose cotransporter. Reevaluation of function and distribution of expression. J Biol Chem 1994; 269: 12032–9. [PubMed] [Google Scholar]
- 120.Tomimatsu T, Horie T. Enhanced glucose absorption in the rat small intestine following repeated doses of 5-fluorouracil. Chem-Biol Interact 2005; 155: 129–39. [DOI] [PubMed] [Google Scholar]
- 121.Van Kampen C, Gauldie J, Collins SM. Proinflammatory properties of IL-4 in the intestinal microenvironment. Am J Physiol-Gastrointest Liver Physiol 2005; 288: G111–17. [DOI] [PubMed] [Google Scholar]
- 122.Vermeire S, Van Assche G, Rutgeerts P. C-reactive protein as a marker for inflammatory bowel disease. Inflamm Bowel Dis 2004; 10: 661–5. [DOI] [PubMed] [Google Scholar]
- 123.Jin XL, Zimmers TA, Zhang ZX, Pierce RH, Koniaris LG. Interleukin-6 is an important in vivo inhibitor of intestinal epithelial cell death in mice. Gut 2010; 59: 186–96. [DOI] [PubMed] [Google Scholar]
- 124.Sun XM, Hsueh W. Bowel necrosis induced by tumor necrosis factor in rats is mediated by platelet-activating factor. J Clin Invest 1988; 81: 1328–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Borman RA, Jewell R, Hillier K. Investigation of the effects of platelet-activating factor (PAF) on ion transport and prostaglandin synthesis in human colonic mucosa in vitro. Br J Pharmacol 1998; 123: 231–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Venkatesha RT, Ahamed J, Nuesch C, Zaidi AK, Ali H. Platelet-activating factor-induced chemokine gene expression requires NF-kappa B activation and Ca2+/calcineurin signaling pathways - Inhibition by receptor phosphorylation and beta-arrestin recruitment. J Biol Chem 2004; 279: 44606–12. [DOI] [PubMed] [Google Scholar]
- 127.Soares PMG, Lima RCP, Mota J, Justino PFC, Brito GAC, Ribeiro RA, Cunha FQ, Souza M. Role of platelet-activating factor in the pathogenesis of 5-fluorouracil-induced intestinal mucositis in mice. Cancer Chemother Pharmacol 2011; 68: 713–20. [DOI] [PubMed] [Google Scholar]
- 128.Liu SXL, Tian RL, Baskind H, Hsueh W, De Plaen IG. Platelet-activating factor induces the processing of nuclear factor-kappa B p105 into p50, which mediates acute bowel injury in mice. Am J Physiol-Gastrointest Liver Physiol 2009; 297: G76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yonekawa H, Murata E, Akita M, Satomi A. Reorganized small intestine from fetal mouse as an in vitro wound healing model. J Gastroenterol 2003; 38: 442–50. [DOI] [PubMed] [Google Scholar]
- 130.Madison BB, Braunstein K, Kuizon E, Portman K, Qiao XT, Gumucio DL. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development 2005; 132: 279–89. [DOI] [PubMed] [Google Scholar]
- 131.Liang R, Morris P, Cho SSC, Abud HE, Jin XQ, Cheng W. Hedgehog signaling displays a biphasic expression pattern during intestinal injury and repair. J Pediatr Surg 2012; 47: 2251–63. [DOI] [PubMed] [Google Scholar]
- 132.Kang DH, Han ME, Song MH, Lee YS, Kim EH, Kim HJ, Kim GH, Kim DH, Yoon S, Baek SY, Kim BS, Kim JB, Oh SO. The role of hedgehog signaling during gastric regeneration. J Gastroenterol 2009; 44: 372–9. [DOI] [PubMed] [Google Scholar]
- 133.Alimonti A, Gelibter A, Pavese I, Satta F, Cognetti F, Ferretti G, Rasio D, Vecchione A, Di Palma M. New approaches to prevent intestinal toxicity of irinotecan-based regimens. Cancer Treat Rev 2004; 30: 555–62. [DOI] [PubMed] [Google Scholar]
- 134.Fittkau M, Voigt W, Holzhausen HJ, Schmoll HJ. Saccharic acid 1.4-lactone protects against CPT-11-induced mucosa damage in rats. J Cancer Res Clin Oncol 2004; 130: 388–94. [DOI] [PubMed] [Google Scholar]
- 135.Yamamoto M, Kurita A, Asahara T, Takakura A, Katono K, Iwasaki M, Ryuge S, Wada M, Onoda S, Yanaihara T, Yokoba M, Mitsufuji H, Nishji Y, Fukui T, Masuda N. Metabolism of irinotecan and its active metabolite SN-38 by intestinal microflora in rats. Oncol Rep 2008; 20: 727–30. [PubMed] [Google Scholar]
- 136.Tryland I, Fiksdal L. Enzyme characteristics of beta-D-galactosidase- and beta-D-glucuronidase-positive bacteria and their interference in rapid methods for detection of waterborne coliforms and Escherichia coli. Appl Environ Microbiol 1998; 64: 1018–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cao SS, Black JD, Troutt AB, Rustum YM. Interleukin 15 offers selective protection from irinotecan-induced intestinal toxicity in a preclinical animal model. Cancer Res 1998; 58: 3270–4. [PubMed] [Google Scholar]
- 138.Logan RM, Gibson RJ, Bowen JM, Stringer AM, Sonis ST, Keefe DMK. Characterisation of mucosal changes in the alimentary tract following administration of irinotecan: implications for the pathobiology of mucositis. Cancer Chemother Pharmacol 2008; 62: 33–41. [DOI] [PubMed] [Google Scholar]
- 139.Logan RM, Stringer AM, Bowen JM, Gibson RJ, Sonis ST, Keefe DMK. Serum levels of NF kappa B and pro-inflammatory cytokines following administration of mucotoxic drugs. Cancer Biol Ther 2008; 7: 1139–45. [DOI] [PubMed] [Google Scholar]
- 140.Al-Dasooqi N, Bowen JM, Gibson RJ, Logan RM, Stringer AM, Keefe DM. Irinotecan-induced alterations in intestinal cell kinetics and extracellular matrix component expression in the dark agouti rat. Int J Exp Pathol 2011; 92: 357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bowen JM, Tsykin A, Stringer AM, Logan RM, Gibson RJ, Keefe DMK. Kinetics and regional specificity of irinotecan-induced gene expression in the gastrointestinal tract. Toxicology 2010; 269: 1–12. [DOI] [PubMed] [Google Scholar]
- 142.Bowen JM, Gibson RJ, Stringer AM, Chan TW, Prabowo AS, Cummins AG, Keefe DMK. Role of p53 in irinotecan-induced intestinal cell death and mucosal damage. Anti-Cancer Drug 2007; 18: 197–210. [DOI] [PubMed] [Google Scholar]
- 143.Al-Dasooqi N, Gibson RJ, Bowen JM, Logan RM, Stringer AM, Keefe DM. Matrix metalloproteinases are possible mediators for the development of alimentary tract mucositis in the dark agouti rat. Exp Biol Med 2010; 235: 1244–56. [DOI] [PubMed] [Google Scholar]
- 144.Manicone AM, McGuire JK. Matrix metalloproteinases as modulators of inflammation. Semin Cell Dev Biol 2008; 19: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wolf M, Albrecht S, Marki C. Proteolytic processing of chemokines: Implications in physiological and pathological conditions. Int J Biochem Cell Biol 2008; 40: 1185–98. [DOI] [PubMed] [Google Scholar]
- 146.Stringer AM, Gibson RJ, Logan RM, Bowen JM, Yeoh ASJ, Laurence J, Keefe DMK. Irinotecan-induced mucositis is associated with changes in intestinal mucins. Cancer Chemother Pharmacol 2009; 64: 123–32. [DOI] [PubMed] [Google Scholar]
- 147.Stringer AM, Gibson RJ, Logan RM, Bowen JM, Yeoh ASJ, Keefe DMK. Faecal microflora and beta-glucuronidase expression are altered in an irinotecan-induced diarrhea model in rats. Cancer Biol Ther 2008; 7: 1919–25. [DOI] [PubMed] [Google Scholar]
- 148.Nakao T, Kurita N, Komatsu M, Yoshikawa K, Iwata T, Utusnomiya T, Shimada M. Irinotecan injures tight junction and causes bacterial translocation in rat. J Surg Res 2012; 173: 341–7. [DOI] [PubMed] [Google Scholar]
- 149.Brandi G, Dabard J, Raibaud P, Di Battista M, Bridonneau C, Pisi AM, Labate AMM, Pantaleo MA, De Vivo A, Biasco G. Intestinal microflora and digestive toxicity of irinotecan in mice. Clin Cancer Res 2006; 12: 1299–307. [DOI] [PubMed] [Google Scholar]
- 150.Drucker DJ, Ehrlich P, Asa SL, Brubaker PL. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci U S A 1996; 93: 7911–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lee SJ, Lee J, Li KK, Holland D, Maughan H, Guttman DS, Yusta B, Drucker DJ. Disruption of the murine Glp2r impairs paneth cell function and increases susceptibility to small bowel enteritis. Endocrinology 2012; 153: 1141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]