Abstract
Cashew apple is a tropical pseudofruit consumed as juice due to its excellent nutritional and sensory properties. In spite of being well known for its important antioxidant properties, the cashew apple has not been thoroughly investigated for its therapeutic potential. Thereby, this study evaluated the antioxidant capacity, anti-inflammatory, and wound-healing activities of cashew apple juice. Juices from ripe and immature cashew apples were analyzed for antioxidant, anti-inflammatory, and wound-healing properties. Those were evaluated in murine models of xylene-induced ear edema and wound excision. Swiss mice were treated with cashew juice by gavage. Edema thickness was measured and skin lesions were analyzed by planimetry and histology. Both antioxidant content and total antioxidant activity were higher in ripe cashew apple juice (RCAJ) than in unripe cashew apple juice (UNCAJ). The UNCAJ presented the main anti-inflammatory activity by a significant inhibition of ear edema (66.5%) when compared to RCAJ (10%). Moreover, UNCAJ also showed the best result for wound contraction (86.31%) compared to RCAJ (67.54%). Despite of higher antioxidant capacity, RCAJ did not promote better anti-inflammatory, and healing responses, which may be explained by the fact that treatment increased antioxidants level leading to a redox “imbalance” turning down the inflammatory response modulation exerted by reactive oxygen species (ROS). The results suggest that UNCAJ presents a greater therapeutic activity due to a synergistic effect of its phytochemical components, which improve the immunological mechanisms as well as an optimal balance between ROS and antioxidants leading to a better wound healing process.
Keywords: Anacardium occidentale, antioxidants, inflammation, wound healing activity
Introduction
The cashew tree (Anacardium occidentale L.) belongs to the family Anacardiaceae. It is a native plant of Brazil and the fruit consists of a cashew nut (the true fruit) and a cashew apple (pseudofruit), which has excellent nutritional and sensory properties.1 The cashew culture is one of the main agronomic activities in Northeast Brazil and almost the whole production is concentrated in the states of Ceará, Piauí, and Rio Grande do Norte.2 However, no more than 10% of potential cashew apple output is at present consumed or utilized in either fresh or processed form such as jam, syrup, chutney, beverage, ice creams, etc.3–5 The economic value of cashew apple juice has increased as the concentrated and processed (called cajuína) forms are among the most popular products in Northeast Brazil.6
Recently, there are an increasing number of reports evaluating the clinical effectiveness of several parts of cashew tree, for example, nuts,7 stem bark,8 leaves,9 and cashew apple juice10 revealing several properties from anti-inflammatory, antiulcer, antibacterial, antifungal to antitumor. However, the potential of cashew apple nutraceutical properties has not been explored as compared to other organs.
Cashew apple is rich in vitamin C, organic acids, antioxidants, minerals, and carbohydrates.1,4,11 Its phytochemical profile reveals a complex source of natural antioxidants as phenolic compounds and carotenoids what makes this pseudofruit an excellent source of antioxidants that can scavenge free radical or reactive oxygen species (ROS), inhibit free radical formation, and prevent damage of cellular components, as well as cellular death.10,12–14 More recently, the interest and research on the nutritional and medicinal properties of cashew apple and its juice have grown,11,15 but none of these studies have evaluated the anti-inflammatory and healing potential of cashew apple or juice. Therefore, the present study evaluated the antioxidant, anti-inflammatory, and healing properties of cashew apple juice.
Methods and materials
Plant material and juice preparation
Ripe (orange reddish) and unripe (yellowish green) cashew apple fruits (A. occidentale L. dwarf clone “CCP76”) were harvested at Pacajus Experimental Station of Embrapa Tropical Agroindustry, Ceará, located at 38° 30′W and 4° 30′S with average temperature of 26.5℃ and 1100 mm of precipitation.
The nuts were removed and the cashew apple was processed in a domestic food processor for 1 min at low speed and then the pulp was filtered through a sieve to concentrate it. The juice was prepared as pulp was diluted in water (1:1) and then stored at –20℃ for further analysis. This solution was then used for all phytochemical analyses and oral treatments of the animals.
Antioxidants analysis
The total antioxidant activity was determined using the 2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt method as described by Re et al.16 Total antioxidant activity was expressed as Trolox equivalent antioxidant capacity, µM Trolox/g of juice.
Total vitamin C was determined by titration with 2,6-dichloroindophenol according to the procedure described by Strohecker and Henning.17 The results were expressed as mg/100 g of juice. Total phenols were measured using a modified Folin–Ciocalteu method as described by Obanda et al.18 The absorbance was measured at 700 nm and results are expressed as mg gallic acid equivalent (GAE)/100 g of juice.
Total anthocyanins and yellow flavonoids were determined as described by Francis.19 The absorbance was measured at 535 and at 374 nm for total anthocyanin and for yellow flavonoid content using absorption coefficients of 98.2 and 76.6, respectively. The results were expressed as mg/100 g of juice.
Animals
Male Swiss mice weighing 25–30 g were individually housed in clean polyethylene cages under standard experimental conditions of humidity (40–45%), temperature (23–25℃), light/dark cycle (12 h), and fed on normal pellet diet and water ad libitum. All animal experiments were carried out in accordance with the guidelines for the ethical use of experimental animals published by the Brazilian National Council for the Control of Animal Experimentation. Moreover, the present research was approved by the Institutional Animal Care Committee at Federal University of Ceará under the protocol number 85/09 (CEPA/UFC).
Anti-inflammatory activity in ear edema model
The anti-inflammatory effect was evaluated by using xylene-induced ear edema model in mice according to Akindele and Adeyemi20 with some modifications. The animals were randomly allocated in four groups (n = 8 per group). Two groups were pretreated by gavage with 0.2 mL of ripe cashew apple juice (RCAJ) and unripe cashew apple juice (UNCAJ), respectively, for 30 consecutive days before the ear edema induced by xylene. A control group was performed with 0.2 mL of water in the same referred conditions while the fourth one was used as a reference standard group, which received dexamethasone doses (DEXA, 5 mg/kg) during three consecutive days prior to the edema induction. The basal ear thickness was measured before the induction of the edema with xylene, as a control. Xylene was applied topically in the inner (25 µL) and outer (25 µL) surfaces of the ear to induce acute edema in all groups. Edema was measured with a micrometer (Mitutoyo Serie 293), 1 h after the xylene application and expressed as the increase in ear thickness due to the inflammatory challenge. The micrometer was applied near the tip of the ear just distal to the cartilaginous ridges and the thickness was recorded in µm.21
Wound healing activity
Mice were randomly divided into four groups of 10 animals each group. Two groups were pretreated daily by gavage with 0.2 mL of RCAJ, and UNCAJ during 14 days. A control group received 0.2 mL of water by gavage, while a reference group was used as a positive control (REF group), which received a nutritional supplement (Decubital®, Nuteral). Both of them were in the same conditions as referred earlier.22 After this time period (14 days), a single wound was made by excising the skin with a 1 cm2 surgical mold on the dorsal region of each mouse (day 0). Mice were anesthetized by subcutaneous injections with 10% ketamine chlorhydrate (115 mg/kg) and 2% xilazine chlorhydrate (10 mg/kg) before their surgical procedure.23 Their dorsal surface was shaved with a sterile blade24 and disinfected with povidone–iodine. All groups carried on their respective treatments (RCAJ, UNCAJ, water, and nutritional supplement) for 21 consecutive days starting from day 0. The wounds were left undressed to the open environment and observed daily until the end of the experiment.
Planimetry
Planimetry was performed on days 0, 3, 7, and 14 on anesthetized animals (n = 6) according to De Oliveira et al.25 The anesthesia protocol used to make the wounds is the same above described. Contractions, which contribute for wound closure, were studied by tracing the raw wound. The wound area on each evaluation day was obtained by tracing the perimeter of the wound onto a sterile piece of clear acetate film with a special marking pen. The wound and one piece of millimeter paper with known area (1 cm2) were digitalized using a scanner. The measuring area was obtained comparing the amount of pixels inside perimeter and inside the known area using the mathematic expression: Wa = (Ka×Nw)/Nk, where Wa =wound area, Ka = known area, Nw = number of pixels inside wound area, and Nk = number of pixels inside known area. Thus, the unhealed wound area and the percentage of wound contraction were calculated and used for statistical analysis.
Histological evaluation
Skin specimens from wounds of each mouse (n = 4) were collected on days 3, 7, 14, and 21 according to De Oliveira et al.25 The specimens were fixed in 10% neutral buffered formalin, processed and embedded in paraffin. Five-micrometer sections were cut and stained with hematoxylin–eosin. In every skin section, an area just beneath the epidermis or crust formation was randomly selected. At each time, the re-epithelialization of the tissues was qualitatively assessed under the light microscope at a magnification of 100 to 400 ×. Re-epithelialization and ulceration of the tissues were scored as present (+) or absent (–). A histological evaluation focused in on the edema, mononuclear (MNC) and polymorphonuclear (PMN) cells, fibroblast proliferation (FP), and neovascularization in dermis were scored as absent (–), mild (+), moderate (++), and severe (+++) for epidermal or dermal re-modeling.26 The same investigator blindly evaluated all histological sections according to the histological scoring system (Table 1).
Table 1.
Histological scoring system
| Parameters | Absent (–) | Mild (+) | Moderate (++) | Intense (+++) |
|---|---|---|---|---|
| Edema* | 0 | <50 | 50 | >50 |
| PMN† | 0 | 1–5 | 6–10 | 11–20 |
| MNC† | 0 | 1–5 | 6–10 | 11–20 |
| FP† | 0 | 10–15 | 16–25 | >26 |
| NV‡ | 0 | <5 | 6–10 | >10 |
Percentage/field/section.
Cells/field/section.
Capillaries/field/section.
Statistical analysis
To evaluate significant differences between the means of different groups, the results of anti-inflammatory activity were analyzed by the non-parametric Mann–Whitney U test. Healing activity results were analyzed by one-way analysis of variance followed by Tukey’s test. All analyses were performed using the program Graph-Pad PRISM 4.0. and were considered as significant at p < 0.05. The results were expressed in mean ± standard error mean.
Results
The antioxidant potential of cashew apple juice
Phytochemical composition and total antioxidant activity of RCAJ and UNCAJ are summarized in Table 2. The total antioxidant capacity of RCAJ (85 ± 7 µM Trolox/g of juice) is twofold greater than UNCAJ (45 ± 4 µM Trolox/g of juice). The phytochemical screening for specific non-enzymatic antioxidants showed that RCAJ had significant higher levels of total phenolics (38.3 mg GAE/100 g of juice), anthocyanins (2.05 mg/100 g of juice), yellow flavonoids (3.92 mg/100 g of juice), total vitamin C (86.22 mg/100 g of juice), except for carotenoids (0.39 mg/100 g of juice) and for tannins, 0.42, 0.34, 0.37 mg/100 g of juice to dimers, oligomers, and polymers, respectively.
Table 2.
Phytochemical composition (mg/100 g of juice) of ripe cashew apple juice (RCAJ) and unripe cashew apple juice (UNCAJ)
| Bioactive compounds (mg/100 g of juice) | RCAJ | UNCAJ |
|---|---|---|
| Total vitamin C | 86.22 ± 0.21* | 60.45 ± 0.55 |
| Carotenoids | 0.39 ± 0.02 | 0.21 ± 0.01 |
| Total phenolics† | 38.30 ± 1.78* | 14.84 ± 0.21 |
| Anthocyanins | 2.05 ± 0.03* | 0.69 ± 0.07 |
| Yellow flavonoids | 3.92 ± 0.03* | 2.00 ± 0.17 |
| Tannin | ||
| Dimeric | 0.42 ± 0.07 | 0.27 ± 0.06 |
| Oligomeric | 0.34 ± 0.02 | 0.25 ± 0.01 |
| Polymeric | 0.37 ± 0.06 | 0.24 ± 0.02 |
Each value is the mean ± standard deviation.
mg GAE/100 g of juice.
GAE: galic acid equivalent.
p < 0.05 when compared to bioactive compounds between RCAJ and UNCAJ.
Anti-inflammatory effect of cashew apple juice on ear edema
The results of the anti-inflammatory activity of cashew apple juice in xylene-induced ear edema are presented in Table 3. The anti-inflammatory potential found for groups treated with RCAJ and UNCAJ was compared to the negative (animals treated with water) and positive controls (animals treated with DEXA). The group treated with UNCAJ presented a higher edema inhibition rate (66.5%) when compared to the group treated with RCAJ (10%) with p < 0.05. UNCAJ and DEXA treatments attenuated ear edema to a similar extent, i.e. 66.5 and 75%, respectively.
Table 3.
Anti-inflammatory activity of cashew apple juice in xylene-induced ear edema in mice
| Treatment groups | Edema (µm) | Inhibition (%) |
|---|---|---|
| Water | 56.70 ± 14.90 | – |
| DEXA | 14.40 ± 6.90* | 75.0 ± 4.46 |
| RCAJ | 51.00 ± 7.20 | 10.01 ± 3.81** |
| UNCAJ | 19.00 ± 7.15 * | 66.5 ± 3.69 |
DEXA: dexamethasone; RCAJ: ripe cashew apple juice; UNCAJ: unripe cashew apple juice.
Results are expressed as means ± SEM (n = 8).
p < 0.05 compared to water group, ** p < 0.05 compared to DEXA group.
Effect of cashew apple juice on wound healing
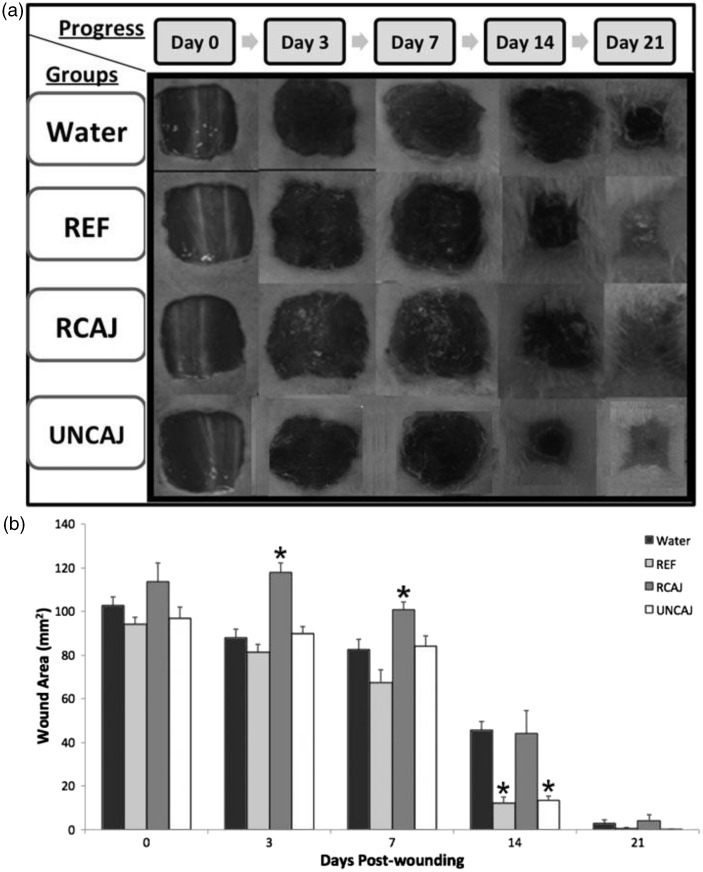
Figure 1 shows macroscopic analysis of the progress of wound on mice dorsal surface carried out 21 days after the wound excision (a) and the unhealed wound area (mm2) at 0, 3, 7, 14, and 21 days (b) as in Figure 1(a). In the wound-healing model (Figure 1(a)), all analyzed groups showed clean and exudate-free wound margins. Particularly, UNCAJ and REF groups showed apparently less swollen and hyperemic wounds excision, as well as a thinner granulation tissue compared to water and RCAJ groups (Figure 1(a)). The healing process of all the groups followed a normal evolution showing the unhealed wound area diminished throughout 21 days (Figure 1(b)). In day 0, all groups presented average value of the wound areas (100 mm2). Already, on the third day, all groups presented granulation tissue formation, which was easily noticeable at wound edges. The unhealed wound area did not show any significant difference among water, REF, and UNCAJ groups. However, RCAJ group showed a higher wound area when compared to the other groups (p < 0.05). The unhealed wound area profile, on the seventh day, was similar to third day, excepting that RCAJ which was comparable to water group. On the 14th day the UNCAJ was similar to the REF group and revealed smaller unhealed wound areas when compared to water and RCAJ groups (p < 0.05). This result could be expressed as wound contraction corresponding to 85 and 86% for the REF and UNCAJ groups, respectively. At the end of the experiment (21st day), the unhealed wound areas were negligible for all groups revealing 100% of wound contraction.
Figure 1.
Effect of cashew apple juice on wound healing. Macroscopic analysis of the progress of wound healing (a) and development of the unhealed wound area (mm2) over time (b). Groups: Water, Reference (REF), Ripe Cashew Apple Juice (RCAJ), and Unripe Cashew Apple Juice (UNCAJ) in 0, 3, 7, 14, and 21 days. * p < 0.05 compared to water group
Histological evaluation of wound healing process
Histological evaluation of wound healing process at 0, 3, 7, 14, and 21 days for all tested groups is shown in Table 4. On the third day, REF group presented only a slight edema formation and the moderate presence of PMN and MNC cells in the infiltrate as well as a moderate FP. When compared to REF, only the UNCAJ group presented a similar pattern when considering edema formation and FP. On the seventh day, the differences in edema formation and the re-epithelialization were even more pronounced between RCAJ and UNCAJ groups. However, on the 14th day, all groups presented re-epithelialization and 21 days after the wound excision, no apparent differences were found for the evaluated parameters (Table 4). On the 14th day, the histological analysis of the wound tissues of all tested groups (Figure 2) shows (arrows) the re-epithelized epidermis with keratin, new capillary vessels formation, and the reorganization of collagen fibers.
Table 4.
Histological evaluation of wound healing process in different groups of treatment per biopsy day
| Treatment groups per biopsy day | Wound healing process |
||||||
|---|---|---|---|---|---|---|---|
| Ed | PMN | MNC | FP | NV | RE | U | |
| Day 3 | |||||||
| Water | +++ | ++ | +++ | + | – | – | + |
| REF | + | ++ | ++ | ++ | – | – | + |
| RCAJ | +++ | ++ | +++ | + | – | – | + |
| UNCAJ | ++ | +++ | +++ | ++ | – | – | + |
| Day 7 | |||||||
| Water | ++ | + | ++ | +++ | + | – | – |
| REF | – | – | +++ | +++ | +++ | + | – |
| RCAJ | ++ | + | ++ | ++ | ++ | – | + |
| UNCAJ | – | – | +++ | +++ | ++ | + | – |
| Day 14 | |||||||
| Water | + | – | ++ | +++ | ++ | + | – |
| REF | – | – | + | +++ | +++ | + | – |
| RCAJ | ++ | + | ++ | +++ | ++ | + | – |
| UNCAJ | – | – | + | +++ | +++ | + | – |
| Day 21 | |||||||
| Water | – | – | + | +++ | ++ | + | – |
| REF | – | – | + | +++ | +++ | + | – |
| RCAJ | – | – | ++ | +++ | +++ | + | – |
| UNCAJ | – | – | + | +++ | +++ | + | – |
Hematoxylin and eosin stained sections were scored as mild (+), moderate (++), and severe (+++) for epidermal and/or dermal re-modeling. Re-epithelialization and ulceration were scored as present (+) and absent (–).
Ed: edema; FP: fibroblast proliferation; MNC: mononuclear cells; NV: neovascularization; PMN: polymorphonuclear cells; RCAJ: ripe cashew apple juice; RE: re-epithelialization; REF: reference; U: ulceration; UNCAJ: unripe cashew apple juice.
Figure 2.
Microscopic view of hematoxylin and eosin stained sections of wound tissues on day 14. (I) Water, (II) Reference (REF), (III) Ripe Cashew Apple Juice (RCAJ), and (IV) Unripe Cashew Apple Juice (UNCAJ). (a) Re-epithelized epidermis with keratin, (b) new capillary vessels, (c) mononuclear cells, (d) fibroblast proliferation, (e) reorganization fibers collagens. Original magnification: 100 ×(Scale bar: 200 µm)
Discussion
This study is the first demonstration of the potential of RCAJ and UNCAJ as anti-inflammatory treatments to promote wound healing. Their antioxidant profiles are characterized.
The protective effect of cashew apple against free radicals has been attributed to the presence of phytochemicals with antioxidant properties.27 Total phenolics and vitamin C are bioactive compounds that directly contribute to the total antioxidant activity of such fruit28 and therefore, the significant differences in the antioxidant composition observed between UNCAJ and RCAJ may be related to ripening metabolism.29 Similar data were observed by De Figueiredo et al.30 during the development and maturation of cashew apple.
The phytochemical evaluation of RCAJ showed greater levels (1.5-fold) of all constituents when compared to UNCAJ (Table 2), as the total antioxidant activity which was two times higher. Those results indicate that RCAJ has a higher free-radical scavenging activity supported by its greater total antioxidant capacity due to the antioxidants level.
Several compounds present in plants have demonstrated an anti-inflammatory potential as, for example, phenolics as tannins and flavonoids.31,32 Flavonoids also have the ability to act on the immune system and represent a promising therapeutic alternative to treatment of inflammatory processes.33 Nutritional deficiency is also related to immune system depletion, which may result in delays in wound healing due to a decrease in quality of tissue reparation and the synthesis of new tissues.34 Wound healing is a complex process involving vascular, cellular, and biochemical events that are obviously dependent on the nutritional substrates available35; for instance, the antioxidant vitamin C is directly related to collagen synthesis.36
In spite of UNCAJ lower antioxidant capacity, it presented an anti-inflammatory activity in xylene-induced ear edema in mice similar to DEXA group (Table 3), an anti-inflammatory drug used as reference. This result is unexpected as the anti-inflammatory potential is usually related to flavonoid levels; hence it was not the case for RCAJ, which presented greater antioxidants and negligible anti-inflammatory activity. Inflammation is a good protective response and is associated with the first line of defense. It has been proposed that the release of ROS at the site of injury could play a role in modulating the inflammatory response.37
In the last years, the role of antioxidants as protective substances is always correlated with optimal health conditions; nevertheless, the curative power of antioxidants is sometimes overestimated. It is well known that a satisfactory balance between endogenous and exogenous antioxidant capacity is essential to optimal responses.38 In this context, and as described earlier, the higher antioxidant capacity presented by RCAJ did not promote a better anti-inflammatory response. It suggests that pretreatment with RCAJ for 30 consecutive days increased the antioxidants level leading to a redox “imbalance” turning down the inflammatory response modulation exerted by ROS. Considering that an abundant supply of antioxidants could abolish the signalization of ROS in the inflammation site, the ROS scavenging activity of the unbalanced antioxidants supply could ultimately delay the healing process.39–41 Indeed, it is reported that large amounts of antioxidant supplement consumption may be harmful to health as some vitamins and phenolic phytochemicals with particular structures under certain conditions exhibit prooxidant activity, revealing a potential toxicity.42–44
The wound healing process that includes the inflammation, tissue formation, and tissue remodeling phases is the result of well-orchestrated cellular and biochemical responses.36 In the present study, macroscopic results showed that animals treated with UNCAJ presented a better wound healing process (Figure 1). Those results were later validated by the histological analysis (Table 4), suggesting that some other and not analyzed compound, present only in UNCAJ, would modulate the responses of cells involved in initial phases of the inflammatory process accelerating the repair and therefore, the healing stages (Figure 1). Despite the presence of such hypothesized compound in UNCAJ, the lower antioxidant capacity of UNCAJ alone could promote an optimal balance between ROS and antioxidants inducing a better wound healing process (Figure 1).
ROS may act as cellular messengers to stimulate key processes associated with wound healing, including cell motility, FP, and angiogenesis.45 An overall approach concerning histological evaluation of wound healing process in the present study showed an identical FP in UNCAJ and REF groups (Table 4). That suggests that the presence of PMN and MNC cells in early inflammatory phase in animals treated with UNCAJ led to a better fibroblast activation, thus accelerating the wound healing process. Furthermore, PMN and MNC may also exert an important role in the healing process via cytokines signalization leading to re-epithelialization, angiogenesis, and remodeling and contributing to scar formation.46 PMN cells migration to the lesion site leads to ROS production to fight invading microorganisms. Furthermore, to maintain a controlled ROS tissue concentration, an intricate cellular signalization is required to avoid oxidative stress and further damage.47,48 Furthermore, the nutritional supplementation with UNCAJ led to a better antioxidant balance favoring the healing process without interfering in the signalization process exerted by ROS.48 Even if all tested groups were re-epithelialized within 14 days after the surgery, it should be underlined that UNCAJ was more sensitive to eosin stain, which indicates a better reorganization of collagen fibers, similar to REF (Figure 2). Moreover, it must be emphasized that already after seven days, only UNCAJ and REF groups presented re-epithelialization, and no edema was verified. Studies demonstrated that the administration of antioxidants like vitamins C and E and anthocyanins to mice with skin wounds led to better healing, increased angiogenesis, and fibroblasts and keratinocytes induction48,49 It must also be highlighted the role of other phenolic compounds, such as tannins, which may have a wound healing activity through a complex regulation involving angiogenesis and FP.12,50,
Conclusions
In summary, the UNCAJ showed better anti-inflammatory and wound healing activities probably through a synergistic effect of its phytochemical components by improving the immunological defense mechanisms and poising the ROS scavenging/signalization.
Authors’ contributions
MSV, NFGR, MLMO, and ART carried out the lab work, generation, collection, assembly, interpretation of data, and drafted the manuscript. FYMS, FGMP, and CFHM were involved in the analysis of data and manuscript. DCSNP, MRAM, EFM, and DFM participated in the conception and design of the study, data analyses, and manuscript. All authors read and approved the final manuscript.
Acknowledgements
We thank the Brazilian agencies CAPES, CNPq, and FUNCAP for the financial support. The authors would also gratefully acknowledge Prof. Francisco Gutenberg Albuquerque Filho for the final English revision.
References
- 1.Sivagurunathan P, Sivasankari S, Muthukkaruppan SM. Characterisation of cashew apple (Anacardium occidenale L.) fruits collected from Ariyalur District. J Biosci Res 2010; 1: 101–7. [Google Scholar]
- 2.Santos RP, Santiago AAX, Gadelha CAA, Cajazeiras JB, Cavada BS, Martins JL, Oliveira TM, Bezerra GA, Santos RP, Freire VN. Production and characterization of the cashew (Anacardium occidentale L.) peduncle bagasse ashes. J Food Eng 2007; 79: 1432–7. [Google Scholar]
- 3.Akinwale TO. Cashew apple juice. Its use in fortifying the nutritional quality of some tropical fruits. Eur Food Res Technol 2000; 211: 205–7. [Google Scholar]
- 4.Kubo I, Masuoka N, Ha TJ, Tsujimoto K. Antioxidant activity of anacardic acids. Food Chem 2006; 99: 555–62. [Google Scholar]
- 5.Chagas CMA, Honorato TL, Pinto GAS, Maia GA, Rodrigues S. Dextransucrase production using cashew apple juice as substrate: effect of phosphate and yeast extract addition. Bioprocess Biosyst Eng 2007; 30: 207–15. [DOI] [PubMed] [Google Scholar]
- 6.Oliveira VH. Cashew crop. Rev Bras Frutic 2008; 30: 1–3. [Google Scholar]
- 7.Davis L, Stonehouse W, Loots DT, Mukuddem-Petersen J, van der Westhuizen FH, Hanekom SM, Jerling JC. The effects of high walnut and cashew nut diets on the antioxidant status of subjects with metabolic syndrome. Eur J Nutr 2007; 46: 155–64. [DOI] [PubMed] [Google Scholar]
- 8.Vanderlinde FA, Landim HF, Costa EA, Galdino PM, Maciel MAM, Anjos GC, Malvar DC, Côrtes WS, Rocha FF. Evaluation of the antinociceptive and anti-inflammatory effects of the acetone extract from Anacardium occidentale L. Brazilian. J Pharm Sci 2009; 45: 437–42. [Google Scholar]
- 9.Dharamveer D, Mishra B, Siddiqui HH. Pharmacognostical and phytochemical studies on Anacardium occidentale Linn. leaves. Res J Pharm Technol 2013; 6: 75–79. [Google Scholar]
- 10.Melo-Cavalcante AA, Picada JN, Rubensam G, Henriques JAP. Antimutagenic activity of cashew apple (Anacardium occidentale Sapindales, Anacardiaceae) fresh juice and processed juice (cajuína) against methyl methanesulfonate, 4-nitroquinoline N-oxide and benzo[a]pyrene. Genet Mol Biol 2008; 31: 759–66. [Google Scholar]
- 11.Melo-Cavalcante AA, Dantas SMM de M, Leite A de S, Matos LA, e Sousa JM, Picada JN, da Silva J. In vivo antigenotoxic and anticlastogenic effects of fresh and processed cashew (Anacardium occidentale) apple juices. J Med Food 2011; 14: 792–98. [DOI] [PubMed] [Google Scholar]
- 12.Rodríguez JAM, López JRM, Gutiérrez GC, Coronel M-AG, Mora ES, Rodríguez LAM, Rodríguez FM. Evaluation of the capacity of granulation in surgical wounds with condensed tannins in matrices Tio2. MRS Proc 2012; 1479: 63–68. [Google Scholar]
- 13.Boller S, Soldi C, Marques MCA, Santos EP, Cabrini DA, Pizzolatti MG, Zampronio AR, Otuki MF. Anti-inflammatory effect of crude extract and isolated compounds from Baccharis illinita DC in acute skin inflammation. J Ethnopharmacol 2010; 130: 262–6. [DOI] [PubMed] [Google Scholar]
- 14.Vieira LM, Sousa MSB, Mancini-Filho J, Lima A. Fenólicos totais e capacidade antioxidante in vitro de polpas de frutos tropicais. Rev Bras Frutic 2011; 33: 888–97. [Google Scholar]
- 15.Talasila U, Shaik KB. Quality, spoilage and preservation of cashew apple juice: a review. J Food Sci Technol. Epub ahead of print 2013. http://dx.doi.org/10.1007/s13197-013-0931-0.
- 16.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999; 26: 1231–7. [DOI] [PubMed] [Google Scholar]
- 17.Strohecker RL, Henning HM. Analisis de vitaminas: métodos comprobados, Madrid: Paz Montalvo, 1967, pp. 428–428. [Google Scholar]
- 18.Obanda M, Owuor PO, Taylor SJ. Flavanol composition and caffeine content of green leaf as quality potential indicators of Kenyan black teas. J Sci Food Agric 1997; 74: 209–15. [Google Scholar]
- 19.Francis FJ. Analysis of anthocyanins. In: Markakis P. (ed). Anthocyanins as food colors, New York: Academic Press, 1982, pp. 181–207. [Google Scholar]
- 20.Akindele AJ, Adeyemi OO. Antiinflammatory activity of the aqueous leaf extract of Byrsocarpus coccineus. Fitoterapia 2007; 78: 25–28. [DOI] [PubMed] [Google Scholar]
- 21.Boller S, Soldi C, Marques MCA, Santos EP, Cabrini DA, Pizzolatti MG, Zampronio AR, Otuki MF. Anti-inflammatory effect of crude extract and isolated compounds from Baccharis illinita DC in acute skin inflammation. J Ethnopharmacol 2010;130:262–6. [DOI] [PubMed]
- 22.Collard E, Roy S. Improved function of diabetic wound-site macrophages and accelerated wound closure in response to oral supplementation of a fermented papaya preparation. Antioxid Redox Signal 2010; 13: 599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Figueiredo IST, Ramos MV, Ricardo NMPS, Gonzaga MLC, Pinheiro RSP, De Alencar NMN. Efficacy of a membrane composed of polyvinyl alcohol as a vehicle for releasing of wound healing proteins belonging to latex of Calotropis procera. Process Biochem 2014; 49: 512–9. [Google Scholar]
- 24.Kokane DD, More RY, Kale MB, Nehete MN, Mehendale PC, Gadgoli CH. Evaluation of wound healing activity of root of Mimosa pudica. J Ethnopharmacol 2009; 124: 311–15. [DOI] [PubMed] [Google Scholar]
- 25.De Oliveira MLM, Nunes-Pinheiro DCS, Tomé AR, Mota EF, Lima-Verde IA, Pinheiro FG, Campello CC, de Morais SM. In vivo topical anti-inflammatory and wound healing activities of the fixed oil of Caryocar coriaceum Wittm. seeds. J Ethnopharmacol 2010; 129: 214–9. [DOI] [PubMed] [Google Scholar]
- 26.Akkol EK, Koca U, Pesin I, Yılmazer D, Toker G, Yesilada E. Exploring the wound healing activity of Arnebiadensiflora (Nordm.) Ledeb. by in vivo models. J Ethnopharmacol 2009; 124: 137–41. [DOI] [PubMed] [Google Scholar]
- 27.Vissotto LC, Rodrigues E, Chisté RC, Benassi M de T, Mercadante AZ. Correlation, by multivariate statistical analysis, between the scavenging capacity against reactive oxygen species and the bioactive compounds from frozen fruit pulps. Ciência e Tecnol Aliment 2013; 33: 57–65. [Google Scholar]
- 28.Abreu CRA, Maia GA, Figueredo RW de, Sousa PHM, Alves RE, Moura CFH, Rufino MSM. Bioactive compounds and antioxidant activity of cashew apple (Anacardium occidentale L.) from commercial early dwarf clones. Acta Hortic 2009; 841: 451–4. [Google Scholar]
- 29.Gordon A, Friedrich M, da Matta VM, Herbster Moura CF, Marx F. Changes in phenolic composition, ascorbic acid and antioxidant capacity in cashew apple (Anacardium occidentale L.) during ripening. Fruits 2012; 67: 267–76. [Google Scholar]
- 30.Figueiredo RW de, Lajolo FM, Elesbão Alves R, Filgueiras HAC. Physical–chemical changes in early dwarf cashew pseudofruits during development and maturation. Food Chem 2002; 77: 343–7. [Google Scholar]
- 31.Serafini M, Peluso I, Raguzzini A. Flavonoids as anti-inflammatory agents. Proc Nutr Soc 2010; 69: 273–8. [DOI] [PubMed] [Google Scholar]
- 32.Veitch NC, Grayer RJ. Flavonoids and their glycosides, including anthocyanins. Nat Prod Rep 2011; 28: 1626–95. [DOI] [PubMed] [Google Scholar]
- 33.Coutinho MAS, Muzitano MF, Costa SS. Flavonoids: potential therapeutic agents for the inflammatory process. Rev Virtual Quím 2009; 1: 241–56. [Google Scholar]
- 34.Tazima MFGS, Vicente YAMVA, Moriya T. Wound biology and healing. Med Ribeirão Preto 2008; 41: 259–64. [Google Scholar]
- 35.Wild T, Rahbarnia A, Kellner M, Sobotka L, Eberlein T. Basics in nutrition and wound healing. Nutrition 2010; 26: 862–6. [DOI] [PubMed] [Google Scholar]
- 36.Tsala DE, Amadou D, Habtemariam S. Natural wound healing and bioactive natural products. Phytopharmacology 2013; 4: 532–60. [Google Scholar]
- 37.Khodr B, Khalil Z. Modulation of inflammation by reactive oxygen species: implications for aging and tissue repair. Free Radic Biol Med 2001; 30: 1–8. [DOI] [PubMed] [Google Scholar]
- 38.Brambilla D, Mancuso C, Scuderi MR, Bosco P, Cantarella G, Lempereur L, Di Benedetto G, Pezzino S, Bernardini R. The role of antioxidant supplement in immune system, neoplastic, and neurodegenerative disorders: a point of view for an assessment of the risk/benefit profile. Nutr J 2008; 7: 29–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gutteridge JMC, Halliwell B. Antioxidants: molecules, medicines, and myths. Biochem Biophys Res Commun 2010; 393: 561–4. [DOI] [PubMed] [Google Scholar]
- 40.Hultqvist M, Olsson LM, Gelderman KA, Holmdahl R. The protective role of ROS in autoimmune disease. Trends Immunol 2009; 30: 201–8. [DOI] [PubMed] [Google Scholar]
- 41.Tang SY, Cheah IK, Wang H, Halliwell B. Notopterygium forbesii Boiss extract and its active constituent phenethyl ferulate attenuate pro-inflammatory responses to lipopolysaccharide in RAW 264.7 macrophages. A “protective” role for oxidative stress? Chem Res Toxicol 2009; 22: 1473–82. [DOI] [PubMed] [Google Scholar]
- 42.Bast A, Haenen GRMM. Ten misconceptions about antioxidants. Trends Pharmacol Sci 2013; 34: 430–6. [DOI] [PubMed] [Google Scholar]
- 43.Lee SH, Oe T, Blair IA. Vitamin C-induced decomposition of lipid hydroperoxides to endogenous genotoxins. Science 2001; 292: 2083–6. [DOI] [PubMed] [Google Scholar]
- 44.Lee KW, Lee HJ. Biphasic effects of dietary antioxidants on oxidative stress-mediated carcinogenesis. Mech Ageing Dev 2006; 127: 424–31. [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez PG, Felix FN, Woodley DT, Shim EK. The role of oxygen in wound healing: a review of the literature. Dermatol Surg 2008; 34: 1159–69. [DOI] [PubMed] [Google Scholar]
- 46.Wilgus TA. Immune cells in the healing skin wound: influential players at each stage of repair. Pharmacol Res 2008; 58: 112–16. [DOI] [PubMed] [Google Scholar]
- 47.Archile-Contreras AC, Purslow PP. Oxidative stress may affect meat quality by interfering with collagen turnover by muscle fibroblasts. Food Res Int 2011; 44: 582–8. [Google Scholar]
- 48.Fitzmaurice SD, Sivamani RK, Isseroff RR. Antioxidant therapies for wound healing: a clinical guide to currently commercially available products. Skin Pharmacol Physiol 2011; 24: 113–26. [DOI] [PubMed] [Google Scholar]
- 49.Nizamutdinova IT, Kim YM, Chung J, Il, Shin SC, Jeong YK, Seo HG, Lee JH, Chang KC, Kim HJ. Anthocyanins from black soybean seed coats stimulate wound healing in fibroblasts and keratinocytes and prevent inflammation in endothelial cells. Food Chem Toxicol 2009; 47: 2806–12. [DOI] [PubMed] [Google Scholar]
- 50.Devi P, Meera R. Study of antioxdant, anti-inflammatory and wound healing activity of extracts of Litsea glutinosa. J Pharm Sci Res 2010; 2: 155–63. [Google Scholar]