Abstract
As the most abundant protein in mammals and a major structural component in extracellular matrix, collagen holds a pivotal role in tissue development and maintaining the homeostasis of our body. Persistent disruption to the balance between collagen production and degradation can cause a variety of diseases, some of which can be fatal. Collagen remodeling can lead to either an overproduction of collagen which can cause excessive collagen accumulation in organs, common to fibrosis, or uncontrolled degradation of collagen seen in degenerative diseases such as arthritis. Therefore, the ability to monitor the state of collagen is crucial for determining the presence and progression of numerous diseases. This review discusses the implications of collagen remodeling and its detection methods with specific focus on targeting native collagens as well as denatured collagens. It aims to help researchers understand the pathobiology of collagen-related diseases and create novel collagen targeting therapeutics and imaging modalities for biomedical applications.
Keywords: Drug delivery, collagen binding molecules, collagen mimetic peptide, collagen hybridizing peptide, theranostics, collagen remodeling, denatured collagen, ECM remodeling
Graphical abstract
Introduction
Collagen is the most abundant protein in mammals and is a major component of extracellular matrix (ECM) providing a vital structural matrix for tissue development, maintenance, and regeneration [1]. The three main types of collagen, type I, II, and III, account for nearly 90% of the collagen found in the body; all of these types are fibrous and share a triple-helical molecular structure. Normal tissue development involves a dynamic collagen remodeling process, which includes both collagen production and degradation. An imbalance in these processes causes abnormalities in the structure and metabolism of collagen, leading to various pathological conditions, including fibrosis, arthritis, and cancer [2]. Thus, the ability to detect structural and metabolic anomalies in collagen would be significant in both diagnosing and treating related pathological conditions.
In this review, we highlight the importance of recognizing two different states of collagen–triple helical, intact collagen, and unfolded, denatured collagen (dn-collagen). Methods of detecting and targeting each of these collagens are also reviewed in order to show new approaches in utilizing collagen for delivery of drug molecules and imaging modalities.
Targeting and Monitoring Native Collagen
As the most abundant protein in humans, collagen constitutes approximately 25% of the total protein. It is the major structural component of the extracellular matrix (ECM) supporting cell proliferation, migration, and differentiation [3, 4]. Since collagen is present in the ECM of most tissues, it provides potential deposition sites for therapeutic agents. In addition, the detection of native collagen could be highly useful for monitoring progression and amelioration of diseases that are known to alter collagen remodeling activity such as fibrosis and arthritis.
A. Collagen binding peptides for enhancing growth factor efficacy and for modulating native collagen function
Growth factors (GF) are known to bind to the ECM for prolonged activity and timely function [5, 6]. ECM components, such as collagen acts as a natural repository that controls the presentation and release GFs. GF or GF-mimetics which can bind to collagen has the potential to improve in vivo stability and promote sustained release. Here we discuss collagen binding peptides (Table 1) that can mediate the attachment of GFs or other therapeutics moieties.
Table 1.
Collagen binding peptides and their applications.
| Collagen binding peptides | Source | Target | Kd (μM)* | Application | Ref. |
|---|---|---|---|---|---|
| RRANAALKAGELYKSILYGC | Collagen I platelet receptor | Collagen type I | 0.86 | Modulate collagen fibrillogenesis and inhibit MMP-mediated collagen degradation | 7,8 |
| MIVIELGTNPLKSSGIENGAF QGMKK | Leucine-rich repeats (LRR) 4–5 of Decorin | Collagen type I | 0.394 | Induce collagen mineralization in bone defects | 16 |
| LRELHLNNN | Inner surface of LRR 10 of Decorin | Collagen type I | 0.17 | Promote controlled release of molecules ≥2 KDa | 18 |
| WREPSFCALS | Von Willebrand Factor | Collagen type I | 100 | Promote vascularization and cellularization | 20,21 |
| TKKTLRT | Collagenase | Collagen type I | ≤100 | Promote vascularization and cellularization | 21 |
| WYRGRL | Phage display | Collagen type II | n.d. | Target articular cartilage | 23–25 |
| KLWVLPK | Phage display | Collagen type IV | n.d. | Target sites exposed collagen in blood vessels damaged by balloon angioplasty | 27 |
n.d. = not determined
Many ECM-binding proteins contain specific domains that have affinity to collagen. Inspired by decorin, a collagen binding peptidoglycan was developed which contains a dermatan sulfate peptidoglycan (DS-GAG). DS-GAG utilized a peptide sequence derived from the collagen I platelet receptor [RRANAALKAGELYKSILYGC (SILY); Kd: 0.86 μM] [7, 8]. The original sequence (RRANAALKAGELYKCILY; Kd: 10 nM) [9] has a higher binding affinity to collagen type I than the modified version, SILY, but the modification allowed dermatan sulfate to be conjugated to the terminus of the peptide. Similar to decorin which binds to collagen and inhibit lateral aggregation of collagen fibrils, DS-GAG modulated collagen fibril organization[8]. It is likely that the DS-GAG also had protective effects for collagens against MMP-mediated degradation by blocking MMP binding sites [10] [11]. In addition, DS facilitated cell proliferation by helping FGF receptors dimerize, since N-acetylgalactosamine (GalNAc) and iduronate (IdoA) can interact with FGFs (FGF-2 and FGF-10) and its receptor, FGFR2-IIIb [12, 13]. Further investigation of the original sequence of SILY revealed that only GELYKCILY was responsible for the collagen binding ability [14]. Goldberg research group conjugated collagen binding domain derived from decorin (residues 176–201, MIVIELGTNPLKSSGIENGAFQGMKK; Kd: 0.394 μM) to a recombinant pig bone sialoprotein (BSP), P2S [15], which can bind to HA. The conjugate promoted mineralization of bone defects by binding to collagen and by inducing nucleation of HA [16]. Through mutagenic studies, glutamate (residue 180) was found to be critical for specific binding to collagen type I [17]. Recently, new decorin-based peptide that binds to type I collagen was developed. This peptide has a sequence of LRELHLNNN (Kd: 0.17 μM) [18] and was found to increase the retention of molecules with a molecular weight of less than 2 kDa within type I collagen matrix (e.g., collagen gel). Upon matrix degradation in the biological system, controlled release of molecules was achieved. DARKSEVQK and KELNLVY, also derived from platelets, were found to specifically bind to type III collagen and inhibit type III collagen-induced platelet aggregation in a dose-dependent manner [19].
A collagen binding domain (CBD) WREPSFCALS, which was identified from von Willebrand Factor (vWF), exhibits low binding affinity (Kd: 100 μM) for collagen type I [20]. Another CBD derived from the collagenase is TKKTLRT. Using the recombinant protein technique, Dai and coworkers fused TKKTLRT to various GFs via their C-termini, including brain-derived neutrophic factor (BDNF), vascular endothelial growth factor (VEGF), nerve growth factor β (NGF-β), basic fibroblast growth factor (bFGF), ciliary neurotrophic factor (CTNF), bone morphogenic protein 2 (BMP-2), neurotrophin 3 (NT3), and platelet-derived growth factor (PDGF). The TKKTLRT sequence restricted the GFs’ diffusion and increased the tissue retention of the growth factors which led to enhanced tissue regeneration and wound repair. This sequence was found to promote vascularization and cellularization better than the WREPSFCALS because of its higher collagen binding affinity, which is presumably due to higher ratio of polar amino acids to non-polar amino acids [21]. The TKKTLRT sequence was even fused with single chain fragment variable (CBD-scFv) of cetuximab to target collagen rich carcinoma [22].
In addition to collagen specific domains derived from natural ECM binding proteins, there are other collagen targeting peptides that were identified using phage-display methods. A peptide WYRGRL that binds to α1 chain of type II collagen was discovered via phage-display and found to target articular cartilage [23–25]. Using this collagen binding sequence, Schultz and coworkers achieved targeted drug delivery of the cathepsin D inhibitor for the treatment of OA [24]. Three WYRGRL peptides and one cathepsin D inhibitor were tethered via a tetrapodal DOTAM [26] template, and the drug conjugate was administered intra-articularly to not only allow lipophilic drug molecules to penetrate joint compartments, but also to enhance the retention of inhibitor within cartilage through its multi-valent binding. Another collagen binding peptide, KLWVLPK, was discovered by phage-display and was found to specifically bind to collagen type IV. This peptide was attached to lipid-polymeric nanoparticles to target sites of balloon angioplasty [27]. It was also incorporated into a peptide amphiphile sequence in order to target areas of vascular intervention [28]. When conjugated to a β-sheet forming domain, self-assembled nanofibers were formed, which were shown to bind to exposed collagens on the injured blood vessels for up to two days. Besides the known slow clearance of nanofibers from circulation [29], the multivalent presentation of peptides also contributed to the increased binding to nanofibers.
Considering the importance of type I collagen in mediating homeostasis and biological development processes, the interactions of type I collagen with other extracellular matrix molecules and cell surface proteins have been mapped out [30–32]. For example, collagen has binding domains specific for fibronectin as well as integrins. Different types of glycoproteins and proteoglycans are involved in modulation of collagen fibrilogenesis and in cell attachment. More information about the interactions between collagen, ECM molecules and cells beyond the boundary of detecting and targeting of extracellular matrix molecules for therapeutic applications can be found in recently published review papers [33, 34]. These complex interactions provide a greater understanding about the relationship between ligand-binding sites and functional domains of type I collagen which will be highly useful in the design of collagen targeting molecules for therapeutic modulation of collagen function.
B. Detection of collagen accumulation in fibrosis
Fibrosis is a common pathological outcome of many chronic inflammatory diseases affecting nearly all tissues in the body [35, 36]. Fibrosis is signified by the excessive accumulation of ECM components, particularly type I collagen, in and around the damaged tissues (Fig. 1A) [37, 38]. Tissue repair in response to injury or irritation can progress into an irreversible fibrotic response if the tissue injury is severe or repetitive, leading to permanent scarring, organ malfunction and, ultimately death, as seen in end-stage liver disease [39], kidney disease [40], idiopathic pulmonary fibrosis (IPF) [41] and heart failure [42]. In addition, fibrosis is a major pathological feature of many chronic autoimmune diseases, and is a common outcome of rejection of organ transplant and medical implants (Fig. 1A) [43, 44].
Figure 1.
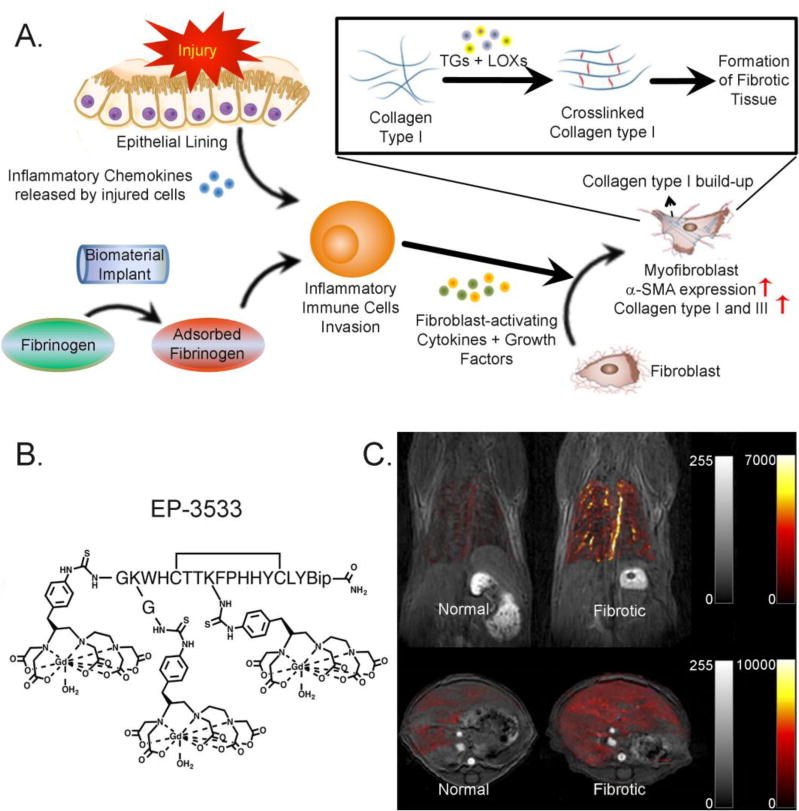
(A) Progression of fibrotic tissue formation caused by chronic inflammation or by incompatibility of biomaterial implants; (B) EP-3533, a Gd-bearing collagen-targeted MRI contrast agent (reprinted with permission from Ref. 55); (C) In vivo molecular imaging of pulmonary (top panel) and liver fibrosis (bottom panel) using EP-3533 (reprinted with permission from Ref. 59 and 60).
Currently, there is no single test that can diagnose fibrosis, and clinical methods for monitoring the disease progression are very limited. There is no established treatment for liver_fibrosis [45], and only a few drugs were approved for treatment of other fibrotic diseases (e.g., both pirfenidone and nintedanib were approved for the treatment of IPF) [46–48]. Biopsy remains the most important, and often the only method for fibrosis detection and diagnosis (e.g., in liver fibrosis); however, biopsy is not suited for routine screening/monitoring because of its cost, and associated morbidity and mortality (rate between 0.01–0.1% in liver [49]), as well as inaccuracy caused by sampling variation. Therefore, there is an unmet need for accurate and non-invasive methods to detect fibrosis. Many imaging methods based on the morphologic or mechanical change of the fibrotic tissue in the late stage are under development, such as high resolution computed tomography (HRCT), magnetic resonance imaging (MRI) [50], ultrasonography (US) [51], as well as biochemical assays for identifying protein degradation fragments using ELISA [52, 53]. HRCT can provide cross-sectional images of the fibrotic tissues with a quality that depends on the radiation exposure [54]. Unfortunately, HRCT can induce cancer and genetic mutations. MRI offers deep tissue penetration and high spatial resolution, however, its use is limited in diagnosing liver fibrosis as most contrast agents cannot distinguish the different stages of liver fibrosis [53]. US is typically used as a diagnostic tool in chest diseases, and as a guide for transthoracic needle biopsies [51]. Biochemical assays are non-invasive and suitable for high-throughput screening either in preclinical or clinical settings for estimating the safety and efficacy of certain therapies [52, 53], but they are neither site-specific nor disease-specific [55]. Scientists and engineers are addressing limitations of early stage detection (e.g., for MRI [56]) and inadequate correlation between the disease and serum markers [52].
Molecular imaging using a specific probe that targets excessive collagen is one appealing strategy for detecting fibrosis in its early stage when excess collagen molecules have been deposited in the tissue without apparent changes in the anatomy of the tissue. Among several collagen binding molecules that were tested [57, 58], EP-3533 (Fig. 1B) which employs a type I collagen-specific peptide (Kd: 1.8 ± 1.0 μM) has shown some promise in imaging fibrosis [56]. Because the concentration of collagen can increase up to 10-folds during fibrosis [59], EP-3533 was able differentiate fibrotic tissues from the healthy ones. A combination of intensity strength of the contrast agent and Ultra-short Echo Time Magnetic Resonance (UTE-MR) allowed early-stage visualization of fibrotic tissue in the lungs and monitoring of the disease progression. The cyclic conformation (achieved by disulfide bridge formation) was responsible for the tight collagen binding. This agent was used to successfully visualize type I collagen in pulmonary [60] and liver fibrosis (Fig. 1C) [53, 61]. In addition, EP-3533 was conjugated to high-density lipoprotein nanoparticles to monitor compositional changes in atherosclerotic plague regression [62].
CNA35, a 35 kDa peptide derived from an adhesion protein of Staphylococcus aureus, has moderate affinity to type I collagen (Kd ~ 0.5 μM) [63] and is frequently employed for collagen imaging [64–66]. For example, in vivo application of CNA35 allowed labeling [66] and imaging [67] of atherosclerotic plagues. By binding directly to both individual fibrillar and non-fibrillar collagens, CNA35 allows for detailed visualizations of different structural hierarchies of collagen organization for both early and mature collagens [63, 64].
C. Functional assemblies based on collagen binding molecules
Multivalent design is an effective way to enhance the affinity of collagen binding molecules. For example, an AB5 dendritic wedge architecture (Figure 2A) was employed to enhance the binding affinity of the HVWMQAP which binds to type I collagen [68]. The dendritic display demonstrated a 100-fold improvement in collagen affinity compared to monovalent HVWMQAP. Significant improvement in the binding of CNA35 to collagen was achieved by employing micelle architecture (Figure 2B) [69]. By varying the ratio of CNA35 to lipid, different amounts of protein could be loaded in micelles. A fluorescent binding experiment showed that the most significant binding was observed with micelles having 5 and 17 proteins per micelle. A multivalent CNA35 micelle was also reported to target collagen type I, II, III, (fiber form) and IV (network-like structure).
Figure 2.
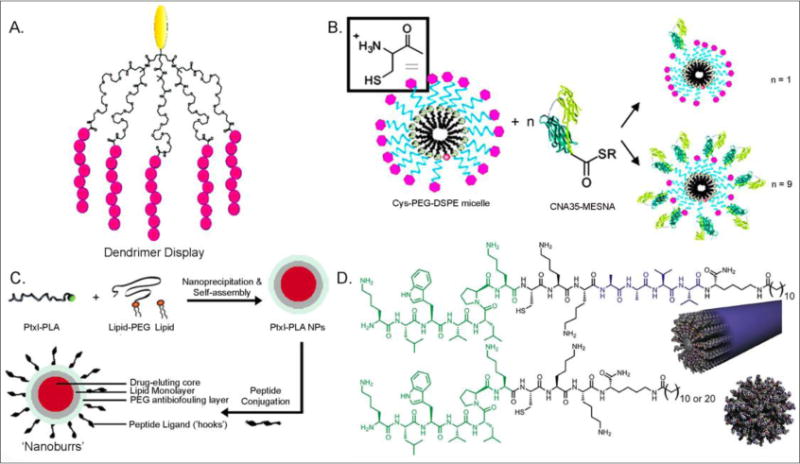
Multivalent display of collagen-targeting moiety: (A) HVWMQAP presented on AB5 dendritic wedge (in pink; reprinted with permission from Ref. 68), (B) CNA35 micelle (reprinted with permission from Ref. 69), (C) HWGSLRA Nanoburrs (reprinted with permission from Ref. 27), (D) Peptide amphiphile nanostructures (reprinted with permission from Ref. 28).
Functionalization of nanoparticle surfaces with collagen binding molecules can lead to collagen-targeted drug delivery or improved molecular imaging of exposed collagen matrix in vivo. Targeting exposed collagen, especially in injured blood vessel, is particularly important due to the fact that cardiovascular disease continues to be the leading cause of death worldwide [70]. Vascular intervention to treat the disease is also challenged by high failure rates from restenosis [71]. In addition, exposed collagen matrix is associated with angiogenesis in tumor formation as well as other angiogenesis-dependent diseases [72]. Langer and coworkers reported micelle-based paclitaxel delivery system that display HWGSLRA on micelle surface as “hooks” for binding to collagen (Figure 2C) [27]. Also, peptide amphiphiles can self-assemble into nanofibers (Figure 2D, top) or nanospheres (Figure 2D, bottom) which disiplay high density of collagen binding peptides on their surfaces [28]. It was shown that nanofibers have preferential binding to injured vessels and are cleared at a slower rate relative to nanospheres of similar dimensions. Conjugation of peptide amphiphile with fluorescent labels provided molecular imaging of the injured tissues.
Targeting and Monitoring Denatured Collagen (dn-collagen)
Collagen degradation is part of normal tissue development and maintenance; however, excessive collagen degradation is closely associated with a wide variety of diseases and pathological conditions. In cancer, collagen degradation caused by high MMP activity is responsible for angiogenesis and tumor progression [73–75]. In atherosclerosis, the thinning and weakening of the fibrous collagen cap due to MMP degradation renders the atherosclerotic plaques susceptible to rupture which can cause acute myocardial infarction and sudden cardiac death [76]. Degradation of type II collagen, the predominant protein in cartilage, is the key pathogenesis step of osteoarthritis [77, 78]. During persistent liver inflammation and injury, the hepatic stellate cells express MMPs to degrade normal liver matrix [79]. These examples highlight the pivotal role of collagen degradation in some of the most prevalent and/or life-threatening diseases in current human population. (A list of diseases associated with collagen remodeling is found in Table 2).
Table 2.
Diseases associated with high level of collagen degradation and remodeling, and their location in the body.
| Distribution | Diseases and medical research areas |
|---|---|
| heart | myocardial infarct |
| blood vessels | atherosclerotic plaques, blood vessel injury and failure (stroke) |
| cartilage | osteoarthritis, rheumatoid arthritis |
| bone | osteoporosis, bone fracture/healing, Marfan syndrome |
| ligament & tendon | mechanical injury, sports medicine, e.g., rotator cuff tear |
| skin | skin damage (photo, aging), skin cancer |
| eye | cornea injury & infection, corneal healing after LASIK, keratoconus, wet AMD |
| lung | lung inflammation, pulmonary fibrosis |
| liver | liver fibrosis, cirrhosis |
| kidney | nephritis, kidney fibrosis |
| uterus | uterine fibroid |
| embryo | ECM developmental biology, heritable connective tissue disorders |
| teeth | oral inflammation |
| uterus | uterine fibroid |
| others | cancer progression & metastasis, foreign body response to implants |
Since the dn-collagen is present at the disease sites in all of the ailments listed in Table 2, it can be targeted similar to intact collagen for the purpose of imaging and drug delivery. The field of targeting dn-collagen is in its infancy; so far most targeting strategies are focused on MMP activities [80], or on targeting markers that signal collagen degradation [80]. Enzymatically digested collagen fragments have been used to develop several types of antibodies. Until recently, these antibodies were the only molecules that can directly target dn-collagen. Recently, researchers have discovered that collagen hybridizing peptide (CHP), also known as collagen mimetic peptides (CMP) can specifically target denatured collagen strands. CHP can recognize and hybridize with dn-collagen by re-forming the collagen triple helix [82]. Targeting dn-collagen is still a relatively new field, but it holds significant potential in advancing therapeutics and diagnostics for numerous human diseases (Table 2).
A. Collagen degradation
Collagen degradation can proceed either on the inside or outside the cells. Intracellular degradation occurs either in the lysosome or in the cisternae of the Golgi apparatus [83]. However, for the purpose of targeting and imaging disease, extracellular degradation is more relevant. Different types of MMPs or collagenases cleave collagen into asymmetrical fragments, which are susceptible to nonspecific proteolytic cleavage [84]. Proteolytic degradation unravels the triple helical structure of the native collagen and exposes additional bioactive sites that enhance cell attachment, migration, and proliferation [85]. Importantly, collagen cleavage also weakens the ECM leading to further degradation and many symptoms seen in diseases involving dn-collagen [86]. Due to the difficulty of directly measuring dn-collagen, MMP activity is often used as a surrogate to detect collagen degradation. Various methods have been developed to detect MMP activity, including reverse transcription polymerization chain reaction (RT-PCR) [87], ELISA assay, and zymography [88, 89]. There are also methods based on fluorescence measurements that employ FRET triple helical peptide (fTHP), and gelatin conjugated with fluorescein isothiocyanate (FITC) or 2-methoxy-2,4-diphenyl-3(2H)-furanone (MDPF), as well as colorimetric assay using horseradish peroxidase and biotinylated gelatin [90, 91]. Recently, our research group discovered that the dn-collagen strands can form triple helices with collagen mimetic peptides (CMP) [82].
B. Disease detection and therapeutics associated with collagen degradation
The ability to detect early stages of collagen remodeling is crucial for understanding the disease mechanism, prevention of the progression of disease, and evaluating the efficacy of available treatments. X-ray radiography, bone scan, magnetic resonance imaging, computed tomography, and histomorphometric analysis are considered gold-standard methods to measure the quality of bone and cartilage. These methods, however, have limitations in terms of sensitivity and reproducibility. Moreover, they lack the ability to detect diseases in their early stages. Therefore, there is a need for reliable methods with which early detection of changes in bone and cartilage quality can be measured with high fidelity.
There is a wealth of research on identifying biochemical markers associated with degradation of bone and cartilage [52, 55, 92–93]. In general, these biochemical markers, or neoepitopes, are by-products of enzymatic processes which are involved in the formation and degradation of bone and cartilage. Detection of these biomarkers in biochemical assays is usually done by measuring the level of the neoepitopes in body fluids, such as serum, plasma, synovial fluid, and urine [92]. There are several methods to detect these biomarkers including ELISA, radioimmunoassay (RIA), electrochemical luminescence, and HPLC analysis. Two notable epitopes for analyzing bone quality are serum C-telopeptide of type I collagen (CTX-I) and serum N-propeptide of type I collagen (PINP), which correlates with bone resorption and bone formation, respectively [55]. In contrast, cartilage quality can be measured by detecting the level of serum C-telopeptide of type II collagen (CTX-II). These are selected as reference markers in clinical settings due to their high sensitivity and reproducibility, as well as low cross-reactivity with other biomolecules. Nevertheless, full analysis of disease progression and selection of optimal treatment option can be best accomplished by a combination of imaging and biochemical techniques.
To date, development of therapeutics against bone diseases with high level of dn-collagen has largely focused on inhibiting the function of osteoclasts. One widely-used class of therapeutics for osteoporosis is bisphosphonates [94]. As anti-resorptive agents, bisphosphonates binds to bone minerals followed by uptake and apoptosis of osteoclasts [95]. Nitrogen-containing bisphosphonates, the most advanced drug in its class, disrupt the maturation of osteoclasts by inhibiting the function of Farnesyl PyroPhosphate Synthase (FPPS) [96]. Denosumab, a monoclonal antibody (mAb) which was recently approved by FDA for treating post-menopausal osteoporosis, binds to RANK-L and inhibits maturation and function of osteoclasts [97]. In 2010, FDA also approved denosumab for bone metastasis treatment. Certolizumab (phase III) is a PEGylated anti-TNFα that is approved for treatment of rheumatoid arthritis and Crohn’s disease [98].
In conclusion, there are therapeutics developed for the treatment of diseases associated with high activity of collagen degradation; however, other than the bone therapy, discovery of drugs for such diseases has been hampered largely by the inability to verify the therapeutic efficacy in ECM remodeling. For example, there is no definitive bone remodeling markers to interpret clinical outcomes or treatment responses in patients with bone metastases [99]. Development of new biomarkers for collagen degradation would be important not only to support the drug discovery processes, but also for detecting and managing disease in patients.
C. Antibody and phage display in discovering dn-collagen targeting molecules
Since the presence of dn-collagen is a symptom of many degenerative diseases, it is highly useful to have molecules that can recognize and specifically bind to the dn-collagen for the purposes of detecting disease or drug delivery. Using thermally denatured type I and IV collagens, in subtractive immunization technique, mAbs HUIV26 and HUI77 were develop and were found to specifically recognize enzymatically digested and denatured collagen [100]. HUIV26 specifically recognized thermally dn-collagen type IV, whereas HUI77 bound to thermally dn-collagens of type I, II, III, IV, and V with a range of affinities. In type IV collagen, the domain responsible for inducing angiogenesis exists as hidden interaction site (cryptic site) that becomes exposed only after proteolytic degradation or denaturation of collagen triple helix. HUIV26 was reported to target such cryptic sites and was therefore used to inhibit tumor growth [101, 102].
D93 (Kd: 6.5 μM), a humanized form of HUI77-derived from monoclonal IgG1 antibody, recognizes dn-collagen type IV by binding to a cryptic site of MMP-processed collagen [85]. The cryptic site was found to mediate adhesion, migration, and proliferation of tumor and endothelial cells [101, 103]. The cryptic site, located on the α1(IV) chain, contained a peptide sequence of PGAKGLPGPPGPPGPY (P1337-Y1352). Binding assays revealed a sequence of OGAKGLPG-POGPOGPY to be the target of D93 binding. Under the name of TRC093 (developed by Tracon Pharmaceuticals Inc.), D93 completed its phase I clinical trial in 2010.
In search for quantitative immunoassays for type II collagen, extensive screenings were performed against structurally different antigens of type II collagen [104]. Screening utilizing an ELISA assay yielded specific monoclonal antibodies E1E5 (IC50: 400 ng/mL) and E4A11 (IC50: 500 ng/mL) that interact with dn-collagen type II by binding to an epitope on the α1 chain [105]. These mAbs can detect the presence of dn-collagen type II with a minimum concentration of 20 ng/mL.
The peptide sequence TLTYTWS which was discovered via phage-display was found to specifically bind to a cryptic site on MMP-2 processed type IV collagen [106]. This cryptic site was reported to serve as an angiogenic signal for endothelial cells. By binding to the cryptic site, TLTYTWS was found to reduce endothelial differentiation in vitro and inhibit angiogenesis in vivo. Nonetheless, the binding mechanism of TLTWTWS to the cryptic site requires further investigation.
Overall, dn-collagen targeting molecules selected by library approach have revealed new knowledge on how changes in collagen structure (which reveal cryptic sites) affect collagen’s biological function. In addition, these molecules are expected to facilitate development of imaging agents and therapeutics that can specifically target dn-collagens.
D. Collagen Hybridizing Peptide
Collagen hybridizing peptide (CHP) is a rationally designed synthetic peptide that has strong propensity to fold into triple helix structure of collagen and is able to target dn-collagen strands both in vitro and in vivo. It is also known as collagen mimetic peptide (CMP) due to its resemblance to natural triple helical collagen. It is mainly composed of 6–10 repeats of triple helicogenic trimeric GlyProPro or GlyProHyp sequence. Since denatured collagen has unfolded single collagen strands, and since CHP has a strong propensity to fold into triple helix, CHP is able to hybridize to denatured collagen strands with high strength and specificity. This is similar to DNA fragments binding to complementary DNA strands [107].
Since CHPs self-assemble into homotrimers which have little driving force for collagen binding, the peptides need to be subjected to a condition that favor monomeric state before it can be allowed to bind to denatured collagen strands. In this regards, our research group has developed two types of CHPs, one based on simple GPO sequence that needs to be heated to create single strands, and a photo-caged version which only hybridizes with dn-collagen after UV irradiation. The latter peptide is made of the same GPO sequence as the former but contains a photo-cleavable nitrobenzyl group attached to the single central glycine which prevents triple helical folding. (Fig. 3A) [108]. Heat inducible CHP can be used when the moiety (e.g. imaging modality or drug molecules) to be conjugate to the CHP is sensitive to light, whereas photo-inducible, caged CHP can be used when those moieties are heat sensitive (e.g. protein). Conjugating imaging agents can turn this peptide into diagnostic imaging agents, and attaching drug molecules can turn it into targeted drug delivery system [108–110].
Figure 3.
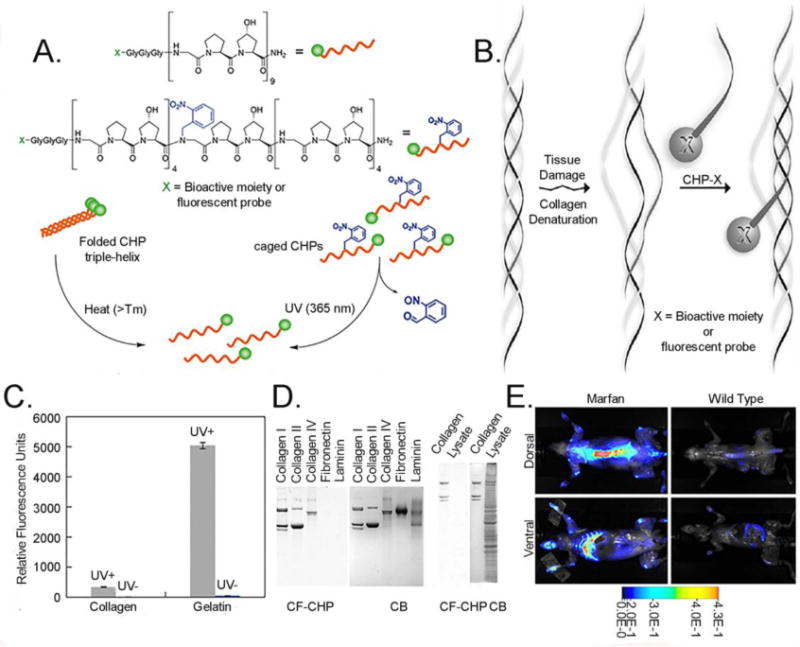
(A) Structures of fluorescently labeled CHP and fluorescently labeled and nitrobenzyl caged CHP, and schematic illustration of the two approaches (heat- vs photo-triggered) of generating single strand CHPs for dn-collagen hybridization (reprinted with permission from Ref. 109), (B) Schematics of CHP hybridizing to dn-collagen, (C) Specific binding of caged-CHP to gelatin after UV irradiation (reprinted with permission from Ref. 108), (D) SDS-PAGE loaded with collagen type I, II, IV, fibronectin, laminin, and endothelial cell lysates (2 μg of each protein or lysates), and stained with CF-CHP and CB (reprinted with permission from Ref. 109), (E) Systemic injection of near-IR fluorophore conjugated CHP into transgenic mouse with Marfan syndrome (reprinted with permission from Ref. 108).
The affinity of CHP to denatured collagen is driven by the availability of single strand collagen like chain (Fig. 3B, C). CHP can not only bind to fibrillar type (type I and II) of collagen, but also network-like collagen (type IV) as long as the triple helical domain of these collagens are denatured by proteolytic or other physico-chemical activity (e.g. heat, denaturants, mechanical stress). In addition, since CHP is hydrophilic and contains no charged amino acid, it is highly inert and shows almost no non-specific binding to other biomolecules (Fig. 3D) [109]. Most importantly, triple helical protein structure is an extremely rare supersecondary protein structure that is hard to find in proteins other than collagen [111]. For this reason, systemic administration of CHP conjugated with a near-IR dye demonstrated remarkably specific imaging of tumor as well as high bone remodeling activity in transgenic mouse with Marfan syndrome (Fig. 3E).
Therapeutic application of CHP was demonstrated by Raines and coworkers where (PPG)7 was conjugated to substance P (SubP), a neuropeptide involved in wound healing [110]. Similar to peptides based on GPO sequence, the (PPG)7 can hybridize with damaged collagen molecules at the wound site, allowing the SubP which is conjugated (PPG)7 to be anchored in the wound bed and prevent scar tissue formation. In addition, a cancer-targeting delivery agent was developed by displaying (PPG)7 on the surface of M13 bacteriophage. [112]. This work took advantage of the fact that cancerous tissues contain high level of denatured collagens due to fast tissue remodeling [113]. The bacteriophage displaying CHP had a 16-fold higher binding affinity to dn-collagen than the wild bacteriophage. In addition, CHP comprised of GPP sequence was used for gene delivery. By incorporating GEKGER peptide sequence into the middle of the CHP sequence [(GPP)3GPRGEKGERGPR(GPP)3GPCCG], Urello and coworkers demonstrated that the CHP could engage the α2β1 integrin for gene internalization into the cell [114].
The CHP was also used to enhance the bioactivity of collagen-based tissue scaffolds [115–117]. Our research group has conjugated CHP, (ProHypGly)9 to a proangiogenic peptide known as QK (KLTWQELYQLKYKGI) which is derived from VEGF. The QK-CHP enhanced early signs of angiogenic activity in HUVECs in scaffolds comprised of both gelatin and collagen which mimic the ECM of healing tissues [118, 119] suggesting that the QK-CHP can potentially be used to promote wound healing response of injured tissues.
Ultimately, CHP, with its simplicity and robust modularity, has the potential to facilitate the development of novel therapeutics targeting dn-collagen. Further discussion on assemblies and functionalization of CHP can be found in cited articles [82, 107, 120–122].
Conclusion
Since collagen is the major component of the ECM, providing structural support and key factors for tissue proliferation and regeneration, abnormal collagen remodeling activity can lead to conditions that result in excessive collagen build-up or collagen degradation, as in the case of fibrosis and arthritis, respectively. To understand, and ultimately remediate such pathologic conditions, researchers have focused on identifying marker molecules that can recognize and bind to either the intact collagens (of accumulated collagen) or the dn-collagen (of degraded collagen). Through the use of these biomakers, a greater understanding of the disease states have been gathered, which in turn, is providing more effective steps in preventing the progression of the disease. In addition, these biological tools can be utilized in both delivering therapeutics and also evaluating their efficacy. They can be an excellent, so called, “theranostics” for common pathologic conditions with high collagen remodeling activity. Although there are many specific biological markers for intact collagen, there are only a few ones specific for dn-collagen, including the newly discovered CHP which is a small peptide that can bind to denatured collagen with high specificity. More efforts are needed from the research community to discover new biomarkers for denatured collagen and at the same time, develop existing markers into useful diagnostics and therapeutics that can address human disease associated with excess collagen degradation.
Table 3.
Dn-collagen targeting molecules and their applications.
| Dn-collagen targeting molecules | Discovery method | Target | Kd (μM)* | Application | Ref. |
|---|---|---|---|---|---|
| mAb HUIV26 | Subtractive immunization | Thermally dn-collagen type IV | n.d. | Inhibit angiogenesis and tumor growth | 100–102 |
| mAb HUI77 | Subtractive immunization | Thermally dn-collagen type I–V | n.d. | 100–102 | |
| D93 | HUI77-derived humanized mAb | MMP-processed dn-collagen type IV | 6.5 | Inhibit tumor growth | 85 |
| mAb E1E5 | Quantitative immunoassay | Dn-collagen type II | n.d. | Detect dn-collagen type II | 105 |
| mAb E4A11 | Quantitative immunoassay | Dn-collagen type II | n.d. | Detect dn-collagen type II | 105 |
| TLTYTWS | Phage display | MMP2-processed collagen type IV | n.d. | Reduce endothelial differentiation and inhibit angiogenesis | 106 |
n.d. = not determined
Acknowledgments
Development of collagen hybridizing peptide discussed in this paper was supported by grants from NIAMS/NIH (R01-AR060484 and R21-AR065124) and DOD (W81XWH-12-1-0555).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Drs. Yu and Li are co-founders of 3Helix Inc that commercializes CHP technology.
References
- 1.Gelse K, Poeschl E, Aigner T. Collagens: Structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Myllyharju J, Kivirikko KI. Collagens and collagen-related diseases. Ann Med. 2001;33:7–21. doi: 10.3109/07853890109002055. [DOI] [PubMed] [Google Scholar]
- 3.Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929–958. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birk DE, Bruckner P. Collagen suprastructures. In: Brinckmann J, Notbohm H, Müller PK, editors. Collagen. Springer; Berlin/Heidelberg, Berlin: 2005. pp. 185–205. [Google Scholar]
- 5.Macri L, Silverstein D, Clark RA. Growth factor binding to the pericellular matrix and its importance in tissue engineering. Adv Drug Deliv Rev. 2007;59:1366–1381. doi: 10.1016/j.addr.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen. 2011;19:134–148. doi: 10.1111/j.1524-475X.2011.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stuart K, Paderi J, Snyder PW, Freeman L, Panitch A. Collagen-Binding Peptidoglycans Inhibit MMP Mediated Collagen Degradation and Reduce Dermal Scarring. PLoS ONE. 2011;6:e22139. doi: 10.1371/journal.pone.0022139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paderi JE, Panitch A. Design of a Synthetic Collagen-Binding Peptidoglycan that Modulates Collagen Fibrillogenesis. Biomacromolecules. 2008;9:2562–2566. doi: 10.1021/bm8006852. [DOI] [PubMed] [Google Scholar]
- 9.Chiang TM, Kang AH. A synthetic peptide derived from the sequence of a type I collagen receptor inhibits type I collagen-mediated platelet aggregation. J Clin Invest. 1997;100:2079–2084. doi: 10.1172/JCI119741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geng YQ, McQuillan D, Roughley PJ. SLRP Interaction can protect collagen fibrils from cleavage by collagenases. Matrix Biol. 2006;25:484–491. doi: 10.1016/j.matbio.2006.08.259. [DOI] [PubMed] [Google Scholar]
- 11.Paderi JE, Sistiabudi R, Ivanisevic A, Panitch A. Collagen-Binding Peptidoglycans: A Biomimetic Approach to Modulate Collagen Fibrillogenesis for Tissue Engineering Applications. Tissue Eng Part A. 2009;15:2991–2999. doi: 10.1089/ten.tea.2009.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Penc SF, Pomahac B, Winkler T, Dorschner RA, Eriksson E, Herndon M, Gallo RL. Dermatan Sulfate released after injury is a potent promoter of fibroblast growth factor-2 function. J Biol Chem. 1998;273:28116–28121. doi: 10.1074/jbc.273.43.28116. [DOI] [PubMed] [Google Scholar]
- 13.Radek KA, Taylor KR, Gallo RL. FGF-10 and specific structural elements of dermatan sulfate size and sulfation promote maximal keratinocyte migration and cellular proliferation. Wound Repair and Regeneration. 2009;17:118–126. doi: 10.1111/j.1524-475X.2008.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiang TM. A synthetic nonapeptide derived from the sequence of a platelet type I collagen receptor inhibits type I collagen-mediated platelet aggregation. Am J Med Sci. 2000;320:362–367. doi: 10.1097/00000441-200012000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Harris NL, Rattray KR, Tye CE, Underhill TM, Somerman MJ, D’Errico JA, Chambers AF, Hunter GK, Goldberg HA. Functional analysis of bone sialoprotein: identification of the hydroxyapatite-nucleating and cell-binding domains by recombinant peptide expression and site-directed mutagenesis. Bone. 2000;27:795–802. doi: 10.1016/s8756-3282(00)00392-6. [DOI] [PubMed] [Google Scholar]
- 16.Hunter GK, Poitras MS, Underhill TM, Grynpas MD, Goldberg HA. Induction of collagen mineralization by a bone sialoprotein-decorin chimeric protein. J Biomed Mater Res. 2001;55:496–502. doi: 10.1002/1097-4636(20010615)55:4<496::aid-jbm1042>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 17.Kresse H, Liszio C, Schönherr E, Fisher LW. Critical role of glutamate in a central leucine-rich repeat of decorin for interaction with type I collagen. J Biol Chem. 1997;272:18404–18410. doi: 10.1074/jbc.272.29.18404. [DOI] [PubMed] [Google Scholar]
- 18.Federico S, Pierce BF, Piluso S, Wischke C, Lendlein A, Neffe AT. Design of Decorin-Based Peptides that Bind to Collagen I and their Potential as Adhesion Moieties in Biomaterials. Angew Chem Int Ed. 2015;54:1–6. doi: 10.1002/anie.201505227. [DOI] [PubMed] [Google Scholar]
- 19.Chiang TM, Cole F, Woo-Rasberry V. Cloning, characterization, and functional studies of a 47-kDa platelet receptor for type III collagen. J Biol Chem. 2002;277:34896–34901. doi: 10.1074/jbc.M205311200. [DOI] [PubMed] [Google Scholar]
- 20.Takagi J, Asai H, Saito Y. A collagen/gelatin-binding decapeptide derived from bovine propolypeptide of von Willebrand factor. Biochemistry. 1992;31:8530–8534. doi: 10.1021/bi00151a021. [DOI] [PubMed] [Google Scholar]
- 21.Zhao W, Chen B, Li X, Lin H, Sun W, Zhao Y, Han Q, Dai J. Vascularization and cellularization of collagen scaffolds incorporated with two different collagen-targeting human basic fibroblast growth factors. J Biomed Mater Res. 2007;82A:630–636. doi: 10.1002/jbm.a.31179. [DOI] [PubMed] [Google Scholar]
- 22.Liang H, Li X, Chen B, Wang B, Zhao Y, Zhuang Y, Shen H, Zhang Z, Dai J. A collagen-binding EGFR single-chain Fv antibody fragment for the targeted cancer therapy. J Control Release. 2015;209:101–109. doi: 10.1016/j.jconrel.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 23.Rothenfluh DA, Bermudez H, O’neil CP, Hubbell JA. Biofunctional polymer nanoparticles for intra-articular targeting and retention in cartilage. Nat Mater. 2008;7:248–254. doi: 10.1038/nmat2116. [DOI] [PubMed] [Google Scholar]
- 24.Hu H-Y, Lim N-H, Ding-Pfennigdorff D, Saas J, Wendt KU, Ritzeler O, Nagase H, Plettenburg O, Schultz C, Nazare M. DOTAM Derivatives as Active Cartilage-Targeting Drug Carriers for the Treatment of Osteoarthritis. Bioconjugate Chem. 2015;26:383–388. doi: 10.1021/bc500557s. [DOI] [PubMed] [Google Scholar]
- 25.Cabanas-Danes J, Nicosia C, Landman E, Karperien M, Huskens J, Jonkheijm P. A fluorogenic monolayer to detect the co-immobilization of peptides that combine cartilage targeting and regeneration. J Mater Chem B. 2013;1:1903–1908. doi: 10.1039/c3tb20109k. [DOI] [PubMed] [Google Scholar]
- 26.De León-Rodríguez LM, Kovacs Z. The synthesis and chelation chemistry of DOTA-peptide conjugates. Bioconjug Chem. 2008;19:391–402. doi: 10.1021/bc700328s. [DOI] [PubMed] [Google Scholar]
- 27.Chan JM, Zhang L, Tong R, Ghosh D, Gao W, Liao G, Yuet KP, Gray D, Rhee JW, Cheng J, Golomb G, Libby P, Langer R, Farokhzad OC. Spatiotemporal controlled delivery of nanoparticles to injured vasculature. Proc Natl Acad Sci U S A. 2010;107:2213–2218. doi: 10.1073/pnas.0914585107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moyer TJ, Kassam HA, Bahnson ESM, Morgan CE, Tantakitti F, Chew TL, Kibbe MR, Stupp SI. Shape-Dependent Targeting of Injured Blood Vessels by Peptide Amphiphile Supramolecular Nanostructures. Small. 2015;11:2750–2755. doi: 10.1002/smll.201403429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2:249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Lullo GA, Sweeney SM, Korkko J, Ala-Kokko L, San Antonio JD. Mapping the ligand-binding sites and disease-associated mutations on the most abundant protein in the human, type I collagen. J Biol Chem. 2002;277:4223–4231. doi: 10.1074/jbc.M110709200. [DOI] [PubMed] [Google Scholar]
- 31.Sweeney SM, Orgel JP, Fertala A, McAuliffe JD, Turner KR, Di Lullo GA, Chen S, Antipova O, Perumal S, Ala-Kokko L, Forlino A, Cabral WA, Barnes AM, Marini JC, San Antonio JD. Candidate Cell and Matrix Interaction Domains on the Collagen Fibril, the Predominant Protein of Vertebrates. J Biol Chem. 2008;283:21187–21197. doi: 10.1074/jbc.M709319200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orgel JP, San Antonio JD, Antipova O. Molecular and structural mapping of collagen fibril interactions. Connect Tissue Res. 2011;52:2–17. doi: 10.3109/03008207.2010.511353. [DOI] [PubMed] [Google Scholar]
- 33.Järveläinen H, Sainio A, Koulu M, Wight TN, Penttinen R. Extracellular Matrix Molecules: Potential Targets in Pharmacotherapy. Pharmacol Rev. 2009;61:198–223. doi: 10.1124/pr.109.001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.An B, Lin YS, Brodsky B. Collagen interactions: Drug design and delivery. Adv Drug Deliv Rev. 2015 doi: 10.1016/j.addr.2015.11.013. http://dx.doi.org/10.1016/j.addr.2015.11.013. [DOI] [PubMed]
- 35.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. 2011;4:165–178. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raghu G, Brown KK, Costabel U, Cottin V, du Bois RM, Lasky JA, Thomeer M, Utz JP, Khandker RK, McDermott L, Fatenejad S. Treatment of idiopathic pulmonary fibrosis with etanercept: an exploratory, placebo-controlled trial. Am J Respir Crit Care Med. 2008;178:948–955. doi: 10.1164/rccm.200709-1446OC. [DOI] [PubMed] [Google Scholar]
- 39.Sørensen HT, Thulstrup AM, Mellemkjar L, Jepsen P, Christensen E, Olsen JH, Vilstrup H. Long-term survival and cause-specific mortality in patients with cirrhosis of the liver: a nationwide cohort study in Denmark. J Clin Epidemiol. 2003;56:88–93. doi: 10.1016/s0895-4356(02)00531-0. [DOI] [PubMed] [Google Scholar]
- 40.Textor SC. Ischemic nephropathy: where are we now? J Am Soc Nephrol. 2004;15:1974–1982. doi: 10.1097/01.ASN.0000133699.97353.24. [DOI] [PubMed] [Google Scholar]
- 41.King TEJ, Schwarz MI, Brown K, Tooze JA, Colby TV, Waldron JAJ, Flint A, Thurlbeck W, Cherniack RM. Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med. 2001;164:1025–1032. doi: 10.1164/ajrccm.164.6.2001056. [DOI] [PubMed] [Google Scholar]
- 42.Khan R, Sheppard R. Fibrosis in heart disease: understanding the role of transforming growth factor-β1 in cardiomyopathy, valvular disease and arrhythmia. Immunology. 2006;118:10–24. doi: 10.1111/j.1365-2567.2006.02336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hertig A, Anglicheau D, Verine J, Pallet N, Touzot M, Ancel PY, Mesnard L, Brousse N, Baugey E, Glotz D, Legendre C, Rondeau E, Xu-Dubois YC. Early epithelial phenotypic changes predict graft fibrosis. J Am Soc Nephrol. 2008;19:1584–1591. doi: 10.1681/ASN.2007101160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang L, Hu W. Molecular determinants of biocompatibility. Expert Rev Med Devices. 2005;2:493–500. doi: 10.1586/17434440.2.4.493. [DOI] [PubMed] [Google Scholar]
- 45.Schuppan D, Kim YO. Evolving therapies for liver fibrosis. J Clin Invest. 2013;123:1887–1901. doi: 10.1172/JCI66028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Selman M, Thannickal VJ, Pardo A, Zisman DA, Martinez FJ, Lynch JP. Idiopathic pulmonary fibrosis: pathogenesis and therapeutic approaches. Drugs. 2004;64:405–430. doi: 10.2165/00003495-200464040-00005. [DOI] [PubMed] [Google Scholar]
- 47.Rafii R, Juarez MM, Albertson TE, Chan AL. A review of current and novel therapies for idiopathic pulmonary fibrosis. J Thorac Dis. 2013;5:48–73. doi: 10.3978/j.issn.2072-1439.2012.12.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, Kim DS, Kolb M, Nicholson AG, Noble PW, Selman M, Taniguchi H, Brun M, Le Maulf F, Girard M, Stowasser S, Schlenker-Herceg R, Disse B, Collard HR. INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 49.Myers RP, Fong A, Shaheen AA. Utilization rates, complications and costs of percutaneous liver biopsy: a population-based study including 4275 biopsies. Liver Int. 2008;28:705–712. doi: 10.1111/j.1478-3231.2008.01691.x. [DOI] [PubMed] [Google Scholar]
- 50.Kersjes W, Mayer E, Buchenroth M, Schunk K, Fouda N, Cagil H. Diagnosis of pulmonary metastases with turbo-SE MR imaging. Eur Radiol. 1997;7:1190–1194. doi: 10.1007/s003300050272. [DOI] [PubMed] [Google Scholar]
- 51.Yang PC. Ultrasound-guided transthoracic biopsy of the chest. Radiol Clin North Am. 2000;38:323–343. doi: 10.1016/s0033-8389(05)70166-4. [DOI] [PubMed] [Google Scholar]
- 52.Karsdal MA, Henriksen K, Leeming DJ, Mitchell P, Duffin K, Barascuk N, Klickstein L, Aggarwal P, Nemirovskiy O, Byrjalsen I, Qvist P, Bay-Jensen AC, Dam EB, Madsen SH, Christiansen C. Biochemical markers and the FDA Critical Path: How biomarkers may contribute to the understanding of pathophysiology and provide unique and necessary tools for drug development. Biomarkers. 2009;14:181–202. doi: 10.1080/13547500902777608. [DOI] [PubMed] [Google Scholar]
- 53.Motola DL, Caravan P, Chung RT, Fuchs BC. Noninvasive Biomarkers of Liver Fibrosis: Clinical Applications and Future Directions. Curr Pathobiol Rep. 2014;2:245–256. doi: 10.1007/s40139-014-0061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clarke DL, Carruthers AM, Mustelin T, Murray LA. Matrix regulation of Idiopathic pulmonary fibrosis: the role of enzymes. Fibrogenesis and Tissue Repair. 2013;6:20. doi: 10.1186/1755-1536-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferreira A, Alho I, Casimiro S, Costa L. Bone remodeling markers and bone metastases: From cancer research to clinical implications. Bonekey Rep. 2015;22 doi: 10.1038/bonekey.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caravan P, Das B, Dumas S, Epstein FH, Helm PA, Jacques V, Koerner S, Kolodziej A, Shen L, Sun WC, Zhang Z. Collagen-Targeted MRI Contrast Agent for Molecular Imaging of Fibrosis. Angew Chem Int Ed. 2007;46:8171–8173. doi: 10.1002/anie.200700700. [DOI] [PubMed] [Google Scholar]
- 57.Muzard J, Sarda-Mantel L, Loyau S, Meulemans A, Louedec L, Bantsimba-Malanda C, Hervatin F, Marchal-Somme J, Michel JB, Le Guludec D, Billiald P, Jandrot-Perrus M. Non-Invasive Molecular Imaging of Fibrosis Using a Collagen-Targeted Peptidomimetic of the Platelet Collagen Receptor Glycoprotein VI. PLoS ONE. 2009;4:e5585. doi: 10.1371/journal.pone.0005585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chettibi S, Newton B, Husbyn M, Solbakken M. Novel imaging agents. Google Patents. 2007 [Google Scholar]
- 59.Jugdutt BI, Joljart MJ, Khan MI. Rate of collagen deposition during healing and ventricular remodeling after myocardial infarction in rat and dog models. Circulation. 1996;94:94–101. doi: 10.1161/01.cir.94.1.94. [DOI] [PubMed] [Google Scholar]
- 60.Caravan P, Yang Y, Zachariah R, Schmitt A, Mino-Kenudson M, Chen HH, Sosnovik DE, Dai G, Fuchs BC, Lanuti M. Molecular magnetic resonance imaging of pulmonary fibrosis in mice. Am J Respir Cell Mol Biol. 2013;49:1120–1126. doi: 10.1165/rcmb.2013-0039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fuchs BC, Wang H, Yang Y, Wei L, Polasek M, Schuhle DT, Lauwers GY, Parkar A, Sinskey AJ, Tanabe KK, Caravan P. Molecular MRI of collagen to diagnose and stage liver fibrosis. J Hepatol. 2013;59:992–998. doi: 10.1016/j.jhep.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen W, Cormode DP, Vengrenyuk Y, Herranz B, Feig JE, Klink A, MulderJ WJ, Fisher EA, Fayad ZA. Collagen-specific peptide conjugated HDL nanoparticles as MRI contrast agent to evaluate compositional changes in atherosclerotic plaque regression. JACC Cardiovasc Imaging. 2013;6:373–384. doi: 10.1016/j.jcmg.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zong Y, Xu Y, Liang X, Keene DR, Hook A, Gurusiddappa S, Hook M, Narayana SV. A ‘Collagen Hug’ Model for Staphylococcus aureus CNA binding to collagen. Embo J. 2005;24:4224–4236. doi: 10.1038/sj.emboj.7600888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boerboom RA, Krahn KN, Megens RT, van Zandvoort MA, Merkx M, Bouten CV. High resolution imaging of collagen organisation and synthesis using a versatile collagen specific probe. J Struct Biol. 2007;159:392–399. doi: 10.1016/j.jsb.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 65.Krahn KN, Bouten CV, van Tuijl S, van Zandvoort MA, Merkx M. Fluorescently labeled collagen binding proteins allow specific visualization of collagen in tissues and live cell culture. Anal Biochem. 2006;350:177–185. doi: 10.1016/j.ab.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 66.Megens RT, Oude Egbrink MG, Cleutjens JP, Kuijpers MJ, Schiffers PH, Merkx M, Slaaf DW, van Zandvoort MA. Imaging Collagen in Intact Viable Healthy and Atherosclerotic Arteries using Fluorescently Labeled CNA35 and Two-Photon Laser Scanning Microscopy. Mol Imaging. 2007;6:247–260. [PubMed] [Google Scholar]
- 67.van Bochove GS, Sanders HMHF, de Smet M, Keizer HM, Mulder WJM, Krams R, Strijkers GJ, Nicolay K. Molecular MR Imaging of Collagen in Mouse Atherosclerosis by Using Paramagnetic CNA35 Micelles. Eur J Inorg Chem. 2012;2012:2115–2125. [Google Scholar]
- 68.Helms BA, Reulen SWA, Nijhuis S, de Graaf-Heuvelmans PTHM, Merkx M, Meijer EW. High-Affinity Peptide-Based Collagen Targeting Using Synthetic Phage Mimics: From Phage Display to Dendrimer Display. J Am Chem Soc. 2009;131:11683–11685. doi: 10.1021/ja902285m. [DOI] [PubMed] [Google Scholar]
- 69.Reulen SWA, Dankers PYW, Bomans PHH, Meijer EW, Merkx M. Collagen Targeting Using Protein-Functionalized Micelles: The Strength of Multiple Weak Interactions. J Am Chem Soc. 2009;131:7304–7312. doi: 10.1021/ja807723p. [DOI] [PubMed] [Google Scholar]
- 70.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 71.Gröschel K, Riecker A, Schulz JB, Ernemann U, Kastrup A. Systematic review of early recurrent stenosis after carotid angioplasty and stenting. Stroke. 2005;36:367–373. doi: 10.1161/01.STR.0000152357.82843.9f. [DOI] [PubMed] [Google Scholar]
- 72.Folkman J. Angiogenesis: An organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 73.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol Mech Dis. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 74.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 75.Seandel M, Noack-Kunnmann K, Zhu D, Aimes RT, Quigley JP. Growth factor-induced angiogenesis in vivo requires specific cleavage of fibrillar type I collagen. Blood. 2001;97:2323–2332. doi: 10.1182/blood.v97.8.2323. [DOI] [PubMed] [Google Scholar]
- 76.Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of Vulnerable/Unstable Plaque. Atertio Thromb Vasc Biol. 2010;30:1282–1292. doi: 10.1161/ATVBAHA.108.179739. [DOI] [PubMed] [Google Scholar]
- 77.Troeberg L, Nagase H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim Biophys Acta. 2012;1824:133–145. doi: 10.1016/j.bbapap.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dejica VM, Mort JS, Laverty S, Percival MD, Antoniou J, Zukor DJ, Poole AR. Cleavage of type II collagen by cathepsin K in human osteoarthritic cartilage. Am J Pathol. 2008;173:161–169. doi: 10.2353/ajpath.2008.070494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Benyon RC, Arthur MJP. Extracellular matrix degradation and the role of hepatic stellate cells. Semin Liver Dis. 2001;21:373–384. doi: 10.1055/s-2001-17552. [DOI] [PubMed] [Google Scholar]
- 80.Nemirovskiy OV, Du DRW, Sunyer T, Aggarwal P, Welsch DJ, Mathews WR. Discovery and development of a type II collagen neoepitope (TIINE) biomarker for matrix metalloproteinase activity: From in vitro to in vivo. Anal Biochem. 2007;361:93–101. doi: 10.1016/j.ab.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 81.Rennie MJ. Teasing out the truth about collagen. J Physiol. 1999;521:1. doi: 10.1111/j.1469-7793.1999.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li Y, Yu SM. Targeting and mimicking collagens via triple helical peptide assembly. Curr Opin Chem Biol. 2013;17:968–975. doi: 10.1016/j.cbpa.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Laurent GJ. Dynamic state of collagen: pathways of collagen degradation in vivo and their possible role in regulation of collagen mass. Am J Physiol. 1987;252:C1–9. doi: 10.1152/ajpcell.1987.252.1.C1. [DOI] [PubMed] [Google Scholar]
- 84.Otto CM, Prendergast B. Aortic-valve stenosis–from patients at risk to severe valve obstruction. N Engl J Med. 2014;371:744–756. doi: 10.1056/NEJMra1313875. [DOI] [PubMed] [Google Scholar]
- 85.Freimark B, Clark D, Pernasetti F, Nickel J, Myszka D, Baeuerle PA, Van Epps D. Targeting of humanized antibody D93 to sites of angiogenesis and tumor growth by binding to multiple epitopes on denatured collagens. Mol Immunol. 2007;44:3741–3750. doi: 10.1016/j.molimm.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 86.Stoop R, van der Kraan PM, Buma P, Hollander AP, Poole AR, van den Berg WB. Denaturation of type II collagen in articular cartilage in experimental murine arthritis. Evidence for collagen degradation in both reversible and irreversible cartilage damage. J Pathol. 1999;188:329–337. doi: 10.1002/(SICI)1096-9896(199907)188:3<329::AID-PATH371>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 87.Morgan H, Hill PA. Human breast cancer cell-mediated bone collagen degradation requires plasminogen activation and matrix metalloproteinase activity. Cancer Cell Int. 2005;5:1. doi: 10.1186/1475-2867-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leppert D, Leib SL, Grygar C, Miller KM, Schaad UB, Holländer GA. Matrix metalloproteinase (MMP)-8 and MMP-9 in cerebrospinal fluid during bacterial meningitis: association with blood-brain barrier damage and neurological sequelae. Clin Infect Dis. 2000;31:80–84. doi: 10.1086/313922. [DOI] [PubMed] [Google Scholar]
- 89.Levine JM, Ruaux CG, Bergman RL, Coates JR, Steiner JM, Williams DA. Matrix metalloproteinase-9 activity in the cerebrospinal fluid and serum of dogs with acute spinal cord trauma from intervertebral disk disease. Am J Vet Res. 2006;67:283–287. doi: 10.2460/ajvr.67.2.283. [DOI] [PubMed] [Google Scholar]
- 90.Krizkovaa S, Zitkaa O, Adama V, Kizeka R, Masarik M, Stiborovad M, Eckschlager T, Chavis GJ. Assays for determination of matrix metalloproteinases and their activity. Trac-Trend Anal Chem. 2011;30:1819–1832. [Google Scholar]
- 91.Ratnikov B, Deryugina E, Leng J, Marchenko G, Dembrow D, Strongin A. Determination of matrix metalloproteinase activity using biotinylated gelatin. Anal Biochem. 2000;286:149–155. doi: 10.1006/abio.2000.4798. [DOI] [PubMed] [Google Scholar]
- 92.Rousseau J-C, Delmas PD. Biological markers in osteoarthritis. Nat Clin Pract Rheumatol. 2007;3:346–356. doi: 10.1038/ncprheum0508. [DOI] [PubMed] [Google Scholar]
- 93.Seibel MJ. Clinical use of markers of bone turnover in metastatic bone disease. Nat Clin Pract Oncol. 2005;2:504–517. doi: 10.1038/ncponc0320. [DOI] [PubMed] [Google Scholar]
- 94.Drake MT, Clarke BL, Khosla S. Bisphosphonates: Mechanism of Action and Role in Clinical Practice. Mayo Clin Proc. 2008;83:1032–1045. doi: 10.4065/83.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McClung M, Harris ST, Miller PD, Bauer DC, Davison KS, Dian L, Hanley DA, Kendler DL, Yuen CK, Lewiecki EM. Bisphosphonate therapy for osteoporosis: benefits, risks, and drug holiday. Am J Med. 2013;126:13–20. doi: 10.1016/j.amjmed.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 96.Dunford JE, Thompson K, Coxon FP, Luckman SP, Hahn FM, Poulter CD, Ebetino FH, Rogers MJ. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther. 2001;296:235–242. [PubMed] [Google Scholar]
- 97.Hanley DA, Adachi JD, Bell A, Brown V. Denosumab: mechanism of action and clinical outcomes. Int J Clin Pract. 2012;66:1139–1146. doi: 10.1111/ijcp.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Goel N, Stephens S. Certolizumab pegol. MAbs. 2010;2:137–147. doi: 10.4161/mabs.2.2.11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Van Poznak CH, Temin S, Yee GC, Janjan NA, Barlow WE, Biermann JS, Bosserman LD, Geoghegan C, Hillner BE, Theriault RL, Zuckerman DS, Von Roenn JH. American Society of Clinical Oncology. American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. J Clin Oncol. 2011;29:1221–1227. doi: 10.1200/JCO.2010.32.5209. [DOI] [PubMed] [Google Scholar]
- 100.Xu J, Rodriguez D, Kim JJ, Brooks PC. Generation of monoclonal antibodies to cryptic collagen sites by using subtractive immunization. Hybridoma. 2000;19:375–385. doi: 10.1089/02724570050198893. [DOI] [PubMed] [Google Scholar]
- 101.Xu J, Rodriguez D, Petitclerc E. Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J Cell Biol. 2001;154:1069–1079. doi: 10.1083/jcb.200103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roth JM, Caunt M, Cretu A, Akalu A, Policarpio D, Li X, Gagne P, Formenti S, Brooks PC. Inhibition of experimental metastasis by targeting the HUIV26 cryptic epitope in collagen. Am J Pathol. 2006;168:1576–1586. doi: 10.2353/ajpath.2006.050176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chang C, Werb Z. The many faces of metalloproteases: cell growth, invasion, angiogenesis and metastasis. Trends Cell Biol. 2001;11:537–543. doi: 10.1016/s0962-8924(01)02122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chichester C, Barrach HJ, Chichester A, Matoney A, Srinivas GR. Evidence for polyreactivity seen with monoclonal antibodies produced against type II collagen. J Immunol Methods. 1991;140:259–267. doi: 10.1016/0022-1759(91)90379-t. [DOI] [PubMed] [Google Scholar]
- 105.Srinivas GR, Barrach HJ, Chichester CO. Quantitative immunoassays for type II collagen and its cyanogen bromide peptides. J Immunol Methods. 1993;159:53–62. doi: 10.1016/0022-1759(93)90141-s. [DOI] [PubMed] [Google Scholar]
- 106.Mueller J, Gaertner FC, Blechert B, Janssen K-P, Essler M. Targeting of Tumor Blood Vessels: A Phage-Displayed Tumor-Homing Peptide Specifically Binds to Matrix Metalloproteinase-2-Processed Collagen IV and Blocks Angiogenesis In vivo. Mol Cancer Res. 2009;7:1078–1085. doi: 10.1158/1541-7786.MCR-08-0538. [DOI] [PubMed] [Google Scholar]
- 107.Yu SM, Li Y, Kim D. Collagen mimetic peptides: Progress toward functional applications. Soft Matter. 2011;7:7927–7938. doi: 10.1039/C1SM05329A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li Y, Foss CA, Summerfield DD, Doyle JJ, Torok CM, Dietz HC, Pomper MG, Yu SM. Targeting collagen strands by photo-triggered triple-helix hybridization. Proc Natl Acad Sci USA. 2012;109:14767–14772. doi: 10.1073/pnas.1209721109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li Y, Ho D, Huan M, Chan TR, An B, Yu H, Brodsky B, Jun AS, Yu SM. Direct detection of collagenous proteins by fluorescently labeled collagen mimetic peptides. Bioconjug Chem. 2013;24:9–16. doi: 10.1021/bc3005842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chattopadhyay S, Guthrie KM, Teixeira L, Murphy CJ, Dubielzig RR, McAnulty JF, Raines RT. Anchoring a cytoactive factor in a wound bed promotes healing. J Tissue Eng Regen Med. 2014 doi: 10.1002/term.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brodsky B, Persikov AV. Molecular structure of the collagen triple helix. Adv Protein Chem. 2005;70:301–339. doi: 10.1016/S0065-3233(05)70009-7. [DOI] [PubMed] [Google Scholar]
- 112.Jin H-E, Farr R, Lee S-W. Collagen mimetic peptide engineered M13 bacteriophage for collagen targeting and mimicking in cancer. Biomaterials. 2014;35:9236–9245. doi: 10.1016/j.biomaterials.2014.07.044. [DOI] [PubMed] [Google Scholar]
- 113.Brinckerhoff CE, Rutter JL, Benbow U. Interstitial collagenases as markers of tumor progression. Clin Cancer Res. 2000;6:4823–4830. [PubMed] [Google Scholar]
- 114.Urello MA, Kiick KL, Sullivan MO. A CMP-based method for tunable, cell-mediated gene delivery from collagen scaffolds. J Mater Chem B. 2014;2:8174–8185. doi: 10.1039/c4tb01435a. [DOI] [PubMed] [Google Scholar]
- 115.Wang AY, Leong S, Liang YC, Huang RC, Chen CS, Yu SM. Immobilization of growth factors on collagen scaffolds mediated by polyanionic collagen mimetic peptides and its effect on endothelial cell morphogenesis. Biomacromolecules. 2008;9:2929–2936. doi: 10.1021/bm800727z. [DOI] [PubMed] [Google Scholar]
- 116.Chan TR, Stahl PJ, Yu SM. Matrix-Bound VEGF Mimetic Peptides: Design and Endothelial-Cell Activation in Collagen Scaffolds. Adv Funct Mater. 2011;21:4252–4262. doi: 10.1002/adfm.201101163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stahl PJ, Chan TR, Shen YI, Sun G, Gerecht S, Yu SM. Capillary Network-Like Organization of Endothelial Cells in PEGDA Scaffolds Encoded with Angiogenic Signals via Triple Helical Hybridization. Adv Funct Mater. 2014;4:3213–3225. doi: 10.1002/adfm.201303217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Grover CN, Cameron RE, Best SM. Investigating the morphological, mechanical and degradation properties of scaffolds comprising collagen, gelatin and elastin for use in soft tissue engineering. J Mech Behav Biomed Mater. 2012;10:62–74. doi: 10.1016/j.jmbbm.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 119.Chan TR, Stahl PJ, Li Y, Yu SM. Collagen–gelatin mixtures as wound model, and substrates for VEGF-mimetic peptide binding and endothelial cell activation. Acta Biomaterialia. 2015;15:164–172. doi: 10.1016/j.actbio.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang AY, Foss CA, Leong S, Mo X, Pomper MG, Yu SM. Spatio-temporal modification of collagen scaffolds mediated by triple helical propensity. Biomacromolecules. 2008;9:1755–1763. doi: 10.1021/bm701378k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Santos JL, Li Y, Culver HR, Yu SM, Herrera-Alonso M. Conducting polymer nanoparticles decorated with collagen mimetic peptides for collagen targeting. Chem Commun. 2014;50:15045–15048. doi: 10.1039/c4cc06056c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li Y, San BH, Kessler JL, Kim JH, Xu Q, Hanes J, Yu SM. Non-covalent photo-patterning of gelatin matrices using caged collagen mimetic peptides. Macromol Biosci. 2015;15:52–62. doi: 10.1002/mabi.201400436. [DOI] [PMC free article] [PubMed] [Google Scholar]


