Abstract
Importance
Posttraumatic stress disorder (PTSD) is a prevalent, serious public health concern, particularly in the military. The identification of genetic risk factors for PTSD may provide important insights into the biological basis of vulnerability and comorbidity.
Objective
To discover genetic loci associated with lifetime PTSD risk in two cohorts from the Army Study To Assess Risk and Resilience in Servicemembers (Army STARRS).
Design, Setting and Participants
Two coordinated genomewide association studies of mental health in the US military: New Soldier Study (NSS, N=3167 cases and 4607 trauma-exposed controls) and Pre/Post Deployment Study (PPDS, N=947 cases and 4969 trauma-exposed controls). The primary analysis compared lifetime DSM-IV PTSD cases to trauma-exposed controls without lifetime PTSD.
Main Outcomes and Measures
Association analyses were conducted for PTSD using logistic regression models within each of 3 ancestral groups (European, African, Latino) by study and then meta-analyzed. Heritability and genetic correlation and pleiotropy with other psychiatric and immune-related disorders were estimated.
Results
We observed a genomewide significant locus in ANKRD55 on chromosome 5 (rs159572; odds ratio [OR] = 1.62, p-value =2.43×10−8; adjusted for cumulative trauma exposure [AOR] = 1.68, p-value = 1.18×10−8) in the African American samples from NSS. We also observed a genomewide significant locus in or near ZNF626 on chromosome 19 (rs11085374; OR = 0.77, p-value = 4.59 ×10−8) in the European American samples from NSS. We did not find similar results for either SNP in the corresponding ancestry group from the PPDS sample, or in other ancestral groups or trans-ancestral meta-analyses. SNP-based heritability was non-significant, and no significant genetic correlations were observed between PTSD and six mental disorders and nine immune-related disorders. Significant evidence of pleiotropy was observed between PTSD and rheumatoid arthritis and, to a lesser extent, psoriasis.
Conclusions and Relevance
In the largest GWAS of PTSD to date, involving a US military sample, we found limited evidence of association for specific loci. Further efforts are needed to replicate the genomewide significant association with ANKRD55 – associated in prior research with several autoimmune and inflammatory disorders – and to clarify the nature of the genetic overlap observed between PTSD and rheumatoid arthritis and psoriasis.
Keywords: genomewide association, genetic, immune, inflammatory, military, posttraumatic stress disorder, pleiotropy, risk, trauma
INTRODUCTION
Posttraumatic stress disorder (PTSD) is a common consequence of exposure to extreme, life threating stress.1,2 PTSD is also frequently associated with other mental health problems such as major depressive disorder,3 substance abuse4 and suicidality5,6 and with other adverse health sequelae such as obesity,7 cardiovascular disease8-10 type 2 diabetes,11,12 and other immune-related disorders such as rheumatoid arthritis.13
Although most Americans (50-85%) experience traumatic events over a lifetime, the lifetime prevalence of PTSD is approximately 7%,14 suggesting differential vulnerability to the disorder. Rates of trauma exposure and PTSD are higher among U.S. military personnel and veterans,15 particularly those exposed to combat.16,17 Much of the research on risk for PTSD has focused on the differential impacts of type,18 frequency, duration, and consequences (e.g., extent of physical injury) of trauma exposures.19 Pre-trauma risk factors, including personality characteristics and early life experiences, have also been extensively scrutinized,18,20,21 as have post-trauma factors such as social support.22
Twin studies have long established that genetic variation contributes to risk for PTSD symptoms, with heritability estimates in the range of .28 - .46.23-26 Genetic association studies have focused on a limited set of candidate genes and have been largely underpowered to detect loci of modest effect.27 More recently, several genomewide association studies (GWAS) of PTSD have been reported in civilian28,29 and military or veteran samples,30-33 yielding several genomewide significant associations that have yet to be widely replicated.
The present investigation makes use of data from the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS),34 a large, coordinated set of study components intended to improve understanding of suicide, PTSD and related mental health risk and resilience in the US Army. Blood samples for DNA were provided by participants in two components of Army STARRS: a study of new soldiers during their first week of basic training (New Soldier Study [NSS]), and a study of three Brigade Combat Teams prior to their deployment to Afghanistan (Pre/Post Deployment Study [PPDS]). Each of these studies has a larger PTSD-affected sample size than any genetic study of PTSD previously published. We report here results from within-ancestral-group and within-study genomewide analyses, followed by meta-analyses across studies.
METHODS
A synopsis of Methods is included here. A more detailed version is available in the online Supplemental Material.
Subjects
Detailed information about the design and conduct of Army STARRS is available in a separate report.34 The recruitment, consent, human subject and data protection procedures were approved by all collaborating organizations.
New Soldier Study (NSS)
The NSS was carried out among new soldiers at the start of their basic training at one of three Army Installations between April 2011 and November 2012. Of 39,784 NSS participants who completed the computerized self-administered questionnaire (SAQ), 33,088 (83.2%) provided blood samples. Genotyping was conducted on samples from the first half of the cohort, from which 7,999 samples were selected based on phenotype and case-control status (NSS1). When the remaining half of the cohort collection was completed, we selected an informative subset (see Supplemental Material) for genotyping (NSS2; N = 2,835).
Pre/Post Deployment Survey (PPDS)
The PPDS is a multi-wave panel survey that collected baseline data (T0) from US Army soldiers in three Brigade Combat Teams (BCTs) during the first quarter of 2012, within approximately six weeks of their deployment to Afghanistan. 7,927 PPDS soldiers with eligible SAQ responses were genotyped for GWAS.
Demographics and Case-Control Status
The population, sex and age composition of our analyzed sample of cases and controls is shown in Table 1. The majority of subjects were male and we analyzed male and female subjects together. A total of 3,167 PTSD cases and 4,607 trauma exposed controls from NSS1 and NSS2, and 947 PTSD cases and 4,969 trauma-exposed controls from PPDS entered the following statistical analyses.
Table 1.
Ancestry and sex and age distributions in the case-control samples
| NSS1 | NSS2 | NSS1 + NSS2 | PPDS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Case | Control | Total | Case | Control | Total | Case | Control | Total | Case | Control | Total | |
| Population | ||||||||||||
| European American | 1245 | 2291 | 3536 | 895 | 618 | 1513 | 2140 | 2909 | 5049 | 672 | 3335 | 4007 |
| African American | 306 | 664 | 970 | 191 | 151 | 342 | 497 | 815 | 1312 | 97 | 570 | 667 |
| Latino American | 306 | 697 | 1003 | 224 | 186 | 410 | 530 | 883 | 1413 | 178 | 1064 | 1242 |
| Sex (%male) | 78% | 84% | 82% | 79% | 78% | 79% | 78% | 82% | 81% | 93% | 95% | 94% |
| Age (SD) | 20.7 (3.0) | 21.3 (3.5) | 21.1 (3.3) | 20.1 (3.1) | 20.6 (3.4) | 20.3 (3.2) | 20.4 (3.0) | 21.1 (3.5) | 20.9 (3.3) | 27.1 (5.9) | 26.4 (6.0) | 26.5 (6.0) |
| Total | 1857 | 3652 | 5509 | 1310 | 955 | 2265 | 3167 | 4607 | 7774 | 947 | 4969 | 5916 |
PTSD Cases are identified through information provided by PCL and CIDI-SC. Controls were exposed to at least 1 non-deployment trauma (NSS1, NSS2 and PPDS) or deployment trauma (PPDS).
Measures
The SAQ included a computerized version of the Composite International Diagnostic Interview screening scales (CIDI-SC)35 and a screening version of the PTSD Checklist (PCL).36 Trauma exposure was assessed from answers pertaining to childhood, adulthood civilian, and, for PPDS participants, military traumatic events (See Supplemental Material:Methods). PTSD diagnosis was assigned using multiple imputation methods that relied on PCL and CIDI-SC data; our clinical reappraisal study found satisfactory concordance with independent clinical diagnoses based on blinded Structured Clinical Interviews for DSM-IV (AUC = 0.70–0.79; κ =0.4–0.6).37
DNA Collection and Genotyping
Whole blood samples were shipped to Rutgers University Cell & DNA Repository (RUCDR), where they were frozen for later DNA extraction using standard methods. NSS1 and PPDS samples were genotyped using the Illumina OmniExpress + Exome array with additional custom content. NSS2 samples were genotyped on the Ilumina PsychChip. (See Supplemental Material:Methods.)
Imputation, Population Assignment and Principal Component Analysis
Statistical Analysis
Lifetime PTSD cases and controls (i.e., individuals without lifetime PTSD) reporting at least 1 traumatic event were included in the association analyses. PLINK (version 1.9)38 was used to perform association tests on imputed SNP dosage with logistic regression adjusted for the top 10 within-population principal components. The meta-analysis of NSS1+NSS2 is the primary analysis. The analysis of PPDS is our internal attempt at replication analysis. We sought external replication with other relevant military published datasets (see Results).
SNP-based heritability was estimated using GCTA.39 We tested the genetic correlation (proportion of variance that phenotypes share due to genetic causes, which considers only causal variants with the same directionality of effects) and pleiotropy (effect of the same gene on multiple phenotypes, which considers causal variants with both same and opposite effects) of PTSD in all European samples with psychiatric disorders (including schizophrenia (SCZ),40 bipolar disorder (BIP),41 attention deficit hyperactivity disorder (ADHD),42 major depressive disorder (MDD),43 autism spectrum disorder (ASD), and a cross-disorder phenotype (XD)44); and with immune-related disorders (including Crohn’s disease (CD),45 ulcerative colitis (UC),46 multiple sclerosis (MS),47 psoriasis (PS),48 rheumatoid arthritis (RA),49 systemic lupus erythematosis (SLE),50 celiac disease (CEL),51 primary biliary cirrhosis (PBC),52 and insulin-dependent diabetes mellitus (T1D)53) using LD score regression (LDSC)54 and the GPA (Genetic analysis incorporating Pleiotropy and Annotation) R package,55 respectively.
We followed up the significant pleiotropic outcomes with enrichment analysis using DEPICT v1 (Data-driven Expression Prioritized Integration for Complex Traits)56 and DAVID v6.7 (Database for Annotation, Visualization and Integrated Discovery),57 respectively. See Supplemental Material:Methods for further details.
RESULTS
Genomewide Association Analyses
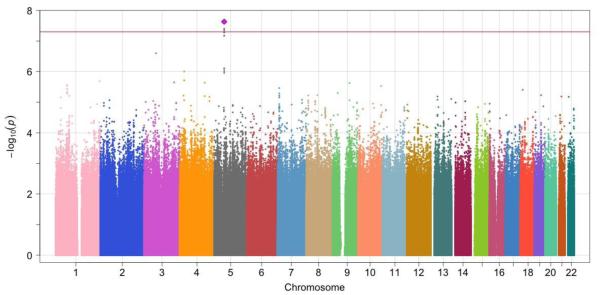
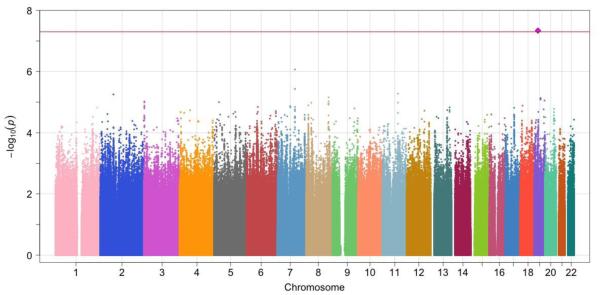
The λGC and the QQ plot showed negligible inflation of association p-values in NSS (meta-analysis of NSS1/NSS2) or PPDS (Supplemental eFigure 6). A SNP on chromosome 19 was significantly associated with PTSD in the NSS results in European American samples (rs11085374; OR=0.77, p-value=4.59 ×10−8). A SNP on chromosome 5 was significantly associated with PTSD in African American subjects in the NSS results (rs159572; OR=1.62, p-value=2.34 ×10−8). We did not find similar results for either SNP in the corresponding ancestry group from the PPDS sample. The individual study and meta-analysis results are presented in Table 2 and the Manhattan plots in NSS African American and European American samples are shown in Figure 1. We further created regional plots58 for 500 Kb regions around the two top hit SNPs (Supplemental eFigure 7). No significant associations were observed in the Latino NSS or PPDS samples or in any of the trans-ethnic meta-analyses.
Table 2.
Genome-wide significant loci in the NSS1, NSS2 and PPDS individual analyses and meta-analyses from the standard GWAS analysis
| Chr. | Position | SNP |
Nearest
gene |
A1 | A2 | Population | Study | FRQ | INFO | P-value | OR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 55507046 | rs159572 | ANKRD55 | A | C | European A. |
NSS1 | 0.75 | 1.00 | 0.9327 | 1.00 |
| NSS2 | 0.73 | 0.98 | 0.04543 | 0.84 | |||||||
| NSS-meta | 0.2337 | 0.94 | |||||||||
| PPDS | 0.73 | 1.00 | 0.3608 | 0.94 | |||||||
| All-meta | 0.1334 | 0.94 | |||||||||
| 5 | 55507046 | rs159572 | ANKRD55 | A | C | African A. | NSS1 | 0.46 | 1.04 | 4.91×10−6 | 1.57 |
| NSS2 | 0.44 | 0.95 | 1.16×10−3 | 1.74 | |||||||
| NSS-meta | 2.34×10−8 | 1.62 | |||||||||
| PPDS | 0.47 | 1.03 | 0.284 | 1.18 | |||||||
| All-meta | 6.13 ×10−8 | 1.51 | |||||||||
| 5 | 55507046 | rs159572 | ANKRD55 | A | C | Latino A. | NSS1 | 0.68 | 1.03 | 0.5213 | 1.07 |
| NSS2 | 0.67 | 0.96 | 0.2131 | 0.82 | |||||||
| NSS-meta | 0.8814 | 0.99 | |||||||||
| PPDS | 0.69 | 1.09 | 0.07136 | 0.81 | |||||||
| All-meta | 0.2281 | 0.92 | |||||||||
|
| |||||||||||
|
| |||||||||||
| Chr. | Position | SNP |
Nearest
gene |
A1 | A2 | Population | Study | FRQ | INFO | P-value | OR |
|
| |||||||||||
| 19 | 20906220 | rs11085374 | ZNF626 | A | T | European A. |
NSS1 | 0.32 | 0.98 | 3.67×10−6 | 0.77 |
| NSS2 | 0.30 | 0.81 | 3.62×10−3 | 0.77 | |||||||
| NSS-meta | 4.59×10−8 | 0.77 | |||||||||
| PPDS | 0.31 | 0.97 | 0.496 | 1.05 | |||||||
| All-meta | 5.40×10−5 | 0.86 | |||||||||
| 19 | 20906220 | rs11085374 | ZNF626 | A | T | African A. | NSS1 | 0.51 | 0.90 | 0.6318 | 0.95 |
| NSS2 | 0.50 | 0.67 | 0.6213 | 1.10 | |||||||
| NSS-meta | 0.8513 | 0.98 | |||||||||
| PPDS | 0.51 | 0.85 | 0.7949 | 0.96 | |||||||
| All-meta | 0.7733 | 0.98 | |||||||||
| 19 | 20906220 | rs11085374 | ZNF626 | A | T | Latino A. | NSS1 | 0.27 | 0.92 | 0.0799 | 1.23 |
| NSS2 | 0.25 | 0.76 | 0.6498 | 1.09 | |||||||
| NSS-meta | 0.08197 | 1.19 | |||||||||
| PPDS | 0.26 | 0.93 | 0.342 | 0.87 | |||||||
| All-meta | 0.3765 | 1.08 | |||||||||
A1: risk allele; A2: non-risk allele; FRQ: overall risk allele frequency; INFO: imputation quality score; OR: odds ratio; NSS-meta: meta-analysis between NSS1 and NSS2 results; All-meta: meta-analysis between NSS1, NSS2 and PPDS results
Position is in NCBI Build 37/UCSC hg19 coordinates.
Figure 1.
Manhattan plots of NSS1 and NSS2 meta-analysis in European American and African American samples
(a). NSS meta-analysis, African American samples, identifying genome-wide significant association for PTSD with rs159572 on Chr 5
(b). NSS meta-analysis, European American samples, identifying a genome-wide significant association for PTSD with rs11085374 on Chr 19
Adjustment for lifetime trauma exposure slightly strengthened the genomewide significant associations for the two lead SNPs (Supplemental eTable 1) whereas simultaneous adjustment for lifetime trauma exposure, sex, and age slightly attenuated the associations (Supplemental eTable 2 and eFigure 8).
The top SNP rs159572 (eFigure 7a) on chromosome 5 is intronic to ANKRD55, and multiple other SNPs in this region were in LD with this SNP. ANKRD55 has been associated with several autoimmune diseases, including multiple sclerosis,59,60 type 2 diabetes,61 celiac disease62 and rheumatoid arthritis.63
The top SNP rs11085374 on chromosome 19 is located near ZNF626. There was minimal LD between this SNP and surrounding SNPs (eFigure 7b), and no other SNPs in the region showed evidence of association.
Meta-analysis with other Military Datasets
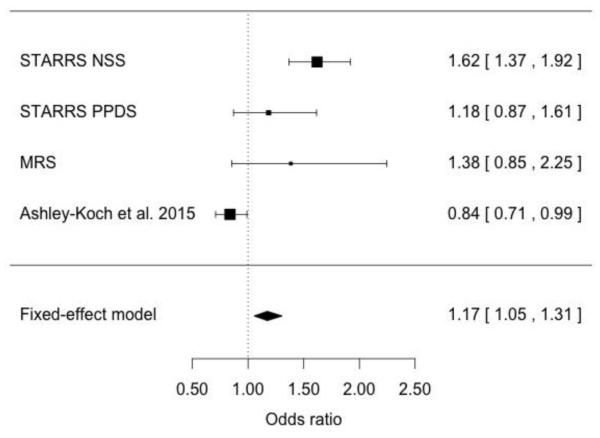
We meta-analyzed results for SNP rs159572 on chromosome 5 between three GWAS of African American military samples, including data reported here from Army STARRS (current analysis), Marine Resiliency Study (MRS)31 and a recently published genetic study of Iraq-Afghanistan US Veterans32 (Figure 2). The results were directionally consistent in the Army STARRS NSS and PPDS samples as well as the MRS, but not in the PTSD Veteran GWAS (meta-analysis OR=1.17, 95% CI: 1.05-1.31).
Figure 2.
Meta-analysis for SNP rs159572 between African American samples from Army STARRS, Marine Resiliency Study31 and Ashley-Koch et al. 201532
Alternate Phenotypic Characterization
To examine the robustness of our findings, we tested for association of the top two SNPs at the chromosome 19 and 5 loci with an alternate phenotypic characterization of PTSD; all subjects in the respective ancestral groups were included. For this purpose we chose a dimensional measure of lifetime (“at its worst”) PTSD severity, a 6-item version (range: 0-24) of the PTSD Checklist (PCL)64 that we have used in other published Army STARRS research.65 Among European Americans rs11085374 was significantly associated with lifetime PCL-6 severity in NSS1 (p-value=0.007) and NSS2 (p-value <0.001), but not PPDS (p-value=0.82). Among African Americans rs159572 was significantly associated with lifetime PCL-6 severity in NSS1 (p-value=0.002) and NSS2 (p-value=0.028) but not PPDS (p-value=0.419).
Heritability of Lifetime PTSD Phenotype
We estimated SNP-based heritability (h2g) using GCTA39 in European American samples for NSS[1,2], PPDS, and both cohorts pooled together. We found no significant h2g estimates, either in overall (h2g=0.062 [SE=0.049], p=0.104) or gender-specific analyses (Supplemental eTable 3).
Pleiotropy and Genetic Correlation
We tested the pleiotropy shared by PTSD and six psychiatric disorders and nine immune-related disorders in the European American samples (Table 3). Significant pleiotropy was observed for PTSD and two immune-related disorders: rheumatoid arthritis (RA) (p-value=3.04 ×10−9) and psoriasis (PS) (p-value=2.41 ×10−3). No significant pleiotropy was observed between PTSD and the other psychiatric disorders tested. No significant genetic correlations were found in the same datasets (Supplemental eTable 5).
Table 3.
Genetic Pleiotropy Analysis between PTSD and Other Disorders
| Disorder | p-value | |
|---|---|---|
| Immune-related Disorders |
Crohn’s Disease | 0.636 |
| Multiple Sclerosis | 0.961 | |
| Psoriasis | 2.41 ×10−3 | |
| Rheumatoid Arthritis | 3.04 ×10−9 | |
| SLE (Lupus) | 0.874 | |
| Type 1 Diabetes | 0.128 | |
| Ulcerative Colitis | 0.382 | |
| Celiac Disease | 0.049 | |
| Primary Biliary Cirrhosis | 0.09 | |
| Psychiatric Disorders | Schizophrenia | 0.123 |
| Bipolar Disorder | 0.78 | |
| Attention Deficit/ Hyperactivity Disorder |
0.887 | |
| Major Depressive Disorder | 0.783 | |
| Autism Spectrum Disorder | 0.838 | |
| PGC Cross-Disorder | 0.294 |
Considering the pleiotropy results, we performed an enrichment analysis of SNPs with pleiotropy posterior probability > 0.5. For PTSD-RA, we observed several significant enrichments for MeSH tissue and cell type annotations (Supplemental eTable 6) and Gene Ontology (GO) terms (Supplemental eTable 7) related to several immune systems and functions. No enrichment was present for PTSD-PS.
Finally, we estimated that the probability for a SNP associated with PTSD to be a CNS SNP is 2.28 (s.e.=0.24) times the probability for a SNP not associated with PTSD to be a CNS SNP. Similarly, the enrichment ratio for immune-related eQTLs in PTSD is 2.36 (s.e.=0.27).
DISCUSSION
This study is, to the best of our knowledge, the largest GWAS of PTSD conducted to date. As it is representative of the US Army, the composition of the samples was ethnically diverse, obligating us to initially conduct association analyses within ancestral groups and then to attempt trans-ancestral meta-analyses. We found no genomewide significant loci at the level of the trans-ancestral meta-analyses, but did find two genomewide significant loci, one each in the African American and European American samples from the New Soldier Study (NSS).
In the African American NSS sample, we observed genomewide significant association with PTSD for SNPs on chromosome 5 in ANKRD55 (ankyrin repeat domain 55). Importantly, inclusion of data from African Americans from additional military cohorts continued to yield a meta-analytically genomewide significant result at this locus, albeit attenuated compared to NSS alone (Figure 2). This gene, whose function is currently unknown, has been reported to be associated with a range of autoimmune and inflammatory disorders including multiple sclerosis,59,60 type 2 diabetes,61 celiac disease62 and rheumatoid arthritis.63 We believe we were able to observe this genetic overlap in European Americans (while the ANKRD55 finding was in African Americans) because human populations can present ancestry-specific risk alleles in the context of similar underlying biological mechanisms of disease predisposition.66
Remarkably, we also found evidence of significant pleiotropy between PTSD and two immune-related disorders, namely rheumatoid arthritis and, to a lesser extent, psoriasis. These novel findings are consistent with recent reports of pleiotropy between other mental disorders such as schizophrenia and immune disorders such as rheumatoid arthritis and multiple sclerosis.67,68 In the context of new evidence that schizophrenia involves allelic variation in the major histocompatibility complex (MHC), these observations suggest that intensive scrutiny of immune factors, and perhaps especially complement component 4,69 should be the subject of further study in other mental disorders such as PTSD.
A hypothetical immune-related or inflammatory etiology for PTSD has, in fact, gained some empirical support.70 Two recent studies have found elevation in levels of the inflammatory biomarker C-Reactive Protein (CRP) in individuals at risk71 or suffering from PTSD.72 Others studies have found abnormal cytokine regulation73 or other evidence of a pro-inflammatory milieu in PTSD.74 It is also noteworthy that PTSD is itself highly comorbid with several of the disorders associated with ANKRD55, including type 2 diabetes12,75 and rheumatoid arthritis.76. Moreover, a recent epidemiological study of Iraq and Afghanistan military veterans found PTSD to be associated with a broad range of autoimmune disorders, including inflammatory bowel disease, rheumatoid arthritis, and multiple sclerosis.77 Further research is needed to determine whether variation in ANKRD55 – or other genes contributing to the observed pleiotropy – accounts for these associations. It will also be important to determine why the association of ANKRD55 with PTSD is apparently restricted to African Americans, although this may be explainable on the basis of differing LD block structure and increased minor allele frequency.
We also detected in the European American NSS samples one genomewide significant SNP on chromosome 19 near ZNF626 (zinc finger protein 626), a gene thought to be involved in regulation of RNA transcription. The regional plot showed no other associated SNPs in LD with this result. This may represent spurious association, but it also may simply reflect a lack of nearby variants in LD with the index SNP; this requires further study.
Genomewide significant results from NSS were not replicated in PPDS. Of note, the PPDS sample was smaller and distinct in important ways from NSS. NSS participants were younger (mostly 18-20 years of age) and their trauma exposure and resultant PTSD were entirely pre-military. In contrast, PPDS participants were older, their non-military trauma exposure was on average higher than in NSS (reflecting the accrual of traumatic exposures over time) and many had additionally experienced deployment-related traumas. This finding, i.e., consistency of results in identically ascertained samples (i.e., NSS1 and NSS2) but inconsistency in a second military sample with different rates and types of trauma exposure, serves as a reminder of the challenges this field will face in working across and combining datasets that include individuals with heterogeneous trauma experiences.78
In this regard, it is noteworthy that adjustment for trauma exposure tended to increase the statistical significance of genomewide or near-genomewide significant SNPs. We know, however, that certain types of trauma have higher conditional risks for PTSD than others.15 Accordingly, adjustment for trauma on the basis of tallying exposure to different trauma types – without taking into account differential conditional risks of PTSD for certain trauma types – might inadequately model these relationships. Our results underscore the need for additional work to determine the appropriate metrics for trauma exposure and the optimal functional forms for modeling these outcomes in genetic datasets. It is unclear, for instance, when these effects should be modeled by covarying for trauma exposure or when interactions – with overall trauma severity or with specific trauma types – should be considered. Well-powered gene-environment-wide interaction studies (GEWIS) may be especially illuminating, particularly given observations that the interaction of PTSD susceptibility genes with early trauma (e.g., childhood maltreatment) exposure may be paramount.79
The cross-phenotype LDSC results failed to provide evidence of genetic correlation between PTSD and the other mental disorders examined. Previous studies have reported evidence for shared genetic risk between PTSD and bipolar disorder.31,80 Clinical and genetic epidemiological studies have found considerable comorbidity – at least some of which is explained by shared genetic vulnerability26,81,82 – between PTSD and major depressive disorder and attention-deficit/hyperactivity disorder.83,84 Insufficient power is a possible explanation for our study’s failure to find evidence of shared genetic risk across these disorders. Importantly, however, enrichment analysis suggested that risk variants for PTSD aggregate in many of the same biological pathways shared with other neuropsychiatric disorders, notably those involved in immune regulation.85 In light of the recent observation of
Our results should be interpreted in light of several additional limitations. First, samples sizes – especially within ancestral groups – are likely to be insufficiently powered to detect loci of modest effect. Given our total sample size, we would have 80% power to detect associations for SNPs with 20% minor allele frequency with an OR of 1.2 or higher. Second, it is well established that risk for trauma exposure is genetically correlated with risk for PTSD.24,86,87 Therefore, although exclusion of trauma-unexposed control subjects should have improved our power to detect PTSD risk loci given trauma exposure, it may have reduced our ability to detect loci that contribute to PTSD by increasing risk of trauma. Third, our finding of no apparent heritability emanating from the GCTA analyses may be due to insufficient power or other limitations in this approach.88 Fourth, the use of GPA to detect pleiotropy is quite novel, and heretofore unappreciated limitations in this approach may exist.
In summary, we found no genomewide significant findings that transcended ancestry and replicated across studies. We did, however, find genomewide significant evidence of an association of ANKRD55, a gene previously associated with inflammatory and immune disorders, with PTSD in African Americans that was observed in a sample of pre-military PTSD (NSS), not replicated in a sample of mixed pre-military and military PTSD (PPDS), but showing similar effect size and directionality in an independent sample of Marines with PTSD (Marine Resiliency Study). This association is small in magnitude and, even if replicated, would be of no obvious clinical utility at present. Its value may lie, however, in eventual elucidation of the nature of PTSD and its relationship to other illnesses. The finding of pleiotropy between PTSD and rheumatoid arthritis and psoriasis should further motivate the study of immune-related factors in PTSD, their potential contribution to comorbidity with inflammatory disorders and, indeed, a possible role for anti-inflammatory treatments in PTSD.89
Supplementary Material
ACKNOWLEDGMENTS
Funding:
Army STARRS was sponsored by the Department of the Army and funded under cooperative agreement number U01MH087981 (MBS and RJU) with the U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Mental Health (NIH/NIMH). R01 DA12690 (JG) provided support for the GPA analyses. The contents are solely the responsibility of the authors and do not necessarily represent the views of the Department of Health and Human Services, NIMH, the Veterans Administration, Department of the Army, or the Department of Defense.
Footnotes
Access to Data and Data Analysis:
Murray B. Stein MD, MPH had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Stein, Chen, Gelernter, Jain, Maihofer, Polimanti, Ripke, Smoller, and Wang, as well as Ms. Sun, conducted and are jointly responsible for the data analysis.
Conflict of Interest Disclosure:
Dr. Stein has in the last 3 years been a consultant for Healthcare Management Technologies, and Actelion, Dart Neuroscience, Janssen, Oxeia Biopharmaceuticals, Pfizer, Resilience Therapeutics, and Tonix Pharmaceuticals. Dr. Kessler has in the last 3 years been a consultant for Hoffman-La Roche, Inc., Johnson & Johnson Wellness and Prevention, and Sanofi-Aventis Groupe. Dr. Kessler has served on advisory boards for Mensante Corporation, Plus One Health Management, Lake Nona Institute, and U.S. Preventive Medicine. Dr. Kessler owns 25% share in DataStat, Inc. Dr. Smoller is an unpaid member of the Scientific Advisory Board of PsyBrain, Inc. The remaining authors report nothing to disclose.
Army STARRS Collaborative Team Members:
The Army STARRS Team consists of Co-Principal Investigators: Robert J. Ursano, MD (Uniformed Services University of the Health Sciences) and Murray B. Stein, MD, MPH (University of California San Diego and VA San Diego Healthcare System); Site Principal Investigators: Steven G. Heeringa, PhD (University of Michigan) and Ronald C. Kessler, PhD (Harvard Medical School); National Institute of Mental Health (NIMH) collaborating scientists: Lisa J. Colpe, PhD, MPH and Michael Schoenbaum, PhD; Army liaisons/consultants: COL Steven Cersovsky, MD, MPH (USAPHC) and Kenneth Cox, MD, MPH (USAPHC). Other team members: Pablo A. Aliaga, MA (Uniformed Services University of the Health Sciences); COL David M. Benedek, MD (Uniformed Services University of the Health Sciences); K. Nikki Benevides, MA (Uniformed Services University of the Health Sciences); Paul D. Bliese, PhD (University of South Carolina); Susan Borja, PhD (NIMH); Evelyn J. Bromet, PhD (Stony Brook University School of Medicine); Gregory G. Brown, PhD (University of California San Diego); Christina Buckley, BA (Uniformed Services University of the Health Sciences); Laura Campbell-Sills, PhD (University of California San Diego); Tianxi Cai, ScD (Harvard T.H. Chan School of Public Health); Chia-Yen Chen, ScD (Massachusetts General Hospital); Catherine L. Dempsey, PhD, MPH (Uniformed Services University of the Health Sciences); Julie O. Denenberg MA (University of California San Diego); Carol S. Fullerton, PhD (Uniformed Services University of the Health Sciences); Nancy Gebler, MA (University of Michigan); Joel Gelernter MD (Yale University); Robert K. Gifford, PhD (Uniformed Services University of the Health Sciences); Stephen E. Gilman, ScD (Harvard School of Public Health); Feng He (University of California San Diego); Marjan G. Holloway, PhD (Uniformed Services University of the Health Sciences); Paul E. Hurwitz, MPH (Uniformed Services University of the Health Sciences); Sonia Jain, PhD (University of California San Diego); Tzu-Cheg Kao, PhD (Uniformed Services University of the Health Sciences); Karestan C. Koenen, PhD (Columbia University); Kevin P. Jensen, PhD (Yale University); Lisa Lewandowski-Romps, PhD (University of Michigan); Holly Herberman Mash, PhD (Uniformed Services University of the Health Sciences); Adam X. Maihofer MS (University of California San Diego); James E. McCarroll, PhD, MPH (Uniformed Services University of the Health Sciences); James A. Naifeh, PhD (Uniformed Services University of the Health Sciences); Tsz Hin Hinz Ng, MPH (Uniformed Services University of the Health Sciences); Matthew K. Nock, PhD (Harvard University); Rema Raman, PhD (University of California San Diego); Holly J. Ramsawh, PhD (Uniformed Services University of the Health Sciences); Anthony Joseph Rosellini, PhD (Harvard Medical School); Nancy A. Sampson, BA (Harvard Medical School); LCDR Patcho Santiago, MD, MPH (Uniformed Services University of the Health Sciences); Michaelle Scanlon, MBA (NIMH); Jordan W. Smoller, MD, ScD (Massachusetts General Hospital); Amy Street, PhD (Boston University School of Medicine); Xiayong Sun, MS (University of California San Diego); Michael L. Thomas, PhD (University of California San Diego); Patti L. Vegella, MS, MA (Uniformed Services University of the Health Sciences); Leming Wang, MS (Uniformed Services University of the Health Sciences); Erin Ware, PhD (University of Michigan); Christina L. Wassel, PhD (University of Pittsburgh); Simon Wessely, FMedSci (King’s College London); Hongyan Wu, MPH (Uniformed Services University of the Health Sciences); LTC Gary H. Wynn, MD (Uniformed Services University of the Health Sciences); Alan M. Zaslavsky, PhD (Harvard Medical School); Bailey G. Zhang, MS (Uniformed Services University of the Health Sciences); and Lei Zhang, PhD (Uniformed Services University of the Health Sciences).
REFERENCES
- 1.Thomas JL, Wilk JE, Riviere LA, McGurk D, Castro CA, Hoge CW. Prevalence of mental health problems and functional impairment among active component and National Guard soldiers 3 and 12 months following combat in Iraq. Arch Gen Psychiatry. 2010;67(6):614–623. doi: 10.1001/archgenpsychiatry.2010.54. [DOI] [PubMed] [Google Scholar]
- 2.Santiago PN, Ursano RJ, Gray CL, et al. A systematic review of PTSD prevalence and trajectories in DSM-5 defined trauma exposed populations: intentional and non-intentional traumatic events. PLoS One. 2013;8(4):e59236. doi: 10.1371/journal.pone.0059236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stander VA, Thomsen CJ, Highfill-McRoy RM. Etiology of depression comorbidity in combat-related PTSD: a review of the literature. Clin Psychol Rev. 2014;34(2):87–98. doi: 10.1016/j.cpr.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Zatzick D, Donovan D, Dunn C, et al. Substance use and posttraumatic stress disorder symptoms in trauma center patients receiving mandated alcohol screening and brief intervention. J Subst Abuse Treat. 2012;43(4):410–417. doi: 10.1016/j.jsat.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramsawh HJ, Fullerton CS, Mash HB, et al. Risk for suicidal behaviors associated with PTSD, depression, and their comorbidity in the U.S. Army. J Affect Disord. 2014;161:116–122. doi: 10.1016/j.jad.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Nock MK, Hwang I, Sampson N, et al. Cross-national analysis of the associations among mental disorders and suicidal behavior: findings from the WHO World Mental Health Surveys. PLoS Med. 2009;6(8):e1000123. doi: 10.1371/journal.pmed.1000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kubzansky LD, Bordelois P, Jun HJ, et al. The Weight of Traumatic Stress: A Prospective Study of Posttraumatic Stress Disorder Symptoms and Weight Status in Women. JAMA Psychiatry. 2013 doi: 10.1001/jamapsychiatry.2013.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wentworth BA, Stein MB, Redwine LS, et al. Post-traumatic stress disorder: a fast track to premature cardiovascular disease? Cardiol Rev. 2013;21(1):16–22. doi: 10.1097/CRD.0b013e318265343b. [DOI] [PubMed] [Google Scholar]
- 9.Crum-Cianflone NF, Bagnell ME, Schaller E, et al. Impact of combat deployment and posttraumatic stress disorder on newly reported coronary heart disease among US active duty and reserve forces. Circulation. 2014;129(18):1813–1820. doi: 10.1161/CIRCULATIONAHA.113.005407. [DOI] [PubMed] [Google Scholar]
- 10.Sumner JA, Kubzansky LD, Elkind MS, et al. Trauma Exposure and Posttraumatic Stress Disorder Symptoms Predict Onset of Cardiovascular Events in Women. Circulation. 2015;132(4):251–259. doi: 10.1161/CIRCULATIONAHA.114.014492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lukaschek K, Baumert J, Kruse J, et al. Relationship between posttraumatic stress disorder and type 2 diabetes in a population-based cross-sectional study with 2970 participants. J Psychosom Res. 2013;74(4):340–345. doi: 10.1016/j.jpsychores.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Roberts AL, Agnew-Blais JC, Spiegelman D, et al. Posttraumatic stress disorder and incidence of type 2 diabetes mellitus in a sample of women: a 22-year longitudinal study. JAMA Psychiatry. 2015;72(3):203–210. doi: 10.1001/jamapsychiatry.2014.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Donovan A, Cohen BE, Seal KH, et al. Elevated Risk for Autoimmune Disorders in Iraq and Afghanistan Veterans with Posttraumatic Stress Disorder. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kessler RC, Wang PS. The descriptive epidemiology of commonly occurring mental disorders in the United States. Annu Rev Public Health. 2008;29:115–129. doi: 10.1146/annurev.publhealth.29.020907.090847. [DOI] [PubMed] [Google Scholar]
- 15.Wisco BE, Marx BP, Wolf EJ, Miller MW, Southwick SM, Pietrzak RH. Posttraumatic stress disorder in the US veteran population: results from the National Health and Resilience in Veterans Study. J Clin Psychiatry. 2014;75(12):1338–1346. doi: 10.4088/JCP.14m09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kessler RC, Heeringa SG, Stein MB, et al. Thirty-day prevalence of DSM-IV mental disorders among nondeployed soldiers in the US Army: results from the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS) JAMA Psychiatry. 2014;71(5):504–513. doi: 10.1001/jamapsychiatry.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramchand R, Rudavsky R, Grant S, Tanielian T, Jaycox L. Prevalence of, risk factors for, and consequences of posttraumatic stress disorder and other mental health problems in military populations deployed to Iraq and Afghanistan. Curr Psychiatry Rep. 2015;17(5):37. doi: 10.1007/s11920-015-0575-z. [DOI] [PubMed] [Google Scholar]
- 18.Kessler RC, Rose S, Koenen KC, et al. How well can post-traumatic stress disorder be predicted from pre-trauma risk factors? An exploratory study in the WHO World Mental Health Surveys. World Psychiatry. 2014;13(3):265–274. doi: 10.1002/wps.20150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandweiss DA, Slymen DJ, Leardmann CA, et al. Preinjury psychiatric status, injury severity, and postdeployment posttraumatic stress disorder. Arch Gen Psychiatry. 2011;68(5):496–504. doi: 10.1001/archgenpsychiatry.2011.44. [DOI] [PubMed] [Google Scholar]
- 20.Bomyea J, Risbrough V, Lang AJ. A consideration of select pre-trauma factors as key vulnerabilities in PTSD. Clin Psychol Rev. 2012;32(7):630–641. doi: 10.1016/j.cpr.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DiGangi JA, Gomez D, Mendoza L, Jason LA, Keys CB, Koenen KC. Pretrauma risk factors for posttraumatic stress disorder: a systematic review of the literature. Clin Psychol Rev. 2013;33(6):728–744. doi: 10.1016/j.cpr.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Polusny MA, Erbes CR, Murdoch M, Arbisi PA, Thuras P, Rath MB. Prospective risk factors for new-onset post-traumatic stress disorder in National Guard soldiers deployed to Iraq. Psychol Med. 2011;41(4):687–698. doi: 10.1017/S0033291710002047. [DOI] [PubMed] [Google Scholar]
- 23.True WR, Rice J, Eisen SA, et al. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry. 1993;50(4):257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- 24.Stein MB, Jang KL, Taylor S, Vernon PA, Livesley WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. Am J Psychiatry. 2002;159(10):1675–1681. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- 25.Afifi TO, Asmundson GJ, Taylor S, Jang KL. The role of genes and environment on trauma exposure and posttraumatic stress disorder symptoms: a review of twin studies. Clin Psychol Rev. 2010;30(1):101–112. doi: 10.1016/j.cpr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Sartor CE, Grant JD, Lynskey MT, et al. Common heritable contributions to low-risk trauma, high-risk trauma, posttraumatic stress disorder, and major depression. Arch Gen Psychiatry. 2012;69(3):293–299. doi: 10.1001/archgenpsychiatry.2011.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smoller JW. The Genetics of Stress-Related Disorders: PTSD, Depression, and Anxiety Disorders. Neuropsychopharmacology. 2016;41(1):297–319. doi: 10.1038/npp.2015.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie P, Kranzler HR, Yang C, Zhao H, Farrer LA, Gelernter J. Genome-wide association study identifies new susceptibility loci for posttraumatic stress disorder. Biol Psychiatry. 2013;74(9):656–663. doi: 10.1016/j.biopsych.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guffanti G, Galea S, Yan L, et al. Genome-wide association study implicates a novel RNA gene, the lincRNA AC068718.1, as a risk factor for post-traumatic stress disorder in women. Psychoneuroendocrinology. 2013;38(12):3029–3038. doi: 10.1016/j.psyneuen.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Logue MW, Baldwin C, Guffanti G, et al. A genome-wide association study of post-traumatic stress disorder identifies the retinoid-related orphan receptor alpha (RORA) gene as a significant risk locus. Mol Psychiatry. 2013;18(8):937–942. doi: 10.1038/mp.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nievergelt CM, Maihofer AX, Mustapic M, et al. Genomic predictors of combat stress vulnerability and resilience in U.S. Marines: A genome-wide association study across multiple ancestries implicates PRTFDC1 as a potential PTSD gene. Psychoneuroendocrinology. 2015;51:459–471. doi: 10.1016/j.psyneuen.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 32.Ashley-Koch AE, Garrett ME, Gibson J, et al. Genome-wide association study of posttraumatic stress disorder in a cohort of Iraq-Afghanistan era veterans. J Affect Disord. 2015;184:225–234. doi: 10.1016/j.jad.2015.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Almli LM, Stevens JS, Smith AK, et al. A genome-wide identified risk variant for PTSD is a methylation quantitative trait locus and confers decreased cortical activation to fearful faces. Am J Med Genet B Neuropsychiatr Genet. 2015;168B(5):327–336. doi: 10.1002/ajmg.b.32315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ursano RJ, Colpe LJ, Heeringa SG, et al. The Army study to assess risk and resilience in servicemembers (Army STARRS) Psychiatry. 2014;77(2):107–119. doi: 10.1521/psyc.2014.77.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kessler RC, Ustun TB. The World Mental Health (WMH) Survey Initiative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI) Int J Methods Psychiatr Res. 2004;13(2):93–121. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilkins KC, Lang AJ, Norman SB. Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depress Anxiety. 2011;28(7):596–606. doi: 10.1002/da.20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kessler RC, Santiago PN, Colpe LJ, et al. Clinical reappraisal of the Composite International Diagnostic Interview Screening Scales (CIDI-SC) in the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS) Int J Methods Psychiatr Res. 2013;22(4):303–321. doi: 10.1002/mpr.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88(1):76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schizophrenia Working Group of the Psychiatric Genomics C Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Psychiatric GWAS Consortium Bipolar Disorder Working Group Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nature genetics. 2011;43(10):977–983. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neale BM, Medland SE, Ripke S, et al. Meta-analysis of genome-wide association studies of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(9):884–897. doi: 10.1016/j.jaac.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Major Depressive Disorder Working Group of the Psychiatric GWAS Consortium. Ripke S, Wray NR, et al. A mega-analysis of genome-wide association studies for major depressive disorder. Mol Psychiatry. 2013;18(4):497–511. doi: 10.1038/mp.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cross-Disorder Group of the Psychiatric Genomics Consortium Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381(9875):1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Franke A, McGovern DP, Barrett JC, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet. 2010;42(12):1118–1125. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson CA, Boucher G, Lees CW, et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011;43(3):246–252. doi: 10.1038/ng.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.International Multiple Sclerosis Genetics C. Hafler DA, Compston A, et al. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med. 2007;357(9):851–862. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- 48.Feng BJ, Sun LD, Soltani-Arabshahi R, et al. Multiple Loci within the Major Histocompatibility Complex Confer Risk of Psoriasis. PLoS genetics. 2009;5(8) doi: 10.1371/journal.pgen.1000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okada Y, Wu D, Trynka G, et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. 2014;506(7488):376. doi: 10.1038/nature12873. + [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harley JB, Alarcon-Riquelme ME, Criswell LA, et al. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nature genetics. 2008;40(2):204–210. doi: 10.1038/ng.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dubois PCA, Trynka G, Franke L, et al. Multiple common variants for celiac disease influencing immune gene expression. Nature genetics. 2010;42(4):295–U242. doi: 10.1038/ng.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cordell HJ, Han YH, Mells GF, et al. International genome-wide meta-analysis identifies new primary biliary cirrhosis risk loci and targetable pathogenic pathways. Nat Commun. 2015:6. doi: 10.1038/ncomms9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barrett JC, Clayton DG, Concannon P, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41(6):703–707. doi: 10.1038/ng.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bulik-Sullivan B, Finucane HK, Anttila V, et al. An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47(11):1236–1241. doi: 10.1038/ng.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chung D, Yang C, Li C, Gelernter J, Zhao H. GPA: a statistical approach to prioritizing GWAS results by integrating pleiotropy and annotation. PLoS Genet. 2014;10(11):e1004787. doi: 10.1371/journal.pgen.1004787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pers TH, Karjalainen JM, Chan Y, et al. Biological interpretation of genome-wide association studies using predicted gene functions. Nat Commun. 2015;6:5890. doi: 10.1038/ncomms6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang DW, Sherman BT, Tan Q, et al. DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 2007;35:W169–175. doi: 10.1093/nar/gkm415. Web Server issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pruim RJ, Welch RP, Sanna S, et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26(18):2336–2337. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alloza I, Otaegui D, de Lapuente AL, et al. ANKRD55 and DHCR7 are novel multiple sclerosis risk loci. Genes Immun. 2012;13(3):253–257. doi: 10.1038/gene.2011.81. [DOI] [PubMed] [Google Scholar]
- 60.Lill CM, Schjeide BM, Graetz C, et al. Genome-wide significant association of ANKRD55 rs6859219 and multiple sclerosis risk. J Med Genet. 2013;50(3):140–143. doi: 10.1136/jmedgenet-2012-101411. [DOI] [PubMed] [Google Scholar]
- 61.Harder MN, Ribel-Madsen R, Justesen JM, et al. Type 2 diabetes risk alleles near BCAR1 and in ANK1 associate with decreased beta-cell function whereas risk alleles near ANKRD55 and GRB14 associate with decreased insulin sensitivity in the Danish Inter99 cohort. J Clin Endocrinol Metab. 2013;98(4):E801–806. doi: 10.1210/jc.2012-4169. [DOI] [PubMed] [Google Scholar]
- 62.Zhernakova A, Stahl EA, Trynka G, et al. Meta-analysis of genome-wide association studies in celiac disease and rheumatoid arthritis identifies fourteen non-HLA shared loci. PLoS Genet. 2011;7(2):e1002004. doi: 10.1371/journal.pgen.1002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Viatte S, Plant D, Bowes J, et al. Genetic markers of rheumatoid arthritis susceptibility in anti-citrullinated peptide antibody negative patients. Ann Rheum Dis. 2012;71(12):1984–1990. doi: 10.1136/annrheumdis-2011-201225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dickstein BD, Weathers FW, Angkaw AC, et al. Diagnostic Utility of the Posttraumatic Stress Disorder (PTSD) Checklist for Identifying Full and Partial PTSD in Active-Duty Military. Assessment. 2015;22(3):289–297. doi: 10.1177/1073191114548683. [DOI] [PubMed] [Google Scholar]
- 65.Stein MB, Kessler RC, Heeringa SG, et al. Prospective Longitudinal Evaluation of the Effect of Deployment-Acquired Traumatic Brain Injury on Posttraumatic Stress and Related Disorders: Results From the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS) Am J Psychiatry. 2015;172(11):1101–1111. doi: 10.1176/appi.ajp.2015.14121572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gelernter J, Kranzler HR, Sherva R, et al. Genome-wide association study of alcohol dependence:significant findings in African- and European-Americans including novel risk loci. Mol Psychiatry. 2014;19(1):41–49. doi: 10.1038/mp.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Andreassen OA, Harbo HF, Wang Y, et al. Genetic pleiotropy between multiple sclerosis and schizophrenia but not bipolar disorder: differential involvement of immune-related gene loci. Mol Psychiatry. 2015;20(2):207–214. doi: 10.1038/mp.2013.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Q, Yang C, Gelernter J, Zhao H. Pervasive pleiotropy between psychiatric disorders and immune disorders revealed by integrative analysis of multiple GWAS. Hum Genet. 2015;134(11-12):1195–1209. doi: 10.1007/s00439-015-1596-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sekar A, Bialas AR, de Rivera H, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016 doi: 10.1038/nature16549. (ePub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Segman RH, Stein MB. C-reactive protein: a stress diathesis marker at the crossroads of maladaptive behavioral and cardiometabolic sequelae. Am J Psychiatry. 2015;172(4):307–309. doi: 10.1176/appi.ajp.2015.15010063. [DOI] [PubMed] [Google Scholar]
- 71.Eraly SA, Nievergelt CM, Maihofer AX, et al. Assessment of Plasma C-Reactive Protein as a Biomarker of Posttraumatic Stress Disorder Risk. JAMA Psychiatry. 2014;71(4):9. doi: 10.1001/jamapsychiatry.2013.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Michopoulos V, Rothbaum AO, Jovanovic T, et al. Association of CRP Genetic Variation and CRP Level With Elevated PTSD Symptoms and Physiological Responses in a Civilian Population With High Levels of Trauma. Am J Psychiatry. 2015;172(4):353–362. doi: 10.1176/appi.ajp.2014.14020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith AK, Conneely KN, Kilaru V, et al. Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet. 2011;156B(6):700–708. doi: 10.1002/ajmg.b.31212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lindqvist D, Wolkowitz OM, Mellon S, et al. Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain Behav Immun. 2014 doi: 10.1016/j.bbi.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 75.Miller-Archie SA, Jordan HT, Ruff RR, et al. Posttraumatic stress disorder and new-onset diabetes among adult survivors of the World Trade Center disaster. Prev Med. 2014;66:34–38. doi: 10.1016/j.ypmed.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 76.Boscarino JA, Forsberg CW, Goldberg J. A twin study of the association between PTSD symptoms and rheumatoid arthritis. Psychosom Med. 2010;72(5):481–486. doi: 10.1097/PSY.0b013e3181d9a80c. [DOI] [PubMed] [Google Scholar]
- 77.O'Donovan A, Cohen BE, Seal KH, et al. Elevated risk for autoimmune disorders in iraq and afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry. 2015;77(4):365–374. doi: 10.1016/j.biopsych.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Logue MW, Amstadter AB, Baker DG, et al. The Psychiatric Genomics Consortium Posttraumatic Stress Disorder Workgroup: Posttraumatic Stress Disorder Enters the Age of Large-Scale Genomic Collaboration. Neuropsychopharmacology. 2015;40(10):2287–2297. doi: 10.1038/npp.2015.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liberzon I, King AP, Ressler KJ, et al. Interaction of the ADRB2 gene polymorphism with childhood trauma in predicting adult symptoms of posttraumatic stress disorder. JAMA Psychiatry. 2014;71(10):1174–1182. doi: 10.1001/jamapsychiatry.2014.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Solovieff N, Roberts AL, Ratanatharathorn A, et al. Genetic Association Analysis of 300 Genes Identifies a Risk Haplotype in SLC18A2 for Post-traumatic Stress Disorder in Two Independent Samples. Neuropsychopharmacology. 2014;39(8):1872–1879. doi: 10.1038/npp.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koenen KC, Fu QJ, Ertel K, et al. Common genetic liability to major depression and posttraumatic stress disorder in men. J Affect Disord. 2008;105(1-3):109–115. doi: 10.1016/j.jad.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wolf EJ, Miller MW, Krueger RF, Lyons MJ, Tsuang MT, Koenen KC. Posttraumatic stress disorder and the genetic structure of comorbidity. J Abnorm Psychol. 2010;119(2):320–330. doi: 10.1037/a0019035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Elhai JD, Contractor AA, Tamburrino M, et al. Structural relations between DSM-5 PTSD and major depression symptoms in military soldiers. J Affect Disord. 2015;175:373–378. doi: 10.1016/j.jad.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 84.Bajor LA, Lai Z, Goodrich DE, et al. Posttraumatic stress disorder, depression, and health-related quality of life in patients with bipolar disorder: review and new data from a multi-site community clinic sample. J Affect Disord. 2013;145(2):232–239. doi: 10.1016/j.jad.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Network, Pathway Analysis Subgroup of Psychiatric Genomics C Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat Neurosci. 2015;18(2):199–209. doi: 10.1038/nn.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lyons MJ, Goldberg J, Eisen SA, et al. Do genes influence exposure to trauma? A twin study of combat. Am J Med Genet. 1993;48(1):22–27. doi: 10.1002/ajmg.1320480107. [DOI] [PubMed] [Google Scholar]
- 87.Kremen WS, Koenen KC, Afari N, Lyons MJ. Twin studies of posttraumatic stress disorder: differentiating vulnerability factors from sequelae. Neuropharmacology. 2012;62(2):647–653. doi: 10.1016/j.neuropharm.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krishna Kumar S, Feldman MW, Rehkopf DH, Tuljapurkar S. Limitations of GCTA as a solution to the missing heritability problem. Proc Natl Acad Sci U S A. 2016;113(1):E61–70. doi: 10.1073/pnas.1520109113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Michopoulos V, Jovanovic T. Chronic inflammation: a new therapeutic target for post-traumatic stress disorder? The Lancet. Psychiatry. 2015;2(11):954–955. doi: 10.1016/S2215-0366(15)00355-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.