Abstract
The PR1 peptide, derived from the leukemia-associated antigens proteinase 3 and neutrophil elastase, is overexpressed on HLA-A2 in acute myeloid leukemia (AML). We developed a high affinity T cell receptor-like murine monoclonal antibody, 8F4, which binds to the PR1/HLA-A2 complex, mediates lysis of AML, and inhibits leukemia colony formation. Here, we explored whether 8F4 was active in vivo against chemotherapy-resistant AML, including secondary AML. In a screening model, co-incubation of AML with 8F4 ex vivo prevented engraftment of all tested AML subtypes in immunodeficient NSG mice. In a treatment model of established human AML, administration of 8F4 significantly reduced or eliminated AML xenografts and extended survival compared with isotype antibody-treated mice. Moreover, in secondary transfer experiments, mice inoculated with bone marrow from 8F4-treated mice showed no evidence of AML engraftment, supporting possible activity of 8F4 against the subset of AML with self-renewing potential. Our data provide evidence that 8F4 antibody is highly active in AML, including chemotherapy-resistant disease, supporting its potential use as a therapeutic agent in patients with AML.
INTRODUCTION
Monoclonal antibodies (mAbs) against tumor-specific or lineage-specific antigens are effective treatments for a growing number of cancers. Most of the mAbs used in the clinical setting target surface proteins that although expressed by normal cells, have distinct expression patterns on the malignant cells. However, the majority of onco-mutated proteins and tumor-specific antigens are expressed within the tumor cell, in the nucleus or cytoplasm; targeting such proteins with mAbs has proven to be a difficult task. Nevertheless, intracellular proteins can be valuable targets for immunotherapy. In acute myeloid leukemia (AML), a neoplasm largely resistant to conventional therapies, the potential of allogeneic hematopoietic stem cell transplantation (HSCT), a proven potentially curative therapy, is due to its graft-versus-leukemia effect that is mediated by donor cytotoxic T lymphocytes (CTL). 1 Specifically, peptides from intracellular proteins within the AML blasts are processed and presented on cell surface major histocompatibility class I (MHC-I) antigens. These peptide/MHC-I complexes are recognized by the T cell receptor (TCR) on CD8+ CTL, which in the appropriate tumor environment can eliminate the malignant cells.2, 3 TCR-like mAbs that target peptide/MHC-I on the tumor cell surface have been developed and are promising as novel cancer immunotherapies.4–7 While the TCR binds to cognate peptide/MHC ligands with low affinity because of rapid off-rates,8, 9 TCR-like mAbs bind to surface peptide/MHC-I with several orders of magnitude higher affinity and therefore may have therapeutic advantages.4, 5, 10–12 Despite the technical challenges of developing mAbs with specificity for peptides in the context of MHC-I, a number of TCR-like mAbs targeting intracellular tumor-associated antigens have been investigated, and a few have shown promising activity against tumor cell lines,6, 7, 13 including leukemia cell lines.14, 15
PR1 is a human leukocyte antigen HLA-A2 restricted 9-mer peptide derived from the myeloid serine proteases proteinase 3 (P3) and neutrophil elastase (NE),10 which are normally contained intracellularly within azurophilic granules in normal granulocytes. NE and P3 have been shown to be aberrantly expressed in AML and chronic myeloid leukemia (CML).2, 16, 17 PR1-specific CTL have been shown to lyse malignant and dysplastic cells in AML, CML, and myelodysplastic syndrome (MDS), and were also have been shown to contribute to cytogenetic remission in CML.3, 18–20 We developed a TCR-like mouse mAb, 8F4, which binds to the PR1/HLA-A2 complex on the surface of AML.21 8F4 mediates both complement-dependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity (ADCC) of AML. Importantly, 8F4 inhibits leukemia stem cells (i.e. LSC) but not normal hematopoietic progenitor cells in in vitro colony forming assays.21 However, the in vivo effect of 8F4 on primary leukemia cells has not been explored.
Here, we studied the in vivo effects of 8F4 in a patient-derived xenograft (PDX) model. Specifically, primary cells from patients with a variety of AML subtypes were inoculated into NOD scid IL2 receptor gamma-chain knock out (NSG) mice.22 We show that treatment of established AML xenografts with 8F4 reduced human AML. In secondary transfer experiments, we found that 8F4 depleted AML, including cells with self-renewing potential. Taken together, our findings justify the further development of 8F4 as a potential therapeutic agent for patients with AML.
MATERIALS AND METHODS
Patients and donors
Human AML samples were collected from patients treated at the University of Texas MD Anderson Cancer Center (MDACC) after obtaining written informed consent under protocols approved by MDACC Institutional Review Board (IRB). The HLA status of the patients and other data, including previous treatments and outcome, were obtained from the patients' electronic medical record. The HLA testing was conducted at the MDACC HLA typing Laboratory. Patients UPN1–4, UPN7 and UPN8 were molecularly typed as HLA-A02:01:01; patient UPN5 had serologic typing only and was identified as HLA-A2. Mononuclear cells were separated by gradient density centrifugation using histopaque 1077 (Sigma-Aldrich).
Assessment of PR1/HLA-A2 expression and susceptibility to 8F4-mediated cytotoxicity
8F4 mAb was generated in BALB/c mice as previously described. 21 8F4 was affinity purified from hybridoma supernatant and directly conjugated to Alexa-647 fluorochrome (Invitrogen). To assess PR1/HLA-A2 expression, samples were stained in the presence of blocking antibody bb7.2, as described. 21 To account for variance in staining conditions performed on different days, 8F4 median fluorescence intensity (MFI) was normalized to the MFI of IgG-binding compensation beads (eBioscience), stained with 8F4 (maximum [Max] MFI); PR1/HLA-A2 expression was reported as % Max MFI.
To study susceptibility of samples to 8F4-mediated killing flow cytometry-based CDC assay was used as described. 21
Mouse model
NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG), NOD.Cg-Prkdcscid Tg(HLA-A2.1)1Enge/DvsJ (NOD scid/HLA-A2), NOD.CB17-Prkdcscid/J (NOD scid), C57BL/6-Tg(HLA-A2.1)1Enge/J (B6/HLA-A2), and C57BL/6J (B6) female mice were purchased from the Jackson Lab and housed at MDACC with the Institutional Animal Care and Use Committee approved protocol. AML cells were intravenously (IV) injected into mice as previously described.22, 23 Briefly, 6–10 weeks old mice received sub-lethal irradiation before tail vein injection with 1–10 × 106 human AML cells per mouse. For ex vivo treatment, 8F4 or isotype-matched mouse IgG2a specific to keyhole limpet hemocyanin (20 μg/ml, R&D Systems) were added to AML cell suspensions and incubated on ice for 30 min before injection. AML engraftment was assessed intermittently in the peripheral blood (PB) by tail bleeding and analyzing the % of AML cells by flow cytometry. For treatment, mice were allocated to treatment or control groups by matching %AML in PB. 8F4 or mouse IgG (IgG, Jackson ImmunoResearch) were injected using varying schedules.
Animals were sacrificed 3–4 days after the last mAb treatment and tissues were harvested. Secondary transplantation was performed according to published protocol.24 Briefly, cells, isolated from the BM or spleen of the mAb-treated primary mice, were IV transplanted into secondary recipient. BM, liver, and spleen were harvested 8–16 weeks following transplant and were analyzed for engraftment. Human cells in mouse blood or in the single-cell suspensions from mouse tissues were reported as the percentage of mouse (mo) CD45−/human (hu) CD45+/ HLA-A2+ cells within live singlet cell populations. For HLA-A2− samples, HLA-DR, CD13, or HLA-ABC were used as secondary markers to confirm the presence of human cells. Human lineage (Lin) antibodies targeting CD3, CD4, CD8, CD19, CD20, CD14, and CD16, as well as CD34 and CD38 fluorescently conjugated antibodies were used for analysis of AML LSC, defined as CD34+CD38−Lin−. Data were acquired on an LSR Fortessa flow cytometer (BD Biosciences) and analyzed using FlowJo software Ver. 9.3.2 (Tree Star). For histologic analysis of mouse tissue, formalin-fixed tissues were embedded in paraffin, sectioned and stained with hematoxylin and eosin (H&E) stain. Pathology, bacteriology and virology studies were performed at the MDACC Department of Veterinary Medicine and Surgery.
To study 8F4 pharmacokinetics, NSG mice (female, 10 months old) received a single intravenous injection of 8F4 (10 mg/kg). At the indicated time points, tail blood was drawn, serum was separated, frozen, and later analyzed by ELISA, as described, 21 for 8F4 concentration.
To model 8F4 effect on normal HLA-A2+ hematopoiesis, we injected 8F4 (10 mg/kg, 3 times per week for total 10 injections) into HLA-A2 transgenic B6 mice (B6/HLA-A2). Complete blood count (CBC) was monitored using SCIL Vet ABC plus hematology analyzer (Scil animal care company Ltd., Gurnee, IL) before 8F4 and, starting 1 week after last injection, as indicated. At week 16, bone marrow cells were stained with antibodies specific for mouse GR-1 and analyzed by flow cytometry.
Statistical analysis
Data are presented as mean ± SEM. Student’s t test (unpaired, two-sided) was used to study differences between groups; Kaplan-Meier analyses and log-rank tests were used in the survival comparisons; two-phase exponential decay model was used for analyzing 8F4 pharmacokinetics. Statistical analysis was performed with GraphPad Prism 6 (GraphPad Software, Inc). P values of <0.05 were considered to be significant.
RESULTS
Pre-treatment with 8F4 prevents AML engraftment in mouse models
We previously showed that HLA-A2+ AML cells are susceptible to 8F4-mediated CDC in vitro. 21 To determine whether 8F4 is active against leukemia in vivo, we set up xenotransplantation models of primary leukemia cells from AML patients using NSG mice. 25 We screened available cryopreserved AML blasts from peripheral blood (PB) or leukapheresis (LP) samples for their ability to initiate leukemia in mouse models. 22, 24 Mouse BM was analyzed for the presence of human AML three to four weeks after injections. We identified 10 patient samples that successfully engrafted into NSG mice (UPN1–5, UPN7 and UPN8) or NOD scid/HLA-A2 mice (UPN 6, 9–10); engraftment was defined as the detection of 0.25 – 76% AML in the mouse BM. These samples were subsequently used in in vivo experiments. Patient characteristics, including AML FAB subtype, cytogenetics, molecular abnormalities and response to therapy are shown in Table 1. Samples were acquired from patients with primary refractory or relapsed AML who had >65% circulating blasts. Furthermore, with the exception of 1 patient (UPN4), all patients died of progressive disease within 3 months of sample collection. The selected samples were studied for PR1/HLA-A2 expression and susceptibility to 8F4-mediated CDC in vitro (Supplemental Figure S1). Consistent with our previous report, PR1/HLA-A2 expression was variable in HLA-A2+ AML samples.
Table 1. Patient characteristics.
Summarized clinical and laboratory data of patients at the time of sample withdrawal. All samples used in this study were from patients with advanced leukemia, with high blast counts, and most of patients were refractory to therapy.
| UPN | Age/Sex | Leukemia Type | WBC Count, K/ul | % Blast | Cytogenetic and Molecular1 Abnormalities | Prior Treatment | Treatment Status | |
|---|---|---|---|---|---|---|---|---|
| PB | BM | |||||||
| 1 | 76/M | 2° AML (MF), M1 | 58.6 | 81 | 87 | 47 XY,+8, JAK2+ | For MF: Hydrea and Decitabine. No AML treatment |
Newly diagnosed |
| 2 | 38/M | 2° AML (MDS), M7 | 4.0 | 65 | 64 | 49 XY, 51 XY complex cytogenetics, del(5),del(7) |
|
1° Ref |
| 3 | 61/M | M2 | 77 | 66 | 31 | Hyperdiploid clone FLT3-ITD |
|
Rel, ref |
| 4 | 47/F | AML/ALL2 | 116 | 48 | 68 | Diploid t(9;22) BCR-ABL b2a2 |
No prior treatment at time of sample. | Newly diagnosed |
| 5 | 73/F | 2° AML (MDS), M5 | 39.5 | 72 | 74 | Pseudodiploid del(5),add(12) RAS+ |
ARA-C/Daunorubicin | 1° Ref |
| 6A2− | 42/F | M1 | 132 | 91 | 98 | Diploid FLT3+ |
N/A | Newly diagnosed |
| 7 | 81/M | 2° AML (CMML) | 14 | 95 | 70 | Cytogenetics, ND No molecular abnormalities |
|
1° Ref |
| 8 | 47/F | M1 | 26 | 96 | 94 | 44 XX Complex cytogenetics FLT3+ NPM1+ |
|
Rel, ref |
| 9A2− | 71/F | 2° AML (MDS) | 19 | 95 | 92 | 47 XX, 46 XX del (5) +8, +17 |
|
1° Ref |
| 10A2− | 28/F | Ph+ ALL | 238 | 77 | 79 | 46XX t(9;22) 190 kDa BCR-ABL1 fusion protein; Monoclonal IgG gene rearrangements |
Untreated Newly | diagnosed |
- HLA-A2 negative patients
Molecular analysis included PCR analysis for FLT-3, NPMP1, C-KIT, and Ras.,
Ph+ ALL 90%; AML 10%
Abbreviations: FAB, French-American-British morphological criteria; M, male; F, female; MF, myelofibrosis; MDS, myelodysplastic syndrome; WBC, white blood cell, PB, peripheral blasts; BM, bone marrow, ARA-C, cytarabine; Ref, refractory; Rel/ref, relapsed refractory; CR, compete remission; del, deletion; add, addition.
Nevertheless, all the HLA-A2+ AML samples that were examined were susceptible to 8F4-mediated CDC with 22 – 99 % specific lysis, and their lysis correlated with PR1/HLA-A2 expression (Supplementary Figure S1).
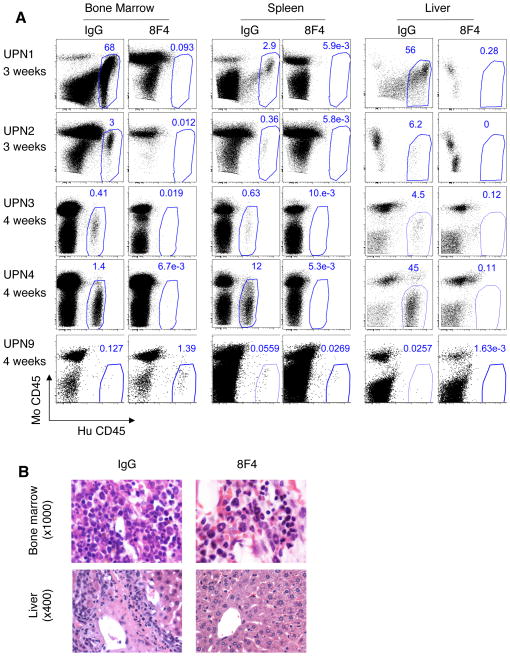
Next, we tested samples in a pre-treatment model24 in which AML cells were incubated with 8F4 or isotype-control antibody IgG2a (IgG) prior to injection into NSG mice. Co-incubation of AML specimens from UPN1–4 with 8F4 ex vivo prevented the engraftment of the AML in the mouse BM, spleen, or liver (Figure 1). 8F4 did not inhibit the engraftment of HLA-A2− control UPN9. These results were confirmed with H&E staining of mouse tissues (representative data shown in Figure 1B). Similar results were shown in HLA-A2 transgenic mice (NOD scid/HLA-A2, Supplementary Figure S2). Engraftment of UPN1 and UPN5, but not of HLA-A2− sample UPN6, was prevented when the sample was pre-mixed with 8F4. Because NOD scid/HLA-A2 mice have a low level of PR1/HLA-A2 expression on BM cells (Supplementary Figure S3A), similar to that observed in HLA-A2+ leukemia patients, 21 this is a highly relevant model. Unfortunately, NOD scid/HLA-A2 is much less permissive in terms of engraftment of primary leukemia and thus we used the more permissive NSG model26 to conduct subsequent experiments.
Figure 1. 8F4 Ex vivo treatment prevents engraftment of primary AML in NSG mice.
AML cells were incubated with 20μg 8F4 or isotype control mouse IgG2a monoclonal antibody (IgG) and intravenously transplanted in sublethally-irradiated NSG recipient mice. At indicated time points tissues were analyzed for the presence of leukemia cells. (A) Representative flow cytometry dot plots depict leukemia cells in tissues of IgG-treated mice transplanted with four HLA-A2+ (UPN1–4) and one HLA-A2- (UPN9) patient samples. The percentages of human cells (hu CD45+/mo CD45−) are shown within the gate in each plot. (B) Representative H&E stained bone marrow (upper panels) and liver (lower panels) slides from mice that were transplanted with UPN1 patient sample. Left panels - AML infiltration is seen in tissues of control mice following injection of AML cells treated with control IgG isotype antibody. Right panels - no AML infiltration is seen in corresponding tissues of mice injected with AML cells that were treated with 8F4.
8F4 reduces established AML in NSG mice
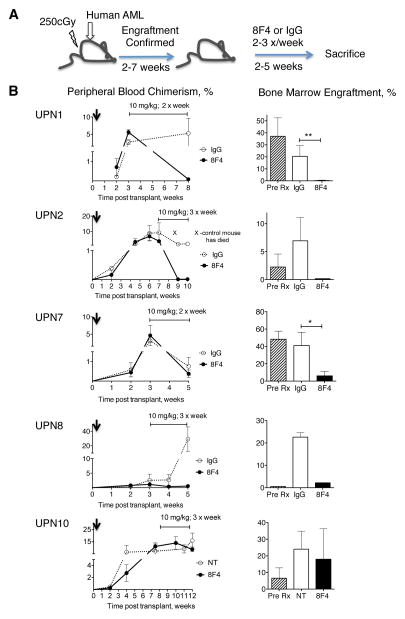
To determine whether 8F4 can eliminate established leukemia, we injected AML cells from 4 patients (UPN1, 2, 7, and 8) into NSG mice (Figure 2). After leukemia engraftment for each individual sample was confirmed in PB of all mice, and in BM of one or two mice in each group, we administered 8F4 until the time of sacrifice (Figure 2A). The treatment schedule was chosen based on a two-phase exponential elimination of 8F4 in NSG mice with a relatively short initial fast phase serum half-life of 3-hours, followed by a slow terminal phase with a half life of 93 hours (Supplementary Figure S4). After 5 weeks of treatment, UPN1 leukemia was undetectable (<0.01%) in the BM of four out of five 8F4-treated mice (mean, 0.19 ± 0.19% cells, n=5) compared to control animals (20.4 ± 4.1%, n=5; p=0.0011) (Figure 2B). Correspondingly, UPN1 leukemia infiltrated the liver (7.7 ± 0.16%) and spleen (1.4 ± 0.17%) of IgG-treated mice, but was undetectable in these same organs of 8F4-treated mice. Similarly reduced levels of AML were observed in BM (Figure 2B), liver and spleen (data not shown) in the 3 other AML samples, treated with 8F4 (data not shown). In a control experiment, mice that were transplanted with the HLA-A2-negative sample UPN10 showed similar levels of BM leukemia and blood chimerism as untreated mice after ten 8F4 injections (Figure 2B). Growth of leukemia was also seen with HLA-A2-negative AML sample, UPN9, after treatment with 8F4 (Supplementary Figure S5).
Figure 2. 8F4 treatment reduces established leukemia in NSG mice.
(A) The schedule of 8F4 treatment in mice transplanted with primary AML cell from patients. (B) Leukemia engraftment in mice, transplanted with four HLA-A2+ patient samples (UPN1, UPN2, UPN7, and UPN8) and one HLA-A2- patient sample (UPN10) was monitored by flow cytometry analysis of human cells in the peripheral blood (left panels). Increasing percentage of human AML cells in the PB of mice pre-treatment shows established and growing leukemia. Mice with established leukemia were treated with 8F4 or control antibody (IgG). Horizontal capped line in each graph in the left panel indicates the treatment period and schedule. Right panels show percent bone marrow engraftment at time of sacrifice. To confirm the BM engraftment of each sample, mice from each leukemia group were sacrificed and analyzed before treatment (Pre Rx). The percentages of human cells are shown. Data shown as mean percent human cells ± SEM of the chimerism for each treatment group (n=2–5); * p< .05; ** p< .005. We were unable to perform statistical testing on UPN 2 and 8 samples because of sample numbers in the group.
Additionally, the effect of low doses of 8F4 on established leukemia from sample UPN1 was investigated. Low-dose weekly injections of 8F4 at 2 mg/kg and 0.5 mg/kg eliminated peripheral blood AML in mice with established disease (Supplementary Figure S6). Taken together, these results show that 8F4 is highly active against treatment-refractory primary human HLA-A2-positive AML in established AML treatment NSG model.
8F4 treatment prolongs survival of mice with established AML
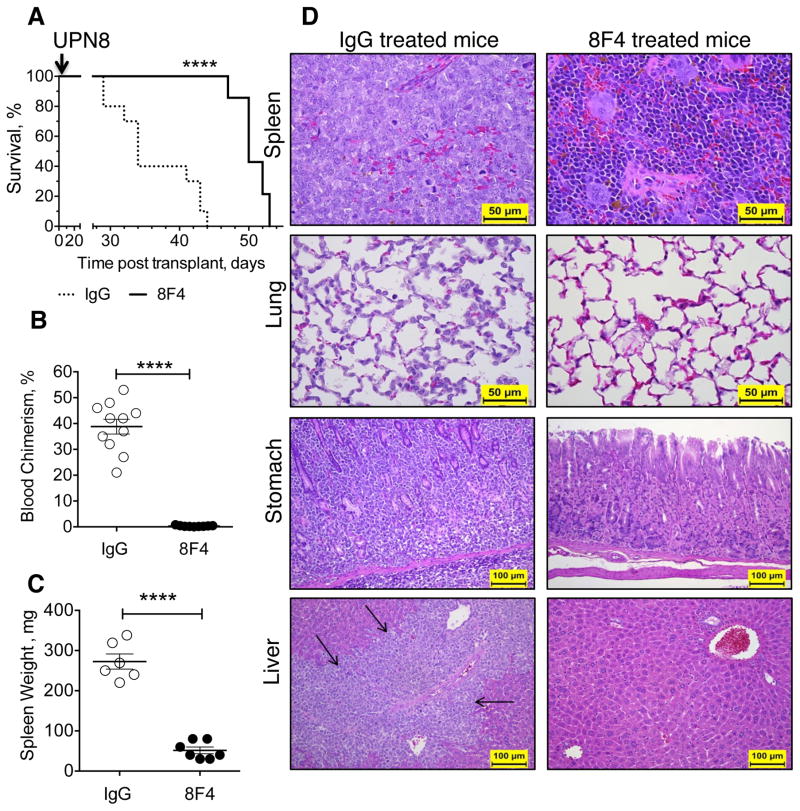
To determine the effect of 8F4 on the survival of mice with AML xenografts, we studied AML from UPN8, a patient with a high leukemia burden who had hemi-paralysis from leptomeningeal AML involvement. In untreated mice, circulating blasts reached mean 35% ± 8% (n=3) by week 4, with a concurrent 23.3% weight loss. At week 5, approximately 50% of mice containing UPN8 AML developed hind limb paresis and all mice became moribund with a mean of 59.2% ± 7% AML in the PB (n=4, data not shown). At week four, 3 x 106 splenocytes containing > 50% blasts from untreated mice were transferred to secondary recipient mice. After engraftment was established at 1 week, mice were treated with 10 mg/kg 8F4 or IgG 3-times weekly. Median survival of 8F4-treated mice was 50 (n=9, range 47 to 53) days compared to 34 days in the IgG-treated group (n=10, range 29 to 44; p < 0.0001; Figure 3A). In addition, the leukemia burden was significantly lower in the 8F4-treated group, compared to the isotype-treated group at week 4, as shown by fewer circulating blasts in Figure 3B (mean 0.33 ± 0.08%, n=9, versus 38.8 ± 2.3%, n=11; p < 0.001). Furthermore, 8F4-treated mice had a normal spleen size (51.4 ± 8.3 mg, n=7 versus 272.7 ± 19.0 mg, n=7; p < 0.01 Figure 3C) and, in contrast to isotype-treated mice, histological analysis of 8F4-treated mice showed absence or significant reduction of AML in spleen, lungs, stomach and liver (Figure 3D), as well as kidney, adrenal glands, heart, bone marrow, intestine, lymph nodes and pancreas (data not shown). Although 8F4-treated mice showed a significant survival advantage and lower disease burden, the animals succumbed from leukemia accumulation in the central nervous system (CNS), suggested by the observed paresis in the mice, and confirmed by histological analysis, which demonstrated extensive AML tumor infiltration in the brain, head tissues and in the spinal cord (Supplementary Figure S7). This is not surprising since IgG do not efficiently penetrate the blood-brain barrier.27 These data therefore confirm that 8F4 treatment can significantly reduce highly aggressive human leukemia xenograft and as a result can extend survival.
Figure 3. 8F4 treatment prolongs survival of NSG mice with established leukemia.
AML from UPN8 was expended in vivo by serial subsequent transplants of spleen cells in NSG recipients. Transplanted mice were treated with 8F4 or control IgG starting on day 7. (A) Kaplan-Meier survival curves show longer median survival in 8F4 treated mice (median 50 days, n=10) in comparison with IgG treated mice (34 days, n=9). (B) AML was present in the peripheral blood only in IgG-treated animals at week 4. (C) Spleens were enlarged only in IgG-treated mice. (B-C) Each data point represents one mouse; mean ± SEM for each group is also presented; **** p< .0001. (D) Representative H&E-stained sections of mice, treated with 8F4 or IgG. Spleen: the extramedullary hematopoiesis and all other tissue structures present in 8F4-treated mice are completely effaced by infiltration of AML in the control mice. Lung: AML cells are diffusely infiltrated into the alveolar walls of IgG treated mice, but not in the 8F4-treated mice. Stomach: AML infiltrated diffusely between the glands of gastric mucosa and in the sub-mucosa of glandular stomach of IgG-treated mice, but not in the stomach of 8F4-treated mice. Liver: the AML cells infiltrated severely into the portal triads of liver (black arrows) of IgG-treated mice, and there was minimal or no infiltration of AML in the liver of 8F4-treated mice.
8F4 reduces leukemia-initiating potential of AML
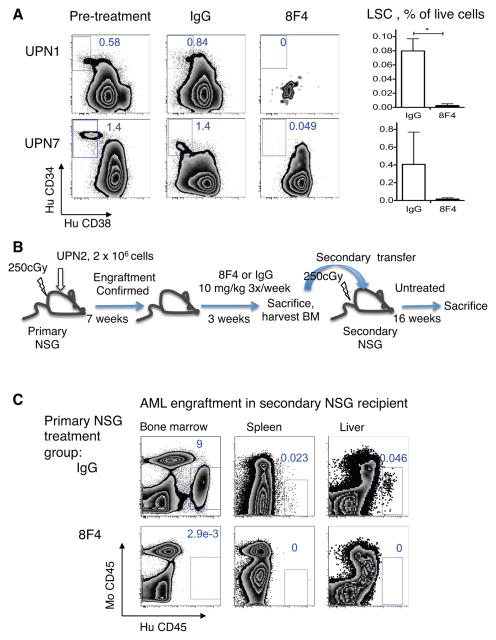
Critical to the success of AML therapy is the complete elimination of the malignant clone from which the AML is derived. Previous reports have suggested that AML evolves from a population of cells having CD34+CD38−Lin− phenotype that are enriched for leukemia-initiating cells or leukemia stem cells (LSC).28 Self-renewing capacity of LSC can be confirmed using serial transfer mouse models.25, 28 Previously, we reported that PR1/HLA-A2 is expressed on LSC.21 We also showed that 8F4 mediated CDC lysis of LSC and inhibited leukemia colony growth. 21 Thus, to test whether 8F4 is active against LSC in vivo, we enumerated CD34+CD38−Lin− LSC in the BM of 8F4- and isotype-treated NSG mice that were engrafted with AML from either UPN1 or UPN7. Experiments were performed according to the scheme shown in Figure 2A. CD34+CD38−Lin− LSC were present prior to treatment in mice with UPN1 and UPN7, as shown in Figure 4A. However, after only 4 doses of 8F4 (twice weekly), overall leukemia burden in the BM decreased and LSC were undetectable in 8F4-treated mice with established UPN1 and UPN7 compared with IgG-treated mice (Figure 4A).
Figure 4. 8F4 treatment depletes leukemia-initiating cells in vivo.
(A) Mice with established leukemia UPN1, and UPN7 were treated with 8F4 or control antibody (IgG) as indicated in Figure 2. LSC were defined as Lin−CD34+CD38−. Zebra plots (left panel) show a representative staining of Lin- human cells in mouse BM before and after treatment with 8F4 or IgG, gated on LSC. Numbers show LSC percentage within human Lin- cells. Right panels show LSC percent (mean± SEM) of all live cells (including mouse and human) in bone marrow for each treatment group. (B) Secondary transfer model was used to test the effect of treatment on LSC as measured by leukemia self-renewing capacity. Mice with established leukemia (UPN2) were treated with 8F4 three times per week starting weeks 7. At week 10, residual leukemia in bone marrow from 8F4-treated mice were transplanted into secondary recipient mice. (C) Representative zebra flow cytometry plots of tissues from the transfer recipient mice at week 16 show no detectable leukemia in the bone marrow, spleen and liver of mice that received the AML transplant from 8F4-treated animals, indicating elimination of LSC populations (n=2–4 mice per treatment group).
To confirm that 8F4 targets a leukemia cell population with self-renewing capacity, we performed secondary transfer experiments. Primary NSG mice were engrafted with UPN2 and then, starting on week 7, injected with 10 mg/kg of 8F4 or IgG three times weekly for 3 weeks, as summarized in Figure 4B. Because a residual non-LSC population was still detectable in the transferred bone marrow from 8F4-treated mice (0.11%, see Supplementary Figure S8), we performed secondary transfer experiment to determine whether persistent leukemia would grow in untreated secondary recipient mice. At 16 weeks after secondary transfer, AML was undetectable in blood (not shown), BM, spleen and liver (Figure 4C) of mice that received BM from 8F4-treated animals while AML was observed in recipients that received BM from IgG-treated mice. Therefore, both in vitro and in vivo experiments demonstrate the activity of 8F4 against primary human AML, including cells with self-renewing potential, such as LSC.
8F4-induced granulocytopenia in HLA-A2 transgenic mice is reversible
Although PR1/HLA-A2 is overexpressed in AML blasts and LSC, it also can be detected on normal PB monocytes and BM myeloblasts, including normal hematopoietic stem cells. 21 Consistent with PR1/HLA-A2 expression, we have shown that 8F4 mediates relatively low-level CDC in normal BM samples, including Lin−CD34+CD38− populations; however, as described previously, it did not show inhibition of colony-formation from healthy donor BM. 21
Because the human PR1 sequence is conserved in both human and mouse NE and P3, 29 we tested whether 8F4 treatment prevents normal HLA-A2+ hematopoiesis in vivo using HLA-A2 transgenic B6 mice (B6/HLA-A2). We first confirmed PR1/HLA-A2 expression in B6/HLA-A2 BM and showed a similar expression level of PR1/HLA-A2 between mouse and normal HLA-A2+ human BM cells (Supplementary Figure S3B).21
B6/HLA-A2 mice were injected with 8F4 (10 mg/kg) 3 times weekly for a total of 10 injections, the dosing schedule in which 8F4 showed effective elimination of established AML in NSG treatment model (Figure 2–3). As shown in Supplementary Figure S9A, white blood cell (WBC) counts decreased from mean of 10.9 ± 1.2x103/mm3 to mean 1.9 ± 0.32x103/mm3 during the period of 8F4 administration, and then steadily recovered to 6.20 ± 2.43x103/mm3 by week 6 post last 8F4 injection. Granulocytes decreased from a mean of 3.63 ± 0.38x103/mm3 to 0.97 ± 0.09x103/mm3, and then rebounded to 2.43 ± 0.78x103/mm3. Additionally, BM granulocytes fully rebounded by week 8 post last 8F4 injection – the termination point of the experiment (Supplementary Figure S9B), suggesting that BM myeloblasts were not depleted by 8F4. Mice treated with 8F4 maintained normal weight and did not show physical abnormalities. A detailed histologic analysis of tissues immediately after the final dose of 8F4, as well as at the recovery time point, could further explore other potential off-target effects of 8F4 and are thus planned for future studies. Overall, these data indicate that 8F4 induces reversible mild granulocytopenia with limited adverse effects on long-term hematopoiesis.
DISCUSSION
In this study, we showed that the anti-PR1/HLA-A2 mAb, 8F4, is highly active against human AML in vivo. 8F4 reduced a variety of AML subtypes, including relapsed and refractory AML, and, significantly, secondary AML. In addition, 8F4 treatment prolonged the survival of AML xenograft mice compared to isotype control-treated mice. Moreover, 8F4 depleted the subset of cells with self-renewing potential in secondary transfer experiments. Thus, our data support the clinical development of 8F4 as a therapeutic agent with a potent activity against aggressive leukemia.
Here we demonstrated activity of 8F4 against primary AML in vivo. While this study was not designed to explore the mechanism of action of 8F4 in vivo, we previously showed that 8F4 mediates lysis of AML in vitro by utilizing both CDC21,30 and antibody-dependent cellular cytotoxicity (ADCC) mechanisms.21 However, because NSG mice lack a fully competent complement system,31 ADCC is a more likely predominant mechanism of action of 8F4 in the NSG model. In the absence of NK cells,31 characteristic of this model, neutrophils, monocytes and macrophages, which have been demonstrated to be active in anti tumor ADCC, 32–34 may play a role as effector cells. This is consistent with the mechanism of action reported for the WT1/HLA-A2 TCR-mimic antibody, which showed ADCC activity toward acute lymphocytic leukemia (ALL) cells in NSG mice. 14, 15 In clinical studies, including patients with FcγR gene polymorphisms, ADCC was also shown to be a primary mechanism in the FDA-approved antibodies Trastuzumab35 and Rituximab. 36, 37 Nevertheless the possibility of another mechanism, such as effector-independent apoptosis, which has been demonstrated for other TCR mimics 6 is also possible and currently is being explored.
To date, the primary treatment modality for AML remains chemotherapy-based; however, the prognosis of AML remains poor with a 20–30% 5-year survival rate, and median survival duration of 10–14 months. Although HSCT can be curative in AML, it carries a high rate of treatment-related mortality and morbidity, primarily a result of graft-versus-host disease.38 Nevertheless, myeloid leukemia is susceptible to immunotherapy, specifically HSCT, which is used as a potentially curative treatment for patients with aggressive and chemotherapy refractory disease. TCR-like antibodies provide a novel approach to the treatment of malignancies. Since they are specific for distinct tumor antigens, they have the potential of sparing normal tissues from the toxicities seen with standard cancer therapies. However, the development of TCR-like antibodies has been impeded by the difficulty in generating antibodies that target peptide/HLA class I complexes, since the HLA molecule makes up a large portion of the binding surface and therefore the antibody binds with high affinity to epitopes on the HLA molecule, compared to the peptide in the conformational epitope complex.39 Despite this difficulty, our group, as well as others, has been able to develop TCR-like mAbs that specifically target tumor cells.4, 6, 7, 14, 15, 21 And, although standard therapies can induce complete remission, relapse is common, and is postulated to be due to the outgrowth of chemotherapy-resistant LSC. Therefore, targeting the LSC is a critical component of the development of curative leukemia treatments. Cells enriched for the LSC phenotype have been defined 25, 28 and several investigational monoclonal antibodies have shown promising effects by eliminating LSC in vivo. 24, 40 TCR-like antibodies, however, have not been investigated for activity against LSC in in vivo models. Here, using primary AML cells from high-risk patients, we demonstrate via phenotyping and secondary transplant experiments that 8F4 reduced cells with leukemia-initiating potential such as LSC, consistent with our in vitro results.21 This finding validates further discovery and development of this class of antibodies as potential therapeutic agents.
The use of primary patient samples in this study allowed us to study the action of 8F4 on LSC and AML blasts. It must be recognized, however, that the use of PDX models has limitations. For example, there is heterogeneity of engraftment between different patient samples and within each sample, and the number of patients cells available for study is limited, necessitating the use of limited numbers of mice in each treatment group. We have tried to address this limitation by studying a relatively large number of patients with various forms of disease and by using cells after in vivo expansion by serial transplant of splenocytes in NSG recipients (UPN8, Figure 3). While our data supports potent anti-leukemia activity of 8F4, potential resistance mechanisms will be important to study. For example, clonal evolution of AML can result in the loss of HLA alleles via uniparental disomy after allogeneic stem cell transplantation,41, 42 which could result in loss of susceptibility of the AML clone to 8F4-induced killing. Another potential limitation common for therapeutic antibodies is their inability to cross the blood-brain barrier. 27 This can explain the limited effect of 8F4 on AML blasts from patient sample UPN8, which infiltrated the brain and CNS of the mice and the corresponding patient from which the AML was derived (Supplemental Figure S7). Although CNS involvement is uncommon at presentation in adult AML,43 various strategies have been developed to deliver antibodies to the CNS.27, 44 Although this study did not formally test whether PR1 is presented on normal cells or whether non-PR1 peptides could be targeted by 8F4, we showed previously that PR1 is presented on myeloid precursors in the bone marrow at a low level, 21 which supports the likelihood that PR1 also may be presented on normal leukocytes. Thus, granulocytopenia after treatment with 8F4 could present a potential risk for humans, as supported by toxicity observed in the HLA-A2 transgenic B6 mouse model (Supplemental Figure S9).
Finally, in view of the heterogeneity of AML, it is unlikely that any single antigen will be consistently expressed in all leukemia. Furthermore, loss of tumor antigens is a well-known phenomenon used by tumor cells to evade recognition by the immune system.45 Other investigators have studied antibodies targeting other surface antigens in AML, which are expressed on LSC.24, 40, 46 Taken together with our data, this suggests a rationale for combination therapies of antibodies that have different mechanisms of action, which can target different pathways in leukemia and ultimately eradicate the disease.
In conclusion, this study shows that the TCR-like antibody 8F4 has potent activity against primary human AML in vivo. Based on these results, we have begun to produce and characterize a humanized 8F4 antibody, which could be developed for clinical testing. Our findings here also justify the future development of TCR-like antibodies specific to intracellular leukemia-associated antigens for treatment of patients with AML.
Supplementary Material
Acknowledgments
This study was supported by research funding from NCI CA100632 (to JJM); NCI P01 CA148600-05 (to JJM); Leukemia and Lymphoma Society 6030-12 (to JJM); Leukemia and Lymphoma Society 7262-08 (to JJM); Gillson Longenbaugh Foundation (to JJM), NCI CA16672 Core Grant (Monoclonal Antibody Core Facility; Flow Cytometry and Cellular Imaging Facility; Research Animal Support Facility; Histopathology Facility); NCI CA164346 (to MJY), Developmental Research Awards in Leukemia SPORE CA100632 (to MJY); and Ladies Leukemia League (to MJY), Center for Inflammation and Cancer, Center for Genetics and Genomics, IRG, Sister Institution Network fund of UT MD Anderson Cancer Center (to MJY). In particular, we wish to acknowledge Dr. Long Vien in the Monoclonal Antibody Core Facility for providing purified 8F4. We acknowledge Dr. Gregory Lizee for critical reading of manuscript.
Footnotes
Conflict of interest disclosure: JM and AS are inventors on a related patent and they receive royalty payments.
CONFLICT OF INTERESTS
Dr. Jeffrey Molldrem and Dr. Anna Sergeeva are inventors on a related patent and they receive royalty payments.
References
- 1.Champlin R, Khouri I, Kornblau S, Marini F, Anderlini P, Ueno NT, et al. Allogeneic hematopoietic transplantation as adoptive immunotherapy. Induction of graft-versus-malignancy as primary therapy. Hematol Oncol Clin North Am. 1999 Oct;13(5):1041–1057. vii–viii. doi: 10.1016/s0889-8588(05)70108-8. [DOI] [PubMed] [Google Scholar]
- 2.Molldrem J, Dermime S, Parker K, Jiang YZ, Mavroudis D, Hensel N, et al. Targeted T-cell therapy for human leukemia: cytotoxic T lymphocytes specific for a peptide derived from proteinase 3 preferentially lyse human myeloid leukemia cells. Blood. 1996 Oct 1;88( 7):2450–2457. [PubMed] [Google Scholar]
- 3.Rusakiewicz S, Molldrem JJ. Immunotherapeutic peptide vaccination with leukemia-associated antigens. Curr Opin Immunol. 2006 Oct;18(5):599–604. doi: 10.1016/j.coi.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Denkberg G, Cohen CJ, Lev A, Chames P, Hoogenboom HR, Reiter Y. Direct visualization of distinct T cell epitopes derived from a melanoma tumor-associated antigen by using human recombinant antibodies with MHC- restricted T cell receptor-like specificity. Proc Natl Acad Sci U S A. 2002 Jul 9;99(14):9421–9426. doi: 10.1073/pnas.132285699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weidanz JA, Hawkins O, Verma B, Hildebrand WH. TCR-like biomolecules target peptide/MHC Class I complexes on the surface of infected and cancerous cells. Int Rev Immunol. 2011 Oct-Dec;30(5–6):328–340. doi: 10.3109/08830185.2011.604880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verma B, Jain R, Caseltine S, Rennels A, Bhattacharya R, Markiewski MM, et al. TCR mimic monoclonal antibodies induce apoptosis of tumor cells via immune effector-independent mechanisms. Journal of immunology. 2011 Mar 1;186(5):3265–3276. doi: 10.4049/jimmunol.1002376. [DOI] [PubMed] [Google Scholar]
- 7.Jain R, Rawat A, Verma B, Markiewski MM, Weidanz JA. Antitumor activity of a monoclonal antibody targeting major histocompatibility complex class I-Her2 peptide complexes. Journal of the National Cancer Institute. 2013 Feb 6;105(3):202–218. doi: 10.1093/jnci/djs521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis MM, Boniface JJ, Reich Z, Lyons D, Hampl J, Arden B, et al. Ligand recognition by alpha beta T cell receptors. Annu Rev Immunol. 1998;16:523–544. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 9.Krogsgaard M, Davis MM. How T cells 'see' antigen. Nat Immunol. 2005 Mar;6(3):239–245. doi: 10.1038/ni1173. [DOI] [PubMed] [Google Scholar]
- 10.Reiter Y, Di Carlo A, Fugger L, Engberg J, Pastan I. Peptide-specific killing of antigen-presenting cells by a recombinant antibody-toxin fusion protein targeted to major histocompatibility complex/peptide class I complexes with T cell receptor-like specificity. Proc Natl Acad Sci U S A. 1997 Apr 29;94(9):4631–4636. doi: 10.1073/pnas.94.9.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar P, Vahedi-Faridi A, Saenger W, Ziegler A, Uchanska-Ziegler B. Conformational changes within the HLA-A1:MAGE-A1 complex induced by binding of a recombinant antibody fragment with TCR-like specificity. Protein Sci. 2009 Jan;18(1):37–49. doi: 10.1002/pro.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dao T, Liu C, Scheinberg DA. Approaching untargetable tumor-associated antigens with antibodies. Oncoimmunology. 2013 Jul 1;2(7):e24678. doi: 10.4161/onci.24678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klechevsky E, Gallegos M, Denkberg G, Palucka K, Banchereau J, Cohen C, et al. Antitumor activity of immunotoxins with T-cell receptor-like specificity against human melanoma xenografts. Cancer Res. 2008 Aug 1;68(15):6360–6367. doi: 10.1158/0008-5472.CAN-08-0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dao T, Yan S, Veomett N, Pankov D, Zhou L, Korontsvit T, et al. Targeting the intracellular WT1 oncogene product with a therapeutic human antibody. Science translational medicine. 2013 Mar 13;5(176):176ra133. doi: 10.1126/scitranslmed.3005661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubrovsky L, Pankov D, Brea EJ, Dao T, Scott A, Yan S, et al. A TCR-mimic antibody to WT1 bypasses tyrosine kinase inhibitor resistance in human BCR-ABL+ leukemias. Blood. 2014 Apr 10;123(21) doi: 10.1182/blood-2014-01-549022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molldrem JJ, Clave E, Jiang YZ, Mavroudis D, Raptis A, Hensel N, et al. Cytotoxic T lymphocytes specific for a nonpolymorphic proteinase 3 peptide preferentially inhibit chronic myeloid leukemia colony-forming units. Blood. 1997 Oct 1;90(7):2529–2534. [PubMed] [Google Scholar]
- 17.Molldrem JJ, Lee PP, Wang C, Felio K, Kantarjian HM, Champlin RE, et al. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat Med. 2000 Sep;6(9):1018–1023. doi: 10.1038/79526. [DOI] [PubMed] [Google Scholar]
- 18.Qazilbash MH, Wieder ED, Thall PF, Wang X, Rios RL, Lu S, et al. PR1 Peptide Vaccine-Induced Immune Response Is Associated with Better Event-Free Survival in Patients with Myeloid Leukemia. ASH Annual Meeting Abstracts 2007 November 16. 2007;110(11):283. [Google Scholar]
- 19.Qazilbash MH, Wieder ED, Thall PF, Wang X, Rios RL, Lu S, et al. PR1 Vaccine Elicited Immunological Response after Hematopoietic Stem Cell Transplantation Is Associated with Better Clinical Response and Event-Free Survival. ASH Annual Meeting Abstracts 2007 November 16. 2007;110(11):577. [Google Scholar]
- 20.QuintasCardama A, Kantarjian HM, Rios R, Wieder ED, Molldrem JJ, Cortes J. Randomized Phase II Study of Proteinase 3-Derived PR1 Peptide Vaccine and GM-CSF with or without PEG-Interferon ALFA-2B to Eradicate Minimal Residual Disease in Chronic Myeloid Leukemia. ASH Annual Meeting Abstracts 2008 November 16. 2008;112(11):3219. [Google Scholar]
- 21.Sergeeva A, Alatrash G, He H, Ruisaard K, Lu S, Wygant J, et al. An anti-PR1/HLA-A2 T-cell receptor-like antibody mediates complement-dependent cytotoxicity against acute myeloid leukemia progenitor cells. Blood. 2011 Apr 21;117(16):4262–4272. doi: 10.1182/blood-2010-07-299248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez PV, Perry RL, Sarry JE, Perl AE, Murphy K, Swider CR, et al. A robust xenotransplantation model for acute myeloid leukemia. Leukemia. 2009 Nov;23(11):2109–2117. doi: 10.1038/leu.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma Q, Wang C, Jones D, Quintanilla KE, Li D, Wang Y, et al. Adoptive transfer of PR1 cytotoxic T lymphocytes associated with reduced leukemia burden in a mouse acute myeloid leukemia xenograft model. Cytotherapy. 2010 Dec;12(8):1056–1062. doi: 10.3109/14653249.2010.506506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin L, Lee EM, Ramshaw HS, Busfield SJ, Peoppl AG, Wilkinson L, et al. Monoclonal antibody-mediated targeting of CD123, IL-3 receptor alpha chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell. 2009 Jul 2;5(1):31–42. doi: 10.1016/j.stem.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 25.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994 Feb 17;367(6464):645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 26.Agliano A, Martin-Padura I, Mancuso P, Marighetti P, Rabascio C, Pruneri G, et al. Human acute leukemia cells injected in NOD/LtSz-scid/IL-2Rgamma null mice generate a faster and more efficient disease compared to other NOD/scid-related strains. International journal of cancer Journal international du cancer. 2008 Nov 1;123(9):2222–2227. doi: 10.1002/ijc.23772. [DOI] [PubMed] [Google Scholar]
- 27.Jones AR, Shusta EV. Antibodies and the Blood-Brain Barrier. In: An Z, editor. Therapeutic Monoclonal Antibodies: From Bench to Clinic. John Wiley & Sons, Inc; Hoboken, NJ, USA: 2009. [Google Scholar]
- 28.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997 Jul;3(7):730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 29.Lacey SF, La Rosa C, Kaltcheva T, Srivastava T, Seidel A, Zhou W, et al. Characterization of immunologic properties of a second HLA-A2 epitope from a granule protease in CML patients and HLA-A2 transgenic mice. Blood. 2011 Aug 25;118(8):2159–2169. doi: 10.1182/blood-2011-04-349951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alatrash G, Mittendorf EA, Sergeeva A, Sukhumalchandra P, Qiao N, Zhang M, et al. Broad cross-presentation of the hematopoietically derived PR1 antigen on solid tumors leads to susceptibility to PR1-targeted immunotherapy. Journal of immunology. 2012 Dec 1;189(11):5476–5484. doi: 10.4049/jimmunol.1201221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shultz LD, Schweitzer PA, Christianson SW, Gott B, Schweitzer IB, Tennent B, et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. Journal of immunology. 1995 Jan 1;154(1):180–191. [PubMed] [Google Scholar]
- 32.Hernandez-Ilizaliturri FJ, Jupudy V, Ostberg J, Oflazoglu E, Huberman A, Repasky E, et al. Neutrophils contribute to the biological antitumor activity of rituximab in a non-Hodgkin's lymphoma severe combined immunodeficiency mouse model. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003 Dec 1;9(16 Pt 1):5866–5873. [PubMed] [Google Scholar]
- 33.de la Fuente M, Alonso MC, Solana R, Pena J. Macrophage and lymphocyte antibody-dependent cellular cytotoxicity in spontaneous leukemogenesis of AKR/J mice. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 1989;10(6):310–315. doi: 10.1159/000217630. [DOI] [PubMed] [Google Scholar]
- 34.Mufson RA, Aghajanian J, Wong G, Woodhouse C, Morgan AC. Macrophage colony-stimulating factor enhances monocyte and macrophage antibody-dependent cell-mediated cytotoxicity. Cellular immunology. 1989 Mar;119(1):182–192. doi: 10.1016/0008-8749(89)90234-7. [DOI] [PubMed] [Google Scholar]
- 35.Boero S, Morabito A, Banelli B, Cardinali B, Dozin B, Lunardi G, et al. Analysis of in vitro ADCC and clinical response to trastuzumab: possible relevance of FcgammaRIIIA/FcgammaRIIA gene polymorphisms and HER-2 expression levels on breast cancer cell lines. Journal of translational medicine. 2015;13(1):324. doi: 10.1186/s12967-015-0680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bowles JA, Wang SY, Link BK, Allan B, Beuerlein G, Campbell MA, et al. Anti-CD20 monoclonal antibody with enhanced affinity for CD16 activates NK cells at lower concentrations and more effectively than rituximab. Blood. 2006 Oct 15;108(8):2648–2654. doi: 10.1182/blood-2006-04-020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiner GJ. Rituximab: mechanism of action. Seminars in hematology. 2010 Apr;47(2):115–123. doi: 10.1053/j.seminhematol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hahn T, McCarthy PL, Jr, Zhang MJ, Wang D, Arora M, Frangoul H, et al. Risk factors for acute graft-versus-host disease after human leukocyte antigen-identical sibling transplants for adults with leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008 Dec 10;26(35):5728–5734. doi: 10.1200/JCO.2008.17.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krogsgaard M, Wucherpfennig KW, Cannella B, Hansen BE, Svejgaard A, Pyrdol J, et al. Visualization of myelin basic protein (MBP) T cell epitopes in multiple sclerosis lesions using a monoclonal antibody specific for the human histocompatibility leukocyte antigen (HLA)-DR2-MBP 85–99 complex. The Journal of experimental medicine. 2000 Apr 17;191(8):1395–1412. doi: 10.1084/jem.191.8.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, Jr, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009 Jul 23;138(2):286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vago L, Perna SK, Zanussi M, Mazzi B, Barlassina C, Stanghellini MT, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med. 2009 Jul 30;361(5):478–488. doi: 10.1056/NEJMoa0811036. [DOI] [PubMed] [Google Scholar]
- 42.Villalobos IB, Takahashi Y, Akatsuka Y, Muramatsu H, Nishio N, Hama A, et al. Relapse of leukemia with loss of mismatched HLA resulting from uniparental disomy after haploidentical hematopoietic stem cell transplantation. Blood. 2010 Apr 15;115(15):3158–3161. doi: 10.1182/blood-2009-11-254284. [DOI] [PubMed] [Google Scholar]
- 43.Stewart DJ, Keating MJ, McCredie KB, Smith TL, Youness E, Murphy SG, et al. Natural history of central nervous system acute leukemia in adults. Cancer. 1981 Jan 1;47(1):184–196. doi: 10.1002/1097-0142(19810101)47:1<184::aid-cncr2820470130>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 44.Atwal JK, Chen Y, Chiu C, Mortensen DL, Meilandt WJ, Liu Y, et al. A therapeutic antibody targeting BACE1 inhibits amyloid-beta production in vivo. Science translational medicine. 2011 May 25;3(84):84ra43. doi: 10.1126/scitranslmed.3002254. [DOI] [PubMed] [Google Scholar]
- 45.Olson BM, McNeel DG. Antigen loss and tumor-mediated immunosuppression facilitate tumor recurrence. Expert review of vaccines. 2012 Nov;11(11):1315–1317. doi: 10.1586/erv.12.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Majeti R. Monoclonal antibody therapy directed against human acute myeloid leukemia stem cells. Oncogene. 2011 Mar 3;30(9):1009–1019. doi: 10.1038/onc.2010.511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.