Abstract
Burkholderia pseudomallei is an intracellular Gram-negative bacterial pathogen and the causative agent of melioidosis, a widespread disease in Southeast Asia. Reactive nitrogen, in an intermediate form of nitric oxide (NO), is one of the first lines of defense used by host cells to eliminate intracellular pathogens, through the stimulation of inducible nitric oxide synthase (iNOS). Studies in phagocytotic cells have shown that the iNOS response is muted in B. pseudomallei infection, and implicated the rpoS sigma factor as a key regulatory factor mediating suppression. The liver is a main visceral organ affected by B. pseudomallei, and there is little knowledge about the interaction of liver cells and B. pseudomallei. This study investigated the induction of iNOS, as well as autophagic flux and light-chain 3 (LC3) localization in human liver (HC04) cells in response to infection with B. pseudomallei and its rpoS deficient mutant. Results showed that the rpoS mutant was unable to suppress iNOS induction and that the mutant showed less induction of autophagy and lower co-localization with LC3, and this was coupled with a lower intracellular growth rate. Combining these results suggest that B. pseudomallei rpoS is an important factor in establishing infection in liver cells.
Keywords: autophagy, Burkholderia pseudomallei, hepatocyte, inducible nitric oxide synthase (iNOS), melioidosis, RpoS
This study investigated the induction of nitric oxide synthase (iNOS) in human hepatocyte cells (HC-04) infected with wild-type Burkholderia pseudomallei or an rpoS mutant. RpoS was implicated in the ability to suppress iNOS induction and with the induction of autophagy, indicating that it plays an important role in B. pseudomallei interactions with liver cells.
INTRODUCTION
The intracellular Gram-negative bacterium Burkholderia pseudomallei is the causative agent of melioidosis (Woods et al.1999; White 2003; Cheng and Currie 2005), a tropical disease that is present throughout Southeast Asia (Woods et al.1999; White 2003; Cheng and Currie 2005). Clinical symptoms of infection are commonly characterized by pneumonia and multiple abscesses, which are mostly found at bacteria dissemination sites such as the lungs, liver and spleen (Cheng and Currie 2005), with the liver being implicated as a primary target for B. pseudomallei (Ben et al.2004; Lee et al.2006; Pal et al.2014). The liver is a critical organ that plays an important role in maintaining homeostasis (Parker and Picut 2005) and is comprised of several cell types including hepatocytes, which can generate and secrete inflammatory cytokines and chemokines in response to bacterial infections (Parker and Picut 2005).
The rpoS gene of B. pseudomallei encodes for the important transcription factor, sigma S, which plays a role in transcriptional regulation and which responds during stress conditions (Lange and Hengge-Aronis 1991; Hengge-Aronis et al.1993; Hengge-Aronis 2000; Osiriphun et al.2009). Notably, it has been reported that the RpoS of B. pseudomallei regulates many factors involved in pathogenesis such as multinucleated giant cell formation and the induction of apoptosis in the RAW264.7 murine macrophage cell line (Utaisincharoen et al.2006; Lengwehasatit et al.2008).
As previously reported, B. pseudomallei can interfere with the induction of nitric oxide synthase (iNOS) expression, which is involved in the elimination of intracellular bacteria via the reactive nitrogen intermediate nitric oxide (NO) in the RAW264.7 murine macrophage cell line (Utaisincharoen et al.2001, 2003). However, a recent publication by Bast et al. (2011) showed that iNOS-derived NO might not be the main mechanism that eliminates the bacteria in human hepatocytes. Besides iNOS-derived NO production, the induction of autophagy has been associated with the elimination of intracellular pathogens (Campoy and Colombo 2009; Gomes and Dikic 2014).
Autophagy is the catabolic pathway which cells use to degrade unnecessary or dysfunctional cellular components through the action of lysosomes (Mizushima 2007; Glick, Barth and Macleod 2010). Moreover, it also acts as an antimicrobial response found in many cell types (Campoy and Colombo 2009; Gomes and Dikic 2014). Autophagy has been reported as a mechanism that is used to eliminate several bacteria such as Mycobacterium tuberculosis and Group A Streptococcus (Campoy and Colombo 2009; Mostowy and Cossart 2012). Recently, several studies have reported that the LC3 (light-chain 3)-associated phagosome helps in the elimination of B. pseudomallei from infected mouse macrophages (Gong et al.2011; Randow, MacMicking and James 2013; Romao and Münz 2014). However, any relationship between iNOS expression and the stimulation of autophagy in host cells infected with B. pseudomallei has not been shown.
In this study, HC04 cells were used to determine any relationship between iNOS expression and the induction of autophagy as a consequence of infection with B. pseudomallei PP844, a wild-type strain, and an rpoS-deficient mutant strain. Evidence was obtained at both the transcriptional and the translational levels, and showed an inverse correlation between expression of iNOS and the induction of autophagy in B. pseudomallei-infected HC04 cells.
MATERIALS AND METHODS
Cells and culture conditions
The human hepatocyte cell line HC04, which was established and characterized by Sattabongkot et al. (2006), was maintained in equal volumes of DMEM and Ham's F-12 media (Gibco, ThermoFisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco) and incubated in a 37°C-humidified incubator in a 5% CO2 atmosphere.
Bacterial strains
Burkholderia pseudomallei PP844 was originally isolated from a melioidosis patient at Srinagarind Hospital, Khon Kaen, Thailand (Wuthiekanun et al.1996; Anuntagool et al.1998). The B. pseudomallei rpoS mutant strain used in this study was originally constructed and verified by Subsin et al. (2003). Escherichia coli B was purchased from Coli Genetic Stock Center (CGSC, Yale University, CT, USA).
Infection
The HC04 cell line was infected at an interested multiplicity of infection (MOI) following a previously established standard methodology (Utaisincharoen et al.2001). Briefly, a total of 5 × 105 cells were seeded into wells of a 6-well plate and incubated overnight under standard conditions, after which cells were counted before co-culture with bacteria at MOI 10 or 100 and incubation for the times specified. Mock infections (no bacteria) were undertaken in parallel.
Evaluation of intracellular survival of Burkholderia pseudomallei
Burkholderia pseudomallei-infected HC04 cells were pelleted by centrifugation at 130 × g and lysed with 0.1% TritonX-100 (Sigma-Aldrich, St. Louis, MO, USA) in PBS pH 7.4. Ten-fold of serial dilution were undertaken and the number of bacteria was determined as colony forming units at each time point post infection using the drop plate method. The colony forming unit (CFU) calculations were determined using the standard method (Herigstad, Hamilton and Heersink 2001).
Immunofluorescence analysis
Burkholderia pseudomallei-infected and mock-infected HC04 cells were incubated with 4% paraformaldehyde for 10 min and permeabilized with 0.3% TritonX-100 in 1× PBS pH 7.4. After washing with 1× PBS pH 7.4, the cells were incubated with a 1:1000 dilution of a rabbit anti-NOS2 polyclonal antibody (sc651; Santa Cruz Biotechnology, Inc. Dallas, TX, USA) or a 1:500 dilution of a rabbit anti-LC3 polyclonal antibody (2775S; Cell Signaling Technology, Danvers, MA, USA) as appropriate for 1 h. Slides were subsequently incubated with a 1:500 dilution of a goat anti-rabbit IgG, conjugated with Alexa fluor®488 (A11008; Invitrogen, ThermoFisher Scientific, Waltham, MA, USA) and were additionally stained with DAPI (4′,6-diamidino-2-phenylindole) (D1306; Invitrogen, ThermoFisher Scientific) to detect bacteria under an Olympus FV10i confocal microscope.
RNA extraction and gene expression
Total RNA was extracted from B. pseudomallei-infected and mock-infected HC04 cells using TRIzol® reagent (Invitrogen, ThermoFisher Scientific) and was subsequently treated with DNaseI (Promega, Madison, WI, USA). The cDNA was synthesized using gene-specific reverse primers and Moloney Murine Leukemia Virus (M-MLV) reverse transcriptase (Promega). The sequences of iNOS primers are (forward) 5′-ATGCCAGATGGCAGCATCAGA-3′ and (reverse) 5′-ATCTCCTCCAGGATGTTGTA-3′. The sequences of β-actin primer are (forward) 5′-CTCTTCCAGCCTTCCTTCCT-3′ and (reverse) 5′-AGCACTGTGTTGGCGTACAG-3′. The total cDNA of each sample were used to quantitate the levels of gene expression using KAPA SYBR FAST qPCR Kit (Kapabiosystems Co.). The expression of iNOS and β-actin was analyzed using the qPCR_Mx3000P software (Agilent Technologies, CA, USA).
Autophagic flux analysis
HC04 cells were either not treated or treated with 125 nM bafilomycin A1 for 2 h before mock infection or infection with B. pseudomallei at MOI 10. Cells and bacteria were co-cultured under standard conditions for the times indicated after which cells were washed with 1× PBS pH 7.4, and total proteins extracted. The expression levels of LC3-II and Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) were determined by western blotting using a rabbit anti-LC3 polyclonal antibody (2775S; Cell Signaling Technology) and a mouse anti-GAPDH monoclonal antibody (611463; BD Pharmacia, San Jose, CA, USA) followed by a Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG antibody (sc2004; Santa Cruz Biotechnology, Inc.) and a HRP-conjugated goat anti-mouse IgG antibody (sc2005; Santa Cruz Biotechnology, Inc.). Signal was observed using Luminata Forte Western HRP substrate (WBLUF0100; Merck Millipore, Darmstadt, Germany). Band intensities were quantitated using the ImageJ software.
Statistical analysis
Graphs, means and standard deviations were calculated using SigmaPlot 11.0 (Informer Technologies, Inc.). Statistical analyses were determined by one-way Analysis of Variance (ANOVA) using SigmaPlot 11.0 (Informer Technologies, Inc.). A P-value ≤0.05 was considered statistically significant.
RESULTS
The intracellular survival of Burkholderia pseudomallei in HC04 cells
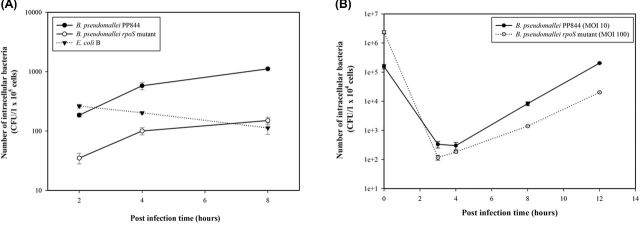
While it has been established that Burkholderia pseudomallei PP844 and its rpoS mutant do not show differences in growth rate when grown in Luria-Bertani (LB) medium (Subsin et al.2003), we initially sought to determine whether there were differences in intracellular survival in liver cells. HC04 cells were therefore infected with either B. pseudomallei PP844, its rpoS mutant or Escherichia coli B and at selected times post infection the intracellular bacterial numbers determined by the drop plate method with results expressed in terms of CFUs of the intracellular bacteria (Herigstad, Hamilton and Heersink 2001). Results showed that B. pseudomallei PP844 produced significantly higher levels of intracellular bacteria than the rpoS mutant strain at all time points examined (Fig. 1A), suggesting that RpoS plays a role in the invasion and/or intracellular survival of B. pseudomallei.
Figure 1.
The survivability of B. pseudomallei PP844 and its rpoS mutant in HC04 cell. (A) The intracellular replication of B. pseudomallei PP844, B. pseudomallei rpoS mutant and E. coli B in HC04 cells starting with the same MOI 10 at 2, 4 and 8 h.p.i. The number of intracellular bacteria was determined using the drop plate technique and calculated by using a CFU method. Data are Means ± SEMs from experiments carried out in duplicate. (B) The intracellular replication of B. pseudomallei PP844 (MOI 10) and B. pseudomallei rpoS mutant (MOI100) in HC04 cells. The number of intracellular bacteria was determined using the drop plate technique and calculated by using a CFU method. Data are Means ± SEMs from experiments carried out in duplicate.
To confidently conclude whether the rpoS gene influence on the intracellular survival of B. pseudomallei inside the HC04 cells, the increasing MOI of the rpoS-deficient B. pseudomallei was performed at MOI 100 to properly assess the ability of bacteria replication. Results showed that B. pseudomallei rpoS mutant still exhibited lower level of intracellular replication than its parental strain (Fig. 1B) (Table 1), which indicated that rpoS influence on the intracellular survivability of B. pseudomallei both invasion and replication inside the cell.
Table 1.
The representative of data from Fig. 1B.
| B. pseudomallei strains | |||
|---|---|---|---|
| Post infection time (hours) | PP844 (wild type) (CFU) | rpoS mutant (CFU) | P-value |
| 0 | 156 666 ± 26 870 | 2366 667 ± 268 701 | ND |
| 3 | 333 ± 85 | 117 ± 21 | * |
| 4 | 300 ± 85 | 183 ± 17 | * |
| 8 | 8333 ± 1273 | 1400 ± 85 | 0.019 |
| 12 | 203 333 ± 8485 | 20 167 ± 778 | <0.001 |
ND means not determined.
Asterisk (*) means there is no significant different.
Expression of iNOS in HC04 cells after infection with Burkholderia pseudomallei
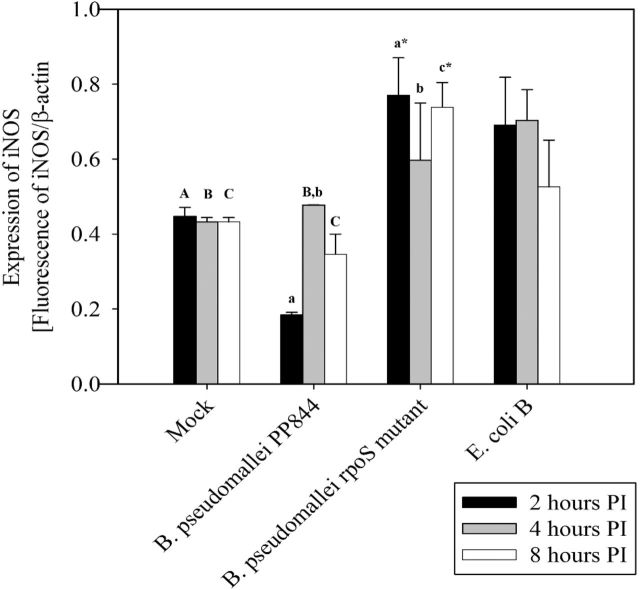
To investigate the expression of iNOS in response to infection, HC04 cells were mock infected or infected with B. pseudomallei PP844 and its rpoS mutant under standard conditions and at appropriate time points, cells were examined under a confocal microscope after staining with appropriate antibodies. Results showed the clear induction of iNOS starting from 4 h post infection (h.p.i.) in response to B. pseudomallei infection of the rpoS mutant, but not by the parental PP844 strain (Fig. 2A and B).
Figure 2.
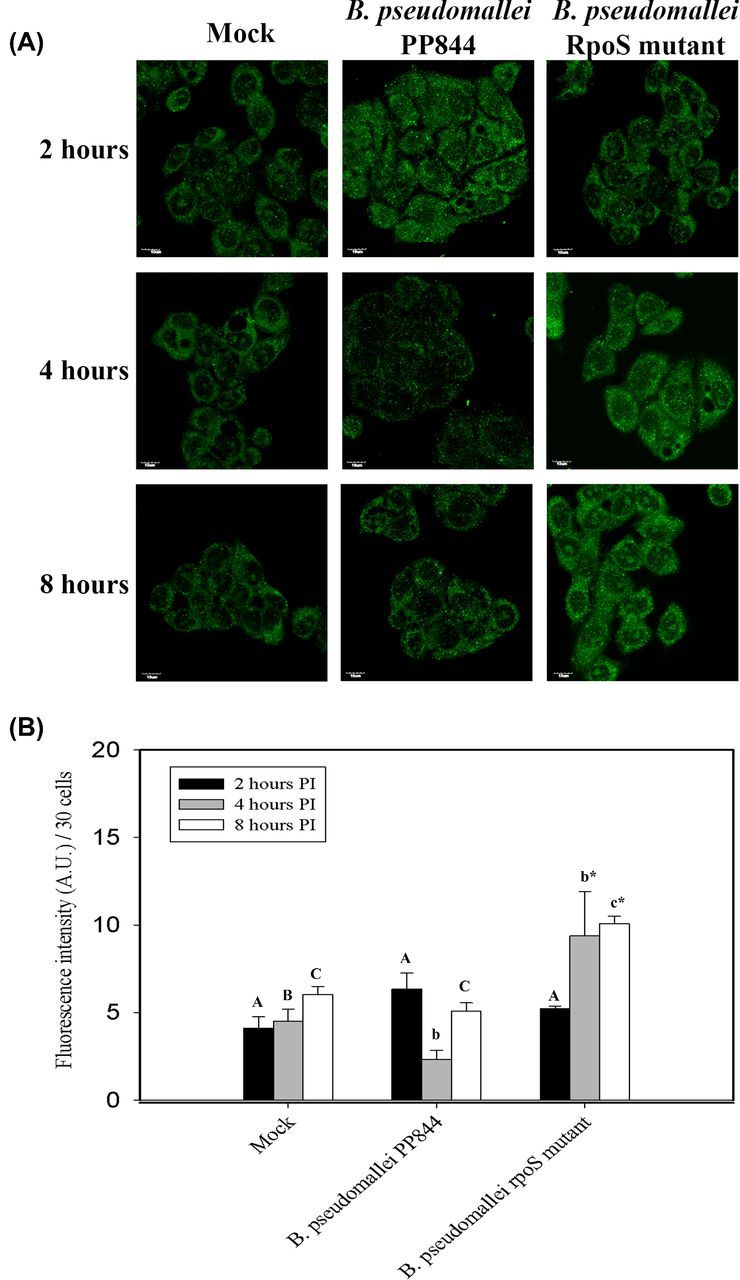
Expression of iNOS in response to B. pseudomallei infection of HC04 cells. (A) Representative fluorescence microscope images of human liver HC04 cells mock infected or infected with B. pseudomallei or its rpoS mutant at MOI 10. Images were captured at 2, 4 and 8 h.p.i. The scale bar in each panel is 10 μm. (B) Measurement of fluorescence intensity was undertaken using ImageJ software. Statistical analyses were determined using SigmaPlot 11.0 one-way ANOVA. Lower case letters indicate a significant difference from the corresponding upper case letters (P-value <0.05). Lower case letters with a star indicate that rpoS mutant was statistically different from wild type.
To confirm this result, levels of iNOS mRNA expression were investigated using quantitative RT-PCR (qRT-PCR). HC04 cells were therefore mock infected or infected with B. pseudomallei PP844 and its rpoS mutant under standard conditions, in parallel with infection with E. coli B which is known to induce iNOS expression (Titheradge 1997) as a positive control. Results showed that iNOS mRNA expression was significantly higher in cells infected with the rpoS mutant at all time points examined compared to mock, and higher at 2 and 8 h.p.i compared to the parental PP844 strain. At 4 h.p.i, expression of iNOS in response to rpoS mutant infection was increased as compared to the parental strain, but this did not reach statistical significance. Levels of iNOS expression in the rpoS mutant infection were not statistically different from the levels of iNOS expression seen in response to infection with E. coli B (Fig. 3). Interestingly, a significant inhibition of iNOS expression was seen at 2 h.p.i after infection by the parental B. pseudomallei PP844 strain (Fig. 3). Importantly, the results were consistent with the results of the immunofluorescence assay, although increased expression of the iNOS message was seen somewhat earlier than the concomitant increase in protein expression in B. pseudomallei rpoS mutant infection. However, these results are consistent with previous reports that RpoS is involved in inhibition of iNOS expression in response to infection by B. pseudomallei (Utaisincharoen et al.2006).
Figure 3.
Quantification of iNOS expression. Human liver HC04 cells were mock infected or infected with B. pseudomallei or its rpoS mutant or E. coli B at MOI 10 and at 2, 4 and 8 h.p.i. RNA was extracted and expression of iNOS determined by qRT-PCR. The level of iNOS expression was normalized against the expression of β-actin. Statistical analyses were undertaken using SigmaPlot 11.0 one-way ANOVA. Lower case letters indicate a significant difference from the corresponding upper case letters (P-value <0.05). Lower case letters with a star indicate that the rpoS mutant was statistically different from wild type.
Autophagic flux analysis
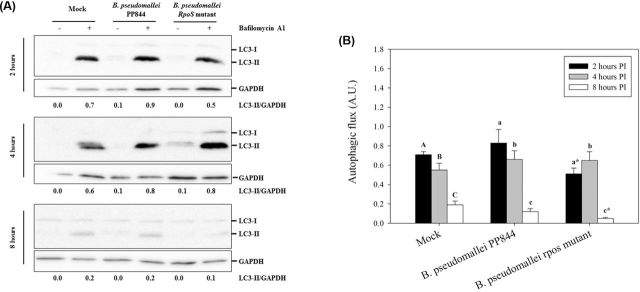
To determine whether the level of iNOS expression might be related to the induction of autophagy, the expression of the microtubule-associated protein light-chain 3 (LC3), a key autophagy-related protein (Tanida, Ueno and Kominami 2004), was determined and used to calculate the autophagic flux. After infecting HC04 cells in the presence or absence of bafilomycin A1 to inhibit fusion between autophagosomes and lysosomes (Yamamoto et al.1998), total proteins were collected at 2, 4 and 8 h.p.i and the levels of LC3-II determined by western blotting. The expression of GAPDH was additionally determined as an internal control to normalize the LC3 expression.
Results (Fig. 4) indicated that at 2 h.p.i. B. pseudomallei PP844 showed increased autophagic flux as compared to the rpoS mutant, while at 4 and 8 h.p.i. the autophagic flux in B. pseudomallei PP844-infected cells was slightly higher than in cells infected by the rpoS mutant. A comparison with the results for iNOS expression suggests that the suppression of iNOS might be associated with the induction of autophagy in the host cell.
Figure 4.
Assessment of autophagic flux in HC04 cells. (A) Human liver HC04 cells were mock infected or infected with B. pseudomallei or its rpoS mutant at MOI 10 in the presence or absence of bafilomycin A1 and at 2, 4 and 8 h.p.i. expression of LC3-I (14kDa) and II (16kDa) determined by western blotting. GAPDH were used as a loading control for each condition. The intensity of LC3-II and GAPDH were determined using ImageJ software and the numbers below the blots show the ratio between LC3-II and GAPDH. (B) The plot of autophagic flux in HC04 cells as determined from A. Statistical analyses were undertaken using SigmaPlot 11.0 one-way ANOVA. Lower case letters indicate a significant difference from the corresponding upper case letters (P-value <0.05). Lower case letters with a star indicate that the rpoS mutant was statistically different from wild type.
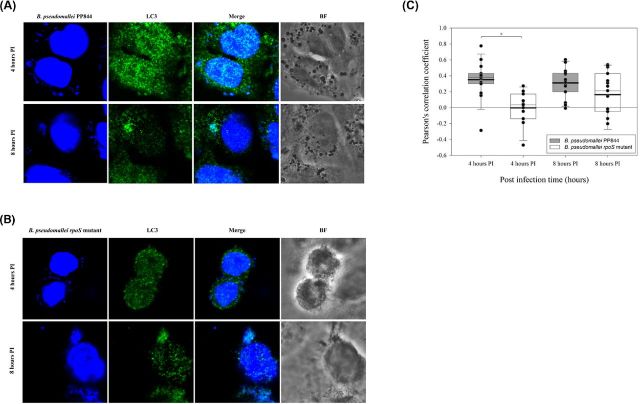
Co-localization of LC3 and Burkholderia pseudomallei
The previous results showed decreased autophagic flux in the rpoS mutant as compared to B. pseudomallei PP844. To further investigate this, the expression of LC3 was examined by confocal microscopy, and cells were counterstained with DAPI to allow visualization of the nucleus and the bacterial genome. Results (Fig. 5) showed a markedly high level of expression of LC3 in B. pseudomallei PP844 as compared to the rpoS mutant, and moreover analysis of co-localization between LC3 and the bacterial genome at 4 h.p.i. showed a significantly higher co-localization between B. pseudomallei PP844 and LC3 than between the rpoS mutant and LC3 (Fig. 5). By 8 h.p.i., the co-localization between the bacterial genome and LC3 was still higher for B. pseudomallei PP844 than for the rpoS mutant, although this was no longer statistically significant.
Figure 5.
Co-localization of B. pseudomallei and LC3 in HC04 cells. Human liver HC04 cells were mock infected or infected with (A) B. pseudomallei or (B) its rpoS mutant at MOI 10 and at 4 and 8 h.p.i. examined for LC3 expression using an Alexa Fluor 488 antibody (green) and the presence of bacteria through DAPI (blue) staining and examination under a fluorescent microscope. (C) Pearson's correlation coefficients (PCC) for LC3 and bacteria (extra nuclei DAPI signal) were determined using the ImageJ software with the Pearson-spearman correlation (PSC) co-localization plug-in. PCC values for each condition are shown in a box chart which shows the mean (black line) and median (gray line) values. The lower and the upper borders of the box are 5th and 95th percentiles, respectively.
DISCUSSION
Previous studies have implicated the liver as one of the visceral organs infected by Burkholderia pseudomallei, resulting in the formation of abscesses and contributing to the pathology of melioidosis (Ben et al.2004; Lee et al.2006; Pal et al.2014). However, the interaction between B. pseudomallei and liver cells remains poorly characterized. To better understand the pathophysiology of liver cells under B. pseudomallei infection, this study used the human hepatocyte HC04 cell line which has previously been shown to be an appropriate model cell line for liver cell pathogens (Sattabongkot et al.2006).
The rpoS gene encodes the alternative RNA polymerase sigma factor, and previous studies have implicated RpoS as a global regulatory factor induced in response to a variety of cellular stresses conditions (Lange and Hengge-Aronis 1991; Hengge-Aronis et al.1993; Hengge-Aronis 2000; Osiriphun et al.2009). In B. pseudomallei, it has been shown that lack of rpoS does not affect bacterial growth in LB medium (Subsin et al.2003). As shown in the result, B. pseudomallei parental strain exhibited higher invasive ability into HC04 than the rpoS-deficient strain (Fig. 1A). Moreover, the results in Fig. 1B and Table 1 show that B. pseudomallei deficient rpoS gene markedly reduced numbers of bacteria in liver cells, suggesting that rpoS plays a role in the intracellular survival of B. pseudomallei PP844 (Fig. 1B and Table 1).
The exact mechanism by which the pathogen survives the intracellular environment is still unclear. However, Escherichia coli DpsA has been studied, and it has been suggested that DpsA acts as a regulator of the cell-cycle check point during oxidative stress to reduce cell growth, providing an opportunity for repairing oxidative DNA damage (Chodavarapu et al.2008). Consistent with the role of B. pseudomallei DpsA in enhancing intracellular survival, a decreased level of dpsA expression in the rpoS-mutant strain under oxidative stress results in a reduced intracellular survival (Jangiam et al.2010; Al-Maleki et al.2014).
Host cells have a number of innate immune responses to suppress or eliminate invading pathogens, and the pathogens adapt to suppress or subvert these responses, allowing their survival and replication. Inducible nitric oxide synthase (iNOS) is one such system (Bogdan 2015) and previous studies in the mouse macrophage RAW264.7 cell line showed early suppression of iNOS by B. pseudomallei (Utaisincharoen et al.2001), a result consistent with our present study in liver cells. Markedly however, suppression of iNOS expression was limited to B. pseudomallei PP844 and the rpoS mutant showed no inhibition of iNOS expression. Indeed, robust induction of iNOS expression was observed in the rpoS mutant, consistent with the levels of induction of iNOS expression seen with the control E. coli B infection.
Autophagy is a cellular degradation pathway that delivers sequestered cytoplasmic constituents such as damaged cell organelles to lysosomes for degradation and the subsequent recycling of cellular constituents (Klionsky and Emr 2000). However, autophagy has additionally been shown to be an essential component of the host response to a number of different pathogens (Levine 2005). Previous studies in RAW264.7 cells have shown that activation of autophagy could decrease the intracellular survival of B. pseudomallei, and the secreted protein BopA was suggested to be the factor mediating B. pseudomallei escape from autophagy (Cullinane et al.2008). As shown here, there was an increase in autophagic flux in response to infection of liver cells with B. pseudomallei PP844, but not with the rpoS mutant (Fig. 4A and B).
The link between iNOS and autophagy is somewhat contradictory. In macrophages, stimulation of iNOS-derived NO by Lipopolysaccharide (LPS) leads to a triggering of autophagy via MAPK/NF-κB/iNOS signaling (Han et al.2013), whereas in Hela cells it was shown that inhibition of iNOS increased autophagic flux (Sarkar et al.2011). Our results suggest that the rpoS mutant which induces a strong induction of iNOS blocks increased autophagic flux seen with B. pseudomallei PP844.
Several studies have reported the co-localization of LC3 and B. pseudomallei containing phagosomes and it is suggested that the autophagic machinery is recruited to B. pseudomallei containing phagosomes through the action of the B. pseudomallei Bsa type III secretion system (D'Cruze et al.2011; Gong et al.2011; Rinchai et al.2015). However, as observed here, co-localization of LC3 and B. pseudomallei was significantly reduced in early infection in the rpoS mutant as compared to B. pseudomallei PP844. Interestingly, the hydrophobic affinity chromatography technique which was used to investigate the interaction between LC3 and bacterial protein found that there is some bacterial protein that interacts with LC3 and it is under the RpoS regulation (Joompa, pers. comm.). This supports our finding in Figs 4 and 5. Collectively our results show an inverse relationship between iNOS expression and autophagy, and show that rpoS is pivotal in suppressing the host cell induction of iNOS in response to B. pseudomallei in liver cells.
Acknowledgments
We would like to thank the Yoshimori laboratory at the Department of Genetics, Osaka University Graduate School of Medicine for providing the reagents and equipment used in this study. Thanks to Prof. Duncan R. Smith at the Institute of Molecular Biosciences and Center for Emerging and Neglected Infectious Disease, Mahidol University for comments about the experiment and language editing.
FUNDING
This work was supported by the [BRG5680010]. SP was supported by and . SS was supported by the [].
Conflict of interest. None declared.
REFERENCES
- Al-Maleki AR, Mariappan V, Vellasamy KM, et al. Enhanced intracellular survival and epithelial cell adherence abilities of Burkholderia pseudomallei morphotypes are dependent on differential expression of virulence-associated proteins during mid-logarithmic growth phase. J Proteomics. 2014;106:205–20. doi: 10.1016/j.jprot.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Anuntagool N, Intachote P, Wuthiekanun V, et al. Lipopolysaccharide from nonvirulent Ara+ Burkholderia pseudomallei isolates is immunologically indistinguishable from Lipopolysaccharide from virulent ara− clinical isolates. Clin Diagn Lab Immun. 1998;5:225–9. doi: 10.1128/cdli.5.2.225-229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast A, Schmidt IHE, Brauner P, et al. Defense mechanisms of hepatocytes against Burkholderia pseudomallei. Front Microbiol. 2011;2:277. doi: 10.3389/fmicb.2011.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben R-J, Tsai Y-Y, Chen J-C, et al. Non-septicemic Burkholderia pseudomallei liver abscess in a young man. J Microbiol Immunol. 2004;37:254–7. [PubMed] [Google Scholar]
- Bogdan C. Nitric oxide synthase in innate and adaptive immunity: an update. Trends Immunol. 2015;36:161–78. doi: 10.1016/j.it.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Campoy E, Colombo MI. Autophagy in intracellular bacterial infection. BBA-Mol Cell Res. 2009;1793:1465–77. doi: 10.1016/j.bbamcr.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Cheng AC, Currie BJ. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005;18:383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodavarapu S, Gomez R, Vicente M, et al. Escherichia coli Dps interacts with DnaA protein to impede initiation: a model of adaptive mutation. Mol Microbiol. 2008;67:1331–46. doi: 10.1111/j.1365-2958.2008.06127.x. [DOI] [PubMed] [Google Scholar]
- Cullinane M, Gong L, Li X, et al. Stimulation of autophagy suppresses the intracellular survival of Burkholderia pseudomallei in mammalian cell lines. Autophagy. 2008;4:744–53. doi: 10.4161/auto.6246. [DOI] [PubMed] [Google Scholar]
- D'Cruze T, Gong L, Treerat P, et al. Role for the Burkholderia pseudomallei type three secretion system cluster 1 bpscN gene in virulence. Infect Immun. 2011;79:3659–64. doi: 10.1128/IAI.01351-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes LC, Dikic I. Autophagy in antimicrobial immunity. Mol Cell. 2014;54:224–33. doi: 10.1016/j.molcel.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Gong L, Cullinane M, Treerat P, et al. The Burkholderia pseudomallei type III secretion system and BopA are required for evasion of LC3-associated phagocytosis. PLoS One. 2011;6:e17852. doi: 10.1371/journal.pone.0017852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengge-Aronis R. A role for the sigma S subunit of RNA polymerase in the regulation of bacterial virulence. Adv Exp Med Biol. 2000;485:85–93. doi: 10.1007/0-306-46840-9_11. [DOI] [PubMed] [Google Scholar]
- Hengge-Aronis R, Lange R, Henneberg N, et al. Osmotic regulation of rpoS-dependent genes in Escherichia coli. J Bacteriol. 1993;175:259–65. doi: 10.1128/jb.175.1.259-265.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herigstad B, Hamilton M, Heersink J. How to optimize the drop plate method for enumerating bacteria? J Microbiol Meth. 2001;44:121–9. doi: 10.1016/s0167-7012(00)00241-4. [DOI] [PubMed] [Google Scholar]
- Han H-E, Kim T-K, Son H-J, et al. Activation of Autophagy Pathway Suppresses the Expression of iNOS, IL6 and Cell Death of LPS-Stimulated Microglia Cells. Biomol Ther (Seoul) 2013;21:21–8. doi: 10.4062/biomolther.2012.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jangiam W, Loprasert S, Smith DR, et al. Burkholderia pseudomallei RpoS regulates OxyR and the katG-dpsA operon under conditions of oxidative stress. Microbiol Immunol. 2010;54:389–97. doi: 10.1111/j.1348-0421.2010.00230.x. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–21. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange R, Hengge-Aronis R. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol Microbiol. 1991;5:49–59. doi: 10.1111/j.1365-2958.1991.tb01825.x. [DOI] [PubMed] [Google Scholar]
- Lee Y-L, Lee SS-J, Tsai H-C, et al. Pyogenic liver abscess caused by Burkholderia pseudomallei in Taiwan. J Formos Med Assoc. 2006;105:689–93. doi: 10.1016/s0929-6646(09)60171-6. [DOI] [PubMed] [Google Scholar]
- Lengwehasatit I, Nuchtas A, Tungpradabkul S, et al. Involvement of B. pseudomallei RpoS in apoptotic cell death in mouse macrophages. Microb Pathogenesis. 2008;44:238–45. doi: 10.1016/j.micpath.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Levine B. Eating oneself and uninvited guests: autophagy-related pathways in cellular defense. Cell. 2005;120:159–62. doi: 10.1016/j.cell.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Mizushima N. Autophagy: process and function. Gene Dev. 2007;21:2861–73. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- Mostowy S, Cossart P. Bacterial autophagy: restriction or promotion of bacterial replication? Trends Cell Biol. 2012;22:283–91. doi: 10.1016/j.tcb.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Osiriphun Y, Wongtrakoongate P, Sanongkiet S, et al. Identification and characterization of RpoS regulon and RpoS-dependent promoters in Burkholderia pseudomallei. J Proteome Res. 2009;8:3118–31. doi: 10.1021/pr900066h. [DOI] [PubMed] [Google Scholar]
- Pal P, Ray S, Moulick A, et al. Liver abscess caused by Burkholderia pseudomallei in a young man: a case report and review of literature. World J Clin Cases. 2014;2:604–7. doi: 10.12998/wjcc.v2.i10.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker GA, Picut CA. Liver immunobiology. Toxicol Pathol. 2005;33:52–62. doi: 10.1080/01926230590522365. [DOI] [PubMed] [Google Scholar]
- Randow F, MacMicking JD, James LC. Cellular self-defense: how cell-autonomous immunity protects against pathogens. Science. 2013;340:701–6. doi: 10.1126/science.1233028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinchai D, Riyapa D, Buddhisa S, et al. Macroautophagy is essential for killing of intracellular Burkholderia pseudomallei in human neutrophils. Autophagy. 2015;11:748–55. doi: 10.1080/15548627.2015.1040969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romao S, Münz C. LC3-associated phagocytosis. Autophagy. 2014;10:526–8. doi: 10.4161/auto.27606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Korolchuk VI, Renna M, et al. Complex inhibitory effects of nitric oxide on autophagy. Mol Cell. 2011;43:19–32. doi: 10.1016/j.molcel.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattabongkot J, Yimamnuaychoke N, Leelaudomlipi S, et al. Establishment of a human hepatocyte line that supports invitro development of the exo-erythrocytic stages of the malaria parasites Plasmodium falciparum and P. vivax. Am J Trop Med Hyg. 2006;74:708–15. [PubMed] [Google Scholar]
- Subsin B, Thomas MS, Katzenmeier G, et al. Role of the stationary growth phase sigma factor RpoS of Burkholderia pseudomallei in response to physiological stress conditions. J Bacteriol. 2003;185:7008–14. doi: 10.1128/JB.185.23.7008-7014.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanida I, Ueno T, Kominami E. LC3 conjugation system in mammalian autophagy. Int J Biochem Cell B. 2004;36:2503–18. doi: 10.1016/j.biocel.2004.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titheradge MA. Nitric Oxide Protocols. Vol. 100. New Jersey: Humana Press; 1997. [Google Scholar]
- Utaisincharoen P, Anuntagool N, Limposuwan K, et al. Involvement of beta interferon in enhancing inducible nitric oxide synthase production and antimicrobial activity of Burkholderia pseudomallei-infected macrophages. Infect Immun. 2003;71:3053–7. doi: 10.1128/IAI.71.6.3053-3057.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utaisincharoen P, Arjcharoen S, Limposuwan K, et al. Burkholderia pseudomallei RpoS regulates multinucleated giant cell formation and inducible nitric oxide synthase expression in mouse macrophage cell line (RAW 264.7) Microb Pathogenesis. 2006;40:184–9. doi: 10.1016/j.micpath.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Utaisincharoen P, Tangthawornchaikul N, Kespichayawattana W, et al. Burkholderia pseudomallei interferes with inducible nitric oxide synthase (iNOS) production: a possible mechanism of evading macrophage killing. Microbiol Immunol. 2001;45:307–13. doi: 10.1111/j.1348-0421.2001.tb02623.x. [DOI] [PubMed] [Google Scholar]
- White NJ. Melioidosis. Lancet. 2003;361:1715–22. doi: 10.1016/s0140-6736(03)13374-0. [DOI] [PubMed] [Google Scholar]
- Woods DE, DeShazer D, Moore RA, et al. Current studies on the pathogenesis of melioidosis. Microbes Infect. 1999;1:157–62. doi: 10.1016/s1286-4579(99)80007-0. [DOI] [PubMed] [Google Scholar]
- Wuthiekanun V, Smith MD, Dance DA, et al. Biochemical characteristics of clinical and environmental isolates of Burkholderia pseudomallei. J Med Microbiol. 1996;45:408–12. doi: 10.1099/00222615-45-6-408. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Tagawa Y, Yoshimori T, et al. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct. 1998;23:33–42. doi: 10.1247/csf.23.33. [DOI] [PubMed] [Google Scholar]