Abstract
Increasing evidence suggests that certain types of cancers are more common in people with diabetes mellitus (DM). This study aimed to investigate the risk of skin cancer in patients with DM in Taiwan. In this retrospective cohort study using data from the Taiwan Longitudinal Health Insurance Research Database, the risk of developing overall skin cancer, including nonmelanoma skin cancer (NMSC) and melanoma, was compared by Poisson regression analysis and Cox regression analysis between the DM and non-DM cohorts. The DM cohort with newly diagnosed DM (n = 41,898) and a non-DM cohort were one-to-one matched by age, sex, index date, and comorbidities (coronary artery disease, hyperlipidemia, hypertension, chronic kidney disease, chronic obstructive pulmonary disease, and obesity). Compared with non-DM cohort statistically, for the people with DM aged ≥60 years, the incidence rates of overall skin cancer and NMSC were significantly higher (overall: DM/non-DM: number [n] = 99/76, incidence rate ratio [IRR] = 1.44, P = 0.02; NMSC: DM/non-DM: n = 94/66, IRR = 1.57, P = 0.005). By Cox regression analysis, the risk of developing overall skin cancer or NMSC was significantly higher after adjusting for sex, comorbidities, and overall diseases with immunosuppression status (overall: adjusted hazard ratio [AHR] = 1.46, P = 0.01; NMSC: AHR = 1.6, P = 0.003). Other significant risk factors were older males for skin cancer (overall: AHR = 1.68, P = 0.001; NMSC: AHR = 1.59, P = 0.004; melanoma: AHR = 3.25, P = 0.04), chronic obstructive pulmonary disease for NMSC (AHR = 1.44, P = 0.04), and coronary artery disease for melanoma (AHR = 4.22, P = 0.01). The risk of developing melanoma was lower in the DM cohort than in the non-DM cohort, but without significance (AHR = 0.56, P = 0.28; DM/non-DM: n = 5/10). The incidence rate and risk of developing overall skin cancer, including NMSC, was significantly higher in older adults with DM. Other significant risk factors for older adults with DM were males for NMSC and melanoma, chronic obstructive pulmonary disease for NMSC, and coronary artery disease for melanoma.
Keywords: diabetes mellitus, melanoma, National Health Insurance Research Database (NHIRD), nationwide retrospective cohort study, nonmelanoma skin cancer, risk, skin cancer
1. Introduction
The increased risk of certain types of cancer in people with diabetes mellitus (DM) has been reported, including cancer of the liver, biliary tract, pancreas, stomach, colorectum, kidney, bladder, breast, and endometrium, but conversely a decreased risk of prostate cancer.[1,2] Continuous hyperglycemia and high serum levels of insulin or insulin-like growth factor (IGF) have been proposed to be possible mechanisms for the carcinogenesis in DM patients. Persistent hyperglycemia may contribute to malignant cells growth, and the overproduction of superoxide and reactive oxygen species.[3] IGF modulates the epidermal cells proliferation. High serum levels of insulin and IGFs in DM patients have been shown to increase cellular proliferation and activation of the oncogenic epidermal growth factor receptors, resulting in mitogenic and antiapoptotic effects and inducing malignant cell transformation.[3–6]
The major categories of skin cancer include melanoma and nonmelanoma skin cancer (NMSC) (squamous cell carcinoma, basal cell carcinoma, and malignant neoplasm of sebaceous glands and sweat glands). These were among the top 10 most common types of cancers in Taiwan.[7–9] However, the risk of skin cancer in patients with DM has seldom been explored. Epidemiological studies in other countries have demonstrated inconsistent associations between type 2 DM and malignant melanoma or NMSC,[10–20] with only a few reports about the association of NMSC and DM.[13,15–17]
The aim of this study was to investigate the risk of overall skin cancers, NMSC, and melanoma among a DM cohort and non-DM cohort with no history of skin cancer after controlling for confounding factors, including age, sex, comorbidities, and overall diseases with immunosuppression status using data from National Health Insurance Research Database (NHIRD).
2. Methods
2.1. Data sources
The National Health Insurance (NHI) program in Taiwan is a compulsory, single-payer, tax-financed healthcare system that was launched in 1995 and provides health care to 99% of the country's 23.75 million people.[21] It comprises information of demographic data, dates of clinical visits, and diagnostic codes from the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9 CM codes) of the beneficiaries, and it has contractual agreements with 97% of the hospitals and clinics in Taiwan. This database has been the source of many epidemiological studies published in peer-reviewed journals.[7,9,22–24] In this study, the Longitudinal Health Insurance Database 2000 (LHID2000) was used, which contains all of the original claims data of one million individuals randomly sampled from the registry for beneficiaries of the NHIRD from 1996 to 2000. No significant differences in the age and sex distribution have been shown between the patients in the LHID2000 and the original NHIRD. The randomly sampled one million beneficiaries were enrolled from 2000 and followed up until the end of 2012. The NHIRD contains de-identified data to ensure patient anonymity. The Institutional Review Board of Kaohsiung Veterans General Hospital approved this study.
2.2. Design
The ICD-9-CM codes were used to define diagnosis of diseases, comorbidities, and overall diseases with immunosuppression status in this study, as shown in Table 1. This retrospective population-based cohort study was conducted with 2 study groups: a DM cohort and a comparison cohort without DM matched at a 1-to-1 ratio. The flowchart of patient selection of the study cohorts was presented in Fig. 1.
Table 1.
ICD-9-CM codes used for diseases diagnosis and ATC codes for immunosuppressant in the this study.
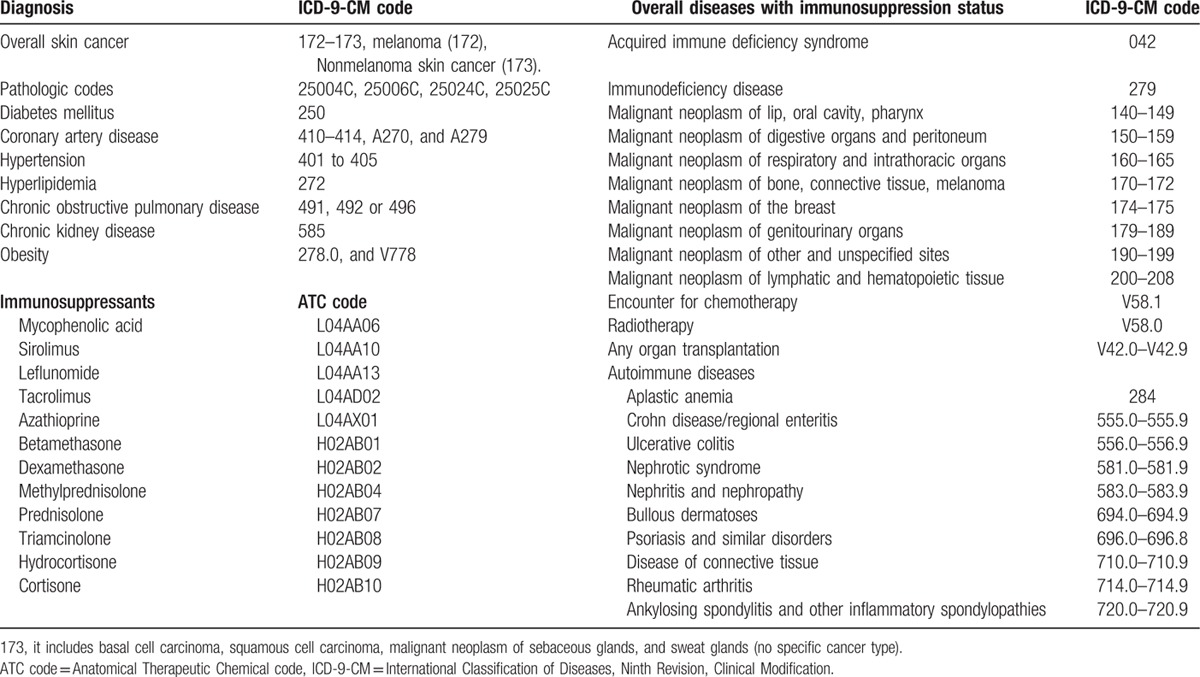
Figure 1.
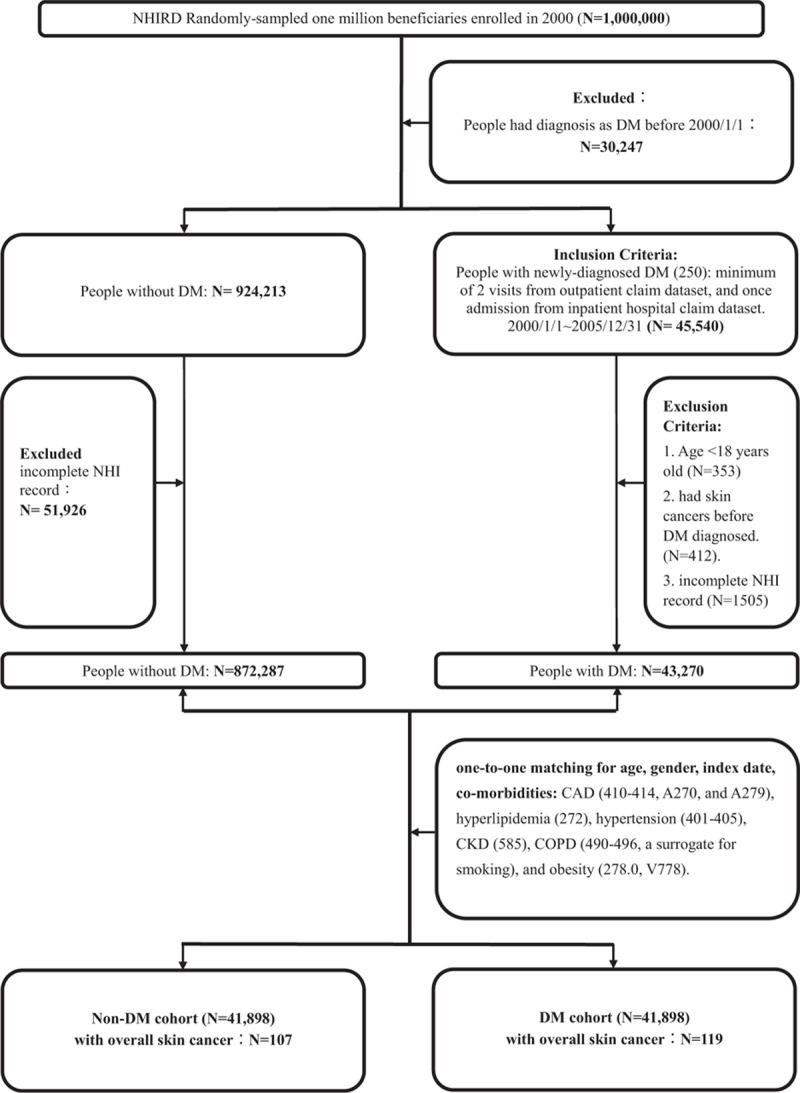
Study design and flowchart of patient selection of the study cohorts. CAD = coronary artery disease, CKD = chronic kidney disease, COPD = chronic obstructive pulmonary disease, DM = diabetes mellitus, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database.
The DM cohort consisted of adults patients (age ≥18 years) with a first-time diagnosis of DM (ICD-9-CM code 250.0–250.9) from 2000 to 2005. The patients who had a diagnosis of diabetes before the year 2000 were excluded (n = 30,247). The newly diagnosed DM was based on a minimum of 2 visits from outpatient claim dataset or once admission from inpatient hospital claim dataset. The index date for the patients in the DM cohort was the date of their first-time diagnosis of DM. The patients with newly diagnosed skin cancer presented after the index date of DM were then identified (overall skin cancers [172–173], including melanoma [172], and NMSC [173]). (ICD-9-CM code 173: it includes basal cell carcinoma [BCC], squamous cell carcinoma [SCC], malignant neoplasm of sebaceous glands and sweat glands, but skin cancer type is not specified in 173.) Information that identified patients with skin cancer was based on a minimum of 2 outpatient visits and once admission along with pathologic codes (25004C, 25006C, 25024C, or 25025C) at those visits, and registry in the Taiwan Catastrophic Illness Patient Database. The registry of the Catastrophic Illness Patient Database may not contain all of the patients with skin cancer because some with small lesions would have been cured after wide excision.
In all, 41,898 patients were identified as newly diagnosed DM from 2000 to 2005 after excluding those aged <18 years (n = 353), those with a diagnosis of skin cancer before the index date of DM patients (n = 41), and those who had incomplete NHI information (n = 1505). The comparison non-DM cohort was 1-to-1 matched with the DM cohort by age, sex, index date, and comorbidities with a minimum 2 visits.[23] The comorbidities included coronary artery disease (CAD) (410–414, A270, and A279), hyperlipidemia (272), hypertension (401–405), chronic kidney disease (CKD) (585), chronic obstructive pulmonary disease (COPD) (490–496, a surrogate for smoking), and obesity (278.0 and V778). The immunosuppression status is also an important risk factor of skin cancer. The ICD-9 codes of major categories of diseases with immunosuppression status and Anatomical Therapeutic Chemical (ATC) codes of immunosuppressants (ATC code L04A: immunosuppressant and H02AB: systemic glucocorticoids) are shown in Table 1. The overall diseases with immunosuppression status was based on a minimum of 3 visits from outpatient claim dataset or once admission from inpatient hospital claim dataset for those diseases in Table 1, using immunosuppressive therapy at 3 visits for the autoimmune diseases or any organ transplantation, malignant neoplasm under 3 times chemotherapy or radiation therapy, acquired immunodeficiency syndrome, and immunodeficiency in 1 year duration before the index date of DM in this study (in DM/non-DM cohort: n = 252/214).
The Taiwan NHI Bureau regularly reviews the diagnoses in the NHIRD to maintain consistency at every referral teaching hospital and medical center. This study is retrospective because all the data had been collected before this study was conducted; however, this study would predict future outcomes. To determine the incidence of skin caner, each patient was assessed from the index date until the end of follow-up on December 31, 2012, or until the patient was censored because of death.
2.3. Statistical analysis
Descriptive statistical analyses using Pearson chi-square tests were performed to compare the differences of the incidence of skin cancer between the DM and non-DM cohorts. The distribution of overall diseases with immunosuppression status in DM and non-DM cohorts was also compared by Pearson chi-square tests. The incidence rate (IR) was calculated as the incident number of skin cancer identified during follow-up, divided by the total person-years for each cohort, and stratified analysis by NMSC, melanoma, and subgroups of sex and age. The incidence rate ratio (IRR) of skin cancer between 2 cohorts was compared using Poisson regression analysis. The risk of developing skin cancer (the hazard of overall skin cancer, NMSC, or melanoma) in the 2 cohorts in age subgroups were calculated using Cox proportional-hazards regression univariate analysis, and adjustment for comorbidities and overall diseases with immunosuppression status was calculated by Cox regression multivariate analysis. Differences between the survival curves of cumulative incidences in the 2 cohorts were determined by using Kaplan–Meier survival analysis and the log-rank test.
Data extraction from the LHID2000 was performed by SAS version 9.4 (SAS Institute Inc., Cary, NC). IBM SPSS statistical software version 20 (IBM Institute Inc, NY) was used for all analyses. Statistics significance was set at a 2-tailed P value of less than 0.05.
3. Results
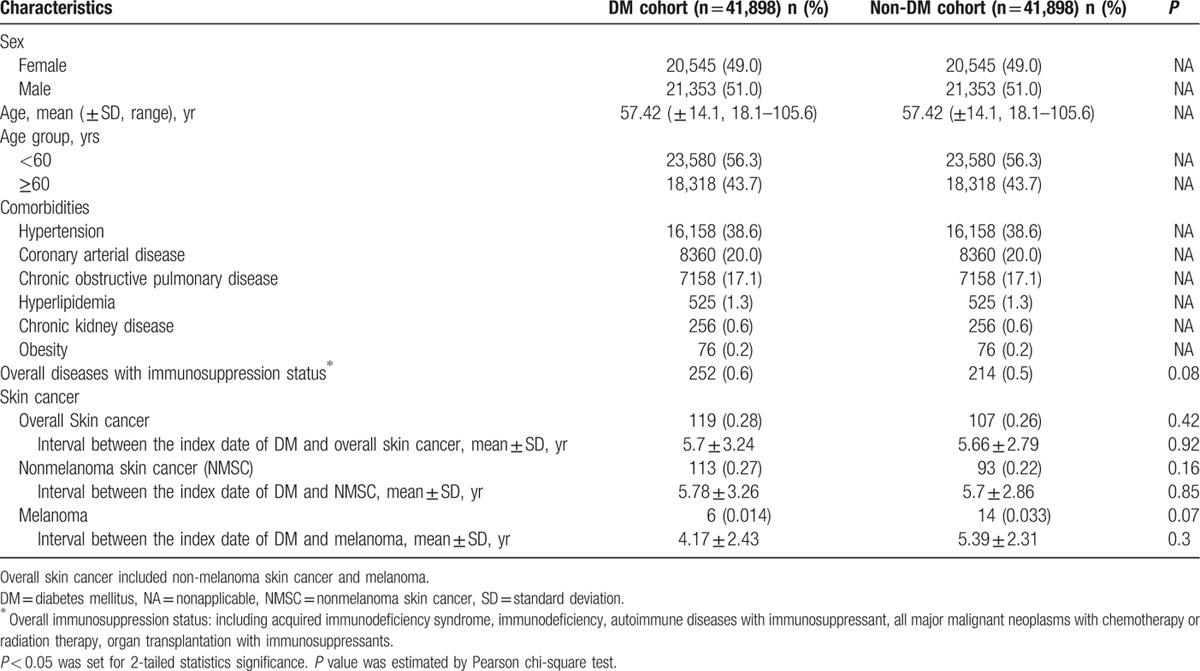
The DM and non-DM cohorts had comparable demographic characteristics and comorbidities as these factors were matched in the study design (Table 2). The number of overall skin cancer was 119 in the DM cohort and 107 in the non-DM cohort from 2000 to 2005. The mean age (±SD, range) in the 2 cohorts at the diagnosis of skin cancer was 57.41 (±14.1, 18.1–105.6) years, with a male-to-female ratio of 1.04:1. The mean intervals between the index date and the occurrence of skin cancer were 5.7 ± 3.24 years for the DM cohort and 5.66 ± 2.79 years for the non-DM cohort. There were no statistically significant differences in the mean interval from the index date to the occurrence of overall skin cancer, NMSC, and melanoma between the 2 cohorts, respectively.
Table 2.
Demographic characteristics, comorbidities, and skin cancer of the DM and non-DM cohorts.
The IRRs of overall skin cancer, NMSC, and melanoma between the DM and non-DM cohorts, and stratified analysis by the subgroup of age and sex are presented in Table 3. By the end of the follow-up, the IR of overall skin cancer was 3.2 per 10,000 person-years in the DM cohort, which was 1.18 times higher than in the non-DM cohort (IRR = 1.18, P = 0.22). The IR of NMSC was 3.04 per 10,000 person-years in the DM cohort, which was 1.29 times higher than in the non-DM cohort with borderline significance. (IRR = 1.29, P = 0.07). The IR of melanoma was 0.16 per 10,000 person-years in the DM cohort, which was 0.46 times lower than in the non-DM cohort, but without significance (IRR = 0.46, P = 0.11).
Table 3.
The incidence rate ratios of skin cancer in the DM and non-DM cohorts by age and sex subgroups.
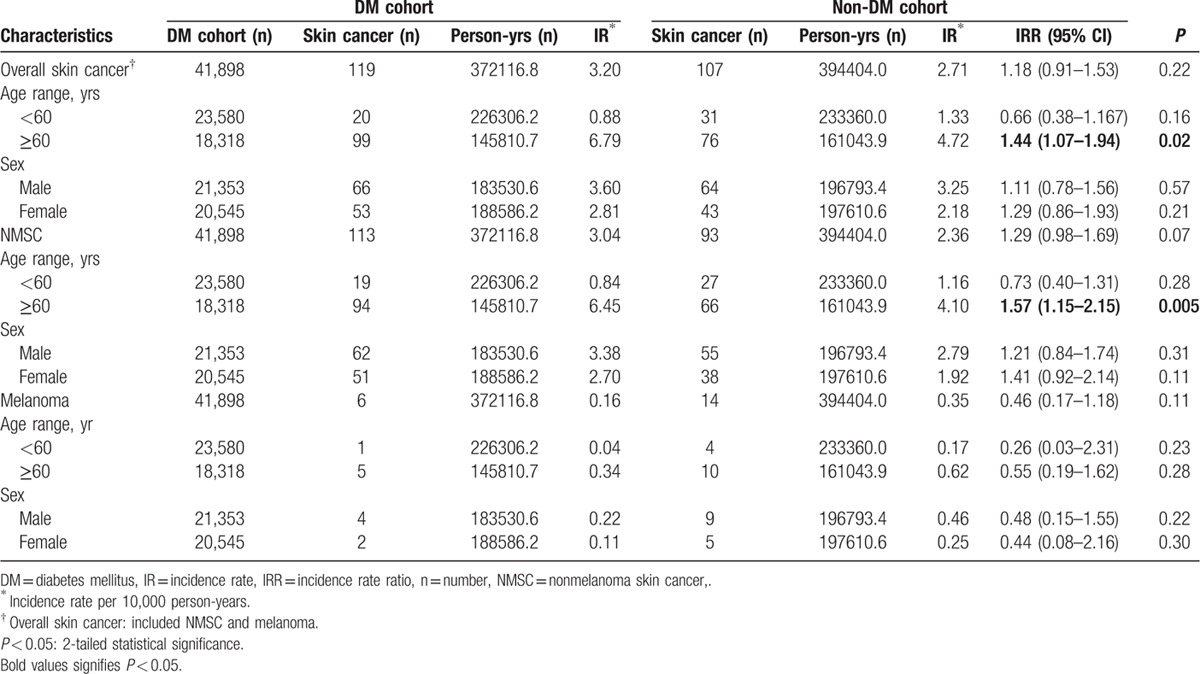
In the statistical analysis stratified by age or sex subgroup, those aged ≥60 years in the DM cohort had a significantly higher IR of overall skin cancers than the non-DM cohort. (IRR = 1.44, 95% confidence interval [CI] 1.07–1.94, P = 0.02; DM/non-DM: n = 99/76). The IR of NMSC was 1.57 times higher in the DM cohort than that in the non-DM cohort, which was statistically significant. (IRR = 1.57, 95% CI 1.15–2.15, P = 0.005; DM/non-DM: n = 94/66). For those aged <60 years, the IRs of overall skin cancer and NMSC were not significantly different between the 2 cohorts, respectively.
In age subgroup analysis, the IRRs of melanoma were not significantly different between the 2 cohorts. In sex subgroup analysis, IRRs of overall skin cancers, NMSC, or melanoma were also not significantly different between the 2 cohorts, respectively.
To investigate the potential risk factors for skin cancer in the age subgroups, Cox proportional-hazard regression analysis was performed, including DM status, sex, comorbidities, and overall diseases with immunosuppression status (Table 4). Kaplan–Meier analysis was also performed for cumulative incidence of skin cancer (Figs. 2 and 3).
Table 4.
The crude and adjusted hazard ratios for skin cancer in the DM and non-DM cohorts by age subgroup and adjustment for sex and comorbidities.
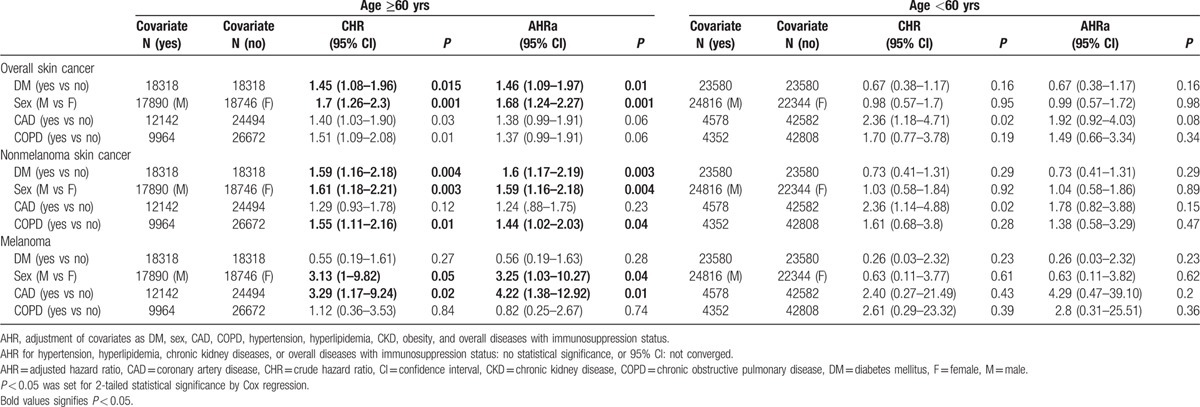
Figure 2.
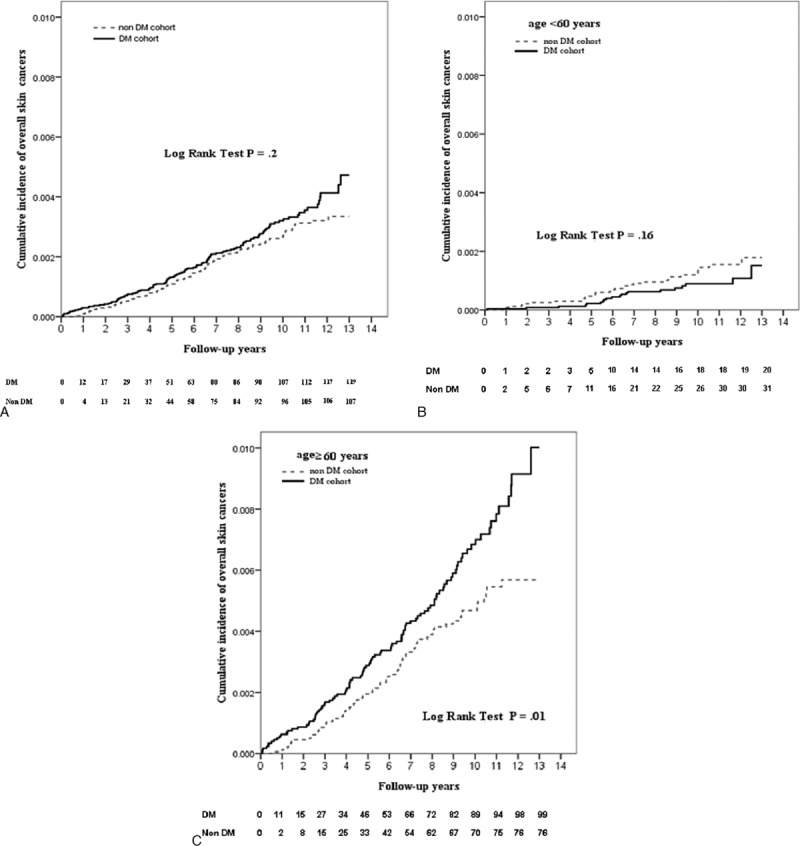
Kaplan–Meier survival curves of overall skin cancer after index date of DM. A, Comparison of cumulative incidence of overall skin cancer in all patients with and without DM. B, In subgroup of age <60 years, comparison of cumulative incidence of overall skin cancer of patients with and without DM. C, In subgroup of age ≥60 years, comparison of cumulative incidence of overall skin cancer in patients with and without DM. Each patient was assessed from the index date until the end of the follow-up on December 31, 2012, or until the patient was censored because of death.
Figure 3.

Kaplan–Meier survival curve of nonmelanoma skin cancer after index date of DM. A, Comparison of cumulative incidence of nonmelanoma skin cancer in all patients with and without DM. B, In subgroup of age <60 years, comparison of cumulative incidence of nonmelanoma skin cancers in patients with and without DM. C, In subgroup of age ≥60 years, comparison of cumulative incidence of nonmelanoma skin cancer in patients with and without DM. Each patient was assessed from the index date until the end of the follow-up on December 31, 2012, or until the patient was censored because of death.
In the subgroup of those aged ≥60 years, the risk of developing overall skin cancer was 1.46 times higher in the DM cohort than in the non-DM cohort after adjusting for sex, comorbidities, and overall diseases with immunosuppression status. (Adjusted hazard ratio [AHR] 1.46, 95% CI 1.09–1.97, P = 0.01) The DM cohort had a 60% increase of NMSC than in the non-DM cohort after adjusting for sex, comorbidities, and overall diseases with immunosuppression status. (AHR 1.6, 95% CI 1.17–2.19, P = 0.003) during follow-up period, and the difference was statistically significant. The hazard of melanoma in the DM cohort was 0.56 times lower in the DM cohort than in the non-DM cohort, but this difference did not reach significance, even after adjusting for sex, comorbidities, and overall diseases with immunosuppression status (AHR 0.56, 95% CI 0.19–1.63, P = 0.28). In the subgroup of age <60 years, there were no significant differences in any of these factors.
Other significant risks factors for developing skin cancer included sex, COPD, and CAD. The risk of developing overall skin cancer, NMSC, and melanoma was significantly higher in the older male patients than in the older female patients, respectively, after adjustment for DM, comorbidities, and overall diseases with immunosuppression status (in those age ≥60 years: overall: AHR 1.68, 95% CI 1.24–2.27, P = 0.001; NMSC: AHR 1.59, 95% CI 1.16–2.18, P = 0.004; melanoma: AHR 3.25, 95% CI 1.03–10.27, P = 0.04). In addition, the risk of developing NMSC was significantly higher in patients with COPD than in those without COPD (age ≥60 years: AHR 1.44, 95% CI 1.02–2.03, P = 0.04). Furthermore, the risk of developing melanoma was significantly higher in the patients with CAD than those without CAD (age ≥60 years: AHR 4.22, 95% CI 1.38–12.92, P = 0.01).
However, there were no statistically significant differences in the distribution of overall diseases with immunosuppression status between DM and non-DM cohorts (Table 2), even in age subgroups. In addition, in age subgroups, the risk of developing overall skin cancer and NMSC was not significantly higher in patients with overall diseases with immunosuppression status than in those without immunosuppression status, even after adjustments (data not shown).
Kaplan–Meier analysis showed that there was statistically significant difference between 2 curves of the cumulative incidence of overall skin cancer or NMSC in 2 cohorts in those aged ≥60 years (log-rank test: overall: P = 0.01; NMSC: P = 0.004) (Figs. 2C and 3C). DM cohort had a lower cumulative IR of melanoma compared with the non-DM cohort in those aged ≥60 years, but the difference did not reach statistical significance (log-rank test P = 0.27).
4. Discussion
In this study, compared with the non-DM cohort, in the subgroup of those aged ≥60 years, the IRRs of overall skin cancers or NMSC were significantly higher in the DM cohort. By Cox regression analysis, the risk of developing overall skin cancer or NMSC was significantly higher in the DM cohort after adjusting for sex, comorbidities, and overall diseases with immunosuppression status. Other significant risk factors for older adults with DM were males for skin cancer (overall, NMSC, and melanoma), COPD for NMSC, and CAD for melanoma. The IRRs or risk of melanoma were lower in the DM cohort in both age and sex subgroups or after adjustments, although without significance, which could be related to the small number.
In this study, the average incidence of DM in each year from age 18 to 105 was 0.7% from 2000 to 2005, which is similar to the study by Jiang et al.[22] In Taiwan, skin cancer has been among the 10 leading cancers in males or females since 2000.[7–9] The IR in this study is similar to the age-standardized IR of NMSC in the Taiwan cancer registry from 2000 to 2006 (7.3: males 7.81, females 6.78) per 100,000 people and those of melanoma (0.67: males 0.70, females 0.63) per 100,000 people.[9] The male-to-female ratio in this cohort was 1.04:1, which is similar to the Taiwan cancer registry database (male:female = 1.1:1).[8] In USA, the IR of melanoma was exceeding 22 cases over 100,000 people.[25,26]
According to the consensus report by the American Diabetes Association,[1] the relationship between diabetes and cancer was discussed with regard to 4 major aspects. First was the association between diabetes and the incidence of cancer.[1] Previous studies have reported inconsistent associations between DM and malignant melanoma or NMSC, with some articles reporting a higher,[10–14] and others a lower IRR[15,17,19] of melanoma in patients with DM than reference populations with or without significance. Hemminki et al[13] reported that the incidence ratios of cutaneous squamous cell carcinoma was higher in patients with type 2 DM. However, other studies have reported a significantly lower rate ratio of NMSC in people with DM.[15,16] Differences in ethnicity or environmental factors may be related to these discrepancies.
Second was risk factors for both diabetes and cancer.[1] Shared common risks factors, diabetes itself, or metabolic dysfunction could all increase the risk for some types of cancer. However, it is difficult to evaluate these factors because of inter-relationship.[1]
Some studies have reported that the lifestyle risk factors for the development of DM,[27,28] such as obesity and occupational physical inactivity, were also related to an increased risk of melanoma.[29,30] Chronic exposure to sun or ultraviolet ray is also an important risk factor for skin cancer.[31–33] In addition, occupations that were male predominant and involved chronic exposure to the sun, such as farm workers, fisherman, and construction laborers, have also been reported to carry a higher risk for skin cancer.[34–37] Other possibly associated risk factors for skin cancer include lifestyle, physical activity, and environmental factors.
The possible mechanism of the association between NMSC and COPD may be environmental exposure to chemicals, such as polycyclic aromatic hydrocarbons (PAH). Tobacco smoke and air pollution are also major risk factors for COPD patients, both of which contain PAH that are potential human carcinogens.[38] Occupational or environmental exposure of PAH has also been reported to increase the risk for squamous cell carcinoma of the skin.[39,40]
The possible mechanism of the association of melanoma and CAD may be insulin resistance. Insulin resistance is an additional risk factor for the pathogenesis of cardiovascular disease in patients with type 2 DM,[41] and it has been proposed to be a potentially independent risk factor for melanoma.[42] Further studies are needed to elucidate these hypotheses.
Immunosuppression status is a potential risk factor for skin cancer. The associated diseases mentioned in the literatures and previous studies were organ transplantation with long-term immunosuppressive therapy (corticosteroids, azathioprine, and cyclosporine),[43,44] leukemia and lymphoma,[45,46] and acquired immunodeficiency syndrome.[47,48] The autoimmune diseases with immunosuppressive therapy, such as rheumatoid arthritis, ulcerative colitis, or inflammatory bowel disease, were also reported to be associated with skin cancer.[49–51] In this study, the immunosuppression status was not present to be a risk factor for overall skin cancer, NMSC, and melanoma. The probable causes were the complexity of the diseases with immunosuppression status, the low incidence of those diseases, and the extremely limited number of skin cancer in the diseases with immunosuppression status. Therefore, it was hard to obtain statistically significant results.
Third was possible physiological links between diabetes and the risk for cancer.[1] In vitro studies have shown that human keratinocytes irradiated with ultraviolet B light undergo premature stress-induced senescence or apoptosis,[32,52] and that this response to ultraviolet B irradiation is dependent on the functional activation of the IGF receptor.[53] The IGF system is an integral part of growth regulation by the body, and abnormalities at all levels of the IGF system have been implicated in carcinogenesis and cellular transformation.[54–56] The regulation between the IGF system and melanoma cells have been investigated in several articles. Abnormality in insulin binding and receptor phosphorylation were found in an insulin-resistant melanoma cell line.[57] The resistance to apoptosis in melanoma cells has been shown to be promoted by IGF1 through an increased expression of antiapoptosis proteins (BCL2, BCL-X(L), and Survivin).[58] The long-term effect of hyperinsulinemia was another possible mechanism of the cancer development in patients with DM.[59] Hyperglycemia may increase the production of reactive oxygen species,[60] decrease the expression of antioxidants,[61] lead to DNA damage,[62] and promote cancer cells proliferation.[63]
Fourth was the influences of diabetes treatment on the risk for cancer.[1] The medication of DM may have a role on the risk for skin cancer. Using insulin or rosiglitazone had been reported to possibly reduce the incidence of NMSC in DM patients.[24,64] Due to the complexity of multidrug regimens and the effect of the duration and cumulative dosage of diabetes medication, the effect of diabetes treatment was not considered in this study.
There are still many unanswered questions with regards to these 4 aspects, and why the different direction of IRRs of NMSC and melanoma between the DM and non-DM cohorts in this study is unclear. Further studies were warranted to clarify this issue.
The major strengths of the present study are the large study population from the Taiwan's NHIRD, the chronological relationship from the cohort study, the method of 1:1 matching by age, sex, and comorbidities for the patients in both cohorts, and analysis by subgroup stratification and confounders adjustment. This increases the statistical power for the evaluation of the risk in the analyses. Nevertheless, there were some limitations to this study. Even though the study population included one million people, and searching for more skin cancer over a 6-year period, the total number of skin cancer in the DM cohort, and especially those with melanoma, was still low. In addition, several factors were not available from the insurance claims data, including body mass index, details of smoking, and alcohol consumption,[65] laboratory data, occupation, and physical activity. For example, data on several suspected risk factors for skin cancer, such as chronic exposure to sunlight or ultraviolet ray, occupation, and obesity were lacking. Furthermore, the misclassification may exist because a certain proportion of patients with DM, skin cancer, or obesity may have been undiagnosed. The factor of DM treatment should try to be included in the future studies. However, the lack of those factors might have resulted in some degree of bias. Generalizability of this study may be limited due to above reasons, environmental factors, or ethnicity.
5. Conclusions
The present study indicated that there were a significantly higher incidence and risk of developing overall skin cancer, including NMSC, in older adults with DM. The risk of melanoma was lower in the DM cohort, but without significance. Other significant risk factors for older adults with DM included males for NMSC and melanoma, CAD for melanoma, and COPD for NMSC, and they should be investigated further in the future studies.
Acknowledgments
The authors are grateful for the use of the National Health Insurance Research Database provided by the Statistic Center of the Department of Health and Welfare. The authors wish to thank the staffs in the Research Center of Medical Informatics, Kaohsiung Veterans General Hospital, for data extraction and management; Dr Ching-Chih Lee, an ENT physician of our hospital and a PhD at the Division of Epidemiology, Institute of Public Health, National Yang-Ming University; Dr Chung Chang, Assistant Professor at Division of Statistics, Department of Applied Mathematics, National Sun Yat-Sen University; and Dr Hsing Chao, Associate Professor of School of Public Health, Taipei Medical University, for statistical consultation.
Footnotes
Abbreviations: AHR = adjusted hazard ratio, ATC code = Anatomical Therapeutic Chemical code, CAD = coronary heart disease, CHR = crude hazard ratio, CI = confidence interval, CKD = chronic kidney disease, COPD = chronic obstructive pulmonary disease, DM = diabetes mellitus, ICD-9 CM = International Classification of Diseases, Ninth Revision, Clinical Modification, IGF = insulin-like growth factor, IR = incidence rate, IRR = incidence rate ratio, LHID = Longitudinal Health Insurance Database, n = number, NA = nonapplicable, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, NMSC = nonmelanoma skin cancer, PAH = polycyclic aromatic hydrocarbon, SD = standard deviation.
Author contributions: Study concept and design—H-CL, H-WT); acquisition of data—P-L T; analysis and interpretation of data and statistical analysis—H-WT; drafting of the manuscript—H-WT; critical revision of the manuscript for important intellectual content—K-WT, W-CH, Y-LS; study supervision—H-CL, Y-LS.
Funding: This study was supported by grants from Kaohsiung Veterans General Hospital (VGHKS15-EM4-01, VGHKS103-009, VGHKS104-020) and the Taiwan Health Promotion Administration.
The authors have no conflicts of interest to disclose.
References
- 1.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care 2010; 33:1674–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sasazuki S, Charvat H, Hara A, et al. Diabetes mellitus and cancer risk: pooled analysis of eight cohort studies in Japan. Cancer Sci 2013; 104:1499–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001; 414:813–820. [DOI] [PubMed] [Google Scholar]
- 4.Adams TE, McKern NM, Ward CW. Signalling by the type 1 insulin-like growth factor receptor: interplay with the epidermal growth factor receptor. Growth Factors 2004; 22:89–95. [DOI] [PubMed] [Google Scholar]
- 5.Valentinis B, Baserga R. IGF-I receptor signalling in transformation and differentiation. Mol Pathol 2001; 54:133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Renehan AG, Zwahlen M, Minder C, et al. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet 2004; 363:1346–1353. [DOI] [PubMed] [Google Scholar]
- 7.Chen CJ, You SL, Lin LH, et al. Cancer epidemiology and control in Taiwan: a brief review. Jpn J Clin Oncol 2002; 32 (suppl):S66–81. [DOI] [PubMed] [Google Scholar]
- 8.Taiwan Cancer Registry. Taiwan Cancer Registry Annual Report. Available at: http://tcr.cph.ntu.edu.tw/ (accessed August 23, 2015). [Google Scholar]
- 9.Chiang CJ, Chen YC, Chen CJ, et al. Taiwan Cancer Registry Task Force. Cancer trends in Taiwan. Jpn J Clin Oncol 2010; 40:897–904. [DOI] [PubMed] [Google Scholar]
- 10.Ragozzino M, Melton LJ, 3rd, Chu CP, et al. Subsequent cancer risk in the incidence cohort of Rochester, Minnesota, residents with diabetes mellitus. J Chronic Dis 1982; 35:13–19. [DOI] [PubMed] [Google Scholar]
- 11.Stattin P, Bjor O, Ferrari P, et al. Prospective study of hyperglycemia and cancer risk. Diabetes Care 2007; 30:561–567. [DOI] [PubMed] [Google Scholar]
- 12.Ulcickas Yood M, Oliveria S, Campbell U, et al. Incidence of cancer in a population-based cohort of patients with type 2 diabetes. Diabetes AND Metabolic Syndrome. Clin Res Rev 2009; 3:12–16. [Google Scholar]
- 13.Hemminki K, Li X, Sundquist J, et al. Risk of cancer following hospitalization for type 2 diabetes. Oncologist 2010; 15:548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atchison EA, Gridley G, Carreon JD, et al. Risk of cancer in a large cohort of U.S. veterans with diabetes. Int J Cancer 2011; 128:635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wotton CJ, Yeates DG, Goldacre MJ. Cancer in patients admitted to hospital with diabetes mellitus aged 30 years and over: record linkage studies. Diabetologia 2011; 54:527–534. [DOI] [PubMed] [Google Scholar]
- 16.Wideroff L, Gridley G, Mellemkjaer L, et al. Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst 1997; 89:1360–1365. [DOI] [PubMed] [Google Scholar]
- 17.Adami HO, McLaughlin J, Ekbom A, et al. Cancer risk in patients with diabetes mellitus. Cancer Causes Control 1991; 2:307–314. [DOI] [PubMed] [Google Scholar]
- 18.Rousseau MC, Parent ME, Pollak MN, et al. Diabetes mellitus and cancer risk in a population-based case-control study among men from Montreal. Canada Int J Cancer 2006; 118:2105–2109. [DOI] [PubMed] [Google Scholar]
- 19.Harding JL, Shaw JE, Peeters A, et al. Cancer risk among people with type 1 and type 2 diabetes: disentangling true associations, detection bias, and reverse causation. Diabetes Care 2015; 38:264–270. [DOI] [PubMed] [Google Scholar]
- 20.Qi L, Qi X, Xiong H, et al. Type 2 diabetes mellitus and risk of malignant melanoma: a systematic review and meta-analysis of cohort studies. Iran J Public Health 2014; 43:857–866. [PMC free article] [PubMed] [Google Scholar]
- 21.National Health Insurance Administration, Ministry of Health, Executive Yuan. 2014–2015 National Health Insurance Annual Report. National Health Insurance Administration, Ministry of Health, Executive Yuan; December 2014. http://www.nhi.gov.tw. [Google Scholar]
- 22.Jiang YD, Chang CH, Tai TY, et al. Incidence and prevalence rates of diabetes mellitus in Taiwan: analysis of the 2000–2009 Nationwide Health Insurance database. J Formos Med Assoc 2012; 111:599–604. [DOI] [PubMed] [Google Scholar]
- 23.Tseng KS, Lin C, Lin YS, et al. Risk of head and neck cancer in patients with diabetes mellitus: a retrospective cohort study in Taiwan. JAMA Otolaryngol Head Neck Surg 2014; 140:746–753. [DOI] [PubMed] [Google Scholar]
- 24.Tseng CH. Rosiglitazone may reduce non-melanoma skin cancer risk in Taiwanese. BMC Cancer 2015; 15:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Surveillance Research Program NCI. Cancer Statistics Review by Surveillance, Epidemiology, and End Results (SEER) Program. 2016. Available at: http://seer.cancer.gov/statistics/summaries.html (accessed February 2, 2016). [Google Scholar]
- 26.Linos E, Swetter SM, Cockburn MG, et al. Increasing burden of melanoma in the United States. J Invest Dermatol 2009; 129:1666–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Admiraal WM, van Valkengoed IG, LdM JS, et al. The association of physical inactivity with Type 2 diabetes among different ethnic groups. Diabet Med 2011; 28:668–672. [DOI] [PubMed] [Google Scholar]
- 28.Garg SK, Maurer H, Reed K, et al. Diabetes and cancer: two diseases with obesity as a common risk factor. Diabetes Obes Metab 2014; 16:97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sergentanis TN, Antoniadis AG, Gogas HJ, et al. Obesity and risk of malignant melanoma: a meta-analysis of cohort and case-control studies. Eur J Cancer 2013; 49:642–657. [DOI] [PubMed] [Google Scholar]
- 30.Lee TK, MacArthur AC, Gallagher RP, et al. Occupational physical activity and risk of malignant melanoma: the Western Canada Melanoma Study. Melanoma Res 2009; 19:260–266. [DOI] [PubMed] [Google Scholar]
- 31.Hull AP. Melanoma and solar radiation. Hosp Pract (Off Ed) 1983; 18:17–18. [PubMed] [Google Scholar]
- 32.Cotton J, Spandau DF. Ultraviolet B-radiation dose influences the induction of apoptosis and p53 in human keratinocytes. Radiat Res 1997; 147:148–155. [PubMed] [Google Scholar]
- 33.Kraemer KH. Sunlight and skin cancer: another link revealed. Proc Natl Acad Sci U S A 1997; 94:11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blair A, Zahm SH, Pearce NE, et al. Clues to cancer etiology from studies of farmers. Scand J Work Environ Health 1992; 18:209–215. [DOI] [PubMed] [Google Scholar]
- 35.Surdu S. Non-melanoma skin cancer: occupational risk from UV light and arsenic exposure. Rev Environ Health 2014; 29:255–264. [DOI] [PubMed] [Google Scholar]
- 36.Kaskel P, Lange U, Sander S, et al. Ultraviolet exposure and risk of melanoma and basal cell carcinoma in Ulm and Dresden, Germany. J Eur Acad Dermatol Venereol 2015; 29:134–142. [DOI] [PubMed] [Google Scholar]
- 37.Dlugosz A, Merlino G, Yuspa SH. Progress in cutaneous cancer research. J Investig Dermatol Symp Proc 2002; 7:17–26. [DOI] [PubMed] [Google Scholar]
- 38.Ding YS, Zhang L, Jain RB, et al. Levels of tobacco-specific nitrosamines and polycyclic aromatic hydrocarbons in mainstream smoke from different tobacco varieties. Cancer Epidemiol Biomarkers Prev 2008; 17:3366–3371. [DOI] [PubMed] [Google Scholar]
- 39.Markey AC. Etiology and pathogenesis of squamous cell carcinoma. Clin Dermatol 1995; 13:537–543. [DOI] [PubMed] [Google Scholar]
- 40.Boffetta P, Jourenkova N, Gustavsson P. Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes Control 1997; 8:444–472. [DOI] [PubMed] [Google Scholar]
- 41.Patel TP, Rawal K, Bagchi AK, et al. Insulin resistance: an additional risk factor in the pathogenesis of cardiovascular disease in type 2 diabetes. Heart Fail Rev 2016; 21:11–23. [DOI] [PubMed] [Google Scholar]
- 42.Antoniadis AG, Petridou ET, Antonopoulos CN, et al. Insulin resistance in relation to melanoma risk. Melanoma Res 2011; 21:541–546. [DOI] [PubMed] [Google Scholar]
- 43.Berg D, Otley CC. Skin cancer in organ transplant recipients: epidemiology, pathogenesis, and management. J Am Acad Dermatol 2002; 47:1–17. [DOI] [PubMed] [Google Scholar]
- 44.Jiyad Z, Olsen CM, Burke MT, et al. Azathioprine and risk of skin cancer in organ transplant recipients: systematic review and meta-analysis. Am J Transplant 2016; May 10. doi: 10.1111/ajt.13863. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 45.Brewer JD, Shanafelt TD, Khezri F, et al. Increased incidence and recurrence rates of nonmelanoma skin cancer in patients with non-Hodgkin lymphoma: a Rochester Epidemiology Project population-based study in Minnesota. J Am Acad Dermatol 2015; 72:302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Famenini S, Martires KJ, Zhou H, et al. Melanoma in patients with chronic lymphocytic leukemia and non-Hodgkin lymphoma. J Am Acad Dermatol 2015; 72:78–84. [DOI] [PubMed] [Google Scholar]
- 47.Crum-Cianflone N, Hullsiek KH, Satter E, et al. Cutaneous malignancies among HIV-infected persons. Arch Intern Med 2009; 169:1130–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brugnaro P, Morelli E, Cattelan F, et al. Non-AIDS defining malignancies among human immunodeficiency virus-positive subjects: epidemiology and outcome after two decades of HAART era. World J Virol 2015; 4:209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Long MD, Martin CF, Pipkin CA, et al. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology 2012; 143:390–399.e391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abbas AM, Almukhtar RM, Loftus EV, Jr, et al. Risk of melanoma and non-melanoma skin cancer in ulcerative colitis patients treated with thiopurines: a nationwide retrospective cohort. Am J Gastroenterol 2014; 109:1781–1793. [DOI] [PubMed] [Google Scholar]
- 51.Chakravarty EF, Michaud K, Wolfe F. Skin cancer, rheumatoid arthritis, and tumor necrosis factor inhibitors. J Rheumatol 2005; 32:2130–2135. [PubMed] [Google Scholar]
- 52.Began D, Hurwitz A, Spandau F. Aged keratinocytes fail to undergo apoptosis following UVB irradiation. J Invest Dermatol 1999; 112:614. [Google Scholar]
- 53.Kuhn C, Hurwitz SA, Kumar MG, et al. Activation of the insulin-like growth factor-1 receptor promotes the survival of human keratinocytes following ultraviolet B irradiation. Int J Cancer 1999; 80:431–438. [DOI] [PubMed] [Google Scholar]
- 54.Grimberg A, Cohen P. Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J Cell Physiol 2000; 183:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khandwala HM, McCutcheon IE, Flyvbjerg A, et al. The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocr Rev 2000; 21:215–244. [DOI] [PubMed] [Google Scholar]
- 56.LeRoith D, Roberts CT., Jr The insulin-like growth factor system and cancer. Cancer Lett 2003; 195:127–137. [DOI] [PubMed] [Google Scholar]
- 57.Haring HU, White MF, Kahn CR, et al. Abnormality of insulin binding and receptor phosphorylation in an insulin-resistant melanoma cell line. J Cell Biol 1984; 99:900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hilmi C, Larribere L, Giuliano S, et al. IGF1 promotes resistance to apoptosis in melanoma cells through an increased expression of BCL2, BCL-X(L), and survivin. J Invest Dermatol 2008; 128:1499–1505. [DOI] [PubMed] [Google Scholar]
- 59.Novosyadlyy R, LeRoith D. Hyperinsulinemia and type 2 diabetes: impact on cancer. Cell Cycle 2010; 9:1449–1450. [DOI] [PubMed] [Google Scholar]
- 60.Robertson R, Zhou H, Zhang T, et al. Chronic oxidative stress as a mechanism for glucose toxicity of the beta cell in type 2 diabetes. Cell Biochem Biophys 2007; 48:139–146. [DOI] [PubMed] [Google Scholar]
- 61.Turturro F, Friday E, Welbourne T. Hyperglycemia regulates thioredoxin-ROS activity through induction of thioredoxin-interacting protein (TXNIP) in metastatic breast cancer-derived cells MDA-MB-231. BMC Cancer 2007; 7:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lorenzi M, Montisano DF, Toledo S, et al. High glucose induces DNA damage in cultured human endothelial cells. J Clin Invest 1986; 77:322–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer 2004; 4:891–899. [DOI] [PubMed] [Google Scholar]
- 64.Chuang TY, Lewis DA, Spandau DF. Decreased incidence of nonmelanoma skin cancer in patients with type 2 diabetes mellitus using insulin: a pilot study. Br J Dermatol 2005; 153:552–557. [DOI] [PubMed] [Google Scholar]
- 65.Varela-Rey M, Woodhoo A, Martinez-Chantar ML, et al. Alcohol, DNA methylation, and cancer. Alcohol Res 2013; 35:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]


