Abstract
Pancreatic ductal adenocarcinomas (PDAC) are aggressive with frequent lymphatic spread. By analysis of data from The Cancer Genome Atlas, we determined that ∼35% of PDACs have a pro-angiogenic gene signature. We now show that the same PDACs exhibit increased expression of lymphangiogenic genes and lymphatic endothelial cell (LEC) markers, and that LEC abundance in human PDACs correlates with endothelial cell microvessel density. Lymphangiogenic genes and LECs are also elevated in murine PDACs arising in the KRC (mutated Kras; deleted RB) and KIC (mutated Kras; deleted INK4a) genetic models. Moreover, pancreatic cancer cells (PCCs) derived from KRC tumors express and secrete high levels of lymphangiogenic factors, including the EGF receptor ligand, amphiregulin. Importantly, TGF-β1 increases lymphangiogenic genes and amphiregulin expression in KRC PCCs but not in murine PCCs that lack SMAD4, and combinatorial targeting of the TGF-β type I receptor (TβRI) with LY2157299 and EGFR/HER2 with lapatanib suppresses tumor growth and metastasis in a syngeneic orthotopic model, and attenuates tumor lymphangiogenesis and angiogenesis while reducing lymphangiogenic genes and amphiregulin and enhancing apoptosis. Therefore, this combination could be beneficial in PDACs with lymphangiogenic or angiogenic gene signatures.
Keywords: pancreatic cancer, lymphangiogenesis, TCGA, mouse model, TGF-β
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer-related death in the United States, with a 5-year survival rate of 7% [1]. PDAC is associated with frequent of major driver mutations (KRAS, TP53, CDKN2A and SMAD4), numerous low frequency driver mutations, marked chemoresistance, and extensive desmoplasia [2-4]. Moreover, PDAC is often diagnosed at advanced stages that are locally invasive and/or widely metastatic which precludes the possibility for potentially curative resection. In cases that are resectable, lymph node involvement is common, and cancer cell invasion or spread into peripancreatic or distant lymph nodes is associated with increased risk of disease recurrence and a poor prognosis [5, 6].
PDACs overexpress vascular endothelial growth factors (VEGFs) including VEGF-C and VEGF-D, and several [7-10] but not all studies [11] have correlated high VEGF-C and VEGF-D levels with increased intratumoral lymphatic vessel density, and a propensity for lymph node invasion and metastasis. Both growth factors exert effects through VEGF-receptor 2 (VEGF-R2) or VEGF-receptor 3 (VEGF-R3), and VEGF-R2 is overexpressed in PDAC, is present in both cancer cells and stromal cells, and is associated with tumor aggressiveness and decreased patient survival [8, 12-14]. By contrast, VEGF-R3 expression is restricted to stromal and tumor lymphatic endothelial cells (LECs), and does not correlate with shorter survival times [8, 15]. Nonetheless, studies using subcutaneous or orthotopic models of PDAC have suggested that targeting VEGF-R2 with vatalanib, VEGF-R2 and VEGF-R3 with lenvatinib, or all three VEGF receptors with axitinib suppresses tumor growth and lymphatic metastasis [16-18]. Moreover, suppression of VEGF-C or VEGF-D expression in vivo impedes tumor growth and cancer cell spread to lymph nodes [19, 20].
VEGF receptor signaling is also pro-angiogenic [21] but in contrast to findings in preclinical models, the benefit of targeting the lymphangiogenic or angiogenic effects of these pathways in PDAC patients is ambiguous. For example, inhibiting VEGF receptor kinase activity with axitinib, or the angiogenic actions of VEGF-A with bevacizumab or VEGF Trap does not prolong patient survival [22-24], yet in a recent Phase II study, vatalanib slightly extended the survival of patients with metastatic PDAC [25]. Although the reasons for the overall failure of these therapies are not completely understood, they could conceivably be due to the heterogeneous nature of PDAC [3, 26] and/or the dense stroma that impedes intratumoral drug delivery as suggested by studies using certain genetically engineered mouse models (GEMMs) of PDAC [27-29]. Moreover, PDACs overexpress additional lymphangiogenic and angiogenic factors [21, 30] that may interfere with the effectiveness of single pathway-targeted therapies.
Based on an analysis of PDAC data from The Cancer Genome Atlas (TCGA), we previously reported that ∼35% of patients have a strong angiogenic signature [31]. We now show that these same PDACs as well as murine PDACs from the KRC and KIC GEMMs in which mutated Kras is expressed in the pancreas together with deleted RB or INK4a, respectively, due to Pdx1-driven Cre recombination are enriched in lymphangiogenic genes and LECs. Lymphangiogenic genes are also up-regulated in pancreatic cancer cells (PCCs) derived from KRC tumors, and these cells secrete high levels of lymphangiogenic factors. Moreover, in an orthotopic model these PCCs produce tumors that frequently metastasize and exhibit lymphangiogenesis and angiogenesis, and these events are suppressible by targeting TGF-β together with EGFR/HER2. Therefore, this combination could be useful in PDAC patients whose tumors exhibit a lymphangiogenic gene expression profile.
2. Materials and Methods
2.1 TCGA and array analyses
Analysis of TCGA PDAC data was previously described [31, 32]. DESeq was performed for differential expression analysis [33], and genes were identified based on lymphangiogenesis gene ontology (GO) terms and recent reviews on tumor lymphangiogenesis [34, 35].
Arrays from KRC and KPC tumors was performed by Miltenyi Biotec as described [32]. Briefly, whole genome microarrays were hybridized with RNA from tumors or normal pancreata. Intensities were converted to log2-scale and LOESS normalization was performed. Heatmaps reflect normalized intensity values. Unpaired t-tests with equal variance were used to test for significant differences. P-values were subjected to multiple testing (Benjamini-Hochberg) correction to reduce false discovery rate (FDR). Fold change >1.5, P<0.01 and FDR<0.05 was considered statistically significant.
2.2 Mice
KRC, KPC, KIC and KSC GEMMs were generated and maintained as described [32, 36-38]. For orthotopic, 200,000 KRC cells were injected into the pancreata of 40 (20 males; 20 females) 8 week-old syngeneic mice. After 10 days, mice were randomized into four groups (10 mice/group) and imaged using a Vevo2100 high resolution ultrasound (Visual Sonics, Inc.) to confirm that tumors had similar volumes before treatment. Volumes were calculated using 3D abdominal scans and Vevo2100 System software (v1.6.0). The mean tumor volume/group was ∼35±5.1 mm3 before treatment when there were no significant differences between the groups (one-way ANOVA; P>0.8). Mice were gavaged daily with LY2157299 [50 mg/kg], lapatinib [50 mg/kg], LY2157299 and lapatinib, or an equivalent volume of vehicle (0.5% hydroxypropylmethyl cellulose). All mice were re-imaged 10 days later to assess for changes in tumor volume. Mice were sacrificed when moribund and tissues were subjected to immunohistochemistry as described below. All studies were approved by the Institutional Animal Care and Use Committee of Indiana University.
2.3 Immunohistochemistry
Tissues were fixed (10% formalin), paraffin-embedded and 4 μm sections were prepared using a HM355S microtome (Thermo Scientific). The tissue microarray (TMA) of 54 different human PDACs was previously described [32]. After de-paraffinization and tissue rehydration, antigen retrieval was performed with antigen unmasking solution (Vector Labs). Following overnight incubation with primary antibodies, immunohistochemical detection was performed using biotinylated secondary antibodies and a NOVA RED detection kit (Vector Labs), or Alexa-Fluor conjugated secondary antibodies for immunofluorescence (Life Technologies). Antibodies: LYVE-1, CD31 (Abcam); CK19 (DSHB); p-Histone H3, Cleaved Caspase-3 (Cell Signaling Technology). For quantitation, images from 5 fields/mouse with at least 4 mice/group were acquired at 20× magnification using an Olympus BX60 microscope and QImaging ExiBlue camera. Images were analyzed using CellProfiler (v.2.1.1) and a DAB Pipeline [39]. Proliferating cells were counted using ImageProPlus software (v7.0Media Cybernetics).
2.4 Elisa
KRC cells were cultured [36], confirmed as mycoplasma-free (Mycoalert kit; Lonza) and seeded in 6-well plates. After 24h, cells were serum-starved overnight, and incubated with control media or TGF-β1 ([0.5nM], 24h). Medium was collected, centrifuged, filtered through a 0.22 μm filter, and cytokine levels were determined using a Milliplex ELISA kit (Millipore).
2.5 Quantitative PCR and Immunoblotting
Total RNA was isolated from cells using Trizol (Life Technologies) or tissues using guanidine thiocyanate [36]. cDNA was prepared with a high capacity RNA-to-cDNA kit, and quantitative PCR (qPCR) was performed for the indicated mRNAs using Taqman gene expression assays (Life Technologies). Rps6 served as the endogenous control. Immunoblotting was performed using flash-frozen human PDAC tissues or cell lysates as described [32]. Antibodies: LYVE-1, CD31 (Abcam); PARP, Caspase-3, Cleaved Caspase-3 (Cell Signaling Technology); ERK2 (Santa Cruz).
2.6 Statistical Analysis
One-way ANOVA with Tukey's post-hoc test or one-tailed Student's t-test was used to test for significant differences (Sigma Plot v.11.0; Systat Software). P<0.05 was considered statistically significant, and asterisks denote significant differences.
3. Results
3.1 Lymphangiogenesis is associated with angiogenesis in PDAC
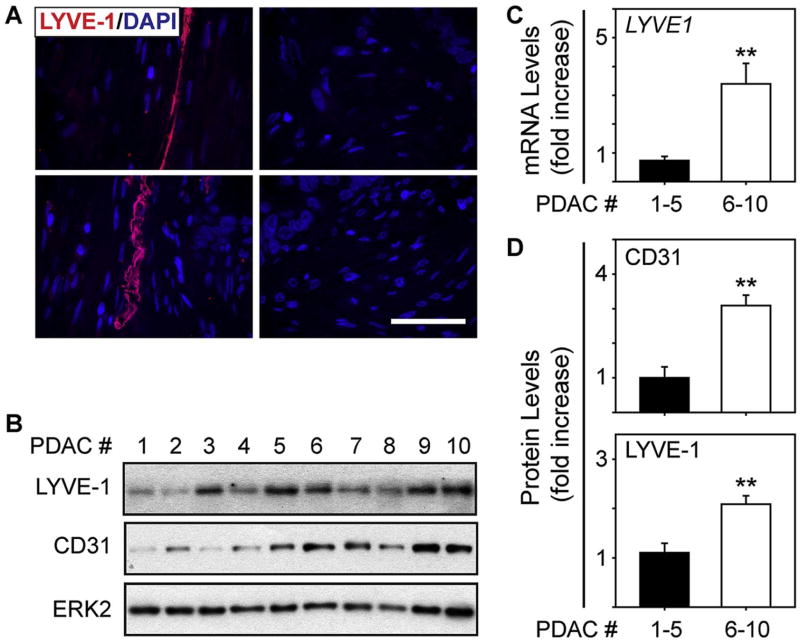
By analyzing 135 PDAC transcriptomes from TCGA, we recently determined that ∼35% of patient tumors harbor an angiogenic gene signature in which multiple pro-angiogenic genes are increased [31]. Moreover, within this gene set, the lymphangiogenic receptors VEGF-R2 (KDR) and VEGF-R3 (FLT4) are up-regulated by 3-fold and 2.5-fold, respectively [31]. We therefore sought to determine whether PDACs with an angiogenic signature exhibit increased lymphangiogenic gene expression. Accordingly, we compared the transcriptomes of angiogenic PDACs with those that lacked this signature, and assessed the levels of lymphangiogenesis-associated genes. This analysis revealed that 30 lymphangiogenesis genes were significantly up-regulated in angiogenic PDACs, including lymphangiogenic factors and the lymphatic endothelial cell (LEC) markers LYVE1, PDPN and PROX1 (Table 1). Thus, angiogenic PDACs could be lymphangiogenic and harbor more LECs than PDACs without these signatures. To explore this possibility, we used a TMA and assessed for the presence of LECs in 54 PDACs. 27 PDACs (50%) contained vessels that were positive for the LEC marker, LYVE-1 (Fig. 1A). Moreover, in these tumors, higher LYVE-1 mRNA and protein levels were associated with increased expression of the blood vessel endothelial marker, CD31 (Fig. 1B-D), pointing to a correlation between lymphatic and blood vessel endothelial cell density in PDAC.
Table 1.
Lymphangiogenic genes are up-regulated in PDACs with an angiogenic signature.
| Number | Gene Symbol | Fold Change | P-value | FDR |
|---|---|---|---|---|
| 1 | IGF1 | 12.2 | 1.92E-18 | 2.31E-16 |
| 2 | LYVE1 | 6.23 | 3.24E-15 | 2.08E-13 |
| 3 | VEGFD | 6.08 | 1.01E-05 | 8.95E-05 |
| 4 | CXCL12 | 5.96 | 1.62E-33 | 6.47E-30 |
| 5 | HGF | 5.95 | 6.49E-19 | 8.30E-17 |
| 6 | ZAP70 | 4.71 | 5.67E-06 | 5.34E-05 |
| 7 | EDNRB | 4.04 | 4.99E-24 | 1.95E-21 |
| 8 | S1PR1 | 3.82 | 3.13E-24 | 1.42E-21 |
| 9 | PDGFRA | 3.69 | 3.37E-20 | 5.81E-18 |
| 10 | CXCR4 | 3.41 | 1.93E-06 | 2.06E-05 |
| 11 | LPAR | 3.21 | 1.09E-15 | 7.90E-14 |
| 12 | CCBE1 | 2.79 | 1.44E-02 | 4.69E-02 |
| 13 | S1PR3 | 2.59 | 1.04E-12 | 4.19E-11 |
| 14 | STAB1 | 2.52 | 4.05E-13 | 1.76E-11 |
| 15 | NFATC1 | 2.46 | 1.27E-01 | 2.67E-01 |
| 16 | ELMO1 | 2.46 | 1.36E-05 | 1.17E-04 |
| 17 | FLT4 | 2.13 | 2.20E-07 | 2.91E-06 |
| 18 | EDNRA | 2.05 | 1.36E-08 | 2.38E-07 |
| 19 | VASH1 | 2.04 | 1.87E-08 | 3.19E-07 |
| 20 | NRP1 | 2.03 | 2.69E-09 | 5.43E-08 |
| 21 | PDPN | 1.96 | 4.95E-05 | 3.64E-04 |
| 22 | ACVRL1 | 1.94 | 1.72E-07 | 2.34E-06 |
| 23 | PROX1 | 1.94 | 6.54E-04 | 3.46E-03 |
| 24 | PDGFRB | 1.94 | 1.92E-08 | 3.26E-07 |
| 25 | STAB2 | 1.93 | 3.11E-01 | 5.16E-01 |
| 26 | FGF2 | 1.89 | 2.49E-03 | 1.09E-02 |
| 27 | VEGFC | 1.83 | 2.63E-04 | 1.55E-03 |
| 28 | NOTCH2 | 1.67 | 2.24E-05 | 1.81E-04 |
| 29 | S1PR2 | 1.63 | 5.30E-04 | 2.87E-03 |
| 30 | BMPR2 | 1.53 | 3.56E-04 | 2.03E-03 |
Genes are ranked by fold change (FC), P-value and false discovery rate (FDR). A FC>1.5, P<0.01 and FDR<0.05 was considered statistically significant.
Fig. 1. Correlation between lymphatic and blood vessel endothelial cells in PDAC.
(A) Immunofluorescence for LYVE-1 (red) on a TMA of human PDAC tissues shows that LYVE-1-positive lymphatic vessels are present in some PDACs (left) but absent in others (right). Shown are representative images from 4 different PDACs, and nuclei are marked with DAPI (blue). Scale bar, 50 μm. (B) Immunoblots using lysates from 10 different LYVE-1 positive PDACs show that higher LYVE-1 levels (PDACs 6-10) tend to associate with higher CD31 levels. ERK2 served as a loading control. (C) qPCR with RNA from the same tissues as in (B) shows that LYVE1 mRNA levels are significantly higher in PDACs 6-10 (open bar). (D) Quantification of the immunoblots in (B) confirms that CD31 (top) and LYVE-1 (bottom) protein levels are high in PDACs 6-10 (open bars) compared with PDACs 1-5 (closed bar). **, P<0.01.
3.2 Lymphangiogenic genes are elevated in KRC tumors
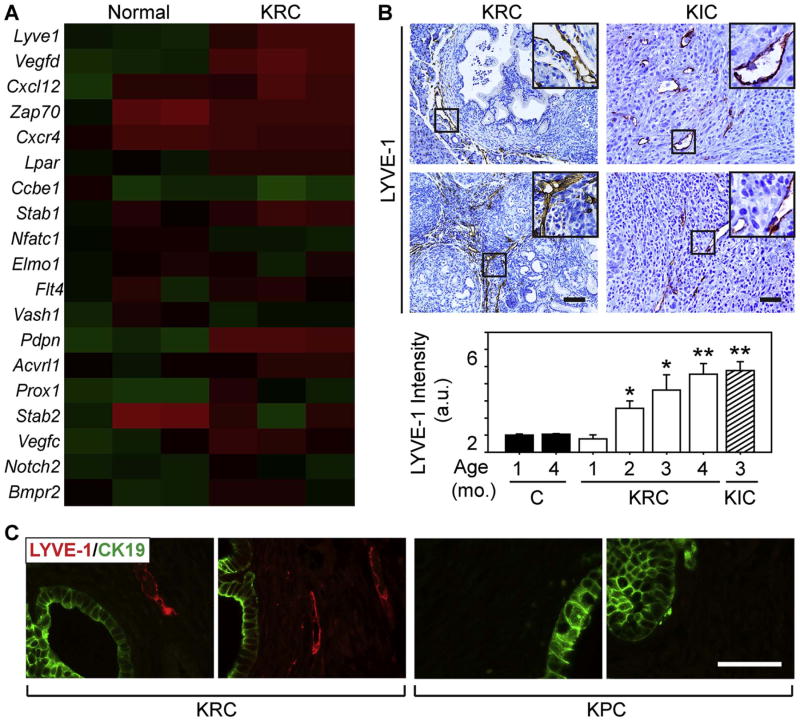
Oncogenic KRAS and inactivating CDKN2A/INK4a mutations are frequent in PDAC (95% and 90%, respectively) along with overexpression of CyclinD1 and multiple tyrosine kinase receptors and their corresponding ligands that together could facilitate RB dysfunction [40-43]. Indeed, RB inactivation is common in PDAC and in several genetically engineered mouse models (GEMMs) of PDAC [44]. In a GEMM that combines oncogenic Kras with RB deletion (KRC mice) PDACs form rapidly, and are rich in CD31-positive endothelial cells and functional blood vessels [32, 36]. Moreover, these tumors and tumors from KIC mice (mutated Kras; deleted INK4a) have pro-angiogenic transcriptomes that resemble the signature present in angiogenic PDACs [32, 37]. Thus, we sought to determine whether KRC or KIC tumors exhibit enhanced lymphangiogenic gene expression. Overall, 12/30 lymphangiogenic genes were up-regulated in KRC tumors, including the same LEC markers and lymphangiogenic genes that were increased in angiogenic human PDAC (Table 2; Fig. 2A). Quantitative PCR (qPCR) confirmed that LEC markers (Lyve1; Pdpn) and lymphangiogenic genes (Nrp1; Vegfc; Vegfd; Vegfr3) were significantly increased in KRC and KIC tumors (Supplementary Fig. 1A-B). By contrast, no lymphangiogenic genes were up-regulated in the KPC GEMM of PDAC (mutated Kras and p53) which lacks an angiogenic signature [32].
Table 2.
Lymphangiogenic genes are up-regulated in KRC tumors.
| KRC Tumors | ||||
|---|---|---|---|---|
|
| ||||
| Number | Symbol | FC | P-value | FDR |
| 1 | Pdpn | 9.83 | 7.25E-06 | 9.41E-04 |
| 2 | Vegfd | 8.91 | 9.15E-06 | 1.00E-03 |
| 3 | Igf1 | 7.40 | 8.00E-05 | 2.54E-03 |
| 4 | Pdgfra | 6.49 | 6.82E-07 | 4.38E-04 |
| 5 | Pdgfrb | 5.02 | 1.36E-05 | 1.18E-03 |
| 6 | Lyve1 | 4.65 | 2.89E-04 | 4.92E-03 |
| 7 | S1pr2 | 3.96 | 2.32E-04 | 4.36E-03 |
| 8 | Ednra | 2.67 | 3.16E-02 | 1.10E-01 |
| 9 | Vegfc | 2.55 | 4.42E-03 | 2.63E-02 |
| 10 | Prox1 | 2.35 | 2.18E-03 | 1.62E-02 |
| 11 | Ednrb | 2.12 | 5.13E-03 | 2.91E-02 |
| 12 | Nrp1 | 1.82 | 1.36E-03 | 1.20E-02 |
Genes are ranked by fold change (FC), P-value and false discovery rate (FDR), and genes with FC>1.5; P<0.01; FDR<0.05 were considered statistically significant.
Fig. 2. KRC and KIC tumors exhibit lymphangiogenesis.
(A) A heatmap of array data comparing KRC mice with normal controls shows that out of 19 lymphangiogenic genes, 12 are up-regulated in the tumors. (B) LYVE-1 immunoreactivity is abundant in KRC (left) and KIC pancreata (right). Insets show magnified images of boxed areas. Quantification (bottom) shows that the overall intensity of LYVE-1 immunoreactivity increases significantly in KRC mice (open bars) from postnatal months 2 to 4, and in KIC mice at postnatal month 3 (hatched bar), whereas there is no difference in age-matched controls (closed bars). *P<0.05; **P<0.01. (C) Double immunofluorescence for CK19 (green) and LYVE-1 (red) shows that tumors (left panels) in 4 month-old KRC mice harbor LYVE-1-positive vessels adjacent to CK19-positive cells, but these vessels are absent in age-matched KPC tumors (right panels). Shown are representative images from two different mice per GEMM. Scale bars, 50 μm.
Because LEC-specific mRNAs were elevated in KRC and KIC tumors, we next assessed these GEMMs for the presence of LECs. At postnatal month 1, KRC mice exhibited LYVE-1 immunoreactivity on sinusoidal-like vessels surrounding the stroma adjacent to pancreatic intraepithelial neoplasia (PanIN), and by 4 months these vessels were abundant and often present within the stroma adjacent to CK19-positive PanIN lesions and cancer cells (Fig. 2B-C). A similar pattern occurred in KIC mice in which intratumoral LYVE-1-positive LECs were abundant (Fig. 2B). By contrast, LECs were not detectable in the stroma of KPC tumors (Fig. 2C). Therefore, KRC and KIC tumors harbor LECs and have lymphangiogenic profiles that resemble the signature in 35% of human PDACs.
3.3 KRC cells produce lymphangiogenic factors
Pancreatic cancer cells (PCCs) derived from KRC tumors express and secrete angiogenic factors, and akin to KRC tumors and angiogenic human PDACs, they have a transcriptome that reflects constitutive TGF-β pathway activation [32]. Moreover, they secrete high levels of VEGF-C under TGF-β-stimulated conditions [32], suggesting that TGF-βs could be involved in PDAC lymphangiogenesis. Therefore, we compared KRC PCCs with PCCs from KC mice which express oncogenic Kras, but retain RB and do not exhibit TGF-β activation [44]. This analysis revealed that 6 lymphangiogenic factors (Ccbe1; Cxcl12; Edn1; Pdgfa; Pdgfb; Vegfc) [34, 35], were up-regulated in KRC PCCs (Supplementary Table 1), and qPCR confirmed that Pdgfa, Vegfc and Vegfd mRNA levels were higher in KRC PCCs compared with KC PCCs (Supplementary Fig. 2A). Some genes were also elevated in human PCCs, but only Hs766T cells which originated from a lymph node metastasis [45] exhibited up-regulation of all 6 (Supplementary Fig. 3). By contrast, PCCs from KSC mice (mutated Kras; deleted SMAD4) lack canonical TGF-β signaling [38] and had a paucity of PanIN-associated and intratumoral LECs, did not exhibit Pdgfa, Vegfc or Vegfd up-regulation (Supplementary Fig. 2A-B). Moreover, these genes were not up-regulated by TGF-β 1 in KSC cells, whereas all 3 were TGF-β 1-responsive in KRC cells (Supplementary Fig. 2C). Together, these data suggest that TGF-βs contribute to enhanced lymphangiogenic gene expression.
3.4 Targeting TGF-β and EGFR/HER2 attenuates tumor growth and metastasis
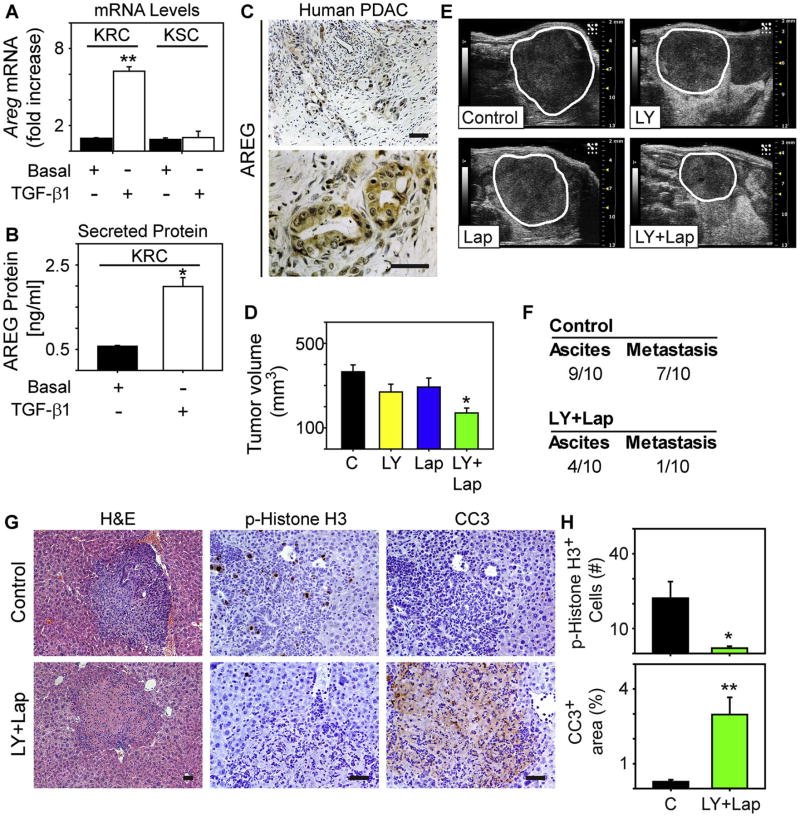
In addition to TGF-β, the EGF receptor (EGFR) is lymphangiogenic [46] and KRC PCCs expressed and secreted the EGFR ligand, amphiregulin in the basal state (Fig. 3A-B). Moreover, TGF-β 1 up-regulated amphiregulin in KRC PCCs but not in KSC PCCs, and markedly increased amphiregulin levels in KRC conditioned medium (Fig. 3A-B). By contrast, other EGFR ligands (EGF, HB-EGF and betacellulin) were not detectable in KRC medium under basal or TGF-β 1-stimulated conditions. Amphiregulin also localized to PCCs in human PDACs (Fig. 3C) which commonly exhibit active canonical TGF-β signaling [44]. Therefore, inhibiting TGF-βs could suppress lymphangiogenesis by reducing expression of amphiregulin or other lymphangiogenic factors, or by blocking direct effects of TGF-βs on LECs [47].
Fig. 3. LY2157299 and lapatinib suppress tumor growth and metastasis.
(A) qPCR shows that TGF-β1 (open bars, [0.5nM], 24 h) up-regulates amphiregulin mRNA levels (Areg) in KRC cells but not in KSC cells. (B) An ELISA for amphiregulin (AREG) shows that compared with basal levels (closed bar), TGF-β1 ([0.5 nM], open bar) significantly increases AREG in conditioned media from KRC cells (PCCs). Data in (A-B) are mean±SEM from three independent experiments. *P<0.05, **P<0.01. (C) Human PDAC tissues exhibit strong AREG immunoreactivity in the PCCs. Shown are representative low (top) and high (bottom) magnification images. (D-E) In a syngeneic orthotopic model with KRC PCCs, the combination of LY2157299 and lapatinib (LY+Lap, green bar) significantly decreases the growth of established tumors compared with mice receiving vehicle control (C, closed bar), or LY2157299 (yellow bar) or lapatinib (blue bar) alone. *P<0.05. The panels in (E) are representative ultrasound images showing that tumors (outlined) in mice receiving LY+Lap are smaller than in mice receiving vehicle (C), or LY or Lap alone. (F) A table showing the total number of control or LY+Lap mice that developed ascites or gross metastasis. (G-H) Metastatic lesions in livers from control mice (top) are highly proliferative, as evidenced by an abundance of p-Histone H3-positive cells (middle), but have a paucity of cleaved caspase-3 immunoreactivity (CC3, right). Shown are representative images from 1 of 9 mice. By contrast, in the one LY+Lap mouse with metastasis (bottom), p-Histone H3-positive cells are mostly absent, and there is strong CC3 immunoreactivity (right). Quantification (H) confirms that p-Histone H3 is decreased in LY+Lap metastatic lesions, whereas CC3 is significantly increased. *P<0.05, **P<0.01. Scale bars, 50 μm.
In order to assess the role of TGF-β in tumor lymphangiogenesis, we used KRC PCCs in a syngeneic orthotopic model to target this pathway in a metastatic model [44]. We initiated therapy after tumor formation and used LY2157299 to inhibit TGF-βs. LY2157299 is a TGF-β type I receptor (TβRI) kinase inhibitor that is currently in clinical trials for several cancers, and is tolerable and stable unlike other TβRI inhibitors that also target other TGF-β-related receptors [48]. Although tumor size was not reduced by LY2157299 or by lapatinib which is an FDA-approved dual EGFR/HER2 kinase inhibitor that could interfere with EGFR activation by amphiregulin or the lymphangiogenic effects of both receptors [46, 49-51], the combination of LY2157299 together with lapatanib markedly attenuated tumor growth (Fig. 3D-E). Moreover, LY2157299 and lapatinib suppressed ascites formation and the development of metastatic disease since only one mouse exhibited hepatic metastasis, whereas 90% of mice in the control group had metastatic lesions (P=0.018) that contained phospho-Histone H3 (p-H3)-positive cancer cells (Fig. 3F-H). By contrast, metastatic cells in the mouse receiving LY2157299 and lapatinib were rarely positive for p-H3 but exhibited strong immunoreactivity for cleaved caspase-3 (Fig. 3G-H), suggesting that TβRI and EGFR/HER2 inhibition suppresses metastatic cancer cell proliferation while enhancing their apoptosis.
3.4 LY2157299 and lapatanib suppress lymphangiogenesis and angiogenesis
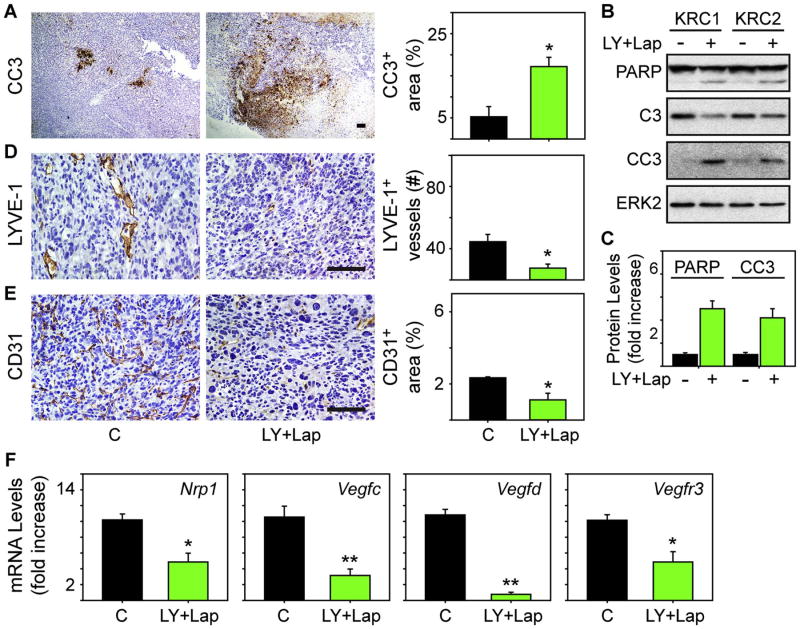
Cleaved caspase-3 immunoreactivity was also abundant in tumors from mice receiving LY2157299 and lapatinib, whereas tumors in the control group contained small areas of cleaved caspase-3 positivity (Fig. 4A). Moreover, LY2157299 together with lapatinib enhanced cleaved PARP and cleaved caspase-3 levels in KRC cells (Fig. 4B-C), suggesting that this combination enhances PCC apoptosis. To determine whether LY2157299 and lapatinib decreased intratumoral angiogenesis or lymphangiogenesis, we next assessed for differences in vessel density. LYVE-1-positive vessels were present in control tumors along with an abundance of CD31 -positive cells (Fig. 4D-E), indicating that these tumors harbor lymphatic and blood vessel endothelial cells. By contrast, tumors from LY2157299 and lapatinib-treated mice had a paucity of lymphatic vessels, and exhibited decreased CD31 immunoreactivity and Nrp1, Vegfc, Vegfd and Vegfr3 mRNA levels (Fig. 4D-F). Moreover, the combination of LY2157299 and lapatinib markedly attenuated amphiregulin mRNA levels and immunoreactivity in the PCCs (Fig. 5A-B). Analysis of serial sections revealed that lymphatic vessels and blood vessels in control tumors contained p-H3-positive endothelial cells that were mostly absent in these vessel types in LY2157299 and lapatinib-treated tumors (Fig. 6A-C). Therefore, targeting TGF-β together with EGFR/HER2 increases apoptosis while suppressing tumor angiogenesis and lymphangiogenesis, and attenuating lymphangiogenic gene expression and LEC and blood vessel endothelial cell proliferation.
Fig. 4. LY2157299 and lapatinib enhances apoptosis and decreases lymphangiogenesis and angiogenesis.
(A) Immunohistochemistry and quantification of cleaved caspase-3 (CC3) immunoreactivity shows that LY+Lap tumors have large CC3-positive areas (green bar) by comparison with control tumors (closed bar). (B-C) Immunoblots show that LY+Lap ([100 nM] each, 48h) enhances PARP cleavage, and decreases total caspase-3 (C3) while increasing cleaved caspase-3 (CC3) levels. ERK2 confirms equivalent lane loading. Shown are representative immunoblots. Quantification (C) of two experiments with two different KRC cell lines confirms that LY+Lap (green bars) significantly increases cleaved PARP and CC3 levels. (D-E) Immunohistochemistry for LYVE-1 (D) and CD31 (E) shows that tumors from control mice have an abundance of LYVE-1-positive vessels and CD31-positive endothelial cells. By contrast, tumors from mice receiving LY+Lap have weak LYVE-1 and CD31 immunoreactivity. Quantification (right panels) confirms that LY+Lap (green bars) decreases the number of LYVE-1-positive vessels and CD31-positive cells. Scale bars in (A, D-E), 100 μm. (F) qPCR reveals that tumors from LY+Lap mice (green bars) have attenuated expression of the indicated mRNAs by comparison with controls (C, closed bars). Data are mean±SEM of five tumors/group. *P<0.05, **P<0.01.
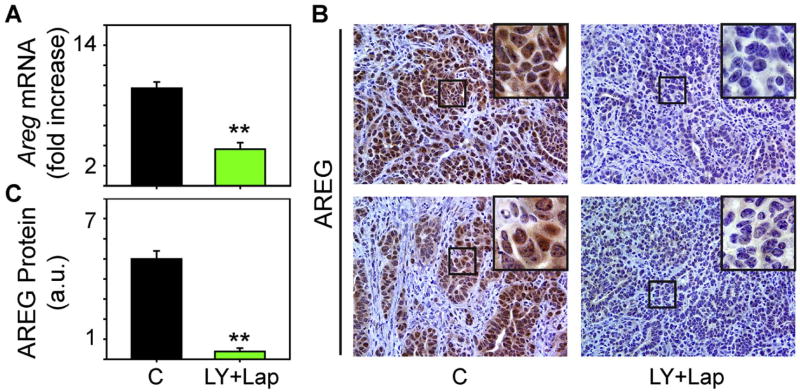
Fig. 5. LY2157299 and lapatinib suppress amphiregulin expression.
(A-B) qPCR and immunohistochemistry for amphiregulin (AREG) reveals that tumors from LY+Lap mice have markedly decreased amphiregulin mRNA levels (A) and protein expression in the PCCs (B) by comparison with control (C) tumors. Insets in (B) show magnified images of boxed areas. Scale bars, 50 μm. (C) Quantification confirms that amphiregulin immunoreactivity is markedly decreased in LY+Lap tumors. Data are mean±SEM of two mice/group. **P<0.01.
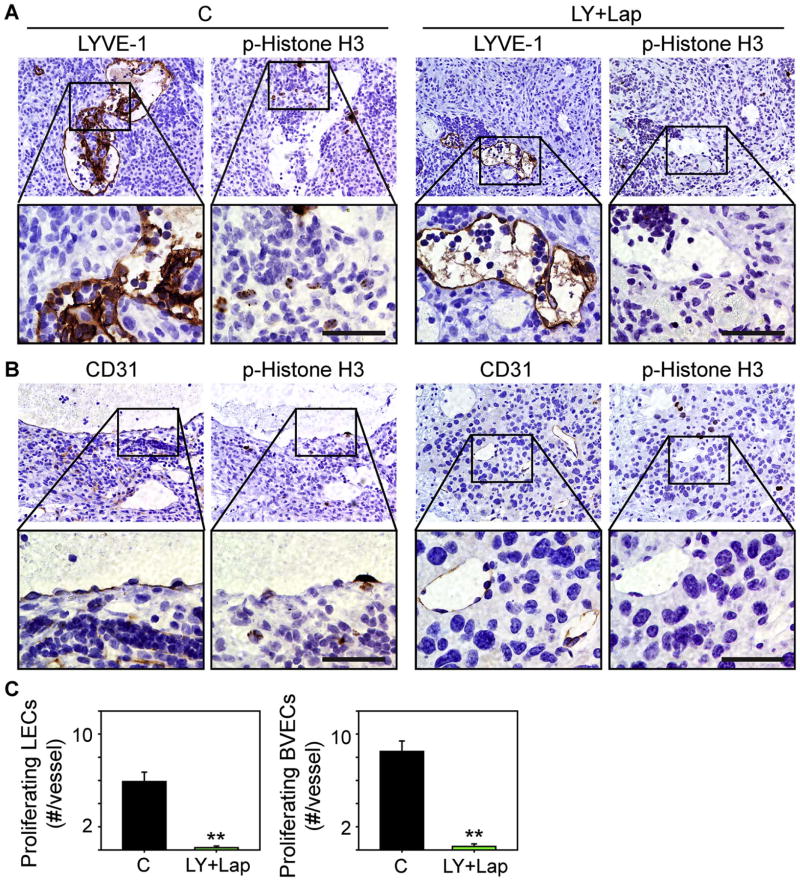
Fig. 6. LY2157299 and lapatinib suppress lymphatic and blood vessel endothelial cell proliferation.
(A-B) Immunohistochemistry for LYVE-1 and p-Histone H3 (A), or CD31 and p-Histone H3 (B) in serial sections from control tumors (C, left panels) or tumors from mice receiving LY2157299 and lapatinib (LY+Lap, right panels) shows that control tumors harbor p-Histone H3-positive endothelial cells in regions that exhibit LYVE-1 or CD31 immunoreactivity, whereas p-Histone H3 is absent in similar regions in LY+Lap tumors. The lower set of panels in (A) and (B) are high magnification images of the boxed areas. Scale bars, 50 μm. (C) Quantification confirms that the number of proliferating (p-Histone H3-positive) LECs (left) and blood vessel endothelial cells (BVECs, right) are attenuated in LY+Lap tumors. **P<0.01.
Discussion
Increased lymphangiogenesis in pancreatic tumors or their draining nodes is generally believed to relate directly to the extent of lymphatic involvement [52, 53]. However, some studies suggest that intratumoral lymphatic vessel density (LVD) is not associated with lymphatic invasion or metastasis [11, 54]. Moreover, LVD is heterogeneous and PDACs can have collapsed lymphatic vessels which may interfere with cancer cell infiltration of lymph nodes [15, 54]. Nevertheless, some patients develop lymphatic metastases in the absence of robust lymphangiogenesis [11]. Thus, LVD does not necessarily predict potential for lymphatic spread, which underscores the need for a better understanding of lymphangiogenesis and lymphatic metastasis-promoting pathways in PDAC.
In two independent but complementary studies [31, 32], we analyzed PDAC RNA-seq data from TCGA and determined that there are three groups of PDAC that differ in relation to angiogenic gene expression. Whereas some PDACs (∼35%) exhibit increased expression of many angiogenic genes, others (∼18%) do not. In the present study, we determined that the tumors enriched in angiogenic genes also exhibit increased expression of lymphangiogenic genes and all three lymphatic endothelial cell (LEC) makers, pointing to congruence between angiogenesis and lymphangiogenesis in PDAC. In support of this conclusion, we determined that there is a correlation between LEC and blood vessel endothelial cell density in PDAC tissues. Moreover, patients with lymphangiogenic tumors tended to have a higher frequency of positive lymph nodes and an overall higher lymph node ratio (Supplementary Table 2). Therefore, genes within this signature could be useful for identifying patients who are likely to develop lymphatic metastases.
Lymphangiogenic genes were also elevated in murine PDACs from the KRC and KIC GEMMs, and several were the same as those up-regulated in angiogenic human PDACs. For example, VEGF-C, VEGF-D and VEGF-R3 which stimulate lymphatic vessel growth and are often associated with lymphatic invasion and metastasis in PDAC [7-9] were high in KRC, KIC and human PDACs, along with NRP1 which interacts with VEGFs and VEGF-R3 to enhance VEGF receptor signaling and LEC motility and vessel maturation [55-57]. By contrast, none of these genes were elevated in KPC tumors. Given that KRC and KIC tumors harbor an abundance of lymphatic and blood vessels and have an angiogenic profiles [32, 37], these findings highlight similarities between the angiogenic/lymphangiogenic subgroup of PDACs and KRC and KIC mice, and the usefulness of these GEMMs for studying lymphangiogenic pathways.
To assess for factors contributing to enhanced PDAC lymphangiogenesis, we utilized KRC PCCs and determined that several lymphangiogenic factors were up-regulated, including Ccbe1 and Cxcl12. CCBE1 facilitates VEGF-C cleavage into its active form thereby stimulating LEC sprouting [58], whereas CXCL12 enhances LEC migration [34]. Importantly, both genes along with the CXCL12 receptor, CXCR4, were up-regulated in lymphangiogenic human PDACs. Moreover, KRC and KIC PCCs expressed high levels Pdgfa, Vegfc and Vegfd which enhance tumor lymphangiogenesis and lymphatic metastasis [34, 35]. Together, these findings point to the presence of multiple lymphangiogenic pathways in PDAC, which may explain why VEGF-targeted therapies were unsuccessful in patients [22-24].
Angiogenic PDACs have a pro-inflammatory and TGF-β-activated transcriptome [31], and these pathways intersect in relation to lymphangiogenesis and metastasis in several ways. First, TGF-β can directly stimulate LEC sprouting and these effects depend on intact receptor signaling [47]. Second, TGF-β enhances cancer cell epithelial-to-mesenchymal transition (EMT) and up-regulates CCR7 and CCL21 through non-canonical pathways to promote lymphatic metastasis [59]. TGF-β activates non-canonical pathways in PCCs and its ability to induce EMT depends on SMAD4 or intact canonical signaling, and angiogenic PDACs tend to have cancer cells with wild-type SMAD4 and increased expression of CCR7 [31, 38, 44, 60]. Thus, these PDACs could have enhanced lymphangiogenesis. Indeed, both EMT and a CCR7/CCL21 axis are associated with lymphatic invasion and metastasis in PDAC [61-63]. Third, TGF-β increases sphingosine-1 phosphate (S1P) production [64, 65], and in the present study we determined that three S1P receptors (S1PR1-3) were part of the lymphangiogenic signature. S1P contributes to lymphatic metastasis by activating NF-kB and STAT3, the latter of which primes draining nodes for cancer cell seeding [66, 67]. Fourth, TGF-β is immune-modulating and activates regulatory T-cells (Tregs) while polarizing neutrophils and macrophages into their respective (N2 and M2) tumor-promoting phenotypes [68]. Treg, neutrophil and M2 macrophage markers (FOXP3, ELANE and CD163) are elevated in angiogenic PDACs [31], and each of these cells are associated with increased PDAC lymphatic metastasis [69-71]. Moreover, Tregs suppress cancer-directed immunity in pancreatic cancer-associated lymph nodes [72]. Together, these observations further underscore the complexity of lymphangiogenesis in PDAC, and highlight the importance of TGF-β in this process.
KRC tumors and their PCCs also have intact and activated TGF-β signaling, and overexpress many pro-inflammatory cytokines, some of which are enhanced by TGF-β 1 [32, 44]. Here, we determined that TGF-β 1 up-regulated lymphangiogenic genes and enhanced amphiregulin expression and secretion from KRC PCCs, and that amphiregulin was expressed by the cancer cells in human PDACs which often exhibit activated TGF-β pathways [44]. By contrast, none of these genes were TGF-β-inducible in SMAD4-deficient KSC PCCs, underscoring the importance of TGF-β in lymphangiogenesis. Previous studies have suggested that inhibiting TGF-β suppresses human and murine PCC EMT and invasion, and growth and metastasis in orthotopic tumor models [44, 73], most likely due to suppression of TGF-β-dependent paracrine actions within the tumor microenvironment. In this study, however, LY2157299 monotherapy did not suppress tumor growth in a syngeneic orthotopic model using KRC PCCs. While the reasons for these differences are not entirely clear, it is conceivable that suppressing TβRI alone leads to up-regulation of deleterious signaling pathways. In support of this conclusion, combining lapatinib with LY2157299 markedly inhibited tumor growth and suppressed lymphangiogenesis and lymphangiogenic gene expression, while attenuating tumor angiogenesis, ascites and metastasis and interfering with the growth-promoting effects of amphiregulin [74]. Thus our findings underscore the need to consider the benefits of combinatorial strategies for suppressing TGF-β actions in PDAC.
In summary, our study highlights overlap between angiogenic and lymphangiogenic pathways in PDAC, and suggests that targeting pathways common to several lymphangiogenic factors is beneficial. Thus, in an immune-competent orthotopic model generated with murine PCCs from a GEMM that reflects lymphangiogenic human PDACs, targeting TGF-β pathways together with EGFR and HER2 attenuated tumor growth, and tumor angiogenesis and lymphangiogenesis while suppressing cancer cell spread. EGFR correlates with lymphatic metastasis [49], but such an association has not been reported for HER2 in PDAC. Nevertheless, HER2 is associated with increased lymphatic metastasis in gastric and esophageal cancers [50, 51], and a poor prognosis in lymph-node positive breast cancers [75]. Moreover, HER2 is overexpressed in PDAC and correlates with metastasis and decreased patient survival [41]. Therefore, this combination could be advantageous in PDAC, and be especially useful in patients whose tumors have angiogenic or lymphangiogenic gene signatures.
Supplementary Material
Highlights.
PDACs with an angiogenic gene signature are enriched in lymphangiogenic genes.
Tumors from the KRC PDAC model harbor LECs and have a lymphangiogenic profile.
Pancreatic cancer cells from KRC tumors express and secrete lymphangiogenic factors.
Targeting TβRI and EGFR/HER2 pathways together is beneficial in KRC mice.
Acknowledgments
We thank the IU Simon Cancer Center (IUSCC) at IU School of Medicine for use of the Bio-Plex Core which assisted with ELISAs, the Tissue Procurement & Distribution Core which provided the TMA, and the Chemical Genomics core which synthesized the LY2157299 for in vivo use. This work was supported, in part, by an award from the Pancreatic Cancer Signature Center to J.G., by a Diversity in Health-related Research Supplement from the National Cancer Institute (NCI) of the National Institutes of Health (NIH) to I.E.I-W., by the NCI under award number F30CA200301 to K.E.C, and by a Shared Resources grant from the IUSCC and a US Public Health Service Grant from the NCI under award number CA-75059 to M.K.
Abbreviations
- AREG
Amphiregulin
- EGFR
EGF receptor
- GEMM
Genetically engineered mouse model
- HER2
Human epidermal growth factor receptor 2
- LEC
Lymphatic endothelial cell
- LVD
Lymphatic vessel density
- PCC
Pancreatic cancer cell
- PDAC
Pancreatic ductal adenocarcinoma
- TCGA
The Cancer Genome Atlas
- TβRI
Transforming growth factor-β type I receptor
- TGF-β
Transforming growth factor-β
- TMA
Tissue microarray
- VEGF
Vascular endothelial growth factor
Footnotes
Conflicts of interest: None
Conflicts of Interest Statement: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: A Cancer Journal for Clinicians. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J, Chang DK, Cowley MJ, Gardiner BB, Song S, Harliwong I, Idrisoglu S, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491(7424):399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, Johns AL, Miller D, Nones K, Quek K, Quinn MCJ, Robertson AJ, Fadlullah MZH, Bruxner TJC, Christ AN, Harliwong I, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518(7540):495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiong HQ, Carr K, Abbruzzese JL. Cytotoxic chemotherapy for pancreatic cancer: Advances to date and future directions. Drugs. 2006;66(8):1059–1072. doi: 10.2165/00003495-200666080-00003. [DOI] [PubMed] [Google Scholar]
- 5.Delcore R, Rodriguez FJ, Forster J, Hermreck AS, Thomas JH. Significance of lymph node metastases in patients with pancreatic cancer undergoing curative resection. The American Journal of Surgery. 1996;172(5):463–469. doi: 10.1016/S0002-9610(96)00237-1. [DOI] [PubMed] [Google Scholar]
- 6.Pai RK, Beck AH, Mitchem J, Linehan DC, Chang DT, Norton JA, Pai RK. Pattern of Lymph Node Involvement and Prognosis in Pancreatic Adenocarcinoma: Direct Lymph Node Invasion Has Similar Survival to Node-Negative Disease. The American Journal of Surgical Pathology. 2011;35(2):228–234. doi: 10.1097/PAS.0b013e318206c37a. [DOI] [PubMed] [Google Scholar]
- 7.Kurahara H, Takao S, Maemura K, Shinchi H, Natsugoe S, Aikou T. Impact of Vascular Endothelial Growth Factor-C and -D Expression in Human Pancreatic Cancer: Its Relationship to Lymph Node Metastasis. Clinical Cancer Research. 2004;10(24):8413–8420. doi: 10.1158/1078-0432.CCR-04-0379. [DOI] [PubMed] [Google Scholar]
- 8.Tang RF, Itakura J, Aikawa T, Matsuda K, Fujii H, Korc M, Matsumoto Y. Overexpression of Lymphangiogenic Growth Factor VEGF-C in Human Pancreatic Cancer. Pancreas. 2001;22(3):285–292. doi: 10.1097/00006676-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Zhang B, Zhao WH, Zhou WY, Yu WS, Yu JM, Li S. Expression of vascular endothelial growth factors-C and -D correlate with evidence of lymphangiogenesis and angiogenesis in pancreatic adenocarcinoma. Cancer Detection and Prevention. 2007;31(6):436–442. doi: 10.1016/j.cdp.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Cheng P, Jin G, Hu X, Shi M, Zhang Y, Liu R, Zhou Y, Shao C, Zheng J, Zhu M. Analysis of tumor-induced lymphangiogenesis and lymphatic vessel invasion of pancreatic carcinoma in the peripheral nerve plexus. Cancer Science. 2012;103(10):1756–1763. doi: 10.1111/j.1349-7006.2012.02364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sipos B, Kojima M, Tiemann K, Klapper W, Kruse ML, Kalthoff H, Schniewind B, Tepel J, Weich H, Kerjaschki D, Klöppel G. Lymphatic spread of ductal pancreatic adenocarcinoma is independent of lymphangiogenesis. The Journal of Pathology. 2005;207(3):301–312. doi: 10.1002/path.1840. [DOI] [PubMed] [Google Scholar]
- 12.Büchler P, Reber HA, Büchler MW, Friess H, Hines OJ. VEGF-RII Influences the Prognosis of Pancreatic Cancer. Annals of Surgery. 2002;236(6):738–749. doi: 10.1097/00000658-200212000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itakura J, Ishiwata T, Shen B, Kornmann M, Korc M. Concomitant over-expression of vascular endothelial growth factor and its receptors in pancreatic cancer. International journal of cancer. 2000;85(1):27–34. doi: 10.1002/(sici)1097-0215(20000101)85:1<27::aid-ijc5>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 14.Doi Y, Yashiro M, Yamada N, Amano R, Ohira G, Komoto M, Noda S, Kashiwagi S, Kato Y, Fuyuhiro Y, Hirakawa K. Significance of phospho-vascular endothelial growth factor receptor-2 expression in pancreatic cancer. Cancer Science. 2010;101(6):1529–1535. doi: 10.1111/j.1349-7006.2010.01547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider M, Buchler P, Giese N, Giese T, Wilting J, Buchler MW, Friess H. Role of lymphangiogenesis and lymphangiogenic factors during pancreatic cancer progression and lymphatic spread. International Journal of Oncology. 2006;28(4):889–890. [PubMed] [Google Scholar]
- 16.Solorzano CC, Baker CH, Bruns CJ, Killion JJ, Ellis LM, Wood J, Fidler IJ. Inhibition of growth and metastasis of human pancreatic cancer growing in nude mice by PTK 787/ZK222584, and inhibitor of the vascular endothelial growth factor receptor tyrosine kinases. Cancer Biotherapy and Radiopharmaceuticals. 2001;16(5):359–370. doi: 10.1089/108497801753354267. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto Y, Matsui J, Matsushima T, Obaishi H, Miyazaki K, Nakamura K, Tohyama O, Semba T, Yamaguchi A, Hoshi SS, Mimura F, Haneda T, Fukuda Y, Kamata Ji, Takahashi K, Matsukura M, et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vascular Cell. 2014;6:18–18. doi: 10.1186/2045-824X-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu-Lowe DD, Zou HY, Grazzini ML, Hallin ME, Wickman GR, Amundson K, Chen JH, Rewolinski DA, Yamazaki S, Wu EY, McTigue MA, Murray BW, Kania RS, O'Connor P, Shalinsky DR, Bender SL. Nonclinical Antiangiogenesis and Antitumor Activities of Axitinib (AG-013736), an Oral, Potent, and Selective Inhibitor of Vascular Endothelial Growth Factor Receptor Tyrosine Kinases 1, 2, 3. Clinical Cancer Research. 2008;14(22):7272–7283. doi: 10.1158/1078-0432.CCR-08-0652. [DOI] [PubMed] [Google Scholar]
- 19.Shi Y, Tong M, Wu Y, Yang Z, Hoffman RM, Zhang Y, Tian Y, Qi M, Lin Y, Liu Y, Dai L, Sun Y, Wang Z. VEGF-C ShRNA Inhibits Pancreatic Cancer Growth and Lymphangiogenesis in an Orthotopic Fluorescent Nude Mouse Model. Anticancer Research. 2013;33(2):409–417. [PubMed] [Google Scholar]
- 20.Koch M, Dettori D, Van Nuffelen A, Souffreau J, Marconcini L, Wallays G, Moons L, Bruyère F, Oliviero S, Noel A, Foidart JM, Carmeliet P, Dewerchin M. VEGF-D deficiency in mice does not affect embryonic or postnatal lymphangiogenesis but reduces lymphatic metastasis. The Journal of Pathology. 2009;219(3):356–364. doi: 10.1002/path.2605. [DOI] [PubMed] [Google Scholar]
- 21.Korc M. Pathways for aberrant angiogenesis in pancreatic cancer. Molecular Cancer. 2003;2(1):8. doi: 10.1186/1476-4598-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kindler HL, Ioka T, Richel DJ, Bennouna J, Letourneau R, Okusaka T, Funakoshi A, Furuse J, Park YS, Ohkawa S, Springett GM, Wasan HS, Trask PC, Bycott P, Ricart AD, Kim S, et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. The lancet oncology. 2011;12(3):256–262. doi: 10.1016/S1470-2045(11)70004-3. [DOI] [PubMed] [Google Scholar]
- 23.Kindler HL, Friberg G, Singh DA, Locker G, Nattam S, Kozloff M, Taber DA, Karrison T, Dachman A, Stadler WM, Vokes EE. Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. Journal of Clinical Oncology. 2005;23(31):8033–8040. doi: 10.1200/JCO.2005.01.9661. [DOI] [PubMed] [Google Scholar]
- 24.Rougier P, Riess H, Manges R, Karasek P, Humblet Y, Barone C, Santoro A, Assadourian S, Hatteville L, Philip PA. Randomised, placebo-controlled, double-blind, parallel-group phase III study evaluating aflibercept in patients receiving first-line treatment with gemcitabine for metastatic pancreatic cancer. European journal of cancer. 2013;49(12):2633–2642. doi: 10.1016/j.ejca.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Dragovich T, Laheru D, Dayyani F, Bolejack V, Smith L, Seng J, Burris H, Rosen P, Hidalgo M, Ritch P, Baker AF, Raghunand N, Crowley J, Von Hoff DD. Phase II trial of vatalanib in patients with advanced or metastatic pancreatic adenocarcinoma after first-line gemcitabine therapy (PCRT O4-001) Cancer Chemother Pharmacol. 2014;74(2):379–387. doi: 10.1007/s00280-014-2499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samuel N, Hudson TJ. The molecular and cellular heterogeneity of pancreatic ductal adenocarcinoma. Nat Rev Gastroenterol Hepatol. 2012;9(2):77–87. doi: 10.1038/nrgastro.2011.215. [DOI] [PubMed] [Google Scholar]
- 27.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic Targeting of the Stroma Ablates Physical Barriers to Treatment of Pancreatic Ductal Adenocarcinoma. Cancer cell. 2012;21(3):418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, Feig C, Nakagawa T, Caldwell ME, Zecchini HI, Lolkema MP, Jiang P, Kultti A, Thompson CB, Maneval DC, Jodrell DI, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62(1):112–120. doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, DeNicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, et al. Inhibition of Hedgehog Signaling Enhances Delivery of Chemotherapy in a Mouse Model of Pancreatic Cancer. Science. 2009;324(5933):1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korc M. Role of growth factors in pancreatic cancer. Surgical oncology clinics of North America. 1998;7(1):25–41. [PubMed] [Google Scholar]
- 31.Craven KE, Gore J, Wilson JL, Korc M. Angiogenic gene signature in human pancreatic cancer correlates with TGF-beta and inflammatory transcriptomes. Oncotarget. 2015;7(1):323–341. doi: 10.18632/oncotarget.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gore J, Craven KE, Wilson JL, Cote GA, Cheng M, Nguyen HV, Cramer HM, Sherman S, Korc M. TCGA data and patient-derived orthotopic xenografts highlight pancreatic cancer-associated angiogenesis. Oncotarget. 2015;6(10):7504–7521. doi: 10.18632/oncotarget.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biology. 2010;11(10):R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer. 2014;14(3):159–172. doi: 10.1038/nrc3677. [DOI] [PubMed] [Google Scholar]
- 35.Zheng W, Aspelund A, Alitalo K. Lymphangiogenic factors, mechanisms, and applications. The Journal of Clinical Investigation. 2014;124(3):878–887. doi: 10.1172/JCI71603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carrière C, Gore AJ, Norris AM, Gunn JR, Young AL, Longnecker DS, Korc M. Deletion of Rb Accelerates Pancreatic Carcinogenesis by Oncogenic Kras and Impairs Senescence in Premalignant Lesions. Gastroenterology. 2011;141(3):1091–1101. doi: 10.1053/j.gastro.2011.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whipple CA, Young AL, Korc M. A KrasG12D-driven genetic mouse model of pancreatic cancer requires glypican-1 for efficient proliferation and angiogenesis. Oncogene. 2012;31(20):2535–2544. doi: 10.1038/onc.2011.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bardeesy N, Cheng KH, Berger JH, Chu GC, Pahler J, Olson P, Hezel AF, Horner J, Lauwers GY, Hanahan D, DePinho RA. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 2006;20(22):3130–3146. doi: 10.1101/gad.1478706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamprecht MR, Sabatini DM, Carpenter AE. CellProfiler: free, versatile software for automated biological image analysis. BioTechniques. 2007;42(1):71–75. doi: 10.2144/000112257. [DOI] [PubMed] [Google Scholar]
- 40.Maitra A, Kern SE, Hruban RH. Molecular pathogenesis of pancreatic cancer. Best Practice & Research Clinical Gastroenterology. 2006;20(2):211–226. doi: 10.1016/j.bpg.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Preis M, Korc M. Kinase signaling pathways as targets for intervention in pancreatic cancer. Cancer Biololgy and Therapy. 2010;9(10) doi: 10.4161/cbt.9.10.11534. [DOI] [PubMed] [Google Scholar]
- 42.Gansauge S, Gansauge F, Ramadani M, Stobbe H, Rau B, Harada N, Beger HG. Overexpression of Cyclin D1 in Human Pancreatic Carcinoma Is Associated with Poor Prognosis. Cancer Res. 1997;57(9):1634–1637. [PubMed] [Google Scholar]
- 43.Schutte M, Hruban RH, Geradts J, Maynard R, Hilgers W, Rabindran SK, Moskaluk CA, Hahn SA, Schwarte-Waldhoff I, Schmiegel W, Baylin SB, Kern SE, Herman JG. Abrogation of the Rb/p16 Tumor-suppressive Pathway in Virtually All Pancreatic Carcinomas. Cancer Research. 1997;57(15):3126–3130. [PubMed] [Google Scholar]
- 44.Gore AJ, Deitz SL, Palam LR, Craven KE, Korc M. Pancreatic cancer–associated retinoblastoma 1 dysfunction enables TGF-β to promote proliferation. The Journal of Clinical Investigation. 2014;124(1):338–352. doi: 10.1172/JCI71526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Owens RB, Smith HS, Nelson-Rees WA, Springer EL. Epithelial cell cultures from normal and cancerous human tissues. Journal of the National Cancer Institute. 1976;56(4):843–849. doi: 10.1093/jnci/56.4.843. [DOI] [PubMed] [Google Scholar]
- 46.Marino D, Angehrn Y, Klein S, Riccardi S, Baenziger-Tobler N, Otto VI, Pittelkow M, Detmar M. Activation of the epidermal growth factor receptor promotes lymphangiogenesis in the skin. Journal of dermatological science. 2013;71(3):184–194. doi: 10.1016/j.jdermsci.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.James JM, Nalbandian A, Mukouyama Ys. TGFβ signaling is required for sprouting lymphangiogenesis during lymphatic network development in the skin. Development (Cambridge, England) 2013;140(18):3903–3914. doi: 10.1242/dev.095026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nature reviews Drug discovery. 2012;11(10):790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pryczynicz A, Guzinska-Ustymowicz K, Kemona A, Czyzewska J. Expression of EGF and EGFR Strongly Correlates with Metastasis of Pancreatic Ductal Carcinoma. Anticancer Research. 2008;28(2B):1399–1404. [PubMed] [Google Scholar]
- 50.Bozzetti C, Negri FV, Lagrasta CA, Crafa P, Bassano C, Tamagnini I, Gardini G, Nizzoli R, Leonardi F, Gasparro D, Camisa R, Capelli S, Silini EM, Ardizzoni A. Comparison of HER2 status in primary and paired metastatic sites of gastric carcinoma. British Journal of Cancer. 2011;104(9):1372–1376. doi: 10.1038/bjc.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Konig AM, Reeh M, Dancau A, Rathjens M, Gros S, Uzunoglu FG, Bockhorn M, Simon R, Sauter G, Marx A, Izbicki JR. Concordance of HER2 Status in Primary Tumour and Lymph Node Metastases in Patients with Esophageal Carcinoma. Anticancer Research. 2013;33(11):4975–4982. [PubMed] [Google Scholar]
- 52.Fink DM, Steele MM, Hollingsworth MA. The lymphatic system and pancreatic cancer. Cancer Letters. 2015 doi: 10.1016/j.canlet.2015.11.048. ePub(Dec. 29) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kurahara H, Takao S, Shinchi H, Maemura K, Mataki Y, Sakoda M, Hayashi T, Kuwahata T, Minami K, Ueno S, Natsugoe S. Significance of lymphangiogenesis in primary tumor and draining lymph nodes during lymphatic metastasis of pancreatic head cancer. Journal of Surgical Oncology. 2010;102(7):809–815. doi: 10.1002/jso.21744. [DOI] [PubMed] [Google Scholar]
- 54.Wang Z, Wu J, Li G, Zhang X, Tong M, Wu Z, Liu Z. Lymphangiogenesis and biological behavior in pancreatic carcinoma and other pancreatic tumors. Molecular Medicine Reports. 2012;5(4):959–963. doi: 10.3892/mmr.2012.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Herbert SP, Stainier DYR. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol. 2011;12(9):551–564. doi: 10.1038/nrm3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kärpänen T, Heckman CA, Keskitalo S, Jeltsch M, Ollila H, Neufeld G, Tamagnone L, Alitalo K. Functional interaction of VEGF-C and VEGF-D with neuropilin receptors. The FASEB Journal. 2006;20(9):1462–1472. doi: 10.1096/fj.05-5646com. [DOI] [PubMed] [Google Scholar]
- 57.Deng Y, Zhang X, Simons M. Molecular controls of lymphatic VEGFR3 signaling. Arteriosclerosis, thrombosis, and vascular biology. 2015;35(2):421–429. doi: 10.1161/ATVBAHA.114.304881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jeltsch M, Jha SK, Tvorogov D, Anisimov A, Leppänen VM, Holopainen T, Kivelä R, Ortega S, Kärpanen T, Alitalo K. CCBE1 Enhances Lymphangiogenesis via A Disintegrin and Metalloprotease With Thrombospondin Motifs-3–Mediated Vascular Endothelial Growth Factor-C Activation. Circulation. 2014;129(19):1962–1971. doi: 10.1161/CIRCULATIONAHA.113.002779. [DOI] [PubMed] [Google Scholar]
- 59.Pang MF, Georgoudaki AM, Lambut L, Johansson J, Tabor V, Hagikura K, Jin Y, Jansson M, Alexander JS, Nelson CM, Jakobsson L, Betsholtz C, Sund M, Karlsson MCI, Fuxe J. TGF-[beta]1-induced EMT promotes targeted migration of breast cancer cells through the lymphatic system by the activation of CCR7/CCL21-mediated chemotaxis. Oncogene. 2016;35(6):748–760. doi: 10.1038/onc.2015.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ijichi H, Chytil A, Gorska AE, Aakre ME, Fujitani Y, Fujitani S, Wright CVE, Moses HL. Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-β signaling in cooperation with active Kras expression. Genes & Development. 2006;20(22):3147–3160. doi: 10.1101/gad.1475506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamada S, Fuchs BC, Fujii T, Shimoyama Y, Sugimoto H, Nomoto S, Takeda S, Tanabe KK, Kodera Y, Nakao A. Epithelial-to-mesenchymal transition predicts prognosis of pancreatic cancer. Surgery. 2013;154(5):946–954. doi: 10.1016/j.surg.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 62.Nakata B, Fukunaga S, Noda E, Amano R, Yamada N, Hirakawa K. Chemokine Receptor CCR7 Expression Correlates with Lymph Node Metastasis in Pancreatic Cancer. Oncology. 2008;74(1-2):69–75. doi: 10.1159/000139126. [DOI] [PubMed] [Google Scholar]
- 63.Guo J, Lou W, Ji Y, Zhang S. Effect of CCR7, CXCR4 and VEGF-C on the lymph node metastasis of human pancreatic ductal adenocarcinoma. Oncology Letters. 2013;5(5):1572–1578. doi: 10.3892/ol.2013.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gellings Lowe N, Swaney JS, Moreno KM, Sabbadini RA. Sphingosine-1-phosphate and sphingosine kinase are critical for transforming growth factor-β-stimulated collagen production by cardiac fibroblasts. Cardiovascular Research. 2009;82(2):303–312. doi: 10.1093/cvr/cvp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller AV, Alvarez SE, Spiegel S, Lebman DA. Sphingosine Kinases and Sphingosine-1-Phosphate Are Critical for Transforming Growth Factor β-Induced Extracellular Signal-Regulated Kinase 1 and 2 Activation and Promotion of Migration and Invasion of Esophageal Cancer Cells. Molecular and Cellular Biology. 2008;28(12):4142–4151. doi: 10.1128/MCB.01465-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Deng J, Liu Y, Lee H, Herrmann A, Zhang W, Zhang C, Shen S, Priceman Saul J, Kujawski M, Pal Sumanta K, Raubitschek A, Hoon Dave SB, Forman S, Figlin Robert A, Liu J, Jove R, et al. S1PR1-STAT3 Signaling Is Crucial for Myeloid Cell Colonization at Future Metastatic Sites. Cancer Cell. 2012;21(5):642–654. doi: 10.1016/j.ccr.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang WC, Nagahashi M, Terracina KP, Takabe K. Emerging Role of Sphingosine-1-phosphate in Inflammation, Cancer, and Lymphangiogenesis. Biomolecules. 2013;3(3):408–434. doi: 10.3390/biom3030408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Massagué J. TGFβ in Cancer. Cell. 2008;134(2):215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang Y, Du Z, Yang F, Di Y, Li J, Zhou Z, Pillarisetty VG, Fu D. FOXP3(+) Lymphocyte Density in Pancreatic Cancer Correlates with Lymph Node Metastasis. PLoS ONE. 2014;9(9):e106741. doi: 10.1371/journal.pone.0106741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hasegawa S, Eguchi H, Tomokuni A, Tomimaru Y, Asaoka T, Wada H, Hama N, Kawamoto K, Kobayashi S, Marubashi S, Konnno M, Ishii H, Mori M, Doki Y, Nagano H. Pre-treatment neutrophil to lymphocyte ratio as a predictive marker for pathological response to preoperative chemoradiotherapy in pancreatic cancer. Oncology Letters. 2016;11(2):1560–1566. doi: 10.3892/ol.2015.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kurahara H, Takao S, Maemura K, Mataki Y, Kuwahata T, Maeda K, Sakoda M, Iino S, Ishigami S, Ueno S, Shinchi H, Natsugoe S. M2-Polarized Tumor-Associated Macrophage Infiltration of Regional Lymph Nodes Is Associated With Nodal Lymphangiogenesis and Occult Nodal Involvement in pN0 Pancreatic Cancer. Pancreas. 2013;42(1):155–159. doi: 10.1097/MPA.0b013e318254f2d1. [DOI] [PubMed] [Google Scholar]
- 72.Keenan BP, Saenger Y, Kafrouni MI, Leubner A, Lauer P, Maitra A, Rucki AA, Gunderson AJ, Coussens LM, Brockstedt DG, Dubensky TW, Hassan R, Armstrong TD, Jaffee EM. A Listeria Vaccine and Depletion of T-Regulatory Cells Activate Immunity Against Early Stage Pancreatic Intraepithelial Neoplasms and Prolong Survival of Mice. Gastroenterology. 2014;146(7):1784–1794.1786. doi: 10.1053/j.gastro.2014.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rowland-Goldsmith MA, Maruyama H, Kusama T, Ralli S, Korc M. Soluble Type II Transforming Growth Factor-beta (TGF-B) Receptor Inhibits TGF-B Signaling in COLO-357 Pancreatic Cancer Cells in Vitro and Attenuates Tumor Formation. Clinical Cancer Research. 2001;7(9):2931–2940. [PubMed] [Google Scholar]
- 74.Yokoyama M, Ebert M, Funatomi H, Friess H, Buchler MW, Johnson GR, Korc M. Amphiregulin is a potent mitogen in human pancreatic cancer cells: correlation with patient survival. International Journal of Oncology. 1995;6:625–631. doi: 10.3892/ijo.6.3.625. [DOI] [PubMed] [Google Scholar]
- 75.Weigelt B, Peterse JL, van't Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5(8):591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.