Abstract
Importance
Effective, practical, non-pharmacological treatments are needed to treat menopause related insomnia symptoms in primary and women’s specialty care settings. Objective: To evaluate the treatment efficacy of telephone-based cognitive behavioral therapy for insomnia (CBT-I) versus menopause education control (MEC).
Design
Single-site, randomized, controlled trial. Participants were recruited from November 2013 to September 2014. Blinded assessments were conducted at baseline, 8 weeks (post-intervention), and 24-week follow-up.
Setting
Community sample recruited in Western Washington State with mailed postcards.
Participants
106 peri- or postmenopausal women aged 40–65 years, reporting at least moderate insomnia symptoms (Insomnia Severity Index [ISI] >12) and 2 or more daily hot flashes.
Interventions
Six CBT-I or MEC telephone sessions over 8 weeks. All participants submitted weekly electronic sleep diaries and received group-specific written educational materials. CBT-I included sleep restriction, stimulus control, sleep hygiene education, and cognitive restructuring components with behavioral homework. MEC provided general information about menopause and women’s health.
Main Outcomes and Measures
The primary outcome was the ISI. The secondary outcome was the Pittsburgh Sleep Quality Index (PSQI). Additional outcomes included sleep and hot flash diary variables and hot flash interference.
Results
From baseline to 8 weeks, ISI decreased 9.9 points in women receiving CBT-I and 4.7 points in women receiving MEC (P<0.001). PSQI decreased 4.0 points in women receiving CBT-I and 1.4 points in women receiving MEC (P<0.001). Significant group differences were sustained at 24 weeks. At 8 and 24 weeks, 70% and 84% of women in CBT-I had ISI scores in the no-insomnia range compared to 24% and 43% of MEC women, respectively. Women in CBT-I also had greater improvements in diary-reported sleep latency, wake time, and sleep efficiency. There were no differences between groups in daily hot flash frequency, but hot flash interference was significantly (p=0.03) decreased at 8 and 24 weeks for CBT-I compared to MEC.
Conclusions and Relevance
Telephone-delivered CBT-I was effective for improving sleep in peri- and postmenopausal women with insomnia and hot flashes. Results support further development and testing of centralized CBT-I programs for treatment of midlife insomnia in women.
Trial Registration
Clinicaltrials.gov #NCT01936441
INTRODUCTION
Sleep complaints are a common and often bothersome menopausal symptom1,2 which increase throughout the menopausal transition and early postmenopause.3,4 Insomnia is associated with increased depression, impaired daytime function, reduced libido, and increased health care utilization, creating a substantial burden for women and society.5–10 Women with combined vasomotor and insomnia symptoms have more emergency room visits, and lower scores on physical and mental quality of life than women without sleep complaints.11 Insomnia is also associated with increased risk for obesity, diabetes, stroke, and coronary artery disease,12,13 conditions which increase longterm disease and economic burdens in menopausal women.14
Evidence-based behavioral treatments for insomnia symptoms in peri- and postmenopausal women are lacking. In routine practice, insomnia is most often treated with medications.15–17 However, because of side effects,18 not all women desire or benefit from medications.19 Cognitive-behavior therapy for insomnia (CBT-I) is a well-established evidence-based approach.20–25 However, in-person CBT-I is rarely available in settings where most women receive care. Practical considerations such as cost, transportation, time required for most in-person therapies, and scheduling challenges further impact in-person CBT-I accessibility.
This paper presents results from a single-site, randomized controlled trial of a telephone CBT-I intervention versus telephone-delivered menopause education (MEC). We hypothesized that CBT-I would be more efficacious than MEC for improving sleep 8 and 24 weeks post-randomization. The study was approved by the Fred Hutchinson Cancer Research Center and University of Washington institutional review boards.
METHODS
Participants
The study was conducted within the Menopause Strategies Finding Lasting Answers for Symptoms and Health (MsFLASH) research network.26 Women in western Washington State were mailed recruitment postcards (November 2013–September 2014) including a telephone screening contact number. Respondents aged 40–65 years, endorsing significant insomnia symptoms, and reporting 2 or more hot flashes daily the previous 2 weeks were mailed a consent form and questionnaires, including 2-week sleep and hot flash diaries. Menopausal status was defined as: postmenopausal, no menstrual periods within the past 12 months, bilateral oophorectomy, or age 55+ years with hysterectomy or endometrial ablation; and perimenopausal, at least one menses in the last 12 months, or <age 55 with hysterectomy or endometrial ablation without bilateral oophorectomy.
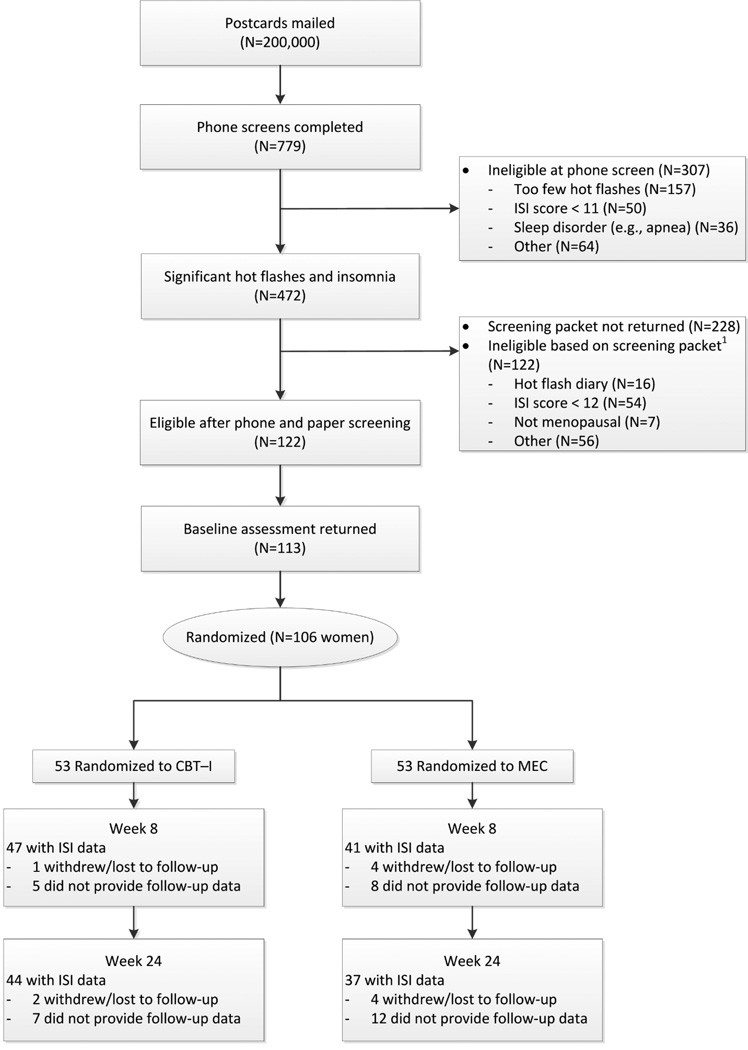
Eligible women scored 12 or higher on the Insomnia Severity Index (ISI)27 at both phone screening and on mailed questionnaires. Women were excluded if they had a primary sleep disorder diagnosis, consumed >3 alcoholic drinks daily, had a current major illness interfering with sleep, had a job involving shift work (>3 times a week), or routinely (>3 times a week) used prescription sleeping medications. Women reporting use of over-the-counter sleep aids, melatonin, or herbal sleep remedies were not excluded. Screening, eligibility, and participation are shown in Figure 1.
Figure 1.
Participant recruitment and retention of telephone delivered cognitive-behavioral therapy for insomnia (CBT-I) versus menopause education (MEC)
Randomization
Eligible women were block-randomized to CBT-I or MEC. Participants were told the study compared two educational treatments for sleep problems in women with hot flashes, but treatments differed in their approach. Participants were not informed how their group differed from the other.
Interventions
The CBT-I and MEC interventions both consisted of six, 20–30 minute telephone sessions over eight weeks (weeks 1–4, 6, 8). Participants were invited to have their first session in-person at a research office, but women were permitted to have Session 1 by phone. Treatment materials, including the “Menopause: Time for a Change” booklet,28 and additional group-specific reading materials were distributed at Session 1 or mailed before the first telephone session. In Session 1, women were taught how to complete an on-line daily sleep diary which was submitted to study interventionists (called “coaches”) the day before each telephone session.
CBT-I
The CBT-I protocol provided information about age-related sleep changes, sleep hygiene, sleep restriction, and stimulus control procedures (Table 1).27 Participants were instructed to keep a compressed schedule of bed and rising times. Initial sleep restriction windows were set to match the average sleep time reported in baseline screening logs but no less than 5.5 hours in bed. The sleep window was extended by 15 minutes per week when the electronic sleep diary indicated an average 85% sleep efficiency (time asleep divided by time in bed) or greater during the past week. Stimulus control instructions strengthened the association between bed and sleep by reducing time spent in bed on non-sleep activities. Sleep hygiene education included information about improving bedtime routines and identifying behavioral and environmental factors negatively impacting sleep. Cognitive techniques were taught to reduce physiological arousal at bedtime and to change unrealistic beliefs about sleep loss.29
Table 1.
Six session content of CBT-I and MEC telephone sessions*
| Session | CBT-I | MEC |
|---|---|---|
| 1 | Sleep changes during menopause Rationale for behavioral approach Sleep scheduling/bed restriction |
Introduction to menopause: What to expect Sleep hygiene strategies |
| 2 | Review of behavioral sleep plan Stimulus control instructions |
Hot flashes: Self-management techniques |
| 3 | Review of behavioral sleep plan Sleep stages and cycles across the age span |
Pharmacological supplements and natural remedies |
| 4 | Review of behavioral sleep plan Changing beliefs/attitudes about sleep |
Benefits of exercise in menopause |
| 5 | Review of behavioral sleep plan Constructive worry Sleep hygiene recommendations |
Post-menopausal health concerns and nutrition |
| 6 | Review of behavioral sleep plan Maintenance/relapse prevention plan |
Sexuality, urinary, and vaginal tract health |
| Treatment components |
Education Sleep monitoring Sleep scheduling and goal setting Behavioral homework and problem-solving |
Education Sleep monitoring Support |
CBT-I = Cognitive Behavior Therapy for Insomnia; MEC = Menopause Education Condition
MEC
The MEC protocol included educational content and readings relevant to women’s health and quality of life. Sessions were designed to reduce uncertainty about changes occurring during menopause and to help women identify symptom self-management strategies. Sessions explicitly excluded active ingredients hypothesized to mediate CBT-I treatment impact on sleep.30 Individual sessions were conducted in an informative, supportive, non-directive format. Weekly sleep logs were submitted. There was no practice or instruction in CBT-I principles (e.g., no recommendations to restrict time in bed).
Study Coaches, Training, and Treatment Fidelity
Telephone sessions were led by two female Master’s-level coaches (one social worker, one psychologist) without prior experience in menopause education or CBT-I. Coaches received one day trainings for each intervention, led by experts in CBT-I (CMM) and menopause education (NFW).
Both coaches delivered both interventions; all telephone sessions were recorded. Training included PI review of all six recordings for two pilot cases (one CBT-I, one MEC) for each coach. Thereafter, two sessions for each participant (one randomly selected, one chosen by either the coach or PI) were reviewed to maintain treatment fidelity and ensure there was no contamination between treatment conditions. Coaches completed weekly content checklists to ensure adherence to key session components. Feedback on reviewed audio-recordings was discussed in weekly team meetings.
Measures
Blinded assessments were conducted at baseline, 8 weeks (post-intervention), and 24-week follow-up. They included primary and secondary sleep outcomes, and additional sleep and hot flash outcomes described below. Treatment satisfaction was measured at 8 weeks. Assessment packets and diaries were mailed to women with a prepaid return envelope. Women who failed to return packets within 4 weeks of the scheduled collection date were contacted by telephone to gather primary and secondary sleep outcome data. Research staff involved in data collection and analysis had no knowledge of treatment group assignment.
Baseline Characteristics
Variables included age, race, education, marital status, menopausal stage, depression symptoms,31 sleep medication use, and duration of sleep disturbances.
Primary and Secondary Sleep Outcomes
The primary outcome was the Insomnia Severity Index (ISI),27,32 a 7-item questionnaire assessing global insomnia severity. Items are rated 0–4 (total score range 0–28); 15 or higher is considered moderate to severe insomnia in clinical populations.27 A score >10 is considered optimal for detecting insomnia cases in community samples,32 and a 6-point within-group reduction is a clinically meaningful change.33
The 19-item Pittsburgh Sleep Quality Index (PSQI)34 was a secondary sleep outcome. Total scores range from 0–21; higher scores indicate worse sleep. A decrease to PSQI <5 or 3-point reduction in score is considered clinically meaningful.35,36 Both the ISI and PSQI have been used in previous MsFLASH network trials.16,37
Additional Outcomes
Daily sleep diaries included: bed and rise time, sleep latency (time to fall asleep), and number and duration of nighttime awakenings.38 On a separate diary, participants recorded the frequency, severity, and bother of nighttime and daytime hot flashes. Sleep and hot flash diary results were calculated from two weeks of baseline data, and one week of data at 8 and 24 weeks.
The Hot Flash Related Daily Interference Scale (HFRDIS)39 includes 10 areas of daily functioning that may be affected by hot flashes. Items are rated on a 10-point scale; higher scores indicate worse interference.
Treatment satisfaction - At 8 weeks, participants rated the credibility, acceptability, and perceived effectiveness of their intervention.27 Rated items were: 1) Did this treatment and its rationale make sense to you? 2) How acceptable did you consider this treatment? 3) How suitable was this treatment for improving your quality of life despite having menopausal symptoms? 4) How effective did you expect this treatment to be? 5) How well were you able to adhere to this treatment program? And 6) How would you rate the quality of your working relationship with your menopause counselor? All items were rated on a 7-point scale; higher scores indicate greater satisfaction.
Statistical Analyses
The intent-to-treat analysis included all participants who provided follow-up data, regardless of adherence to treatment assignment. Baseline characteristics were compared between arms using t- or chi-square tests. Treatment contrasts for the ISI, PSQI, sleep diary, and hot flash outcomes were computed as Wald statistics from repeated measures linear regression models of each outcome by intervention arm, time, and baseline value of the outcome. Repeated measures logistic regression models were performed to compare incidence of good sleep quality (PSQI <5) by arm. Participants who contributed baseline and either 8- or 24-week data were included in these analyses. Robust standard errors were calculated via generalized estimating equations to account for correlations between repeated measures from each participant. Treatment effect sizes (difference in mean outcome between groups, divided by the pooled standard deviation) were computed for the ISI and PSQI. Treatment satisfaction ratings were compared by arm using t-tests.
Two sensitivity analyses of the ISI and PSQI were conducted. First, outcome data submitted more than 4 weeks past due were excluded from analysis. Second, missing outcome data for both groups were imputed based on the observed MEC data, using multiple imputation under the assumption that data from both CBT-I and control participants who discontinued follow-up early would mirror that of control participants after discontinuation.40 All analyses were conducted using SAS Version 9.4.
A sample size of 45 participants per group was chosen to provide 90% power to detect a 4-point difference in ISI change between the randomized groups, assuming a standard deviation of 5.6 based on observed scores in an earlier MsFLASH study16 and a t-test with 2-sided significance level of 5%. We planned to enroll 50 women per group to compensate for up to 10% loss to follow-up.
RESULTS
There were 106 participants (mean age=54.8 years) randomly assigned to the two intervention arms. The two arms did not differ significantly by age, race, education, marital status, menopausal stage, sleep medication use, duration of sleep disturbances (Table 2), or any baseline sleep or hot flash outcome measure.
Table 2.
Baseline characteristics by intervention group
| CBT-I (N=53) | MEC (n=53) | |||
|---|---|---|---|---|
| Baseline Characteristic* | n | % | n | % |
| Age, years, mean (SD) | 55.0 (3.5) | 54.7 (4.7) | ||
| Race | ||||
| White | 49 | 92.5 | 48 | 90.6 |
| African American | 0 | 0.0 | 1 | 1.9 |
| Other / Unknown | 4 | 7.5 | 4 | 7.5 |
| Education | ||||
| ≤ High school diploma / GED | 3 | 5.7 | 2 | 3.8 |
| School after high school | 9 | 17.0 | 10 | 18.9 |
| College graduate | 41 | 77.4 | 41 | 77.4 |
| Married / Marriage like relationship | 44 | 83.0 | 39 | 73.6 |
| Alcohol use, drinks/day | ||||
| 0 | 19 | 35.8 | 21 | 39.6 |
| 1 | 27 | 50.9 | 27 | 50.9 |
| ≥2 | 7 | 13.2 | 5 | 9.4 |
| Smoking | ||||
| Never | 40 | 75.5 | 42 | 79.2 |
| Past | 13 | 24.5 | 10 | 18.9 |
| Current | 0 | 0.0 | 1 | 1.9 |
| Menopause status | ||||
| Postmenopausal | 34 | 64.2 | 34 | 64.2 |
| Perimenopausal | 16 | 30.2 | 15 | 28.3 |
| Indeterminate | 3 | 5.7 | 4 | 7.5 |
| Hot flashes per day, mean (SD) | 7.3 (4.5) | 7.8 (4.1) | ||
| Patient Health Questionnaire depression scale (PHQ-8), mean (SD) |
7.4 (3.4) | 8.1 (4.8) | ||
| Increase in sleep problems at menopause |
52 | 98.1 | 52 | 98.1 |
| Yes | 1 | 1.9 | 0 | 0.0 |
| No | 0 | 0.0 | 1 | 1.9 |
| Missing | ||||
| Sleep problem start time | ||||
| Within the past 6 months | 2 | 3.8 | 4 | 7.6 |
| About 6–12 months ago | 7 | 13.2 | 6 | 11.3 |
| 1 – 5 years ago | 28 | 52.8 | 20 | 37.7 |
| More than 5 years ago | 15 | 28.3 | 22 | 41.5 |
| Missing | 1 | 1.9 | 1 | 1.9 |
CBT-I = Cognitive Behavior Therapy for Insomnia; MEC = Menopause Education Condition
No significant differences by intervention arm for any variable
Adherence, Treatment Discontinuation, and Completeness of Outcomes Ascertainment
Participants in both CBT-I and MEC attended an average of 5.7 sessions (range 1–6). Sessions averaged 22.8 minutes (range 16.4–32.6). There were no significant differences in number of telephone sessions or session length by intervention arm or coach.
There were no between-group differences in the number of drop-outs or reasons for treatment discontinuation (Figure 1). Follow-up ISI data were collected on 88 participants (83%) at 8 weeks and 81 (76%) at 24 weeks; 10 women (3 CBT, 7 MEC) completed telephone ISI and PSQI forms at week 8, and 7 women (3 CBT, 4 MEC) completed telephone forms at week 24.
Sleep Outcomes
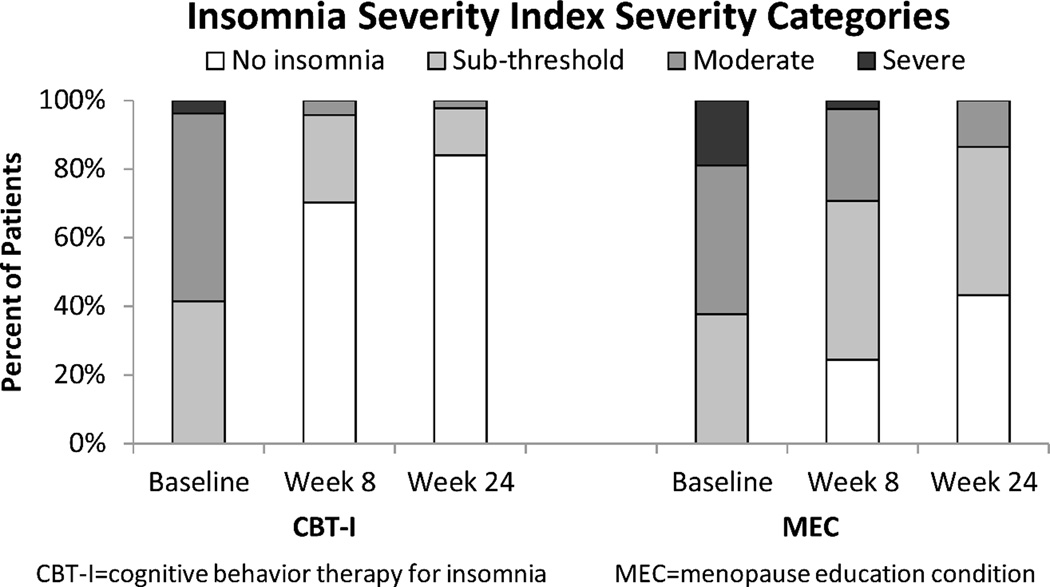
At baseline, 58% of women in CBT-I and 62% in MEC had ISI scores in the moderate (ISI=15–21) to severe (ISI=22–28) insomnia range (Figure 2). From baseline to 8 weeks, ISI decreased 9.9 points in women receiving CBT-I and 4.7 points in women receiving MEC, a mean between-group difference of 5.2 points [95% CI −6.1, −3.3] (P<0.001). Significant group differences were sustained at 24 weeks (Table 3).
Figure 2.
Percentage of ISI total scores categorized by insomnia category at baseline, 8 weeks, and 24-week follow-up (ISI categories represent the following score ranges: No insomnia = 0 – 7; Subthreshold insomnia = 8 – 14; Moderate insomnia = 15 – 21; Severe insomnia = 22 – 28)
Table 3.
Sleep outcome results by treatment condition
| CBT-I | MEC | Difference | ||||
|---|---|---|---|---|---|---|
| Outcome | n | Mean (95% CI) | n | Mean (95% CI) | Mean (95% CI) | p-value* |
| Insomnia Severity Index | ||||||
| Baseline | 53 | 15.6 (14.8, 16.4) | 53 | 16.8 (15.8, 17.9) | −1.2 (−2.6, 0.1) | |
| Week 8 - baseline | 47 | −9.9 (−11.2, −8.7) | 41 | −4.7 (−6.1, −3.3) | −5.2 (−6.1, −3.3) | <0.001 |
| Week 24 - baseline | 44 | −10.7 (−11.9, −9.4) | 37 | −6.7 (−8.4, −5.0) | −4.0 (−6.0, −1.9) | <0.001 |
| Pittsburgh Sleep Quality Index | ||||||
| Baseline | 51 | 8.9 (8.2, 9.6) | 53 | 9.4 (8.6, 10.3) | 0.5 (−1.6, 0.6) | |
| Week 8 - baseline | 47 | −4.0 (−5.0, −3.1) | 41 | −1.4 (−2.1, −0.7) | −2.7 (−3.9, −1.5) | <0.001 |
| Week 24 - baseline | 44 | −4.3 (−5.1, −3.5) | 38 | −2.7 (−3.5, −1.9) | −1.6 (−2.7, −0.5) | <0.001 |
| Diary Sleep Latency (mins) | ||||||
| Baseline | 51 | 54.4 (43.8, 65.0) | 52 | 51.1 (42.0, 60.2) | 3.3 (−10.5, 17.1) | |
| Week 8 – baseline | 43 | −31.5 (−39.2, −23.8) | 33 | −12.2 (−22.8, −1.7) | −19.3 (−31.8, −6.8) | <0.001 |
| Week 24 – baseline | 39 | −25.3 (−32.8, −17.8) | 29 | −13.4 (−25.1, −1.8) | −11.9 (−24.9, 1.2) | 0.007 |
| Diary Wake Time After Sleep Onset (mins)** |
||||||
| Baseline | 51 | 71.7 (60.7, 82.7) | 52 | 83.0 (69.2, 96.8) | −11.3 (−28.8, 6.2) | |
| Week 8 – baseline | 43 | −37.4 (−48.3, −26.6) | 33 | −17.0 (−33.5, −0.5) | −20.4 (−39.1, −1.7) | <0.001 |
| Week 24 - baseline | 39 | −32.8 (−43.9, −21.7) | 29 | −26.0 (−40.3, −11.8) | −6.7 (−24.2, 10.7) | 0.02 |
| Diary Total Sleep Time (hrs) | ||||||
| Baseline** | 51 | 6.6 (6.3, 6.8) | 52 | 6.4 (6.1, 6.7) | 0.1 (−0.3, 0.5) | |
| Week 8 – baseline | 43 | 0.4 (−0.1, 0.9) | 33 | 0.2 (−0.1, 0.5) | 0.2 (−0.3, 0.8) | 0.14 |
| Week 24 - baseline | 39 | 0.7 (0.4, 0.9) | 29 | 0.5 (0.1, 0.9) | 0.1 (−0.3, 0.6) | 0.17 |
| Diary Sleep Efficiency** | ||||||
| Baseline | 51 | 75.8 (73.1, 78.6) | 52 | 74.6 (71.5, 77.7) | 1.3 (−2..8, 5.3) | |
| Week 8 – baseline | 43 | 12.1 (9.4, 14.8) | 33 | 5.1 (1.3, 8.9) | 7.0 (2.6, 11.5) | <0.001 |
| Week 24 - baseline | 39 | 10.7 (8.3, 13.0) | 29 | 7.5 (3.5, 11.6) | 3.1 (−1.5, 7.8) | 0.007 |
CBT-I = Cognitive Behavior Therapy for Insomnia; MEC = Menopause Education Condition
p-values from contrasts of CBTI vs. MEC in a repeated measures linear model of outcome as a function of intervention arm, week (8, 24), and baseline outcome value.
Daily sleep diary variables were computed as following: Time in Bed (TIB) = difference between final bed and final rise time the following morning; Sleep Latency (SL) = estimated time to fall asleep at night after turning out the light; Wake Time After Sleep Onset (WASO) = estimated total time awake each night; Total Sleep Time (TST) = TIB – (SL + WASO); Sleep Efficiency = TST / TIB
At baseline, 92% of women (49 in each arm) had PSQI levels ≥5, indicating poor sleep quality. PSQI decreased 4.0 points at post-treatment in women receiving CBT-I and 1.4 points in women receiving MEC, a mean between-group difference of 2.7 points [95% CI −3.9, −1.5] (P<0.001), approaching a 3-point clinically significant difference.35 Significant group differences were sustained at 24 weeks (Table 3). Women in the CBT-I group were significantly more likely than those in MEC to have good sleep quality (PSQI ≤5) at week 8 (OR 5.6; 95% CI 2.3–14.8; P<0.001) and week 24 (OR 3.7; 95% CI 1.4–9.5; P=0.006).
Women in CBT-I also had significantly greater 8- and 24-week improvements in diary-reported sleep latency, wake time, and sleep efficiency compared to MEC, although relative differences between treatment groups were attenuated at 24 weeks.
Standardized mean differences (i.e., effect sizes) for ISI and PSQI at 8 weeks were 1.04 and 0.84 standard deviation units, respectively, indicating large treatment effects for CBT-I. At 8 weeks, 70% of women in CBT-I had total scores in a “no clinically significant insomnia” range (ISI 0–7) compared to only 24% of women randomized to MEC; at 24 weeks 84% of women in the CBT-I group versus 43% in MEC were in the no insomnia range (Figure 2).
Study results for ISI and PSQI were not significantly different than the primary analyses when protocol violators were excluded, and were also robust to sensitivity analyses for missing data (eTables 1–2).
Hot Flash Outcomes
There were no significant differences between treatment group ratings of hot flash frequency (daily or nighttime), severity, or bother at either 8 or 24 weeks. The HFRDIS was significantly (p=0.03) decreased at 8 weeks for CBT-I participants (−15.7, 95% CI −20.4, −11.0) compared to MEC (−7.1, 95% CI −14.6, 0.4). Significant between-group differences (p=0.003) were maintained at 24 weeks (CBTI: −22.8, 95% CI −28.6, −16.9; MEC: −11.6, 95% CI −19.4, −3.8). When the HFRDIS was analyzed excluding the single sleep item, results were comparable.
Patient Reported Satisfaction Outcomes
Average ratings of perceived suitability, acceptability, effectiveness, and trainer quality for both intervention arms at the post-treatment assessment were high (mean range 4.2–6.7 on the 1–7 point scale). There were no differences between CBT-I and MEC in acceptability, treatment adherence, or relationship quality with the menopause coach. CBT-I ratings were significantly higher than MEC for whether the treatment made sense (p=0.005), whether it was suitable for improving quality of life despite having menopausal symptoms (p=0.009), and perceived treatment effectiveness (p<0.001).
DISCUSSION
Behavioral interventions for women with moderate menopause-associated insomnia and vasomotor symptoms are lacking. In this randomized, controlled trial, brief, telephone-delivered CBT-I resulted in significant 8- and 24-week improvements in self-reported insomnia symptoms, overall sleep quality, sleep latency, wake time after sleep onset, and sleep efficiency compared to the MEC control. Although CBT-I has been found to be efficacious for improving sleep in populations with other co-morbid conditions,41 this is one of the first studies to show that CBT-I helps healthy women with hot flashes sleep better. A recent small trial found that six sessions of CBT-I significantly improved sleep outcomes compared to placebo control in middle-aged breast cancer survivors with chronic insomnia.42 A few other small studies reported psychologist-led groups and self-help cognitive-behavioral strategies for improving hot flashes and night sweats, but did not target sleep.43,44
This study found no between-group differences in self-reported hot flash frequency, severity, or bother, but CBT-I reduced self-reported hot flash interference at 8 and 24 weeks relative to MEC. This may indicate that for women in CBT-I, the cognitive strategies taught to reduce daytime dysfunction associated with sleep loss generalized to how they responded to vasomotor symptoms. Alternatively, improved sleep could have improved tolerance of hot flashes.
A study strength was the telephone-delivered menopause education control. The MEC controlled for non-specific treatment effects including therapist attention and treatment duration but explicitly excluded active ingredients hypothesized to mediate treatment impact on sleep.30 MEC had high ratings of acceptability and adherence, and low dropout treatment rates equivalent to CBT-I, suggesting that it was a well-received attention control intervention.
This study does not provide a comparison to placebo or active medication treatments for insomnia. CBT-I and pharmacotherapy are considered effective for treating chronic insomnia,45,46 with medications offering an advantage due to immediate treatment effects, but CBT-I produces superior long-term outcomes.47,48 There have been no head-to-head trials comparing CBT-I versus medication for peri- and early postmenopausal women with insomnia symptoms. CBT-I reductions in ISI in the current trial approached those observed in previous studies examining the effect of eszopiclone on insomnia symptoms in menopausal women,49 and were larger than have been reported in placebo-controlled trials of escitalopram, venlafaxine, or low dose estradiol effects on sleep in this population.16,50 Future direct comparison of outcomes and cost-effectiveness with pharmacotherapies for insomnia and hot flashes are warranted.
Current findings support the potential for training non-sleep specialists to deliver telephone-based CBT-I to women with insomnia and vasomotor symptoms in a variety of primary and women’s health care settings. Telephone-based CBT-I allows upscaling to reach large populations of midlife women seeking treatment for sleep problems. Centralized telephone CBT-I should be tested as a dissemination model, similar to effective telephone-based counseling programs for smoking cessation.
Study limitations are acknowledged. The program was delivered in the Seattle area, and women responding to recruitment mailings tended were predominantly college educated and Caucasian, limiting generalizablity. Women did not undergo formal evaluation for primary sleep disorders so we were unable to examine whether the effect of CBT-I was consistent across women with and without these conditions. In studies of this type, it is not possible to mask interventionists to treatment assignment. However, all outcomes were collected by research staff blinded to treatment assignment. As expected, lower MEC post-treatment ratings of treatment effectiveness indicated some nonequivalence between treatment groups in perceived impact for insomnia symptoms.
Sleep and vasomotor outcomes were based on self-report, the most salient and relevant efficacy indicators for clinical practice and women themselves. However, future studies incorporating PSG as a screening and outcome measure would have value. Changes in ISI and PSQI scores among women receiving CBT-I were significant and clinically robust at post-treatment although the differences relative to MEC were somewhat attenuated at 24-weeks. Trials of the efficacy of CBT-I used in conjunction with other treatments to manage hot flashes are warranted to identify the optimal strategy for achieving long-term improvement in sleep-related symptoms among menopausal women.
In conclusion, telephone-based CBT-I effectively improved sleep in peri- and postmenopausal women with insomnia and vasomotor symptoms, both immediately post-treatment and at 24-weeks of follow-up. These results support further development and testing of centralized CBT-I programs for treatment of midlife insomnia in women.
Supplementary Material
Acknowledgments
Funding/Support: The MsFLASH research network was established under an NIH cooperative agreement to conduct studies of the efficacy of treatments for the management of menopausal hot flashes. The studes were sponsored by the National Institute on Aging (NIA), in collaboration with the Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD), the National Center for Complementary and Alternative Medicine (NCCAM), and the Office of Research on Women’s Health (ORWH). This study was supported by grant #U01AG032699.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: The authors wish to thank Martha Cagley, MS and Amy Cunningham, MS at the University of Washington Northwest Research Group on Aging for their invaluable assistance in conducting this study as intervention coaches. We would also like to acknowledge Janet Carpenter, PhD, RN, FAAN for her early support and intellectual contributions to the development of this research project.
Footnotes
Author contributions: K.A. Guthrie had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors made substantial contributions to the study and this manuscript. None were compensated for the manuscript preparation.
Conflict of Interest Disclosures: Dr. Morin reports personal fees for consultancy from Merck and grants from Novartis. Dr. Joffe reports grant support from Merck, and consulting fees from NeRRe Therapeutics, Merck, SAGE Therapeutics, and Mitsubishi Tanabe. None of the other authors report any conflicts of interest.
REFERENCES
- 1.Shaver JL, Woods NF. Sleep and menopause: A narrative review. Menopause. 2015;22(8):899–915. doi: 10.1097/GME.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 2.Woods NF, Hohensee C, Carpenter JS, et al. Symptom clusters among MsFLASH clinical trial participants. Menopause. 2015 Oct 26; doi: 10.1097/GME.0000000000000516. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kravitz HM, Joffe H. Sleep during the perimenopause: A SWAN story. Obstet Gynecol Clin North Am. 2011;38(3):567–586. doi: 10.1016/j.ogc.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Q, Lang CP. Examining the relationship between subjective sleep disturbacne and menopause: A systematic review and meta-analysis. Menopause. 2014;21(12):1301–1318. doi: 10.1097/GME.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 5.Utian WH. Psychosocial and socioeconomic burden of vasomotor symptoms in menopause: A comprehensive review. Health Qual Life Outcomes. 2005;5(3):47. doi: 10.1186/1477-7525-3-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reed SD, Newton KM, LaCroix AZ, Grothaus LC, Ehrlich K. Night sweats, sleep disturbance, and depression associated with diminished libido in late menopausal transition and early postmenopause: Baseline data from the Herbal Alternatives for Menopause Trial (HALT) Am J Obstet Gynecol. 2007;196(6):593. doi: 10.1016/j.ajog.2007.03.008. e591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daley M, Morin CM, LeBlanc M, Grégoire JP, Savard J. The economic burden of insomnia: Direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms, and good sleepers. Sleep. 2009;32(1):55–64. [PMC free article] [PubMed] [Google Scholar]
- 8.Woods N, Mitchell ES. Sleep symptoms during the menopausal transition and early menopause: Observations from the Seattle Midlife Women’s Health Study. Sleep. 2010;33(4):539–549. doi: 10.1093/sleep/33.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartz A, Ross JJ, Noyes R, Williams P. Somatic symptoms and psychological characteristics associated with insomnia in postmenopausal women. Sleep Med. 2013;14(1):71–78. doi: 10.1016/j.sleep.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Woods NF, Mitchell ES. Symptom interference with work and relationships during the menopausal transition and early postmenopause: Observations from the Seattle Midlife Women’s Health Study. Menopause. 2011;18(6):654–661. doi: 10.1097/gme.0b013e318205bd76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolge SC, Balkrishnan R, Kannan H, Seal B, Drake CL. Burden associated with chronic sleep maintenance insomnia characterized by nighttime awakenings among women with menopausal symptoms. Menopause. 2010;17(1):80–86. doi: 10.1097/gme.0b013e3181b4c286. [DOI] [PubMed] [Google Scholar]
- 12.Grandner MA, Jackson NJ, Pak VM, Gehrman PR. Sleep disturbance is associated with cardiovascular and metabolic disorders. J Sleep Res. 2012;21(4):427–433. doi: 10.1111/j.1365-2869.2011.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sands-Lincoln M, Loucks EB, Lu B, et al. Sleep duration, insomnia, and coronary heart disease among postmenopausal women in the Women’s Health Initiative. Journal of Women’s Health. 2013;22(6):477–486. doi: 10.1089/jwh.2012.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan JG. Cost and policy implications from the increasing prevalence of obesity and diabetes mellitus. Gender Med. 2009;6(Suppl 1):96–108. doi: 10.1016/j.genm.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 15.National Institutes of Health. NIH state-of the-science conference statement on manifestations and management of chronic insomnia in adults. Vol. 2005. National Institutes of Health; 2005. Jun 13–15, p. 22. [PubMed] [Google Scholar]
- 16.Ensrud KE, Joffe H, Guthrie KA, et al. Effect of escitalopram on insomnia symptoms and subjective sleep quality in healthy perimenopausal and postmenopausal women with hot flashes: A randomized controlled trial. Menopause. 2012;19(8):848–855. doi: 10.1097/gme.0b013e3182476099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joffe H, Massler A, Sharkey KM. Evaluation and management of sleep disturbance during the menopause transition. Semin Reprod Med. 2010;28(5):404–421. doi: 10.1055/s-0030-1262900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brower KJ, McCammon RJ, Wojnar M, Ilgen MA, Wojnar J, Valenstein M. Prescription sleeping pills, insomnia, and suicidality in the National Comorbidity Survey Replication. J Clin Psychiatry. 2011;72(4):515–521. doi: 10.4088/JCP.09m05484gry. [DOI] [PubMed] [Google Scholar]
- 19.Davidson JR. Insomnia treatment options for women. Obstet Gynecol Clin North Am. 2009;36(4):831–846. doi: 10.1016/j.ogc.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Ellis JG, Gehrman P, Espie CA, Riemann D, Perlis ML. Acute insomnia: Current conceptualizations and future directions. Sleep Med Rev. 2012;16(1):5–14. doi: 10.1016/j.smrv.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Morin CM, Benca R. Chronic insomnia. Lancet. 2012;379(9821):1129–1141. doi: 10.1016/S0140-6736(11)60750-2. [DOI] [PubMed] [Google Scholar]
- 22.Okajima I, Komada Y, Inque Y. A meta-analysis on the treatment effectiveness of cognitive behavioral therapy for primary insomnia. Sleep Biol Rhythms. 2011;9(1):24–34. [Google Scholar]
- 23.Riemann D, Spiegelhalder K, Espie CA, et al. Chronic insomnia: Clinical and research challenges - an agenda. Pharmacopsychiatry. 2011;44(1):1–14. doi: 10.1055/s-0030-1267978. [DOI] [PubMed] [Google Scholar]
- 24.Smith MT, Huang MI, Manber R. Cognitive behavior therapy for chronic insomnia occurring within the context of medical and psychiatric disorders. Clin Psychol Rev. 2005;25(5):559–592. doi: 10.1016/j.cpr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive behavioral therapy for chronic insomnia: A systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191–204. doi: 10.7326/M14-2841. [DOI] [PubMed] [Google Scholar]
- 26.Newton KM, Carpenter JS, Guthrie KA, et al. Methods for the design of vasomotor symptom trials: the menopausal strategies: Finding lasting answers to symptoms and health network. Menopause. 2014;21(1):45–58. doi: 10.1097/GME.0b013e31829337a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morin CM. Insomnia: Psychological assessment and management. New York:NY: Guilford Press; 1993. [Google Scholar]
- 28.National Institute on Aging. Menopause: Time for a change. Vol. 2008. Gaithersburg, MD: National Institutes of Health, Department of Health and Human Services; 2008. Jan, p. 2008. [Google Scholar]
- 29.Morin CM, Beulieu-Bonneau S. Cognitive-behavioral therapy for insomnia in peri-and post-menopausal women. Quebec, QC, Canada: Universite Laval; 2013. p. 32. [Google Scholar]
- 30.Balderson B, McCurry SM, Vitiello MV, et al. Information without implementation: A practical example for developing a best practice education control group. Behav Sleep Med. 2015 Oct 20;:1–14. doi: 10.1080/15402002.2015.1036271. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114(1–3):163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 32.Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang M, Morin CM, Schaefer K, Wallenstein GV. Interpreting score differences in the Insomnia Severity Index: Using health-related outcomes to define the minimally important difference. Curr Med Res Opin. 2009;25(10):2487–2494. doi: 10.1185/03007990903167415. [DOI] [PubMed] [Google Scholar]
- 34.Buysse DJ, Reyholds CF, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI) Sleep. 1991;14(1):331–338. [PubMed] [Google Scholar]
- 35.Buysse DJ, Germain A, Moul DE, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011;171(10):887–895. doi: 10.1001/archinternmed.2010.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Surman CB, Roth T. Impact of stimulant pharmacotherapy on sleep quality: Post hoc analyses of 2 large, double-blind, randomized, placebo-controlled trials. J Clin Psychiatry. 2011;72(7):903–908. doi: 10.4088/JCP.11m06838. [DOI] [PubMed] [Google Scholar]
- 37.Ensrud KE, Stone KL, Blackwell TL, et al. Frequency and severity of hot flashes and sleep disturbance in postmenopausal women with hot flashes. Menopause. 2009;16(2):286–292. doi: 10.1097/gme.0b013e31818c0485. [DOI] [PubMed] [Google Scholar]
- 38.Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: Standardizing prospective sleep self-monitoring. Sleep. 2012;35(2):287–302. doi: 10.5665/sleep.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carpenter JS. The Hot Flash Related Daily Interference Scale: a tool for assessing the impact of hot flashes on quality of life following breast cancer. J Pain Symptom Manage. 2001;22(6):979–989. doi: 10.1016/s0885-3924(01)00353-0. [DOI] [PubMed] [Google Scholar]
- 40.Mallinckrodt CH, Lin Q, Molenberghs M. A structured framework for assessing sensitivity to missing data assumptions in longitudinal clinical trials. Pharm Stat. 2013;12(1):1–6. doi: 10.1002/pst.1547. [DOI] [PubMed] [Google Scholar]
- 41.Wu JQ, Appleman ER, Salazar RD, Ong JC. Cognitive behavioral therapy for insomnia comorbid with psychiatric and medical conditions: A meta-analysis. JAMA Internal Medicine. 2015;175(9):1461–1472. doi: 10.1001/jamainternmed.2015.3006. [DOI] [PubMed] [Google Scholar]
- 42.Matthews EE, Berger AM, Schmiege SJ, et al. Cognitive behavioral therapy for insomnia outcomes in women after primary breast cancer treatment: A randomized, controlled trial. Oncol Nurs Forum. 2014;41(3):241–253. doi: 10.1188/14.ONF.41-03AP. [DOI] [PubMed] [Google Scholar]
- 43.Ayers B, Smith M, Hellier J, Mann E, Hunter MS. Effectiveness of group and self-help cognitive behavior therapy in reducing problematic menopausal hot flushes and night sweats (MENOS 2): A randomized controlled trial. Menopause. 2012;19(7):749–759. doi: 10.1097/gme.0b013e31823fe835. [DOI] [PubMed] [Google Scholar]
- 44.Norton S, Chilcot J, Hunter MS. Cognitive-behavior therapy for menopausal symptoms (hot flushes and night sweats): Moderators and mediators of treatment effects. Menopause. 2014;21(6):574–578. doi: 10.1097/GME.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 45.NIH state-of the-science conference statement on manifestations and management of chronic insomnia in adults. NIH Consens Sci Statements. 2005 Jun 13–15;22(2):1–30. [PubMed] [Google Scholar]
- 46.Morin CM. Combined therapeutics for insomnia: Should our first approach be behavioral or pharmacological? Sleep Med. 2006;7(S1):S15–S19. doi: 10.1016/j.sleep.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Morin CM, Vallières A, Guay B, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009;301(19):2005–2015. doi: 10.1001/jama.2009.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morin CM, Beaulieu-Bonneau S, Ivers H, et al. Speed and trajectory of changes of insomnia symptoms during acute treatment with cognitive-behavioral therapy, singly and combined with medication. Sleep Med. 2014;15(6):701–707. doi: 10.1016/j.sleep.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soares CN, Joffe H, Rubens R, Caron J, Roth T, Cohen L. Eszopiclone in patients with insomnia during perimenopause and early postmenopause: a randomized controlled trial. Obstet Gynecol. 2006;108(6):1402–1410. doi: 10.1097/01.AOG.0000245449.97365.97. [DOI] [PubMed] [Google Scholar]
- 50.Ensrud KE, Guthrie KA, Hohensee C, et al. Effects of estradiol and venlafaxine on insomnia symptoms and sleep quality in women with hot flashes. Sleep. 2015;38(1):97–108. doi: 10.5665/sleep.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.