Abstract
Liver X receptors are in the nuclear receptor superfamily and are contained in the regulation of lipid and cholesterol metabolism. Besides, liver X receptors are considered crucial regulators of the inflammatory response and innate immunity. The current study evaluates the in vivo effects that the synthetic liver X receptor agonist TO901317 protects against cisplatin-induced kidney injury in mice. Mice received cisplatin administration through a single intraperitoneal injection (20 mg/kg in saline). And then the mice were treated with the TO901317 by daily gavage (10 mg/kg/day) 12 h postcisplatin administration, and cisplatin nephrotoxicity was evaluated. At 72 h after cisplatin treatment, elevated plasma urea and creatinine levels (P < 0.05) were evidenced which indicates the renal dysfunction of the vehicle-treated mice, consistent with tubular necrosis, protein cast, dilation of renal tubules, and desquamation of epithelial cells in renal tubules. In contrast, the severity of renal dysfunction and histological damage was reduced in TO901317 treated mice (P < 0.05). In accordance, circulating tumor necrosis factor alpha levels, renal tumor necrosis factor alpha, p47phox, gp91phox, and protein expression levels and COX-2 mRNA, renal monocyte chemoattractant protein 1, VACAM-1 mRNA and intercellular adhesion molecule-1 contents, and renal prostaglandin E2 amounts, were higher in samples from cisplatin-treated mice in comparison with controls (P < 0.05) but attenuated in the TO901317 treatment group (P < 0.05). Taken together, treatment with the liver X receptor agonist TO901317 ameliorated the inflammatory response and oxidative stress in cisplatin-induced kidney injury in mice.
Keywords: Liver X receptor agonist, cisplatin nephrotoxicity, acute kidney injury
Introduction
Cisplatin is universally used as anticancer agent1; nonetheless, dose-related side effects such as nephrotoxicity and neurotoxicity have limited its clinical application.2 Multiple studies have examined the mechanisms of cisplatin-induced kidney injury and found that inflammation plays a significant role in cisplatin-induced acute kidney injury.3,4 Indeed, growing evidence suggests that a stark activation of tumor necrosis factor-α (TNF-α) and other inflammatory indicators in kidney are induced by cisplatin, and it is capable of attenuating cisplatin-induced renal injury by suppressing the inflammatory gene expression.5 It is widely accepted that the progression of cisplatin-induced nephrotoxicity implicates oxidative stress via over-generation of reactive oxygen species (ROS).6 Unfortunately, cisplatin-induced nephrotoxicity has no specific treatments yet; hydration with saline is the sole treatment administered to patients.7 Therefore, a new and effective treatment against cisplatin-induced kidney injury is called upon urgently.
Liver X receptors (LXRs) exist in two forms: LXRα and LXRβ. The former one expressed mostly in the liver, adrenal tissue, kidney, adipose tissue, and macrophages; the latter one expressed ubiquitously.8 They are participants of the nuclear receptor superfamily involved in the regulation of cholesterol and lipid metabolism. To further their importance in cholesterol homeostasis, LXRs are also considered great regulators for both inflammatory response and innate immunity. In vitro, the over-expression of inflammatory mediators such as interleukin-6 cyclooxygenase (COX)-2, and inducible nitric oxide synthase stimulated by TNF-α, bacterial lipopolysaccharide (LPS), or interleukin-1β (IL-1β) is decreased by LXR agonists.9 Moreover, activation of LXR reduces matrix metalloproteinase-9 mRNA expression and blunts its induction by pro-inflammatory stimuli through antagonism of the NF-kappaB signaling pathway.10 Interestingly, LXR agonists were shown to strikingly decrease the LPS-induced production of TNF-α and prostaglandin E2 (PGE2) in rat Kupffer cells.11 In agreement with in vitro data, LXR agonists reduce inflammation and inhibit inflammatory gene expression in contact dermatitis and atherosclerosis animal models.9,12,13 Besides the anti-inflammatory effects, LXR agonists also exert protective effects against high glucose-induced oxidative stress by inhibiting ROS production.14 Importantly, LXR mRNA and protein expressions are suppressed by LPS in kidney and interleukin-1β or TNF-α in human proximal tubular (HK-2) cells.15 To date, the biological function of LXR receptors in the kidney is barely known, and whether LXR agonists have beneficial effect on acute kidney injury is largely unknown. The current study urges to examine the potential therapeutic effects of the LXR agonist TO901317 in cisplatin-induced renal injury.
Materials and methods
Materials
Cisplatin [Cis-diamminedichloro-platinum (II)] from Sigma-Aldrich (St. Louis, MO, USA). The TO901317 compound from the Cayman Chemical Company (Ann Arbor, MI, USA) and dissolved in dimethylsulfoxide (DMSO).
Animal experiments
Male C57BL/6 mice (8–10 week old) were feed on standard rodent chow with adequate water. The mice were grouped into three groups: control (Cont, n = 8), cisplatin-induced kidney injury (Cisplatin; n = 12), and cisplatin-induced kidney injury with TO901317 treatment (Cisplatin + TO901317; n = 14) groups. Cisplatin was dissolved in saline at 2 mg/mL and used to treat mice by single intraperitoneal (i.p.) injection (20 mg/kg), both in cisplatin and TO901317 + cisplatin groups. The LXR agonist TO901317 was administered at 10 mg/kg/day (TO901317 + cisplatin), and DMSO alone was given to vehicle control animals (cisplatin vehicle) by daily gavage starting 12 h after cisplatin administration. The control mice received an i.p. saline injection. After mouse euthanasia (72 h after cisplatin treatment), both kidneys were excised and blood samples were drawn from the inferior vena cava. All procedures employing mice were conducted in accordance with the principles and guidance of the Ethics Committee of the Shandong University.
Renal function
Plasma urea (BUN) and creatine levels were determined to assess the renal function. After blood collection, serum levels of these toxicity markers were measured immediately using a blood chemistry analyzer.
Renal histology
Mouse kidneys were fixed in 4% paraformaldehyde overnight. After embedding in paraffin, 4-µm sections were prepared and stained with periodic acid-Schiff and hematoxylin and eosin, respectively. The renal histological damage was valued by the tubular lysis, disruption, cast formation, and dilation which indicated the tissue damage, and measured by the percentage of damaged tubules as described: 0, no damage; Lv.1, <25%; Lv.2, 25–50%; Lv.3, 50–75%; Lv.4, >75%.16
Real-time RT-PCR
The kidneys were obtained and preserved in the RNA later solution (Sangong Biotech, China) at − 20℃ until extraction of RNA. Total RNA was isolated using TRIzol (Invitrogen, CA, USA), and cDNA was synthesized with Superscript (TaKaRa, Japan). Real-time RT-PCR was implemented by a Quanti Tect SYBR Green kit (Qiagen, Germany) on an ABI Prism 7500 real-time PCR instrument equipped with suitable software (Applied Biosystems, CA, USA). The oligonucleotide sequences used for real-time PCR (RT-PCR) were as follows (Sangong Biotech, China): GAPDH, sense 5′-GTCTTCACTACCATGGAGAGG-3′ and antisense 5′-TCATGGATGACCTTGGCCAG-3′; LXRα, sense 5′-TCCATCAACCACCCCCAC GAC-3′ and antisense 5′-CAGCCAGAAAACACCCAACCT-3′; LXRβ, sense 5′-GCTCAGGAGCTGATGATCCA-3′ and antisense 5′-GCGCTTGATC-CTCGTGTAG-3′; ABCA1, sense 5′-TGTCCAGTCCAGTAATGGTTCTGT-3′ and antisense 5′-AAGCGAGATATGGTCCGGATT-3′; ABCG1, sense 5′-CCAGAAGTCGGAGGCCATC-3′ and antisense 5′-AAGTCCAGGTACAGCTTGGCA-3′, TNF-α, sense 5′-TCCCCAAAGGGATGAGAAG-3′ and antisense 5′–CACTTGGTGGTTTGCTACGA-3′; IL-1β, sense 5′-ACTGTGAAATGCCACCTTTTG-3′ and antisense 5′-TGTTGATGTGCTGCTGTGAG-3′; P47phox, sense 5′-GTCGTGGAGAAGAGCGAGAG-3′ and antisense 5′-CGCTTTGATGGTTACATACGG-3′; gp91phox, sense 5′-CCGTATTGTGGGAGACTGGA-3′ and antisense 5′-CTTGAGAATGGAGGCAAAGG-3′; COX-2, sense 5′-AGGACTCTGCTCACGAAGGA-3′ and antisense 5′-TGACATGGATTGGAACAGCA-3′; MCP-1, sense 5′-GCTCTCTCTTCCTCCACCAC-3′ and antisense 5′-ACAGCTTCTTTGGGACACCT-3′; ICAM-1, sense 5′-CGCTTCCGCTACCATCAC-3′ and antisense GGCGGCTCAGTATCCTC-3′; VCAM-1, sense 5′-GGAGAGACAAAGCAGAAGTGG-3′ and antisense 5′-AACACAAGCGTGGATTTGG-3′.
Western blotting
Total protein samples in kidney tissues were extracted using a lysis buffer, and the concentrations were measured using the Coomassie reagent. Equal amounts of tissue protein (50 µg) were denatured in 10 min at 100℃, separated by SDS-PAGE, and then transferred onto nitrocellulose membranes. Nonfat dry milk (5%) was used in Tris-buffered saline (TBS) to block the blots overnight and then incubated for 1 h with anti-mouse TNF-α and β-actin monoclonal antibodies (Santa Cruz, USA) or rabbit polyclonal antibody raised against mouse COX-2 (Cayman Chemical, MI, USA). Then the blots were washed with TBS and were incubated with horseradish peroxidase-conjugated secondary antibody, and immune complexes were detected using the Electro-Chemi-Luminescence (ECL) system (Amersham). And then the Bio-Rad electrophoresis image analyzer (Bio-Rad, UK) was used to qualify the protein signal.
Measurement of renal PGE2 content
Whole kidneys were quick-frozen in liquid nitrogen at once upon collection and stored at − 80℃ environment. Then, kidney tissues were lysed and homogenized as previously described,17 and PGE2 contents were measured using a regular PGE2 kit (Cayman Chemical) under the guidance of manufacturer’s instructions.
Quantification of thiobarbituric acid-reactive substances
The thiobarbituric acid-reactive substances (TBARS) of mouse kidney were measured based on malondialdehyde (MDA) formation. The measurement was carried out with a TBARS Assay kit (Cell Biolabs, USA) just following the manufacturer’s instructions.
Statistical analysis
Values shown represent means ± SEM. Data were analyzed using one-way analysis of variance followed by a Bonferroni post-test. A P < 0.05 value was considered statistical significance.
Results
Effects of the LXR agonist TO901317 on renal gene regulation
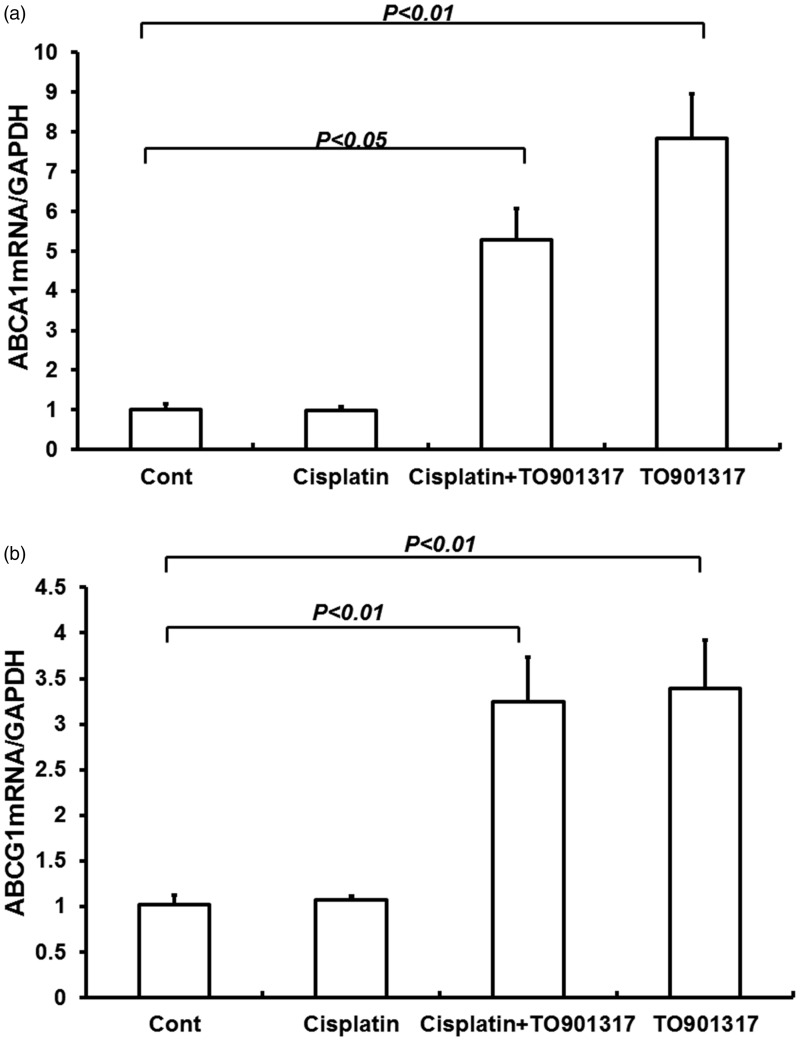
Both the LXRα and LXRβ are expressed in kidney; the LXRα is widely expressed in the kidney including every nephron segment and the glomeruli, while the LXRβ is ubiquitously expressed.18 To date, many LXR target genes have been identified, including ATP-binding cassette transporter (ABC) A1, ABCG8, ABCG5, cholesterol ester transport protein, and lipoprotein lipase. These LXR target genes play an important role for LXRα and LXRβ in the regulation of cholesterol and fatty acid metabolism. The ABCA1 and ABCG1 were chosen as the target genes to evaluate the activation of LXRs. The result indicated that TO901317 increased both ABCA1 and ABCG1 mRNA expression levels (Figure 1(a–b)) (P < 0.01). These results reached to an agreement with previous data which had indicated the activation of LXR target genes in mouse kidney after treatment with TO901317.19
Figure 1.
Effects of the LXR agonist TO901317 on renal ABCA1 (a) and ABCG1 (b) gene expression in control, Cisplatin + vehicle, Cisplatin + TO901317, and TO901317 mice. Cont: n = 8; Cisplatin: n = 12; Cisplatin + TO901317: n = 14; TO901317: n = 6. Data are mean ± SEM
TO901317 ameliorates cisplatin-induced renal dysfunction
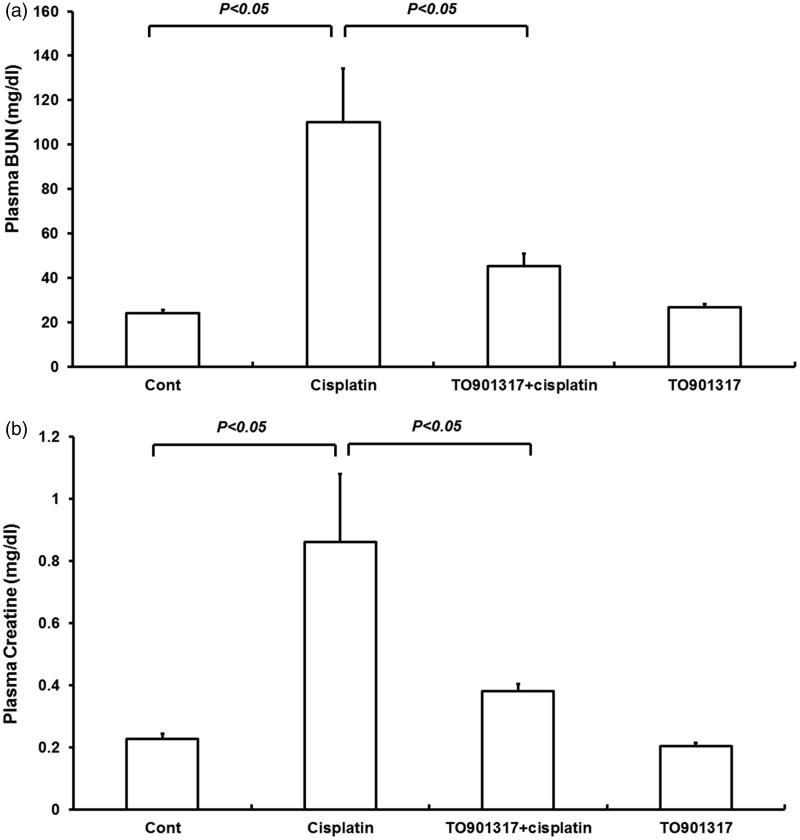
The renal function was assessed by measuring plasma BUN and creatinine levels. Treatment with TO901317 alone had no effect on renal function. Cisplatin injection induced severe renal dysfunctions, increasing plasma BUN levels, from 24.2 ± 1.46 to 110.1 ± 24.1 mg/dL (P < 0.05) (Figure 2(a)), and plasma creatinine amounts, from 0.22 ± 0.02 to 0.86 ± 0.22 mg/dL (P < 0.05) (Figure 2(b)). Interestingly, treatment with the LXR agonist TO901317 12 h after cisplatin injection resulted in reduced plasma BUN (45.2 ± 5.7 vs. 110.1 ± 24.1 mg/dL, P < 0.05) (Figure 2(a)) and creatinine (0.38 ± 0.02 vs. 0.86 ± 0.22 mg/dL, P < 0.05) levels, by contrast with the Cisplatin group (Figure 2(b)).
Figure 2.
Plasma BUN (a) and creatinine (b) levels in control, Cisplatin vehicle, Cisplatin + TO901317, and TO901317 mice. Cont: n = 8; Cisplatin: n = 12; Cisplatin + TO901317: n = 14; TO901317: n = 6. Data are mean ± SEM
TO901317 attenuates cisplatin-induced histological changes in the kidney
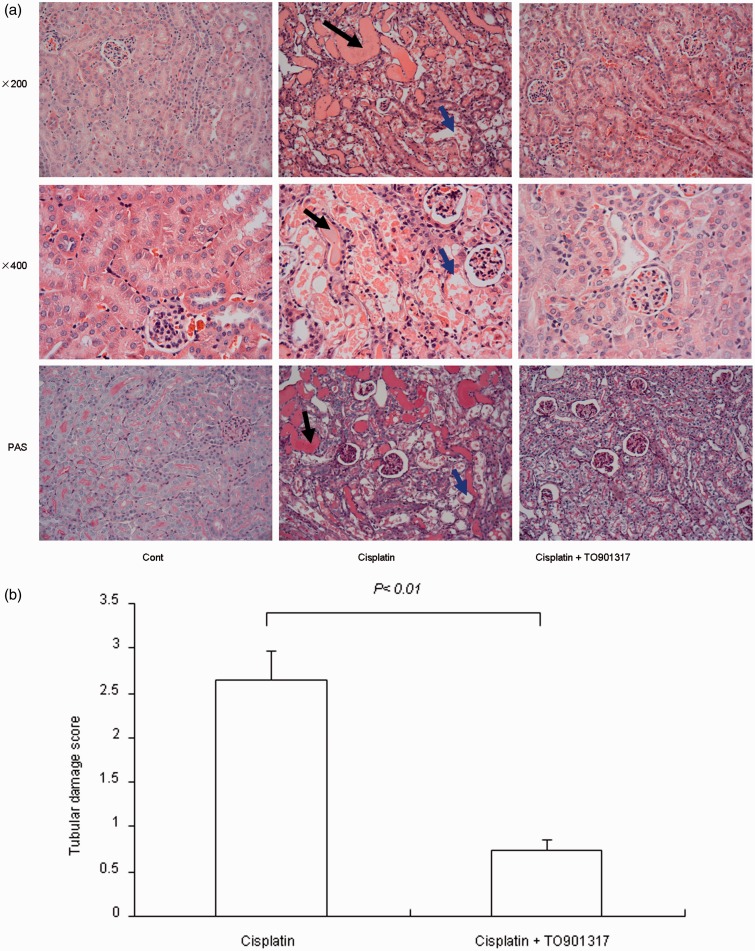
Cisplatin injection caused obvious renal histological damage such as tubular necrosis, dilation of renal tubules, protein cast, and desquamation of epithelial cells in the renal tubules (Figure 3(a)). These histological changes were remarkably alleviated after treatment with the LXR agonist TO901317. Indeed, mice in the TO901317 treatment group displayed significantly reduced semi-quantitative histological damage scores, compared with the cisplatin group (P < 0.01) (Figure 3(b)).
Figure 3.
Morphological analysis of cisplatin-induced renal injury in control, Cisplatin vehicle, Cisplatin + TO901317, and TO901317 mice. (a) Representative photomicrographs with hematoxylin and eosin staining (200 × and 400×) and periodic acid-Schiff staining (200×) of renal cortex of kidneys. The black arrow indicates intratubular cast formation and the blue arrow indicates damaged tubular cells. (b) Semi-quantitative analysis of histological appearance. Cont: n = 8; Cisplatin: n = 12; Cisplatin + TO901317: n = 14; TO901317: n = 6. Data are mean ± SEM. (A color version of this figure is available in the online journal.)
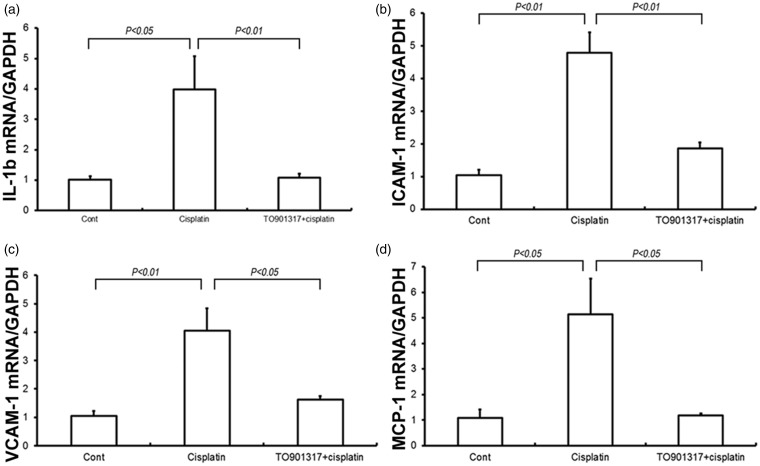
TO901317 reduces cisplatin-induced renal inflammation
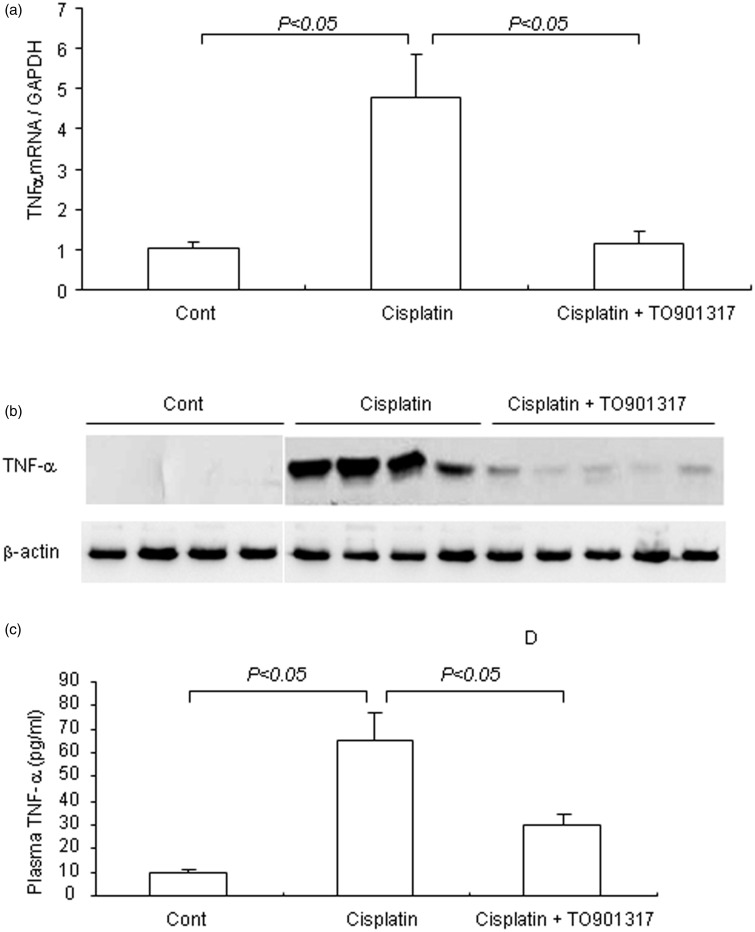
TNF-α is well known to play a key role in the inflammatory responses in cisplatin-induced kidney injury. Therefore, we measured the renal TNF-α mRNA and protein amounts as well as circulating TNF-α. Renal TNF-α mRNA and protein amounts were increased by 4.7- and 4.5-fold (P < 0.05), respectively, in the cisplatin-treatment group compared with control animals, and the LXR agonist TO901317 remarkably reduced the increase in TNF-α mRNA and protein expression levels (P < 0.05) (Figure 4(a) and (b)). Similarly, circulating TNF-α levels were elevated (6.9-fold) (P < 0.05) in cisplatin-treated mice, while only a 3.0-fold (P < 0.05) was observed after treatment with the LXR agonist TO901317 (Figure 4(c)). Cisplatin induced renal mRNA expression of adhesion molecules and chemokines, including intercellular adhesion molecule-1 (ICAM-1) (P < 0.01), vascular cell adhesion molecule-1 (VCAM-1) (P < 0.05), and monocyte chemoattractant protein 1 (MCP-1) (P < 0.05); this effect was remarkably attenuated by TO901317 (P < 0.05) (Figure 5(a–d)). Taken together, TO901317 treatment alleviated the expression of pro-inflammatory cytokines, chemokines, and adhesion molecules in cisplatin-induced kidney injury.
Figure 4.
Renal TNF-α mRNA and protein expression levels and circulating TNF-α amounts in control, Cisplatin vehicle, and Cisplatin + TO901317 mice. (a) qRT-PCR analysis of renal TNF-α mRNA; (b) Immunoblotting of TNF-α; (c) Enzyme-linked immunosorbent assay analysis of circulating TNF-α. Cont: n = 8; Cisplatin: n = 12; Cisplatin + TO901317: n = 14. Data are mean ± SEM
Figure 5.
Levels of pro-inflammatory cytokines and adhesion molecules in control, Cisplatin vehicle, and Cisplatin + TO901317 mice. (a) Real-time RT-PCR of renal IL-1βexpression; (b) Real-time RT-PCR of renal ICAM-1 expression; (c) Real-time RT-PCR of renal VCAM-1 expression; (d) Real-time RT-PCR of renal MCP-1 expression. The expression was normalized to GAPDH levels. Cont: n = 8; Cisplatin: n = 12; Cisplatin + TO901317: n = 14. Data are mean ± SEM
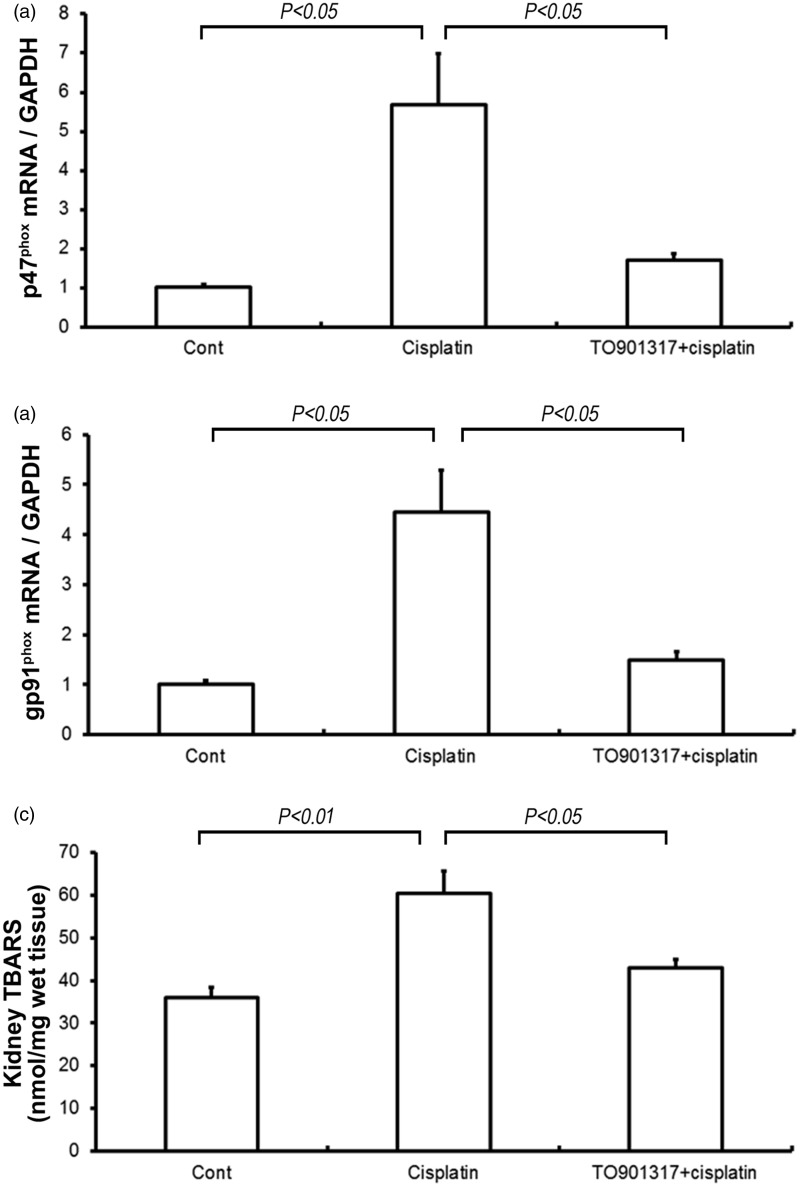
TO901317 attenuates cisplatin-induced renal oxidative stress
Both renal p47phox and gp91phox were examined on the mRNA level. At 72 h post-treatment with cisplatin, renal p47phox and gp91phox mRNA levels were 5.6- and 4.4-fold higher, respectively, in cisplatin vs. control mice(P < 0.05) (Figure 6(a) and (b)). However, the increases of the p47phox and gp91phox mRNA levels were reduced by the TO901317 treatment in comparison with cisplatin vehicle mice (P < 0.05). The levels of kidney TBARS displayed a similar pattern of changes as the renal p47phox and gp91phox expression (P < 0.05) (Figure 6(c)).
Figure 6.
Real-time RT-PCR analysis of p47phox (a) and gp91phox (b) gene expression and measurement of thiobarbituric acid-reactive substances (TBARS) (c) in the kidneys of control, Cisplatin vehicle, and Cisplatin + TO901317 mice. Cont: n = 8; Cisplatin: n = 12; Cisplatin + TO901317: n = 14. Data are mean ± SEM
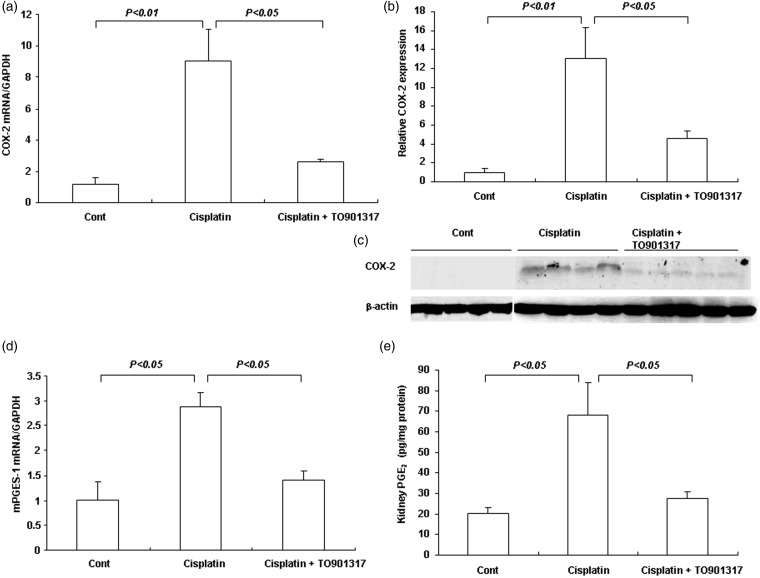
TO901317 reduced cisplatin-induced over-expression of renal COX-2
COX-2 is another critical marker of inflammatory response in cisplatin-induced kidney injury. Renal COX-2 mRNA and protein levels were 9.0- and 13.2-fold higher, respectively, in the cisplatin group compared with control mice (P < 0.01); after TO901317 treatment, these levels were only 2.6- and 4.6-fold higher compared with control mice (Figure 7(a)–(c)). The renal mRNA expression of mPGES-1 followed the same pattern of COX-2 (P < 0.05) (Figure 7(d)). The renal content of PGE2, which reflects COX-2 enzyme activity, was increased by 3.4-fold after cisplatin injection, an enhancement almost completely abolished by treatment with TO901317 (P < 0.05) (Figure 7(e)).
Figure 7.
COX-2 pathway in the kidneys of control, Cisplatin vehicle, and Cisplatin + TO901317 mice. (a) Real-time RT-PCR of renal COX-2 expression; (b) Densitometric value of the COX-2 protein; (c) Immunoblotting of COX-2; (d) Real-time RT-PCR of renal mPGES-1 expression; (e) Enzyme-linked immunosorbent assay analysis of renal PGE2 content. Cont: n = 8; Cisplatin: n = 12; Cisplatin + TO901317: n = 14. Data are mean ± SEM
Discussion
In the present study, mice were treated with the LXR agonist TO901317 12 h postcisplatin administration. We demonstrated that the TO901317 reduced renal inflammation and oxidative stress to attenuate cisplatin-induced kidney injury. These findings indicated that LXR agonists are potential therapeutic agents for cisplatin-induced kidney injury.
In clinical practice, several therapeutic agents produce functional impairment and toxic injury to kidney which may lead to acquired acute renal failure; indeed, the kidney plays a major role in excretion and is exposed to large amounts of parent and active metabolites of drugs.20 Cisplatin is commonly used in the treatment of different types of human tumor. Kidneys represent the main route of cisplatin excretion, with proximal tubule cells considered the primary site for cisplatin accumulation.21 Therefore, nephrotoxicity is one of the most severe side effects of cisplatin chemotherapy. Thus, researchers have focused on the mechanisms of cisplatin-induced kidney injury in order to find potential nephron-protective agents after cisplatin treatment.
Inflammation is an important factor in the pathogenesis of cisplatin nephrotoxicity. It has been demonstrated that cisplatin administration frequently results in the up-regulation of pro-inflammatory mediators in kidney22 and activates the signaling pathway for NF-κB. Of all the inflammatory cytokines, TNF-α is considered as a central one in mediating renal injury. TNF-α, in particular, is up-regulated in cisplatin-induced kidney injury and coordinates the activation of a large range of chemokines and cytokines in the kidney under cisplatin administration.18,23,24 Interestingly, inhibition of TNF-α production attenuates cisplatin-induced kidney injury but does not impact anti-tumor efficacy.25 Moreover, TNF-α knockout mice were less likely to sustain renal injury than wild-type animals and displayed markedly higher survival rates following cisplatin injection.24 In the current study, we demonstrated that cisplatin increased renal expression of TNF-α, IL-1β, MCP-1, and ICAM-1, in agreement with previous reports. In vitro, LXR agonist could reduce LPS-induced inflammation by reducing the expression of TNF-α and other inflammatory mediators.9 Consistent with in vitro data, treatment with the LXR agonist TO901317 blunted the induction of these factors in the kidney and also reduced serum TNF-α levels, indicating its anti-inflammatory effect.18
Oxidative stress has been acknowledged as an important factor which contributes to cisplatin-induced kidney injury. Multiple cellular sources of increased superoxide production may include NADH/NADPH and xanthine oxidases in the mitochondrial respiratory chain. NADPH oxidase is made up of six subunits. These subunits are GTPase (usually Rac1 or Rac2) and “phox” units: p22phox, gp91phox, p47phox, p67phox, and p40phox. Among these subunits, gp91phox and p47phox are the most important elements: the former contains the catalytic domain, while the latter is indispensable for cytosolic subunit translocation and initiation of NADPH oxidase assembly.26–28 Cisplatin increases superoxide production, especially ROS, as shown with cultured renal tubular cells, kidney tissues, and animal models. Interestingly, the ROS production induced by cisplatin in the kidney is significantly accompanied by over-expression of ROS generating NADPH oxidase enzyme isoforms NOX2 (gp91phox) and p47 phox mRNA.29 Meanwhile, the augmented production of MDA is favored with the generation of ROS, the end product of lipid peroxidation and/or ROS generation and an indicator of ROS production. On the other hand, activation of LXR by TO901317 attenuates oxidative stress and decreases the TNF-α-induced superoxide and nitrotyrosine production under normolipemic conditions. It was shown that the LXR agonist TO901317 decreases the expression of TNF-α-induced oxidative stress markers NOX4 and p22phox and normalizes NOX activity to basal levels in human umbilical vein endothelial cells.30 These findings indicate that the LXR agonist possesses anti-oxidative properties independently of its cholesterol-modulating effects. In the current study, we found increased MDA production, and enhanced renal mRNA and protein levels for the NADPH oxidase enzyme isoforms NOX2 (gp91phox) and p47phox three days after cisplatin administration. These data indicate that the LXR agonist TO901317 significantly ameliorates the oxidative stress induced by cisplatin.
COX contains two distinct membrane-anchored iso-enzymes, COX-1 and COX-2. COX-1 has the most abundant isoform in the kidney, where it is constitutively expressed. However, COX-2 is expressed at a minimized level in variable kidney regions despite of species and inducible in macrophages, endothelial cells, and monocytes.31 The inflammation in response to cytokines and reactive oxygen intermediates could induce the expression of COX-2, which implies that COX-2 is related to inflammation.32 COX-2 is a crucial factor participated in the pathogenesis of cisplatin nephrotoxicity. In a previous study, we found that cisplatin induces the up-regulation of COX-2 mRNA and immuno-reactivity in the kidney after three days treatment.33 These findings are in accordance with a recent study that indicated an increase in COX-2 mRNA levels and strong detection of the COX-2 protein in kidneys of cisplatin-treated mice. These findings were recognized by the amelioration of cisplatin-induced injury after treatment with COX-2 inhibitor.34 Herein, cisplatin induced the up-regulation of COX-2 mRNA and protein, consistent with our previous findings. Furthermore, we examined the kidney PGE2, which belongs to the major pro-inflammatory PGs derived from the coordinated action of COX-2. We found that kidney PGE2 content is markedly increased by cisplatin and reversed to normal levels by TO901317. The inhibition of COX-2 expression and PGE2 production by LXR agonists is supported by other studies. The activation of LXR inhibits the LPS-induced COX-2 expression and PGE2 production in murine peritoneal macrophages,15 while in vivo, the LXR agonist reduces global ischemic brain injury by inhibiting the production of COX-2 in mice.16
LXRs are acknowledged to play an important role in cholesterol metabolism which shows beneficial effects limiting atherogenesis. In addition, LXRs are also regulators of the innate immune response. In the present study, we found the TNF-α, COX-2/mPGES-1/PGE2 pathway and other inflammatory mediators are inhibited for the LXR agonist TO901317 attenuates the cisplatin-induced kidney injury in mice. We also found that TO901317 ameliorates the cisplatin-induced oxidative stress. Herein, the effects that LXR agonist TO901317 protects against cisplatin nephrotoxicity were demonstrated. These pre-clinical findings pave the way for future clinical trials involving LXRs to suppress the adverse effects of cisplatin. However, the detailed mechanism of the inhibitory effects of the LXR agonist on inflammation and oxidative stress is unclear and need further exploration. And further studies are warranted to evaluate the protective effects of the LXR agonist TO901317 on other models of nephrotoxicity, for example, the endotoxin-induced endotoxemia and renal injury.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (Grant No. 81200530) (to HW).
Author contributions
All authors participated in conceiving and outlining the manuscript. HW and MY provided the title and the general outline of the manuscript. The entire team proof-read and edited the manuscript.
References
- 1.Giaccone G. Clinical perspectives on platinum resistance. Drugs 2000; 59(Suppl 4): 9–17; discussion 37–8. [DOI] [PubMed] [Google Scholar]
- 2.Jia Z, Wang N, Aoyagi T, Wang H, Liu H, Yang T. Amelioration of cisplatin nephrotoxicity by genetic or pharmacologic blockade of prostaglandin synthesis. Kidney Int 2011; 79: 77–88. [DOI] [PubMed] [Google Scholar]
- 3.Cheng O, Ostrowski RP, Liu W, Zhang JH. Activation of liver X receptor reduces global ischemic brain injury by reduction of nuclear factor-kappaB. Neuroscience 2010; 166: 1101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 2008; 73: 994–1007. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Liu H, Jia Z, Olsen C, Litwin S, Guan G, Yang T. Nitro-oleic acid protects against endotoxin-induced endotoxemia and multiorgan injury in mice. Am J Physiol Renal Physiol 2010; 298: F754–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsuruya K, Ninomiya T, Tokumoto M, Hirakawa M, Masutani K, Taniguchi M, Fukuda K, Kanai H, Kishihara K, Hirakata H, Iida M. Direct involvement of the receptor-mediated apoptotic pathways in cisplatin-induced renal tubular cell death. Kidney Int 2003; 63: 72–82. [DOI] [PubMed] [Google Scholar]
- 7.Cornelison TL, Reed E. Nephrotoxicity and hydration management for cisplatin, carboplatin, and ormaplatin. Gynecol Oncol 1993; 50: 147–58. [DOI] [PubMed] [Google Scholar]
- 8.Calkin AC, Tontonoz P. Liver x receptor signaling pathways and atherosclerosis. Arterioscler Thromb Vasc Biol 2010; 30: 1513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joseph SB, McKilligin E, Pei L, Watson MA, Collins AR. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc Natl Acad Sci U S A 2002; 99: 7604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castrillo A, Joseph SB, Marathe C, Mangelsdorf DJ, Tontonoz P. Liver X receptor-dependent repression of matrix metalloproteinase-9 expression in macrophages. J Biol Chem 2003; 278: 10443–9. [DOI] [PubMed] [Google Scholar]
- 11.Wei Q, Dong G, Yang T, Megyesi J, Price PM, Dong Z. Activation and involvement of p53 in cisplatin-induced nephrotoxicity. Am J Physiol Renal Physiol 2007; 293: F1282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fowler AJ, Sheu MY, Schmuth M, Kao J, Fluhr JW, Rhein L, Collins JL, Willson TM, Mangelsdorf DJ, Elias PM, Feingold KR. Liver X receptor activators display anti-inflammatory activity in irritant and allergic contact dermatitis models: liver-X-receptor-specific inhibition of inflammation and primary cytokine production. J Invest Dermatol 2003; 120: 246–55. [DOI] [PubMed] [Google Scholar]
- 13.Kelly KJ, Meehan SM, Colvin RB, Williams WW, Bonventre JV. Protection from toxicant-mediated renal injury in the rat with anti-CD54 antibody. Kidney Int 1999; 56: 922–31. [DOI] [PubMed] [Google Scholar]
- 14.Cheng Y, Feng Y, Zhu M, Yan B, Fu S, Guo J, Hu J, Song X, Guo S, Liu G. Synthetic liver X receptor agonist T0901317 attenuates high glucose-induced oxidative stress, mitochondrial damage and apoptosis in cardiomyocytes. Acta Histochem 2014; 116: 214–21. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. Downregulation of liver X receptor-alpha in mouse kidney and HK-2 proximal tubular cells by LPS and cytokines. J Lipid Res 2005; 46: 2377–87. [DOI] [PubMed] [Google Scholar]
- 16.Ramesh G, Reeves WB. Salicylate reduces cisplatin nephrotoxicity by inhibition of tumor necrosis factor-alpha. Kidney Int 2004; 65: 490–9. [DOI] [PubMed] [Google Scholar]
- 17.Harris RC. Cyclooxygenase-2 in the kidney. J Am Soc Nephrol 2000; 11: 2387–94. [DOI] [PubMed] [Google Scholar]
- 18.Wang YY, Dahle MK, Agren J, Myhre AE, Reinholt FP, Foster SJ, Collins JL, Thiemermann C, Aasen AO, Wang JE. Activation of the liver X receptor protects against hepatic injury in endotoxemia by suppressing Kupffer cell activation. Shock 2006; 25: 141–6. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Zhang X, Chen L, Wu J, Su D, Lu WJ, Hwang MT, Yang G, Li S, Wei M, Davis L, Breyer MD, Guan Y. Liver X receptor agonist TO-901317 upregulates SCD1 expression in renal proximal straight tubule. Am J Physiol Renal Physiol 2006; 290: F1065–73. [DOI] [PubMed] [Google Scholar]
- 20.Ekor M, Emerole GO, Farombi EO. Phenolic extract of soybean (Glycine max) attenuates cisplatin-induced nephrotoxicity in rats. Food Chem Toxicol 2010; 48: 1005–12. [DOI] [PubMed] [Google Scholar]
- 21.Ninomiya Y, Yasuda T, Kawamoto M, Yuge O, Okazaki Y. Liver X receptor ligands inhibit the lipopolysaccharide-induced expression of microsomal prostaglandin E synthase-1 and diminish prostaglandin E2 production in murine peritoneal macrophages. J Steroid Biochem Mol Biol 2007; 103: 44–50. [DOI] [PubMed] [Google Scholar]
- 22.Khan KN, Venturini CM, Bunch RT, Brassard JA, Koki AT, Morris DL, Trump BF, Maziasz TJ, Alden CL. Interspecies differences in renal localization of cyclooxygenase isoforms: implications in nonsteroidal antiinflammatory drug-related nephrotoxicity. Toxicol Pathol 1998; 26: 612–20. [DOI] [PubMed] [Google Scholar]
- 23.Deng J, Kohda Y, Chiao H, Wang Y, Hu X, Hewitt SM, Miyaji T, McLeroy P, Nibhanupudy B, Li S, Star RA. Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int 2001; 60: 2118–28. [DOI] [PubMed] [Google Scholar]
- 24.Honma S, Takahashi N, Shinohara M, Nakamura K, Mitazaki S, Abe S, Yoshida M. Amelioration of cisplatin-induced mouse renal lesions by a cyclooxygenase (COX)-2 selective inhibitor. Eur J Pharmacol 2013; 715: 181–8. [DOI] [PubMed] [Google Scholar]
- 25.Ramesh G, Reeves WB. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest 2002; 110: 835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Babior BM. Activation of the respiratory burst oxidase. Environ Health Perspect 1994; 102(Suppl 10): 53–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallin JI. Delineation of the phagocyte NADPH oxidase through studies of chronic granulomatous diseases of childhood. Int J Tissue React 1993; 15: 99–103. [PubMed] [Google Scholar]
- 28.Geiszt M, Leto TL. The Nox family of NAD(P)H oxidases: host defense and beyond. J Biol Chem 2004; 279: 51715–8. [DOI] [PubMed] [Google Scholar]
- 29.Horvath B, Mukhopadhyay P, Kechrid M, Patel V, Tanchian G, Wink DA, Gertsch J, Pacher P. Beta-caryophyllene ameliorates cisplatin-induced nephrotoxicity in a cannabinoid 2 receptor-dependent manner. Free Radic Biol Med 2012; 52: 1325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srivastava RC, Farookh A, Ahmad N, Misra M, Hasan SK, Husain MM. Evidence for the involvement of nitric oxide in cisplatin-induced toxicity in rats. Biometals 1996; 9: 139–42. [DOI] [PubMed] [Google Scholar]
- 31.Lu TT, Repa JJ, Mangelsdorf DJ. Orphan nuclear receptors as eLiXiRs and FiXeRs of sterol metabolism. J Biol Chem 2001; 276: 37735–8. [DOI] [PubMed] [Google Scholar]
- 32.Hartmann JT, Lipp HP. Toxicity of platinum compounds. Expert Opin Pharmacother 2003; 4: 889–901. [DOI] [PubMed] [Google Scholar]
- 33.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med 2003; 9: 213–9. [DOI] [PubMed] [Google Scholar]
- 34.Spillmann F, Van Linthout S, Miteva K, Lorenz M, Stangl V, Schultheiss HP, Tschope C. LXR agonism improves TNF-alpha-induced endothelial dysfunction in the absence of its cholesterol-modulating effects. Atherosclerosis 2014; 232: 1–9. [DOI] [PubMed] [Google Scholar]