Abstract
Average and maximal lifespan are important biological characteristics of every species, but can be modified by mutations and by a variety of genetic, dietary, environmental, and pharmacological interventions. Mutations or disruption of genes required for biosynthesis or action of growth hormone (GH) produce remarkable extension of longevity in laboratory mice. Importantly, the long-lived GH-related mutants exhibit many symptoms of delayed and/or slower aging, including preservation of physical and cognitive functions and resistance to stress and age-related disease. These characteristics could be collectively described as “healthy aging” or extension of the healthspan. Extension of both the healthspan and lifespan in GH-deficient and GH-resistant mice appears to be due to multiple interrelated mechanisms. Some of these mechanisms have been linked to healthy aging and genetic predisposition to extended longevity in humans. Enhanced insulin sensitivity combined with reduced insulin levels, cell senescence in the adipose tissue and central nervous system inflammation, and increased levels of adiponectin represent such mechanisms. Further progress in elucidation of mechanisms that link reduced GH action to delayed and healthy aging should identify targets for lifestyle and pharmacological interventions that could benefit individuals as well as society.
Introduction
Human life expectancy at birth increased dramatically during the past 200 years due to progress in medicine, increased access to safe sources of drinking water, massive childhood vaccination campaigns, and other public health measures. Improved chances of survival to advanced age combined with a declining birth rate result in a progressive, and often very rapid, increase in the proportion of elderly individuals in most countries. These developments help revive and sustain the universal interest in combating the impact of advancing age on physical and mental function and on disease burden, in various forms of “anti-aging medicine” and in the possibilities of (chances at) extending life.
Although the average and the maximal longevity are often regarded as more or less fixed phenotypic characteristics of a species, there is increasing evidence that human maximal longevity is slowly but detectably increasing (Vaupel 2010) and it is well documented that both average and maximal longevity can be increased by various environmental factors as well as by dietary, genetic, and pharmacological interventions in species ranging from yeast and worms to insects and mammals. There is also increasing appreciation of the fact that many of the fundamental mechanisms of aging are evolutionarily conserved (Tatar et al. 2003; Longo et al. 2015) and shared by most if not all living organisms [also including plants (Minina et al. 2013)] and that interventions that extend life in experimental animals can be expected to have similar effects in our own species.
Among the various anti-aging interventions, life-extending mutations (sometimes referred to as “longevity genes”) attract particular attention because they appear to be uniquely suitable for elucidating the mechanisms of aging. In most animals with these mutations, the expression of one gene is affected with the resulting deletion (or reduction of the levels) of a particular hormone, receptor, enzyme, or signaling molecule. Consequently, extension of longevity can be related to a defined functional change at the cellular level. Moreover, the genetic basis of extended longevity in these animals allows comparing long-lived and normal (control) animals when they are young and their phenotypes are not impacted by age-related pathology.
It is of obvious and indeed paramount significance to know whether extensions of lifespan achieved by genetic or other means are associated with extensions of “healthspan,” a period of life free of frailty and age-related disease. There is evidence that exceptional human longevity, exemplified by survival to age of 100 or 110 years is associated with compression of morbidity (Fries et al. 2011; Andersen et al. 2012; Sebastiani et al. 2013). However, some of the life-extending mutations in Caenorhabditis elegans were recently reported to lead to an increase in the relative duration of the period of frailty (Bansal et al. 2015). Calorie restriction extends both lifespan and healthspan in different mammalian species (Weindruch 1992; Mattison et al. 2012; Colman et al. 2014). In mice with several of the growth hormone (GH)-related mutations which extend longevity, healthspan is also extended (Flurkey et al. 2001; Ikeno et al. 2009; Bartke et al. 2013; Brown-Borg 2015) but the potential impact of these mutations on the absolute or relative duration of frailty has not been explored. In this article, we will summarize the information on phenotypes of long-lived GH-related mouse mutants with emphasis on characteristics linked to frailty and risk of chronic disease.
Growth hormone deficiency and GH resistance extend lifespan and healthspan of laboratory mice
In 1996, Brown-Borg et al. (1996) reported a major extension of longevity in mice homozygous for Ames dwarf (df) mutation [subsequently renamed, Prophet of pituitary 1, Prop1df (Sornson et al. 1996)]. This recessive loss-of-function mutation interferes with differentiation of adenohypophyseal cells expressing pituitary factor 1 (Pit-1) leading to a deficiency of somatotrophs, lactotrophs, and thyrotrophs and their hormonal products: growth hormone (GH), prolactin (PRL), and thyroid-stimulating hormone (thyrotropin, TSH) (Bartke 1964; Sornson et al. 1996; Bartke 1998). Interestingly, studies of Snell dwarf mice, Pit-1 mutants with identical (or nearly identical) endocrine phenotype conducted in the ‘60s and ‘70s produced conflicting results with reports of accelerated aging and drastically shortened longevity (Fabris et al. 1972), normal or possibly extended lifespan (Shire 1973) as well as markedly extended longevity with some individuals surviving beyond 40 months of age (Silberberg 1972). More recent work provided evidence that Snell dwarf mice, similar to Ames dwarf mice, are remarkably long lived (Flurkey et al. 2001) and it is now believed that opposite findings in one of the earlier studies (Fabris et al. 1972) must have been due to husbandry practices detrimental to these slow-growing and diminutive mutants (such as weaning them at an early age and/or feeding them a low-fat (“maintenance”) diet immediately after weaning) or to pathogens.
The role of GH deficiency in the extended longevity of Ames dwarf mice proposed by Brown-Borg et al. (1996) was strongly supported by the demonstration of extended longevity in mice with an isolated GH deficiency due to a spontaneous mutation of the GH-releasing hormone (GHRH) receptor (Flurkey et al. 2001), with targeted deletion of the GHRH gene (Sun et al. 2013) and in mice with GH resistance produced by disruption of the GH receptor/GH-binding protein gene (Coschigano et al. 2003). It deserves particular emphasis that the extended longevity of mice lacking GH or GH receptors is reproducible in different laboratories, that it applies to both females and males and that it is not limited to a particular genetic background or diet (reviewed in Bartke 2011; Bartke et al. 2013). However, association of reduced GH signaling and extended longevity is not universal. In contrast to the effects of GH deficiency and GH resistance, interfering with GH actions by transgenic expression of an antagonistic GH analog failed to extend longevity (Coschigano et al. 2003), although it produced some beneficial metabolic effects (Berryman et al. 2014).
Recent elegant studies by List and his colleagues provided evidence that the major anti-aging impact of disrupting GH receptors in all tissues cannot be reproduced by disrupting this receptor selectively in the liver, skeletal and cardiac muscle, or adipose tissue. Longevity was not altered in Liver GHR-KO (Li et al. 2014; List et al. 2014), reduced in Fat GHR-KO mice (List et al. 2013), and slightly increased in males from one of the two experimental cohorts of Muscle GHR-KO mice (List et al. 2015). Further studies in these novel lines of mice should provide mechanistic insights into the unexpected findings concerning their longevity. Without additional information we can only conclude that the remarkably long lives of animals with “global” GHR disruption either require suppression of GH action in multiple organ systems or are due to GH resistance at a site other than liver, muscles, or adipose tissue. Available (largely indirect) evidence leads us to speculate that the effects of GH signals on the immune system (Wang et al. 2006; Masternak et al. 2012; Dixit et al. unpublished) and/or the brain (Miller et al. 1995; Hascup 2015; Sadagurski 2015) might be involved in the control of aging.
Can findings on the role of GH in the control of aging in mice be extrapolated to other species?
Body size, a GH-dependent trait is negatively related to longevity not only in laboratory mice, but also rats (Rollo 2002), domestic dogs (Patronek et al. 1997; Greer et al. 2007), domestic cats (Kienzle and Moik 2011), and horses (Brosnahan and Paradis 2003). A similar relationship was uncovered in the studies of numerous human cohorts (Samaras et al. 2007). However, opposite and negative findings concerning the relationship between human height and longevity have also been reported (Samaras et al. 2007). Of particular interest is the recent report by He et al. (2014), who demonstrated a negative correlation of height to longevity in a large cohort of American men of Japanese ancestry. Importantly, increased old age survival in this group was related to insulin levels and to genetic polymorphism of FOXO3, two traits that have already been mechanistically linked to both GH action and control of human aging (Wijsman et al. 2011; Kahn 2015).
Studies in humans with various hereditary dwarfing syndromes (including Prop1-related hypopituitarism, isolated GH deficiency, and GH resistance) produced inconsistent findings with examples of reduced, unaltered, or possibly extended longevity (Krzisnik et al. 1999; Besson et al. 2003; Oliveira et al. 2007; Laron 2008; Guevara-Aguirre 2011). However, both GH-resistant and GH-deficient individuals are remarkably protected from age-related chronic disease (Oliveira et al. 2007; Shechter et al. 2007; Guevara-Aguirre 2011; Steuerman et al. 2011) in spite of changes in body composition and serum lipids that would normally predict increased rather than reduced risk of cardiovascular disease, diabetes, and cancer.
It should be pointed out that in contrast to the negative association between adult body size and longevity within species (as discussed earlier in this section), larger species generally live longer than smaller species. Thus, whales and elephants live longer than horses or cows, horses and cows live longer than cats or dogs which, in turn, live longer than rats or mice. However, there are some notable exceptions with some bats, naked mole rats, and humans living much longer than would be expected from their body size. Recent studies suggest that longevity differences between species, although generally opposite from the intra-specific differences, are also related to the GH/IGF-1/mTOR signaling (Fushan et al. 2015). Moreover, among species belonging to the same taxonomical family, the smallest animals can have the longest rather than the shortest lifespan (Keil et al. 2015).
Mechanisms of extended longevity of GH-related mutants
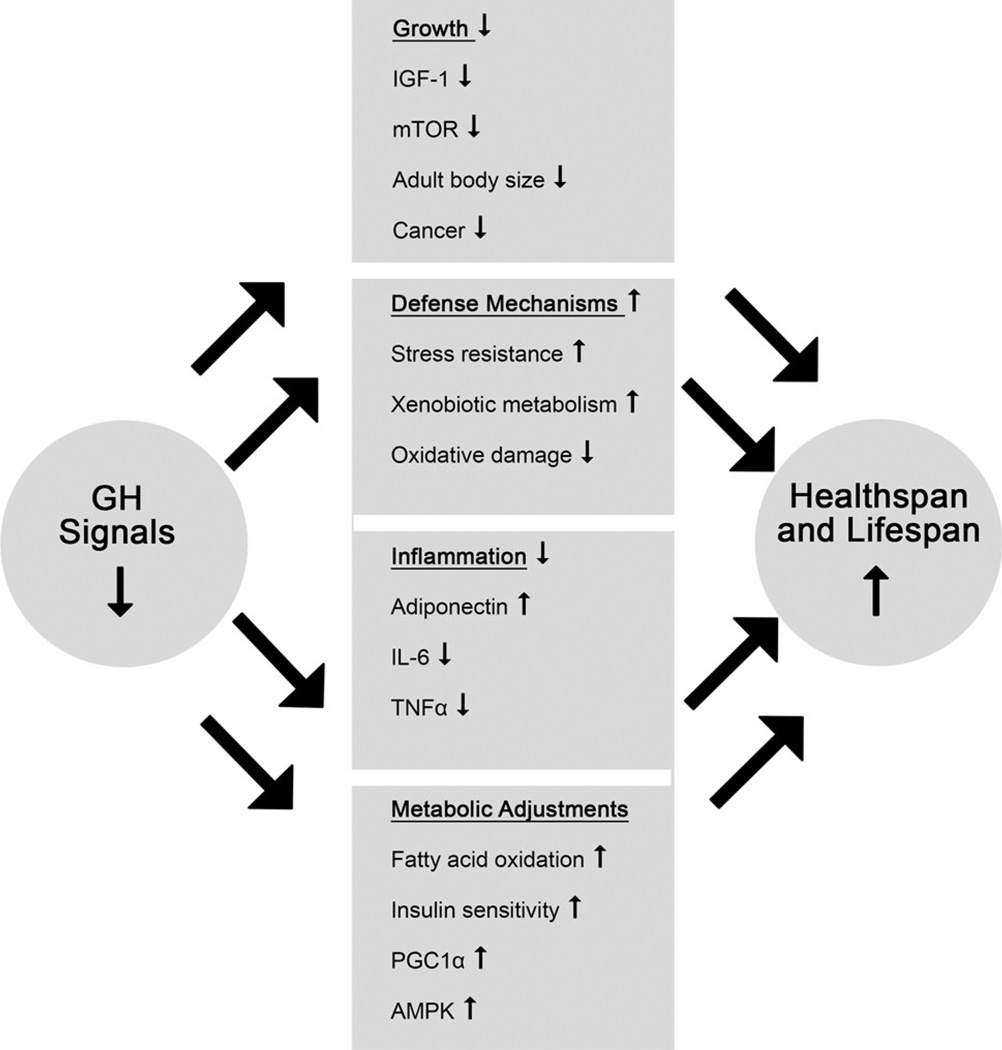
Following the demonstration that hypopituitary Ames and Snell dwarf mice and GH-resistant GHR-KO mice are long lived, much work was directed at identifying the mechanisms responsible for this somewhat counterintuitive benefit of the absence of GH action. Most of the studies that have been conducted to date involved comparisons of characteristics known or suspected to be causally related to aging in long-lived mutants and in the normal (wild type) animals of the same chronological age. In interpreting the results of these studies, it is important to remember that the detected difference may represent not only putative mechanisms of aging but also markers of a different biological age in animals that are almost certainly aging at a different rate. This potential difficulty was addressed directly in the study of Panici et al. (2009), who compared selected characteristics of GHR-KO mice to normal animals of the same age and also to normal animals that lived approximately the same percentage of their life expectancy as the “knockouts.” The results revealed that for the hepatic expression of genes related to GH and insulin signaling, the differences between GHR-KO and normal animals were not due to comparing animals of different biological age. Candidate mechanisms of delayed and/or slower aging of mice with GH-related mutations were recently reviewed (Bartke 2011; Bartke et al. 2013; Brown-Borg 2015) and their detailed analysis is outside the scope of this article. Key findings are listed in Table 1 and Fig. 1. The picture that emerges from the available evidence is that reduced GH action and extended longevity are mechanistically linked by multiple direct and indirect mechanisms. These mechanisms may interact in a complex way. For example, reduced inflammation, reduced mTOR (particularly mTORCI), and reduced insulin levels in the long-lived GH-related mutants have all been proposed to represent important mechanisms of slower/delayed aging, while they also contribute to improved insulin sensitivity which represents yet another mechanism of slowing the aging process and extending longevity. Differential impact of reduced GH signaling on the expression of the same genes in different tissues (Bonkowski et al. 2009; Masternak et al. 2009; Gesing et al. 2011; Masternak and Bartke 2012; Masternak et al. 2012) represents yet another level of complexity. We do not know which of the many consequences of the absence of GH action in these animals may represent the primary mechanisms of extended longevity. At this juncture, it appears equally plausible that it is the summation and interaction of these multiple effects that produces the phenotype of healthy aging and long life.
Table 1.
Proposed mechanisms of slower and/or delayed aging in mice with growth hormone (GH)-related mutations
| Mechanism | Ames dwarf Prop1df |
Snell dwarf Pit1dw |
Little Ghrhrlit |
Ghrh−/− | Ghr−/− |
|---|---|---|---|---|---|
| Reduced oxidative stress | X | X | |||
| Improved anti-oxidant defenses | X | ||||
| Stress resistance | X | X | X | X | |
| Reduced IGF-1 levels and adult body size | X | X | X | X | X |
| Reduced mTOR signaling | X | X | X | ||
| Increased mTORC2 signaling | X | X | X | ||
| Increased adiponectin levels | X | X | X | X | |
| Reduced proinflammatory cytokines | X | X | |||
| Reduced insulin levels | X | X | X♂ | X | |
| Increased insulin sensitivity | X | X | X | X | X |
| Reduced glucose levels | X | X | X | X | |
| Reduced serum lipids | X | X | X | ||
| Reduced body temperature | X | X | X | ||
| Reduced respiratory exchange ratio (RER) | X | X | |||
| Reduced blood pressure | X | X | |||
| Reduced number of senescent cells in adipose tissue | X | X | X | ||
| Increased number of very small embryonic-like stem cells (VSELs) |
X | ||||
| Improved genome maintenance | X |
Please note that many of the suspected anti-aging mechanisms were examined in only two or three of the listed mutants. Thus, most of the blank spaces represent lack of data rather than lack of relevances of the corresponding mechanism
Fig. 1.
Mechanisms believed to be involved in linking GH signaling with healthspan and lifespan. Adapted from Bartke et al. (2013)
Is aging “programmed” by GH actions during development?
Attempts to understand how major defects in normal endocrine function (absence of GH or GH receptors) can be beneficial for life expectancy, include speculations that the detrimental (“pro-aging”) aspects of GH signaling have not been eliminated by natural selection because of the importance of the beneficial impact of GH on characteristics closely related to evolutionary fitness: growth, sexual maturation, and fertility. This reasoning would imply a divergent role of GH in the control of aging at different stages of the life history. Combined with controversial but persistent reports that GH treatment can have various “anti-aging” or “rejuvenating” effects in elderly individuals (Rudman et al. 1990; Sonksen 2013; Ashpole et al. 2015, Krantz et al. 2015), this concept provided impetus for examining the effects of GH in juvenile as compared to adult animals.
To determine how GH signaling at different stages of life history influences aging and lifespan, we are using relatively brief (6 weeks) periods of GH replacement therapy in animals with congenital GH deficiency starting at different ages. Results available to date indicate that the pre- and peri-pubertal period which is normally characterized by rapid somatic growth is particularly important in the control of aging and longevity by GH. Following GH treatment between 1 and 7 weeks or 2 and 8 weeks of age, multiple phenotypic characteristics measured 7–18 months later were partially or completely normalized, and longevity was markedly reduced (Panici et al. 2010, Sun et al. unpublished, Sadagurski et al. 2015). Importantly, the characteristics that are normalized in Ames dwarf mice by GH treatment during development include levels of insulin and adiponectin, oxygen consumption, respiratory quotient, and markers of hypothalamic inflammation (Sadagurski et al. 2015, Sun et al. unpublished), traits that are believed responsible for extended longevity of these animals. Apparently, GH actions during the first 10 weeks of postnatal life are important for programing the mechanisms and the trajectory of aging, most likely by epigenetic means.
Which characteristics of long-lived GH-related mutants may account for their healthy aging?
As described earlier in this article, there is considerable evidence that the incidence of age-related disease is reduced and/or delayed in GH-deficient and GH-resistant mice and that these animals have a longer “healthspan” (Flurkey et al. 2001; Ikeno et al. 2009; Bartke 2011). At an advanced age, these mutants exhibit better neuromuscular function, including strength and coordination (Arum et al. 2013), improved learning and memory (Kinney et al. 2001; Kinney-Forshee et al. 2004), more effective glucose homeostasis (Arum et al. 2014), and various indices of delayed reproductive aging (Sluczanowska-Glabowska et al. 2012; Schneider et al. 2014, and unpublished data). These characteristics could be interpreted as symptoms of delayed and/or slower aging and we believe that they represent causal relationships. More specifically, we hypothesize that the delay and slowing of the biological process of aging is an important, and most likely the key mechanism linking reduced GH signaling with extension of healthspan. This hypothesis may also apply to other examples of delayed aging. As was already mentioned, increased lifespan is associated with longer healthspan in experimental animals exposed to chronic calorie restriction (Weindruch 1992; Mattison et al. 2012; Colman et al. 2014), and in humans with exceptional longevity (Fries et al. 2011; Andersen et al. 2012; Sun et al. 2013) and, with the exception of some characteristics, this may apply also to rapamycin-treated mice which live longer than untreated control animals (Halloran et al. 2012 Zhang et al. 2014).
Aging and chronic disease are intertwined and very difficult to separate, fueling persistent discussions whether aging indeed exists as a process distinct from pathology and disease. The fact that chronological age is a major (and generally the leading) risk factor for multiple ailments has been known for millennia and is amply confirmed by modern epidemiological studies. Symptoms of various chronic diseases overlap with many symptoms of aging with diabetes providing the best example. Indeed, many consequences of diabetes can be interpreted as symptoms of accelerated aging and various aspects of general health status and functionality of individuals with diabetes resemble those in otherwise healthy individuals who are chronologically older (Cigolle et al. 2011). Interestingly, some functional characteristics of the long-lived GH-related mouse mutants are roughly opposite to those encountered in individuals with diabetes, and even more clearly opposite to those present in individuals with metabolic syndrome or “pre-diabetes.” The most striking of these characteristics are improved glucose homeostasis with enhanced insulin sensitivity and a modest reduction in insulin levels, reduced markers of inflammation, and increased levels of adiponectin.
Improved insulin signaling
Enhanced insulin sensitivity is one of the consistent physiological characteristics of GH-deficient and GH-resistant mice (Hsieh and Papaconstantinou 2009; Bartke 2011; Bartke et al. 2013, Brown-Borg 2015). In Snell dwarf, Ames dwarf, GHRH −/−, and GHR−/− mice peripheral levels of insulin, glucose or, most commonly, both are significantly lower than in their normal siblings studied under identical conditions. Enhanced insulin sensitivity in these animals was confirmed by following the time course of changes in blood glucose levels after intraperitoneal insulin injection (insulin tolerance test), by calculating the HOMA score and, in the case of Ames dwarf mice, also by hyperinsulinemic-euglycemic clamps (Wiesenborn et al. 2014). Glucose tolerance in long-lived GH-related mutants was reported as enhanced, normal, or reduced, apparently depending on the sex and age of the animals (Dominici et al. 2002; Panici et al. 2010; Bartke 2011; Bartke et al. 2013). Limited ability to “clear” injected glucose, particularly within the first 15 min after administration, is undoubtedly related to the reduced development of pancreatic beta cells in animals with severely reduced circulating IGF-1 levels (Parsons et al. 1995; Liu et al. 2004). We have shown that Ames dwarf and −/− mice have reduced ability to release insulin in response to acute stimulation by intraperitoneally injected glucose load or refeeding after a fast (Bartke 2011; Bartke et al. 2013, and unpublished data). Importantly, in Ames dwarf mice, enhanced insulin sensitivity was shown to persist into advanced age (Arum et al. 2014).
Although a rigorous proof of the causal relationship of glucose homeostasis to the risk of age-related disease is difficult to obtain, the existence of such a relationship is indirectly supported by countless epidemiological studies and by the observations in calorie-restricted animals. In mammalian species ranging from mice to men, reduced insulin levels and enhanced insulin sensitivity are among the most striking and consistent physiological responses to calorie restriction, the most effective anti-aging intervention (Weindruch and Walford 1988; Fontana et al. 2010; Ravussin 2015). Reduced insulin levels and enhanced insulin sensitivity have been found in the offspring of long-lived humans (Rozing 2010; Wijsman et al. 2011a, 2011b). These individuals are significantly healthier than their spouses or partners and have reduced mortality (Westendorp 2009), presumably reflecting enrichment in genetic polymorphisms favoring slower aging. Moreover, opposite changes in glucose homeostasis, hyperinsulinemia and insulin resistance, as well as its extreme dysregulation in diabetes represent known risk factors for cardiovascular disease, cancer, and almost certainly dementia, as well as premature death (Cigolle et al. 2011; Collaboration et al. 2011; Musi and Bartke 2016). Mechanistically, both hyperinsulinemia and hyperglycemia have been related to age-related pathology and aging (Baynes and Monnier 1989; Parr 1996; Lambert et al. 2004).
In further support of the importance of tight glucose control in healthy aging, longevity in mice can be extended by antidiabetic drugs including metformin (Martin-Montalvo et al. 2013) which reduces insulin resistance and inhibits hepatic gluconeogenesis, by closely related phenformin (Anisimov et al. 2003; Anisimov and Bartke 2013) and by acarbose which delays absorption of ingested carbohydrates (Harrison et al. 2014). Moreover, both phenformin and metformin reduced the incidence of cancer in aging laboratory rodents (Anisimov et al. 2003; Anisimov and Bartke 2013; Martin-Montalvo et al. 2013).
It needs to be mentioned that increased insulin sensitivity is not a universal finding in mice in which longevity of one or both sexes has been extended by disruption or overexpression of various genes (Kurosu et al. 2005; Taguchi et al. 2007; Selman et al. 2009). However, it is not clear whether the discrepancy between these findings is not more apparent than real. It was suggested that insulin resistance can be beneficial by protecting the animals or particular organ systems from detrimental actions of hyperinsulinemia (Barzilai et al. 2012; Kim et al. 2015). It can also be speculated that the anti-aging action of reduced insulin signaling can be achieved either by reducing insulin levels or by developing insulin resistance (Kurosu et al. 2005; Taguchi et al. 2007; Selman et al. 2009; Barzilai et al. 2012).
Reduced inflammation
Chronic low-grade sterile inflammation “inflammaging” is considered as both a common symptom and an important mechanism of aging and has been linked to the risk of multiple chronic non-infectious diseases. In groundbreaking studies, the D. Cai laboratory provided evidence that links hypothalamic inflammation with obesity, insulin resistance, and the progression of aging (Tang et al. 2015). In the long-lived GH-related mutant mice, expression of proinflammatory markers is reduced in the perigonadal fat, circulating blood, and various brain regions including the hypothalamus (Wang et al. 2006, Dixit, et al. in press, Masternak et al. 2012; Hascup et al. 2015; Sadagurski et al. 2015) and glial activation is also suppressed (Sadagurski et al. 2015). In most, although not all cases, reduced levels of mRNA coding for proinflammatory cytokines are accompanied by the corresponding changes in the levels of these proteins (Wang et al. 2006; Masternak et al. 2012, Baquedano et al. 2014; Hascup et al. 2015). In addition to reduced expression of proinflammatory cytokines IL-6, TNFα, and IL-1β, GH-deficient and GH-resistant mice have increased circulating levels of adiponectin, a very important anti-inflammatory factor (Berryman et al. 2004; Bartke et al. 2013; Sun et al. 2013; Brown-Borg 2015).
Increased levels of adiponectin
Improved insulin signaling and reduced inflammation, two of the presumed key mechanisms of healthy aging of GH-related mutants can be related to increased levels of adiponectin in these animals. Both total adiponectin and the high molecular weight fraction believed to be responsible for most of its biological effects are elevated in GHRH−/−, GHR−/−, and in the hypopituitary dwarf mice (Berryman et al. 2004; Lubbers et al. 2013). In these long-lived GH-related mutants, adiponectin levels are elevated in both sexes. In Ames dwarf mice, elevation of plasma adiponectin levels was shown to persist as the animals age (Louis et al. 2010). Interestingly, adiponectin levels were normal or reduced rather than increased in mice with selective deletion of GH receptors in the adipose tissue which reduced rather than extended longevity (List et al. 2013). In addition to its anti-inflammatory and anti-atherogenic effects and its ability to enhance insulin sensitivity, adiponectin exerts antifibrotic and cardio-protective actions (Park and Sweeney 2013; Jahng et al. 2015; Matsuda et al. 2015), reduces detrimental influence of high-fat diet on glucose homeostasis (Liu et al. 2013, 2015) and improves lipid metabolism in insulinopenic animals (Ye et al. 2014). In humans, low adiponectin levels increase susceptibility to metabolic syndrome, diabetes, and cardiovascular disease (Chedraui et al. 2014; Ahl et al. 2015; Matsuda et al. 2015). The suspected causal link between adiponectin and longevity is consistent with the findings of increased adiponectin levels in centenarians from different, genetically distinct populations (Paolisso et al. 1996; Bonafe et al. 2003; Kojima et al. 2004; Bik et al. 2006).
Other mechanisms of reduced risk of chronic disease in GH-related mutants
Our emphasis on increased adiponectin levels, reduced inflammation, and improved insulin signaling as likely mechanisms of reduced risk of chronic disease in GH-deficient and GH-resistant mice is not meant to distract from the likely importance of multiple other mechanisms (Table 1; Fig. 1). Severe reduction of circulating IGF-1 levels in all long-lived GH-related mutants undoubtedly contributes to the reduced incidence and/or delayed onset of neoplastic disease in these animals (Vergara et al. 2004; Ikeno et al. 2009, reviewed in Bartke 2011; Bartke et al. 2013; Brown-Borg 2015). Improved anti-oxidant defenses (Hsieh et al. 2002; Romanick et al. 2004;Brown-Borg 2015) and stress resistance (Bokov et al. 2009; Bartke et al. 2013; Brown-Borg 2015), reduced blood pressure (Egecioglu et al. 2007), reduced accumulation of senescent cells (Stout et al. 2014), improved maintenance of stem cells, and thus presumably the potential for tissue repair (Ratajczak et al. 2011), as well as reduced mTORC1 and enhanced mTORC2 signaling (Sharp and Bartke 2005, Dominick et al. 2015) and profound alterations in lipid homeostasis (Boylston et al. 2004; Westbrook 2012) represent other potential mechanisms of increased disease resistance and “healthy aging” of GH-deficient and GH-resistant animals.
At the molecular level, the suspected mechanisms of delayed aging in hypopituitary, GH-deficient and GH-resistant mice include organ-specific increases in the expression and/or activation of PPARγ, PPARα, PGC1α, and AMPK, reduced inhibitory (serine) phosphorylation of IRS-1, and multiple changes in the expression of genes related to carbohydrate, lipid, and energy metabolism (reviewed in Bartke et al. 2013, Brown-Borg 2015), as well as expression of various micro RNAs (Bates et al. 2010; Victoria et al. 2015).
Could reduced GH signaling have any advantages under natural conditions?
It is often difficult to determine to what extent findings obtained in domesticated laboratory animals living under highly controlled and artificial conditions of a research facility may apply to their wild ancestors living in the natural environment.
In the context of this article, we want to consider the question of how the characteristics of animals with GH-related mutations could relate to individual and population survival “in the wild” and to the process of natural selection. The extreme phenotypes of Ames dwarf, Snell dwarf, or Ghr−/− mice establish causal association of reduced GH signaling with delayed puberty, reduced fecundity, slowed aging, and reduced risk of age-related frailty and disease. This indicates that individuals at the “low end” of the normal range of variation in GH levels and action will exhibit similar, although less pronounced characteristics. We hypothesize that under natural conditions, these individuals would be less successful in producing offspring in the first year of their life but will be more likely to survive winter and reproduce in the second year. Proposing a winter survival advantage of smaller individuals may seem counterintuitive but it is based on observations in other species of small non-hibernating rodents. Djungarian hamsters (Phodopus sungorus) are approximately the same size as mice, live in the harsh environment of central Asia, and every fall undergo a major loss of body weight (Niklowitz and Hoffmann 1988). Apparently the benefits of reduced energy requirements for the maintenance of a smaller body outweigh the likely disadvantages of altered surface to mass ratio and reduced insulation which promote heat loss.
Under the proposed scenario, the combination of delayed sexual maturation, reduced reproductive effort early in life, increased stress and disease resistance, reduced adult body weight, and slower aging of animals with lower GH signaling would improve their chances of surviving the winter which represents a major challenge for small mammals living at intermediate or high geographical latitudes. Thus these animals would have a better chance to produce offspring in the ensuing spring and genes favoring somewhat lower GH signaling would persist in the population. The evidence for a delay in reproductive aging of GH-related mutants which was mentioned earlier would seem consistent with the proposed hypothesis. The broader issue of balancing evolutionary adaptation to the environment with the advantages of maintaining diversity of responses to environmental challenges by preserving underlying genetic variation within the population and question of the mechanisms (or indeed existence) of individual versus group selection are outside the scope of this brief review.
Acknowledgments
Work described in this article was supported by NIH P01AG031736 and R01AG019899. We thank Dr. O. Arum, A. Spong, and other members of our laboratory who were importantly involved in various stages of this research and we apologize to those whose work pertinent to the discussed issues we have failed to mention or cite.
References
- Ahl S, Guenther M, Zhao S, James R, Marks J, Szabo A, Kidambi S. Adiponectin levels differentiate metabolically healthy vs unhealthy among obese and nonobese white individuals. J Clin Endocrinol Metab. 2015;100(11):4172–4180. doi: 10.1210/jc.2015-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Sebastiani P, Dworkis DA, Feldman L, Perls TT. Health span approximates life span among many supercentenarians: compression of morbidity at the approximate limit of life span. J Gerontol A. 2012;67(4):395–405. doi: 10.1093/gerona/glr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN, Bartke A. The key role of growth hormone-insulin-IGF-1 signaling in aging and cancer. Crit Rev Oncol Hematol. 2013;87(3):201–223. doi: 10.1016/j.critrevonc.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN, Semenchenko AV, Yashin AI. Insulin and longevity: antidiabetic biguanides as geroprotectors. Biogeron-tology. 2003;4(5):297–307. doi: 10.1023/a:1026299318315. [DOI] [PubMed] [Google Scholar]
- Arum O, Rasche ZA, Rickman DJ, Bartke A. Prevention of neuromusculoskeletal frailty in slow-aging ames dwarf mice: longitudinal investigation of interaction of longevity genes and caloric restriction. PLoS ONE. 2013;8(10):e72255. doi: 10.1371/journal.pone.0072255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arum O, Saleh JK, Boparai RK, Kopchick JJ, Khardori RK, Bartke A. Preservation of blood glucose homeostasis in slow-senescing somatotrophism-deficient mice subjected to intermittent fasting begun at middle or old age. Age (Dordr) 2014;36(3):9651. doi: 10.1007/s11357-014-9651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashpole NM, Sanders JE, Hodges EL, Yan H, Sonntag WE. Growth hormone, insulin-like growth factor-1 and the aging brain. Exp Gerontol. 2015;68:76–81. doi: 10.1016/j.exger.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal A, Zhu LJ, Yen K, Tissenbaum HA. Uncoupling lifespan and healthspan in Caenorhabditis elegans longevity mutants. Proc Natl Acad Sci U S A. 2015;112(3):E277–E286. doi: 10.1073/pnas.1412192112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baquedano E, Ruiz-Lopez AM, Sustarsic EG, Herpy J, List EO, Chowen JA, Frago LM, Kopchick JJ, Argente J. The absence of GH signaling affects the susceptibility to high-fat diet-induced hypothalamic inflammation in male mice. Endocrinology. 2014;155(12):4856–4867. doi: 10.1210/en.2014-1367. [DOI] [PubMed] [Google Scholar]
- Bartke A. Histology of the anterior hypophysis, thyroid and gonads of two types of dwarf mice. Anat Rec. 1964;149:225–235. doi: 10.1002/ar.1091490206. [DOI] [PubMed] [Google Scholar]
- Bartke A. Growth hormone and aging. Endocrine. 1998;8(2):103–108. doi: 10.1385/ENDO:8:2:103. [DOI] [PubMed] [Google Scholar]
- Bartke A. Single-gene mutations and healthy ageing in mammals. Philos Trans R Soc Lond B. 2011;366(1561):28–34. doi: 10.1098/rstb.2010.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A, Sun LY, Longo V. Somatotropic signaling: tradeoffs between growth, reproductive development, and longevity. Physiol Rev. 2013;93(2):571–598. doi: 10.1152/physrev.00006.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61(6):1315–1322. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates DJ, Li N, Liang R, Sarojini H, An J, Masternak MM, Bartke A, Wang E. MicroRNA regulation in Ames dwarf mouse liver may contribute to delayed aging. Aging Cell. 2010;9(1):1–18. doi: 10.1111/j.1474-9726.2009.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baynes JW, Monnier VM. In: The maillard reaction in aging, diabetes and nutrition. Liss Alan R., editor. New York: 1989. [Google Scholar]
- Berryman DE, List EO, Coschigano KT, Behar K, Kim JK, Kopchick JJ. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm IGF Res. 2004;14(4):309–318. doi: 10.1016/j.ghir.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Berryman DE, Lubbers ER, Magon V, List EO, Kopchick JJ. A dwarf mouse model with decreased GH/IGF-1 activity that does not experience life-span extension: potential impact of increased adiposity, leptin, and insulin with advancing age. J Gerontol A. 2014;69(2):131–141. doi: 10.1093/gerona/glt069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson A, Salemi S, Gallati S, Jenal A, Horn R, Mullis PS, Mullis PE. Reduced longevity in untreated patients with isolated growth hormone deficiency. J Clin Endocrinol Metab. 2003;88(8):3664–3667. doi: 10.1210/jc.2002-021938. [DOI] [PubMed] [Google Scholar]
- Bik W, Baranowska-Bik A, Wolinska-Witort E, Martynska L, Chmielowska M, Szybinska A, Broczek K, Baranowska B. The relationship between adiponectin levels and metabolic status in centenarian, early elderly, young and obese women. Neuro Endocrinol Lett. 2006;27(4):493–500. [PubMed] [Google Scholar]
- Bokov AF, Lindsey ML, Khodr C, Sabia MR, Richardson A. Long-lived ames dwarf mice are resistant to chemical stressors. J Gerontol A. 2009;64(8):819–827. doi: 10.1093/gerona/glp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonafe M, Barbieri M, Marchegiani F, Olivieri F, Ragno E, Giampieri C, Mugianesi E, Centurelli M, Franceschi C, Paolisso G. Polymorphic variants of insulin-like growth factor I (IGF-I) receptor and phosphoinositide 3-kinase genes affect IGF-I plasma levels and human longevity: cues for an evolutionarily conserved mechanism of life span control. J Clin Endocrinol Metab. 2003;88(7):3299–3304. doi: 10.1210/jc.2002-021810. [DOI] [PubMed] [Google Scholar]
- Bonkowski MS, Dominici FP, Arum O, Rocha JS, Al Regaiey KA, Westbrook R, Spong A, Panici J, Masternak MM, Kopchick JJ, Bartke A. Disruption of growth hormone receptor prevents calorie restriction from improving insulin action and longevity. PLoS ONE. 2009;4(2):e4567. doi: 10.1371/journal.pone.0004567. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Boylston WH, Gerstner A, DeFord JH, Madsen M, Flurkey K, Harrison DE, Papaconstantinou J. Altered cholesterologenic and lipogenic transcriptional profile in livers of aging Snell dwarf (Pit1dw/dwJ) mice. Aging Cell. 2004;3(5):283–296. doi: 10.1111/j.1474-9728.2004.00115.x. [DOI] [PubMed] [Google Scholar]
- Brosnahan MM, Paradis MR. Demographic and clinical characteristics of geriatric horses: 467 cases (1989–1999) J Am Vet Med Assoc. 2003;223(1):93–98. doi: 10.2460/javma.2003.223.93. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM. The somatotropic axis and longevity in mice. Am J Physiol Endocrinol Metab. 2015;309(6):E503–E510. doi: 10.1152/ajpendo.00262.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384(6604):33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- Chedraui P, Perez-Lopez FR, Escobar GS, Palla G, Montt-Guevara M, Cecchi E, Genazzani AR, Simoncini TP. Research Group for the Omega Women’s Health. Circulating leptin, resistin, adiponectin, visfatin, adipsin and ghrelin levels and insulin resistance in postmenopausal women with and without the metabolic syndrome. Maturitas. 2014;79(1):86–90. doi: 10.1016/j.maturitas.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Cigolle CT, Lee PG, Langa KM, Lee YY, Tian Z, Blaum CS. Geriatric conditions develop in middle-aged adults with diabetes. J Gen Intern Med. 2011;26(3):272–279. doi: 10.1007/s11606-010-1510-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaboration ERF. Seshasai SR, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, Njolstad I, Fletcher A, Nilsson P, Lewington S, Collins R, Gudnason V, Thompson SG, Sattar N, Selvin E, Hu FB, Danesh J. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun. 2014;5:3557. doi: 10.1038/ncomms4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144(9):3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- Dominici FP, Hauck S, Argentino DP, Bartke A, Turyn D. Increased insulin sensitivity and upregulation of insulin receptor, insulin receptor substrate (IRS)-I and IRS-2 in liver of Ames dwarf mice. J Endocrinol. 2002;173:81–94. doi: 10.1677/joe.0.1730081. [DOI] [PubMed] [Google Scholar]
- Dominick G, Berryman DE, List EO, Kopchick JJ, Li X, Miller RA, Garcia GG. Regulation of mTOR activity in Snell dwarf and GH receptor gene-disrupted mice. Endocrinology. 2015;156(2):565–575. doi: 10.1210/en.2014-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Andersson IJ, Bollano E, Palsdottir V, Gabrielsson BG, Kopchick JJ, Skott O, Bie P, Isgaard J, Bohlooly YM, Bergstrom G, Wickman A. Growth hormone receptor deficiency in mice results in reduced systolic blood pressure and plasma renin, increased aortic eNOS expression, and altered cardiovascular structure and function. Am J Physiol Endocrinol Metab. 2007;292(5):E1418–E1425. doi: 10.1152/ajpendo.00335.2006. [DOI] [PubMed] [Google Scholar]
- Fabris N, Pierpaoli W, Sorkin E. Lymphocytes, hormones, and ageing. Nature. 1972;240:557–559. doi: 10.1038/240557a0. [DOI] [PubMed] [Google Scholar]
- Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci USA. 2001;98(12):6736–6741. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Klein S, Holloszy JO. Effects of long-term calorie restriction and endurance exercise on glucose tolerance, insulin action, and adipokine production. Age (Dordr) 2010;32(1):97–108. doi: 10.1007/s11357-009-9118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries JF, Bruce B, Chakravarty E. Compression of morbidity 1980–2011: a focused review of paradigms and progress. J Aging Res. 2011;2011:261702. doi: 10.4061/2011/261702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fushan AA, Turanov AA, Lee SG, Kim EB, Lobanov AV, Yim SH, Buffenstein R, Lee SR, Chang KT, Rhee H, Kim JS, Yang KS, Gladyshev VN. Gene expression defines natural changes in mammalian lifespan. Aging Cell. 2015;14(3):352–365. doi: 10.1111/acel.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesing A, Masternak MM, Wang F, Joseph AM, Leeuwenburgh C, Westbrook R, Lewinski A, Karbownik-Lewinska M, Bartke A. Expression of key regulators of mitochondrial biogenesis in growth hormone receptor knockout (GHRKO) mice is enhanced but is not further improved by other potential life-extending interventions. J Gerontol A. 2011;66(10):1062–1076. doi: 10.1093/gerona/glr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer KA, Canterberry SC, Murphy KE. Statistical analysis regarding the effects of height and weight on life span of the domestic dog. Res Vet Sci. 2007;82(2):208–214. doi: 10.1016/j.rvsc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3(70) doi: 10.1126/scitranslmed.3001845. 70ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halloran J, Hussong SA, Burbank R, Podlutskaya N, Fischer KE, Sloane LB, Austad SN, Strong R, Richardson A, Hart MJ, Galvan V. Chronic inhibition of mammalian target of rapamycin by rapamycin modulates cognitive and non-cognitive components of behavior throughout lifespan in mice. Neuroscience. 2012;223:102–113. doi: 10.1016/j.neuroscience.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Allison DB, Ames BN, Astle CM, Atamna H, Fernandez E, Flurkey K, Javors MA, Nadon NL, Nelson JF, Pletcher S, Simpkins JW, Smith D, Wilkinson JE, Miller RA. Acarbose, 17-alpha-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13(2):273–282. doi: 10.1111/acel.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascup ER, Wang F, Kopchick JJ, Bartke A. Inflammatory and glutamatergic homeostasis are involved in successful aging. J Gerontol A. 2015 doi: 10.1093/gerona/glv010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Morris BJ, Grove JS, Petrovitch H, Ross W, Masaki KH, Rodriguez B, Chen R, Donlon TA, Willcox DC, Willcox BJ. Shorter men live longer: association of height with longevity and FOXO3 genotype in American men of Japanese ancestry. PLoS ONE. 2014;9(5):e94385. doi: 10.1371/journal.pone.0094385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CC, Papaconstantinou J. Dermal fibroblasts from long-lived Ames dwarf mice maintain their in vivo resistance to mitochondrial generated reactive oxygen species (ROS) Aging (Albany NY) 2009;1(9):784–802. doi: 10.18632/aging.100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CC, DeFord JH, Flurkey K, Harrison DE, Papaconstantinou J. Effects of the Pit 1 mutation on the insulin signaling pathway: implications on the longevity of the long-lived Snell dwarf mouse. Mech Ageing Dev. 2002;123:1244–1255. doi: 10.1016/s0047-6374(02)00037-4. [DOI] [PubMed] [Google Scholar]
- Ikeno Y, Hubbard GB, Lee S, Cortez LA, Lew CM, Webb CR, Berryman DE, List EO, Kopchick JJ, Bartke A. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J Gerontol A. 2009;64(5):522–529. doi: 10.1093/gerona/glp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahng JW, Turdi S, Kovacevic V, Dadson K, Li RK, Sweeney G. Pressure overload-induced cardiac dysfunction in aged male adiponectin knockout mice is associated with autophagy deficiency. Endocrinology. 2015;156(7):2667–2677. doi: 10.1210/en.2015-1162. [DOI] [PubMed] [Google Scholar]
- Kahn AJ. FOXO3 and related transcription factors in development, aging, and exceptional longevity. J Gerontol A. 2015;70(4):421–425. doi: 10.1093/gerona/glu044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil G, Cummings E, de Magalhaes JP. Being cool: how body temperature influences ageing and longevity. Biogerontology. 2015;16(4):383–397. doi: 10.1007/s10522-015-9571-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienzle E, Moik K. A pilot study of the body weight of purebred client-owned adult cats. Br J Nutr. 2011;106(Suppl 1):S113–S115. doi: 10.1017/S0007114511001802. [DOI] [PubMed] [Google Scholar]
- Kim KS, Lee YM, Lee IK, Kim DJ, Jacobs DR, Jr, Lee DH. Paradoxical associations of insulin resistance with total and cardiovascular mortality in humans. J Gerontol A. 2015;70(7):847–853. doi: 10.1093/gerona/glu194. [DOI] [PubMed] [Google Scholar]
- Kinney BA, Meliska CJ, Steger RW, Bartke A. Evidence that Ames dwarf mice age differently from their normal siblings in behavioral and learning and memory parameters. Horm Behav. 2001;39(4):277–284. doi: 10.1006/hbeh.2001.1654. [DOI] [PubMed] [Google Scholar]
- Kinney-Forshee BA, Kinney NE, Steger RW, Bartke A. Could a deficiency in growth hormone signaling be beneficial to the aging brain? Physiol Behav. 2004;80(5):589–594. doi: 10.1016/j.physbeh.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Kojima T, Kamei H, Aizu T, Arai Y, Takayama M, Nakazawa S, Ebihara Y, Inagaki H, Masui Y, Gondo Y, Sakaki Y, Hirose N. Association analysis between longevity in the Japanese population and polymorphic variants of genes involved in insulin and insulin-like growth factor 1 signaling pathways. Exp Gerontol. 2004;39(11–12):1595–1598. doi: 10.1016/j.exger.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Krantz E, Trimpou P, Landin-Wilhelmsen K. Effect of growth hormone treatment on fractures and quality of life in post-menopausal osteoporosis: a 10-year follow-up study. J Clin Endocrinol Metab. 2015;100(9):3251–3259. doi: 10.1210/jc.2015-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzisnik C, Kolacio Z, Battelino T, Brown M, Parks JS, Laron Z. The “Little People” of the island of Krk—revisited. Etiology of hypopituitarism revealed. J Endocr Genet. 1999;1:9–19. [Google Scholar]
- Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005;309(5742):1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert AJ, Wang B, Merry BJ. Exogenous insulin can reverse the effects of caloric restriction on mitochondria. Biochem Biophys Res Commun. 2004;316(4):1196–1201. doi: 10.1016/j.bbrc.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Laron Z. The GH-IGF1 axis and longevity. The paradigm of IGF1 deficiency. Hormones (Athens) 2008;7(1):24–27. doi: 10.14310/horm.2002.1111034. [DOI] [PubMed] [Google Scholar]
- Li W, Li X, Miller RA. ATF4 activity: a common feature shared by many kinds of slow-aging mice. Aging Cell. 2014;13(6):1012–1018. doi: 10.1111/acel.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- List EO, Berryman DE, Funk K, Gosney ES, Jara A, Kelder B, Wang X, Kutz L, Troike K, Lozier N, Mikula V, Lubbers ER, Zhang H, Vesel C, Junnila RK, Frank SJ, Masternak MM, Bartke A, Kopchick JJ. The role of GH in adipose tissue: lessons from adipose-specific GH receptor gene-disrupted mice. Mol Endocrinol. 2013;27(3):524–535. doi: 10.1210/me.2012-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- List EO, Berryman DE, Funk K, Jara A, Kelder B, Wang F, Stout MB, Zhi X, Sun L, White TA, LeBrasseur NK, Pirtskhalava T, Tchkonia T, Jensen EA, Zhang W, Masternak MM, Kirkland JL, Miller RA, Bartke A, Kopchick JJ. Liver-specific GH receptor gene-disrupted (LiGHRKO) mice have decreased endocrine IGF-I, increased local IGF-I, and altered body size, body composition, and adipokine profiles. Endocrinology. 2014;155(5):1793–1805. doi: 10.1210/en.2013-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- List EO, Berryman DE, Ikeno Y, Hubbard GB, Funk K, Comisford R, Young JA, Stout MB, Tchkonia T, Masternak MM, Bartke A, Kirkland JL, Miller RA, Kopchick JJ. Removal of growth hormone receptor (GHR) in muscle of male mice replicates some of the health benefits seen in global GHR−/− mice. Aging (Albany NY) 2015;7(7):500–512. doi: 10.18632/aging.100766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JL, Coschigano KT, Robertson K, Lipsett M, Guo Y, Kopchick JJ, Kumar U, Liu YL. Disruption of growth hormone receptor gene causes diminished pancreatic islet size and increased insulin sensitivity in mice. Am J Physiol Endocrinol Metab. 2004;287(3):E405–E413. doi: 10.1152/ajpendo.00423.2003. [DOI] [PubMed] [Google Scholar]
- Liu Y, Turdi S, Park T, Morris NJ, Deshaies Y, Xu A, Sweeney G. Adiponectin corrects high-fat diet-induced disturbances in muscle metabolomic profile and whole-body glucose homeostasis. Diabetes. 2013;62(3):743–752. doi: 10.2337/db12-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Palanivel R, Rai E, Park M, Gabor TV, Scheid MP, Xu A, Sweeney G. Adiponectin stimulates autophagy and reduces oxidative stress to enhance insulin sensitivity during high-fat diet feeding in mice. Diabetes. 2015;64(1):36–48. doi: 10.2337/db14-0267. [DOI] [PubMed] [Google Scholar]
- Longo VD, Antebi A, Bartke A, Barzilai N, Brown-Borg HM, Caruso C, Curiel TJ, de Cabo R, Franceschi C, Gems D, Ingram DK, Johnson TE, Kennedy BK, Kenyon C, Klein S, Kopchick JJ, Lepperdinger G, Madeo F, Mirisola MG, Mitchell JR, Passarino G, Rudolph KL, Sedivy JM, Shadel GS, Sinclair DA, Spindler SR, Suh Y, Vijg J, Vinciguerra M, Fontana L. Interventions to slow aging in humans: are we ready? Aging Cell. 2015;14(4):497–510. doi: 10.1111/acel.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis A, Bartke A, Masternak MM. Effects of growth hormone and thyroxine replacement therapy on insulin signaling in Ames dwarf mice. J Gerontol A. 2010;65(4):344–352. doi: 10.1093/gerona/glq018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubbers ER, List EO, Jara A, Sackman-Sala L, Cordoba-Chacon J, Gahete MD, Kineman RD, Boparai R, Bartke A, Kopchick JJ, Berryman DE. Adiponectin in mice with altered GH action: links to insulin sensitivity and longevity? J Endocrinol. 2013;216(3):363–374. doi: 10.1530/JOE-12-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, Schwab M, Pollak M, Zhang Y, Yu Y, Becker KG, Bohr VA, Ingram DK, Sinclair DA, Wolf NS, Spindler SR, Bernier M, de Cabo R. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masternak MM, Bartke A. Growth hormone, inflammation and aging. Pathobiol Aging Age Relat Dis. 2012 doi: 10.3402/pba.v2i0.17293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masternak MM, Panici JA, Bonkowski MS, Hughes LF, Bartke A. Insulin sensitivity as a key mediator of growth hormone actions on longevity. J Gerontol A. 2009;64(5):516–521. doi: 10.1093/gerona/glp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masternak MM, Bartke A, Wang F, Spong A, Gesing A, Fang Y, Salmon AB, Hughes LF, Liberati T, Boparai R, Kopchick JJ, Westbrook R. Metabolic effects of intra-abdominal fat in GHRKO mice. Aging Cell. 2012;11(1):73–81. doi: 10.1111/j.1474-9726.2011.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K, Fujishima Y, Maeda N, Mori T, Hirata A, Sekimoto R, Tsushima Y, Masuda S, Yamaoka M, Inoue K, Nishizawa H, Kita S, Ranscht B, Funahashi T, Shimomura I. Positive feedback regulation between adiponectin and T-cadherin impacts adiponectin levels in tissue and plasma of male mice. Endocrinology. 2015;156(3):934–946. doi: 10.1210/en.2014-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489(7415):318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB, Bartke A, O’Callaghan JP. Increased glial fibrillary acidic protein (GFAP) levels in the brains of transgenic mice expressing the bovine growth hormone (bGH) gene. Exp Gerontol. 1995;30(3–4):383–400. doi: 10.1016/0531-5565(94)00064-a. [DOI] [PubMed] [Google Scholar]
- Minina EA, Sanchez-Vera V, Moschou PN, Suarez MF, Sundberg E, Weih M, Bozhkov PV. Autophagy mediates caloric restriction-induced lifespan extension in Arabidopsis. Aging Cell. 2013;12(2):327–329. doi: 10.1111/acel.12048. [DOI] [PubMed] [Google Scholar]
- Musi N, Bartke A. Diabetes and aging. In: Sierra F, Kohanski R, editors. Advances in geroscience. New York: Springer International Publishing; 2016. pp. 355–376. [Google Scholar]
- Niklowitz P, Hoffmann K. Pineal and pituitary involvement in the photoperiodic regulation of body weight, coat color and testicular size of the Djungarian hamster, Phodopus sungorus. Biol Reprod. 1988;39(2):489–498. doi: 10.1095/biolreprod39.2.489. [DOI] [PubMed] [Google Scholar]
- Oliveira JL, Aguiar-Oliveira MH, D’Oliveira A, Jr, Pereira RM, Oliveira CR, Farias CT, Barreto-Filho JA, Anjos-Andrade FD, Marques-Santos C, Nascimento-Junior AC, Alves EO, Oliveira FT, Campos VC, Ximenes R, Blackford A, Parmigiani G, Salvatori R. Congenital growth hormone (GH) deficiency and atherosclerosis: effects of GH replacement in GH-naive adults. J Clin Endocrinol Metab. 2007;92(12):4664–4670. doi: 10.1210/jc.2007-1636. [DOI] [PubMed] [Google Scholar]
- Panici JA, Wang F, Bonkowski MS, Spong A, Bartke A, Pawlikowska L, Kwok PY, Masternak MM. Is altered expression of hepatic insulin-related genes in growth hormone receptor knockout mice due to GH resistance or a difference in biological life spans? J Gerontol A. 2009;64(11):1126–1133. doi: 10.1093/gerona/glp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panici JA, Harper JM, Miller RA, Bartke A, Spong A, Masternak MM. Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long-lived mutant mice. FASEB J. 2010;24(12):5073–5079. doi: 10.1096/fj.10-163253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolisso G, Gambardella A, Ammendola S, D’Amore A, Balbi V, Varricchio M, D’Onofrio F. Glucose tolerance and insulin action in healty centenarians. Am J Physiol. 1996;270(5 Pt 1):E890–E894. doi: 10.1152/ajpendo.1996.270.5.E890. [DOI] [PubMed] [Google Scholar]
- Park M, Sweeney G. Direct effects of adipokines on the heart: focus on adiponectin. Heart Fail Rev. 2013;18(5):631–644. doi: 10.1007/s10741-012-9337-8. [DOI] [PubMed] [Google Scholar]
- Parr T. Insulin exposure controls the rate of mammalian aging. Mech Ageing Dev. 1996;88(1–2):75–82. doi: 10.1016/0047-6374(96)01723-x. [DOI] [PubMed] [Google Scholar]
- Parsons JA, Bartke A, Sorenson RL. Number and size of islets of Langerhans in pregnant, human growth hormone-expressing transgenic, and pituitary dwarf mice: effect of lactogenic hormones. Endocrinology. 1995;136:2013–2021. doi: 10.1210/endo.136.5.7720649. [DOI] [PubMed] [Google Scholar]
- Patronek GJ, Waters DJ, Glickman LT. Comparative longevity of pet dogs and humans: implications for gerontology research. J Gerontol A. 1997;52(3):B171–B178. doi: 10.1093/gerona/52a.3.b171. [DOI] [PubMed] [Google Scholar]
- Ratajczak J, Shin DM, Wan W, Liu R, Masternak MM, Piotrowska K, Wiszniewska B, Kucia M, Bartke A, Ratajczak MZ. Higher number of stem cells in the bone marrow of circulating low Igf-1 level Laron dwarf mice-novel view on Igf-1, stem cells and aging. Leukemia. 2011;25(4):729–733. doi: 10.1038/leu.2010.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin E, Redman LM, Rochon J, Das SK, Fontana L, Kraus WE, Romashkan S, Williamson DA, Meydani SN, Villareal DT, Smith SR, Stein RI, Scott TM, Stewart TM, Saltzman E, Klein S, Bhapkar M, Martin CK, Gilhooly CH, Holloszy JO, Hadley EC, Roberts SB C. S. Group. A 2-year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. J Gerontol A. 2015;70(9):1097–1104. doi: 10.1093/gerona/glv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollo CD. Growth negatively impacts the life span of mammals. Evol Dev. 2002;4(1):55–61. doi: 10.1046/j.1525-142x.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- Romanick MA, Rakoczy SG, Brown-Borg HM. Long-lived Ames dwarf mouse exhibits increased antioxidant defense in skeletal muscle. Mech Ageing Dev. 2004;125(4):269–281. doi: 10.1016/j.mad.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Rozing MP, Westendorp RG, de Craen AJ, Frolich M, de Goeij MC, Heijmans BT, Beekman M, Wijsman CA, Mooijaart SP, Blauw GJ, Slagboom PE, van Heemst DG Leiden Longevity Study. Favorable glucose tolerance and lower prevalence of metabolic syndrome in offspring without diabetes mellitus of nonagenarian siblings: the Leiden longevity study.”. J Am Geriatr Soc. 2010;58(3):564–569. doi: 10.1111/j.1532-5415.2010.02725.x. [DOI] [PubMed] [Google Scholar]
- Rudman D, Feller AG, Nagraj HS, Gergans GA, Lalitha PY, Goldberg AF, Schlenker RA, Cohn L, Rudman IW, Mattson DE. Effects of human growth hormone in men over 60 years old. N Engl J Med. 1990;323(1):1–6. doi: 10.1056/NEJM199007053230101. [DOI] [PubMed] [Google Scholar]
- Sadagurski M, Landeryou T, Cady G, Kopchick JJ, List EO, Berryman DE, Bartke A, Miller RA. Growth hormone modulates hypothalamic inflammation in long-lived pituitary dwarf mice. Aging Cell. 2015;14(6):1045–1054. doi: 10.1111/acel.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaras TT, Bartke A, Rollo CD. Human body size and the laws of scaling: physiological, performance, growth, longevity and ecological ramifications. New York: Nova Science Publishers; 2007. [Google Scholar]
- Schneider A, Zhi X, Moreira F, Lucia T, Jr, Mondadori RG, Masternak MM. Primordial follicle activation in the ovary of Ames dwarf mice. J Ovarian Res. 2014;7:120. doi: 10.1186/s13048-014-0120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastiani P, Sun FX, Andersen SL, Lee JH, Wojczynski MK, Sanders JL, Yashin A, Newman AB, Perls TT. Families enriched for exceptional longevity also have increased healthspan: findings from the long life family study. Front Public Health. 2013;1:38. doi: 10.3389/fpubh.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D, Withers DJ. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326(5949):140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp ZD, Bartke A. Evidence for down-regulation of phosphoinositide 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR)-dependent translation regulatory signaling pathways in Ames dwarf mice. J Gerontol A. 2005;60(3):293–300. doi: 10.1093/gerona/60.3.293. [DOI] [PubMed] [Google Scholar]
- Shechter M, Ginsberg S, Scheinowitz M, Feinberg MS, Laron Z. Obese adults with primary growth hormone resistance (Laron Syndrome) have normal endothelial function. Growth Horm IGF Res. 2007;17(2):165–170. doi: 10.1016/j.ghir.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Shire JG. Growth hormone and premature ageing. Nature. 1973;245(5422):215–216. doi: 10.1038/245215a0. [DOI] [PubMed] [Google Scholar]
- Silberberg R. Articular aging and osteoarthrosis in dwarf mice. Pathol Microbiol (Basel) 1972;38(6):417–430. doi: 10.1159/000162458. [DOI] [PubMed] [Google Scholar]
- Sluczanowska-Glabowska S, Laszczynska M, Piotrowska K, Glabowski W, Kopchick JJ, Bartke A, Kucia M, Ratajczak MZ. Morphology of ovaries in laron dwarf mice, with low circulating plasma levels of insulin-like growth factor-1 (IGF-1), and in bovine GH-transgenic mice, with high circulating plasma levels of IGF-1. J Ovarian Res. 2012;5:18. doi: 10.1186/1757-2215-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonksen P. Idiopathic growth hormone deficiency in adults, Ben Johnson and the somatopause. J Clin Endocrinol Metab. 2013;98(6):2270–2273. doi: 10.1210/jc.2013-2025. [DOI] [PubMed] [Google Scholar]
- Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O’Connell SM, Gukovsky I, Carriere C, Ryan AK, Miller AP, Zuo L, Gleiberman AS, Andersen B, Beamer WG, Rosenfeld MG. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature. 1996;384(6607):327–333. doi: 10.1038/384327a0. [DOI] [PubMed] [Google Scholar]
- Steuerman R, Shevah O, Laron Z. Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies. Eur J Endocrinol. 2011;164(4):485–489. doi: 10.1530/EJE-10-0859. [DOI] [PubMed] [Google Scholar]
- Stout MB, Tchkonia T, Pirtskhalava T, Palmer AK, List EO, Berryman DE, Lubbers ER, Escande C, Spong A, Masternak MM, Oberg AL, LeBrasseur NK, Miller RA, Kopchick JJ, Bartke A, Kirkland JL. Growth hormone action predicts age-related white adipose tissue dysfunction and senescent cell burden in mice. Aging (Albany NY) 2014;6(7):575–586. doi: 10.18632/aging.100681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun LY, Spong A, Swindell WR, Fang Y, Hill C, Huber JA, Boehm JD, Westbrook R, Salvatori R, Bartke A. Growth hormone-releasing hormone disruption extends lifespan and regulates response to caloric restriction in mice. Elife. 2013;2:e01098. doi: 10.7554/eLife.01098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317(5836):369–372. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- Tang Y, Purkayastha S, Cai D. Hypothalamic microinflammation: a common basis of metabolic syndrome and aging. Trends Neurosci. 2015;38(1):36–44. doi: 10.1016/j.tins.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299(5611):1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- Vaupel JW. Biodemography of human ageing. Nature. 2010;464(7288):536–542. doi: 10.1038/nature08984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara M, Smith-Wheelock M, Harper JM, Sigler R, Miller RA. Hormone-treated snell dwarf mice regain fertility but remain long lived and disease resistant. J Gerontol A. 2004;59(12):1244–1250. doi: 10.1093/gerona/59.12.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victoria B, Dhahbi JM, Nunez Lopez YO, Spinel L, Atamna H, Spindler SR, Masternak MM. Circulating microRNA signature of genotype-by-age interactions in the long-lived Ames dwarf mouse. Aging Cell. 2015;14(6):1055–1066. doi: 10.1111/acel.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Al-Regaiey KA, Masternak MM, Bartke A. Adipocytokines and lipid levels in Ames dwarf and calorie-restricted mice. J Gerontol A. 2006;61(4):323–331. doi: 10.1093/gerona/61.4.323. [DOI] [PubMed] [Google Scholar]
- Weindruch R. Effect of caloric restriction on age-associated cancers. Exp Gerontol. 1992;27(5–6):575–581. doi: 10.1016/0531-5565(92)90012-o. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford RL. In: The retardation of aging and disease by dietary restriction. Thomas Charles C., editor. Springfield; 1988. [Google Scholar]
- Westbrook R. Dissertation. Southern Illinois University Carbondale; 2012. The effects of altered growth hormone signaling on murine metabolism. [Google Scholar]
- Westendorp RG, van Heemst D, Rozing MP, Frolich M, Mooijaart SP, Blauw GJ, Beekman M, Heijmans BT, de Craen AJ, Slagboom PE The Leiden Longevity Study. Nonagenarian siblings and their offspring display lower risk of mortality and morbidity than sporadic nonagenarians: The Leiden Longevity Study. J Am Geriatr Soc. 2009;57(9):1634–1637. doi: 10.1111/j.1532-5415.2009.02381.x. [DOI] [PubMed] [Google Scholar]
- Wiesenborn DS, Ayala JE, King E, Masternak MM. Insulin sensitivity in long-living Ames dwarf mice. Age (Dordr) 2014;36(5):9709. doi: 10.1007/s11357-014-9709-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijsman CA, Rozing MP, Streefland TC, le Cessie S, Mooijaart SP, Slagboom PE, Westendorp RG, Pijl H, van Heemst DG Leiden Longevity Study. Familial longevity is marked by enhanced insulin sensitivity. Aging Cell. 2011a;10(1):114–121. doi: 10.1111/j.1474-9726.2010.00650.x. [DOI] [PubMed] [Google Scholar]
- Wijsman CA, van Heemst D, Rozing MP, Slagboom PE, Beekman M, de Craen AJ, Maier AB, Westendorp RG, Blom HJ, Mooijaart SP. Homocysteine and familial longevity: the Leiden Longevity Study. PLoS ONE. 2011b;6(3):e17543. doi: 10.1371/journal.pone.0017543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R, Holland WL, Gordillo R, Wang M, Wang QA, Shao M, Morley TS, Gupta RK, Stahl A, Scherer PE. Adiponectin is essential for lipid homeostasis and survival under insulin deficiency and promotes beta-cell regeneration. Elife. 2014;3:e.03851. doi: 10.7554/eLife.03851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bokov A, Gelfond J, Soto V, Ikeno Y, Hubbard G, Diaz V, Sloane L, Maslin K, Treaster S, Rendon S, van Remmen H, Ward W, Javors M, Richardson A, Austad SN, Fischer K. Rapamycin extends life and health in C57BL/6 mice. J Gerontol A. 2014;69(2):119–130. doi: 10.1093/gerona/glt056. [DOI] [PMC free article] [PubMed] [Google Scholar]