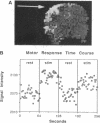
Abstract
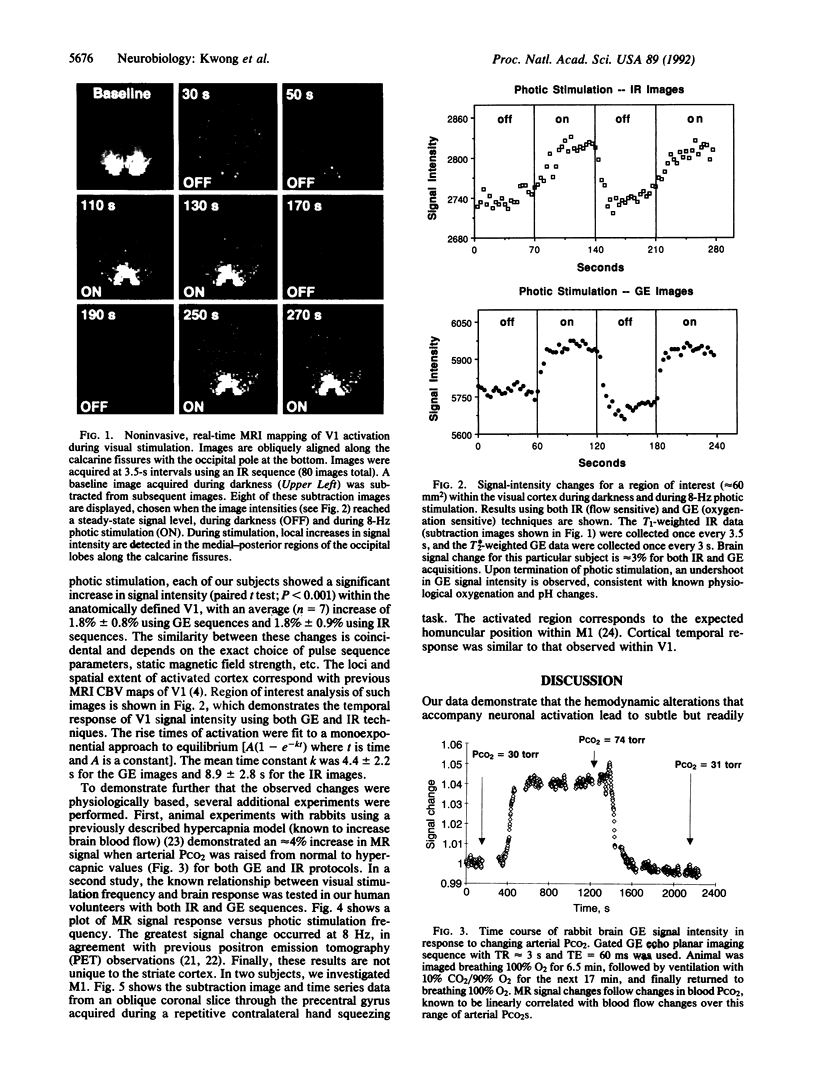
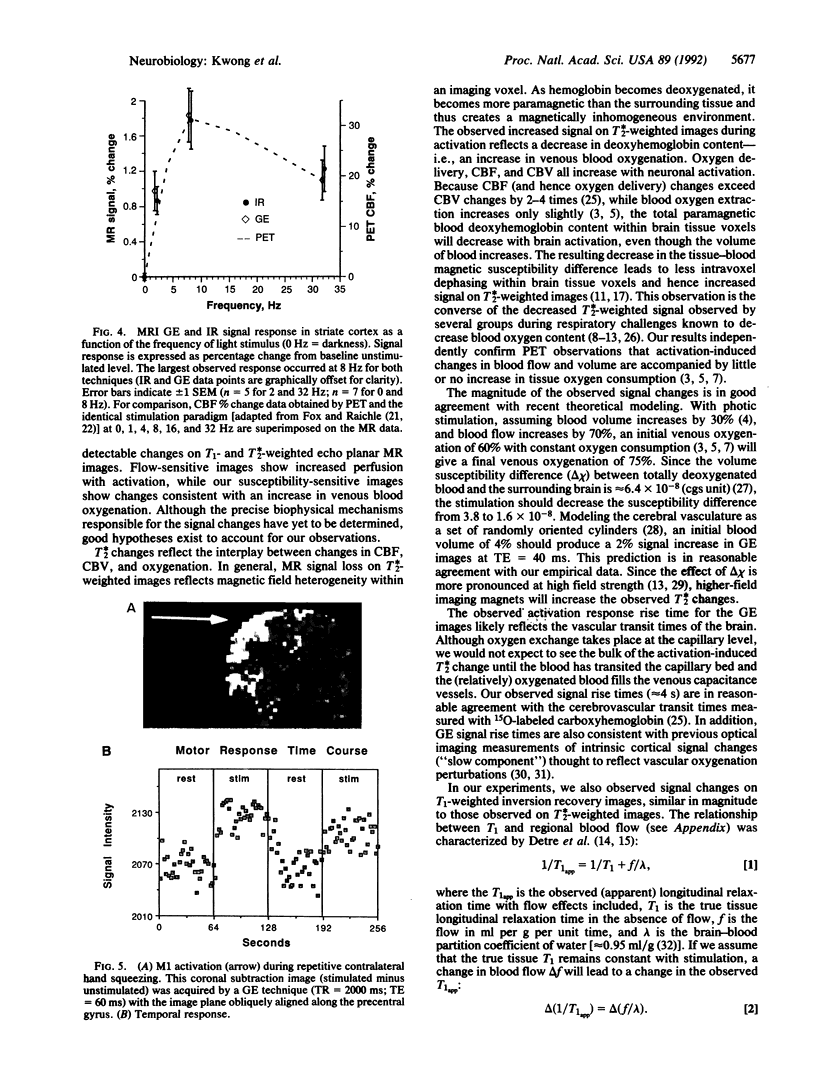
Neuronal activity causes local changes in cerebral blood flow, blood volume, and blood oxygenation. Magnetic resonance imaging (MRI) techniques sensitive to changes in cerebral blood flow and blood oxygenation were developed by high-speed echo planar imaging. These techniques were used to obtain completely noninvasive tomographic maps of human brain activity, by using visual and motor stimulus paradigms. Changes in blood oxygenation were detected by using a gradient echo (GE) imaging sequence sensitive to the paramagnetic state of deoxygenated hemoglobin. Blood flow changes were evaluated by a spin-echo inversion recovery (IR), tissue relaxation parameter T1-sensitive pulse sequence. A series of images were acquired continuously with the same imaging pulse sequence (either GE or IR) during task activation. Cine display of subtraction images (activated minus baseline) directly demonstrates activity-induced changes in brain MR signal observed at a temporal resolution of seconds. During 8-Hz patterned-flash photic stimulation, a significant increase in signal intensity (paired t test; P less than 0.001) of 1.8% +/- 0.8% (GE) and 1.8% +/- 0.9% (IR) was observed in the primary visual cortex (V1) of seven normal volunteers. The mean rise-time constant of the signal change was 4.4 +/- 2.2 s for the GE images and 8.9 +/- 2.8 s for the IR images. The stimulation frequency dependence of visual activation agrees with previous positron emission tomography observations, with the largest MR signal response occurring at 8 Hz. Similar signal changes were observed within the human primary motor cortex (M1) during a hand squeezing task and in animal models of increased blood flow by hypercapnia. By using intrinsic blood-tissue contrast, functional MRI opens a spatial-temporal window onto individual brain physiology.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belliveau J. W., Cohen M. S., Weisskoff R. M., Buchbinder B. R., Rosen B. R. Functional studies of the human brain using high-speed magnetic resonance imaging. J Neuroimaging. 1991 Feb;1(1):36–41. doi: 10.1111/jon19911136. [DOI] [PubMed] [Google Scholar]
- Belliveau J. W., Kennedy D. N., Jr, McKinstry R. C., Buchbinder B. R., Weisskoff R. M., Cohen M. S., Vevea J. M., Brady T. J., Rosen B. R. Functional mapping of the human visual cortex by magnetic resonance imaging. Science. 1991 Nov 1;254(5032):716–719. doi: 10.1126/science.1948051. [DOI] [PubMed] [Google Scholar]
- Belliveau J. W., Rosen B. R., Kantor H. L., Rzedzian R. R., Kennedy D. N., McKinstry R. C., Vevea J. M., Cohen M. S., Pykett I. L., Brady T. J. Functional cerebral imaging by susceptibility-contrast NMR. Magn Reson Med. 1990 Jun;14(3):538–546. doi: 10.1002/mrm.1910140311. [DOI] [PubMed] [Google Scholar]
- Brooks R. A., Di Chiro G. Magnetic resonance imaging of stationary blood: a review. Med Phys. 1987 Nov-Dec;14(6):903–913. doi: 10.1118/1.595994. [DOI] [PubMed] [Google Scholar]
- Churchland P. S., Sejnowski T. J. Perspectives on cognitive neuroscience. Science. 1988 Nov 4;242(4879):741–745. doi: 10.1126/science.3055294. [DOI] [PubMed] [Google Scholar]
- Cohen M. S., Weisskoff R. M. Ultra-fast imaging. Magn Reson Imaging. 1991;9(1):1–37. doi: 10.1016/0730-725x(91)90094-3. [DOI] [PubMed] [Google Scholar]
- Deiber M. P., Passingham R. E., Colebatch J. G., Friston K. J., Nixon P. D., Frackowiak R. S. Cortical areas and the selection of movement: a study with positron emission tomography. Exp Brain Res. 1991;84(2):393–402. doi: 10.1007/BF00231461. [DOI] [PubMed] [Google Scholar]
- Detre J. A., Leigh J. S., Williams D. S., Koretsky A. P. Perfusion imaging. Magn Reson Med. 1992 Jan;23(1):37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- Fox P. T., Miezin F. M., Allman J. M., Van Essen D. C., Raichle M. E. Retinotopic organization of human visual cortex mapped with positron-emission tomography. J Neurosci. 1987 Mar;7(3):913–922. doi: 10.1523/JNEUROSCI.07-03-00913.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox P. T., Mintun M. A., Raichle M. E., Miezin F. M., Allman J. M., Van Essen D. C. Mapping human visual cortex with positron emission tomography. 1986 Oct 30-Nov 5Nature. 323(6091):806–809. doi: 10.1038/323806a0. [DOI] [PubMed] [Google Scholar]
- Fox P. T., Raichle M. E. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox P. T., Raichle M. E., Mintun M. A., Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988 Jul 22;241(4864):462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Fox P. T., Raichle M. E. Stimulus rate dependence of regional cerebral blood flow in human striate cortex, demonstrated by positron emission tomography. J Neurophysiol. 1984 May;51(5):1109–1120. doi: 10.1152/jn.1984.51.5.1109. [DOI] [PubMed] [Google Scholar]
- Fox P. T., Raichle M. E. Stimulus rate determines regional brain blood flow in striate cortex. Ann Neurol. 1985 Mar;17(3):303–305. doi: 10.1002/ana.410170315. [DOI] [PubMed] [Google Scholar]
- Frostig R. D., Lieke E. E., Ts'o D. Y., Grinvald A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6082–6086. doi: 10.1073/pnas.87.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald A., Lieke E., Frostig R. D., Gilbert C. D., Wiesel T. N. Functional architecture of cortex revealed by optical imaging of intrinsic signals. 1986 Nov 27-Dec 3Nature. 324(6095):361–364. doi: 10.1038/324361a0. [DOI] [PubMed] [Google Scholar]
- Grubb R. L., Jr, Raichle M. E., Eichling J. O., Ter-Pogossian M. M. The effects of changes in PaCO2 on cerebral blood volume, blood flow, and vascular mean transit time. Stroke. 1974 Sep-Oct;5(5):630–639. doi: 10.1161/01.str.5.5.630. [DOI] [PubMed] [Google Scholar]
- Lueck C. J., Zeki S., Friston K. J., Deiber M. P., Cope P., Cunningham V. J., Lammertsma A. A., Kennard C., Frackowiak R. S. The colour centre in the cerebral cortex of man. Nature. 1989 Aug 3;340(6232):386–389. doi: 10.1038/340386a0. [DOI] [PubMed] [Google Scholar]
- Mintun M. A., Fox P. T., Raichle M. E. A highly accurate method of localizing regions of neuronal activation in the human brain with positron emission tomography. J Cereb Blood Flow Metab. 1989 Feb;9(1):96–103. doi: 10.1038/jcbfm.1989.13. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Lee T. M. Magnetic resonance imaging of blood vessels at high fields: in vivo and in vitro measurements and image simulation. Magn Reson Med. 1990 Oct;16(1):9–18. doi: 10.1002/mrm.1910160103. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Lee T. M., Nayak A. S., Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990 Apr;14(1):68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- Perlmutter J. S., Powers W. J., Herscovitch P., Fox P. T., Raichle M. E. Regional asymmetries of cerebral blood flow, blood volume, and oxygen utilization and extraction in normal subjects. J Cereb Blood Flow Metab. 1987 Feb;7(1):64–67. doi: 10.1038/jcbfm.1987.9. [DOI] [PubMed] [Google Scholar]
- Phelps M. E., Kuhl D. E., Mazziota J. C. Metabolic mapping of the brain's response to visual stimulation: studies in humans. Science. 1981 Mar 27;211(4489):1445–1448. doi: 10.1126/science.6970412. [DOI] [PubMed] [Google Scholar]
- Prichard J., Rothman D., Novotny E., Petroff O., Kuwabara T., Avison M., Howseman A., Hanstock C., Shulman R. Lactate rise detected by 1H NMR in human visual cortex during physiologic stimulation. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5829–5831. doi: 10.1073/pnas.88.13.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M. E., Eichling J. O., Straatmann M. G., Welch M. J., Larson K. B., Ter-Pogossian M. M. Blood-brain barrier permeability of 11C-labeled alcohols and 15O-labeled water. Am J Physiol. 1976 Feb;230(2):543–552. doi: 10.1152/ajplegacy.1976.230.2.543. [DOI] [PubMed] [Google Scholar]
- Thulborn K. R., Waterton J. C., Matthews P. M., Radda G. K. Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field. Biochim Biophys Acta. 1982 Feb 2;714(2):265–270. doi: 10.1016/0304-4165(82)90333-6. [DOI] [PubMed] [Google Scholar]
- Turner R., Le Bihan D., Moonen C. T., Despres D., Frank J. Echo-planar time course MRI of cat brain oxygenation changes. Magn Reson Med. 1991 Nov;22(1):159–166. doi: 10.1002/mrm.1910220117. [DOI] [PubMed] [Google Scholar]
- Weisskoff R. M., Kiihne S. MRI susceptometry: image-based measurement of absolute susceptibility of MR contrast agents and human blood. Magn Reson Med. 1992 Apr;24(2):375–383. doi: 10.1002/mrm.1910240219. [DOI] [PubMed] [Google Scholar]
- Williams D. S., Detre J. A., Leigh J. S., Koretsky A. P. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):212–216. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S., Watson J. D., Lueck C. J., Friston K. J., Kennard C., Frackowiak R. S. A direct demonstration of functional specialization in human visual cortex. J Neurosci. 1991 Mar;11(3):641–649. doi: 10.1523/JNEUROSCI.11-03-00641.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]