Abstract
Background
We previously showed that bortezomib (BTZ) partially depletes plasma cells, yet has limited efficacy for desensitization in kidney transplant (KTx) candidates when up to 16 doses is given.
Methods
This study aimed to determine the safety and efficacy of 32 doses of BTZ (1.3mg/m2 of body surface area [BSA]) in 10 highly sensitized KTx candidates with alloantibodies against their intended living donor.
Results
Dose reduction was needed in 2 patients and 2 others completely discontinued therapy for adverse events. Anti- human leukocyte antigens (HLA) antibodies mean fluorescence intensity (MFI) values were stable prior to BTZ (p=0.96) but decreased after therapy (mean decrease of 1916 [standard error, SE 425] MFI, p<0.01). No patient developed a negative crossmatch against their original intended donor, and the calculated panel reactive antibodies (cPRA) based on MFI of 2000, 4000, and 8000 was unchanged in all patients.
Conclusions
These data suggest that 32 doses of BTZ monotherapy was not well tolerated and resulted in only a modest reduction in anti-HLA antibodies.
Introduction
Donor specific antibodies (DSA) are a major barrier to successful KTx and are associated with an increased risk of early and late antibody associated graft loss. Avoiding DSA is especially difficult in highly sensitized transplant candidates with cPRA greater than 90%. Intense efforts to increase transplantation for these patients including kidney paired donation and priority for compatible deceased donor organs have been successful, but are not adequate. Currently, 6% of patients on the KTx waiting list have a cPRA of a 100 % (1). The current therapy available to decrease alloantibody is largely ineffective, and positive crossmatch kidney transplantation is associated with antibody mediated rejection and early allograft loss (2-11). DSA also is a problem for other solid organ transplantation including heart, lung, and pancreas transplant candidates. Thus, developing therapy to decrease alloantibody production for the highly sensitized patient is a major unmet need in organ transplantation.
We and others have hypothesized that treatment with the proteasome inhibitor BTZ might deplete antibody-secreting long-lived plasma cells and have an impact on DSA production (12-14). We demonstrated in vitro that proteasome inhibition using BTZ caused apoptosis of bone marrow-derived plasma cells (15). In a pilot clinical study, we found that up to 16 doses of BTZ depleted bone marrow plasma cells, but the effect on serum alloantibody was modest (16).
Based on these encouraging, but limited results, we hypothesized that more doses of BTZ were needed for a meaningful reduction in antibody. Thus, the aim of this study was to evaluate the efficacy and safety of 32 doses of BTZ in highly sensitized KTx candidates.
Materials and Methods
Study Design
This was a prospective, open-labeled, nonrandomized, trial conducted with informed consent using a protocol approved by the institutional review board (IRB) of Mayo Foundation and Clinic, Rochester, Minnesota (IRB approval numbers 08-000556 and 15-002637; protocol number X05261; ClinicalTrials.gov Identifier: NCT00722722). The patients in this study met the following criteria: 1) B-cell flow cytometric cross match channel shift (BFXM) of greater than 300 against their intended living donor, 2) evidence of DSA (defined by identifiable antibodies with specificities for at least 1 donor HLA type by single antigen bead assay [SAB]); 3) cPRA ≥ 90%; 4) end stage renal disease and 5) otherwise meeting criteria for KTx at our program. Ten patients met the above inclusion criteria between October 2008 and June 2013. See SDC, Table 1 for exclusion criteria.
The primary endpoint was the reduction in serum alloantibody levels following BTZ treatment to reach a BFXM of less than 300.
Desensitization schedule
BTZ was given in cycles (4 doses = 1 cycle). The initial dose for all patients was 1.3 mg/m2 BSA intravenously on days 1, 4, 8, and 11 with at least 10 days in between the last dose of a cycle and the first dose of the next cycle (Figure 1). The dose administered remained the same throughout each cycle unless an adverse event occurred. No dose adjustment was made for renal function. We planned to give up to 8 cycles of BTZ (32 doses) unless the patient received a transplant.
Figure 1.
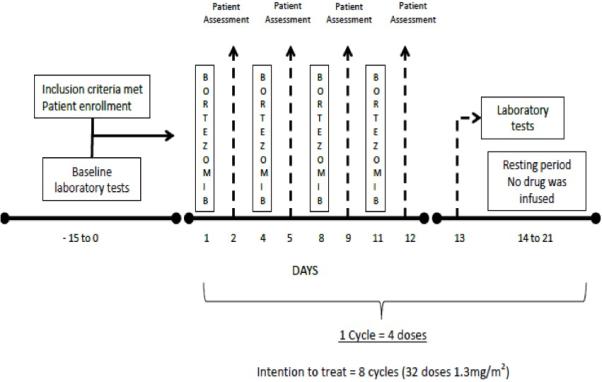
Treatment protocol.
Adverse events assessment
After each BTZ dose, patients had a complete physical examination with emphasis on the neurological evaluation to detect potential toxicities, and adverse event data was collected prospectively. Patients also had to complete a Functional Assessment of Cancer Therapy Scale/Gynecologic Oncology Group—Neurotoxicity (FACT/GOG-Ntx) questionnaire (17). Toxicities were assessed according to the NCI Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0 (18). All previously established or new toxicities observed any time (with the exception of neuropathic pain and peripheral sensory neuropathy), were managed according to recommendations by the manufacturer as outlined in SDC, Table 2. BTZ-related neuropathic pain and/or peripheral sensory neuropathy were managed as presented in SDC, Table 3.
Alloantibody quantification
The T and B flow cytometric crossmatch (TFXM, BFXM) and SAB (LABScreen, One Lambda, Canoga Park, CA) assays that we used have been previously described (19). SAB assays were obtained prior to and after bortezomib monotherapy. SAB results following any other therapy (i.e. plasmapheresis) were not included in this analysis. Single serum dilutions (1/8) were performed at least once pre and posttreatment to detect prozone phenomenon.
SAB results from solid phase assays are semi-quantitative and yield multiple data points. Detecting a meaningful change in alloantibody is difficult, and thus we examined several measures including: 1) MFI values for randomly selected individual antibody specificities pre and posttreatment, 2) BFXM and TFXM mean channel shifts against original intended donor pre and posttreatment, and 3) cPRA pre and posttreatment (as determined by thresholds of 2000, 4000 and 8000 MFI and the UNOS cPRA calculator [20]). Beads for comparison were categorized as low (MFI <2000), medium (MFI 2000-8000), and high (MFI>8000) based on their pretreatment serum results in order to determine whether antibodies at various levels are more or less likely to change with BTZ therapy.
Statistical Analyses
Patient demographics, BFXM, TFXM and cPRA data was summarized using means and standard deviation (SD) for continuous variables as well as the count and proportion for discrete variables; this information was analyzed using t-tests, paired or unpaired, according to the data compared.
A linear mixed model in the form of a segmented regression was used to evaluate significance of treatment effect on MFI while controlling for time (21, 22). In this case, the first time point posttreatment centers the time scale for the mixed model. Therefore, time pretreatment was recorded as negative time. The fixed effects used were time, treatment, and time posttreatment (equal to zero before treatment and simply time after treatment). Random effects were used to control for alloantibody specificity and repeated measures on patients. The same model structure was also used to analyze treatment effect within class I and class II alloantibodies. Data points of MFI versus time were averaged over patient and alloantibody specificity at each time point. Superimposed lines are associated with the respective slopes and intercepts for the fixed effects in the segmented regression formula. Mean alloantibody levels before and after treatment as well as average treatment effect are given as parameter estimates corresponding to intercepts and the slope associated with the categorical treatment variable. Therefore, these are summarized as parameter estimate and SE. A p-value of 0.05 or less was considered statistically significant.
All analyses were carried out using JMP Pro 10.0 and SAS 9.4 (SAS Inst., Cary, NC). Plots of the mixed effects models were created using the R statistical package (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study population
Most patients were female (7/10; 70%) and had at least 1 previous KTx (8/10; 80%). The most common cause of end stage renal disease was glomerulonephritis (5/10; 50%) and the mean age at enrollment was 39.5 (SD 6.6) years (Table 1). The baseline BFXM mean channel shift was 532 (SD 78), and 90% (9/10) of patients had both anti HLA- class I and class II antibodies. The mean cPRA2000 before treatment was 98.2% (SD 3.5).
Table 1.
Baseline demographics.
| n=10 | |
|---|---|
| Gender Female n( %) | 7 (70) |
| Age at treatment (mean±SD) | 39.5 ± 6.6 |
| Race; n (%) | |
| Caucasian | 9 (90) |
| Hispanic | 1 (10) |
| Cause of end stage renal disease; n (%) | |
| Glomerulonephritis | 5 (50) |
| Alport's syndrome | 1 (10) |
| Cystic kidney disease | 2 (20) |
| Congenital | 2 (20) |
| Baseline dialysis status; n (%) | |
| Hemodialysis | 7 (70) |
| Peritoneal Dialysis | 3 (30) |
| Prior kidney transplants; n (%) | |
| 0 | 2 (20) |
| 1 | 4 (40) |
| 2 | 1 (10) |
| 3 | 2 (20) |
| more than 3 | 1 (10) |
| Donor Specific Antibody (class); n (%) | |
| Both class I and II | 9 (90) |
| Anti-Class II only | 1 (10) |
| Anti-Class I only | 0 |
| B- FXM with original intended donor (mean channel shift ±SD) | 532 ± 78 |
| Baseline cPRA (MFI ± 2000)- (mean±SD) | 98.2 ± 3.5 |
| Transplantation posttreatment; n (%) | 8 (80) |
| Time to transplantation (mean month ±SD) | 31 ± 19 |
Tolerability
Five patients (50%) completed the 32-dose regimen without dose-reduction or discontinuation. One patient (10%) received a positive crossmatch KTx (BFXM of 431 post BTZ monotherapy and 273 following plasmapheresis prior to transplant) after dose 20 and BTZ was discontinued. Dose reduction was required in 2 patients who developed severe peripheral neuropathy but eventually completed the 32-dose course. In 1 of these cases, the severe neuropathy was manifested by left scapular myofascial pain and upper extremity edema. BTZ was suspended for 9 months before restarting at 1mg/m2BSA. The other patient experienced progressive severe bilateral lower extremity neuropathy, anorexia and insomnia. BTZ was held for 42 days and restarted at 1 mg/m2 BSA.
Two patients (20%) discontinued therapy prior to the planned 32 doses for adverse effects. One patient stopped after 16 doses for personal reasons and severe fatigue. The other patient developed disseminated varicella zoster, severe local herpes recurrence, peritonitis in the setting of peritoneal dialysis, encephalopathy, ataxia and visual hallucinations which required hospitalization. BTZ was initially discontinued for 38 days and restarted at 1 mg/m2 BSA. The drug was again reduced to 0.7mg/m2 BSA but eventually stopped after 24 doses for peripheral neuropathy. Other commonly observed BTZ-related toxicities are presented on SDC, Table 4.
Effect of BTZ on individual anti-HLA specificities
We compared the pretreatment and posttreatment MFIs of a random representative sample of anti-HLA antibodies (nonprozone) using a linear mixed model. We used data from a mean of 6.9 (SD 4.1) pretreatment assays and 4.8 (SD 6.5) posttreatment assays obtained from a mean of 484 (SD 328) days before BTZ (pretreatment) and a mean of 274 (SD 484) days after completion of BTZ (posttreatment). A mean of 5.0 (SD 1.2) individual class I specificities and 4.4 (SD 1.6) class II specificities were compared per patient.
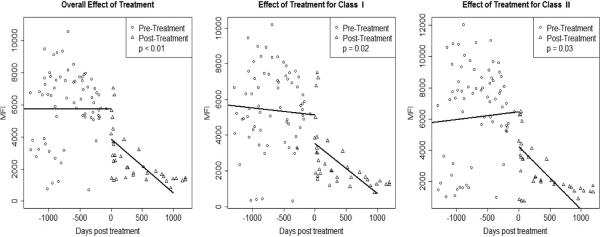
In this representative sample, the MFIs of individual specificities were stable pretreatment p=0.96, but decreased posttreatment p<0.01 (Figure 2A). The mean decrease in MFI overall was 1916 (SE 425). Both anti-class I and anti-class II antibodies decreased (p=0.02 and p=0.03, respectively) with baseline MFI in the 2000-8000 or >8000 range (class I: MFI 2000-8000, p<0.01; MFI > 8000, p<0.01 and class II: MFI 2000-8000, p<0.01; MFI > 8000, p<0.01). There was also a numeric reduction in MFI in specificities with baseline MFI <2000, but this did not reach statistical significance (p=0.44 for class I and p=0.54 for class II) (Figure 2B).
Figure 2A. Level of anti-HLA antibody decreased following bortezomib.
Linear mixed model performed: each data point represents the mean of all MFI values recorded at that time point per patient. The mean MFI of individual specificities were stable pretreatment p=0.96, but decreased posttreatment p<0.01. This effect was also detected in both anti-class I and anti-class II specificities (p=0.02 and p=0.03, respectively).
Figure 2B. Change in MFI postbortezomib stratified by antibody class and baseline MFI.
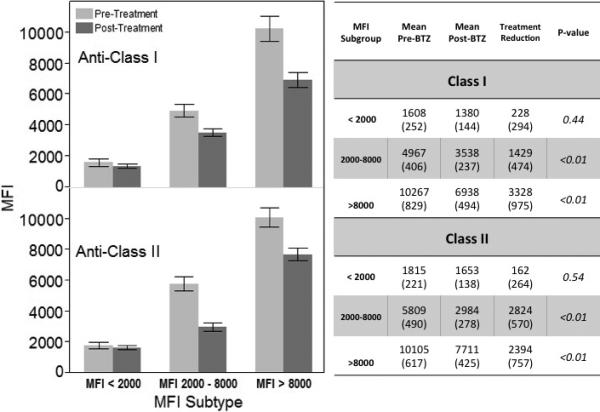
Bars and text are given as mean (standard error). Grey = prebortezomib; black= postbortezomib.
Effect of BTZ on cPRA and unacceptable antigens
Despite the decrease in MFI after BTZ, the mean cPRA calculated based on MFI of 2000, 4000, and 8000 was unchanged (Figure 2C). The mean cPRA2000 went from 98.2% (SD 3.5) to 98.3% (SD 2.9) posttreatment, p=0.83. The cPRA4000 was 93.8% (SD 12.3) pretreatment compared to 95.8% (SD 5.0) posttreatment (p=0.47), and the cPRA8000 was 86% (SD 20) pretreatment compared to 83.2% (SD 24) posttreatment (p=0.13). At baseline, we identified 423 different unacceptable antigens. The mean number of unacceptable antigens per patient decreased following treatment from 47 (SD 18) to 41 (SD 17), p=0.03. For class I, the mean number of unacceptable antigens went from 30 (SD 18) pretreatment to 25 (SD 17) posttreatment, p=0.07. For class II, the mean number of unacceptable antigens decreased from 17 (SD 4) prior to BTZ to 16 (SD 4) after treatment, p=0.06.
Figure 2C. No change in cPRA following bortezomib monotherapy.
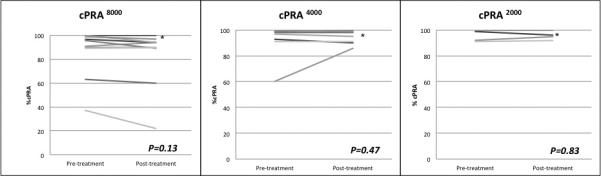
There was no significant decrease in cPRA when MFI 2000, 4000, or 8000 used as alloantibody positivity threshold. (*) More than 1 patient had cPRA =100% that was unchanged posttreatment.
The mean BFXM decreased from 532 (SD 78) pretreatment to 465 (SD 90) posttreatment (p=0.02) towards their original intended donor; but no patient reached a BFXM of less than 300. The mean TFXM remained unchanged from 270 (SD 199) to 259 (SD 197), p=0.58.
Transplant Outcomes
Eight out of 10 (80%) of the treated patients received a KTx at a mean of 938 (SD 566) days postbortezomib (16 doses=1, 20 doses=1 and 32 doses=6). The mean cPRA of these patients immediately prior to transplant was also similar to what it was prior to BTZ treatment (cPRA2000: 97.5% [SD 3.7], p=0.67; cPRA4000: 96.1% [SD 4.8], p=0.42; cPRA8000 89.1% [SD 15], p=0.63).
Three of the transplants (37.5%) were negative crossmatch from deceased donors, while 5 (62.5%) were positive crossmatch. Of the positive crossmatch transplants, only 2 were with the recipient's original intended living donor. Four (80%) of the positive crossmatch transplant recipients received plasmapheresis immediately prior to transplant to achieve a BFXM < 300 (mean BFXM of 209 [SD 81]). Patient and graft survival is 100% at a mean follow up of 815 (SD 732) days.
Discussion
Therapy to reduce alloantibody is an unmet need in transplantation. Bortezomib has been the subject of much interest in this area, but data establishing its clinical efficacy is limited (23, 24). Our current study shows that 32 doses of bortezomib monotherapy given to a highly sensitized patient cohort resulted in only a modest reduction in both class I and II antibodies at various MFI levels, but it is not well tolerated. However, it is unlikely that the decrease in antibody was clinically meaningful because no consistent change in cPRA at various MFI levels was detected, and none of the patients achieved a BFXM that was considered acceptable for transplantation with their original intended donor (BFXM <300). Ultimately, 80% of the patients in this cohort did receive a kidney transplant, but it is unknown whether their chance for transplantation was increased because of this therapy. The patient's cPRA was unchanged with therapy, but there was a modest decrease in the number of unacceptable antigens per patient. However, most patients waited nearly 3 years following therapy for a transplant. Our findings are consistent and extend the findings of our own work and the work of others (25, 26).
Importantly, in the current study we found that prior to bortezomib treatment, antibody levels were stable. Thus, any reduction in alloantibody was likely due to treatment and not due to chance variability. This therapy may have been more effective in a less sensitized patient cohort, but the highly sensitized cohort we targeted is the most in need of therapy. National registry data shows that despite the new kidney allocation system in the United States, 6% of the patients on the deceased donor waitlist have a cPRA of 100% (1). These patients have the longest transplant waiting time and are subject to increased morbidity and mortality on dialysis. Less sensitized patients are more likely to receive benefit from paired donor programs (27) or recent changes from the kidney allocation system.
The reasons for the limited efficacy remain unclear. In vivo, normal PCs may be more resistant to proteasome inhibition than myeloma cells (i.e. malignant plasma cells) (28). It is also possible that combining BTZ with other agents or plasmapheresis might increase its efficacy in a manner similar to combination therapy has been shown to improve its efficacy in myeloma (29). BTZ combined with other therapy is associated with a reduction in anti-HLA antibodies in a less sensitized patient cohort (30). In that cohort, it remains unclear how much of the treatment effect was from BTZ or the other therapies used (ie. plasmapheresis). Other approaches to PC depletion with blockade of IL-5 or IL-6 are areas are of particular interest.
Our study was limited because it was nonrandomized and small. It is possible that we did not have enough power to detect a significant change in cPRA or flow cytometric crossmatch. It is important to emphasize, that although there was no change in cPRA, there was a decrease in the total number of the unacceptable antigens posttreatment, which could have increased the access to transplantation for some patients. Another limitation of our study was the quantification of anti-HLA antibody. We performed 1 serum dilution to detect bead saturation, but we did not obtain antibody titers. Determining the change in the antibody titer as opposed to examining the MFI alone may have provided more information about the actual change in antibody level, but it is unlikely that this information would change clinical management. Most of the antibody levels remained too high for negative crossmatch transplantation regardless of treatment.
In conclusion, up to 32 doses of bortezomib monotherapy is associated with a decrease in anti-HLA antibodies, but we do not recommend it routinely for desensitization. This therapy was not well tolerated, and the reduction in antibody did not appear to translate into important clinical outcomes such negative crossmatch kidney transplant or even a reduction in cPRA.
Supplementary Material
Acknowledgments
The authors thank R. Dierkhising and W. Kremers for assistance with statistical analyses, S. De Goey and R. Knauer for support in antibody characterization at the tissue-typing laboratory. An abstract of this project was accepted for poster presentation at the 2014 World Transplant Congress- San Francisco, CA, USA.
Abbreviations
- BFXM
B-cell flow cytometric crossmatch
- BTZ
bortezomib
- BSA
body surface area
- cPRA
calculated panel reactive antibodies
- DSA
donor specific alloantibodies
- HLA
human leukocyte antigens
- IRB
institutional review board
- KTx
kidney transplant
- MFI
mean fluorescence intensity
- SAB
single antigen bead assay
- SD
standard deviation
- SE
standard error
- TFXM
T-cell flow cytometric crossmatch
Footnotes
The impact of Velcade™ on antibody secreting cells in sensitized renal allograft candidates; ClinicalTrials.gov Identifier: NCT00722722
Author Contributions
Manuel A. Moreno Gonzales
Participated in research design;
Participated in the writing of the paper;
Participated in the performance of the research;
Participated in data analysis
MAMG declares no conflicts of interest
Manish J. Gandhi
Participated in research design;
Participated in the writing of the paper;
Participated in the performance of the research;
MJG declares no conflicts of interest
Carrie A. Schinstock
Participated in research design;
Participated in the writing of the paper;
Participated in the performance of the research;
Participated in data analysis
CAS declares no conflicts of interest
Natalie A. Moore
Participated in the performance of the research
Participated in data analysis
NAM declares no conflicts of interest
Byron H. Smith
Participated in the writing of the paper;
Participated in the performance of the research
Participated in data analysis
BHS declares no conflicts of interest
Nong Y. Braaten
Participated in the performance of the research
NYB declares no conflicts of interest
M.D. Stegall
Participated in research design;
Participated in the writing of the paper;
Participated in data analysis
MDS has research contracts with Alexion Pharmaceuticals and Takeda Pharmaceuticals.
References
- 1.Massie AB, Luo X, Lonze BE, et al. Early Changes in Kidney Distribution under the New Allocation System. J Am Soc Nephrol. doi: 10.1681/ASN.2015080934. [published online ahead of print December 17th, 2015] doi:10.1681/ASN.2015080934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montgomery RA, Lonze BE, King KE, et al. Desensitization in HLA incompatible kidney recipients and survival. N Engl J Med. 2011;365:318–326. doi: 10.1056/NEJMoa1012376. [DOI] [PubMed] [Google Scholar]
- 3.Bentall A, Cornell LD, Gloor JM, et al. Five-year outcomes in living donor kidney transplants with a positive crossmatch. Am J Transplant. 2013;13:76–85. doi: 10.1111/j.1600-6143.2012.04291.x. [DOI] [PubMed] [Google Scholar]
- 4.Taner T, Gandhi MJ, Sanderson SO, et al. Prevalence, course and impact of HLA donor-specific antibodies in liver transplantation in the first year. Am J Transplant. 2012;12:1504–1510. doi: 10.1111/j.1600-6143.2012.03995.x. [DOI] [PubMed] [Google Scholar]
- 5.Daly RC, Topilsky Y, Joyce L, et al. Combined heart and liver transplantation: protection of the cardiac graft from antibody rejection by initial liver implantation. Transplantation. 2013;95:e2–e4. doi: 10.1097/TP.0b013e318277226d. [DOI] [PubMed] [Google Scholar]
- 6.Ciurea SO, Thall PF, Wang X, et al. Donor-specific anti-HLA Abs and graft failure in matched unrelated donor hematopoietic stem cell transplantation. Blood. 2011;118:5957–5964. doi: 10.1182/blood-2011-06-362111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vo AA, Peng A, Toyoda M, et al. Use of intravenous immune globulin and rituximab for desensitization of highly HLA-sensitized patients awaiting kidney transplantation. Transplantation. 2010;89:1095–1102. doi: 10.1097/TP.0b013e3181d21e7f. [DOI] [PubMed] [Google Scholar]
- 8.Gloor JM, DeGoey SR, Pineda AA, et al. Overcoming a positive crossmatch in living-donor kidney transplantation. Am J Transplant. 2003;3:1017–1023. doi: 10.1034/j.1600-6143.2003.00180.x. [DOI] [PubMed] [Google Scholar]
- 9.Montgomery RA, Zachary AA. Transplanting patients with a positive donor-specific crossmatch: a single center's perspective. Pediatr Transplant. 2004;8:535–542. doi: 10.1111/j.1399-3046.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 10.Perry DK, Pollinger HS, Burns JM, et al. Two novel assays of antibody secreting cells demonstrating resistance to desensitization with IVIG and rATG. Am J Transplant. 2008;8:133–143. doi: 10.1111/j.1600-6143.2007.02039.x. [DOI] [PubMed] [Google Scholar]
- 11.Stegall MD, Gloor J, Winters JL, Moore SB, Degoey S. A comparison of plasmapheresis versus high-dose IVIG desensitization in renal allograft recipients with high levels of donor specific alloantibody. Am J Transplant. 2006;6(2):346–351. doi: 10.1111/j.1600-6143.2005.01178.x. [DOI] [PubMed] [Google Scholar]
- 12.Everly MJ, Everly JJ, Susskind B, et al. Bortezomib provides effective therapy for antibody- and cell-mediated acute rejection. Transplantation. 2008;86:1754–1761. doi: 10.1097/TP.0b013e318190af83. [DOI] [PubMed] [Google Scholar]
- 13.Walsh RC, Everly JJ, Brailey P, et al. Proteasome inhibitor-based primary therapy for antibody-mediated renal allograft rejection. Transplantation. 2010;89:277–284. doi: 10.1097/TP.0b013e3181c6ff8d. [DOI] [PubMed] [Google Scholar]
- 14.Wong W, Lee RA, Saidman SL, et al. Bortezomib in kidney transplant recipients with antibody mediated rejection: three case reports. Clinical transplants. 2009:401–405. [PMC free article] [PubMed] [Google Scholar]
- 15.Perry DK, Burns JM, Pollinger HS, et al. Proteasome inhibition causes apoptosis of normal human plasma cells preventing alloantibody production. Am J Transplant. 2009;9:201–209. doi: 10.1111/j.1600-6143.2008.02461.x. [DOI] [PubMed] [Google Scholar]
- 16.Diwan TS, Raghavaiah S, Burns JM, et al. The impact of proteasome inhibition on alloantibody-producing plasma cells in vivo. Transplantation. 2011;91:536–541. doi: 10.1097/TP.0b013e3182081333. [DOI] [PubMed] [Google Scholar]
- 17.Cella DF, Tulsky DS, Gray G, Sarafian B, Lloyd S, Linn E, et al. The functional assessment of cancer therapy (FACT) scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–79. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 18.National Cancer Istitue [May 11th, 2016];Cancer Therapy Evaluation Program. http://ctep.cancer.gov/reporting/ctc.html. Updated March 3rd, 2016.
- 19.Burns JM, Cornell LD, Perry DK, et al. Alloantibody levels and acute humoral rejection early after positive crossmatch kidney transplantation. Am J Transplant. 2008;8:2684–2694. doi: 10.1111/j.1600-6143.2008.02441.x. [DOI] [PubMed] [Google Scholar]
- 20.Organ Procurement and Transplantation Network [May 11th, 2016];Professional Education. http://optn.transplant.hrsa.gov/resources/professionalResources.asp?index=78. Updated 2016.
- 21.Naumova EN, Must A, Laird NM. Tutorial in biostatistics: Evaluating the impact of ‘critical periods' in longitudinal studies of growth using piecewise mixed effects models. Int. J. Epi. 2001;30:1332–1341. doi: 10.1093/ije/30.6.1332. [DOI] [PubMed] [Google Scholar]
- 22.Wagner AK, Soumerai SB, Zhang F, et al. Segmented regression analysis of interrupted time series studies in medication use research. J. Clin. Pharm. Ther. 2002;27:299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 23.Sberro-Soussan R, Zuber J, Suberbielle-Boissel C, et al. Bortezomib alone fails to decrease donor specific anti-HLA antibodies: 4 case reports. Clinical transplants. 2009:433–438. [PubMed] [Google Scholar]
- 24.Sberro-Soussan R, Zuber J, Suberbielle-Boissel C, et al. Bortezomib as the sole post-renal transplantation desensitization agent does not decrease donor-specific anti-HLA antibodies. Am J Transplant. 2010;10:681–686. doi: 10.1111/j.1600-6143.2009.02968.x. [DOI] [PubMed] [Google Scholar]
- 25.Sberro-Soussan R, Zuber J, Suberbielle-Boissel C, et al. Bortezomib alone fails to decrease donor specific anti-HLA antibodies: even after one year post-treatment. Clinical transplants. 2010:409–414. [PubMed] [Google Scholar]
- 26.Schmid N, Alloway RR, Walsh RC, et al. Prospective evaluation of the toxicity profile of proteasome inhibitor-based therapy in renal transplant candidates and recipients. Transplantation. 2012;94:352–361. doi: 10.1097/TP.0b013e318257acf6. [DOI] [PubMed] [Google Scholar]
- 27.Li H, Stegall MD, Dean PG, et al. Assessing the efficacy of kidney paired donation--performance of an integrated three-site program. Transplantation. 2014;98:300–305. doi: 10.1097/TP.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 28.Stegall MD, Moore N, Taner T, et al. Down-regulating humoral immune responses: implications for organ transplantation. Transplantation. 2014;97:247–257. doi: 10.1097/TP.0b013e3182a72115. [DOI] [PubMed] [Google Scholar]
- 29.Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116:679. doi: 10.1182/blood-2010-02-268862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woodle ES, Shields AR, Ejaz NS, et al. Prospective iterative trial of proteasome inhibitor-based desensitization. Am J Transplant. Jan. 2015;15:101–108. doi: 10.1111/ajt.13050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.