Abstract
Pbx1 controls chromatin accessibility to a large number of genes and is entirely conserved between mice and humans. The Pbx1-d dominant negative isoform is more frequent in the CD4+ T cells from lupus patients than from healthy controls. Pbx1-d is associated with the production of autoreactive T cells in mice carrying the Sle1a1 lupus susceptibility locus. Transgenic expression of Pbx1-d in CD4+ T cells reproduced the phenotypes of Sle1a1 mice, with increased inflammatory functions of CD4+ T cells and impaired regulatory T cell homeostasis. Pbx1-d Tg also expanded the number of follicular helper T cells in a cell-intrinsic and antigen-specific manner that was enhanced in recall responses, and resulted in TH1-biased antibodies. Moreover, Pbx1-d Tg CD4+ T cells upregulated the expression of miR-10a, miR-21 and miR-155, which have been implicated in Treg and TFH cell homeostasis. Our results suggest that Pbx1-d impacts lupus development by regulating effector T cell differentiation and promoting TFH cells at the expense of Treg cells. In addition, our results identify Pbx1 as a novel regulator of CD4+ T cell effector function.
Keywords: Pbx1, regulatory T cells, follicular helper T cells, SLE, microRNA
Introduction
The NZM2410 murine strain was derived from the classical (NZB x NZW) F1 model of systemic lupus erythematosus (SLE), which is an autoimmune disease characterized by the production of autoantibodies (autoAbs) to nuclear antigens (Ag), immune activation, and immune-complex mediated inflammation in various tissues. We have mapped three major NZM2410 lupus susceptibility loci, and generated three congenic strains, B6.NZMSle1, -Sle2, and -Sle3, each carrying the corresponding NZM2410-derived genomic interval on the C57BL/6J (B6) genome (1, 2). Analysis of immunological properties of each congenic strain in comparison to B6 showed that Sle1 and Sle3 increase T cell activation and effector functions, and that Sle1 and Sle2 promote the development of autoreactive B cells and B cell hyperactivity (3).
Sle1 on telomeric chromosome 1 was the locus with the strongest linkage to lupus nephritis, and its expression is required for the development of systemic autoimmunity and pathogenesis in the NZM2410 model (4, 5). Sle1 expression results in the production of autoAbs specific for chromatin (6) through intrinsic defects in B and CD4+ T cells (7). Three Sle1 independent sub-loci, Sle1a, Sle1b, and Sle1c, contribute to autoimmune phenotypes (8). Sle1a and Sle1c induce the production of activated autoreactive T cells and decrease the number and function of Foxp3+ regulatory CD4+ (Treg) cells (9–11). Sle1b is associated with extensive polymorphisms between two divergent haplotypes of the SLAM family, and it regulates B cell (12) as well as T cell (13) tolerance. Sle1a has been mapped to two interacting loci, Sle1a1 and Sle1a2 (14). Sle1a1 contains only one functional known gene, pre-B cell leukemia homeobox 1 (Pbx1), and the lupus-associated allele corresponds to the increased expression of a novel splice isoform, Pbx1-d, in CD4+ T cells (15). Pbx1, a member of the three-amino-acid-loop-extension (TALE) class of homeodomain proteins, is a transcriptional factor that regulates chromatin access of multimeric complexes that include Hox factors, as well as myeloid ecotropic viral integration site (Meis) and Pbx-regulating protein-1 (Prep-1), two other TALE proteins that regulate chromatin remodeling and coactivator access (16). The interactions of Pbx-Meis/Prep-1 complexes with both Hox and non-Hox factors ultimately contribute to either gene activation or repression (17). Pbx1-d lacks exons 6 and 7 corresponding to the DNA binding domain and the Hox binding domain, respectively, which confers this splice isoform a dominant-negative function (18).
Sle1a1 expands the number of activated and autoreactive CD4+ T cells, and reduces the number of peripheral Treg (pTreg) cells in a CD4+ T cell intrinsic manner (15). These T cell phenotypes were not sufficient however to induce a robust production of autoAbs in B6.Sle1a1 mice, which requires the expression of Sle1 in B cells (14). In addition, Pbx1-d expression is associated with abnormal responses to TGF-β and retinoic acid (RA) in both murine and human T cells (19). Pbx1 expression is necessary for B cell development (20), but its function in T cells is unknown. The goal of this study was to directly address the role of Pbx1-d over-expression in CD4+ T cells. We showed that transgenic B6 mice that overexpress Pbx1-d in their CD4+ T cells (Pbx1-d Tg mice) reproduce the phenotypes of B6.Sle1a1 mice, with increased inflammatory functions of CD4+ T cells and impaired Treg cells homeostasis. In addition, Pbx1-d Tg mice showed a follicular helper T (TFH) cell population that expanded in an Ag-specific and T-cell intrinsic manner, with an enhanced capacity to locate in B cell follicles and to promote affinity maturation of TH1-associated Ab isotypes. These results suggest that Pbx1 regulates the balance between pTreg and TFH cell maintenance or differentiation, and that Pbx1-d contributes to autoimmunity by tilting the balance in favor on TFH over Treg cells.
Materials and Methods
Mice
B6.CD4-Pbx1-d Tg (Pbx1-d Tg) mice were generated at the University of Florida transgenic core using a bicistronic Pbx1-d/GFP cDNA controlled by the CD4 promoter (Sup. Fig. 1A) in a C57BL/6J background. Four Tg lines were obtained, with a Tg copy number of 8–13 in the A886, C855 and A872 lines, and 30 in the C861 line (Sup. Fig. 1B). GFP protein expression was not achieved in any of the lines. The following primers were used to detect Pbx1-d Tg are: Forward (spanning exons 5 and 8): ATCACAGTCTCCCAGGTGGA, and Reverse (in exon 9): ATCCTGCCAACCTCCATTAG. Pbx1-d expression was restricted to CD4+ T cells (Sup. Fig. 1B and C). Primer and Taqman probe sequences used to measure Pbx1-d message expression have been described (21). Mice from all four lines were healthy at least up to one year of age. Results reported in this study were obtained with mice from the first three lines, without any difference observed between lines. C861 mice were not included due to poor breeding performance. Initial characterization was performed with Tg-negative littermates, which presented phenotypes identical to that of B6 controls. No difference was observed between hemizygous and homozygous lines, indicating that within the observed range, the Tg copy number was not critical for the phenotypes. The results reported in this study were obtained with homozygous mice. B6, B6.SJL-PtprcaPep3b/BoyJ (B6.Ly5a), B6.Cg-Tg(TcraTcrb)425Cbn/J (B6.OT-II) and B6.129S7-Rag1tm1Mom/J (B6.Rag-1−/−) mice were originally obtained from the Jackson Laboratory. B6.Thy1a.OT-II mice were graciously provided by Dr. Stephen Schoenberger. The B6.Sle1a.1NZW/NZW (B6.Sle1a1) and B6.NZM-Sle1NZM2410/AegSle2NZM2410/AegSle3NZM2410/Aeg/LmoJ (B6.TC) congenic mice have been previously described (5, 14). B6.Foxp3-enhanced GFP (B6.Foxpegfp) mice (22) were kindly provided by Dr. V. J. Kuchroo. Pbx1-d Tg.Foxp3egfp mice were bred from the A886 line and Pbx1-d Tg.OT-II were bred from the C855 line. All mice were bred and maintained at the University of Florida in specific pathogen-free conditions. Only female mice, except for the colitis experiment, were used in this study under a protocol approved by the Institutional Animal Care and Use Committee of the University of Florida.
T cell polarization
In vitro induced Treg (iTreg) cells were differentiated as previously described (15). Briefly, CD4+CD25− cells were negatively selected from B6 or Pbx1-d Tg splenocytes using the CD4+CD25+ Treg isolation kit (Miltenyi Biotech), and then 5 × 105 cells were stimulated with mouse T-activator CD3/CD28 beads (Life Technologies) at the concentration of 1 × 106 beads/mL in the presence of 100 U IL-2, 20 ng/ml TGF-β (Pepro Tech), and 0–5 nM all-trans retinoic acid (RA) for 5 d. TH1 and TH17 polarization was performed as previously described (23).
Flow cytometry
Single-cell suspensions were prepared using standard procedures from spleen, thymus, and mesenteric lymph node (mLN). After red blood cell lysis, cells were blocked with anti-CD16/32 Ab (2.4G2), and stained in FACS staining buffer (2.5% FBS, 0.05% sodium azide in PBS). Fluorochrome-conjugated Abs used were to B220 (RA3-6B2), BCL6 (K112-91), CD4 (RAM4-5), CD8 (53-6.7), CD25 (PC61.5), CD44 (IM7), CD45.1 (A20), CD45.2 (104), CD62L (MEL-14), CD69 (H1.2F3), CD95 (Jo2), CD90.1 (HIS51), CD90.2 (53-2.1), Foxp3 (FJK-16s), Ly-77 (GL7), Neuropilin-1 (761705), PD-1 (RMP1-30), IFN-γ (XMG1.2), IL-2 (JES6-5H4), IL-17a (eBio17B7), and IL-21 (FFA21) purchased from BD Biosciences, eBioscience, BioLegend, and R&D Systems. Follicular T cells were stained as previously described (55) in a three-step process using purified CXCR5 (2G8) followed by biotinylated anti-rat IgG (Jackson ImmunoResearch), then PerCP5.5-labeled streptavidin in FACS staining buffer on ice. Dead cells were excluded with fixable viability dye (eFluor780; eBioscience). Data were collected on LSRFortessa (BD Biosciences) and analyzed with FlowJo software (Tree Star).
Mixed bone marrow (BM) chimera and adoptive cell transfers
Chimeras were prepared as previously described (9) with the following modifications. 8–10 week-old (B6 x B6.Ly5a)F1 recipient mice were lethally irradiated with two doses of 452 rad (4 h apart) using an X-RAD 320 irradiator (Precision X-Ray). Donor BM cells were depleted of mature T cells using CD5 MicroBeads (Miltenyi Biotech). Mixed 1:1 BM cells (1 × 107 cells) were given to the recipient mice by tail-vein injection. Treg cells were analyzed in chimeric mice 8 weeks later. For T-dependent (TD) immune responses, chimeric mice were immunized with 100 μg of NP23-KLH (Biosearch Tech.) in alum 8 weeks after BM cell transplantation, and TFH cells were analyzed 1 week after immunization. CD4+ T cells from Pbx1-d Tg.OT-II, B6.Sle1a1.OT-II and B6.OT-II mice were purified by negative selection using MACS MicroBeads, then 0.5 × 106 cells were tail-vein injected into B6.Ly5a mice, followed by subcutaneous immunization with 50 μg NP16-OVA in alum 4 h after cell transfer. For mixed adoptive transfer, CD4+ T cells from Pbx1-d Tg.OT-II or B6.Sle1a1.OT-II and B6.Thy1a.OT-II mice were mixed at 1:1 ratio, and 1 × 106 cells were injected into (B6 x B6.Thy1a)F1 recipient mice prior to immunization with NP16-OVA.
To assess the function of effector T (TEff) cells in vivo, 4 × 105 purified CD4+CD25−TEff cells from 2 month-old Pbx1-d Tg and B6 mice were tail-vein injected into B6.Rag-1−/−mice. The function of Treg cells was evaluated in co-injections of 1 × 105 CD4+CD25+ Treg cells from either Pbx1-d Tg or B6 mice along with 4 × 105 CD4+CD25−B6 TEff cells. Recipient mice were monitored for clinical signs of colitis for up to 8 weeks and body weight was monitored weekly. The recipient mice that lost more than 15% body weight or showed overt clinical signs of disease were sacrificed. Colon histology was ranked blindly in a semi-quantitative fashion as previously described (9).
Immunohistochemistry
To stain splenic germinal centers (GCs) in B6.Ly5a mice that received Pbx1-d Tg.OT-II or B6.OT-II CD4+ T cells, 7 μm thick OCT-embedded cryosections were prepared on super-frost glass, washed with PBS, fixed with 4% paraformaldehyde for 15 min at 4°C, and subsequently permeabilized with 0.1% Triton X-100 for 5 min at 4°C. The sections were washed with cold PBS and incubated overnight in the dark at 4°C with anti-CD45.2 conjugated to PE, anti-GL7 conjugated to AF488, and anti-IgD conjugated to APC (all from eBioscience). Sections were washed three times with cold PBS, mounted with cytoseal, and covered with glass coverslip. The stained sections were analyzed using an EVOS FL digital inverted fluorescence microscope (Fisher Scientific, Waltham, MA), images were captured by ×10, ×20, and ×40 objectives, keeping all of the conditions of microscope and settings of software identical for all treatments and controls.
Immunization and Ab measurements
For TD responses, 8–10 wk-old mice were immunized intra-peritoneally with 100 μg NP23KLH in alum, and boosted at week 2 and 6. Serum samples were collected 1, 3, 5, and 7 weeks after the first immunization. NP-specific Abs were measured by ELISA using NP4-or NP25-BSA (Biosearch Tech.) coated plates, followed by incubation with 1:1000 diluted serum samples and developed with alkaline phosphatase-conjugated goat anti-mouse IgG1, IgG2a, IgG2c, IgG3, or IgM (Southern Biotech). All samples were run in duplicate. Anti-dsDNA and anti-chromatin IgG were measured as previously described (6) in sera diluted 1:100, and relative units were standardized using serial dilutions of a positive serum from B6.TC mice, setting the 1:100 dilution reactivity to 100 U. Serum anti-nuclear Ab (ANA) were measured by applying 1:40 diluted sera to fixed Hep-2 cells (Inova) and revealed with anti-mouse IgG-FITC (Invitrogen).
miRNA analysis
Splenic CD4+ T cells from Pbx1-d Tg.Foxp3egfp, B6.Sle1a1.Foxp3egfp, and B6.Foxp3egfp mice were first enriched by negative selection using MACS MicroBeads, then GFP+ and GFP−cells were sorted with a FACSAria cytometer with a purity ≥ 95%. miRNAs were isolated and reverse transcribed using Life Technologies reagents. Quantification of miRNAs expression was performed using TaqMan MicroRNA assays (Life Technologies) using the Applied Biosystems StepOne™ real-time PCR machine. U6 snRNA was used as internal control. Comparisons were made using the 2−ΔΔCt method and values were normalized to the B6 average values.
Luciferase assay
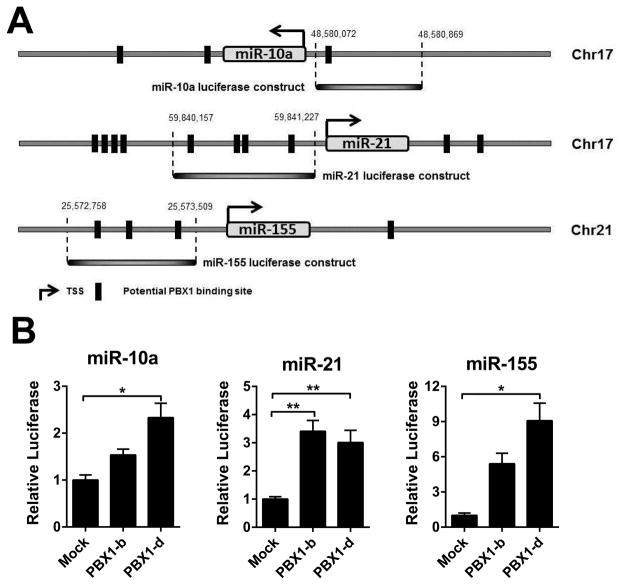
Putative human PBX1 binding sites in the ~ 2 Kb flanking miR-10a, miR-21 and miR-155 precursors were identified using Jaspar3 (set for 75% accuracy). We generated luciferase reporter constructs containing the 798-bp, 1071-bp and 850-bp region upstream of miR-10a, miR-21 and miR-155, respectively (Fig. 8A) cloned into the pGL4.25 Luciferase vector (Promega). PBX1-b or PBX1-d expression plasmids were generated by inserting cDNAs (15) into the pHAGE-CMV-MCS-IzsGreen plasmid. The luciferase reporter constructs and the pGL3-basic plasmid were then each co-transfected with either PBX1-b or PBX1-d expression plasmid into HEK293T cells. Cells were harvested after 48 h. After lysis with the Passive Lysis Buffer (Promega), luciferase activity was measured using a dual luciferase reporter assay system (Promega) according to the manufacturer’s instructions with a Lumat LB 9507 luminometer (Berthold Technologies).
Figure 8. Human PBX1 binds to the promoter of miR-10a, miR-21, and miR-155.
A. miRNA luciferase constructs containing predicted PBX1 binding sites were selected using Jaspar3. The regions used in the luciferase assays are indicated for each locus. B. Dual luciferase analyses of miR-10a, miR-21 and miR-155 expression in the presence of either PBX1-b or PBX1-d, showing fold change relative to the absence of PBX1 expression plasmid. All results are expressed as mean ± SD from at least three independent experiments.
Statistical analysis
Differences between groups were evaluated by two-tailed statistics: unpaired t tests or Mann-Whitney U tests depending on whether the data was normally distributed, paired t tests for mixed BM-chimeras, χ2 tests to compare distributions, and two-way ANOVA tests for time-course experiments. Unless specified, graphs show means and standard deviations of the mean. *P < 0.05, **P < 0.01, and ***P < 0.001.
Results
Pbx1-d Tg mice replicated the phenotypes of B6.Sle1a1 mice
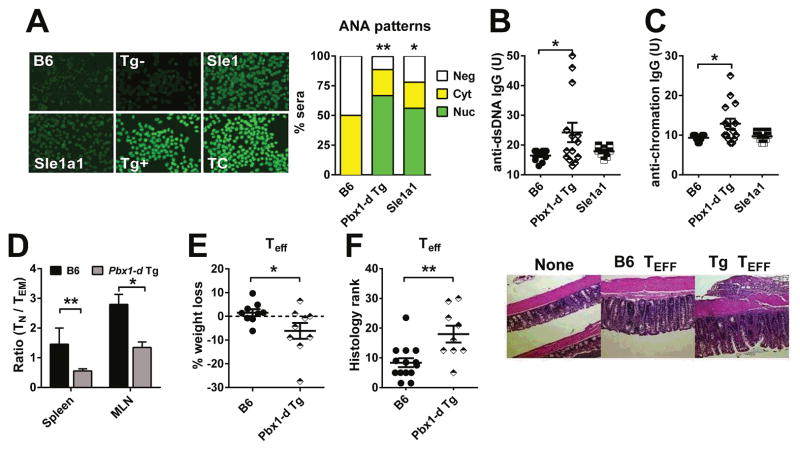
To address whether the overexpression of Pbx1-d in CD4+ T cells was sufficient to induce their activation and loss of tolerance, we generated Pbx1-d Tg mice that express Pbx1-d driven by the Cd4 promoter (Supl. Fig. 1). Pbx1-d Tg overexpression in CD4+ T cells resulted in the production of serum ANA with the characteristic homogenous nuclear staining pattern (Fig. 1A) as well as anti-dsDNA and anti-chromatin IgG in 7 to 12 month old mice (Fig. 1B and C). A similar nuclear staining pattern was observed in the serum of B6.Sle1a1 mice, although with a lower intensity, which is consistent with a low level of anti-dsDNA and anti-chromatin IgG (Fig. 1B and C), as we have previously reported for this strain (14, 15). This suggested that Pbx1-d overexpression results in the production of autoreactive CD4+ T cells that are sufficient to induce humoral autoimmunity against nuclear Ag. B6.Sle1a1 mice were characterized by the expansion of CD44+CD62L−effector memory T (TEM) cells relative to CD44−CD62L+ naïve T (TN) cells (15). Aged Pbx1-d Tg mice also presented a skewed TN/TEM ratio (Fig. 1D). We examined the consequence of Pbx1-d overexpression in CD4+CD25−TEff cells in vivo with the experimental colitis model that we have used with B6.Sle1a1 T cells (9). B6.Rag1−/− mice that received Pbx1-d Tg T cells showed a greater body weight loss and a Eff more severe colitis than the mice that received B6 TEff cells (Fig. 1E–F). These results suggest that Pbx1-d expression is sufficient to induce an intrinsic activation in CD4+ T cells. However, we observed a similar ability of Pbx1-d Tg or B6 CD4+CD25+ T cells to suppress B6 TEff cells in the same colitis model (data not shown), suggesting that Pbx1-d Tg Treg in vivo functions were less affected than that of B6.Sle1a1 Treg cells (14).
Figure 1. Pbx1-d overexpression in CD4+ T cells reproduced the phenotypes of B6.Sle1a1 mice.
A. Representative serum ANA staining patterns in B6, Pbx1-d Tg− (Tg−), B6.Sle1, B6.Sle1a1, Pbx1-d Tg (Tg+), and TC mice as positive control. The pattern distribution (nuclear, cytoplasmic or negative) was significantly different between Pbx1-d Tg+ or B6.Sle1a1 and (B6 + Tg−) combined mice (n = 10–18 per strain). Serum anti-dsDNA (B) and anti-chromatin (C) IgG (n = 10–15). D. TN/TEM ratio in the spleen and mLN from B6 and Pbx1-d Tg mice (n = 3–4). Mice were 7–12 month old for A–D. E. Maximum weekly percentage of body weight loss by B6.Rag1−/ − mice up to 8 weeks after transfer of Pbx1-d Tg or B6 TEff cells. F. Colitis pathology score 8 weeks after transfer with representative images of colon histology taken with the same 40X magnification shown on the right. Each symbol represents a mouse. *P < 0.05, **P < 0.01.
Pbx1-d overexpression impaired Treg cell homeostasis
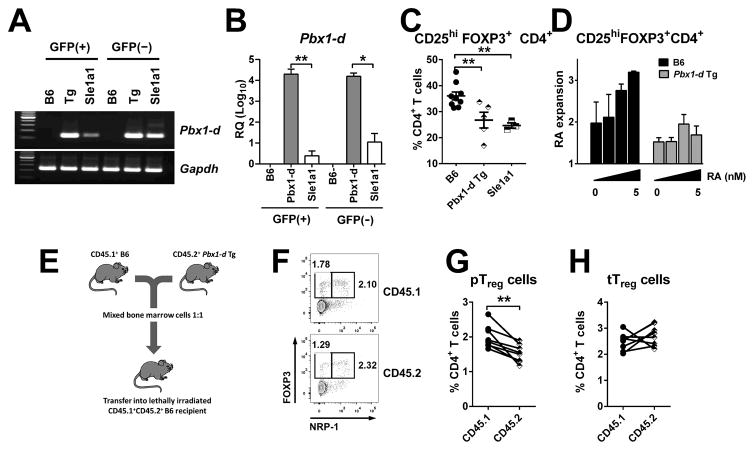
To assess the role of Pbx1-d in Treg cells as compared to conventional CD4+ T cells, we bred the EGFP-Foxp3 reporter construct to both B6.Sle1a1 and Pbx1-d Tg mice. Pbx1-d expression in Pbx1-d Tg CD4+ T cells was ~ 10,000 folds higher than in B6 T cells, and was similar in Foxp3+ and Foxp3− cells (Fig. 2A–B). Pbx1-d expression in the Pbx1-d Tg T cells was also much higher (~100 folds) than in B6.Sle1a1 mice in either Foxp3+ or Foxp3−T cells (Fig 2A–B). As previously reported for B6.Sle1a1 mice (14, 15), the frequency of CD4+Foxp3+ Treg cells was reduced in Pbx1-d Tg mice with the largest difference in the mLN (Fig. 2C). In addition, in vitro iTreg cell polarization by TGF-β and RA was reduced in Pbx1-d Tg CD4+CD25−T cells as compared to B6 cells (Fig. 2D). These results indicate that, as Sle1a1, Pbx1-d overexpression in CD4+ T cells impairs iTreg differentiation by interfering with the response to RA in the presence of TGF-β.
Figure 2. Pbx1-d overexpression impaired pTreg cell induction.
bx1-d mRNA expression in Foxp3+ and Foxp3−CD4+ T cells purified from B6.Foxp3egfp, Pbx1-d Tg.Foxp3egfp, and B6.Sle1a1.Foxp3egfp mice analyzed by conventional (A) and quantitative (B) RT-PCR with the data presented relative to B6 (RQ; n = 3). C. Frequency of Foxp3+ Treg cells in the mLN of 10–12 month-old B6, Pbx1-d Tg, and B6.Sle1a1 mice. Each symbol represents a mouse. D. Foxp3 induction in CD4+CD25−T cells in the presence of TGF-β, presented as the ratio of induction with over without RA. E–H. Mixed-BM chimera analysis of Treg cells. E. Experimental design. F. Gates for Foxp3+Nrp-1+ tTreg and Foxp3+Nrp-1−pTreg cells within the total CD4+CD8−CD45.1+ or CD45.2+ thymocytes. Percentage of pTreg (G) and tTreg cells (H) in the chimeras with each linked symbol representing a mouse. *P < 0.05, ** P < 0.01.
To further evaluate the impact of Pbx1-d on Treg differentiation, we reconstituted lethally irradiated (B6 x B6.Ly5a)F1 mice with T cell-depleted BM cells mixed from Pbx1-d Tg and B6.Ly5a mice (Fig. 2E). Pbx1-d Tg BM yielded to a decreased percentage of CD4+Foxp3+Nrp-1−pTreg cells as compared to the B6.Ly5a BM in the thymus of the recipient mice, whereas the proportion of CD4+Foxp3+Nrp-1+ thymic Treg (tTreg) cells was similar between the two genotypes (Fig. 2F–H). In these conditions, however, the proportions of Treg cells in the peripheral organs of recipient mice were similar for BM-derived cells of either genotypes (data not shown), possibly due to the relatively short time between BM transfer and phenotype readout. Together these data indicate that Pbx1-d Tg overexpression in CD4+ T cells impairs the induction or maintenance of pTreg cells in a cell-intrinsic manner.
Pbx1-d Tg overexpression in CD4+ T cells expanded the number of TFH cells
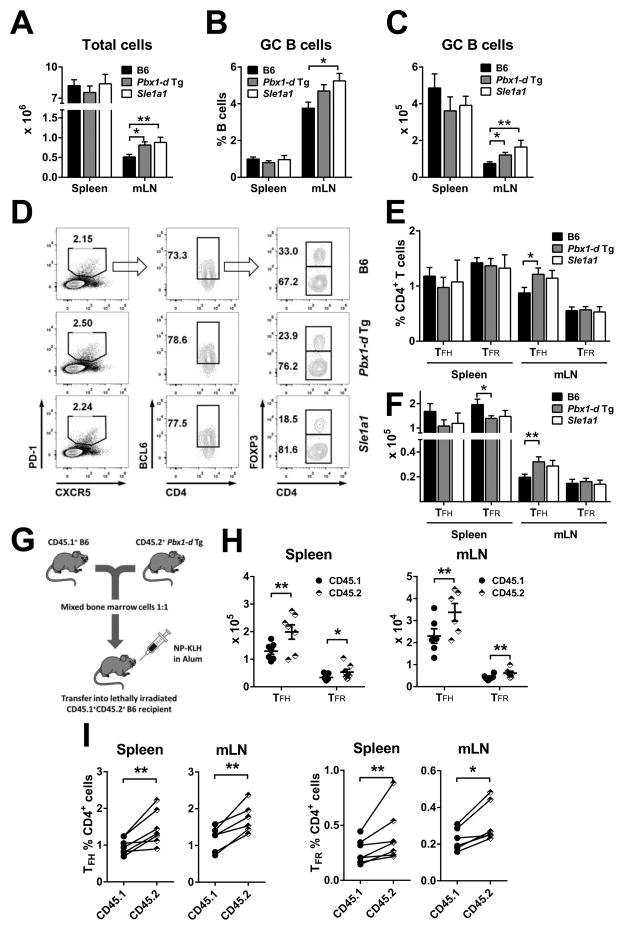
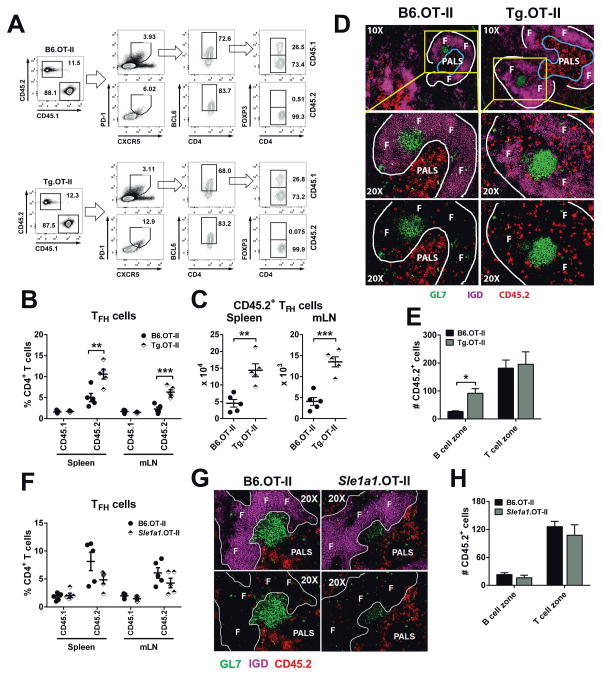
Since Pbx1-d overexpressing CD4+ T cells showed an activated phenotype (Fig. 1D), we next examined their cytokine production. Ex vivo Pbx1-d Tg CD4+ T cells showed a significantly increased IL-21 expression (Sup. Fig. 2A–B) as compared to B6, but there was no difference for the level of IL-2, IL-17A or IFN-γ production (Sup. Fig. 2A, C–E), or the frequency of IL-17A+ or IFN-γ+ CD4+ T cells (data not shown). Under in vitro TH1 polarization conditions, a small but significant difference was observed with Pbx1-d Tg CD4+ T cells producing more IFN-γ than B6 cells, while there was no difference for IL-17A (Sup. Fig. 2F and G). These results prompted us to investigate TFH cell differentiation in Pbx1-d Tg mice. TFH cells secrete high levels of IL-21, and IL-21 signaling up-regulates Bcl6 expression, which is required for the development of TFH cells and germinal center (GC) formation (24–27). At steady state, young (6–8 weeks) Pbx1-d Tg and B6.Sle1a1 mice showed an increased cellularity in the mLN, where the numbers and percentages of GC B cells were also increased as compared to B6 (Fig. 3A–C). The percentages and absolute numbers of CD4+PD1+CXCR5+Bcl6+Foxp3−TFH cells were significantly higher in the mLN from Pbx1-d Tg mice, but not in their spleen (Fig. 3D–F). A similar trend was observed for B6.Sle1a1 TFH cells. On the other hand, the number of CD4+PD1+CXCR5+Bcl6+Foxp3+ follicular regulatory T (TFR) cells was significantly reduced in the spleen of Pbx1-d Tg mice (Fig. 3F). We next tested the differentiation of TFH and TFR cells 7 d after administrating a TD Ag into chimeric mice reconstituted with a 1:1 mix of Pbx1-d Tg and B6 BM (Fig. 3G). The number and percentage of both Pbx1-d Tg-derived TFH and TFR cells were significantly increased as compared to B6-derived cells (Fig. 3H and I). The discrepancy between the unchanged frequency or a decreased number of Pbx1-d Tg TFR cells in unmanipulated mice (Fig. 3E and F) on one hand and the increased number and frequency of Pbx1-d Tg-derived TFR cells in chimeric mice (Fig. 3H and I) on the other hand is likely due to homeostatic expansion that favors all Bcl6-expressing Pbx1-d Tg-derived T cells. The numbers of B6 and Pbx1-d Tg-derived total CD4+ T cells were similar before immunization, indicating an Ag-driven enhancement of TFH cell differentiation by Pbx1-d overexpression. These steady-state and immunization results are consistent with Pbx1-d overexpression in CD4+ T cells increasing TFH cell differentiation in a cell-intrinsic manner.
Figure 3. Pbx1-d Tg expression in CD4+ T cells expanded GC lymphocytes.
Absolute numbers of lymphocytes (A), percentages (B) and absolute numbers (C) of B220+GL-7+FAS+ GC B cells in the spleen and mLN from 2 month-old B6, Pbx1-d Tg, and B6.Sle1a1 mice. D. Representative FACS plots for CD4+CXCR5+PD-1+Bcl6+Foxp3+ TFR and CD4+CXCR5+PD-1 +Bcl6+Foxp3−TFH cells. Percentages (E) and absolute numbers (F) of TFH and TFR cells. n = 5–10. G–I: Follicular T cell expansion in response to NP-KLH immunization in mixed BM chimeras. G: Experimental strategy. Absolute numbers (H) and percentages (I) of TFH and TFR cells 7 d after immunization gated according to their strain of origin: CD45.1: B6, CD45.2: Pbx1-d Tg. Each symbol (linked in I) represents a mouse. *P < 0.05, **P < 0.01.
Pbx1-d Tg overexpression enhanced Ag-specific TFH cell differentiation and follicular localization
To track TFH cells in an Ag-specific manner, Pbx1-d Tg mice were bred with B6.OT-II mice, which express an MHC class II-restricted T cell receptor specific for ovalbumin (OVA) (28). Pbx1-d Tg.OT-II or B6.OT-II CD4+ T cells were transferred into B6.Ly5a mice, which were then immunized with NP-OVA. By d 5 after immunization, Pbx1-d Tg.OT-II and B6.OT-II total CD4+ T cells were expanded to a similar extent, and there was no difference for the recipient CD45.1+ T cells (Fig. 4A). However, the CD45.2+ Pbx1-d Tg.OT-II CD4+ T cells contained a higher frequency and number of TFH cells (Fig. 4B and C) than the B6.OT-II CD4+ T cells. Either Pbx1-d Tg.OT-II or B6.OT-II transferred CD4+ T cells produced very low numbers of TFR cells (Fig. 4A). Homing of primed TFH cells to B cell follicles is a critical step to generate GCs. The number of transferred Pbx1-d Tg.OT-II CD4+ T cells within the B cell zone was significantly higher than the number of B6.OT-II CD4+ T cells, whereas there was no difference in the number of transferred T cells in the T cell zone (Fig. 4D and E). In addition, CXCR5 and Bcl6 expression was significantly higher in the transferred Pbx1-d Tg.OT-II than the transferred B6.OT-II CD4+ T cells (CXCR5: 483 ± 18.14 vs. 430.20 ± 10.14, P < 0.001; Bcl6: 507.20 ± 23.21 vs. 436.60 ± 13.86, P < 0.05). At d 7 after immunization, there was a similar frequency of Pbx1-d Tg.OT-II and B6.OT-II TFH cells (Supplemental Fig. 3A), and a similar number of these cells in the spleen (Supplemental Fig. 3B), although the number ofPbx1-d Tg.OT-II TFH cells was still higher in the mLN (Supplemental Fig. 3B). Interestingly, the number of total Pbx1-d Tg.OT-II T cells was increased in both spleen and mLN (Supplemental Fig. 3C). This expanded population was largely absent from the GC (Supplemental Fig. 3D), suggesting that they may correspond to extrafollicular helper T cells, a population that is expanded in lupus-prone mice (29).
Figure 4. Pbx1-d Tg expression in CD4+ T cells regulated Ag-specific TFH cell differentiation.
TFH cells were analyzed 5 d after NP-OVA immunization of CD45.1+ B6 mice transferred with CD45.2+ naïve B6.OT-II or Pbx1-d Tg.OT-II cells. A. Representative flow cytometric plots showing mLN CD4+ CD45.1+ (endogenous) and CD45.2+ (transferred) gated CXCR5+PD-1+ TFH and TFR cells. B. Percentages of donor and recipient TFH cells. C. Absolute numbers of donor TFH cells. Each symbol represents a recipient mouse. D. Localization of donor OT-II CD4+ T cells (CD45.2+, red) relative to B cell follicles (IgD+, F), GCs (GL7+, green), and periarteriolar lymphoid sheaths (PALS). The framed areas in the 10x magnification are shown below at 20x, with the bottom panels showing only the outline of the IgG+ B cell follicles for a better comparison of the number and location of CD45.2+ T cells. E. Numbers of B6.OT-II or Pbx1-d Tg.OT-II CD4+ T cells in B or T cell zones, showing mean ± SEM from 3–7 GC regions per mouse from 3 mice per strain. F. Percentages of donor and recipient TFH cells 5 d after NP-OVA immunization of CD45.1+ B6 mice transferred with CD45.2+ naïve B6.OT-II or Sle1a1.OT-II cells. G. Representative 20x spleen sections of B6.OT-II or Sle1a1.OT-II cell recipients, as in D. H. Numbers of B6.OT-II or Sle1a1.OT-II CD4+ T cells in B or T cell zones, showing mean ± SEM from 3–7 GC regions per mouse from 5 mice per strain. *P < 0.05, **P < 0.01.
We performed the same experiment with B6.Sle1a1.OT-II CD4+ T cells, which generated a similar frequency and number of TFH cells as well as a similar number of B6.Sle1a1.OT-II CD4+ T cells located in the B cell follicles than B6.OT-II CD4+ T cells 5 d after immunization (Fig. 4F–G). At d 7 after immunization, the number and frequency of TFH cells were also similar between cells of Sle1a1 or B6 origin (Supplemental Fig. 3E and F). The number of total Sle1a1.OT-II CD4+ T cells was however higher in the spleen than B6.OT-II T cells and there was a trend in the mLN (Supplemental Fig. 3G). Therefore, these results suggest that a high level of Pbx1-d expression in CD4+ T cells increased Ag-specific TFH cell differentiation as well as early entry in B cell follicles, while the more modest increased expression of Pbx1-d in Sle1a1 CD4+ T cells increased their Ag-specific expansion.
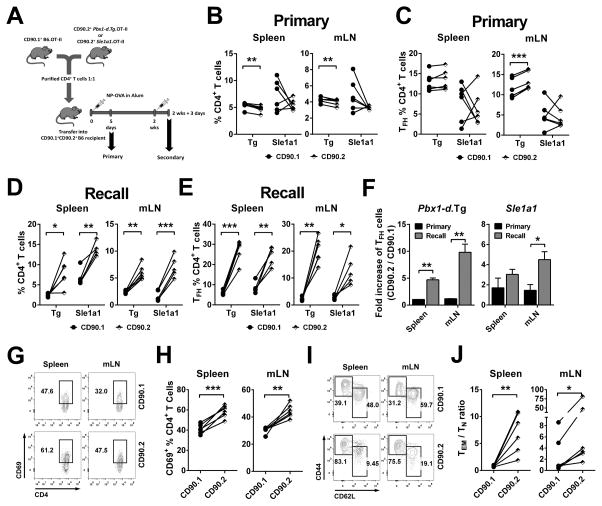
We then examined the effect of Pbx1-d overexpression on TFH cell differentiation in a competitive setting by co-transferring B6.Thy1a.OT-II and Pbx1-d Tg.OT-II (1:1) CD4+ T cells into (B6 x B6.Thy1a)F1 recipient mice. Five days after primary NP-OVA immunization (Fig. 5A), the percentage of total B6.Thy1a.OT-II CD4+ T cells was higher than that of Pbx1-d.OT-II Tg CD4+ T cells in the spleen and mLN, and the same trend was observed for Sle1a1.OT-II CD4+ T cells (Fig. 5B). A higher percentage of TFH cells was only found with Pbx1-d Tg origin in the mLN (Fig. 5C). We next asked whether Pbx1-d overexpression influenced the recall Ag-response in the same model (Fig. 5A). In contrast to the primary response, the percentages of Pbx1-d.OT-II Tg and Sle1a1.OT-II total CD4+ T cells (Fig. 5D) and TFH cells (Fig 5E) were higher than that of corresponding cells of B6 origin in the spleen and mLN at 3 d after secondary immunization. Moreover, the relative expansion of the TFH subset was significantly greater in cells from Pbx1-d Tg or Sle1a1 origin as compared to cells from B6 origin in the recall as compared to the primary response (Fig. 5F). Finally, the percentage of Pbx1-d Tg activated (Fig. 5G–H) and CD44hi memory CD4+ T cells (Fig 5I–J) was enhanced, which we did not observed during the primary response. Taken together, these results indicate that Pbx1-d overexpression conferred a cell-intrinsic competitive advantage during Ag-specific TFH cell differentiation that was enhanced during recall responses.
Figure 5. Pbx1-d intrinsically regulated TFH cell differentiation and amplified recall responses.
A. Experimental design for primary and recall response after mixed adoptive transfer of CD90.1+ B6.OT-II and CD90.2+ Pbx1-d Tg.OT-II or Sle1a1.OT-II CD4+ T cells into (B6 x B6.Thy1a)F1 mice. Percentages of CD90.1+ B6.OT-II and CD90.2+ Pbx1-d Tg.OT-II or Sle1a1.OT-II CD4+ T cells (B and D) and TFH cells (C and E) from each genotype in the primary (B and C) and recall (D and E) responses. F. Fold increase of TFH cells in recall vs. primary response, calculated as the ratio of CD90.2+ /CD90.1+ TFH cells. Representative flow cytometric plots and corresponding percentages of CD4+CD69+ activated T (G and H) and TEM / TN CD4+ T cells ratios (I and J) in CD90.1+ B6.OT-II and CD90.2+ Pbx1-d.OT-II Tg donor cells in the recall response. Each linked symbol represents a recipient mouse. n = 5–6, *P < 0.05, **P < 0.01, ***P < 0.001.
Pbx1-d Tg mice responded to TD Ags with an increased TH1-related Ig isotypes
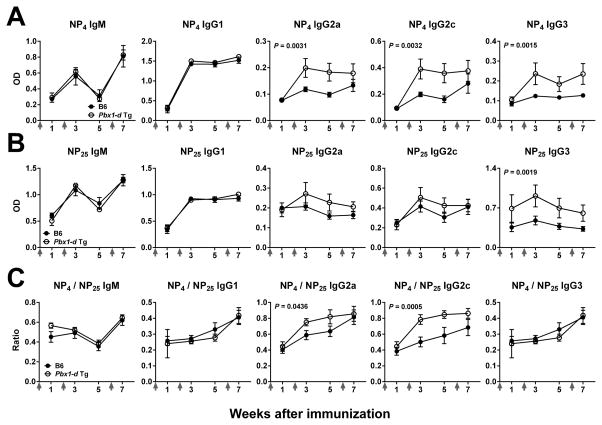
To assess the effect of Pbx1-d overexpression on affinity maturation and class-switching, B6 and Pbx1-d Tg mice were boosted 2 and 6 weeks after primary immunization with NP-KLH. Pbx1-d Tg mice produced significantly more high and low affinity anti-NP-IgG3 and high-affinity anti-NP IgG2a and IgG2c than B6 mice (Fig. 6A–B). Moreover, the affinity of anti-NP IgG2a and IgG2c as measured by the NP4/NP25 ratio was significantly increased in Pbx1-d Tg mice after boosting (Fig. 6C), suggesting that Pbx1-d overexpression increases affinity maturation of TH1-associated antibodies.
Figure 6. Pbx1-d Tg expression in CD4+ T cells increased affinity maturation of TH1-related isotypes.
B6 and Pbx1-d Tg mice were immunized with NP-KLH then boosted 2 and 6 weeks later (arrows on X axes). Serum levels of NP-specific Abs 1 wk before and after boosting measured in plates coated with NP4 (A) or NP25 (B) BSA. C. Affinity measured as the NP4 / NP25 ratio for each isotype, n = 5.
Pbx1-d overexpression increased the expression of miR-10, miR-21 and miR-155 in CD4+ T cells
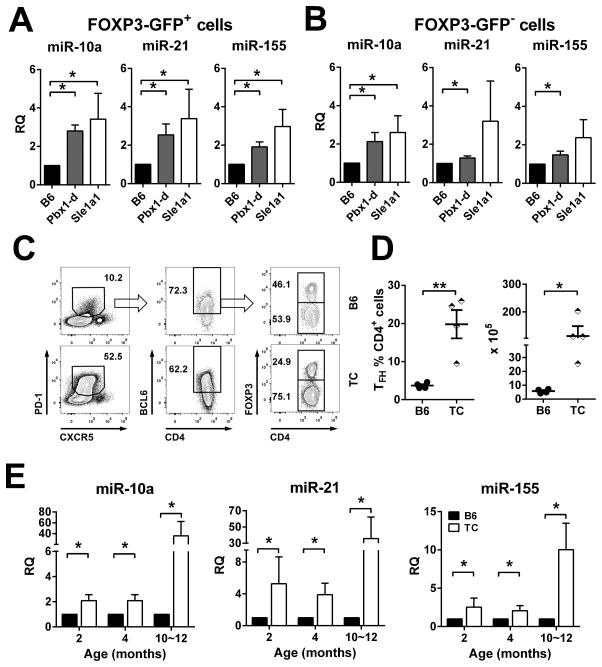
Numerous studies have implicated microRNAs (miRNAs) in Treg and TFH cell differentiation and functions (30). We have analyzed the effect of Pbx1-d overexpression on candidate miRNAs selected for their role in this process (miR-10a, -17, -19a, -19b, -20a, -21, -92, -155, -181a, and -181b) in Foxp3-GFP+ and Foxp3-GFP−CD4+ T cells from young Pbx1-d Tg.Foxp3egfp, B6.Sle1a1.Foxp3egfp, and B6.Foxp3egfp mice. miR-10a, miR-21 and miR-155 expression was 2–3 fold higher in either Pbx1-d Tg or B6.Sle1a1 than in B6.Foxp3-GFP+ Treg cells (Fig. 7A). The same result was obtained for Pbx1-d Tg Foxp3-GFP−conventional CD4+ T cells, while expression of miR-21 and miR-155 was more variable in B6.Sle1a1 Foxp3-GFP−CD4+ T cells, although going in the same direction (Fig. 7B). The expression of miRNAs from the miR17~92 and miR181 families was significantly increased in Foxp3-GFP+ Treg cells from B6.Sle1a1 mice, but not in Treg cells from Pbx1-d Tg mice and there was no consistent difference between strains in Foxp3-GFP−conventional T cells (Supplemental Table 1). These findings indicate that Pbx1-d affects the expression of specific miRNAs that have been associated with CD4+ Treg and TFH cell differentiation and function, either directly or indirectly.
Figure 7. Pbx1-d Tg in CD4+ T cells increased the expression of miR-10a, miR-21, and miR-155.
miRNA expression was analyzed by qPCR in FOXP3+-GFP+ (A) and FOXP3-GFP− (B) CD4+ T cells from Pbx1-d Tg.Foxp3egfp, B6.Sle1a1.Foxp3egfp, and B6.Foxp3egfp spleens. Representative FACS plots for follicular T cells in spleens from 4 month old B6 and TC mice (C) and corresponding percentages and absolute numbers of TFH cells (D). E. miRNAs expression in CD4+ T cells from B6 and TC mice at 2, 4, and 10–12 month of age, n = 3–4.
To address whether the increased expression of miR-10a, miR-21 and miR-155 found in Pbx1-d Tg and B6.Sle1a1 CD4+ T cells was maintained in mice with clinical lupus phenotypes, we analyzed B6.Sle1.Sle2.Sle3 (TC) mice, which carry the Sle1a1 allele of Pbx1 (5). TC mice present spontaneously expanded subsets of TFH cells (Fig. 7C and D). miR-10a, miR-21 and miR-155 were expressed at a higher level in TC CD4+ T cells starting at 2 months of age, before the mice produce autoAbs and show overt signs of autoimmune activation (Fig. 7E). These results suggest that the up-regulation of these three miRNAs driven by Pbx1-d overexpression in the Tg mice contributes to the autoimmune phenotype in lupus mice that express the Pbx1-d allele.
miR-21 expression is regulated by the PBX1/HOX9 complex in leukemia (31), suggesting that Pbx1 could directly regulate the transcription of miR-10a, miR-21 and miR-155. An in silico analysis identified several putative PBX1 binding sites for each of the three miRNAs that are overexpressed in Pbx1-d Tg T cells. We co-transfected HEK293T cells with one of three luciferase constructs containing the upstream regulatory region for miR-10a, miR-21 or miR-155 that contained the most proximal putative PBX1 binding sites (Fig. 8A) and plasmids expressing either the normal allele PBX1-b or the lupus allele PBX1-d cDNA. As shown in Fig. 8B, PBX1-b and PBX1-d increased the transcription of each of the three miRNA, with higher levels obtained for PBX1-d than PBX1-b for miR-10a and miR-155. These data strongly support transcriptional regulation of miR-10a, miR-21 and miR-155 by PBX1.
Discussion
This study examined the role of Pbx1-d expression in CD4+ T cells relative to the phenotypes induced by the Sle1a1 lupus susceptibility locus. Sle1a1 increases CD4+ T cell effector functions and reduces the number and function of Treg cells, resulting in the production of autoreactive CD4+ T cells (9, 11). Pbx1-d over expression in the CD4+ T cells of B6 mice resulted in autoAb production and T cell activation as well as a decreased number of pTreg cells and a reduced iTreg differentiation in vitro. These results are fully consistent with Pbx1-d overexpression being responsible for Sle1a1 CD4+ T cell activation and impaired iTreg cell differentiation or maintenance (9). The most striking phenotype of Pbx1-d Tg mice was however the expansion of TFH cells and associated TD immune responses. BM chimera and adoptive transfer studies showed that Pbx1-d overexpression resulted in a cell-intrinsic Ag-specific expansion of TFH cells. This phenotype was accentuated in recall responses, which represent the chronic stimulation by autoantigens better than primary responses. This suggests that Pbx1-d expression contributes to lupus by promoting TFH cells at the expense of iTreg differentiation, resulting in the production of class-switched affinity matured autoantibodies that are the hallmark of the Sle1 susceptibility locus and lupus pathogenesis.
Lupus patients present high levels of circulating TFH-like cells that correlate with disease activity (32, 33) and functional TFH cells have been found in the kidneys of patients with lupus nephritis (34). TFH cells are the limiting factor for GC size and function, and there is abundant evidence from several mouse models that unrestricted TFH expansion leads to systemic autoimmunity (35, 36). Multiple studies have also found an association between decreased numbers or impaired function of Treg cells in lupus patients and mouse models (37). The differentiation of effector CD4+ T cell subsets is a dynamic and plastic process, and cross-talks between the TFH and Treg pathways have been described (30, 38). Although rapid progress has been made in recent years in mapping out the molecular pathways leading to Treg (39) and TFH cell differentiation (40), many questions still remain, including the mechanisms by which the balance between these two subsets is maintained. We propose that PBX1 is involved in this process through its ability to regulate gene expression in a cell specific manner through complex interactions with its co-factors. The over-expression of dominant-negative Pbx1-d leads to an imbalance in T cell homeostasis: a modest Pbx1-d over-expression limits the spontaneous expansion or maintenance of the Treg subset in B6.Sle1a1 mice, while a high over-expression of Pbx1-d expands TFH cells Pbx1-d Tg mice. Homeostatic expansion in the context of BM chimera revealed a role for Pbx1-d Tg overexpression for Treg maintenance. Similarly, a recall response in a competitive setting revealed a much stronger response of TFH cells expressing Pbx1-d either as a transgene or endogenously. This could be due to a better survival of memory TFH cells after the primary response, or to a better ability to respond to the challenge, two possibilities that we will explore in future experiments.
Interestingly, we found that the imbalance between Treg and TFH cells was enhanced in the mLN. We have previously associated Pbx1-d expression with an impaired response to RA ((15) and this report), and the major effect of RA in vivo occurs in gut-associated lymphoid tissue. It is therefore possible that the anatomy of TFH cell expansion in Pbx1-d Tg mice reflects the relative response to RA and commensal microbiota, an issue that will be explored in future studies. Pbx1-d led to TFH cell expansion in spite of a relatively expanded TFR subset, which is reminiscent of a recent study of mice with a specific deletion of PTEN in Foxp3+ T cells, in which TFH cell expansion occurred as a result of impaired Treg cell functions but in the presence of unchanged or expanded TFR cell numbers (41). In addition to Pbx1-d expression level, other factors may contribute to the differences between B6.Sle1a1 and Pbx1-d Tg mice. B6.Sle1a1 mice express Pbx1-d in other cell types, such as mesenchymal stem cells, leading to an increased production of pro-inflammatory cytokines (21). Pbx1 also regulates the production of inflammatory cytokines in macrophages in response to apoptotic cells (42). Although we have shown that the Sle1a1 T cell phenotypes are cell-intrinsic (15), we cannot rule out that Pbx1-d expression in other cell types may have additional modulatory effects on CD4+ T cell differentiation
MicroRNAs are essential mediators of TH cell plasticity, including the balance between Treg and TFH cells (30). Numerous studies have documented different miRNA profiles in human and murine SLE (43–45), and causal relationships between overexpression of either miR-21 or miR-155 and lupus have been established (46, 47). We showed here that increased expression of miR-10a, miR-21 and miR-155 occurs in CD4+ T cells before TC mice show autoimmune manifestations. Importantly, increased Pbx1-d expression in both the B6.Sle1a1 congenic and Pbx1-d Tg CD4+ T cells showed the same pattern of miRNA expression, implicating Pbx1-d as a primary driver regulating the intrinsic expression of these three miRNAs in T cells, either directly or indirectly. The existence of PBX1 binding sites in the distal promoter of each of these three miRNAs that are sufficient to increase transcription in the presence of Pbx1 supports this hypothesis. If Pbx1-d functions as a dominant negative transcription factor, it suggests that Pbx1-b is a negative regulator of these three miRNAs, especially for miR-10a and miR-155. Pbx1 can however bind to DNA indirectly through numerous co-factors (48, 49). Detailed studies will be necessary to unravel the molecular mechanisms by which Pbx1 isoforms regulate the expression of miR-10a, miR-21 and miR-155.
The exact role of each of these miRNAs in T cells overexpressing Pbx1-d could provide important cues on the mechanisms by which Pbx1 regulates T cell differentiation. miR-10a is over-expressed in B cells from lupus patients (44) and miR-10a stabilizes Treg cells (50) and prevents the conversion of pTreg into TFH cells by targeting Bcl6 and its co-repressor Ncor2 in a TGF-β and RA-dependent manner (51). It was therefore unexpected to find consistently high levels of miR-10a expression in the CD4+ T cells, including Foxp3+ T cells, of the lupus congenic mice characterized here. Moreover, the level of miR-10a was similar between Pbx1-d Tg and TC CD4+ T cells, arguing that Pbx1-d overexpression is the main determinant of this dysregulation. miR-21 is elevated in lupus (52) and it regulates aberrant T cell responses by blocking PDCD4 expression (45). miR-21 expression has not been directly linked to either Treg or TFH cells, but it is activated by STAT3 in T cells (53), and it is possible that its increased expression in Pbx1-d Tg CD4+ T cells is secondary to their increased IL-21 expression. miR-155 is a central modulator of T cell functions (54). Receptor activation increases miR-155 expression in T cells, which regulates effector subset differentiation and maintenance. miR-155 deficiency has been associated with decreased TH1 responses as well as low Treg cell numbers, which fits with Pbx1-d Tg expressing T cells showing high miR-155 expression, skewed TH1 humoral responses and a reduced Treg population. Specific deletion of miR-155 in T cells showed an intrinsic requirement of miR-155 for TFH cell differentiation (55). miR-155 deficiency normalized B cell functions and autoAb production in FAS-deficient mice (47). The role of miR-155 expression in T cells was however not addressed in this model. Our results demonstrate consistent high levels of miR-155 expression in the CD4+ T cells of the lupus congenic mice examined in this study, including both Foxp3+ and Foxp3−Pbx1-d Tg CD4+ T cells. This is consistent with a model in which miR-155 favors TFH over Treg cell differentiation and demonstrates that Pbx1-d overexpression is sufficient to drive this process. Interestingly, we found an overexpression of the miR-17~92 cluster in Sle1a1 Treg cells but no difference in Pbx1-d Tg Treg cells, or in non-Treg cells in either strain. miR-17~92 prevents pTreg cell differentiation (56), which fits with our findings that Treg cells are more affected in Sle1a1 than Pbx1-d Tg T cells. Moreover, miR-17~92 over-expression in lymphocytes leads to autoimmune phenotypes in mice (57), suggesting that it may be a mechanism by which Pbx1-d overexpression contributes to autoimmunity.
In conclusion, the phenotypes and CD4+ T cell differentiation in Pbx1-d Tg mice are similar to that of B6.Sle1a1 mice, which establishes Pbx1 as the gene responsible for the Sle1a1 phenotype. It also highlights critical roles for the lupus development in regulating Treg and TFH cell differentiation, and developing humoral autoimmunity. The Pbx1-d Tg mice provide a novel model to investigate T cell intrinsic mechanisms that regulate Treg/TFH homeostasis. Further studies are required to identify the Pbx1/Pbx1-d-regulated genes in T cells and their contribution to CD4+ T cell differentiation and effector functions.
Supplementary Material
Acknowledgments
We are indebted to Dr. Edward Chan for his advice on miRNAs. We thank Leilani Zeumer, Nathalie Kanda, Shun Lu, and Yuxin Niu for outstanding technical help.
Abbreviations
- Ab
antibody
- Ag
antigen
- ANA
anti-nuclear Ab
- B6
C57BL/6J mice
- B6.Ly5a
B6.SJL-PtprcaPep3b/BoyJ
- BM
bone marrow
- GC
germinal center
- iTreg
induced Treg
- mLN
mesenteric lymph node
- OVA
ovalbumin
- pTreg
peripheral Treg
- SLE
systemic lupus erythematosus
- TC
B6.NZM-Sle1NZM2410/AegSle2NZM2410/AegSle3NZM2410/Aeg/LmoJ
- TD
T-dependent
- Teff
effector T cells
- Tg
transgene
- TEM
CD44+CD62L-CD4+ effector memory T cells
- TFH
CXCR5+PD-1+BCL6+FOXP3-CD4+ follicular helper T cells
- TFR
CD4+PD1+CXCR5+Bcl6+Foxp3+ follicular regulatory T cells
- TN
CD44−CD62L+ naïve T cells
- Treg
regulatory T cells
- tTreg
thymic Treg
Footnotes
Author Contributions
Author contribution: SCC, TMB, S-SA and LM designed experiments; SCC, TEH, AAT, HRS, SL, BPC carried out experiments; SCC, TEH, AAT, and LM analyzed data; and SCC, S-SA and LM wrote the paper.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Morel L, Rudofsky UH, Longmate JA, Schiffenbauer J, Wakeland EK. Polygenic control of susceptibility to murine systemic lupus erythematosus. Immunity. 1994;1:219–229. doi: 10.1016/1074-7613(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 2.Morel L, Yu Y, Blenman KR, Caldwell RA, Wakeland EK. Production of congenic mouse strains carrying genomic intervals containing SLE-susceptibility genes derived from the SLE-prone NZM2410 strain. Mammalian genome. 1996;7:335–339. doi: 10.1007/s003359900098. [DOI] [PubMed] [Google Scholar]
- 3.Morel L. Mapping lupus susceptibility genes in the NZM2410 mouse model. Adv Immunol. 2012;115:113–139. doi: 10.1016/B978-0-12-394299-9.00004-7. [DOI] [PubMed] [Google Scholar]
- 4.Morel L, Tian XH, Croker BP, Wakeland EK. Epistatic modifiers of autoimmunity in a murine model of lupus nephritis. Immunity. 1999;11:131–139. doi: 10.1016/s1074-7613(00)80088-6. [DOI] [PubMed] [Google Scholar]
- 5.Morel L, Croker BP, Blenman KR, Mohan C, Huang G, Gilkeson G, Wakeland EK. Genetic reconstitution of systemic lupus erythematosus immunopathology with polycongenic murine strains. Proc Natl Acad Sci USA. 2000;97:6670–6675. doi: 10.1073/pnas.97.12.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohan C, Alas E, Morel L, Yang P, Wakeland EK. Genetic dissection of SLE pathogenesis. Sle1 on murine chromosome 1 leads to a selective loss of tolerance to H2A/H2B/DNA subnucleosomes. J Clin Invest. 1998;101:1362–1372. doi: 10.1172/JCI728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sobel ES, Satoh M, Chen WF, Wakeland EK, Morel L. The major murine systemic lupus erythematosus susceptibility locus Sle1 results in abnormal functions of both B and T cells. J Immunol. 2002;169:2694–2700. doi: 10.4049/jimmunol.169.5.2694. [DOI] [PubMed] [Google Scholar]
- 8.Morel L, Blenman KR, Croker BP, Wakeland EK. The major murine systemic lupus erythematosus susceptibility locus, Sle1, is a cluster of functionally related genes. Proc Natl Acad Sci USA. 2001;98:1787–1792. doi: 10.1073/pnas.031336098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuda CM, Wan S, Sobel ES, Croker BP, Morel L. Murine lupus susceptibility locus Sle1a controls regulatory T cell number and function through multiple mechanisms. J Immunol. 2007;179:7439–7447. doi: 10.4049/jimmunol.179.11.7439. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Perry D, Boackle SA, Sobel ES, Molina H, Croker BP, Morel L. Several genes contribute to the production of autoreactive B and T cells in the murine lupus susceptibility locus Sle1c. J Immunol. 2005;175:1080–1089. doi: 10.4049/jimmunol.175.2.1080. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Cuda C, Morel L. Genetic determination of T cell help in loss of tolerance to nuclear antigens. J Immunol. 2005;174:7692–7702. doi: 10.4049/jimmunol.174.12.7692. [DOI] [PubMed] [Google Scholar]
- 12.Wandstrat AE, Nguyen C, Limaye N, Chan AY, Subramanian S, Tian XH, Yim YS, Pertsemlidis A, Garner HR, Jr, Morel L, Wakeland EK. Association of extensive polymorphisms in the SLAM/CD2 gene cluster with murine lupus. Immunity. 2004;21:769–780. doi: 10.1016/j.immuni.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Keszei M, Detre C, Rietdijk ST, Munoz P, Romero X, Berger SB, Calpe S, Liao G, Castro W, Julien A, Wu YY, Shin DM, Sancho J, Zubiaur M, Morse HC, III, Morel L, Engel P, Wang N, Terhorst C. A novel isoform of the Ly108 gene ameliorates murine lupus. J Exp Med. 2011;208:811–822. doi: 10.1084/jem.20101653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuda CM, Zeumer L, Sobel ES, Croker BP, Morel L. Murine lupus susceptibility locus Sle1a requires the expression of two sub-loci to induce inflammatory T cells. Genes and immunity. 2010;11:542–553. doi: 10.1038/gene.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuda CM, Li S, Liang S, Yin Y, Potula HH, Xu Z, Sengupta M, Chen Y, Butfiloski E, Baker H, Chang LJ, Dozmorov I, Sobel ES, Morel L. Pre-B cell leukemia homeobox 1 is associated with lupus susceptibility in mice and humans. J Immunol. 2012;188:604–614. doi: 10.4049/jimmunol.1002362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sagerstrom CG. PbX marks the spot. Dev Cell. 2004;6:737–738. doi: 10.1016/j.devcel.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Laurent A, Bihan R, Omilli F, Deschamps S, Pellerin I. PBX proteins: much more than Hox cofactors. Intl J Dev Biol. 2008;52:9–20. doi: 10.1387/ijdb.072304al. [DOI] [PubMed] [Google Scholar]
- 18.Sengupta M, Liang S, Potula HHS, Chang LJ, Morel L. The SLE-associated Pbx1-d isoform acts as a dominant-negative transcriptional regulator. Genes and Immunity. 2012;13:653–657. doi: 10.1038/gene.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sobel ES, Brusko TM, Butfiloski EJ, Hou W, Li S, Cuda CM, Abid AN, Reeves WH, Morel L. Defective response of CD4+ T cells to retinoic acid and TGFbeta in systemic lupus erythematosus. Arthritis Res Ther. 2011;13:R106. doi: 10.1186/ar3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanyal M, Tung JW, Karsunky H, Zeng H, Selleri L, Weissman IL, Herzenberg LA, Cleary ML. B-cell development fails in the absence of the Pbx1 proto-oncogene. Blood. 2007;109:4191–4199. doi: 10.1182/blood-2006-10-054213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu S, Zeumer L, Sorensen H, Yang H, Ng Y, Yu F, Riva A, Croker B, Wallet S, Morel L. The murine Pbx1-d lupus susceptibility allele accelerates mesenchymal stem cell differentiation and impairs their immunosuppressive function. J Immunol. 2015;194:43–55. doi: 10.4049/jimmunol.1401851. [DOI] [PubMed] [Google Scholar]
- 22.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 23.Yin Y, Choi SC, Xu Z, Perry DJ, Seay H, Croker BP, Sobel ES, Brusko TM, Morel L. Normalization of CD4+ T cell metabolism reverses lupus. Sci Transl Med. 2015;7:274ra218. doi: 10.1126/scitranslmed.aaa0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Spolski R, Leonard WJ. Interleukin-21: a double-edged sword with therapeutic potential. Nat Rev Drug Disc. 2014;13:379–395. doi: 10.1038/nrd4296. [DOI] [PubMed] [Google Scholar]
- 26.Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF, Hwu P, Shaffer DJ, Akilesh S, Roopenian DC, Morse HC, 3rd, Lipsky PE, Leonard WJ. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173:5361–5371. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- 27.Luthje K, Kallies A, Shimohakamada Y, Belz GT, Light A, Tarlinton DM, Nutt SL. The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nat Immunol. 2012;13:491–498. doi: 10.1038/ni.2261. [DOI] [PubMed] [Google Scholar]
- 28.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 29.Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C, Flavell RA, Craft J. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med. 2008;205:2873–2886. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baumjohann D, Ansel KM. MicroRNA-mediated regulation of T helper cell differentiation and plasticity. Nat Rev Immunol. 2013;13:666–678. doi: 10.1038/nri3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Velu CS, Chaubey A, Phelan JD, Horman SR, Wunderlich M, Guzman ML, Jegga AG, Zeleznik-Le NJ, Chen J, Mulloy JC, Cancelas JA, Jordan CT, Aronow BJ, Marcucci G, Bhat B, Gebelein B, Grimes HL. Therapeutic antagonists of microRNAs deplete leukemia-initiating cell activity. J Clin Invest. 2014;124:222–236. doi: 10.1172/JCI66005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi J-Y, Hsi-enHo J, Pasoto SG, Bunin V, Kim S, Carrasco S, Borba EF, Gonçalves CR, Costa PR, Kallas EG, Bonfa E, Craft J. Circulating follicular helper-like T cells in systemic lupus erythematosus: Association with disease activity. Arth Rheumatol. 2015;4:988–999. doi: 10.1002/art.39020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L, Zhao P, Ma L, Shan Y, Jiang Z, Wang J, Jiang Y. Increased interleukin 21 and follicular helper T-like cells and reduced interleukin 10+ B cells in patients with new-onset systemic lupus erythematosus. J Rheumatol. 2014;41:1781–1792. doi: 10.3899/jrheum.131025. [DOI] [PubMed] [Google Scholar]
- 34.Liarski VM, Kaverina N, Chang A, Brandt D, Yanez D, Talasnik L, Carlesso G, Herbst R, Utset TO, Labno C, Peng Y, Jiang Y, Giger ML, Clark MR. Cell distance mapping identifies functional T follicular helper cells in inflamed human renal tissue. Sci Transl Med. 2014;6:230ra246. doi: 10.1126/scitranslmed.3008146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Craft JE. Follicular helper T cells in immunity and systemic autoimmunity. Nat Rev Rheumatol. 2012;8:337–347. doi: 10.1038/nrrheum.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pratama A, Vinuesa CG. Control of TFH cell numbers: why and how? Immunol Cell Biol. 2014;92:40–48. doi: 10.1038/icb.2013.69. [DOI] [PubMed] [Google Scholar]
- 37.Ohl K, Tenbrock K. Regulatory T cells in systemic lupus erythematosus. Europ J Immunol. 2015;45:344–355. doi: 10.1002/eji.201344280. [DOI] [PubMed] [Google Scholar]
- 38.Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, Hori S, Fagarasan S. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 39.Ohkura N, Kitagawa Y, Sakaguchi S. Development and maintenance of regulatory T cells. Immunity. 2013;38:414–423. doi: 10.1016/j.immuni.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shrestha S, Yang K, Guy C, Vogel P, Neale G, Chi H. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat Immunol. 2015;16:178–187. doi: 10.1038/ni.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chung EY, Liu J, Homma Y, Zhang Y, Brendolan A, Saggese M, Han J, Silverstein R, Selleri L, Ma X. Interleukin-10 expression in macrophages during phagocytosis of apoptotic cells is mediated by homeodomain proteins Pbx1 and Prep-1. Immunity. 2007;27:952–964. doi: 10.1016/j.immuni.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dai Y, Huang YS, Tang M, Lv TY, Hu CX, Tan YH, Xu ZM, Yin YB. Microarray analysis of microRNA expression in peripheral blood cells of systemic lupus erythematosus patients. Lupus. 2007;16:939–946. doi: 10.1177/0961203307084158. [DOI] [PubMed] [Google Scholar]
- 44.Martinez-Ramos R, Garcia-Lozano JR, Lucena JM, Castillo-Palma MJ, Garcia-Hernandez F, Rodriguez MC, Nunez-Roldan A, Gonzalez-Escribano MF. Differential expression pattern of microRNAs in CD4+ and CD19+ cells from asymptomatic patients with systemic lupus erythematosus. Lupus. 2014;23:353–359. doi: 10.1177/0961203314522335. [DOI] [PubMed] [Google Scholar]
- 45.Stagakis E, Bertsias G, Verginis P, Nakou M, Hatziapostolou M, Kritikos H, Iliopoulos D, Boumpas DT. Identification of novel microRNA signatures linked to human lupus disease activity and pathogenesis: miR-21 regulates aberrant T cell responses through regulation of PDCD4 expression. Ann Rheum Dis. 2011;70:1496–1506. doi: 10.1136/ard.2010.139857. [DOI] [PubMed] [Google Scholar]
- 46.Pan W, Zhu S, Yuan M, Cui H, Wang L, Luo X, Li J, Zhou H, Tang Y, Shen N. MicroRNA-21 and microRNA-148a contribute to DNA hypomethylation in lupus CD4+ T cells by directly and indirectly targeting DNA methyltransferase 1. J Immunol. 2010;184:6773–6781. doi: 10.4049/jimmunol.0904060. [DOI] [PubMed] [Google Scholar]
- 47.Thai TH, Patterson HC, Pham DH, Kis-Toth K, Kaminski DA, Tsokos GC. Deletion of microRNA-155 reduces autoantibody responses and alleviates lupus-like disease in the Fas(lpr) mouse. Proc Natl Acad Sci USA. 2013;110:20194–20199. doi: 10.1073/pnas.1317632110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bjerke GA, Hyman-Walsh C, Wotton D. Cooperative transcriptional activation by Klf4, Meis2, and Pbx1. Mol Cell Biol. 2011;31:3723–3733. doi: 10.1128/MCB.01456-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Penkov D, Mateos San Martin D, Fernandez-Diaz LC, Rossello CA, Torroja C, Sanchez-Cabo F, Warnatz HJ, Sultan M, Yaspo ML, Gabrieli A, Tkachuk V, Brendolan A, Blasi F, Torres M. Analysis of the DNA-binding profile and function of TALE homeoproteins reveals their specialization and specific interactions with Hox genes/proteins. Cell Rep. 2013;3:1321–1333. doi: 10.1016/j.celrep.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 50.Jeker LT, Zhou X, Gershberg K, de Kouchkovsky D, Morar MM, Stadthagen G, Lund AH, Bluestone JA. MicroRNA 10a Marks Regulatory T Cells. PLoS ONE. 2012;7:e36684. doi: 10.1371/journal.pone.0036684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi H, Kanno T, Nakayamada S, Hirahara K, Sciume G, Muljo SA, Kuchen S, Casellas R, Wei L, Kanno Y, O’Shea JJ. TGF-beta and retinoic acid induce the microRNA miR-10a, which targets Bcl-6 and constrains the plasticity of helper T cells. Nat Immunol. 2012;13:587–595. doi: 10.1038/ni.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan W, Zhu S, Yuan M, Cui H, Wang L, Luo X, Li J, Zhou H, Tang Y, Shen N. MicroRNA-21 and MicroRNA-148a Contribute to DNA Hypomethylation in Lupus CD4+ T Cells by Directly and Indirectly Targeting DNA Methyltransferase 1. J Immunol. 2010;184:6773–6781. doi: 10.4049/jimmunol.0904060. [DOI] [PubMed] [Google Scholar]
- 53.Sawant DV, Wu H, Kaplan MH, Dent AL. The Bcl6 target gene microRNA-21 promotes Th2 differentiation by a T cell intrinsic pathway. Mol Immunol. 2013;54:435–442. doi: 10.1016/j.molimm.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lind EF, Ohashi PS. Mir-155, a central modulator of T-cell responses. Eu J Immunol. 2014;44:11–15. doi: 10.1002/eji.201343962. [DOI] [PubMed] [Google Scholar]
- 55.Hu R, Kagele DA, Huffaker TB, Runtsch MC, Alexander M, Liu J, Bake E, Su W, Williams MA, Rao DS, Moller T, Garden GA, Round JL, O’Connell RM. miR-155 promotes T follicular helper cell accumulation during chronic, low-grade inflammation. Immunity. 2014;41:605–619. doi: 10.1016/j.immuni.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang S, Li C, Olive V, Lykken E, Feng F, Sevilla J, Wan Y, He L, Li QJ. Molecular dissection of the miR-17-92 cluster’s critical dual roles in promoting Th1 responses and preventing inducible Treg differentiation. Blood. 2011;118:5487–97. doi: 10.1182/blood-2011-05-355644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, Kuchroo VK. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-β-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol. 2008;181:2277–2284. doi: 10.4049/jimmunol.181.4.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.