Abstract
Metals can have a number of detrimental or beneficial effects in the cell, but first they must get in. Organisms have evolved transport mechanisms to get metals that are required, or essential into the cell. Nonessential metals often enter the cell through use of the machinery provided for essential metals. Much work has been done to advance our understanding of how these metals are transported across the plasma and organelle membranes. This review provides an overview of these metal transport processes.
Introduction
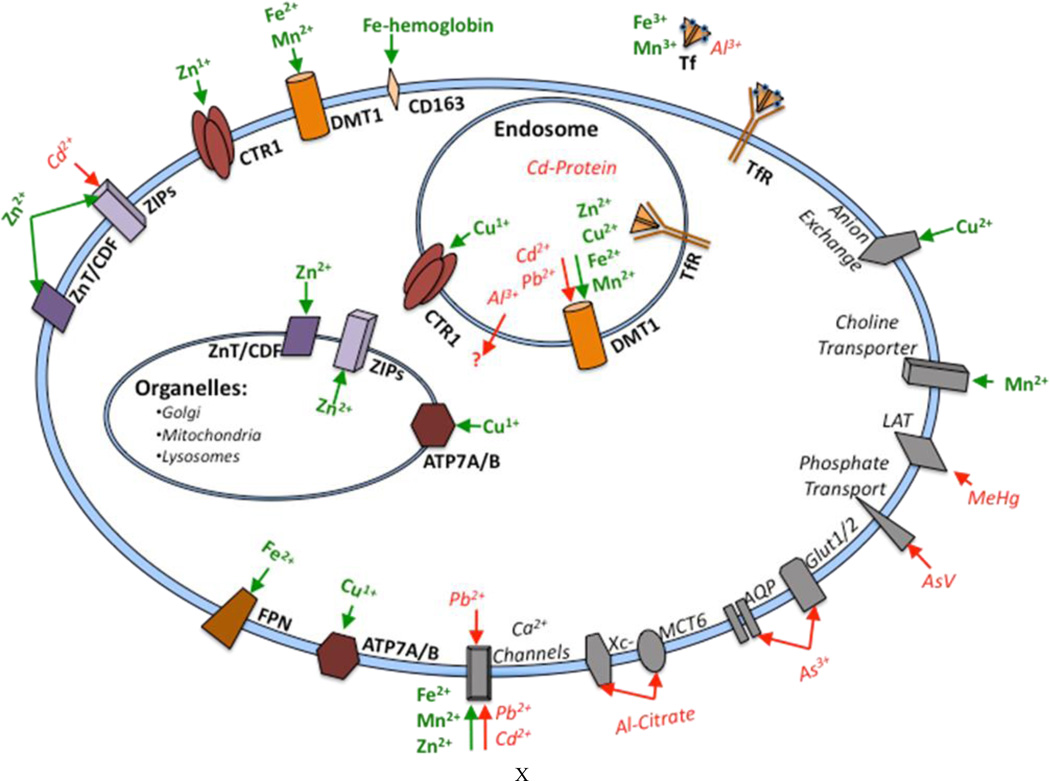
Metals are ubiquitously present in the environment and many can bioaccumulate. Human exposure to metals has risen due to use of these elements in industrial products as well as use of deeper sources of water and erosion. Additionally, many occupations involve exposure to metals and metal conjugates. Metals are systemic toxins and interact with specific systems to produce teratogenic, neurotoxic, cardiotoxic, and/or nephrotoxic effects, to name a few. Metals are taken into the body via inhalation, ingestion and dermal routes, can accumulate and can be stored in both soft and hard tissues. Metals disrupt metabolic processes by altering a number of homeostatic processes including antioxidant balance, binding to free sulfhydryl groups, competing for binding sites on a vast array of enzymes, receptors and transport proteins. Nearly one-third of proteins require metals, with approximately 47% of enzymes requiring metals, and 41% requiring metals at their catalytic centers1, 2. Metalloenzymes make up approximately 44% of oxidoreductases, 40% of transferases, 39% of hydrolases, 36% of lyases, 36% of isomerases and 59% of ligases1, 2. Iron (Fe), copper (Cu), manganese (Mn), and zinc (Zn) are essential metals and as such cells have mechanisms to acquire these nutrients from their extracellular environment. However, many of the transporters for these metals are hijacked by nonessential and toxic metals [i.e., cadmium (Cd), lead (Pb), mercury (Hg)]. As of yet, no known transporter for nonessential metals has been identified. Many metal ions can pass through the cell membrane alone or in complex with other proteins, a process referred to as molecular mimicry. Oftentimes metals in complexes can enter the cell more readily than the metal alone, especially if the latter is charged. Ion pumps can be hijacked by metals, leaving essential metals to compete for entry. Calcium channels and anion transporters represent another mechanism of entry as well as transport by amino acid and organic anion transporters when the metal is bound to amino acids or organic ions. Intracellular transport mechanisms are also present. Cells containing these transporters are found throughout the body or can be restricted to particular cell types. Increased body burden may occur when metals enter and accumulate in body tissue faster than the body’s detoxification pathways3 can dispose of them and the balance between uptake and efflux is tipped. The biological half-lives for metals can be as long as decades and many are readily transferred across the placental and blood-brain barriers and are known to have serious damaging effects on the developing nervous system. In adults, chronic symptoms frequently associated with accumulation of metals include fatigue, neurological disorders and allergic hypersensitivity. Metal entry into the cell is the topic of this minireview (Figure 1).
Fig 1.
Cellular Metal Transport. Essential metals are represented in green and non-essential metals are represented in red. Primary metal transporters are illustrated in bold, while secondary metal transporters are italicized.
Part I: Essential Metals
Copper (Cu)
Elemental copper (Cu) occurs naturally in the earth’s crust, soil, and mineral compounds. Cu metal is used in many industrial and commercial products such as coins, wire, sheet metal and pipes, as well as to remove and prevent mildew in agriculture and wood and leather products. Cu is found in all plants and animals as an essential electron donor and acceptor in cuproproteins. Humans acquire Cu primarily through dietary intake, which normally greatly exceeds biological demand; the Institute of Medicine (IOM) recommends 0.9mg/day intake and estimates a biological need for 0.7mg/day4. 20–50% of ingested Cu is absorbed through the enterohepatic circulation depending on intake levels4. In the liver, Cu is either stored bound to metallothionines, incorporated into cerruloplasmin and secreted into plasma, or excreted into bile5.
Transport Mechanisms
Enterocytes take up Cu from the intestine through copper transporter 1 (CTR1). Divalent metal transporter 1 (DMT1) is also capable of absorbing Cu; however, in normal conditions CTR1 is the primary uptake mechanism6. CTR1 is a ubiquitously expressed high affinity Cu transporter that exists as a homotrimer. A methionine and histidine rich extracellular N-terminal domain facilitates copper binding in CTR17. CTR1 is localized at the plasma membrane, and is responsible for transport of Cu from endosomes into the cytosol.
In addition to CTR1, a low affinity Cu transporter (CTR2) shares significant homology with CTR1 and may also mediate iron uptake. In contrast to CTR1, however, only a small amount of CTR2 is localized at the plasma membrane and the majority is located at intracellular compartments8. The exact role of CTR2 in Cu handling is not well understood, although current evidence suggests that it may serve to release Cu stores from lysosomes or other vesicles.
Intracellularly, several Cu binding proteins and chaperones aid in Cu trafficking and prevent free Cu from reaching high concentrations in the cytosol. Free Cu can cause cellular damage by producing reactive oxygen species (ROS) through the Fenton reaction and by competitively displacing other metals from proteins. Cu transport within the cell utilizes affinity gradients of copper chaperones9. Metallothionine and glutathione (GSH) have high affinity for Cu and act to buffer Cu in the cytosol and provide Cu reserves; however, metallothionines cannot transfer Cu to cuproproteins. Chaperone proteins facilitate Cu incorporation into cuproproteins. Ccs1 is a chaperone located in the cytosol, which delivers and facilitates incorporation of Cu into superoxide dismutase 1 (SOD1)10, 11. Cytosolic Cox17, and membrane associated Cox11 and Sco1/2 act together to provide Cu for cytochrome c oxidase12. Atox1 is a multifunctional chaperone which has been proposed to receive Cu from CTR1, exchange Cu with other chaperones and Cu-glutathione, transfer Cu to the exporters ATP7A and ATP7B, and play a role in regulating expression and localization of Cu handling proteins in the cell13.
P-type ATPases, ATP7A and ATP7B, are responsible for Cu transport into the trans Golgi network (TGN), and for excretion from the cell. With low levels of Cu, ATP7A and ATP7B are both localized at the trans-Golgi network (TGN) to provide Cu for incorporation into cuproproteins. However, in conditions of excess Cu, both ATP7A and ATP7B localize to vesicles near the plasma membrane and release intracellular of Cu through exocytosis. In polarized cells, such as enterocytes and hepatocytes, ATP7A is located at the basolateral membrane and ATP7B is located at the apical membrane14. In most tissues ATP7B can compensate for mutations in ATP7A, however, this is not the case in the intestine, brain or kidney. A recent study identified an ATP7B isoform lacking exon1 in renal cells that remains associated with the TGN in high intracellular Cu conditions, leading to speculation that it may have a role in storing Cu15.
Biological/Toxicological Functions
Cuproproteins are involved in a wide array of essential biological functions. For instance, cytochrome c oxidase is involved in electron transport and ATP production, cerruloplasmin is a ferroreductase required for iron uptake, dopamine (DA)-β dehydroxylase synthesizes norepinephrine from DA, tyrosinase produces melanin, and lysil oxidases form connective tissue and remodel extracellular matrix. Mutations in ATP7A result in Menkes disease in which enterocytes are unable to export Cu, causing severe Cu deficiency. A recently described condition similar to Charcot-Marie-Tooth Disease Type 2 is also associated with ATP7A mutations, although it is distinct from Menkes disease and indicates an additional role for ATP7A in maintaining motor neurons16. ATPA7B mutations, on the other hand, cause Wilson’s disease in which hepatocytes are unable to excrete Cu into the bile, resulting in Cu overload.
Iron (Fe)
Iron (Fe) is the fourth most common element found in earth’s crust, and is typically found as an iron oxide mineral17. Due to its low costs and heavy-duty physical and chemical properties, Fe is readily used in several industrial applications, including steel production and the manufacturing of tools, machinery, ships and structural frame components of buildings. Additionally, iron (III) chloride is used in water purification and sewage treatments, paint and animal feed additives, fabric dyes. Iron (II) sulfate is the form of Fe used in fortified foods, whereas iron(III) sulfate can be used to pull down sewage minerals from tank water18. Fe is essential for normal physiological processes, considering Fe-bound hemoglobin is necessary for the transport of oxygen in blood. For this reason, the IOM has determined a recommended daily allowance (RDA) of 8 mg Fe/day for men and 18 mg Fe/day for women. Fe-rich food sources include meat and poultry, fruits and vegetables (e.g., spinach), fortified bread, cereal and grain products19. Approximately 15–35% of ingested Fe is absorbed into the body through the digestive tract20.
Transport Mechanisms
Upon absorption, there is tight homeostatic control over Fe uptake, storage and intracellular distribution. The type of regulation is determined by the Fe oxidation state, with Fe readily participating in a redox reaction between ferric (3+) and ferrous (2+) states. Typically, extracellular Fe usually is mostly bound to the transferrin (Tf) protein in plasma in its ferric state, followed by binding to the ubiquitously expressed transferrin receptor 1 (TfR1) protein21. As with Mn3+, the bound Fe-Tf complex is internalized into endocytic vesicles, followed by reacidification of the vesicle to release Fe3+, followed by ferroreductase-mediated reduction to Fe(II)22 for DMT1-mediated import into the cytoplasm23. There is also a homologous TfR2-associated complex, but TfR2 shows lower affinity for Fe-Tf and specific localization to hepatocytes, erythroid cells and duodenum crypt cells24. Any non-Tf bound Fe is directly absorbed through intestinal cells via DMT1-mediated uptake, similar to Mn2+25. Additionally, some evidence suggests that iron may also be taken up under certain conditions (e.g., iron overload) through L-type Ca2+ channels under certain conditions26.
Upon import into the cell, there are certain storage capabilities that are unique to Fe. One of the most important Fe store mechanisms is ferritin. This protein is ubiquitously expressed and serves to both detoxify and store Fe that is not immediately required for use by the body, followed by degradation in order to release the Fe needed by cells27. Another direct mechanism of Fe uptake involves Fe bound to hemoglobin in the plasma, which can then be taken into cells through the hemoglobin scavenger receptor CD163, found on monocytes and macrophages. This mechanism occurs in conditions such as in sickle cell anemia28. Fe-bound heme accounts for nearly 80% of the Fe found in our bodies, not just in the plasma29, but also in our brains30.
In addition to the diversity in storage and uptake mechanisms, there are several regulatory mechanisms that serve to maintain proper Fe homeostasis. For example, iron regulatory proteins (IRPs) can bind to iron responsive elements (IREs) found in the 5’ untranslated region of iron-associated proteins, allowing for translational control of Fe-associated proteins in a manner dependent on the current cellular Fe status. In particular, under conditions of low intracellular Fe levels and/or increased oxidative stress, IRP1 and IRP2 can bind to IREs in the mRNA of iron-associated genes (e.g., ferritin, TfR, DMT1) to stabilize the IRE-possessing isoforms, assuring the import of Fe to compensate for the low Fe status and/or increased presence of ROS31. Additionally, inflammatory cytokines have also been associated with iron regulation in monocytes and macrophages32, demonstrating the stabilization of Fe stores in conditions of inflammation to fight off infection. Moreover, a more systemic regulator of Fe stores and homeostasis is hepcidin, an Fe-induced peptide hormone synthesized in the liver that mediates intestinal Fe absorption and recycling of iron through macrophages33, 34. Recent evidence has found hepcidin-mediated regulation of iron homeostasis that is triggered upon conditions of ischemia in the brain35.
Additionally, iron also has a specific export mechanism through the ferroportin (Fpn) transporter, further regulating intracellular Fe levels to maintain proper homeostasis. Ferroportin is an iron exporter found on the surface of duodenal enterocytes, macrophages, hepatocytes and neurons36, 37. Fpn acts as a receptor for hepcidin. Once bound to Fpn, hepcidin induces its internalization and degradation38. In fact, recent evidence has found hepcidin-mediated regulation not just on Fpn, but also on DMT1 and another Fe transport protein called ceruloplasmin39. In addition to the hepcidin ligand, Fpn levels are also controlled by intracellular Fe levels through IREs40, as well as the inflammatory status of the cell41. Moreover, proper Fe homeostasis would not be possible without ferroreductases that convert Fe3+ to Fe2+ for high-affinity transport into the cell and within endosomes. This regulation allows for a single-electron reduction of Fe via the mammalian ferrireductase family of Steap proteins (Steap1–4), with expression of these proteins consistent with their necessity at the plasma membrane and within endocytic vesicles42.
Biological/Toxicological Functions
Despite such a complex and interweaved network of regulatory and buffering mechanisms, both Fe deficiency and overload disorders have become a pressing public health concern. Iron-deficiency anemia can result from several Fe loss, including from insufficient dietary Fe intake, a decrease in Fe levels from the loss of blood, or an inability to properly absorb Fe from the diet43. Moreover, inflammatory reactions can lead to alterations in hepcidin-mediated control of Fe release from macrophages44; pathogenically elevated levels of hepcidin arising from other diseases can also result in anemic conditions45. Conversely, Fe overload disorders are can be either genetic or environmentally induced. Acute Fe overload mostly arises from overdose of Fe supplement tablets. However, a chronic condition of Fe overload has been associated with mutations in particular iron-associated genes. A hereditary form of Fe overload known as hemochromatosis has been connected to mutations in genes encoding ferroportin, hepcidin, TfR2, and the human hemochromatosis protein (HFE) protein. Mutations in the gene encoding the hereditary hemochromatosis protein HFE result in an autosomal recessive form of hemochromatosis (HH). Although the precise function of HFE (a major histocompatibility class I-like protein) is unknown, evidence has shown that it can compete with Tf for TfR1 binding capabilities46, subsequently regulating hepcidin expression downstream47. Although rare, HH can also arise from heterozygous mutations in the gene encoding Fpn48, in addition to the gene encoding TfR249. Interestingly a juvenile form of HH has been connected to mutations in the gene encoding HFE2, named hemojuvelin. This form of the disease has an age-of-onset in the teen years to the early 20’s, and also involves mediation of hepcidin, but through the HFE2 protein50.
Additionally, Fe accumulation is also associated with neurodegenerative disorders in the brain. Iron preferentially accumulates in the mitochondria for heme biosynthesis51, and due to its highly redox-reactive nature, can participate in the Fenton reaction to create increased ROS production29. Moreover, recent evidence has found an increase in DMT1 expression in the brains of PD patients, concomitant with a rise in Fe accumulation and dopaminergic (DAergic) cell death in the substantia nigra52. Another disease linked to Fe accumulation is Freidrich’s Ataxia, arising from mutations in frataxin, a protein involved in mitochondrial Fe transport53. Evidence also points to the contribution of Fe dysregulation and subsequent oxidative stress as significant factors in the pathophysiology of Alzheimer’s disease54. Such evidence illustrates the importance of maintaining tight homeostatic regulation over the mechanisms responsible for proper Fe transport and storage.
Manganese (Mn)
Manganese (Mn) is the 12th most abundant element and composes approximately 0.1% of the earth’s crust. The human population is readily exposed to Mn through soil erosion, resulting in the presence of Mn in food, air and waterways. In its natural form in the environment, Mn exists usually as oxides, carbonates, and silicates, with Mn dioxide being the most commonly found natural form. Organic Mn is highly present in the environment through common human uses, such as in smoke inhibitors and the antiknock gasoline additive methylcyclopentadienyl manganese tricarbonyl (MMT). It also has a limited use as a medical magnetic resonance imaging contrast reagent. Moreover, inorganic forms of Mn can be found in high concentrations in various industrial settings, as it is used in the manufacturing of dry-cell batteries, fireworks, ceramics, glass, leather and textiles. Additionally, inorganic Mn is also commonly found in fertilizers, in addition to paint and cosmetics as an additive known as manganese violet55. However, the primary route of Mn exposure in humans is through diet, as Mn is a necessary micronutrient that is found in several foods. Whole grains, rice, nuts, legumes and tea contain the highest Mn content, in addition to Mn-containing nutritional supplements and Mn-enriched infant and neonate formulas that contain a trace element solution56. Mn is also found in leafy green vegetables and in fruits like blueberries. The high prevalence of these types of foods in daily diet makes it relatively easy to acquire the adequate intake (AI) set by the Food and Nutrition Board of the IOM: 2.3 mg/day for men and 1.8 mg/day for women. Additionally, the Food and Drug Administration (FDA) has sent a maximum Mn concentration in bottled drinking water at 0.05 mg/L, though the Environmental Protection Agency (EPA) has noted that lifetime exposure to 0.3 mg/L Mn in drinking water should not cause any harmful effects. Despite daily diet being the main source of Mn exposure in humans, only about 3–5% of Mn is absorbed into the body through the digestive tract57.
Transport Mechanisms
The mechanisms through which Mn is absorbed into the digestive tract are not fully understood. It is thought that Mn can enter the digestive tract either through a passive diffusion process58 or through an active-transport process59 involving the divalent metal transporter 1 (DMT1). DMT1 is responsible for the uptake of a variety of divalent metals, and is coupled to a proton gradient dependent on the cell’s membrane potential25. Meanwhile, blood manganese is primarily (80%) bound to albumin and β-globulin, with a minority bound to transferrin in the trivalent form60. Upon circulation, distribution of Mn is diffuse throughout the body, with bone, brain, kidneys, and liver accumulating the most Mn. The liver is especially important in this distribution, as Mn is excreted through the biliary system. Moreover, Mn characteristically accumulates in the brain, specifically in the basal ganglia region57. Several transport mechanisms are thought to play a role in transporting Mn across the capillary endothelial cells of the blood-brain barrier (BBB), or through the cerebral spinal fluid via the choroid plexus.
Although Mn(III) is not found as prevalently as Mn(II)61, trivalent Mn can form a complex with the Tf protein, which can then bind to the Fe- and pH-dependent TfR to cross the BBB62. Additionally, another primary transport mechanism of Mn in the divalent state is through DMT125. In addition to its expression on the surface of enterocytes, DMT1 is also highly expressed in the olfactory epithelium63, suggesting another route of metal uptake. In the brain, DMT1 shows high expression in the basal ganglia64, in addition to the cerebellum, thalamus65, and hippocampus and cortex66. In addition to transporting divalent Mn directly from the extracellular space into the cytoplasm, DMT1 expression has also been shown to co-localize with TfR86, indicating an overlap with the TfR-mediated transport of Mn. In the latter pathway, Mn3+ complexed with Tf binds to its TfR, allowing the uptake of Mn3+ into cellular endosomes that then recruit v-ATPases to reacidify the endosome, causing the metal to dissociate and convert to the divalent state. This then allows DMT1 localized to the endosomal membrane to transport Mn2+ into the cytosol23. Additionally, the choline transporter has also been linked with Mn uptake at the BBB, showing the highest affinity for Mn even over choline and other metal cations. This active transport process could be another mechanism for preferential Mn accumulation in the brain73, 74. Moreover, evidence also exists for uptake of Mn2+ across the BBB through voltage-gated and store-operated calcium channels70, 87, ionotropic glutatmate receptor Ca2+ channels71, and a citrate transporter72. Additionally, crossover between Zn and Mn exists with another transport mechanism through the Zn transporters ZIP8 and ZIP1475, 76, 88. Recent data has also identified the HIP14 (Huntingtin-interacting protein 14) magnesium transporter to also be a novel, putative Mn transporter77. Additionally, a P-type transmembrane ATPase protein (ATP13A2), whose exact function remains unknown, has also been recently shown to transport Mn across cell membranes78.
In addition to these transport processes, Mn is also found in several metalloproteins. Mn serves as a necessary cofactor for several vital enzymes that are required for proper development, digestion, reproduction, immune function, energy metabolism and antioxidant defenses, including glutamine synthetase, pyruvate carboxylase and arginase56. Mn3+ is also a very important cofactor for manganese superoxide dismutase (MnSOD), involved in the dismutation of superoxide in mitochondria to decrease oxidative stress-induced cell death89. In addition to these metalloenzymes, other metalloproteins requiring Mn2+ include lectins and integrins, indicating the vital role of Mn in proper cytoskeletal organization and structure90. Recent evidence has also found a metalloprotein involved in the efflux of Mn known as ferroportin. Although previously known as an Fe exporter, these recent findings reveal that Fpn expression not only decreases Mn accumulation within cells, but also reduces Mn-induced cytotoxicity79.
Biological/Toxicological Functions
Upon uptake and interaction with such a broad range of transporters and metalloproteins, Mn can bioaccumulate to a higher degree in the brain. Cases of excess Mn exposure can arise from the environment, in both urban (steeling, mining, MMT in the air from gas combustion, etc.) and rural (agricultural runoff with pesticides and fertilizers) settings91. Additionally, neonates given Mn-supplemented total parenteral nutrition are also at risk for Mn intoxication, due to their undeveloped biliary system that does not allow for proper excretion. A similar risk of Mn toxicity occurs in the patient population suffering from liver dysfunction or Fe deficiency56. Severe Mn poisoning can result in an irreversible condition known as “manganism,” a disorder that resembles the neurodegenerative movement disorder Parkinson’s disease (PD) in both symptomatology and shared cellular mechanisms in the same general brain region92. This includes Mn-induced oxidative stress from preferential accumulation within mitochondria93, in addition to selective toxicity towards DAergic neurons94 – the same cell-type affected in PD. Thus, in addition to the necessity of Mn for metabolic and antioxidant function, proper homeostasis must be maintained through regulation by the many Mn-containing metalloprotein and transport mechanisms to avoid toxicity-induced cell death.
Zinc (Zn)
As a very common element found in Earth’s crust, the essential metal zinc (Zn) is found in air, soil, water and all foods. The most natural form of Zn found in the environment is zinc oxide or sphalerite. However, inorganic zinc compounds are extensively used in industrial processes, such as the creation of ceramics, paints and rubber, in addition to manufacturing and dyeing fabrics. Zn compounds are also used to preserve wood. Usage of Zn compounds is also significant in the pharmaceutical industry, with zinc-containing products including vitamins, sun block and diaper rash lotions, athlete’s foot and poison ivy treatments, as well as acne and anti-dandruff medications. Zn metal is primarily used as a coating of metals such as iron and steel to prevent corrosion, known as galvanization95.
Zn primarily enters the human body through the digestive tract from ingestion of Zn-containing food and water. The average Zn intake in the U.S. ranges from 5.2–16.2 mg on a daily basis. The National Academy of Sciences has set an RDA of 8 mg/day for women and 11 mg/day for men, whereas the EPA has set a maximum of 5 mg/L of Zn in drinking water. Upon ingestion, approximately 20–30% of Zn is absorbed, with uptake from the intestinal lumen requiring passive diffusion and a carrier-mediated process in a homeostatic manner. Upon absorption, zinc is mostly found within the muscle, bone, gastrointestinal tract, kidney, brain, skin, lung, heart and pancreas, and can be readily excreted in urine and feces. In blood plasma, a majority of Zn is localized in erythrocytes95.
Transport Mechanisms
In the blood plasma, 80% of zinc is bound to albumin, while the rest binds to α2-macrogloblulin96. NMR spectroscopy and molecular modeling studies have found a high-affinity Zn-binding site on albumin, localized to an overlap region between the first and second domains of the human serum albumin protein. This region involves amino acid ligands in Domain I (His67 and Asn99) that overlap with His247 and Asp240in Domain II to form the “A site” high-affinity Zn-binding site97.
The uptake of Zn into cells falls under the responsibility of two major transporter families: the ZIP (Zrt-, Irt-like Protein) family and the ZnT/CDF (cation diffusion facilitator) family. The ZIP family of Zn transporters is named after the Zrt1 yeast transporter, and is given the mammalian SLC39 family designation. All ZIP transporters are unique in that they function to transport Zn from extracellular space (or intestinal lumen) into the cytoplasm of the cell. However, the mechanism behind mammalian ZIP transporter-mediated Zn uptake remains unclear, though data suggests that the human Zip2 transporter may require co-transport of Zn (II) and HCO3− for proper uptake81. In addition to localization on the cellular plasma membrane, ZIP family of Zn transporters can also be found on the surface of intracellular organelles, e.g., Zip7-mediated Zn efflux out of the Golgi and into the cytoplasm80. Meanwhile, the function of ZnT/CDF family of transporters (the SLC30 family) opposes that of the ZIP family in promoting Zn efflux out of the cytoplasm and into extracellular space or into the lumen of organelles. Notably, despite the CDF designation, these transporters do not operate through diffusion, but rather through an active transport mechanism that uses a gradient dependent on other ions in order to properly extrude Zn ions98. This family of transporters can be found on the plasma membrane for Zn efflux out of the cell, but some ZnT transporters can also be found on the surface of vesicles that mediate Zn trafficking into organelles. For example, ZnT3 is predominantly found on synaptic vesicles83, 84. Such tight regulation by ZIP and ZnT transporters is required for proper cellular Zn homeostasis, a necessary control for several physiological processes. Recent evidence has shown that a Zn transporter of the ZIP family, ZIP1482, regulates G-protein-coupled receptor (GPCR)-mediated signaling required for mammalian systemic growth and energy metabolism99, a crucial process that necessitates Zn in our diets.
Additionally, recent studies have also shown the involvement of DMT1 in Zn uptake. DMT1, originally named DCT1 for divalent cation transporter, is responsible for the uptake of a variety of divalent metals, including Zn (II). Its mechanism of transport involves an active transport mode that is coupled to a proton gradient dependent on the cell’s membrane potential25. Recent evidence has confirmed the involvement of DMT1 in transporting Zn, although Zn shows a different relative capacity of transport compared to other divalent metals like Fe and Cu6. This is most likely due to the contribution of the several other Zn transporters previously mentioned that serve a more prominent role in Zn uptake. Moreover, new findings have also shown the human CTR1 can additionally play a role in Zn uptake6. Therefore, the complex system of transport mechanisms involved in Zn homeostasis clearly indicates the need for tight regulation of this metal to keep its essentiality from becoming cytotoxic.
Biological/Toxicological Functions
Homeostatic regulation of Zn is especially significant in the brain, where Zn has been found to bind several different receptors outside of ZIP and ZnT transporters. For example, Zn can also bind and inhibit GABAA receptors in the brain100. Zn (II) may be taken up through Ca (II)-permeable kainate/AMPA receptors in postsynaptic neurons85, resulting in the damaging production of ROS. Additionally, aberrant Zn (and Cu) homeostasis has also been linked to β-amyloid aggregation that is characteristic of Alzheimer’s disease (AD)-associated mutations in presenilin, the enzyme that cleaves β-amyloid precursor proteins to release β-amyloid. Interestingly, new data shows the involvement of presenilin in promoting Zn turnover, in addition to regulating Cu/Zn SOD activity, thereby suggesting the role of presenilin in preventing AD-associated β-amyloid aggregation through Zn clearance101. Zn is also a major cofactor in various enzymes, such as Cu/Zn SOD. Mutations in Cu/Zn SOD have been linked to amyotrophic lateral sclerosis (ALS), a disorder that involves the debilitating degeneration of motor neurons. In particular, the loss of Zn in connection to mutant SOD1 has been considered a primary event in the pathophysiology behind ALS102, with recent findings revealing a significant Zn-binding site that may be responsible for its ability to participate in necessary redox reactions103. Additionally, another major Zn-binding protein is metallothionein, which binds Zn with high stability and provides a shuttle system for Zn transfer104. Metallothionein levels have been associated to exacerbate and quicken mutant SOD1-associated ALS105. These studies show the significance and necessity of proper Zn homeostasis in the brain to prevent oxidative stress-induced neurodegeneration.
Part II: Nonessential Metals
Aluminum (Al)
Aluminum (Al) is the most common metal in the earth’s crust. Elemental Al is highly reactive and never found in nature, Al is found in insoluble mineral compounds such as bauxite and clay. Al metal is non-reactive and produced largely from bauxite. Al metal is used to manufacture beverage cans, cookware, roofing material, fireworks and explosives. Al compounds are also used as water treatment agents, as catalysts in acid production and rubber manufacturing and are found in many pharmaceuticals and consumer products including hemodialysis medications, antacids, antiperspirants, cosmetics and food additives. Soluble Al is trivalent and not capable of redox reactions. Depending on pH Al may be present in solution as Al3+, Al(OH)2+, Al(OH)2+, and Al(OH)4−106. Although Al has no known biological function, it is present in plants and animals. Human populations are exposed to Al through drinking water, food, inhalation and pharmaceuticals. In the United States, the average adult ingests 7–9mg Al each day through food, and very little from air or drinking water106.
Transport Mechanisms
The majority of ingested Al rapidly forms insoluble complexes with phosphate in the acidic environment of the stomach and as a result <1% is able to enter the bloodstream107. Al uptake from pharmaceuticals, particularly hemodialysis medications, is much more significant. In the blood, most Al3+ is bound to Tf, Al3+ can also replace calcium and magnesium in citrate complexes108. Transferrin-Al is taken up by cells through the TfR cycle. Within the cell, Al3+ is released from Tf and can stabilize IRP-2, resulting in increased diferric Tf and Tf-Al uptake due to upregulation of iron import and storage proteins such as TfR, DMT1, and ferritin. Al-citrate complexes are transported by monocarbolylate transporter 6 (MCT6), a lactate and pyruvate transporter109. More recent studies have demonstrated additional transport of Al-citrate through the sodium-independent glutamate transporter family Xc−110. Human studies show that the vast majority of Al is excreted within several days following absorption, primarily through the urine with a very small amount in feces. The remaining Al is deposited in the skeleton, liver and kidneys108.
Biological/Toxicological Functions
Al does not have any known natural biological function, although excessive exposure has toxic consequences. In a double-blind, developmental study ingestion of Al salts in rats from gestation through 12 months predominately caused renal failure, there were no observed cognitive deficits107. Exposure to Al-Citrate, on the other hand, caused neurological effects in a Drosophila model, which were attributed to secondary increases in Fe rather than direct effects of Al111. The minimal risk exposure level (MRL) for Al in adults is 1mg/kg/day106.
Arsenic (As)
Arsenic originates from volcanic tuff and can form inorganic complexes with oxygen, chlorine and sulfur. It has several oxidation states but those of greatest toxicological importance are inorganic arsenite (AsIII) and arsenate (AsV), the two forms that are also most commonly found in drinking water. A common source of human exposure to inorganic arsenic is through groundwater and drinking water contamination. The EPA maximum contaminant level for arsenic in drinking water is established at 10 parts per billion (ppb 0.01mg/L). Industrial sources of inorganic arsenic compounds are insecticides, weed killers, fungicides, antifouling paints and wood preservatives. Historical uses include use as a pigment for wallpaper and clothing and as an agent for homicide and suicide.
Transport Mechanisms
Inorganic arsenic is transformed in the body via one-carbon metabolism through a series of methylation and reduction reactions, utilizing S-adenosylmethionine (SAM) as a donor of methyl groups. Methylarsonic acid (MMA) and dimethylarsinic acid (DMA) are the main metabolites of this pathway and are excreted in urine. Up until recently, this pathway was thought to be the detoxification pathway, but several studies have now shown that the metabolites themselves can also produce toxicity112.
Uptake of inorganic arsenite [As(III)] is mediated by the aquaglyceroporins (AQPs) which are normally responsible for transporting small uncharged molecules such as glycerol, urea and water113–115. AQP9, the major subtype found in liver and astrocytes, has been shown to handle both inorganic As(III) and its intermediate monomethylarsonous acid [MMA(III)]116–118. AQP7, the major kidney, testis and adipose tissue aquaglyceroporin, has been implicated in uptake of As(III)119, 120. Inorganic arsenate [As(V)] is a phosphate analogue and therefore can be taken into the cell via phosphate transporters121, 122. Glucose transporter isoform 1 (GLUT1) is a glucose permease (class of multipass transmembrane proteins that facilitate the diffusion of a specific molecule in or out of the cell by passive transport) found in erythrocytes and epithelial cells forming the BBB that has also been implicated in As(III) and MMA(III) uptake123, 124. Human hepatocytes exposed to high micromolar concentrations of As(III) showed a positive correlation between the expression of high-capacity GLUT2 transporters and cellular retention of As(III) and methylated arsenicals125.
Biological/Toxicological Functions
Epidemiologic evidence links inorganic arsenic exposure to an increased risk of a variety of cancers (i.e., lung, bladder, skin, kidney), type 2 diabetes, vascular and cardiovascular disease, hypertension, genotoxicity and reproductive and developmental anomalies108. Many of these disease states can be linked to problems with steroid or nuclear receptor-medicated gene regulation126. Arsenic is also a neurotoxin. The gamut of health effects after arsenic exposure can be attributed to whole body organ distribution of arsenic after an oral exposure, such as via drinking water. Both inorganic arsenic and its metabolites easily pass the placental and blood-brain barriers127. For example, arsenic is likely to cause a number of cellular changes, including disruption of zinc-associated proteins128, inhibit DNA repair129, induce chromosomal aberrations130, alter apoptosis131, induce oxidative stress through hydroxyl radical formation132, inhibiting sulfhydryl enzyme systems required for cell metabolism as well as other mechanisms.
Cadmium (Cd)
An environmental source of Cd is the earth’s crust. It is often associated with zinc, lead and copper ores and is extracted as a byproduct during the production of zinc, lead and copper. Anthropogenic sources include batteries and to a lesser extent pigments, coatings, stabilizers for plastics and nonferrous alloys, and photovoltaic devices. Sources of cadmium in the food supply include shellfish, leafy vegetables, potatoes and grains, peanuts, soybeans and sunflower seeds as well as tobacco and organ meats. Cd bioaccumulates and this represents the primary source of cadmium exposure aside from occupational exposure and tobacco smoke. Occupational exposure is the result of smelting and electroplating, which require heating of Cd-containing materials. Tobacco smokers experience nearly double the cadmium exposure compared to nonsmokers. Routes of exposure include inhalation and ingestion.
Transport Mechanisms
Cd uptake is influenced by levels of Fe, as well as other nutrients. Upon absorption, Cd is transported to the kidney and liver where it is biotransformed and remains for a number of years, while a small portion is excreted in the urine and feces. Divalent DMT1 is likely to transport free Cd133–136. Cd bound to protein can be taken up by endocytosis, especially in hepatocytes137. Cd, Pb and Hg displace Zn and Cu from metallothionein, which serves as an intracellular storage protein for Zn and Cu (see above). Cd (2+) is a calcium (Ca2+) analogue and has the ability to impair calcium transport by competitive binding and selective inhibition of Ca2+-ATPase transporters138–140. Cd absorption appears to occur in part by voltage-gated mechano-sensitive L-type calcium channels, but at slower rates than calcium141. The ZIP transporters have also been shown to transport Cd in mammalian systems, particularly the ZIP8 and ZIP14 (Zn2+/HCO3- symporters)142. Cd, like many other nonessential metals, has been shown to form sulfur-conjugates with thiols, such as cysteine and GSH and these conjugates can act as molecular homologues which can be transported by the amino acid or organic cation/anion transporters143.
Biological/Toxicological Functions
Under levels of Fe deficiency there is a higher uptake and therefore increased Cd toxicity. High and/or chronic inhalational exposure results in kidney disease and death. Symptoms following oral exposure are irritation of the stomach, vomiting, diarrhea, Cd buildup in the kidneys resulting in kidney damage, brittle bones and in severe cases death. Lung cancer has been documented in workers exposed to Cd in the air and Cd is listed as a probable human carcinogen. Cd can cross the placental barrier and is found in breast milk. Children are expected to experience symptoms similar to adults, but animal studies indicate higher absorption in children and an increased susceptibility to bone fragility. Cd in drinking water at 0.04mg/L for up to 10 days is not expected to produce adverse effects in children and lifetime exposure to 0.005mg/L is not expected to have adverse effects. National Institute for Occupational Safety and Health regulates air Cd levels at 5µg/m3 Cd over and 8-hr work-day.
Lead (Pb)
Lead (Pb) is an easily molded, corrosion resistant heavy metal found in the earth’s crust and has many industrial uses. Pb is used to manufacture pipes, vehicle batteries, ammunition, and radiation protection equipment, it is also found as a pigment in paints and dyes. Pb compounds were used as gasoline additives in the United States until 1996, and are still in use in some developing countries. Pb is naturally released into the air by weathering and erosion, although the majority of environmental Pb results from burning gas, coal, oil and waste, and from release of lead into acidic water and soil from pipes, paints, and waste144. Humans are exposed to inorganic Pb through ingestion of contaminated drinking water, soil, and Pb-based paints and through inhalation of Pb containing dust particles which can be significant in many manufacturing and construction occupations144.
Transport Mechanisms
Nearly 95% of inhaled Pb is absorbed into the bloodstream, absorption of ingested Pb, however, depends on several factors including the age of the individual and when the individual last ate. In adults that have recently eaten, only 6% of ingested Pb is absorbed compared to 60–80% if the last meal was a day ago. In children, 50% of ingested Pb is absorbed. The precise mechanisms of Pb absorption by enterocytes is not know, although a recent in vitro study using intestinal segments from mice suggests that intestinal Pb uptake occurs through both DMT1-dependent and independent mechanisms145. This is consistent with findings that Pb uptake is modulated by dietary iron status which alters expression of iron transport proteins such as DMT1146, 147.
Once in the bloodstream, Pb is taken up by red blood cells (RBC) through anion exchange148 and Ca2+ uptake channels149 where it binds primarily to δ-aminolevulinic acid dehydratase (ALAD). Pb is actively exported from red blood cells (RBCs) via Ca2+. In plasma, the majority of Pb2+ a ATPases150ssociates with albumin or sulfhydryl compounds such as cysteine, citrate, glutathione, and histidine151. Following exposure Pb is distributed in soft tissues, and within weeks adults excrete nearly 99%. Pb is transported across the plasma membrane mainly by calcium and iron transport systems. Pb transport into cells can be mediated by DMT1. Rats exposed to both Pb and Cd demonstrate upregulation of DMT1 protein in brain tissue152. Similarly, an in vitro blood brain barrier model showed competitive uptake of Pb through the IRE+ DMT1 isoform153. Pb can also be transported through calcium channels including L-type Ca2+ channels154 and Ca2+ transport mechanisms stimulated by depletion of intracellular Ca2+ stores155, 156. Stimulation of cerebellar granule cells results in increased Pb2+ uptake though N-methyl-D-aspartate (NMDA) receptors which conduct Ca2+157. Within the cell calbindin, a calcium binding protein, binds Pb and may mediate its intracellular transport158. As with several other metals discussed in this review, Pb has a high affinity for sulfhydryl groups which increases the accumulation and retention of Pb in rat C6 glioma cells159. Furthermore, Pb exposure causes oxidative stress in rat astroglia by increasing intracellular Cu through higher levels of uptake and competitive binding of Cu exporter ATP7A159, 160.
Biological/Toxicological Functions
Pb has no known natural biological function and is highly toxic. Pb exposure has detrimental effects on renal, cardiovascular, and reproductive function, causes blood and brain pathologies and disrupts neurodevelopment in fetus and child144. The toxic nature of Pb is caused by several biochemical consequences of cellular Pb. Pb inhibits ALAD, an enzyme critical for the incorporation of iron into heme in RBCs, resulting in zinc rather than heme protoporphyrins and causing in microcytic anemia161. Pb also acts as a calcium analog, and can interfere with neuronal signaling by blocking NMDA receptors and altering calcium-dependent signaling pathways such as PKC161. Pb also causes ROS production, binds sulfhydryl groups similar to methylmercury (MeHg), and can displace iron, zinc, and calcium from proteins. Studies have consistently demonstrated that even low levels Pb have toxic effects in children and adults, and therefore there is no MRL or reference dose (RfD) for Pb exposure144. Pb exposure is commonly treated with chelation therapy to increase Pb excretion and repletion of iron, calcium and zinc, deficiencies which are known to increase Pb uptake.
Mercury (Hg)
Elemental mercury combines with other elements (i.e. chlorine, sulfur, or oxygen) to form white powders/crystals, known as inorganic mercury salts. Elemental mercury also combines with carbon to form organic mercury compounds. In the environment, mercury is released from natural and anthropogenic sources163. Inorganic mercury enters the air from mining ore deposits, burning coal and waste, and from manufacturing plants. It enters the water or soil from natural deposits, disposal of wastes, and the use of mercury-containing fungicides. Mercury is maintained in the upper sedimentary layers of sea and lake-beds and is methylated and thus transformed to the toxic species MeHg by sulfate-reducing bacteria. Historical use of mercury compounds includes fungicides, topical antiseptics, vaccine preservatives, disinfectants, laxatives, diuretics, nasal sprays, dental amalgams, batteries, thermometers, skin-lightening creams, cosmetics and other biomedical applications. Due to the information on the toxic effects of mercury its use has been reduced163.
Transport Mechanisms
Elemental mercury vaporizes at room temperature, and is readily absorbed by the lungs and distributed by the blood. MeHg that is ingested is efficiently absorbed by the gastrointestinal tract. After ingestion, its distribution to the blood is complete within approximately 30 hours, and the blood level accounts for about 7% of the ingested dose. MeHg predominantly accumulates in erythrocytes bound to cysteine residues on the beta-chain of the hemoglobin molecule. The brain is the primary target site for MeHg and approximately 10% is retained in the brain with the remainder transported to the liver and kidneys where it is excreted through bile and urine.
MeHg has a high affinity for the anionic form of –SH groups. Despite the high thermodynamic stability of the MeHg-SH bond, very rapid exchange of MeHg between –SH groups is known to occur. In cells, MeHg can form a complex with the –SH containing amino acid cysteine. The MeHg-S-Cys complex closely mimics the structure of the neutral amino acid, methionine, and is a substrate of the L-neutral amino acid transporter system, which is responsible for its uptake into cells. Uptake can be passive or energy-dependent, depending on the Hg species162. MeHg readily binds metallothionines and metalloproteins with cysteine residues displacing Zn2+162, 164. The primary mechanism of MeHg as well as its specificity has yet to be identified. MeHg-cysteine and -cystine conjugates have shown increased efflux, presumably due to the generation and involvement of GSH.
Biological/Toxicological Functions
Two other major anti-oxidative enzymes that are inhibited by mercury are SOD and catalase. Hormones are also vulnerable to mercury as it binds cofactors and inhibits their formation. Progesterone uptake by cells is inhibited when mercury binds to an important free thiol group on the progesterone receptor. It is also well documented that cadmium, lead, and mercury disrupt intracellular transport in neurons by inhibiting microtubule polymerization and assembly. Mercury can also decrease the production of neurotransmitters and GSH by binding and sequestering cysteine.
Due to bioaccumulation, concentrations of MeHg are magnified within the food chain, reaching concentrations in fish 10,000–100,000 times greater than in the surrounding water163. EPA drinking water limit is 2 ppb. Levels of mercury should not exceed 5.0 µg/L in whole blood or 1.0 µg/g in hair, corresponding to a RfD of 0.1 µg/kg body weight/day162. Inorganic mercury salts are extremely toxic to kidneys, causing severe renal dysfunctions including tubular necrosis and glomerulonephritis163. Adults exposed to MeHg exhibit symptoms of paresthesias of the circumoral area and hands and feet, acrodynia, constriction of the visual-field, memory problems and ataxia. The neuropathology in adults is characterized by region-specific degeneration of neurons in the visual cortex and cerebellar internal granule cells165. Symptoms often appear following a latent phase of weeks or even months. Mercury can cross the placental and blood-brain barriers and is secreted through breast milk. Exposure to MeHg is worse for young children than for adults, as damage in adult brains is focal, whereas in fetal and neonatal brains the damage is widespread and diffuse162, 165. The brain levels of MeHg in the fetus are generally higher than in the mother and in newborns or infants MeHg exposure leads to cerebral palsy-like effects, characterized by ataxic motor and mental symptoms with hypoplastic and symmetrical atrophy of the cerebrum and cerebellum. Mechanisms associated with MeHg neurotoxicity in the developing as well the adult CNS include: (1) oxidative stress, (2) microtubule disruption, (3) increase in intracellular calcium (Ca2+) with disturbance of neurotransmitter function, (4) inhibition of macromolecule synthesis (DNA, RNA and protein), and (5) excitotoxicity, as a result of altered glutamate homeostasis. MeHg exposure is associated with increased brain lipid peroxidation, superoxide and hydrogen peroxide amounts, impaired levels of SOD, GSH reductase and GSH peroxidase activities, as well as a general decrease in GSH levels164.
Summary
Several protein families play important roles in cell entry of both essential and nonessential metals, including the ZIP, DMT1, Fe/Cu reductases, and the P-type ATPases. Although homeostatic regulation exists to control physiological levels of essential metals, this regulation can be disrupted by presence of nonessential metals. Cell detoxification mechanisms also exist within the cell to extrude unwanted metals. These play an important balance with the cell entry mechanisms to control uptake and deposition within the cell compartment. In order to more fully understand how metals produce detrimental effects within the cell, we first must understand their entry and exit keeping in mind nutrition, genetics and biochemical/molecular effects on these processes.
Table 1.
Essential Metal Transport and Regulation
| Metal | Gene | Function | Subcellular location | Reference |
|---|---|---|---|---|
| Copper | CTR1 | Cu release from endosome | Plasma membrane | 67 |
| CTR2 | Cu release from endosome/from other cellular compartments |
Intracellular compartments, plasma membrane |
8, 68 | |
| Metallothionine, GSH |
Cu binding | Cytosol | 12 | |
| Ccs1 | SOD1 Cu chaperone | Cytosol | 10, 11 | |
| Cox17 | Cytochrome c Oxidase chaperone |
Cytosol | 12 | |
| Cox 11, Sco1/2 | Cytochrome c Oxidase chaperone |
Membrane | 12 | |
| Atox1 | Multifunctional Cu chaperone |
Cytosol | 13 | |
| ATPase7A | Cu export | TGN or Basolateral membrane vesicles |
69 | |
| ATPase7B | Cu export | TGN or Apical membrane vesicles |
69 | |
| Iron | Tf | Serum iron binding protein | Plasma membrane | 21, 23 |
| DMT1 | Fe transporter | Plasma membrane | 25 | |
| L-type Ca2+channels | Calcium Entry | Plasma membrane | 26 | |
| Hemoglobin scavenger receptor CD163 |
Hemoglobin uptake | Plasma membrane | 28 | |
| Manganese | DMT1 | Fe transporter | Plasma membrane | 25, 63 |
| Voltage-gated and store- operated calcium channels |
Calcium entry | Plasma membrane | 70 | |
| Ionotropic glutamate receptor Ca channels |
Calcium Entry | Plasma membrane | 71 | |
| Citrate Transporter | Citrate Transport | Plasma membrane | 72 | |
| Choline Transporter | Choline Transport | Plasma membrane | 73, 74 | |
| ZIP8/ZIP14 | Zinc transport | Plasma membrane and intracellular organelles |
75, 76 | |
| HIP14 (Huntingtin-interacting protein 14) |
Magnesium transporter | Cytoplasmic vesicle membranes and Golgi |
77 | |
| P-type transmembrane ATPase protein (ATP13A2) |
Unknown | Plasma membrane | 78 | |
| Ferroportin | Fe exporter | Plasma membrane | 79 | |
| Zinc | ZIP (Zrt-, Irt-like Protein) | Zinc transporter | Plasma membrane and intracellular organelles |
80–82 |
| ZnT/CDF | Cation diffusion facilitator | Plasma membrane and surface of vesicles that mediate Zn trafficking into organelles |
83, 84 | |
| Human Cu transporter 1 (hCTR1) |
Copper Transporter | Plasma membrane | 6 | |
| DMT1 | Iron transporter | Plasma membrane | 6 | |
| Ionotropic glutamate receptor Ca channels |
Calcium Entry | Plasma membrane | 85 |
Table 2.
Nonessential Metal Transport and Regulation
| Metal | Gene | Function | Subcellular location | Reference |
|---|---|---|---|---|
| Aluminum | Monocarboxylate Transporter 6 (MCT6) |
Lactate/pyruvate transporter | Plasma membrane | 109 |
| Tf | Serum iron binding protein | Plasma membrane | 108 | |
| Xc- | Na-independent glutamate transporter |
Plasma membrane | 110 | |
| Arsenic | Aquaglyceroporins (AQPs) | Transport small uncharged molecules |
Plasma membrane | 114, 115, 117 |
| Glucose transporter isoform 1 (GLUT 1) and isoform 2 (GLUT 2) |
Glucose transport | Plasma membrane | 123–125 | |
| Cadmium | Divalent metal transporter 1 (DMT1) |
Iron transporter | Plasma membrane | 134–136 |
| Zinc transporters (Zrt/Irt- related proteins (ZIP) |
Zinc transport | Plasma membrane | 142 | |
| Amino Acid or organic cation/anion transporters |
Transport of amino acid/cation/anions |
Plasma membrane | 143 | |
| Lead | DMT1 | Fe transporter | Plasma membrane | 137, 145, 152, 153 |
| Ca-ATPases | Ca export | Plasma membrane | 150 | |
| Calbindin | Calcium binding protein | Cytosol | 158 | |
| Voltage-gated and store- operated calcium channels |
Calcium entry | Plasma membrane | 155–157 | |
| Mercury | Neutral amino acid transport system –L type (LAT) |
Transport of neutral amino acids |
Plasma membrane | 162 |
Acknowledgments
This review was supported in part by Grants from the National Institute of Environmental Health Sciences ES R01-10563, R01-07331, ES T32-007028 and the Molecular Toxicology Center ES P30 000267.
Notes and references
- 1.Waldron KJ, Rutherford JC, Ford D, Robinson NJ. Nature. 2009;460:823–830. doi: 10.1038/nature08300. [DOI] [PubMed] [Google Scholar]
- 2.Andreini C, Bertini I, Cavallaro G, Holliday GL, Thornton JM. J Biol Inorg Chem. 2008;13:1205–1218. doi: 10.1007/s00775-008-0404-5. [DOI] [PubMed] [Google Scholar]
- 3.Martinez-Finley EJ, Aschner M. J Toxicol. 2011;2011:895236. doi: 10.1155/2011/895236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.I. o. M. Food and Nutrition Board. 2006:304–311. [Google Scholar]
- 5.Syracuse Research Corporation., United States. Dept. of Health and Human Services., United States. Agency for Toxic Substances and Disease Registry. and United States. Atlanta, GA: Public Health Service., Agency for Toxic Substances and Disease Registry; p. v. [Google Scholar]
- 6.Espinoza A, Le Blanc S, Olivares M, Pizarro F, Ruz M, Arredondo M. Biol Trace Elem Res. 2011 doi: 10.1007/s12011-011-9243-2. [DOI] [PubMed] [Google Scholar]
- 7.De Feo CJ, Aller SG, Unger VM. Biometals. 2007;20:705–716. doi: 10.1007/s10534-006-9054-7. [DOI] [PubMed] [Google Scholar]
- 8.Bertinato J, Swist E, Plouffe LJ, Brooks SP, L'Abbe M R. Biochem J. 2008;409:731–740. doi: 10.1042/BJ20071025. [DOI] [PubMed] [Google Scholar]
- 9.Banci L, Bertini I, Ciofi-Baffoni S, Kozyreva T, Zovo K, Palumaa P. Nature. 2010;465:645–648. doi: 10.1038/nature09018. [DOI] [PubMed] [Google Scholar]
- 10.Culotta VC, Klomp LW, Strain J, Casareno RL, Krems B, Gitlin JD. J Biol Chem. 1997;272:23469–23472. doi: 10.1074/jbc.272.38.23469. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt PJ, Kunst C, Culotta VC. J Biol Chem. 2000;275:33771–33776. doi: 10.1074/jbc.M006254200. [DOI] [PubMed] [Google Scholar]
- 12.Robinson NJ, Winge DR. Annu Rev Biochem. 2010;79:537–562. doi: 10.1146/annurev-biochem-030409-143539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutsenko S, Bhattacharjee A, Hubbard AL. Metallomics. 2010;2:596–608. doi: 10.1039/c0mt00006j. [DOI] [PubMed] [Google Scholar]
- 14.Linz R, Lutsenko S. J Bioenerg Biomembr. 2007;39:403–407. doi: 10.1007/s10863-007-9101-2. [DOI] [PubMed] [Google Scholar]
- 15.Barnes N, Bartee MY, Braiterman L, Gupta A, Ustiyan V, Zuzel V, Kaplan JH, Hubbard AL, Lutsenko S. Traffic. 2009;10:767–779. doi: 10.1111/j.1600-0854.2009.00901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaler SG. Nat Rev Neurol. 2011;7:15–29. doi: 10.1038/nrneurol.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan JW, Anders E. Proc Natl Acad Sci U S A. 1980;77:6973–6977. doi: 10.1073/pnas.77.12.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wildermuth E, Stark H, Friedrich G, Ebenhoch F, Kuhborth B, Silver J, Rituper R. Ullmann's Encyclopedia of Industrial Chemistry. 2000 [Google Scholar]
- 19.I. o. M. Food and Nutrition Board. 2001:162–196. [Google Scholar]
- 20.Monsen ER, Hallberg L, Layrisse M, Hegsted DM, Cook JD, Mertz W, Finch CA. Am J Clin Nutr. 1978;31:134–141. doi: 10.1093/ajcn/31.1.134. [DOI] [PubMed] [Google Scholar]
- 21.Cheng Y, Zak O, Aisen P, Harrison SC, Walz T. Cell. 2004;116:565–576. doi: 10.1016/s0092-8674(04)00130-8. [DOI] [PubMed] [Google Scholar]
- 22.Sendamarai AK, Ohgami RS, Fleming MD, Lawrence CM. Proc Natl Acad Sci U S A. 2008;105:7410–7415. doi: 10.1073/pnas.0801318105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Touret N, Furuya W, Forbes J, Gros P, Grinstein S. J Biol Chem. 2003;278:25548–25557. doi: 10.1074/jbc.M212374200. [DOI] [PubMed] [Google Scholar]
- 24.Kawabata H, Yang R, Hirama T, Vuong PT, Kawano S, Gombart AF, Koeffler HP. J Biol Chem. 1999;274:20826–20832. doi: 10.1074/jbc.274.30.20826. [DOI] [PubMed] [Google Scholar]
- 25.Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 26.Oudit GY, Sun H, Trivieri MG, Koch SE, Dawood F, Ackerley C, Yazdanpanah M, Wilson GJ, Schwartz A, Liu PP, Backx PH. Nat Med. 2003;9:1187–1194. doi: 10.1038/nm920. [DOI] [PubMed] [Google Scholar]
- 27.Harrison PM, Arosio P. Biochim Biophys Acta. 1996;1275:161–203. doi: 10.1016/0005-2728(96)00022-9. [DOI] [PubMed] [Google Scholar]
- 28.Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 29.Hentze MW, Muckenthaler MU, Andrews NC. Cell. 2004;117:285–297. doi: 10.1016/s0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- 30.Biagioli M, Pinto M, Cesselli D, Zaninello M, Lazarevic D, Roncaglia P, Simone R, Vlachouli C, Plessy C, Bertin N, Beltrami A, Kobayashi K, Gallo V, Santoro C, Ferrer I, Rivella S, Beltrami CA, Carninci P, Raviola E, Gustincich S. Proc Natl Acad Sci U S A. 2009;106:15454–15459. doi: 10.1073/pnas.0813216106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haile DJ. Am J Med Sci. 1999;318:230–240. doi: 10.1097/00000441-199910000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Torti FM, Torti SV. Blood. 2002;99:3505–3516. doi: 10.1182/blood.v99.10.3505. [DOI] [PubMed] [Google Scholar]
- 33.Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, Loreal O. J Biol Chem. 2001;276:7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- 34.Nicolas G, Bennoun M, Porteu A, Mativet S, Beaumont C, Grandchamp B, Sirito M, Sawadogo M, Kahn A, Vaulont S. Proc Natl Acad Sci U S A. 2002;99:4596–4601. doi: 10.1073/pnas.072632499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding H, Yan CZ, Shi H, Zhao YS, Chang SY, Yu P, Wu WS, Zhao CY, Chang YZ, Duan XL. PLoS One. 2011;6:e25324. doi: 10.1371/journal.pone.0025324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC. Cell Metab. 2005;1:191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Moos T, Rosengren Nielsen T. Semin Pediatr Neurol. 2006;13:149–157. doi: 10.1016/j.spen.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Nemeth E, Preza GC, Jung CL, Kaplan J, Waring AJ, Ganz T. Blood. 2006;107:328–333. doi: 10.1182/blood-2005-05-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li L, Holscher C, Chen BB, Zhang ZF, Liu YZ. Biol Trace Elem Res. 2011;143:1581–1593. doi: 10.1007/s12011-011-8967-3. [DOI] [PubMed] [Google Scholar]
- 40.Zhang DL, Hughes RM, Ollivierre-Wilson H, Ghosh MC, Rouault TA. Cell Metab. 2009;9:461–473. doi: 10.1016/j.cmet.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang F, Liu XB, Quinones M, Melby PC, Ghio A, Haile DJ. J Biol Chem. 2002;277:39786–39791. doi: 10.1074/jbc.M201485200. [DOI] [PubMed] [Google Scholar]
- 42.Ohgami RS, Campagna DR, McDonald A, Fleming MD. Blood. 2006;108:1388–1394. doi: 10.1182/blood-2006-02-003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Cell. 2010;142:24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 44.Theurl I, Schroll A, Nairz M, Seifert M, Theurl M, Sonnweber T, Kulaksiz H, Weiss G. Haematologica. 2011;96:1761–1769. doi: 10.3324/haematol.2011.048926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ganz T, Nemeth E. Annu Rev Med. 2011;62:347–360. doi: 10.1146/annurev-med-050109-142444. [DOI] [PubMed] [Google Scholar]
- 46.Feder JN, Penny DM, Irrinki A, Lee VK, Lebron JA, Watson N, Tsuchihashi Z, Sigal E, Bjorkman PJ, Schatzman RC. Proc Natl Acad Sci U S A. 1998;95:1472–1477. doi: 10.1073/pnas.95.4.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmidt PJ, Toran PT, Giannetti AM, Bjorkman PJ, Andrews NC. Cell Metab. 2008;7:205–214. doi: 10.1016/j.cmet.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montosi G, Donovan A, Totaro A, Garuti C, Pignatti E, Cassanelli S, Trenor CC, Gasparini P, Andrews NC, Pietrangelo A. J Clin Invest. 2001;108:619–623. doi: 10.1172/JCI13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fleming RE, Ahmann JR, Migas MC, Waheed A, Koeffler HP, Kawabata H, Britton RS, Bacon BR, Sly WS. Proc Natl Acad Sci U S A. 2002;99:10653–10658. doi: 10.1073/pnas.162360699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Papanikolaou G, Samuels ME, Ludwig EH, MacDonald ML, Franchini PL, Dube MP, Andres L, MacFarlane J, Sakellaropoulos N, Politou M, Nemeth E, Thompson J, Risler JK, Zaborowska C, Babakaiff R, Radomski CC, Pape TD, Davidas O, Christakis J, Brissot P, Lockitch G, Ganz T, Hayden MR, Goldberg YP. Nat Genet. 2004;36:77–82. doi: 10.1038/ng1274. [DOI] [PubMed] [Google Scholar]
- 51.Flatmark T, Romslo I. J Biol Chem. 1975;250:6433–6438. [PubMed] [Google Scholar]
- 52.Salazar J, Mena N, Hunot S, Prigent A, Alvarez-Fischer D, Arredondo M, Duyckaerts C, Sazdovitch V, Zhao L, Garrick LM, Nunez MT, Garrick MD, Raisman-Vozari R, Hirsch EC. Proc Natl Acad Sci U S A. 2008;105:18578–18583. doi: 10.1073/pnas.0804373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen OS, Hemenway S, Kaplan J. Proc Natl Acad Sci U S A. 2002;99:12321–12326. doi: 10.1073/pnas.192449599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gallagher JJ, Finnegan ME, Grehan B, Dobson J, Collingwood JF, Lynch MA. J Alzheimers Dis. 2011 doi: 10.3233/JAD-2011-110614. [DOI] [PubMed] [Google Scholar]
- 55.A. f. T. S. a. D. Research. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service; 2008. [Google Scholar]
- 56.Aschner JL, Aschner M. Mol Aspects Med. 2005;26:353–362. doi: 10.1016/j.mam.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.U. S. D. o. H. a. H. Services, P. H. Service and A. f. T. S. a. D. Registry. 2008 [Google Scholar]
- 58.Bell JG, Keen CL, Lonnerdal B. J Toxicol Environ Health. 1989;26:387–398. doi: 10.1080/15287398909531263. [DOI] [PubMed] [Google Scholar]
- 59.Garcia-Aranda JA, Wapnir RA, Lifshitz F. J Nutr. 1983;113:2601–2607. doi: 10.1093/jn/113.12.2601. [DOI] [PubMed] [Google Scholar]
- 60.Dobson AW, Erikson KM, Aschner M. Ann N Y Acad Sci. 2004;1012:115–128. doi: 10.1196/annals.1306.009. [DOI] [PubMed] [Google Scholar]
- 61.Gunter TE, Miller LM, Gavin CE, Eliseev R, Salter J, Buntinas L, Alexandrov A, Hammond S, Gunter KK. J Neurochem. 2004;88:266–280. doi: 10.1046/j.1471-4159.2003.02122.x. [DOI] [PubMed] [Google Scholar]
- 62.Aschner M, Gannon M. Brain Res Bull. 1994;33:345–349. doi: 10.1016/0361-9230(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 63.Thompson K, Molina RM, Donaghey T, Schwob JE, Brain JD, Wessling-Resnick M. FASEB J. 2007;21:223–230. doi: 10.1096/fj.06-6710com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang E, Ong WY, Connor JR. Neuroscience. 2004;128:487–496. doi: 10.1016/j.neuroscience.2004.06.055. [DOI] [PubMed] [Google Scholar]
- 65.Burdo JR, Menzies SL, Simpson IA, Garrick LM, Garrick MD, Dolan KG, Haile DJ, Beard JL, Connor JR. J Neurosci Res. 2001;66:1198–1207. doi: 10.1002/jnr.1256. [DOI] [PubMed] [Google Scholar]
- 66.Wang XS, Ong WY, Connor JR. J Neurocytol. 2001;30:353–360. doi: 10.1023/a:1014464514793. [DOI] [PubMed] [Google Scholar]
- 67.Zhou B, Gitschier J. Proc Natl Acad Sci U S A. 1997;94:7481–7486. doi: 10.1073/pnas.94.14.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van den Berghe PV, Folmer DE, Malingre HE, van Beurden E, Klomp AE, van de Sluis B, Merkx M, Berger R, Klomp LW. Biochem J. 2007;407:49–59. doi: 10.1042/BJ20070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gupta A, Lutsenko S. Future Med Chem. 2009;1:1125–1142. doi: 10.4155/fmc.09.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Riccio A, Mattei C, Kelsell RE, Medhurst AD, Calver AR, Randall AD, Davis JB, Benham CD, Pangalos MN. J Biol Chem. 2002;277:12302–12309. doi: 10.1074/jbc.M112313200. [DOI] [PubMed] [Google Scholar]
- 71.Kannurpatti SS, Joshi PG, Joshi NB. Neurochem Res. 2000;25:1527–1536. doi: 10.1023/a:1026602100160. [DOI] [PubMed] [Google Scholar]
- 72.Crossgrove JS, Allen DD, Bukaveckas BL, Rhineheimer SS, Yokel RA. Neurotoxicology. 2003;24:3–13. doi: 10.1016/s0161-813x(02)00089-x. [DOI] [PubMed] [Google Scholar]
- 73.Lockman PR, Roder KE, Allen DD. J Neurochem. 2001;79:588–594. doi: 10.1046/j.1471-4159.2001.00589.x. [DOI] [PubMed] [Google Scholar]
- 74.Lorkovic H, Feyrer A. Neurosci Lett. 1984;51:331–335. doi: 10.1016/0304-3940(84)90398-7. [DOI] [PubMed] [Google Scholar]
- 75.He L, Girijashanker K, Dalton TP, Reed J, Li H, Soleimani M, Nebert DW. Mol Pharmacol. 2006;70:171–180. doi: 10.1124/mol.106.024521. [DOI] [PubMed] [Google Scholar]
- 76.Girijashanker K, He L, Soleimani M, Reed JM, Li H, Liu Z, Wang B, Dalton TP, Nebert DW. Mol Pharmacol. 2008;73:1413–1423. doi: 10.1124/mol.107.043588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goytain A, Hines RM, Quamme GA. J Biol Chem. 2008;283:33365–33374. doi: 10.1074/jbc.M801469200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gitler AD, Chesi A, Geddie ML, Strathearn KE, Hamamichi S, Hill KJ, Caldwell KA, Caldwell GA, Cooper AA, Rochet JC, Lindquist S. Nat Genet. 2009;41:308–315. doi: 10.1038/ng.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yin Z, Jiang H, Lee ES, Ni M, Erikson KM, Milatovic D, Bowman AB, Aschner M. J Neurochem. 2010;112:1190–1198. doi: 10.1111/j.1471-4159.2009.06534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang L, Kirschke CP, Zhang Y, Yu YY. J Biol Chem. 2005;280:15456–15463. doi: 10.1074/jbc.M412188200. [DOI] [PubMed] [Google Scholar]
- 81.Gaither LA, Eide DJ. J Biol Chem. 2000;275:5560–5564. doi: 10.1074/jbc.275.8.5560. [DOI] [PubMed] [Google Scholar]
- 82.Pinilla-Tenas JJ, Sparkman BK, Shawki A, Illing AC, Mitchell CJ, Zhao N, Liuzzi JP, Cousins RJ, Knutson MD, Mackenzie B. Am J Physiol Cell Physiol. 2011;301:C862–C871. doi: 10.1152/ajpcell.00479.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cole TB, Wenzel HJ, Kafer KE, Schwartzkroin PA, Palmiter RD. Proc Natl Acad Sci U S A. 1999;96:1716–1721. doi: 10.1073/pnas.96.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Palmiter RD, Cole TB, Quaife CJ, Findley SD. Proc Natl Acad Sci U S A. 1996;93:14934–14939. doi: 10.1073/pnas.93.25.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sensi SL, Yin HZ, Carriedo SG, Rao SS, Weiss JH. Proc Natl Acad Sci U S A. 1999;96:2414–2419. doi: 10.1073/pnas.96.5.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gruenheid S, Canonne-Hergaux F, Gauthier S, Hackam DJ, Grinstein S, Gros P. J Exp Med. 1999;189:831–841. doi: 10.1084/jem.189.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lucaciu CM, Dragu C, Copaescu L, Morariu VV. Biochim Biophys Acta. 1997;1328:90–98. doi: 10.1016/s0005-2736(97)00039-4. [DOI] [PubMed] [Google Scholar]
- 88.Fujishiro H, Doi M, Enomoto S, Himeno S. Metallomics. 2011;3:710–718. doi: 10.1039/c1mt00020a. [DOI] [PubMed] [Google Scholar]
- 89.Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ. Nat Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- 90.Weatherburn D. Manganese-Containing Enzymes and Proteins. 2001 [Google Scholar]
- 91.Benedetto A, Au C, Aschner M. Chem Rev. 2009;109:4862–4884. doi: 10.1021/cr800536y. [DOI] [PubMed] [Google Scholar]
- 92.Milatovic D, Zaja-Milatovic S, Gupta RC, Yu Y, Aschner M. Toxicol Appl Pharmacol. 2009;240:219–225. doi: 10.1016/j.taap.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gavin CE, Gunter KK, Gunter TE. Toxicol Appl Pharmacol. 1992;115:1–5. doi: 10.1016/0041-008x(92)90360-5. [DOI] [PubMed] [Google Scholar]
- 94.Stredrick DL, Stokes AH, Worst TJ, Freeman WM, Johnson EA, Lash LH, Aschner M, Vrana KE. Neurotoxicology. 2004;25:543–553. doi: 10.1016/j.neuro.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 95.U. S. D. o. H. a. H. Services, P. H. Service and A. f. T. S. a. D. Registry, editor. Draft Toxicological Profile for Zinc. 2005. [PubMed] [Google Scholar]
- 96.Foote JW, Delves HT. J Clin Pathol. 1984;37:1050–1054. doi: 10.1136/jcp.37.9.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stewart AJ, Blindauer CA, Berezenko S, Sleep D, Sadler PJ. Proc Natl Acad Sci U S A. 2003;100:3701–3706. doi: 10.1073/pnas.0436576100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liuzzi JP, Cousins RJ. Annu Rev Nutr. 2004;24:151–172. doi: 10.1146/annurev.nutr.24.012003.132402. [DOI] [PubMed] [Google Scholar]
- 99.Hojyo S, Fukada T, Shimoda S, Ohashi W, Bin BH, Koseki H, Hirano T. PLoS One. 2011;6:e18059. doi: 10.1371/journal.pone.0018059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hosie AM, Dunne EL, Harvey RJ, Smart TG. Nat Neurosci. 2003;6:362–369. doi: 10.1038/nn1030. [DOI] [PubMed] [Google Scholar]
- 101.Greenough MA, Volitakis I, Li QX, Laughton K, Evin G, Ho M, Dalziel AH, Camakaris J, Bush AI. J Biol Chem. 2011;286:9776–9786. doi: 10.1074/jbc.M110.163964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Estevez AG, Crow JP, Sampson JB, Reiter C, Zhuang Y, Richardson GJ, Tarpey MM, Barbeito L, Beckman JS. Science. 1999;286:2498–2500. doi: 10.1126/science.286.5449.2498. [DOI] [PubMed] [Google Scholar]
- 103.Son M, Srikanth U, Puttaparthi K, Luther C, Elliott JL. J Neurochem. 2011;118:891–901. doi: 10.1111/j.1471-4159.2011.07360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Robbins AH, McRee DE, Williamson M, Collett SA, Xuong NH, Furey WF, Wang BC, Stout CD. J Mol Biol. 1991;221:1269–1293. [PubMed] [Google Scholar]
- 105.Puttaparthi K, Gitomer WL, Krishnan U, Son M, Rajendran B, Elliott JL. J Neurosci. 2002;22:8790–8796. doi: 10.1523/JNEUROSCI.22-20-08790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.U. S. D. o. H. a. H. Services, P. H. Service and A. f. T. S. a. D. Registry. 2008 [Google Scholar]
- 107.Poirier J, Semple H, Davies J, Lapointe R, Dziwenka M, Hiltz M, Mujibi D. Neuroscience. 2011;193:338–362. doi: 10.1016/j.neuroscience.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 108.Priest ND. J Environ Monit. 2004;6:375–403. doi: 10.1039/b314329p. [DOI] [PubMed] [Google Scholar]
- 109.Yokel RA, Wilson M, Harris WR, Halestrap AP. Brain Res. 2002;930:101–110. doi: 10.1016/s0006-8993(02)02234-5. [DOI] [PubMed] [Google Scholar]
- 110.Nagasawa K, Ito S, Kakuda T, Nagai K, Tamai I, Tsuji A, Fujimoto S. Toxicol Lett. 2005;155:289–296. doi: 10.1016/j.toxlet.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 111.Wu Z, Du Y, Xue H, Wu Y, Zhou B. Neurobiol Aging. 2012;33:199, e191–e199, e112. doi: 10.1016/j.neurobiolaging.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 112.Vahter M. Annu Rev Nutr. 2009;29:381–399. doi: 10.1146/annurev-nutr-080508-141102. [DOI] [PubMed] [Google Scholar]
- 113.Liu Z. Adv Exp Med Biol. 2010;679:71–81. doi: 10.1007/978-1-4419-6315-4_6. [DOI] [PubMed] [Google Scholar]
- 114.Leung J, Pang A, Yuen WH, Kwong YL, Tse EW. Blood. 2007;109:740–746. doi: 10.1182/blood-2006-04-019588. [DOI] [PubMed] [Google Scholar]
- 115.Shinkai Y, Sumi D, Toyama T, Kaji T, Kumagai Y. Toxicol Appl Pharmacol. 2009;237:232–236. doi: 10.1016/j.taap.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 116.Yoshino Y, Yuan B, Kaise T, Takeichi M, Tanaka S, Hirano T, Kroetz DL, Toyoda H. Toxicol Appl Pharmacol. 2011;257:198–208. doi: 10.1016/j.taap.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu Z, Shen J, Carbrey JM, Mukhopadhyay R, Agre P, Rosen BP. Proc Natl Acad Sci U S A. 2002;99:6053–6058. doi: 10.1073/pnas.092131899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tsukaguchi H, Weremowicz S, Morton CC, Hediger MA. Am J Physiol. 1999;277:F685–F696. doi: 10.1152/ajprenal.1999.277.5.F685. [DOI] [PubMed] [Google Scholar]
- 119.Ishibashi K, Kuwahara M, Kageyama Y, Tohsaka A, Marumo F, Sasaki S. Biochem Biophys Res Commun. 1997;237:714–718. doi: 10.1006/bbrc.1997.7219. [DOI] [PubMed] [Google Scholar]
- 120.Kageyama Y, Ishibashi K, Hayashi T, Xia G, Sasaki S, Kihara K. Andrologia. 2001;33:165–169. doi: 10.1046/j.1439-0272.2001.00443.x. [DOI] [PubMed] [Google Scholar]
- 121.Persson BL, Petersson J, Fristedt U, Weinander R, Berhe A, Pattison J. Biochim Biophys Acta. 1999;1422:255–272. doi: 10.1016/s0304-4157(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 122.Willsky GR, Malamy MH. J Bacteriol. 1980;144:366–374. doi: 10.1128/jb.144.1.366-374.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu Z, Sanchez MA, Jiang X, Boles E, Landfear SM, Rosen BP. Biochem Biophys Res Commun. 2006;351:424–430. doi: 10.1016/j.bbrc.2006.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vannucci SJ, Gibbs EM, Simpson IA. Am J Physiol. 1997;272:E267–E274. doi: 10.1152/ajpendo.1997.272.2.E267. [DOI] [PubMed] [Google Scholar]
- 125.Drobna Z, Walton FS, Paul DS, Xing W, Thomas DJ, Styblo M. Arch Toxicol. 2010;84:3–16. doi: 10.1007/s00204-009-0499-7. [DOI] [PubMed] [Google Scholar]
- 126.Barr FD, Krohmer LJ, Hamilton JW, Sheldon LA. PLoS One. 2009;4:e6766. doi: 10.1371/journal.pone.0006766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jin Y, Xi S, Li X, Lu C, Li G, Xu Y, Qu C, Niu Y, Sun G. Environ Res. 2006;101:349–355. doi: 10.1016/j.envres.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 128.Beyersmann D, Hartwig A. Arch Toxicol. 2008;82:493–512. doi: 10.1007/s00204-008-0313-y. [DOI] [PubMed] [Google Scholar]
- 129.Gentry PR, McDonald TB, Sullivan DE, Shipp AM, Yager JW, Clewell HJ., 3rd Environ Mol Mutagen. 2010;51:1–14. doi: 10.1002/em.20505. [DOI] [PubMed] [Google Scholar]
- 130.Salnikow K, Zhitkovich A. Chem Res Toxicol. 2008;21:28–44. doi: 10.1021/tx700198a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pi EH, Simpson GM. Psychiatr Serv. 2005;56:31–33. doi: 10.1176/appi.ps.56.1.31. [DOI] [PubMed] [Google Scholar]
- 132.Shi H, Shi X, Liu KJ. Mol Cell Biochem. 2004;255:67–78. doi: 10.1023/b:mcbi.0000007262.26044.e8. [DOI] [PubMed] [Google Scholar]
- 133.Bannon DI, Abounader R, Lees PS, Bressler JP. Am J Physiol Cell Physiol. 2003;284:C44–C50. doi: 10.1152/ajpcell.00184.2002. [DOI] [PubMed] [Google Scholar]
- 134.Kwong RW, Andres JA, Niyogi S. Aquat Toxicol. 2010;99:343–350. doi: 10.1016/j.aquatox.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 135.Kwong RW, Andres JA, Niyogi S. Aquat Toxicol. 2011;102:1–9. doi: 10.1016/j.aquatox.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 136.Park JD, Cherrington NJ, Klaassen CD. Toxicol Sci. 2002;68:288–294. doi: 10.1093/toxsci/68.2.288. [DOI] [PubMed] [Google Scholar]
- 137.Bressler JP, Olivi L, Cheong JH, Kim Y, Bannona D. Ann N Y Acad Sci. 2004;1012:142–152. doi: 10.1196/annals.1306.011. [DOI] [PubMed] [Google Scholar]
- 138.Niyogi S, Kent R, Wood CM. Comp Biochem Physiol C Toxicol Pharmacol. 2008;148:305–314. doi: 10.1016/j.cbpc.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 139.Niyogi S, Wood CM. J Comp Physiol B. 2004;174:243–253. doi: 10.1007/s00360-003-0409-x. [DOI] [PubMed] [Google Scholar]
- 140.Verbost PM, Flik G, Pang PK, Lock RA, Wendelaar Bonga SE. J Biol Chem. 1989;264:5613–5615. [PubMed] [Google Scholar]
- 141.Klinck JS, Wood CM. Aquat Toxicol. 2011;102:58–72. doi: 10.1016/j.aquatox.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 142.Liu N, Yue Q, Wang YQ, Cheng AL, Gao EQ. Dalton Trans. 2008:4621–4629. doi: 10.1039/b804996c. [DOI] [PubMed] [Google Scholar]
- 143.Zalups RK, Ahmad S. Toxicol Appl Pharmacol. 2003;186:163–188. doi: 10.1016/s0041-008x(02)00021-2. [DOI] [PubMed] [Google Scholar]
- 144.U. S. D. o. H. a. H. Services, P. H. Service and A. f. T. S. a. D. Registry. 2007 [Google Scholar]
- 145.Elsenhans B, Janser H, Windisch W, Schumann K. Toxicology. 284:7–11. doi: 10.1016/j.tox.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 146.Bradman A, Eskenazi B, Sutton P, Athanasoulis M, Goldman LR. Environ Health Perspect. 2001;109:1079–1084. doi: 10.1289/ehp.011091079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Barton JC, Conrad ME, Nuby S, Harrison L. J Lab Clin Med. 1978;92:536–547. [PubMed] [Google Scholar]
- 148.Bannon DI, Olivi L, Bressler J. Toxicology. 2000;147:101–107. doi: 10.1016/s0300-483x(00)00187-6. [DOI] [PubMed] [Google Scholar]
- 149.Calderon-Salinas JV, Quintanar-Escorcia MA, Gonzalez-Martinez MT, Hernandez-Luna CE. Hum Exp Toxicol. 1999;18:327–332. doi: 10.1191/096032799678840138. [DOI] [PubMed] [Google Scholar]
- 150.Simons TJ. J Physiol. 1988;405:105–113. doi: 10.1113/jphysiol.1988.sp017323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Al-Modhefer AJ, Bradbury MW, Simons TJ. Clin Sci (Lond) 1991;81:823–829. doi: 10.1042/cs0810823. [DOI] [PubMed] [Google Scholar]
- 152.Gu C, Chen S, Xu X, Zheng L, Li Y, Wu K, Liu J, Qi Z, Han D, Chen G, Huo X. Neurochem Res. 2009;34:1150–1156. doi: 10.1007/s11064-008-9891-6. [DOI] [PubMed] [Google Scholar]
- 153.Wang Q, Luo W, Zhang W, Liu M, Song H, Chen J. Toxicol In Vitro. 2011;25:991–998. doi: 10.1016/j.tiv.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 154.Tomsig JL, Suszkiw JB. Biochim Biophys Acta. 1991;1069:197–200. doi: 10.1016/0005-2736(91)90124-q. [DOI] [PubMed] [Google Scholar]
- 155.Kerper LE, Hinkle PM. Toxicol Appl Pharmacol. 1997;146:127–133. doi: 10.1006/taap.1997.8234. [DOI] [PubMed] [Google Scholar]
- 156.Kerper LE, Hinkle PM. J Biol Chem. 1997;272:8346–8352. doi: 10.1074/jbc.272.13.8346. [DOI] [PubMed] [Google Scholar]
- 157.Mazzolini M, Traverso S, Marchetti C. J Neurochem. 2001;79:407–416. doi: 10.1046/j.1471-4159.2001.00557.x. [DOI] [PubMed] [Google Scholar]
- 158.Habermann E, Crowell K, Janicki P. Arch Toxicol. 1983;54:61–70. doi: 10.1007/BF00277816. [DOI] [PubMed] [Google Scholar]
- 159.Qian Y, Mikeska G, Harris ED, Bratton GR, Tiffany-Castiglioni E. Toxicol Appl Pharmacol. 1999;158:41–49. doi: 10.1006/taap.1999.8657. [DOI] [PubMed] [Google Scholar]
- 160.Qian Y, Zheng Y, Ramos KS, Tiffany-Castiglioni E. Neurochem Res. 2005;30:429–438. doi: 10.1007/s11064-005-2677-1. [DOI] [PubMed] [Google Scholar]
- 161.Goering PL. Neurotoxicology. 1993;14:45–60. [PubMed] [Google Scholar]
- 162.Aschner M, Onishchenko N, Ceccatelli S. Met Ions Life Sci. 2010;7:403–434. doi: 10.1039/BK9781847551771-00403. [DOI] [PubMed] [Google Scholar]
- 163.Clarkson TW, Magos L. Crit Rev Toxicol. 2006;36:609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- 164.Farina M, Rocha JB, Aschner M. Life Sci. 2011;89:555–563. doi: 10.1016/j.lfs.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Clarkson TW, Magos L, Myers GJ. N Engl J Med. 2003;349:1731–1737. doi: 10.1056/NEJMra022471. [DOI] [PubMed] [Google Scholar]