Mechanistic links between cell autonomous clocks and metabolic processes remain largely unknown. The current study shows that cell autonomous clocks influence protein biotinylation, which in turn impacts fatty acid oxidation and protein synthesis. These observations may explain why disruption of circadian functions (e.g., shift workers) increases risk of cardiometabolic diseases.
Keywords: biotin, chronobiology, heart, liver, metabolism, nutrition
Abstract
Circadian clocks are critical modulators of metabolism. However, mechanistic links between cell autonomous clocks and metabolic processes remain largely unknown. Here, we report that expression of the biotin transporter slc5a6 gene is decreased in hearts of two distinct genetic mouse models of cardiomyocyte-specific circadian clock disruption [i.e., cardiomyocyte-specific CLOCK mutant (CCM) and cardiomyocyte-specific BMAL1 knockout (CBK) mice]. Biotinylation is an obligate posttranslational modification for five mammalian carboxylases: acetyl-CoA carboxylase α (ACCα), ACCβ, pyruvate carboxylase (PC), methylcrotonyl-CoA carboxylase (MCC), and propionyl-CoA carboxylase (PCC). We therefore hypothesized that the cardiomyocyte circadian clock impacts metabolism through biotinylation. Consistent with decreased slc5a6 expression, biotinylation of all carboxylases is significantly decreased (10–46%) in CCM and CBK hearts. In association with decreased biotinylated ACC, oleate oxidation rates are increased in both CCM and CBK hearts. Consistent with decreased biotinylated MCC, leucine oxidation rates are significantly decreased in both CCM and CBK hearts, whereas rates of protein synthesis are increased. Importantly, feeding CBK mice with a biotin-enriched diet for 6 wk normalized myocardial 1) ACC biotinylation and oleate oxidation rates; 2) PCC/MCC biotinylation (and partially restored leucine oxidation rates); and 3) net protein synthesis rates. Furthermore, data suggest that the RRAGD/mTOR/4E-BP1 signaling axis is chronically activated in CBK and CCM hearts. Finally we report that the hepatocyte circadian clock also regulates both slc5a6 expression and protein biotinylation in the liver. Collectively, these findings suggest that biotinylation is a novel mechanism by which cell autonomous circadian clocks influence metabolic pathways.
NEW & NOTEWORTHY
Mechanistic links between cell autonomous clocks and metabolic processes remain largely unknown. The current study shows that cell autonomous clocks influence protein biotinylation, which in turn impacts fatty acid oxidation and protein synthesis. These observations may explain why disruption of circadian functions (e.g., shift workers) increases risk of cardiometabolic diseases.
a hallmark of energy homeostasis is the fluctuation of metabolic processes within a physiologic range, in response to both endogenous and exogenous stimuli/stresses. This is illustrated by alterations in energy homeostatic parameters on a daily basis. More specifically, sleep/wake and fasting/feeding cycles impact daily oscillations in both energy supply and demand, which normally remain in synchrony with time-of-day-dependent perturbations in metabolic fluxes (5, 6). Classically, rhythms in metabolism have been attributed to factors extrinsic to cells/tissues, including behavior-induced fluctuations in neurohumoral factors (e.g., postprandial rise in circulating insulin levels). More recently it has become apparent that an intrinsic, cell autonomous mechanism, known as the circadian clock, orchestrates the timing between extracellular stimuli and metabolic processes (5, 6, 17). The physiological significance of this molecular mechanism has been highlighted in animal models of genetic disruption of the circadian clock, which often present cardiometabolic disease phenotypes (33, 44, 57). It is noteworthy that behavioral (e.g., shift work) and/or genetic (e.g., polymorphisms) perturbations in the synchrony between the environment and the intrinsic clock mechanism are associated with increased risk for obesity, diabetes, and hypertension in humans (29, 47, 58).
The circadian clock is a transcriptionally based mechanism, composed of a series of positive and negative feedback loops (53). At the heart of the mechanism reside two transcription factors, BMAL1 and CLOCK, which, upon heterodimerization, bind to E-boxes within target genes, resulting in induction (18, 25). Several target genes encode for negative clock components, such as PERIOD 1/2/3, CRYPTOCHROME 1/2, and REV-ERBα; these negative components repress transcriptional activity of BMAL1/CLOCK (30, 40, 48). The latter repression is relieved when levels of the negative components decrease; one cycle takes ∼24 h. It has been estimated that circadian clocks modulate expression of up to 13% of the cellular transcriptome, which in turn carries the potential to perturb distinct biological processes in a time-of-day-dependent manner (51). However, a recent comparison of gene expression microarray data sets with proteomic analyses suggests that only ∼50% of the proteins that oscillate over the course of the day in liver and heart can be explained by fluctuations in gene expression (39, 42). Such observations suggest an important role for posttranscriptional mechanisms. Posttranslational modifications (PTMs) have emerged not only as being integral to the circadian clock mechanism but also as key mechanisms by which the circadian clock influences biological processes, including metabolism. Clock controlled PTMs identified to date include phosphorylation, ubiquitination, SUMoylation, ADP-ribosylation, O-GlcNAcylation, and acetylation (3, 4, 9, 14, 23, 28, 35). In the latter case, recent studies by Peek et al. (37) in the liver suggest that direct BMAL1/CLOCK regulation of nampt [encoding for nicotinamide phosphoribosyltransferase (NAMPT)] influences hepatic NAD+ levels, which in turn modulate acetylation of mitochondrial β-oxidation enzymes via NAD+-dependent sirtuins. Accordingly, livers from germline BMAL1 null mice have been reported to exhibit decreased NAMPT and NAD+ levels, associated with increased acyl-CoA dehydrogenase acetylation and decreased rates of fatty acid β-oxidation (37).
In an attempt to elucidate novel mechanisms by which cell autonomous circadian clocks influence metabolic pathways, we have generated various mouse models of cell-type specific circadian clock dysfunction. Two of these models have focused on the heart, given that this organ: 1) has a high metabolic rate; 2) is responsive to fluctuations in energy supply and demand; and 3) is sensitive to various homeostatic neurohumoral factors (e.g., insulin). These models are cardiomyocyte-specific CLOCK mutant (CCM) and cardiomyocyte-specific BMAL1 knockout (CBK) mice (7, 15, 60). Using these complementary models, we have reported that the cardiomyocyte circadian clock influences myocardial ketone body, glucose, and fatty acid metabolism (14, 55, 60). In the cases of glucose and fatty acids, both oxidative and nonoxidative metabolism appear to be clock regulated. Unlike livers from germline BMAL1 knockout mice, hearts from CCM and CBK mice both exhibit increased fatty acid oxidation (7, 60). Furthermore, despite decreased NAMPT expression in CCM and CBK hearts, protein acetylation is decreased (38). These data suggest that cell autonomous circadian clocks may influence fatty acid β-oxidation through multiple mechanisms.
A key regulatory site for β-oxidation is the carnitine shuttle, which is essential for the entry of long chain fatty acids into the mitochondrial matrix. Acetyl-CoA carboxylase (ACC) synthesizes malonyl-CoA, an allosteric inhibitor of the carnitine shuttle (binding to carnitine acyltransferase 1) (34). ACCβ, the important regulatory isoform in metabolically active tissues, is phosphorylated and inactivated by AMP-activated protein kinase (AMPK) (43). Recent investigation of ACCβ phosphorylation revealed no difference in CCM hearts, relative to littermate control hearts (38). ACCβ is subject to biotinylation, a distinct PTM that is essential (i.e., obligate requirement) for enzymatic activity of all mammalian carboxylases [i.e., ACCα, ACCβ, pyruvate carboxylase (PC), methylcrotonyl-CoA carboxylase (MCC), and propionyl-CoA carboxylase (PCC)] (31, 54). Interestingly, a recently published gene expression microarray interrogating genes that are differentially expressed in a common manner in both CCM and CBK hearts (relative to littermate controls) suggested decrease levels of slc5a6, encoding for sodium-dependent multivitamin transporter (SMVT) (60). SMVT transports biotin into cells (41). We therefore hypothesized that the cardiomyocyte circadian clock influences myocardial fatty acid oxidation through biotinylation of ACCβ. Here, we confirm decreased slc5a6 expression in CCM and CBK hearts and highlight biotinylation as a novel connection between the cardiomyocyte circadian clock with regulation of myocardial fatty acid and amino acid metabolism. We also reveal that the hepatocyte circadian clock similarly regulates protein biotinylation in the liver, suggesting that this mechanistic link is present in multiple organs.
MATERIALS AND METHODS
Mice.
The present study utilized: 1) CCM (α-MHC-dnCLOCK+/−) and littermate controls (α-MHC-dnCLOCK−/−) on the FVB/N background; 2) CBK (BMAL1flox/flox/α-MHC-CRE+/−) and littermate controls (BMAL1flox/flox/α-MHC-CRE−/−) on the C57BL/6J background; and 3) HBK (BMAL1flox/flox/Albumin-CRE+/−) and littermate controls (BMAL1flox/flox/Albumin-CRE−/−) on the C57BL/6J background. Both CBK and CCM mice have been described previously (7, 15, 60). All experimental mice were male, 12–16 wk old (at the time of euthanasia), and were housed at the Animal Resource Program at the University of Alabama at Birmingham (UAB), under temperature-, humidity-, and light- controlled conditions. A strict 12:12-h light-dark cycle regime was enforced (lights on at 6 AM; zeitgeber time 0); the light/dark cycle was maintained throughout these studies. As such, physiologic diurnal variations were investigated in mice (as opposed to circadian rhythms). All mice were housed within regular microisolator cages and had free access to food and water. All animal experiments were approved by the Institutional Animal Care and Use Committee of UAB.
Diets.
Unless otherwise indicated, mice were given a standard rodent chow. For a subset of mice, 10-wk-old mice were fed a biotin-supplemented diet (Teklad TD.02458; biotin content 100 ppm); a calorically matched control diet was also utilized, which differed only in the biotin content (Teklad TD.97126; biotin content 1 ppm). Mice were fed these specialized diets for a 6-wk duration (i.e., mice euthanized at 16 wk of age). Biotin-supplemented and control diets have been utilized previously to study the impact of biotin on glucose homeostasis in mice (32).
Quantitative RT-PCR.
RNA was extracted from hearts using standard procedures (11). Candidate gene expression analysis was performed by quantitative RT-PCR, using methods described previously (21, 24). For quantitative RT-PCR, specific Taqman assays were designed for target mRNA species from mouse sequences available in GenBank. Quantitative RT-PCR data are presented as fold change from the trough value in littermate controls.
Cell culture and transfections.
HepG2 cells (ATCC) were grown and maintained in DMEM supplemented with 10% fetal bovine serum and 1% penicillin streptomycin. For transfections, 1 μg of each plasmid (BMAL1 and/or CLOCK) and the total DNA (2 μg) per well was maintained with empty vector per well of a 12-well plate. Cells were transfected using Lipofectamine 3000 (Invitrogen) according to manufacturer's instructions and RNA was isolated ∼48 h posttransfection.
Immunoblotting.
Qualitative analysis of protein expression and posttranslational modifications (e.g., biotinylation, phosphorylation) was performed as described previously (20, 38). Lysates (5–30 μg) were separated on a 6 or 10% bis-acrylamide gel by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a PVDF membrane and initially stained with ponceau for assessment of total protein loading. Membranes were subsequently probed with a streptavidin-horseradish peroxidase (HRP) conjugate (GE Healthcare Life Cciences RPN1231) for assessment of protein biotinylation or with anti-SLC38A9 (Abcam 81687), anti-RRAGD (Abcam187679), anti-p62 (Abnova H00008878-M01), anti-mammalian target of rapamycin (anti-mTOR) (Cell Signaling 2983), anti-p-mTOR (Cell Signaling 2971), anti-4E binding protein 1 (4E-BP1) (Cell Signaling 9452), anti-p-4E-BP1 (Cell Signaling 2855), or anti-calsequestrin (Abcam 3516). In the case of protein biotinylation, three prominent bands are detected in the murine heart at ∼265, 130, and 75 kDa, representing ACCα/β, PC, and PCC/MCC, respectively (20). It is important to note that due to similarities in molecular weight, these blots cannot distinguish between ACCα and ACCβ; the same is true for PCC and MCC, which co-migrate with one another. Bands were visualized with Luminata Forte Western HRP Substrate (Millipore) on PVDF membranes with a Bio-Rad Chemidoc and quantified using ImageJ (National Institutes of Health). Biotinylated carboxylases were normalized to ponceau stain, whereas SLC38A9, RRAGD, p62, p-4E-BP1, and p-mTOR were normalized to calsequestrin.
Working mouse heart perfusions.
Myocardial substrate utilization and contractile function were measured ex vivo through isolated working mouse heart perfusions, as described previously (7, 14, 55, 56). All hearts were perfused in the working mode (nonrecirculating manner) for 30 min with a preload of 12.5 mmHg and an afterload of 50 mmHg. Standard Krebs-Henseleit buffer was supplemented with 8 mM glucose, 0.4 mM oleate conjugated to 3% BSA (fraction V, fatty acid-free; dialyzed), 5 mM lactate, 0.5 mM pyruvate, 10 μU/ml insulin (basal/fasting concentration), 0.05 mM l-carnitine, 0.13 mM glycerol, and a cocktail of all 20 amino acids at physiologic concentrations (0.1 mM alanine, 0.6 mM arginine, 0.1 mM asparagine, 0.1 mM aspartic acid, 0.1 mM cystine, 0.1 mM glutamic acid, 2.0 mM glutamine, 0.1 mM glycine, 0.2 mM histidine, 0.4 mM isoleucine, 0.4 mM leucine, 0.4 mM lysine, 0.1 mM methionine, 0.2 mM phenylalanine, 0.1 mM proline, 0.1 mM serine, 0.4 mM threonine, 0.5 mM tryptophan, 0.2 mM tyrosine, and 0.4 mM valine). Metabolic fluxes were assessed through the use of distinct radiolabeled tracers: 1) [9,10-3H]oleate (0.067 mCi/l; β-oxidation); and 2) [U-14C]leucine (0.08 mCi/l; leucine oxidation and net protein synthesis). Measures of cardiac metabolism (e.g., oxygen consumption) and function (e.g., cardiac power) were determined as described previously (7, 14, 55, 56). At the end of the perfusion period, hearts were snap-frozen in liquid nitrogen and stored at −80°C before analysis. It is important to note that previous studies have shown that similar perfusion conditions provide alternative substrates, such that fatty acid oxidation rates are attenuated (50, 52).
Statistical analysis.
Statistical analyses were performed using student t-tests and two-way ANOVA, as described previously (7, 8). Briefly, Excel 2010 was used to perform Student's t-tests (only when 2 experimental groups were investigated). Stata version IC10.0 (Stata, San Antonio, TX) was used to perform two-way ANOVA to investigate main effects of time, genotype, and/or diet, followed by Bonferroni post hoc analyses for pair-wise comparisons (indicated in figures). In all analyses, the null hypothesis of no model effects was rejected at P < 0.05.
RESULTS
Decreased expression of slc5a6 in the heart following cardiomyocyte circadian clock disruption.
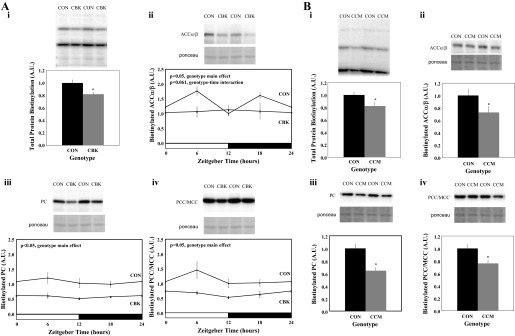
Unbiased gene expression microarray studies have been performed for hearts isolated from CBK and CCM, vs. littermate control, mice (7, 60). Review of this data for alterations in gene expression that might influence fatty acid metabolism identified slc5a6 (encoding SMVT) as being decreased in both CBK and CCM hearts. Given that SMVT transports biotin, and that biotinylation is essential for carboxylases within multiple metabolic pathways, we decided to validate these findings through RT-PCR (Fig. 1, A and B). This analysis confirmed decreased expression of slc5a6 in hearts of CBK (27% decrease; Fig. 1Aii) and CCM mice (22% decrease; Fig. 1Bii) in a time-of-day-independent manner (i.e., main effect of genotype). In contrast, no significant time main effect was observed in control hearts. Unfortunately, an antibody specific for murine SMVT is not commercially available; several antibodies specific for the human protein were tested in mouse tissues but failed to detect the protein (data not shown).
Fig. 1.
Time-of-day-dependent variations in slc5a6 gene expression in cardiomyocyte-specific BMAL1 knockout (CBK; A) and cardiomyocyte-specific CLOCK mutant (CCM; B) hearts, as well as littermate control hearts. Gene expression was assessed through microarray (i) and RT-PCR (ii) approaches. Hearts were isolated at 3-h intervals. Data are shown as mean ± SE for 6 separate observations/group.
Decreased protein biotinylation in heart following circadian clock disruption.
Since SMVT is known to transport biotin into cells, protein biotinylation was next assessed in CBK, CCM, and littermate control hearts. Consistent with decreased slc5a6 gene expression, total protein biotinylation was decreased by 18% in CBK hearts (Fig. 2Ai); based on molecular weight, biotinylation of ACCα/β, PC, and PCC/MCC was significantly decreased by 19, 46, and 42% (respectively) in CBK hearts in a time-of-day-independent manner (Fig. 2, Aii-Aiv). Given that no significant main effect of time was observed for biotinylation of the carboxylases in control hearts (Fig. 2A), we assessed protein biotinylation in CCM and control littermate hearts isolated only at zeitgeber time 6 (Fig. 2B). Total protein biotinylation was decreased by 18% in CCM hearts; biotinylation of ACCα/β, PC, and PCC/MCC was significantly decreased by 27, 35, and 24% (respectively) in CCM hearts (Fig. 2B).
Fig. 2.
Protein biotinylation in the heart following genetic disruption of the cardiomyocyte circadian clock. Hearts were isolated from CBK (Ai-Aiv) mice, as well as littermate controls (CON), at 3-h intervals. Hearts were isolated from CCM (Bi-Biv) mice, as well as littermate controls (CON), at zeitgeber time (ZT) 6. Carboxylases identified based on molecular weight were ACCα/β (ii), PC (iii), and MCC/PCC (iv). Data are shown as mean ± SE for 6 separate observations/group. *P < 0.05 for CON vs. CBK/CCM.
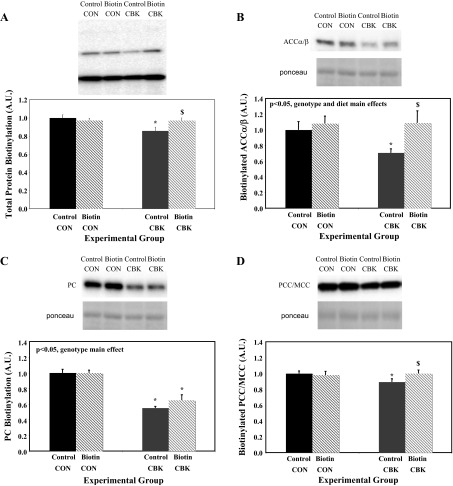
Dietary biotin supplementation increases protein biotinylation in CBK hearts.
We next investigated whether a biotin-supplemented diet could normalize protein biotinylation in the heart in a mouse model of cardiomyocyte-specific circadian clock disruption. Given that the slc5a6 mRNA and protein biotinylation were similarly affected in both CBK and CCM hearts (Figs. 1 and 2), only one model was chosen for these feeding studies (namely CBK mice). Accordingly, CBK and littermate control mice were fed either a biotin-supplemented or control diet for 6 wk. Consistent with the data in Fig. 2B, biotinylation of ACCα/β, PC, and PCC/MCC was decreased in hearts isolated from CBK mice fed a control diet (relative to littermate controls; Fig. 3). Biotin supplementation had no significant effect on protein biotinylation in littermate controls (Fig. 3). In contrast, biotin supplementation increased protein biotinylation in CBK hearts; biotinylation of ACCα/β and PCC/MCC was essentially normalized, while PC biotinylation was only partially normalized (Fig. 3).
Fig. 3.
Protein biotinylation in the heart of CBK and littermate control (CON) mice fed either a biotin supplemented or control diet. Hearts were isolated from mice at ZT6. Carboxylases identified based on molecular weight were acetyl-CoA carboxylase α (ACCα; B), pyruvate carboxylase (PC; C), and methylcrotonyl-CoA carboxylase/propionyl-CoA carboxylase (MCC/PCC; D). Data are shown as mean ± SE for 6 separate observations/group. *P < 0.05 for CON vs. CBK for the same diet. $P < 0.05 for control vs. biotin for the same genotype.
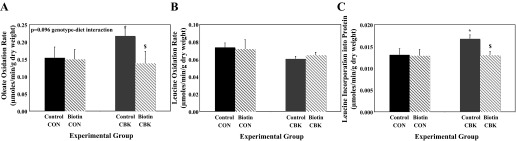
Dietary biotin supplementation partially normalized oxidative metabolism in CBK hearts.
We next determined whether 6-wk dietary biotin supplementation influenced oxidative metabolism in hearts isolated from CBK mice. Consistent with decreased biotinylation of ACCα/β (Figs. 2 and 3), previous studies have reported increased oleate oxidation in both CCM and CBK hearts (7, 60). The present study confirms increased oleate oxidation rates in hearts of CBK mice fed a control diet (relative to littermate controls), which are normalized following biotin supplementation (Fig. 4A). Consistent with decreased biotinylation of MCC (Figs. 2 and 3), rates of leucine oxidation are decreased in hearts of CBK (0.074 ± 0.005 vs. 0.060 ± 0.003 μmol·min−1·g dry wt−1 in control vs. CBK hearts, respectively; P < 0.05) and CCM (0.074 ± 0.004 vs. 0.052 ± 0.005 μmol·min−1·g dry wt−1 in control vs. CCM hearts, respectively; P < 0.05) mice fed a control diet (relative to littermate controls). Although the biotin supplemented diet tended to increase leucine oxidation rates in CBK hearts, this effect was not statistically significant (Fig. 4B). Biotin supplementation did not significantly influence either fatty acid or leucine oxidation rates in littermate control hearts (Fig. 4, A and B). It is noteworthy that oxygen consumption, cardiac power, and rate pressure product were not significantly different between the four experimental groups (Table 1).
Fig. 4.
Rates of oleate oxidation (A), leucine oxidation (B), and net protein synthesis (C) in the heart of CBK and littermate control (CON) mice fed either a biotin supplemented or control diet. Hearts were isolated from mice at ZT6. Data are shown as mean ± SE for 6 separate observations/group. *P < 0.05 for CON vs. CBK for the same diet. $P < 0.05 for control vs. biotin for the same genotype.
Table 1.
Gravimetric and ex vivo heart perfusion parameters for CBK and littermate control mice fed either a biotin supplemented or control diet
| Control Littermate (CON) |
CBK |
|||
|---|---|---|---|---|
| Parameter | Control diet | Biotin supplementation | Control diet | Biotin supplementation |
| Body weight (BW), g | 28.1 ± 0.8 | 28.4 ± 1.3 | 28.6 ± 1.5 | 30.8 ± 1.1 |
| Biventricular weight (BVW), g | 0.160 ± 0.003 | 0.176 ± 0.007 | 0.179 ± 0.007 | 0.197 ± 0.011 |
| BVW/BW ratio | 5.70 ± 0.07 | 6.20 ± 0.14 | 6.30 ± 0.23 | 6.40 ± 0.24 |
| Myocardial oxygen consumption (V̇O2), μmol·min−1·g dry wt−1 | 10.2 ± 1.1 | 8.7 ± 1.3 | 7.45 ± 0.86 | 8.6 ± 1.0 |
| Cardiac power (CP), mW | 1.18 ± 0.10 | 1.10 ± 0.19 | 1.05 ± 0.11 | 1.12 ± 0.10 |
| Cardiac efficiency (CP/MV̇O2) | 0.12 ± 0.01 | 0.13 ± 0.01 | 0.14 ± 0.01 | 0.13 ± 0.01 |
| Developed pressure (DP), mmHg | 22.3 ± 1.7 | 22.3 ± 2.1 | 25.2 ± 1.3 | 21.3 ± 2.1 |
| Heart rate (HR), beats/min | 303 ± 22 | 256 ± 19 | 244 ± 16 | 290 ± 12 |
| Rate pressure rroduct (DP × HR) | 6,677 ± 542 | 5,804 ± 906 | 6,223 ± 680 | 6,109 ± 612 |
Data are shown as mean ± SE for 6 separate observations/group.
CBK, cardiomyocyte-specific BMAL1 knockout mice.
Hearts were isolated from mice at ZT6. Biventricular weights are wet heart weights following the described ex vivo perfusion protocol.
CBK hearts exhibit increased rates of protein synthesis, which are normalized by dietary biotin supplementation.
In addition to oxidative metabolism, leucine can be incorporated into protein (i.e., protein synthesis). Accordingly, rates of [14C]leucine incorporation into protein (i.e., net protein synthesis) were determined in hearts isolated from CBK and control littermates fed either a control or biotin-supplemented diet. Net protein synthesis was significantly increased in CBK hearts relative to littermate control hearts (Fig. 4C). Biotin supplementation had no effect on net protein synthesis in control littermate hearts (Fig. 4C). In contrast, biotin supplementation normalized rates of net protein synthesis in CBK hearts (Fig. 4C).
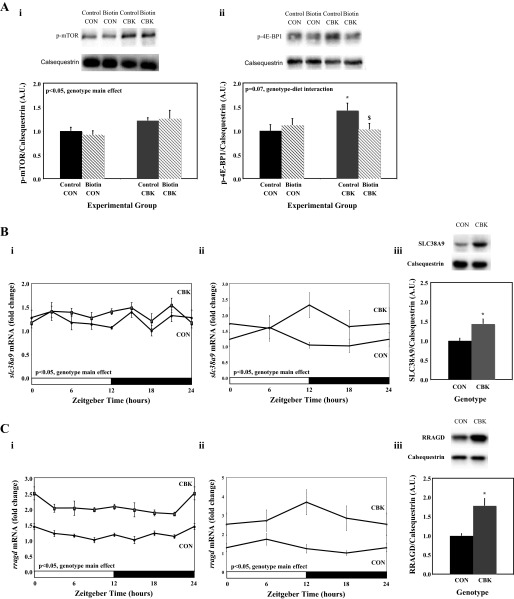
Accumulating evidence suggests that leucine promotes protein synthesis through activation of mTOR and downstream targets (e.g., 4E-BP1) (22). We therefore hypothesized that decreased leucine oxidation in CBK hearts may increase the availability of “free” leucine for mTOR activation, thus resulting in increased protein synthesis, and that partial normalization of leucine oxidation through biotin supplementation may be sufficient to attenuate protein synthesis. Consistent with this hypothesis, both p-mTOR and its downstream mediator p-4E-BP1 were increased in CBK hearts (genotype main effect; Fig. 5A). Importantly, biotin supplementation normalized p-4E-BP1 levels in CBK hearts (Fig. 5A), consistent with protein synthesis normalization (Fig. 4C). Interestingly, leucine activates mTOR in a SLC38A9/RRAGD-dependent manner, and our previous microarray study suggested increased slc38a9 and rragd gene expression in CBK heart (Fig. 5, Bi and Ci), suggesting that CBK hearts may exhibit increased sensitivity to leucine-mediated mTOR activation (27, 45, 46). RT-PCR and immunoblotting confirmed increased gene and protein expression SLC38A9 and RRAGD in CBK hearts (isolated from mice fed a standard rodent chow; Fig. 5, B and C).
Fig. 5.
Impact of biotin supplementation on phosphorylated (p)-mammalian target of rapamycin (mTOR) and p-4E binding protein 1 (4E-BP1) (Ai and Aii), as well as differential gene and protein expression of SLC38A9 (Bi-Biii) and RRADG (Ci-Ciii), in CBK vs. littermate control (CON) hearts. Hearts were isolated from mice at ZT6. Gene expression was assessed through microarray (Bi and Ci) and RT-PCR (Bii and Cii) approaches. Data are shown as mean ± SE for 6 separate observations/group. *P < 0.05 for CON vs. CBK for the same diet. $P < 0.05 for control vs. biotin for the same genotype.
Chronic activation of the RRAGD/mTOR/4E-BP1 signaling axis in CCM hearts.
To address the question whether perturbations in the SLC38A9/RRAGD/mTOR/4E-BP1 signaling axis are a consequence of cardiomyocyte circadian clock disruption, or are specific to the CBK model, we next investigated these components in CCM hearts. Examination of microarray data revealed increased expression of rragd but not slc38a9, in CCM hearts, relative to littermate controls (Fig. 6, Ai and Bi). RT-PCR and immunoblotting confirmed increased gene and protein expression of RRAGD, but not SLC38A9, in CCM hearts (Fig. 6, A and B). Similarly, both p-mTOR and p-E4-BP1 were increased in CCM hearts (Fig. 6C). Collectively, these data suggest that the RRAGD/mTOR/4E-BP1 signaling axis is chronically activated in CCM hearts.
Fig. 6.
Differential gene and protein expression of SLC38A9 (Ai-Aiii) and RRADG (Bi-Biii), as well as p-mTOR and p-4E-BP1 levels (Ci and Cii) in CCM vs. littermate control (CON) hearts. Hearts were isolated from mice at ZT6. Gene expression was assessed through microarray (Ai and Bi) and RT-PCR (Aii and Bii) approaches. Data are shown as mean ± SE for 6 separate observations/group. *P < 0.05 for CON vs. CCM.
Evidence suggesting hepatocyte clock control of slc5a6 and protein biotinylation in the liver.
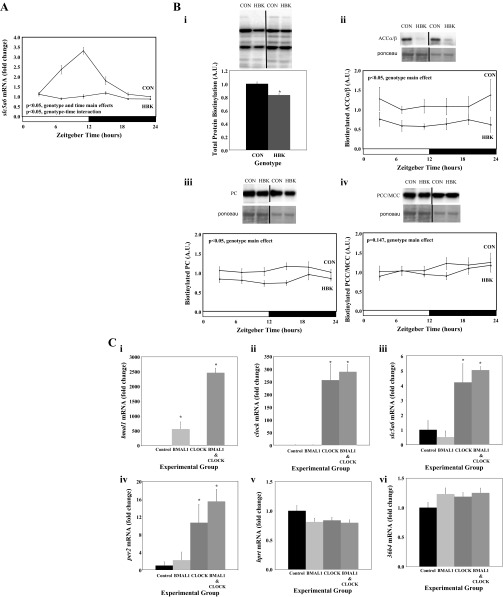
We next investigated whether circadian clock regulation of slc5a6 was unique to the heart, or more broadly applicable to metabolically active tissues. Accordingly, livers from HBK and littermate control mice were investigated. Unlike control hearts, control livers exhibit a robust time-of-day-dependent rhythm in slc5a6 expression, which is absent in HBK livers (Fig. 7A). Independent of time-of-day, slc5a6 was repressed by 44% in HBK livers (relative to littermate control livers; Fig. 7A). Consistent with decreased slc5a6 expression, total protein biotinylation was decreased by 17% in HBK livers; biotinylation of ACCα/β and PC was significantly decreased by 43 and 24%, respectively, in HBK livers in a time-of-day-independent manner, while a slight (10%), but nonsignificant (P = 0.147), decrease in biotinylated PCC/MCC was also observed in HBK livers (Fig. 7B). Similar to control hearts, control livers did not exhibit a significant time-of-day-dependent oscillation in protein biotinylation (Fig. 7B).
Fig. 7.
Time-of-day-dependent variations in slc5a6 gene expression in HBK and littermate control livers (A), protein biotinylation in the liver following genetic disruption of the hepatocyte circadian clock (Bi-Biv), and expression of slc5a6 in HepG2 cells following CLOCK and BMAL1 overexpression (Ci-Cvi). Livers were isolated from HBK and littermate control (CON) mice at 4-h intervals. Carboxylases identified based on molecular weight were ACCα/β (Bii), PC (Biii), and MCC/PCC (Biv). Data are shown as mean ± SE for 6 separate observations/group. *P < 0.05 for CON vs. other experimental groups.
Given the loss of the slc5a6 oscillation in the liver following knockout of BMAL1 (Fig. 7A), we next investigated whether BMAL1 and/or CLOCK overexpression could induce slc5a6 expression. Overexpression of CLOCK alone, or CLOCK and BMAL1 in combination, significantly increased slc5a6 mRNA levels in HepG2 cells; per2 and hprt/36b4 serve as positive and negative controls, respectively (Fig. 7C). Interestingly, transfection of cells with both CLOCK and BMAL1 led to a greater induction of BMAL1, relative to transfection with BMAL1 alone (Fig. 7Ci), consistent with the feedforward actions of the CLOCK/BMAL1 heterodimer (53). Collectively, these data suggest that the hepatocyte circadian clock regulates slc5a6 expression and protein biotinylation in the liver.
DISCUSSION
The purpose of the present study was to identify a novel mechanistic link between the cardiomyocyte circadian clock and myocardial metabolism. Genetic disruption of the cardiomyocyte circadian clock (i.e., CBK/CCM models) resulted in decreased gene expression of the biotin transporter slc5a6 and a global decrease in protein biotinylation in the heart. Rates of fatty acid oxidation were increased in CBK hearts, consistent with decreased ACCα/β biotinylation, and both parameters were normalized by feeding CBK mice a biotin supplemented diet. Similarly, decreased MCC biotinylation in CBK (and CCM) hearts was associated with decreased leucine oxidation, while biotin supplementation increased MCC biotinylation and partially restored leucine oxidation rates in CBK hearts. We also observed elevated net protein synthesis rates in CBK hearts, which were normalized by biotin supplementation, and provide evidence for clock control of the RRAGD/mTOR/4E-BP1 signaling axis in the heart. Finally, we extend upon our findings in the heart to include another metabolically active tissue and report that disruption of the hepatocyte circadian clock (i.e., HBK model) decreases slc5a6 expression and protein biotinylation in the liver. Collectively, these findings highlight biotinylation as a potential mechanistic link between cell autonomous clocks and metabolism.
Circadian clocks undoubtedly influence metabolism. This is highlighted by multiple studies reporting that germline genetic manipulation of numerous circadian clock components invariably impacts metabolic homeostasis. For example, CLOCK mutant mice exhibit altered day-night differences in behaviors such as food intake, as well as in metabolically relevant neurohumoral factors, which is associated with an obesity phenotype (57). BMAL1 knockout mice also exhibit disrupted diurnal variations in behaviors, associated with increased adiposity at a young age (49). In addition, both CLOCK mutant and BMAL1 knockout mice have been described as models of diabetes, due in part to impairments in insulin secretion (33). CRY1/2 knockout mice exhibit alterations in gluconeogenesis, while REV-ERBα knockout mice display augmented cold-induced energy expenditure (i.e., thermogenesis) (19, 61). Multiple discrete mechanisms by which clock components influence metabolic processes have been proposed. For example, Pan and Hussain (36) have reported BMAL1/CLOCK control of genes critical for lipoprotein assembly and secretion in intestinal epithelial cells as a mechanism contributing towards circadian regulation of lipid homeostasis. REV-ERBα is known to influence the expression of a number of genes encoding for enzymes in β-oxidation in numerous tissues, while evidence suggests that BMAL1/CLOCK influences β-oxidation in the liver via an NAMPT/SIRT/acetylation axis (13, 37). Given that cell autonomous circadian clocks influence ∼13% of the transcriptome, it is likely that additional mechanisms exist by which the clock governs metabolic processes.
We have recently developed multiple models of cell-type specific circadian clock disruption. Two such models, namely CCM and CBK mice, target the cardiomyocyte circadian clock. Using these models, we have previously reported clock control of ketone body, glucose, and fatty acid metabolism (7, 14, 55, 60). In the case of ketone body utilization, we reported decreased gene and protein expression, as well as enzymatic activity, of β-hydroxybutyrate dehydrogenase in CCM and CBK hearts, which was associated with decreased β-hydroxybutyrate oxidation (60). Time-of-day-dependent oscillations in dgat2 (diacylglycerol acyltransferase 2) expression and triglyceride synthesis in control hearts, are both abolished in CCM hearts (55). Similarly, diurnal variations in both oxidative and nonoxidative glucose metabolism are markedly attenuated in CCM hearts (14). Previous studies have also observed increased rates of fatty acid oxidation in both CCM and CBK hearts, independent of the time-of-day and/or feeding status (i.e., fed vs. fasted) (7, 60). This is in striking contrast to decreased rates of β-oxidation reported in livers of germline BMAL1 knockout mice (37). In an attempt to delineate the mechanism(s) responsible for increased fatty acid oxidation in CCM/CBK hearts, we recently interrogated the NAMPT/NAD+/acetylation axis, as well as the phosphorylation status of AMPK and ACC. However, despite decreased NAMPT and NAD+ levels in CCM hearts, protein acetylation was decreased (not increased) (38); similar observations have been made in CBK hearts (unpublished observations). It is also noteworthy that recent reports indicate acetylation promotes fatty acid oxidation in the heart (1, 2). In addition, ACCβ phosphorylation status is identical between CCM and control hearts, while AMPK phosphorylation and activity tends to be decreased (14, 38); again, similar observations have been made in CBK hearts (unpublished observations). Collectively, these data are not consistent with the hypothesis that the cardiomyocyte circadian clock influences myocardial β-oxidation through the NAMPT/NAD+/acetylation or AMPK/ACC axes.
In an attempt to identify mechanisms contributing towards increased fatty acid oxidation rates in CCM and CBK hearts, we turned to a recently published microarray study involving 120 ventricular samples (isolated at different times of day from CCM, CBK, and littermate control mice) (60). Initial examination of this data set did not readily identify genes encoding for proteins directly involved in fatty acid uptake or the β-oxidation pathway. Interestingly, the gene (slc5a6) encoding for the biotin transporter (SMVT) was decreased in both CCM and CBK hearts. Mammalian carboxylases have an obligate requirement for biotin, due to an active involvement of this vitamin in the catalytic addition of CO2 to a substrate (termed a “ping-pong” reaction) (31, 54). Importantly, biotin deficiency results in decreased biotinylation of carboxylases, which in turn decreases their enzymatic activities; this is rapidly reversible by biotin supplementation (10, 16). The mammalian heart possesses five main carboxylases, involved in fatty acid metabolism (ACCα/β), leucine catabolism (MCC), and anaplerosis (PC and PCC) (20). Consistent with decreased slc5a6 expression (Fig. 1, A and B), biotinylation of all carboxylases was decreased in both CBK and CCM hearts (relative to littermate controls; Fig. 2, A and B). Interestingly, protein biotinylation did not exhibit an apparent time-of-day-dependent oscillation in control hearts. This may be due to the relatively slow turnover rates of this posttranslational modification and/or maintenance of a constant rate of biotin uptake over the course of the day. It is noteworthy that previous studies in both rat and mouse hearts indicate that unlike glucose utilization, fatty acid oxidation rates do not exhibit a diurnal variation (7, 55, 59). Importantly, decreased ACCβ biotinylation in CCM and CBK hearts is consistent with increased rates of fatty acid oxidation, while decreased MCC biotinylation is consistent with decreased leucine oxidation (Figs. 3 and 4). Furthermore, biotin supplementation increased protein biotinylation in CBK hearts, normalized rates of fatty acid oxidation, and partially restored leucine oxidation rates (Figs. 3 and 4). To determine whether circadian clock regulation of biotinylation was a heart-specific phenomenon or a more general mechanism employed by multiple metabolically active tissues, we also investigated livers from HBK mice. Interestingly, slc5a6 gene expression exhibits a marked diurnal variation in control livers, which is completely abolished in HBK livers (Fig. 7A). Consistent with lower slc5a6 expression, HBK livers exhibit decreased biotinylation of carboxylases (relative to control livers), in a time-of-day-independent manner (Fig. 7B). Given critical roles of ACCα, ACCβ, PC, MCC, and PCC in fatty acid synthesis, fatty acid oxidation, gluconeogenesis, leucine catabolism, and analpleorsis, respectively, clock control of biotinylation in the liver has the potential of profoundly influencing metabolic homeostasis. Collectively, these observations are consistent with the hypothesis that cell autonomous circadian clocks influence metabolism through protein biotinylation, in a time-of-day-independent manner.
Both CCM and CBK hearts exhibit prohypertrophic phenotypes, which decompensates in CBK (but not CCM) mice with age (i.e., age onset cardiomyopathy) (15, 60). The mechanism(s) responsible for this phenotype is(are) currently unknown. The present study also investigated rates of leucine incorporation into protein, as an indicator of net protein synthesis. Consistent with a prohypertrophic phenotype, CBK hearts exhibit increased rates of net protein synthesis (Fig. 4C). Somewhat surprisingly, biotin supplementation normalized rates of protein synthesis in CBK hearts (Fig. 4C). Leucine is known to promote protein synthesis through activation of mTOR (27, 45, 46). Given that leucine oxidation is decreased in CBK (and CCM) hearts, we hypothesized that an increase in leucine (and its intermediates) within the cardiomyocyte would lead to activation of mTOR, thereby promoting protein synthesis. Accordingly, partial restoration of leucine oxidation following biotin supplementation may be sufficient to reverse mTOR activation. Consistent with this hypothesis, phosphorylation of mTOR, and its downstream target 4E-BP1, is increased in CBK hearts; biotin supplementation decreased p-4E-BP1 (but not p-mTOR) levels (Fig. 5A). Previous studies have reported that leucine-mediated activation of mTOR and its downstream targets is dependent on the lysosomal amino acid transporter SLC38A9 and a RAS-related GTP binding protein (RRAGD) (45, 46). Both SLC38A9 and RRAGD exhibit increased expression in CBK hearts (Fig. 5, B and C), suggesting increased sensitivity to leucine-induced mTOR activation. Similarly, RRAGD, p-mTOR, and p-4E-BP1 (but not SLC38A9) are elevated in CCM hearts (Fig. 6). We speculate that the combination of decreased leucine oxidation concomitant with increased leucine-sensitivity (due to increased RRAGD expression) contributes to the prohypertrophic phenotype observed in the heart following genetic disruption of the cardiomyocyte circadian clock (through chronic activation of mTOR). Interestingly, recent studies have reported chronic activation of mTOR signaling in livers of germline BMAL1 knockout mice and that the decreased lifespan observed in these mice is attenuated following treatment with Rapatar (mTOR inhibitor) (26).
It is important to note that the current study has a number of limitations/shortcomings. Due to similarities in molecular weight, the strategies employed for assessment of protein biotinylation cannot differentiate between ACCα and ACCβ, nor between MCC and PCC. Likewise, the possibility exists that proteins other than carboxylases are subject to biotinylation in the heart; if of similar molecular weight to the carboxylases, these proteins could theoretically contribute towards the three primary signals classically described for streptavidin blots. Future immunoprecipitation studies are therefore required to address these concerns. Although biotin supplementation (i.e., rescue) studies were performed in CBK mice, a similar approach was not taken for CCM and HBK mice; whether biotin supplementation normalizes distinct metabolic signatures in HBK mice is of particular interest. Due to constraints associated with radiolabeled tracer studies, the metabolic fates of only two substrates were assessed in the biotin supplementation studies; the possibility remains that biotin supplementation may also influence the glucose utilization perturbations previously reported in CBK hearts (60). Similarly, PC and PCC play critical roles in anaplerosis, a pivotal process in myocardial oxidative metabolism (12, 20); future studies are required to assess whether the cardiomyocyte circadian clock influences anaplerosis in the heart through biotinylation of these carboxylases.
In summary, the present study reports that cell autonomous circadian clocks influence protein biotinylation. In the case of the heart, alterations in biotinylation are associated with predicted perturbations in fatty acid and leucine oxidation. Furthermore, we report that biotinylation appears to influence myocardial protein synthesis. Whether biotinylation-dependent alterations in metabolism and/or protein synthesis contribute towards increased susceptibility to cardiometabolic disease following circadian disruption (e.g., shift work) requires further investigation.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-106199, HL-074259, HL-123574, and HL-122975.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
L.H., J.A.H., A.R., D.S., R.A.P.-G., G.R.M., S.M.B., C.-W.C., G.C.R., J.C.C., and M.E.Y. performed experiments; L.H., J.A.H., A.R., D.S., R.A.P.-G., G.R.M., S.M.B., C.-W.C., G.C.R., J.C.C., and M.E.Y. edited and revised manuscript; L.H., J.A.H., A.R., D.S., R.A.P.-G., G.R.M., S.M.B., C.-W.C., G.C.R., J.C.C., and M.E.Y. approved final version of manuscript; A.R., D.S., G.R.M., G.C.R., and M.E.Y. analyzed data; M.E.Y. conception and design of research; M.E.Y. interpreted results of experiments; M.E.Y. prepared figures; M.E.Y. drafted manuscript.
ACKNOWLEDGMENTS
We thank Maximiliano H. Grenett for technical assistance.
REFERENCES
- 1.Abo Alrob O, Lopaschuk GD. Role of CoA and acetyl-CoA in regulating cardiac fatty acid and glucose oxidation. Biochem Soc Trans 42: 1043–1051, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Alrob OA, Sankaralingam S, Ma C, Wagg CS, Fillmore N, Jaswal JS, Sack MN, Lehner R, Gupta MP, Michelakis ED, Padwal RS, Johnstone DE, Sharma AM, Lopaschuk GD. Obesity-induced lysine acetylation increases cardiac fatty acid oxidation and impairs insulin signalling. Cardiovasc Res 103: 485–497, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 134: 317–328, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Asher G, Reinke H, Altmeyer M, Gutierrez-Arcelus M, Hottiger MO, Schibler U. Poly(ADP-ribose) polymerase 1 participates in the phase entrainment of circadian clocks to feeding. Cell 142: 943–953, 2010. [DOI] [PubMed] [Google Scholar]
- 5.Bailey SM, Udoh US, Young ME. Circadian regulation of metabolism. J Endocrinol 222: R75–96, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science 330: 1349–1354, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bray M, Shaw C, Moore M, Garcia R, Zanquetta M, Durgan D, Jeong W, Tsai J, Bugger H, Zhang D, Rohrwasser A, Rennison J, Dyck J, Litwin S, Hardin P, Chow C, Chandler M, Abel E, Young M. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function; metabolism; and gene expression. Am J Physiol Heart Circ Physiol 294: H1036–H1047, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Bray MS, Ratcliffe WF, Grenett MH, Brewer RA, Gamble KL, Young ME. Quantitative analysis of light-phase restricted feeding reveals metabolic dyssynchrony in mice. Int J Obes (Lond) 37: 843–852, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardone L, Hirayama J, Giordano F, Tamaru T, Palvimo JJ. Circadian clock control by SUMOylation of BMAL1. Science 309: 1390–1394, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Chiang GS, Mistry SP Sassone-Corsi P. Activities of pyruvate carboxylase and propionyl CoA carboxylase in rat tissues during biotin deficiency and restoration of the activities after biotin administration. Proc Soc Exp Biol Med 146: 21–24, 1974. [DOI] [PubMed] [Google Scholar]
- 11.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987. [DOI] [PubMed] [Google Scholar]
- 12.Des Rosiers C, Labarthe F, Lloyd SG, Chatham JC. Cardiac anaplerosis in health and disease: food for thought. Cardiovasc Res 90: 210–219, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duez H, Staels B. Rev-erb-alpha: an integrator of circadian rhythms and metabolism. J Appl Physiol 107: 1972–1980, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durgan DJ, Pat BM, Laczy B, Bradley JA, Tsai JY, Grenett MH, Ratcliffe WF, Brewer RA, Nagendran J, Villegas-Montoya C, Zou C, Zou L, Johnson RL Jr, Dyck JR, Bray MS, Gamble KL, Chatham JC, Young ME. O-GlcNAcylation, novel post-translational modification linking myocardial metabolism and cardiomyocyte circadian clock. J Biol Chem 286: 44606–44619, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durgan DJ, Tsai JY, Grenett MH, Pat BM, Ratcliffe WF, Villegas-Montoya C, Garvey ME, Nagendran J, Dyck JR, Bray MS, Gamble KL, Gimble JM, Young ME. Evidence suggesting that the cardiomyocyte circadian clock modulates responsiveness of the heart to hypertrophic stimuli in mice. Chronobiol Int 28: 187–203, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eng WK, Giraud D, Schlegel VL, Wang D, Lee BH, Zempleni J. Identification and assessment of markers of biotin status in healthy adults. Br J Nutr 110: 321–329, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gamble KL, Young ME. Metabolism as an integral cog in the mammalian circadian clockwork. Crit Rev Biochem Mol Biol 48: 317–331, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gekakis N, Staknis D, Nguyen H, Davis F, Wilsbacher L, King D, Takahashi J, Weitz C. Role of the CLOCK protein in the mammalian circadian mechanism. Science 280: 1564–1569, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Gerhart-Hines Z, Feng D, Emmett MJ, Everett LJ, Loro E, Briggs ER, Bugge A, Hou C, Ferrara C, Seale P, Pryma DA, Khurana TS, Lazar MA. The nuclear receptor Rev-erbalpha controls circadian thermogenic plasticity. Nature 503: 410–413, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibala MJ, Young ME, Taegtmeyer H. Anaplerosis of the citric acid cycle: role in energy metabolism of heart and skeletal muscle. Acta Physiol Scand 168: 657–665, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Gibson UE, Heid CA, Williams PM. A novel method for real time quantitative RT-PCR. Genome Res 6: 995–1001, 1996. [DOI] [PubMed] [Google Scholar]
- 22.Hara K, Yonezawa K, Weng QP, Kozlowski MT, Belham C, Avruch J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem 273: 14484–14494, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Hardin PE, Yu W. Circadian transcription: passing the HAT to CLOCK. Cell 125: 424–426, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res 6: 986–994, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Hogenesch J, Gu Y, Jain S, Bradfield C. The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc Natl Acad Sci USA 95: 5474–5479, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khapre RV, Kondratova AA, Patel S, Dubrovsky Y, Wrobel M, Antoch MP, Kondratov RV. BMAL1-dependent regulation of the mTOR signaling pathway delays aging. Aging 6: 48–57, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol 10: 935–945, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kloss B, Price J, Saez L, Blau J, Rothenfluh A, Wesley C, Young M. The Drosophila clock gene double-time encodes a protein closely related to human casein kinase Iepsilon. Cell 94: 97–107, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Knutsson A, Kempe A. Shift work and diabetes–a systematic review. Chronobiol Int 31: 1146–1151, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Kume K, Zylka M, Sriram S, Shearman L, Weaver D, Jin X, Maywood E, Hastings M, Reppert S. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell 98: 193–205, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Lane MD. The biotin connection: Severo Ochoa, Harland Wood, and Feodor Lynen. J Biol Chem 279: 39187–39194, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Lazo de la Vega-Monroy ML, Larrieta E, German MS, Baez-Saldana A, Fernandez-Mejia C. Effects of biotin supplementation in the diet on insulin secretion, islet gene expression, glucose homeostasis and beta-cell proportion. J Nutr Biochem 24: 169–177, 2013. [DOI] [PubMed] [Google Scholar]
- 33.Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, Lopez JP, Philipson LH, Bradfield CA, Crosby SD, JeBailey L, Wang X, Takahashi JS, Bass J. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 466: 627–631, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGarry J. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 51: 7–18, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Millar AJ. Clock proteins: turned over after hours? Curr Biol 10: R529–531, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Pan X, Hussain MM. Clock is important for food and circadian regulation of macronutrient absorption in mice. J Lipid Res 50: 1800–1813, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peek CB, Affinati AH, Ramsey KM, Kuo HY, Yu W, Sena LA, Ilkayeva O, Marcheva B, Kobayashi Y, Omura C, Levine DC, Bacsik DJ, Gius D, Newgard CB, Goetzman E, Chandel NS, Denu JM, Mrksich M, Bass J. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science 342: 1243417, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peliciari-Garcia RA, Goel M, Aristorenas JA, Shah K, He L, Yang Q, Shalev A, Bailey SM, Prabhu SD, Chatham JC, Gamble KL, Young ME. Altered myocardial metabolic adaptation to increased fatty acid availability in cardiomyocyte-specific CLOCK mutant mice. Biochim Biophys Acta pii: S1388-1981(15)00234-6, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Podobed P, Pyle WG, Ackloo S, Alibhai FJ, Tsimakouridze EV, Ratcliffe WF, Mackay A, Simpson J, Wright DC, Kirby GM, Young ME, Martino TA. The day/night proteome in the murine heart. Am J Physiol Regul Integr Comp Physiol 307: R121–R137, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110: 251–260, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Quick M, Shi L. The sodium/multivitamin transporter: a multipotent system with therapeutic implications. Vitam Horm 98: 63–100, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reddy AB, Karp NA, Maywood ES, Sage EA, Deery M, O'Neill JS, Wong GK, Chesham J, Odell M, Lilley KS, Kyriacou CP, Hastings MH. Circadian orchestration of the hepatic proteome. Curr Biol 16: 1107–1115, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Ruderman N, Prentki M. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov 3: 340–351, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Sadacca LA, Lamia KA, deLemos AS, Blum B, Weitz CJ. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia 54: 120–124, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141: 290–303, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320: 1496–1501, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scott EM, Carter AM, Grant PJ. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int J Obes (Lond) 32: 658–662, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Shearman L, Sriram S, Weaver D, Maywood E, Chaves I, Zheng B, Kume K, Lee C, van d Horst GT, Hastings M, Reppert S. Interacting molecular loops in the mammalian circadian clock. Science 288: 1013–1019, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Shi SQ, Ansari TS, McGuinness OP, Wasserman DH, Johnson CH. Circadian disruption leads to insulin resistance and obesity. Curr Biol 23: 372–381, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spitzer JJ. Effect of lactate infusion on canine myocardial free fatty acid metabolism in vivo. Am J Physiol 226: 213–217, 1974. [DOI] [PubMed] [Google Scholar]
- 51.Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature 417: 78–83, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Stowe KA, Burgess SC, Merritt M, Sherry AD, Malloy CR. Storage and oxidation of long-chain fatty acids in the C57/BL6 mouse heart as measured by NMR spectroscopy. FEBS Lett 580: 4282–4287, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet 9: 764–775, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tong L. Structure and function of biotin-dependent carboxylases. Cell Mol Life Sci 70: 863–891, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsai JY, Kienesberger PC, Pulinilkunnil T, Sailors MH, Durgan DJ, Villegas-Montoya C, Jahoor A, Gonzalez R, Garvey ME, Boland B, Blasier Z, McElfresh TA, Nannegari V, Chow CW, Heird WC, Chandler MP, Dyck JR, Bray MS, Young ME. Direct regulation of myocardial triglyceride metabolism by the cardiomyocyte circadian clock. J Biol Chem 285: 2918–2929, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsai JY, Villegas-Montoya C, Boland BB, Blasier Z, Egbejimi O, Gonzalez R, Kueht M, McElfresh TA, Brewer RA, Chandler MP, Bray MS, Young ME. Influence of dark phase restricted high fat feeding on myocardial adaptation in mice. J Mol Cell Cardiol 55: 147–155, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turek F, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen D, Eckel R, Takahashi J, Bass J. Obesity and metabolic syndrome in Clock mutant mice. Science 308: 1043–1045, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woon PY, Kaisaki PJ, Braganca J, Bihoreau MT, Levy JC, Farrall M, Gauguier D. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc Natl Acad Sci USA 104: 14412–14417, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Young M, Razeghi P, Cedars A, Guthrie P, Taegtmeyer H. Intrinsic diurnal variations in cardiac metabolism and contractile function. Circ Res 89: 1199–1208, 2001. [DOI] [PubMed] [Google Scholar]
- 60.Young ME, Brewer RA, Peliciari-Garcia RA, Collins HE, He L, Birky TL, Peden BW, Thompson EG, Ammons BJ, Bray MS, Chatham JC, Wende AR, Yang Q, Chow CW, Martino TA, Gamble KL. Cardiomyocyte-specific BMAL1 plays critical roles in metabolism, signaling, and maintenance of contractile function of the heart. J Biol Rhythms 29: 257–276, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang EE, Liu Y, Dentin R, Pongsawakul PY, Liu AC, Hirota T, Nusinow DA, Sun X, Landais S, Kodama Y, Brenner DA, Montminy M, Kay SA. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med 16: 1152–1156, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]