Abstract
Changes to patterns of wind and ocean currents are tightly linked to climate change and have important implications for cost of travel and energy budgets in marine vertebrates. We evaluated how El Niño-Southern Oscillation (ENSO)-driven wind patterns affected breeding Laysan and black-footed albatross across a decade of study. Owing to latitudinal variation in wind patterns, wind speed differed between habitat used during incubation and brooding; during La Niña conditions, wind speeds were lower in incubating Laysan (though not black-footed) albatross habitat, but higher in habitats used by brooding albatrosses. Incubating Laysan albatrosses benefited from increased wind speeds during El Niño conditions, showing increased travel speeds and mass gained during foraging trips. However, brooding albatrosses did not benefit from stronger winds during La Niña conditions, instead experiencing stronger cumulative headwinds and a smaller proportion of trips in tailwinds. Increased travel costs during brooding may contribute to the lower reproductive success observed in La Niña conditions. Furthermore, benefits of stronger winds in incubating habitat may explain the higher reproductive success of Laysan albatross during El Niño conditions. Our findings highlight the importance of considering habitat accessibility and cost of travel when evaluating the impacts of climate-driven habitat change on marine predators.
Keywords: El Niño-Southern Oscillation, wind, seabird, movement, central place forager, albatross
1. Background
Climate change is rapidly altering marine systems and is predicted to have increasing and widespread effects in future years [1–7]. The mechanisms through which climate change influences marine organisms are diverse, and include changes to physical habitat characteristics, prey availability, trophic interactions and phenology [1–3,8,9]. Distributional shifts across a broad range of marine taxa have been linked with changes in a number of oceanographic variables [10–12]. Shifts in suitable habitats can cause habitats to become inaccessible and/or can increase energetic costs of travel, but climate impacts on the accessibility of habitats have received less attention.
The intensity and location of dominant wind patterns and ocean currents have been significantly impacted by climate change and these changes are predicted to accelerate in the future [13–19]. These changes will directly impact the movement and energetic costs of transport for animals that fly or swim [20–24]. Large marine vertebrates such as tuna, sharks, cetaceans, seabirds and sea turtles are likely to be particularly sensitive to changes in fluid flow as they experience high drag while swimming and/or flying through marine habitats due to their large body sizes and rapid travel speeds [25–30]. Variability in wind and ocean currents may be especially influential on the population-level processes of marine vertebrates like seabirds and pinnipeds that function as central place foragers while breeding, returning to the breeding colony to incubate eggs and/or feed their young. Energy acquisition and allocation of these central place foragers have direct consequences for fitness and reproductive performance [31–34]. In the light of ongoing environmental change, it is therefore important to consider the effects of wind or ocean currents when examining and interpreting the movement patterns, distribution and habitat use of these species (e.g. [25,28,35–38]). While a growing body of work has examined how environmental change in foraging habitat influences the distribution of marine vertebrates (e.g. [39,40]) there is a dearth of information about the consequences of climate-driven changes to wind patterns and ocean currents for migrations and energy budgets (e.g. [41]).
Wind speed and direction are major factors that influence seabird movement [42,43] and distribution [44], particularly for albatrosses whose foraging strategy of exploiting ephemeral, widely separated prey patches requires energetically efficient gliding flight (e.g. [45–48]). The energetic cost of flight for albatrosses is among the lowest measured for flying animals; this allows albatrosses to cover extensive distances, flying thousands of kilometres during a single foraging trip and achieving speeds of more than 100 km h−1, while expending very little energy (e.g. [45,46,49–53]). Albatrosses require sufficient wind for energetically efficient flight and albatross ranges are typically limited to regions with some of the highest winds speeds globally [41,44,48,54,55]. The magnitude and direction of the wind field can have important influences on where albatrosses forage; birds often use flight paths that maximize travel with favourable winds [41,46,48,56].
El Niño-Southern Oscillation (ENSO) events cause widespread biological changes in marine systems [57–60] and seabird species often serve as useful indicators of the extent and severity of the biological effects of these events (e.g. [57,61,62]). Driven by changes in wind patterns in the equatorial Pacific, ENSO events are a major source of climatic and oceanographic interannual variability and can impact marine ecosystems throughout the world (e.g. [63–67]). Studies examining ENSO effects on seabird populations have largely focused on diet, foraging behaviour and oceanographic aspects of foraging habitat (e.g. [57,62,68–70]), while the impacts of the underlying wind patterns on seabirds have not been investigated in detail. We postulate that changes in wind patterns associated with ENSO events will influence seabird fitness and reproduction by impacting the energetic costs of travel and foraging (sensu [41]).
El Niño events typically have negative impacts on marine organisms in the eastern North Pacific (e.g. [66,71–73]), but can have positive effects on marine predators in the central Pacific [70,74]. Laysan (Phoebastria immutabilis) and black-footed albatrosses (P. nigripes) experience substantial declines in reproductive success (chicks fledged per eggs laid) during La Niña conditions when the contraction of the subarctic gyre displaces suitable foraging habitat further north [70]. However, stronger trade winds during La Niña events (e.g. [75,76]) may decrease foraging costs of albatross by facilitating at-sea travel rates.
Here we examine how ENSO conditions influence wind available to foraging Laysan and black-footed albatrosses during breeding, and how this in turn impacts albatross movement and habitat use.
2. Material and methods
2.1. Study species
Laysan and black-footed albatrosses are long-lived species with slow reproductive rates [77] that are faced with a number of anthropogenic threats (particularly bycatch) [78] in addition to impacts of climate-driven environmental change [70]. These species breed in subtropical waters of the North Pacific, with the vast majority breeding in the Northwest Hawaiian Islands [79]. Both species exhibit prolonged breeding periods; egg laying occurs in November and December, and chicks fledge in June or July. Parents take turns foraging and incubating eggs or brooding chicks. Foraging trips of adult albatrosses are relatively long during the 65 day incubation period (typically 10–31 days) [80–82]. However, during brooding, rapidly growing chicks must be fed frequently and foraging trips of the parents are much more constrained (typically 1–3 days) [70,80,81,83]. During chick-rearing, chicks are left unattended while both parents forage, alternating between short (1–2 days) and long (more than 12 days) foraging trips [80,83]. Previous work has suggested that climate-driven environmental variability has the greatest impacts during brooding when parents are most constrained [70]. Here, we compare the effects of wind on albatross movement during more constrained (brooding) and less constrained (incubating) breeding stages.
2.2. Study area
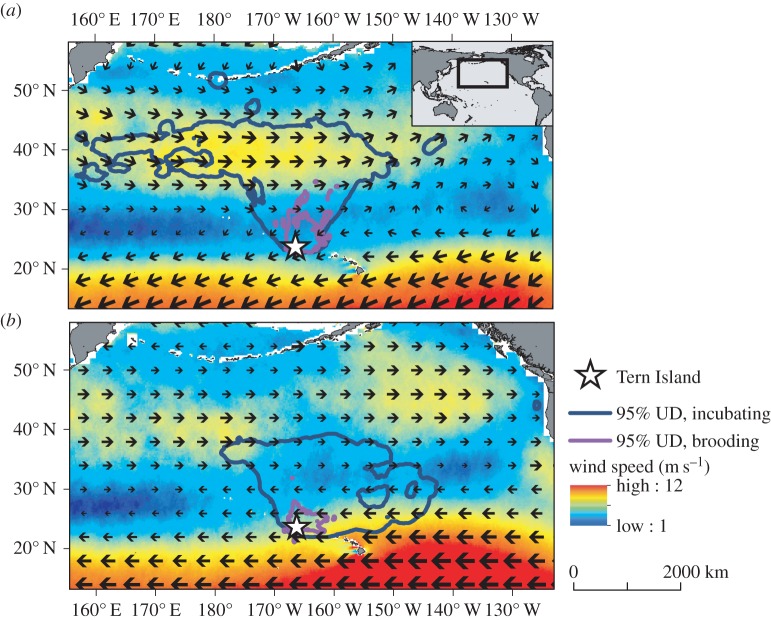
Albatross telemetry studies were conducted from Tern Island at French Frigate Shoals in the Northwest Hawaiian Islands (23.87°N, 166.28°W; figure 1), where the US Fish and Wildlife Service (USFWS) maintained a field station until 2012. Tern Island provides breeding substrate for thousands of seabirds, as well as monk seals and green sea turtles [84]. Easterly (from the east) trade winds are prevalent at Tern Island and are strongest during summer months and during La Niña conditions (figure 1).
Figure 1.
Wind speed and direction in the North Pacific during El Niño (a) and La Niña (b) conditions relative to Tern Island and albatross foraging habitat. Foraging habitat is shown as the 95% utilization distribution (UD) during the incubating and brooding phases for Laysan (a) and black-footed albatrosses (b), respectively. Wind speed and direction represent mean seasonal (December–February) values during incubation and brooding phases from 2003 to 2012 for El Niño conditions (MEI > 0.5) and La Niña conditions (MEI < −0.5). Wind speed and direction is represented by the size and rotation of arrows, respectively.
2.3. Albatross telemetry and mass data
Laysan and black-footed albatross were tracked from Tern Island during 2002/2003–2005/2006 and 2007/2008–2011/2012 breeding seasons using satellite platform terminal transmitters (PTTs; 30 g Pico-100, Microwave Telemetry, Columbia, MD, USA, 42 g SPOT4 and SPOT5, Wildlife Computers, Redmond, WA, USA) and GPS data loggers (40 g Technosmart GPS, 35 g TechnoSmart GiPSy, 32 g E&O Technologies, and 30 g igotU, GT-120, Mobile Action Technology Inc, Taiwan), all well below the recommended mass threshold of 3% of body weight [85]. PTT tags are accurate to less than 10 km [86], while GPS tags are accurate to less than 12 m [87]. Tags were attached to three to five dorsal feathers using Tesa cloth adhesive tape (Tesa, Hamburg, Germany; electronic supplementary material, figure S1). At the end of each trip, the tag and tape were gently removed from the feathers. No albatrosses were lost during the study and all tagged birds returned to the nest site, though a small proportion of tags deployed (4.2%) were lost at sea during foraging trips. GPS data were offloaded as raw binary data which were converted into latitude and longitude coordinates by commercial software (E&O Technologies, TechnoSmart Software and Mobile Action software, respectively), while positions from PTT tags were downloaded as latitude and longitude coordinates from Argos system software. Body mass was measured during tag deployment and retrieval using a spring-loaded Pesola scale (Pesola AG, Baar, Switzerland). During tag retrieval in the brooding period, we could not ensure that body mass measurements occurred before adults offloaded food to their chicks; therefore, analyses of body mass change across foraging trips focused on incubating birds only.
We tracked a total of 107 Laysan and 108 black-footed albatrosses (table 1). Only one foraging trip was tracked and analysed for each individual bird. Telemetry data were resampled to equal time steps (a 3-h time scale) in the R statistical package using the Minimum Covariance Determinant robust estimator in the MASS library. For each albatross foraging trip, we assessed cumulative trip distance and the duration of each trip using the ArgosFilter (v. 0.63) and MASS libraries in R. Albatross tracks have been contributed to Bird Life International's seabird tracking database (www.seabirdtracking.org; see Hawaii/ French Frigate Shoals datasets).
Table 1.
Number of tracks used in analyses by species, ENSO conditions and breeding stage.
| species | ENSO conditions | breeding stage | no. tracks |
|---|---|---|---|
| Laysan | El Niño | incubation | 26 |
| brooding | 21 | ||
| La Niña | incubation | 23 | |
| brooding | 38 | ||
| black-footed | El Niño | incubation | 33 |
| brooding | 20 | ||
| La Niña | incubation | 25 | |
| brooding | 29 |
2.4. Wind patterns in albatross habitat during El Niño and La Niña conditions
Wind data at a spatial resolution of 0.25 degrees were obtained from NOAA's ERDDAP server (www.http://coastwatch.pfeg.noaa.gov/erddap/griddap). Rainy areas are removed from this dataset as rain can alter the ocean surface and can lead to unreliable wind speeds measurements [88,89]. Data from the QuikSCAT satellite, in operation until November 2009, were used for the 2003–2009 breeding periods, whereas data from the METOP/ASCAT satellite (operational after October 2009) were used for 2010–2012. We evaluated the zonal (east–west) and meridional (north–south) components of wind separately, as well as the overall magnitude of the wind. Raw daily satellite wind data did not provide complete and consistent coverage for analysis of albatross tracks due to data gaps in between satellite orbits, particularly for data from the METOP/ASCAT satellite at lower latitudes [90], and removal of rain-flagged data. We therefore used daily composites of zonal and meridional wind components and wind speed (calculated daily using an 8-day moving window).
We compared wind conditions between El Niño and La Niña events in two ways. We first examined overarching trends in albatross habitat using seasonal climatologies. Monthly wind composites were calculated during the albatross incubating and brooding periods (mean wind speed and zonal and meridional wind, respectively, from December through February). Next, we examined zonal wind, meridional wind and wind speed during albatross foraging trips. We sampled wind speed and direction for each location on albatross tracks using daily maps of wind components described above. We used the Multivariate El Niño Southern Oscillation (ENSO) Index (MEI) to assess El Niño conditions (http://www.esrl.noaa.gov/psd/enso/mei/table.html).
2.5. Influence of wind on albatross habitat use
We examined albatross movement during incubation and brooding relative to cumulative wind using the following metrics: maximum distance travelled to the north, south, east and west from Tern Island, and the ratio of latitudinal to longitudinal travel. Cumulative wind values (zonal, meridional and wind speed) represent wind in albatross foraging habitat summed for each day of the foraging trip. We used 95% utilization distributions (UDs) of Laysan and black-footed albatross tracks during incubation and brooding, respectively, to represent foraging habitat. UDs were produced in ArcGIS using the Kernel Density Spatial Analyst tool and were calculated using a 100 km radius for habitat used during incubation, and a 50 km radius for habitat used during brooding.
The speed and direction of wind experienced by albatrosses varied dramatically with latitude (figures 1 and 2). Albatrosses travelling farther north experience more westerly zonal winds as a result of wind patterns, leading to a spurious strong positive correlation between albatross trip distance and zonal winds. Therefore, albatross movement was examined relative to wind variables at the centroid of albatross foraging habitat for each day an albatross was at sea.
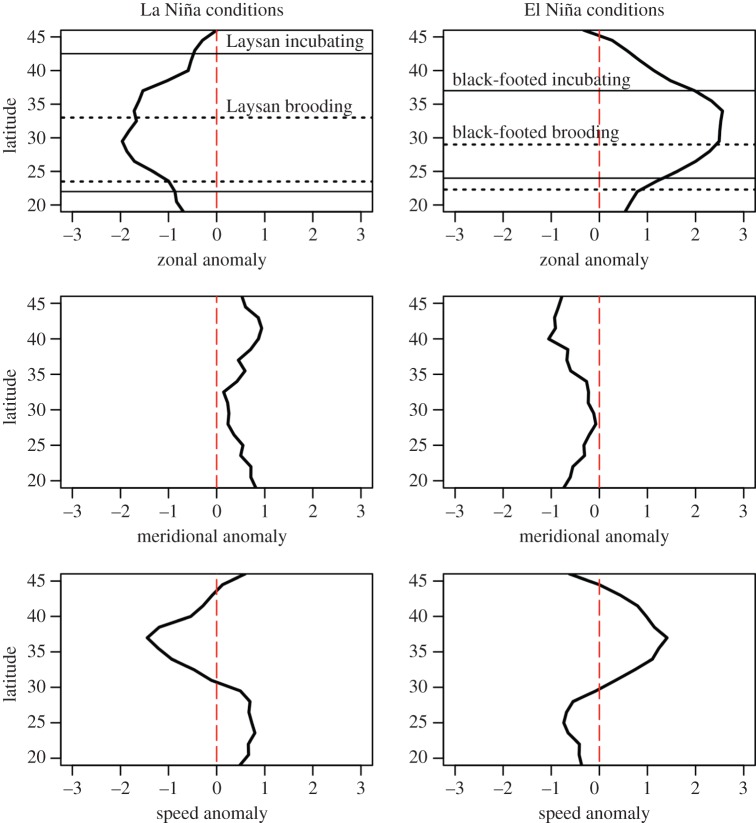
Figure 2.
Latitudinal variability in zonal and meridional wind components and wind speed during strong El Niño and strong La Niña conditions (MEI > 0.5 and < −0.5, respectively). Values represent deviations from seasonal (December–February) means values during incubation and brooding phases from 2003 to 2012, sampled every 0.2 degrees latitude north of Tern Island. The dashed vertical line represents average conditions from 2003 to 2012. The mean latitudinal range of Laysan and black-footed albatross during incubation and brooding phases is shown relative to zonal anomalies in La Niña conditions as a reference. (Online version in colour.)
We used generalized additive models (GAMs) to evaluate the influence of wind variables on Laysan and black-footed albatross movement parameters as exploratory data analyses indicated nonlinear relationships between these variables. GAMs are particularly useful in ecological studies because they allow nonlinear relationships to be evaluated by incorporating both categorical and continuous variables [91,92]. To avoid over-fitting, we used cubic spline smoothers with 3 or fewer degrees of freedom. Significantly correlated variables were not included in the same model (table 2). GAMs were performed in the R statistical package (v. 3.1.3) using the mgcv (v. 1.8–7) library, and model selection was performed by comparing generalized cross-validation scores.
Table 2.
Pearson's correlation coefficients between zonal and meridional wind and wind speed in Laysan and black-footed albatross habitat during incubation and brooding, respectively. p-values are shown in brackets; bold values represent significant correlations (p < 0.05). We used the absolute value of zonal or meridional wind to calculate linear correlations with wind speed.
| zonal wind | meridional wind | wind speed | |
|---|---|---|---|
| incubation | |||
| Laysan | |||
| zonal wind | — | −0.11 (0.23) | −0.65 (7.1 × 10−7) |
| meridional wind | — | 0.34 (0.019) | |
| wind speed | — | ||
| black-footed | |||
| zonal wind | — | 0.19 (0.14) | −0.69 (1.6 × 10−9) |
| meridional wind | — | 0.40 (2.0 × 10−3) | |
| wind speed | — | ||
| brooding | |||
| Laysan | |||
| zonal wind | — | 0.26 (0.04) | 0.93 (2.2 × 10−16) |
| meridional wind | — | 0.51 (4.3 × 10−5) | |
| wind speed | — | ||
| black-footed | |||
| zonal wind | — | 0.16 (0.26) | 0.97 (2.2 × 10−16) |
| meridional wind | — | 0.28 (0.05) | |
| wind speed | — | ||
2.6. Effects of El Niño-Southern Oscillation-driven wind patterns on albatross movement and wind conditions
To gain insight into the effects of ENSO-driven wind patterns on energetic demands of foraging albatrosses, we assessed the angle between the flight direction of the bird and the direction of winds at each 3 h track location. Tail winds were defined as angles less than 60°; side winds as angles between 60° and 120° and head winds as angles greater than 120° [46]. We quantified average and cumulative wind speed experienced by foraging albatrosses during head, side and tail winds; assessed the proportion of each track spent in each of these three categories of wind; and compared values during El Niño and La Niña conditions using Wilcoxon rank sum tests. As foraging trips varied in duration, particularly during incubation, cumulative wind speeds experienced by albatrosses in different wind conditions were standardized by trip length (cumulative head, side or tail wind speeds experienced by albatrosses in a given trip were divided by the total number of observations in that trip and then multiplied by average incubating or brooding trip length for each species).
To examine how wind conditions influenced albatross speed, we calculated mean travel speed over ground during a foraging trip (calculated from the travel speed at each 3-h time step), and the overall travel rate (total distance travelled per trip/ trip duration). We then compared travel rates in head, side and tail winds for trips when albatrosses were incubating or brooding in El Niño and La Niña conditions, respectively. Lastly, we compared mean travel speeds and overall rates of travel between El Niño and La Niña conditions for incubating and brooding Laysan and black-footed albatrosses using Wilcoxon rank sum tests.
Wind climatologies were calculated in ArcGIS 10 using the Spatial Analyst extension, while the raster (v. 2.4–18), rgdal (v. 1.0–7), geosphere (v. 1.4–3) and Imap (v. 1.32) R packages were used to sample wind rasters and for distance and bearing calculations.
3. Results
3.1. Wind patterns in albatross foraging habitat in relation to El Niño-Southern Oscillation conditions
El Niño and La Niña conditions produced strong anomalies in wind patterns within albatross foraging habitat that varied with latitude (figure 2). Positive (negative) zonal wind anomalies were observed during El Niño conditions (La Niña conditions) and were particularly large between latitudes of approximately 25°–40°N. Anomalies in meridional wind were weaker than those of zonal wind and showed the opposite pattern (positive (negative) anomalies during La Niña (El Niño) conditions). During El Niño (La Niña) conditions, wind speed showed positive (negative) anomalies in latitudes typically used by incubating albatrosses (foraging habitat during incubation was centred at approximately 30°–37°N), and negative (positive) anomalies in latitudes typically used by albatrosses during brooding (foraging habitat during brooding was centred at approximately 24°–26°N).
Differences in the latitudinal range of foraging albatrosses during incubation and brooding resulted in differences in wind experienced across a breeding season. For example, the incubating trips of Laysan albatrosses extend further north than the short trips made during brooding. During La Niña conditions, incubating Laysan albatrosses experienced weaker westerly winds, stronger northerly winds and weaker overall wind speeds (figure 3). Conversely, during brooding, albatrosses experienced stronger easterly winds, weaker northerly winds and higher overall wind speeds in La Niña conditions (figure 3).
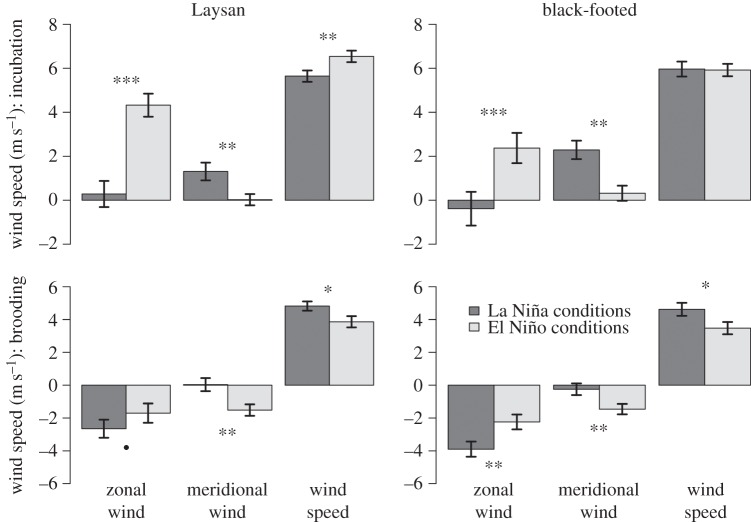
Figure 3.
Mean zonal wind, meridional wind and wind speeds (±s.e.) experienced during Laysan and black-footed albatross incubating and brooding trips in El Niño and La Niña conditions (MEI > 0.5 and <−0.5, respectively). Positive zonal wind speeds represent westerly winds, while positive meridional wind speeds represent southerly winds. filled circle indicates p < 0.1, *p < 0.05, **p < 0.01, ***p < 0.001, Wilcoxon rank sum test.
Prevailing westerlies at mid-latitudes vary dramatically with ENSO conditions and Laysan albatrosses foraged further north into these prevailing westerlies than black-footed albatrosses during incubation (figures 1 and 2). As a result, wind speeds experienced by incubating Laysan albatrosses were significantly higher during El Niño conditions than La Niña conditions, whereas wind speeds for incubating black-footed albatrosses did not differ between El Niño and La Niña conditions (figure 3).
3.2. Effects of wind on albatross movement
Wind variables were significant predictors of the spatial extent of Laysan and black-footed albatross foraging patterns during incubation and brooding, with GAMs explaining 11–47% of the variance in albatross movement metrics, respectively (table 3; electronic supplementary material, figure S2). Increased wind speed was associated with a larger foraging range for incubating Laysan albatrosses (increased movement in all directions). For brooding albatrosses and incubating black-footed albatrosses, zonal wind was associated with longitudinal shifts in foraging range; birds travelled further east when zonal wind was higher (i.e. during weaker easterlies) and generally travelled further west when zonal wind was lower (i.e. stronger easterlies).
Table 3.
Results of generalized additive models (GAMs) examining the effects of zonal wind, meridional wind and wind speed on directional movement of incubating and brooding Laysan and black-footed albatross. Note that a negative relationship with zonal wind represents a positive relationship with the magnitude of easterly winds.
| dependent variable | explanatory variable | relationship | intercept p value | variable p-value | prop. deviance (%) | R2-value |
|---|---|---|---|---|---|---|
| incubation | ||||||
| Laysan albatross | ||||||
| maximum north | wind speed | positive | <2×10−16 | 9.54×10−6 | 40.90 | 0.37 |
| maximum south | NSD | |||||
| maximum east | wind speed | positive | <2×10−16 | 7.30×10−3 | 14.80 | 0.15 |
| maximum west | wind speed | positive | <2×10−16 | 5.87×10−4 | 28.50 | 0.27 |
| lat. : long. travel | wind speed | negative | <2×10−16 | 1.54×10−5 | 33.20 | 0.32 |
| black-footed albatross | ||||||
| maximum north | wind speed | positive | <2×10−16 | 8.10×10−6 | 29.30 | 0.28 |
| maximum south | NSD | |||||
| maximum east | zonal wind | positive | 1.35×10−12 | 0.016 | 14.60 | 0.13 |
| maximum west | zonal wind | negative | 4.85×10−7 | 1.34×10−8 | 47.20 | 0.45 |
| lat. : long. travel | NSD | |||||
| brooding | ||||||
| Laysan albatross | ||||||
| maximum north | wind speed | positive | 9.14×10−12 | 3.18×10−4 | 21.80 | 0.20 |
| maximum south | NSD | |||||
| maximum east | zonal wind | positive | 2.24×10−12 | 0.0045 | 18.00 | 0.17 |
| maximum west | zonal wind | negative | 1.22×10−9 | 0.011 | 12.30 | 0.10 |
| lat. : long. travel | zonal wind | negative | 1.00×10−14 | 3.43×10−3 | 20.90 | 0.19 |
| black-footed albatross | ||||||
| maximum north | wind speed | positive | 3.60×10−11 | 1.40×10−3 | 19.60 | 0.18 |
| maximum south | NSD | |||||
| maximum east | zonal wind | positive | 7.08×10−11 | 0.02 | 10.60 | 0.088 |
| maximum west | NSD | |||||
| lat. : long. travel | zonal wind | negative | 3.78×10−7 | 4.53×10−3 | 18.60 | 0.17 |
3.3. Effects of El Niño-Southern Oscillation conditions on albatross movement relative to wind
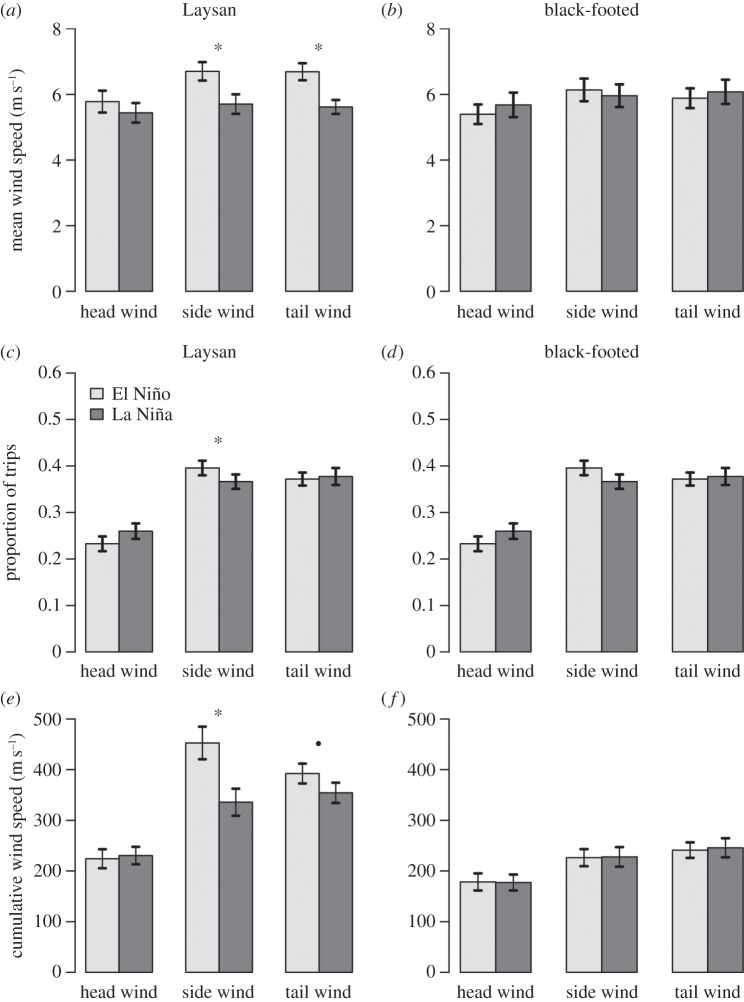
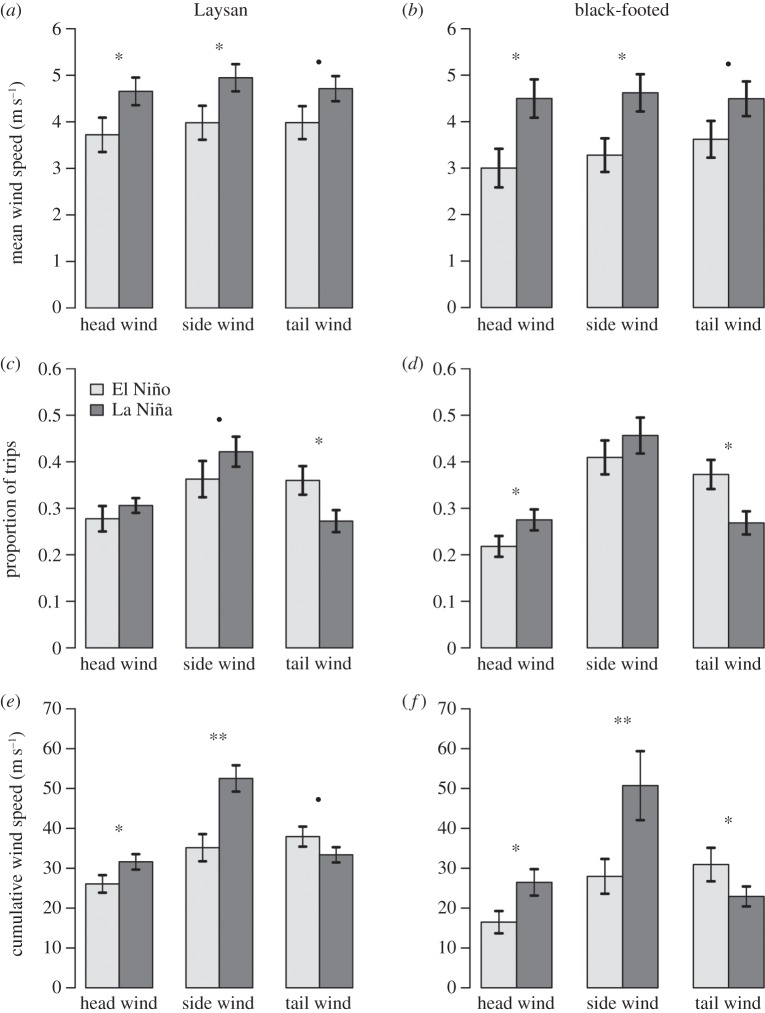
During the incubation phase, Laysan albatrosses experienced higher wind speeds during El Niño conditions in side and tail winds (figure 4a), whereas winds experienced by black-footed albatrosses did not differ between El Niño and La Niña conditions (figure 4b). During El Niño conditions, Laysan albatrosses spent significantly more time in side winds and experienced higher cumulative wind speeds in side and tail winds (figure 4c,e). Brooding albatrosses experienced higher wind speeds during La Niña conditions in head, side and tail winds (figure 5a,b). Both brooding Laysan and black-footed albatrosses spent significantly less time in tailwinds during La Niña conditions, while Laysan albatrosses spent more time in side winds and black-footed albatrosses spent significantly more time in head winds (figure 5c,d). During La Niña conditions, brooding albatrosses experienced significantly higher cumulative head wind and side wind speeds, while cumulative tail wind speeds were lower (figure 5e,f).
Figure 4.
(a,b) Mean wind speed experienced during incubating trips in head winds, side winds and tail winds during El Niño and La Niña conditions (means ± s.e.). (c,d) Proportion of incubating trips spent in head winds, side winds and tail winds during El Niño and La Niña conditions (means ± s.e.). (e,f) Cumulative wind speed experienced by incubating Laysan and black-footed albatrosses in head wind, side wind and tail winds during El Niño and La Niña conditions (means ± s.e.). filled circle indicates p < 0.1, *p < 0.05, Wilcoxon rank sum test.
Figure 5.
(a,b) Mean wind speed experienced during brooding trips in head winds, side winds and tail winds during El Niño and La Niña conditions (means ± s.e.). (c,d) Proportion of brooding trips spent in head winds, side winds and tail winds during El Niño and La Niña conditions (means ± s.e.). (e,f) Cumulative wind speed experienced by brooding Laysan and black-footed albatrosses in head wind, side wind and tail winds during El Niño and La Niña conditions (means ± s.e.). filled circle indicates p < 0.10, *p < 0.05, Wilcoxon rank sum test.
3.4. Effect of wind speed on foraging albatrosses
During higher wind speeds, brooding albatrosses spent proportionally more time in side winds but less time in tail winds (figure 6). Incubating Laysan albatrosses spent proportionally more time in side winds during high wind speeds, while incubating black-footed albatrosses showed no significant differences in the proportion of trips spent in different wind conditions. Change in body mass adjusted for trip duration was positively and significantly correlated with wind speed for incubating Laysan albatrosses (r = 0.48, p < 0.01) but not for incubating black-footed albatrosses (r = −0.18, p = 0.24).
Figure 6.
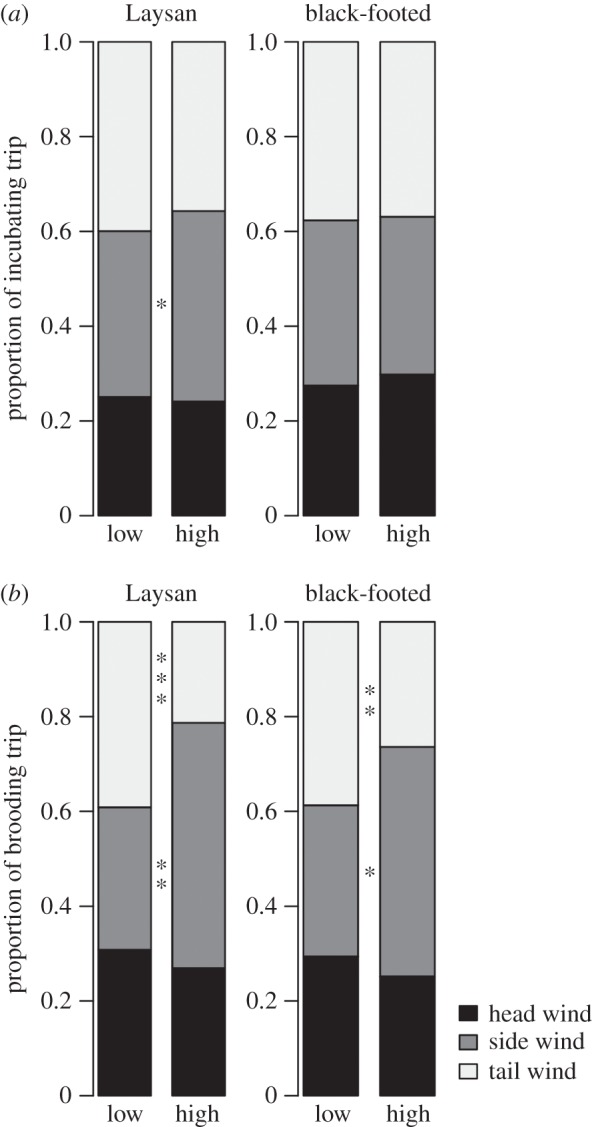
(a) Proportion of incubating trips spent in head winds, side winds and tail winds during high and low wind speeds, respectively (means ± s.e.). (b) Proportion of brooding trips spent in head winds, side winds and tail winds during high and low wind speeds, respectively (means ± s.e.). Asterosls indicates p < 0.05, **p < 0.01, ***p < 0.001, Wilcoxon rank sum test.
3.5. El Niño-Southern Oscillation effects on albatross travel speed
During brooding trips in La Niña conditions (when wind speeds were higher), Laysan and black-footed albatrosses travelled more slowly in head winds than in tail or side winds and birds travelled into stronger head winds (figures 5a and 7); this was also true for incubating Laysan albatrosses during El Niño conditions (associated with increased wind speeds). During ENSO conditions with lower wind speeds (La Niña conditions for incubating trips and El Niño conditions for brooding trips), speeds travelled in head winds and tail winds were not significantly different. Both the mean speed travelled and overall travel rate were significantly higher for Laysan albatrosses during El Niño conditions than in La Niña conditions, but only during incubating trips (electronic supplementary material, figure S3). ENSO conditions did not affect mean speed travelled or overall travel rates for black-footed albatrosses.
Figure 7.
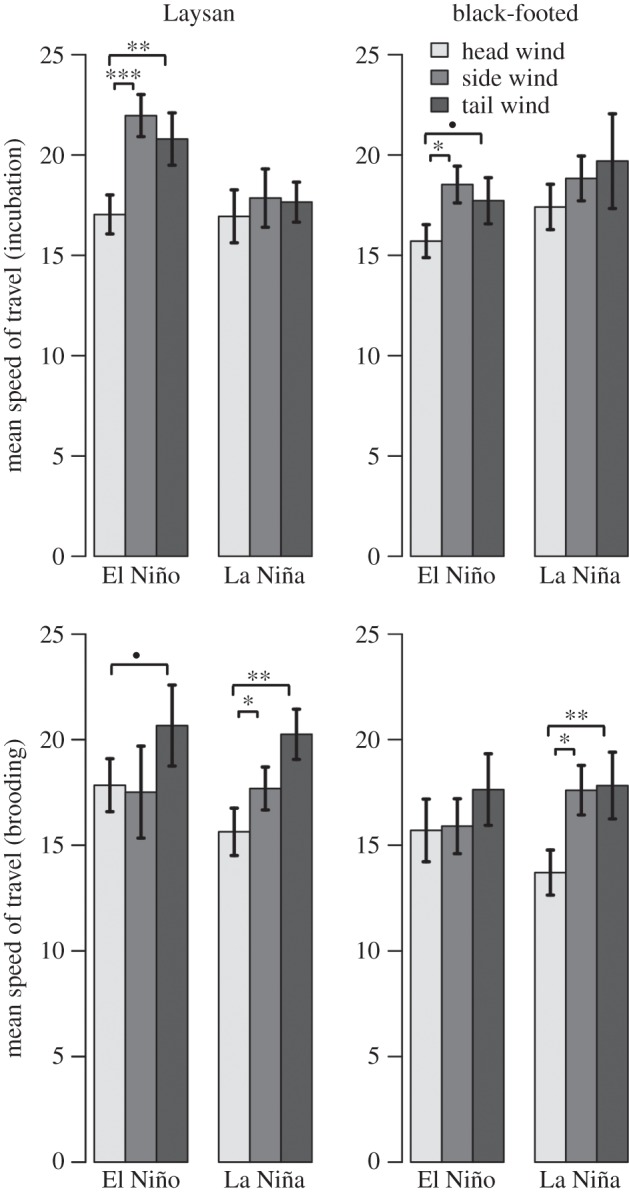
Mean speed travelled for Laysan and black-footed albatrosses during incubating and brooding trips in head winds, side winds and tail winds during El Niño and La Niña conditions, respectively (means ± s.e.). Filled circle indicates p < 0.1, *p < 0.05, **p < 0.01, ***p < 0.001, Wilcoxon rank sum test.
4. Discussion
4.1. Effects of El Niño-Southern Oscillation-driven wind patterns on albatross habitat, movement and energetics
Changes in wind patterns had significant impacts on the spatial distribution of foraging albatrosses, and these effects differed between species and breeding phases. During the more flexible incubating trips, Laysan albatrosses foraged in northerly waters with high wind speeds and were able to exploit strong prevailing winds to expand their foraging range (table 3; electronic supplementary material, figure S2). The range of incubating black-footed albatrosses is too limited to allow these birds to fully take advantage of prevailing winds to the north, while brooding albatrosses were constrained to forage in prevailing winds in proximity to the nest site (figure 1). The foraging range of brooding albatrosses and incubating black-footed albatrosses shifted longitudinally with zonal winds (table 3).
Furthermore, we found notable effects of climate-driven changes in wind patterns on foraging albatrosses. ENSO conditions are tightly linked with changes in wind patterns; during El Niño events, easterly trade winds decrease in the central Pacific, while trade winds increase during La Niña events (e.g. [75,76]). ENSO conditions dramatically influence the wind field available to albatrosses during incubation and brooding, and latitudinal trends in wind patterns created differences in the winds experienced by foraging albatrosses within a given year (figures 2 and 3). Variability in the wind field has important implications for habitat accessibility [48] and the energetic costs of foraging trips, and, therefore, on the ability of adult albatrosses to maintain their own body condition and provision chicks (e.g. [47]). Increases in wind speed can positively affect foraging albatrosses, resulting in increases in flight speeds, mass gained during foraging trips and breeding success [41,48,53]. Our results show that Laysan albatrosses putatively benefited from increased wind speeds in incubating habitat during El Niño conditions by experiencing faster travel rates (electronic supplementary material, figure S3). Mass gain of incubating Laysan albatrosses was positively and significantly correlated with wind speed, providing further support for an energetic benefit of increased wind speed during incubation. For brooding birds, however, stronger trade winds during La Niña conditions appeared to incur increased energetic costs. Wind direction can influence the cost of travel in albatrosses, with foraging costs decreasing from head winds to side winds to tail winds [46,48]. During La Niña conditions, brooding albatrosses of both species experienced higher cumulative head wind speeds and lower cumulative tail wind speeds (figure 5e,f), suggesting higher overall travel costs. Differences in flight direction relative to wind between El Niño and La Niña conditions appear to be due primarily to changes in wind speed; when examining the impacts of high versus low wind speed alone, differences in proportional time spent in side and tail winds during brooding was even more pronounced (figure 6).
Several studies have demonstrated that albatrosses avoid travelling in headwinds for extended periods of time, often making long, looping flights to exploit large-scale wind patterns (e.g. [46,48,93–95]). However, Wakefield et al. [48] suggest that this strategy may be less effective during brooding, when birds are spatially and temporally constrained. Our results are consistent with this finding, and suggest that less constrained foraging albatrosses benefit from large-scale wind patterns while more constrained brooding albatrosses have limited capacity to avoid headwinds while foraging. Increased wind speeds may be beneficial when albatrosses are travelling with tail winds, but may result in increased energetic costs when birds travel into headwinds. Brooding albatrosses spent proportionally more time flying in side winds as wind speed increased (figure 6), perhaps as a means of offsetting energetics costs by flying less directly into strong winds rather than facing winds head-on.
4.2. Cost of transport may amplify adverse effects of La Niña conditions on foraging habitat
Thorne et al. [70] found that La Niña conditions and associated oceanographic patterns shifted foraging habitat northward for Laysan and black-footed albatrosses breeding at Tern Island. This resulted in longer foraging trips and decreased reproductive success. We expected that stronger trade winds and increased wind speeds in brooding habitat during La Niña conditions would result in energetic benefits, but our results suggest the opposite. Thus, the negative impacts of La Niña conditions may be amplified, rather than mediated, by ENSO-driven wind patterns. Long-lived seabirds like albatrosses are thought to maximize their lifetime reproductive output by minimizing annual fecundity, and adults will abandon their chicks in order to maintain their own body condition during poor environmental conditions [96–98]. Increased energetic costs of brooding trips could contribute to the decreases in reproductive success observed for both species during La Niña conditions. ENSO-driven wind patterns may also play a role in observed differences in reproductive success between species. Incubating Laysan albatrosses experience increased wind speeds during El Niño conditions, while the more southerly foraging black-footed albatrosses do not. This may contribute to the higher reproductive success of Laysan albatrosses during El Niño conditions.
4.3. Importance of climate-driven changes to wind and ocean currents for marine vertebrates
We demonstrated impacts of wind on the movement of Laysan and black-footed albatrosses. Many other highly mobile marine vertebrates are known to exploit patterns of prevailing wind and ocean currents during movements and migrations (e.g. [36,37,99,100]). Influences of currents and wind can translate into energetic costs or benefits in central place foragers such as breeding pinnipeds and seabirds that can have consequences for reproduction and life history (e.g. [23,41]). Our findings suggest that in addition to impacts on the productivity and location of foraging habitat (e.g. [40,70,101,102]), climate-driven environmental variability may influence whether or not it is energetically feasible for central place foragers to reach these habitats.
Significantly, our results suggest that climate-driven changes in wind patterns can result in changes in habitat use, energy budgets and potentially reproductive success of albatrosses over relatively short time periods (annual time scales). Climate models predict much broader and more long-term changes to wind patterns and ocean currents that will probably amplify the short-term impacts of environmental variability demonstrated here. For example, trade winds are projected to weaken while mid-latitude westerlies are projected to strengthen and shift poleward [76,103–105]. Breeding Laysan and black-footed albatrosses could initially benefit from these changes, which would decrease wind speed in the foraging habitat of brooding birds and increase wind speed in habitat used during incubation. However, in the long term these benefits may be offset by increased energetic costs of reaching distant foraging grounds; northward shifts in westerlies would increase the distance from the breeding colony to regions of favourable wind speed (sensu [41]).
5. Conclusion
Shifts in species distributions in response to climate change have been well documented (e.g. [10,12,106]), but rapid rates of change suggest that some species may not be able to keep pace with changing climates [107,108]. Scenarios of future climate change suggest important implications for movement and cost of transport in marine vertebrates in addition to impacts on the productivity and location of foraging areas (e.g. [41,109]). Climate-driven environmental variability will directly impact the intensity, temporal variability and spatial location of ocean currents and wind patterns (e.g. [14,16,103–105,110]). These changes will be particularly relevant for marine vertebrates whose movement through air and ocean currents is likely to be directly impacted by oceanographic and atmospheric change, and for central place foragers that may be less flexible in their responses to climate change [111]. We suggest that wind and ocean currents are influential metrics of habitat connectivity and accessibility that are tightly linked to patterns of global change and should be accounted for when considering the ecological impacts of projected climate-driven environmental change.
Supplementary Material
Supplementary Material
Acknowledgements
We thank the Papahānaumokuākea Marine National Monument, the Hawaiian Islands National Wildlife Refuge, the US Fish and Wildlife Service and the Department of the Interior for permission to conduct research on Tern Island. We also thank Jill Awkerman, Yann Tremblay, Daniel Barton, Sarah Chisholm, Brian Erickson, Ian L Jones, Kirsten Lindquist, Scott Seganti, Colleen Siudzinski, Sabrina Luecht, Kelli Burkinshaw, Sarah Thomsen, Sarah Youngren, Ruth Brown, Caitlin Kroeger, Dan Rapp, Jimmy and Kristina Macaulay, and Morgan Gilmour.
Ethics
All protocols in this study were approved by the Institutional Animal Care and Use Committees at UCSC.
Authors' contributions
L.T. conducted movement and wind analyses and drafted the manuscript. M.C., M.K. and S.S. carried out fieldwork, assisted with data management and, along with E.H. and S.B., helped to draft the manuscript. D.C. assisted with data collection and coordination. S.S. lead and organized the fieldwork component of the study and coordinated the study. All authors read and approved the final manuscript.
Competing interests
We declare we have no competing interests.
Funding
This research was part of the Tagging of Pacific Pelagics (TOPP) program, supported by the Moore Foundation, Packard Endowment Grant to UCSC, the National Ocean Partnership Program (N00014-02-1-1012), the Office of Naval Research (N00014-00-1-0880 and N00014-03-1-0651). The Pacific Islands Climate Change Cooperative (PICCC) provided additional support for data analysis and synthesis (12170-B-G104 to S.A. Shaffer and E.L. Hazen).
References
- 1.Hays GC, Richardson AJ, Robinson C. 2005. Climate change and marine plankton. Trends Ecol. Evol. 20, 337–344. (doi:10.1016/j.tree.2005.03.004) [DOI] [PubMed] [Google Scholar]
- 2.Harley CD, Randall Hughes A, Hultgren KM, Miner BG, Sorte CJ, Thornber CS, Rodriguez LF, Tomanek L, Williams SL. 2006. The impacts of climate change in coastal marine systems. Ecol. Lett. 9, 228–241. (doi:10.1111/j.1461-0248.2005.00871.x) [DOI] [PubMed] [Google Scholar]
- 3.Hoegh-Guldberg O, et al. 2007. Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742. (doi:10.1126/science.1152509) [DOI] [PubMed] [Google Scholar]
- 4.Intergovernmental Panel on Climate Change 2007. Climate change 2007: synthesis report. Geneva, Switzerland: Intergovernmental Panel on Climate Change. [Google Scholar]
- 5.Belkin IM. 2009. Rapid warming of large marine ecosystems. Prog. Oceanogr. 81, 207–213. (doi:10.1016/j.pocean.2009.04.011) [Google Scholar]
- 6.Cheung WW, Lam VW, Sarmiento JL, Kearney K, Watson R, Pauly D. 2009. Projecting global marine biodiversity impacts under climate change scenarios. Fish Fish. 10, 235–251. (doi:10.1111/j.1467-2979.2008.00315.x) [Google Scholar]
- 7.Hoegh-Guldberg O, Bruno JF. 2010. The impact of climate change on the world's marine ecosystems. Science 328, 1523–1528. (doi:10.1126/science.1189930) [DOI] [PubMed] [Google Scholar]
- 8.Edwards M, Richardson AJ. 2004. Impact of climate change on marine pelagic phenology and trophic mismatch. Nature 430, 881–884. (doi:10.1038/nature02808) [DOI] [PubMed] [Google Scholar]
- 9.Winder M, Schindler DE. 2004. Climate change uncouples trophic interactions in an aquatic ecosystem. Ecology 85, 2100–2106. (doi:10.1890/04-0151) [Google Scholar]
- 10.Perry AL, Low PJ, Ellis JR, Reynolds JD. 2005. Climate change and distribution shifts in marine fishes. Science 308, 1912–1915. (doi:10.1126/science.1111322) [DOI] [PubMed] [Google Scholar]
- 11.Pörtner HO, Knust R. 2007. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315, 95–97. (doi:10.1126/science.1135471) [DOI] [PubMed] [Google Scholar]
- 12.Nye JA, Link JS, Hare JA, Overholtz WJ. 2009. Changing spatial distribution of fish stocks in relation to climate and population size on the Northeast United States continental shelf. Mar. Ecol. Progr. Ser. 393, 111–129. (doi:10.3354/meps08220) [Google Scholar]
- 13.Fyfe JC, Saenko OA. 2006. Simulated changes in the extratropical Southern Hemisphere winds and currents. Geophys. Res. Lett. 33, L06701 (doi:10.1029/2005GL025332) [Google Scholar]
- 14.Lovenduski NS, Gruber N. 2005. Impact of the Southern Annular Mode on Southern Ocean circulation and biology. Geophys. Res. Lett. 32, L11603 (doi:10.1029/2005GL022727) [Google Scholar]
- 15.Böning CW, Dispert A, Visbeck M, Rintoul S, Schwarzkopf FU. 2008. The response of the Antarctic Circumpolar Current to recent climate change. Nat. Geosci. 1, 864–869. (doi:10.1038/ngeo362) [Google Scholar]
- 16.Sallée J, Speer K, Morrow R. 2008. Response of the Antarctic Circumpolar Current to atmospheric variability. J. Clim. 21, 3020–3039. (doi:10.1175/2007JCLI1702.1) [Google Scholar]
- 17.Polovina JJ, Howell EA, Abecassis M. 2008. Ocean's least productive waters are expanding. Geophys. Res. Lett. 35, L03618 (doi:10.1029/2007GL031745) [Google Scholar]
- 18.Polovina JJ, Dunne JP, Woodworth PA, Howell EA. 2011. Projected expansion of the subtropical biome and contraction of the temperate and equatorial upwelling biomes in the North Pacific under global warming. ICES J. Mar. Sci. 68, 986–995. (doi:10.1093/icesjms/fsq198) [Google Scholar]
- 19.Burrows MT, et al. 2011. The pace of shifting climate in marine and terrestrial ecosystems. Science 334, 652–655. (doi:10.1126/science.1210288) [DOI] [PubMed] [Google Scholar]
- 20.Polovina JJ, Kobayashi DR, Parker DM, Seki MP, Balazs GH. 2000. Turtles on the edge: movement of loggerhead turtles (Caretta caretta) along oceanic fronts, spanning longline fishing grounds in the central North Pacific, 1997–1998. Fish. Oceanogr. 9, 71–82. (doi:10.1046/j.1365-2419.2000.00123.x) [Google Scholar]
- 21.Luschi P, Hays GC, Papi F. 2003. A review of long-distance movements by marine turtles, and the possible role of ocean currents. Oikos 103, 293–302. (doi:10.1034/j.1600-0706.2003.12123.x) [Google Scholar]
- 22.Gaspar P, Georges J-Y, Fossette S, Lenoble A, Ferraroli S, Le Maho Y. 2006. Marine animal behaviour: neglecting ocean currents can lead us up the wrong track. Proc. R. Soc. B 273, 2697–2702. (doi:10.1098/rspb.2006.3623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hindle AG, Rosen DA, Trites AW. 2010. Swimming depth and ocean currents affect transit costs in Steller sea lions Eumetopias jubatus. Aquat. Biol. 10, 139–148. (doi:10.3354/ab00279) [Google Scholar]
- 24.Chapman JW, Klaassen RH, Drake VA, Fossette S, Hays GC, Metcalfe JD, Reynolds AM, Reynolds DR, Alerstam T. 2011. Animal orientation strategies for movement in flows. Curr. Biol. 21, R861–R870. (doi:10.1016/j.cub.2011.08.014) [DOI] [PubMed] [Google Scholar]
- 25.Feldkamp SD. 1987. Swimming in the California sea lion: morphometrics, drag and energetics. J. Exp. Biol. 131, 117–135. [DOI] [PubMed] [Google Scholar]
- 26.Lovvorn JR. 2001. Upstroke thrust, drag effects, and stroke-glide cycles in wing-propelled swimming by birds. Am. Zool. 41, 154–165. (http://dx.doi.org/10.1093/icb/41.2.) [Google Scholar]
- 27.Lovvorn JR, Watanuki Y, Kato A, Naito Y, Liggins GA. 2004. Stroke patterns and regulation of swim speed and energy cost in free-ranging Brünnich's guillemots. J. Exp. Biol. 207, 4679–4695. (doi:10.1242/jeb.01331) [DOI] [PubMed] [Google Scholar]
- 28.Heath JP, Gilchrist HG, Ydenberg RC. 2006. Regulation of stroke pattern and swim speed across a range of current velocities: diving by common eiders wintering in polynyas in the Canadian Arctic. J. Exp. Biol. 209, 3974–3983. (doi:10.1242/jeb.02482) [DOI] [PubMed] [Google Scholar]
- 29.Goldbogen JA, Pyenson ND, Shadwick RE. 2007. Big gulps require high drag for fin whale lunge feeding. Mar. Ecol. Prog. Ser. 349, 289–301. (doi:10.3354/meps07066) [Google Scholar]
- 30.Goldbogen JA, Calambokidis J, Croll DA, Harvey JT, Newton KM, Oleson EM, Schorr G, Shadwick RE. 2008. Foraging behavior of humpback whales: kinematic and respiratory patterns suggest a high cost for a lunge. J. Exp. Biol. 211, 3712–3719. (doi:10.1242/jeb.023366) [DOI] [PubMed] [Google Scholar]
- 31.Wilson R, Culik B. 1992. Packages on penguins and device-induced data. Wildlife telemetry: remote monitoring and tracking of animals. Chichester, UK: Ellis Horward, 573–580. [Google Scholar]
- 32.Sæther B-E, Andersen R, Pedersen HC. 1993. Regulation of parental effort in a long-lived seabird an experimental manipulation of the cost of reproduction in the Antarctic petrel, Thalassoica antarctica. Behav. Ecol. Sociobiol. 33, 147–150. (doi:10.1007/BF00216594) [Google Scholar]
- 33.Chastel O, Weimerskirch H, Jouventin P. 1995. Body condition and seabird reproductive performance: a study of three petrel species. Ecology 76, 2240–2246. (doi:10.2307/1941698) [Google Scholar]
- 34.Navarro J, González-Solís J. 2007. Experimental increase of flying costs in a pelagic seabird: effects on foraging strategies, nutritional state and chick condition. Oecologia 151, 150–160. (doi:10.1007/s00442-006-0559-0) [DOI] [PubMed] [Google Scholar]
- 35.Brill RW, Holts D, Chang R, Sullivan S, Dewar H, Carey F. 1993. Vertical and horizontal movements of striped marlin (Tetrapturus audax) near the Hawaiian Islands, determined by ultrasonic telemetry, with simultaneous measurement of oceanic currents. Mar. Biol. 117, 567–574. (doi:10.1007/BF00349767) [Google Scholar]
- 36.Luschi P, Sale A, Mencacci R, Hughes G, Lutjeharms J, Papi F. 2003. Current transport of leatherback sea turtles (Dermochelys coriacea) in the ocean. Proc. R. Soc. Lond. B 270, S129–S132. (doi:10.1098/rsbl.2003.0036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lambardi P, Lutjeharms JR, Mencacci R, Hays GC, Luschi P. 2008. Influence of ocean currents on long-distance movement of leatherback sea turtles in the Southwest Indian Ocean. Mar. Ecol. Prog. Ser. 353, 289–301. (doi:10.3354/meps07118) [Google Scholar]
- 38.Shiomi K, Sato K, Mitamura H, Arai N, Naito Y, Ponganis PJ. 2008. Effect of ocean current on the dead-reckoning estimation of 3-D dive paths of emperor penguins. Aquat. Biol. 3, 265–270. (doi:10.3354/ab00087) [Google Scholar]
- 39.Grémillet D, Boulinier T. 2009. Spatial ecology and conservation of seabirds facing global climate change: a review. Mar. Ecol. Prog. Ser. 391, 121–137. (doi:10.3354/meps08212) [Google Scholar]
- 40.Hazen EL, et al. 2013. Predicted habitat shifts of Pacific top predators in a changing climate. Nat. Clim. Change 3, 234–238. (doi:10.1038/nclimate1686) [Google Scholar]
- 41.Weimerskirch H, Louzao M, De Grissac S, Delord K. 2012. Changes in wind pattern alter albatross distribution and life-history traits. Science 335, 211–214. (doi:10.1126/science.1210270) [DOI] [PubMed] [Google Scholar]
- 42.Pennycuick C. 1982. The flight of petrels and albatrosses (Procellariiformes), observed in South Georgia and its vicinity. Phil. Trans. R. Soc. Lond. B 300, 75–106. (doi:10.1098/rstb.1982.0158) [Google Scholar]
- 43.Spear LB, Ainley DG. 1997. Flight behaviour of seabirds in relation to wind direction and wing morphology. Ibis 139, 221–233. (doi:10.1111/j.1474-919X.1997.tb04620.x) [Google Scholar]
- 44.Suryan RM, et al. 2008. Wind, waves, and wing loading: morphological specialization may limit range expansion of endangered albatrosses. PLoS ONE 3, e4016 (doi:10.1371/journal.pone.0004016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weimerskirch H, Salamolard M, Sarrazin F, Jouventin P. 1993. Foraging strategy of wandering albatrosses through the breeding season: a study using satellite telemetry. Auk, 110, 325–342. [Google Scholar]
- 46.Weimerskirch H, Guionnet T, Martin J, Shaffer SA, Costa D. 2000. Fast and fuel efficient? Optimal use of wind by flying albatrosses. Proc. R. Soc. Lond. B 267, 1869–1874. (doi:10.1098/rspb.2000.1223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaffer SA, Costa DP, Weimerskirch H. 2003. Foraging effort in relation to the constraints of reproduction in free-ranging albatrosses. Funct. Ecol. 17, 66–74. (doi:10.1046/j.1365-2435.2003.00705.x) [Google Scholar]
- 48.Wakefield ED, Phillips RA, Matthiopoulos J, Fukuda A, Higuchi H, Marshall GJ, Trathan PN. 2009. Wind field and sex constrain the flight speeds of central-place foraging albatrosses. Ecol. Monogr. 79, 663–679. (doi:10.1890/07-2111.1) [Google Scholar]
- 49.Costa D, Prince P. 1987. Foraging energetics of Grey-headed Albatrosses Diotnedea chrysostoma at Bird Island, South Georgia. Ibis 129, 149–158. (doi:10.1111/j.1474-919X.1987.tb03196.x) [Google Scholar]
- 50.Shaffer SA, Costa DP, Weimerskirch H. 2001. Behavioural factors affecting foraging effort of breeding wandering albatrosses. J. Anim. Ecol. 70, 864–874. (doi:10.1046/j.0021-8790.2001.00548.x) [Google Scholar]
- 51.Costa DP, Shaffer SA. 2012. Seabirds and marine mammals. In Metabolic ecology: a scaling approach, pp. 225–233. Chichester, UK: John Wiley & Sons. [Google Scholar]
- 52.Prince P, Wood A, Barton T, Croxall J. 1992. Satellite tracking of wandering albatrosses (Diomedea exulans) in the South Atlantic. Antarct. Sci. 4, 31–36. (doi:10.1017/S0954102092000075) [Google Scholar]
- 53.Weimerskirch H, Bonadonna F, Bailleul F, Mabille G, Dell'Omo G, Lipp H-P. 2002. GPS tracking of foraging albatrosses. Science 295, 1259–1259 (doi:10.1126/science.1068034) [DOI] [PubMed] [Google Scholar]
- 54.Sachs G. 2005. Minimum shear wind strength required for dynamic soaring of albatrosses. Ibis 147, 1–10. (doi:10.1111/j.1474-919x.2004.00295.x) [Google Scholar]
- 55.Sachs G, Traugott J, Nesterova AP, Dell'Omo G, Kümmeth F, Heidrich W, Vyssotski AL, Bonadonna F. 2012. Flying at no mechanical energy cost: disclosing the secret of wandering albatrosses. PLoS ONE 7, e41449 (doi:10.1371/journal.pone.0041449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Catry P, Phillips RA, Croxall JP, Burger A. 2004. Sustained fast travel by a gray-headed albatross (Thalassarche chrysostoma) riding an Antarctic storm. Auk 121, 1208–1213. (doi:10.1642/0004-8038(2004)121[1208:SFTBAG]2.0.CO;2) [Google Scholar]
- 57.Barber RT, Chavez FP. 1983. Biological consequences of El Niño. Science 222, 1203–1210. (doi:10.1126/science.222.4629.1203) [DOI] [PubMed] [Google Scholar]
- 58.Schreiber RW, Schreiber EA. 1984. Central Pacific seabirds and the El Niño southern oscillation: 1982 to 1983 perspectives. Science 225, 713–716. (doi:10.1126/science.225.4663.713) [DOI] [PubMed] [Google Scholar]
- 59.Lehodey P, Bertignac M, Hampton J, Lewis A, Picaut J. 1997. El Niño Southern Oscillation and tuna in the western Pacific. Nature 389, 715–718. (doi:10.1038/39575) [Google Scholar]
- 60.Stenseth NC, Ottersen G, Hurrell JW, Mysterud A, Lima M, Chan KS, Yoccoz NG, Ådlandsvik B. 2003. Studying climate effects on ecology through the use of climate indices: the North Atlantic Oscillation, El Nino Southern Oscillation and beyond. Proc. R. Soc. Lond. B 270, 2087–2096. (doi:10.1098/rspb.2003.2415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cairns D. 1988. Seabirds as indicators of marine food supplies. Biol. Oceanogr. 5, 261–271. [Google Scholar]
- 62.Velarde E, Ezcurra E, Cisneros-Mata MA, LavÍn MF. 2004. Seabird ecology, El Niño anomalies, and prediction of sardine fisheries in the Gulf of California. Ecol. Appl. 14, 607–615. (doi:10.1890/02-5320) [Google Scholar]
- 63.Philander SG. 1990. El Niño, La Niña and the Southern Oscillation. Int. Geophys. Ser. 46, 35–38. [Google Scholar]
- 64.Karl D, Letelier R, Hebel D, Tupas L, Dore J, Christian J, Winn C. 1995. Ecosystem changes in the North Pacific subtropical gyre attributed to the 1991–92 El Nino. Nature 373, 230–234. (doi:10.1038/373230a0) [Google Scholar]
- 65.Chavez F, Strutton P, Friederich G, Feely R, Feldman G, Foley D, McPhaden M. 1999. Biological and chemical response of the equatorial Pacific Ocean to the 1997–98 El Niño. Science 286, 2126–2131. (doi:10.1126/science.286.5447.2126) [DOI] [PubMed] [Google Scholar]
- 66.Chavez F, et al. 2002. Biological and chemical consequences of the 1997–1998 El Niño in central California waters. Prog. Oceanogr. 54, 205–232. (doi:10.1016/S0079-6611(02)00050-2) [Google Scholar]
- 67.Hollowed AB, Hare SR, Wooster WS. 2001. Pacific Basin climate variability and patterns of Northeast Pacific marine fish production. Prog. Oceanogr. 49, 257–282. (doi:10.1016/S0079-6611(01)00026-X) [Google Scholar]
- 68.Hodder J, Graybill MR. 1985. Reproduction and survival of seabirds in Oregon during the 1982–1983 El Nino. Condor 87, 535–541. (doi:10.2307/1367954) [Google Scholar]
- 69.Wilson UW. 1991. Responses of three seabird species to El Niño events and other warm episodes on the Washington coast, 1979–1990. Condor 93, 853–858. (doi:10.2307/3247719) [Google Scholar]
- 70.Thorne LH, et al. 2015. Foraging behavior links climate variability and reproduction in North Pacific albatrosses. Mov. Ecol. 3, 1–15. (doi:10.1186/s40462-015-0050-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marinovic B, Croll D, Gong N, Benson S, Chavez F. 2002. Effects of the 1997–1999 El Niño and La Niña events on zooplankton abundance and euphausiid community composition within the Monterey Bay coastal upwelling system. Prog. Oceanogr. 54, 265–277. (doi:10.1016/S0079-6611(02)00053-8) [Google Scholar]
- 72.Sanchez-Velasco L, Valdez-Holguın J, Shirasago B, Cisneros-Mata MA, Zarate A. 2002. Changes in the spawning environment of Sardinops caeruleus in the Gulf of California during El Nino 1997–1998. Estuar. Coast. Shelf Sci. 54, 207–217. (doi:10.1006/ecss.2001.0840) [Google Scholar]
- 73.Crocker DE, Costa DP, Le Boeuf BJ, Webb PM, Houser DS. 2006. Impact of El Niño on the foraging behavior of female northern elephant seals. Mar. Ecol. Prog. Ser. 309, 1–10. (doi:10.3354/meps309001) [Google Scholar]
- 74.Antonelis GA, Baker JD, Polovina JJ. 2003. Improved body condition of weaned Hawaiian monk seal pups associated with El Niño events: potential benefits to an endangered species. Mar. Mammal Sci. 19, 590–598. (doi:10.1111/j.1748-7692.2003.tb01323.x) [Google Scholar]
- 75.Schwing F, Murphree T, Dewitt L, Green PM. 2002. The evolution of oceanic and atmospheric anomalies in the northeast Pacific during the El Niño and La Niña events of 1995–2001. Prog. Oceanogr. 54, 459–491. (doi:10.1016/S0079-6611(02)00064-2) [Google Scholar]
- 76.Collins M, et al. 2010. The impact of global warming on the tropical Pacific Ocean and El Niño. Nat. Geosci. 3, 391–397. (doi:10.1038/ngeo868) [Google Scholar]
- 77.Warham J. 1990. The petrels: their ecology and breeding systems. San Diego, CA: Academic Press. [Google Scholar]
- 78.Lewison RL, Crowder LB, Read AJ, Freeman SA. 2004. Understanding impacts of fisheries bycatch on marine megafauna. Trends Ecol. Evol. 19, 598–604. (doi:10.1016/j.tree.2004.09.004) [Google Scholar]
- 79.Arata JA, Sievert PR, Naughton MB. 2009. Status assessment of Laysan and black-footed albatrosses, North Pacific Ocean, 1923–2005. Reston, VA: US Geological Survey. [Google Scholar]
- 80.Kappes MA, Shaffer SA, Tremblay Y, Foley DG, Palacios DM, Bograd SJ, Costa DP. 2015. Reproductive constraints influence habitat accessibility, segregation, and preference of sympatric albatross species. Mov. Ecol. 3, 1–24. (doi:10.1186/s40462-015-0063-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kappes MA, Shaffer SA, Tremblay Y, Foley DG, Palacios DM, Robinson PW, Bograd SJ, Costa DP. 2010. Hawaiian albatrosses track interannual variability of marine habitats in the North Pacific. Prog. Oceanogr. 86, 246–260. (doi:10.1016/j.pocean.2010.04.012) [Google Scholar]
- 82.Rice DW, Kenyon KW. 1962. Breeding distribution, history, and populations of North Pacific albatrosses. Auk 79, 365–386. (doi:10.2307/4082822) [Google Scholar]
- 83.Hyrenbach KD, Fernández P, Anderson DJ. 2002. Oceanographic habitats of two sympatric North Pacific albatrosses during the breeding season. Mar. Ecol. Prog. Ser. 233, 283–301. (doi:10.3354/meps233283) [Google Scholar]
- 84.Amerson AB. 1971. The natural history of French Frigate Shoals north-western Hawaiian Islands: vegetation. Atoll Res Bull 150, 62–79. (doi:10.5479/si.00775630.150.1) [Google Scholar]
- 85.Phillips RA, Xavier JC, Croxall JP, Burger A. 2003. Effects of satellite transmitters on albatrosses and petrels. Auk 120, 1082–1090. (doi:10.1642/0004-8038(2003)120[1082:EOSTOA]2.0.CO;2) [Google Scholar]
- 86.Costa DP, et al. 2010. Accuracy of ARGOS locations of pinnipeds at-sea estimated using Fastloc GPS. PLoS ONE 5, e8677 (doi:10.1371/journal.pone.0008677) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.GiPSy. 2007. Micro GPS datalogger for tracking free-moving birds and mammals. Rome, Italy: Technosmart. [Google Scholar]
- 88.Portabella M, Stoffelen A. 2001. Rain detection and quality control of SeaWinds. J. Atmos. Ocean. Technol. 18, 1171–1183. (doi:10.1175/1520-0426(2001)018<1171:RDAQCO>2.0.CO;2) [Google Scholar]
- 89.Pickett MH, Tang W, Rosenfeld LK, Wash CH. 2003. QuikSCAT satellite comparisons with nearshore buoy wind data off the US west coast. J. Atmos. Ocean. Technol. 20, 1869–1879. (doi:10.1175/1520-0426(2003)020<1869:QSCWNB>2.0.CO;2) [Google Scholar]
- 90.Bentamy A, Fillon DC. 2012. Gridded surface wind fields from Metop/ASCAT measurements. AAPG Bull. 33, 1729–1754. (doi:10.1080/01431161.2011.600348) [Google Scholar]
- 91.Yee TW, Mitchell ND. 1991. Generalized additive models in plant ecology. J. Veg. Sci. 2, 587–602. (doi:10.2307/3236170) [Google Scholar]
- 92.Guisan A, Edwards TC, Hastie T. 2002. Generalized linear and generalized additive models in studies of species distributions: setting the scene. AAPG Bull. 157, 89–100. (doi:10.1016/S0304-3800(02)00204-1) [Google Scholar]
- 93.Jouventin P, Weimerskirch H. 1990. Satellite tracking of wandering albatrosses. Nature 343, 746–748. (doi:10.1038/343746a0) [Google Scholar]
- 94.Phillips R, Silk J, Phalan B, Catry P, Croxall J. 2004. Seasonal sexual segregation in two Thalassarche albatross species: competitive exclusion, reproductive role specialization or foraging niche divergence? Proc. R. Soc. Lond. B 271, 1283–1291. (doi:10.1098/rspb.2004.2718) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Phillips R, Silk J, Croxall J. 2005. Foraging and provisioning strategies of the light-mantled sooty albatross at South Georgia: competition and co-existence with sympatric pelagic predators. Mar. Ecol. Prog. Ser. 285, 259–270. (doi:10.3354/meps285259) [Google Scholar]
- 96.Chaurand T, Weimerskirch H. 1994. Incubation routine, body mass regulation and egg neglect in the blue petrel Halobaena caerulea. Ibis 136, 285–290. (doi:10.1111/j.1474-919X.1994.tb01097.x) [Google Scholar]
- 97.Erikstad KE, Asheim M, Fauchald P, Dahlhaug L, Tveraa T, Dahlhaug P. 1997. Adjustment of parental effort in the puffin; the roles of adult body condition and chick size. Behav. Ecol. Sociobiol. 40, 95–100. (doi:10.1007/s002650050320) [Google Scholar]
- 98.Weimerskirch H, Zimmermann L, Prince PA. 2001. Influence of environmental variability on breeding effort in a long-lived seabird, the yellow-nosed albatross. Behav. Ecol. 12, 22–30. (doi:10.1093/oxfordjournals.beheco.a000374) [Google Scholar]
- 99.Dadswell M, Spares A, Reader J, Stokesbury M. 2010. The North Atlantic subpolar gyre and the marine migration of Atlantic salmon Salmo salar: the ‘Merry-Go-Round'hypothesis. J. Fish Biol. 77, 435–467. (doi:10.1111/j.1095-8649.2010.02673.x) [DOI] [PubMed] [Google Scholar]
- 100.Felicísimo ÁM, Muñoz J, González-Solis J. 2008. Ocean surface winds drive dynamics of transoceanic aerial movements. PLoS ONE 3, e2928 (doi:10.1371/journal.pone.0002928) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bost C-A, Cotté C, Bailleul F, Cherel Y, Charrassin J-B, Guinet C, Ainley DG, Weimerskirch H. 2009. The importance of oceanographic fronts to marine birds and mammals of the southern oceans. J. Mar. Sys. 78, 363–376. (doi:10.1016/j.jmarsys.2008.11.022) [Google Scholar]
- 102.Block BA, et al. 2011. Tracking apex marine predator movements in a dynamic ocean. Nature 475, 86–90. (doi:10.1038/nature10082) [DOI] [PubMed] [Google Scholar]
- 103.Yin JH. 2005. A consistent poleward shift of the storm tracks in simulations of 21st century climate. Geophys. Res. Lett. 32, L18701 (doi:10.1029/2005GL023684) [Google Scholar]
- 104.Toggweiler J, Russell JL, Carson S. 2006. Midlatitude westerlies, atmospheric CO2, and climate change during the ice ages. Paleoceanography 21, PA2005 (doi:10.1029/2005PA001154) [Google Scholar]
- 105.Toggweiler J, Russell J. 2008. Ocean circulation in a warming climate. Nature 451, 286–288. (doi:10.1038/nature06590) [DOI] [PubMed] [Google Scholar]
- 106.Walther G-R, Post E, Convey P, Menzel A, Parmesan C, Beebee TJ, Fromentin J-M, Hoegh-Guldberg O, Bairlein F. 2002. Ecological responses to recent climate change. Nature 416, 389–395. (doi:10.1038/416389a) [DOI] [PubMed] [Google Scholar]
- 107.Visser ME. 2008. Keeping up with a warming world; assessing the rate of adaptation to climate change. Proc. R. Soc. B 275, 649–659. (doi:10.1098/rspb.2007.0997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Loarie SR, Duffy PB, Hamilton H, Asner GP, Field CB, Ackerly DD. 2009. The velocity of climate change. Nature 462, 1052–1055. (doi:10.1038/nature08649) [DOI] [PubMed] [Google Scholar]
- 109.Cai W, Shi G, Cowan T, Bi D, Ribbe J. 2005. The response of the Southern Annular Mode, the East Australian Current, and the southern mid-latitude ocean circulation to global warming. Geophys. Res. Lett. 32, L23706 (doi:10.1029/2005GL024701) [Google Scholar]
- 110.Péron C, Weimerskirch H, Bost C-A. 2012. Projected poleward shift of king penguins’(Aptenodytes patagonicus) foraging range at the Crozet Islands, southern Indian Ocean. Proc. R. Soc. B 279, 2515–2523. (doi:10.1098/rspb.2011.2705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hays GC, et al. 2016. Key questions in marine megafauna movement ecology. Trends Ecol. Evol. 2076, 1–13. (doi:10.1016/j.tree.2016.02.015) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.