Abstract
BACKGROUND
Eating a diet high in fat can lead to obesity, chronic metabolic disease, and increased inflammation in both the central and peripheral nervous systems. Dietary supplements that are high in omega-3 polyunsaturated fatty acids can reduce or prevent these negative health consequences in rats. Eating high fat chow also increases the sensitivity of rats to behavioral effects of drugs acting on dopamine systems (e.g., cocaine), and this effect is greatest in adolescent females.
METHODS
The present experiment tested the hypothesis that dietary supplementation with fish oil prevents high fat chow induced increases in sensitivity to cocaine in adolescent female rats. Female Sprague-Dawley rats (post-natal day 25–27) ate standard laboratory chow (5.7% fat), high fat chow (34.4% fat), or high fat chow supplemented with fish oil (20% w/w). Cocaine dose dependently (1–17.8 mg/kg) increased locomotion and induced sensitization across 6 weeks of once-weekly testing in all rats; however, these effects were greatest in rats eating high fat chow.
RESULTS
Dietary supplementation with fish oil prevented enhanced locomotion and sensitization in rats eating high fat chow. There were no differences in inflammatory markers in plasma or the hypothalamus among dietary conditions.
CONCLUSIONS
These results demonstrate that dietary supplementation with fish oil can prevent high fat diet-induced sensitization to cocaine, but they fail to support the view that these effects are due to changes in proinflammatory cytokines. These data add to a growing literature on the relationship between diet and drug abuse and extend the potential health benefits of fish oil to stimulant drug abuse prevention.
Keywords: dopamine, high-fat chow, cocaine, sensitization, rat, fish oil
1. INTRODUCTION
Feeding conditions (i.e., type and amount of food consumed) can impact sensitivity of rats to the behavioral effects of drugs acting on dopamine systems (Baladi et al., 2012a; Collins et al., 2008). For example, eating high fat chow increases yawning induced by direct-acting dopamine receptor agonists (i.e., quinpirole; Baladi et al., 2011b; Baladi and France, 2010). Similarly, eating high fat chow enhances the sensitivity of rats to cocaine-induced locomotion and sensitization (Baladi et al., 2012b, 2015). Females are more sensitive than males to cocaine (Anker and Carroll, 2011; Lynch and Carroll, 1999; Chin et al., 2001) and the impact of eating high fat chow on sensitivity to cocaine is greater in females than in males (Baladi et al., 2015, 2011; Serafine et al., 2014b) and is more dramatic in adolescent rats compared with adults (Baladi et al., 2012b, 2015).
The mechanism underlying this diet-induced enhancement in drug sensitivity is not known; however, eating high fat chow also causes several other negative health consequences, including obesity and insulin resistance (Baladi et al., 2011; Liu et al., 2013; Serafine et al., 2014a). Insulin signaling can impact dopamine systems (Daws et al., 2011); for example, dopamine transporter expression and function are decreased in obese rats and in rats that are insulin resistant (Narayanswami et al., 2013; Owens et al., 2012; South and Huang, 2008; Speed et al., 2011; Williams et al., 2007). However, enhanced sensitivity to drugs acting on dopamine systems occurs even in the absence of diet-induced obesity (see Baladi et al., 2012b, 2015) and insulin resistance (Serafine et al., 2014a). Thus, eating a high fat diet in the absence of obesity or insulin resistance is sufficient to alter sensitivity to drugs.
Eating high fat chow also increases inflammation, both in adipose tissue and in the hypothalamus (Thaler et al., 2012; Wang et al., 2012). For example, the proinflammatory cytokines tumor necrosis factor (TNF)-alpha and interleukin (IL)-6 are significantly increased in hypothalamic tissue from rats eating high fat chow (Maric et al., 2014; Wang et al., 2012). Diet-induced elevations in proinflammatory markers might underlie the diet-induced enhancement in drug sensitivity; however, this hypothesis has not been systematically examined.
Dietary supplements that are high in omega-3 polyunsaturated fatty acids (e.g., fish oil) can prevent high fat chow-induced obesity, insulin resistance, and hypothalamic inflammation (Cintra et al., 2012; Pimentel et al., 2013). To examine whether fish oil prevents high fat chow-induced enhanced sensitivity to the behavioral effects of cocaine, the present experiment investigated the locomotor stimulating effects of cocaine in female rats eating standard chow, high fat chow, or high fat chow supplemented with fish oil. To examine whether proinflammatory markers (e.g., TNF-alpha and IL-6) that are increased in male rats eating high fat chow are also elevated in female rats eating high fat chow, protein levels of 27 different chemokines and cytokines were analyzed using a Luminex-based assay from plasma and hypothalamic samples taken 24 hours after the last cocaine test.
2. MATERIALS AND METHODS
2.1 Subjects
Female Sprague–Dawley rats (n = 38; Harlan, Indianapolis, IN, USA; postnatal day [PND] 20 upon arrival) weighing 70–80 g at the beginning of the experiment (PND 25–27), were housed individually in cages measuring 21.5 × 24 × 20.5 cm high in an environmentally controlled room (24 ± 1°C, 50 ± 10% relative humidity) that was maintained under a 12:12-h light/dark cycle (light period 0700–1900 hours). Rats had free access to food and water in the home cage throughout the experiment (dietary conditions outlined in section 1.2.2.). Rats were weighed daily at 0800 hours. Rats were maintained and experiments were conducted in accordance with the Institutional Animal Care and Use Committee, the University of Texas Health Science Center at San Antonio, and with the 2011 Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, the National Research Council, and the National Academy of Sciences).
2.2. Feeding Conditions
Rats were habituated to the laboratory as well as to the experimental procedures (PND 21–24). All subjects had free access to standard chow upon arrival and during initial tests with cocaine and saline (baseline; PND 25–27) before being randomly assigned to different dietary conditions. Thereafter, and for the duration of the experiment, separate groups of rats had free access to standard laboratory chow (n = 12), free access to a high fat chow (n = 13), or free access to a high fat chow supplemented with 20% (w/w) fish oil (n = 13). Half of the subjects in each diet group were treated with cocaine (n = 20), and the other half were treated with saline (n = 18). All other handling and activity recording were the same among the three groups with tests occurring once per week. The standard chow (Harlan Teklad 7912) had a calculated gross energy content of 3.1 kcal/g, with 17% kcal from fat. The high fat chow (Harlan Teklad 06414) had a calculated energy content of 5.1 kcal/g, with 60% kcal from fat. The supplemented chow contained the same high fat chow, but with 20% (w/w) fish oil (Nordic Naturals Omega-3 Pet), resulting in a calculated energy content of 5.9 kcal/g with 68% kcal from fat. The fish oil was manufactured with d-alpha tocopherol (a preservative) to limit oxidation. The product manufacturer guarantees a minimum of 31% total omega-3 fatty acid content per serving size (1 teaspoon), containing 15% eicosapentaenoic acid and 9% docosahexaenoic acid (www.nordicnaturals.com). Fish oil, and high fat food prepared with fish oil, were refrigerated; daily any uneaten fish oil or high fat chow was discarded and replaced with fresh food.
2.3. Insulin Sensitivity
A sample of blood was collected from the tip of the tail following a small incision (using a sterile scalpel blade) and was expressed on a blood-glucose test strip. Glucose values were measured with a commercially available glucose meter (Accu-Chek Aviva; CVS). Based on previous work in this laboratory using female rats (Serafine et al., 2014a), glucose was measured prior to as well as 15, 30, 45, and 75 min after an i.p. injection of 2.0 U/kg insulin. Insulin sensitivity was measured once during the experiment, 4 weeks after assignment to dietary conditions, on a day when no cocaine or saline tests occurred.
2.4. Locomotor Activity
Experiments were conducted in Plexiglas® chambers, measuring 26×61×23 cm high (Instrumentation Services, The University of Texas Health Science Center, San Antonio, TX), equipped with metal floors and located within ventilated sound-attenuating cubicles (MED Associates Inc., St. Albans, VT, USA). Horizontal activity was measured using four pairs of infrared photo beams (Multi-Varimex, Columbus Instruments, Columbus, OH, USA) positioned 4 cm above the floor of the chamber. Photo beams were separated by 15 cm with two photo beams located 8 cm from the ends of the chamber. An interface and computer monitored the experiments and recorded data.
For three consecutive days beginning on PND 21, rats were placed in locomotor chambers for 30 min, after which an i.p. injection of saline (0.2 mL) was administered every 15 min for a total of 5 injections (105 min). Beginning the following week (on PND 25–27), rats were again placed in locomotor chambers for 30 min, after which injections of saline (0.2 mL; n = 18) or cumulative doses of cocaine (1.0, 3.2, 10, 17.8 mg/kg; i.p.; n = 20) preceded by an injection of saline (0.2 mL) were administered every 15 min for a total of 5 injections (105 min). These doses include the ascending limb of the cocaine dose-response curve for locomotor stimulation (Baladi et al., 2012b). Only the ascending limb was included because it was expected that the curve would shift leftward after repeated injections of cocaine. Cocaine tests occurred once per week on the same day and time for a total of 5 weeks (i.e., until PND 60–62). For all locomotor activity experiments, the data are presented for 5-min periods beginning 10 min after injections.
2.5. Brain and Plasma Inflammation
Plasma and brain tissue were collected from all subjects 24 hours following the last saline or cocaine test. Plasma was collected via extracting the trunk blood of the animal, funneling it into an EDTA-coated tube, and centrifuging the tube at room temperature at 10000 rmp for 10 minutes. The plasma supernatant was removed and stored in centrifuge tubes at −80°C until analysis. Brain tissue was homogenized in a buffer containing 0.32 M sucrose, 1 mM EDTA, and a protease inhibitor cocktail (cOmplete™ Mini 11836153001, Roche, Indianapolis, IN), and the supernatants were stored at −80°C until analysis. Concentrations of epidermal growth factor (EGF), eotaxin, fractalkine, granulocyte-colony stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), GRO/KC/CINC-1, interferon gamma gamma (IFNγ), IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12(p70), IL-13, IL-17A, IL-18, interferon gamma-induced protein 10 (IP-10), leptin, LIX, monocyte chemoattractant protein-1 (MCP-1), macrophage inhibitory protein 1-alpha (MIP-1α), macrophage inhibitory protein-2 (MIP-2), regulated on activation, normal T expressed and secreted (RANTES), TNFα, and vascular endothelial growth factor (VEGF), were simultaneously quantified using a single sample of plasma or hypothalamic tissue from each subject, using a Milliplex MAP rat cytokine/chemokine magnetic bead panel (EMD Millipore Corp., St. Charles, MO, USA). Protein concentrations were determined using antibodies for each analyte covalently immobilized to a set of microspheres according to the protocol developed and validated by Millipore. The analytes were then detected using a cocktail of biotinylated antibodies. Following streptavidin-phycoerythrin conjugate binding, the reporter florescent signal was measured with a Luminex FlexMap 3D™ platform (Luminex Corp., Austin, TX, USA) at the University of Texas Health Science Center at San Antonio Core for Advanced Translational Technologies. Data were collected using a calibration curve obtained in each plate (one for plasma and one for brain tissue homogenates) using the respective recombinant proteins diluted in the kit matrix for plasma samples, or lysis buffer for tissue samples. When the raw results from the Luminex FlexMap 3D™ platform for a specific protein fell below detectable levels, they were not included in the results.
2.6. Data Analyses
Results are expressed as the mean (± 1 SEM) locomotor activity counts in the last 5 min of each 15-min observation period for each group and plotted as a function of cocaine dose or saline injection, or as mean (± 1 SEM) area under the dose-response curve (AUC) and plotted as a function of week. Data are also expressed as the average (± 1 SEM) change in glucose concentration (mg/dL) from pre-insulin injection levels as a function of week, and as the average (± 1 SEM) amount of proinflammatory cytokine level (pg/mL) in each group 24 hours after the last injection of cocaine or saline. To examine differences among groups dose-response curves for locomotion were analyzed with a two-way repeated measures ANOVA (feeding condition by dose) using GraphPad Prism (GraphPad Software Inc., San Diego, CA, USA). AUC for each dose-response curve was also calculated and differences between groups over time were analyzed using a two-way repeated measures ANOVA (feeding condition by week; GraphPad Prism). Multiple comparisons were made with the Bonferroni test (GraphPad Prism) where appropriate. Differences in proinflammatory markers were analyzed using two-way ANOVAs (feeding condition by drug). A two-way ANOVA (group by time) was used to examine changes in blood glucose concentration before and after administration of insulin to quantify insulin sensitivity. A two-tailed paired-t test with Bonferroni multiple comparisons was used to examine maximal changes in blood glucose (45 min after insulin administration; Durham et al., 2006) between groups. For all tests, p<0.05.
2.7. Drugs
Cocaine hydrochloride (NIDA Research Technology Branch, Rockville, MD, USA) was dissolved in sterile 0.9% saline and administered i.p. in a volume of 1 ml/kg body weight. Insulin (protamine zinc recombinant human insulin; Boehringer Ingelheim Vetmedica, Inc., St. Joseph, MO) was dissolved in sterile 0.9% saline and injected i.p. in a volume of 1 ml/kg body weight.
3. RESULTS
3.1. Body Weight
At the beginning of the experiment when all rats were eating standard chow, the average (± 1 SEM) body weight for all 38 rats was 70.6 ± 0.9 g. One week later, rats were assigned to dietary conditions. Rats gained weight throughout the experiment such that on the last day they weighed, on average, 216 ± 3.2 g (Figure 1). Specifically, for the groups that were tested with cocaine, those eating standard chow weighed 213.0 ± 5.3 g, those eating high fat chow weighed 222.3 ± 5.9 g, and those eating high fat chow with fish oil weighed 215.3 ± 15.0 g. For the groups that were tested with saline, those eating standard chow weighed 213.2 ± 4.6 g, those eating high fat chow weighed 219.5 ± 4.9 g, and those eating high fat chow with fish oil weighed 220.5 ± 6.7 g. There were no significant differences in body weight among groups at any point in the experiment.
Figure 1.
Body weight of rats eating standard chow (circles), high fat chow (squares), or high fat chow with fish oil (triangles) and tested with saline (left panel) or cocaine (right panel). Ordinate: body weight in g. Abscissa: week in study.
3.2. Food Consumption
A two-way ANOVA examining food consumption as a function of day for rats that received only saline revealed a significant main effect of day (i.e., food intake increased during the experiment [F(34,595)=7.125, p<0.0001]), a significant main effect of diet [F(2,525)=969.2, p<0.0001], and a significant day by diet interaction [F(68,525)=1.634, p= 0.0018] (see Figure 2 top left panel). Tukey’s multiple comparisons revealed that saline-treated rats eating standard chow ate significantly more (g) than saline-treated rats eating either high fat chow (p<0.0001) or high fat chow with fish oil (p<0.0001).
Figure 2.
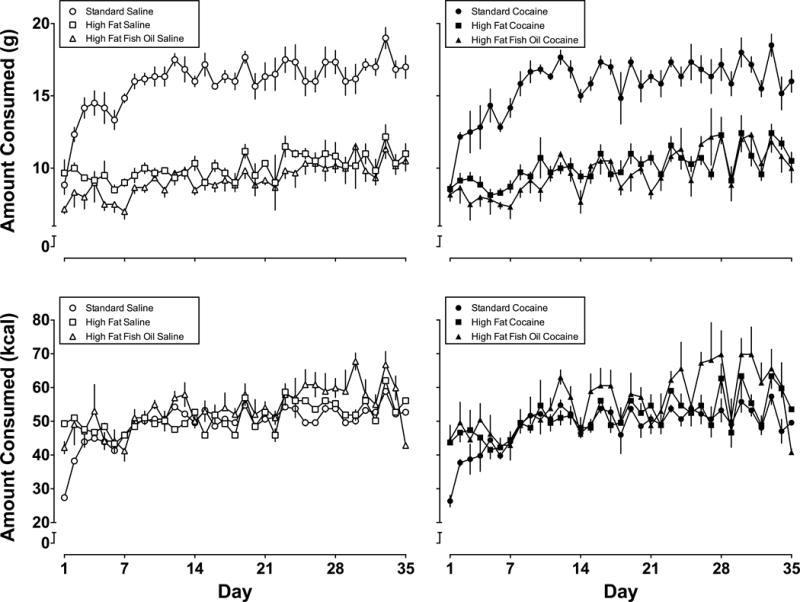
Mean (± 1 SEM) daily food intake in g (top panels) and in kcal (bottom panels) for rats tested with saline (left panels) or cocaine (right panels) and eating standard chow (circles), high fat chow (squares), or high fat chow with fish oil (triangles). Ordinates: amount consumed in g and kcal. Abscissa: week in study.
A two-way ANOVA examining food consumption as a function of day for rats that received cocaine revealed a significant main effect of day [F(34,595)=8.904, p<0.0001], a significant main effect of diet [F(2,595)=619.1], and a significant diet by day interaction [F(68,595)=1.491, p=0.0089]. Tukey’s multiple comparisons revealed that cocaine-treated rats eating standard chow ate significantly more (g) than cocaine-treated rats eating either high fat chow only (p < 0.0001) or high fat chow with fish oil (p < 0.0001; Figure 2, top right panel).
Two-way ANOVAs for total kcal/g consumed yielded main effects of day and diet for cocaine-treated rats (main effect of day [F(34,595)= 6.725, p<0.0001] and main effect of diet [F(2,595)= 25.74, p<0.0001] and for saline-treated rats (main effect of day [F(34,525)= 12.83, p<0.0001] and main effect of diet [F(2,525)=32.45, p<0.0001]) There was no interaction among groups eating different diets for cocaine-treated rats (see Figure 2, bottom right panel) but there was a significant interaction between diet and day for saline-treated rats eating different diets [F(68,525)=2.729, p<0.0001] with Tukey’s multiple comparisons indicating that all three groups were significantly different from each other (p<0.005; Figure 2, bottom left panel).
3.3. Insulin Sensitivity
Under control conditions (no insulin) there were no differences in blood glucose concentration among groups of rats eating different diets (data not shown). For groups that were tested with cocaine, baseline glucose values were as follows: 111.8 ± 1.5 mg/dl for rats eating standard chow; 119.1 ± 2.4 mg/dl for rats eating high fat chow; and 118.1 ± 3.8 mg/dl for rats eating high fat chow with fish oil. For groups that were tested with saline, baseline glucose values were as follows: 112.7 ± 3.3 mg/dl for rats eating standard chow; 115.7 ± 2.2 mg/dl for rats eating high fat chow; and 117.3 ± 2.5 mg/dl for rats eating high fat chow with fish oil. There was a significant main effect of time (before or after insulin) indicating a significant hypoglycemic response after insulin administration in all groups [F(4,128) = 53.85, p<0.0001). A two-way ANOVA and paired t-test for maximum decreases in blood glucose (i.e., 45 minutes after 2 U/kg insulin; Figure 3) failed to detect any significant difference among groups regardless of diet of drug condition.
Figure 3.
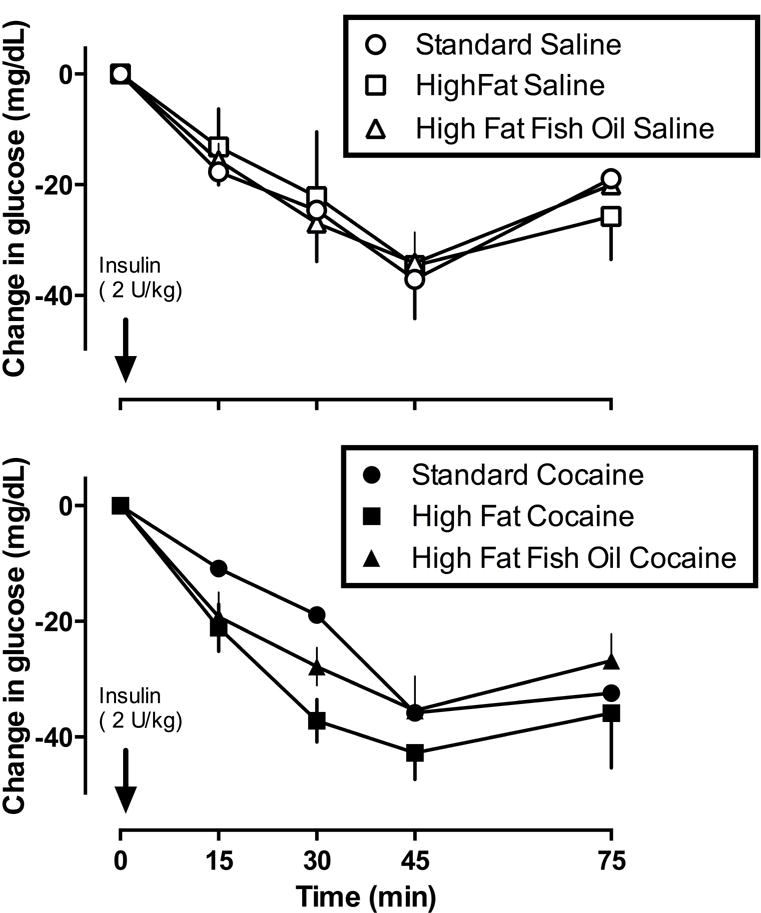
Change in blood glucose (mean ± 1 SEM, mg/dL; ordinate) determined during week 4 of the study after injection of 2 U/kg insulin in rats eating standard chow (circles), high fat chow (squares), or high fat chow with fish oil (triangles). Abscissae: time (min) after administration of insulin.
3.4. Locomotor Activity
Cocaine dose-dependently increased locomotor activity, with peak activity occurring at a dose of 17.8 mg/kg. There were no differences among the three dietary conditions with regard to initial sensitivity to cocaine-induced locomotion when all rats were eating standard chow (Figure 4A). The following week, rats eating high fat chow were more sensitive than rats eating standard chow to the locomotor-stimulating effects of cocaine (Figure 4B; 17.8 mg/kg). A two-way ANOVA with diet and dose as factors revealed a main effect of diet [F(2,85) = 4.662, p = 0.0340], a main effect of dose [F(4, 85) = 16.96, p<0.0001], as well as a significant interaction between diet and dose (F(8,85) = 2.215, p = 0.034). Since there was a significant interaction, Bonferroni multiple comparisons were conducted and revealed that rats eating high fat chow were significantly more active than rats eating standard chow, but only at the largest dose of cocaine (17.8 mg/kg, p<0.001). This difference persisted the following week (e.g., 2 weeks after assignment to dietary conditions; main effect of diet [F(2,85) = 4.778, p=0.0108], dose [F(4,85) = 11.28, p<0.0001], and a significant interaction [F(8,85) = 2.664, p=0.0117]) with Bonferroni multiple comparisons revealing that rats eating high fat chow were significantly more active than rats eating standard chow at a dose of 17.8 mg/kg (p<0.01). Moreover, rats eating high fat chow were also more active than rats eating high fat chow with fish oil (17.8 mg/kg; Figure 4C; p<0.01). The following week (e.g., 3 weeks after assignment to dietary conditions), a two-way ANOVA revealed significant main effects of diet [F(2,85) =4.755, p=0.0110], dose [F(4,85) = 14.75, p<0.0001], and a significant diet by dose interaction [F(8,85) = 2.581, p=0.0142]. Bonferroni multiple comparisons revealed that rats eating high fat chow were more active than rats eating high fat chow with fish oil, although only at the largest dose of cocaine (17.8 mg/kg; p<0.0001; Figure 4D). The next week (e.g., 4 weeks after assignment to dietary conditions), a two-way ANOVA revealed significant main effects of diet [F(2,85) =6.137, p=0.0032], dose [F(4,85) = 33.50, p<0.0001], and a significant diet by dose interaction [F(8,85) = 2.542, p=0.0156], with Bonferroni multiple comparisons revealing that rats eating high fat chow were more active than rats eating high fat chow with fish oil at two doses of cocaine (10.0 mg/kg, p<0.001; 17.8 mg/kg, p<0.05; Figure 4E). On the last week of testing (e.g., 5 weeks after assignment to dietary conditions), a two-way ANOVA revealed significant main effects of diet [F(2,85) =9.307, p=0.0003], dose [F(4,85) = 24.23, p<0.0001], and a significant diet by dose interaction [F(8,85) = 2.643, p=0.0123]. Bonferroni multiple comparisons revealed that rats eating high fat chow were more active than rats eating standard chow (p<0.05) and rats eating high fat chow with fish oil (10 mg/kg; p<0.0001; Figure 4F).
Figure 4.
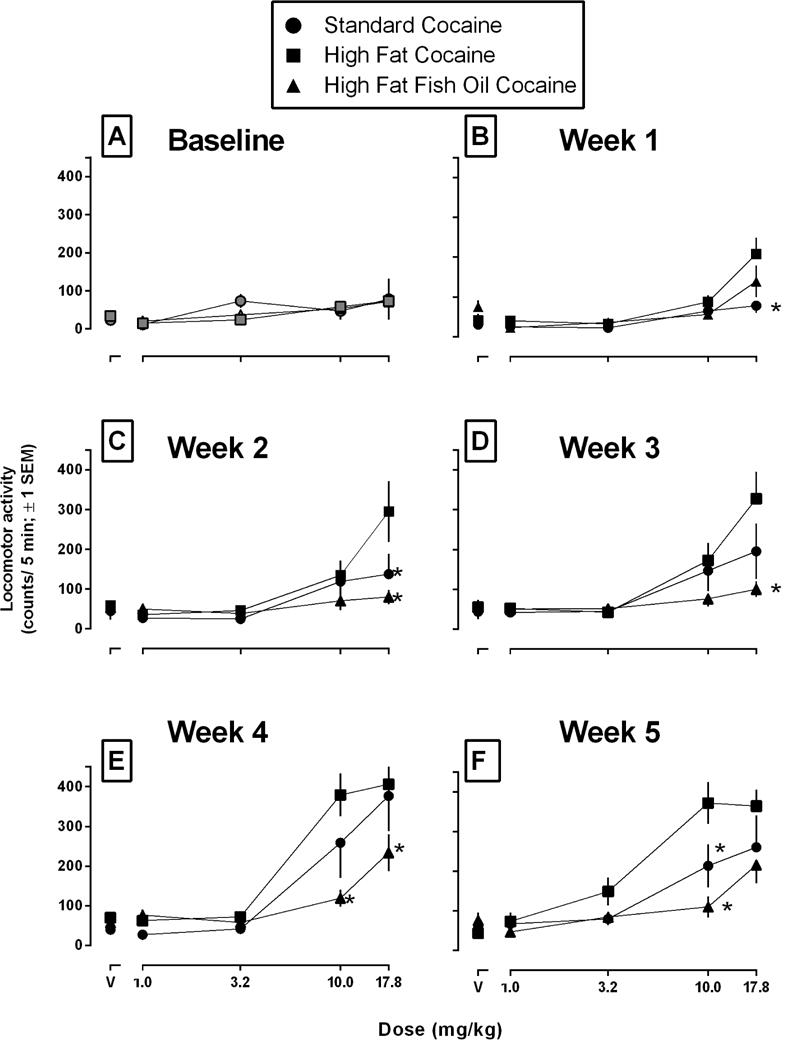
Locomotor activity counts for a 5-min period following the administration of cumulative doses of cocaine in female adolescent rats. During the baseline condition (Panel A) all rats ate standard chow. Thereafter (Panels B–F), different groups of rats ate standard chow (circles), high fat chow (squares), or high fat chow with fish oil (triangles) over 5 weeks of once weekly behavioral testing. Ordinates: mean (± 1 SEM) locomotor activity counts. Abscissae: dose of cocaine in mg/kg body weight (V = vehicle). *Significantly different from rats eating high fat chow.
Sensitization to cocaine emerged in all groups (Figure 4F; see also Figure 5). A two-way repeated measures ANOVA analyzing the AUC for cocaine dose-response curves revealed main effects of week ([F (15,85) = 30.99; p <0.0001]; see Figure 5), diet [F (2, 17) = 5.703; p = 0.0127], as well as a significant diet by week interaction [F (10,85) = 3.332; p = 0.0011]. Bonferonni multiple comparisons revealed that on weeks 4 and 5, the AUC was greater in rats eating high fat chow compared with those eating high fat chow with fish oil. Moreover, in week 5, the AUC was greater in rats eating high fat chow compared with those eating standard chow (all p values <0.05; Figure 5). Data are not shown from rats treated with saline.
Figure 5.
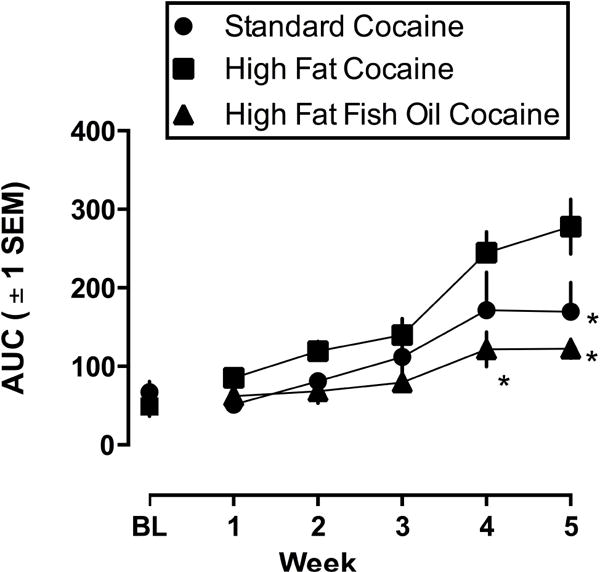
Area under the cocaine dose-response curves (AUC) in rats eating standard chow (circles), high fat chow (squares), or high fat chow with fish oil (triangles) over 5 once weekly cocaine tests. Ordinate: mean (± 1 SEM) AUC. Abscissa: week in study. BL refers to the initial (baseline; top left panel, Figure 4) cocaine test when all rats were eating standard chow.
3.5. Inflammatory Markers
Although two-way ANOVAs revealed significant differences for several cytokines and chemokines between saline- and cocaine-treated rats and among dietary conditions, there was no significant interaction between diet and drug. For example, there was a main effect of drug for plasma protein levels of IL-6, IL-1α, and MIP-1α and a significant main effect of diet for leptin and EFG (all p values < 0.05; see Table 1). In the hypothalamus, there was a main effect of diet for protein levels of IL-18 and fractalkine only (all p values < 0.05; see Table 2).
Table 1.
Plasma protein concentration (average pg/ml ± 1 SEM) for cytokines and chemokines that were significantly changed and fell within the detectable range.
| Cytokine/Chemokine | Group | Main effect | Statistics | |||||
|---|---|---|---|---|---|---|---|---|
| Standard Saline | High Fat Saline | High Fat Fish Oil Saline | Standard Cocaine | High Fat Cocaine | High Fat Fish Oil Cocaine | |||
| IL-6 | 1618.8 ± 3254 | 1548.3 ± 276.9 | 1551.2 ± 144.5 | 2186.0 ± 257.7 | 1951.8 ± 382.9 | 2436.4 ± 308.9 | Drug | F(1,32) = 6.337, p= 0.017 |
| Leptin | 6476.5 ± 836.3 | 12632.7 ± 1291.0 | 12230.3 ± 1250.2 | 6087.7 ± 873.3 | 11291.0 ± 1972.2 | 9863.1 ± 1938.1 | Diet | F(2,32)= 8,238, p= 0.0013 |
| IL-1alpha | 74.3 ± 11.6 | 73.9 ±11.32 | 68.1 ± 9.9 | 90.0 ± 13.3 | 94.3 ± 12.8 | 98.1 ± 11.3 | Drug | F(1,32)= 5.168, p= 0.0299 |
| EGF | 258.9 ± 79.7 | 388.0 ± 107.2 | 228.8 ± 26.9 | 590.1 ± 71.4 | 441.3 ± 73.2 | 183.8 ± 55.5 | Diet | F(2,32) = 5.022, p= 0.0127 |
| MIP-1alpha | 12.6 ± 2.1 | 14.06 ±1.8 | 13.6 ±0.5 | 15.5 ± 1.6 | 16.3 ± 2.4 | 19.6 ± 1.6 | Drug | F(1,32) = 6.191, p= 0.0182 |
Table 2.
Hypothalamic protein concentration average pg/ml ± 1 SEM) for cytokines and chemokines that were significantly changed and fell within the detectable range.
| Cytokine/Chemokine | Group | Main effect | Statistics | |||||
|---|---|---|---|---|---|---|---|---|
| Standard Saline | High Fat Saline | High Fat Fish Oil Saline | Standard Cocaine | High Fat Cocaine | High Fat Fish Oil Cocaine | |||
| IL-18 | 67.6 ± 13.5 | 138.1 ± 24.4 | 110.6 ± 18.7 | 71.4 ± 12.1 | 107.0 ± 20.2 | 120.8 ± 22.1 | Diet | F(2,32) = 4.431, p= 0.02 |
| Fractalkine | 6.2 ± 0.4 | 7.0 ± 0.5 | 7.0 ± 0.3 | 5.8 ± 0.5 | 6.8 ± 0.4 | 7.0 ± 0.3 | Diet | F(2,32) = 3.707, p= 0.0356 |
4. DISCUSSION
Eating high fat chow enhances the sensitivity of rats to behavioral effects of drugs acting on dopamine systems (Gosnell, 2005; Avena and Hoebel, 2003; Baladi et al., 2012b; Serafine et al., 2014b). Although eating high fat chow also causes adverse health consequences, such as obesity, insulin resistance, and inflammation, eating high fat chow can alter sensitivity to drugs even in the absence of those adverse consequences (Baladi et al., 2011; Serafine et al., 2014b). This study examined the impact of dietary supplementation with fish oil, which is rich in omega-3 polyunsaturated fatty acids, on changes in body weight, insulin sensitivity, inflammation, and sensitivity to the locomotor effects of cocaine as a result of eating high fat chow. Cocaine dose-dependently increased locomotor activity in rats, and induced sensitization after repeated administration. Consistent with previous reports, these effects were largest in rats eating high fat chow (Figure 5; see also Baladi et al., 2011; Serafine et al., 2014b). Dietary supplementation with fish oil (20% w/w) prevented high fat chow-induced enhancement of sensitivity to the behavioral effects of cocaine.
As reported previously (Baladi et al., 2015; Serafine et al., 2014b), unlimited access to high fat chow did not alter body weight in adolescent female rats, compared with adolescent female rats eating standard chow. Adolescent rats gain body weight very rapidly and eating high fat chow does not further accelerate that weight gain, in contrast to the dramatic weight gain (e.g., obesity) that occurs in adult rats eating high fat chow (Baladi et al., 2011; Serafine et al., 2014a). Although body weight was not significantly different among groups of rats under different dietary conditions, nonsystematic observation of tissue during dissection suggested that body fat pads appeared to be larger and more widespread in rats eating high fat chow compared with rats eating standard chow or high fat chow with fish oil. Further studies on body fat composition might provide insights with regards to the impact of fish oil on high fat diet induced changes in sensitivity to drugs.
Insulin resistance can develop in adult rats after only two weeks of eating high fat chow (see Baladi et al., 2011; Serafine et al., 2014a). In the present study, adolescent female rats did not develop insulin resistance despite 5 weeks of eating exclusively high fat chow. While it is possible that longer access to high fat chow might have resulted in the development of insulin resistance in adolescent female rats, it is also possible that insulin signaling was altered by eating high fat chow despite a normal response to insulin challenge. For example, eating high fat chow can alter activation of the insulin-activated signaling kinase Akt, thereby decreasing dopamine transporter expression on the cell surface and reducing dopamine clearance (Speed et al., 2011). Thus, increased sensitivity to cocaine in rats eating high fat chow might result, in part, from changes in insulin signaling; conversely, attenuation of high fat chow induced increases in sensitivity to cocaine in rats eating high fat chow with fish oil might occur from a normalization of insulin signaling.
Other feeding-related hormones, such as leptin, might also play a role in the behavioral effects that were observed in the current study. For example, leptin increases dopamine transporter expression in rats eating standard chow (Perry et al., 2010) and leptin resistance develops in rats eating high fat chow (Kim et al., 2006). Consistent with previous reports, plasma leptin concentration was elevated in rats eating high fat chow; however, dietary supplementation with fish oil did not prevent this increase in leptin (Table 1).
Eating high fat chow can also increase proinflammatory markers both in peripheral tissues (Maric et al., 2014) and in the central nervous system (Maric et al., 2014; Wang et al., 2012). In the current study, there was no interaction between diet and drug treatment for any inflammatory marker. However, the present study used an assay to examine multiple inflammatory markers simultaneously in samples collected from individual subjects. While this approach detects changes in inflammation caused by injury (e.g., Fox et al., 2005), changes in inflammation due to consumption of high fat chow might not be sufficiently robust to be detected using this technique. Changes in mRNA levels might be more sensitive to diet-induced inflammation (see Maric et al., 2014; Wang et al., 2012; Thaler et al., 2012). Moreover, in the current study rats ate high fat chow for 5 weeks and showed increased sensitivity to cocaine-induced locomotion after as little as 2 weeks, whereas in previous studies reporting significant effects of diet on hypothalamic inflammatory markers subjects ate high fat chow for up to 10 weeks (Maric et al., 2014; Wang et al., 2012). Since the current study focused on adolescence as a potential period of vulnerability to dietary effects on sensitivity to drugs (adolescence is also a period of increased intake of high fat foods in humans [Poti et al., 2013]), it was not feasible to significantly extend the period of access to high fat chow without infringing on adulthood. Another difference between the current study and previous studies is the type of high fat chow. Some studies used coconut oil or butter to create a high fat diet (e.g., Maric et al., 2014) while others used commercially available chow similar to the chow used in the present study (e.g., Wang et al., 2012). Because different chows were used across studies, the total kcal from fat in the various diets likely was not the same. Moreover, some studies differentiated rats based on whether obesity developed in individual rats eating a high fat diet (Wang et al., 2012). In the current study, adolescent rats did not become obese, perhaps precluding significant changes in cytokines that occur only with obesity. Finally, the majority of studies on inflammatory effects of eating high fat chow in rats used adult male subjects. It is possible that the effects of diet (and drug) on inflammatory markers differ between males and females.
In the present study, rats eating high fat chow with fish oil were significantly less sensitive than rats eating high fat chow without fish oil to the locomotor-stimulating effects of cocaine and showed less sensitization to cocaine over repeated testing. These changes in sensitivity to cocaine were not accompanied by obesity, insulin resistance, or changes in inflammatory markers in the plasma or hypothalamus. Previous studies using adult male rats have reported that consuming fish oil attenuates high fat chow-induced obesity, insulin resistance, and hypothalamic inflammation (Cintra et al., 2012; Pimentel et al., 2013). Eating fish oil attenuated changes in sensitivity to behavioral effects of cocaine without attenuating changes in inflammatory markers, indicating that other factors mediate diet-induced sensitization to cocaine. It appears as though diet can impact dopamine signaling independently from changes in body weight (i.e., obesity) and other metabolic factors (Hryhorczuk et al., 2015). These results demonstrate that eating a high fat diet can increase sensitivity to drugs of abuse in ways that might be relevant to vulnerability; the results also suggest that dietary supplementation with fish oil might prevent these effects.
Highlights.
High fat diet enhances sensitivity to the locomotor effects of cocaine
Eating fish oil prevents enhanced sensitization to cocaine
Changes in cytokines do not mediate this effect of eating fish oil
Acknowledgments
The work was supported, in part, by the National Institutes on Drug Abuse of the National Institutes of Health under award numbers K05DA017918 and T32DA031115. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. CL was supported by a SURF award from the American Society for Pharmacology and Experimental Therapeutics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
All three authors made significant contributions to the conduct of the study (KMS, CL), data analyses (KMS, CL, CPF), and preparation of the manuscript (KMS, CL, CPF).
Conflict of Interest
No conflict declared
Contributor Information
Katherine M. Serafine, Email: kserafine@gmail.com.
Charles P. France, Email: france@uthscsa.edu.
References
- Anker JJ, Carroll ME. Responding during signaled availability and nonavailability of iv cocaine and food in rats: age and sex differences. Psychopharmacology. 2011;215:785–799. doi: 10.1007/s00213-011-2181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena NM, Hoebel BG. A diet promoting sugar dependency causes behavioral cross-sensitization to a low dose of amphetamine. Neuroscience. 2003;122:17–20. doi: 10.1016/s0306-4522(03)00502-5. [DOI] [PubMed] [Google Scholar]
- Baladi MG, Daws LC, France CP. You are what you eat: influence of type and amount of food consumed on central dopamine systems and the behavioral effects of direct- and indirect-acting dopamine receptor agonists. Neuropharmacology. 2012a;63:76–86. doi: 10.1016/j.neuropharm.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, France CP. High fat diet and food restriction differentially modify the behavioral effects of quinpirole and raclopride in rats. Eur J Pharmacol. 2009;610:55–60. doi: 10.1016/j.ejphar.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, France CP. Eating high-fat chow increases the sensitivity of rats to quinpirole-induced discriminative stimulus effects and yawning. Behav Pharmacol. 2010;21:615–620. doi: 10.1097/FBP.0b013e32833e7e5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, Horton RE, Owens WA, Daws LC, France CP. Eating high fat chow decreases dopamine clearance in adolescent and adult male rats but selectively enhances the locomotor stimulating effects of cocaine in adolescents. Int J Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyv024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, Koek W, Aumann M, Velasco F, France CP. Eating high fat chow enhances the locomotor-stimulating effects of cocaine in adolescent and adult female rats. Psychopharmacology. 2012b;222:447–457. doi: 10.1007/s00213-012-2663-7. [DOI] [PubMed] [Google Scholar]
- Baladi MG, Newman AH, France CP. Influence of body weight and type of chow on the sensitivity of rats to the behavioral effects of the direct-acting dopamine-receptor agonist quinpirole. Psychopharmacology. 2011;217:573–585. doi: 10.1007/s00213-011-2320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J, Sternin O, Wu HB, Fletcher H, Perrotti LI, Jenab S, Quinones-Jenab V. Sex differences in cocaine-induced behavioral sensitization. Cell Mol Biol (Noisy-le-grand) 2001;47:1089–1095. [PubMed] [Google Scholar]
- Cintra DE, Ropelle ER, Moraes JC, Pauli JR, Morari J, Souza CT, Grimaldi R, Stahl M, Caravalheira JB, Saad MJ, Velloso LA. Unsaturated fatty acids revert diet-induced hypothalamic inflammation in obesity. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Calinski DM, Newman AH, Grundt P, Woods JH. Food restriction alters N′-propyl-4,5,6,7-tetrahydrobenzothiazole-2,6-diamine dihydrochloride (pramipexole)-induced yawning, hypothermia, and locomotor activity in rats: evidence for sensitization of dopamine D2 receptor-mediated effects. J Pharmacol Exp Ther. 2008;325:691–697. doi: 10.1124/jpet.107.133181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC, Avison MJ, Robertson SD, Niswender KD, Galli A, Saunders C. Insulin signaling and addiction. Neuropharmacology. 2011;61:1123–1129. doi: 10.1016/j.neuropharm.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham HA, Truett GE. Development of insulin resistance and hyperphagia in Zucker fatty rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R652–R658. doi: 10.1152/ajpregu.00428.2004. [DOI] [PubMed] [Google Scholar]
- Fox C, Dingman A, Derugin N, Wendland MF, Manabat C, Ji S, Ferriero DM, Vexler ZS. Minocycline confers early but transient protection in the immature brain following focal cerebral ischemia-reperfusion. J Cereb Blood Flow Metab. 2005;25:1138–1149. doi: 10.1038/sj.jcbfm.9600121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosnell BA. Sucrose intake enhances behavioral sensitization produced by cocaine. Brain Res. 2005;1031:194–201. doi: 10.1016/j.brainres.2004.10.037. [DOI] [PubMed] [Google Scholar]
- Hryhorczuk C, Florea M, Rodaros D, Poirier I, Daneault C, Des Rosiers C, Arvanitogiannis A, Alquier T, Fulton S. Dampened mesolimbic function and signaling by saturated but not monounsaturated dietary lipids. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YW, Kim JY, Park SY, Won KC, Choi KH, Huh JY, Moon KH. Metformin restores leptin sensitivity in high-fat-fed obese rats with leptin resistance. Diabetes. 2006;55:716–724. doi: 10.2337/diabetes.55.03.06.db05-0917. [DOI] [PubMed] [Google Scholar]
- Larson N, Neumark-Sztainer D, Laska MN, Story M. Young adults and eating away from home: associations with dietary intake patterns and weight status differ by choice of restaurant. J Am Diet Assoc. 2011;111:1696–1703. doi: 10.1016/j.jada.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Labouebe G, Karunakaran S, Clee SM, Borgland SL. Effect of insulin on excitatory synaptic transmission onto dopamine neurons of the ventral tegmental area in a mouse model of hyperinsulinemia. Nutr Diabetes. 2013;3:e97. doi: 10.1038/nutd.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology. 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Maric T, Woodside B, Lucheshi GN. The effects of dietary saturated fat on basal hypothalamic neuroinflammation in rats. Brain Behav Immun. 2014;36:35–45. doi: 10.1016/j.bbi.2013.09.011. [DOI] [PubMed] [Google Scholar]
- McGuire BA, Baladi MG, France CP. Eating high-fat chow enhances sensitization to the effects of methamphetamine on locomotion in rats. Eur J Pharmacol. 2011;658:156–159. doi: 10.1016/j.ejphar.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanswami V, Thompson AC, Cassis LA, Bardo MT, Dwoskin LP. Diet-induced obesity: dopamine transporter function, impulsivity and motivation. Int J Obes. 2013;8:1095–1103. doi: 10.1038/ijo.2012.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens WA, Williams JM, Saunders C, Avison MJ, Galli A, Daws LC. Rescue of dopamine transporter function in hypoinsulinemic rats by a D2 receptor-ERK-dependent mechanism. J Neurosci. 2012;8:2637–2647. doi: 10.1523/JNEUROSCI.3759-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel GD, Lira FS, Rosa JC, Oller do Nascimento CM, Oyama LM, Harumi Watanabe RL, Ribeiro EB. High-fat fish oil diet prevents hypothalamic inflammatory profile in rats. ISRN Inflamm. 2013 doi: 10.1155/2013/419823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry ML, Lenninger GM, Chen R, Luderman KD, Yang H, Gnegy ME, Myers MG, Jr, Kennedy RT. Leptin promotes dopamine transporter and tyrosine hydroxylase activity in the nucleus accumbens of Sprague-Dawley rats. J Neurochem. 2010;114:666–674. doi: 10.1111/j.1471-4159.2010.06757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poti JM, Slining MM, Popkin BM. Solid fat and added sugar intake among U.S. children: The role of stores, schools, and fast food, 1994–2010. Am J Prev Med. 2013;45:551–559. doi: 10.1016/j.amepre.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafine KM, Bentley TA, Grenier AE, France CP. Eating high fat chow and the behavioral effects of direct-acting and indirect-acting dopamine receptor agonists in female rats. Behav Pharmacol. 2014a;25:287–295. doi: 10.1097/FBP.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafine KM, Bentley TA, Koek W, France CP. Eating high fat chow, but not drinking sucrose or saccharin, enhances the development of sensitization to the locomotor stimulating effects of cocaine in adolescent female rats. Behav Pharmacol. 2014b;26:321–325. doi: 10.1097/FBP.0000000000000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South T, Huang XF. High-fat diet exposure increases dopamine D2 receptor and decreases dopamine transporter receptor binding density in the nucleus accumbens and caudate putamen of mice. Neurochem Res. 2008;33:598–605. doi: 10.1007/s11064-007-9483-x. [DOI] [PubMed] [Google Scholar]
- Speed N, Saunders C, Davis AR, Owens WA, Matthies HJ, Saadt S, Kennedy JP, Vaughan RA, Neve RL, Lindsley CW, Russo SJ, Daws LC, Niswender KD, Galli A. Impaired striatal Akt signaling disrupts dopamine homeostasis and increases feeding. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschop MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ge A, Cheng M, Guo F, Zhao M, Zhou X, Liu L, Yang N. Increased hypothalamic inflammation associated with the susceptibility to obesity in rats exposed to high-fat diet. Exp Diabetes Res. 2012 doi: 10.1155/2012/847246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Owens WA, Turner GH, Saunders C, Dipace C, Blakely RD, France CP, Gore JC, Daws LC, Avison MJ, Galli A. Hypoinsulinemia regulates amphetamine-induced reverse transport of dopamine. PLoS Biol. 2007;5 doi: 10.1371/journal.pbio.0050274. [DOI] [PMC free article] [PubMed] [Google Scholar]


