Abstract
Waxy maize is prevalently grown in China and other countries due to the excellent characters and economic value. However, its low content of lysine can’t meet the nutritional requirements of humans and livestock. In the present study, we introgressed the opaque2 (o2) allele into waxy maize line Zhao OP-6/O2O2 by using marker-assisted selection (MAS) technique and successfully improved the lysine content and quality of waxy maize. Transcript abundance analysis indicated that the wx1 expression levels had no difference between Zhao OP-6/o2o2 and Zhao OP-6/O2O2. However, Zhao OP-6/o2o2 was characterized by a phenotype of hard and vitreous kernels and accumulation of protein bodies at smaller size (one third of that of parents) but in larger numbers. Biochemical analyses showed that Zhao OP-6/o2o2 had 16.7% less free amino acids than Zhao OP-6/O2O2, especially those derived from glycolytic intermediates, but its content of lysine was increased by 51.6% (0.47% vs. 0.31%). The content of amylopectin was 98.5% in Zhao OP-6/o2o2, significantly higher than that in Zhao OP-6/O2O2 (97.7%). Proteomic analyses indicated that o2 introgression not only decreased the accumulation of various zein proteins except for 27-kDa γ-zein, but also affected other endosperm proteins related to amino acid biosynthesis, starch-protein balance, stress response and signal transduction. This study gives us an intriguing insight into the metabolism changes in endosperm of waxy maize introgressed with opaque2.
Introduction
Waxy maize (Zea mays L. sinensis Kulesh), also known as sticky maize, is one sub-type of maize that was first discovered in Southwestern China and then prevalently grown in other Asian countries [1–3]. The endosperm of waxy maize has a high content of amylopectin (nearly 100%), and is thus characterized by high viscosity, easy digestion, and good light transmittance [4]. These excellent characters and fresh harvest make waxy maize widely used in frozen food processing, paper-making and livestock feeding industries. However, due to the limited levels and types of essential amino acids, especially lysine, the nutritional value of waxy maize is relatively low. Generally, the lysine content in maize grain should be more than 0.5% (>51 mg per gram of protein) to meet human and livestock requirements [5], but waxy maize has a lysine content of only 0.24–0.34%. By introgression of opaque2 (o2) and opaque2 modifier (o2m) alleles into elite maize inbred lines with marker-assisted selection (MAS) technique, the genetically modified opaque2 maize, also known as quality protein maize (QPM), shows an improved lysine content of approximately 0.4% [6–9]. Therefore, it is of importance to breed a novel waxy maize line with high lysine content by introgressing the o2 and o2m traits with MAS.
Accumulation of starch and storage proteins occurs in the developing endosperm of maize, the quality of which is contributed to by the action of the Waxy1 (Wx1) and Opaque2 (O2) genes [10]. The single copy 3.8-kb Wx1 contains 14 exons and is mapped on the short arm of chromosome 9 [11,12]. Previous studies have shown that transposable elements Ac/Ds and En/Spm, deletion mutation and mutagenic ethylmethane sulfonate (EMS) mutagenesis account for the pre-mRNA splicing or translation errors and result in a low expression level of Wx1 [13]. As a result, the granule-bound starch synthase I (GBSS I) activity of the wx1 mutant has a decreased activity (5–95%) in amylose synthesis, leading to the low level of amylose but high level of amylopectin in maize endosperm and pollen [14,15]. O2 is a transcriptional factor that contains a basic leucine-zipper (bZIP) motif. It is specifically expressed in the developing endosperm and directly regulates the expression of 22-kDa α-zeins [16–18]. The substantial reduction of α-zeins is concomitant with increased accumulation of non-zeins, consequently accounting for the increased contents of lysine and tryptophan in maize mutants [19, 20]. In addition, a large number of studies have shown that O2 also has pleiotropic effects on the expression of non-storage proteins including ribosome-inactivating protein b-32 (RIP), cytosolic pyruvate phosphate dikinase 1 (cyPPDK1), lysine ketoglutarate reductase-saccaropine dehydrogenase (LKR-SDH), acetohydroxyacid synthase (AHAS) and Opaque2 heterodimerizing protein 1 (OHP1) [21–23]. Thus O2 as a regulator plays a crucial role in maize endosperm development by influencing the storage protein and nitrogen/carbon metabolism. Although the individual functions of Wx1 and O2 are well known, how these genes interact to maintain the starch-protein balance is yet unknown.
It has been reported that o2 mutation can alter the transcriptional patterns of Wx1 in varying degrees [24–26], but no evidence revealed the regulatory effect of o2 on the expression of wx1. By backcrossing of o2 and o16 traits with MAS, the quality and lysine content of waxy maize have been successfully improved [27,28]. However, the molecular mechanism underlying the ameliorated amino acid composition of maize endosperm and specific kernel phenotype is yet unknown. In o2 mutants, genes associated with glycolytic pathway, endoplasmic reticulum (ER) stress responses and amino acid synthesis demonstrated differences in transcript profiles [23], thus offering an unbiased hypothesis that some novel mechanisms play a vital role in the modification of waxy maize endosperm.
To disclose the extensive changes of endosperm metabolism when introgressed o2 into waxy maize Zhao OP-6/O2O2, we constructed a set of near-isogenic lines (NILs) with QPM as the donor, and performed submicroscopic observations of the endosperm structure and biochemical analysis of the protein bodies and nutrient contents. Further proteomic analysis of immature seeds identified several specific proteins involved in metabolic pathways, such as synthesis of starch and protein, composition of amino acids and carbohydrate metabolism. Combined analysis of the results gives us an intriguing insight into the effect of integrating o2 and wx1 on the metabolism of maize developing endosperm.
Materials and Methods
Plant materials
QPM CA339 derived from pool33 (Centro Internacional de Mejoramientode Maizy Trigo, Mexico DF, Mexico) was used as the non-recurrent parent (donor). The elite waxy inbred line Zhao OP-6 (Maize Research Center of Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing, China) was used as a recurrent parent (receptor). The o2-wx NILs, Zhao OP-6/o2o2, were selected from the two parents by using MAS technique. Co-dominant SSR markers phi057 and phi027 were used to select heterozygous (O2WX/o2wx) individuals, while the dominant SSR marker phi112 was used to detect transposable element rbg at BC6F1 [29]. All heterozygous genotypes were converted to the recurrent parent Zhao OP-6 through six backcrossing cycles, followed by three rounds of self-pollination. Theoretically, the o2-wx NILs had 99% of the recurrent parent genome, and were phenotypically uniform and genetically homogeneous after six generations of backcrossing. All of the maize materials were grown in adjacent plots in the experimental station (N40°36′ and E116°34′) of Chinese Academy of Agricultural Sciences during the summer of 2013.
A minimum of three well-filled ears of each genotype were sampled at 18 day after pollination (DAP), when the conversion of importing sucrose and amino acids into starch and storage proteins reached a high level. Ears were picked up at approximately noon, and kernels were collected from the centre of each ear. Embryos and surrounding pericarps were dissected, frozen immediately in liquid nitrogen, and stored at -80°C before use.
Mature kernels were harvested after physiological maturity and dried in a green house. To avoid biological variations, equal numbers of well-filled ears (≥ 3) were pooled and treated as one sample, and each experiment had two or more replicates.
DNA extraction, PCR amplification, electrophoresis and genotype analysis
Seedling leaves of parents and offsprings were collected and used for DNA extraction with CTAB method. The integrity and quality of DNA were detected by electrophoresis in 1% agarose gel, and the DNA concentration was adjusted to ~100 ng/μL. Primers specific for phi057, phi027, phi112 and 100 SSR markers were adopted from the maize genome database MaizeGDB (http://www.maizegdb.org) and synthesized by AuGCT (Beijing, China). PCR amplification and product analysis were performed as reported previously [29].
To establish a genotype database of each individual, band patterns A, B, H, and U of the 100 SSR markers distributed on ten maize chromosomes were adopted. Pattern A indicated the origin of recurrent parent Zhao OP-6, B for non-recurrent parent CA339, H for heterozygous genotype, and U for unidentified genotype. According to the statistical analysis, the genetic background recovery rate of BC6F3 individuals was calculated with the formula G (g) = [L + X (g)] / (2L), in which G indicates the number of backcross generations, X indicates the number of molecular markers with the same pattern as parent Zhao OP-6, and L is the number of polymorphic SSR markers. The formula E [G(g)] = 1 - (1/2) g+1 was used to calculate the theoretical genetic background recovery rate, and G referred to the number of backcross generations.
Abundance analysis of o2 and wx transcripts
Developing maize endosperm was collected from the center of three well-filled ears at 18 DAP. Total RNA was individually extracted with TRIZol kit (Thermo Fisher) and pooled together at the same amount. After DNA removal with RNase-free DNase I (Sigma-Aldrich), the cDNA was synthesized using an oligo d(T) primer kit (Promega). Two pairs of specific primers that spanned the exons of o2 and wx1 (O2RT-F: 5′-TCAGGAATAATCCAGTGCAGAA-3′ and O2RT-R: 5′-TCGACGTTAGCGTCGTTGTA-3′, and WXRT-F: 5′-TGTAGCTGCTTGCTTGTGCT-3′ and WXRT-R: 5′-CACCGAACAGCAGGGATTAT-3′) and one primer pair specific for the glyceraldehyde-3-posphate dehydrogenase (GAPDH) gene as the reference (GAPDH-F: 5′-CCCTTCATCACCACGGACTAC-3′ and GAPDH-R: 5′-AACCTTCTTGGCACCACCCT-3′) were designed for the analysis of transcript abundance. The PCR amplification products were separated on 1.5% agarose gels for analysis.
Kernel characteristics and structure observation
The characteristics and appearance of intact kernels were collected by a SONY α700 camera (mode A). Endosperm hardness was graded from 1 to 5 following the CIMMYT universal standards, i.e., grade 1 (completely vitreous) to grade 5 (completely opaque), with a 25% difference between grades [30]. The kernel density was calculated by dividing the weight of 50 kernels by the volume (displacement of 95% ethanol in a cylinder) [31]. Mature kernels were peeled with a blade at the peripheral region, spray-coated with platinum, and observed under a Hitachi scanning electron microscope (SEM, S3400N). Developing kernels at 21 DAP were prepared as below: kernels (including partial pericarp) (1 mm × 3 mm × 1 mm) were sequentially fixed in 2.5% (w/v) glutaraldehyde and paraformaldehyde, followed by post-fixation in osmium tetraoxide. After dehydration in a gradient of ethanol (75−100%), samples were transferred to a propylene oxide solution and gradually embedded in paraffin. Sections of samples were prepared by a diamond knife microtome and observed under a Hitachi H7600 transmission electron microscope (TEM). Submicroscopic structure analysis was performed at the Institute of Food Science and Technology of Chinese Academy of Agricultural Sciences.
Biochemical characterization and protein quantification
Mature kernels were dried at 65°C to constant weight and pulverized to a fine powder for the analysis of lysine and crude protein contents (at least two replicates and 40 g powder per sample). Crude protein content was measured with the Kjeldahl method according to the Chinese National Standard GB2905-82 (Nitrogen-to-protein conversion factor, K = 6.25). To determine the contents of 17 free amino acids (FAAs), all samples were pretreated following the Chinese National Standard GB7649-87, and analyzed by the S433D full-automatic amino acid analyzer (Beckman Coulter). The content of kernel crude oil was determined by using the Bruker Minispec MQ20 NMR Analyzer. Total starch was extracted, the content of amylose was measured by using the AUTOPOL III Polarimeter (Rudolph), and the percentage of amylopectin was calculated.
Zein and non-zein proteins were extracted according to the previous study with some modifications [32]. For each sample, a total of 15 mature kernels were collected from the centre of three well-filled ears (5 kernels from each ear), mixed, and soaked in distilled water for 6 h. Pericarps were then removed without damaging endosperm, and endosperm was further ground into a fine powder in liquid nitrogen. Powdered endosperm (50 mg per sample) was transferred to a 2 mL Eppendorf tube and incubated in 0.4 mL of extraction buffer (70% ethanol, 2% 2-mercaptoethanol, and 1% SDS) at 37°C with agitation for 2~3 h. After 10-min centrifugation (12,000 rpm) at room temperature (Centrifuge 5424R, Eppendorf), the supernatant comprised the zein fraction, and the sediment consisted of nonzein proteins. Extracted protein were measured using a bicinchoninic acid protein assay kit (Solarbio) according to the instructions. In order to identify the change of zein composition, aliquots of each supernatant (100 μL) were transferred to a fresh tube and dried at 50°C until the liquid evaporated absolutely. Distilled water (100 μL) was then added to dissolve the pellets. The same amounts of samples based on protein concentration were loaded onto a polyacrylamide gel (4% stacking gel and 15% separation gel), followed by staining with Coomassie Brilliant Blue R250 (Bio-Rad). Each sample had two replicates.
2D SDS-PAGE
Developing kernels at 18 DAP were used for protein extraction and 2-D SDS-PAGE analysis. The pooled endosperm powder (50 mg) was subjected to protein extraction using the acetone precipitation method [21] and dried in a lyophilizer at -20°C. The freeze-dried samples were then resuspended in IPG lysate buffer (0.2 M urea, 5% CHAPS, 50 mM thiocarbamide, 0.7% DTT, and 40% Bio-lyte) and incubated at 4°C for 1 h with frequent shaking. After removal of the insoluble fraction (12,000 rpm, 4°C and 15 min), soluble proteins were quantified by using a 2-D Quant Kit (GE Healthcare) with bovine serum albumin as a standard. IPG rehydration buffer (0.2 M urea, 5% CHAPS, 0.05 M thiocarbamide, 0.7% DTT, 40% Bio-lyte, and 1% bromophenol blue) was then added to adjust the final volume to 350~400 μL. The first dimension separation was performed using the Ettan IPG-phor-II (GE Healthcare). Aliquots of protein extract (~600 μg) were separated on 18 cm Immobiline Dry Strips (pH 5–8, Bio-Rad), with three technical replicates per sample. After rehydration for 14 h at 50 V, isoelectric focusing (IEF) was performed at 18°C following the procedures as shown below: 50 mA per strip and 250 V STEP for 1 h, 500 V STEP for 1 h, 2000 V GRAD for 30 min, 2000 V STEP for 1 h, 5000 V GRAD for 30 min, 5000 V STEP for 1 h, 8000 V GRAD for 2 h, 30000 V for focus and finally 500 V STEP for 10 h. The strips were then immersed in 8 mL of two types of equilibration buffer for 15 min with agitation. The immobilized proteins were subjected to second dimension separation on 12% SDS-PAGE gel at 16°C using the Mini-Protein II vertical gel apparatus (Bio-Rad). The conditions were: 1 W for approximately 30 min until the blue line reached the separation gel, and 10 W until the electrophoresis was finished. Proteins were visualized with Coomassie brilliant blue R250. Each sample had three technical replicates. Gels were compared, and spot intensities were quantified using the ImageMaster 2D Platinum 5.0 analysis software (Bio-Rad). Student’s t-test was used to determine the spot intensity changes between samples after normalization, and those with more than 1.4-fold changes were defined as significant difference (p < 0.05).
Identification of protein spots by mass spectrometry
Protein spots that consistently appeared in three replicates and showed significant difference between samples were excised manually for mass spectrometry (MS) analysis. Briefly, gel pieces were destained in 25 mM NH4HCO3 and 50% (v/v) acetonitrile for 3 to 15 min at room temperature, freeze-dried, and rehydrated in 15 ng/μL sequencing-grade trypsin (Promega) at 4°C for 1 h. NH4HCO3 (25 mM) was then added to completely cover the gel pieces. After 16 h incubation at 37°C, the gel pieces were transferred to fresh Eppendorf tubes containing 5% trifluoroacetic acid and incubated at 37°C for another 1 h. Acetonitrile (50%) and trifluoroacetic acid (2.5%) were then added, and the mixtures were incubated at 37°C for 1 h. The freeze-dried peptides were analyzed by a Matrix Assisted Laser Desorption Ionization Time of Flight Mass Spectrometry (ABI-4800, AB Scienx) with the positive ion reflector mode and the sweep range of 900 to 4000 Da. Peaks derived from the mass spectra with a value of S/N > 10 were searched against the NCBI database by using the Mascot search engine. Only the protein spots with a score of over 70 (p < 0.05) were considered to be a putative protein and identified at Lab Assistant Biotechnology Company (Beijing, China).
Results
Polymorphism of SSR markers at target loci and MAS of o2-wx NILs
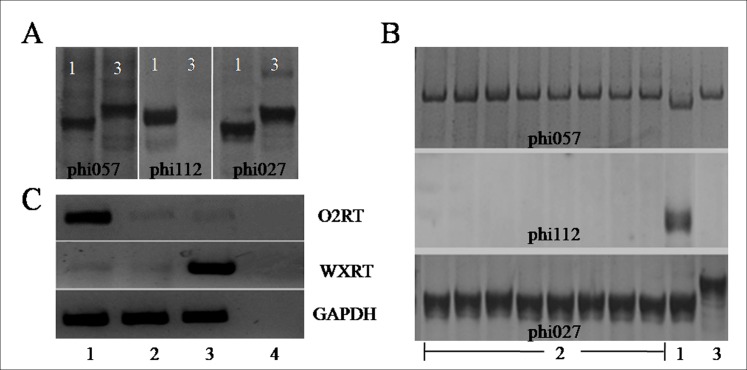
Three polymorphic markers were tested in this study. As shown in Fig 1A, two markers (phi057 and phi112) specific for the o2 allele were found to be polymorphic between the waxy maize Zhao OP-6/O2O2 and the QPM CA339/o2o2, and phi027 of the wx1 allele had polymorphism between the parents. Therefore, these polymorphic markers can be used for the MAS of corresponding target alleles: co-dominant markers phi057 and phi027 for the selection of heterozygous genotype O2WX/o2wx and recessive homozygous genotype o2wx/o2wx, while phi112 for the genotypes O2O2 and O2o2. Genotype O2WX/o2wx was selected from the progeny and backcrossed to the recurrent parent Zhao OP-6/O2O2. After six backcrosses, recurrent parents and corresponding heterozygous plants had similar agronomic traits including stature, leaf, seed and flower. Finally, the recessive o2wx/o2wx plants were selected following two to three generations of self-pollination of O2WX/o2wx plants. The electrophoretic patterns of eight o2-wx NILs are shown in Fig 1B. The band pattern of Zhao OP-6/o2o2 was consistent with that of CA339/o2o2 and Zhao OP-6/O2O2, indicating that the o2 allele has been successfully introgressed into the genetic background of Zhao OP-6/O2O2. Transcript abundance analysis (Fig 1C) indicated that the expression levels of wx1 in Zhao OP-6/o2o2 and Zhao OP-6/O2O2 were identically low, while O2 showed different expression levels. The transcript abundance of o2 in Zhao OP-6/o2o2 was similar to that in CA339.
Fig 1. Electrophoresis analysis of SSR markers for MAS and transcript abundance of target genes.
(A) SSR markers phi057, phi112 and phi027 specific for o2 and transposable elements rbg and wx1, respectively. (B) The genotyping of different individuals from BC6 F3 family. (C) Transcript abundance analysis of o2 and wx1 in developing endosperm of 18 DAP by RT-PCR. O2RT and WXRT are two specific primers spanning the exons of o2 and wx1 allele, and GAPDH is the reference gene. 1, Zhao OP-6/O2O2; 2, o2-wx NIL individuals; 3, CA339; 4, blank control of distilled H2O.
To calculate the recovery rate of original genetic background of Zhao OP-6/o2o2 individuals, 100 SSR markers were selected for polymorphism analysis. Of them, 54 were found to be polymorphic between parent plants. In the BC6F3 generation, the average recovery rate of selected individuals was 91.5%, 7.7% lower than the theoretical value (Table 1).
Table 1. Background analysis of two selected BC6F3 families.
| BC6F3 family number | Recovery rate (%) | Donor parent genome (%) | Heterozygote genome (%) | Unidentified genome (%) |
|---|---|---|---|---|
| Zhao OP-6/o2o2-1 | 91.5 | 4.8 | 3.2 | 0.5 |
| Zhao OP-6/o2o2-2 | 91.5 | 5.6 | 2.4 | 0.5 |
Kernel characteristics and submicroscopic structure
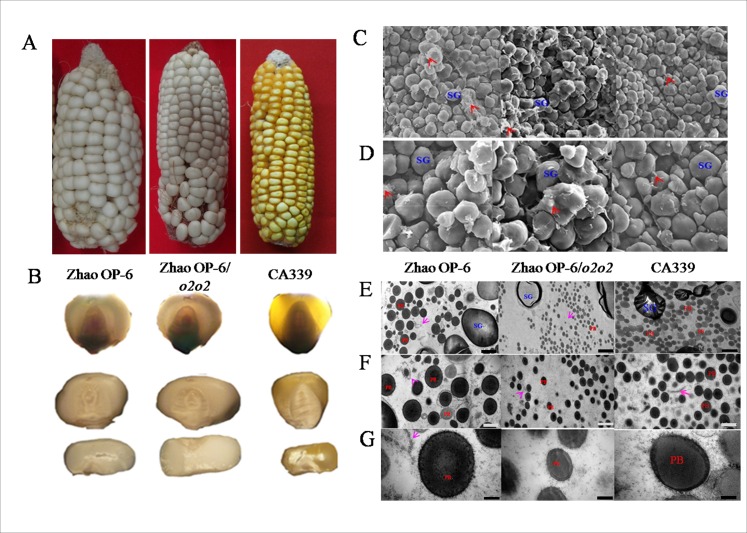
Under normal and transmitted light, the kernels of Zhao OP-6/o2o2 and Zhao OP-6/O2O2 were completely vitreous indicated that the hardness of the two lines were 1. (Fig 2A and 2B). In addition, no significant difference was detected in the hundred-kernel weight and kernels density (S1 Table). Under SEM, the starch granules of Zhao OP-6/o2o2 were compact and embedded in a dense proteinaceous matrix (Fig 2C), while those of Zhao OP-6/O2O2 and CA339 had relative smooth, loosely packed starch granules (Fig 2C) with little contact with protein bodies (Fig 2D). The dense packing of protein bodies around starch grains may account for the more vitreous endosperm [33]. Immature endosperm cells of Zhao OP-6/o2o2 and Zhao OP-6/O2O2 at 21 DAP showed similar micro- and ultra-structures (Fig 2E and 2F), in which protein bodies were regularly shaped, well separated from each other, and evenly surrounded by starch granules. Notably, smaller protein bodies accumulated in Zhao OP-6/o2o2 endosperm cells at the volume of one third of that of the parents, but at higher amount (Fig 2G). Moreover, numerous small and cisternal ERs were dilated in the endosperm cells of recurrent parent Zhao OP-6/O2O2 and accumulated on the peripheral cell walls with polygonal ring or vesicle-like structures (Fig 2F). These structures may originate from the endo-membrane system.
Fig 2. Phenotypic features of o2-wx NILs and the two parents.
(A) Photographs of intact ears taken under normal light. (B) Light transmission of mature kernels on a light box. Kernels were randomly selected from the intact ears. (C and D) SEM of the peripheral region of mature endosperm at 1000 × and 2000 × magnification, respectively. SG, starch granule; red arrows, protein body. (E, F and G) TEM of the developing endosperms of 21 DAP at low (bars = 2 μm), moderate (bars = 1 μm) and high (bars = 200 nm) magnifications, respectively. PB, protein body; SG, starch granule; purple arrows, dilated ER in Zhao OP-6/O2O2.
Changes of zeins and FAA composition in o2-wx NILs endosperm
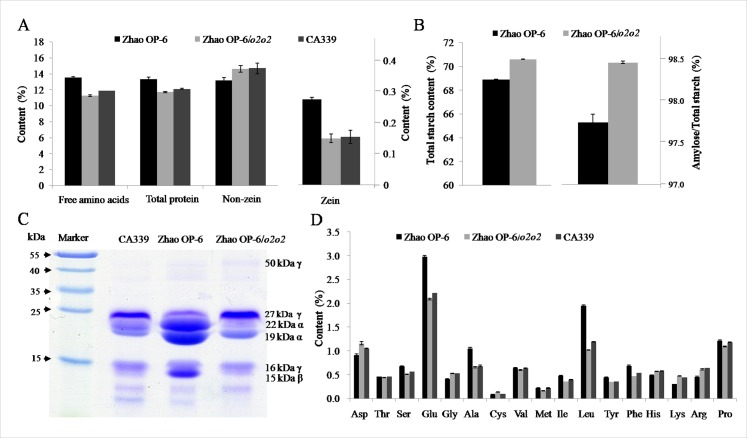
To investigate the potential biochemical differences between Zhao OP-6/o2o2 and the parents, we studied the major characters and components of mature kernels. No significant difference was found in the contents of moisture and oil between Zhao OP-6/O2O2 and Zhao OP-6/o2o2 (S1 Table), although these lines showed difference from CA339 in moisture and oil contents and hardness. However, in comparison to parent plants, Zhao OP-6/o2o2 showed reduced FAAs but increased total starch contents (Fig 3A and 3B). Moreover, the percentage of amylopectin of Zhao OP-6/o2o2 was 98.5%, higher than that of Zhao OP-6/O2O2 (97.7%, p < 0.01, Fig 3B).
Fig 3. Biochemical characterization of o2-wx NILs and the two parents.
(A) The contents of FAAs, total protein, zein and non-zein and in mature kernels. The data were calculated based on the percentage per milligram of dried mature kernel. (B) Contents of total starch and amylopectin in the mature kernels of Zhao OP-6/O2O2 and Zhao OP-6/o2o2. The data were calculated based on per milligram of dried mature kernels. (C) SDS-PAGE analysis of zein proteins extracted from mature endosperm. (D) The contents of 17 FAAs in mature kernels. All data are shown as mean ± S. E. (n = 3).
The qualitative and quantitative differences of zein, non-zein proteins and FAAs of different maize lines were also assessed and compared. A shown in Fig 3A, there is no significant difference in the total protein contents of Zhao OP-6/o2o2 and Zhao OP-6/O2O2. However, decreased amounts of zeins and increased amounts of non-zeins were found in Zhao OP-6/o2o2. SDS-PAGE (Fig 3C) indicated that Zhao OP-6/O2O2 had five major polypeptides in endosperm, i.e. 27-kDa γ-zein, 22-kDa α-zein, 19-kDa α-zein, 16-kDa γ-zein, and 15-kDa β-zein. Four of them (except for the 27-kDa γ-zein) were also found in Zhao OP-6/o2o2 but with decreased amounts, especially the 22-kDa α-zein and 15-kDa β-zein. The reduced accumulation of 19-kDa α-zein had also been reported in a previous study [16]. These two α-zeins showed considerably reduced accumulation in the donor parent CA339. Notably, in comparison with Zhao OP-6/O2O2, the content of 27-kDa γ-zein was significantly increased in Zhao OP-6/o2o2, which is similar to CA339 [34]. The unchanged level of 16-kDa γ-zein ruled out the possibility that expression of zein gene was generally affected in Zhao OP-6/o2o2. Comparison of kernel FAA composition also revealed differences between Zhao OP-6/o2o2 and the parents (Fig 3D). In total, Zhao OP-6/o2o2 had 16.7% less FAAs than recurrent parent Zhao OP-6/O2O2. In the mature kernels of Zhao OP-6/o2o2, the contents of lysine and glycine were most significantly increased by 51.6% and 26.9%, respectively, while threonine, cysteine, and methionine derived from the aspartic acid pathway had no change in amounts. Though the two glutamic acid-derived amino acids, histidine and arginine, showed no difference between Zhao OP-6/o2o2 and Zhao OP-6/O2O2, the content of glutamic acid decreased in Zhao OP-6/o2o2. Besides, the contents of leucine, serine and alanine were significantly decreased by 47.8%, 24.9% and 38.1%, respectively. And Zhao OP-6/o2o2 had slightly decreased contents of isoleucine, tyrosine, and phenylalanine.
Proteomic comparison of parent plants and o2-wx NILs
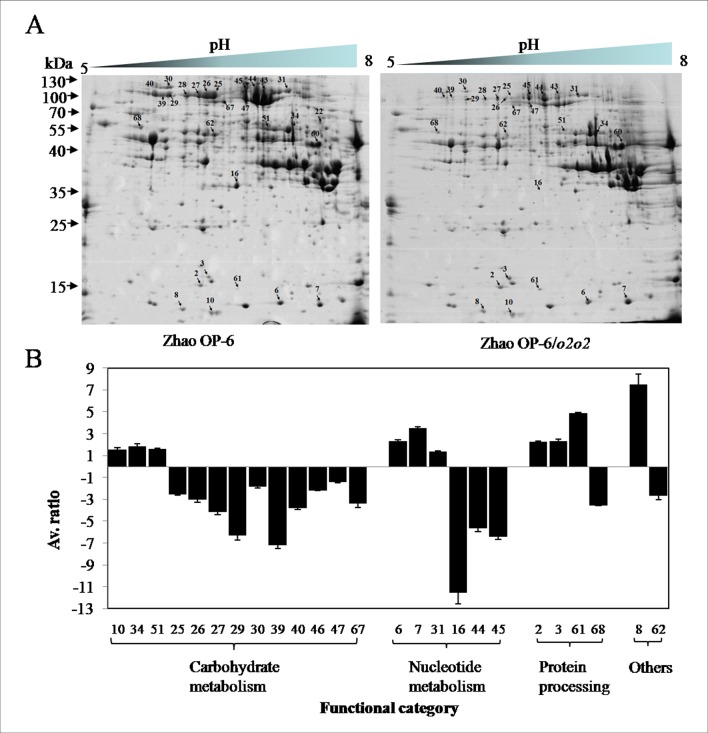
2-D SDS-PAGE was used to compare the proteins in maize endosperms. As results, the molecular weights of maize endosperm proteins ranged from 10 to 130 kDa with the pH gradient from 5 to 8 (Fig 4A). A total of 40 protein spots displayed significant abundance differences or showed altered accumulations at the protein level (Fig 4B). Except for unidentified protein spots (due to weak spectra or unsuccessful database searches), 25 protein spots were identified by MS (S2 Table). A comparison of protein abundance between Zhao OP-6/o2o2 and parent plants showed that fewer protein species (10 of 25 protein spots) were up-regulated in Zhao OP-6/o2o2. Of them, proteins involved in the maintenance and folding of proteins in the ER and defense to biotic and abiotic stresses were enriched, including the 17.5 kDa class II heat shock protein (spot 2), 17.4 kDa class I heat shock protein (spot 3), nucleoside diphosphate kinase 1 (NDPK1, spots 6 and 7) and 17.0 kDa class II heat shock protein (spot 61). Moreover, the 14 kDa zinc-binding protein (ZBP14, spot 8), trypsin/factor XIIA inhibitor (TPA, spot 10) and glucose-1-phosphate adenylyltransferase (ADPase, spots 34 and 51) showed increased abundances in Zhao OP-6/o2o2, which were probably related to the improved zinc site binding and starch synthesis. On the other hand, the down-regulated proteins in Zhao OP-6/o2o2 might be involved in different metabolic pathways. The cyPPDK1-related proteins (spots 25, 27, 29, 39, and 40) were predominant with similar fold changes and accounted for 24% of the differential proteins. The significant reduction in the accumulation of sucrose synthase 1 (SH1, spots 46 and 47), a key enzyme in glycometabolism, indicated the changes of sugar metabolism in Zhao OP-6/o2o2. The α-glucan phosphorylase 1 (PHS1, spot 30) and 1,4-α-glucan-branching IIb (SBE IIb, spot 67) that are involved in starch metabolism were both down-regulated in Zhao OP-6/o2o2. Although several proteins involved in protein folding and plant defense were enriched in Zhao OP-6/o2o2, the expression of Chaperonin 60 (CPN60, spot 68) and RIP (spot 16) was completely suppressed. The suppression of RIP might be associated with the low expression levels of EF2-related proteins (spots 31, 44, and 45). S-adenosyl-L-homocysteine hydrolase (SAHH, spot 62) that takes part in methylation by regulating methyl transferase reactions was also down-regulated in Zhao OP-6/o2o2. The differential expression of these proteins in Zhao OP-6/o2o2 and parent plants may explain the principle biochemical and morphological variations of o2-wx NILs.
Fig 4. 2-D SDS-PAGE analysis of the polypeptides in developing endosperms of o2-wx NILs and parent plants at 18 DAP.
(A) Profiles of Zhao OP-6/O2O2 (left) and Zhao OP-6/o2o2 (right). Protein spots showing significant differences (1.4-fold, p < 0.05) were numbered and identified by MS. (B) Quantitative analysis of the proteins of differential abundance. Values are shown as mean ± S.E. (n = 3).
Discussion
In the present study, MAS technique was used to produce a set of o2-wx NILs by introgressing o2 allele into waxy maize line Zhao OP-6/O2O2. Recurrent parents and the o2-wx NILs (Zhao OP-6/o2o2) had similar agronomic traits including stature, leaf, seed and flower. However, some specific fragments of CA339 were also found in Zhao OP-6/o2o2 by calculating the recovery rate of original genetic background. The result indicated that it is impossible to produce NILs harboring single recessive allele o2-wx only based on recurrent phenotypes and MAS using a single marker. Therefore, a larger number of markers are indispensable to create single allele NILs by MAS for foreground and background selections [27].
Endosperm of o2 mutants is usually soft, fragile, rich in water, and susceptible to pests [19]. However, some endosperm modifier alleles having capacity of changing opaque endosperm into vitreous one can mitigate these weaknesses [35]. In the present study, Zhao OP-6/o2o2 was found to share similar kernel phenotype to the recurrent parent, such as vitreousness, hardness, density and hundred-kernel weight, suggesting that the endosperm modifier o2m allele may play a crucial role in the waxy genetic background (S1 Table). Moreover, the wx1 allele has been identified to be positively correlated with the vitreousness of maize endosperm and thus may represent another endosperm modifier allele [36]. SEM analysis indicated that starch grains, protein bodies, and viscous cytoplasm in combination formed a more compact matrix in the vitreous region of Zhao OP-6/o2o2 than the parents. Normally, maize starch granules do not form cohesive contacts with one another. However, there are physical connections between starch granules of Zhao OP-6/o2o2, which may account for the hard, vitreous phenotype. Meanwhile, accumulation of protein bodies contributes to a more vitreous and harder endosperm of maize [37]. TEM further revealed the configuration and distribution of protein bodies. In Zhao OP-6/o2o2 endosperms at 21 DAP, smaller and regularly shaped protein bodies were found to prominently aggregate into clumps. Interestingly, though Zhao OP-6/o2o2 endosperm cells accumulated smaller protein bodies at one third of the parent volume, the number of the protein bodies was higher than that of the parents. This observation corresponds to the putative role of increased 27-kDa γ-zein in CA339 endosperm [34]. The 27-kDa γ-zein is located on the periphery of protein bodies and is widely cross-linked by disulfide bonds and covalent linkages between protein bodies; these interactions provide a mechanism for cementing protein bodies around starch grains [38]. Moreover, the 27-kDa γ-zein has been demonstrated to play a key role in endosperm modification by RNAi and γ-radiation; its accumulation contributes to a rigid matrix vitreous endosperm as well [34,39]. Notably, dilated rough ER was observed surrounding the protein bodies in Zhao OP-6 endosperm cells, indicating that ER stress is probably induced in plant cells by a variety of factors, including agents affecting calcium homeostasis, abiotic and biotic stresses, and inhibitors of glycosylation [40,41]. ER stress usually occurs in o2 mutants, and leads to a non-homeostasis environment for protein folding and formation of disulfide bonds in ER lumen [42,43]. However, ER stress was rarely found in Zhao OP-6/o2o2. The reason might be that several proteins involved in the maintenance and folding of proteins in the ER are up-regulated due to the alteration of protein body structure in ER.
The major pathways regulated by O2 are amino acid biosynthesis and starch-protein balance. Typically, FAAs constitute about 1 to 3% of the non-protein nitrogen in wild-type maize endosperm whereas exhibit increased levels in o2 mutants [44–46]. In the present study, the contents of FAAs in Zhao OP-6/o2o2, especially leucine, serine and alanine derived from the glycolytic intermediates and most abundant in the early endosperm development, appeared to have a genetic predisposition for down-accumulation. This result is against the previous study [45], indicating that the wx1 as an opaque2 modifier may influence the amino acid composition in maize endosperm [36]. Reduction of these abundant amino acids, especially leucine, is largely responsible for the relative increase of minor amino acids, especially lysine and glycine, as the endosperm matures. The increased level of lysine in Zhao OP-6/o2o2 largely depended on the reduction of α-zein synthesis which excludes lysine while several non-zein proteins which accounts for most of the higher percentage of lysine are associated with varying degrees of increased accumulation [47]. In addition, O2 has the ability to regulate the activity of cyPPDK1 and AHAS [48]. AHAS catalyzes the first step of branched amino acid synthesis, and cyPPDK1 is a key regulator of the glycolytic pathway derived from pyruvate; both enzymes were also down-regulated by o2 and finally accounted for the reduction of leucine concentration [21,49].
Pyramiding of recessive alleles, especially double-recessive or triple-recessive mutations, has specific genetic effects on ameliorating the quantity and quality of starch, sugar, oil, and protein in endosperm [50]. A previous study reported that the double-recessive o2 and wx1 mutations had no effect on the grain starch content [26]. However, in the present study, the total starch content of Zhao OP-6/o2o2 kernels was significantly higher than the recurrent parent Zhao OP-6. Interestingly, the amylopectin content of Zhao OP-6/o2o2 was also very significantly higher than that in Zhao OP-6/O2O2. These results demonstrated that the altered starch structure by completely suppressing the expression level of wx1 in o2-wx NILs may elucidate the role of GBSS I in production of amylopectin. Compared with Zhao OP-6/O2O2 and soft o2 genotypes, amylopectin in Zhao OP-6/o2o2 with reduced intermediate-length α-1,4-linked glucose chains is associated with increased swelling in water and formation of tight contacts between starch granules [33]. In addition, several starch biosynthesis genes, especially ADPase and SBE IIb, showed altered expression levels between Zhao OP-6/o2o2 and Zhao OP-6/O2O2 based on 2D SDS-PAGE. ADPase [51] that catalyzes the first committed step of starch synthesis was up-regulated, while SBE IIb [52] that catalyzes the formation of α-1,6-linked glucan and is required for amylopectin synthesis at the surface of the starch granule was down-regulated. Previous studies indicated that the deficiency of SBE Ia has no impact on endosperm starch structure, whereas SBE IIb is closely related to the reduced level of amylopectin [53]. But there is no evidence to verify the predominant function of SBE IIb over SBE Ia, and SBE Ia might have effect on amylopectin structure only in the absence of SBE IIb [54]. In vitro assay, SBE Ia preferentially produces longer polymers (> 16) while SBE IIb produced shorter ones (< 12) [55]. Thus the increased amylopectin level may largely depend on the SBE Ia activity. In addition, expression changes of one or more starch biosynthesis enzymes probably results in the starch modification of Zhao OP-6/o2o2, or altering the expression or mutation of one starch biosynthetic enzyme has multiple effects on enzyme activities.
As highlighted before, the development of maize endosperm is complex, which is driven by qualitative and quantitative coordinate expression of endosperm protein asset of different genotypes [21]. To better clarify the role of O2 in endosperm protein expression and to investigate their possible interactions in waxy genetic background, 2D SDS-PAGE analysis of 18 DAP endosperms was performed. Of 25 differentially expressed protein spots, those putative cyPPDK1 spots represents an exception that showed similar down-regulation (up to 3-fold or higher). Although cyPPDK1 isoforms had slight changes in pIs and molecular weights, they were all controlled by O2 as shown before [56]. A close examination of the expression patterns of proteins involved in sugar and starch metabolism shows that Zhao OP-6/o2o2 has perturbations in sucrose metabolism and environmental response. Sucrose has been shown to act as signals to trigger changes in the expression of a broad range of post-transcriptional modifications [57]. Therefore, the differential expression of SH1, a key enzyme in sucrose synthesis pathway, indicated that genetic backgrounds of different lines or different environment responses may indirectly affect the expression of SH1 in o2 mutants. Moreover, the expression levels of several proteins involved in maintenance and folding of proteins in ER showed cross-occurrence between Zhao OP-6/o2o2 and Zhao OP-6/O2O2. For example, the small cytoplasmic chaperones (heat shock protein) were significantly up-regulated in Zhao OP-6/o2o2, whereas the expression of Chaperonin 60 was down regulated. These proteins are related to the protein unfolding, and their interaction is likely related to protein body distribution and structure in the ER [42]. Stress response and defense-related proteins including NDPK1 and RIP also showed differential expression in Zhao OP-6/o2o2. NDPK1 is a master regulator of diverse pathways in the cell and plays a important role in plant defense responses [58], while RIP has a defensive role against pathogens and viruses and represents a well-known target of O2 regulation [59]. In Zhao OP-6/o2o2, the expression level of NDPK1 was increased by about 2-fold, while RIP was completely suppressed. The up-regulation of NDPK1 is more likely related to pleiotropic responses instead of resistance to pests since evidences have shown that o2 mutants are much more sensitive to pests [19]. The decrease in RIP abundance may be associated with the low expression of EF2; as a result, the efficiency of translation was suffocated. Regulation of protein expression is mainly controlled by signal transducers, which is central to a myriad of biological processes at the molecular level [24]. In the present study, two proteins, SAHH and ZBP14, were identified to involve in the signal transduction. SAHH is an NAD+-dependent tetrameric enzyme which catalyzes the breakdown of S-adenosyl homocysteine to homocysteine and adenosine. The decreased accumulation of SAHH in Zhao OP-6/o2o2 may affect cell growth and regulation of gene expression [60]. ZBP14 as one of the key enzymes in cellular signal transduction has a central role in the control of many cellular processes, and its activity is modified by activators such as diacylglycerol [61]. Although further studies are required to provide convictive proofs that O2 regulates the differential expression of proteins directly or indirectly, the interaction of these proteins is a strong indicator to facilitate the construction of specific phenotype and influence the functions of endosperm cells.
Supporting Information
(XLSX)
(XLSX)
Acknowledgments
The authors thank Dr. Zhiguo Tian for kindly providing waxy maize inbred line Zhao OP-6. We also thank the reviewers for their hard work on reviewing our manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the China Agriculture Research System (CARS-02-01) and the funding was received by MSL. The Beijing Municipal Natural Science Foundation (31401390) and the funding was received by LYS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Zeng MQ. The Relationship of waxy maize in China. Crop breed resource 1987; 3: 8. [Google Scholar]
- 2.Kuleshov NN. Some peculiarities in the maize of Asia. (Original version in Russian, St-Petersbourg, 1928.). Ann Mo Bot Gard 1954; 41: 271–299. [Google Scholar]
- 3.Zheng HJ, Wang H, Yang H, Wu JH, Shi B, Cai R, et al. Genetic Diversity and Molecular Evolution of Chinese Waxy Maize Germplasm. PLoS ONE 2013; 8: e66606 10.1371/journal.pone.0066606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu D, Lu W. Effects of protein removal on the physico-chemical properties of waxy maize flours. Starch/Stärke 2012; 64: 874–881. [Google Scholar]
- 5.Tian QZ, Li XH, Li MS, Jiang W, Zhang SH. Molecular markers assisted selection to quality protein maize. J Maize Sci 2004; 12: 108–110, 113. [Google Scholar]
- 6.Yang YF, Guo Q, Cheng J, Zheng XY, Lin CM. Analysis of genetic and quality traits of waxy corn inbred lines in China temperate zone. Acta Botan Boreali-Occident Sin 2009; 29: 2213–2220. [Google Scholar]
- 7.Hospital F, Chevalet C, Mulsant P. Using markers in gene introgression breeding programs. Genetics 1992; 132: 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibbon BC, Larkins BA. Molecular genetic approaches to developing quality protein maize. Trend Genet 2005; 21: 227–233. [DOI] [PubMed] [Google Scholar]
- 9.Meng ZD. Discussion on waxy corn breeding strategies. J Maize Sci 2001; 9: 14–17. [Google Scholar]
- 10.Paulis JW, Bietz JA, Bogyo TP, Nelsen TC, Darrah LL, Zuber MS. Expression of A/B zeins in single and double maize endosperm mutants. Theor Appl Genet 1992; 85: 407–414. 10.1007/BF00222321 [DOI] [PubMed] [Google Scholar]
- 11.Klosgen RB, Gierl A, Schwarz-Sommer Z, Saedler H. Molecular analysis of the waxy locus of Zea mays. Mol Gen Genet 1986; 227: 91–96. [Google Scholar]
- 12.Mason-Gamer RJ, Weil CF, Kellogg EA. Granule-bound starch synthase: structure, function and phylogenetic utility. Mol Biol Evol 1998; 15: 1658–1673. [DOI] [PubMed] [Google Scholar]
- 13.Briggs RW, Amano E, Smith HH. Genetic recombination with ethylmethane sulphonate induced waxy mutants in maize. Nature 1965; 207: 890–891. [Google Scholar]
- 14.Nelson OE, Rines HW. The enzymatic deficiency in the waxy mutation of maize. Biochem Bioph Res Co 1962; 9: 297–300. [DOI] [PubMed] [Google Scholar]
- 15.Sprague GF, Brimhall B, Nixon RM. Some affects of the waxy gene in corn on properties of the endosperm starch. J Am Soc Agron 1943; 35: 817–822. [Google Scholar]
- 16.Hartings H, Maddaloni M, Lazzaroni N, Fonzo ND, Motto M, Salamini F, et al. The O2 gene which regulates zein deposition in maize endosperm encodes a protein with structural homologies to transcriptional activators. EMBO J 1989; 8: 2795–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt RJ, Ketudat M, Aukerman MJ, Hoschek G. Opaque2 is a transcriptional activator that recognizes a specific target site in 22 kD zein genes. Plant Cell 1992; 4: 689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt RJ, Burr FA, Aukerman MJ, Burr B. Maize regulatory gene opaque-2 encodes a protein with a "leucine-zipper" motif that binds to zein DNA. Proc Nat Acad Sci USA 1990; 87: 46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mertz ET, Bates LS, Nelson OE. Mutant gene that changes protein composition and increases lysine content of maize endosperm. Science 1964; 145: 270–280. [DOI] [PubMed] [Google Scholar]
- 20.Paulis J, Wall J, Kwolek W, Donaldson G. Selection of high lysine corns with varied kernel characteristics and compositions by a rapid turbidimetric assay for zein. J Agric Food Chem 1974; 22: 318–323. [DOI] [PubMed] [Google Scholar]
- 21.Damerval C, Guilloux ML. Characterization of novel target proteins of the o2 mutation expressed during maize endosperm development. Mol Gen Genet 1998; 257: 354–361. [DOI] [PubMed] [Google Scholar]
- 22.Hunter BG, Beatty MK, Singletary GW, Hamaker BR, Dilkes BP, Larkins BA, et al. Maize opaque endosperm mutations create extensive changes in patterns of gene expression. Plant Cell 2002; 14: 2591–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jia M, Wu H, Clay KL, Jung R, Larkins BA, Gibbon BC. Identification and characterization of lysine-rich proteins and starch biosynthesis genes in the opaque2 mutant by transcriptional and proteomic analysis. BMC Plant Biol 2013; 13: 60 10.1186/1471-2229-13-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartings H, Lauria M, Lazzaroni N, Pirona R, Motto M. The Zea mays mutants opaque-2 and opaque-7 disclose extensive changes in endosperm metabolism as revealed by protein, amino acid, and transcriptome-wide analyses. BMC Genomics 2011; 12: 41 10.1186/1471-2164-12-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia H, Nettleton D, Peterson JM, Vazquez-Carrillo G, Jannink JL, Scott MP. Comparison of transcript profiles in wild-type and o2 maize endosperm in different genetic backgrounds. Crop Sci 2007; 47: S45–S59. [Google Scholar]
- 26.Li XY, Liu JL. The effects of maize endosperm mutant genes and gene interactions on kernel components II. The interactions of o2 with su1, sh2, bt2 and wx genes. Acta Agronom Sin 1993; 19: 460–467. [Google Scholar]
- 27.Zhang WL, Yang WP, Wang MC, Wang W, Zeng GP, Chen ZW, et al. Increasing lysine content of waxy maize through introgression of opaque-2 and opaque-16 genes using molecular assisted and biochemical development. PLoS ONE 2013; 8: e56227 10.1371/journal.pone.0056227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang L, Wang W, Yang W, Wang M. Marker-assisted selection for pyramiding the waxy and opaque-16 genes in maize using cross and backcross schemes. Mol Breeding 2013; 31: 767–775. [Google Scholar]
- 29.Chen Y, Zhou ZQ, Zhao G, Li XH, Song LY, Yan N, et al. Transposable element rbg induces the differential expression of opaque-2 mutant gene in two maize o2 NILs derived from the same inbred line. PLoS ONE 2014; 9: e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prasanna B, Vasal S, Kassahun B, Singh N. Quality protein maize. Curr Sci 2001; 81: 1308–1319. [Google Scholar]
- 31.Zhao G, Li M, Zhang D, Li X, Wu Z, Ci X, et al. Kernel lysine content does not increase in some maize opaque2 mutants. Planta 2011; 235: 205–215. 10.1007/s00425-011-1491-z [DOI] [PubMed] [Google Scholar]
- 32.Wallace JC, Lopes MA, Paiva E, Larkins BA. New methods for extraction and quantitation of zeins reveal a high content of γ-zein in modified opaque-2 maize. Plant Physiol 1990; 92: 191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gibbon BC, Wang X, Larkins BA. Altered starch structure is associated with endosperm modification in Quality Protein Maize. Proc Nat Acad Sci USA 2003; 100: 15329–15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan L, Dou Y, Kianian SF, Zhang C, Holding DR. Deletion mutagenesis identifies a haploinsufficient role for gamma-zein in opaque2 endosperm modification. Plant Physiol 2014; 164: 119–130. 10.1104/pp.113.230961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geetha KB, Lending CR, Lopes MA, Wallace JC, Larkin BA. Opaque-2 modifiers increase gamma-zein synthesis and alter its spatial distribution in maize endosperm. Plant Cell 1991: 1207–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Babu BK, Agrawal PK, Saha S, Gupta HS. Mapping QTLs for opaque2 modifiers influencing the tryptophan content in quality protein maize using genomic and candidate gene-based SSRs of lysine and tryptophan metabolic pathway. Plant Cell Rep 2015; 34: 37–45. 10.1007/s00299-014-1685-5 [DOI] [PubMed] [Google Scholar]
- 37.Holding DR, Larkins BA. The development and importance of zein protein bodies in maize endosperm. Maydica 2006; 51: 243–254. [Google Scholar]
- 38.Lopes MA, Larkins BA. γ-Zeins are essential for endosperm modification in Quality Protein Maize. Crop Sci 1991; 31: 1655–1662. [Google Scholar]
- 39.Wu Y, Messing J. RNA interference-mediated change in protein body morphology and seed opacity through loss of different zein proteins. Plant Physiol 2010; 153: 337–347. 10.1104/pp.110.154690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huizen VR, Martindale JL, Gorospe M, Holbrook NJ. P58IPK, a novel endoplasmic reticulum stress-inducible protein and potential negative regulator of eIF2a signaling. J Biol Chem 2003; 278: 15558–15564. [DOI] [PubMed] [Google Scholar]
- 41.Iwata Y, Fedoroff NV, Koizumi N. Arabidopsis bZIP60 is a proteolysis-activated transcription factor involved in the endoplasmic reticulum stress response. Plant Cell 2008; 20: 3107–3121. 10.1105/tpc.108.061002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol 2004; 14: 20–28. [DOI] [PubMed] [Google Scholar]
- 43.Kirst ME, Meyer DJ, Gibbon BC, Jung R, Boston RS. Identification and characterization of endoplasmic reticulum-associated degradation proteins differentially affected by endoplasmic reticulum stress. Plant Physiol 2005; 138: 218–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sodek L, Wilson CM. Incorporation of leucine-14C and lysine-14C into protein in the developing endosperm of normal and opaque-2 corn. Arch Biochem Bioph 1970; 140: 29–38. [DOI] [PubMed] [Google Scholar]
- 45.Sodek L, Wilson CM. Amino acid composition of proteins isolated from normal, opaque-2, and floury-2 corn endosperms by a modified Osborne procedure. J Agric Food Chem 1971; 19: 1144–1149. [Google Scholar]
- 46.Moro GL, Habben JE, Hamaker BR, Larkins BA. Characterization of the variability for lysine content in normal and opaque-2 maize endosperm. Crop Sci 1996; 36: 1651–1659. [Google Scholar]
- 47.Kemper EL, Neto GC, Papes F, Moraes KCM, Leite A. The role of Opaque-2 in the control of lysine-degrading activities in developing maize endosperm. Plant Cell 1999; 11: 1981–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brochetto-Braga MR, Leite A, Arruda P. Partial purification and characterization of lysine-ketoglutarate reductase in normal and opaque-2 maize endosperm. Plant Physiol 1992; 98: 1139–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maddaloni M, Donini G, Balconi C, Rizzi E, Gallusci P, Forlani F, et al. The transcriptional activator Opaque-2 controls the expression of cytosolic form of pyruvate orthophosphate dikinase-1 in maize endosperms. Mol Gen Genet 1996; 250: 647–654. [DOI] [PubMed] [Google Scholar]
- 50.Xia T, Dou MA, Liu JL. Studies on gene action of several endosperm mutants in maize (Zea mays L.). Acta Agronom Sin 1997; 23: 753–758. [Google Scholar]
- 51.Smith AM, Denyer K, Martin C. The synthesis of the starch granule. Annu Rev Plant Biol 1997; 48: 65–87. [DOI] [PubMed] [Google Scholar]
- 52.Boyer C, Preiss J. Multiple forms of (1,4)-α-D-glucan-6-glucosyl transferase from developing Zea mays L. kernels. Carbohydr Res 1978; 61: 321–334. [Google Scholar]
- 53.Klucinec JD, Thompson DB. Structure of amylopectins from ae-containing maize starches. Cereal Chem 2002; 79: 19–23. [Google Scholar]
- 54.Yao Y. Maize starch-branching enzyme isoforms and amylopectin structure. In the absence of starch-branching enzyme IIb, the further absence of starch-branching enzyme Ia leads to increased branching. Plant Physiol 2004; 136: 3515–3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guan HP, Li P, ImparlRadosevich J, Preiss J, Keeling P. Comparing the properties of Escherichia coli branching enzyme and maize branching enzyme. Arch Biochem Bioph 1997; 342: 92–98. [DOI] [PubMed] [Google Scholar]
- 56.Gallusci P, Varotto S, Matsuoko M, Maddaloni M, Thompson RD. Regulation of cytosolic pyruvate, orthophosphate dikinase expression in developing maize endosperm. Plant Mol Biol 1996; 31: 45–55. [DOI] [PubMed] [Google Scholar]
- 57.Price J, Laxmi A, Martin SKS, Jang JC. Global transcription profiling reveals multiple sugar signal transduction mechanisms in Arabidopsis. Plant Cell 2004; 16: 2128–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pereira CA, Reigada C, Sayé M, Digirolamo FA, Miranda MR. Cytosolic Trypanosoma cruzi nucleoside diphosphate kinase generates large granules that depend on its quaternary structure. Exp Parasitol 2014; 142: 43–50. 10.1016/j.exppara.2014.04.009 [DOI] [PubMed] [Google Scholar]
- 59.Lohmer S, Maddaloni M, Motto M, N. DF, Hartings H, Salamini F, et al. The maize regulatory locus Opaque-2 encodes a DNA-binding protein which activates the transcriptio of the b-32 gene. EMBO J 1991; 10: 617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Y, Kavran JM, Chen Z, Karukurichi KR, Leahy DJ, Cole PA. Regulation of S-adenosyl-L-homocysteine hydrolase by lysine acetylation. J Biol Chem 2014; 289: 31361–31372. 10.1074/jbc.M114.597153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robinson K, Jones D, Howell S, Soneji Y, Martint S, Aitken A. Expression and characterization of maize ZBP14, a member of a new family of zinc-binding proteins. Biochem J 1995; 307: 267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.