Abstract
Curcumin, traditionally used as food and medicinal purposes, has recently been reported to have protective efficacy against hypoxia. Hypoxia is one of the important reactive factors in tumor metastasis, which is a key problem in clinical thyroid cancer therapy. In present study, we investigate the anti-metastatic effect of curcumin on the K1 papillary thyroid cancer cells as well as its potential mechanisms. The results show that curcumin effectively inhibits hypoxia-induced reactive oxygen species (ROS) upregulation and significantly decreases the mRNA and protein expression levels of hypoxia-inducible factor-1α (HIF-1α) in K1 cells. Curcumin also decreases the DNA binding ability of HIF-1α to hypoxia response element (HRE). Furthermore, curcumin enhances E-cadherin expression, inhibits metalloproteinase-9 (MMP-9) enzyme activity, and weakens K1 cells migration under hypoxic conditions. In summary, these results indicate that curcumin possesses a potent anti-metastatic effect and might be an effective tumoristatic agent for the treatment of aggressive papillary thyroid cancers.
Keywords: Curcumin, K1 papillary thyroid cancer cell, hypoxia-inducible factor-1α, metastasis
Introduction
Recently, extensive research has been carried out on the health-promoting properties of different phytochemicals.1 Traditional herbal polyphenols have attracted the interest of many researchers due to their anti-oxidant, anti-inflammatory, anti-bacterial, anti-mutagenicity, and anti-cancer properties.2,3 Curcumin (bis(4-hydroxy-3-methoxy-phenyl)-1,6-heptadiene-3,5-dione), derived from the plant turmeric, is one of the best characterized polyphenols in several South Asian countries. Curcumin also has a long history of being used to treat inflammation and cancer in India and China. However, its anticancer actions are complicated and the mechanisms responsible for its anticancer effects remain incompletely understood.
Thyroid cancer is the most common endocrine tumor.4 Papillary thyroid cancer (PTC) is the most common thyroid malignancy. Current clinical treatments for thyroid cancer include surgery, radioactive iodine (131I) therapy, chemotherapy, or a combination of all of these treatments. However, more than 6% of thyroid cancers are invasive and metastatic.5 The metastasis of thyroid cancer is serious and regarded as the first sign of a potentially lethal outcome.6 Thus, preventing metastasis is essential in thyroid cancer therapy. A few studies have demonstrated curcumin plays an important role in inhibiting invasion.7 In human laryngeal cancer Hep2 cells, curcumin has been shown to inhibit tumor cell invasion and metastasis by down-regulating metalloproteinase-2 (MMP-2) expression and reducing the activity and expression of integrins and focal adhesion kinase (FAK).8 Given the results of our previous studies, curcumin is a plausible candidate for preventing metastasis. In this study, we investigate whether curcumin can inhibit tumor metastasis in PTC K1 cells.
Hypoxia is a common condition in solid tumors. One major regulator used by cancer cells to adapt to hypoxia is the transcription factor hypoxia-inducible factor-1 (HIF-1). HIF-1 is composed of an oxygen-regulated HIF-1α and a constitutively expressed HIF-1β subunit. HIF-1β is constitutively expressed and not responsive to oxygen concentrations while HIF-1α is the most ubiquitously expressed, and functions as the master oxygen homeostasis regulator in many cell types.9 HIF-1α is hydroxylated and rapidly degraded under normoxic conditions. In contrast, under hypoxic conditions, HIF-1α rapidly accumulates and transactivates many genes, including angiogenic and extracellular proteases such as the MMPs.10 Among the MMPs, MMP-9 has been positively correlated with a higher incidence of metastases in thyroid cancer.11 In addition, HIF-1α also regulates E-cadherin expression. A previous study by Imai et al. demonstrated that hypoxia decreased the expression of E-cadherin in ovarian cancer cells.12 Further study13 has confirmed that E-cadherin is lost from the human breast cancer cell (MDA-MB-468) surface after hypoxia. It is well known that MMP-9 and E-cadherin are the hallmarks of tumor metastasis and invasion. The extent of hypoxia in a tumor may, therefore, represent an independent indicator of a poor prognosis for the tumor.
In present study, we demonstrate that curcumin inhibits hypoxia-induced migration in K1 PTC cells via regulation the expression and DNA binding activity of HIF-1.
Materials and methods
Chemicals
Curcumin, dichloro-dihydro-fluorescein diacetate (DCFH-DA), dimethyl sulfoxide (DMSO), gelatine, phenylmethylsulfonylphenylmethylsulfonyl fluoride (PMSF), Nonidet P-40 (NP-40), and propidium iodide (PI) were purchased from Sigma (St. Louis, MO). Anti-HIF-1α was purchased from Novus and the antibodies of E-cadherin, BNIP3, and β-actin were purchased from Santa Cruz. Other drugs and reagents used in this study were as follows: M-MLV reverse transcriptase, RNase inhibitor, dNTP, and Taq polymerase (Promega, USA). All of the other chemicals were of the highest analytical grade and purchased from common sources. The curcumin was dissolved in DMSO at 10 mg/mL and stored at −20°C until diluted before use.
Cell culture and drug treatment
K1 PTC cells were obtained from the European Collection of Cell Cultures and maintained in a complete medium (DMEM:F-12:MCDB105 = 2:1:1) containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 U/mL streptomycin in a humid atmosphere of 5% (v/v) CO2 and 95% (v/v) air at 37°C. The K1 cells in the log phase were plated in cell culture dishes (Corning, NY) at a density of 3 × 105 cells per well for the 35 mm dish and 6 × 105 cells per well for the 60 mm dish and then left for overnight culture. The cells were pretreated with different concentrations (12.5, 25, and 50 µmol/L) of curcumin for 1 h. Then, after the medium containing curcumin was removed, the cells were exposed to hypoxia (2% O2, 93% N2, and 5% CO2, v/v) for an additional 12 h. The control cells were treated with the same medium without curcumin and exposed to normal oxygen levels. The solvent control (SC) contained an amount of DMSO equivalent to the highest concentration used in the curcumin-treated group.
Hypoxic conditions (2% O2, 93% N2, and 5% CO2 v/v) were achieved in a humidified variable aerobic workstation (Theom-3131#, USA). Before the experiment, the media were pre-equilibrated overnight at this oxygen level.
Cell viability assay
The effects of hypoxia and curcumin on cell viability were determined by the MTT assay. In brief, for hypoxia experiment, cells (10,000 cells per well) underwent 6, 12, 24, 48, and 72 h hypoxia in quadruplicate in a 96-well plate. And for curcumin experiment, cells were pretreated with curcumin in quadruplicate in a 96-well plate for 1 h at normal oxygen. After washed once with phosphate-buffered saline (PBS) and replaced with serum-containing medium without curcumin, the cells were exposed to hypoxia for an additional 24 h. Then, for both hypoxia and curcumin pretreated groups, 40 µL of MTT solution (5 mg/mL) was added to each well and incubated for another 4 h at 37°C. The supernatants were aspirated carefully and 150 µL of DMSO was added, and then the plate was holding on vibrator for 20 s. The optical density of the cell suspension was measured at 570 nm using a microplate reader (Bio-Tek instruments Inc., Winooski, VT). Cell viability was expressed as a percentage of MTT reduction, assuming that the absorbance of untreated cells was 100%.
Western blot analysis
Cellular total protein prepared from the cells was used to analyze the HIF-1α and E-cadherin protein. In brief, K1 cells, pretreated with curcumin under hypoxic conditions, were collected and washed with PBS. After centrifugation, the cells were collected and lysed in 20 µL lysis buffer (150 mmol/L NaCl, 1% [w/v] NP-40, 0.02% [w/v] sodium azide, 10 µg/mL PMSF, 50 mmol/L Tris–HCl [pH 8.0]) supplemented with additional protease inhibitor PMSF. The lysate was frozen and thawed three times, then centrifuged at 12,000×g for 5 min at 4°C. The supernatant was collected and the protein concentration was determined using the Bradford assay. After the addition of a sample loading buffer, the protein samples were electrophoresed on a 10% SDS-PAGE and subsequently transferred onto a polyvinylidene fluoride membrane (Millipore, USA). Next, the membrane was incubated in a fresh blocking buffer (0.1% [v/v] Tween 20 in Tris-buffered saline, pH 7.4, containing 5% [w/v] skim milk) at room temperature for 1 h and then probed with the following antibodies: anti-β-actin (1:1000, v/v), anti-HIF-1α (1:1000, v/v), anti-BNIP3 (1:500), and anti-E-cadherin (1:1000, v/v) in a blocking buffer at 4°C overnight. After being washed three times (5 min per a wash) with Tris-buffered saline with 0.1% (v/v) Tween 20 (TBST), the membrane was incubated again at room temperature for 1 h with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibody (Goat anti-mouse IgG, 1:500 v/v, or Goat anti-rabbit IgG, 1:500 v/v, Santa Cruz Biotechnology, CA) and then washed three times with a TBST buffer. The membrane was then incubated with enhanced chemiluminescence substrate solution (Santa Cruz Biotechnology) for 5 min, according to the manufacturer’s instructions and then visualized with X-ray film.
Reverse transcriptase-polymerase chain reaction (RT-PCR)
After cell collection, the total RNA was extracted from the cells using the Tripure reagent (Roche, USA), as described by the manufacturer. First-strand cDNAs were generated by reverse transcription using oligo (dT) from RNA samples. The primer sequences (Sangon, Shanghai, China) were as follows: HIF-1α, forward 5'-CTTCTGGATGCTGGTGATT-3', reverse, 5'-TCCTCGGCTAGTTAGGGTA-3'; BNIP3, forward 5'-CCACCTCGCTCGCAGACACCAC-3', reverse, 5'-GAGAGCAGCAGAGATGGAAGGAAAAC-3' and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), forward, 5'-AATGACCCCTTCATTGAC-3', reverse, 5'-TCCACGACGTACTCAGCGC-3'. The PCR was performed using an initial step of denaturation at 94°C for 5 min, with 35 cycles of amplification at 94°C for 30 s, annealing at 60°C for 30 s, elongation at 72°C for 30 s, and extension at 72°C for 5 min, annealing at 58°C for 30 s for BNIP3 exceptionally. The PCR products were electrophoresed in 2% agarose gel and visualized by ethidium bromide (EB) dying. The relative expression was quantified densitometrically using the GIS-2019 system (Tanon, Shanghai, China), and calculated according to the reference bands of GAPDH.
Electrophoretic mobility shift assay (EMSA)
The cells were pretreated with three different concentrations (0, 25, and 50 µmol/L) of curcumin for 1 h. Then, the cells were washed with PBS three times and exposed to hypoxia for 12 h. Nuclear extracts were prepared and used to detect further HIF-1α translocation according to the manufacturer’s protocol for the Thermo Scientific LightShift Chemiluminescent EMSA Kit (Cat.No.20148). In brief, the probe containing the HIF-1α oligonucleotide consensus sequence was labeled with biotin. The sequence of the oligonucleotide was forward, 5'-BIOTIN-TCTGTACGTGACCACACTCACCTC-3', reverse, 3'-AGACATGCACTGGTGTGAGTGGAG-BIOTIN-5'. The unlabeled probe sequence was forward, 5'-TCTGTACGTGACCACACTCACCTC-3', reverse, 5'-GAGGTGAGTGTGGTCACGTACAGA-3', and the mutant probe sequence was forward, 5'-TCTGTAAAAGACCACACTCACCTC-3', reverse, 5'-GAGGTGAGTGTGGTCTTTTACAGA-3'. The DNA–protein complex was separated on a non-denaturating 6% polyacrylamide gel. After electrophoresis, the DNAs were transferred onto a nylon membrane at 100 V for 30 min. Then the transferred DNAs were cross-linked to the nylon membrane by two 45 s ultraviolet ray exposures at 120 mJ/cm2. Subsequently, the blocked nylon membrane, which was soaked in blocking buffer and shaken at 25°C for 15 min, was probed with streptavidin–HRP conjugate (1:300 dilution). After the probing, the membrane was soaked in substrate working solution (2 mL Luminol/enhancer solution and 2 mL stable peroxide solution) for 5 min, and exposed with X-ray film. The band in the film was analyzed using the GIS-2019 system. The sequence specificity of the nuclear protein binding to all of the oligonucleotides was confirmed by competition studies in which the nuclear extracts were incubated for 10 min at room temperature with a 100-fold molar excess unlabeled probe and a mutant probe before the addition of labeled oligonucleotides.
Zymography
The K1 cells were incubated in serum-free media under normoxic or hypoxic conditions for 0, 6, 12, and 24 h. The curcumin-treated cells were also incubated in serum-free media for 12 h under hypoxic conditions. The cells’ culture medium samples were then collected and concentrated using Amicon Ultra centrifugal filter devices (Millipore). The samples containing an equal amount of total proteins were loaded on denaturing 10% polyacrylamide-SDS gels containing 0.1% gelatin. After electrophoresis, the gels were washed twice in 50 mmol/L Tris-HCl buffer (pH 7.5) for 30 min, which contained 2.5% Triton X-100. Then, the gels were incubated in an activation buffer (50 mmol/L Tris-HCl [pH 7.5], 200 nmol/L NaCl, 1 µmol/L ZnCl2, and 10 mmol/L CaCl2) for 24 h at 37°C until enzymatic degradation of the substrate had taken place. The gels were stained with 0.1% Coomassie blue R-250 (Sigma, St. Louis, MO) and then destained. Gelatinolytic bands were observed as clear zones against the blue background, and the intensity of the bands was quantified using the GIS-2019 system, to provide a semi-quantitative assay of the enzymatic activity.
In vitro wounding-healing experiment
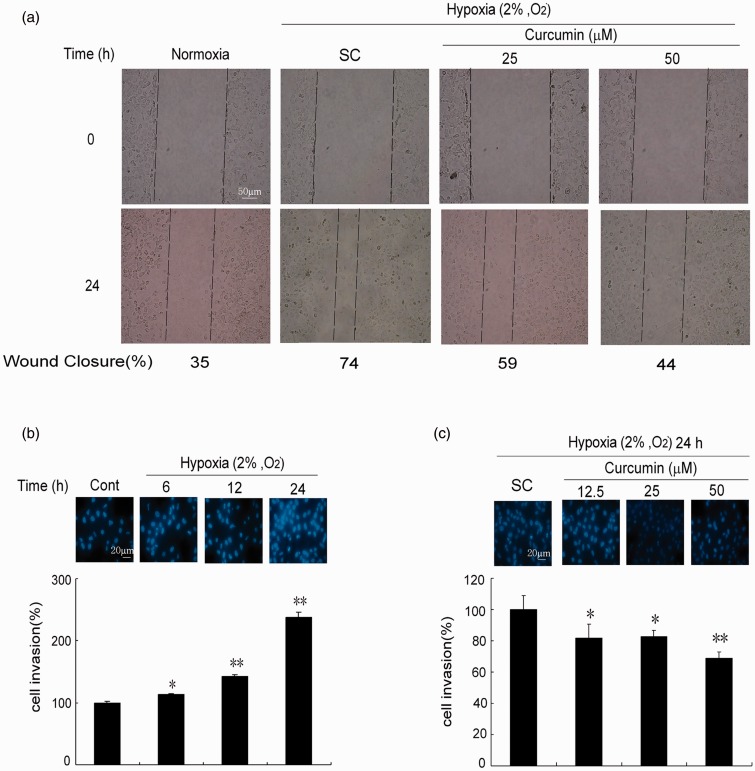
In vitro wounding-healing was performed using a modified version of the scratch assay. The K1 cells were grown to confluence in 60 mm dishes; then, after being washed with serum-free medium, the monolayers were scratched with a plastic pipette tip to create cell-free areas (“wounds”). The cultures were washed twice in a serum-free medium to remove cell debris and the marked area of the wound was photographed under phase-contrast microscopy. The cells were covered with serum-free medium. For the curcumin treatment, the cells were incubated in curcumin (25 and 50 µmol/L) for 1 h before the “wound,” and were then washed and photographed. Subsequently, the dishes underwent normoxia or hypoxia for 24 h, after which the marked areas of the wound were re-photographed. The recovery of the wound areas were determined by multiplying the length by the average width of the recovered areas. The wound closure was the recovered area divided by the initial area devoid of cells. The experiment was independently repeated in triplicate.
Invasion assay
The cancer cell invasive ability with or without curcumin treatment was examined by a transwell membrane culture system (8 µm pore size, 6.5 mm diameter, Corning Costar Corporation) precoated with Matrigel (2.5 mg/mL). Briefly, the K1 cells were harvested in complete medium at density of 5 × 105 cells/mL. To the lower wells of the chambers, 600 µL of conditional medium were added. Hundred microliters of cells were seeded onto the upper pre-coated wells, then the chamber was incubated at hypoxia for 0, 6, 12, and 24 h or pretreatment with curcumin (12.5, 25, and 50 µmol/L) for 1 h and hypoxia for 24 h. After hypoxic treatment, the cells in the upper well were removed with a cotton swab, and the remaining cells beneath the membrane were stained with Hoechst 33342 (10 µg/mL) and inspected and counted under a fluorescence microscope. The experiments were performed in triplicate.
Measurement of ROS
The reactive oxygen species (ROS) generation in cells was assessed using the fluorescent probe DCFH-DA. DCFH-DA is a cell-permeable indicator for ROS that is nonfluorescent until the acetate groups are removed by intracellular esterases and oxidation occurs within the cell. After varying amounts of time (0, 1, 2, 4, and 24 h) of exposure to hypoxia, the cells were washed with PBS three times and loaded with DCFDA-DH (10 µmol/L) for 30 min in MEM without phenol red. The cells were then collected by centrifugation, washed twice with PBS, and analyzed by flow cytometry through the FL1 channel.
Statistical analysis
The results were expressed as mean ± SEM. Two group comparisons were evaluated using the Student’s t-test and the differences were considered statistically significant when P < 0.05.
Results
The effects of hypoxia and curcumin pretreatment on the cell viability of K1 cells
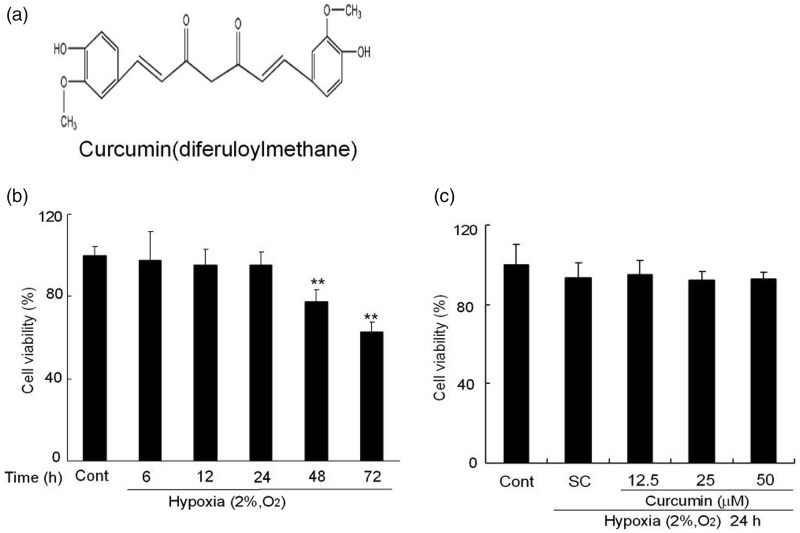
K1 cells were exposed to hypoxia condition for different time periods and cell survival was assessed by MTT assay. As shown in Figure 1b, cells viability slightly decreased after hypoxia, but there were no significant decrease within 24 h hypoxia compared to control cells. However, longer period hypoxia upon 48 h markedly decreased cells survival. There were only 77.6 ± 5.5% at 48 h and 62.7 ± 4.8% at 72 h viable cells as compared to control cells, respectively. Therefore, the treatment of hypoxia for 24 h was used in the subsequent experiments. As shown in Figure 1c, pretreatment of curcumin (12.5, 25, and 50 µmol/L) for 1 h had no obvious effects on the cells viability compared to SC cells under hypoxia of 24 h. These results demonstrated exposure of K1 cells to hypoxia condition for 24 h in the presence or absence of curcumin pretreatment had no significant effects on its cell viability.
Figure 1.
The effects of hypoxia and curcumin pretreatment on the cell viability of K1 cells. (a) Chemical structure of curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione). (b) Effects of hypoxia on K1 cell viability. K1 cells were treated with hypoxia (2% O2, v/v) for 6, 12, 24, 48, and 72 h. Then, the cells and Cont were determined by MTT assay. (c) Effects of curcumin on K1 cell viability. K1 cells were exposed to various concentrations of curcumin (12.5, 25, and 50 µmol/L) for 1 h. Subsequently, the cells underwent hypoxia (2% O2, v/v) for 24 h. Then, these cells and Cont were determined by MTT assay. All of the data were expressed as the mean ± SEM of the three experiments and each included triplicate sets. Cont: control, SC: solvent control. *P < 0.05 and **P < 0.01 versus Cont (Student’s two-tailed t-test)
Curcumin down-regulates the mRNA and protein expression levels of HIF-1α
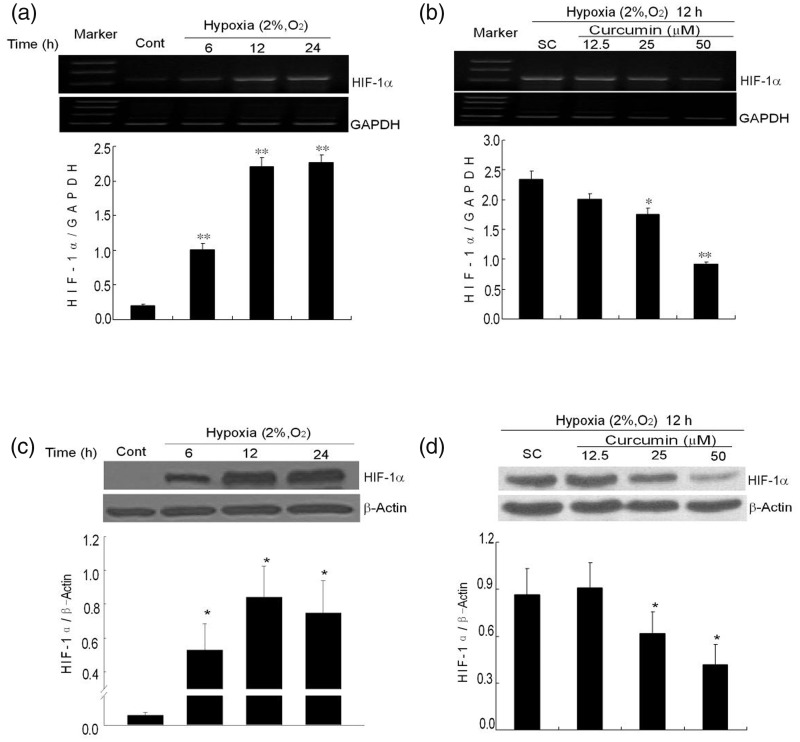
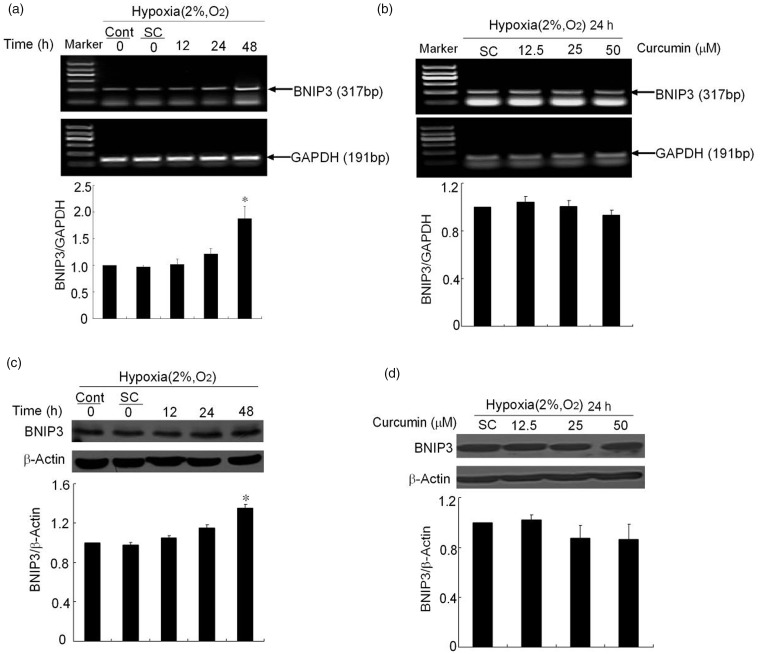
It is well known that hypoxia induces the expression of HIF-1α. However, HIF-1α expression in K1 papillary thyroid carcinoma cells under hypoxia is unclear. In this study, HIF-1α mRNA noticeably increased after a period of hypoxia. Compared to normoxic condition, the mRNA level increased by 5.1-fold after hypoxia incubation for 6 h. At 12 h and 24 h, it increased by 11.17- and 11.45-folds, respectively (Figure 2a). Pretreatment with curcumin for 1 h significantly decreased the expression of HIF-1α mRNA to 74.9% and 39.3% at 25 and 50 µmol/L, respectively (Figure 2b). It indicates that curcumin could inhibit the mRNA expression of HIF-1α in a dose-dependent manner. Consistent with mRNA expression, the protein expression of HIF-1α was up-regulated in a similar manner. After K1 cells were exposed to hypoxia for different time periods, the expression of HIF-1α protein dramatically increased after hypoxic incubation compared to normoxia, and reached maximum at 12 h, and then decreased at 24 h (Figure 2c). Pretreatment with curcumin (25 and 50 µmol/L) for 1 h significantly down-regulated the expression of HIF-1α in the K1 cells (Figure 2d).
Figure 2.
Effects of curcumin on the mRNA and protein expression levels of HIF-1α. (a) Expression of HIF-1α mRNA induced by hypoxia (2% O2, v/v) for different time periods (6, 12 and 24 h). GAPDH is used as internal control. The graph shows the densitometry analysis of HIF-1α/GAPDH. (b) The effects of curcumin on HIF-1α mRNA expression. After pretreatment with curcumin (12.5, 25, and 50 µmol/L), the cells were exposed to hypoxia for 12 h. Subsequently, the HIF-1α mRNA was analyzed with RT-PCR. GAPDH was detected for internal control. (c) Expression of HIF-1α protein induced by hypoxia (2% O2, v/v) for different time periods (6, 12, and 24 h). The bottom graph shows the densitometry analysis of HIF-1α/β-actin. (d) Curcumin-decreased HIF-1α protein expression. The cells were treated with or without curcumin (12.5, 25, and 50 µmol/L) and were exposed to hypoxia for 12 h. They were then analyzed with a Western blot of total cellular protein. Equal amounts of total cellular protein (60 µg) were loaded and β-actin was used as an internal control. The densitometry analysis is also shown below. All of the data represent the means ± SEM of three independent experiments. Cont: control, SC: solvent control. *P < 0.05, **P < 0.01 versus SC (Student’s two-tailed t-test)
Curcumin decreases the DNA-binding ability to the HRE of HIF-1α
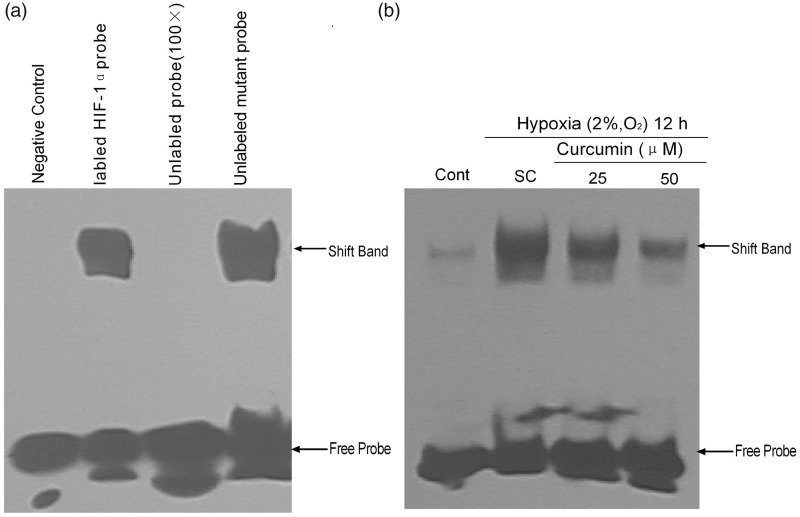
EMSA was performed to confirm the HIF-1α binding to the putative hypoxia response element (HRE) consensus. Nuclear proteins extracted from cells pretreated with curcumin (25 and 50 µmol/L) for 1 h and incubated under hypoxia for 12 h were harvested for further EMSA assay. The shift-band indicated that the HIF-1α was bound with the HRE element. The DNA-binding activity of HIF-1α in these samples was significantly higher than in the normoxic cells (Figure 3a, lane 2). To identify the specificity of the labeled probe, an unlabeled probe or a mutated probe were used to compete with the labeled probe. The shift-band was competitively blocked by a 100-fold excess of unlabeled specific probes, but not by 100-fold of mutant HIF-1α probe. These results confirmed that HIF-1α binds specifically to the HRE probe (Figure 3a, lanes 3 and 4). Meanwhile, curcumin pretreatment (25 and 50 µmol/L) noticeably decreased the DNA-binding activity of HIF-1α (Figure 3b).
Figure 3.
Effects of curcumin on HIF-1α protein binding with HRE. (a) The EMSA control system was used to identify the specificity of HIF-1α binding to a biotin-probe. The K1 cell was treated with or without hypoxia for 12 h, and then the nuclear extracts (10 µg) were analyzed by EMSA. The negative control indicates the sample without hypoxia. The labeled HIF-1α probe, unlabeled probe (100×), and mutant probe were samples that had undergone hypoxia for 12 h. (b) Effects of curcumin pretreatment on HIF-1 DNA binding activity in K1 cells. After curcumin (25 and 50 µmol/L) pretreatment for 1 h followed by hypoxia incubation for 12 h, the nuclear extracts (10 µg) were analyzed by EMSA. Cont: control, SC: solvent control
Curcumin suppresses hypoxia-induced secretion of MMP-9
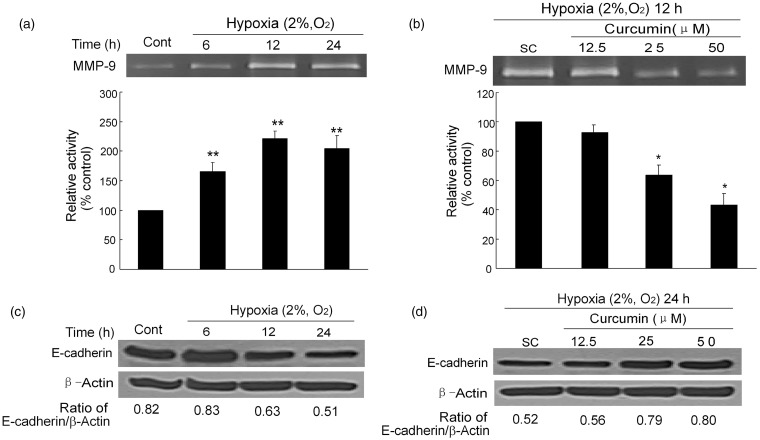
HIF regulates the expression of over 100 genes that modulate key aspects of tumorigenesis, including angiogenesis, metabolism, proliferation, invasion, and metastasis.14 MMP-9, which plays a key role in tumor metastasis, is one of the most important downstream genes regulated by HIF. To investigate the effects of curcumin on tumor invasion, MMP-9 gelatinase activity was analyzed using zymography. Densitometry analysis indicated that MMP-9 activities under hypoxic conditions were significantly increased compared to its activity under normoxic conditions. The MMP-9 activities increased to about 2-fold under hypoxia within 12 or 24 h (Figure 4a). However, pretreatment with curcumin at 25 and 50 µmol/L significantly suppressed the secretion of MMP-9. The relative activities of the drug treatment groups were 0.64 - and 0.43-fold, respectively, compared to that of the SC (P < 0.01). These results demonstrate that curcumin suppresses MMP-9 secretions in a dose-dependent manner (Figure 4b).
Figure 4.
Curcumin inhibits MMP-9 activity and restores E-cadherin expression. (a) Hypoxia increases the MMP-9 activity. MMP-9 was measured by gelatin zymography after exposure to hypoxia for different times (6, 12, and 24 h). The densitometry analysis of MMP-9 bands is listed below. (b) Inhibition effects of curcumin on MMP-9 activity. After pretreatment with curcumin (12.5, 25, and 50 µmol/L), the cells underwent hypoxia for 12 h, and then the activity of MMP-9 was analyzed, the bottom graph is also the densitometry analysis. (c) Hypoxia-diminished E-cadherin protein expression after hypoxia (2% O2, v/v) for different times (6, 12, and 24 h). (d) Curcumin-restored E-cadherin protein expression. Cells were treated with or without curcumin (12.5, 25, and 50 µmol/L) and then were exposed to hypoxia for 12 h. E-cadherin protein levels were analyzed by Western blot. SC: solvent control. All of the data represent the means ± SEM of three independent experiments. Cont: control, SC: solvent control. *P < 0.05, **P < 0.01 versus SC (Student′s two-tailed t-test)
Curcumin up-regulates E-cadherin expression
In addition to MMP-9, E-cadherin also plays a critical role in tumor metastasis.15 As shown in Figure 4c, when K1 cells were exposed to hypoxia for 12 h, the decline tendency of E-cadherin expression continued for 24 h. Pretreatment with curcumin (25 and 50 µmol/L) effectively elevated the expression of E-cadherin, which was down-regulated by hypoxia (Figure 4d).
Curcumin blocks the migration of K1 cells
The results of the zymography (Figure 4) and the E-cadherin (Figure 4) test suggest that curcumin may possess the potential to inhibit cell migration under hypoxic conditions. As shown in Figure 5a, hypoxia promoted the migration of K1 cells and significantly accelerated wound closure. However, curcumin dose-dependently slowed down the wound closure at 25 and 50 µmol/L.
Figure 5.
Curcumin decreases K1 cells migration and invasion. (a) Curcumin inhibits scratch wound healing. The cells were scratch-wounded with a sterile 200 µL pipette tip and then incubated under hypoxia (2% O2, v/v) or normoxia (21% O2, v/v). Representative wounds were photographed after scratch wounding (0 h) and after 24 h of healing. (b) Hypoxia enhances cells invasion. After hypoxia (2% O2, v/v) over various times (6, 12, and 24 h), the cancer cell invasive ability was examined by membrane transwell culture system precoated with Matrigel (2.5 mg/mL). Morphology of cells was determined by staining with Hoechst 33342 (10 µg/mL) and inspected with a fluorescence microscope. (c) Curcumin decreases cells invasion induced by hypoxia. After pretreatment with curcumin (12.5, 25, and 50 µmol/L), the cells were exposed to hypoxia for 24 h, subsequently, the cells invasion was examined. The wound closure was calculated in three independent experiments. Cont: control, SC: solvent control. *P < 0.05, **P < 0.01 versus SC (Student’s two-tailed t-test). (A color version of this figure is available in the online journal.)
Curcumin inhibits cell invasion
Cell invasion assay was performed using the transwell membrane culture system. As shown in Figure 5b, cell invasion were significantly increased in a time-dependent manner under hypoxic conditions compared to normoxic. However, pretreatment with curcumin at 12.5 and 25 µmol/L significantly suppressed the cell invasion compared to that of the SC (P < 0.05), and 50 µmol/L of curcumin pretreatment group showed more significant effects against cell invasion (P < 0.01) (Figure 5c).
Curcumin effects BNIP3 expression
BNIP3 is one of the HIF target genes and plays an important role for hypoxia-induced apoptosis and autophagy.16 Therefore, the BNIP3 expression under hypoxia was investigated. Both mRNA and protein levels of BNIP3 were only slightly increased within 24 h of hypoxia. For 48 h of hypoxia, there was a significant increase of BNIP3 expression compared to control cells (Figure 6a, c). However, to investigate curcumin effect on BNIP3, pretreatment with curcumin (25 and 50 µmol/L) for 1 h slightly down-regulated the mRNA and protein expression of BNIP3 to about 90% that of SC, but there was no significant difference between these groups (Figure 6b, d).
Figure 6.
Curcumin decreases the expression of BNIP3. (a) Expression of BNIP3 mRNA induced by hypoxia (2% O2, v/v) over various times (12, 24, and 48 h). The GAPDH band is shown to confirm the equal loading of RNA. The graph shows the densitometry analysis of BNIP3/GAPDH. (b) The effects of curcumin on BNIP3 mRNA expression. After pretreatment with curcumin (12.5, 25, and 50 µmol/L), the cells were exposed to hypoxia for 24 h; subsequently, the BNIP3 mRNA was analyzed with RT-PCR. GAPDH was detected for internal control. (c) Expression of BNIP3 protein induced by hypoxia (2% O2, v/v) over various times (12, 24 and 48 h). The bottom graph shows the densitometry analysis of BNIP3/β-actin. (d) Curcumin decreases BNIP3 protein expression. The cells were treated with or without curcumin (12.5, 25 and 50 µmol/L) and were exposed to hypoxia for 24 h. They were then analyzed with a Western blot of total cellular protein. Equal amounts of total cellular protein (60 µg) were loaded and β-actin was used as an internal control. The densitometry analysis is also shown below. All of the data represent the means ± SEM of three independent experiments. Cont: control, SC: solvent control. *P < 0.05, **P < 0.01 versus SC (Student’s two-tailed t-test)
Curcumin inhibits hypoxia-induced ROS production
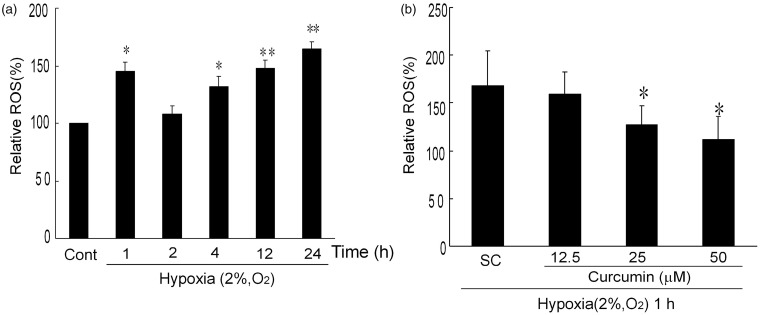
The effect of hypoxia on intracellular ROS production was investigated by flow cytometry. Cells cultured in 2% O2 for different time periods were incubated with DCFH-DA to detect the intracellular ROS level. As shown in Figure 7a, after culture under hypoxia condition for different times, the ROS level in the K1 cells significantly increased; compared to the normoxia control, the relative ROS levels were 145 ± 7.9% at 1 h, 131 ± 8.7% at 4 h, 147 ± 7.2% at 12 h, and 164 ± 6.6% at 24 h, respectively. Hypoxia induced a rapid ROS production within 1 h, therefore the samples that had undergone hypoxia for 1 h were selected for further experiments. Pretreatment with curcumin (25 and 50 µmol/L) for 1 h significantly attenuated the generation of ROS under hypoxia condition. As illustrated in Figure 7b, the relative level of ROS decreased from 168 ± 36.7% of SC group to 127 ± 20% and 113 ± 24.9% in 25 and 50 µmol/L curcumin of pretreatment groups, respectively. However, further study showed that curcumin was unable to attenuate ROS generated by hypoxia over 4 h or a longer period (data not shown). Taken together, these results indicated that curcumin decreased the level of hypoxia-induced ROS in K1 cells in a dose-dependent manner.
Figure 7.
Curcumin inhibits hypoxia-induced ROS in K1 cells. (a) Time-dependent effects of hypoxia-induced ROS in K1 cells. K1 cells were exposed to hypoxia for indicated times (2% O2, v/v), subsequently, cells were stained with DCFA-DH (10 µmol/L) for 20 min and analyzed with flow cytometry. *P < 0.05 and **P < 0.01 versus control (Student’s two-tailed t-test). (b) Effects of curcumin on hypoxia-induced ROS in K1 cells. K1 cells were exposed to various concentrations of curcumin (0, 12.5, 25, and 50 µmol/L) for 1 h. Then the cells underwent hypoxia (2% O2, v/v) for 1 h. After pretreatment, the cells were detected by DCFA-DH. All of the data were expressed as the mean ± SEM. of the three experiments and each included triplicate sets. Cont: control, SC: solvent control. *P < 0.05 versus SC (Student’s two-tailed t-test)
Discussion
PTC accounts for 80% of all thyroid cancers and the overall 10-year survival rate is more than 90%. However, there is only a 60% survival rate in 10-year follow-ups for PTC with extensive metastasis.17 Thus, metastasis presents particular difficulties in thyroid cancer therapy.
HIF-1 is an oxygen-dependent transcriptional activator, which plays crucial roles in tumor progression and metastasis. Consistent with previous reports,9,18 a significant increase in HIF-1α mRNA levels was observed in K1 cells under hypoxia condition (Figure 1a). However, some studies have come to controversial conclusions regarding the hypoxia-induced alterations of HIF-1α mRNA levels. Kallio et al. found that HIF-1α expression increases under hypoxic environment, but that the greatest change is in the protein level rather than in the transcription level.19 Other study reported that cobalt ions can stabilize HIF-1α protein levels by inhibiting prolyl hydroxylase activity, but have no effect on HIF-1α mRNA.20 It should be noticed that Kallio’s hypoxic model of cobalt chloride-inducing hypoxia differed from our hypoxia method. Therefore, the different results with regard to HIF-1α may be due to the different cell types or different experiment methods used in each system. Treatment of curcumin dose-dependently decreased the mRNA levels of HIF-1α which is induced by hypoxia (Figure 1b). Similar results were obtained in HIF-1α protein expression by Western blot analysis. We observed that the pretreatment of curcumin down-regulated the protein expression of HIF-1α induced by hypoxia (Figure 2d). The result was similar to previous reports,21 which showed that curcumin down-regulated HIF-1α protein levels and activity and led to the inhibition of vascular endothelial growth factor (VEGF) gene expression.
Reversing HIF-1α protein expression had a positive effect in anti-hypoxia experiments, as did decreasing the binding activity of HIF-1α to HRE. When O2 is absent, stabilized HIF-1α is translocated to the nucleus, where it forms a heterodimer with HIF-1β. The HIF-1 heterodimer is a transcription factor recognized by many HRE sites on chromosomes.22 By EMSA method, a significant HIF-1α/HRE shift-band was observed indicating the binding of HIF-1α to HRE element in hypoxic conditions. Curcumin significantly inhibited the DNA binding ability of HIF-1α (Figure 3a, b).
HIF-1α is a hallmark of tumor invasion and metastasis. Our data verified that curcumin inhibited the expression and DNA binding activity of hypoxia-induced HIF-1α, we next asked whether curcumin had any effect on inhibiting/suppressing tumor metastasis. Epithelial-mesenchymal transition (EMT) is considered as a pivotal event in the metastatic cascade that allows cells to acquire migratory and invasive ability.23 It has been reported that HIF-1 promotes EMT through direct regulation of EMT-related proteins, such as TWIST.24 During EMT process, epithelial cell layers lose polarity together with cell-to-cell contacts. The expression of proteins that promote cell-to-cell contact, such as E-cadherin, may be lost, and the cells may acquire mesenchymal markers such as vimentin and the MMPs MMP-9, resulting in an enhanced ability for cell migration and invasion.25 It has been reported that curcumin disrupted EMT process via inhibition of NF-kB-Snail signaling pathway as well as upregulation of E-cadherin expression.26 In our study, cell migration was augmented after hypoxic treatment (Figure 5a). Pretreatment of curcumin significantly attenuated the K1 cells hypoxia-induced motor ability.
E-cadherin plays a critical role in tumor invasion and metastasis. In normal tissue, E-cadherin and β-catenin form an E-cadherin/β-catenin complex that maintains cell polarity and organizes structure integrity. In contrast, E-cadherin expression decreases in tumor tissue, which attenuates a cell’s adhere ability and enhances its motor ability, leading to cell ablate and migration.27 E-cadherin also correlates with hypoxia-induced tumor cell migration and invasion.28 Consistent with published reports, our results demonstrated that E-cadherin was down-regulated (Figure 4d).
Interfering E-cadherin-mediated cell–cell adhere is an important factor in tumor invasion and a disorganizing extracellular matrix (ECM) coordinates the effect. ECM is the extracellular part of animal tissue that usually provides structural support for the cells and a mechanical barrier to tumor cell invasion. MMP-9 is the primary type of MMP, which involve a large family of zinc-dependent endopeptidases that degrade ECM proteins. By zymographic examination, we demonstrated that pretreatment with curcumin decreased the MMP-9 activity induced by hypoxia in K1 cells (Figure 4b). Other studies have confirmed the hypoxia-stimulated MMP-9 expression using the isolated rat lung mast cells model29 and the breast cancer cells model.30 Choi et al. further demonstrated that HIF-1α regulated MMP-9 expression via HIF-1α RNA interference. Furthermore, suppressing MMP-9 expression and activity inhibits tumor cell migration and invasion.31 Therefore, the migration experiment proved that curcumin inhibited the hypoxia-induced migration of K1 cells and Transwell experiment further displayed curcumin’s effect of suppressing K1 cells invasion (Figure 5). All of these results demonstrated the involvement of MMP-9 in the process through which curcumin inhibits K1 cell migration and invasion. Together, these studies demonstrate that curcumin can effectively inhibit hypoxia-induced K1 cell migration and invasion by suppressing MMP-9 activity.
Considering that HIF-1 could also affect BNIP3 expression and regulates cell viability via autophagy and apoptosis pathways,16 we next investigate the mRNA and protein expressions of BNIP3 under hypoxia condition. Our results indicated no significant differences were detected in the mRNA and protein expressions of BNIP3 within 24 h under hypoxia (2% oxygen). For long period of hypoxia up to 48 h, both mRNA and protein expressions of BNIP3 were significantly induced. Curcumin treatment also had no effect on the mRNA and protein expressions of BNIP3 under hypoxia (Figure 6). Indeed, in our model, the cell viability only slightly decreased and no significant apoptotic cells were detected after hypoxia for 24 h (Figure 1b). Based on these data, we conclude that curcumin inhibited hypoxia-induced migration in K1 cells in a BNIP3-independent way.
Accumulated evidence indicated that ROS stimulates HIF-1α expression.32 Consistent with previous reports,33 we found that pretreatment with curcumin inhibited ROS production (Figure 7). These results support the hypothesis that curcumin affects HIF-1α by scavenging the intracellular biological free radicals in K1 cells. Other mechanism needs to be further investigated.
Overall, our study confirms that curcumin is a promising anticancer and anti-metastasis compound. Our results reveal that curcumin probably affects the HIF-1α levels, including the pre-transcription and post-transcription levels. Further experiments clarified the relationship between curcumin and two tumor EMT hallmarks (E-cadherin and MMP-9), and demonstrated that curcumin inhibited hypoxia-induced K1 cells migration and invasion. This knowledge may be useful for the development of combination therapies that use curcumin with other anti-metastasis drugs to treat papillary thyroid carcinomas.
Conclusion
The present study demonstrated that curcumin significantly inhibits the ROS production as well as the mRNA and protein expression of HIF-1α in hypoxic K1 cells, blocks the binding activity of HIF-1α to HRE, enhances E-cadherin expression, inhibits MMP-9 activity, and suppresses the migration ability of K1 cells under hypoxic conditions. Therefore, curcumin possesses a distinct anti-metastatic property and may be used for the development of combination therapies with other anti-metastasis drugs to treat papillary thyroid carcinomas.
ACKNOWLEDGMENTS
This study was supported by grants from the National Natural Science Foundation of China (Nos. 31071491 and 31171627), the Ministry of Health Foundation of China (W201304), the Natural Science Foundation of Jiangsu Province (No. BK2010156), the Science and Research Foundation of the Health Bureau of Jiangsu Province (No. H2010027), and the Public Service Platform for Science and Technology Infrastructure Construction Project of Jiangsu Province (No. BM2012066).
Author contributions
All authors participated in the interpretation and review of this manuscript. CT and LZ designed and performed the research, they contributed equally to this work.
References
- 1.Lantto TA, Colucci M, Závadová V, Hiltunen R, Raasmaja A. Cytotoxicity of curcumin, resveratrol and plant extracts from basil, juniper, laurel and parsley in SH-SY5Y and CV1-P cells. Food Chem 2009; 117: 405–11. [Google Scholar]
- 2.Parvathy KS, Negi PS, Srinivas P. Antioxidant, antimutagenic and antibacterial activities of curcumin-β-diglucoside. Food Chem 2009; 115: 265–71. [Google Scholar]
- 3.Song F, Zhang L, Yu H-X, Lu R-R, Bao J-D, Tan C, Sun Z. The mechanism underlying proliferation-inhibitory and apoptosis-inducing effects of curcumin on papillary thyroid cancer cells. Food Chem 2012; 132: 43–50. [DOI] [PubMed] [Google Scholar]
- 4.Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer 2006; 6: 292–306. [DOI] [PubMed] [Google Scholar]
- 5.Tuttle RM, Ball DW, Byrd D, Dilawari RA, Doherty GM, Duh QY, Ehya H, Farrar WB, Haddad RI, Kandeel F, Kloos RT, Kopp P, Lamonica DM, Loree TR, Lydiatt WM, McCaffrey JC, Olson JA, Jr, Parks L, Ridge JA, Shah JP, Sherman SI, Sturgeon C, Waguespack SG, Wang TN, Wirth LJ. Thyroid carcinoma. J Natl Compr Canc Netw 2010; 8: 1228–74. [DOI] [PubMed] [Google Scholar]
- 6.Robie DK, Dinauer CW, Tuttle RM, Ward DT, Parry R, McClellan D, Svec R, Adair C, Francis G. The impact of initial surgical management on outcome in young patients with differentiated thyroid cancer. J Pediatr Surg 1998; 33: 1134–8; discussion 9–40. [DOI] [PubMed] [Google Scholar]
- 7.Yang CL, Liu YY, Ma YG, Xue YX, Liu DG, Ren Y, Liu XB, Li Y, Li Z. Curcumin blocks small cell lung cancer cells migration, invasion, angiogenesis, cell cycle and neoplasia through Janus kinase-STAT3 signalling pathway. PLoS ONE 2012; 7: e37960–e37960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitra A, Chakrabarti J, Banerji A, Chatterjee A, Das BR. Curcumin, a potential inhibitor of MMP-2 in human laryngeal squamous carcinoma cells HEp2. J Environ Pathol Toxicol Oncol 2006; 25: 679–90. [DOI] [PubMed] [Google Scholar]
- 9.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer 2003; 3: 721–32. [DOI] [PubMed] [Google Scholar]
- 10.Semenza GL. Surviving ischemia: adaptive responses mediated by hypoxia-inducible factor 1. J Clin Invest 2000; 106: 809–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajoria S, Suriano R, George A, Shanmugam A, Schantz SP, Geliebter J, Tiwari RK. Estrogen induced metastatic modulators MMP-2 and MMP-9 are targets of 3,3'-diindolylmethane in thyroid cancer. PLoS ONE 2011; 6: e15879–e15879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imai T, Horiuchi A, Wang C, Oka K, Ohira S, Nikaido T, Konishi I. Hypoxia attenuates the expression of E-cadherin via up-regulation of SNAIL in ovarian carcinoma cells. Am J Pathol 2003; 163: 1437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lester RD, Jo M, Montel V, Takimoto S, Gonias SL. uPAR induces epithelial-mesenchymal transition in hypoxic breast cancer cells. J Cell Biol 2007; 178: 425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amelio I, Melino G. The “Sharp” blade against HIF-mediated metastasis. Cell Cycle 2012; 11: 4530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rachagani S, Senapati S, Chakraborty S, Ponnusamy MP, Kumar S, Smith LM, Jain M, Batra SK. Activated KrasG(1)(2)D is associated with invasion and metastasis of pancreatic cancer cells through inhibition of E-cadherin. Br J Cancer 2011; 104: 1038–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sowter HM, Ratcliffe PJ, Watson P, Greenberg AH, Harris AL. HIF-1-dependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors. Cancer Res 2001; 61: 6669–73. [PubMed] [Google Scholar]
- 17.Davies L, Welch HG. Thyroid cancer survival in the United States: observational data from 1973 to 2005. Arch Otolaryngol Head Neck Surg 2010; 136: 440–4. [DOI] [PubMed] [Google Scholar]
- 18.Li R, Wang Y, Liu Y, Chen Q, Fu W, Wang H, Cai H, Peng W, Zhang X. Curcumin inhibits transforming growth factor-beta1-induced EMT via PPARgamma pathway, not Smad pathway in renal tubular epithelial cells. PLoS ONE 2013; 8: e58848–e58848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kallio PJ, Pongratz I, Gradin K, McGuire J, Poellinger L. Activation of hypoxia-inducible factor 1alpha: posttranscriptional regulation and conformational change by recruitment of the Arnt transcription factor. Proc Natl Acad Sci U S A 1997; 94: 5667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 2001; 107: 43–54. [DOI] [PubMed] [Google Scholar]
- 21.Bae MK, Kim SH, Jeong JW, Lee YM, Kim HS, Kim SR, Yun I, Bae SK, Kim KW. Curcumin inhibits hypoxia-induced angiogenesis via down-regulation of HIF-1. Oncol Rep 2006; 15: 1557–62. [PubMed] [Google Scholar]
- 22.Weidemann A, Klanke B, Wagner M, Volk T, Willam C, Wiesener MS, Eckardt KU, Warnecke C. Hypoxia, via stabilization of the hypoxia-inducible factor HIF-1alpha, is a direct and sufficient stimulus for brain-type natriuretic peptide induction. Biochem J 2008; 409: 233–42. [DOI] [PubMed] [Google Scholar]
- 23.Cowin P, Welch DR. Breast cancer progression: controversies and consensus in the molecular mechanisms of metastasis and EMT. J Mammary Gland Biol Neoplasia 2007; 12: 99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Zhou BP. Activation of beta-catenin and Akt pathways by Twist are critical for the maintenance of EMT associated cancer stem cell-like characters. BMC Cancer 2011; 11: 49–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene 2008; 27: 6958–69. [DOI] [PubMed] [Google Scholar]
- 26.Huang T, Chen Z, Fang L. Curcumin inhibits LPS-induced EMT through downregulation of NF-kappaB-Snail signaling in breast cancer cells. Oncol Rep 2013; 29: 117–24. [DOI] [PubMed] [Google Scholar]
- 27.Beavon IR. The E-cadherin-catenin complex in tumour metastasis: structure, function and regulation. Eur J Cancer 2000; 36: 1607–20. [DOI] [PubMed] [Google Scholar]
- 28.Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci U S A 2008; 105: 6392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maxova H, Novotna J, Vajner L, Tomasova H, Vytasek R, Vizek M, Bacakova L, Valouskova V, Eliasova T, Herget J. In vitro hypoxia increases production of matrix metalloproteinases and tryptase in isolated rat lung mast cells. Physiol Res 2008; 57: 903–10. [DOI] [PubMed] [Google Scholar]
- 30.Choi JY, Jang YS, Min SY, Song JY. overexpression of MMP-9 and HIF-1alpha in breast cancer cells under hypoxic conditions. J Breast Cancer 2011; 14: 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SJ, Lim JH, Choi YH, Kim WJ, Moon SK. Interleukin-28A triggers wound healing migration of bladder cancer cells via NF-kappaB-mediated MMP-9 expression inducing the MAPK pathway. Cell Signal 2012; 24: 1734–42. [DOI] [PubMed] [Google Scholar]
- 32.Bonello S, Zahringer C, BelAiba RS, Djordjevic T, Hess J, Michiels C, Kietzmann T, Gorlach A. Reactive oxygen species activate the HIF-1alpha promoter via a functional NFkappaB site. Arterioscler Thromb Vasc Biol 2007; 27: 755–61. [DOI] [PubMed] [Google Scholar]
- 33.Zhao X-C, Zhang L, Yu H-X, Sun Z, Lin X-F, Tan C, Lu R-R. Curcumin protects mouse neuroblastoma Neuro-2A cells against hydrogen-peroxide-induced oxidative stress. Food Chem 2011; 129: 387–94. [DOI] [PubMed] [Google Scholar]