Abstract
CD47 engagement by the macrophage signal regulatory protein alpha (SIRPα) inhibits phagocytic activity and protects red blood cells (RBCs) from erythrophagocytosis. The role of CD47-SIRPα in the innate immune response to Plasmodium falciparum infection is unknown. We hypothesized that disruption of SIRPα signaling may enhance macrophage uptake of malaria parasite-infected RBCs. To test this hypothesis, we examined in vivo clearance in CD47-deficient mice infected with Plasmodium berghei ANKA and in vitro phagocytosis of P. falciparum-infected RBCs by macrophages from SHP-1-deficient (Shp-1−/−) mice and NOD.NOR-Idd13.Prkdcscid (NS-Idd13) mice, as well as human macrophages, following disruption of CD47-SIRPα interactions with anti-SIRPα antibodies or recombinant SIRPα-Fc fusion protein. Compared to their wild-type counterparts, Cd47−/− mice displayed significantly lower parasitemia, decreased endothelial activation, and enhanced survival. Using macrophages from SHP-1-deficient mice or from NS-Idd13 mice, which express a SIRPα variant that does not bind human CD47, we showed that altered SIRPα signaling resulted in enhanced phagocytosis of P. falciparum-infected RBCs. Moreover, disrupting CD47-SIRPα engagement using anti-SIRPα antibodies or SIRPα-Fc fusion protein also increased phagocytosis of P. falciparum-infected RBCs. These results indicate an important role for CD47-SIRPα interactions in innate control of malaria and suggest novel targets for intervention.
INTRODUCTION
Macrophages can recognize altered cell surface ligands on aged, malignant, or infected cells, triggering their phagocytic uptake. CD47 is a glycoprotein “marker of self” that is expressed on cell membranes in humans and mice (1, 2). Decreased levels of CD47 are present on senescent and apoptotic cells and are associated with increased macrophage clearance. Conversely, high levels of CD47 expressed on cancer cells and leukemic stem cells may prevent the cells from being efficiently cleared in vivo (2, 3).
CD47 exerts its inhibitory effect on phagocytosis through its interaction with macrophage signal-regulatory protein alpha (SIRPα). SIRPα and CD47 constitute a cell-cell communication system that plays essential roles in hematopoietic and immunological regulation. Following CD47 engagement, SIRPα cytoplasmic-region immunoreceptor tyrosine-based inhibitory motifs (ITIMs) recruit Src homology-2 domain-containing protein tyrosine phosphatases SHP-1 and SHP-2, resulting in decreased macrophage uptake and reduced production of proinflammatory mediators (2, 4–7). A critical role of CD47-SIRPα signaling in negatively regulating erythrophagocytosis has been demonstrated by the rapid macrophage clearance of CD47-deficient red blood cells (RBCs) when transfused into wild-type animals (2).
CD47 has been implicated in mediating the outcome of several infectious processes. CD47-deficient mice succumb to bacterial peritonitis (8) but are less susceptible to Staphylococcus aureus-induced arthritis (9) and are protected from lipopolysaccharide (LPS)-induced acute lung injury and Escherichia coli pneumonia (10). SIRPα is polymorphic and highly expressed on hepatic Kupffer cells and splenic red pulp macrophages, cell populations important in the innate control of malaria (11, 12). A recent study of Plasmodium yoelii malaria in a nonlethal murine model reported that CD47 was an important determinant of age-specific RBC invasion and parasite burden (13). Although Plasmodium falciparum is responsible for the majority of cases of severe and cerebral malaria (CM), the role of CD47-SIRPα in falciparum malaria has not been reported. In this study, we used a combined genetic and functional approach to investigate the contribution of CD47-SIRPα interactions to innate clearance of P. falciparum-infected RBCs in vitro and in a lethal model of experimental cerebral malaria (ECM). We show that CD47 on infected RBCs and SIRPα on macrophages are important determinants of the outcome in vivo and of macrophage phagocytosis of P. falciparum-infected RBCs in vitro. A direct role for SIRPα was established by functional disruption of receptor signaling using anti-SIRPα antibodies and recombinant SIRPα-Fc fusion proteins.
MATERIALS AND METHODS
Ethics statement and Plasmodium berghei ANKA ECM infection.
The animal use protocols were reviewed and approved by the Faculty of Medicine Advisory Committee on Animal Services at the University of Toronto according to the Guide to the Care and Use of Experimental Animals of the Canadian Council on Animal Care (53).
C57BL/6 J-Ptpn6<me-v>/J (Shp1−/−), C57BL/6 129-cd47<tm1Fpl>/J (Cd47−/−), and wild-type counterpart C57BL/6J mice (6 to 9 weeks old) were obtained from Jackson Laboratories (West Grove, PA). P. berghei ANKA parasites (MR4) were cultivated by passage through C57BL/6J mice, and experimental infections were performed as described previously (14).
Reagents.
Endotoxin-free RPMI 1640 culture medium was obtained from Life Technologies (Burlington, Canada). Fetal calf serum (FCS) was obtained from Wisent (Mississauga, Canada) and was heat inactivated at 56°C for 30 min before use. Mouse anti-human CD47 antibodies (clone B6H12) with isotype control mouse IgG1, anti-CD47 monoclonal antibody (MAb) (clone miap301) with isotype control, and fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD47 antibodies (clone B6H12) with FITC-conjugated mouse IgG isotype control were from BD PharMingen, San Diego, CA. Ficoll-Paque and Percoll were obtained from Pharmacia (Peapack, NJ). Monoclonal anti-SIRPα/CD172a and anti-mouse IgG conjugated to alkaline phosphatase were from Sigma (St. Louis, MO). All other reagents were obtained from Sigma-Aldrich (Oakville, Canada).
Cytokine ELISA.
Gamma interferon (IFN-γ), tumor necrosis factor (TNF), von Willebrand factor (vWF), angiopoietin-1 (Ang-1), and soluble intracellular adhesion molecule-1 (sICAM-1) were measured by enzyme-linked immunosorbent assay (ELISA) (R&D Systems). Briefly, plates were coated with the respective cytokine capture antibodies in phosphate-buffered saline (PBS) overnight at 4°C and then blocked with 1% bovine serum albumin (BSA) in PBS for 1 h. Plasma from infected and uninfected mice was diluted 1:50, added to the plates, and incubated for 2 h at room temperature (RT). Detection antibody (1:10,000) and streptavidin-horseradish peroxidase (HRP) (1:200) were then incubated at RT, followed by substrate solution, stop solution, and reading at 450 nm.
RBC CD47 assays.
Percoll-washed RBCs were infected with P. falciparum. Uninfected and ring-stage- and mature-stage-infected RBCs were purified with sorbitol and Percoll as described previously (15). The purified cells were washed with PBS supplemented with 0.5% BSA and 0.1% sodium azide. Five million cells were incubated at 4°C with mouse anti-human CD47 (clone B6H12), as well as the isotype control mouse IgG1. Fifty million RBCs from wild-type or CD47-deficient mice were incubated with anti-CD47 MAb (clone miap301) or with an isotype control. All the antibodies were diluted 1:500 in PBS–0.1% BSA. RBCs were washed and incubated with anti-mouse IgG conjugated to alkaline phosphatase. After three washings, the RBC membranes were extracted with Tris-EDTA buffer (1 mM Tris, 5 mM EDTA, pH 8) supplemented with 1 mM phenylmethanesulfonyl fluoride (PMSF) and then solubilized in PBS–0.1% Tween 20 at 37°C. CD47 levels were analyzed with the alkaline phosphatase-conjugated substrate nitroblue tetrazolium chloride–5-bromo-4-chloro-3′-indolylphosphate p-toluidine salt (NBT-BCIP) and expressed as the optical density at 450 nm (OD450). The values of CD47 were brought to 100% parasitemia using the following calculation, as described previously (15): I = (Tot − N × n)/(1 − n), where I is the concentration of CD47 on 100% infected RBCs, Tot is the anti-CD47 bound on uninfected RBCs and infected RBCs, N is the anti-CD47 bound on uninfected RBCs, and n is the fraction of uninfected RBCs.
Flow cytometric analysis of CD47 on RBCs.
Direct fluorescent staining was measured on uninfected or ring-stage- or mature-stage-P. falciparum-infected RBCs in darkness at 4°C for 20 min with anti-human CD47-FITC (clone B6H12) or isotype control. CD47 on murine RBCs was analyzed using anti-mouse CD47-FITC (clone miap301) or isotype control diluted 1:25 (vol/vol). To detect membrane glycophorin, RBCs were stained with phycoerythrin anti-human CD235ab antibody (clone HIR2; BioLegend) for 30 min at RT. The RBCs were washed and resuspended in 500 μl 1× PBS containing 2% formaldehyde supplemented with Vibrant (Life Technologies) diluted 3:1,000 (vol/vol) to detect parasite DNA. All samples were analyzed by flow cytometry (FACSCalibur; BD), and the data were analyzed using FlowJo (Tree Star).
Phagocytosis assays with P. falciparum-infected RBCs.
Human peripheral blood mononuclear cells were isolated and purified from the peripheral blood of healthy donors as previously described (16). Resident peritoneal macrophages from Shp1 −/−, NOD.NOR-Idd13.Prkdcscid (NS-Idd13), and wild-type mice were harvested as described previously (17, 18). Macrophages used in phagocytosis assays were examined with and without prestimulation with LPS and IFN-γ (17). Briefly, seeded macrophages were incubated with 100 ng/ml IFN-γ (R&D Systems) for 24 h and 0.3 μg/ml LPS (Sigma-Aldrich) for 1 h and washed three times with RPMI 1640. P. falciparum clones ITG and 3D7 (mycoplasma free) were maintained in continuous culture, synchronized, and purified as described previously (15). Uninfected and P. falciparum-infected RBCs were incubated with 50% fresh autologous serum for 30 min at 37°C; resuspended in RPMI 1640 with 20 μg/ml anti-SIRPα antibody (Sigma-Aldrich), 10 μg/ml SIRPα-Fc (human SIRPα-Fc fusion protein) (17), or the respective isotype control antibodies at 10% hematocrit; and incubated with macrophages (adhered to glass coverslips at a target/effector ratio of 40:1 [ring stage] or 20:1 [mature stage]) at 37°C for 120 min. Phagocytosis assays were conducted as described previously (16). All experiments were performed in duplicate and repeated at least 3 times.
Statistical analysis.
Statistical significance for the survival study was assessed by a log-rank test and parasitemia and cytokine levels by two-way repeated-measures analysis of variance (ANOVA). Other comparisons were assessed by a Mann-Whitney test. Statistical analysis was performed using GraphPad (La Jolla, CA) Prism software.
RESULTS
CD47 deficiency is associated with improved survival in a preclinical model of ECM.
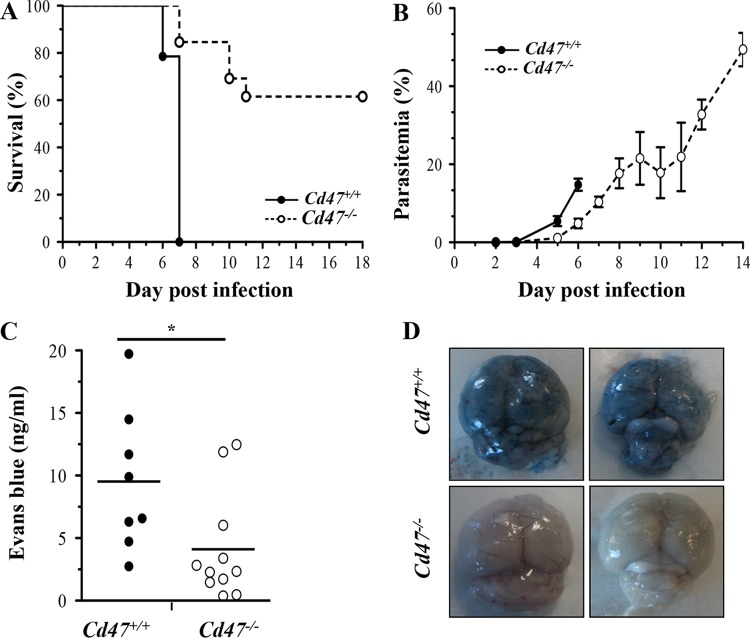
To determine whether CD47 participates in the innate control of malaria in vivo, Cd47−/− mice and their wild-type Cd47+/+ counterparts were inoculated intraperitoneally with 1 × 106 P. berghei ANKA-infected RBCs and monitored for survival and parasitemia in the ECM model (Fig. 1). Compared to Cd47+/+ mice, Cd47−/− mice developed significantly lower parasite burdens over the course of infection (Fig. 1B) and displayed improved survival (Fig. 1A). To rule out a preferential invasion of RBCs from Cd47+/+ mice, we conducted in vitro assays of P. berghei ANKA invasion of RBCs from Cd47+/+ mice and Cd47−/− mice. We did not observe significant differences in invasion in these experiments (see Fig. S2 in the supplemental material). However, in other ongoing studies, we have observed significantly impaired invasion of Cd47−/− RBCs by Plasmodium chabaudi chabaudi AS (see Fig. S3 in the supplemental material), suggesting species-specific differences in mechanisms of invasion. All Cd47+/+ mice developed ECM and succumbed to infection, whereas the majority of Cd47−/− mice survived without developing signs of ECM until the completion of the experiment. In addition, the rapid murine coma and behavior scale (RMCBS) for quantitative assessment of murine ECM (19) showed that Cd47−/− mice are less susceptible to ECM and neurological injury (see Fig. S1 in the supplemental material).
FIG 1.
Cd47−/− mice are less susceptible to P. berghei ANKA ECM. (A) Survival curves following challenge with 1 million P. berghei ANKA-infected erythrocytes by intraperitoneal (i.p.) injection for Cd47+/+ mice (solid line) versus Cd47−/− mice (dashed line). P < 0.0001 by log-rank test; n = 13 mice/group. (B) Parasite burdens as assessed by peripheral parasitemia were significantly decreased in Cd47−/− versus Cd47+/+ P. berghei ANKA-infected mice but increased progressively. P < 0.001 by two-way ANOVA; P < 0.05 by Sidak's multiple test comparing Cd47+/+ versus Cd47−/− on day 5 and day 6; n = 10 mice/group. The parasitemia and survival curves are from one representative experiment out of the three performed. The error bars represent standard errors of the mean (SEM). (C and D) An Evans blue dye extravasation assay was performed on days 6 and 7 postinfection to assess microvascular leakage into the brain parenchyma. Shown are photographs of representative brains from P. berghei ANKA-infected Cd47+/+ mice (top) and Cd47−/− mice (bottom) (D) and quantification of Evans blue dye in the brains (C) (*, P = 0.0232; Mann-Whitney test; n = 8 for Cd47+/+ mice and n = 11 for Cd47−/− mice). The points represent individual animals, and the lines are group means.
To assess whether improved survival in Cd47−/− mice was associated with preservation of the blood-brain barrier (BBB), we assessed vascular integrity at day 6 postinfection using the Evans blue dye assay (14). Compared to Cd47+/+ mice, Cd47−/− mice had significantly reduced cerebral microvascular leakage and preserved BBB function, as assessed by decreased dye extravasation into the brain parenchyma (Fig. 1C and D). Although P. berghei ANKA-infected Cd47−/− mice did not develop ECM, a proportion did succumb to anemia during the 2nd and 3rd weeks of infection.
CD47-SIRPα signaling modulates malaria-induced inflammation and endothelial activation.
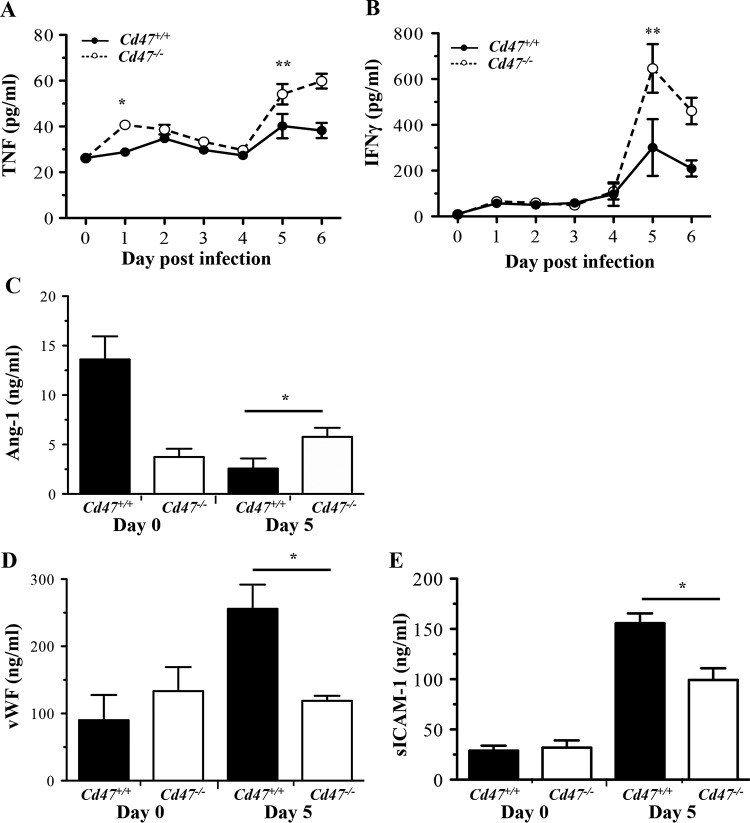
Increased proinflammatory cytokines are associated with poor clinical outcomes in both murine malaria models and human malaria parasite infection (12, 20, 21). To determine whether CD47-SIRPα interactions modify malaria-induced inflammation, we assessed levels of proinflammatory cytokines and endothelial activation biomarkers in plasma following P. berghei ANKA infection (Fig. 2). Of note, at days 5 and 6 postinfection, when Cd47+/+ mice began showing signs of ECM, Cd47−/− mice had significantly higher levels of TNF and IFN-γ in plasma (Fig. 2A and B) than their wild-type counterparts. Although high levels of proinflammatory cytokines are characteristic of cerebral malaria, production of these mediators early in the course of infection (∼24 h postinfection) is associated with subsequent protection from ECM (22). Increased levels of circulating TNF were observed as early as 24 h after infection.
FIG 2.
P. berghei ANKA infection of Cd47−/− mice is associated with increased systemic inflammation but decreased endothelial activation. The levels of inflammatory and endothelial markers in plasma were determined by ELISA in Cd47+/+ and Cd47−/− mice at day 0 (prior to infection) and following infection with P. berghei ANKA. (A and B) TNF (A) and IFN-γ (B) (*, P < 0.001; **, P < 0.0001; two-way ANOVA comparing infection and CD47 genotype with Sidak posttest comparisons; means and SEM; n ≥ 4). (C to E) Ang-1(C), vWF (D), and sICAM-1 (E) (*, P = 0.0379, P = 0.0006, and P = 0.0059, respectively; Mann-Whitney test; means and SEM for groups; n ≥ 7).
Endothelial activation is also a central feature of both human CM and murine ECM (14, 23). Ang-1 functions to maintain a quiescent endothelium, while elevated circulating levels of vWF are associated with endothelial activation and severe malaria parasite infections (24, 25). Despite higher levels of proinflammatory cytokines, P. berghei ANKA-infected Cd47−/− mice had significantly higher levels of Ang-1 in plasma than infected Cd47+/+ mice (Fig. 2C), and significantly lower levels of vWF and sICAM-1 in plasma (Fig. 2D and E) at day 5 postinfection. Collectively, these data indicate that disruption of SIRPα signaling is associated with preserved endothelial quiescence and BBB integrity (Fig. 1D) despite increased circulating levels of proinflammatory cytokines during malaria parasite infection.
CD47 levels decrease on P. falciparum-infected RBCs compared to uninfected RBCs.
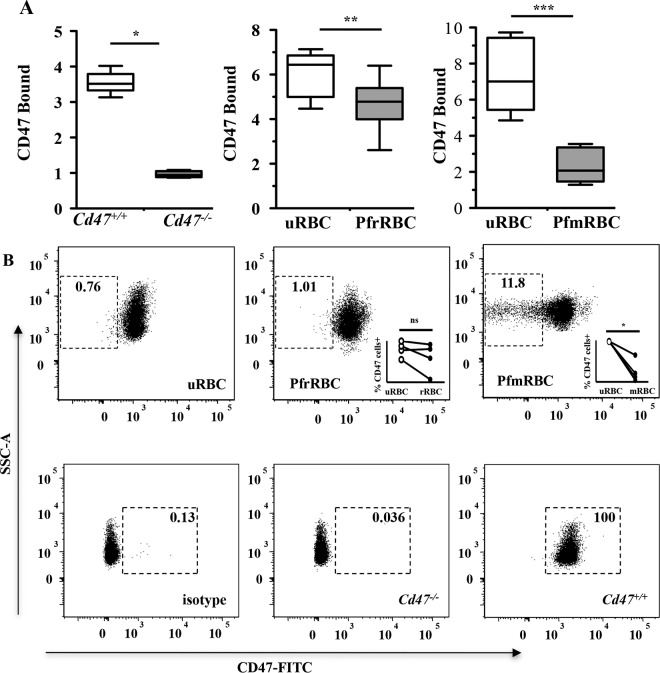
To extend our observations to human malaria, we investigated the role of CD47-SIRPα interactions in P. falciparum malaria. To determine whether CD47 expression is altered on malaria parasite-infected RBCs, we measured CD47 on human uninfected RBCs (uRBCs) and P. falciparum-infected RBCs (PfRBCs) (Fig. 3). As a control, we examined levels of CD47 on uninfected Cd47−/− and Cd47+/+ murine RBCs (Fig. 3A, left, and B, bottom). As determined by an immunoenzymatic assay (15), we observed decreased levels of CD47 on ring-stage (PfrRBCs) and mature-stage (PfmRBCs) P. falciparum-infected human RBCs compared to uninfected RBCs (Fig. 3A). Similarly, flow cytometric analysis demonstrated decreased levels of anti-CD47 bound on PfmRBCs compared to uninfected RBCs and PfrRBCs (Fig. 3B, top). CD47 levels measured with anti-CD47 antibody (clone B6H12) decreased proportionally to the degree of maturation of the parasite in the RBCs (uninfected > ring-infected > mature-infected RBCs).
FIG 3.
P. falciparum infection of RBCs alters CD47 levels. (A) An immunoenzyme assay was used to measure mouse CD47 (clone miap-301) on uninfected RBCs from Cd47+/+ and Cd47−/− mice (left) and human CD47 (clone B6H12) on uRBCs and PfrRBCs (middle) and PfmRBCs (right). The data are expressed as the ratio of anti-CD47-stained cells to isotype control-stained cells and are presented as the medians and ranges of two independent experiments with miap-301 (*, P < 0.0001, n = 10 Cd47+/+ mice versus n = 6 Cd47−/− mice; Mann-Whitney test) and of eight experiments with B6H12 (**, P = 0.022, and ***, P = 0.0001; Mann-Whitney test). (B) Flow cytometry was also used to measure the levels of CD47 on uRBCs, PfrRBCs, PfmRBCs, Cd47+/+ RBCs, and Cd47−/− RBCs. Uninfected RBCs were identified as vibrant negative, while infected RBCs (ring and mature stages) were identified as vibrant positive. Representative dot plots showing CD47 versus side scatter (SSC-A) are shown. The gates were based on isotype controls. The numbers indicate the percentages of cells within the CD47-negative gate (top) or the percentages of cells within the CD47-positive gate (bottom). The data are representative of the results of 4 experiments. Statistical comparisons were by Mann-Whitney matched-pair tests; *, P = 0.013; ns, not significant.
Disrupted CD47-SIRPα interactions enhance phagocytosis of Plasmodium falciparum-infected RBCs.
Based on the in vivo observations that parasite burdens were lower in P. berghei ANKA-infected Cd47−/− mice than in their wild-type counterparts, we examined the impact of disruption of the CD47-SIRPα pathway on macrophage uptake of PfRBCs.
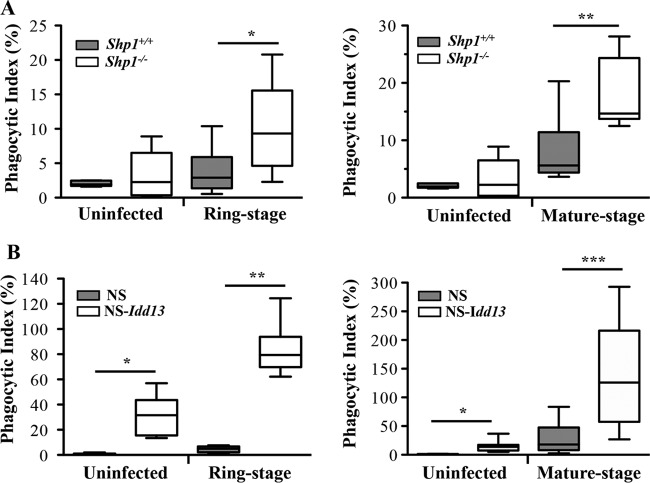
Following ligation of CD47, SIRPα recruits and activates SHP-1, an important negative regulator of the macrophage effector function (5–7, 26). We examined macrophage uptake of both PfrRBCs and PfmRBCs using macrophages from SHP-1-deficient mice (Shp1−/− [mev/mev mice]) (27, 28). Compared to wild-type control macrophages, Shp1−/− macrophages showed significantly increased phagocytosis of both PfrRBCs and PfmRBCs (Fig. 4A).
FIG 4.
Genetic disruption of CD47-SIRPα interactions increases phagocytosis of P. falciparum-infected RBCs by murine macrophages. Uninfected RBCs and P. falciparum-infected ring-stage (left) and mature-stage (right) RBCs were incubated with macrophages from mice with SHP1 deficiency (Shp1−/−) (A) and variant SIRPα mice (NS-Idd13) (white boxes) compared to their wild-type counterparts (gray boxes) (B). Differential uptake of parasitized RBCs at ring stage and mature stage by macrophages from wild-type mice versus Shp1−/− mice (A) (*, P = 0.022, and **, P < 0.0001; Mann-Whitney test) and macrophages from NS versus NS-Idd13 mice (B) (*, P = 0.005; **, P = 0.0022; ***, P = 0.0006; Mann-Whitney test) was observed. The results are the combined data from three experiments and are shown as box-and-whisker plots, representing interquartile and complete ranges, with the horizontal line in each box indicating the median level of phagocytic index.
In order to obtain evidence of a direct role for SIRPα in PfRBC uptake, we performed PfRBC phagocytic assays (Fig. 4B) with bone marrow-derived macrophages from NOD.NOR-Idd13.Prkdcscid (NS-Idd13) mice, which express a SIRPα variant that does not bind to human CD47, or NS mice as controls (29). NS-Idd13 mice were previously shown to eliminate xenotransplanted human hematopoietic stem cells via altered CD47-SIRPα interactions (29). We hypothesized that if macrophages also clear PfRBCs via a SIRPα-dependent pathway, NS-Idd13 variants would display increased phagocytotic uptake of PfRBCs compared to NS control macrophages (17, 29). Indeed, macrophages from NS-Idd13 mice showed significantly enhanced phagocytosis of both PfrRBCs and PfmRBCs compared to NS macrophages (Fig. 4B), supporting a direct role for SIRPα in mediating uptake of malaria parasite-infected RBCs.
Importantly, the phagocytic index for infected RBCs was significantly higher than that for uninfected RBCs for both Shp1−/− macrophages (Fig. 4A) and NS-Idd13 macrophages (Fig. 4B), demonstrating preferential uptake of malaria parasite-infected RBCs over uninfected, normal RBCs by macrophages upon disruption of CD47-SIRPα signaling. These data, combined with the observation of uptake when CD47 is altered (see Fig. S4 in the supplemental material), establish the relevance of CD47-SIRP signaling for the uptake of malaria parasite-infected RBCs by macrophages.
Disruption of CD47-SIRPα engagement by SIRPα-Fc and anti-human SIRPα antibody induces phagocytosis of P. falciparum-infected RBCs.
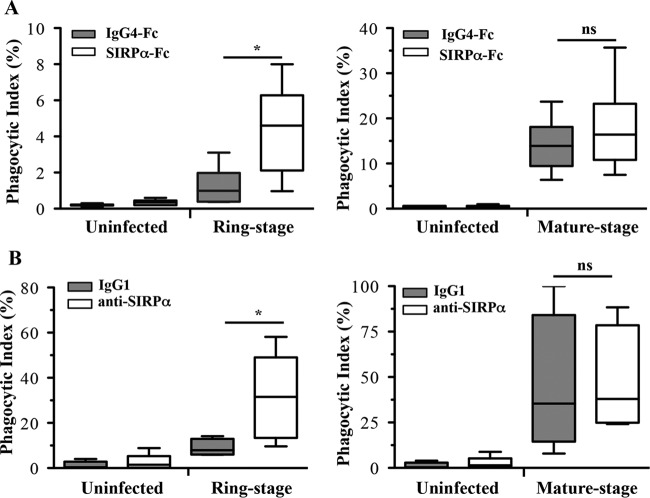
As a complementary strategy to establish a role for CD47 on PfRBCs and SIRPα on murine or human monocyte-derived macrophages (MDMs) in the uptake of infected RBCs, we examined the consequences of functional blockade of SIRPα signaling in phagocytosis assays using human SIRPα-Fc protein (SIRPα-Fc) and anti-human SIRPα antibodies (anti-SIRPα). SIRPα-Fc is a fusion protein composed of the CD47-binding IgV domain of human SIRPα fused to a human IgG4-Fc moiety and has been previously shown to induce preferential macrophage uptake of acute myeloid leukemia (AML) stem cells (17).
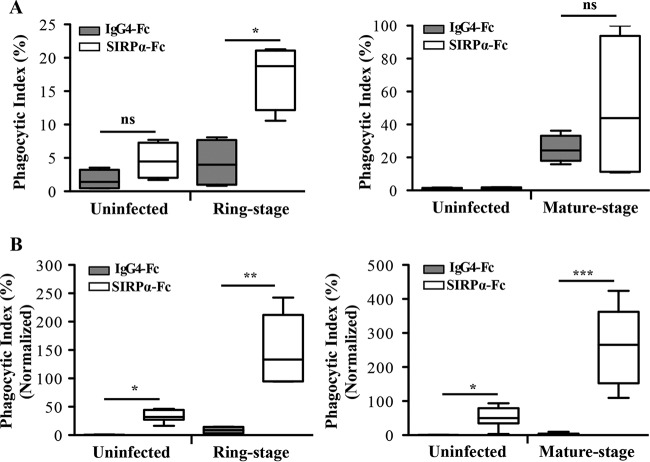
Human MDMs incubated with infected RBCs in the presence of anti-SIRPα or SIRPα-Fc displayed significantly increased uptake of ring-stage (Fig. 5), but not mature-stage, PfRBCs. When human MDMs were prestimulated with LPS and IFN-γ, uptake increased 2-fold at ring stage and mature stage (Fig. 6A); however, similar to unprimed human MDMs, only ring-stage PfRBCs were significantly different from isotype controls. In contrast, macrophages from NS mice primed with LPS and IFN-γ exhibited significantly increased uptake of both ring- and mature-stage PfRBCs in the presence of SIRPα-Fc compared to the isotype control (Fig. 6B). Notably, and in contrast to mouse MDMs, human MDMs incubated with uninfected RBCs in the presence of either SIRPα-Fc or anti-SIRPα did not show increased phagocytosis. Two P. falciparum lines (ItG and 3D7) were used in this study and displayed the same phenotype in macrophage uptake assays, indicating that our findings were not limited to a single parasite clone.
FIG 5.
Functional disruption of CD47-SIRPα interactions with human SIRPα-Fc proteins and anti-SIRPα antibodies increases phagocytosis of P. falciparum-infected RBCs by human macrophages not LPS or IFN-γ prestimulated. Shown are human SIRPα-Fc recombinant protein treatment (white boxes) or control IgG4-Fc treatment (gray boxes) (A) and anti-SIRPα antibody treatment (white boxes) or control IgG1 treatment (gray boxes) (B) of uninfected RBCs and P. falciparum-infected RBCs at ring stage (left) and mature stage (right) incubated with human monocyte-derived macrophages. Comparisons of phagocytic indexes in control IgG4-Fc- versus SIRPα-Fc-treated macrophages (A) (*, P = 0.026; Mann-Whitney test) or control IgG1- versus anti-SIRPα antibody-treated macrophages (B) (*, P = 0.019; Mann-Whitney test) for ring-stage P. falciparum-infected-RBC phagocytosis are shown. The results are the combined data from three experiments and are shown as box-and-whisker plots, representing interquartile and complete ranges, with the horizontal line in each box indicating the median level of phagocytic index. ns, not significant.
FIG 6.
Functional blockade of SIRPα signaling by human SIRPα-Fc in phagocytosis assays. LPS- and IFN-γ-prestimulated macrophages were incubated with uninfected or ring-stage- or mature-stage-P. falciparum-infected RBCs in the presence of SIRPα-Fc (white boxes) and the isotype IgG4-Fc control (gray boxes). (A) Human MDMs prestimulated with LPS and IFN-γ showed increased uptake of both ring-stage- and mature-stage-P. falciparum-infected RBCs. Comparisons of phagocytic indexes between control IgG4-Fc- versus SIRPα-Fc-treated macrophages with ring-stage-P. falciparum-infected RBCs are shown. *, P = 0.005; ns, not significant; n = 4; t test. The data are the combined results from two experiments. (B) Mouse SN macrophages showed a significant increase of both ring-stage and mature-stage parasites. The values of the phagocytic indexes were normalized to 100% infected RBCs as described previously (15) using the following calculation: I = (Tot − N × n)/(1 − n), where I is the concentration of CD47 on 100% infected RBCs, Tot is the anti-CD47 bound on uninfected RBCs and infected RBCs, N is the anti-CD47 bound on uninfected RBCs, and n is the fraction of uninfected RBCs. Comparisons for uninfected-RBC and infected-RBC uptake for ring-stage- and mature-stage-P. falciparum-infected RBCs are shown (*, P = 0.005; **, P = 0.0022; ***, P < 0.0001). The data were analyzed by the Mann-Whitney test and are the combined results of at least three experiments. The results are shown as box-and-whisker plots, representing interquartile and complete ranges, with the horizontal line in each box indicating the median level of phagocytic index.
Thus, as shown using genetic approaches, functional disruption of SIRPα signaling by SIRPα-Fc fusion protein or anti-SIRPα antibodies resulted in significant and preferential phagocytosis of malaria parasite-infected RBCs over uninfected RBCs.
DISCUSSION
This study provides direct evidence for the involvement of the CD47-SIRPα pathway in the host innate immune response to falciparum malaria. We show that CD47 deficiency is associated with decreased parasite burdens and improved survival in a preclinical model of experimental cerebral malaria. Despite increased levels of proinflammatory mediators, endothelial integrity was preserved in Cd47−/− infected mice, as indicated by higher circulating levels of Ang-1, lower levels of vWF and sICAM-1, and enhanced BBB integrity. Experiments using P. falciparum-infected RBCs suggest that the mechanism of protection is via enhanced macrophage phagocytosis of both ring-stage and mature-stage malaria parasite-infected RBCs. P. falciparum infection of RBCs was associated with altered CD47 expression, and evidence for a direct role for SIRPα in regulating macrophage phagocytosis was demonstrated by disrupting SIRPα signaling using both genetic (e.g., NS-Idd13) and functional (e.g., SIRPα-Fc) strategies.
Contrary to our data, Hempel et al. (30) recently showed no alteration of infected-RBC CD47 expression. In this study, Albumax (Life Technologies, Paisley, United Kingdom) was used to grow the parasites. Compared to the use of human serum, parasites grown with Albumax display altered expression of over 500 genes (31). For example, the cytoadhesion of infected RBCs to various endothelial receptors is greatly reduced in the presence of Albumax compared to human serum. Therefore, the results obtained with Albumax use may not represent the actual state of CD47 expression on infected RBCs.
As part of homeostasis, senescent and modified RBCs are recognized as “altered self” and removed by phagocytes of the reticuloendothelial system. Several molecules on the RBC membrane may be modified during aging, including band 3 and phosphatidylserine (32–35), targeting RBCs for macrophage uptake. In addition, CD47 on RBCs also interacts with SIRPα on macrophages to regulate erythrophagocytosis. Alterations in CD47 or SIRPα abundance, structure, or function may disrupt signaling through SIRPα, resulting in enhanced macrophage clearance of RBCs (2, 36). Previous studies have linked modification of CD47 on senescent RBCs to increased phagocytic uptake (13, 37–39). P. falciparum infection exerts substantial oxidative stress on host RBCs, resulting in membrane alterations similar to those observed on senescent RBCs (40, 41). Moreover, malaria parasite infection induces RBC rigidity that has recently been implicated in modifying CD47-SIRPα interactions (42). Consequently, we hypothesized that P. falciparum infection may result in an alteration in the levels or conformation of CD47 and its interaction with macrophage SIRPα, resulting in preferential innate clearance of malaria parasite-infected RBCs.
We initially investigated this hypothesis in vivo in the ECM model using CD47-deficient mice. Cd47−/− mice are viable and have normal RBC parameters (8). However, when Cd47−/− RBCs are transfused into Cd47+/+ mice, they are rapidly cleared by splenic macrophages, whereas Cd47+/+ RBCs transfused into Cd47−/− mice circulate normally (2). In our experimental system, challenge of Cd47+/+ mice with P. berghei ANKA resulted in high parasite burdens and 100% lethality due to ECM. In contrast, Cd47−/− mice displayed progressively increased parasitemia, and the majority survived without developing ECM (neurological manifestations between days 5 and 10 postinfection), consistent with our hypothesis that impaired CD47-SIRPα signaling would result in enhanced phagocytosis of malaria parasite-infected RBCs.
Endothelial activation and loss of vascular integrity play important mechanistic roles in the pathophysiology of CM, and vascular leakage has been shown to directly correlate with the onset of neurological signs in ECM (43). Of interest, P. berghei ANKA-infected Cd47−/− mice were able to maintain relative endothelial quiescence despite circulating levels of proinflammatory cytokines higher than those observed in Cd47+/+ mice. While high levels of proinflammatory cytokines may contribute to severe malaria (20, 21), early proinflammatory cytokine production (as observed here) has been shown to be protective in murine malaria models (22). TNF has anti-parasitic activity, and TNF-deficient mice have increased parasite burdens (44, 45). Of note, SIRPα signaling negatively regulates cytokine production, with CD47 engagement by SIRPα attenuating production of interleukin-12 (IL-12), TNF, and IL-6 (7, 46). Collectively, these data support the hypothesis that CD47 deficiency confers a survival advantage by enhancing preferential clearance of malaria parasite-infected RBCs and by permitting early proinflammatory cytokine responses to malaria parasite infection.
Murine preclinical models, while informative in genetic studies, may have limitations with respect to translation to human disease. Moreover, CD47 may interact with multiple different ligands in vivo, including integrins, thrombospondin, and G proteins (17). Therefore, to extend these observations to human malaria and to establish a direct role for SIRPα, we utilized genetic and functional approaches with P. falciparum-infected RBCs and murine and human MDMs. CD47 binding to SIRPα results in the recruitment of SHP1 in order to induce intracellular signaling. Therefore, to investigate the role of SIRPα signaling in malaria parasite-infected-RBC uptake, we used Shp1−/− macrophages in P. falciparum-infected-RBC phagocytosis assays. Disruption of SIRPα signaling in Shp1−/− macrophages resulted in increased phagocytosis of both ring- and mature-stage-parasite-infected RBCs. Since a number of receptors utilize SHP1, we used an alternative genetic approach to directly implicate SIRPα. A number of SIRPα variants with differing capacities to bind human CD47 have been identified in mice (17, 29). We used bone marrow-derived macrophages from NS-Idd13 mice, which express a SIRPα variant that does not bind to human CD47, to specifically implicate disruption of CD47-SIRPα interactions in mediating enhanced uptake of malaria parasite-infected RBCs. Compared to NS controls, NS-Idd13 macrophages displayed increased phagocytosis of both ring- and mature-stage P. falciparum-infected RBCs. For NS-Idd13 macrophages, increased phagocytosis of infected RBCs in vitro was observed only when macrophages were activated (i.e., LPS and IFN-γ prestimulated), indicating that disruption of CD47-SIRPα alone is insufficient to enhance uptake of infected RBCs. This is in agreement with recent models of AML, where prestimulation of macrophages, through TLRs or IFN-γ receptor, is required for phagocytosis of AML target cells (17). Our data are also consistent with reports that other activating signals through Fcγ or complement receptors may also be required to induce phagocytosis when SIRPα-inhibitory signals are absent (2, 47). Overall, these data fit a model where the cellular commitment to phagocytose a target is determined by integrated signals generated from both activating receptors and inhibitory receptors, such as SIRPα (47).
To generate an independent line of evidence, we also employed functional strategies to disrupt CD47-SIRPα signaling using anti-SIRPα antibodies and a SIRPα-Fc fusion protein. SIRPα-Fc is a fusion protein designed to bind and block CD47 on target cells and to enhance their phagocytic clearance. This product is currently under evaluation as a therapeutic in AML (17). Blockade of CD47-SIRPα interactions with either of these reagents resulted in increased phagocytosis of P. falciparum-infected RBCs by activated human and murine macrophages.
Notably, treatment with SIRPα-Fc did not increase human macrophage uptake of uninfected RBCs, but rather, conferred preferential macrophage phagocytosis of infected RBCs over uninfected RBCs. These results are consistent with those observed in AML models and support the development of therapeutics that antagonize SIRPα signaling.
During P. falciparum invasion and maturation, parasite proteins are deposited on and exported to the RBC surface, inducing structural, morphological, and functional changes in RBC proteins and membranes (48, 49). We investigated whether P. falciparum RBC infection might modify CD47 on the RBC membrane. Using ELISA and flow cytometry, we showed decreased levels of anti-CD47 binding on P. falciparum-infected RBCs. Although these decreases in antibody reactivity may indicate decreased surface levels of CD47, it is also possible that they are the result of conformational changes in CD47 induced by parasite invasion and growth within the RBC. How malaria might alter CD47 levels or conformation is unknown. Recently, it has been reported that experimental aging of erythrocytes induces a conformational change in CD47 (39). However, the mechanism proposed to explain CD47 involvement in clearance of experimental RBC aging is unclear (50). Plasmodium infection may induce a form of accelerated RBC aging by the generation of reactive oxygen species that oxidize RBC hemoglobin to hemichrome. Hemichrome has a high affinity for band 3 proteins (32) that form complexes with CD47 (51). Damage, mutations, or deficiency in band 3 can alter CD47 levels and/or conformation (51). Taking these data together, we propose a model where malaria parasite infection induces oxidant stress, with the deposition of hemichromes on RBC band 3, thereby altering CD47 levels and/or conformation, resulting in disrupted SIRPα interaction and enhanced phagocytosis of infected RBCs (52). Alternatively, malaria-induced changes in RBC rigidity may also alter CD47-SIRPα interactions (42). These putative mechanisms warrant further study.
Notably, SIRPα is polymorphic and displays geographic variation without a clear mechanistic explanation for this heterogeneity. Takenaka and colleagues sequenced the SIRPα domain encoding IgV from 37 individuals of Caucasian, African, Chinese, and Japanese descent and identified 4 distinct SIRPα IgV-encoding alleles (V1 to V4), with V1 and V2 being the most common (29). These observations suggest that SIRPα variants with altered CD47 binding may have been retained or selected in areas where malaria is endemic if they confer enhanced innate clearance of infected RBCs, a hypothesis that can now be formally tested.
In summary, our data suggest that the CD47-SIRPα pathway may play a role in the innate immune response to malaria by regulating prophagocytic and proinflammatory processes essential to the control of blood-stage malaria parasite infection. Disrupting SIRPα signaling represents a potential and rational pharmacological target, and current therapeutics being developed for cancer may inform the choice of agents with potential utility as adjunctive treatments for malaria.
Supplementary Material
ACKNOWLEDGMENTS
This study was funded in part by Canadian Institutes of Health Research grants MOP-13721, MOP-115160 (K.C.K.), and MOP136813 and CIHR Canada Research Chairs (K.C.K. and W.C.L.).
K.A., Z.L., and J.M.H. performed the experiments; K.A., Z.L., L.S., and C.F. analyzed the data; K.A., L.S., C.F., and K.C.K. created figures and wrote the paper; K.A. and K.C.K. conceived and designed the research; Z.L., L.S., J.M.H., C.F., J.C.Y.W., and W.C.L. reviewed and revised the manuscript.
We declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Statement
CIHR Canada Research Chair in Molecular Parasitology to K.C.K. and Canada Research Chair in Infection and Inflammation to W. Conrad Liles.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01426-15.
REFERENCES
- 1.Lindberg FP, Gresham HD, Schwarz E, Brown EJ. 1993. Molecular cloning of integrin-associated protein: an immunoglobulin family member with multiple membrane-spanning domains implicated in alpha V beta 3 dependent ligand binding. J Cell Biol 123:485–496. doi: 10.1083/jcb.123.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD. 2000. Role of CD47 as a marker of self on red blood cells. Science 288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 3.Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL. 2009. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matozaki T, Murata Y, Okazawa H, Ohnishi H. 2009. Functions and molecular mechanisms of the CD47-SIRP alpha signalling pathway. Trends Cell Biol 19:72–80. doi: 10.1016/j.tcb.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Ishikawa-Sekigami T, Kaneko Y, Okazawa H, Tomizawa T, Okajo J, Saito Y, Okuzawa C, Sugawara-Yokoo M, Nishiyama U, Ohnishi H, Matozaki T, Nojima Y. 2006. SHPS-1 promotes the survival of circulating erythrocytes through inhibition of phagocytosis by splenic macrophages. Blood 107:341–348. doi: 10.1182/blood-2005-05-1896. [DOI] [PubMed] [Google Scholar]
- 6.Okazawa H, Motegi S, Ohyama N, Ohnishi H, Tomizawa T, Kaneko Y, Oldenborg PA, Ishikawa O, Matozaki T. 2005. Negative regulation of phagocytosis in macrophages by the CD47-SHPS-1 system. J Immunol 174:2004–2011. doi: 10.4049/jimmunol.174.4.2004. [DOI] [PubMed] [Google Scholar]
- 7.Neznanov N, Neznanova L, Kondratov RV, Burdelya L, Kandel ES, O'Rourke DM, Ullrich A, Gudkov AV. 2003. Dominant negative form of signal-regulatory protein-alpha (SIRPalpha/SHPS-1) inhibits tumor necrosis factor-mediated apoptosis by activation of NF-kappa B. J Biol Chem 2003 278:3809–3815. [DOI] [PubMed] [Google Scholar]
- 8.Lindberg FP, Bullard DC, Caver TE, Gresham HD, Beaudet AL, Brown EJ. 1996. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science 274:795–798. doi: 10.1126/science.274.5288.795. [DOI] [PubMed] [Google Scholar]
- 9.Verdrengh M, Lindberg FP, Ryden C, Tarkowski A. 1999. Integrin-associated protein (IAP)-deficient mice are less susceptible to developing Staphylococcus aureus-induced arthritis. Microbes Infect 1:745–751. doi: 10.1016/S1286-4579(99)80076-8. [DOI] [PubMed] [Google Scholar]
- 10.Su X, Johansen M, Looney MR, Brown EJ, Matthay MA. 2008. CD47 deficiency protects mice from lipopolysaccharide-induced acute lung injury and Escherichia coli pneumonia. J Immunol 180:6947–6953. doi: 10.4049/jimmunol.180.10.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujioka Y, Matozaki T, Noguchi T, Iwamatsu A, Yamao T, Takahashi N, Tsuda M, Takada T, Kasuga M. 1996. A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol Cell Biol 16:6887–6899. doi: 10.1128/MCB.16.12.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevenson MM, Riley EM. 2004. Innate immunity to malaria. Nat Rev Immunol 4:169–180. doi: 10.1038/nri1311. [DOI] [PubMed] [Google Scholar]
- 13.Banerjee R, Khandelwal S, Kozakai Y, Sahu B, Kumar S. 2015. CD47 regulates the phagocytic clearance and replication of the Plasmodium yoelii malaria parasite. Proc Natl Acad Sci U S A 112:3062–3067. doi: 10.1073/pnas.1418144112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serghides L, Kim H, Lu Z, Kain DC, Miller C, Francis RC, Liles WC, Zapol WM, Kain KC. 2011. Inhaled nitric oxide reduces endothelial activation and parasite accumulation in the brain, and enhances survival in experimental cerebral malaria. PLoS One 6:e27714. doi: 10.1371/journal.pone.0027714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ayi K, Turrini F, Piga A, Arese P. 2004. Enhanced phagocytosis of ring-parasitized mutant erythrocytes: a common mechanism that may explain protection against falciparum malaria in sickle trait and beta-thalassemia trait. Blood 104:3364–3371. doi: 10.1182/blood-2003-11-3820. [DOI] [PubMed] [Google Scholar]
- 16.McGilvray ID, Serghides L, Kapus A, Rotstein OD, Kain KC. 2000. Nonopsonic monocyte/macrophage phagocytosis of Plasmodium falciparum-parasitized erythrocytes: a role for CD36 in malarial clearance. Blood 66:3231–3240. [PubMed] [Google Scholar]
- 17.Theocharides AP, Jin L, Cheng PY, Prasolava TK, Malko AV, Ho JM, Poeppl AG, van Rooijen N, Minden MD, Danska JS, Dick JE, Wang JC. 2012. Disruption of SIRPa signalling in macrophages eliminates human acute myeloid leukemia stem cells in xenografts. J Exp Med 209:1883–1899. doi: 10.1084/jem.20120502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel SN, Serghides L, Smith TG, Silverstein RL, Kurtz TW, Pravenec M, Kain KC. 2004. CD36 mediates the phagocytosis of Plasmodium falciparum-infected erythrocytes by rodent macrophages. J Infect Dis 189:204–213. doi: 10.1086/380764. [DOI] [PubMed] [Google Scholar]
- 19.Carroll RW, Wainwright MS, Kim KY, Kidambi T, Gómez ND, Taylor T, Haldar K. 2010. A rapid murine coma and behavior scale for quantitative assessment of murine cerebral malaria. PLoS One 5:e13124. doi: 10.1371/journal.pone.0013124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day NP, Hien TT, Schollaardt T, Loc PP, Chuong LV, Chau TT, Mai NT, Phu NH, Sinh DX, White NJ, Ho M. 1999. The prognostic and pathophysiologic role of pro- and anti-inflammatory cytokines in severe malaria. J Infect Dis 180:1288–1297. doi: 10.1086/315016. [DOI] [PubMed] [Google Scholar]
- 21.Grau GE, Taylor TE, Molyneux ME, Wirima JJ, Vassalli P, Hommel M, Lambert PH. 1989. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med 320:1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell AJ, Hansen AM, Hee L, Ball HJ, Potter SM, Walker JC, Hunt NH. 2005. Early cytokine production is associated with protection from murine cerebral malaria. Infect Immun 73:5645–5653. doi: 10.1128/IAI.73.9.5645-5653.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turner GD, Morrison H, Jones M, Davis TM, Looareesuwan S, Buley ID, Gatter KC, Newbold CI, Pukritayakamee S, Nagachinta B, White NJ, Berendt AR. 1994. An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intracellular adhesion molecule-1 in cerebral sequestration. Am J Pathol 145:1057–1069. [PMC free article] [PubMed] [Google Scholar]
- 24.Lovegrove FE, Tangpukdee N, Opoka RO, Lafferty EI, Rajwans N, Hawkes M, Krudsood S, Looareesuwan S, John CC, Liles WC, Kain KC. 2009. Serum angiopoietin-1 and -2 levels discriminate cerebral malaria from uncomplicated malaria and predict clinical outcome in African children. PLoS One 4:e4912. doi: 10.1371/journal.pone.0004912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erdman LK, Dhabandia A, Musoke C, Conroy AL, Hawkes M, Higgins S, Rajwans N, Wolofsky KT, Streiner DL, Liles WC, Cserti-Gazdewich CM, Kain KC. 2011. Combinations of host biomarkers predict mortality among Uganda children with severe malaria: a retrospective case-control study. PLoS One 6:e17440. doi: 10.1371/journal.pone.0017440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veillette A, Thibaudeau E, Latour S. 1998. High expression of inhibitory receptor SHPS-1 and its association with protein-tyrosine phosphatase SHP-1 in macrophages. J Biol Chem 273:22719–22728. doi: 10.1074/jbc.273.35.22719. [DOI] [PubMed] [Google Scholar]
- 27.Shultz LD, Schweitzer PA, Rajan TV, Yi T, Ihle JN. 1993. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell 73:1445–1454. doi: 10.1016/0092-8674(93)90369-2. [DOI] [PubMed] [Google Scholar]
- 28.Kozlowski M, Mlinaric-Rascan I, Feng GS, Shen R, Pawson T, Siminovitch KA. 1993. Expression and catalytic activity of the tyrosine phosphatase PTP1C is severely impaired in motheaten and viable motheaten mice. J Exp Med 178:2157–2163. doi: 10.1084/jem.178.6.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takenaka K, Prasolava TK, Wang JC, Mortin-Toth SM, Khalouei S, Gan OI, Dick JE, Danska JS. 2007. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol 8:1313–1323. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- 30.Hempel C, Kohnke H, Maretty L, Jensen PØ, Staalsø T, Kurtzhals JA. 2014. Plasmodium falciparum avoids change in erythrocytic surface expression of phagocytosis markers during inhibition of nitric oxide synthase activity. Mol Biochem Parasitol 198:29–36. doi: 10.1016/j.molbiopara.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Tilly AK, Thiede J, Metwally N, Lubiana P, Bachmann A, Roeder T, Rockliffe N, Lorenzen S, Tannich E, Gutsmann T, Bruchhaus I. 2015. Type of in vitro cultivation influences cytoadhesion, knob structure, protein localization and transcriptome profile of Plasmodium falciparum. Sci Rep 5:16766. doi: 10.1038/srep16766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turrini F, Giribaldi G, Carta F, Mannu F, Arese P. 2003. Mechanisms of band 3 oxidation and clustering in the phagocytosis of Plasmodium falciparum-infected erythrocytes. Redox Rep 8:300–303. doi: 10.1179/135100003225002943. [DOI] [PubMed] [Google Scholar]
- 33.Bratosin D, Mazurier J, Tissier JP, Estaquier J, Huart JJ, Ameisen JC, Aminoff D, Montreuil J. 1998. Cellular and molecular mechanism of senescent erythrocyte phagocytosis by macrophages. A review. Biochimie 80:173–195. doi: 10.1016/S0300-9084(98)80024-2. [DOI] [PubMed] [Google Scholar]
- 34.Lutz HU, Fasler S, Stammler P, Bussolino F, Arese P. 1988. Naturally occurring anti-band 3 antibodies and complement in phagocytosis of oxidatively-stressed and in clearance of senescent red cells. Blood Cells 14:175–203. [PubMed] [Google Scholar]
- 35.Low PS, Waugh SM, Zinke K, Drenckhahn D. 1985. The role of hemoglobin denaturation and band 3 clustering in red blood cell aging. Science 227:531–533. doi: 10.1126/science.2578228. [DOI] [PubMed] [Google Scholar]
- 36.Olsson M, Nilsson A, Oldenborg PA. 2006. Target cell CD47 regulates macrophage activation and erythrophagocytosis. Transfus Clin Biol 13:39–43. doi: 10.1016/j.tracli.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Olsson M, Oldenborg PA. 2008. CD47 on experimentally senescent murine RBCs inhibits phagocytosis following Fcgamma receptor-mediated but not scavenger receptor-mediated recognition by macrophages. Blood 112:4259–4267. doi: 10.1182/blood-2008-03-143008. [DOI] [PubMed] [Google Scholar]
- 38.Khandelwal S, van Rooijen N, Saxena RK. 2007. Reduced expression of CD47 during murine red blood cell (RBC) senescence and its role in RBC clearance from the circulation. Transfusion 47:1725–1732. doi: 10.1111/j.1537-2995.2007.01348.x. [DOI] [PubMed] [Google Scholar]
- 39.Burger P, Hilarius-Stokman P, de Korte D, van den Berg TK, van Bruggen R. 2012. CD47 functions as a molecular switch for erythrocyte phagocytosis. Blood 119:5512–5521. doi: 10.1182/blood-2011-10-386805. [DOI] [PubMed] [Google Scholar]
- 40.Golenser J, Chevion M. 1989. Oxidative stress and malaria: host-parasite relationships in normal and abnormal erythrocytes. Semin Hematol 26:313–325. [PubMed] [Google Scholar]
- 41.Hunt NH, Stocker R. 1990. Oxidative stress and the redox status of malaria-infected erythrocytes. Blood Cells 16:499–526. [PubMed] [Google Scholar]
- 42.Sosale NG, Rouhiparkouhi T, Bradshaw AM, Dimova R, Lipowsky R, Discher DE. 2015. Cell rigidity and shape override CD47's “self” signalling in phagocytosis by hyperactivating myosin-II. Blood 125:542–552. doi: 10.1182/blood-2014-06-585299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nacer A, Movila A, Baer K, Mikolajczak SA, Kappe SH, Frevert U. 2012. Neuroimmunological blood brain barrier opening in experimental cerebral malaria. PLoS Pathog 8:e1002982. doi: 10.1371/journal.ppat.1002982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hernandez-Valladares M, Naessens J, Musoke AJ, Sekikawa K, Rihet P, Ole-Moiyoi OK, Busher P, Iraqi FA. 2006. Pathology of Tnf-deficient mice infected with Plasmodium chabaudi adami 408XZ. Exp Parasitol 114:271–278. doi: 10.1016/j.exppara.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 45.Körner H, McMorran B, Schlüter D, Fromm P. 2010. The role of TNF in parasitic diseases: still more questions than answers. Int J Parasitol 40:879–888. doi: 10.1016/j.ijpara.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 46.Demeure CE, Tanaka H, Mateo V, Rubio M, Delespesse G, Sarfati M. 2000. CD47 engagement inhibits cytokine production and maturation of human dendritic cells. J Immunol 164:2193–2199. doi: 10.4049/jimmunol.164.4.2193. [DOI] [PubMed] [Google Scholar]
- 47.Oldenborg PA, Gresham HD, Lindberg FP. 2001. CD47-signal regulatory protein α (SIRPalpha) regulates Fcgamma and complement receptor-mediated phagocytosis. J Exp Med 193:855–862. doi: 10.1084/jem.193.7.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waller KL, Stubberfield LM, Dubljevic V, Nunomura W, An X, Mason AJ. 2007. Interactions of Plasmodium falciparum erythrocyte membrane protein 3 with the red blood cell membrane skeleton. Biochim Biophys Acta 1768:2145–2156. doi: 10.1016/j.bbamem.2007.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pei X, Guo X, Coppel R, Bhattacharjee S, Haldar K. 2007. The ring-infected erythrocyte surface antigen (RESA) of Plasmodium falciparum stabilizes spectrin tetramers and suppresses further invasion. Blood 110:1036–1042. doi: 10.1182/blood-2007-02-076919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lutz HU. 2013. Comment concerning the role of CD47 and signal regulatory protein alpha in regulating the clearance of aged red blood cells. Transfus Med Hemother 40:140–141. doi: 10.1159/000350507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bruce LJ, Beckmann R, Ribeiro ML, Peters LL, Chasis JA. 2003. A band 3-based macrocomplex of integral and peripheral proteins in the RBC membrane. Blood 101:4180–4188. doi: 10.1182/blood-2002-09-2824. [DOI] [PubMed] [Google Scholar]
- 52.Lutz HU, Bogdanova A. 2013. Mechanisms tagging senescent red blood cells for clearance in healthy humans. Front Physiol 4:387. doi: 10.3389/fphys.2013.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Canadian Council on Animal Care. 1993. Guide to the care and use of experimental animals, 2nd ed, vol 1 Canadian Council on Animal Care, Ottawa, ON, Canada. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.