Abstract
Context
Thrombospondin 1 (THBS1 or TSP-1) is an adipose-derived matricellular protein, which has recently been highlighted as a potential mediator of insulin resistance and adipose inflammation in obesity.
Objective
In this study, we aimed to determine the clinical significance of THBS1 as a novel biological marker of visceral obesity, metabolic syndrome, and diabetes.
Methods
The THBS1 mRNA level was quantified with real-time PCR in human adipose tissues obtained from 16 non-obese subjects. The relationships between serum THBS1 level and obesity/diabetes traits as well as the diagnostic components of metabolic syndrome were assessed in 164 normal-weight or overweight/obese subjects (78 males and 86 females; mean age, 50.4; mean BMI, 29.8) with analysis of covariance (ANCOVA) and regression analyses.
Results
THBS1 was predominantly expressed in visceral adipose tissues relative to subcutaneous adipose tissues (P < 0.001). The visceral THBS1 expression was positively associated with the body mass index (BMI; γs = 0.54, P = 0.033). ANCOVA demonstrated that the THBS1 level is associated with abdominal obesity (P < 0.001), hyperglycemia (P = 0.02), and hypertension (P = 0.04). Multivariable regression analysis suggested an association between serum THBS1 and fasting plasma glucose levels. The associations between serum THBS1 levels and obesity/diabetes traits were found preferentially in women (BMI, γs = 0.30, P = 0.05; FPG, γs = 0.26, P = 0.016). Subanalyses demonstrated that the association with obesity traits was predominantly found in premenopausal women (BMI, γs = 0.41, P = 0.007), whereas the association with diabetes traits was predominant in postmenopausal women (HbA1c, γs = 0.38, P = 0.01). During medical weight reduction treatment, the change in the serum THBS1 level was associated with the change in BMI and HbA1c in pre- and postmenopausal women, respectively.
Conclusions
Serum THBS1 is a useful biological marker of obesity and metabolic syndrome in Japanese subjects, particularly in women. THBS1 may act as a critical circulating factor that couples obesity with metabolic syndrome and diabetes in humans.
Keywords: Thrombospondin 1, Biological marker, Obesity, Diabetes, Metabolic syndrome
1. Introduction
Thrombospondin 1 (THBS1 or TSP-1) is a multifunctional glycoprotein released from various types of cell, including platelets, macrophages, and adipocytes [1–3]. The THBS1 precursor (MW = 145 kDa) is assembled into a disulfide-linked homotrimer and secreted into the extracellular milieu [4]. THBS1 exerts its diverse biological effects through binding to extracellular matrix (ECM) proteins and cell surface receptors, thereby regulating cell–cell and cell–matrix interactions [5]. THBS1 is also known to regulate the activation of transforming growth factor-β1 (TGF-β1) [6–8]. THBS1 interacts with a cohort of target molecules through its multiple functional domains and participates in a wide range of physiological and pathological processes such as tissue remodeling, wound healing, angiogenesis, and inflammation [6].
Recent findings suggest a causal role played by adipose-derived THBS1 in the pathogenesis of insulin resistance and adipose tissue inflammation [9–11]. In a mouse model, THBS1 was highly expressed in visceral adipose tissues, and the serum THBS1 protein level increased in response to high-fat diet challenge [10]. Targeted disruption of Thbs1 in mice ameliorated diet-induced insulin resistance, adipose tissue inflammation, and muscle fibrosis [10]. In humans, adipose THBS1 expression was increased in obese and insulin-resistant individuals [3,12].
We hypothesized that circulating THBS1 may serve as a novel biological marker of metabolic syndrome and adipose tissue inflammation associated with human obesity. While the link between adipose THBS1 expression and obesity in humans has been demonstrated by others [3], the significance of serum THBS1 as a biological marker of human obesity, diabetes, and metabolic syndrome has not been fully examined to date. We determined the clinical significance of the serum THBS1 level in defining the complex phenotypes of human obesity, diabetes, and metabolic syndrome. Moreover, we assessed the fat depot-dependent expression of THBS1 in Japanese subjects, whose body composition may differ from that of Caucasians and African-Americans [13,14].
2. Methods
2.1. Human Subjects
2.1.1. THBS1 Gene Expression in Adipose Tissues
Paired samples of visceral (omental) and subcutaneous adipose tissues were obtained from 16 patients (11 males and 5 females; mean age, 69.1 years; mean body mass index [BMI], 22.8 kg/m2) who underwent abdominal surgery. Samples were frozen in liquid nitrogen immediately after resection and stored at −80 °C for RNA extraction. The study protocol was approved by the human research ethics committee of Kyoto Medical Center, and written informed consent forms were obtained from all participants.
2.1.2. Correlation Analyses of THBS1 Levels in Circulation
A total of 164 Japanese obese patients and non-obese volunteers (78 males and 86 females; mean age, 50.4 years; mean BMI, 29.8 kg/m2) were consecutively enrolled at the National Hospital Organization Kyoto Medical Center. Blood samples were collected from the antecubital vein in the morning after a 12-h fast. The study protocol was approved by the human research ethics committee of Kyoto Medical Center and all participants agreed to the study by providing signed documents of informed consent.
2.2. Quantitative Real-Time PCR
Total RNA was isolated from adipose tissue samples with the RNeasy Lipid Tissue Mini Kit (QIAGEN), and reverse transcribed to cDNA using the High-Capacity RNA-to-cDNA Kit (Life Technologies). Gene expression was quantitated using the Power SYBR Green PCR Master Mix and ABI PRISM 7000 Sequence Detection System (Applied Biosystems). The respective gene expression is shown as the relative ratio to 36B4 (Rplp0) expression, which was used as an internal reference for normalization. The sequences of the primers were as follows: 36B4, 5′-AGCCCAGAACACTGGTCTC-3′ and 5′-ACTCAGGATTTCAATGGTGCC-3′; THBS1, 5′-TCAGGACCCATCTATGATAAAACCTA-3′ and 5′-TCAGGTCAGAGAAGAACACCATTTC-3′ [3]; IL-6, 5′-AAATGCCAGCCTGCTGACGAAG-3′ and 5′-AACAACAATCTGAGGTGCCCATGCTAC-3′ [15].
2.3. Data Collection and Laboratory Assay Methods
The BMI, waist circumference (WC), systolic blood pressure (SBP), and diastolic blood pressure (DBP) were measured as described previously [16]. The fat distribution was measured with the dual bioelectrical impedance analysis (Dual-BIA) method (Omron Healthcare Corporation) [17]. Serum levels of fasting plasma glucose (FPG), hemoglobin A1c (HbA1c), immunoreactive insulin (IRI), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transpeptidase (γ-GTP), leptin, adiponectin, and high sensitivity C-reactive protein (hsCRP) were determined according to standard procedures [16]. The homeostasis model assessment of insulin resistance (HOMA-IR) was used as an index of insulin resistance [18]. The human THBS1 concentration in serum was determined with an enzyme-linked immunosorbent assay using the Quantikine Human Thrombospondin-1 Immunoassay kit (R&D Systems).
2.4. Weight Reduction Program
A total of 78 patients (34 males and 44 females) were subjected to 6-month weight reduction therapy consisting of dietary modification with reduced energy intake and increased physical activity, as previously described [16]. Briefly, along with the prescribed diet consisting of 25 kcal/kg of ideal body weight per day, patients were instructed to exercise at a moderate intensity for at least 30 min a day, 3 days/week.
2.5. Statistical Analysis
Data are presented as means ± SD or medians with interquartile ranges. Statistical significance was defined by a P value < 0.05 (two-tailed). For paired samples, the differences between two groups were evaluated with a paired t-test or Wilcoxon signed-rank test. Welch’s t-test or Mann–Whitney U test was used for comparison between unpaired groups. Multiplicity adjustment was performed by the Bonferroni method. Independent predictors of serum THBS1 levels were identified using analysis of covariance (ANCOVA) and multivariable regression analysis. The following variables were forced entered into the multivariate models: presence or absence of hypertension, hyperglycemia, hypertriglycemia, reduced HDL-C, and abdominal obesity. Spearman’s rank correlation coefficient was used to determine the relationships between the serum THBS1 level and clinical variables and the effects of weight reduction therapy. Statistical analyses were performed with SPSS ver. 22.0 for Windows (IBM Japan, Tokyo, Japan).
3. Results
3.1. THBS1 Transcripts are Predominantly Found in Visceral Adipose Tissues
Recent studies with animal models suggest the differential expression of THBS1 between the visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) [10,11]. To determine whether there is a difference in THBS1 expression between these two fat depots among Japanese subjects, we assessed THBS1 expression in paired samples of SAT and VAT obtained from 16 individuals who underwent surgical procedures for medical conditions unrelated to obesity (5 females and 11 males; mean age, 69.1; mean BMI, 22.8). The baseline characteristics of the subjects are provided in Table S1. The relative expression of THBS1 was significantly higher in VAT than SAT (P < 0.001, Fig. 1A). In this metabolically healthy population, no significant difference in interleukin-6 (IL-6) expression was observed between the two groups (Fig. 1B).
Fig. 1.
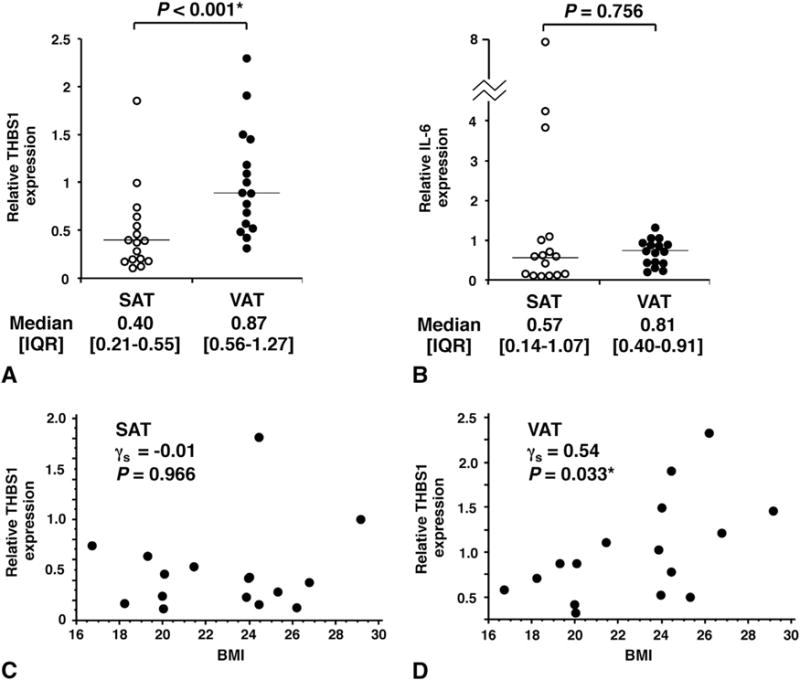
(A, B) The expression levels of THBS1 (A) and IL-6 (B) in human adipose tissues were quantified with real-time PCR. Data are expressed relative to the 36B4 gene used as an internal control for normalization. Horizontal bars represent median values. SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue. The Wilcoxon signed-rank test was used to compare the difference in gene expression between SAT and VAT. (C, D) Correlations between THBS1 expression levels and obesity traits. Spearman’s rank correlation coefficient (γs) was used to determine the association of THBS1 expression with BMI in SAT (C) or VAT (D). *P < 0.05 (statistically significant).
3.2. Visceral fat THBS1 Expression is Associated with Metabolic Syndrome
To determine whether THBS1 expression in each fat depot is associated with the type and degree of obesity, we examined the correlation between the fat depot-specific THBS1 expression level and physical parameters of adiposity as well as the quantitative traits of metabolic diseases. The VAT THBS1 expression, but not SAT THBS1, was positively associated with BMI (γs = 0.54, P = 0.033, Fig. 1C) and WC (γs = 0.54, P = 0.029, Table S2). Conversely, a negative association was observed between VAT THBS1 expression and the HDL-C level (γs = −0.56, P = 0.024, Table S2), suggesting that VAT THBS1 expression may reflect visceral obesity and a low HDL-C level, two key components of metabolic syndrome. No correlation was observed between SAT THBS1 expression and the quantitative traits of obesity and metabolic syndrome (BMI, γs = −0.01, P = 0.966; Fig. 1C and Table S2). These results suggest that THBS1 expression in VAT but not SAT is likely to be associated with obesity and metabolic syndrome in Japanese subjects.
3.3. The Association Between Serum THBS1 and Metabolic Syndrome, Obesity, and Diabetes
Observing the higher expression of VAT THBS1 to be associated with a higher BMI, we determined the potential significance of serum THBS1 as a biological marker of visceral obesity and metabolic syndrome. To determine whether there was a significant correlation between the serum THBS1 level and clinical parameters, at least 109 subjects were needed when we estimated the sample size with an assumed effect size of 0.3, alpha error of 0.05, and 90% power (two-tailed). In this study, we assessed the correlations between serum THBS1 levels and obesity traits as well as the diagnostic components of metabolic syndrome in 164 Japanese subjects with and without diabetes (78 males and 86 females, Table 1). Firstly, we screened for a potential association between the serum THBS1 level and diagnostic components of metabolic syndrome using ANCOVA. Metabolic syndrome was defined according to the definition of the International Diabetes Federation (IDF), i.e., TG ≥ 150 mg/dL; HDL ≤ 40 mg/dL; systolic BP ≥ 130, diastolic BP ≥ 85 mg/dL, or previously diagnosed hypertension; fasting plasma glucose level ≥ 100 mg/dL; waist circumference ≥85 cm in males, ≥90 cm in females (Table 2). In this study group, the serum THBS1 level was found to reflect abdominal obesity (F = 13.2, P < 0.001, B = 5.6, Table 2). The significance of THBS1 in reflecting abdominal obesity was similar to that of serum leptin, which displays a positive association (F = 13.8, P < 0.001, B = 8.7), and adiponectin, which displays an inverse association (F = 17.8, P < 0.001, B = −3.5). Of note, unlike serum leptin or adiponectin, serum THBS1 was associated with hypertension (F = 4.1, P = 0.04) and hyperglycemia (F = 5.5, P = 0.02), two additional components of metabolic syndrome. Adiponectin displayed a significant inverse correlation with hypertriglyceridemia (F = 5.3, P = 0.02, B = −1.6) in addition to abdominal obesity, but no additional associations were observed for leptin (Table 2). These results suggest the significance of serum THBS1 as a potential biological marker of obesity and metabolic syndrome. Next, we performed multivariable regression analysis (stepwise) to screen for potential associations between the serum THBS1 level and age, sex, and the independent variables of metabolic diseases. In this analysis, we screened for hypertension (SBP), obesity (BMI), diabetes (FPG), hypertriglyceridemia (TG), hypercholesterolemia (TC), fatty liver (ALT), and inflammation (hsCRP). In this analysis, a significant correlation between the serum THBS1 and FPG was identified (P = 0.04).
Table 1.
Baseline characteristics and metabolic parameters of the study subjects.
| Variable
|
Total
|
Male
|
Female
|
|---|---|---|---|
| n | 164 | 78 | 86 |
| Age (years) | 50.4 ± 13.6 | 50.6 ± 14.4 | 50.3 ± 12.9 |
| BMI (kg/m2) | 29.8 ± 6.7 | 28.3 ± 6.4 | 31.1 ± 6.7 |
| WC (cm) | 96.9 ± 15.5 | 94.3 ± 16.2 | 99.3 ± 14.5 |
| VAT area (cm2) | 102.6 ± 50.5 | 106.6 ± 51.1 | 98.7 ± 49.9 |
| SAT area (cm2) | 250.0 ± 117.3 | 219.8 ± 114.3 | 279.3 ± 113.3 |
| SBP (mmHg) | 134.9 ± 18.9 | 133.8 ± 17.7 | 135.9 ± 20.0 |
| DBP (mmHg) | 82.1 ± 12.0 | 81.6 ± 11.7 | 82.5 ± 12.4 |
| FPG (mmol/L) | 6.0 ± 1.8 | 6.2 ± 2.0 | 5.9 ± 1.7 |
| HbA1c (%) | 6.2 ± 1.2 | 6.1 ± 1.2 | 6.2 ± 1.2 |
| IRI (pmol/L) | 60.0 [33.6–105.3] | 45.6 [31.2–82.2] | 69.6 [40.8–121.2] |
| HOMA-IR | 2.6 [1.4–5.4] | 2.2 [1.2–4.0] | 2.9 [1.6–6.3] |
| TG (mmol/L) | 1.4 [0.9–2.1] | 1.5 [0.9–2.3] | 1.4 [1.0–2.0] |
| HDL-C (mmol/L) | 1.5 ± 0.4 | 1.3 ± 0.4 | 1.6 ± 0.4 |
| LDL-C (mmol/L) | 3.1 ± 0.8 | 2.9 ± 0.9 | 3.3 ± 0.6 |
| AST (IU/L) | 26.3 ± 12.6 | 27.6 ± 13.1 | 25.1 ± 11.9 |
| ALT (IU/L) | 33.3 ± 24.9 | 39.0 ± 29.2 | 28.2 ± 18.9 |
| γGTP (IU/L) | 47.5 ± 52.8 | 57.0 ± 63.1 | 39.5 ± 41.0 |
| Leptin (ng/mL) | 9.4 [4.3–18.7] | 5.6 [3.0–9.8] | 16.2 [8.8–24.7] |
| Adiponectin (μg/mL) | 6.9 [4.7–9.1] | 5.8 [4.4–8.3] | 7.8 [5.4–9.9] |
| hsCRP (μg/mL) | 0.8 [0.4–1.8] | 0.7 [0.4–1.0] | 0.8 [0.4–2.3] |
| THBS1 (μg/mL) | 14.0 [7.8–19.7] | 13.1 [8.0–17.5] | 15.9 [6.4–21.7] |
Data are shown as the mean ± SD or median and interquartile ranges. BMI, body mass index; WC, waist circumference; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose, HbA1c, hemoglobin A1c; IRI, immunoreactive insulin; HOMA-IR, homeostasis model assessment of insulin resistance; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ALT, alanine aminotransferase; AST, aspartate aminotransferase; γ-GTP, γ-glutamyl transpeptidase; hsCRP, high sensitivity C-reactive protein; THBS1, thrombospondin 1.
Table 2.
The association between metabolic syndrome and serum THBS1, leptin, or adiponectin.
| Clinical component | THBS1
|
Leptin
|
Adiponectin
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| F | P | B | F | P | B | F | P | B | |
| Hypertension | 4.1 | 0.04 | −2.6 | 0.3 | 0.58 | 1.1 | 2.7 | 0.10 | 1.2 |
| Hyperglycemia | 5.5 | 0.02 | 3.2 | 0.3 | 0.55 | 1.2 | 0.1 | 0.74 | −0.2 |
| Hypertriglyceridemia | 0.1 | 0.74 | 0.4 | 2.1 | 0.14 | 2.8 | 5.3 | 0.02 | −1.6 |
| Reduced HDL | 1.1 | 0.28 | −2.3 | 2.4 | 0.11 | −4.9 | 0.4 | 0.49 | −0.7 |
| Abdominal obesity | 13.2 | <0.001 | 5.6 | 13.8 | <0.001 | 8.7 | 17.8 | <0.001 | −3.5 |
Based on these analyses, we assessed the individual correlation between the serum THBS1 level and a series of metabolic traits (Table 3). In the total population, we observed the positive correlations of serum THBS1 with BMI, VAT area, FPG, HbA1c, HOMA-IR, leptin, and hsCRP. Subanalyses demonstrated significant correlations particularly in females. In males, age was associated with the level of THBS1 (P = 0.022, Table 3). Of note, in females, the serum THBS1 level was correlated positively with serum leptin level (γs = 0.25, P = 0.023) and inversely with the serum adiponectin level (γs = −0.28, P = 0.011), suggesting the similarities and differences of these three adipokines in reflecting obesity and the clinical components of metabolic syndrome.
Table 3.
Correlation analyses of serum THBS1 levels and metabolic parameters.
| Variable | Total (n = 164)
|
Male (n = 78)
|
Female (n = 86)
|
|||
|---|---|---|---|---|---|---|
| γs | P | γs | P | γs | P | |
| Age | 0.04 | 0.590 | 0.26 | 0.022* | −0.11 | 0.307 |
| BMI | 0.17 | 0.033* | −0.14 | 0.232 | 0.30 | 0.005* |
| WC | 0.14 | 0.067 | −0.04 | 0.704 | 0.24 | 0.029* |
| VAT area | 0.17 | 0.032* | 0.12 | 0.290 | 0.22 | 0.060 |
| SAT area | 0.16 | 0.054 | −0.05 | 0.657 | 0.22 | 0.058 |
| FPG | 0.19 | 0.018* | 0.06 | 0.587 | 0.26 | 0.016* |
| HbA1c | 0.24 | 0.002* | 0.20 | 0.083 | 0.22 | 0.039* |
| IRI | 0.14 | 0.082 | −0.08 | 0.533 | 0.23 | 0.034* |
| HOMA-IR | 0.17 | 0.044* | −0.05 | 0.697 | 0.26 | 0.015* |
| TG | 0.10 | 0.226 | 0.07 | 0.528 | 0.16 | 0.135 |
| HDL-C | −0.06 | 0.466 | −0.06 | 0.609 | −0.20 | 0.072 |
| LDL-C | 0.07 | 0.357 | −0.12 | 0.291 | 0.16 | 0.144 |
| AST | 0.09 | 0.255 | −0.08 | 0.497 | 0.21 | 0.052 |
| ALT | 0.14 | 0.082 | −0.05 | 0.637 | 0.30 | 0.004* |
| γGTP | −0.08 | 0.392 | −0.21 | 0.101 | 0.11 | 0.358 |
| Leptin | 0.18 | 0.022* | −0.09 | 0.426 | 0.25 | 0.023* |
| Adiponectin | −0.15 | 0.070 | −0.02 | 0.863 | −0.28 | 0.011* |
| hsCRP | 0.22 | 0.007* | −0.01 | 0.920 | 0.33 | 0.002* |
| Estradiol | – | – | – | – | 0.14 | 0.271 |
| FSH | – | – | – | – | −0.13 | 0.300 |
Spearman’s rank correlation coefficient (γs) was used to assess the relationships among the study variables. FSH, follicle-stimulating hormone.
P < 0.05 (statistically significant).
Subanalyses demonstrated that among males, no significant correlation was observed between THBS1 levels and obesity/diabetes traits except for age (Table 3). However, HbA1c showed a trend toward a positive association with the THBS1 level (P = 0.083, Table 3). The effect size of the relationship between the THBS1 level and HbA1c level in men (n = 48) was relatively low (γs = 0.20); therefore, the statistical power might have been insufficient to detect the potentially positive association between the serum THBS1 level and diabetes traits in men (post hoc calculation of power = 0.49).
3.4. Serum THBS1 Levels are Significantly Higher in Obese Female Subjects with Greater BMI
To further define the association between blood THBS1 levels and obesity, we compared the serum THBS1 levels of male and female subjects who were ranked according to the severity of obesity. Subjects were divided into four groups based on BMI: group 1 (normal weight), BMI <25; group 2 (overweight), 25 ≤ BMI <30; group 3 (obese, class I), 30 ≤ BMI <35; group 4 (obese, classes II and III), BMI ≥35. The circulating THBS1 levels in females were significantly higher in obese subjects with greater BMI (groups 2–4) than in the non-obese group (group 1, P < 0.001, Fig. 2A). In male subjects, however, we did not observe any positive relationship between serum THBS1 levels and BMI (Fig. 2B). Notably, the average serum THBS1 level of non-obese males was significantly higher than that of non-obese females (P < 0.001: Mann–Whitney U test, Fig. 2A, B), suggesting the presence of a male-specific mechanism that may increase serum THBS1 levels.
Fig. 2.
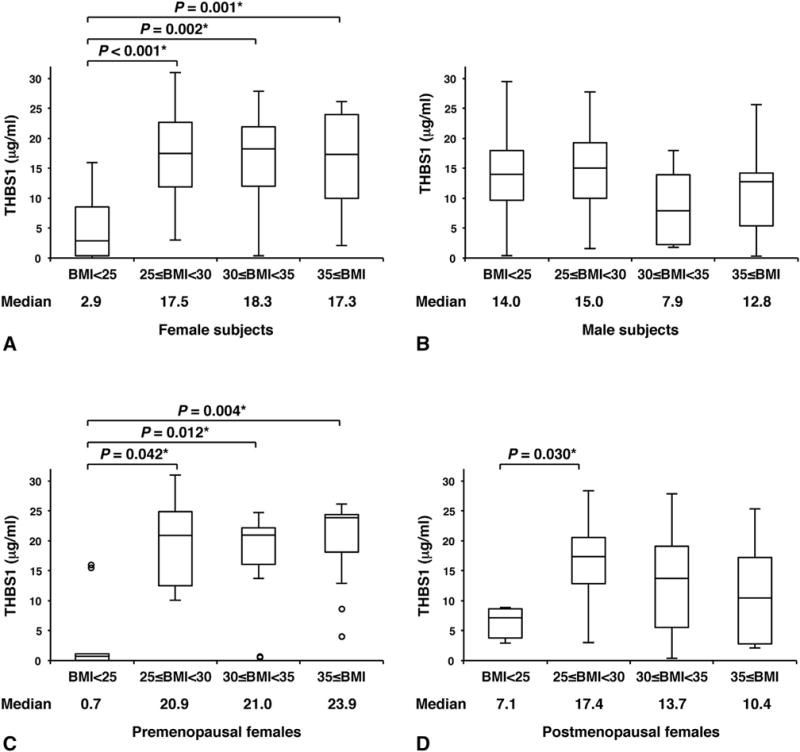
Box and whisker plots of circulating THBS1 levels. Study subjects (A, females, n = 86; B, males, n = 78; C, premenopausal females, n = 42; D, postmenopausal females, n = 44) were divided into four groups based on BMI: group 1, BMI <25; group 2, 25 ≤ BMI <30; group 3, 30 ≤ BMI <35; group 4, 35 ≤ BMI. A, females, n = 86 (group 1 = 15; group 2 = 22; group 3 = 28; group 4 = 21). (B) Males, n = 78 (group 1 = 28; group 2 = 25; group 3 = 15; group 4 = 10). (C) Premenopausal females, n = 42 (group 1 = 9; group 2 = 7; group 3 = 14; group 4 = 12). (D) Postmenopausal females, n = 44 (group 1 = 6; group 2 = 15; group 3 = 14; group 4 = 9). The horizontal line within the box indicates the median, and boundaries of the box represent the 25th and 75th percentile. Groups were compared by the Mann–Whitney U test with Bonferroni correction. *P < 0.05.
We speculated that the sex-dependent dichotomy in the association between THBS1 and obesity/diabetes traits might be due to higher estrogen levels in women. Indeed, the positive association between serum THBS1 levels and BMI was more evident in premenopausal females. However, we were not able to detect any significant correlation between serum estradiol/follicle-stimulating hormone (FSH) and THBS1 in females (Tables 3 and 4). When females were divided into premenopausal and postmenopausal groups (Table S3), serum THBS1 levels tended to be lower in the postmenopausal group that displays a clear increase of FSH and decrease of estradiol levels (Table S3). Among non-obese females, however, baseline THBS1 levels tended to be lower in premenopausal than postmenopausal females (median, 0.7 versus 7.1, P = 0.07, Fig. 2C, D). In premenopausal females, a significant increase of serum THBS1 levels was observed in overweight/obese groups (Fig. 2C). In the postmenopausal group, serum THBS1 levels were still higher in the overweight/obese groups than non-obese group; however, the incremental increase of the serum THBS1 level was not clearly associated with increased BMI (Fig. 2D). As observed in the total female population, no significant correlations between THBS1 and FSH/estradiol levels were noted in premenopausal and postmenopausal females (Table 4). Moreover, unlike in males, age was not associated with the levels of serum THBS1 in females (Table 4).
Table 4.
Correlation analyses of serum THBS1 levels and metabolic parameters in female subjects.
| Variable | Premenopausal (n = 42) |
Postmenopausal (n = 44) |
||
|---|---|---|---|---|
| γs | P | γs | P | |
| Age | −0.11 | 0.479 | 0.16 | 0.308 |
| BMI | 0.41 | 0.007* | 0.04 | 0.813 |
| WC | 0.35 | 0.023* | 0.05 | 0.730 |
| VAT area | 0.36 | 0.033* | 0.05 | 0.742 |
| SAT area | 0.37 | 0.029* | 0.06 | 0.728 |
| FPG | 0.27 | 0.090 | 0.35 | 0.019* |
| HbA1c | 0.19 | 0.240 | 0.38 | 0.010* |
| IRI | 0.18 | 0.249 | 0.25 | 0.096 |
| HOMA-IR | 0.24 | 0.136 | 0.31 | 0.042* |
| TG | 0.23 | 0.136 | 0.05 | 0.765 |
| HDL-C | −0.33 | 0.033* | −0.02 | 0.896 |
| LDL-C | 0.13 | 0.419 | 0.05 | 0.769 |
| AST | 0.21 | 0.188 | 0.21 | 0.174 |
| ALT | 0.27 | 0.080 | 0.32 | 0.033* |
| γGTP | 0.28 | 0.108 | 0.03 | 0.857 |
| Leptin | 0.36 | 0.026* | 0.13 | 0.400 |
| Adiponectin | −0.29 | 0.081 | −0.21 | 0.171 |
| hsCRP | 0.34 | 0.035* | 0.23 | 0.141 |
| Estradiol | −0.09 | 0.635 | −0.14 | 0.437 |
| FSH | 0.15 | 0.398 | 0.03 | 0.875 |
Spearman’s rank correlation coefficient (γs) was used to assess the relationships among the study variables.
P < 0.05.
3.5. Premenopausal Association Between Serum THBS1 Levels and Obesity Traits in Women
Thus, the association between serum THBS1 levels and obesity traits was clearer in premenopausal females regardless of the levels of FSH and estradiol. Subanalyses in pre- and postmenopausal females suggest that serum THBS1 levels are associated with multiple traits of obesity in premenopausal females (BMI, WC, VAT area, SAT area, HDL-C, leptin, and hsCRP; Table 4). While the associations between serum THBS1 levels and obesity traits were less obvious in postmenopausal females, THBS1 levels demonstrated positive correlations with insulin resistance and diabetes as well as fatty liver (FPG, HbA1c, HOMA-IR, and ALT; Table 4) in postmenopausal females. The potential association between the serum THBS1 level and FPG was also noted in premenopausal females as well (γs = 0.27, P = 0.09).
3.6. The Effects of Weight Reduction Therapy on Serum THBS1 Levels and Metabolic Variables
Finally, we assessed the effect of body weight reduction on serum THBS1 levels and metabolic variables. In this group, 34 males (mean BMI, 29.0) and 44 females (mean BMI, 30.3) underwent weight reduction therapy. Significant decreases in BMI, visceral fat area and serum leptin levels were observed after 6 months of behavioral and dietary intervention [16] in both male and female subjects (Table S4). Although the therapy successfully resulted in a mild to moderate reduction of BMI, there was no significant difference in serum THBS1 levels after the therapy (Table S4). Notably, however, serum THBS1 levels in female subjects showed a declining trend during weight reduction therapy (median value: from 18.1 to 15.6 μg/mL, P = 0.203, Table S4). In premenopausal females, the decrease in the serum THBS1 level in each patient was correlated with the reduction of BMI (γs = 0.47, P = 0.035, Table 5). In addition, the reduction of serum THBS1 levels displayed a positive trend with the loss of visceral fat volume (γs = 0.38, P = 0.135) in premenopausal females. Conversely, in postmenopausal females, the changes in THBS1 levels were negatively associated with the changes of HbA1c and LDL-cholesterol levels, suggesting a potential biological coupling between THBS1 and metabolic syndrome in postmenopausal females.
Table 5.
Correlation analyses of changes in serum THBS1 and metabolic parameters during weight reduction therapy.
| Variable | Male n = 34 |
Female n = 44 |
Premenopausal n = 20 |
Postmenopausal n = 24 |
||||
|---|---|---|---|---|---|---|---|---|
| γs | P | γs | P | γs | P | γs | P | |
| ΔBMI | −0.16 | 0.357 | 0.09 | 0.543 | 0.47 | 0.035* | −0.15 | 0.493 |
| ΔWC | 0.09 | 0.631 | −0.01 | 0.970 | 0.25 | 0.283 | −0.28 | 0.186 |
| ΔVAT area | −0.03 | 0.875 | 0.13 | 0.433 | 0.38 | 0.135 | −0.15 | 0.516 |
| ΔSAT area | −0.02 | 0.910 | 0.02 | 0.895 | 0.13 | 0.633 | 0.01 | 0.970 |
| ΔFPG | −0.16 | 0.355 | 0.00 | 0.978 | 0.30 | 0.204 | −0.28 | 0.189 |
| ΔHbA1c | −0.17 | 0.323 | −0.06 | 0.703 | 0.32 | 0.175 | −0.41 | 0.044* |
| ΔIRI | −0.08 | 0.689 | −0.07 | 0.672 | 0.16 | 0.535 | −0.20 | 0.365 |
| ΔHOMA-IR | −0.10 | 0.600 | −0.05 | 0.759 | 0.22 | 0.400 | −0.25 | 0.260 |
| ΔTG | −0.28 | 0.112 | −0.11 | 0.486 | 0.06 | 0.806 | −0.28 | 0.182 |
| ΔHDL-C | −0.09 | 0.597 | 0.12 | 0.435 | 0.08 | 0.728 | 0.08 | 0.714 |
| ΔLDL-C | 0.07 | 0.712 | −0.21 | 0.170 | 0.18 | 0.440 | −0.58 | 0.003* |
| ΔAST | 0.15 | 0.387 | −0.15 | 0.347 | 0.02 | 0.947 | −0.29 | 0.172 |
| ΔALT | 0.17 | 0.331 | 0.03 | 0.865 | 0.17 | 0.474 | −0.11 | 0.619 |
| ΔγGTP | −0.06 | 0.748 | −0.20 | 0.215 | −0.10 | 0.736 | −0.23 | 0.284 |
| ΔLeptin | −0.20 | 0.270 | −0.10 | 0.530 | 0.11 | 0.658 | −0.18 | 0.406 |
| ΔAdiponectin | 0.30 | 0.097 | 0.06 | 0.700 | 0.06 | 0.819 | 0.07 | 0.764 |
| ΔhsCRP | −0.25 | 0.175 | −0.04 | 0.795 | −0.01 | 0.977 | −0.04 | 0.861 |
The parameter change (Δ) was defined as the level after 6 months minus the level at the baseline. Spearman’s rank correlation coefficient (γs) was used to assess the relationships among the study variables.
P < 0.05.
4. Discussion
In this study, we demonstrated that THBS1 was expressed more in visceral fat tissues than in subcutaneous fat tissues and that the serum THBS1 level may serve as a useful biological marker that reflects metabolic syndrome, obesity, and diabetes. THBS1 expression in visceral adipose tissues is correlated positively with both BMI and WC but negatively with HDL cholesterol levels, suggesting that VAT THBS1 expression may represent a biological process underlying visceral obesity and metabolic syndrome. Moreover, we newly demonstrated that the serum THBS1 level is positively correlated with quantitative traits of obesity and diabetes, particularly in females.
In this study, we were able to demonstrate the usefulness of the serum THBS1 level as a promising biological marker in reflecting metabolic syndrome, obesity, and diabetes, particularly in females. Serum THBS1 reflects abdominal obesity to a degree similar to that of serum leptin and adiponectin. Serum leptin concentrations are strongly correlated with the percentage body fat [19]. On the other hand, an inverse correlation between serum adiponectin and the fat mass has been shown by others [20,21]. We were able to replicate those findings in our cohort (Table 2) and we newly demonstrated the positive correlation between serum THBS1 and abdominal obesity/metabolic syndrome in the same subjects. The ANCOVA data and multivariable regression analysis suggested an additional correlation between serum THBS1 levels and hyperglycemia, which is not observed for serum leptin or adiponectin. Given the significant correlation observed between serum THBS1 levels and metabolic syndrome as well as hyperglycemia, we used regression analyses for multiple quantitative traits. Regression analyses suggested a significant correlation between serum THBS1 and obesity/diabetes traits, which is more prominent in females. Subanalyses of females further suggested the presence of a robust association between THBS1 and obesity in premenopausal women and diabetes in postmenopausal females.
Our study is limited because of its: 1) relatively small size for subanalyses, 2) cross-sectional and retrospective study design, and 3) lack of a cause–effect relationship between THBS1 and metabolic diseases. Firstly, our study to define the significance of THBS1 as a biological marker was conducted with a relatively small group (164 subjects). We were able to replicate the significant correlations between serum THBS1 and the waist circumference, BMI, and hyperglycemia using ANCOVA and multivariable regression analysis as well as single correlation analyses. However, the statistical power of single correlation analyses for multiple parameters was limited for gender-specific subanalyses. In the total population as well as premenopausal females, we were able to detect significant associations between serum THBS1 levels and BMI and VAT areas. Our subanalyses, however, pointed to a gender-dependent dichotomy in the association between serum THBS1 and obesity/diabetes traits. We observed a trend of association between serum THBS1 and HbA1c in men as well (γs = 0.20, P = 0.083). However, the statistical power for this subanalysis was not robust (power = 52%); therefore, we were not able to determine the potential association between TBHS1 and diabetes traits in men. Unlike premenopausal females showing the associations between serum THBS1 and quantitative traits of metabolic syndrome/obesity/inflammation (BMI, WC, VAT area, SAT area, HDL-C, hsCRP), postmenopausal females mostly display the associations of THBS1 with diabetes traits (FPG, HbA1c, HOMA-IR) and fatty liver (ALT). Conversely, premenopausal females demonstrated a borderline association between serum THBS1 levels and FPG (γs = 0.27, P = 0.09). Nonetheless, we may require a larger sample size to determine the potential association in this subgroup. A future study that focuses on female subjects with a larger sample size may clarify how serum THBS1 levels represent the phenotypes of obesity, diabetes, dyslipidemia, fatty liver, and chronic inflammation in women. Secondly, our study was cross-sectional in determining the significance of serum THBS1 as a biological marker of metabolic syndrome, obesity, and diabetes, and was retrospective in assessing the association between serum THBS1 levels and the effect of weight reduction therapy. Serum THBS1 levels as well as their increase in obesity might be genetically determined [22,23]. As such, our study is limited in assessing the role of the serum THBS1 level as a prognostic marker to predict the progression of obesity-related metabolic syndrome and diabetes. To determine the role of THBS1 as a prognostic marker in obesity, diabetes, and metabolic syndrome, a prospective study stratified by the baseline serum THBS1 level might be necessary. Thirdly, this study does not clearly demonstrate a causal role of THBS1 in human obesity, metabolic syndrome, and diabetes. Based on mouse models [9–11], we hypothesize that the individuals who maintain relatively lower serum THBS1 levels during nutritional challenges might be protected from diet-induced obesity, diabetes, and metabolic syndrome. This hypothesis should be addressed in a prospective cohort study.
Adipose THBS1 expression was significantly higher in VAT than in SAT in our study population, which is consistent with previous reports by others [10,12]. In a previous report, THBS1 expression was marginally higher in VAT than SAT in obese American subjects, and the difference between the two adipose depots was actually non-significant [3]. They showed that THBS1 expression in SAT was positively correlated with obesity, and the study did not mention the association between VAT THBS1 expression and obese phenotypes [3]. In our study with Japanese subjects, no correlation was found between SAT THBS1 expression and the degree of obesity. Varma et al. used adipose tissues obtained from obese American patients with a BMI of 29 and higher [3], whereas the subjects of our study were surgical patients with normal BMI. In addition to the difference in the average BMI, an ethnicity-dependent difference in body composition and the presence or absence of obesity-related metabolic diseases might have contributed to the difference in fat depot-dependent association of THBS1 expression with obesity and diabetes.
A sex-dependent difference in the degree of association between the serum THBS1 level and obesity/diabetes traits might be due to the sexual dimorphism of adipose tissue biology, including differential fat tissue distribution and function [24–26]. The biological mechanism underlying the menopausal status-dependent association between THBS1 and diabetes is unclear at this time. Accumulating evidence suggests that postmenopausal biological change might lead to a decrease in the ability to expand adipose tissue for additional fat storage and oxidation [27,28]. The reduced expandability of adipose tissue in postmenopausal females may impair the lipid-buffering capacity of the body, rendering postmenopausal women more susceptible to lipotoxicity through elevated free fatty acids in circulation and ectopic lipid accumulation in non-adipose tissues [29]. The menopausal status-related changes in adipose tissue expandability may underlie the significant association between serum THBS1 and diabetes traits in postmenopausal women. Age itself and the levels of FSH/estradiol did not show any significant correlations with THBS1 levels in females. As such, postmenopausal biological change, including an altered fat distribution [30], may modify the association between serum THBS1 and the metabolic phenotype (obesity and diabetes). A decrease in the serum THBS1 level was associated with weight loss in premenopausal women; however, serum THBS1 levels were paradoxically increased in postmenopausal women who showed improvement in diabetes control with weight reduction. The cause of this paradoxical relationship between a change in the serum THBS1 level and the improvement of diabetes traits in postmenopausal women is unclear at this time. We speculate that in diabetic postmenopausal women, the amelioration of lipotoxicity after weight loss might have restored the population of preadipocytes that express higher THBS1 than adipocytes [31]. Further studies in relation to weight reduction therapy are necessary to understand the role of THBS1 in the regulation of human adipose tissue biology and metabolism during obesity progression and regression.
Recent studies identified THBS1 as a mediator of insulin resistance and adipose inflammation in animal models of diet-induced obesity [9–11]. These findings in animal models of obesity are consistent with the previously observed association between adipose THBS1 expression and inflammation in humans [3]. Although potential target organs of THBS1 have not been fully defined in humans, the obesity-induced circulation of THBS1 may provide a molecular link between visceral adiposity and metabolic dysfunction in obese subjects [10]. The activity of THBS1 depends on the repertoire of downstream molecules expressed in the target organs, including ECM proteins and cell surface receptors, such as CD36 and CD47 [32,33]. One of the other downstream targets of THBS1 is transforming growth factor-β1 (TGF-β1), which plays a critical role in tissue remodeling and inflammation [8]. THBS1 is capable of converting latent TGF-β1 to a biologically active form [8]. It is plausible that the THBS1-dependent activation of TGF-β1 may induce a series of inflammatory mediators including plasminogen activator inhibitor-1 (PAI-1) [34] and then contribute to obesity-induced tissue inflammation and the development of metabolic syndrome.
In summary, our results suggest the significant role of THBS1 in reflecting the complex phenotypes of human obesity and diabetes. The circulating THBS1 level is associated with obesity traits in premenopausal females and with diabetes in postmenopausal females. Understanding the pathological role played by THBS1 in modifying the complex relationship between obesity and metabolic syndrome or diabetes in humans should help us develop new diagnostic tools and treatment for obesity-related metabolic disorders.
Supplementary Material
Acknowledgments
We thank Drs. Rodney A. Hayward and William H. Herman (University of Michigan) for discussion.
Funding
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, JSPS KAKENHI, Grant Number 24590719 and 15K08634 and Danone Institute of Japan Foundation for a financial support of the 2015 DIJF to NS-A, JSPS KAKENHI Grant Number 26893335 to YM, and grant P30DK092926 to Michigan Center of Diabetes Translational Research (MCDTR) Methods and Measurement Core.
Abbreviations
- THBS1
thrombospondin 1
- BMI
body mass index
- WC
waist circumference
- VAT
visceral adipose tissue
- SAT
subcutaneous adipose tissue
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- FPG
fasting plasma glucose
- HbA1c
hemoglobin A1c
- IRI
immunoreactive insulin
- HOMA-IR
homeostasis model assessment of insulin resistance
- TG
triglycerides
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- γ-GTP
γ-glutamyl transpeptidase
- hsCRP
high sensitivity C-reactive protein
- FSH
follicle-stimulating hormone
- ECM
extracellular matrix
- TGF-β
transforming growth factor-β
Appendix A. Supplementary Data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.metabol.2015.07.016.
Footnotes
Clinical Trial Registration Number: UMIN000016823.
Author Contributions
All authors had full access to all of the data in the study and gave final approval of the submitted version. MI, T-HC, and NS-A conceived and designed the study. YM, MT, YS, and KM carried out the experiment. YM, YS, HY, KM, and NS-A analyzed the data. YM, HY, HH, II, AS, MI, T-HC, and NS-A interpreted the results. YM, MI, T-HC, and NS-A wrote the manuscript. T-HC and NS-A supervised the study.
Disclosure Statement
The authors have nothing to disclose.
References
- 1.Baenziger NL, Brodie GN, Majerus PW. Isolation and properties of a thrombin-sensitive protein of human platelets. J Biol Chem. 1972;247:2723–31. [PubMed] [Google Scholar]
- 2.Jaffe EA, Ruggiero JT, Falcone DJ. Monocytes and macrophages synthesize and secrete thrombospondin. Blood. 1985;65:79–84. [PubMed] [Google Scholar]
- 3.Varma V, Yao-Borengasser A, Bodles AM, Rasouli N, Phanavanh B, Nolen GT, et al. Thrombospondin-1 is an adipokine associated with obesity, adipose inflammation, and insulin resistance. Diabetes. 2008;57:432–9. doi: 10.2337/db07-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawler JW, Slayter HS, Coligan JE. Isolation and characterization of a high molecular weight glycoprotein from human blood platelets. J Biol Chem. 1978;253:8609–16. [PubMed] [Google Scholar]
- 5.Lawler J. The functions of thrombospondin-1 and-2. Curr Opin Cell Biol. 2000;12:634–40. doi: 10.1016/s0955-0674(00)00143-5. [DOI] [PubMed] [Google Scholar]
- 6.Sweetwyne MT, Murphy-Ullrich JE. Thrombospondin1 in tissue repair and fibrosis: TGF-beta-dependent and independent mechanisms. Matrix Biol. 2012;31:178–86. doi: 10.1016/j.matbio.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schultz-Cherry S, Murphy-Ullrich JE. Thrombospondin causes activation of latent transforming growth factor-beta secreted by endothelial cells by a novel mechanism. J Cell Biol. 1993;122:923–32. doi: 10.1083/jcb.122.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy-Ullrich JE, Poczatek M. Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev. 2000;11:59–69. doi: 10.1016/s1359-6101(99)00029-5. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Tong X, Rumala C, Clemons K, Wang S. Thrombospondin1 deficiency reduces obesity-associated inflammation and improves insulin sensitivity in a diet-induced obese mouse model. PLoS One. 2011;6:e26656. doi: 10.1371/journal.pone.0026656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue M, Jiang Y, Barnes RH, II, Tokunaga M, Martinez-Santibanez G, Geletka L, et al. Thrombospondin 1 mediates high-fat diet-induced muscle fibrosis and insulin resistance in male mice. Endocrinology. 2013;154:4548–59. doi: 10.1210/en.2013-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong P, Gonzalez-Quesada C, Li N, Cavalera M, Lee DW, Frangogiannis NG. Thrombospondin-1 regulates adiposity and metabolic dysfunction in diet-induced obesity enhancing adipose inflammation and stimulating adipocyte proliferation. Am J Physiol Endocrinol Metab. 2013;305:E439–50. doi: 10.1152/ajpendo.00006.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramis JM, Franssen-van Hal NL, Kramer E, Llado I, Bouillaud F, Palou A, et al. Carboxypeptidase E and thrombospondin-1 are differently expressed in subcutaneous and visceral fat of obese subjects. Cell Mol Life Sci. 2002;59:1960–71. doi: 10.1007/PL00012518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banerji MA, Faridi N, Atluri R, Chaiken RL, Lebovitz HE. Body composition, visceral fat, leptin, and insulin resistance in Asian Indian men. J Clin Endocrinol Metab. 1999;84:137–44. doi: 10.1210/jcem.84.1.5371. [DOI] [PubMed] [Google Scholar]
- 14.He Q, Horlick M, Thornton J, Wang J, Pierson RN, Jr, Heshka S, et al. Sex and race differences in fat distribution among Asian, African-American, and Caucasian prepubertal children. J Clin Endocrinol Metab. 2002;87:2164–70. doi: 10.1210/jcem.87.5.8452. [DOI] [PubMed] [Google Scholar]
- 15.Bruun JM, Lihn AS, Pedersen SB, Richelsen B. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): implication of macrophages resident in the AT. J Clin Endocrinol Metab. 2005;90:2282–9. doi: 10.1210/jc.2004-1696. [DOI] [PubMed] [Google Scholar]
- 16.Satoh N, Shimatsu A, Kato Y, Araki R, Koyama K, Okajima T, et al. Evaluation of the cardio-ankle vascular index, a new indicator of arterial stiffness independent of blood pressure, in obesity and metabolic syndrome. Hypertens Res. 2008;31:1921–30. doi: 10.1291/hypres.31.1921. [DOI] [PubMed] [Google Scholar]
- 17.Yamakage H, Ito R, Tochiya M, Muranaka K, Tanaka M, Matsuo Y, et al. The utility of dual bioelectrical impedance analysis in detecting intra-abdominal fat area in obese patients during weight reduction therapy in comparison with waist circumference and abdominal CT. Endocr J. 2014;61:807–19. doi: 10.1507/endocrj.ej14-0092. [DOI] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 19.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 20.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 21.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–5. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 22.Winton HL, Bidwell JL, Armitage WJ. Thrombospondin-1 polymorphisms influence risk of corneal allograft rejectionthrombospondin-1 polymorphisms. Invest Ophthalmol Vis Sci. 2014;55:2115–20. doi: 10.1167/iovs.13-13681. [DOI] [PubMed] [Google Scholar]
- 23.Stenina OI, Byzova TV, Adams JC, McCarthy JJ, Topol EJ, Plow EF. Coronary artery disease and the thrombospondin single nucleotide polymorphisms. Int J Biochem Cell Biol. 2004;36:1013–30. doi: 10.1016/j.biocel.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Kotani K, Tokunaga K, Fujioka S, Kobatake T, Keno Y, Yoshida S, et al. Sexual dimorphism of age-related changes in whole-body fat distribution in the obese. Int J Obes Relat Metab Disord. 1994;18:207–212. [PubMed] [Google Scholar]
- 25.Saad MF, Damani S, Gingerich RL, Riad-Gabriel MG, Khan A, Boyadjian R, et al. Sexual dimorphism in plasma leptin concentration. J Clin Endocrinol Metab. 1997;82:579–84. doi: 10.1210/jcem.82.2.3739. [DOI] [PubMed] [Google Scholar]
- 26.Rosenquist KJ, Massaro JM, Pedley A, Long MT, Kreger BE, Vasan RS, et al. Fat quality and incident cardiovascular disease, all-cause mortality, and cancer mortality. J Clin Endocrinol Metab. 2015;100:227–34. doi: 10.1210/jc.2013-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray SL, Vidal-Puig AJ. Adipose tissue expandability in the maintenance of metabolic homeostasis. Nutr Rev. 2007;65:S7–S12. doi: 10.1111/j.1753-4887.2007.tb00331.x. [DOI] [PubMed] [Google Scholar]
- 28.Tan CY, Vidal-Puig A. Adipose tissue expandability: the metabolic problems of obesity may arise from the inability to become more obese. Biochem Soc Trans. 2008;36:935–40. doi: 10.1042/BST0360935. [DOI] [PubMed] [Google Scholar]
- 29.Tardif N, Salles J, Guillet C, Tordjman J, Reggio S, Landrier JF, et al. Muscle ectopic fat deposition contributes to anabolic resistance in obese sarcopenic old rats through eIF2alpha activation. Aging Cell. 2014;13:1001–11. doi: 10.1111/acel.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ley CJ, Lees B, Stevenson JC. Sex- and menopause-associated changes in body-fat distribution. Am J Clin Nutr. 1992;55:950–4. doi: 10.1093/ajcn/55.5.950. [DOI] [PubMed] [Google Scholar]
- 31.Guo W, Pirtskhalava T, Tchkonia T, Xie W, Thomou T, Han J, et al. Aging results in paradoxical susceptibility of fat cell progenitors to lipotoxicity. Am J Physiol Endocrinol Metab. 2007;292:E1041–51. doi: 10.1152/ajpendo.00557.2006. [DOI] [PubMed] [Google Scholar]
- 32.Dawson DW, Pearce SF, Zhong R, Silverstein RL, Frazier WA, Bouck NP. CD36 mediates the In vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol. 1997;138:707–17. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao AG, Lindberg FP, Finn MB, Blystone SD, Brown EJ, Frazier WA. Integrin-associated protein is a receptor for the C-terminal domain of thrombospondin. J Biol Chem. 1996;271:21–4. doi: 10.1074/jbc.271.1.21. [DOI] [PubMed] [Google Scholar]
- 34.Song CZ, Siok TE, Gelehrter TD. Smad4/DPC4 and Smad3 mediate transforming growth factor-beta (TGF-beta) signaling through direct binding to a novel TGF-beta-responsive element in the human plasminogen activator inhibitor-1 promoter. J Biol Chem. 1998;273:29287–90. doi: 10.1074/jbc.273.45.29287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


