ABSTRACT
CD137 and its ligand, CD137L, are expressed on activated T cells and antigen-presenting cells (APC), respectively, and are powerful inducers of cellular, type 1 immune responses. CD137 is ectopically expressed by Hodgkin and Reed-Sternberg (HRS) cells, the malignant cells in Hodgkin lymphoma (HL). Here we report that CD137 transmits signals into HRS cells, which induce the secretion of IL-13. IL-13 in conditioned supernatants of HRS cell lines inhibits the secretion of IFNγ by peripheral blood mononuclear cells (PBMC). Since IFNγ is essential for the development of a type 1 immune response, CD137-induced IL-13 secretion facilitates escape from immune surveillance. Further, CD137-induced IL-13 enhances the growth of HRS cell lines. CD137, IL-13 double-positive cells could be detected in the majority (58%) of HL patient samples, providing clinical evidence for a role of IL-13 induction by CD137 during HL pathogenesis. This study validates CD137 as a candidate target for immunotherapy of HL.
KEYWORDS: CD137, Hodgkin Lymphoma, IL-13, immune deviation, immune escape, proliferation
Abbreviations
- CLL
chronic lymphocytic leukemia
- HL
Hodgkin Lymphoma
- HRS cells
Hodgkin and Reed-Sternberg cells
- PBMC
peripheral blood mononuclear cells
Introduction
HL is a malignancy of the lymphatic system which has the rare feature of consisting overwhelmingly of tumor stroma that is made up primarily of infiltrating leukocytes. The malignant, tumor-causing cells in HL are the Hodgkin and Reed-Sternberg (HRS) cells which constitute merely a small percentage of the tumor mass. Complex interactions between HRS cells and tumor stroma support tumor growth and escape from immune surveillance, by mechanisms which are only partly understood.1,2 Cytokines such as IL-13 3,4 and IL-15 5 are secreted by the HRS cells, and play an important role in HL pathogenesis as they provide autocrine and paracrine growth stimulation for HRS cells. Further, these cytokines contribute to the establishment of the extensive tumor stroma which is essential for HL development. Also, due to the rarity of HRS cells, the biology and the pathogenesis of HL are incompletely characterized.
Recently, it has been shown that HRS cells ectopically express the cytokine receptor CD137 (TNFRSF9, 4-1BB). Two independent studies found that in 86% of HL cases, HRS cells stain positive for CD137.6,7 This was a surprising finding since HRS cells are generally derived from germinal center B cells, on which CD137 could not be detected. This high correlation of CD137 expression and malignant transformation suggested that CD137 expression is selected for, because it provides a growth and/or selection advantage to HRS cells and HL.
CD137, a member of the tumor necrosis factor receptor superfamily, is a costimulatory molecule on T cells, and its ligand CD137L is expressed on APC.8-10 APC use the CD137/CD137L system to costimulate T-cell activity. The CD137 signal is a powerful inducer of T-cell activity. CD137 signaling into T cells promotes their survival, cytotoxicity and proliferation, and polarizes the T cells toward a type 1, cell-mediated immune response.11 For cancer cells, CD137L expression on APC may turn into a disadvantage, as CD137L can stimulate CD137 on infiltrating T cells, and thereby enhance an antitumor T-cell response. Accordingly, eliminating the T-cell costimulatory activity of CD137L translates into a growth and selection advantage. One possible mechanism to eliminate CD137L, is to neutralize it using soluble CD137, which is generated by differential splicing, and is inhibitory to cell-surface expressed CD137.12-14 This mechanism may be active in chronic lymphocytic leukemia (CLL), as CLL patients have significantly elevated levels of soluble CD137.15
Another possibility to eliminate the costimulatory activity of CD137L is found in HL, where HRS cells express CD137 ectopically. CD137 binds to CD137L on the HRS cells, and the complex gets internalized and degraded. In addition, CD137 gets transferred from HRS cells to adjacent CD137L-expressing cells through trogocytosis (a process by which patches of cell membrane including membrane proteins get transferred from one cell to another during cell to cell contact16), which eliminates CD137L also on surrounding APC and HRS cells. This significantly reduces T-cell costimulation and the secretion of IFNγ, the key cytokine for a type 1, cell-mediated immune response, thereby aiding HL in escaping immune surveillance.6,17,18
Results and discussion
Besides blunting an anti-HL immune response through trogocytosis, CD137 may have other functions in the pathogenesis of HL. In order to analyze the effects of ectopic CD137 expression on the biology of HRS cells, we compared cytokine secretion between CD137-expressing HRS cell lines (L-428-CD137 and L-1236-CD137 and KM-H2-control) and corresponding cell lines that do not express CD137 (L-428-control, L-1236-control and KM-H2-CD137−) (Fig. S1). CD137 expression resulted in an increase in the release of TNF, IL-6, and IL-13 in the L-428-CD137 and L-1236-CD137 cell lines (Fig. 1). CD137 was most likely crosslinked by CD137L that is endogenously expressed by the HRS cell lines (Fig. S2).6
Figure 1.
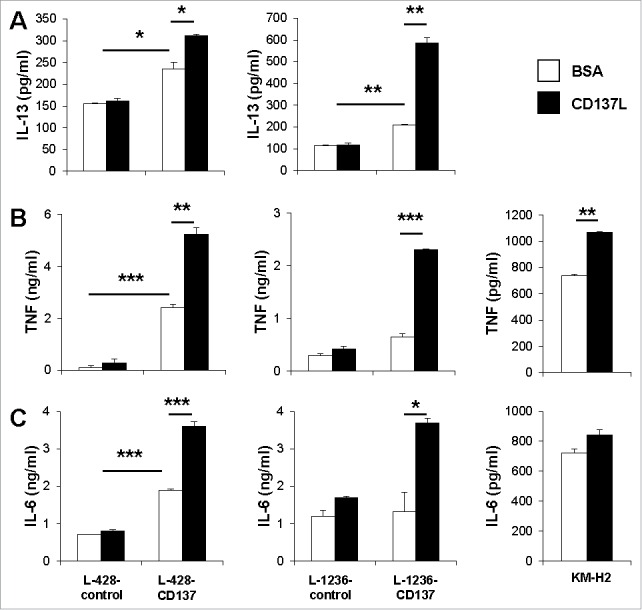
CD137 signaling induces IL-13, TNF and IL-6 secretion by HRS cell lines. 5 × 105 L-428-control, L-1236-control, L-428-CD137, L-1236-CD137 or KM-H2 cells were cultured on plates coated with 5 μg/mL of recombinant CD137L or BSA for 24 h. Levels of IL-13 (A), TNF (B) and IL-6 (C) were measured by ELISA. Depicted are means ± SD of triplicate measurements. *p <0.05; **p <0.01; ***p <0.005. Data are representative of three independent experiments.
Enhancement of CD137 signaling by treatment with a recombinant CD137L protein further increased the secretion of TNF, IL-6 and IL-13 in L428-CD137 and L-1236-CD137 cells. In contrast, treatment of L-428-control and L-1236-control cells with recombinant CD137L protein had no effect on the release of TNF, IL-6 and IL-13 (Fig. 1A).
IL-13 could not be detected in the supernatants of KM-H2 cells, also not upon treatment with CD137L protein. The absence of IL-13 in KM-H2 cells is in agreement with some,19 but contradictory to other studies3,20 that report secretion of IL-13 in KM-H2 cells. The divergent findings on IL-13 secretion by KM-H2 cells by different groups could be due to the culture conditions such as seeding cell densities, serum source and concentration. HDLM-2 cells, which endogenously express CD137 (Fig. S1) also secrete IL-13 at around 160 pg/mL (data not shown). However, there was no increase in IL-13 secretion upon treatment with recombinant CD137L protein. This could be due to a number of reasons including different downstream signaling mechanisms of CD137 in the individual HRS cell lines.
A powerful immune escape mechanism employed by tumors is immune deviation, for example, the secretion of Th2 cytokines such as IL-4 and/or IL-13, which inhibit type 1, cell-mediated and anti-cancer immune responses.21 IL-4 is not expressed in HL and by HRS cell lines, but IL-13 has been associated with classical HL.3,22, 23 The differences observed in the extent of IL-13 induction between these cell lines could be due to the type of HL, these cell lines originated from. L-1236 is derived from mixed cellularity HL, whereas L-428 is from nodular sclerosis.24
We confirmed IL-13 induction by CD137 signaling in a cellular system by culturing the L-1236-control or L-1236-CD137 cells with the monocytic THP-1 cells which express CD137L endogenously and constitutively (Fig. S2). CD137-expressing L-1236 cells, but not control cells responded with a significant increase in IL-13 secretion, demonstrating that engagement of CD137 on HRS cells by CD137L on APC induces IL-13 secretion in the HRS cells (Fig. 2A). The expression of CD137 on HRS cells, which enables communication with CD137L-expressing monocytes, may not only induce IL-13, but also the secretion of other soluble factors, that enhance HRS cell proliferation and survival. Some of these factors may also be secreted by the monocytes since CD137L is expressed as a transmembrane protein on the cell surface of monocytes, and via reverse signaling can induce activation of monocytes that results in the secretion of cytokines such as TNF, IL-6, IL-8 and M-CSF.25,26
Figure 2.
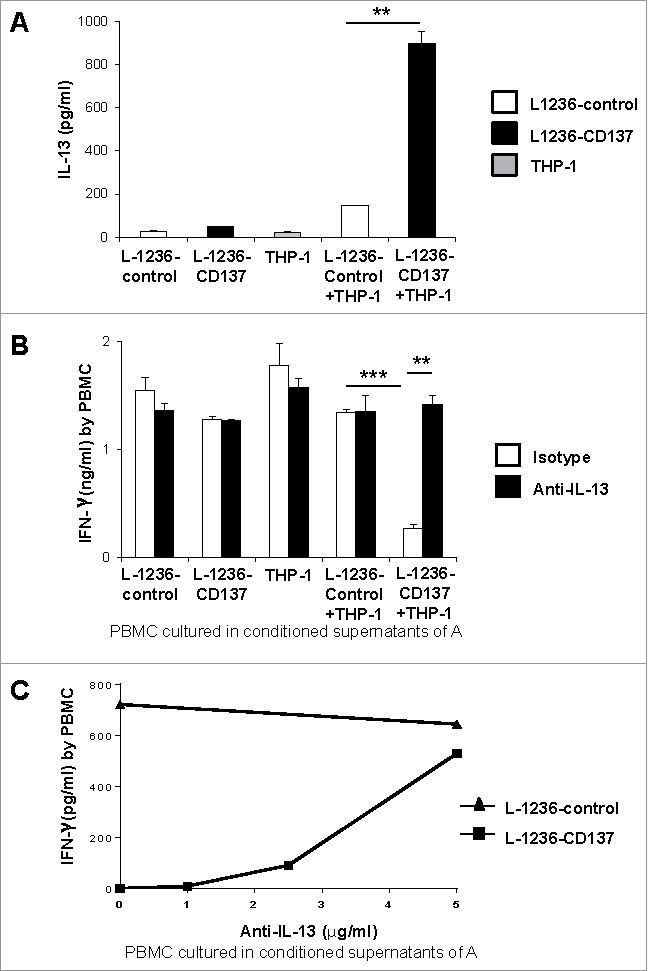
(A) 5 × 105 L-1236-control or L-1236-CD137 cells were co-cultured with 5 × 105 THP-1 cells for 24 h. Supernatants from the cocultures were tested for IL-13 levels by ELISA. (B) PBMC were sub-optimally activated with 2 ng/mL of anti-CD3 (clone OKT3) and cultured in 50% conditioned supernatants of the cocultures of (A) for 24 h. 5 μg/mL of polyclonal goat IgG or goat polyclonal IL-13 antibody were added. (C) Anti-IL-13 antibody has been added at indicated concentrations to activated PBMC cultured in 50% conditioned supernatants of (A). IFNγ secretion by was measured by ELISA after 24 h. Depicted are means ± SD of triplicate measurements. *p <0.05; **p <0.01; ***p <0.005. Data are representative of three independent experiments.
Next, we investigated the biological relevance of HRS cell-secreted IL-13 on PBMC by testing whether conditioned supernatant from CD137-stimulated HRS cells inhibits the secretion of IFNγ. PBMC were sub-optimally activated with 2 ng/mL of anti-CD3 for 24 h and cultured in the conditioned supernatants from co-cultures of L-1236-control or L-1236-CD137 cells with THP1 cells. Conditioned supernatants from cells in which CD137 signaling was induced, i.e. in which IL-13 was present, reduced IFNγ secretion by PBMC, indicating that soluble factor(s) which are induced upon CD137 signaling in HRS cells inhibit a type 1, cell-mediated immune response (Fig. 2B). No secretion of IFNγ could be detected by THP-1, L-1236-control or L-1236-CD137 cells alone.
To verify that IL-13 in the conditioned supernatants caused the reduction in IFNγ secretion by PBMC, a neutralizing anti-IL-13 antibody was added to the conditioned supernatants. Neutralization of IL-13 in the conditioned supernatants reversed the inhibition of IFNγ secretion by PBMC in a dose dependent manner (Fig. 2C), demonstrating that IL-13 which is induced by CD137 signaling in HRS cells, blunts type 1 immune responses (Fig. 2B).
IL-13 not only supports escape from immune surveillance but it has also been identified as a potent growth factor for HRS cells, including L-428 and L-1236 cells.3,4 Comparing the growth rates of CD137-expressing and control HRS cell lines, we could indeed measure a faster expansion of the cell populations that expressed CD137 (Fig. 3). In line with earlier reports,27,28 neutralization of IL-13 significantly reduced proliferation of L-1236 cells in a dose dependent manner (Fig. S3). Also, addition of recombinant IL-13 to L-1236 cells has been reported to enhance proliferation.27 Although, no IL-13 could be detected in KM-H2 cells, the CD137-expressing KM-H2 cells proliferate faster than KM-H2-CD137− cells, suggesting that CD137 signaling upregulates other growth factors, in addition to IL-13, which enhance proliferation of HRS cells.
Figure 3.
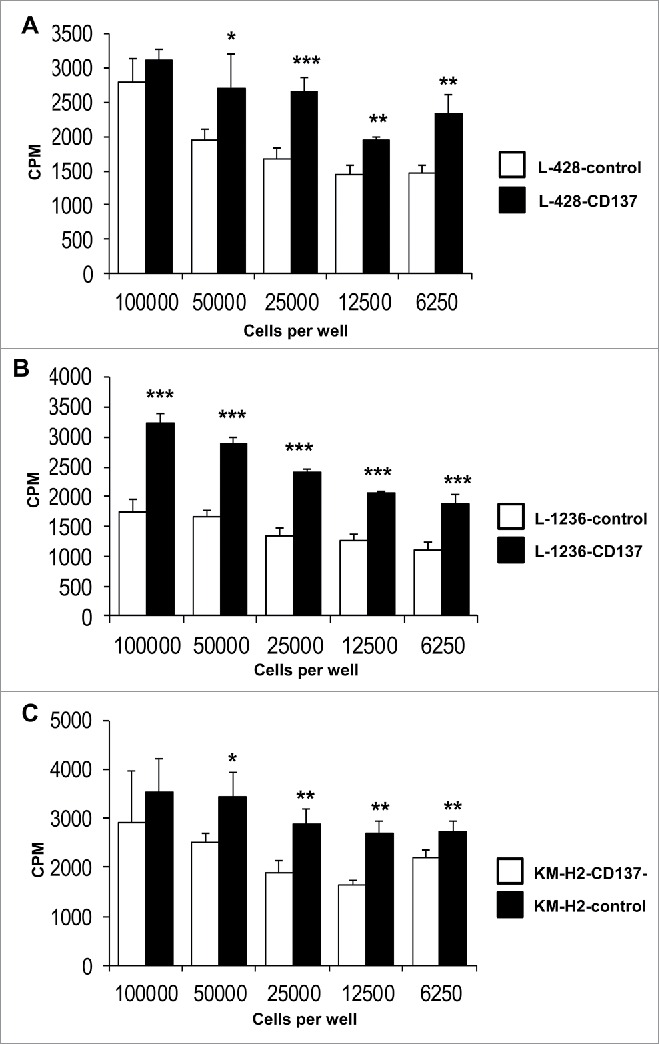
CD137 expression increases HRS cell proliferation. Indicated numbers of L-428 (A) or L-1236 (B) or KM-H2 (C) cells were seeded (white bar: non-CD137-expressing cells, black bar: CD137-expressing cells), and pulsed with 0.5 μCi of 3H-thymidine at the same time. The cells were harvested 24 h later, and 3H-thymidine incorporation was quantified. Depicted are means ± SD. *p <0.05, **p <0.01, ***p < 0.001.
In order to provide clinical evidence for a role of IL-13 induction by CD137 during HL pathogenesis, we performed immunohistochemical double staining for CD137 and IL-13 in HL tissues. Using a tissue microarray of 48 HL cases, we detected IL-13 in 41 (85%) and CD137 in 31 (65%) of them (Table 1). CD137, IL-13 double-positive cells were at least three times bigger than most other cells, which is a morphological feature of HRS cells. Double-positive HRS cells were present in 28 out of 48 HL cases (58%) (Fig. 4). CD137 staining was cytoplasmic as well as on the cell surface which confirms earlier observations.6,7 IL-13 staining was found mainly in the cytoplasm. The percentage of IL-13-positive HRS cells in the tissue samples varied widely which is in agreement with an earlier report that found a range of 25% to almost 100% positive HRS cells.4 Thus, the association of IL-13 and CD137 could also be confirmed in the majority of HL tissues.
Table 1.
Expression of CD137 and IL-13 in HL tumor microarray determined by immunohistochemical double staining. For CD137: + at least 5 cells, ++ at least 10 cells, +++ more than 15 cells stained in the section. For IL-13 staining: + at least 10%, ++ at least 30% and +++ more than 50% of the section stained.
| Number | Case | CD137 | IL-13 | Diagnosis |
|---|---|---|---|---|
| 1 | A1 | + | ++ | − |
| 2 | A2 | + | + | Nodular sclerosis |
| 3 | A3 | − | +++ | Mixed cellularity |
| 4 | A4 | − | ++ | Nodular sclerosis |
| 5 | A5 | − | + | Mixed cellularity |
| 6 | A6 | + | + | Mixed cellularity |
| 7 | A7 | ++ | ++ | Mixed cellularity |
| 8 | A8 | − | +++ | Mixed cellularity |
| 9 | B1 | + | + | Mixed cellularity |
| 10 | B2 | + | − | Lymphocyte predominant |
| 11 | B3 | − | + | Nodular sclerosis |
| 12 | B4 | +++ | +++ | Mixed cellularity |
| 13 | B5 | +++ | ++ | Mixed cellularity |
| 14 | B6 | +++ | ++ | Lymphocyte predominant |
| 15 | B7 | ++ | +++ | Lymphocyte predominant |
| 16 | B8 | +++ | +++ | Mixed cellularity |
| 17 | C1 | + | + | − |
| 18 | C2 | − | − | Mixed cellularity |
| 19 | C3 | − | + | Mixed cellularity |
| 20 | C4 | + | ++ | Mixed cellularity |
| 21 | C5 | + | + | Mixed cellularity |
| 22 | C6 | − | ++ | Mixed cellularity |
| 23 | C7 | ++ | +++ | Mixed cellularity |
| 24 | C8 | ++ | +++ | − |
| 25 | D1 | + | − | Lymphocyte predominant |
| 26 | D2 | +++ | − | Lymphocyte predominant |
| 27 | D3 | + | + | Lymphocyte predominant |
| 28 | D4 | + | ++ | Lymphocyte predominant |
| 29 | D5 | + | +++ | Lymphocyte predominant |
| 30 | D6 | ++ | +++ | Nodular sclerosis |
| 31 | D7 | +++ | +++ | − |
| 32 | D8 | ++ | +++ | Mixed cellularity |
| 33 | E1 | + | + | Lymphocyte predominant |
| 34 | E2 | + | + | Mixed cellularity |
| 35 | E3 | − | + | Mixed cellularity |
| 36 | E4 | − | ++ | Nodular sclerosis |
| 37 | E5 | − | − | Mixed cellularity |
| 38 | E6 | + | ++ | Mixed cellularity |
| 39 | E7 | − | + | Lymphocyte predominant |
| 40 | E8 | + | +++ | Nodular sclerosis |
| 41 | F1 | − | + | Mixed cellularity |
| 42 | F2 | − | − | Lymphocyte predominant |
| 43 | F3 | − | − | Mixed cellularity |
| 44 | F4 | ++ | + | Nodular sclerosis |
| 45 | F5 | − | + | Nodular sclerosis |
| 46 | F6 | − | ++ | Nodular sclerosis |
| 47 | F7 | ++ | ++ | Lymphocyte depletion |
| 48 | F8 | + | ++ | Lymphocyte depletion |
Figure 4.
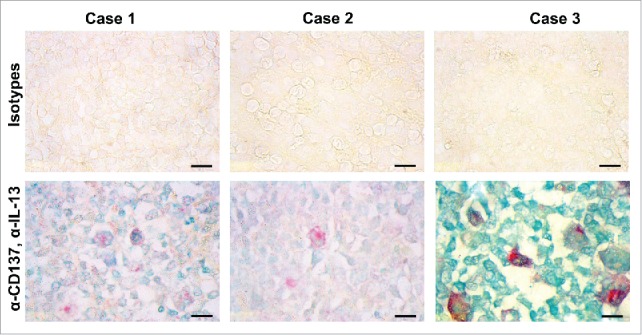
CD137-positive cells in HL express IL-13. Immunohistochemical detection of IL-13 (green) and CD137 (red). Shown are three cases of classical HL of the mixed cellularity type. Size bar: 10 μm.
In this study, we used a microarray consisting of tissue cores of 2 mm diameter, which represent only a small section of the tumor area. This may explain why we detected CD137 expression only in 65% of the samples, while previously we and others found CD137 in 86% of HL samples.6,7 This further implies that the HL cases with IL-13, CD137 double-positive cells may be significantly higher than the 58% found by utilizing a tissue microarray.
Interestingly, not only were 86% of the HL cases found to have CD137-positive HRS cells, but in a different study the same percentage (86%) was found to express IL-13.4 The IL-13 receptor was detectable in 89% of the cases, and an auto or paracrine stimulation by IL-13 was demonstrated for HRS cells, including the L-428 and L-1236 cells used in this study.3,27
In our initial study, we found that ectopic CD137 expression helps HRS cells and HL to escape from immune surveillance by eliminating the immunostimulatory CD137L from HRS cells and from adjacent APC.6 The current study identifies a hitherto unknown immune escape mechanism of HL by demonstrating that ectopic CD137 expression on HRS cells enables the secretion of IL-13, which deviates the immune response toward a type 2 response, leading to a further reduction of IFNγ, and a weakening of a cellular anti-HL immune response (Fig. 5).
Figure 5.
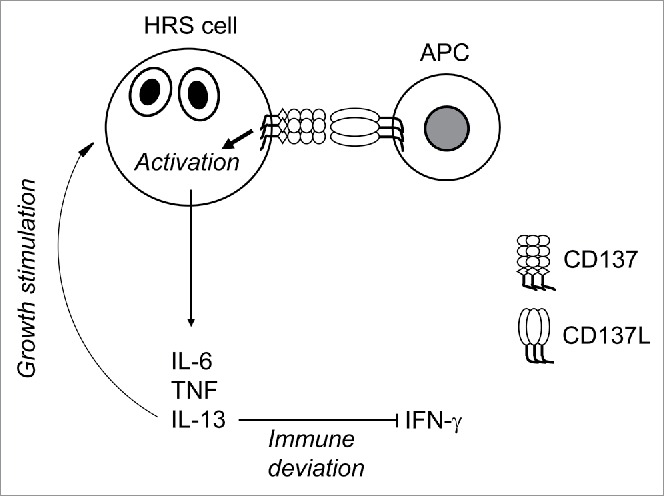
Schematic representation of the role of CD137 in immune deviation and growth stimulation of HRS cells.
CD137 agonists are powerful stimulators of antitumor immune responses, and are among the most promising new drugs for tumor immunotherapy.11,29 However, in the case of HL one would have to caution the use of CD137 agonists, as they may not only costimulate an anti-HL immune response but may also enhance HL growth, and support escape from immune surveillance. Also, CD137 agonists could provide growth selection or advantage to a sub-clinical HL.
A novel therapeutic strategy could be the neutralization of CD137 on HRS cells. This may not only prevent the downregulation of CD137L on APC which is pivotal for inducing a type 1 immune response, but could also prevent IL-13-driven immune deviation and slow down tumor growth.
Material and methods
Cells
The HRS cell lines L-428, L-1236, KM-H2 and HDLM-2 were purchased from the German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany), and were cultured in RPMI 1640 (Sigma, St. Louis, MO) supplemented with 10% or 20% fetal bovine serum (Biowest, Kansas City, MO) at 37°C with 5% CO2. Stable cell lines expressing CD137 were generated by lentiviral transduction. Full length cDNA of CD137 was cloned into pLenti6 vector (Invitrogen, Carlsbad, CA). The cells were transduced with CD137 viral supernatant and subsequently selected with Blasticidin (100 µg/mL). KM-H2 cells, which express CD137 constitutively, were transfected with an empty vector (KM-H2-control) or a siRNA construct that knocks down CD137 (KMH2-CD137−). Stable lines were generated by selection with 400 μg/mL G418.
Buffy coats from healthy donors were obtained from the National University Hospital, Singapore. Human PBMC were isolated by density gradient centrifugation using Ficoll-Paque Plus (GE Healthcare, Chalfont St Giles, UK).
Cell culture
Wells of 96-well plate were coated with 5 μg/mL of recombinant human CD137L (R&D Systems, Minneapolis, MN) or BSA diluted in PBS overnight at 4°C. Wells were washed with PBS twice. L-428-control or L-428-CD137 or L-1236-control or L-1236-CD137 or KM-H2 or HDLM-2 cells were seeded at a density of 5 × 105 cells/mL and cultured for 24 h. Cell free supernatants were collected and tested for cytokines by ELISA.
Co-cultures: HRS cell lines L-1236-control or L-1236-CD137 were co-cultured with CD137L expressing THP-1 cells at a ratio of 1:1 at a density of 5 × 105 cells/mL at 37°C for 24 h. Cell-free conditioned supernatants were collected. PBMC were cultured at a density of 106 cells/mL with RPMI + 10% fetal bovine serum (Biowest, Kansas city, MO) and conditioned supernatants from the co-culture (ratio of 1:1) together with 2 ng/mL of anti-CD3 (clone OKT3) for 24 h. 5 μg/mL of polyclonal goat IgG or polyclonal anti-IL-13 was added to the cultures. Supernatants were tested for IFNγ.
ELISA
Concentrations of IL-6, IL-13, TNF and IFNγ in the cell-free supernatants were determined by ELISA. Duoset kits for Human IL-6, Human IL-13, Human TNF and Human IFNγ were purchased from R&D Systems (Minneapolis, MN) and were used according to the manufacturer's instructions.
Proliferation assay
L-428-control or L-428-CD137 or L-1236-control or L-1236-CD137 or KM-H2-CD137− or KM-H2-control cells were seeded into a 96-well plate and incubated for 24 h. 0.5 μCi of 3H-thymidine were added to each well for the final 18 h. Plates were frozen at −20°C, and radioactivity was measured by beta scintillation counter.
Immunohistochemistry
5 μm tissue microarray slides containing 48 cases of HL (HL481, US Biomax, Rockville, MD) were deparaffinized in Histochoice (Sigma–Aldrich) and hydrated in an alcohol gradient. Antigen demasking was performed by pressure cooking slides in Accel retrieval solution (GBI Labs, Bothell, WA) for 20 min. After 15 min of incubation with dual enzyme block (GBI Labs), slides were blocked in 5% milk + 5% bovine calf serum (Sigma) for 30 min at room temperature. The slides were incubated overnight with mouse anti-human CD137 antibody (clone BBK-2, Neomarkers, Fremont, CA) and polyclonal rabbit anti-human IL-13 (Millipore, Billerica, MA) at 10 μg/mL and 5 μg/mL, respectively, in TBS at 4°C. Same concentrations of mouse IgG (clone MOPC-21, Sigma) and rabbit IgG (Cell Signaling, Danvers, MA) were used as controls. Double staining kit DS202C (GBI labs) was used to detect CD137 (red) and IL-13 (green). Secondary antibodies were incubated for 2 h at room temperature, and detection was carried out using GBI permanent red substrate for 10 min, followed by emerald green substrate for 5 min. Slides were mounted with an organic mountant (DPX, Sigma).
Flow cytometry
Cells were stained with PE-conjugated anti-CD137 antibody (clone 4B4-1, BD Biosciences, San Diego, CA) or anti-CD137L (clone 5F4, BD Biosciences) or mouse-IgG1κ-PE (eBioscience, San Diego, CA) at 4°C in the dark for 15 min. Flow cytometry was performed with Fortessa flow cytometry (BD Biosciences) and analyzed with Flow Jo software (Version 6.1)
Statistics
Statistical significance was determined by a two-tailed unpaired Student's t-test.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
We thank the Life Sciences Institute flow cytometry core facility for excellent assistance.
Funding
This research was supported by a grant (NMRC/BnB/018b/2015) from the National Medical Research Council, Singapore.
References
- 1.Kuppers R, Engert A, Hansmann ML. Hodgkin lymphoma. J Clinical Invest 2012; 122:3439-47; PMID:23023715; http://dx.doi.org/ 10.1172/JCI61245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldinucci D, Celegato M, Casagrande N. Microenvironmental interactions in classical Hodgkin lymphoma and their role in promoting tumor growth, immune escape and drug resistance. Canc Lett 2015. PMID:26474544; http://dx.doi.org/ 10.1016/j.canlet.2015.10.007 [DOI] [PubMed] [Google Scholar]
- 3.Kapp U, Yeh WC, Patterson B, Elia AJ, Kagi D, Ho A, Hessel A, Tipsword M, Williams A, Mirtsos C et al.. Interleukin 13 is secreted by and stimulates the growth of Hodgkin and Reed-Sternberg cells. J Exp Med 1999; 189:1939-46; PMID:10377189; http://dx.doi.org/ 10.1084/jem.189.12.1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skinnider BF, Elia AJ, Gascoyne RD, Trumper LH, von Bonin F, Kapp U, Patterson B, Snow BE, Mak TW. Interleukin 13 and interleukin 13 receptor are frequently expressed by Hodgkin and Reed-Sternberg cells of Hodgkin lymphoma. Blood 2001; 97:250-5; PMID:11133768; http://dx.doi.org/ 10.1182/blood.V97.1.250 [DOI] [PubMed] [Google Scholar]
- 5.Ullrich K, Blumenthal-Barby F, Lamprecht B, Kochert K, Lenze D, Hummel M, Mathas S, Dörken B, Janz M. The IL-15 cytokine system provides growth and survival signals in Hodgkin lymphoma and enhances the inflammatory phenotype of HRS cells. Leukemia 2015; 29:1213-8; PMID:25486870; http://dx.doi.org/ 10.1038/leu.2014.345 [DOI] [PubMed] [Google Scholar]
- 6.Ho WT, Pang WL, Chong SM, Castella A, Al-Salam S, Tan TE, Moh MC, Koh LK, Gan SU, Cheng CK et al.. Expression of CD137 on Hodgkin and Reed-Sternberg cells inhibits T-cell activation by eliminating CD137 ligand expression. Canc Res 2013; 73:652-61; PMID:23204227; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-3849 [DOI] [PubMed] [Google Scholar]
- 7.Anderson MW, Zhao S, Freud AG, Czerwinski DK, Kohrt H, Alizadeh AA, Houot R, Azambuja D, Biasoli I, Morais JC et al.. CD137 is expressed in follicular dendritic cell tumors and in classical Hodgkin and T-cell lymphomas: diagnostic and therapeutic implications. Am J Path 2012; 181:795-803; PMID:22901750; http://dx.doi.org/ 10.1016/j.ajpath.2012.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang S, Chen L. Immunobiology of cancer therapies targeting CD137 and B7-H1/PD-1 cosignal pathways. Curr Top Microbiol Immunol 2011; 344:245-67; PMID:20582531; http://dx.doi.org/ 10.1007/82_2010_81 [DOI] [PubMed] [Google Scholar]
- 9.Thum E, Shao Z, Schwarz H. CD137, implications in immunity and potential for therapy. Front Biosci 2009; 14:4173-88; PMID:19273343; http://dx.doi.org/ 10.2741/3521 [DOI] [PubMed] [Google Scholar]
- 10.Wang C, Lin GH, McPherson AJ, Watts TH. Immune regulation by 4-1BB and 4-1BBL: complexities and challenges. Immunol Rev 2009; 229:192-215; PMID:19426223; http://dx.doi.org/ 10.1111/j.1600-065X.2009.00765.x [DOI] [PubMed] [Google Scholar]
- 11.Dharmadhikari B, Wu M, Abdullah S, Rajendran S, Ishak D, Nickles E et al.. CD137 and CD137L signals are main drivers of type 1, cell-mediated immune responses. Oncoimmunol 2015. 5(4); page e1113367; PMID:27141396; http://dx.doi.org/ 10.1080/2162402X.2015.1113367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michel J, Langstein J, Hofstadter F, Schwarz H. A soluble form of CD137 (ILA/4-1BB), a member of the TNF receptor family, is released by activated lymphocytes and is detectable in sera of patients with rheumatoid arthritis. Eur J Immunol 1998; 28:290-5; PMID:9485208; http://dx.doi.org/ 10.1002/(SICI)1521-4141(199801)28:01%3c290::AID-IMMU290%3e3.0.CO;2-S [DOI] [PubMed] [Google Scholar]
- 13.Shao Z, Sun F, Koh DR, Schwarz H. Characterisation of soluble murine CD137 and its association with systemic lupus. Mol Immunol 2008; 45:3990-9; PMID:18640726; http://dx.doi.org/ 10.1016/j.molimm.2008.05.028 [DOI] [PubMed] [Google Scholar]
- 14.Setareh M, Schwarz H, Lotz M. A mRNA variant encoding a soluble form of 4-1BB, a member of the murine NGF/TNF receptor family. Gene 1995; 164:311-5; PMID:7590349; http://dx.doi.org/ 10.1016/0378-1119(95)00349-B [DOI] [PubMed] [Google Scholar]
- 15.Furtner M, Straub RH, Kruger S, Schwarz H. Levels of soluble CD137 are enhanced in sera of leukemia and lymphoma patients and are strongly associated with chronic lymphocytic leukemia. Leukemia 2005; 19:883-5; PMID:15744355; http://dx.doi.org/ 10.1038/sj.leu.2403675 [DOI] [PubMed] [Google Scholar]
- 16.Caumartin J, Lemaoult J, Carosella ED. Intercellular exchanges of membrane patches (trogocytosis) highlight the next level of immune plasticity. Transpl Immunol 2006; 17:20-2; PMID:17157208; http://dx.doi.org/ 10.1016/j.trim.2006.09.032 [DOI] [PubMed] [Google Scholar]
- 17.Pang WL, Ho WT, Schwarz H. Ectopic CD137 expression facilitates the escape of Hodgkin and Reed-Sternberg cells from immunosurveillance. Oncoimmunol 2013; 2:e23441; PMID:23734307; http://dx.doi.org/ 10.4161/onci.23441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao Z, Harfuddin Z, Pang WL, Nickles E, Koh LK, Schwarz H. Trogocytic CD137 transfer causes an internalization of CD137 ligand on murine APCs leading to reduced T cell costimulation. J Leukoc Biol 2015; PMID:25765680; http://dx.doi.org/ 10.1189/jlb.3A0213-079RRR [DOI] [PubMed] [Google Scholar]
- 19.Natoli A, Lupertz R, Merz C, Muller WW, Kohler R, Krammer PH, Li-Weber M. Targeting the IL-4/IL-13 signaling pathway sensitizes Hodgkin lymphoma cells to chemotherapeutic drugs. Int J Canc 2013; 133:1945-54; PMID:23553437; http://dx.doi.org/ 10.1002/ijc.28189 [DOI] [PubMed] [Google Scholar]
- 20.Celegato M, Borghese C, Casagrande N, Mongiat M, Kahle XU, Paulitti A, Spina M, Colombatti A, Aldinucci D et al.. Preclinical activity of the repurposed drug auranofin in classical Hodgkin lymphoma. Blood 2015; 126:1394-7; PMID:26228484; http://dx.doi.org/ 10.1182/blood-2015-07-660365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terabe M, Park JM, Berzofsky JA. Role of IL-13 in regulation of anti-tumor immunity and tumor growth. Canc Immunol Immunother 2004; 53:79-85; PMID:14610620; http://dx.doi.org/ 10.1007/s00262-003-0445-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu SM, Xie SS, Hsu PL, Waldron JA Jr.. Interleukin-6, but not interleukin-4, is expressed by Reed-Sternberg cells in Hodgkin's disease with or without histologic features of Castleman's disease. Am J Pathol 1992; 141:129-38; PMID:1632458 [PMC free article] [PubMed] [Google Scholar]
- 23.Skinnider BF, Kapp U, Mak TW. The role of interleukin 13 in classical Hodgkin lymphoma. Leuk Lymph 2002; 43:1203-10; PMID:12152987; http://dx.doi.org/ 10.1080/10428190290026259 [DOI] [PubMed] [Google Scholar]
- 24.Drexler HG. Recent results on the biology of Hodgkin and Reed-Sternberg cells. II. Continuous cell lines. Leuk Lymph 1993; 9:1-25; PMID:7682880; http://dx.doi.org/ 10.3109/10428199309148499 [DOI] [PubMed] [Google Scholar]
- 25.Shao Z, Schwarz H. CD137 ligand, a member of the tumor necrosis factor family, regulates immune responses via reverse signal transduction. J Leukoc Biol 2011; 89:21-9; PMID:20643812; http://dx.doi.org/ 10.1189/jlb.0510315 [DOI] [PubMed] [Google Scholar]
- 26.Langstein J, Michel J, Fritsche J, Kreutz M, Andreesen R, Schwarz H. CD137 (ILA/4-1BB), a member of the TNF receptor family, induces monocyte activation via bidirectional signaling. J Immunol 1998; 160:2488-94; PMID:9498794 [PubMed] [Google Scholar]
- 27.Oshima Y, Puri RK. Suppression of an IL-13 autocrine growth loop in a human Hodgkin/Reed-Sternberg tumor cell line by a novel IL-13 antagonist. Cell Immunol 2001; 211:37-42; PMID:11585386; http://dx.doi.org/ 10.1006/cimm.2001.1828 [DOI] [PubMed] [Google Scholar]
- 28.Skinnider BF, Kapp U, Mak TW. Interleukin 13: a growth factor in hodgkin lymphoma. International archives of allergy and immunology 2001; 126:267-76; PMID:11815733; http://dx.doi.org/ 10.1159/000049523 [DOI] [PubMed] [Google Scholar]
- 29.Yonezawa A, Dutt S, Chester C, Kim J, Kohrt HE. Boosting Cancer Immunotherapy with Anti-CD137 Antibody Therapy. Clin Canc Res 2015; 21:3113-20; PMID:25908780; http://dx.doi.org/ 10.1158/1078-0432.CCR-15-0263 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


