Abstract
Clinical data from human chronic wounds implicates biofilm formation with the onset of wound chronicity. Despite the development of novel antimicrobial agents, the cost and complexity of treating chronic wound infections associated with biofilms remain a serious challenge, which necessitates the development of new and alternative approaches for effective anti-biofilm treatment. Recent advancement in nanotechnology for developing a new class of nanoparticles that exhibit unique chemical and physical properties holds promise for the treatment of biofilm infections. Over the last decade, nanoparticle-based approaches against wound biofilm infection have been directed toward developing nanoparticles with intrinsic antimicrobial properties, utilizing nanoparticles for controlled antimicrobials delivery, and applying nanoparticles for antibacterial hyperthermia therapy. In addition, a strategy to functionalize nanoparticles towards enhanced penetration through the biofilm matrix has been receiving considerable interest recently by means of achieving an efficient targeting to the bacterial cells within biofilm matrix. This review summarizes and highlights the recent development of these nanoparticle-based approaches as potential therapeutics for controlling wound biofilm infection, along with current challenges that need to be overcome for their successful clinical translation.
Keywords: biofilm infection, nanoparticles, extracellular polymeric substances, antimicrobial elivery, hyperthermia, wound healing
1. Introduction
The U.S. Centers for Disease Control and Prevention (CDC) reports that up to 70% of infections in the western world are associated with biofilms. Biofilms are consortia of microbial cells adhered to a surface and embedded in a self-produced extracellular matrix that is primarily composed of extracellular polymeric substances (EPS). The EPS is composed of exopolysaccharides, proteins, dead bacteria, bacterial DNA, and enzymes and acts as a protective barrier against antibiotic penetration and cellular attack by host innate immune cells [1–4]. A critical challenge in treatment of biofilm infection is the alarming rate by which they frequently develop a resistance to traditional antimicrobial therapies as well as to host immune responses [5–7]. Biofilm formation in the cutaneous wounds especially raises challenging problems for the management of wound infection. Indeed, many clinical data from human chronic wounds implicates the formation of biofilm in the wound as a major mechanism that contributes to the wound chronicity [3, 8–12].
A number of biochemical and biophysical approaches have been studied as potential therapeutic strategies to control wound biofilm formation. Biochemical approaches include the use of quorum sensing inhibitors [13], new classes of antimicrobial peptides [14], and enzymes that dissolve biofilms [15]. Biophysical approaches include the application of infrared and light pulsing [16, 17], direct-current electrical stimulation [18, 19], ultrasound [20, 21], and alternating electric fields [22]. However, many of these approaches have demonstrated a modest antimicrobial efficacy and still have limitations in successfully controlling biofilm infection.
The use of materials at the nanometer or submicron scale has shown to be promising for biomedical applications including diagnosis and therapy. This is attributed to the increased reactivity of nanomaterial due to large surface area to volume ratio, as well as the flexibility in controlling its chemical and physical properties [23]. In view of this, nanoparticle-based approaches have received considerable attention over the last decade as a new therapy for the treatment of wound biofilm infection. Approaches have been directed toward developing a new class of nanoparticles that can confer antimicrobial effects. For example, nanoparticles made of metal or metal oxide can be synthesized to exhibit intrinsic antimicrobial properties. These nanoparticles exhibit antimicrobial mechanisms against bacterial pathogens by disrupting the cell membrane directly or producing free radicals [24]. Properties of certain nanoparticles that enable controlled and sustained delivery of drugs, such as liposomes or polymeric nanoparticles, can be used for controlled delivery of therapeutic dose of antimicrobial drugs to the target site of biofilm infection [25]. The strategy can overcome the current limitations of conventional antibiotic treatments associated with toxicity, inefficient delivery or enzymatic inactivation of drugs in vivo. In addition, new approaches of physically disrupting bacterial cells within biofilms, by applying external energy sources, have been introduced recently. For example, nanoparticles such as gold nanoparticles or magnetic nanoparticles (MNPs, such as γFe2O3 maghemite or Fe3O4 magnetite nanoparticles) can be excited to generate heat on their surfaces when they receive external energy such as near-infrared (NIR) light [26] or alternate magnetic field (AMF) [27], which can impose an irreversible thermal damage to the target cell when they are properly targeted. These approaches can provide a promising opportunity for treating wound biofilm infections in minimally invasive and on-demand manners. Lastly, a strategy to engineer the size and surface functionality of nanoparticles towards a facilitated penetration through the biofilm matrix can be effective in targeting bacterial cells [28]. This review article highlights recent studies on the application of nanoparticle-based strategies as potential therapeutics for controlling wound biofilm infections.
2. Why biofilm infections are difficult to treat in the cutaneous wound
The deleterious influence of microbial infection on wound healing has been recognized for centuries and has motivated a plethora of approaches to alleviate bacterial burden within a wound site and promote a normal healing. The loss of skin integrity and barrier to cutaneous wounding elicits the exposure of open wounds to bacterial colonization and proliferation. The pathogens including Staphylococcus aureus (S. aureus), Pseudomonas aeruginosa (P. aeruginosa), Staphylococcus epidermidis (S. epidermidis) and Enterococcus spp. have been identified as predominant ones in skin wound infections in hospitalized patients [29]. Skin wounding and infection triggers a cascade of inflammatory events that lead to rapid recruitment of phagocytes (neutrophils and macrophages) from the blood circulation to the local site of injury [30]. In normal physiological conditions, microbes are successfully cleared by the host's immune system, which leads to a normal wound healing process. However, under conditions associated with immune deficiency or dysregulation, microbes frequently attach to the wound surface and a biofilm then begin to develop with the secretion of EPS [31]. Accumulating evidence supports that the chronicity of non-healing wounds, including venous leg ulcers, pressure ulcers, and diabetic foot ulcers, are associated with increased incidences of biofilm formation [32–35].
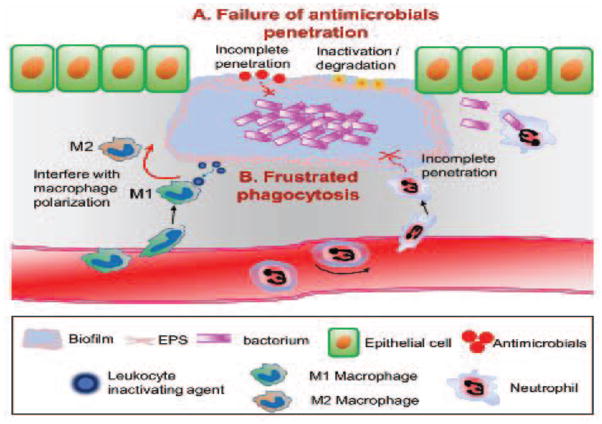
A critical challenge in treatment of infections associated with biofilms is that they are resistant to antibiotics and readily evade innate immune attacks by phagocytes. It has been shown that bacteria within biofilms are 10–1000 times less sensitive to treatments with antimicrobial agents compared to planktonic cells [36]. Current topical and systemic antibiotics are minimally effective in the treatment of chronic biofilm infections in wounds [8]. This has been associated with slow or incomplete penetration of antimicrobials to bacterium since the presence of EPS forms protective barrier for the diffusion of antimicrobials [37]. In addition, antimicrobial agents may react chemically with the extracellular components of the biofilm or attach to the anionic polysaccharides without reaching to the target bacterial cells [38] (Figure 1A).
Figure 1.
Schematic on the potential mechanisms by which biofilm formation in the wound lead to the infection persistency and wound chronicity. The presence of EPS in the biofilm creates a protective environment for the residing microorganisms against antibiotics treatments and host innate immune response. A. The presence of EPS forms protective barrier for the diffusion of antimicrobials or results in inactivation of antimicrobials at the biofilm matrix. B. In addition, the formation of biofilm structures substantially diminishes the phagocytic activities of innate immune cells (neutrophils and macrophages), by not only providing a shielding mechanism from the penetration of neutrophils, but also promoting the production of leukocyte-inactivating substances, which impairs bactericidal activity of macrophages by skewing macrophage polarization towards M2-like phenotype.
Importantly, the advent of a bacterially secreted EPS may also act as a protective barrier against cellular attacks by phagocytic macrophages and neutrophils [1–4]. It has been well characterized that neutrophils and macrophages play a crucial role in innate immune protection against infectious pathogens [39, 40]; their defense mechanisms are highly effective against planktonic bacteria. However, the formation of biofilm structures provides a shielding mechanism that results in protection from the phagocytic activity of neutrophils and macrophages [10]. In addition, biofilms have the capacity to promote the production of leukocyte-inactivating substances, which results in a phenomenon termed “frustrated phagocytosis” [41] (Figure 1B). The quorum sensing compounds derived from EPS, including alginate and rhamnolipids, have been attributed to the inhibitory capacity as the potential to resist the leukocyte attack [42, 43]. Although the phenomenon of frustrated phagocytosis has been well studied in a model of P. aeruginosa biofilm associated with pulmonary infection, S. aureus biofilm has also shown to utilize a similar mechanism by interfering with macrophage polarization towards enhancing their own survival. The classically activated profile of macrophage (M1) is necessary to confer an appropriate host defense against invading bacterial pathogens by producing pro-inflammatory cytokines. A recent study using a cutaneous biofilm infection with S. aureus revealed that macrophages exhibited limited phagocytosis and killing of bacteria in the presence of biofilm matrix, which was associated with skewing of gene expression patterns from M1 to an alternatively activated phenotype (M2) [44].
Since treatment options for clinicians fighting biofilm infections are limited, there is, therefore, an urgent need for an alternative antimicrobial treatment that efficiently and rapidly disrupts biofilms and is non-toxic to host tissue in infection clearance and wound resolution.
3. Nanoparticle-based strategies for controlling biofilm infections
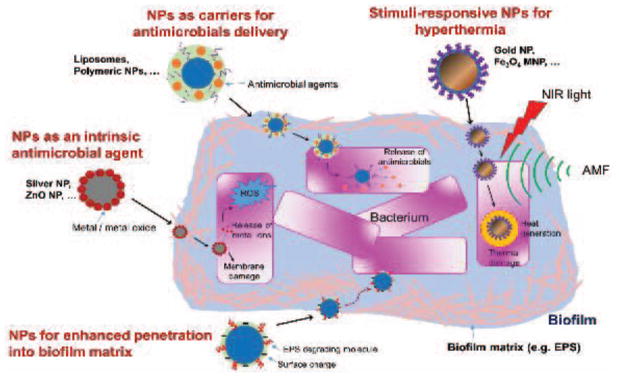
Recent advances in nanoparticle-based technology provide new and promising opportunities for effectively defending wound infection associated with biofilms. Over the last decade, nanoparticle-based approaches against biofilm infection have been directed towards designing nanoparticles to exhibit specific chemical and physical properties towards anti-biofilm activities. This section is focused on highlighting recent studies on the application of various nanoparticle-based approaches as potential antimicrobial therapeutics for controlling biofilm infection in the cutaneous wounds (Table 1). The schematic on the mechanism of actions for various nanoparticle-based approaches for anti-biofilm activities is depicted in Figure 2. For more detailed understanding on the design and synthesis of these nanoparticles for anti-microbial effects, readers may refer to recently published review articles [24–26, 45–51]
Table 1.
The mechanism of actions and limitations for various nanoparticle (NP)-based approaches for anti-biofilm activities.
| Types of NPs used | Mechanism of actions / advantages | Limitations | ||
|---|---|---|---|---|
| NPs as intrinsic antimicrobial agents | Silver NP [63, 64], Zinc oxide NP [71–76] |
|
Toxicity at high concentration | |
| NPs for controlled delivery of antimicrobial agents | Antibiotics delivery | Liposomes [84, 85, 87], Polymeric NP [89, 90], Lipid-polymer hybrid NP [92] |
|
Insufficient drug loading |
| NO delivery | Silica NP [97], Silane hydrogel- based NP [99–101] |
|
Difficulty of controlling the release kinetics of physiologically optimal concentrations of NO in the wound bed | |
| Photosensitizer delivery | Porphyrin [109], Methylene blue [110], Rose bengals [111] |
|
Non-specific cytotoxic reactive oxygen species damage to host cells | |
| Responsive NPs for anti-bacterial hyperthermia treatment | NIR light- triggered hyperthermia | Gold NP [112], Fe3O4 MNP [113], Graphene NP [114] |
|
Non-specific thermal damage to host cells |
| AMF-triggered hyperthermia | Fe3O4 MNP [119–121] |
|
Non-specific thermal damage to host cells | |
| NPs for enhanced penetration to the biofilm matrix | Surface charge functionalization Surface coating with EPS degrading molecules |
[127] [129] |
|
Figure 2.
Schematic on the mechanism of actions for various nanoparticle (NP)-based approaches for treating biofilm infections.
3.1. Nanoparticles as an intrinsic antimicrobial agent
Conventional antimicrobial therapy relies on antibiotics to either kill or interfere with the growth of infectious pathogens. However, the use of antibiotics has been limited by toxicity or allergic reactions to host cells [52]. In addition, the frequent administration of sub-lethal dose of antibiotics has been shown to lead to a multi-drug resistance [53]. The antimicrobial activity of metals or metal oxides has been well reported [54]. As such, nanoparticles made of metals and metal oxides have been employed as topical antimicrobial agents.
Metal nanoparticles such as silver, copper, gold, titanium, and zinc have shown to exhibit an antimicrobial activity against wound biofilm infection. Among them, silver-based nanoparticles have received considerable attentions. The mechanism of the antimicrobial action of silver ions results from their interaction with sulfhydryl groups [55, 56], which interferes with bacterial cell membrane integrity, respiratory chains, enzyme activities, and cell proliferation [57]. Moreover, silver has shown to destabilize the biofilm matrix by compromising intermolecular forces [58, 59]. However, the use of free silver ions in the wound bed resulted in rapid sequestration by proteins and other cellular components, which reduced bioavailability of silver ions and substantially diminished their antibacterial effects [60]. The advantage of using silver-based nanoparticles for the wound biofilm management lies in achieving the prolonged release of silver ions in the target cells, which in turn triggers an antibacterial activity by generation of reactive oxygen species (ROS) [61]. Silver nanoparticles can be synthesized by either chemical method involving the reduction of silver nitrate using a reducing agent or physical method utilizing thermal or electric power energy [62]. The silver nanoparticles were effective in reducing formation of biofilm from both gram-positive and gram-negative bacteria in vitro [63]. The study showed that the 24 hours treatment of silver nanoparticles could result in more than 95% inhibition in biofilm formed by P. aeruginosa and S. epidermidis. The coating of silver nanoparticles on the material surface could successfully restrict biofilm formation by methicillin-resistant S. aureus (MRSA) and methicillin-resistant S. epidermidis (MRSE) isolated from human wounds [64]. However, despite the considerable interest in the use of silver nanoparticles as an antimicrobial agent, their use has been challenged with diminished therapeutic effects for prolonged treatment [65]. This is in line with the reported silver resistance for prolonged treatment in clinical isolates of methicillin-resistant S. aureus (MRSA) [66]. Another limitation is that high dose of silver nanoparticles may delay wound repair by triggering a toxic effect on keratinocytes and fibroblasts [67]. The cytotoxic effect of silver nanoparticles has been shown to be dependent on the size, concentration, and the rate of intracellular silver ion release [68]. However, it is interesting to note that biofilm bacteria, which survived the prolonged treatment of silver nanoparticles, were susceptible to antibiotics [65]. This implicates that combined treatment with antibiotics or antimicrobials can be synergistic for enhancing the anti-biofilm efficacy of silver nanoparticles while reducing the cytotoxic effect [69, 70].
Along with well-reported antibacterial activities of metal oxides, there have been considerable interests in developing nanoparticles made of metal oxides including zinc oxide (ZnO), magnesium oxide (MgO), iron oxide (Fe2O3), aluminum oxide (Al2O3), and copper oxide (CuO). Among them, ZnO nanoparticle has been widely used for the treatment of wound infection [24]. In a rat model of skin wound infection, the delivery of ZnO nanoparticle in combination with β-chitin dressing exhibited an improved wound healing and caused a reduction in the growth of bacteria [71]. Importantly, in a comparative study examining the antimicrobial activity of different types of metal oxide nanoparticles made of ZnO, CuO, and Fe2O3, ZnO nanoparticles were shown to exhibit the most antibacterial effect against multiple species of bacteria including gram positive strains of S. aureus and Bacillus subtilis (B. subtilis) and gram negative strains of E. coli and P. aeruginosa bacteria [72, 73]. It was further revealed that ZnO nanoparticles can confer antimicrobial activities against biofilms formed by P. aeruginosa [74, 75] and S. aureus [76] as well. Although the exact mechanism of antibacterial activity of ZnO nanoparticle has not been well understood, the generation of hydrogen peroxide [77] and cell membrane damage [78] have been suggested as possible mechanisms of antibacterial activity. However, despite its potent anti-biofilm effect, the use of ZnO nanoparticle for clinical translation may be limited by their cytotoxic effects to human cells. For example, concentrations of ZnO nanoparticle at 10 μg/ml exhibited a substantial decrease in viability of human epithelial cells [79].
3.2. Nanoparticles for controlled delivery of antimicrobial agents
The major limitation of conventional antibiotic or antimicrobial treatment against biofilm infections in non-healing chronic wounds relates to the insufficient delivery of the desired concentration of antibiotics to the target bacterial cells, which is largely associated with the presence of a complex matrix network of biofilms, along with the avascular nature in the chronic wounds [80]. This could not only substantially diminish the therapeutic effect of systemically or topically administered drugs, but also lead to the induction of drug resistance. The presence of EPS forming biofilm can not only limit the diffusion of antimicrobial agents to the individual cells of bacteria, but also result in the binding of soluble antimicrobials to matrix components, which prevents them from reaching target cells [81]. Over the last few decades, considerable efforts have been directed to achieve controlled and sustained release of drugs by utilizing nanoparticles in pharmaceutical science. The strategy of delivering antimicrobials using nanoparticles offers several advantages that can substantially increase antimicrobial activity compared to the strategy of delivering free drugs only, which include the release of drugs at a sustained and controlled manner, protection of drugs from enzymatic inactivation, and targeted delivery of drugs to the target tissues [82].
3.2.1. Controlled release of antibiotics
Antibiotics that are encapsulated within nanoparticles can efficiently penetrate EPS to reach target cells compared to free drugs, which can facilitate the delivery of a therapeutic dose of antibiotics to the bacterial cells. Many biocompatible and biodegradable nanoparticles have been used as carriers for antibiotics to promote sustained antimicrobial effects. Among them, liposomes and polymeric nanoparticles have been widely used as carriers for antibiotics delivery against biofilms [45]. Liposome nanoparticle is composed of phospholipid bilayer and has been widely used as a drug delivery carrier in many biomedical applications due to its lipid bilayer structure that mimics cell membranes [83]. Liposome nanoparticle can readily fuse with bacterial cell wall and the encapsulated drug can be released to the cell membranes or the inside of the bacteria [84]. Recent studies have demonstrated the enhanced therapeutic efficacy of delivering antibiotics with liposomes to the biofilms compared to the delivery of free antibiotics. For example, the encapsulation of the β-lactam antibiotic, piperacillin, within liposomes could protect the drug from hydrolysis by staphylococcal β-lactamase [85], which was correlated with a higher activity for piperacillin encapsulated-liposomes against S. aureus compared to the free piperacillin. In another study, Mugabe et al. [86] demonstrated that the strategy of encapsulating gentamycin within liposomes could result in a significantly higher antimicrobial activity against P. aeruginosa biofilms. Importantly, in a mouse model of subcutaneous infection, the delivery of daptomycin encapsulated-liposomes was effective in inhibiting S. aureus biofilm growth at the site of infection, which was comparable to the treatment of intravenous injection of daptomycin [87]. However, despite its therapeutic potential as a topical anti-biofilm agent, the use of antibiotics-encapsulated liposome has been challenged by their chemical and physical instability that can lead to drug leakage during the storage [25].
Among polymeric nanoparticles, nanoparticles made of poly(lactic-co-glycolic acid) (PLGA) have been widely studied. The main advantage of PLGA nanoparticle lies in their biocompatibility and biodegradability, as well as flexibility for controlling the release kinetics of loaded drugs by tuning the degradation profile of PLGA [88]. The use of PLGA nanoparticles for the delivery of antibiotics has shown to be effective in treating multiple species strains of bacteria including P. aeruginosa [89], S. aureus [89], and E. coli [90]. In addition, lipid-polymer hybrid nanoparticles that combine the advantages of both liposomes and polymeric nanoparticles have recently emerged as a new class of drug delivery platform [91]. The hybrid nanoparticle is composed of polymeric nanoparticles core surrounded by lipid layers, which combine the advantages of the highly biocompatible characteristic of lipids with the structural stability and controllable biodegradability imparted by polymeric nanoparticles [92]. Although there have been very few studies of using lipid-polymeric hybrid nanoparticles for controllable release of antibiotics [92], the use of hybrid nanoparticles have been demonstrated to exhibit an enhanced cellular delivery efficacy compared to that obtained from liposomes or polymeric nanoparticles for targeting cancer cells [93]. As such, the hybrid nanoparticles can be an alternative to liposomes or polymeric nanoparticles as an antibiotics delivery platform to treat wound biofilm infections.
Taken together, these studies support that a strategy of loading and delivering antibiotics using nanoparticles has a great therapeutic potential in that desirable doses of antimicrobial agents can be delivered directly into the bacteria, which will overcome current limitations in conventional antibiotic treatment involving low water-solubility, cytotoxicity to healthy tissues, and rapid degradation in the tissue [25, 45]. However, despite its potential for anti-biofilm activity, only few nanoparticle-based antimicrobial agents have been approved for clinical use, which appears to be associated with high cost and inefficient drug loading [94]. Achieving the higher encapsulation efficiency of antibiotics in the nanoparticles may reduce potential toxicity issues that might result from the use of high concentrations of nanoparticles. In addition, premature drug release from the antibiotic-loaded nanoparticles remains another challenge and a novel method to achieve a site specific release of antibiotics, for example by developing infectious environment-sensitive nanoparticles, will be beneficial [45].
3.2.2. Controlled release of nitric oxide
Nitric oxide (NO) has been well reported to exhibit an broad-spectrum of antimicrobial activity by interfering the process of DNA replication and respiration in bacteria [95]. More importantly, NO was shown to be effective in dispersing the formation of biofilm [96]. However, the use of NO as a therapeutic agent has been mainly challenged due to its short half-life and instability in the tissue in vivo. In view of this, a strategy for utilizing nanoparticles as a vehicle for sustained and controlled release of NO has been proposed for the antimicrobial treatment of wound infections. The treatment of NO-releasing silica nanoparticles could demonstrate an enhanced bactericidal efficacy against planktonic P. aeruginosa cells compared to the treatment of small molecule NO donors [97]. In another study, it was further demonstrated that NO-releasing silica nanoparticles are effective in killing bacterial cells within established biofilms [98]. The therapeutic efficacy of NO-releasing nanoparticles was further demonstrated from in vivo studies using murine models of wound infection, in which NO-releasing nanoparticles made of silane hydrogels were effective in reducing bacterial burden in wounds infected with MRSA [99], Acinetobacter baumannii [100], and Candida albicans (C. albicans) [101]. Another advantage for NO-releasing nanoparticles is that it can potentially accelerate wound healing not only by conferring bactericidal activity, but also by promoting angiogenesis and tissue remodeling in the wound bed [102]. Taken together, these studies support that NO-releasing nanoparticles can be a potent antimicrobial therapeutic for topical treatment in cutaneous wound infection. However, a critical challenge for NO-based therapy for wound healing has been associated with maintaining an optimal concentration of NO in the wound bed since either too high or too low levels of NO may hinder the normal process of wound healing [103, 104]. Thus, important design criteria for NO-releasing nanoparticles should ensure the release kinetics of physiologically desirable concentration of NO is precisely controlled in a sustained fashion [102].
3.2.3. Controlled delivery of photosensitizer
Photodynamic therapy (PDT) is a therapeutic approach to kill pathogens using a combination of light and photosensitizer (PS) (e.g., phenothiazine dyes, porphyrin, methylene blue, or rose bengals). Recently, there has been increasing interests in applying PDT as a treatment for various types of localized infections [105]. The application of PDT against wound infection is based on the mechanism that activation of PS by the exposure of light of the appropriate wavelength can trigger the transfer of energy to molecular oxygen, which generates cytotoxic ROS that triggers a necrotic cell death [106]. Although planktonic bacteria were susceptible to PDT, the extent of antimicrobial activity by PDT was shown to be substantially reduced in bacteria in biofilm [107]. One mechanism responsible for the reduced susceptibility of biofilms to PDT was attributed to the failure of PS drug penetration. The strategy for encapsulating PS agents within nanoparticles confers advantages by preventing potential inactivation of the drugs by EPS matrix, over treatment of free photosensitizing molecules. In addition, the characteristic of large surface to volume ratio of nanoparticles can increase the amount of PS that can be delivered to the target cells [108]. Nanoscale carriers including liposomes and biodegradable polymeric nanoparticles have been widely proposed as PS delivery vehicles for PDT [108]. The controlled delivery of PS, porphyrin, by liposome could result in significantly enhanced inactivation of MRSA compared with free dye treatment [109]. Nanoparticles functionalized with methylene blue [110], or rose bengals [111] have demonstrated an efficacy for reducing biofilm formation. However, although the use of PS carrying nanoparticles has a potential for the treatment and management of wound infections, it still has several limitations that need to be overcome for its successful clinical translation. It is necessary to ensure the selectivity of PS carrying-nanoparticles to the target bacterial cells to avoid any potential non-specific PDT damage to the host cells at the site of wound infection [105]. In addition, to be used in the clinical setting, several factors, including the physiochemical properties of the PS, dose of PS to be delivered, and rate of release, and right dosimetry of light, should be carefully understood [108].
3.3. Responsive nanoparticles for anti-bacterial hyperthermia treatment
Approaches of triggering bacterial damage using a combination of externally triggered energy sources and energy absorbing nanoparticles have been developed as new therapeutic options for antimicrobial treatments. The basic principle is to induce irreversible thermal damages to the bacterial cells by activating nanoparticles with externally applied energy source such as NIR light or high frequency AMF. The absorbed energy on nanoparticles can be quickly converted into heat energy, which triggers temperature increase on the surface of nanoparticles. As far as an appropriate targeting strategy is achieved, the nanoparticle-based hyperthermia may hold promise for future clinical application in that the method is non-invasive, tissue-specific, and capable of generating precisely localized heating in the target pathogens for eradication [27].
3.3.1. NIR light-triggered hyperthermia
Nanoparticles made of gold, iron oxide, and graphene have been used as photothermal agents that are responsive to NIR light illumination. Among them, gold nanoparticles have been widely studied for this purpose due to their excellent responsivity to NIR light [26]. Previously, Zharov et al. [112] first demonstrated that NIR light-trigged activation of gold nanoparticles was sufficient to thermally inactivate S. aureus, by achieving a high affinity targeting of gold nanoparticles to S. aureus by conjugating nanoparticles with anti-protein A antibodies. An alumina-coated iron oxide magnetic nanoparticle was also used as a photothermal agent, which demonstrated to be effective in bacterial killing [113]. The temperature of the nanoparticles suspension under illumination with NIR light increased by 20°C for 5 minutes, which resulted in inhibition of the growth of antibiotic resistant strain of both gram-positive and gram-negative bacteria by 95%. Recently, Wu et al. [114] have used a graphene nanoparticles for NIR light-triggered hyperthermia against S. aureus and E.coli. They utilized a photothermal property of reduced graphene oxide upon NIR laser irradiation, in which 80 ppm solution of graphene nanoparticles were sufficient in inducing a rapid killing of both gram-positive and gram-negative bacteria by 99%, within 10 minutes upon NIR laser irradiation.
However, since the mechanism of antibacterial activity for NIR light responsive nanoparticles relies on hyperthermia, their use can be limited due to their potential to impose off-target thermal effects to host cells, unless a suitable targeting strategy is achieved. In view of this, a strategy to combine with other antimicrobial therapeutic modality will be advantageous to achieve a successful eradication of pathogens, which could reduce potential for non-specific thermal damage to host cells by enabling the use of reduced level of NIR light intensity. Recently, Chiang et al. [115] successfully applied a dual modalities of therapeutic platform for treating wound infection by combining a platform for NIR light-triggered hyperthermia with one for controlled release of antibiotics. For this, they have developed a hybrid microsphere made of a shell of PLGA and aqueous cores composed of polypyrrole nanoparticles and vancomycin, in which polypyrrole nanoparticles were used as a photothermal agent. The combination of photothermally-induced hyperthermia and antibiotic delivery could result in synergistic effect of eradicating bacteria in wound abscesses of mice, to an extent that is greater than the sum of the two treatments alone.
3.3.2. AMF-triggered hyperthermia
Recent advancements in developing MNPs with high heating efficiency have improved the efficacy of hyperthermia treatment on pathogens [49]. The MNP hyperthermia utilizes MNPs in conjunction with high frequency AMF (>100kHz), in which MNPs absorb electromagnetic radiation and efficiently transmit energy in the form of highly localized heat (i.e. nanometer range of distance) on the surface of MNPs [48]. This technology has recently proven successful in the treatment of various cancers, with therapies in clinical trials to treat glioblastoma, prostate carcinoma, and breast carcinoma [116–118].
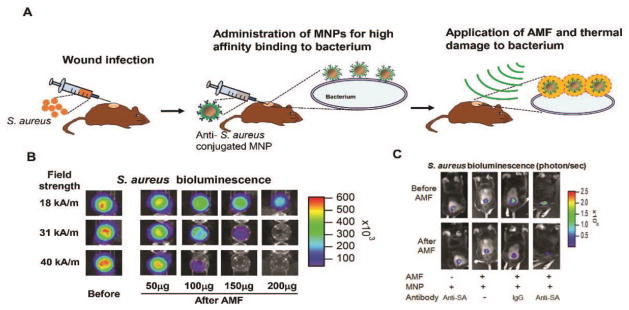
Despite extensive studies on the use of MNPs for cancer hyperthermia treatment, relatively little attention has been devoted to the use of MNP hyperthermia as a potential for antimicrobial therapy. Thomas et al. [119] recently demonstrated that magnetic fluid hyperthermia could be successfully used for bacterial destruction from in vitro culture model of S. aureus. The effect of magnetic fluid hyperthermia on the eradication of bacterial biofilms was further demonstrated in vitro, in which MNPs were added to P. aeruginosa biofilm, and heat was generated by placing the nanoparticle-containing biofilm in an AMF [120]. More than 4 log inactivation of the P. aeruginosa biofilm was observed within 8 minutes when relatively high concentrations of MNPs (60 mg/mL) were used. Recently, Kim et al. [121] successfully validated the ability for MNP heating to effectively disrupt S. aureus biofilms using both in vitro culture assay and in vivo study of a mouse model of S. aureus cutaneous wound infection. The schematic on the strategy of applying MNP hyperthermia for the treatment of mouse model of wound S. aureus infection is depicted in Figure 3A. In an in vitro study, the brief AMF exposure (~3 min) disrupted biofilms in proportion to the amount of MNPs and the applied magnetic field amplitude. A 2-log reduction in S. aureus bioluminescence (about 99% killing) was achieved at a low dose of 1 mg/mL and magnetic field magnitude of 31 kA/m (Figure 3B). The study implicates that the extent of bacterial inactivation in response to AMF excitation of MNPs is highly dependent on MNP dosing and AMF field parameters. In a subsequent in vivo study using a mouse model of S. aureus cutaneous wound infection, the subcutaneous injection of anti-S. aureus antibody conjugated MNPs followed by the application of short duration AMF (3 minutes) could result in a significantly enhanced S. aureus inactivation (80%) compared with non-specific IgG (50%) or compared with MNPs with no linked antibody (Figure 3C).
Figure 3.
In vitro and in vivo studies on the use of magnetic nanoparticle (MNP) targeted hyperthermia against S. aureus biofilm. A. Schematic on the strategy of MNP hyperthermia treatment in a mouse model of wound S. aureus infection. B. Representative bioluminescent image of S. aureus biofilm on 96 well plate before and after alternating magnetic field (AMF) application (for 3 min at 31 kA/m field amplitude) in vitro. The extent of bacterial killing was quantified based on the level of bioluminescence quenching that directly correlates with number of live bacteria (CFUs). C. Representative bioluminescent images of S. aureus in a mouse skin wound in vivo. Mice were infected with S. aureus (SA, 1 × 107 CFU) at wound sites and MNP-anti-S. aureus antibody (MNP-anti-SA mAb) conjugates were locally injected into wound at day 2 post-infection. Then, an AMF was applied for 3 min (31 kA/m field amplitude) for four experimental groups: (1) mice injected with MNP-anti-SA mAb conjugate but without AMF treatment, (2) mice injected with MNP only and with AMF treatment, (3) mice injected with MNP-IgG conjugate and with AMF treatment, (4) mice injected with MNP-anti-SA mAb conjugate and with AMF treatment. Reprinted with permission from reference [121].
However, it should be noted that although magnetic nanoparticle hyperthermia can generate a rapid temperature increase at the surface of a single MNP [122], a theoretical study also showed that overdosing presents the potential for disseminated tissue heating due to simultaneous heat dissipation from a large number of MNPs dispersed in a macroscopic region of tissue [123]. In addition, the application of high intensity AMF on any conductive biological medium can induce Eddy-currents that result in non-specific inductive heating in the body. Therefore, critical design criteria for the safe application are necessary, which has been a major drawback in related therapies based on MNP hyperthermia for cancer treatment. In particular, the ability to safely heat bacterial cells and prevent tissue damage depends upon high affinity targeting of pathogen cells, facilitated by proper customization of treatment parameters of nanoparticles (size and surface coating) as well as AMF (field frequency, amplitude, and duration of exposure).
3.4. Nanoparticles for efficient penetration to the biofilm matrix
Since the presence of EPS matrix in the biofilm structure is a major limiting factor that hinders the free diffusion of antibiotics or therapeutic drugs into the biofilm, strategies to achieve an enhanced penetration of nanoparticles into the biofilm matrix, while ensuring an effective entrapment of antibiotics, are critical for successful anti-biofilm activity. Although nanoparticles are considered to be transported into the biofilm via diffusional movement, both the size and surface chemistry of nanoparticles might alter the nature of interaction between nanoparticles and biofilm matrix [25], which will influence the penetration efficiency of nanoparticles into the biofilm matrix.
Many earlier studies for developing nanoparticles for enhanced penetration through the biofilm matrix have been performed using an experimental model of pulmonary biofilm infections, in which the EPS produced by P. aeruginosa is essential for the formation of thick and mature biofilms [124]. It has been shown that the presence of functional groups on the EPS (e.g., carboxylate or sulfate group) can cause the biofilms to generally exhibit an overall negative charge [125]. For example, the EPS synthesized by P. aeruginosa has shown to contain an anionic polysaccharide of alginate and negatively charged biomolecules including extracellular DNA, which implicates that either neutral or negatively charged nanoparticles can be efficient in diffusing through the biofilm matrix. Indeed, in a recent study for delivering antibiotic encapsulated liposome against Burkholderia cepacia complex (Bcc) biofilms, negatively charged particles were observed to be directed towards bacterial cell clusters, while positively charged particles became immobilized by interactions with extracellular DNA-like structures in the biofilm matrix [126]. The surface coating of antibiotics-loaded PLGA nanoparticles with DNase I could result in significantly enhanced anti-biofilm activity against P. aeruginosa biofilms than that of free free-soluble antibiotics. This appears due to the increased degradation of the extracellular DNA that stabilize the biofilm matrix [127]. Although relatively little attention has been devoted to the use of EPS penetrating nanoparticles for wound biofilm infection, similar strategies used for pulmonary biofilm models can be applied for enhancing the penetration of nanoparticles through the biofilm matrix in the wound in that the EPS comprises integral part of the biofilm structures in skin wounds as well [31].
For nanoparticles with same surface chemistry, smaller size of particles appear to be efficient in achieving the penetration through the biofilm structure. Slomberg et al. [128] evaluated the efficacy of NO-releasing nanoparticles made of silica against P. aeruginosa and S. aureus biofilms as a function of particle size. The study revealed that the extent of NO delivery and eradication of P. aeruginosa and S. aureus biofilms were significantly higher with decreasing size of particles (~14 nm vs 50 nm and 150 nm), which was correlated with increased penetration depth of nanoparticles.
Taken together, the rational design for the control of surface functionality by means of targeting the EPS matrix can be synergistic with various nanoparticle-based anti-biofilm approaches described above, by increasing the efficiency of nanoparticle delivery to target cells. For example, the combined treatment of silver nanoparticles with biofilm dispersing enzymes could result in a synergistic inhibitory effect on biofilm-embedded MRSA in the study using a mouse model of MRSA infection in a cutaneous wound [129].
4. Future perspectives
Despite the development of novel antimicrobial agents, the cost and complexity of treating chronic wound infections remain a serious challenge, which necessitates the development of new and alternative approaches for an effective treatment of wound infection associated with biofilms. As reviewed here, recent advancement in nanotechnology for developing and applying a new class of nanoparticles that exhibit unique chemical and physical properties holds promise as an alternative to conventional antibiotic treatment for controlling biofilm infections.
However, there are several key issues that remain to be resolved for the successful clinical translation of nanoparticle-based approaches for treating biofilm infections. First, most of reported studies that demonstrate anti-biofilm activities of nanoparticles were based on single species strain of bacteria and mostly relying on in vitro cell culture studies. However, recent clinical data implicate that human chronic infectious wounds are colonized with polymicrobial biofilms composed of multiple species of both gram positive and gram negative pathogens, which is now considered to be a primary impediment to the healing of chronic wounds [3, 8, 9]. The polymicrobial biofilms can exhibit a higher level of antimicrobial tolerance than monospecies [130]. Thus, it is critical to develop a novel strategy that can target multiple gram positive and gram negative bacterial species simultaneously. In addition, the therapeutic efficacy of nanoparticles should be carefully evaluated using clinically relevant polymicrobial biofilm models in vivo.
Second, despite extensive studies on the evaluation of various nanoparticles in anti-biofilm efficacy, limited studies have been performed on how these nanoparticles interact with biofilm structures. The ability of nanoparticles to effectively penetrate into the biofilm is critical for achieving successful biofilm eradication [128]. More detailed understanding on the important parameters that influence the penetration efficiency of nanoparticles into the biofilms will lead to an improved design of nanoparticles that increase anti-biofilm effects [131].
Third, there have been concerns over the potential health impacts of engineered nanoparticles, along with our limited knowledge of how nanoparticles interact with host cells and the subsequent biological pathways impacted [132]. Although nanoparticle-based approaches offer new opportunities for antimicrobial treatments, the treatment by engineered nanoparticles must not inhibit the tightly regulated functions of phagocytes (neutrophils, macrophages, etc.) that comprise a critical element of innate immune protection against invading pathogens. In addition, nanoparticles can be cleared from tissue in large by mononuclear phagocytic system, however, local host immune status in the site of infection alters the clearance process of nanoparticles [133]. It is necessary to have a complete understanding of the ultimate fate of nanoparticles aggregates that remain in the wound as well as an understanding of the physical interactions between the nanoparticles and host immune cells that they encounter.
In conclusion, although many challenges remain to be addressed for the successful translation into clinics, the extensive ongoing efforts in development and evaluation of nanoparticle-based therapeutics may lead to a needed tool for the treatment of patients who cannot be successfully treated with standard-of-care antibiotic treatment and establish a novel platform for smart treatments of antibiotic-resistant chronic wound infections.
Acknowledgments
This study was supported by the National Institute of Health (NIH 1R01 NR015674-01) and Farris Family Innovation Award.
References
- 1.Fux CA, Costerton JW, Stewart PS, Stoodley P. Survival strategies of infectious biofilms. Trends Microbiol. 2005 Jan;13:34–40. doi: 10.1016/j.tim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Klausen M, Aaes-Jorgensen A, Molin S, Tolker-Nielsen T. Involvement of bacterial migration in the development of complex multicellular structures in Pseudomonas aeruginosa biofilms. Mol Microbiol. 2003 Oct;50:61–8. doi: 10.1046/j.1365-2958.2003.03677.x. [DOI] [PubMed] [Google Scholar]
- 3.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002 Apr;15:167–93. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costerton JW, Stewart PS, Greenberg PE. Bacterial biofilms: a common cause of persistent infections. Science. 1999 May 21;284:1318–22. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 5.Celli J, Finlay BB. Bacterial avoidance of phagocytosis. Trends Microbiol. 2002 May;10:232–7. doi: 10.1016/s0966-842x(02)02343-0. [DOI] [PubMed] [Google Scholar]
- 6.Foster TJ. Immune evasion by staphylococci. Nat Rev Microbiol. 2005 Dec;3:948–58. doi: 10.1038/nrmicro1289. [DOI] [PubMed] [Google Scholar]
- 7.Rooijakkers SH, van Kessel P, van Strijp JA. Staphylococcal innate immune evasion. Trends Microbiol. 2005 Dec;13:596–601. doi: 10.1016/j.tim.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Parsek MR, Singh PK. Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol. 2003;57:677–701. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- 9.Dowd SE, Wolcott RD, Sun Y, McKeehan T, Smith E, Rhoads D. Polymicrobial nature of chronic diabetic foot ulcer biofilm infections determined using bacterial tag encoded FLX amplicon pyrosequencing (bTEFAP) PLoS One. 2008;3:e3326. doi: 10.1371/journal.pone.0003326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjarnsholt T, Kirketerp-Moller K, Jensen PO, Madsen KG, Phipps R, Krogfelt K, et al. Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regen. 2008 Jan-Feb;16:2–10. doi: 10.1111/j.1524-475X.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 11.Davies D. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov. 2003 Feb;2:114–22. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- 12.James GA, Swogger E, Wolcott R, Pulcini E, Secor P, Sestrich J, et al. Biofilms in chronic wounds. Wound Repair Regen. 2008 Jan-Feb;16:37–44. doi: 10.1111/j.1524-475X.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 13.Brackman G, Cos P, Maes L, Nelis HJ, Coenye T. Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob Agents Chemother. 2011 Jun;55:2655–61. doi: 10.1128/AAC.00045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batoni G, Maisetta G, Brancatisano FL, Esin S, Campa M. Use of antimicrobial peptides against microbial biofilms: advantages and limits. Curr Med Chem. 2011;18:256–79. doi: 10.2174/092986711794088399. [DOI] [PubMed] [Google Scholar]
- 15.Donelli G, Francolini I, Romoli D, Guaglianone E, Piozzi A, Ragunath C, et al. Synergistic activity of dispersin B and cefamandole nafate in inhibition of staphylococcal biofilm growth on polyurethanes. Antimicrob Agents Chemother. 2007 Aug;51:2733–40. doi: 10.1128/AAC.01249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Poto A, Sbarra MS, Provenza G, Visai L, Speziale P. The effect of photodynamic treatment combined with antibiotic action or host defence mechanisms on Staphylococcus aureus biofilms. Biomaterials. 2009 Jun;30:3158–66. doi: 10.1016/j.biomaterials.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 17.Zolfaghari PS, Packer S, Singer M, Nair SP, Bennett J, Street C, et al. In vivo killing of Staphylococcus aureus using a light-activated antimicrobial agent. BMC Microbiol. 2009;9:27. doi: 10.1186/1471-2180-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caubet R, Pedarros-Caubet F, Chu M, Freye E, de Belem Rodrigues M, Moreau JM, et al. A radio frequency electric current enhances antibiotic efficacy against bacterial biofilms. Antimicrob Agents Chemother. 2004 Dec;48:4662–4. doi: 10.1128/AAC.48.12.4662-4664.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Borden AJ, Maathuis PG, Engels E, Rakhorst G, van der Mei HC, Busscher HJ, et al. Prevention of pin tract infection in external stainless steel fixator frames using electric current in a goat model. Biomaterials. 2007 Apr;28:2122–6. doi: 10.1016/j.biomaterials.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Ensing GT, Roeder BL, Nelson JL, van Horn JR, van der Mei HC, Busscher HJ, et al. Effect of pulsed ultrasound in combination with gentamicin on bacterial viability in biofilms on bone cements in vivo. J Appl Microbiol. 2005;99:443–8. doi: 10.1111/j.1365-2672.2005.02643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pitt WG, McBride MO, Lunceford JK, Roper RJ, Sagers RD. Ultrasonic enhancement of antibiotic action on gram-negative bacteria. Antimicrob Agents Chemother. 1994 Nov;38:2577–82. doi: 10.1128/aac.38.11.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spinowitz BS, Schwenk MH, Jacobs PM, Bolton WK, Kaplan MR, Charytan C, et al. The safety and efficacy of ferumoxytol therapy in anemic chronic kidney disease patients. Kidney Int. 2005 Oct;68:1801–7. doi: 10.1111/j.1523-1755.2005.00598.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008 May;83:761–9. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 24.Dizaj SM, Lotfipour F, Barzegar-Jalali M, Zarrintan MH, Adibkia K. Antimicrobial activity of the metals and metal oxide nanoparticles. Materials Science & Engineering C-Materials for Biological Applications. 2014 Nov 1;44:278–284. doi: 10.1016/j.msec.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 25.Forier K, Raemdonck K, De Smedt SC, Demeester J, Coenye T, Braeckmans K. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J Control Release. 2014 Sep 28;190:607–23. doi: 10.1016/j.jconrel.2014.03.055. [DOI] [PubMed] [Google Scholar]
- 26.Pattani VP, Tunnell JW. Nanoparticle-mediated photothermal therapy: A comparative study of heating for different particle types. Lasers in Surgery and Medicine. 2012 Oct;44:675–684. doi: 10.1002/lsm.22072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vallejo-Fernandez G, Whear O, Roca AG, Hussain S, Timmis J, Patel V, et al. Mechanisms of hyperthermia in magnetic nanoparticles. Journal of Physics D-Applied Physics. 2013 Aug 7;46 [Google Scholar]
- 28.Ikuma K, Decho AW, Lau BL. When nanoparticles meet biofilms-interactions guiding the environmental fate and accumulation of nanoparticles. Front Microbiol. 2015;6:591. doi: 10.3389/fmicb.2015.00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dryden MS. Complicated skin and soft tissue infection. J Antimicrob Chemother. 2010 Nov;65(Suppl 3):iii35–44. doi: 10.1093/jac/dkq302. [DOI] [PubMed] [Google Scholar]
- 30.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999 Sep 2;341:738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 31.Percival SL, McCarty SM, Lipsky B. Biofilms and Wounds: An Overview of the Evidence. Adv Wound Care (New Rochelle) 2015 Jul 1;4:373–381. doi: 10.1089/wound.2014.0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fonder MA, Lazarus GS, Cowan DA, Aronson-Cook B, Kohli AR, Mamelak AJ. Treating the chronic wound: A practical approach to the care of nonhealing wounds and wound care dressings. J Am Acad Dermatol. 2008 Feb;58:185–206. doi: 10.1016/j.jaad.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 33.Consensus Development Conference on Diabetic Foot Wound Care: 7–8 April 1999, Boston, Massachusetts. American Diabetes Association. Diabetes Care. 1999 Aug;22:1354–60. doi: 10.2337/diacare.22.8.1354. [DOI] [PubMed] [Google Scholar]
- 34.Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care. 1990 May;13:513–21. doi: 10.2337/diacare.13.5.513. [DOI] [PubMed] [Google Scholar]
- 35.Reiber GE. The epidemiology of diabetic foot problems. Diabet Med. 1996;13(Suppl 1):S6–11. [PubMed] [Google Scholar]
- 36.Mah TF, O'Toole GA. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001 Jan;9:34–9. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- 37.Singh R, Ray P, Das A, Sharma M. Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J Antimicrob Chemother. 2010 Sep;65:1955–8. doi: 10.1093/jac/dkq257. [DOI] [PubMed] [Google Scholar]
- 38.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001 Jul 14;358:135–8. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 39.Miller LS, O'Connell RM, Gutierrez MA, Pietras EM, Shahangian A, Gross CE, et al. MyD88 mediates neutrophil recruitment initiated by IL–1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity. 2006 Jan;24:79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 40.Molne L, Verdrengh M, Tarkowski A. Role of neutrophil leukocytes in cutaneous infection caused by Staphylococcus aureus. Infect Immun. 2000 Nov;68:6162–7. doi: 10.1128/iai.68.11.6162-6167.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bjarnsholt T, Jensen PO, Burmolle M, Hentzer M, Haagensen JA, Hougen HP, et al. Pseudomonas aeruginosa tolerance to tobramycin, hydrogen peroxide and polymorphonuclear leukocytes is quorum-sensing dependent. Microbiology. 2005 Feb;151:373–83. doi: 10.1099/mic.0.27463-0. [DOI] [PubMed] [Google Scholar]
- 42.Jensen PO, Bjarnsholt T, Phipps R, Rasmussen TB, Calum H, Christoffersen L, et al. Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum-sensing-controlled production of rhamnolipid by Pseudomonas aeruginosa. Microbiology. 2007 May;153:1329–38. doi: 10.1099/mic.0.2006/003863-0. [DOI] [PubMed] [Google Scholar]
- 43.Pedersen SS, Kharazmi A, Espersen F, Hoiby N. Pseudomonas aeruginosa alginate in cystic fibrosis sputum and the inflammatory response. Infect Immun. 1990 Oct;58:3363–8. doi: 10.1128/iai.58.10.3363-3368.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thurlow LR, Hanke ML, Fritz T, Angle A, Aldrich A, Williams SH, et al. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J Immunol. 2011 Jun 1;186:6585–96. doi: 10.4049/jimmunol.1002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang L, Pornpattananangku D, Hu CM, Huang CM. Development of nanoparticles for antimicrobial drug delivery. Curr Med Chem. 2010;17:585–94. doi: 10.2174/092986710790416290. [DOI] [PubMed] [Google Scholar]
- 46.Gao W, Thamphiwatana S, Angsantikul P, Zhang L. Nanoparticle approaches against bacterial infections. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014 Nov-Dec;6:532–47. doi: 10.1002/wnan.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chaloupka K, Malam Y, Seifalian AM. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010 Nov;28:580–8. doi: 10.1016/j.tibtech.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 48.Pankhurst QACJ, Jones SK, Dobson J. Applications of magnetic nanoparticles in biomedicine. J Phys D: Appl Phys. 2003;36:R167–R181. [Google Scholar]
- 49.Thiesen B, Jordan A. Clinical applications of magnetic nanoparticles for hyperthermia. Int J Hyperthermia. 2008 Sep;24:467–74. doi: 10.1080/02656730802104757. [DOI] [PubMed] [Google Scholar]
- 50.Taylor E, Webster TJ. Reducing infections through nanotechnology and nanoparticles. Int J Nanomedicine. 2011;6:1463–73. doi: 10.2147/IJN.S22021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanchez DA, Nosanchuk J, Friedman A. The purview of nitric oxide nanoparticle therapy in infection and wound healing. Nanomedicine (Lond) 2012 Jul;7:933–6. doi: 10.2217/nnm.12.67. [DOI] [PubMed] [Google Scholar]
- 52.Barnhill AE, Brewer MT, Carlson SA. Adverse effects of antimicrobials via predictable or idiosyncratic inhibition of host mitochondrial components. Antimicrob Agents Chemother. 2012 Aug;56:4046–51. doi: 10.1128/AAC.00678-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yap MN. The double life of antibiotics. Mo Med. 2013 Jul-Aug;110:320–4. [PMC free article] [PubMed] [Google Scholar]
- 54.Lemire JA, Harrison JJ, Turner RJ. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Microbiol. 2013 Jun;11:371–84. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- 55.Lansdown AB. Silver in health care: antimicrobial effects and safety in use. Curr Probl Dermatol. 2006;33:17–34. doi: 10.1159/000093928. [DOI] [PubMed] [Google Scholar]
- 56.Jung WK, Koo HC, Kim KW, Shin S, Kim SH, Park YH. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl Environ Microbiol. 2008 Apr;74:2171–8. doi: 10.1128/AEM.02001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lansdown AB. Silver. I: Its antibacterial properties and mechanism of action. J Wound Care. 2002 Apr;11:125–30. doi: 10.12968/jowc.2002.11.4.26389. [DOI] [PubMed] [Google Scholar]
- 58.Chaw KC, Manimaran M, Tay FE. Role of silver ions in destabilization of intermolecular adhesion forces measured by atomic force microscopy in Staphylococcus epidermidis biofilms. Antimicrob Agents Chemother. 2005 Dec;49:4853–9. doi: 10.1128/AAC.49.12.4853-4859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klueh U, Wagner V, Kelly S, Johnson A, Bryers JD. Efficacy of silver-coated fabric to prevent bacterial colonization and subsequent device-based biofilm formation. J Biomed Mater Res. 2000;53:621–31. doi: 10.1002/1097-4636(2000)53:6<621::aid-jbm2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 60.Xiu ZM, Ma J, Alvarez PJ. Differential effect of common ligands and molecular oxygen on antimicrobial activity of silver nanoparticles versus silver ions. Environ Sci Technol. 2011 Oct 15;45:9003–8. doi: 10.1021/es201918f. [DOI] [PubMed] [Google Scholar]
- 61.Brayner R, Ferrari-Iliou R, Brivois N, Djediat S, Benedetti MF, Fievet F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006 Apr;6:866–70. doi: 10.1021/nl052326h. [DOI] [PubMed] [Google Scholar]
- 62.Huang Z, Jiang X, Guo D, Gu N. Controllable synthesis and biomedical applications of silver nanomaterials. J Nanosci Nanotechnol. 2011 Nov;11:9395–408. doi: 10.1166/jnn.2011.5317. [DOI] [PubMed] [Google Scholar]
- 63.Kalishwaralal K, BarathManiKanth S, Pandian SR, Deepak V, Gurunathan S. Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids Surf B Biointerfaces. 2010 Sep 1;79:340–4. doi: 10.1016/j.colsurfb.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 64.Ansari MA, Khan HM, Khan AA, Cameotra SS, Alzohairy MA. Anti-biofilm efficacy of silver nanoparticles against MRSA and MRSE isolated from wounds in a tertiary care hospital. Indian J Med Microbiol. 2015 Jan-Mar;33:101–9. doi: 10.4103/0255-0857.148402. [DOI] [PubMed] [Google Scholar]
- 65.Kostenko V, Lyczak J, Turner K, Martinuzzi RJ. Impact of silver-containing wound dressings on bacterial biofilm viability and susceptibility to antibiotics during prolonged treatment. Antimicrob Agents Chemother. 2010 Dec;54:5120–31. doi: 10.1128/AAC.00825-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Loh JV, Percival SL, Woods EJ, Williams NJ, Cochrane CA. Silver resistance in MRSA isolated from wound and nasal sources in humans and animals. Int Wound J. 2009 Feb;6:32–8. doi: 10.1111/j.1742-481X.2008.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Toy LW, Macera L. Evidence-based review of silver dressing use on chronic wounds. J Am Acad Nurse Pract. 2011 Apr;23:183–92. doi: 10.1111/j.1745-7599.2011.00600.x. [DOI] [PubMed] [Google Scholar]
- 68.Gliga AR, Skoglund S, Wallinder IO, Fadeel B, Karlsson HL. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: the role of cellular uptake, agglomeration and Ag release. Part Fibre Toxicol. 2014;11:11. doi: 10.1186/1743-8977-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krychowiak M, Grinholc M, Banasiuk R, Krauze-Baranowska M, Glod D, Kawiak A, et al. Combination of silver nanoparticles and Drosera binata extract as a possible alternative for antibiotic treatment of burn wound infections caused by resistant Staphylococcus aureus. PLoS One. 2014;9:e115727. doi: 10.1371/journal.pone.0115727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hwang IS, Hwang JH, Choi H, Kim KJ, Lee DG. Synergistic effects between silver nanoparticles and antibiotics and the mechanisms involved. J Med Microbiol. 2012 Dec;61:1719–26. doi: 10.1099/jmm.0.047100-0. [DOI] [PubMed] [Google Scholar]
- 71.Kumar S, Lakshmanan V, Raj M, Biswas R, Hiroshi T, Nair SV, et al. Evaluation of wound healing potential of beta-chitin hydrogel/nano zinc oxide composite bandage. Pharm Res. 2013 Feb;30:523–37. doi: 10.1007/s11095-012-0898-y. [DOI] [PubMed] [Google Scholar]
- 72.Jones N, Ray B, Ranjit KT, Manna AC. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol Lett. 2008 Feb;279:71–6. doi: 10.1111/j.1574-6968.2007.01012.x. [DOI] [PubMed] [Google Scholar]
- 73.Azam A, Ahmed AS, Oves M, Khan MS, Habib SS, Memic A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study. Int J Nanomedicine. 2012;7:6003–9. doi: 10.2147/IJN.S35347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee JH, Kim YG, Cho MH, Lee J. ZnO nanoparticles inhibit Pseudomonas aeruginosa biofilm formation and virulence factor production. Microbiol Res. 2014 Dec;169:888–96. doi: 10.1016/j.micres.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 75.Dwivedi S, Wahab R, Khan F, Mishra YK, Musarrat J, Al-Khedhairy AA. Reactive oxygen species mediated bacterial biofilm inhibition via zinc oxide nanoparticles and their statistical determination. PLoS One. 2014;9:e111289. doi: 10.1371/journal.pone.0111289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seil JT, Webster TJ. Reduced Staphylococcus aureus proliferation and biofilm formation on zinc oxide nanoparticle PVC composite surfaces. Acta Biomater. 2011 Jun;7:2579–84. doi: 10.1016/j.actbio.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 77.Lipovsky A, Tzitrinovich Z, Friedmann H, Applerot G, Gedanken A, Lubart R. EPR Study of Visible Light-Induced ROS Generation by Nanoparticles of ZnO. Journal of Physical Chemistry C. 2009 Sep 10;113:15997–16001. [Google Scholar]
- 78.Xie Y, He Y, Irwin PL, Jin T, Shi X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl Environ Microbiol. 2011 Apr;77:2325–31. doi: 10.1128/AEM.02149-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heng BC, Zhao X, Xiong S, Ng KW, Boey FY, Loo JS. Toxicity of zinc oxide (ZnO) nanoparticles on human bronchial epithelial cells (BEAS-2B) is accentuated by oxidative stress. Food Chem Toxicol. 2010 Jun;48:1762–6. doi: 10.1016/j.fct.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 80.Al-Omari A, Cameron DW, Lee C, Corrales-Medina VF. Oral antibiotic therapy for the treatment of infective endocarditis: a systematic review. BMC Infect Dis. 2014;14:140. doi: 10.1186/1471-2334-14-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bordi C, de Bentzmann S. Hacking into bacterial biofilms: a new therapeutic challenge. Ann Intensive Care. 2011;1:19. doi: 10.1186/2110-5820-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meers P, Neville M, Malinin V, Scotto AW, Sardaryan G, Kurumunda R, et al. Biofilm penetration, triggered release and in vivo activity of inhaled liposomal amikacin in chronic Pseudomonas aeruginosa lung infections. J Antimicrob Chemother. 2008 Apr;61:859–68. doi: 10.1093/jac/dkn059. [DOI] [PubMed] [Google Scholar]
- 83.Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013 Jan;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 84.Malam Y, Loizidou M, Seifalian AM. Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol Sci. 2009 Nov;30:592–9. doi: 10.1016/j.tips.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 85.Nacucchio MC, Bellora MJ, Sordelli DO, D'Aquino M. Enhanced liposome-mediated activity of piperacillin against staphylococci. Antimicrob Agents Chemother. 1985 Jan;27:137–9. doi: 10.1128/aac.27.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mugabe C, Azghani AO, Omri A. Liposome-mediated gentamicin delivery: development and activity against resistant strains of Pseudomonas aeruginosa isolated from cystic fibrosis patients. J Antimicrob Chemother. 2005 Feb;55:269–71. doi: 10.1093/jac/dkh518. [DOI] [PubMed] [Google Scholar]
- 87.Li C, Zhang X, Huang X, Wang X, Liao G, Chen Z. Preparation and characterization of flexible nanoliposomes loaded with daptomycin, a novel antibiotic, for topical skin therapy. Int J Nanomedicine. 2013;8:1285–92. doi: 10.2147/IJN.S41695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bala I, Hariharan S, Kumar MN. PLGA nanoparticles in drug delivery: the state of the art. Crit Rev Ther Drug Carrier Syst. 2004;21:387–422. doi: 10.1615/critrevtherdrugcarriersyst.v21.i5.20. [DOI] [PubMed] [Google Scholar]
- 89.Esmaeili F, Hosseini-Nasr M, Rad-Malekshahi M, Samadi N, Atyabi F, Dinarvand R. Preparation and antibacterial activity evaluation of rifampicin-loaded poly lactide-co-glycolide nanoparticles. Nanomedicine. 2007 Jun;3:161–7. doi: 10.1016/j.nano.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 90.Cheow WS, Chang MW, Hadinoto K. Antibacterial efficacy of inhalable levofloxacin-loaded polymeric nanoparticles against E. coli biofilm cells: the effect of antibiotic release profile. Pharm Res. 2010 Aug;27:1597–609. doi: 10.1007/s11095-010-0142-6. [DOI] [PubMed] [Google Scholar]
- 91.Hadinoto K, Sundaresan A, Cheow WS. Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review. Eur J Pharm Biopharm. 2013 Nov;85:427–43. doi: 10.1016/j.ejpb.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 92.Cheow WS, Hadinoto K. Factors affecting drug encapsulation and stability of lipid-polymer hybrid nanoparticles. Colloids Surf B Biointerfaces. 2011 Jul 1;85:214–20. doi: 10.1016/j.colsurfb.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 93.Krishnamurthy S, Vaiyapuri R, Zhang L, Chan JM. Lipid–coated polymeric nanoparticles for cancer drug delivery. Biomater Sci. 2015 Jul;3:923–36. doi: 10.1039/c4bm00427b. [DOI] [PubMed] [Google Scholar]
- 94.Gao P, Nie X, Zou M, Shi Y, Cheng G. Recent advances in materials for extended-release antibiotic delivery system. J Antibiot (Tokyo) 2011 Sep;64:625–34. doi: 10.1038/ja.2011.58. [DOI] [PubMed] [Google Scholar]
- 95.Englander L, Friedman A. Nitric oxide nanoparticle technology: a novel antimicrobial agent in the context of current treatment of skin and soft tissue infection. J Clin Aesthet Dermatol. 2010 Jun;3:45–50. [PMC free article] [PubMed] [Google Scholar]
- 96.Barraud N, Hassett DJ, Hwang SH, Rice SA, Kjelleberg S, Webb JS. Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa. J Bacteriol. 2006 Nov;188:7344–53. doi: 10.1128/JB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hetrick EM, Shin JH, Stasko NA, Johnson CB, Wespe DA, Holmuhamedov E, et al. Bactericidal efficacy of nitric oxide-releasing silica nanoparticles. ACS Nano. 2008 Feb;2:235–46. doi: 10.1021/nn700191f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hetrick EM, Shin JH, Paul HS, Schoenfisch MH. Anti-biofilm efficacy of nitric oxide-releasing silica nanoparticles. Biomaterials. 2009 May;30:2782–9. doi: 10.1016/j.biomaterials.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martinez LR, Han G, Chacko M, Mihu MR, Jacobson M, Gialanella P, et al. Antimicrobial and healing efficacy of sustained release nitric oxide nanoparticles against Staphylococcus aureus skin infection. J Invest Dermatol. 2009 Oct;129:2463–9. doi: 10.1038/jid.2009.95. [DOI] [PubMed] [Google Scholar]
- 100.Mihu MR, Sandkovsky U, Han G, Friedman JM, Nosanchuk JD, Martinez LR. The use of nitric oxide releasing nanoparticles as a treatment against Acinetobacter baumannii in wound infections. Virulence. 2010 Mar-Apr;1:62–7. doi: 10.4161/viru.1.2.10038. [DOI] [PubMed] [Google Scholar]
- 101.Macherla C, Sanchez DA, Ahmadi MS, Vellozzi EM, Friedman AJ, Nosanchuk JD, et al. Nitric oxide releasing nanoparticles for treatment of Candida albicans burn infections. Front Microbiol. 2012;3:193. doi: 10.3389/fmicb.2012.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Han G, Nguyen LN, Macherla C, Chi Y, Friedman JM, Nosanchuk JD, et al. Nitric oxide-releasing nanoparticles accelerate wound healing by promoting fibroblast migration and collagen deposition. Am J Pathol. 2012 Apr;180:1465–73. doi: 10.1016/j.ajpath.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 103.Schaffer MR, Tantry U, Thornton FJ, Barbul A. Inhibition of nitric oxide synthesis in wounds: pharmacology and effect on accumulation of collagen in wounds in mice. Eur J Surg. 1999 Mar;165:262–7. doi: 10.1080/110241599750007153. [DOI] [PubMed] [Google Scholar]
- 104.Schaffer MR, Tantry U, Gross SS, Wasserburg HL, Barbul A. Nitric oxide regulates wound healing. J Surg Res. 1996 Jun;63:237–40. doi: 10.1006/jsre.1996.0254. [DOI] [PubMed] [Google Scholar]
- 105.Dai T, Huang YY, Hamblin MR. Photodynamic therapy for localized infections--state of the art. Photodiagnosis Photodyn Ther. 2009 Sep-Dec;6:170–88. doi: 10.1016/j.pdpdt.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Master A, Livingston M, Sen Gupta A. Photodynamic nanomedicine in the treatment of solid tumors: perspectives and challenges. J Control Release. 2013 May 28;168:88–102. doi: 10.1016/j.jconrel.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fontana CR, Abernethy AD, Som S, Ruggiero K, Doucette S, Marcantonio RC, et al. The antibacterial effect of photodynamic therapy in dental plaque-derived biofilms. J Periodontal Res. 2009 Dec;44:751–9. doi: 10.1111/j.1600-0765.2008.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lucky SS, Soo KC, Zhang Y. Nanoparticles in photodynamic therapy. Chem Rev. 2015 Feb 25;115:1990–2042. doi: 10.1021/cr5004198. [DOI] [PubMed] [Google Scholar]
- 109.Ferro S, Ricchelli F, Mancini G, Tognon G, Jori G. Inactivation of methicillin-resistant Staphylococcus aureus (MRSA) by liposome-delivered photosensitising agents. J Photochem Photobiol B. 2006 May 1;83:98–104. doi: 10.1016/j.jphotobiol.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 110.Khan S, Alam F, Azam A, Khan AU. Gold nanoparticles enhance methylene blue-induced photodynamic therapy: a novel therapeutic approach to inhibit Candida albicans biofilm. Int J Nanomedicine. 2012;7:3245–57. doi: 10.2147/IJN.S31219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shrestha A, Kishen A. Antibiofilm efficacy of photosensitizer-functionalized bioactive nanoparticles on multispecies biofilm. J Endod. 2014 Oct;40:1604–10. doi: 10.1016/j.joen.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 112.Zharov VP, Mercer KE, Galitovskaya EN, Smeltzer MS. Photothermal nanotherapeutics and nanodiagnostics for selective killing of bacteria targeted with gold nanoparticles. Biophysical Journal. 2006 Jan;90:619–627. doi: 10.1529/biophysj.105.061895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yu TJ, Li PH, Tseng TW, Chen YC. Multifunctional Fe(3)O(4)/alumina core/shell MNPs as photothermal agents for targeted hyperthermia of nosocomial and antibiotic-resistant bacteria. Nanomedicine (Lond) 2011 Oct;6:1353–63. doi: 10.2217/nnm.11.34. [DOI] [PubMed] [Google Scholar]
- 114.Wu MC, Deokar AR, Liao JH, Shih PY, Ling YC. Graphene-based photothermal agent for rapid and effective killing of bacteria. ACS Nano. 2013 Feb 26;7:1281–90. doi: 10.1021/nn304782d. [DOI] [PubMed] [Google Scholar]
- 115.Chiang WL, Lin TT, Sureshbabu R, Chia WT, Hsiao HC, Liu HY, et al. A rapid drug release system with a NIR light-activated molecular switch for dual-modality photothermal/antibiotic treatments of subcutaneous abscesses. J Control Release. 2015 Feb 10;199:53–62. doi: 10.1016/j.jconrel.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 116.Dudeck O, Bogusiewicz K, Pinkernelle J, Gaffke G, Pech M, Wieners G, et al. Local arterial infusion of superparamagnetic iron oxide particles in hepatocellular carcinoma: A feasibility and 3.0 T MRI study. Invest Radiol. 2006 Jun;41:527–35. doi: 10.1097/01.rli.0000209601.15533.5a. [DOI] [PubMed] [Google Scholar]
- 117.Jordan A, Scholz R, Maier-Hauff K, van Landeghem FK, Waldoefner N, Teichgraeber U, et al. The effect of thermotherapy using magnetic nanoparticles on rat malignant glioma. J Neurooncol. 2006 May;78:7–14. doi: 10.1007/s11060-005-9059-z. [DOI] [PubMed] [Google Scholar]
- 118.Maier–Hauff K, Rothe R, Scholz R, Gneveckow U, Wust P, Thiesen B, et al. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: results of a feasibility study on patients with glioblastoma multiforme. J Neurooncol. 2007 Jan;81:53–60. doi: 10.1007/s11060-006-9195-0. [DOI] [PubMed] [Google Scholar]
- 119.Thomas L, Dekker L, Kallumadil M, Southern P, Wilson M, Nair S, et al. Carboxylic acid-stabilised iron oxide nanoparticles for use in magnetic hyperthermia. J Mater Chem. 2009;19:6529–6535. [Google Scholar]
- 120.Park H, Park HJ, Kim JA, Lee SH, Kim JH, Yoon J, et al. Inactivation of Pseudomonas aeruginosa PA01 biofilms by hyperthermia using superparamagnetic nanoparticles. J Microbiol Methods. 2011 Jan;84:41–5. doi: 10.1016/j.mimet.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 121.Kim MH, Yamayoshi I, Mathew S, Lin H, Nayfach J, Simon SI. Magnetic nanoparticle targeted hyperthermia of cutaneous Staphylococcus aureus infection. Ann Biomed Eng. 2013 Mar;41:598–609. doi: 10.1007/s10439-012-0698-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dana B, Gannot I. An analytic analysis of the diffusive-heat-flow equation for different magnetic field profiles for a single magnetic nanoparticle. Journal of Atomic, Molecular, and Optical Physics. 2012 doi: 10.1155/2012/135708. [DOI] [Google Scholar]
- 123.Keblinski P, Cahill DG, Bodapati A, Sullivan CR, Taton TA. Limits of localized heating by electromagnetically excited nanoparticles. Journal of Applied Physics. 2006;100:054305. [Google Scholar]
- 124.Cunha MV, Sousa SA, Leitao JH, Moreira LM, Videira PA, Sa-Correia I. Studies on the involvement of the exopolysaccharide produced by cystic fibrosis-associated isolates of the Burkholderia cepacia complex in biofilm formation and in persistence of respiratory infections. J Clin Microbiol. 2004 Jul;42:3052–8. doi: 10.1128/JCM.42.7.3052-3058.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sutherland I. Biofilm exopolysaccharides: a strong and sticky framework. Microbiology. 2001 Jan;147:3–9. doi: 10.1099/00221287-147-1-3. [DOI] [PubMed] [Google Scholar]
- 126.Messiaen AS, Forier K, Nelis H, Braeckmans K, Coenye T. Transport of nanoparticles and tobramycin-loaded liposomes in Burkholderia cepacia complex biofilms. PLoS One. 2013;8:e79220. doi: 10.1371/journal.pone.0079220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Baelo A, Levato R, Julian E, Crespo A, Astola J, Gavalda J, et al. Disassembling bacterial extracellular matrix with DNase-coated nanoparticles to enhance antibiotic delivery in biofilm infections. J Control Release. 2015 Jul 10;209:150–8. doi: 10.1016/j.jconrel.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 128.Slomberg DL, Lu Y, Broadnax AD, Hunter RA, Carpenter AW, Schoenfisch MH. Role of size and shape on biofilm eradication for nitric oxide–releasing silica nanoparticles. ACS Appl Mater Interfaces. 2013 Oct 9;5:9322–9. doi: 10.1021/am402618w. [DOI] [PubMed] [Google Scholar]
- 129.Gawande PV, Clinton AP, LoVetri K, Yakandawala N, Rumbaugh KP, Madhyastha S. Antibiofilm Efficacy of DispersinB((R)) Wound Spray Used in Combination with a Silver Wound Dressing. Microbiol Insights. 2014;7:9–13. doi: 10.4137/MBI.S13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dalton T, Dowd SE, Wolcott RD, Sun Y, Watters C, Griswold JA, et al. An in vivo polymicrobial biofilm wound infection model to study interspecies interactions. PLoS One. 2011;6:e27317. doi: 10.1371/journal.pone.0027317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li X, Yeh YC, Giri K, Mout R, Landis RF, Prakash YS, et al. Control of nanoparticle penetration into biofilms through surface design. Chem Commun (Camb) 2015;51:282–5. doi: 10.1039/c4cc07737g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sahay G, Alakhova DY, Kabanov AV. Endocytosis of nanomedicines. J Control Release. 2010 Aug 3;145:182–95. doi: 10.1016/j.jconrel.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jones SW, Roberts RA, Robbins GR, Perry JL, Kai MP, Chen K, et al. Nanoparticle clearance is governed by Th1/Th2 immunity and strain background. J Clin Invest. 2013 Jul 1;123:3061–73. doi: 10.1172/JCI66895. [DOI] [PMC free article] [PubMed] [Google Scholar]