Abstract
Gestational testosterone (T) excess, acting via both the androgenic and estrogenic pathways, advances puberty and disrupts the neuroendocrine estradiol (E) feedback and periovulatory hormonal dynamics in female sheep. These prenatally programmed defects may be subject to postnatal modifications by continued organizational and/or activational effects of steroids. The present study investigated 1) the organizational contribution of prenatal estrogen excess and 2) the impact of postnatal exposure to E in modulating the effects of prenatal androgen excess (T and dihydrotestosterone [DHT]) on puberty, neuroendocrine feedback mechanisms, and periovulatory hormonal dynamics in sheep. Pregnant Suffolk sheep were treated with T, DHT, E, or E plus DHT (ED) from days 30 to 90 of gestation. A subset of the control (C), T, and DHT female offspring received a constant-release E implant postnatally. Findings revealed that 1) prenatal E-treatment failed to reproduce the neuroendocrine disruptions predicted to be programmed by the estrogenic pathway and 2) prenatal ED-treatment did not adequately replicate the reproductive neuroendocrine defects induced by prenatal T excess. More importantly, continuous postnatal E-treatment, while delaying the onset of puberty and reducing the inhibitory effects of E on tonic luteinizing hormone (LH) release, failed to amplify the E positive feedback and periovulatory defects induced by prenatal T-treatment. Our results indicate that disruptions in E positive feedback mechanisms and periovulatory gonadotropin secretion induced by prenatal T-treatment are programmed predominantly during the prenatal life with postnatal exposure to E excess not contributing further to these disruptions.
Keywords: female reproduction, infertility, androgen, estrogen, organizational effects, activational effects, LH, FSH
Introduction
Many diseases in adults, such as obesity, metabolic syndrome, and polycystic ovary syndrome (PCOS), may have their origins during fetal life. As proposed by Barker's developmental origins of adult disease hypothesis (Barker 2004), adverse prenatal insults can cause permanent changes in the physiology of the developing fetus leading to pathology during adulthood. These insults include inadvertent exposure to hormonal, nutritional, and environmental agents (Rhind et al. 2001, Tang & Ho 2007, Dumesic et al. 2014). The effects of increased exposure to native steroids in disease states (New 2006, Rosenfield 2007) and to environmental endocrine-disrupting chemicals (EDC) that can bind to steroid receptors (Vaiserman 2014) have been the focus of intense research. Due to the high rate of tissue differentiation and metabolism, a developing fetus is extremely sensitive to EDC exposures even at concentrations far below those detrimental to adults (Diamanti-Kandarakis et al. 2009, Schug et al. 2011).
Congenital adrenal hyperplasia (CAH) (New 2006) and PCOS (Sir-Petermann et al. 2002) are disease states characterized by elevated testosterone levels, among other endocrine imbalances. Experimentally, prenatal T-treatment in various species, including sheep, leads to dysfunctions during adult life that resemble those seen in women with PCOS (Padmanabhan & Veiga-Lopez 2013a, 2013b, Dumesic et al. 2014). For instance, prenatal T-treated sheep show progressive loss of cyclicity (Clarke et al. 1977, Birch et al. 2003, Manikkam et al. 2006) and disrupted periovulatory hormonal dynamics (Veiga-Lopez et al. 2008), with defects evident at both the neuroendocrine and ovarian levels (Padmanabhan & Veiga-Lopez 2013b). The neuroendocrine disruptions induced by prenatal T excess include reduced sensitivity to estradiol (E) and progesterone (P) negative feedback (Wood & Foster 1998, Robinson et al. 2002, Sarma et al. 2005), dampened or absent E positive feedback, and disrupted periovulatory hormonal dynamics (Sharma et al. 2002, Unsworth et al. 2005, Veiga-Lopez et al. 2009). Comparative studies with dihydrotestosterone (DHT), a non-aromatizable androgen (Veiga-Lopez et al. 2009), as well as T+ androgen antagonist (Jackson et al. 2008) treated sheep provided evidence that E negative, but not positive, feedback perturbations are programmed by androgenic actions of T. This led to the premise that E positive feedback and periovulatory hormonal dynamics are likely programmed by the estrogenic actions of T (Foster et al. 2006).
Although the organizational period may extend beyond birth (Jackson et al. 2013), evidence suggests that activational effects of the postnatal environment can modify the impact of such organizational changes. For instance, the organizational effects of excess prenatal steroid hormone exposure in masculinizing the behavioral circuits in the males become only apparent during puberty when gonadal steroid output determines the expression of sex-typical behavior (Schulz et al. 2009). This can be explained by the two-hit hypothesis, which states that an early-life adverse event (“first-hit”) programs a pathological condition that may be revealed only later in life by a subsequent exposure to an adverse influence or the so called “second hit” (Bayer et al. 1999, Tang et al. 2008, Puttabyatappa et al. 2015). From an adverse exposure standpoint, humans are exposed throughout their lifespan to steroids involuntarily and sometimes unknowingly through food consumption (phytoestrogens), industrial byproducts (bisphenol A), diseases (CAH, PCOS), and/or voluntarily (contraception and anabolic steroids) (Bahrke et al. 1998, Jefferson et al. 2012, Pignatelli 2013, Peretz et al. 2014, Jensen et al. 2015). Such exposure(s) can be deleterious to fertility and may serve to mask/unmask or reduce/amplify prenatally programmed functions. For example, postnatal overfeeding (second hit) of prenatally T-treated (first hit) sheep exacerbated the reproductive cyclicity defects, with a majority of animals ending their breeding season early (Steckler et al. 2009). Similarly, postnatal exposure to E (second hit) amplified the ovarian defects induced by prenatal T excess (first hit) (Veiga-Lopez et al. 2014).
The objective of this study was to investigate the contributions of prenatal and postnatal E in programming and amplifying reproductive neuroendocrine defects. Specifically, this study tested the following hypotheses: 1) prenatal E-treatment alone induces neuroendocrine defects that have been postulated to be programmed by the estrogenic effects of prenatal T-treatment, 2) prenatal E+DHT-treatment replicates the effects of prenatal T-treatment, and 3) continuous postnatal E exposure amplifies the effects of prenatal androgen excess.
Materials and Methods
All procedures used in this study were approved by the University of Michigan Animal Care and Use Committee and are consistent with National Research Council's Guide for the Care and Use of Laboratory Animals.
Generation of experimental animals
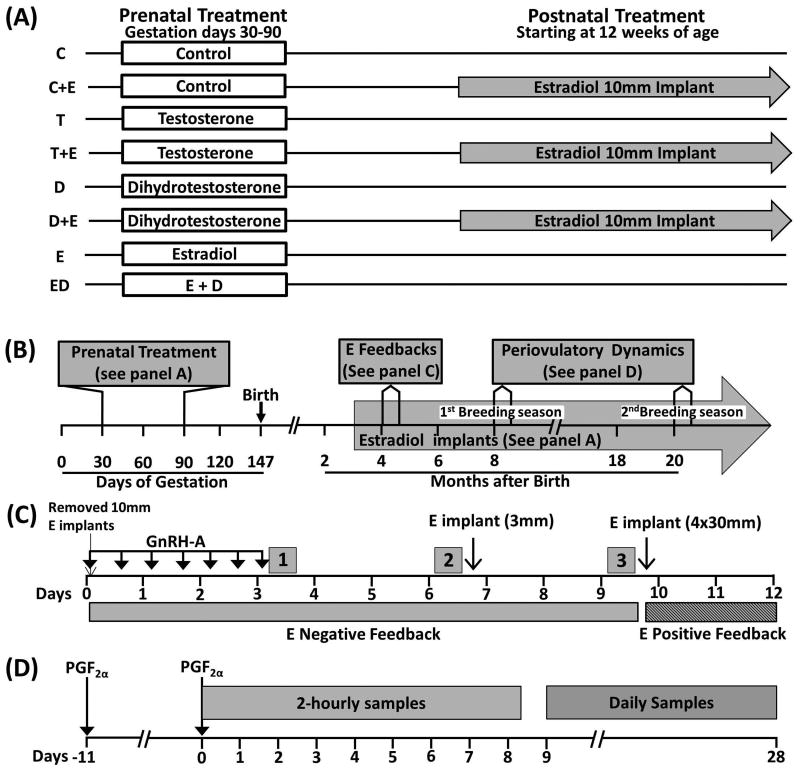
The study was conducted at the University of Michigan Sheep Research Facility, Ann Arbor, MI. Adult Suffolk ewes purchased from local farmers were mated with Suffolk rams of proven fertility. General husbandry and nutrition have been described previously (Manikkam et al. 2004). Starting at 2–3 weeks prior to breeding, ewes were group-fed daily with 0.5 kg shelled corn and 1.0–1.5 kg alfalfa hay/ewe. Once mated, females were assigned randomly to the different treatment groups and housed under a natural photoperiod in the pasture and group-fed with a daily maintenance diet of 1.25 kg alfalfa/brome mix hay/ewe. Lambs born to these ewes were fed a pelleted diet (Shur-Gain, Strykersville, NY) consisting of 3.6 MCal/kg digestible energy and 18% crude protein. At ∼8 weeks of age, lambs were weaned and maintained outdoors. They were fed ad libitum until they attained ∼40 kg of body weight, at which point they were switched to a diet consisting of 2.3MCal/kg digestible energy and 11.3% crude protein. The treatment groups used in the study and the various studies performed are summarized in Figure 1.
Figure 1.
Schematic showing the study design. (A) Prenatal and postnatal treatment groups. (B) Timeline of studies carried out. (C) Timeline of the E negative and positive feedback studies. Periods 1, 2 and 3 indicate pulse bleed at the end of the GnRH-A treatment, 72h after cessation of the GnRH-A (Pre-E) treatment and 72h after the E-treatment (Post-E), respectively. Hatched bar indicates timing of E positive feedback. (D) Timeline of the assessment of the periovulatory hormonal dynamics carried out during the first and second breeding seasons. Arrows indicate the time of estrous synchronization with PGF2α injections and grey boxes show periods of 2-hourly samples and daily sample collection.
The lambs used in the study received either no treatment prenatally (control; C) or were treated prenatally from gestational days 30 to 90 with T (T-treated), DHT (D-treated), estradiol (E-treated) or E+DHT (ED-treated) (Fig. 1A). The number of offspring born in each treatment group was C = 13, E = 11, ED = 6 and 7 each for T and D prenatal treatment groups. A subset of prenatal C, T-, and D-treated animals received E implants postnatally starting at ∼12 weeks of age. Sample size of postnatal groups was 7 for C+E and T+E, and 6 for the D+E group (Fig. 1A). There were insufficient animals to generate postnatal treatment groups from prenatal E and ED-treated animals. Changes in ovarian follicular dynamics in this cohort of animals have been reported previously (Veiga-Lopez et al. 2014).
Prenatal groups
Prenatal T- and D-treated female sheep were generated as previously reported (Wood et al. 1991). Briefly, pregnant Suffolk ewes were injected (intramuscular) twice weekly from 30 to 90 days of gestation with either 100mg T propionate (1.2mg/kg; Sigma–Aldrich, St. Louis, MO) or 100mg DHT propionate (Steraloids, Newport, RI) suspended in 2ml cottonseed oil. Females in the C group did not receive vehicle because no differences in reproductive parameters were found between vehicle-treated and untreated controls in our previous study (Veiga-Lopez et al. 2008). Prenatal E-treated animals were generated by placing a 30mm SILASTIC implant (Dow Corning, Midland, MI) filled with crystalline E subcutaneously in the axillary region for the same duration as described before (Jackson et al. 2008). Control females did not receive sham implants. The efficacy of this approach in suppressing LH secretion in sheep has been well established (Karsch et al. 1993). The 30mm-implant produces circulating E concentrations of ∼3pg/ml, similar to those seen during the follicular phase in the female sheep (Goodman et al. 1980, Goodman et al. 1981, Jackson et al. 2013). The prenatal ED-treated group received E implants along with twice weekly injections of DHT.
Postnatal treatment
Approximately 12 weeks after birth, half of the prenatal C-, T-, and D-treated female lambs received 10mm E implants, which produces circulating concentrations of E at ∼1pg/ml (Goodman et al. 1980, Goodman et al. 1981, Jackson et al. 2013). Figure 1 summarizes the treatment groups and the type and sequence of studies conducted.
Assessment of puberty
Beginning at ∼21 weeks of age, P concentrations in blood samples collected twice a week were assessed in all animals. Age at puberty was defined as the age at the first sample having circulating concentrations of P greater than 0.5ng/ml.
Testing E-negative feedback responses
This test was conducted during the prepubertal period (∼18 weeks of age; Fig. 1B), when female lambs are extremely sensitive to the E-negative feedback (Foster & Jackson 2006). Details of the E-negative feedback test are summarized in Fig. 1C. The gonadotropin-releasing hormone antagonist (GnRH-A), acyline (Contraception and Reproductive Health Branch, National Institutes of Health, Bethesda, MD), was administered subcutaneously (10μg/kg) every 12h for 72h to abolish LH pulsatility and reduce endogenous E concentrations (Sarma et al. 2005, Steckler et al. 2008). Blood samples were collected at 20min intervals for 6h after the last GnRH-A injection to determine the GnRH-A ablation of LH pulsatility (Period 1; Fig. 1C). To establish the impact of reduced endogenous E on LH pulse frequency, blood samples were collected for 6h beginning 72h after cessation of the GnRH-A treatment (Period 2; Pre E; Fig. 1C). The E-negative feedback was then evaluated by inserting a single 3mm SILASTIC E implant (subcutaneously) to produce circulating concentrations of <1pg/ml (Goodman et al. 1980, Goodman et al. 1981, Jackson et al. 2013). Blood samples were collected beginning 72h after insertion of the E implant for 6h at 20min intervals (Period 3; Post E; Fig. 1C).
Testing E-positive feedback responses
This test was carried out following the E negative feedback testing (Fig. 1C) in prepubertal females at ∼19 weeks of age in the absence of P priming, as reported previously (Sharma et al. 2002). All animals received four 30mm SILASTIC E implants (subcutaneously) to provide late follicular phase concentrations of E (Goodman et al. 1980, Goodman et al. 1981, Jackson et al. 2013). Blood samples were collected every 2h for 72h starting 1h before insertion of E implants.
Periovulatory hormonal dynamics
The impact of experimental treatments on periovulatory hormonal dynamics was studied during the first and second breeding season at ∼8 and ∼20 months of age, respectively (Fig. 1B). Studies were carried out during two breeding seasons to determine whether these animals show a progressive loss in cyclicity as observed previously in prenatal T-treated animals (Manikkam et al. 2006). Estrous was synchronized with two injections of prostaglandin F2α (PGF2α, 10 mg, intramuscular; Lutalyse, Pfizer Animal Health, Florham Park, NJ) administered 11 days apart (Fig. 1D). Following the second PGF2α injection, blood samples were collected every 2h for 192h during the first breeding season and for 120h during the second breeding season to assess the periovulatory hormonal changes. This was followed by daily blood sampling for 19 additional days to assess luteal P secretion. LH and FSH were assayed every 2h, E every 4h, and P in daily samples.
Hormone measurements
Plasma LH, FSH, E and P concentrations were measured in duplicate using validated radioimmunoassays (RIA) (Niswender et al. 1969, Rozell & Keisler 1990, Padmanabhan et al. 1995, Padmanabhan et al. 1997). The sensitivity of LH RIA was 0.1 ± 0.01 ng/ml (n = 46 assays; mean ± SEM). Mean intra-assay coefficient of variation (CV) based on four quality control pools measuring 3.1 ± 0.1, 7.2 ± 0.1, 13.4 ± 0.1, and 23.8 ± 0.01 ng/ml were 10.0, 6.0, 6.8, and 6.0%, respectively. The corresponding inter-assay CVs averaged 20.5, 7.2, 5.6, and 4.5%. The sensitivity of FSH RIA was 0.06 ± 0.01 ng/ml (n = 31 assays). The intra-assay CVs based on two quality control pools measuring 5.0 ± 0.1 and 11.2 ± 0.2 ng/ml were 9.9 and 4.8%, respectively. The corresponding inter-assay CVs averaged 13.9 and 8.7%. The sensitivity of E RIA was 0.3 ± 0.1pg/ml (n = 36 assays). Mean intra-assay CVs based on four quality control pools measuring 0.53 ± 0.02, 3.12 ± 0.18, 1.17 ± 0.07, and 2.75 ± 0.29 pg/ml were 12.1, 12.6, 7.5, and 10.2%, respectively. The corresponding inter-assay CVs averaged 24.8, 32.92, 33.28 and 46.96. The sensitivity of P RIA (Coat-a-Count, DPC/Siemens, Los Angeles, CA) was 0.001 ± 0.0002 ng/ml (n = 8 assays). Mean intra-assay CVs based on three quality control pools measuring 0.2 ± 0.006, 1.7 ± 0.02 and 14.3 ± 0.2 ng/ml were 4.8, 5.1 and 2.4% respectively. The corresponding inter-assay CVs averaged 12.2, 3.6 and 3.8%. Sample sets from experimental groups were randomly distributed such that each assay included samples from all treatment groups.
Statistical Analysis
Age at puberty was assessed in all animals. Results from twin females were averaged for assessment of puberty prior to analysis to ensure mother was the experimental unit. Only a subset of animals was used for E negative and positive feedback studies (n = 7 each for C, T, D, E, C+E and T+E and, n = 6 each for ED and D+E) and for assessment of periovulatory hormonal dynamics (n = 6 each for C, T, D, ED, C+E and D+E groups, n = 7 for E and n = 5 for T+E). During the second breeding season, only animals from C, T and E for prenatal treatment groups and C+E and T+E for postnatal groups were available for this study. The number of animals in each treatment group during their second year were C = 9, T = 5, E = 9, C+E = 7 and T+E = 4. For the feedback and periovulatory dynamic studies, when twin female births were involved only one offspring (selected randomly) was used.
For E-negative feedback, LH values from serially collected samples were subjected to pulse analysis using the Cluster algorithm (Veldhuis & Johnson 1986). For cluster analysis, the minimum number of data points to identify either a peak (the highest concentration reached during a pulse) or a nadir (basal level) for the 20min sampling frequency was set at one. The Student's t statistic values used to identify significant increases from preceding nadirs and decreases to following nadirs were set at 1.0. Increases in LH concentrations more than two times the assay sensitivity over the preceding nadir were considered as a pulse. The number of pulses was assessed over a 6h period. For both E-positive feedback and periovulatory hormonal characterization, the LH and FSH surges were defined based on the circulating gonadotropin concentrations being above the baseline plus two times the assay sensitivity and remaining high for at least 8h (Padmanabhan et al. 2015). For synchronization of the estrous cycle following PGF2α injection, daily P concentrations had to fall below 0.5ng/ml. For all statistical analysis, p<0.05 was considered significantly different.
Age at puberty
The age at puberty was analyzed by one-way ANOVA for prenatal treatment group comparisons and a two-way ANOVA for testing postnatal E modulation. In view of the large number of comparisons that reduces power, the magnitude of treatment effects on age at puberty was also examined by effect size analysis (Cohen 1992, Nakagawa & Cuthill 2007, Padmanabhan et al. 2015). This analysis allows comparison of the means between two treatments with respect to the magnitude of difference between them. The computed statistic is Cohen's d value, and values above 0.2, 0.5, and 0.8 were considered as small, medium, and large effect sizes, respectively (Cohen 1992, Nakagawa & Cuthill 2007).
E negative feedback
Variables compared were E concentrations and LH pulse frequency. Number of pulses during the 6h period was square-root transformed prior to analysis. For comparison among prenatal groups, ANOVA was used followed by post-hoc analyses adjusting for multiple comparisons. For postnatal comparisons, paired t-test was used to compare postnatal groups with corresponding prenatal-only treated groups. To test whether number of pulses changed significantly between pre-E (72h Post-GnRH-A) and post-E period in each treatment groups, Wilcoxon Signed-rank test was used. In addition, Cohen's effect size analysis was used as secondary analysis to relate magnitude of differences.
E positive feedback and periovulatory dynamics
Comparisons involved peak time, peak hormone concentration, total hormone released during the surge, the duration of surge, and time from E peak to LH peak. All continuous variables were log-transformed before analysis. For comparing categorical variables (% synchronized and % showing LH surges in the periovulatory dynamics study and % responded in the positive feedback study) between prenatal groups and each of the postnatal pairs, Fisher's exact test was used. For comparison of LH and FSH surge dynamics among only prenatal treatment groups, ANOVA was used followed by post-hoc tests after adjusting for multiple comparisons. For variables with only one T subject providing data in E positive feedback test or two T subjects in periovulatory hormonal dynamics examination, given all other prenatal groups are not significantly different, the mean and standard deviation (SD) of the outcome using combined data from the C, D, E, and ED groups were first calculated. Then the probability of observing a value larger or equal to the value observed in the single T subject assuming it is drawn from a normal distribution with the above derived mean and SD was calculated. A smaller p-value indicates that T subject was less likely to have the same distribution as other groups. For prenatal vs. postnatal treatment comparisons, unpaired t-test was used.
Results
Effects of Prenatal Treatments
Estradiol negative feedback responses
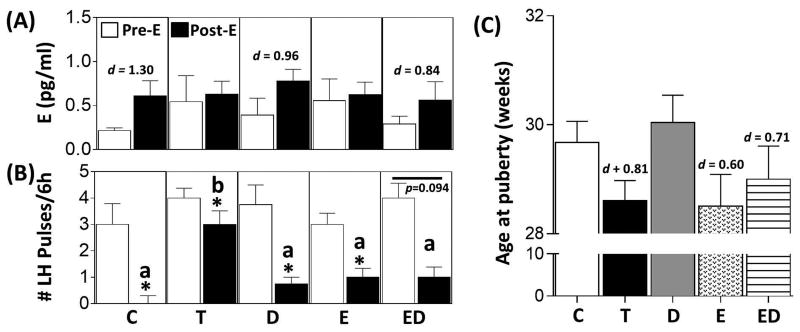
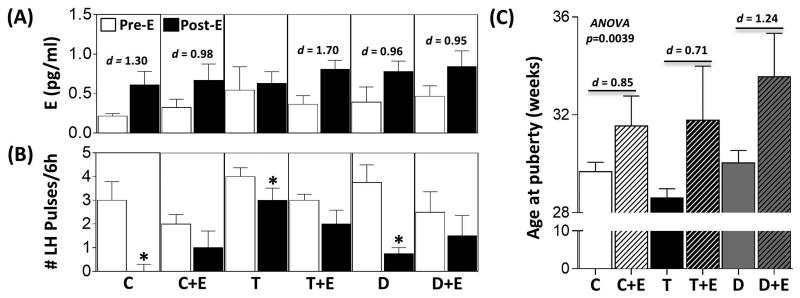
The results of the E negative feedback study in animals that received only prenatal treatments are summarized in Fig. 2A and B, with representative circulating LH profiles shown in Supplemental Fig. 1. Administration of GnRH-A reduced E concentrations to ∼0.3pg/ml in all groups (data not shown). Approximately 72h after the last injection of GnRH-A, endogenous E concentrations had increased slightly but were not statistically different among prenatal treatment groups (Fig. 2A, Pre E). Insertion of a 3mm E implant caused the concentration of E to increase to ∼0.6pg/ml across all prenatal treatment groups (Fig. 2A, Post E). Effect size analysis found a large size effect of E implants in C, D, and ED groups but not in T and E groups. Administration of GnRH-A reduced the LH pulsatility in all prenatal treatment groups (data not shown). 72h after cessation of GnRH-A injection, LH pulsatility increased in all prenatal treatment groups, and LH pulse frequency did not differ among groups (Fig. 2B, Pre E). E-treatment ablated LH pulses in C animals (Fig. 2B, Post E). Prenatal T-treated animals had significantly more LH pulses during the E-treatment period compared to C females (Fig. 2B, Post E). A reduction in LH pulses was evident in T, D, and E-treated animals relative to pre E period, albeit to a lesser degree compared to C animals (Fig. 2B, Post E). While not reaching statistical significance, 5 of 6 animals showed suppression following E-treatment in the ED group (Fig. 2B, Post E).
Figure 2.
Results (mean ± SEM) of the E-negative feedback test in prenatal groups are shown in panels A and B and age of puberty in panel C. Panel A shows the circulating concentrations of E pre- and post-E administration. Panel B shows the number of LH pulses over a 6-h period pre- and post-E administration. Asterisks indicate significant difference (p < 0.05) within the respective prenatal treatments. Bars with different superscripts in the post E period in panel B are significantly different. Panel C shows age of puberty in weeks. Cohen's d values as determined by effect size analysis are shown in panel A (Pre E versus Post E) and C (prenatal treatment versus C).
Age at puberty
The large number of treatment comparisons relative to the sample size precluded identification of statistical differences among prenatal groups (Fig. 2C). However, Cohen's analysis found a large size effect between C and prenatal T, E, and ED groups, but not D treatment.
E Positive Feedback responses
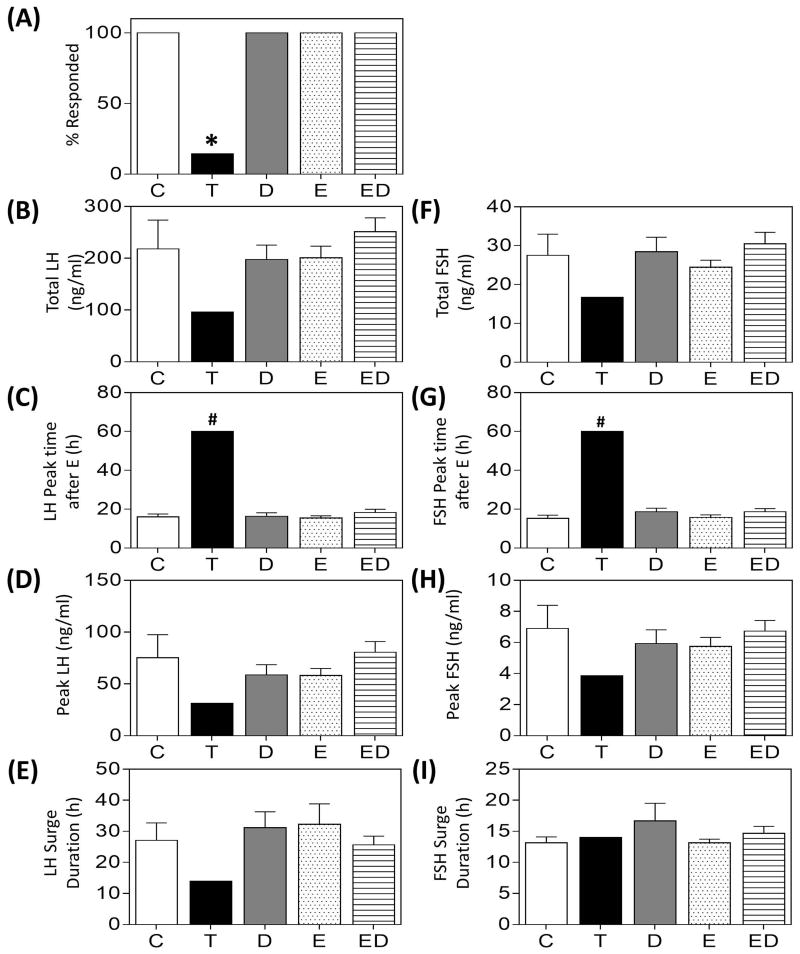
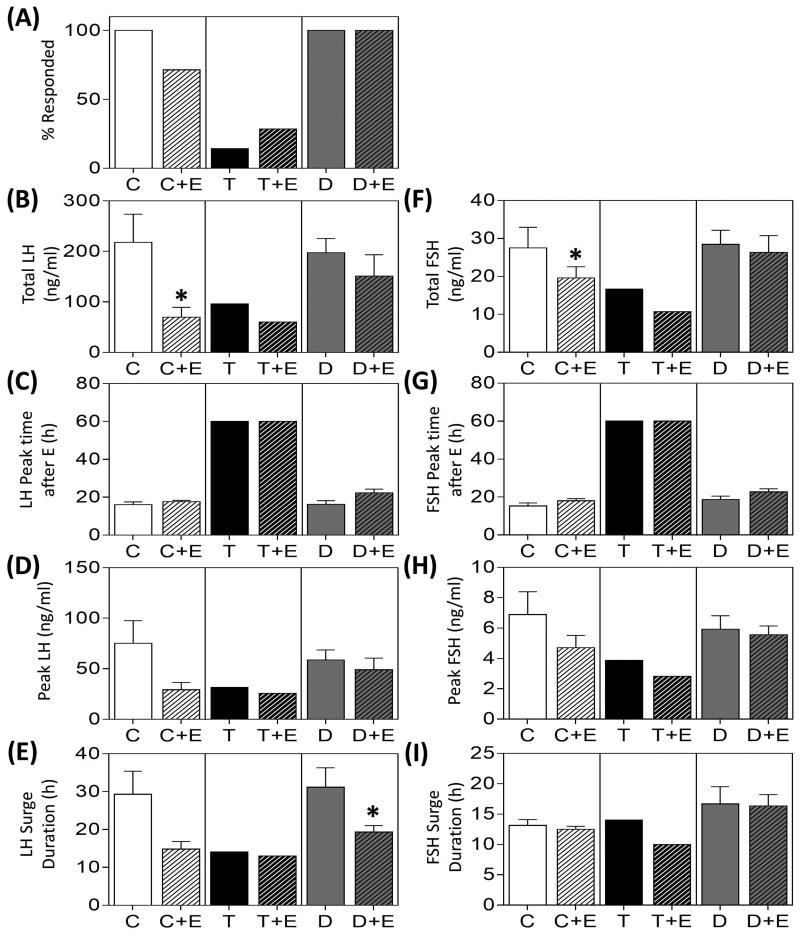
Results of the E positive feedback test in prenatal groups are summarized in Fig. 3 and representative hormonal profiles are depicted in Supplemental Fig. 2. While all females in the C and prenatal D-, E- and ED-treated groups demonstrated gonadotropin surges, only one prenatal T-treated female responded to the E positive feedback challenge (Fig. 3A). Mean total release (Fig. 3B, 3F), peak time (Fig. 3C, 3G), peak concentrations (Fig. 3D, 3H) and duration (Fig. 3E, 3I) of the LH/FSH surges did not differ among C, D, E, and ED groups. The only prenatal T-treated female that responded to the E positive feedback showed a delayed and reduced gonadotropin surge.
Figure 3.
Gonadotropin surge characteristics (mean ± SEM) during the E-positive feedback test in prenatal only treated groups. Observations in T-treated group are from a single animal that responded to the E-positive feedback challenge. Asterisks indicate significant difference from C and # indicates significant difference between the T-animal compared to the composite of all treatment groups (see statistical analysis for modeling).
Periovulatory hormonal dynamics
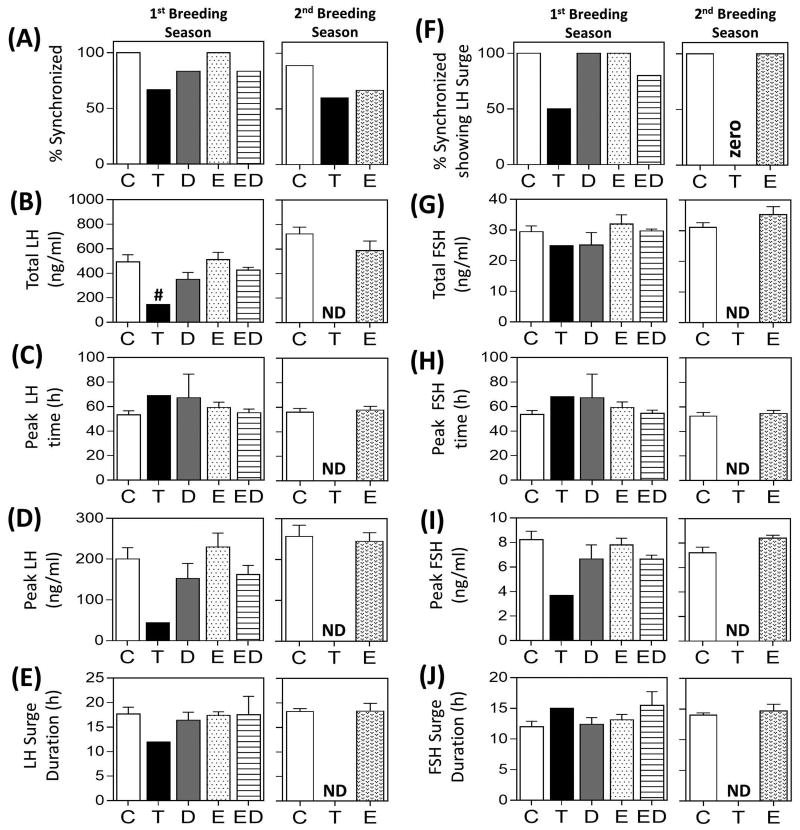
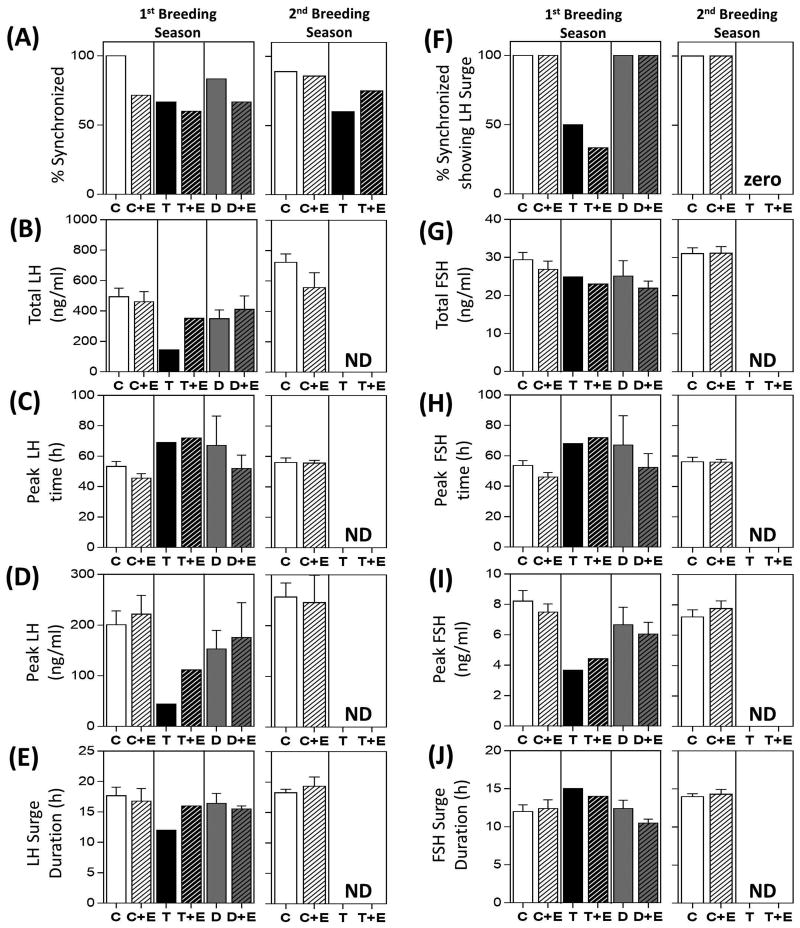
Results of the periovulatory hormonal dynamics during the first and second breeding season in prenatal groups are summarized in Fig. 4 and representative hormonal profiles are depicted in Supplemental Fig. 3 and 4, respectively. During the first breeding season, 100% of the animals were synchronized in the C and E groups, while 66.7%, 83.3%, and 83.3% of the animals showed estrous synchronization following PGF2α administration in T, D and ED groups, respectively (Fig. 4A). Of the female sheep that synchronized, 100% of the animals showed LH surges in C, D and E groups, while only 2 of 4 and 4 of 5 animals showed definable LH surges in the T and ED groups, respectively (Fig. 4F). No differences in mean total release (Fig. 4B, 4G), peak time (Fig. 4C, 4H), peak gonadotropin concentration (Fig. 4D, 4I), and duration (Fig. 4E, 4J) were observed between C, E, D, and ED groups. Of the two prenatal T-treated animals that met the criteria of an LH surge (sustained increase in LH for over 8h), total LH was lower when compared to the control animals but no difference was observed with other surge parameters.
Figure 4.
Results of the estrous synchronization, percentage of synchronized animals showing a definable LH surge and mean ± SEM of LH and primary FSH surge characteristics from periovulatory hormonal studies in prenatal treatment groups during the first and second breeding cycles are shown. Observations in T-treated group are mean from two animals that showed definable LH surge. # indicates significant difference between the T-animal compared to the composite of all treatment groups. ND = No data available as the animals in this group did not exhibit both primary gonadotropin surge.
During the second breeding season, 88.9%, 60.0% and 66.7% of the animals from C, T and E-treatment groups synchronized following PGF2α administration, respectively (Fig. 4A). Of these, 100% of the animals in C and E groups while none of the animals in the T group showed definable LH surges (Fig. 4F). No differences in the gonadotropin surge characteristics were observed between C and E groups (Fig. 4B-E, G-J).
Effects of Postnatal E Exposure
Estradiol negative feedback responses
The impact of postnatal E-treatment on modulating the effects of prenatal steroid-treatment on E negative feedback is summarized in Fig. 5A and B and representative circulating LH profiles in Supplemental Fig. 1. GnRH-A markedly reduced E concentrations in the postnatal groups (C+E, T+E and D+E) to ∼0.3pg/ml (data not shown), and E implant insertion increased concentrations of E to ∼0.6pg/ml (Fig. 5A, Pre E). Effect size analysis comparing postnatal E-treatment groups with corresponding prenatally only treated groups showed large effects following E implants. Suppression of LH pulsatility following GnRH-A treatment and an increase in the number of LH pulses following cessation of GnRH-A treatment in the postnatal groups were similar to changes described for the prenatal treatment only groups. There were no differences in LH pulse frequency among treatment groups during pre-E period (Fig. 5B, Pre E). While E-treatment completely ablated LH pulsatility in the C group, this complete suppression was not achieved in 4 out of 7 animals in the C+E group, which had pulses during the pre-E period (Fig 5B, Post E). The remaining three animals had no LH pulses during the pre-E period, but showed low pulse frequency (1-2 pulses/6h) during E-treatment period. Postnatal E failed to reduce LH pulsatility in T+E and D+E groups (Fig. 5B, Post E) as opposed to suppressive effects being evident in prenatal T and D only females.
Figure 5.
Results (mean ± SEM) of the E-negative feedback test in postnatal groups are shown in panels A and B and age of puberty in panel C. Panel A shows the circulating concentrations of E pre- and post-E administration. Panel B shows the number of LH pulses over a 6-h period pre- and post-E administration. Asterisks indicate significant difference (p < 0.05) between Pre- and Post-E periods within the respective treatment groups. Note the prenatal treatment only groups C, T and D are repeated from respective panels from Fig. 2, to allow comparison between postnatal groups with prenatal only groups. Cohen's d values as determined by effect size analysis are shown in panel A (Pre E versus Post E) and C (prenatal treatment versus postnatal E).
Age at puberty
ANOVA revealed a significant postnatal E-treatment effect in delaying age at puberty (Fig. 5C). Similarly, effect size analysis showed robust effects of postnatal E-treatment in delaying the age at puberty in all treated groups.
E Positive Feedback responses
Results of the E positive feedback test in the postnatal groups are summarized in Fig. 6 and Supplemental Fig. 2. While 5 of 7 C+E and 2 of 7 T+E animals responded to the positive feedback challenge, all D+E females demonstrated a response (Fig. 6A). Except for a reduction in total LH and FSH released in response to postnatal E-treatment in the C+E group (Fig. 6B, 6F), and surge duration in the D+E group relative to corresponding prenatal control groups (Fig. 6E), postnatal E-treatment had no effect on other LH and FSH surge parameters.
Figure 6.
Gonadotropin surge characteristics (mean ± SEM) during the E-positive feedback test in postnatal groups. Data from postnatal groups were compared with the respective prenatal groups (data are repeated from Fig. 3 for comparison purposes). Observations in T-treated group are from a single animal while T+E group is the mean of two animals that responded to the E-positive feedback challenge. Asterisks indicate significant differences (p < 0.05) between postnatal and prenatal groups.
Periovulatory hormonal dynamics
The impact of postnatal E-treatment on periovulatory hormonal dynamics during the first and second breeding season is summarized in Fig. 7, and representative hormonal profiles are depicted in Supplemental Figs. 3 and 4, respectively. Among the animals that received postnatal E-treatment, 71.4% of C+E, 60.0% of T+E and 66.7% of the D+E animals showed estrous synchronization following PGF2α administration during the first breeding season (Fig. 7A). Of those that synchronized, 100% of the animals showed definable LH surges in C+E and D+E groups while only 1 of the 3 animals that synchronized had an LH surge in the T+E group (Fig. 7F). Postnatal E-treatment had no effect on any of the attributes of the preovulatory surge dynamics (Fig. 7). Because only one T+E animal had a definable LH surge, comparison between T and T+E animals was not possible.
Figure 7.
Results of the estrous synchronization, percentage of synchronized animals showing a definable LH surges and mean ± SEM of LH and primary FSH surge characteristics from periovulatory hormonal studies in postnatal groups during the first and second breeding cycles are shown. Data from postnatal groups were compared with the respective prenatal groups (data are repeated from Fig. 4 for comparison purposes). Observations in T-treated group are mean from two animals and one animal in the T+E group that showed definable LH surge. ND = No data available as none of the animals in this group had both primary gonadotropin surge.
During the second breeding season, 85.7% and 75.0% of the animals in C+E and T+E treatment groups synchronized following PGF2α administration, respectively (Fig. 7A). Of these, 100% of the C+E and none of the T+E animals showed definable LH surges (Fig. 7F). Postnatal E-treatment had no effect on any of the parameters of preovulatory surge dynamics in C animals, and since T + E animals did not surge, such comparisons were not possible (Fig. 7).
Discussion
The results from this study confirm our previous findings of neuroendocrine feedback, pubertal timing, and periovulatory disruptions in prenatal T-treated female sheep. However, the data fail to support our hypotheses that 1) prenatal E-treatment replicates the neuroendocrine disruptions attributed to estrogenic programming of E positive feedback and periovulatory hormonal dynamics in prenatal T-treated females; and 2) concomitant prenatal treatment with D and E reproduces the E neuroendocrine feedback and periovulatory defects induced by prenatal T excess. Furthermore, our results indicate that postnatal E-treatment 1) reduces the E inhibitory effects on tonic LH release in prenatal T and D groups, 2) delays the onset of puberty in all groups, and 3) fails to amplify the dampening of LH/FSH surges during the E positive feedback challenge in prenatal T- and D-treated animals. The significance of these findings and their relevance to the understanding of the steroid component in programming these neuroendocrine feedback disruptions are discussed below.
Estrogenic regulation of prenatal T-induced disruptions
Our previous findings showing lack of disruptions in E positive feedback and periovulatory hormonal dynamics in prenatal D-treated sheep (Veiga-Lopez et al. 2009) and failure of androgen antagonist co-treatment to prevent E positive feedback disruptions induced by prenatal T-treatment (Abi Salloum et al. 2012) are consistent with the premise that these defects are mediated by estrogenic programming stemming from aromatization of T to E. Paradoxically, the present observations that prenatal E-treatment did not result in E positive feedback and periovulatory disruptions fail to support this premise. One possibility for this discrepancy is that not sufficient fetal E concentrations were achieved. Alternatively, these findings, in concert with previous findings of partial restoration of preovulatory LH surges in animals co-treated with T and androgen antagonist (Padmanabhan et al. 2015), raise the possibility that both androgens and estrogens synergize in programming the prenatal T-induced disruptions in E feedback and periovulatory hormonal dynamics.
Similarly, the failure of concomitant treatment with E and D to replicate the reproductive neuroendocrine defects might also be a function of inadequate estrogen levels achieved and consequent imbalance in androgen to estrogen ratio at the fetal level relative to what is attained with T-treatment alone. An altered androgen to estrogen ratio could arise from insufficient estrogen levels being achieved due to placental metabolism of the peripherally administered E to lesser active metabolites as seen in monkeys (Slikker et al. 1982) or from increased androgen actions, since D has higher potency than T (Grino et al. 1990). The ratio could also be altered, as D can be metabolized to 3β-diol, an estrogen receptor β agonist (Handa et al. 2008). Therefore additional studies involving the use of estrogen antagonist alone or in combination with an androgen antagonist are required to clearly address the relative role of androgens and estrogens in mediating these effects.
Effects of postnatal E exposure
The lack of response to the E negative feedback challenge in control animals postnatally treated with E may be attributed to the downregulation of the estrogen receptor in the hypothalamus and pituitary gland due to continuous exposure to E. In fact, E-induced downregulation of estrogen receptor expression has been observed in other tissues, such as the uterus (Medlock et al. 1991, Farnell & Ing 2003). Therefore, the interval between the removal of the postnatal E implants and the E negative feedback challenge (7 days) may have been insufficient to allow recovery of estrogen receptor expression in the hypothalamus and pituitary to facilitate responsiveness to the E negative feedback challenge. Considering that puberty was delayed in postnatal E-treated animals, this lack of E negative feedback response was surprising. To what extent this relates to the short interval between the removal of postnatal E implant and the E negative feedback testing remains to be determined. One possibility is that the delay in puberty could be a function of reduced gonadotropic support to the ovary (Foster et al. 1986).
Diminished gonadotropin release in response to E positive feedback was observed in prepubertal female sheep chronically treated with E in this study and in previous studies (Malcolm et al. 2006). However, a reduction in periovulatory release of gonadotropins was not observed when examined during the first and second breeding season. The reason for this difference might relate to the heightened sensitivity of prepubertal females to E negative feedback compared to postpubertal females, resulting in reduced storage of LH in the pituitary that is available for release during the E positive feedback challenge. In contrast to normal periovulatory LH release in our study, postnatal exposure to E was found to block endogenous LH surge in female sheep (Ozturk et al. 1998). The differences in LH surge responses between this study and our study may be a function of the circulating E concentrations achieved by the treatment and/or the timing of treatment.
Postnatal E modulation of prenatal steroid effects
Comparison of the effects of prenatal T plus postnatal E-treatment with prenatal T-treatment alone found postnatal E-treatment does not amplify the effects of prenatal T-treatment in reducing responsiveness to E negative feedback and advancing puberty, a finding consistent with our previous studies (Sarma et al. 2005, Veiga-Lopez et al. 2009, Padmanabhan et al. 2015). Instead, postnatal E-treatment delayed puberty relative to controls, while failing to reduce gonadotropin levels in response to the E negative feedback challenge. Considering that an escape from E negative feedback is an important event controlling the onset of puberty, this dissociation between delayed puberty and reduced sensitivity to E negative feedback is paradoxical. As discussed above, this dissociation may be a function of the short interval between the removal of the E implant and the testing for the E negative feedback.
Furthermore, the finding that E positive feedback and periovulatory hormonal dynamics were impaired in prenatal T-treated sheep, which is in agreement with our previous findings (Sharma et al. 2002, Veiga-Lopez et al. 2009, Padmanabhan et al. 2015), coupled with failure of postnatal E exposure to amplify these disruptions suggest these defects are organized primarily during the prenatal life, with little or no impact of continuous postnatal exposure to excess E, at least at the dose tested. Alternatively, low concentrations of E produced by the ovary during postnatal life may be sufficient to further differentiate (masculinize) the GnRH/gonadotropin surge mechanism in ovary-intact, prenatal T-treated female sheep, with exogenous postnatal E-treatment having no additional impact on the gonadotropin surges. This possibility is supported by the observation that neonatal ovariectomy restores the E-induced LH surge in prenatal T-treated sheep (Jackson et al. 2013).
Contrary to our expectation and in disagreement with our previous studies (Veiga-Lopez et al. 2009), prenatal D-treatment failed to disrupt E negative feedback. The reason for this discripency is unclear but it may involve the different ages at which the E negative feedback tests were carried out, suggesting that the desensitization to the E negative feedback may occur only at a later developmental stage in D-treated compared to T-treated females. As prenatal D-treated animals do not develop a multifollicular ovarian morphology (Smith et al. 2009), the delay in the desensitization of the neuroendocrine axis to the E negative feedback could be due to lower secretion of E and/or other ovarian-derived factors unlike prenatal T-treated females. Failure of postnatal E to alter the E positive feedback or periovulatory hormonal dynamics in prenatal D-treated animals is again consistent with such defects being programmed primarily during the prenatal life.
Conclusions
In conclusion, the results from the present study combined with our earlier findings indicate that disruptions in E feedback mechanisms, timing of puberty, and periovulatory hormonal dynamics seen in prenatal T-treated sheep require activation of both androgenic and estrogenic pathways. Additionally, the present findings indicate that these neuroendocrine disruptions are programmed primarily during the prenatal life and are not amplified or modified by postnatal exposure to E excess, at least at the dose tested. Unfortunately, likely due to a putative metabolism of D into 3β-diol or insufficient fetal E concentrations achieved, the relative role of androgens or estrogens in programming some of these reproductive neuroendocrine defects could not be completely discerned by the present study. In the future, comparative experiments utilizing antagonists of androgen and estrogen receptors separately and in combination need to be undertaken both in the ovariectomized and ovary-intact models. Although postnatal E exposure did not amplify the adverse effects of prenatal T-exposure, it did negatively affect the timing of puberty and preovulatory gonadotropin release in control animals. These data indicate that postnatal exposure to EDC with estrogenic properties, through activational effects, may have potential to adversely affect reproductive neuroendocrine function in females. Therefore, although extrapolation of findings in this sheep model to human pathology should be done cautiously, these observations highlight the potential for gestational and postnatal endocrine imbalances to negatively impact the offspring's reproductive health.
Supplementary Material
Supplemental Figure 1: Circulating pattern of LH from two representative animals from each of the prenatal and postnatal groups during the E-negative feedback test. Arrowheads indicate pulses.
Supplemental Figure 2: Circulating patterns of LH (orange lines) and FSH (blue lines) from three representative animals from each of the prenatal and postnatal groups during the E-positive feedback test.
Supplemental Figure 3: Circulating patterns of LH (orange lines), FSH (blue lines), and E (pink shaded area) from two representative animals from each of the prenatal and postnatal groups following estrous synchronization with PGF2α injections from the first breeding season. Progesterone profiles are depicted in the inset.
Supplemental Figure 4: Circulating patterns of LH (orange lines), FSH (blue lines), and E (pink shaded area) from two representative animals from each of the prenatal and postnatal groups following estrous synchronization with PGF2α injections from the second breeding season. Progesterone profiles are depicted in the inset
Acknowledgments
Funding: This work was supported by National Institutes of Health grant P01 HD44232.
We are grateful to Mr. Douglas Doop for his expert animal care, facility management, and help with generation of the experimental lambs; Drs. Mohan Manikkam and Teresa Steckler, Ms. Jasmine Tavadia, Mr. James Lee, Mr. Rohit Sreedharan, staff and undergraduate students from Dr. Theresa Lee's laboratory and students from University of Michigan Undergraduate Research Opportunity program (UROP) for assistance with prenatal steroid treatment, blood sampling, hormonal assays, and data analyses. We are also thankful to Dr. Aimee K. Wurst from the laboratory of Dr. Keith Inskeep for performing the estradiol measures at West Virginia University, to Dr. Wen Ye for help with statistical analysis and Mr. Jacob Moeller for proof-reading and editing the language.
Footnotes
Declaration of Interest: Authors have no conflicts to disclose.
References
- Abi Salloum B, Herkimer C, Lee JS, Veiga-Lopez A, Padmanabhan V. Developmental programming: prenatal and postnatal contribution of androgens and insulin in the reprogramming of estradiol positive feedback disruptions in prenatal testosterone-treated sheep. Endocrinology. 2012;153:2813–2822. doi: 10.1210/en.2011-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrke MS, Yesalis CE, Brower KJ. Anabolic-androgenic steroid abuse and performance-enhancing drugs among adolescents. Child Adolesc Psychiatr Clin N Am. 1998;7:821–838. [PubMed] [Google Scholar]
- Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;23:588S–595S. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- Bayer TA, Falkai P, Maier W. Genetic and non-genetic vulnerability factors in schizophrenia: the basis of the “two hit hypothesis”. J Psychiatr Res. 1999;33:543–548. doi: 10.1016/s0022-3956(99)00039-4. [DOI] [PubMed] [Google Scholar]
- Birch RA, Padmanabhan V, Foster DL, Unsworth WP, Robinson JE. Prenatal programming of reproductive neuroendocrine function: fetal androgen exposure produces progressive disruption of reproductive cycles in sheep. Endocrinology. 2003;144:1426–1434. doi: 10.1210/en.2002-220965. [DOI] [PubMed] [Google Scholar]
- Clarke I, Scaramuzzi R, Short R. Ovulation in prenatally androgenized ewes. Journal of Endocrinology. 1977;73:385–389. doi: 10.1677/joe.0.0730385. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumesic DA, Goodarzi MO, Chazenbalk GD, Abbott DH. Intrauterine environment and polycystic ovary syndrome. Semin Reprod Med. 2014;32:159–165. doi: 10.1055/s-0034-1371087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnell YZ, Ing NH. The effects of estradiol and selective estrogen receptor modulators on gene expression and messenger RNA stability in immortalized sheep endometrial stromal cells and human endometrial adenocarcinoma cells. J Steroid Biochem Mol Biol. 2003;84:453–461. doi: 10.1016/s0960-0760(03)00066-9. [DOI] [PubMed] [Google Scholar]
- Foster DL, Jackson LM. Puberty in the sheep. Knobil and Neill's Physiology of reproduction. 2006:2127–2176. [Google Scholar]
- Foster DL, Jackson LM, Padmanabhan V. Programming of GnRH feedback controls timing puberty and adult reproductive activity. Mol Cell Endocrinol. 2006;254-255:109–119. doi: 10.1016/j.mce.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Foster DL, Ryan KD, Goodman RL, Legan SJ, Karsch FJ, Yellon SM. Delayed puberty in lambs chronically treated with oestradiol. J Reprod Fertil. 1986;78:111–117. doi: 10.1530/jrf.0.0780111. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Legan SJ, Ryan KD, Foster DL, Karsch FJ. Two effects of estradiol that normally contribute to the control of tonic LH secretion in the ewe. Biol Reprod. 1980;23:415–422. doi: 10.1095/biolreprod23.2.415. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Legan SJ, Ryan KD, Foster DL, Karsch FJ. Importance of variations in behavioural and feedback actions of oestradiol to the control of seasonal breeding in the ewe. J Endocrinol. 1981;89:229–240. doi: 10.1677/joe.0.0890229. [DOI] [PubMed] [Google Scholar]
- Grino PB, Griffin JE, Wilson JD. Testosterone at high concentrations interacts with the human androgen receptor similarly to dihydrotestosterone. Endocrinology. 1990;126:1165–1172. doi: 10.1210/endo-126-2-1165. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L. An alternate pathway for androgen regulation of brain function: activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5alpha-androstane-3beta,17beta-diol. Horm Behav. 2008;53:741–752. doi: 10.1016/j.yhbeh.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LM, Mytinger A, Roberts EK, Lee TM, Foster DL, Padmanabhan V, Jansen HT. Developmental programming: postnatal steroids complete prenatal steroid actions to differentially organize the GnRH surge mechanism and reproductive behavior in female sheep. Endocrinology. 2013;154:1612–1623. doi: 10.1210/en.2012-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LM, Timmer KM, Foster DL. Sexual differentiation of the external genitalia and the timing of puberty in the presence of an antiandrogen in sheep. Endocrinology. 2008;149:4200–4208. doi: 10.1210/en.2007-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson WN, Patisaul HB, Williams CJ. Reproductive consequences of developmental phytoestrogen exposure. Reproduction. 2012;143:247–260. doi: 10.1530/REP-11-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen ET, Daniels JL, Sturmer T, Robinson WR, Williams CJ, Vejrup K, Magnus P, Longnecker MP. Hormonal contraceptive use before and after conception in relation to preterm birth and small for gestational age: an observational cohort study. BJOG. 2015;122:1349–1361. doi: 10.1111/1471-0528.13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsch FJ, Dahl GE, Evans NP, Manning JM, Mayfield KP, Moenter SM, Foster DL. Seasonal changes in gonadotropin-releasing hormone secretion in the ewe: alteration in response to the negative feedback action of estradiol. Biol Reprod. 1993;49:1377–1383. doi: 10.1095/biolreprod49.6.1377. [DOI] [PubMed] [Google Scholar]
- Malcolm KD, Jackson LM, Bergeon C, Lee TM, Padmanabhan V, Foster DL. Long-term exposure of female sheep to physiologic concentrations of estradiol: effects on the onset and maintenance of reproductive function, pregnancy, and social development in female offspring. Biol Reprod. 2006;75:844–852. doi: 10.1095/biolreprod.106.053264. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown MB, Foster DL, Padmanabhan V. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology. 2004;145:790–798. doi: 10.1210/en.2003-0478. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Steckler TL, Welch KB, Inskeep EK, Padmanabhan V. Fetal programming: prenatal testosterone treatment leads to follicular persistence/luteal defects; partial restoration of ovarian function by cyclic progesterone treatment. Endocrinology. 2006;147:1997–2007. doi: 10.1210/en.2005-1338. [DOI] [PubMed] [Google Scholar]
- Medlock KL, Lyttle CR, Kelepouris N, Newman ED, Sheehan DM. Estradiol down-regulation of the rat uterine estrogen receptor. Proc Soc Exp Biol Med. 1991;196:293–300. doi: 10.3181/00379727-196-43191. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc. 2007;82:591–605. doi: 10.1111/j.1469-185X.2007.00027.x. [DOI] [PubMed] [Google Scholar]
- New MI. Extensive clinical experience: nonclassical 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2006;91:4205–4214. doi: 10.1210/jc.2006-1645. [DOI] [PubMed] [Google Scholar]
- Niswender GD, Reichert LE, Jr, Midgley AR, Jr, Nalbandov AV. Radioimmunoassay for bovine and ovine luteinizing hormone. Endocrinology. 1969;84:1166–1173. doi: 10.1210/endo-84-5-1166. [DOI] [PubMed] [Google Scholar]
- Ozturk M, Smith RF, Dobson H. Effect of prolonged exposure to oestradiol on subsequent LH secretion in ewes. J Reprod Fertil. 1998;114:1–9. doi: 10.1530/jrf.0.1140001. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Evans NP, Dahl GE, McFadden KL, Mauger DT, Karsch FJ. Evidence for short or ultrashort loop negative feedback of gonadotropin-releasing hormone secretion. Neuroendocrinology. 1995;62:248–258. doi: 10.1159/000127011. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, McFadden K, Mauger DT, Karsch FJ, Midgley AR., Jr Neuroendocrine control of follicle-stimulating hormone (FSH) secretion. I. Direct evidence for separate episodic and basal components of FSH secretion. Endocrinology. 1997;138:424–432. doi: 10.1210/endo.138.1.4892. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A. Animal models of the polycystic ovary syndrome phenotype. Steroids. 2013a;78:734–740. doi: 10.1016/j.steroids.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A. Sheep models of polycystic ovary syndrome phenotype. Mol Cell Endocrinol. 2013b;373:8–20. doi: 10.1016/j.mce.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Veiga-Lopez A, Herkimer C, Abi Salloum B, Moeller J, Beckett E, Sreedharan R. Developmental Programming: Prenatal and Postnatal Androgen Antagonist and Insulin Sensitizer Interventions Prevent Advancement of Puberty and Improve LH Surge Dynamics in Prenatal Testosterone-Treated Sheep. Endocrinology. 2015;156:2678–2692. doi: 10.1210/en.2015-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz J, Vrooman L, Ricke WA, Hunt PA, Ehrlich S, Hauser R, Padmanabhan V, Taylor HS, Swan SH, VandeVoort CA, Flaws JA. Bisphenol a and reproductive health: update of experimental and human evidence, 2007-2013. Environ Health Perspect. 2014;122:775–786. doi: 10.1289/ehp.1307728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli D. Non-classic adrenal hyperplasia due to the deficiency of 21-hydroxylase and its relation to polycystic ovarian syndrome. Front Horm Res. 2013;40:158–170. doi: 10.1159/000342179. [DOI] [PubMed] [Google Scholar]
- Puttabyatappa M, Cardoso RC, Padmanabhan V. Effect of maternal PCOS and PCOS-like phenotype on the offspring's health. Mol Cell Endocrinol. 2015 doi: 10.1016/j.mce.2015.11.030. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhind SM, Rae MT, Brooks AN. Effects of nutrition and environmental factors on the fetal programming of the reproductive axis. Reproduction. 2001;122:205–214. doi: 10.1530/rep.0.1220205. [DOI] [PubMed] [Google Scholar]
- Robinson JE, Birch RA, Foster DL, Padmanabhan V. Prenatal exposure of the ovine fetus to androgens sexually differentiates the steroid feedback mechanisms that control gonadotropin releasing hormone secretion and disrupts ovarian cycles. Arch Sex Behav. 2002;31:35–41. doi: 10.1023/a:1014075016956. [DOI] [PubMed] [Google Scholar]
- Rosenfield RL. Clinical review: Identifying children at risk for polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:787–796. doi: 10.1210/jc.2006-2012. [DOI] [PubMed] [Google Scholar]
- Rozell TG, Keisler DH. Effects of oestradiol on LH, FSH and prolactin in ovariectomized ewes. J Reprod Fertil. 1990;88:645–653. doi: 10.1530/jrf.0.0880645. [DOI] [PubMed] [Google Scholar]
- Sarma HN, Manikkam M, Herkimer C, Dell'Orco J, Welch KB, Foster DL, Padmanabhan V. Fetal programming: excess prenatal testosterone reduces postnatal luteinizing hormone, but not follicle-stimulating hormone responsiveness, to estradiol negative feedback in the female. Endocrinology. 2005;146:4281–4291. doi: 10.1210/en.2005-0322. [DOI] [PubMed] [Google Scholar]
- Schug TT, Janesick A, Blumberg B, Heindel JJ. Endocrine disrupting chemicals and disease susceptibility. J Steroid Biochem Mol Biol. 2011;127:204–215. doi: 10.1016/j.jsbmb.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Horm Behav. 2009;55:597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma TP, Herkimer C, West C, Ye W, Birch R, Robinson JE, Foster DL, Padmanabhan V. Fetal programming: prenatal androgen disrupts positive feedback actions of estradiol but does not affect timing of puberty in female sheep. Biol Reprod. 2002;66:924–933. doi: 10.1095/biolreprod66.4.924. [DOI] [PubMed] [Google Scholar]
- Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Perez-Bravo F, Recabarren SE. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod. 2002;17:2573–2579. doi: 10.1093/humrep/17.10.2573. [DOI] [PubMed] [Google Scholar]
- Slikker W, Jr, Hill DE, Young JF. Comparison of the transplacental pharmacokinetics of 17 beta-estradiol and diethylstilbestrol in the subhuman primate. J Pharmacol Exp Ther. 1982;221:173–182. [PubMed] [Google Scholar]
- Smith P, Steckler TL, Veiga-Lopez A, Padmanabhan V. Developmental programming: differential effects of prenatal testosterone and dihydrotestosterone on follicular recruitment, depletion of follicular reserve, and ovarian morphology in sheep. Biol Reprod. 2009;80:726–736. doi: 10.1095/biolreprod.108.072801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckler TL, Herkimer C, Dumesic DA, Padmanabhan V. Developmental programming: excess weight gain amplifies the effects of prenatal testosterone excess on reproductive cyclicity—implication for polycystic ovary syndrome. Endocrinology. 2009;150:1456–1465. doi: 10.1210/en.2008-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckler TL, Lee JS, Ye W, Inskeep EK, Padmanabhan V. Developmental programming: exogenous gonadotropin treatment rescues ovulatory function but does not completely normalize ovarian function in sheep treated prenatally with testosterone. Biol Reprod. 2008;79:686–695. doi: 10.1095/biolreprod.108.068643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WY, Ho SM. Epigenetic reprogramming and imprinting in origins of disease. Rev Endocr Metab Disord. 2007;8:173–182. doi: 10.1007/s11154-007-9042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang WY, Newbold R, Mardilovich K, Jefferson W, Cheng RY, Medvedovic M, Ho SM. Persistent hypomethylation in the promoter of nucleosomal binding protein 1 (Nsbp1) correlates with overexpression of Nsbp1 in mouse uteri neonatally exposed to diethylstilbestrol or genistein. Endocrinology. 2008;149:5922–5931. doi: 10.1210/en.2008-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth WP, Taylor JA, Robinson JE. Prenatal programming of reproductive neuroendocrine function: the effect of prenatal androgens on the development of estrogen positive feedback and ovarian cycles in the ewe. Biol Reprod. 2005;72:619–627. doi: 10.1095/biolreprod.104.035691. [DOI] [PubMed] [Google Scholar]
- Vaiserman A. Early-life Exposure to Endocrine Disrupting Chemicals and Later-life Health Outcomes: An Epigenetic Bridge? Aging Dis. 2014;5:419–429. doi: 10.14336/AD.2014.0500419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Lopez A, Astapova OI, Aizenberg EF, Lee JS, Padmanabhan V. Developmental programming: contribution of prenatal androgen and estrogen to estradiol feedback systems and periovulatory hormonal dynamics in sheep. Biol Reprod. 2009;80:718–725. doi: 10.1095/biolreprod.108.074781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Lopez A, Wurst AK, Steckler TL, Ye W, Padmanabhan V. Developmental programming: postnatal estradiol amplifies ovarian follicular defects induced by fetal exposure to excess testosterone and dihydrotestosterone in sheep. Reprod Sci. 2014;21:444–455. doi: 10.1177/1933719113503412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Lopez A, Ye W, Phillips DJ, Herkimer C, Knight PG, Padmanabhan V. Developmental programming: deficits in reproductive hormone dynamics and ovulatory outcomes in prenatal, testosterone-treated sheep. Biol Reprod. 2008;78:636–647. doi: 10.1095/biolreprod.107.065904. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Johnson ML. Cluster analysis: a simple, versatile, and robust algorithm for endocrine pulse detection. Am J Physiol. 1986;250:E486–493. doi: 10.1152/ajpendo.1986.250.4.E486. [DOI] [PubMed] [Google Scholar]
- Wood RI, Ebling FJ, I'Anson H, Bucholtz DC, Yellon SM, Foster DL. Prenatal androgens time neuroendocrine sexual maturation. Endocrinology. 1991;128:2457–2468. doi: 10.1210/endo-128-5-2457. [DOI] [PubMed] [Google Scholar]
- Wood RI, Foster DL. Sexual differentiation of reproductive neuroendocrine function in sheep. Rev Reprod. 1998;3:130–140. doi: 10.1530/ror.0.0030130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Circulating pattern of LH from two representative animals from each of the prenatal and postnatal groups during the E-negative feedback test. Arrowheads indicate pulses.
Supplemental Figure 2: Circulating patterns of LH (orange lines) and FSH (blue lines) from three representative animals from each of the prenatal and postnatal groups during the E-positive feedback test.
Supplemental Figure 3: Circulating patterns of LH (orange lines), FSH (blue lines), and E (pink shaded area) from two representative animals from each of the prenatal and postnatal groups following estrous synchronization with PGF2α injections from the first breeding season. Progesterone profiles are depicted in the inset.
Supplemental Figure 4: Circulating patterns of LH (orange lines), FSH (blue lines), and E (pink shaded area) from two representative animals from each of the prenatal and postnatal groups following estrous synchronization with PGF2α injections from the second breeding season. Progesterone profiles are depicted in the inset