Abstract
Objective
The aim of the study was to characterize patient-reported outcomes of analgesia practices in a population-based surgical collaborative.
Background
Pain control among hospitalized patients is a national priority and effective multimodal pain management is an essential component of postoperative recovery, but there is little understanding of the degree of variation in analgesia practice and patient-reported pain between hospitals.
Methods
We evaluated patient-reported pain scores after colorectal operations in 52 hospitals in a state-wide collaborative. We stratified hospitals by quartiles of average pain scores, identified hospital characteristics, pain management practices, and clinical outcomes associated with highest and lowest case-mix-adjusted pain scores, and compared against Hospital Consumer Assessment of Healthcare Providers and Systems pain management metrics.
Results
Hospitals with the lowest pain scores were larger (503 vs 452 beds; P<0.001), higher volume (196 vs 112; P=0.005), and performed more laparoscopy (37.7% vs 27.2%; P<0.001) than those with highest scores. Their patients were more likely to receive local anesthesia (31.1% vs 12.9%; P<0.001), nonsteroidal anti-inflammatory drugs (33.5% vs 14.4%; P<0.001), and patient-controlled analgesia (56.5% vs 22.8%; P<0.001). Adverse postoperative outcomes were less common in hospitals with lowest pain scores, including complications (20.3% vs 26.4%; P<0.001), emergency department visits (8.2% vs 15.8%; P<0.001), and readmissions (11.3% vs 16.2%; P=0.01).
Conclusions
Pain management after colorectal surgery varies widely and predicts significant differences in patient-reported pain and clinical outcomes. Enhanced postoperative pain management requires dissemination of multimodal analgesia practices. Attention to patient-reported outcomes often omitted from surgical outcomes registries is essential to improving quality from the patient's perspective.
Keywords: analgesia, colectomy, HCAHPS, pain, patient-reported outcomes
The management of pain has become a national priority in US hospitals. Since 2001, the Joint Commission has mandated that hospitals have procedures in place for the assessment and treatment of pain, and for institutional performance improvement in pain control.1 Surgical patients identify adequate pain control as their highest priority in postoperative recovery.2 Patients' experiences with the effectiveness of pain management are highly correlated with their overall ratings of hospital quality and their willingness to recommend a hospital.3 With rising interest in and emphasis on patient-reported outcomes,4 perioperative pain management will be increasingly important to the quality of surgical care.
Nevertheless, pain control after major surgery remains largely inadequate. Despite increasing attention and novel approaches to postoperative pain management, a large majority of patients still experience severe pain after major surgery,5–7 and the incidence of severe pain reported by patients has not improved in recent decades.5,7,8 Poorly controlled pain increases the incidence of postoperative complications, prolongs length of stay, induces readmissions, and significantly impairs patient satisfaction.9 Further, reliance on narcotic analgesia in the perioperative period is associated with prolonged postoperative ileus and length of stay,10 as well as delirium and the risk of respiratory suppression. Thus, practice guidelines for perioperative pain management recommend multimodal approaches, and discourage intermittent use of parenteral narcotics, to reduce the overall quantity of narcotic analgesia required.11,12
Even with increasing use of standardized perioperative care pathways around colorectal resections, there remains substantial variation in perioperative analgesia regimens, and intravenous narcotics remain a mainstay.13 Thus, we sought to evaluate the extent to which multimodal pain management practices are used after major surgery, and how hospitals' perioperative practices affect patient-reported pain levels in real-world surgical practice. In the setting of a population-based, statewide cohort of 52 hospitals participating in a collaborative quality improvement program for general surgery, we evaluated variation in perioperative pain management practices around colorectal surgery, and patient-reported pain scores after surgery.
METHODS
Setting
This is a retrospective cohort study from the Michigan Surgical Quality Collaborative (MSQC), a voluntary network of 52 hospitals that collect data on surgical patients for the purpose of quality improvement.14,15 The MSQC is funded by Blue Cross Blue Shield of Michigan, a private, not-for-profit insurance company. Although Blue Cross Blue Shield provides financial support for the project, it is not involved in the policy recommendations that are developed within the collaborative. MSQC hospitals are predominantly community hospitals. Each participating hospital employs at least 1 trained Surgical Clinical Quality Reviewer to prospectively collect data on general and vascular surgery patients, their operations, and 30-day outcomes. Patient selection uses an algorithm designed to minimize selection bias. MSQC data collection is Institutional Review Board exempt, and the current study was performed with University of Michigan Institutional Review Board review, from a limited data set derived from the MSQC database. Patients with missing data for pain scores were excluded. Participating hospitals with less than 10 cases were excluded from the analysis.
Patients and Procedures
We included all patients who underwent intestinal resection between July 1, 2012 and December 1, 2014, with or without anastomosis and/or stoma. Procedures were selected according to Current Procedural Terminology codes 44120, 44125, 44140, 44141, 44143, 44144, 44145, 44146, 44147, 44150, 44151, 44155, 44156, 44157, 44158, 44160, 44187, 44188, 44202, 44204, 44205, 44206, 44207, 44208, 44210, 44211, 44212, 44227, 44310, 44661, 45110, 45111, 45112, 45113, 45114, 45119, 45135, 45395, 45397, 45402, 45550. We excluded outpatient cases and intraoperative deaths.
Measures
The primary outcome variable was the patient-reported numeric scale pain score closest to 6 am on the first postoperative day. The pain score is a rating from 0 to 10, or from “no pain” to “worst possible, excruciating, unbearable pain.” This scale is used widely, and has good reliability and validity across a variety of postoperative patients.16 Pain scores were obtained from the medical record, and are most typically entered in the nursing records for patients after surgery. We excluded from the analysis all patients (4338, 37.5%) with missing pain scores.
The primary predictors of interest for this study were the institutional characteristics and pain management regimens most associated with differences in pain scores on the first postoperative day. Pain management strategies from both the intraoperative and postoperative phases of care were abstracted prospectively, and categorized as local anesthesia, epidural, intermittent parenteral narcotics, oral narcotics, patient-controlled anesthesia (PCA), nonsteroidal anti-inflammatory drugs (NSAIDs), and/or continuous wound infusions. In addition to the primary variables of interest, additional patient demographic and hospital stay-specific data were analyzed and adjusted to control for individual patient risk, as described below.
To evaluate overall hospital performance on pain management, we obtained patient-reported experience from the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey, administered by the Centers for Medicare and Medicaid Services.17 The HCAHPS survey (viewable at http://www.hcahpsonline.org/surveyinstrument.aspx) is administered to medical and surgical inpatients across all service lines and in several languages, and includes 2 questions about the degree to which pain was well controlled. From these responses, a “star rating” and consistency of pain control are computed for each hospital. We obtained the publicly reported hospital ratings dataset from CMS Hospital Compare,18 including the most recent reporting period, from October 2013 to September 2014, as this most closely mirrored the study period.
Statistical Analyses
Because we were primarily interested in the effect of institutional practice patterns, rather than patient characteristics, on pain scores, we analyzed pain scores at the hospital level. To account for confounding patient and procedural characteristics, pain scores were risk-adjusted for the following parameters: age, sex, race, insurance, body mass index (BMI), alcohol use, smoking, cancer, number of Charlson comorbidities, surgical priority, operation type, procedure duration, and blood loss. We then computed risk and reliability adjusted average pain scores for each hospital by summing model residuals on the overall average followed by reliability adjustment using a Bayesian shrinkage estimator, to minimize the potential for bias due to differences in sample size between hospitals, as we have done in previous studies.19,20 We constructed caterpillar charts to display variation in scores across hospitals, stratified by surgical approach.
To compare hospital structural characteristics, pain management care processes, and outcomes according to pain scores quartiles, we then ranked and grouped hospitals into 4 evenly-sized quartiles by average adjusted pain scores. We compared hospital characteristics and HCAHPS pain management ratings across quartiles using the Cochrane-Armitage chi-square trend test for adjusted proportions, and F probability for adjusted means.
RESULTS
Patient and Procedural Characteristics and Postoperative Pain Scores
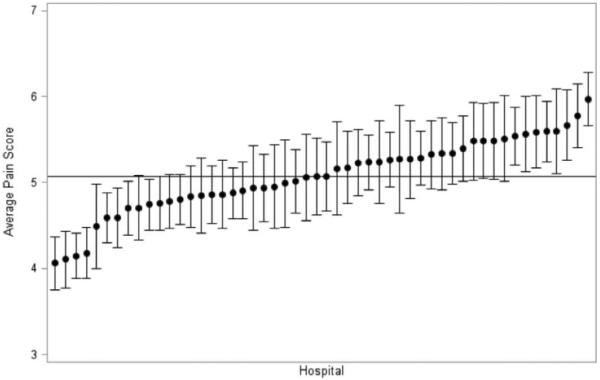
Among the 7221 patients who underwent colorectal resection during the study period, the mean initial pain score reported on postoperative day 1 was 5.1 [standard deviation (SD) 2.44]. There was substantial variation in adjusted pain scores between hospitals, from approximately 4 to 6, out of 10. Hospitals' risk and reliability-adjusted average pain scores are shown in the caterpillar plot in Figure 1. Nine low-ranking hospitals and 8 high-ranking hospitals had average adjusted pain scores that were statistically significantly different from the overall average.
FIGURE 1.
Distribution of risk and reliability adjusted average pain scores on the first day after colorectal resection, by hospital (N=7221).
Average pain scores, according to patient and procedure characteristics, are displayed in Table 1. Significantly higher average pain scores were found among patients who were under age 50 (5.6±0.06) versus over 75 (4.8±0.07; P<0.001), women (5.2±0.04) versus men (4.9±0.04; P<0.001), black race (5.8±0.07) versus white (4.9±0.03) or other (4.9 0.13; P<0.001), smokers (5.6±0.06) versus nonsmokers (4.9±0.03; P<0.001), and uninsured (5.6±0.12) or insured by Medicaid (5.3±0.11) versus Medicare (5.0±0.04) or privately insured (4.9±0.04; P<0.001). There was a significant “J-shaped” distribution21,22 of pain scores by BMI (P<0.001), highest among underweight (BMI <18.5) patients (5.5±0.13) and lowest among overweight (BMI 25–29.9) patients (4.9±0.05). Pain score monotonically increased with number of comorbidities (P<0.001).
TABLE 1.
Patient Characteristics and Unadjusted Pain Scores on the First Day After Colorectal Resection
| N (%) | Postoperative Day #1 Pain Score (Mean±SD) | P | |
|---|---|---|---|
| Age | <0.001 | ||
| <50 | 1476 (20.4) | 5.6±0.06 | |
| 50–65 | 2807 (38.9) | 5.1±0.05 | |
| 65–75 | 1649 (22.8) | 4.9±0.06 | |
| >75 | 1289 (17.9) | 4.8±0.07 | |
| Sex | <0.001 | ||
| Male | 3246 (45.0) | 4.9±0.04 | |
| Female | 3975 (55.1) | 5.2±0.04 | |
| Diagnosis | <0.001 | ||
| Cancer | 1904 (28.2) | 4.7±0.06 | |
| Diverticular disease | 1799 (26.6) | 5.0±0.06 | |
| Inflammatory bowel disease | 307 (4.6) | 5.7±0.14 | |
| Other | 3211 (44.5) | 5.2±0.05 | |
| Race | <0.001 | ||
| White | 5836 (80.8) | 4.9±0.03 | |
| Black | 1056 (14.6) | 5.8±0.07 | |
| Other | 329 (4.6) | 4.9±0.13 | |
| Alcohol >2/d | 0.45 | ||
| Yes | 293 (4.1) | 5.2±0.14 | |
| No | 6928 (95.9) | 5.1±0.03 | |
| Smoking | <0.001 | ||
| Yes | 1740 (24.1) | 5.6±0.06 | |
| No | 5481 (75.9) | 4.9±0.03 | |
| Insurance | <0.001 | ||
| Medicare | 3271 (45.4) | 5.0±0.04 | |
| Medicaid | 499 (6.9) | 5.3±0.11 | |
| Private | 2927 (40.6) | 4.9±0.04 | |
| Self/uninsured/other | 514 (7.1) | 5.6±0.12 | |
| BMI | <0.001 | ||
| <18.5 | 327 (4.5) | 5.5±0.13 | |
| 18.5–24.9 | 2122 (29.4) | 5.2±0.05 | |
| 25–29.9 | 2270 (31.4) | 4.9±0.05 | |
| 30–34.9 | 1415 (19.6) | 5.0±0.06 | |
| ≥35 | 1087 (15.1) | 5.2±0.07 | |
| Charlson comorbidities | <0.001 | ||
| 0 | 2113 (29.3) | 4.9±0.05 | |
| 1–2 | 3897 (54.0) | 5.1±0.04 | |
| 3–4 | 1000 (13.9) | 5.2±0.08 | |
| 5+ | 211 (2.9) | 5.6±0.17 |
The association between pain scores and procedural characteristics are shown in Table 2. Pain scores were significantly lower after minimally invasive (4.8±0.05), compared with open operations (5.2±0.04) and those converted from laparoscopic to open (5.2±0.10; P<0.001).
TABLE 2.
Procedure Characteristics and Unadjusted Pain Scores on the First Day After Colorectal Resection
| N (%) | Postoperative Day #1 pain score (mean±SE) | P † | |
|---|---|---|---|
| Type of operation | <0.001 | ||
| Open segmental colectomy or small bowel resection without ostomy | 3339 (46.2) | 5.2±0.04 | |
| Minimally invasive segmental colectomy or small bowel resection without ostomy | 2137 (29.6) | 4.7± 0.05 | |
| Open segmental colectomy or small bowel resection with ostomy | 975 (13.5) | 5.3± 0.08 | |
| Minimally invasive segmental colectomy or small bowel resection with ostomy | 146 (2.0) | 5.1±0.20 | |
| Open total colectomy or proctocolectomy | 207 (2.9) | 5.3±0.17 | |
| Minimally invasive total colectomy or proctocolectomy | 100 (1.4) | 5.5±0.24 | |
| Open proctectomy, not APR | 58 (0.8) | 4.7±0.32 | |
| Open APR | 100 (1.4) | 5.8±0.24 | |
| Minimally invasive APR | 34 (0.5) | 5.8±0.42 | |
| Other | 125 (1.7) | 5.6 ±0.22 | |
| Approach | <0.001 | ||
| Minimally invasive | 2500 (34.6) | 4.8±0.05 | |
| Minimally invasive, converted to open | 637 (8.8) | 5.2±0.10 | |
| Open | 4084 (56.6) | 5.2±0.04 | |
| Case status | <0.001 | ||
| Emergency | 1452 (20.1) | 5.3± 0.06 | |
| Urgent | 1493 (20.7) | 5.6±0.06 | |
| Elective | 4276 (59.2) | 4.8± 0.04 | |
| Estimated blood loss | 0.07 | ||
| <100 | 3183 (44.1) | 5.0±0.04 | |
| 100–500 | 3524 (48.8) | 5.1±0.04 | |
| 500–1000 | 398 (5.5) | 5.3±0.12 | |
| >1000 | 116 (1.6) | 5.4±0.23 | |
| Operative duration | 0.07 | ||
| <2 h | 3053 (42.3) | 5.0±0.04 | |
| 2–3 h | 2167 (30.0) | 5.0±0.05 | |
| 3–4 h | 1122 (15.5) | 5.2±0.07 | |
| >4 h | 879 (12.2) | 5.2±0.08 |
APR, abdominoperineal resection; SE, standard error.
Abdominoperineal resections (5.8±0.24) incurred higher average pain scores than abdominal $operations (5.2±0.04; P<0.001); and total colectomy (5.5±0.19) incurred higher pain scores than segmental resections (5.1±0.03; P<0.001). Moreover, pain scores were lower after elective operations (4.8±0.04) than after urgent (5.6±0.06) or emergency operations (5.2±0.06; P<0.001). Blood loss and operative duration were not significantly correlated with postoperative pain scores.
Hospital Characteristics and Practices and Postoperative Pain Scores
Examining strata of hospitals by quartiles of average adjusted pain scores, there were significant differences in the institutional characteristics and pain management practices (Table 3). Compared against hospitals with the highest pain scores, those with best pain scores were somewhat larger (mean 503 vs 452 beds; P<0.001), with higher annual volume of colorectal resections (196 vs 112; P=0.005). Mean operative duration was not meaningfully different between the lowest and the highest quartiles.
TABLE 3.
Hospital Characteristics, Analgesia Practices and HCAHPs Scores, by Quartiles of Average Risk and Reliability-adjusted Average Pain Scores on the First Day After Colorectal Resection
| Lowest Quartile (Best Scores) | Second Quartile | Third Quartile | Highest Quartile (Worst Scores) | P | |
|---|---|---|---|---|---|
| Number of hospitals | 12 | 12 | 13 | 12 | |
| Number of patients | 2544 | 1735 | 1480 | 1462 | |
| Pain score, mean±SD | 4.5 (2.2) | 5.0 (2.3) | 5.4 (2.4) | 5.8 (2.4) | <0.001 |
| Hospital characteristics | |||||
| Beds, mean±SD | 503±5 | 544±6 | 371±7 | 452±6 | <0.001 |
| Annual procedure volume, mean±SD | 196±21 | 133±19 | 114±17 | 112±14 | 0.005 |
| Minimally invasive, % | 37.7% | 36.5% | 34.3% | 27.2% | <0.001 |
| Operative duration (min), mean±SD | 151±2 | 159±2 | 144±2 | 153±2 | <0.001 |
| Practices | |||||
| Intraoperative | |||||
| Local anesthesia | 31.1% | 35.9% | 35.6% | 12.9% | <0.001 |
| Epidural | 15.2% | 10.2% | 7.8% | 13.3% | <0.001 |
| Nerve block | 3.3% | 8.9% | 2.7% | 3.6% | 0.22 |
| Postoperative | |||||
| Intermittent parenteral narcotics | 65.8% | 65.4% | 84.4% | 89.2% | <0.001 |
| Oral narcotics | 33.3% | 35.7% | 38.7% | 41.5% | <0.001 |
| PCA | 56.5% | 52.5% | 27.7% | 22.8% | <0.001 |
| Epidural | 11.2% | 7.1% | 7.8% | 12.7% | 0.56 |
| PCEA | 7.0% | 5.2% | 1.3% | 4.5% | <0.001 |
| NSAIDS | 33.5% | 32.1% | 30.7% | 14.4% | <0.001 |
| Continuous local infusion | 0.9% | 3.2% | 0.6% | 0.3% | 0.006 |
| Postoperative | |||||
| 2 or more of the above | 68.3% | 63.3% | 63.1% | 58.5% | <0.001 |
| Epidural + PCA | 2.2% | 1.2% | 0.5% | 2.5% | 0.50 |
| PCA+NSAID | 17.2% | 17.8% | 9.4% | 3.8% | <0.001 |
| HCAHPS pain scores | |||||
| Star rating (mean) | 3.4 (0.8) | 3.2 (0.7) | 3.3 (0.8) | 2.8 (1.1) | 0.23 |
| Pain always controlled | 69.5% | 68.9% | 69.2% | 67.8% | 0.44 |
| Pain usually controlled | 23.9% | 24.0% | 24.1% | 23.3% | 0.63 |
| Pain sometimes/never controlled | 6.6% | 7.1% | 6.7% | 9.0% | 0.02 |
PCEA, patient-controlled epidural analgesia.
Patients in hospitals with best pain scores were significantly more likely than those in hospitals with worst pain scores to receive local anesthesia (31.1% vs 12.9%; P<0.001) and epidural anesthesia (15.2% vs 13.3%; P<0.001) during the operation, and to have PCA (56.5% vs 22.8%, P<0.001), NSAIDs (33.5 vs 14.4%; P<0.001), or a combination of PCA + NSAIDs (17.2% vs 3.8%; P<0.001) postoperatively. Patients in lowest pain score hospitals were significantly less likely to receive intermittent postoperative narcotics, either parenteral (65.8% vs 89.2%; P<0.001) or oral (33.3% vs 41.5%; P<0.001).
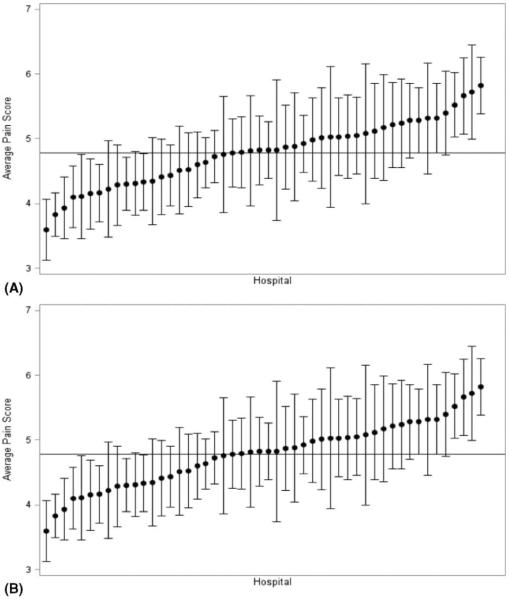
Rates of minimally invasive surgery varied widely between hospitals, with the lowest pain score hospitals performing a significantly higher proportion of operations with a minimally invasive approach (37.7% vs 27.2%; P<0.001). However, even after stratifying by operative approach, there remained substantial variation between hospitals in the adjusted pain scores within both open and minimally invasive operations (Fig. 2A, B). Further, hospitals' adjusted average pain score rankings were correlated between the open and minimally invasive operations (Spearman ρ=0.49, P<0.0001).
FIGURE 2.
A, Distribution of risk and reliability adjusted average pain scores on the first day after minimally-invasive colorectal resection, by hospital (n=2500). B, Distribution of risk and reliability adjusted average pain scores on the first day after open colorectal resection, by hospital (n=4084).
Hospitals' HCAHPS survey results for the pain questions did not differ meaningfully between quartiles of postoperative pain scores. Summary star ratings, on a scale from 1 to 4, ranged from 2.8 in hospitals with the worst scores to 3.4 in those with the best scores, but this difference was not statistically significant (P=0.23). Likewise, there was little difference in the proportions of patients reporting that their pain was always or usually controlled (Table 3).
Association between Pain Scores and Surgical Outcomes
Patients whose operations took place in hospitals in the best quartile of pain scores had significantly shorter mean postoperative length of stay than those in hospitals with the worse pain scores (6.5 vs 7.9 d; P=0.007), and were less likely to have a postoperative complication (20.3% vs 26.4%; P<0.001), emergency department visit (8.2% vs 15.8%; P<0.001), or readmission (11.3% vs 16.2%; P=0.01). Hospitals with best pain scores had significantly lower rates of pulmonary complications including pneumonia and reintubation (3.2% vs 6.6%; P<0.001). There was no difference in the rate of reoperation (Table 4).
TABLE 4.
Clinical Outcomes, by Quartiles of Average Risk and Reliability-adjusted Average Pain Scores on the First Day After Colorectal Resection
| Lowest Quartile (Best Scores) | Second Quartile | Third Quartile | Highest Quartile (Worst Scores) | P | |
|---|---|---|---|---|---|
| Number of hospitals | 12 | 12 | 13 | 12 | |
| Number of patients | 2544 | 1735 | 1480 | 1462 | |
| Pain score, mean±SD | 4.5 (2.2) | 5.0 (2.3) | 5.4 (2.4) | 5.8 (2.4) | <0.001 |
| Length of stay (d), mean±SD | 6.5 (0.1) | 6.9 (0.2) | 6.9 (0.2) | 7.9 (0.2) | 0.007 |
| ED visit | 8.2% | 11.3% | 9.0% | 15.8% | <0.001 |
| Unplanned readmission within 30 d | 11.3% | 13.3% | 10.2% | 16.2% | 0.01 |
| Unplanned reoperation within 30 d | 7.9% | 5.9% | 7.2% | 7.5% | 0.94 |
| Overall complications | 20.3% | 25.7% | 22.8% | 26.4% | <0.001 |
| Pneumonia or reintubation | 3.2% | 4.9% | 4.6% | 6.6% | <0.001 |
| Venous thromboembolism | 2.7% | 3.1% | 2.2% | 2.3% | 0.26 |
| Surgical site infection | 8.8% | 11.9% | 8.2% | 10.7% | 0.32 |
ED, emergency department.
DISCUSSION
Numerous national healthcare quality organizations, including the National Quality Forum, Commonwealth Fund, and the Agency for Healthcare Research and Quality, have made patient-reported outcomes a priority for evaluation and improvement. These efforts face methodological barriers because patient experience, functional outcomes, and quality of life may be rarely measured in clinical practice. Pain, on the contrary, is central to patients' experience of surgery, and is routinely measured in the hospital. Still, few surgical quality improvement organizations have included this outcome in clinical registries.
Within a state-wide surgical collaborative, we have collected postoperative pain scores, and in this first study of these outcomes, we find wide variation in patient-reported levels of postoperative pain after colorectal resection. Pain scores were associated with patients' demographic and clinical characteristics, as well as procedure characteristics. However, even accounting for these factors, there remained substantial differences between hospitals in the average pain scores reported by patients. These differences were associated with distinct practice patterns in perioperative pain management.
At the hospital level, the best performers were significantly less likely to employ intermittent as-needed narcotics, either parenteral or oral. Instead, they tended to use patient-controlled anesthesia, either intravenous or epidural. The superiority of patient-controlled opioid analgesia in postoperative patients is supported by a meta-analysis of 55 trials and over 2000 patients, which found that PCA provides superior pain control and patient satisfaction, though at a cost of increased amount of opioid used.23
Pain scores on the first postoperative day were also predictive of short-term surgical outcomes. Hospitals with higher average pain scores displayed increased rates of overall complications, emergency department visits, and readmissions, suggesting there is consistency in the overall quality performance across both clinical and patient-reported outcomes for colectomy. It is possible that this association is driven by case or patient complexity differences between institutions. However, there may also be a more deterministic relationship at the patient level, whereby patients experiencing more pain have limited mobility, higher rates of ileus, or other adverse consequences that predispose to poorer clinical outcomes. We found that better pain control was associated with a lower rate of pulmonary complications, which could be an indicator of better pulmonary toilet or lesser respiratory depression. In other studies, differences of a single point in the 10-point pain scale have been found to be clinically significant,24 and these associations suggest that differences of this magnitude were associated with key clinical outcomes when averaged across the cohort.
Most likely, however, both pain scores and clinical outcomes reflect more global features of the quality of care in hospitals' surgical performance. Thus, hospitals with the most streamlined, high-quality perioperative care pathways experience the best pain scores, as well as improved clinical outcomes.25–27 On the contrary, hospitals' overall performance on the pain control components of the HCAHPS survey did not differ according to their postoperative pain scores after colectomy. HCAHPS surveys are administered to both medical and surgical patients, without selection for diagnosis or procedure groups. Thus, the lack of correspondence between HCAHPS and postoperative pain scores suggests that the quality of pain management after colorectal resections may be specific to the systems of care surrounding these operations, rather than institution-wide practices for patients with widely varying reasons for admission.
This study is limited by its reliance on patient-reported pain scores, which are by their nature subjective and patient-dependent. Some have even questioned whether lower pain scores are necessarily a good clinical outcome, as excessive attention to reducing pain scores may risk analgesic-related complications such as opioid-related respiratory depression.28 However, the 10-point pain scale is the most widely used clinical metric for postoperative pain management in US hospitals. It is thought to be the most broadly applicable and reliable tool for patient-reported clinical pain assessment,29 and is included in recommendations for perioperative care from the American Society of Anesthesiologists Task Force on Pain Management.30 With increasing interest in patient-reported outcomes in surgery,4,31 the insight into patients' experiences with this measure probably outweighs any loss of clinical precision. Further, by evaluating practices by hospital, rather than by the individual patient level, the reliability of measures is substantially more stable and less susceptible to confounding by indication and selection bias.32
There are also several clinical details we lacked in this analysis. It has been recognized that primary care opioid prescription administration varies by age, race, and insurance status,33 and we do not know about patients' preoperative use of narcotics or chronic pain status—both important predictors of postoperative satisfaction with pain management34—nor whether these characteristics were evenly distributed between hospitals. However, again, our focus on hospital-level practices and outcomes, rather than individual patient pain scores, should minimize bias from these patient-specific factors. At the hospital level, there are likely a variety of pain management practices we could not measure, including the quality of pain-specific care management,35 technical performance of anesthetic blocks, and other factors. We also did not collect data on the use of pre-emptive analgesia in the days before surgery—a practice that has been integrated in some institutions' perioperative care pathways.36,37 Pain scores were missing for many patients, resulting in the exclusion of nearly a third of the sample. However, missingness was distributed relatively equally across hospitals, unrelated to average pain scores reported, and not meaningfully correlated with any demographic or clinical characteristics. Finally, there may have been some differences in the procedures by which pain scores were recorded between hospitals. These unmeasured factors likely account for the substantial residual hospital-level variation that is only partially explained by the practices we describe herein.
Instead, this population-based, statewide collaborative offers a real-world assessment of the range of practices employed for perioperative analgesia around colectomy. The membership of the Michigan Surgical Quality Collaborative is very diverse, including hospitals that are academic and community-based, large and small, urban and rural, some with surgeons who have broad general surgical practices and some with colorectal specialists. Recognizing that there is immediate opportunity for standardization and optimization of pain management practices within our state-wide collaborative, we will use these findings to disseminate best practices for the lowest performers. These efforts will be aided substantially by the close relationship between MSQC and a recently launched state-wide anesthesiology collaborative—the Anesthesiology Performance Improvement and Reporting Exchange (ASPIRE).
In summary, this study shows that hospitals vary significantly in their postoperative pain management practices around colorectal resections, and in their effectiveness in early postoperative pain control, even after accounting for differences in patient mix and complexity. Further, hospitals' success at early postoperative pain management was associated with a variety of other risk-adjusted clinical outcomes. The association between patient-reported pain and other clinical outcomes may reflect the overall quality of perioperative care at high-performing hospitals, or there may be an instrumental effect of pain control on clinical outcomes. Either way, these findings reveal systematic clinical care variation that could be reduced to improve patients' experience of pain after colorectal resections.
Footnotes
Disclosures: The authors have no conflicts of interest.
REFERENCES
- 1.The Joint Commission. Standards FAQ [Accessed January 16, 2015]; Available at: http://www.jointcommission.org/standards_information/jcfaqdetails.aspx?StandardsFAQId=471&StandardsFAQChapterId=78.
- 2.Hughes M, Coolsen MM, Aahlin EK, et al. Attitudes of patients and care providers to enhanced recovery after surgery programs after major abdominal surgery. J Surg Res. 2015;193:102–110. doi: 10.1016/j.jss.2014.06.032. [DOI] [PubMed] [Google Scholar]
- 3.HCAPHPS Patient Level Correlations [Accessed January 16, 2015];Centers for Medicare and Medicaid Services. Available at: http://www.hcahpsonline.org/Files/HCAHPSReport_April_2014_Corrs.pdf.
- 4.Bilimoria KY, Cella D, Butt Z. Current challenges in using patient-reported outcomes for surgical care and performance measurement: everybody wants to hear from the patient, but are we ready to listen? JAMA Surg. 2014;149:505–506. doi: 10.1001/jamasurg.2013.5285. [DOI] [PubMed] [Google Scholar]
- 5.Apfelbaum JL, Chen C, Mehta SS, et al. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97:534–540. doi: 10.1213/01.ANE.0000068822.10113.9E. table of contents. [DOI] [PubMed] [Google Scholar]
- 6.Dolin SJ, Cashman JN, Bland JM. Effectiveness of acute postoperative pain management: I. Evidence from published data. Br J Anaesth. 2002;89:409–423. [PubMed] [Google Scholar]
- 7.Gan TJ, Habib AS, Miller TE, et al. Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin. 2014;30:149–160. doi: 10.1185/03007995.2013.860019. [DOI] [PubMed] [Google Scholar]
- 8.Warfield CA, Kahn CH. Acute pain management. Programs in U.S. hospitals and experiences and attitudes among US adults. Anesthesiology. 1995;83:1090–1094. doi: 10.1097/00000542-199511000-00023. [DOI] [PubMed] [Google Scholar]
- 9.Sexton J, Thomas EJ, Helmreich R. Error, stress, and teamwork in medicine and aviation; cross-sectional surveys. Br Med J. 2000;320:745–749. doi: 10.1136/bmj.320.7237.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed J, Lim M, Khan S, et al. Predictors of length of stay in patients having elective colorectal surgery within an enhanced recovery protocol. Int J Surg. 2010;8:628–632. doi: 10.1016/j.ijsu.2010.07.294. [DOI] [PubMed] [Google Scholar]
- 11.Ashburn MA, Caplan RA, Carr DB, et al. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116:248–273. doi: 10.1097/ALN.0b013e31823c1030. [DOI] [PubMed] [Google Scholar]
- 12.Jin F, Chung F. Multimodal analgesia for postoperative pain control. J Clin Anesthesiol. 2001;13:524–539. doi: 10.1016/s0952-8180(01)00320-8. [DOI] [PubMed] [Google Scholar]
- 13.Mahadevan V, Nadarajan S, Tamilselvan P, et al. Analgesia for laparoscopic colorectal surgery: an international survey. Br J Anaesth. 2012;108:ii414. [Google Scholar]
- 14.Campbell DA, Jr, Kubus JJ, Henke PK, et al. The Michigan Surgical Quality Collaborative: a legacy of Shukri Khuri. Am J Surg. 2009;198:S49–S55. doi: 10.1016/j.amjsurg.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Share DA, Campbell DA, Birkmeyer N, et al. How a regional collaborative of hospitals and physicians in Michigan cut costs and improved the quality of care. Health Aff (Millwood) 2011;30:636–645. doi: 10.1377/hlthaff.2010.0526. [DOI] [PubMed] [Google Scholar]
- 16.Gagliese L, Weizblit N, Ellis W, et al. The measurement of postoperative pain: a comparison of intensity scales in younger and older surgical patients. Pain. 2005;117:412–420. doi: 10.1016/j.pain.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 17.HCAHPS Hospital Survey . Centers for Medicare & Medicaid Services. Baltimore, MD: [Accessed July 31, 2015]. Available at: http://www.hcahpsonline.org. [Google Scholar]
- 18.Hospital Compare [Accessed July 31, 2015];Centers for Medicare and Medicaid Services. Available at: https://data.medicare.gov/data/hospital-compare.
- 19.Krell RW, Staiger DO, Dimick JB. Reliability of surgical outcomes for predicting future hospital performance. Med Care. 2014;52:565–571. doi: 10.1097/MLR.0000000000000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimick JB, Staiger DO, Birkmeyer JD. Ranking hospitals on surgical mortality: the importance of reliability adjustment. Health Serv Res. 2010;45:1614–1629. doi: 10.1111/j.1475-6773.2010.01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 22.Flegal KM, Graubard BI, Williamson DF, et al. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 23.Hudcova J, McNicol E, Quah C, et al. Patient controlled opioid analgesia versus conventional opioid analgesia for postoperative pain. Cochrane Database Syst Rev. 2006 Oct;18(4):CD003348. doi: 10.1002/14651858.CD003348.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Bailey B, Daoust R, Doyon-Trottier E, et al. Validation and properties of the verbal numeric scale in children with acute pain. Pain. 2010;149:216–221. doi: 10.1016/j.pain.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Bradshaw BG, Liu SS, Thirlby RC. Standardized perioperative care protocols and reduced length of stay after colon surgery. J Am Coll Surg. 1998;186:501–506. doi: 10.1016/s1072-7515(98)00078-7. [DOI] [PubMed] [Google Scholar]
- 26.Lassen K, Soop M, Nygren J, et al. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. 2009;144:961–969. doi: 10.1001/archsurg.2009.170. [DOI] [PubMed] [Google Scholar]
- 27.Stephen AE, Berger DL. Shortened length of stay and hospital cost reduction with implementation of an accelerated clinical care pathway after elective colon resection. Surgery. 2003;133:277–282. doi: 10.1067/msy.2003.19. [DOI] [PubMed] [Google Scholar]
- 28.Burgess FW. Pain scores: are the numbers adding up to quality patient care and improved pain control? Pain Med. 2006;7:371–372. doi: 10.1111/j.1526-4637.2006.00219.x. [DOI] [PubMed] [Google Scholar]
- 29.Jensen MP, Karoly P, O'Riordan EF, et al. The subjective experience of acute pain. An assessment of the utility of 10 indices. Clin J Pain. 1989;5:153–159. doi: 10.1097/00002508-198906000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Flum DR, Dellinger EP. Impact of gastric bypass operation on survival: a population-based analysis. J Am Coll Surg. 2004;199:543–551. doi: 10.1016/j.jamcollsurg.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 31.Macefield RC, Avery KN, Blazeby JM. Integration of clinical and patient-reported outcomes in surgical oncology. Br J Surg. 2013;100:28–37. doi: 10.1002/bjs.8989. [DOI] [PubMed] [Google Scholar]
- 32.Dimick JB, Livingston EH. Comparing treatments using observational study designs: what can we do about selection bias? Arch Surg. 2010;145:927. doi: 10.1001/archsurg.2010.223. [DOI] [PubMed] [Google Scholar]
- 33.Olsen Y, Daumit GL, Ford DE. Opioid prescriptions by U.S. primary care physicians from 1992 to 2001. J Pain. 2006;7:225–235. doi: 10.1016/j.jpain.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Danforth RM, Pitt HA, Flanagan ME, et al. Surgical inpatient satisfaction: what are the real drivers? Surgery. 2014;156:328–335. doi: 10.1016/j.surg.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 35.Hammond EJ, Veltman MG, Turner GA, et al. The development of a performance indicator to objectively monitor the quality of care provided by an acute pain team. Anaesth Intensive Care. 2000;28:293–299. [PubMed] [Google Scholar]
- 36.Favuzza J, Brady K, Delaney CP. Transversus abdominis plane blocks and enhanced recovery pathways: making the 23-h hospital stay a realistic goal after laparoscopic colorectal surgery. Surg Endosc. 2013;27:2481–2486. doi: 10.1007/s00464-012-2761-y. [DOI] [PubMed] [Google Scholar]
- 37.Ho KY, Gan TJ, Habib AS. Gabapentin and postoperative pain: a systematic review of randomized controlled trials. Pain. 2006;126:91–101. doi: 10.1016/j.pain.2006.06.018. [DOI] [PubMed] [Google Scholar]