Abstract
Background
The liver immune environment is tightly regulated to balance immune activation with immune tolerance. Understanding the dominant immune pathways initiated in the liver is important since the liver is a site for cell transplantation, such as for islet and hepatocyte transplantation. The purpose of this study is to examine the consequences of alloimmune stimulation when allogeneic cells are transplanted to the liver in comparison to a different immune locale, such as the kidney.
Methods
We investigated cellular and humoral immune responses when allogeneic hepatocytes are transplanted directly to the recipient liver by intraportal injection. A heterotopic kidney engraftment site was used for comparison to immune activation in the liver microenvironment.
Results
Transplantation of allogeneic hepatocytes delivered directly to the liver, via recipient portal circulation, stimulated long-term, high magnitude CD8+ T cell-mediated allocytotoxicity. CD8+ T cells initiated significant in vivo allocytotoxicity as well as rapid rejection of hepatocytes transplanted to the liver even in the absence of secondary lymph nodes or CD4+ T cells. In contrast, in the absence of recipient peripheral lymphoid tissue and CD4+ T cells, CD8-mediated in vivo allocytotoxicity was abrogated and rejection was delayed when hepatocellular allografts were transplanted to the kidney subcapsular site.
Conclusions
These results highlight the CD8-dominant proinflammatory immune responses unique to the liver microenvironment. Allogeneic cells transplanted directly to the liver do not enjoy immune privilege but rather require immunosuppression to prevent rejection by a robust and persistent CD8‐dependent allocytotoxicity primed in the liver.
Introduction
The liver immune environment is of special interest within the field of transplantation due to the observation that MHC-mismatched whole organ liver allografts are accepted without immunotherapy1,2 and that a prior liver transplant can promote acceptance of donor-matched allografts of other organs in animal models3,4. Furthermore, immunosuppression has successfully been withdrawn in some liver transplant recipients without detectable clinical consequences5,6. Exposure of antigens to the portal circulation or the hepatic immune locale may promote a tolerogenic response, which is shared with a few other immune compartments (i.e., anterior chamber of the eye, testis)7–9. Examples include the phenomena of portal venous tolerance, oral tolerance, spontaneous acceptance of whole liver allografts, and the persistent nature of hepatotropic viruses10–12.
Despite these remarkable examples of antigen acceptance in the liver of animals and humans, cell transplantation into the liver has not enjoyed immune privilege in either animal models or in the clinical setting13,14. In fact, despite promising 1-year islet allograft survival rates in diabetic patients, the majority of recipients are not insulin free at 3 years posttransplant15,16. Similarly, allogeneic hepatocyte grafts transplanted into patients with metabolic disorders or liver failure exhibit significant but short-lived benefits ranging 3–26 months posttransplant13,17–19. This disparity, between whole liver transplantation and parenchymal cell transplants implanted into the liver, may be explained by a tightly regulated balance between immune reactivity and tolerance within the liver immune environment10,20. As reported by Kammer et al., it is evident that the liver provides rapid and efficient immunity against pathogens introduced from the gastrointestinal route via splanchnic circulation21. Additionally, there are conflicting reports concerning the propensity of the liver to promote activation or tolerance of CD8+ T cell responses22–24.
In contrast to the tolerogenicity of whole liver transplants, we have reported that hepatocellular allografts are highly immunogenic. Allogeneic hepatocytes initiate CD8-mediated rejection25–29 that is resistant to therapies that readily control CD4-dependent rejection responses25,26,30–32. Hepatocytes also initiate CD4-dependent (CD8-independent) antibody-mediated rejection mechanisms25–27,33–35. The dominant mechanism of rejection, though, differs with the engraftment site. Hepatocyte allografts transplanted to the host liver (via intrasplenic injection) initiate rapid rejection, which features strong allospecific CD8-dependent cytotoxic responses. In contrast, transplantation of donor hepatocytes to the host kidney results in rapid rejection with a dominant humoral effector mechanism36. However, in these prior studies since hepatocytes were transplanted by intrasplenic injection, the failure to tolerize to alloantigens could have occurred due to priming in the spleen before engraftment in the liver. In order to investigate alloantigen-primed immune responses initiated in the liver microenvironment we investigated the kinetics, magnitude, CD4-dependence and requirement for host lymph nodes of CD8-mediated allocytotoxic responses stimulated by allogeneic hepatocytes transplanted to the liver by direct portal injection.
Materials and Methods
Experimental animals
FVB/N (H-2q, Taconic, Hudson, NY), C57BL/6 (H-2b, Jackson, Bar Harbor, ME), CD4 KO (H-2b, Jackson), and LTα KO (H-2b, Jackson) mouse strains were used in this study. Transgenic FVB/N mice expressing human alpha-1 anti-trypsin (hA1AT) were the source of “donor” hepatocytes, as previously described37. Mice that were 6–9 weeks of age were used in experiments. All experiments were performed in compliance with the guidelines of the Institutional Animal Care and Use Committee of the Ohio State University (Protocol 2008A0068-R2).
Hepatocyte transplantation and monitoring of hepatocyte survival
Hepatocyte isolation and purification were performed, as described previously25,37. Hepatocyte viability and purity was >95%. Donor hepatocytes were retrieved from FVB/N mice and transplanted into recipients by 3 different routes in these studies, including intrasplenic injection37, intraportal injection (injection into the portal vein distal to its confluence with the superior mesenteric vein), and kidney subcapsular injection36, as previously described. In all recipients, graft function was determined by serial detection of the secreted transgenic reporter product, hA1AT, in recipient serum25,37. The reporter protein hA1AT does not elicit an immune response and syngeneic, hA1AT-expressing hepatocytes survive long-term37.
Antibodies used for in vivo T cell subset depletion
Recipients were depleted of circulating CD4+ T cells using monoclonal antibody (GK1.5; Bioexpress Cell Culture Services, West Lebanon, New Hampshire) by intraperitoneal injection (250 μg, day -4, -2, 7, 14 relative to transplantation). CD8+ T cells were depleted by intraperitoneal injections (100 μg, day -4, -2, 7, 14 relative to transplantation; clone 53.6.72; Bioexpress Cell Culture Services). Depletion was confirmed through flow cytometric analysis of recipient peripheral blood lymphocytes.
In vivo cytotoxicity assay
Detection of in vivo cytolytic T cell function through clearance of Carboxyfluorescein Diacetate Succinimidyl Ester (CFSE; Molecular Probes, Eugene, OR) stained allogeneic and syngeneic target cells, as previously described38. No difference in cytotoxicity was observed when analyzing targets from the spleen or from the peripheral blood (data not shown).
Donor-reactive alloantibody titer
To quantify alloantibody titer, we analyzed the recipient serum using published methods39.
Statistical analysis
Graft survival between experimental groups was compared using Kaplan Meier survival curves and log-rank statistics (SPSS, Chicago, IL). For all other statistical calculations, a 1-tailed Student’s t test was used. P<0.05 was considered significant. To demonstrate the distribution of the data, results are listed as the mean±standard error.
Results
Allogeneic hepatocytes transplanted into the liver immune environment elicit rapid rejection
To parallel the clinical hepatocyte transplantation route and to evaluate the effect of direct hepatocyte transplant to the recipient liver upon subsequent host immune responses, FVB/N hepatocytes were transplanted into wild-type (WT; C57BL/6) untreated recipient hosts through intraportal injection and monitored for graft survival. Intrasplenic transplant (the preferred route in published studies) was used as a control. Intraportal injection of hepatocytes into recipient mice resulted in rejection with a median survival time (MST) of 10 days, similar to hosts receiving intrasplenic injection of hepatocytes (Figure 1). These data demonstrate that allogeneic hepatocytes transplanted into the liver either directly through the portal vein or indirectly through intrasplenic injection initiate immunostimulatory rather than immunotolerant responses resulting in rapid cell transplant rejection.
Figure 1. Allogeneic hepatocytes elicit rapid rejection whether transplanted directly (intraportal) or indirectly (intrasplenic) to the liver microenvironment.
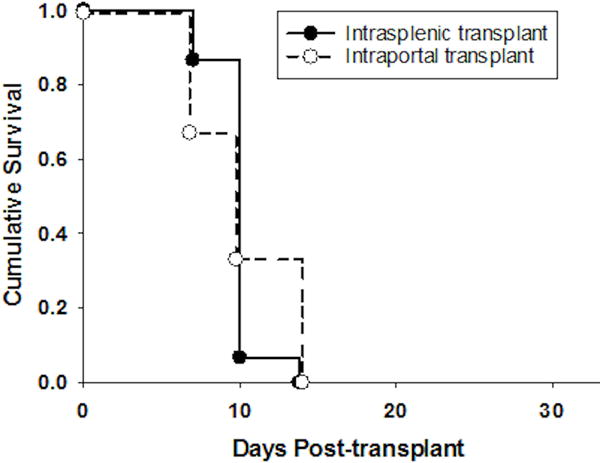
FVB/N (H-2q) hepatocytes were transplanted into untreated wild-type (WT; H-2b) recipients by intraportal injection (n=10) or by intrasplenic (n=15) injection. Graft rejection occurred promptly in both recipients transplanted directly to the liver by intraportal injection and those transplanted by conventional intrasplenic injection (MST=10 days, p=ns).
Kinetics, magnitude and persistence of CD8-mediated allocytotoxicity when allogeneic hepatocytes are transplanted to the liver by intraportal versus intrasplenic injection
Intrasplenic transplantation of allogeneic hepatocytes leads to maximal in vivo cytotoxic activity at 7 days posttransplant (90±2%). Cytotoxicity gradually decreases and returns to baseline levels by day 35 (day 14=26±4%, day 21=16±6%, day 35=7±2%; Figure 2A). Intraportal transplantation also results in high magnitude cell-mediated cytotoxicity at 7 days posttransplant (87±4%) but unlike after intrasplenic injection, allocytotoxicity persists 3 or more weeks beyond allograft rejection (day 14=93±3%, day 21=90±4%, day 35=72±13%). Day 7 allocytotoxicity in recipients of hepatocellular allografts transplanted by intraportal injection was abrogated (6±2%) by depletion of CD8+ T cells (anti-CD8 mAb; day 6 and 7 posttransplant) indicating that CD8+ T cells mediate the observed high cytotoxicity (Figure 2B). These data suggest that the liver immune environment supports the development of persistent, high magnitude CD8+ T cell-mediated cytotoxicity.
Figure 2. Alloimmunity initiated by hepatocellular transplantation directly (intraportal injection) to the liver induces high magnitude and persistent CD8-medated in vivo allocytotoxicity.
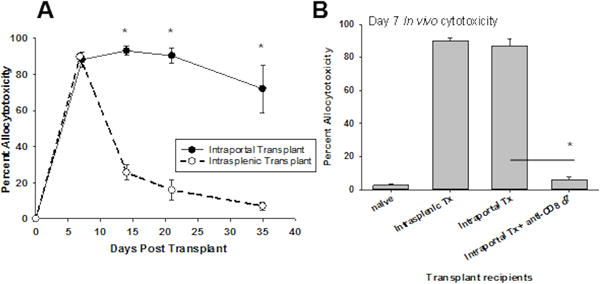
FVB/N hepatocytes (H-2q) were injected into untreated wild-type hosts (WT; H-2b) by intraportal injection or by intrasplenic injection. A) Allospecific in vivo cytotoxic effector activity was measured serially following transplantation, in both recipient groups. Both groups developed high magnitude cytotoxicity by 7 days posttransplant (intraportal=87±4%, intrasplenic=90±2%), but intraportal injection uniquely resulted in sustained cytotoxic activity beyond the time of graft rejection (day 14=93±3%, day 21=90±4%, day 35=72±13%), whereas recipients receiving intrasplenic injection showed a sharp decrease in cytotoxicity following graft rejection (day 14=26±4%, day 21=16±6%, day 35=7±2%; p<0.01 for all time points, signified by “*”). Each data point represents n=4–6 animals per time point. B) CD8+ T cell depletion using monoclonal antibodies on days 6 and 7 posttransplant, 48 hours prior to the in vivo cytotoxicity assay (day 7 posttransplant), eliminated the cytotoxic effector function in hepatocyte recipients (6±2%; p<0.001, signified by “*”; n=4) demonstrating that in vivo effector function in hepatocyte recipients is CD8-mediated. The error bars represent standard error.
CD8-mediated rejection after allogeneic hepatocyte transplant directly to the liver occurs independent of recipient peripheral lymphoid tissue
Previous studies have shown that CD8+ T cell priming by alloantigen in the liver results in apoptotic death of CD8+ T cells and non-productive effector function whereas priming in peripheral lymph nodes promotes competent CD8+ T cell effector function22,40. To determine if rejection of allogeneic hepatocytes transplanted to the liver by direct portal injection requires priming in peripheral lymph nodes, we assessed graft survival in recipients that lack lymph nodes (Ltα KO mice, H-2b). LTα KO recipients rejected hepatocyte allografts with kinetics similar to WT mice (MST=10 days; Figure 3A). To determine if the development of hepatocellular rejection required recipient splenic priming, immune responses in splenectomized recipients were analyzed. A splenectomy was performed at least 7 days prior to intraportal hepatocyte transplantation. Splenectomized hosts (WT and LTα KO) rejected hepatocyte allografts with a MST of 10 days, which is similar to rejection in respective recipients with intact spleens. Thus priming in the recipient spleen is not necessary for prompt rejection of allogeneic hepatocytes (transplanted directly to the liver) in either WT or LTα KO recipients. Furthermore, rejection of allogeneic hepatocytes transplanted directly to the liver occurs despite the absence of both recipient spleen and peripheral lymphoid tissue.
Figure 3. Rejection of allogeneic hepatocytes transplanted directly to the liver (intraportal injection) readily occurs in the absence of recipient peripheral lymphoid tissue.
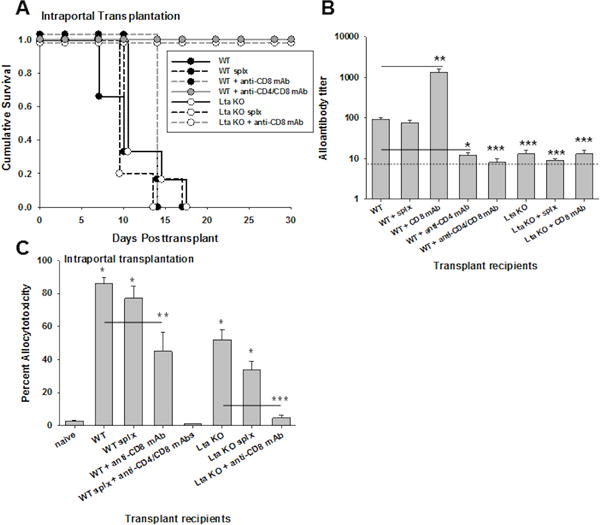
FVB/N hepatocytes (H-2q) were transplanted through intraportal injection into untreated wild-type hosts (WT; n=10), WT hosts with previous splenectomy (splx; n=6), LTα KO hosts (lacking peripheral lymph nodes; n=6), and LTα KO hosts with previous splenectomy (n=6; all H-2b). A) Graft rejection occurred with similar kinetics in all groups (MST=10 days; p=ns). CD8-depletion did not prolong graft survival in WT recipients (MST=day 14, n=4), whereas graft survival was significantly prolonged in CD8-depleted LTα KO recipients (MST>30; p<0.001; n=3). Prolonged graft survival was observed in WT recipients only following both CD4- and CD8-depletion (MST>30; p<0.001; n=4). B) Alloantibody production was evaluated in recipient mice on day 14 following transplantation. Following intraportal transplantation, WT recipients developed significant levels of alloantibody (titer=92±8; n=6) which was abrogated in CD4-deficient recipients (titer=12±2; n=5; p<0.001, signified by “*”) but remained intact despite splenectomy (splx; titer=75±14; n=4; p=ns). Significantly higher levels were observed following CD8-depletion in WT recipients (titer=1300±300, n=3, p<0.001 as compared to WT recipients, as signified by “**”). Alloantibody production was abrogated in WT recipients following CD4- and CD8-depletion (titer=8±2). Alloantibody was not detected in any lymph node deficient hosts (LTα KO; titer=13±3; n=4), LTα KO with splenectomy (splx; titer=9±1; n=4), or CD8-depleted LTα KO recipients (titer=13±3; n=3; p<0.001 for all groups compared to WT recipients, signified by “***”). The dotted line represents naïve serum control. C) CD8-mediated in vivo allocytotoxicity was readily detected in WT (87±4%; n=7), splenectomized WT (77±7%; n=4), LTα KO (53±6%; n=3), and splenectomized LTα KO recipients (34±5%; n=3) as compared to naïve controls (2±0.1%; p<0.01 for all, signified by “*”). In vivo cytotoxicity was significantly reduced but still detectable in CD8-depleted WT recipients (45±12%; n=3; p=0.04 compared to WT, signified by “**”). Cytotoxicity was only abrogated in WT recipients following both CD4- and CD8-depletion (0±0%; p<0.001). In contrast, CD8-depletion abrogated in vivo cytotoxicity in LTα KO recipients (5±2%; n=3; p=0.004, signified by “***”).
To determine the importance of the CD8-dependent rejection pathway in WT and LTα KO recipients which undergo allogeneic hepatocyte transplant by direct portal injection, hepatocellular allograft survival was monitored after depletion of CD8+ T cells. When CD8-depletion occurs prior to transplant and continues posttransplant, this prevents development of CD8+ allo-CTLs and also leads to a heightened alloantibody response41. Under these conditions transplanted hepatocytes are rejected rapidly in CD8-depleted WT recipients (MST=day 14)26,27,33. However, when both CD4+ and CD8+ T cells are depleted in WT recipients, hepatocellular allograft survival is significantly prolonged (MST>30 days; Figure 3A) and no alloantibody (titer=8±2; Figure 3B) nor in vivo cytotoxicity (0±0%; Figure 3C) is detected, consistent with the interpretation that hepatocyte rejection occurs by both alloantibody and CD8-dependent mechanisms in WT recipients. In contrast, CD8-depleted LTα KO recipients exhibit prolonged graft survival (MST>35 days; Figure 3A) and no alloantibody (titer= 13±3; Figure 3B) nor in vivo cytotoxicity (5±2%; Figure 3C). Thus rejection in LTα KO but not WT recipients is solely CD8-dependent.
Further, analysis of alloantibody titers in WT and LTα KO experimental groups revealed that while WT recipients had high alloantibody titers (titer=92±8), LTα KO recipients did not (titer=13±3; Figure 3B). Despite the spleen being an important immune locale of alloantibody-producing B cells41,42, the spleen is not required for alloantibody production as this pattern of alloantibody production in WT (titer=75±14) and absence of alloantibody production in LTα KO (titer=9±1) recipient groups persisted even after splenectomy. CD4+ T cell depletion of WT recipients, as expected, completely inhibited alloantibody production (titer=12±2). These data indicate that both CD4+ T cells and lymph nodes are critical for alloantibody production in response to hepatocyte transplant to the liver. Thus rejection in WT recipients reflects, in part, high alloantibody titers, whereas rejection in LTα KO recipients is entirely mediated by CD8+ T cells.
In vivo allocytotoxicity (day 7) was readily detected in WT (87±4%), splenectomized WT (77±7%), LTα KO (53±6%), and splenectomized LTα KO recipients (34±5%) as compared to naïve controls (2±0.1%; Figure 3C). In vivo cytotoxicity following intrasplenic36 and intraportal transplantation was allospecific as in vivo cytotoxicity was not observed against third party targets (B10.BR, H-2k) (3±1%). In vivo allocytotoxicity was reduced in CD8-depleted (spleen intact) WT recipients (45±12%) with residual allocytotoxicity correlating with high alloantibody levels (titer=1300±300, Figure 3B), as previously reported33. Altogether, these results indicate that secondary lymphoid tissue (lymph nodes and spleen) is not critical for the development of CD8-mediated allocytotoxicity or rejection of allogeneic hepatocytes transplanted directly into the host liver by intraportal injection. In contrast, humoral alloimmunity in response to allogeneic hepatocytes transplanted directly to the liver does not occur in the absence of host lymph nodes.
CD8-mediated rejection after allogeneic hepatocyte transplant directly to the liver occurs independent of recipient CD4+ T cells
We have previously reported using the intrasplenic transplant route that CD8+ T cells efficiently reject hepatocyte allografts in CD4-deficient hosts25,26,28,29. This has been reported using 3 models of CD4+ T cell deficiency, including CD4-depletion, genetically deficient recipients (CD4 KO), and CD8+ T cell reconstitution of SCID or Rag−/− hosts, with similar results25,26,37. To determine if CD4-independent, CD8+ T cell-dependent rejection responses are initiated within the liver, CD4-deficient hosts underwent intraportal transplantation of FVB/N hepatocytes. CD4-depleted WT recipients that underwent intraportal hepatocyte transplant had a mean graft survival time of 10 days (Figure 4A). The spleen is not required for CD8-mediated rejection as splenectomized CD4-depleted WT recipients rejected hepatocyte allografts with a mean survival time of 10 days. Similar graft rejection kinetics were also observed in splenectomized and nonsplenectomized CD4-depleted LTα KO hosts suggesting that recipient lymph nodes and spleen are not required for CD4-independent, CD8-dependent rejection initiated in the liver microenvironment (MST=10 days). Rejection in CD4-depleted lymph node-deficient hosts was mediated by CD8+ T cells, as CD4-deficient LTα KO hosts depleted of CD8+ T cells do not reject hepatocyte allografts (Figure 4A). CD8-mediated in vivo allocytotoxicity (day 7) remained at readily detectable levels in CD4-depleted LTα KO recipients (32±4%) as well as for all other comparison groups, including CD4-depleted WT (68±6%), CD4-depleted splenectomized WT (43±2%), and CD4-depleted splenectomized LTα KO recipients (22±3%; Figure 4B). CD8-depletion in CD4-depleted LTα KO recipients abrogated allocytotoxicity (4±2%). These data are consistent with the interpretation that immunostimulatory responses initiated in the liver by allogeneic cell transplantation result in robust CD8-mediated allocytotoxicity and rejection which occurs even in the absence of lymph nodes and CD4+ T cells. However, in WT recipients which have intact lymph nodes, rejection occurs by CD4-dependent alloantibody production or CD8-dependent rejection since depletion of both CD4+ and CD8+ T cell subsets significantly prolongs graft survival and abrogates in vivo allocytotoxicity.
Figure 4. Hepatocyte transplant directly to the liver (intraportal injection) elicits CD8-dependent rejection (but not alloantibody production) in the absence of CD4+ T cells and peripheral lymphoid tissue.
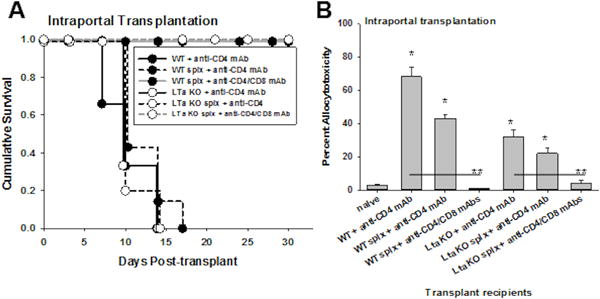
FVB/N hepatocytes (H-2q) were transplanted to the liver by intraportal injection into CD4-deficient wild-type (WT), LTα KO and/or splenectomized (splx) hosts. A) Rejection kinetics were unchanged in the absence of host CD4+ T cells in WT, LTα KO and/or splenectomized hosts. Allogeneic hepatocytes were rejected with similar rapid kinetics in CD4-depleted (wild type) hosts (MST=10 days), CD4-depleted hosts with splenectomy (MST=10 days), or CD4-depleted LTα KO hosts with splenectomy (in the absence of both host lymph nodes and spleen) (MST=10 days; p=ns; n=5–6 samples per condition). Following CD8-depletion, splenectomized CD4-deficient WT (n=4) and LTα KO recipients (n=3) exhibit long-term survival of transplanted hepatocytes (MST>30 days; p=0.002). B) CD8-mediated in vivo allocytotoxicity was detected in CD4-depleted WT (68±6%; n=4), CD4-depleted splenectomized WT (43±2%; n=3), CD4-depleted LTα KO recipients (32±4%; n=5), and CD4-depleted splenectomized LTα KO recipients (22±3%; n=4; p<0.005 for all groups compared to naïve control, signified by “*”). Following CD8-depletion, allocytotoxicity was abrogated in CD4-depleted WT (0±0%; n=4) and CD4-depleted LTα KO recipients (4±2%; n=3; p<0.008, signified by “**” compared to CD4-deficient recipients).
Dominant CD4-dependent humoral immune rejection initiated by kidney subcapsular hepatocellular transplant requires intact peripheral lymph nodes
Kidney subcapsular hepatocellular transplant is known to initiate dominant CD4-dependent humoral alloimmunity and rejection36. In order to determine the importance of peripheral lymph nodes for alloprimed humoral immune responses and rejection after kidney subcapsular hepatocellular transplant, FVB/N hepatocytes (H-2q) were transplanted into the kidney subcapsular space of WT and LTα KO untreated recipients. Some recipients underwent splenectomy prior to transplantation. Spleen-intact and splenectomized WT recipients both developed detectable alloantibody (titer=270±60 and 210±40, respectively; Figure 5A) and rejected hepatocyte allografts with similar kinetics (MST=14 and 10 days, respectively; Figure 5B). These results indicate that the host spleen is not critical for alloantibody production or graft rejection following kidney subcapsular transplantation. However, alloantibody does not develop following kidney subcapsular transplantation in LTα KO recipients (spleen-intact, splenectomized, and CD8-depleted) (Figure 5A). Furthermore, kidney subcapsular (unlike intrahepatic) hepatocyte transplantation in LTα KO hosts resulted in significantly delayed rejection with MST=28 days (LTα KO vs. WT, MST=10 days; Figure 5B). In splenectomized LTα KO recipients, the survival time was also delayed (MST=49 days) but was not statistically different in comparison to spleen intact LTα KO hosts. Whereas CD8-depletion in WT recipients of kidney subcapsular transplants did not delay rejection (MST=13 days), CD8-depletion in LTα KO recipients of kidney subcapsular transplants resulted in long-term survival (MST>50 days, Figure 5B). CD8-depleted WT recipients had high alloantibody levels (titer=3300±600), whereas CD8-depleted LTαKO did not (titer=13±3; Figure 5A). Altogether, these results are consistent with the interpretation that, unlike hepatocytes transplanted to the liver, recipient lymph nodes are critical for priming of immune responses after kidney subcapsular hepatocyte transplant and results in the stimulation of a dominant humoral alloimmune response and a secondary delayed CD8-mediated immune response.
Figure 5. Dominant humoral alloimmunity elicited by kidney subcapsular hepatocyte transplantation is critically dependent on CD4+ T cells and peripheral lymphoid tissue.
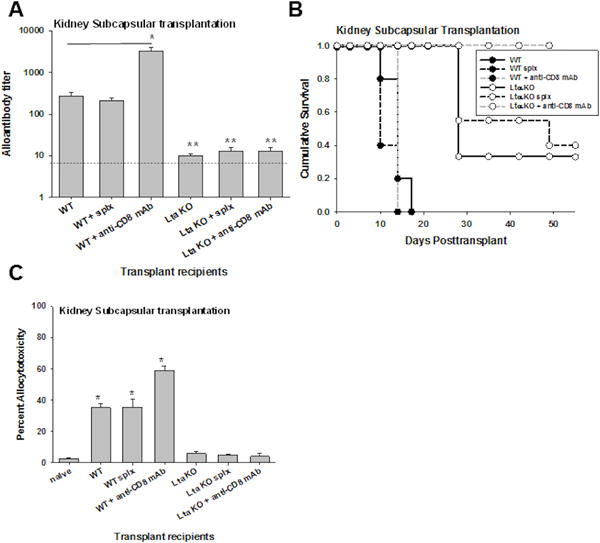
FVB/N hepatocytes (H-2q) were transplanted to the kidney subcapsular site in untreated wild-type (WT) hosts, WT hosts with previous splenectomy, untreated lymph node deficient hosts (LTα KO), and LTα KO hosts with previous splenectomy (all H-2b). A) Alloantibody production was evaluated in recipient mice on day 14 following transplantation. Following kidney subcapsular transplant, WT recipients (titer=270±60; n=5) and splenectomized WT recipients (titer=210±40; n=3) produced significant amounts of alloantibody. Significantly higher alloantibody levels were observed following CD8-depletion in WT recipients (titer=3300±600, n=3, p<0.001 as compared to WT recipients, as signified by “*”). Alloantibody levels were minimal in LTα KO (titer=10±1; n=3), splenectomized LTα KO recipients (titer=13±3; n=3), and CD8-depleted LTα KO recipients (titer=13±3; n=3; p<0.02 for all comparisons, as signified by “**”). The dotted line represents naïve serum control. B) Rejection of allogeneic hepatocytes after transplant to the kidney subcapsular site was not delayed in the absence of host spleen alone (WT splx, MST=10 days compared to MST=14 days for WT; p=ns). Rejection was significantly delayed in the absence of host lymph nodes (LTα KO, MST=28 days; p=0.001 relative to WT), in the absence of both host lymph nodes and spleen (LTα KO splx MST=49 days; p=0.002 relative to WT; p=ns relative to LTα KO; n=5–6 samples per condition), and in CD8-depleted LTα KO recipients (long-term survival, MST>50 days, n=3, p<0.001). C) In vivo allocytotoxicity was detected in WT (35±2%; n=3), splenectomized WT (36±5%; n=3), and CD8-depleted WT kidney subcapsular recipients (59±3%; n=3; p<0.002 compared to naïve control). In vivo cytotoxicity was negligible in all LTα KO recipients (6±1%; n=3), including splenectomized (5±1%; n=3) or CD8-depleted LTα KO recipients (4±2%; n=3; p=ns for all compared to naïve control).
CD8-mediated in vivo cytotoxicity on day 7 was significantly lower following kidney subcapsular transplantation (35±2%; Figure 5C) as compared to intraportal transplantation in WT recipients (87±4%; Figure 3C). Unlike WT recipients which received hepatocyte transplant by intraportal injection (Figure 2B), CD8-depletion did not abrogate peak in vivo cytotoxicity (day 7) in WT kidney subcapsular recipients (59±3%; Figure 5C) and allocytotoxicity in these recipients corresponded with high alloantibody (titer=3300±600, Figure 5B). Allocytotoxicity in WT kidney subcapsular recipients was readily detectable despite the absence of host spleen (36±5% in splenectomized WT recipients). However, in vivo cytotoxicity (day 7) was negligible in all LTα KO kidney subcapsular recipient groups [untreated (6±1%), splenectomized (5±1%), or CD8-depleted (4±2%)]. Collectively, these data suggest that whereas intrahepatic allogeneic cell transplant stimulates robust and unique CD8-mediated allocytotoxicity and rejection which does not depend on priming by CD4+ T cells or host lymph nodes, other sites such as kidney subcapsular transplantation stimulates humoral alloimmunity and rejection, which depends on CD4+ T cells and host lymph nodes. Our data also underscore the importance of, peripheral lymph nodes to the priming of host humoral alloimmune responses but not to CD8-mediated alloimmune responses which are primed in the liver.
Discussion
Both the current experimental studies and clinical experience highlight that hepatocytes can be successfully transplanted either by intraportal or by intrasplenic injection13,17–19. However, despite reports that the liver immune environment may lead to poor CD8+ T cell activation and apoptosis contributing to tolerance induction43, we report here that allogeneic hepatocytes are highly immunogenic and are rejected rapidly, in a CD8-dependent manner. In fact, intraportal transplant of allogeneic hepatocytes results in high magnitude CD8-mediated in vivo allocytotoxicity which persists much longer than in recipients transplanted by intrasplenic injection. However, this enhanced primary response does not necessarily correlate with changes in CD8+ T cell memory responses since CD8+ T cell memory phenotypes are similar for intrasplenic and intraportal hepatocyte transplant recipients (unpublished observations). Intrahepatic CD8-mediated allocytotoxicity (and hepatocyte rejection) following intraportal injection occurs even in the absence of CD4+ T cells and lymphatic structures (including peripheral lymph nodes, Peyer’s patches, and spleen). These data are consistent with the concept that the liver immune environment supports unique (CD4-independent) CD8-mediated alloantigen-specific cytotoxic responses which are high magnitude and long-lived. This was unexpected since a large body of literature underscores that the maturation of conventional cytotoxic CD8+ T cells in response to tumor antigen or infection, is dependent on both CD4+ T cells and priming in lymph nodes44–46. In the current study, the development of this CD4-independent, cytotoxic CD8+ T cell response and rejection primed within the liver immune environment is unique in comparison to T cell-dependent rejection in other transplant models including skin, heart, intestine, islet cell (kidney subcapsular injection), and graft versus host disease. In these other transplant models, graft rejection is highly dependent on peripheral lymphoid tissue as shown by studies which report significantly reduced alloimmune responses and delayed rejection in the absence of secondary lymph nodes and the spleen47–50. These disparate outcomes for hepatocyte compared to other allografts likely reflect the differences in the immune environment of the liver and other sites as well as differences in tissue immunogenicity34. For example, we have previously reported that CD4+ T cell depletion of islet transplant recipients (whether transplanted by intraportal or kidney subcapsular route) in the same MHC recipient/donor combination as hepatocyte transplant recipients in the current study results in prolonged allograft survival (>70 days)51. These long-term surviving islet allografts remain susceptible to rejection since, adoptive transfer of alloreactive CD4-independent CD8+ T cells (stimulated by hepatocyte transplant) results in rejection52.
Unique features of the liver immune environment include the abundance of CD8+ T cells trafficking through the organ, the unusually high proportions of resident immune populations such as NK, NKT cells, and tissue specific antigen presenting populations (hepatic stellate cells, liver sinusoidal endothelial cells, dendritic cells, Kupffer cells), and possible direct interactions of the trafficking CD8+ T cells with engrafted hepatocytes10,53. Pillarisetty et al. reported that while bulk liver dendritic cells are less able to stimulate CD8+ T cell activation than spleen dendritic cells, 20% of the liver’s native dendritic cells are equally capable of T cell activation as their splenic counterparts54. In contrast, the kidney does not harbor large populations of CD8+ T cells or recruit CD8+ T cells, and is generally populated by fewer immune cells in comparison to the liver. Additionally, the rejection response to transplants in the kidney site, in contrast to the liver site, is associated with a dominant humoral alloimmune responses and significantly lower magnitude of CD8+ T cell cytotoxicity36.
Alloantibody production exhibited critical dependence on the presence of both CD4+ T cells and lymph nodes following both intraportal and kidney subcapsular transplantation. This is likely due to the requirement of germinal center formation and cytokine-mediated help for B cell activation and antibody production55,56. These factors account for the differential requirement for lymph nodes in rejection depending on whether allogeneic hepatocytes were directly transplanted to the liver (CD8+ T cell dominant rejection, lymph node-independent) or kidney subcapsular space (alloantibody dominant rejection, lymph node-dependent). Alloantibody also accounts for differences observed for the in vivo allocytotoxicity results in WT and CD8-depleted recipients. When WT recipients are depleted of CD8+ T cells, alloantibody production is heightened and corresponds with in vivo alloantibody-dependent macrophage-mediated cytotoxicity33. We have previously reported that the enhanced amount of alloantibody production observed in CD8-depleted WT recipients occurs due to the depletion of CD8+ T cells which downregulate alloantibody production35 in part by killing antibody producing B cells posttransplant41.
We hypothesize that CD8+ T cells are directly activated in the liver by allogeneic MHC I expressed on transplanted hepatocytes. This is consistent with findings by others that allo-MHC I is sufficient to activate CD8-mediated responses57. Likewise, CD8+ T cell activation within the liver is further supported by in vitro and in vivo studies that suggest hepatocytes directly activate CD8+ T cells in response to bacterial and viral peptides presented by hepatocyte self MHC Class I24,58. Allogeneic hepatocytes could also transfer allogeneic MHC I to liver antigen presenting cells through exosomes, as in reports that hepatocytes release exosomes that can be absorbed by other hepatocytes59 and may enhance the activity of dendritic cells60. Allogeneic MHC I cross-dressed onto host antigen presenting cells could also sufficiently activate CD8+ T cells61 and induce rejection. Another possible explanation for the development of strong CD8+CTL function and CD8-mediated rejection of intrahepatic hepatocellular transplants (despite lack of secondary lymphoid organs and CD4+ T cells) is that the liver can develop tertiary lymphoid tissues called portal tract-associated lymphoid tissue, or PALT. PALT forms as the result of the infiltration of extrahepatic dendritic cells, B cells, T cells, and vascular cells62,63. It has previously been demonstrated that tertiary lymphoid structures can develop rapidly64 and are able to support activation of CD8+ T cells65. Furthermore, Hofmann et al. has hypothesized that, evolutionarily, the liver served as a surrogate lymphoid organ and may represent a remnant before lymph nodes developed66.
Some limitations of this study include the use of LTα KO mice. LTα KO hosts have been shown to be deficient in TNF-α, a proinflammatory cytokine with critical roles in immune stimulation and cell apoptosis67. However, Liepinsh et al. showed that the decreased TNF-α expression in LTα KO mice was limited to myeloid cells, and T cells exhibited normal TNF-α expression67. Since CD8-mediated rejection is not impeded in CD4-depleted LTα KO recipients, it can be inferred that the decreased myeloid expression of TNF-α in LTα KO mice does not significantly interfere with CD8+ T cell development or effector activity. Another consideration is the secretion of hA1AT by transgenic hepatocytes. It has been previously reported that high dose hA1AT administration (4 mg68) can significantly reduce mortality associated with graft versus host disease or delay islet transplant rejection (multiple doses of 1–2 mg69,70). However, this does not appear to be the case with the current studies since transplanted transgenic hepatocytes are readily rejected. Furthermore, transgenic hepatocytes secrete comparatively much smaller amounts of hA1AT into the serum (0.01–0.05 mg/mL) well below the “therapeutic” serum levels of hA1AT (0.5–1 mg/mL) associated with immunoregulatory effects reported in other studies.
Despite experimental evidence for portal venous tolerance induction12, the current studies indicate that robust CD8-dependent alloimmune responses and cell transplant rejection occur when allogeneic cells are transplanted directly to the liver by intraportal injection. Clinical experience to date with hepatocyte or islet transplant to the liver demonstrate the failure to achieve long-term cellular allograft survival despite the use of conventional immunosuppression. Thus, in contrast to the immunotolerant theory of the liver microenvironment which would predict the need for minimal immunosuppression, the current studies highlight the need to develop more effective immunotherapeutic strategies to target both unique CD8-mediated immune responses primed in the liver and conventional alloimmune rejection pathways13,17,18,71.
Nonstandard Abbreviations
- hA1AT
human alpha-1 antitrypsin
- Splx
splenectomy
- MST
median survival time
- Ltα KO
lymphotoxin-alpha knockout
- WT
wild-type
Footnotes
Author contribution, support, and address:
1) Jason M. Zimmerer-
Contribution- Participated in research design, writing of the paper, performance of the research, and data analysis.
Support- National Institutes of Health grants F32 DK082148
Address- Jason.Zimmerer@osumc.edu (The Ohio State University)
2) Phillip H. Horne-
Contribution- Participated in research design, writing of the paper, performance of the research, and data analysis.
Support- American Society of Transplantation Basic Science Physician Scientist Award
Address- Phillip.Horne@duke.edu (Duke University)
3) Mason G. Fisher-
Contribution- Participated in research design and performance of the research.
Address- mfisher@mcw.edu (Medical College of Wisconsin)
4) Thomas A. Pham-
Contribution- Participated in writing of the paper, participated in research design and performance of the research.
Support- ASTS-NKF (National Kidney Foundation) Folkert Belzer, MD, Research Award
Address- tpham03@stanford.edu (Stanford School of Medicine)
5) Keri Lunsford-
Contribution- Participated in research design, data analysis, and performance of the research.
Address- klunsford@mednet.ucla.edu (David Geffen School of Medicine at UCLA)
6) Bryce A. Ringwald- Participated in data analysis and performance of the research.
Address- Ringwald.7@buckeyemail.osu.edu
7) Christina L. Avila- Participated in performance of the research.
Address- Christina.Wright@osumv.edu
8) Ginny L. Bumgardner-
Contribution- Participated in research design, data analysis, writing of the paper, and funding the research.
Support- Roche Organ Transplantation Research Foundation, American Society of Transplant Surgeons, and National Institutes of Health grants DK072262 and AI083456. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Address- Ginny.Bumgardner@osumc.edu (The Ohio State University)
Conflict of Interest
The authors of this manuscript have no conflicts of interest to disclose.
References
- 1.Asakura H, Ku G, Kataoka M, Flye MW. Regulatory cells develop after the spontaneous acceptance of rat liver allografts. Surgery. 2004;136(3):532–536. doi: 10.1016/j.surg.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 2.Li W, Kuhr CS, Zheng XX, et al. New insights into mechanisms of spontaneous liver transplant tolerance: the role of Foxp3-expressing CD25+CD4+ regulatory T cells. Am J Transplant. 2008;8(8):1639–1651. doi: 10.1111/j.1600-6143.2008.02300.x. [DOI] [PubMed] [Google Scholar]
- 3.Wang C, Sun J, Li L, Wang L, Dolan P, Sheil AG. Conversion of pancreas allograft rejection to acceptance by liver transplantation. Transplantation. 1998;65(2):188–192. doi: 10.1097/00007890-199801270-00007. [DOI] [PubMed] [Google Scholar]
- 4.Meyer D, Thorwarth WM, Otto C, et al. Orthotopic liver/small bowel transplantation in rats: a microsurgical model inducing tolerance. Microsurgery. 2001;21(4):156–162. doi: 10.1002/micr.1030. [DOI] [PubMed] [Google Scholar]
- 5.VanBuskirk AM, Burlingham WJ, Jankowska-Gan E, et al. Human allograft acceptance is associated with immune regulation. J Clin Invest. 2000;106(1):145–155. doi: 10.1172/JCI9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starzl TE, Demetris A, Trucco M, et al. Cell Migration and Chimerism After Whole-organ Transplantation: The Basis of Graft Acceptance. Hepatology. 1993;17(6):1127–1152. [PMC free article] [PubMed] [Google Scholar]
- 7.Tokita D, Shishida M, Ohdan H, et al. Liver sinusoidal endothelial cells that endocytose allogeneic cells suppress T cells with indirect allospecificity. J Immunol. 2006;177(6):3615–3624. doi: 10.4049/jimmunol.177.6.3615. [DOI] [PubMed] [Google Scholar]
- 8.Niederkorn JY. Immune privilege in the anterior chamber of the eye. Crit Rev Immunol. 2002;22(1):13–46. [PubMed] [Google Scholar]
- 9.Ren J, Singh AK, Gregerson DS, Shichi H. Induction of immunotolerance in rats by intratesticular administration of an eicosapeptide of bovine S-antigen. Autoimmunity. 1996;25(1):19–31. doi: 10.3109/08916939608994723. [DOI] [PubMed] [Google Scholar]
- 10.Crispe IN. Hepatic T cells and liver tolerance. Nat Rev. 2003;3(1):51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- 11.Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev. 2000;174:21–34. doi: 10.1034/j.1600-0528.2002.017408.x. [DOI] [PubMed] [Google Scholar]
- 12.Crispe IN. Immune tolerance in liver disease. Hepatology. 2014;60(6):2109–2117. doi: 10.1002/hep.27254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker JP, Bumgardner GL. Hepatocyte immunology and transplantation: current status and future potential. Curr Opin Organ Transplant. 2005;10:67–76. [Google Scholar]
- 14.Truong W, Lakey JR, Ryan EA, Shapiro AM. Clinical islet transplantation at the University of Alberta–the Edmonton experience. Clin Transpl. 2005:153–172. [PubMed] [Google Scholar]
- 15.Balamurugan AN, Bottino R, Giannoukakis N, Smetanka C. Prospective and challenges of islet transplantation for the therapy of autoimmune diabetes. Pancreas. 2006;32(3):231–243. doi: 10.1097/01.mpa.0000203961.16630.2f. [DOI] [PubMed] [Google Scholar]
- 16.Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54(7):2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 17.Mitry RR, Hughes RD, Dhawan A. Hepatocyte transplantation. J Clin Exp Hepatol. 2011;1(2):109–114. doi: 10.1016/S0973-6883(11)60129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jorns C, Nowak G, Nemeth A, et al. De Novo Donor-Specific HLA Antibody Formation in Two Patients With Crigler-Najjar Syndrome Type I Following Human Hepatocyte Transplantation With Partial Hepatectomy Preconditioning. Am J Transplant. 2015 doi: 10.1111/ajt.13487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhawan A. Clinical human hepatocyte transplantation-current status and challenges. Liver Transpl. 2015 doi: 10.1002/lt.24226. [DOI] [PubMed] [Google Scholar]
- 20.Selmi C, Mackay IR, Gershwin ME. The immunological milieu of the liver. Semin Liver Dis. 2007;27(2):129–139. doi: 10.1055/s-2007-979466. [DOI] [PubMed] [Google Scholar]
- 21.Kammer AR, van der Burg SH, Grabscheid B, et al. Molecular mimicry of human cytochrome P450 by hepatitis C virus at the level of cytotoxic T cell recognition. J Exp Med. 1999;190(2):169–176. doi: 10.1084/jem.190.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowen DG, Zen M, Holz L, Davis T, McCaughan GW, Bertolino P. The site of primary T cell activation is a determinant of the balance between intrahepatic tolerance and immunity. J Clin Invest. 2004;114(5):701–712. doi: 10.1172/JCI21593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wuensch SA, Pierce RH, Crispe IN. Local intrahepatic CD8+ T cell activation by a non-self-antigen results in full functional differentiation. J Immunol. 2006;177(3):1689–1697. doi: 10.4049/jimmunol.177.3.1689. [DOI] [PubMed] [Google Scholar]
- 24.Wahl C, Bochtler P, Chen L, Schirmbeck R, Reimann J. B7-H1 on hepatocytes facilitates priming of specific CD8 T cells but limits the specific recall of primed responses. Gastroenterology. 2008;135(3):980–988. doi: 10.1053/j.gastro.2008.05.076. [DOI] [PubMed] [Google Scholar]
- 25.Bumgardner GL, Gao D, Li J, Baskin JH, Heininger M, Orosz CG. Rejection responses to allogeneic hepatocytes by reconstituted SCID mice, CD4, KO, and CD8 KO mice. Transplantation. 2000;70(12):1771–1780. doi: 10.1097/00007890-200012270-00017. [DOI] [PubMed] [Google Scholar]
- 26.Bumgardner GL, Li J, Prologo JD, Heininger M, Orosz CG. Patterns of immune responses evoked by allogeneic hepatocytes: evidence for independent co-dominant roles for CD4+ and CD8+ T-cell responses in acute rejection. Transplantation. 1999;68(4):555–562. doi: 10.1097/00007890-199908270-00019. [DOI] [PubMed] [Google Scholar]
- 27.Horne PH, Lunsford KE, Eiring AM, Wang Y, Gao D, Bumgardner GL. CD4+ T-cell-dependent immune damage of liver parenchymal cells is mediated by alloantibody. Transplantation. 2005;80(4):514–521. doi: 10.1097/01.tp.0000168342.57948.68. [DOI] [PubMed] [Google Scholar]
- 28.Horne PH, Koester MA, Jayashankar K, Lunsford KE, Dziema HL, Bumgardner GL. Disparate primary and secondary allospecific CD8+ T cell cytolytic effector function in the presence or absence of host CD4+ T cells. J Immunol. 2007;179(1):80–88. doi: 10.4049/jimmunol.179.1.80. [DOI] [PubMed] [Google Scholar]
- 29.Lunsford KE, Horne PH, Koester MA, et al. Activation and maturation of alloreactive CD4-independent, CD8 cytolytic T cells. Am J Transplant. 2006;6(10):2268–2281. doi: 10.1111/j.1600-6143.2006.01479.x. Epub 2006 Aug 2264. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Gao D, Lunsford KE, Frankel WL, Bumgardner GL. Targeting LFA-1 synergizes with CD40/CD40L blockade for suppression of both CD4-dependent and CD8-dependent rejection. Am J Transplant. 2003;3(10):1251–1258. doi: 10.1046/j.1600-6143.2003.00201.x. [DOI] [PubMed] [Google Scholar]
- 31.Jones ND, Van_Maurik A, Hara M, et al. CD40-CD40 ligand-independent activation of CD8+ T cells can trigger allograft rejection. J Immunol. 2000;165(2):1111–1118. doi: 10.4049/jimmunol.165.2.1111. [DOI] [PubMed] [Google Scholar]
- 32.Bishop DK, Wood SC, Eichwald EJ, Orosz CG. Immunobiology of allograft rejection in the absence of IFN-gamma: CD8+ effector cells develop independently of CD4+ cells and CD40-CD40 Ligand interactions. J Immunol. 2001;166(5):3248–3255. doi: 10.4049/jimmunol.166.5.3248. [DOI] [PubMed] [Google Scholar]
- 33.Horne PH, Zimmerer JM, Fisher MG, et al. Critical role of effector macrophages in mediating CD4-dependent alloimmune injury of transplanted liver parenchymal cells. J Immunol. 2008;181(2):1224–1231. doi: 10.4049/jimmunol.181.2.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bumgardner GL, Orosz CG. Unusual patterns of alloimmunity evoked by allogeneic liver parenchymal cells. Immunol Rev. 2000;174:260–279. doi: 10.1034/j.1600-0528.2002.017409.x. [DOI] [PubMed] [Google Scholar]
- 35.Zimmerer JM, Pham TA, Sanders VM, Bumgardner GL. CD8+ T cells negatively regulate IL-4-dependent, IgG1-dominant posttransplant alloantibody production. J Immunol. 2010;185(12):7285–7292. doi: 10.4049/jimmunol.1001655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horne PH, Lunsford KE, Walker JP, Koester MA, Bumgardner GL. Recipient immune repertoire and engraftment site influence the immune pathway effecting acute hepatocellular allograft rejection. Cell Transplant. 2008;17(7):829–844. doi: 10.3727/096368908786516792. [DOI] [PubMed] [Google Scholar]
- 37.Bumgardner GL, Heininger M, Li J, et al. A Functional Model of Hepatocyte Transplantation for in Vivo Immunologic Studies. Transplantation. 1998;65(1):53–61. doi: 10.1097/00007890-199801150-00011. [DOI] [PubMed] [Google Scholar]
- 38.Lunsford KE, Koester MA, Eiring AM, Gao D, Horne PH, Bumgardner GL. Targeting LFA-1 and CD154 Suppresses the In Vivo Activation and Development of Cytolytic (CD4-Independent) CD8+ T Cells. J Immunol. 2005;175(12):7855–7866. doi: 10.4049/jimmunol.175.12.7855. [DOI] [PubMed] [Google Scholar]
- 39.Bickerstaff A, Nozaki T, Wang JJ, et al. Acute humoral rejection of renal allografts in CCR5(−/−) recipients. Am J Transplant. 2008;8(3):557–566. doi: 10.1111/j.1600-6143.2007.02125.x. [DOI] [PubMed] [Google Scholar]
- 40.Lukens JR, Dolina JS, Kim TS, Tacke RS, Hahn YS. Liver is able to activate naive CD8+ T cells with dysfunctional anti-viral activity in the murine system. PloS one. 2009;4(10):e7619. doi: 10.1371/journal.pone.0007619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zimmerer JM, Pham TA, Wright CL, et al. Alloprimed CD8(+) T Cells Regulate Alloantibody and Eliminate Alloprimed B Cells Through Perforin- and FasL-Dependent Mechanisms. Am J Transplant. 2014;14(2):295–304. doi: 10.1111/ajt.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zimmerer JM, Swamy P, Sanghavi PB, et al. Critical role of NKT cells in posttransplant alloantibody production. Am J Transplant. 2014;14(11):2491–2499. doi: 10.1111/ajt.12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holz LE, Benseler V, Bowen DG, et al. Intrahepatic murine CD8 T-cell activation associates with a distinct phenotype leading to Bim-dependent death. Gastroenterology. 2008;135(3):989–997. doi: 10.1053/j.gastro.2008.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu P, Spiotto MT, Lee Y, Schreiber H, Fu YX. Complementary role of CD4+ T cells and secondary lymphoid tissues for cross-presentation of tumor antigen to CD8+ T cells. J Exp Med. 2003;197(8):985–995. doi: 10.1084/jem.20021804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumamoto Y, Mattei LM, Sellers S, Payne GW, Iwasaki A. CD4+ T cells support cytotoxic T lymphocyte priming by controlling lymph node input. Proc Natl Acad Sci USA. 2011;108(21):8749–8754. doi: 10.1073/pnas.1100567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bennett SR, Carbone FR, Karamalis F, Miller JF, Heath WR. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J Exp Med. 1997;186(1):65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beilhack A, Schulz S, Baker J, et al. Prevention of acute graft-versus-host disease by blocking T-cell entry to secondary lymphoid organs. Blood. 2008;111(5):2919–2928. doi: 10.1182/blood-2007-09-112789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou P, Hwang KW, Palucki D, et al. Secondary lymphoid organs are important but not absolutely required for allograft responses. Am J Transplant. 2003;3(3):259–266. doi: 10.1034/j.1600-6143.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 49.Wang J, Dong Y, Sun JZ, et al. Donor lymphoid organs are a major site of alloreactive T-cell priming following intestinal transplantation. Am J Transplant. 2006;6(11):2563–2571. doi: 10.1111/j.1600-6143.2006.01516.x. [DOI] [PubMed] [Google Scholar]
- 50.Lakkis FG, Arakelov A, Konieczny BT, Inoue Y. Immunologic ‘ignorance’ of vascularized organ transplants in the absence of secondary lymphoid tissue. Nat Med. 2000;6(6):686–688. doi: 10.1038/76267. [DOI] [PubMed] [Google Scholar]
- 51.Lunsford KE, Gao D, Eiring AM, Wang Y, Frankel WL, Bumgardner GL. Evidence for tissue directed immune responses: Analysis of CD4-dependent and CD8-dependent alloimmunity. Transplantation. 2004;78(8):1125–1133. doi: 10.1097/01.tp.0000138098.19429.99. [DOI] [PubMed] [Google Scholar]
- 52.Lunsford KE, Jayanshankar K, Eiring AM, et al. Alloreactive (CD4-Independent) CD8+ T cells jeopardize long-term survival of intrahepatic islet allografts. Am J Transplant. 2008;8(6):1113–1128. doi: 10.1111/j.1600-6143.2008.02219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winau F, Hegasy G, Weiskirchen R, et al. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26(1):117–129. doi: 10.1016/j.immuni.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 54.Pillarisetty VG, Shah AB, Miller G, Bleier JI, DeMatteo RP. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J Immunol. 2004;172(2):1009–1017. doi: 10.4049/jimmunol.172.2.1009. [DOI] [PubMed] [Google Scholar]
- 55.Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol. 2009;10(4):385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Ann Rev Immunol. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 57.Brown K, Sacks SH, Wong W. Coexpression of donor peptide/recipient MHC complex and intact donor MHC: evidence for a link between the direct and indirect pathways. Am J Transplant. 2011;11(4):826–831. doi: 10.1111/j.1600-6143.2011.03437.x. [DOI] [PubMed] [Google Scholar]
- 58.Tay SS, Wong YC, McDonald DM, et al. Antigen expression level threshold tunes the fate of CD8 T cells during primary hepatic immune responses. Proc Natl Acad Sci USA. 2014;111(25):E2540–2549. doi: 10.1073/pnas.1406674111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nojima H, Freeman CM, Schuster RM, et al. Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. J Hepatol. 2015 doi: 10.1016/j.jhep.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakai M, Oshiumi H, Funami K, et al. Interferon (IFN) and Cellular Immune Response Evoked in RNA-Pattern Sensing During Infection with Hepatitis C Virus (HCV) Sensors. 2015;15(10):27160–27173. doi: 10.3390/s151027160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dolan BP, Gibbs KD, Jr, Ostrand-Rosenberg S. Dendritic cells cross-dressed with peptide MHC class I complexes prime CD8+ T cells. J Immunol. 2006;177(9):6018–6024. doi: 10.4049/jimmunol.177.9.6018. [DOI] [PubMed] [Google Scholar]
- 62.Grant AJ, Lalor PF, Hubscher SG, Briskin M, Adams DH. MAdCAM-1 expressed in chronic inflammatory liver disease supports mucosal lymphocyte adhesion to hepatic endothelium (MAdCAM-1 in chronic inflammatory liver disease) Hepatology. 2001;33(5):1065–1072. doi: 10.1053/jhep.2001.24231. [DOI] [PubMed] [Google Scholar]
- 63.Murakami J, Shimizu Y, Kashii Y, et al. Functional B-cell response in intrahepatic lymphoid follicles in chronic hepatitis C. Hepatology. 1999;30(1):143–150. doi: 10.1002/hep.510300107. [DOI] [PubMed] [Google Scholar]
- 64.Yoneyama H, Matsuno K, Zhang Y, et al. Regulation by chemokines of circulating dendritic cell precursors, and the formation of portal tract-associated lymphoid tissue, in a granulomatous liver disease. J Exp Med. 2001;193(1):35–49. doi: 10.1084/jem.193.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thompson ED, Enriquez HL, Fu YX, Engelhard VH. Tumor masses support naive T cell infiltration, activation, and differentiation into effectors. J Exp Med. 2010;207(8):1791–1804. doi: 10.1084/jem.20092454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hofmann J, Greter M, Du Pasquier L, Becher B. B-cells need a proper house, whereas T-cells are happy in a cave: the dependence of lymphocytes on secondary lymphoid tissues during evolution. Trends Immunol. 2010;31(4):144–153. doi: 10.1016/j.it.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 67.Liepinsh DJ, Grivennikov SI, Klarmann KD, et al. Novel lymphotoxin alpha (LTalpha) knockout mice with unperturbed tumor necrosis factor expression: reassessing LTalpha biological functions. Mol Cell Biol. 2006;26(11):4214–4225. doi: 10.1128/MCB.01751-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tawara I, Sun Y, Lewis EC, et al. Alpha-1-antitrypsin monotherapy reduces graft-versus-host disease after experimental allogeneic bone marrow transplantation. Proc Natl Acad Sci USA. 2012;109(2):564–569. doi: 10.1073/pnas.1117665109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ashkenazi E, Baranovski BM, Shahaf G, Lewis EC. Pancreatic islet xenograft survival in mice is extended by a combination of alpha-1-antitrypsin and single-dose anti-CD4/CD8 therapy. PloS one. 2013;8(5):e63625. doi: 10.1371/journal.pone.0063625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lewis EC, Shapiro L, Bowers OJ, Dinarello CA. Alpha1-antitrypsin monotherapy prolongs islet allograft survival in mice. Proc Natl Acad Sci USA. 2005;102(34):12153–12158. doi: 10.1073/pnas.0505579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang F, Zhou L, Ma X, et al. Monitoring of intrasplenic hepatocyte transplantation for acute-on-chronic liver failure: a prospective five-year follow-up study. Transplant Proc. 2014;46(1):192–198. doi: 10.1016/j.transproceed.2013.10.042. [DOI] [PubMed] [Google Scholar]


