Abstract
Theory predicts that bottom-heavy biomass pyramids or ‘stacks’ should predominate in real-world communities if trophic-level increases with body size (mean predator-to-prey mass ratio (PPMR) more than 1). However, recent research suggests that inverted biomass pyramids (IBPs) characterize relatively pristine reef fish communities. Here, we estimated the slope of a kelp forest fish community biomass spectrum from underwater visual surveys. The observed biomass spectrum slope is strongly positive, reflecting an IBP. This is incongruous with theory because this steep positive slope would only be expected if trophic position decreased with increasing body size (consumer-to-resource mass ratio, less than 1). We then used δ15N signatures of fish muscle tissue to quantify the relationship between trophic position and body size and instead detected strong evidence for the opposite, with PPMR ≈ 1650 (50% credible interval 280–12 000). The natural history of kelp forest reef fishes suggests that this paradox could arise from energetic subsidies in the form of movement of mobile consumers across habitats, and from seasonally pulsed production inputs at small body sizes. There were four to five times more biomass at large body sizes (1–2 kg) than would be expected in a closed steady-state community providing a measure of the magnitude of subsidies.
Keywords: ecosystem baseline, fractionation, habitat complexity, species interaction, size spectra, stable isotope analysis
1. Introduction
Half a century of temperate and tropical reef science has yielded a wealth of knowledge regarding how species interactions shape community ecology, yet our ability to predict community size-structure remains constrained by a lack of empirical data and adequate theoretical treatment [1–3]. Inverted biomass pyramids (IBPs), where the biomass of large predatory fishes far outweighs biomass at smaller body sizes and lower trophic-levels, have been reported on relatively pristine reefs in the remote tropical Pacific [1,4]. Such IBPs may be the baseline ecosystem state for reef fish communities in the absence of human exploitation [1]. However, the plausibility of such top-heavy configurations has been debated [2,3]. Recent work demonstrating the equivalence of biomass pyramids and biomass spectra highlights that, in size-structured assemblages, where trophic-level increases with body size, biomass distributions should be ‘stacks’ or bottom-heavy pyramids, and not strongly inverted [3]. Furthermore, natural history suggests that fish communities tend to be strongly size-structured because indeterminate growth and gape-limited size-selective predation predominate among fishes [5,6]. Hence, the empirical evidence of IBPs on reefs presents an interesting paradox.
Biomass spectra (and other forms of individual body-size distributions) provide a powerful means for understanding how size-based energy flow combines with physical and biotic conditions to shape ecological communities [7,8]. Individual metabolic rates (and thus energy requirements) scale predictably with body mass (M) as M0.75 [9], and the energetic equivalence rule constrains energy use to be similar across body sizes within a single trophic-level [10,11]. Hence, the distribution of abundance and biomass across body sizes in multi-trophic-level communities is fundamentally constrained by how efficiently energy is transferred between trophic-levels, and by how trophic-level is related to body size [12]. The first constraint—usually referred to as trophic transfer efficiency (TE)—is not thought to vary widely and typically ranges between 10 and 12% [13,14]. The second constraint—the relationship between trophic-level and body size—is determined by how large, on average, predators are relative to their prey, or the average community predator-to-prey mass ratio (PPMR). These processes are summarized in the following equation, which predicts the equilibrium biomass spectrum slope b that results from a given combination of TE and PPMR:
This model, termed the energetic equivalence hypothesis with trophic transfer correction (EEH with TTC [15]), provides an extremely useful null model for understanding the processes that shape size-structured communities [3,7,12]. Because TE cannot exceed 1 (i.e. production pyramids are bottom-heavy), log(TE)/log(PPMR) will always be negative if PPMR is more than 1, and biomass spectrum slopes are therefore constrained to be less than 0.25 (abundance spectrum slopes <−0.75, as the slope of the biomass spectrum is equal to the slope of the abundance spectrum + 1 [3,12]) if trophic-level increases with body size. The EEH with TTC framework is also applicable for predicting community size-structure in situations where trophic-level decreases rather than increases with increasing body size (e.g. [16]), although in such situations the relationship between trophic-level and body size is more appropriately expressed as a consumer-to-resource mass ratio (CRMR) rather than PPMR.
Biomass pyramids and biomass spectra are equivalent and interchangeable; negative biomass spectrum slopes correspond to bottom-heavy pyramids, positive slopes correspond to inverted pyramids, and slopes of zero (‘flat’ spectra) represent biomass stacks or columns [3]. Hence the ecological processes, summarized by TE and PPMR, that determine the slopes of biomass spectra also determine ecological pattern—the shapes of biomass pyramids. Across body sizes from plankton to fishes, biomass spectra tend to be flat in pelagic marine ecosystems in the absence of exploitation, indicating biomass stacks or columns [17]. Slopes become more negative or less positive as anthropogenic impacts selectively remove large-bodied individuals and species, and often indirectly benefit smaller bodied ones—leading to increasingly bottom-heavy pyramids [7,18].
Fish predators tend to be two to four orders of magnitude larger than their prey [19], but there are few empirical estimates of community PPMR. PPMR can be calculated from the slope of the empirical relationship between trophic position and body size using stable isotope data [20]. Available community-wide PPMR estimates predominantly come from fishes (and in one case, invertebrates) in pelagic and soft-sediment demersal systems, where values have fallen within the expected range of hundreds to thousands [5,7,21]. These PPMRs, combined with TEs of approximately 10%, lead to flat biomass spectra and biomass columns. Because there are no empirical estimates of PPMR for reef fish communities, it is difficult to infer how the process of size-based energy flows underlies observed patterns of community structure on reefs.
Marine communities are energetically open at local to regional scales, owing to both wide dispersal of zooplankton and small fishes and the ranging of larger fishes, across spatial scales ranging from tens to thousands of kilometres [22,23]. The concentration and flow of the smallest and largest size classes can constitute local ecological subsidies (i.e. locally inverted production pyramids), yet despite the longstanding recognition of these phenomena—the relative importance of ecological subsidies has been largely overlooked at community and ecosystem scales [3,24]. Recent developments suggest that the EEH with TTC framework can be used as a null model to detect and measure local subsidies, for example, from Pacific salmon carcasses to streamside soil foodwebs [25].
Here, we seek to understand whether the pattern of observed fish community structure is consistent with the predation process represented by PPMR for fishes in the rocky reef kelp forests of Haida Gwaii, a remote archipelago located off British Columbia, on Canada's northwest coast (electronic supplementary material, figure S1). Kelp forests and coral reefs differ from pelagic systems in that energy is derived from multiple sources of external and local production, and in the presence of habitat-forming foundation species. Temperate kelp forests specifically provide a highly contrasting ecosystem, relative to pelagic and soft-sediment systems that have been studied to date, in which to explore biomass spectra and PPMR. Knowledge of PPMR will illuminate how size-based energy flows underlie community structure in rocky reef kelp forests. A near-zero or weakly negative biomass spectrum slope (stack or pyramid) combined with an estimated community PPMR in the order of hundreds to thousands would be concurrent with theory, and with previous observations in pelagic ecosystems. Alternatively, a high positive biomass spectrum slope (inverted pyramid), in combination with a negative relationship between trophic-level and body size (CRMR between 0 and 1), would be more consistent with what has been observed in detritivorous benthic infauna communities [16]. If neither of these scenarios are supported (i.e. high positive biomass spectrum slope combined with positive PPMR), and assuming EEH with TTC is correct, this implies that the scale of observation does not match the scale at which production enters and moves through the community. Given that off-reef production is often important for sustaining on-reef fish biomass (e.g. [22,26]) such mismatch may be likely. Hence, knowledge of how PPMR corresponds to biomass spectrum slope will provide fundamental new insights into the processes underlying patterns of fish community structure on reefs.
2. Material and methods
This study was undertaken within and around the Gwaii Haanas National Marine Conservation Area Reserve and Haida Heritage Site, on Haida Gwaii, British Columbia, Canada (electronic supplementary material, figure S1). Despite the remote location, both commercial and indigenous food fisheries occur in this area.
(a). Underwater visual census of kelp forest fish size and abundance
Fish communities were surveyed visually using belt-transects at 12 sites; three sites nested within each of four locations (Louise, Lyell, Kunghit East and Kunghit West; electronic supplementary material, figure S1) annually for 4 years (additional detail on survey methodology provided in the electronic supplementary material). Lengths of individual fishes observed on transects were estimated visually to the nearest centimetre. To ensure accuracy of length estimates, observers were trained by estimating the size of known-length objects underwater (following [27]) and carried a measuring pole (an 80 cm length of PVC pipe, labelled with cm increments, and mounted at the end of a 1.5 m pole, as per [28]), which was used to both directly measure fishes where possible, and to self-check visual estimates. Individual weights were then calculated using species-specific length–weight conversions from FishBase (www.fishbase.org).
(b). Biomass spectra
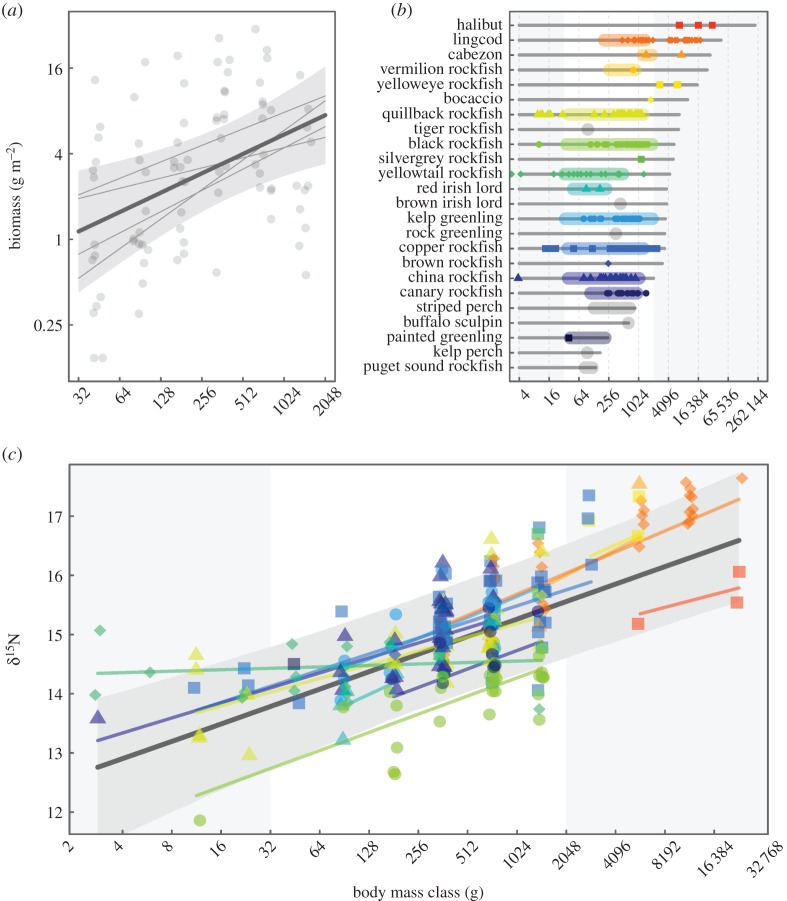
We fit biomass spectra as hierarchical linear models to account for the spatially and temporally nested structure of the survey data using the R package lme4 [29]. Before model fitting, we narrowed the size fraction used for analysis to the range of body sizes that can be surveyed effectively with underwater visual transects. Small fishes (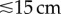 ) are subject to poor detectability in visual surveys [30], hence we used the corresponding mass of 32 g as the lower size cut-off for inclusion in analyses (additional detail provided in [8]). Only three fish larger than 2048 g were observed across all surveys, hence we excluded them and set this mass as our upper cut-off. Between our mass limits of 32–2048 g, we calculated biomass-per log2 size class-unit-area (B) as follows. All biomass within each of the six log2 body-mass classes (32–64 g, 64–128 g, 128–256 g, 256–512 g, 512–1024 g, 1024–2048 g) was summed across all sites for each location–year combination (i.e. binned), and divided by the total area surveyed to give biomass g m−2 in each mass class. We then modelled biomass spectra as hierarchical linear regressions with the midpoints of the log2 size bins (log2 M) as the predictor and log2 B as the response. We centred body-mass class about zero by subtracting the mean prior to model fitting in order to reduce correlations between the slopes and intercepts (following [31]). The spatially and temporally nested structure of the data was accounted for by including both location and year as crossed random effects with slope allowed to vary randomly with location, and intercept allowed to vary randomly with both location and year [8,32].
) are subject to poor detectability in visual surveys [30], hence we used the corresponding mass of 32 g as the lower size cut-off for inclusion in analyses (additional detail provided in [8]). Only three fish larger than 2048 g were observed across all surveys, hence we excluded them and set this mass as our upper cut-off. Between our mass limits of 32–2048 g, we calculated biomass-per log2 size class-unit-area (B) as follows. All biomass within each of the six log2 body-mass classes (32–64 g, 64–128 g, 128–256 g, 256–512 g, 512–1024 g, 1024–2048 g) was summed across all sites for each location–year combination (i.e. binned), and divided by the total area surveyed to give biomass g m−2 in each mass class. We then modelled biomass spectra as hierarchical linear regressions with the midpoints of the log2 size bins (log2 M) as the predictor and log2 B as the response. We centred body-mass class about zero by subtracting the mean prior to model fitting in order to reduce correlations between the slopes and intercepts (following [31]). The spatially and temporally nested structure of the data was accounted for by including both location and year as crossed random effects with slope allowed to vary randomly with location, and intercept allowed to vary randomly with both location and year [8,32].
(c). Stable isotope estimates of individual trophic allometry
We sampled fishes for stable isotope analysis using hook-and-line and spear-fishing and collected a total of 234 individuals of 17 species, spanning a range of 2.6–31 kg mass and 5.8–1.6 m total length (electronic supplementary material, table S1), and used δ15N measurements as a proxy for the trophic position of individual fishes. To ensure we had an adequate range of body sizes to detect relationships between trophic-level and body size [33], we adopted a targeted collection approach. We used both hook-and-line and spear-fishing, with standard commercially available pole spears and custom ‘micro’ spears, to maximize the range of body sizes sampled for the species which dominated the community.
Fishes were weighed, measured and dissected in the field and a sample of white muscle was excised from each fish from the dorsal musculature behind the head. Muscle samples were immediately frozen for transportation and storage. Muscle samples were subsequently processed in the laboratory and analysed for nitrogen and carbon stable isotope composition (δ13C and δ15N; analysed by the UC Davis Stable Isotope Facility; additional details in the electronic supplementary material).
(d). Scaling from individual trophic allometries to the community-wide predator-to-prey mass ratio
We used a Bayesian hierarchical approach to model the community relationship between trophic position and body size, and estimated the community PPMR from the posterior distribution of the slope of this relationship (β) as 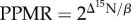 [20]. Adopting a Bayesian hierarchical approach allowed us to account for the nested structure of the data (species sampled within locations [32]) and to explicitly incorporate important sources of uncertainty, including instrument error in measurements of δ15N and uncertainty in the assumed rate of δ15N fractionation (Δ15N). We assumed a normal distribution for Δ15N with a mean of 3.2 and standard deviation of 1. The value of 3.2 has been recommended as an assumed value for fish white muscle tissue [34], and adding a wide standard deviation around this assumed mean encompasses the other widely recommended value of 3.4 [35], as well as making our PPMR estimate robust to emerging evidence that fractionation rate may vary with body size and species (although such variation is likely to be small within the range of body sizes considered here [36,37]). Our model allowed slope to vary randomly with species, and intercept to vary randomly with both species and location. We used non-informative priors on the slope, intercept, and residual standard deviation parameters, and weakly informative priors for random effect standard deviations (half-Cauchy distributions [38]; additional detail on model specification provided in the electronic supplementary material) to account and allow for our limited confidence in species and location effects resulting from our limited sample sizes.
[20]. Adopting a Bayesian hierarchical approach allowed us to account for the nested structure of the data (species sampled within locations [32]) and to explicitly incorporate important sources of uncertainty, including instrument error in measurements of δ15N and uncertainty in the assumed rate of δ15N fractionation (Δ15N). We assumed a normal distribution for Δ15N with a mean of 3.2 and standard deviation of 1. The value of 3.2 has been recommended as an assumed value for fish white muscle tissue [34], and adding a wide standard deviation around this assumed mean encompasses the other widely recommended value of 3.4 [35], as well as making our PPMR estimate robust to emerging evidence that fractionation rate may vary with body size and species (although such variation is likely to be small within the range of body sizes considered here [36,37]). Our model allowed slope to vary randomly with species, and intercept to vary randomly with both species and location. We used non-informative priors on the slope, intercept, and residual standard deviation parameters, and weakly informative priors for random effect standard deviations (half-Cauchy distributions [38]; additional detail on model specification provided in the electronic supplementary material) to account and allow for our limited confidence in species and location effects resulting from our limited sample sizes.
Sampling for isotope samples was not random, and the relative number of samples for each species in each bin did not necessarily reflect the proportional contributions of species to biomass in each size class in the community. Furthermore, some species caught via hook-and-line fishing were not observed on transects. To test whether this biased our results, we conducted a jackknife analysis, excluding one species at a time from the analysis and re-estimating PPMR (electronic supplementary material, figure S3). We also evaluated a hierarchical linear model where individual data points were weighted by the proportional contribution of each species to total biomass in each size-bin for each location, but ignoring measurement error (details provided in the electronic supplementary material).
3. Results
We surveyed a total of 203 transects and observed 4537 fishes between 32 and 2048 g. This included 19 species, predominantly rockfishes (family Sebastidae) and greenlings (family Hexagrammidae; figure 1b; electronic supplementary material, table S1). The estimated biomass spectrum slope (b) was 0.45 (figure 1a, solid yellow line in figure 2). The 95% confidence intervals around the slope estimate were 0.15–0.75 (hatched yellow area in figure 2), and the marginal and conditional R2-values were 0.17 and 0.37, respectively. This positive size spectrum slope implies an ecological pattern consistent with an IBP. The underlying ecological predation process deviated markedly from the observed inverted biomass pattern. Community trophic position, as described by δ15N signatures, increased strongly with body size over four orders of magnitude in size, ranging from 2.6 to 32 kg (figure 1b). Based on the slope (β) of this relationship, the hierarchical Bayesian modelling approach yielded a probability distribution for community PPMR with a median of approximately 1650 (95% credible interval approx. 31–29 × 104; figure 2). The jackknife analysis indicated that no single species had a disproportionate effect on the estimated community relationship, as the probability distribution for estimated PPMR did not change substantially when individual species were excluded from the model (electronic supplementary material, figures S2 and S3). In order to check that fishes with body sizes outside the range included in calculating the biomass spectrum slope did not have a disproportionate effect on our PPMR estimate, we re-estimated PPMR only using isotope data from fishes in the range of body sizes included in visual surveys (64–2048 g), and obtained a very similar estimate (PPMR ∼ 1042). The PPMR estimate from the biomass-weighted hierarchical linear model (5861) had a similar magnitude to the median estimate from the Bayesian model, indicating that the lack of biomass weighting in our Bayesian approach did not bias our estimate of PPMR.
Figure 1.
(a) The biomass spectrum from visual surveys; (b) size ranges observed for individual species in visual surveys (coloured shaded regions) and sampled for isotope analysis (points) relative to lmax (from FishBase, grey bars); and (c) the relationship between δ15N, a proxy for trophic position, and body size for the kelp forest fish community of Haida Gwaii British Columbia, Canada. Dark grey lines in panels (a,c) represent the mean fit for each model (across locations for (a) and across both locations and species for (c)), and grey bands indicate 95% confidence intervals in (a) and 95% credible intervals in (c) about the mean fits. Light grey lines in (a) and coloured lines in (c) represent random effect fits for location and species, respectively. Colours/shapes of points are used to distinguish among species and are consistent between panels (b) and (c), with species that were observed on transects but not sampled for isotope analysis in grey in panel (b). Grey shaded regions in (b,c) indicate body sizes outside the range included in (a).
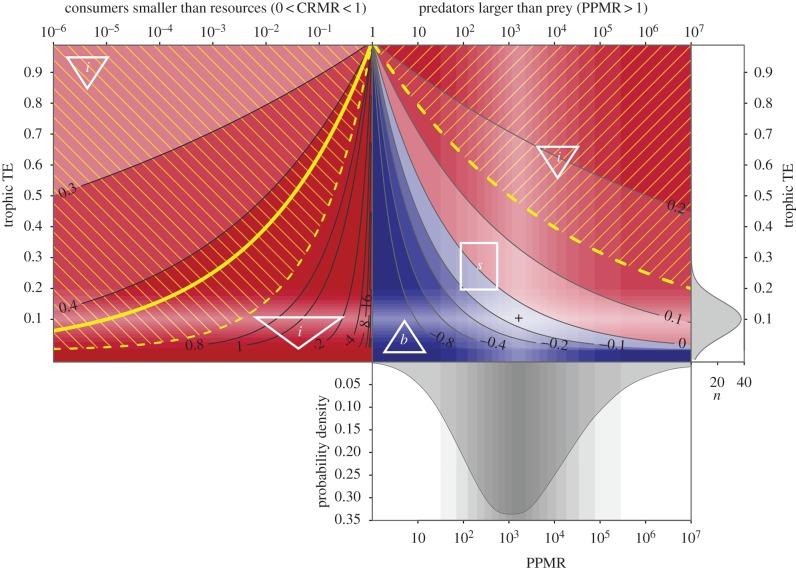
Figure 2.
Expected biomass spectrum slopes (contours and red/blue shading in top panels) resulting from varying combinations of mean community PPMR and TE, shown with reference to the probability density distribution of estimated PPMR for the reef fish community of Haida Gwaii (bottom panel). The top right panel shows scenarios with predators larger than prey (PPMR > 1); top left panel shows scenarios with consumers smaller than resources (0 < CRMR < 1). Positive slopes (red area) correspond to IBPs (represented by triangles labelled i), while negative slopes (blue area) correspond to bottom-heavy pyramids (triangle labelled b) and zero slopes imply stacks/columns (rectangle labelled s). Yellow shading lines indicate the range of slopes corresponding to the 95% confidence bounds around the empirically estimated biomass spectrum slope of 0.45 (solid yellow line). Right vertical axis shows TEs derived from marine food web models (n = 48, mean = 0.101, s.d. = 0.058; [13]). Shaded bands represent 5% quantile increments between 5% and 95% for TE and PPMR, and the black crosshair indicates the highest probability for both distributions (PPMR = 1650, TE = 0.101).
The strongly inverted biomass distribution we observed (yellow area in figure 2) appears to be energetically unfeasible given the hypothesized underlying predation process (PPMR) and reasonable assumed values for TE, unless subsidized from outside the sampling frame. Such a strongly IBP would require one or other of two conditions: either extremely efficient energy transfer (TE > 0.2, pyramid i in right-hand panel of figure 2) or for consumers to be smaller than their resources (CRMR, <1, left-hand panel of figure 2). Neither combination of TE or PPMR/CRMR is likely. Instead, the observed predation-based transfer of energy suggests that only a stacked or bottom-heavy pyramid distribution of biomass is possible (rectangle s in figure 2). The observed range of PPMR estimated here is shown by the bottom panel in figure 2. The intersection of this PPMR distribution with the likely distribution for TE (as derived from foodweb models; [13]) is shown by the crosshair in figure 2, which is consistent with a flat or negative biomass spectrum and hence a stacked or bottom-heavy biomass pyramid with biomass remaining similar or decreasing across size classes. Instead we observed four to five times more biomass in the largest size class (1024–2048 g) than in the smallest (32–64 g; figure 1a), this is clearly unfeasible unless supported by subsidies to the larger size classes.
A biomass spectrum slope of greater than 0.25 is only possible if PPMR is less than one, and there was only a 1.5% probability of PPMR being less than 10 given the posterior distribution on PPMR. Even the lower 95% confidence interval limit on the estimated biomass spectrum slope of 0.15 could only be realized in a closed size-structured community if PPMR was outside the 95% credible interval of the empirical estimate (more than 105), in combination with a TE of more than 0.2 (figure 2), which also falls outside the 95% quantiles for estimated TEs from foodweb models (reported by Pauly & Christensen [13]).
4. Discussion
Here, we report that a temperate reef fish community is structured as an IBP while, paradoxically, the estimated PPMR corresponds to expectations for a stack or a slightly bottom-heavy pyramid. The IBP configuration we observe is similar to what has been reported for other relatively pristine reef fish communities (e.g. [1,39]). However, the natural history of fishes [19,40], our findings here (figure 1), and evidence from other studies that have empirically estimated PPMR for fish communities in other ecosystems (e.g. [6,7,21]) all suggest that fishes tend to be characterized by positive PPMRs (reflecting size-based energy flow), that should result in bottom-heavy pyramids or stacks (biomass spectra with slope ≤ 0). This suggests that other subsidy processes overwrite local size-based energy flows in these systems.
The natural history of temperate reefs suggests that energetic subsidies are the most likely explanation for the mismatch between the observed pattern of community structure and the underlying process of size-based energy flow [3,41]. Processes that are likely to subsidize the fish communities in this study system and facilitate IBPs include the movement, foraging and aggregation of mobile consumers across habitats, seasonally pulsed inputs of production at small body sizes, and multiple energy sources, some of which potentially enter the community at large body sizes. We expand on these hypothesized mechanisms below, but first it is important to emphasize that the extent and magnitude of energy subsidies are relative to the scale of observation [3].
Energetic subsidies have traditionally been defined in terms of the movement of energy across ecosystem boundaries [24,42]. However, in systems that lack clearly defined ecosystem boundaries (such as reefs) and where the assemblage is defined by the scale of observation, it is informative to consider that observed assemblages may be subsidized by production from outside of the scale of observation. In this context, a subsidized assemblage is one for which the sampling scale does not encompass the spatial and temporal scale of the production that supports it [3,24]. Streams where anadromous salmon provide nutrient subsidies to local stream and riparian plant and animal communities provide an example of this concept [25,43]. In this example, when the scale of observation is the stream, the system is subsidized because the production-base for the nutrients that salmon bring to streams spans the ocean-basin scale at which salmon forage. But, if the scale of observation is expanded to encompass both the ocean basins where salmon forage at sea, and the streams where they spawn, the system is no longer subsidized.
A similar (though smaller) mismatch between the spatial scale of observation and the scale of the production-base is likely for the kelp forest fish assemblage of Haida Gwaii (and for reefs in general). Studies of reef fish community structure commonly implicitly assume that diurnal surveys, at a single time of year, are representative. But, if there is an underlying systematic pattern to movements (e.g. diurnal, tidal or seasonal) that is not accounted for in survey designs, abundance and biomass estimates may be biased. Temperate reef-associated fishes typically have ranges with small ‘core areas’ (tens of metres in diameter; [44]) but may undertake brief longer range excursions (hundreds of metres to kilometres) to forage [22,44]. The ‘snapshot’ temporal scale of our surveys means that such foraging excursions are unlikely to be captured and hence observed communities may be ‘subsidized’ by prey production that occurs at a broader spatial scale than is represented by the distribution of fishes on transects. Observations that off-reef production is often important for sustaining on-reef fish biomass lend support to this mechanism (e.g. [22,26]). Because the spatial extent of animal movements scale with body size [23], this spatial subsidy may be more pronounced for larger bodied individuals.
A mismatch in the temporal scale of production versus observation is also likely in this highly seasonal system. Small-bodied pelagic schooling forage fishes, notably Pacific herring (Clupea pallasii), are an important resource base for temperate reef fishes [45] but as they are only ephemerally present for several weeks during the spring each year, they are not captured in surveys outside this window of time. During the time that they are present, juvenile Pacific herring have been observed to dominate rockfish diets (particularly for larger rockfish; [45]), and both rockfishes [46] and greenling [47] have been observed to also prey on herring eggs, which are deposited on macro-algae and rocky substrates in the inter-tidal and shallow sub-tidal zone during seasonal spring-spawning events. Owing to the high calorific content of herring and their eggs, these resources may contribute a substantial proportion of the annual energy budget of reef fish in this study system [45]. Similarly, newly recruited juveniles of reef-associated species are likely to be important prey for larger fishes, both in our study system and for reef fish more generally. Empirical evidence from other systems supports this hypothesis, with significant intra-annual variation in abundance having been shown for other temperate reef fish communities [48].
The manner in which energetic subsidies affect community size-structure depends upon the body size at which the production enters local communities, and on whether the production input is constant or pulsed over time [49]. Subsidies that enter the community at relatively large body sizes will lead to less bottom-heavy/more-inverted pyramids (less-negative/more-positive biomass spectrum slope; [3]). We hypothesize that the short-lived seasonal pulses of forage fishes such as Pacific herring, reef fish recruits and potentially other ephemerally abundant prey resources, have this effect in our study system. Subsidies that enter communities at small body sizes (e.g. zooplankton associated with upwelling) will have the effect of increasing the total amount of energy and biomass available to be propagated to larger body sizes. If such subsidies are constant over time, this will have the effect of broadening the base of biomass pyramids, but may not affect the overall distribution of body sizes (i.e. increase biomass spectrum intercept, with no effect on slope). However, theoretical models suggest that if production is pulsed, there will be a tendency for production and biomass to be transient at small size classes and to accumulate at larger sizes [50]. Hence, snapshot censuses will be more likely to capture less bottom-heavy configurations (less-negative/more-positive biomass spectrum slopes). Given the highly seasonal nature of our high-latitude study system, we suggest that this process is also likely to contribute to the observed IBPs.
We recognize that we cannot quantify the nature or source of subsidies in the current study. Because a single temporally stable resource base is an underlying assumption of the EEH with TTC null model, departures from expectations from EEH with TTC provide a useful means for diagnosing subsidies [25]. However, explicit tests of the hypothetical mechanisms we describe here will be an important goal for future work. In particular, disentangling the combined effects of multiple trophic sources with the temporal and spatial mismatches between the scales of observation and production described above should be a priority. This would require detailed year-round surveys and dietary information, ideally coupled with stable isotope data from fast-turnover tissues (e.g. [51]). Mechanistic models that represent size-based energy flow through multiple coupled trophic pathways (e.g. [52]) will also be useful in addressing these questions. Furthermore, detailed empirical and theoretical consideration of how and when the scaling assumptions of EEH with TTC may be violated in fish communities will be an important avenue for future work.
Our study represents an important methodological advance in estimating PPMR in that we explicitly account for multiple sources of uncertainty to arrive at a probability distribution rather than a point estimate. The probability distribution for PPMR was centred around predators being approximately 1000–2000 times heavier than their prey, reflecting a strong positive relationship between trophic-level and body size both within species and across the whole fish community. Even while accounting for multiple sources of uncertainty, our PPMR estimate is similar to estimates from other size-structured marine communities—as might be expected given that gape-size allometry is highly conserved across fishes. In the North Sea, several point estimates of PPMR for the fish community range from several hundred to several thousand [5,7,53]. Part of this variability stems from the assumptions made in different studies. The only other marine fish community of which we are aware for which PPMR has been estimated is the Western Arabian Sea, where the estimate was 2327 [21].
Our estimation of PPMR allows for uncertainty in Δ15N, but there is also emerging evidence that Δ15N may scale positively with body size [37,54]. Because we did not account for this scaling, the PPMR value we report here (and those from previous studies) may be an over-estimate [54,55], which would strengthen our argument for a paradox. Another assumption which may be flawed, from the EEH with TTC model, is that neither TE nor PPMR vary systematically, and that singe mean values are representative for both of these parameters. While there is some evidence that PPMR and TE may in fact vary with body size it is thought that they vary in a compensatory manner such that the assumption of a single average value is acceptable [6]. Furthermore, the implied departure from (log) linearity in the relationship between trophic position in body size only becomes apparent over a much wider range of body sizes than we consider here. Hence our conclusions should be robust to this assumption, but detailed empirical and theoretical investigation of both the values and implications of TE and PPMR across the full range of body sizes in marine ecosystems will be an important goal for future research.
We found a strong and consistent positive relationship between trophic position and body size at the community scale, but there was variation between slopes among species. Flatter species-level slopes indicate that there is less change in trophic-level with body size (e.g. yellowtail rockfish), while lower intercepts would indicate species tend to forage preferentially on smaller, lower tropic level, prey (e.g. black rockfish; electronic supplementary material, table S2). The species-specific natural history underlying variation among these relationships will also be an interesting avenue for further study.
5. Conclusion
By making the first estimate of community PPMR for a reef ecosystem, this study fundamentally advances our understanding of the processes underlying patterns of reef fish community structure. Several authors have explained the phenomenon of IBPs on reefs using closed-community models (e.g. [1,56]), but our results highlight the importance of recognizing the energetically open nature of reef fish assemblages. In our system, this results in four to five times more biomass of large fishes (1–2 kg) than expected. Subsidies are ubiquitous; in fact, it has been argued that most systems are subsidized, or energetically open [24].
Resource subsidies may lead to patterns of community structure that are inconsistent with models that assume assemblages are energetically ‘closed’—and based solely on in situ productivity [24,25]. Other research has highlighted the importance of the heterogeneity of production in space and time, coupled with sampling scale, when describing the structure of marine communities [57]. We suggest that focusing on how reef communities are shaped by spatial subsidies and temporal subsidies in the form of resource pulses, and on how the scale of observation affects estimates of community structure, will be fruitful lines for future inquiry. Developing new MTE-based models that extend EEH with TTC to explicitly incorporate terms for resource subsidies and multiple sources of energy input would significantly advance the field and will be an important goal for future theoretical work. We hope that our findings will challenge reef ecologists to think carefully about how the energetically open nature of reef fish communities and multiple energetic pathways may play a pivotal role in driving community structure across scales.
Supplementary Material
Acknowledgements
Members of the Dulvy Laboratory (SFU) and attendees of Stats Beerz (SFU) provided valuable feedback and discussion on figures and analyses. For assistance in the field we thank Lynn Lee, Taimen Lee-Vigneault, Leandre Vigneault, Alejandro Frid, Hannah Stewart, Eric White, Matt Drake and Ryan Cloutier. For assistance with laboratory work, we thank Angeleen Olson and Brooke Davis. We also thank the Captain and crew of the CCGV Vector, the Gwaii Haanas Archipelago Management Board and the Council of the Haida Nation's Fisheries Committee for supporting this research. We are grateful to Isabelle Côté, Simon Jennings, Jon Moore, Aaron MacNeil and several anonymous reviewers for constructive comments on previous versions of this manuscript.
Ethics
This study was approved by the SFU Animal Ethics Committee.
Data accessibility
The data used in this publication can be accessed from figshare: https://dx.doi.org/10.6084/m9.figshare.3394792.v1.
Authors' contributions
R.T. designed and performed research, analysed data and wrote the paper; S.C.A. coded Bayesian model and wrote the paper; N.K.D. and A.K.S contributed to project design, supported research and wrote the paper.
Competing interests
We have no competing interests.
Funding
R.T. was supported by an NSERC Vanier Canada Graduate Scholarship, the J. Abbott/M. Fretwell Graduate Fellowship, and an SFU Graduate Fellowship. S.C.A. was supported by the Garfield Weston Foundation/B.C. Packers Ltd. Graduate Fellowship and an SFU Graduate Fellowship. This research was supported by NSERC Discovery grants to N.K.D. and A.K.S., the NSERC Canada Research Chairs program (N.K.D.) and a Parks Canada Contribution Agreement to A.K.S.
References
- 1.Sandin SA, et al. 2008. Baselines and degradation of coral reefs in the northern Line Islands. PLoS ONE 3, e1548 ( 10.1371/journal.pone.0001548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward-Paige C, Flemming JM, Lotze HK. 2010. Overestimating fish counts by non-instantaneous visual censuses: consequences for population and community descriptions. PLoS ONE 5, e11722 ( 10.1371/journal.pone.0011722) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trebilco R, Baum JK, Salomon AK, Dulvy NK. 2013. Ecosystem ecology: size-based constraints on the pyramids of life. Trends Ecol. Evol. 28, 423–431. ( 10.1016/j.tree.2013.03.008) [DOI] [PubMed] [Google Scholar]
- 4.Friedlander A, DeMartini EE. 2002. Contrasts in density, size, and biomass of reef fishes between the northwestern and the main Hawaiian Islands: the effects of fishing down apex predators. Mar. Ecol. Prog. Ser. 230, 253–264. ( 10.3354/meps230253) [DOI] [Google Scholar]
- 5.Jennings S, Pinnegar J, Polunin N, Boon T. 2001. Weak cross-species relationships between body size and trophic level belie powerful size-based trophic structuring in fish communities. J. Anim. Ecol. 70, 934–944. ( 10.1046/j.0021-8790.2001.00552.x) [DOI] [Google Scholar]
- 6.Barnes C, Maxwell D, Reuman D, Jennings S. 2010. Global patterns in predator-prey size relationships reveal size dependency of trophic transfer efficiency. Ecology 91, 222–232. ( 10.1890/08-2061.1) [DOI] [PubMed] [Google Scholar]
- 7.Jennings S, Blanchard JL. 2004. Fish abundance with no fishing: predictions based on macroecological theory. Ecology 73, 632–642 ( 10.1111/j.0021-8790.2004.00839.x). [DOI] [Google Scholar]
- 8.Trebilco R, Dulvy NK, Stewart H, Salomon AK. 2015. The role of habitat complexity in shaping the size-structure of a temperate reef fish community. Mar. Ecol. Prog. Ser. 532, 197–211. ( 10.3354/meps11330) [DOI] [Google Scholar]
- 9.Kleiber M. 1932. Body size and metabolism. Hilgardia 6, 315–332. ( 10.3733/hilg.v06n11p315) [DOI] [Google Scholar]
- 10.Damuth J. 1981. Population density and body size in mammals. Nature 290, 699–700. ( 10.1038/290699a0) [DOI] [Google Scholar]
- 11.Peters R. 1983. The ecological implications of body size. Cambridge studies in ecology. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 12.Brown J, Gillooly J. 2003. Ecological food webs: high-quality data facilitate theoretical unification. Proc. Natl Acad. Sci. USA 100, 1467–1468. ( 10.1073/pnas.0630310100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pauly D, Christensen V. 1995. Primary production required to sustain global fisheries. Nature 374, 255–257. ( 10.1038/374255a0) [DOI] [Google Scholar]
- 14.Ware DM. 2000. Fisheries oceanography: an integrative approach to fisheries ecology and management. Oxford, UK: Blackwell Science. [Google Scholar]
- 15.Reuman D, et al. 2009. Allometry of body size and abundance in 166 food webs. Adv. Ecol. Res. 41, 1–44. ( 10.1016/S0065-2504(09)00401-2) [DOI] [Google Scholar]
- 16.Dinmore TA, Jennings S. 2004. Predicting abundance-body mass relationships in benthic infaunal communities. Mar. Ecol. Prog. Ser. 276, 289–292. ( 10.3354/meps276289) [DOI] [Google Scholar]
- 17.Kerr SR, Dickie LM. 2001. The biomass spectrum: a predator-prey theory of aquatic production. New York, NY: Columbia University Press. [Google Scholar]
- 18.Dulvy NK, Polunin N, Mill AC, Graham N. 2004. Size structural change in lightly exploited coral reef fish communities: evidence for weak indirect effects. Can. J. Fish. Aquat. Sci. 61, 466–475. ( 10.1139/f03-169) [DOI] [Google Scholar]
- 19.Cushing DH. 1975. Marine ecology and fisheries. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 20.Jennings S, Warr KJ, Mackinson S. 2002. Use of size-based production and stable isotope analyses to predict trophic transfer efficiencies and predator-prey body mass ratios in food webs. Mar. Ecol. Prog. Ser. 240, 11–20. ( 10.3354/meps240011) [DOI] [Google Scholar]
- 21.Al-Habsi SH, Sweeting CJ, Polunin NVC, Graham NAJ. 2008. δ15N and δ13C elucidation of size-structured food webs in a Western Arabian Sea demersal trawl assemblage. Mar. Ecol. Prog. Ser. 353, 55–63. ( 10.3354/meps07167) [DOI] [Google Scholar]
- 22.Galván DE, Botto F, Parma AM, Bandieri L, Mohamed N, Iribarne OO. 2009. Food partitioning and spatial subsidy in shelter-limited fishes inhabiting patchy reefs of Patagonia. J. Fish Biol. 75, 2585–2605. ( 10.1111/j.1095-8649.2009.02453.x) [DOI] [PubMed] [Google Scholar]
- 23.Tamburello N, Cote I, Dulvy N. 2015. Energy and the scaling of animal space use. Am. Nat. 186, 196–211. ( 10.1086/682070) [DOI] [PubMed] [Google Scholar]
- 24.Polis GA, Anderson WB, Holt RD. 1997. Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annu. Rev. Ecol. Evol. Syst. 28, 289–316. ( 10.1146/annurev.ecolsys.28.1.289) [DOI] [Google Scholar]
- 25.Hocking MD, Dulvy NK, Reynolds JD, Ring RA, Reimchen TE. 2013. Salmon subsidize an escape from a size spectrum. Proc. R. Soc. B 280, 20122433 ( 10.1098/rspb.2012.2433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wyatt ASJ, Waite AM, Humphries S. 2012. Stable isotope analysis reveals community-level variation in fish trophodynamics across a fringing coral reef. Coral Reefs 31, 1029–1044. ( 10.1007/s00338-012-0923-y) [DOI] [Google Scholar]
- 27.Bell JD, Craik G, Pollard DA, Russell BC. 1985. Estimating length frequency distributions of large reef fish underwater. Coral Reefs 4, 41–44. ( 10.1007/BF00302203) [DOI] [Google Scholar]
- 28.Frid A, Connors B, Cooper AB, Marliave J. 2013. Size-structured abundance relationships between upper- and mid-trophic level predators on temperate rocky reefs. Ethol. Ecol. Evol. 25, 253–268. ( 10.1080/03949370.2013.798350) [DOI] [Google Scholar]
- 29.Bates D, Maechler M, Bolker B, Walker S. 2013. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.0–5. See http://CRAN.R-project.org/package=lme4.
- 30.Ackerman J. 2000. Reef fish assemblages: a re-evaluation using enclosed rotenone stations. Mar. Ecol. Prog. Ser. 206, 227–237. ( 10.3354/meps206227) [DOI] [Google Scholar]
- 31.Daan N, Gislason H, Pope JG, Rice JC. 2005. Changes in the North Sea fish community: evidence of indirect effects of fishing? ICES J. Mar. Sci. 62, 177–188. ( 10.1016/j.icesjms.2004.08.020) [DOI] [Google Scholar]
- 32.Gelman A, Hill J. 2007. Data analysis using regression and multilevel hierarchical models. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 33.Galván D, Sweeting C, Reid W. 2010. Power of stable isotope techniques to detect size-based feeding in marine fishes. Mar. Ecol. Prog. Ser. 407, 271–278. ( 10.3354/meps08528) [DOI] [Google Scholar]
- 34.Sweeting C, Barry J, Barnes C, Polunin N, Jennings S. 2007. Effects of body size and environment on diet-tissue δ15N fractionation in fishes. J. Exp. Mar. Biol. Ecol. 340, 1–10. ( 10.1016/j.jembe.2006.07.023) [DOI] [Google Scholar]
- 35.Post D. 2002. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83, 703–718. ( 10.1890/0012-9658(2002)083%5B0703:USITET%5D2.0.CO;2) [DOI] [Google Scholar]
- 36.Wyatt A, Waite A, Humphries S. 2010. Variability in isotope discrimination factors in coral reef fishes: implications for diet and food web reconstruction. PLoS ONE 5, e13682 ( 10.1371/journal.pone.0013682) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hussey NE, MacNeil MA, McMeans BC, Olin JA, Dudley SFJ, Cliff G, Wintner SP, Fennessy ST, Fisk AT. 2014. Rescaling the trophic structure of marine food webs. Ecol. Lett. 17, 239–250. ( 10.1111/ele.12226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gelman A. 2006. Prior distributions on variance parameters in hierarchical models. Bayesian Anal. 1, 515–533. ( 10.1214/06-BA117A) [DOI] [Google Scholar]
- 39.Sala E, et al. 2012. The structure of Mediterranean rocky reef ecosystems across environmental and human gradients, and conservation implications. PLoS ONE 7, e32742 ( 10.1371/journal.pone.0032742) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scharf FS, Juanes F, Rountree RA. 2000. Predator size–prey size relationships of marine fish predators: interspecific variation and effects of ontogeny and body size on trophic-niche breadth. Mar. Ecol. Prog. Ser. 208, 229–248. ( 10.3354/meps208229) [DOI] [Google Scholar]
- 41.Barneche DR, Kulbicki M, Floeter SR, Friedlander AM, Maina J, Allen AP. 2014. Scaling metabolism from individuals to reef-fish communities at broad spatial scales. Ecol. Lett. 17, 1067–1076. ( 10.1111/ele.12309) [DOI] [PubMed] [Google Scholar]
- 42.Talley DM. 2008. Spatial subsidy. In Encyclopedia of ecology (eds Jorgensen SE, Fath B), pp. 3325–3331. Oxford, UK: Elsevier Science. [Google Scholar]
- 43.Naiman RJ, Bilby RE, Schindler DE, Helfield JM. 2002. Pacific salmon, nutrients, and the dynamics of freshwater and riparian ecosystems. Ecosystems 5, 399–417. ( 10.1007/s10021-001-0083-3) [DOI] [Google Scholar]
- 44.Tolimieri N, Andrews K, Williams G, Katz S, Levin PS. 2009. Home range size and patterns of space use by lingcod, copper rockfish and quillback rockfish in relation to diel and tidal cycles. Mar. Ecol. Prog. Ser. 380, 229–243. ( 10.3354/meps07930) [DOI] [Google Scholar]
- 45.Murie DJ. 1995. Comparative feeding ecology of two sympatric rockfish congeners, Sebastes caurinus (copper rockfish) and S. maliger (quillback rockfish). Mar. Biol. 124, 341–353. ( 10.1007/BF00363908) [DOI] [Google Scholar]
- 46.Keeling BE. 2013. Quantifying the magnitude and mechanisms driving Pacific herring (Clupea pallasi) egg loss on the Central Coast of British Columbia, Canada. Master's thesis, Simon Fraser University, British Columbia, Canada.
- 47.Rooper CN, Haldorson LJ. 2000. Consumption of Pacific herring (Clupea pallasi) eggs by greenling (Hexagrammidae) in Prince William Sound, Alaska. Fish. Bull. 98, 655–659. [Google Scholar]
- 48.Irigoyen AJ, Galvan DE, Venerus LA, Parma AM. 2013. Variability in abundance of temperate reef fishes estimated by visual census. PLoS ONE 8, e61072 ( 10.1371/journal.pone.0061072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson WB, Wait DA, Stapp P. 2008. Resources from another place and time: responses to pulses in a spatially subsidized system. Ecology 89, 660–670. ( 10.1890/07-0234.1) [DOI] [PubMed] [Google Scholar]
- 50.Pope JG, Shepherd JG, Webb J, Stebbing A, Mangel M. 1994. Successful surf-riding on size spectra: the secret of survival in the sea. Phil. Trans. R. Soc. Lond. B 343, 41–49. ( 10.1098/rstb.1994.0006) [DOI] [Google Scholar]
- 51.Perga M, Gerdeaux D. 2005. ‘Are fish what they eat’ all year round? Oecologia 144, 598–606. ( 10.1007/s00442-005-0069-5) [DOI] [PubMed] [Google Scholar]
- 52.Blanchard JL, Jennings S, Law R, Castle MD, Mccloghrie P, Rochet MJ, Benot E. 2009. How does abundance scale with body size in coupled size-structured food webs? J. Anim. Ecol. 78, 270–280. ( 10.1111/j.1365-2656.2008.01466.x) [DOI] [PubMed] [Google Scholar]
- 53.Jennings S, Warr K. 2003. Smaller predator-prey body size ratios in longer food chains. Proc. R. Soc. Lond. B 270, 1413–1417. ( 10.1098/rspb.2003.2392) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reum JCP, Jennings S, Hunsicker ME. 2015. Implications of scaled δ15N fractionation for community predator-prey body mass ratio estimates in size-structured food webs. J. Anim. Ecol. 84, 1618–1627. ( 10.1111/1365-2656.12405) [DOI] [PubMed] [Google Scholar]
- 55.Jennings S, van der Molen J. 2015. Trophic levels of marine consumers from nitrogen stable isotope analysis: estimation and uncertainty. ICES J. Mar. Sci. 72, 2289–2300. ( 10.1093/icesjms/fsv120) [DOI] [Google Scholar]
- 56.Wang H, Morrison W, Singh A. 2009. Modeling inverted biomass pyramids and refuges in ecosystems. Ecol. Model. 220, 1376–1382. ( 10.1016/j.ecolmodel.2009.03.005) [DOI] [Google Scholar]
- 57.Barry JP, Dayton PK. 1991. Physical heterogeneity and organisation of marine communities. In Ecological heterogeneity (eds Kolasa J, Pickett STA), pp. 270–320. New York, NY: Springer. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this publication can be accessed from figshare: https://dx.doi.org/10.6084/m9.figshare.3394792.v1.