Abstract
For the past century, research on neurological disorders has largely focused on the most prominently affected cell types – the neurons. However, with increasing knowledge of the diverse physiological functions of glial cells, their impact on these diseases has become more evident. Thus, many conditions appear to have more complex origins than initially thought.
Since neurological pathologies are often sporadic with unknown etiology, animal models are difficult to create and might only reflect a small portion of patients in which a mutation in a gene has been identified. Therefore, reliable in vitro systems to studying these disorders are urgently needed. They might be a pre-requisite for improving our understanding of the disease mechanisms as well as for the development of potential new therapies. In this review, we will briefly summarize the function of different glial cell types in the healthy central nervous system (CNS) and outline their implication in the development or progression of neurological conditions. We will then describe different types of culture systems to model non-cell autonomous interactions in vitro and evaluate advantages and disadvantages.
Keywords: Direct Conversion, Induced Pluripotent Stem Cells, Neurodegeneration, Non-cell Autonomy, In Vitro Systems
1. Role of glia in the healthy CNS
The term glia is derived from the Greek word “glue” and was used by Virchow in 1856 to describe the filling between neurons in the CNS. Remarkably, despite the persistence of a neuron-centered research for many decades, Virchow had already recognized the importance of glial cells in understanding the functionality of the CNS, as he stated in his lecture in 1858: “Hitherto, gentlemen, in considering the nervous system, I have only spoken of the really nervous parts of it. But if we would study the nervous system in its real relations in the body, it is extremely important to have a knowledge of that substance also which lies between the proper nervous parts, holds them together and gives the whole its form in a greater or less degree”1. Today we are only starting to understand the complexity of the relationship between neurons and glial cells. Improved co-culture techniques have helped to study different aspects in more details.
Classically, three different types of glial cells are distinguished in the CNS (astrocytes, oligodendrocytes and microglia), each possessing distinct functions. However, NG2+ oligodendrocyte precursor cells (OPCs) or polydendrocytes can be counted as a fourth glial cell type due to their various different functions2,3. To different extents and in different combinations, all four cell types have been demonstrated to be involved in either the development or progression of virtually all known pathologic conditions of the CNS including neurodegenerative disorders such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Amyotrophic Lateral Sclerosis (ALS), Huntington’s Disease (HD), Multiple Sclerosis (MS), Spinal Muscular Atrophy (SMA), and other pathologies such as Rett syndrome (RTT), sleep disorders, addiction, epilepsy, depression, migraine and pathological pain4–12. Therefore, robust model systems to unravel the distinct role of each individual glial cell type in a disease state, as well as to study their dynamic interplay, may be very helpful in identifying novel therapies.
Astrocytes are the most abundant cell type in the CNS. Their number and the ratio compared to neurons increases with the complexity of the nervous system, indicating their importance for the development and maintenance of this sophisticated structure13,14. In agreement with the various functions fulfilled by this cell type, the astrocyte population is very heterogeneous in terms of morphology and gene expression15–21. The main role of astrocytes is to provide and maintain homeostasis in the CNS. This includes trafficking of ions, neurotransmitters and neurohormones, metabolic support in accumulating and dispersing energy substrates such as lactate, cellular homeostasis (neurogenesis), as well as organ homeostasis in forming and regulating the blood brain barrier (BBB)12.
Moreover, astrocytes integrate and coordinate synaptic signals with non-synaptic signals and modulate the activity of the surrounding cells in a plastic manner22,23. Initially, astrocytes were thought to overlap with each other, but evidence is now pointing towards an ordered organization, in which individual cells cover independent territories, interfacing with the microvasculature as well as neurons. As modulators of neuronal communication and activity, they form a tripartite synapse with pre- and post-synaptic neurons24. With their multiple processes and branches, a single astrocyte can contact thousands of synapses simultaneously19. In addition, astrocytes are also interconnected via gap junctions forming a complex network that transports signals via Ca2+ waves, although with a much slower speed than neuronal signaling23.
Microglia are long-lived tissue specific macrophages of the CNS that comprise approximately 15–20% of the cells in the brain. Other than the ectodermally produced neurons, astrocytes and oligodendrocytes, they originate from the mesodermal hematopoietic stem cells in the yolk sac. Microglia precursors (myeloid progenitor cells) enter the CNS during early embryonic development before the BBB is established25,26.
As the name indicates, microglia are much smaller than astrocytes. They exist in an amoeboid migratory state while entering the CNS or when activated, and a ramified “resting” state under regular conditions with a small soma and extensive fine processes. In the mature CNS, they are evenly dispersed in all regions and each cell occupies a defined territory (similar to astrocytes). Due to their immobility and absence of activation markers, “resting” microglia where considered quiescent until recent studies demonstrated their tireless and constant monitoring of the environment with their fine ramified processes27. Thus, in the healthy CNS, microglia function as immune surveyors and are mainly responsible for clearing debris. Neurons and astrocytes express receptors and secrete neurotransmitter, and neurotrophins, to constantly signal their good health to the microglia28–31. Likewise, microglia express a broad variety of neurotransmitter receptors that can sense neuronal activity and consequently modulate microglia migration, inflammatory responses, cytokine release, neuroprotection or neurotoxicity29,30. As part of the immune system, microglia secrete modulatory factors such as cytokines and reactive oxygen species (ROS) and express receptors for chemokines, cytokines and complement-factors. They also present antigens to infiltrating T lymphocytes via the major histocompatibility complex (MHC) class II complex. After sensing an injury or a pathological insult, microglial cells rapidly undergo a transformation to an amoeboid morphology and migrate towards the area of insult25,26,32. Interestingly, a recent study suggests that microglia cell migration towards injury or dead neurons is guided via glutamate induced Ca2+ waves32.
In addition to their immunological role, microglia are involved in the elimination of synapses during development (synaptic pruning) via phagocytosis as well as synaptic plasticity33–35.
Oligodendrocytes are responsible for myelination of neuronal axons in the CNS, which is necessary for the fast conduction of electrical signals. During development, OPCs originate in different brain regions and travel long distances to reach their final location. During this process, OPCs undergo complex proliferation and differentiation mechanisms36,37. Myelination is initiated shortly after birth when the OPCs have finished their migration to their site of action. While most extensive myelination takes place within the first year of life in humans, it persists into young adulthood in some regions of the CNS38 and also continues throughout adult life39. Interestingly, myelination in specific regions seems to correlate with the development of corresponding cognitive functions38,40,41. Upon contact with target axons, immature oligodendrocytes undergo a differentiation process and wrap their plasma membranes around the neurons in a complex process whose exact mechanism is still under debate42,43. With increasing membrane layers, the cytoplasm is extruded and the remaining sheets finally consist of up to 160 compact membrane layers of myelin lipids and proteins44,45. The differentiation from oligodendrocyte precursor cells (OPCs) to mature oligodendrocytes, as well as the myelination process, is tightly regulated. The signaling pathways and molecules involved are still poorly understood, partly due to the lack of model systems46,47. It seems that oligodendrocytes only myelinate during a short window during differentiation from OPCs48. While electrical activity of neurons is involved in the initiation of myelination, astrocytes play a role in the efficiency and speed of the wrapping. Oligodendrocytes are thought to be able to support 100 times the weight of their own cell body in membrane44. During peak myelination, an oligodendrocyte can produce 3 times its own weight in membrane per day (up to ~ 5000μm2 of new membrane), which is an enormous metabolic effort requiring high amounts of oxygen and adenosine triphosphate (ATP) and a tremendous capacity of the endoplasmic reticulum45,46,49. Although in the healthy nervous system, these cells are very long-living with a turnover of only ~ 1 cell in 300 per year, they are vulnerable to injury and insults involving inflammation and oxygen deprivation50. Lost oligodendrocytes can be replaced by remaining NG2 positive OPCs who are distributed throughout the adult CNS (see section below for NG2+ cells)51.
Apart from this insulation, oligodendrocytes also provide trophic factors to neurons and regulate the diameter of axons and the distribution of ion-channels among them47.
Oligodendrocytes and astrocytes are also tightly connected via gap junctions (similar to astrocytes among each other), which allow diffusion of ions and small molecules, thus enabling metabolic exchange, spatial buffering as well as electrical coupling52.
In addition, oligodendrocytes produce various immune-regulatory factors and express receptors to receive such signals such as certain MHC subtypes, complement factors, glutamate receptors, chemokines, cytokines and toll like receptors53. This indicates that oligodendrocytes play an active role during inflammation and can closely communicate with microglia.
NG2+ glia (polydendrocytes or OPCs) are the fourth glial cell type found in the CNS. These cells are widely distributed throughout the whole CNS and express two typical markers of the oligodendrocyte lineage, the NG2 chondroitin sulfate proteoglycan (CSPG4) and platelet-derived growth factor α receptor (PDGFαR)2,54. Apart from the restricted zones in the adult brain that generate new neurons, these glial cells are the major dividing cell population. Their best known function is to generate new oligodendrocytes and to a lower extent astrocytes, thus they are frequently called OPCs51. However, this self-renewing cell population is very diverse and has distinct physiological properties. Further, they can receive synaptic input from neurons and subpopulations of NG2+ cells are capable of firing single action potentials2,55,56. Similar to the other glial cell types, these cells have been implicated in many neurological disorders57.
Communication between glial cells among each other as well as with neurons can occur via several mechanisms: i) Direct contact mediated by receptors or via gap junctions, ii) secretion of molecules, iii) secretion of vesicles. Interestingly, all cell types of the CNS have the ability to secrete exosomes53,58–63. Exosomes are small membrane vesicles (50–100nm) containing a wide variety of cargos including proteins, mRNAs and microRNAs. These vesicles can attach to neighboring cells or be internalized. Exosome release is activity-dependent in neurons and has been shown to alter gene expression in the receiving cells as well as to modulate immune reactions. In addition to their function in cellular communication, they are also used for draining obsolete proteins and lipids59,60,63,64.
2. Importance of glial cells in neurological pathology
As previously mentioned, glial cells have been implicated in the development or progression of virtually all neurological pathologies and the consequent impairment of proper neuronal function. It is therefore impossible to give a comprehensive overview of each condition. Since the cell types in the CNS depend on their close connection and communication, the aberrant function of one cell type ultimately may lead to impairment of all other cells in the same environment or circuit. Thus, it is often impossible to discriminate cause and consequence in this complex pathological cascade, especially for sporadic disorders with unknown origin. In vitro systems can help to discern the complexity allowing the study of individual interactions in more detail. This is an advantage as well as a limitation of in vitro systems, since they never represent the whole picture. In the following section, we will try to cover a broad variety of different pathological conditions to give an idea of the huge impact of glial cell types on CNS pathology.
Acute insults to the CNS (ischemia, trauma, stroke, toxins)
Stroke is a major cause of death in the world and current therapeutic strategies mainly focus on neuroprotection. To date, many clinical trials focused on improving neuronal health or survival, have failed. Therefore, combinatorial approaches aiming to target glial cell types might be needed. Astrocytes play an important role in stroke recovery, both positive and negative. If small enough, astrocytes will repair the damage or provide energy to the neurons by breaking down stored glycogen to generate lactate65,66. In the acute phase of stroke, uptake of glutamate and K+ as well as scavenging of reactive oxygen species (ROS) by astrocytes is likely reducing the damage caused by ischemia67. However, if the damage is too severe and astrocytes die, glutamate will be released due to membrane depolarization, which can lead to excitotoxicity in neurons67–70. In vitro studies have also demonstrated that oligodendrocytes are sensitive to excitotoxicity after stroke. Apart from the damage caused by oxidative stress due to the oligodendrocyte’s high metabolic rate, excitotoxicity caused by extracellular glutamate or ATP could be a major component leading to their death under hypoxic-ischemic conditions46,71. In the later phase of recovery after stroke, astrocytes release many neuroprotective agents such as erythropoietin (EPO) or vascular endothelial growth factor (VEGF). However, a major problem in stroke therapy is the timing of a certain treatment, since the same molecule can be detrimental when administered immediately after stroke, but beneficial in the process of recovery12. Two examples are VEGF and MMP9, which both increase the permeability of the BBB, thus leading to brain edema if administered too early after stroke72–74.
Similarly, microglia play a dual role in stroke as they get activated by damaged neurons. Under ischemic conditions, microglia phagocytose debris and secrete pro-inflammatory cytokines75. Galectin-3, a known modifier of immune reactions in the periphery was recently identified to play an important role in the activation and proliferation of microglia following stroke. Microglia deficient of galectin-3 showed impaired up regulation of activation markers following ischemic injury and impaired response to IGF-1 mediated mitotic signaling in a cell culture system as well as the corresponding mouse model76.
Therefore, in stroke, the time window in which a certain treatment can be beneficial or not, is likely crucial. This information on the organ level is lost in culture models and is certainly an aspect that needs to be kept in mind when using these to evaluate potential therapeutics.
Alexander Disease
This rare astrocyte disorder leading to neurodegeneration is caused by mutations in GFAP, the major intermediate filament protein of astrocytes77. Patients suffer from seizures and psychomotor delays, gait disturbances, bulbar signs and autonomic dysfunctions leading to death within the first decade of life12. How these mutations cause disease is currently unknown and could involve loss of regular protein function as well as toxicity of the mutated protein77. Cell culture models have helped to demonstrate that overexpression of wild type (WT) and mutant GFAP causes activation of different stress pathways, disturbance of the proteasome and enhanced autophagy78,79.
Rett syndrome
The neurodevelopmental disorder Rett syndrome (RTT) is another example in which astrocytes and microglia seem to play a major role in the development of the pathology. RTT is caused by dominant mutations in the X-chromosome encoded transcription factor methyl-CpG-binding protein 2 (MeCP2). RTT is a disease from the autism spectrum that affects predominantly females and causes reduced brain growth, loss of motor skills, ataxia, loss of vocalization skills and cognitive abilities, seizures and respiratory dysfunctions80,81. Although earlier studies clearly demonstrated the impact MeCP2 mutations have on neuronal morphology, synaptic transmission and activity82–85, recent research uncovered a major contribution of astrocytes and microglia to the disease phenotype. Cell culture experiments using mutant astrocytes from a RTT mouse model or medium conditioned with such, demonstrated a strong impact of mutant astrocytes on the morphology and health of WT and RTT mutation carrying hippocampal neurons86. More strikingly, the conditioned medium of WT astrocytes was sufficient to rescue the phenotype of the mutant neurons, indicating that trophic support can improve their health86. Similarly, increased glutamate release by RTT microglia was shown to damage hippocampal neurons in culture80. Furthermore, RTT microglia were demonstrated to display reduced phagocytosis in vitro and bone marrow transplants leading to the substitution of RTT microglia with WT microglia in vivo strongly ameliorated the disease phenotype of the RTT mice87. Therefore, both astrocytes and microglia represent valuable targets for future therapeutics. Co-cultures of hippocampal neurons and mutant astrocytes or microglia are ideal settings for testing compounds or shRNA libraries for potential therapeutics.
Epilepsy
Epilepsy is a chronic brain disorder characterized by a predisposition to seizures as well as cognitive and emotional impairments. The cause is largely unknown, although inflammatory processes in the brain are likely involved in the pathology. Activation of microglia and astrocytes associated with secretion of inflammatory cytokines has been extensively described88. Alterations in the expression of glutamate receptors, enzymes, various membrane transporters, and ion channels on astrocytes have been identified in mouse models of epilepsy as well as in human patients89,90. In addition, the organization of the non-overlapping territorial distribution of astrocytes was disrupted in several mouse models as well as in surgically removed tissues from epilepsy patients. The consequences of this loss of organization is not yet fully understood, but could be related to miscommunication caused by the connection of several astrocytes to the same synapse instead of the regular single occupation91.
Neurodegenerative disorders
As previously mentioned, neurodegenerative disorders such as AD, PD, HD and ALS are results of many different pathologies with various underlying causes. Often they remain unidentified until substantial damage has occurred. Therefore, our knowledge of the early stages of these conditions is relatively sparse, which makes it difficult to distinguish causes from consequences and hampers a proper understanding of the underlying mechanisms9. Early intervention is likely a key factor for improvement of therapeutic outcomes. New reprogramming technologies and culture systems are promising tools to investigate this earlier disease time points. Although different subtypes of neurons are affected, they share various hallmarks in disease development. The major risk factor for these disorders is aging, which suggests that cellular maintenance could play a major role in the manifestation of these conditions92. Further, accumulation of protein aggregates, impairment in protein trafficking and energy metabolism, oxidative stress and formation of free radicals are common features for all of them12,93. All these pathways are strongly regulated by glial cell types in the CNS, that are responsible for maintaining homeostasis on the cellular, metabolic, structural and signaling transmission level94. Reactive gliosis characterized by activation and proliferation of glial cells in response to damage can be found in all neurodegenerative conditions95. There is overwhelming evidence for the involvement of all glial cell types in neurodegenerative disorders and their description would go beyond the scope of this review6,8,10–12,96–102. Interestingly, in ALS it has been show that while motor neurons determine the onset of the disease, astrocytes and microglia are mainly involved in the disease progression, thus modulating the reaction of these cell types could lead to substantial benefits for affected patients102–106.
Multiple Sclerosis, Inflammation and injury
Multiple Sclerosis (MS) is a chronic inflammatory disease of the CNS with still unknown etiology that could involve genetic, metabolic and immunological factors107. It is one of the most common inflammatory conditions of the CNS and thought to be caused by auto-immune reactions directed towards myelin108. In combination with a reduction in oligodendrocyte number, accumulation of inflammatory cells, demyelinated axons and reactive glial cells can be observed. In mouse models of MS, microglial activation is detected prior to disease onset and likely plays an important role in modulating the inflammatory response in this disorder109. Astrocytes strongly participate in inflammatory reactions in the CNS by activating microglia, recruiting leukocytes from the periphery, modulating the permeability of the BBB and by secreting chemokines110. Thus, astrocytes play a key role in MS pathology as well as other inflammatory processes in the CNS110–112. Oligodendrocytes, which are vulnerable to inflammation induced damage caused by pro-inflammatory mediators and nitric oxide (NO) become dysfunctional and die during these reactive processes. Mouse models suggest that in earlier stages of MS, NG2+ OPCs can likely compensate for the loss of oligodendrocytes and re-myelinate abolished axons113. Unfortunately, NG2+ cells are highly sensitive to inflammation-induced injury and their number is strongly reduced during later stages of MS in mouse models114. Thus, therapeutics aiming to protect this cell population or to modulate the inflammatory action of astrocytes and microglia, could be highly beneficial for patients suffering from MS or other inflammatory insults.
Other CNS pathologies
Glial cells are also equally involved in other CNS pathologies and conditions including psychiatric disorders, addiction and pain transmission4,5,25,115. Although equally important, the discussion of these conditions lies beyond the scope of this review.
3 In vitro systems to model non-cell autonomy
In vitro systems have tremendously enhanced our knowledge of the different cell types of the CNS. They represent an invaluable, affordable and fast tool to test various hypotheses and provide a platform for screening of potential therapeutics.
Although the goal of in vitro systems is to facilitate research of complex aspects by concentrating on isolated interactions, the setup, interpretation and comparison of data generated in individual studies is not always easy. There are many factors that can change the outcome of an experiment and multiple ways to model different aspects of non-cell autonomy in vitro. Often, several types of cultures are used to confirm an interaction. Also, if available, multiple cell sources should be used to strengthen the observation. Similarly, independent of the cell type used, the culture conditions can substantially influence the outcome of an experiment. The isolation process of primary cells, reprogramming methods, medium composition, growth factors, differentiation protocols, coating substances, as well as cell density are known to alter communication and gene expression in many ways116–121.
Thus, if possible, validation in an animal model or in tissue from patients increases the confidence that the observation is related to the disease condition studied. If not possible, the use of larger sample numbers is strongly advised.
In the following section, we will give an overview over the origin of different cell types used in in vitro studies of neurological disorders and point out their advantages and disadvantages (see also table 1). Afterwards, we will describe different culture methods that can be applied.
Table 1. Cell types used for studying non-cell autonomous aspects of neurological disorders.
Neuronal progenitor cells (NPCs), astrocytes, oligodendrocytes, microglia and different neurons can be isolated from rodent or human tissue. Alternatively, they can be generated by direct conversion with cell type specific factors or by differentiation from embryonic stem cells (ES) or induced pluripotent stem cells (iPS) or from NPCs generated by direct conversion. Exemplary references are listed for each procedure.
| Species | Cell origin | Cell types | ||||
|---|---|---|---|---|---|---|
| NPCs | Astrocytes | Oligodendrocytes | Microglia | Neurons | ||
| Human | Primary | 126,127,169,170 | 130,131,171 | 129–131,172 | 128,129,131 | 127,131 |
| ES/iPS | 173–175 | 134,174,176–179 | 175,180,181 | 146 (macrophages) | 132,135,156,174, 182–184 see also other articles in this issue | |
| Direct conversion | Reviewed in 143,144, see also other articles in this issue and 137,140,141, 185 | 141,186 | Not published | 151 induction of microglia phenotype from blood monocytes | Reviewed in 132,142–144 see also other articles in this issue | |
| Mouse or Rat | Primary | 127,187 | 80,121,188–190 | 43,191,192 | 80,190,193,194 | 189,190,195 |
| ES/iPS | 196–199 | 197,198,200 | 133,201 | 147 (macrophages) | 184,202–204 | |
| Direct Conversion | 139,140,144, 185 | 186 | 138,205 | Not published | Reviewed in 142–144, see also other articles in this issue | |
Human primary cells and cell lines
Many researchers have established human cell lines with characteristics of neurons, astrocytes, oligodendrocytes or microglia which have been shared with the research community upon request, while others are commercially availalbe122–124. Often, these were isolated from primary fetal tissues or biopsies from patients and some were immortalized with oncogenes, or they originate from naturally occurring cancerous tissue that was surgically removed. Most of the time, these cells require further differentiation steps with adequate signaling molecules or growth factors prior to their use in experiments. These cells can be useful tools for research as they are generally easy to work with and are well suited for high throughput analysis due to their fast growth. However, it is important to know the origin of the cells used, as fetal cells might not perfectly represent the situation found in the adult CNS. Furthermore, oncogene immortalization might change the characteristics of the cells and produce a heterogeneous population. Using cell lines, there is also a risk of genetic drift towards specific phenotypes, which can lead to discrepant results between laboratories even when using the same culture conditions. If possible, the use of lower passage numbers is advised since the cells will resemble the parental cell line to a higher extent at that point125.
Isolation of primary cells can be performed from patient post-mortem biopsies within a short time period after death. There are published protocols available for astrocyte and microglia isolation from biopsies126–131. Another strategy for the production of astrocytes, oligodendrocytes or neurons is to isolate primary neuronal progenitor cells (NPCs) from biopsies that can then be differentiated in vitro into the cell types of interest126,127.
While allowing the ability to study original patient derived cells, using such biopsies has several disadvantages. The biopsies are of limited availability and need to be of good quality for successful isolation of cells. Specimen quality and recovery, proper storage and shipment conditions need to be closely monitored. In addition, such biopsies are expensive and the isolation process is time consuming requiring a high degree of expertise in tissue culture. In addition, cells recovered from patients who succumbed to the disorder always represent the end stage of the disease. At this time, substantial inflammation, cell death or other secondary effects might have occurred in the affected CNS region that may complicate the interpretation of the results and influence potential therapeutic drugs.
To circumvent some of these drawbacks of postmortem biopsies, fibroblasts from skin biopsies of patients or endothelial cells from urine samples can be recovered and reprogrammed into induced pluripotent stem cells (iPSCs) and then differentiated into various cell types98,132–135. Alternatively, lineage committed cell types such as induced neuronal precursor cells (iNPCs), induced oligodendrocyte precursors (iOPCs) or induced neurons of different subtypes (iN) can be produced with more direct reprogramming methods that do not include the production of classic stem cells132,136–143. A huge variety of neuronal subtypes has been successfully generated via direct reprogramming methods and can be used for studying cell-autonomous as well as non-cell autonomous aspects of various disorders142–144. Since these new promising reprogramming techniques are covered in other sections of this special issue, they will not be discussed in detail here. There are several advantages of direct reprogramming or direct conversion methods over classical reprogramming to iPSCs: i) These methods are usually faster to create the cell type of interest as they bypass at least the first differentiation step. ii) iNPCs are also easier to culture and maintain compared to iPSCs and still offer the option to generate different cell types such as astrocytes, oligodendrocytes and neurons from the same precursor cells. iii) Many of the direct reprogramming protocols do not involve a clonal selection step, which reduces the impact of clonal variation on the experimental outcome.
Extensive literature and various protocols are available for generating astrocytes, oligodendrocytes and various types of neurons including motor neurons, gabaergic neurons, dopaminergic neurons and hippocampal neurons from human iPSCs and iNPCs or via direct conversion (see Table 1 for examples). These techniques have improved the disease modelling of CNS disorders tremendously. Nonetheless, it is important to keep in mind that although these cells resemble primary cells in many ways, they are only model systems and might not exactly mirror the situation found in the adult CNS. The method chosen to generate the cell type of interest can influence the outcome of experiments. For example, during differentiation of iPSCs or iNPCs to neurons, other cell types such as astrocytes are generated in high numbers. As stated previously, these cells influence the survival of neurons in many neurological conditions. Thus, an unrecognized selection towards more resistant neurons might occur during the differentiation process that could lead to misinterpretation of the results. In a study using mouse embryonic stem cells carrying a human mutation in superoxide dismutase 1 causing ALS, Di Giorgio et al noticed reduced production of motor neurons during differentiation145. This could be due to reduced differentiation efficiency or an intrinsic damage of the motor neurons carrying this mutation. Alternatively, as the authors suggested, the astrocytes that are generated as a side product of motor neuron differentiation, could have influenced the survival of newly generated motor neurons as well. In the latter case, the most susceptible motor neurons would likely have died first. Differentiation techniques generating fewer contaminating other cell types during the process are therefore likely to produce cleaner results in consequent experiments.
Since microglia originate from a different lineage, they cannot be derived from NPCs. To date, no protocols for the direct reprogramming of fibroblasts to macrophages are available, but a few protocols for conventional reprogramming have been described146,147. In many neurological disorders, macrophages migrate into the CNS responding to inflammatory molecules. After migration, they rapidly adapt microglial phenotypes and become indistinguishable from resident cells148–150. Thus, peripheral blood monocytes, which are the precursors of macrophages, can be used in vitro as an alternative to microglia. A recent publication showed conversion of blood monocytes into ramified microglia-like cells using a cocktail of different cytokines151.
Overall, the reprogramming field bears substantial promise in modelling neurological disorders as they allow the continuous production and culture of cells of interest from patients and their use in drug screens and mechanistic studies. Unfortunately, the reprogramming process is still not fully understood and the derived cells do not always reproduce disease relevant phenotypes despite the presence of disease causing mutations152–154.
Three additional considerations need to be kept in mind when using reprogramming methodologies for modelling neurological conditions:
The origin of the biopsy, since the environment and epigenetic memory of skin or endothelial cells might not be identical with primary cells from the CNS. Reprogramming does not always remove these epigenetic marks completely, thus a certain memory of the cell’s past identity remains and might influence the outcome of future experiments155.
If the neurological condition was caused by unknown triggers that act in a tissue specific manner such as neurotoxins or environmental factors affecting cells of the CNS only, reprogrammed skin fibroblasts or other cell types might not be suited to reflect this condition. However, to date, many examples exist where CNS specific disorders of unknown origin were recapitulated when the affected cell types were generated from fibroblasts via reprogramming methods98,132,141,156–158. In fact, skin fibroblasts from ALS patients were demonstrated to display altered expression of genes that are involved in neuronal health159. Three potential mechanisms could be responsible for this phenomenon: i) disease related but so far undiscovered genetic causes, ii) epigenetic imprints that happened in various cell types of the patient, but are only detrimental in the CNS iii) metabolic or inflammatory signals that are distributed via the blood stream during the course of the disease and affect gene expression and epigenetic organization in various cell types.
The time point during the disease course at which the biopsy was taken. Until now, only few groups pay attention to the fact that the skin biopsy or urine sample is taken at one distinct time point during a complex and often progressing neurological disorder without underlying known cause. If metabolic, inflammatory or other kind of disease relevant information is exchanged between the CNS and the blood stream, the fibroblasts would reflect the disease stage of a patient at that moment. Whether these cells, once reprogrammed, are able to reflect more progressive later stages of the same disease, is currently unknown.
In summary, independent of the cell types used, researchers should always clearly authenticate the origin and differentiation protocols they use in their experiments to improve interpretation of data. In addition, when using patient-derived cells, information about the disease stage and course of the patient should be collected and provided whenever possible.
Often, human astrocytes or oligodendrocytes are used in combination with rodent neurons for disease modelling126,141,160. Especially for drug screenings where large amounts of neurons are needed, this might be a good alternative. Valuable insights in several neurodegenerative disorders are collected from these cultures as they recapitulate many disease relevant aspects. However, some interactions might be species specific and can therefore get lost under these conditions. In addition, protocols for the isolation of astrocytes, oligodendrocyte precursors and microglia from rodents are readily available (see table 1 for examples). These cells are generally easier to handle than their human counterparts and can be sufficient to prove the effect of a known disease causing mutation. If no mouse model exists, the gene of interest can be expressed via lentiviral transduction of wild type primary rodent glia or neurons to study the impact on their survival or communication. Moreover, transduction of individual cell types with fluorescent proteins or the use of primary neurons from fluorescent mouse strains is a simple way to monitor survival and health of neurons live.
Culture systems used to study non-cell autonomous interactions in neurological disorders
Co-culture paradigms
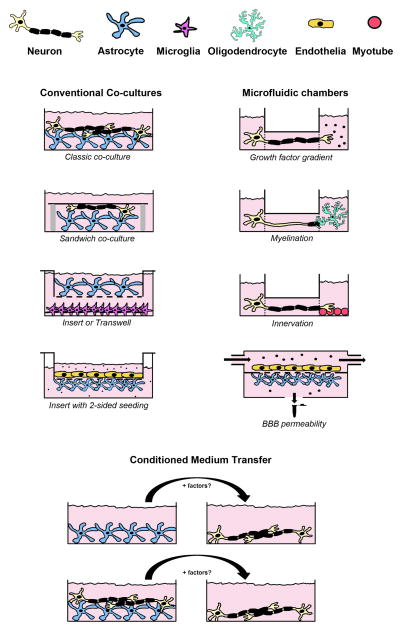
There are multiple ways of setting up co-culture systems in which two or more cell types are cultured in immediate contact with each other (see fig. 1 and table 2). Astrocytes, oligodendrocytes, microglia and neurons attach to cell culture plastic or glass coverslips, although coating with different proteins of the extracellular matrix (such as fibronectin, poly-L-ornithine, laminin, poly-L-lysine) is needed depending on the cell type.
Figure 1. Scheme of different co-culture settings used to model non-cell autonomous aspects of neurological disorders.
Left panel: different cell types can be combined in classic co-cultures with direct contact or in sandwich co-cultures with one cell type on a glass coverslip facing upside down. Alternatively, cells can be seeded in transwells or inserts without direct contact sharing only the secreted factors in the medium. Right panel: Microfluidic chambers can be used to model various aspects of neurological disorders such as axonal growth, myelination, innervation or BBB permeability. Bottom: two systems can be kept in parallel and medium only can be transferred in between with the option of replenishing nutrients or growth factors to avoid starvation. See table 2 for applications and exemplary references for the use of each system.
Table 2. Co-culture paradigms used to model non-cell autonomous aspects of neurological disorders.
Various co-culture systems and media transfer paradigms can be used to model non-cell autonomous aspects of neurological disorders. The utility as well as advantages and disadvantages are listed for each system. Considerations for each system and exemplary references are provided.
| System | Applications | Advantages | Disadvantages | Considerations | Ref. | |
|---|---|---|---|---|---|---|
| Co-cultures | Classic | Assess contact-dependent and contact independent interactions | Easy, affordable, well established | Not possible to discern contact dependent and independent interactions | Density of both cell types needs to be optimized, media composition needs to be permissive for both cell types; media composition, starvation, contact inhibition can alter the outcome of the experiment | 86,141,145,158,160,206,207 |
| Sandwich co-culture | Allows separation of cell types for consequent RNA/protein isolation | More complicated to set-up, increased risk of contamination due to additional equipment. Not possible to discern contact dependent and independent interactions | Glass coverslip and paraffin need to be properly sterilized; glass coating substance can influence gene expression; density of both cell types needs to be optimized, media composition needs to be permissive for both cell types; media composition, starvation, contact inhibition can alter the outcome of the experiment | 162,208 | ||
| Transwell or Insert | Assess contact-independent interactions | Allows separation of cell types for consequent RNA/protein isolation; continuous interaction/secretion of factors | Transwells and Inserts are expensive and usually not re-usable | Density of both cell types needs to be optimized, media composition needs to be permissive for both cell types; media composition, starvation, contact inhibition can alter the outcome of the experiment | 164,206,209 | |
| 2-sided seeding Transwell | Assess permeability of soluble factors through a barrier forming cell type | Easy to set up | Transwells and Inserts are expensive and usually not re-usable | Proper density is crucial for the success of the experiment | 164,209 | |
| Microfluidic chambers | Assess axonal growth or transport, myelination, neuromuscular junctions, permeability of barrier forming cells | Fewer cells needed and reduced reagent consumption, real time analysis possible, automation possible | Complicated setup, less standardized, expensive | Medium turnover, proliferation rate and gas exchange are different in these devices and need to be monitored and optimized | 164–168,209–213 | |
| Multi-cell co-culture | Assess interaction of multiple cell types in combination | Easy to set up, closer to in vivo situation | Not possible to discern contact dependent and independent interactions. Difficult to determine responsible cell type | Density of individual cell types need to be optimized, media composition needs to be permissive for each cell type. Media composition, starvation, contact inhibition can alter the outcome of the experiment | 170,214–216 | |
| Conditioned Media Transfer | From mono-culture | Assess contact-independent effects of soluble factors | Easy to set up, well established straight-forward interpretation of origin of influencing factors. | Does not recapitulate real physiological state, many effectors are only released upon activation or cell-cell contact and will be missed in this setup | Cell density and coating is crucial; media composition can alter the outcome of the experiment; depending on the transfer-protocol and receiving cell-type, supplementation with additional growth factors or serum should be considered | 62,80,86,207 |
| From co-culture | Easy to set up, well established, more physiological | Cell type of interest is needed in larger amounts (2 parallel co-cultures) Interpretation more difficult since different cell types can be responsible for the secretion of molecules | Cell density and coating is crucial; media composition can alter the outcome of the experiment; depending on the transfer-protocol and receiving cell-type, supplementation with additional growth factors or serum should be considered | 126,208 | ||
| Microfluidic chamber | Fewer cells needed and reduced reagent consumption, real time analysis possible, automation possible | complicated setup, expensive | Medium turnover, proliferation rate and gas exchange are different in these devices and need to be monitored and optimized | 164–168,209–213 |
The most commonly used co-culture system is still the classic co-seeding of different cell types. Typically, astrocytes, microglia or oligodendrocytes are seeded first in a monolayer, then the neuronal cell type of interest is added after the first cell type has attached.
In sandwich co-cultures, one cell type (the neuron for example) is plated on a glass coverslip which is then layered face down on a monolayer of a different cell type (astrocyte, oligodendrocyte or microglia). Paraffin dots on the monolayer level ensure the proper placing of the glass coverslip in contact range161. This setup is particularly interesting for RNA sequencing of the individual cell types while maintaining their simultaneous stimulation162,163.
Transwells or inserts allow different cell types to share the same medium while avoiding direct contact. Specialized inserts can be used to model the BBB by plating endothelial cells on one side and pericytes or astrocytes on the other (a detailed review on current methods for modelling the BBB can be found here164).
Microfluidic chambers allow measuring myelination, axonal signaling and transport, BBB modelling, as well as circuit interaction between muscles and motor neurons164–168.
Conditioned medium transfer
In these cultures, the two interacting cell types are kept physically apart from each other and only the medium – or fractions of it - is transferred to the cell type of interest (see fig. 1 and table 2). In such settings, the impact of secreted factors on the health, survival, or gene expression of the other cell type is determined. Although this seems very straight forward, there are some additional thoughts that need to be considered. As mentioned in the introduction, astrocytes, oligodendrocytes and microglia are activated by neuronal activity. As an example, the release of lactate and nerve-growth factor by mouse astrocytes in culture is stimulated by the contact with motor neurons159. Therefore, secretion of certain molecules might only be triggered upon contact and it might be worthwhile to use conditioned medium from a co-culture instead of a mono-culture to test on the cell type of interest. The frequency of transfer is another important aspect, since some molecules might only show an effect when applied several times. Furthermore, the metabolic needs of astrocytes, oligodendrocytes, neurons and microglia are different and therefore when transferring medium from one cell type to the other, growth factors or nutrients might have to be replenished, since the medium could have been depleted by the donating culture.
Conclusions
Non-cell autonomous interactions play a crucial role in virtually all pathologic conditions of the CNS. This aspect that has been neglected for a long time could in part be responsible for the evident lack of effective treatments for many of these diseases. In order to improve the development of new therapeutics, the complex interactions between neurons, astrocytes, oligodendrocytes and microglia have to be taken into account. Co-culture systems are a valuable tool for studying such interactions and can be used for high-throughput drug and effector screens. Although these cultures represent a simplified view of the CNS, they are more complex than usually thought. Many variables can influence the outcome of such experiments and need to be considered and carefully monitored. New reprogramming technologies provide us with exciting new and fast protocols to generate various cell types of the CNS and will have a tremendous impact on disease modelling especially in conditions without known cause. Clearly, the use of these in vitro systems is powerful to improve our understanding of the various glial interactions in the CNS that lead to neurodegeneration and they open the possibility to advance future drug development.
Highlights.
Glial cells and their impact on neurological diseases have become evident.
Reliable in vitro systems to studying these disorders are urgently needed.
In this review, we will briefly summarize the function of different glial cell types.
Glial cells implication in the progression of neurological conditions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kettenmann H, Verkhratsky A. Neuroglia: the 150 years after. Trends Neurosci. 2008;31:653–9. doi: 10.1016/j.tins.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Hill RA, Nishiyama A. NG2 cells (polydendrocytes): listeners to the neural network with diverse properties. Glia. 2014;62:1195–210. doi: 10.1002/glia.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- 4.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji RR, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain. 2013;154(Suppl):S10–28. doi: 10.1016/j.pain.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brambilla L, Martorana F, Rossi D. Astrocyte signaling and neurodegeneration. Prion. 2014;7:28–36. doi: 10.4161/pri.22512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Zhou J. Oligodendrocytes in neurodegenerative diseases. Front Biol (Beijing) 2013;8:127–133. [Google Scholar]
- 8.Verkhratsky A, Parpura V, Pekna M, Pekny M, Sofroniew M. Glia in the pathogenesis of neurodegenerative diseases. Biochem Soc Trans. 2014;42:1291–301. doi: 10.1042/BST20140107. [DOI] [PubMed] [Google Scholar]
- 9.Bossy-Wetzel E, Schwarzenbacher R, Lipton SA. Molecular pathways to neurodegeneration. 2004 doi: 10.1038/nm1067. [DOI] [PubMed] [Google Scholar]
- 10.Lobsiger CS, Cleveland DW. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat Neurosci. 2007;10:1355–60. doi: 10.1038/nn1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heneka MT, Rodríguez JJ, Verkhratsky A. Neuroglia in neurodegeneration. Brain Res Rev. 2010;63:189–211. doi: 10.1016/j.brainresrev.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Verkhratsky A, et al. Neurological diseases as primary gliopathies: a reassessment of neurocentrism. ASN Neuro. 2012;4 doi: 10.1042/AN20120010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banaclocha MAM. Brain Research Bulletin. 2007:21–27. doi: 10.1016/j.brainresbull.2007.01.012. banaclocha2007.pdf. at < http://lib.gen.in/ocean/645f3da1ff78368e7b6b2dd3b417c715/banaclocha2007.pdf>. [DOI] [PubMed]
- 14.Sherwood CC, et al. Evolution of increased glia-neuron ratios in the human frontal cortex. Proc Natl Acad Sci U S A. 2006;103:13606–11. doi: 10.1073/pnas.0605843103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallraff A, Odermatt B, Willecke K, Steinhäuser C. Distinct types of astroglial cells in the hippocampus differ in gap junction coupling. Glia. 2004;48:36–43. doi: 10.1002/glia.20040. [DOI] [PubMed] [Google Scholar]
- 16.Grass D, et al. Diversity of functional astroglial properties in the respiratory network. J Neurosci. 2004;24:1358–65. doi: 10.1523/JNEUROSCI.4022-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthias K, et al. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci. 2003;23:1750–8. doi: 10.1523/JNEUROSCI.23-05-01750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou M, Kimelberg HK. Freshly isolated hippocampal CA1 astrocytes comprise two populations differing in glutamate transporter and AMPA receptor expression. J Neurosci. 2001;21:7901–8. doi: 10.1523/JNEUROSCI.21-20-07901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bushong Ea, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Barres BA. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr Opin Neurobiol. 2010;20:588–94. doi: 10.1016/j.conb.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Oberheim NA, Goldman SA, Nedergaard M. Heterogeneity of astrocytic form and function. Methods Mol Biol. 2012;814:23–45. doi: 10.1007/978-1-61779-452-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirrlinger J, Hülsmann S, Kirchhoff F. Astroglial processes show spontaneous motility at active synaptic terminals in situ. Eur J Neurosci. 2004;20:2235–9. doi: 10.1111/j.1460-9568.2004.03689.x. [DOI] [PubMed] [Google Scholar]
- 23.Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–40. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- 24.Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol. 2010;72:335–55. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci. 2014;15:300–12. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- 26.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 27.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–8. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 28.Harry GJ. Microglia during development and aging. Pharmacol Ther. 2013;139:313–26. doi: 10.1016/j.pharmthera.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biber K, Neumann H, Inoue K, Boddeke HWGM. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci. 2007;30:596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30:527–35. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Biber K, Vinet J, Boddeke HWGM. Neuron-microglia signaling: Chemokines as versatile messengers. J Neuroimmunol. 2008;198:69–74. doi: 10.1016/j.jneuroim.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 32.Sieger D, Moritz C, Ziegenhals T, Prykhozhij S, Peri F. Long-Range Ca2+ Waves Transmit Brain-Damage Signals to Microglia. Dev Cell. 2012;22:1138–1148. doi: 10.1016/j.devcel.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Schafer DP, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tremblay M-È, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhan Y, et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci. 2014;17:400–6. doi: 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]
- 36.Takebayashi H, et al. The basic helix-loop-helix factor olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr Biol. 2002;12:1157–63. doi: 10.1016/s0960-9822(02)00926-0. [DOI] [PubMed] [Google Scholar]
- 37.Lu QR, et al. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- 38.Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–70. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young KM, et al. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron. 2013;77:873–85. doi: 10.1016/j.neuron.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci. 2004;16:1227–33. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- 41.Mabbott DJ, Noseworthy M, Bouffet E, Laughlin S, Rockel C. White matter growth as a mechanism of cognitive development in children. Neuroimage. 2006;33:936–46. doi: 10.1016/j.neuroimage.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 42.Snaidero N, Simons M. Myelination at a glance. J Cell Sci. 2014;127:2999–3004. doi: 10.1242/jcs.151043. [DOI] [PubMed] [Google Scholar]
- 43.Barateiro A, Fernandes A. Temporal oligodendrocyte lineage progression: in vitro models of proliferation, differentiation and myelination. Biochim Biophys Acta. 2014;1843:1917–29. doi: 10.1016/j.bbamcr.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 44.McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. J Neurochem. 2008;107:1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [DOI] [PubMed] [Google Scholar]
- 45.Nave KA, Werner HB. Myelination of the Nervous System: Mechanisms and Functions. Annu Rev Cell Dev Biol. 2014;30:503–533. doi: 10.1146/annurev-cellbio-100913-013101. [DOI] [PubMed] [Google Scholar]
- 46.Bradl M, Lassmann H. Oligodendrocytes: biology and pathology. Acta Neuropathol. 2010;119:37–53. doi: 10.1007/s00401-009-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–40. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 48.Watkins TA, Emery B, Mulinyawe S, Barres BA. Distinct stages of myelination regulated by gamma-secretase and astrocytes in a rapidly myelinating CNS coculture system. Neuron. 2008;60:555–69. doi: 10.1016/j.neuron.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bauer J, et al. Endoplasmic reticulum stress in PLP-overexpressing transgenic rats: gray matter oligodendrocytes are more vulnerable than white matter oligodendrocytes. J Neuropathol Exp Neurol. 2002;61:12–22. doi: 10.1093/jnen/61.1.12. [DOI] [PubMed] [Google Scholar]
- 50.Yeung MSY, et al. Dynamics of Oligodendrocyte Generation and Myelination in the Human Brain. Cell. 2014;159:766–774. doi: 10.1016/j.cell.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 51.Zhu X, Hill RA, Nishiyama A. NG2 cells generate oligodendrocytes and gray matter astrocytes in the spinal cord. Neuron Glia Biol. 2008;4:19. doi: 10.1017/S1740925X09000015. [DOI] [PubMed] [Google Scholar]
- 52.Orthmann-Murphy JL, Abrams CK, Scherer SS. Gap junctions couple astrocytes and oligodendrocytes. J Mol Neurosci. 2008;35:101–16. doi: 10.1007/s12031-007-9027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peferoen L, Kipp M, van der Valk P, van Noort JM, Amor S. Oligodendrocyte-microglia cross-talk in the central nervous system. Immunology. 2014;141:302–13. doi: 10.1111/imm.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trotter J. NG2-positive cells in CNS function and the pathological role of antibodies against NG2 in demyelinating diseases. J Neurol Sci. 2005;233:37–42. doi: 10.1016/j.jns.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 55.Tomassy GS, Fossati V. How big is the myelinating orchestra? Cellular diversity within the oligodendrocyte lineage: facts and hypotheses. Front Cell Neurosci. 2014;8:201. doi: 10.3389/fncel.2014.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–91. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- 57.Xu JP, Zhao J, Li S. Roles of NG2 glial cells in diseases of the central nervous system. Neurosci Bull. 2011;27:413–21. doi: 10.1007/s12264-011-1838-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang S, et al. Synapsin I is an oligomannose-carrying glycoprotein, acts as an oligomannose-binding lectin, and promotes neurite outgrowth and neuronal survival when released via glia-derived exosomes. J Neurosci. 2011;31:7275–90. doi: 10.1523/JNEUROSCI.6476-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frühbeis C, Fröhlich D, Krämer-Albers EM. Emerging roles of exosomes in neuron-glia communication. Front Physiol. 2012;3:119. doi: 10.3389/fphys.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fitzner D, et al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci. 2011;124:447–58. doi: 10.1242/jcs.074088. [DOI] [PubMed] [Google Scholar]
- 61.Guescini M, Genedani S, Stocchi V, Agnati LF. Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. J Neural Transm. 2010;117:1–4. doi: 10.1007/s00702-009-0288-8. [DOI] [PubMed] [Google Scholar]
- 62.Wang G, et al. Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4): potential mechanism of apoptosis induction in Alzheimer disease (AD) J Biol Chem. 2012;287:21384–95. doi: 10.1074/jbc.M112.340513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krämer-Albers EM, et al. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: Trophic support for axons? Proteomics Clin Appl. 2007;1:1446–61. doi: 10.1002/prca.200700522. [DOI] [PubMed] [Google Scholar]
- 64.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–83. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown CE, Li P, Boyd JD, Delaney KR, Murphy TH. Extensive turnover of dendritic spines and vascular remodeling in cortical tissues recovering from stroke. J Neurosci. 2007;27:4101–9. doi: 10.1523/JNEUROSCI.4295-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown AM, Ransom BR. Astrocyte glycogen as an emergency fuel under conditions of glucose deprivation or intense neural activity. Metab Brain Dis. 2015;30:233–9. doi: 10.1007/s11011-014-9588-2. [DOI] [PubMed] [Google Scholar]
- 67.Chen Y, Swanson RA. Astrocytes and brain injury. J Cereb Blood Flow Metab. 2003;23:137–49. doi: 10.1097/01.WCB.0000044631.80210.3C. [DOI] [PubMed] [Google Scholar]
- 68.Giffard RG, Swanson RA. Ischemia-induced programmed cell death in astrocytes. Glia. 2005;50:299–306. doi: 10.1002/glia.20167. [DOI] [PubMed] [Google Scholar]
- 69.Chesler M. Failure and function of intracellular pH regulation in acute hypoxic-ischemic injury of astrocytes. Glia. 2005;50:398–406. doi: 10.1002/glia.20141. [DOI] [PubMed] [Google Scholar]
- 70.Barreto G, White RE, Ouyang Y, Xu L, Giffard RG. Astrocytes: targets for neuroprotection in stroke. Cent Nerv Syst Agents Med Chem. 2011;11:164–73. doi: 10.2174/187152411796011303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dewar D, Underhill SM, Goldberg MP. Oligodendrocytes and Ischemic. Brain Injury. 2003;23:263–274. doi: 10.1097/01.WCB.0000053472.41007.F9. [DOI] [PubMed] [Google Scholar]
- 72.Jelkmann W, Wagner K. Beneficial and ominous aspects of the pleiotropic action of erythropoietin. Ann Hematol. 2004;83:673–86. doi: 10.1007/s00277-004-0911-6. [DOI] [PubMed] [Google Scholar]
- 73.Cunningham LA, Wetzel M, Rosenberg GA. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia. 2005;50:329–39. doi: 10.1002/glia.20169. [DOI] [PubMed] [Google Scholar]
- 74.Zhao BQ, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–5. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 75.Taylor RA, Sansing LH. Microglial responses after ischemic stroke and intracerebral hemorrhage. Clin Dev Immunol. 2013;2013:746068. doi: 10.1155/2013/746068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lalancette-Hébert M, et al. Galectin-3 is required for resident microglia activation and proliferation in response to ischemic injury. J Neurosci. 2012;32:10383–95. doi: 10.1523/JNEUROSCI.1498-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Messing A, Brenner M, Feany MB, Nedergaard M, Goldman JE. Alexander disease. J Neurosci. 2012;32:5017–23. doi: 10.1523/JNEUROSCI.5384-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang G, et al. Autophagy induced by Alexander disease-mutant GFAP accumulation is regulated by p38/MAPK and mTOR signaling pathways. Hum Mol Genet. 2008;17:1540–55. doi: 10.1093/hmg/ddn042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tang G, Xu Z, Goldman JE. Synergistic effects of the SAPK/JNK and the proteasome pathway on glial fibrillary acidic protein (GFAP) accumulation in Alexander disease. J Biol Chem. 2006;281:38634–43. doi: 10.1074/jbc.M604942200. [DOI] [PubMed] [Google Scholar]
- 80.Maezawa I, Jin LW. Rett syndrome microglia damage dendrites and synapses by the elevated release of glutamate. J Neurosci. 2010;30:5346–56. doi: 10.1523/JNEUROSCI.5966-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bienvenu T, Chelly J. Molecular genetics of Rett syndrome: when DNA methylation goes unrecognized. Nat Rev Genet. 2006;7:415–426. doi: 10.1038/nrg1878. [DOI] [PubMed] [Google Scholar]
- 82.Kishi N, Macklis JD. MECP2 is progressively expressed in post-migratory neurons and is involved in neuronal maturation rather than cell fate decisions. Mol Cell Neurosci. 2004;27:306–21. doi: 10.1016/j.mcn.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 83.Armstrong DD. Neuropathology of Rett syndrome. J Child Neurol. 2005;20:747–53. doi: 10.1177/08830738050200090901. [DOI] [PubMed] [Google Scholar]
- 84.Belichenko PV, et al. Widespread changes in dendritic and axonal morphology in Mecp2-mutant mouse models of Rett syndrome: evidence for disruption of neuronal networks. J Comp Neurol. 2009;514:240–58. doi: 10.1002/cne.22009. [DOI] [PubMed] [Google Scholar]
- 85.Moretti P. Learning and Memory and Synaptic Plasticity Are Impaired in a Mouse Model of Rett Syndrome. J Neurosci. 2006;26:319–327. doi: 10.1523/JNEUROSCI.2623-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ballas N, Lioy DT, Grunseich C, Mandel G. Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nat Neurosci. 2009;12:311–7. doi: 10.1038/nn.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Derecki NC, et al. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature. 2012;484:105–9. doi: 10.1038/nature10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vezzani A, Aronica E, Mazarati A, Pittman QJ. Epilepsy and brain inflammation. Exp Neurol. 2013;244:11–21. doi: 10.1016/j.expneurol.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 89.Seifert G, Hüttmann K, Schramm J, Steinhäuser C. Enhanced relative expression of glutamate receptor 1 flip AMPA receptor subunits in hippocampal astrocytes of epilepsy patients with Ammon’s horn sclerosis. J Neurosci. 2004;24:1996–2003. doi: 10.1523/JNEUROSCI.3904-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.De Lanerolle NC, Lee TS, Spencer DD. Astrocytes and epilepsy. Neurotherapeutics. 2010;7:424–38. doi: 10.1016/j.nurt.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Oberheim NA, et al. Loss of astrocytic domain organization in the epileptic brain. J Neurosci. 2008;28:3264–76. doi: 10.1523/JNEUROSCI.4980-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carvalhal Marques F, Volovik Y, Cohen E. The roles of cellular and organismal aging in the development of late-onset maladies. Annu Rev Pathol. 2015;10:1–23. doi: 10.1146/annurev-pathol-012414-040508. [DOI] [PubMed] [Google Scholar]
- 93.Saxena S, Caroni P. Selective neuronal vulnerability in neurodegenerative diseases: from stressor thresholds to degeneration. Neuron. 2011;71:35–48. doi: 10.1016/j.neuron.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 94.Giaume C, Kirchhoff F, Matute C, Reichenbach A, Verkhratsky A. Glia: the fulcrum of brain diseases. Cell Death Differ. 2007;14:1324–35. doi: 10.1038/sj.cdd.4402144. [DOI] [PubMed] [Google Scholar]
- 95.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–34. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 96.Brück D, Wenning GK, Stefanova N, Fellner L. Glia and alpha-synuclein in neurodegeneration: A complex interaction. Neurobiol Dis. 2015 doi: 10.1016/j.nbd.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schlachetzki JCM, Saliba SW, de Oliveira ACP. Studying neurodegenerative diseases in culture models. Rev Bras Psiquiatr. 2013;35(Suppl 2):S92–100. doi: 10.1590/1516-4446-2013-1159. [DOI] [PubMed] [Google Scholar]
- 98.Juopperi TA, et al. Astrocytes generated from patient induced pluripotent stem cells recapitulate features of Huntington’s disease patient cells. Mol Brain. 2012;5:17. doi: 10.1186/1756-6606-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009;187:761–72. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kang SH, et al. Degeneration and impaired regeneration of gray matter oligodendrocytes in amyotrophic lateral sclerosis. Nat Neurosci. 2013;16:571–9. doi: 10.1038/nn.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Philips T, et al. Oligodendrocyte dysfunction in the pathogenesis of amyotrophic lateral sclerosis. Brain. 2013;136:471–482. doi: 10.1093/brain/aws339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nagai M, et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–22. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Boillee S, et al. Onset and Progression in Inherited ALS Determined by Motor Neurons and Microglia. Science (80- ) 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- 104.Yamanaka K, et al. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11:251–3. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Frakes AE, et al. Microglia induce motor neuron death via the classical NF-κB pathway in amyotrophic lateral sclerosis. Neuron. 2014;81:1009–23. doi: 10.1016/j.neuron.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Foust KD, et al. Therapeutic AAV9-mediated Suppression of Mutant SOD1 Slows Disease Progression and Extends Survival in Models of Inherited ALS. Mol Ther. 2013;21:2148–59. doi: 10.1038/mt.2013.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mallucci G, Peruzzotti-Jametti L, Bernstock JD, Pluchino S. The role of immune cells, glia and neurons in white and grey matter pathology in multiple sclerosis. Prog Neurobiol. 2015 doi: 10.1016/j.pneurobio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Duffy SS, Lees JG, Moalem-Taylor G. The contribution of immune and glial cell types in experimental autoimmune encephalomyelitis and multiple sclerosis. Mult Scler Int. 2014;2014:285245. doi: 10.1155/2014/285245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ponomarev ED, Shriver LP, Maresz K, Dittel BN. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J Neurosci Res. 2005;81:374–89. doi: 10.1002/jnr.20488. [DOI] [PubMed] [Google Scholar]
- 110.Claycomb KI, Johnson KM, Winokur PN, Sacino AV, Crocker SJ. Astrocyte regulation of CNS inflammation and remyelination. Brain Sci. 2013;3:1109–27. doi: 10.3390/brainsci3031109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brambilla R, et al. Astrocytes play a key role in EAE pathophysiology by orchestrating in the CNS the inflammatory response of resident and peripheral immune cells and by suppressing remyelination. Glia. 2014;62:452–67. doi: 10.1002/glia.22616. [DOI] [PubMed] [Google Scholar]
- 112.Moreno M, et al. Origins and significance of astrogliosis in the multiple sclerosis model, MOG peptide EAE. J Neurol Sci. 2013;333:55–9. doi: 10.1016/j.jns.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Girolamo F, et al. Cerebral cortex demyelination and oligodendrocyte precursor response to experimental autoimmune encephalomyelitis. Neurobiol Dis. 2011;43:678–89. doi: 10.1016/j.nbd.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 114.Cui QL, et al. Oligodendrocyte progenitor cell susceptibility to injury in multiple sclerosis. Am J Pathol. 2013;183:516–25. doi: 10.1016/j.ajpath.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 115.Somkuwar SS, Staples MC, Galinato MH, Fannon MJ, Mandyam CD. Role of NG2 expressing cells in addiction: a new approach for an old problem. Front Pharmacol. 2014;5:279. doi: 10.3389/fphar.2014.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lyck R, et al. Culture-induced changes in blood-brain barrier transcriptome: implications for amino-acid transporters in vivo. J Cereb Blood Flow Metab. 2009;29:1491–502. doi: 10.1038/jcbfm.2009.72. [DOI] [PubMed] [Google Scholar]
- 117.Halliwell B. Cell culture, oxidative stress, and antioxidants: avoiding pitfalls. Biomed J. 2014;37:99–105. doi: 10.4103/2319-4170.128725. [DOI] [PubMed] [Google Scholar]
- 118.Baqir S, Smith LC. Growth restricted in vitro culture conditions alter the imprinted gene expression patterns of mouse embryonic stem cells. Cloning Stem Cells. 2003;5:199–212. doi: 10.1089/153623003769645866. [DOI] [PubMed] [Google Scholar]
- 119.Portela VM, Zamberlam G, Price CA. Cell plating density alters the ratio of estrogenic to progestagenic enzyme gene expression in cultured granulosa cells. Fertil Steril. 2010;93:2050–2055. doi: 10.1016/j.fertnstert.2009.01.151. [DOI] [PubMed] [Google Scholar]
- 120.Thwaites JW, RLMCDPHNJNSN, WI Influence of Initial Seeding Density on Gene Expression during Neuronal Priming. J Bioprocess Biotech. at < http://www.omicsonline.org/open-access/influence-of-initial-seeding-density-on-gene-expression-during-neuronal-priming-2155-9821.1000195.php&&aid=36538>.
- 121.Foo LC, et al. Development of a method for the purification and culture of rodent astrocytes. Neuron. 2011;71:799–811. doi: 10.1016/j.neuron.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Buntinx M, et al. Characterization of three human oligodendroglial cell lines as a model to study oligodendrocyte injury: morphology and oligodendrocyte-specific gene expression. J Neurocytol. 2003;32:25–38. doi: 10.1023/a:1027324230923. [DOI] [PubMed] [Google Scholar]
- 123.Bello-Morales R, et al. Interaction of PLP with GFP-MAL2 in the human oligodendroglial cell line HOG. PLoS One. 2011;6:e19388. doi: 10.1371/journal.pone.0019388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nagai A, et al. Generation and characterization of immortalized human microglial cell lines: expression of cytokines and chemokines. Neurobiol Dis. 2001;8:1057–68. doi: 10.1006/nbdi.2001.0437. [DOI] [PubMed] [Google Scholar]
- 125.Marx V. Cell-line authentication demystified. Nat Methods. 2014;11:483–488. doi: 10.1038/nmeth.2932. [DOI] [PubMed] [Google Scholar]
- 126.Haidet-Phillips AM, et al. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat Biotechnol. 2011;29:824–8. doi: 10.1038/nbt.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Darbinyan A, Kaminski R, White MK, Darbinian N, Khalili K. Isolation and propagation of primary human and rodent embryonic neural progenitor cells and cortical neurons. Methods Mol Biol. 2013;1078:45–54. doi: 10.1007/978-1-62703-640-5_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Durafourt BA, Moore CS, Blain M, Antel JP. Isolating, culturing, and polarizing primary human adult and fetal microglia. Methods Mol Biol. 2013;1041:199–211. doi: 10.1007/978-1-62703-520-0_19. [DOI] [PubMed] [Google Scholar]
- 129.De Groot CJ, et al. Isolation and characterization of adult microglial cells and oligodendrocytes derived from postmortem human brain tissue. Brain Res Protoc. 2000;5:85–94. doi: 10.1016/s1385-299x(99)00059-8. [DOI] [PubMed] [Google Scholar]
- 130.Whittemore SR, Sanon HR, Wood PM. Concurrent isolation and characterization of oligodendrocytes, microglia and astrocytes from adult human spinal cord. Int J Dev Neurosci. 1993;11:755–64. doi: 10.1016/0736-5748(93)90064-k. [DOI] [PubMed] [Google Scholar]
- 131.Jana M, Jana A, Pal U, Pahan K. A Simplified Method for Isolating Highly Purified Neurons, Oligodendrocytes, Astrocytes, and Microglia from the Same Human Fetal Brain Tissue. Neurochem Res. 2007;32:2015–2022. doi: 10.1007/s11064-007-9340-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Qiang L, Fujita R, Abeliovich A. Remodeling neurodegeneration: somatic cell reprogramming-based models of adult neurological disorders. Neuron. 2013;78:957–69. doi: 10.1016/j.neuron.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 133.Czepiel M, et al. Differentiation of induced pluripotent stem cells into functional oligodendrocytes. Glia. 2011;59:882–92. doi: 10.1002/glia.21159. [DOI] [PubMed] [Google Scholar]
- 134.Emdad L, D’Souza SL, Kothari HP, Qadeer ZA, Germano IM. Efficient differentiation of human embryonic and induced pluripotent stem cells into functional astrocytes. Stem Cells Dev. 2012;21:404–10. doi: 10.1089/scd.2010.0560. [DOI] [PubMed] [Google Scholar]
- 135.Hester ME, et al. Rapid and efficient generation of functional motor neurons from human pluripotent stem cells using gene delivered transcription factor codes. Mol Ther. 2011;19:1905–12. doi: 10.1038/mt.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kim J, et al. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc Natl Acad Sci U S A. 2011;108:7838–43. doi: 10.1073/pnas.1103113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang L, et al. Generation of integration-free neural progenitor cells from cells in human urine. Nat Methods. 2013;10:84–9. doi: 10.1038/nmeth.2283. [DOI] [PubMed] [Google Scholar]
- 138.Yang N, et al. Generation of oligodendroglial cells by direct lineage conversion. Nat Biotechnol. 2013;31:434–9. doi: 10.1038/nbt.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lujan E, Chanda S, Ahlenius H, Südhof TC, Wernig M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc Natl Acad Sci U S A. 2012;109:2527–32. doi: 10.1073/pnas.1121003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cheng L, et al. Generation of neural progenitor cells by chemical cocktails and hypoxia. Cell Res. 2014;24:665–79. doi: 10.1038/cr.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Meyer K, et al. Direct conversion of patient fibroblasts demonstrates non-cell autonomous toxicity of astrocytes to motor neurons in familial and sporadic ALS. Proc Natl Acad Sci U S A. 2014;111:829–32. doi: 10.1073/pnas.1314085111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Faiz M, Nagy A. Induced Pluripotent Stem Cells and Disorders of the Nervous System: Progress, Problems, and Prospects. Neuroscientist. 2013 doi: 10.1177/1073858413493148. [DOI] [PubMed] [Google Scholar]
- 143.Vierbuchen T, Wernig M. Molecular roadblocks for cellular reprogramming. Mol Cell. 2012;47:827–38. doi: 10.1016/j.molcel.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kim J, Ambasudhan R, Ding S. Direct lineage reprogramming to neural cells. Curr Opin Neurobiol. 2012;22:778–84. doi: 10.1016/j.conb.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10:608–14. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Senju S, et al. Generation of dendritic cells and macrophages from human induced pluripotent stem cells aiming at cell therapy. Gene Ther. 2011;18:874–83. doi: 10.1038/gt.2011.22. [DOI] [PubMed] [Google Scholar]
- 147.Senju S, et al. Characterization of dendritic cells and macrophages generated by directed differentiation from mouse induced pluripotent stem cells. Stem Cells. 2009;27:1021–31. doi: 10.1002/stem.33. [DOI] [PubMed] [Google Scholar]
- 148.David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12:388–99. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- 149.Ransohoff RM. Microglia and monocytes: ’tis plain the twain meet in the brain. Nat Neurosci. 2011;14:1098–100. doi: 10.1038/nn.2917. [DOI] [PubMed] [Google Scholar]
- 150.Saederup N, et al. Selective Chemokine Receptor Usage by Central Nervous System Myeloid Cells in CCR2-Red Fluorescent Protein Knock-In Mice. PLoS One. 2010;5:e13693. doi: 10.1371/journal.pone.0013693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ohgidani M, et al. Direct induction of ramified microglia-like cells from human monocytes: dynamic microglial dysfunction in Nasu-Hakola disease. Sci Rep. 2014;4:4957. doi: 10.1038/srep04957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012;481:295–305. doi: 10.1038/nature10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Papp B, Plath K. Reprogramming to pluripotency: stepwise resetting of the epigenetic landscape. Cell Res. 2011;21:486–501. doi: 10.1038/cr.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xu X, Zhong Z. Disease modeling and drug screening for neurological diseases using human induced pluripotent stem cells. Acta Pharmacol Sin. 2013;34:755–64. doi: 10.1038/aps.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liang G, Zhang Y. Embryonic stem cell and induced pluripotent stem cell: an epigenetic perspective. Cell Res. 2013;23:49–69. doi: 10.1038/cr.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cooper O, et al. Differentiation of human ES and Parkinson’s disease iPS cells into ventral midbrain dopaminergic neurons requires a high activity form of SHH, FGF8a and specific regionalization by retinoic acid. Mol Cell Neurosci. 2010;45:258–66. doi: 10.1016/j.mcn.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Marchetto MC, Brennand KJ, Boyer LF, Gage FH. Induced pluripotent stem cells (iPSCs) and neurological disease modeling: progress and promises. Hum Mol Genet. 2011;20:R109–R115. doi: 10.1093/hmg/ddr336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yoshida M, et al. Modeling the Early Phenotype at the Neuromuscular Junction of Spinal Muscular Atrophy Using Patient-Derived iPSCs. Stem cell reports. 2015 doi: 10.1016/j.stemcr.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fang L, et al. MMP-2 and MMP-9 are elevated in spinal cord and skin in a mouse model of ALS. J Neurol Sci. 2010;294:51–6. doi: 10.1016/j.jns.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 160.Re DB, et al. Necroptosis drives motor neuron death in models of both sporadic and familial ALS. Neuron. 2014;81:1001–8. doi: 10.1016/j.neuron.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jones EV, Cook D, Murai KK. A neuron-astrocyte co-culture system to investigate astrocyte-secreted factors in mouse neuronal development. Methods Mol Biol. 2012;814:341–52. doi: 10.1007/978-1-61779-452-0_22. [DOI] [PubMed] [Google Scholar]
- 162.Phatnani HP, et al. Intricate interplay between astrocytes and motor neurons in ALS. Proc Natl Acad Sci U S A. 2013;110:E756–65. doi: 10.1073/pnas.1222361110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ferraiuolo L, et al. Dysregulation of astrocyte-motoneuron cross-talk in mutant superoxide dismutase 1-related amyotrophic lateral sclerosis. Brain. 2011;134:2627–41. doi: 10.1093/brain/awr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wolff A, Antfolk M, Brodin B, Tenje M. In Vitro Blood-Brain Barrier Models-An Overview of Established Models and New Microfluidic Approaches. J Pharm Sci. 2015 doi: 10.1002/jps.24329. [DOI] [PubMed] [Google Scholar]
- 165.Southam KA, King AE, Blizzard CA, McCormack GH, Dickson TC. Microfluidic primary culture model of the lower motor neuron-neuromuscular junction circuit. J Neurosci Methods. 2013;218:164–9. doi: 10.1016/j.jneumeth.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 166.Liu WW, Goodhouse J, Jeon NL, Enquist LW. A microfluidic chamber for analysis of neuron-to-cell spread and axonal transport of an alpha-herpesvirus. PLoS One. 2008;3:e2382. doi: 10.1371/journal.pone.0002382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mehling M, Tay S. Microfluidic cell culture. Curr Opin Biotechnol. 2014;25:95–102. doi: 10.1016/j.copbio.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 168.Halldorsson S, Lucumi E, Gómez-Sjöberg R, Fleming RMT. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens Bioelectron. 2015;63:218–31. doi: 10.1016/j.bios.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 169.Palmer TD, et al. Cell culture. Progenitor cells from human brain after death. Nature. 2001;411:42–3. doi: 10.1038/35075141. [DOI] [PubMed] [Google Scholar]
- 170.Ray B, Chopra N, Long JM, Lahiri DK. Human primary mixed brain cultures: preparation, differentiation, characterization and application to neuroscience research. Mol Brain. 2014;7:63. doi: 10.1186/s13041-014-0063-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sharif A, Prevot V. Isolation and culture of human astrocytes. Methods Mol Biol. 2012;814:137–51. doi: 10.1007/978-1-61779-452-0_11. [DOI] [PubMed] [Google Scholar]
- 172.Zhang X, et al. Induction of oligodendrocytes from adult human olfactory epithelial-derived progenitors by transcription factors. Stem Cells. 2005;23:442–53. doi: 10.1634/stemcells.2004-0274. [DOI] [PubMed] [Google Scholar]
- 173.Nistor G, et al. Derivation of high purity neuronal progenitors from human embryonic stem cells. PLoS One. 2011;6:e20692. doi: 10.1371/journal.pone.0020692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Shi Y, Kirwan P, Smith J, Robinson HPC, Livesey FJ. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci. 2012;15:477–86. S1. doi: 10.1038/nn.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sundberg M, Skottman H, Suuronen R, Narkilahti S. Production and isolation of NG2+ oligodendrocyte precursors from human embryonic stem cells in defined serum-free medium. Stem Cell Res. 2010;5:91–103. doi: 10.1016/j.scr.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 176.Krencik R, Weick JP, Liu Y, Zhang ZJ, Zhang SC. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat Biotechnol. 2011;29:528–34. doi: 10.1038/nbt.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Krencik R, Zhang SC. Directed differentiation of functional astroglial subtypes from human pluripotent stem cells. Nat Protoc. 2011;6:1710–7. doi: 10.1038/nprot.2011.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Juopperi TA, et al. Astrocytes generated from patient induced pluripotent stem cells recapitulate features of Huntington’s disease patient cells. Mol Brain. 2012;5:17. doi: 10.1186/1756-6606-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Roybon L, et al. Human stem cell-derived spinal cord astrocytes with defined mature or reactive phenotypes. Cell Rep. 2013;4:1035–48. doi: 10.1016/j.celrep.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang S, et al. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell. 2013;12:252–64. doi: 10.1016/j.stem.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Douvaras P, et al. Efficient generation of myelinating oligodendrocytes from primary progressive multiple sclerosis patients by induced pluripotent stem cells. Stem cell reports. 2014;3:250–9. doi: 10.1016/j.stemcr.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Karumbayaram S, et al. Directed differentiation of human-induced pluripotent stem cells generates active motor neurons. Stem Cells. 2009;27:806–11. doi: 10.1002/stem.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wang S, et al. Differentiation of human induced pluripotent stem cells to mature functional Purkinje neurons. Sci Rep. 2015;5:9232. doi: 10.1038/srep09232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.López-González R, Velasco I. Therapeutic potential of motor neurons differentiated from embryonic stem cells and induced pluripotent stem cells. Arch Med Res. 2012;43:1–10. doi: 10.1016/j.arcmed.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 185.Ring KL, et al. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell. 2012;11:100–9. doi: 10.1016/j.stem.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Caiazzo M, et al. Direct Conversion of Fibroblasts into Functional Astrocytes by Defined Transcription Factors. Stem Cell Reports. 2014;4:25–36. doi: 10.1016/j.stemcr.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Guo W, Patzlaff NE, Jobe EM, Zhao X. Isolation of multipotent neural stem or progenitor cells from both the dentate gyrus and subventricular zone of a single adult mouse. Nat Protoc. 2012;7:2005–12. doi: 10.1038/nprot.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kerstetter AE, Miller RH. Isolation and culture of spinal cord astrocytes. Methods Mol Biol. 2012;814:93–104. doi: 10.1007/978-1-61779-452-0_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kim HJ, Magrané J. Isolation and culture of neurons and astrocytes from the mouse brain cortex. Methods Mol Biol. 2011;793:63–75. doi: 10.1007/978-1-61779-328-8_4. [DOI] [PubMed] [Google Scholar]
- 190.Gingras M, Gagnon V, Minotti S, Durham HD, Berthod F. Optimized protocols for isolation of primary motor neurons, astrocytes and microglia from embryonic mouse spinal cord. J Neurosci Methods. 2007;163:111–8. doi: 10.1016/j.jneumeth.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 191.Dincman TA, Beare JE, Ohri SS, Whittemore SR. Isolation of cortical mouse oligodendrocyte precursor cells. J Neurosci Methods. 2012;209:219–26. doi: 10.1016/j.jneumeth.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chen Y, et al. Isolation and culture of rat and mouse oligodendrocyte precursor cells. Nat Protoc. 2007;2:1044–51. doi: 10.1038/nprot.2007.149. [DOI] [PubMed] [Google Scholar]
- 193.Yip PK, Kaan TKY, Fenesan D, Malcangio M. Rapid isolation and culture of primary microglia from adult mouse spinal cord. J Neurosci Methods. 2009;183:223–37. doi: 10.1016/j.jneumeth.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 194.Moussaud S, Draheim HJ. A new method to isolate microglia from adult mice and culture them for an extended period of time. J Neurosci Methods. 2010;187:243–53. doi: 10.1016/j.jneumeth.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 195.Gaven F, Marin P, Claeysen S. Primary Culture of Mouse Dopaminergic Neurons. J Vis Exp. 2014:e51751. doi: 10.3791/51751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Brüstle O, et al. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999;285:754–6. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- 197.Benveniste Ronald J, Keller Gordon, Germano Isabelle. Embryonic stem cell—derived astrocytes expressing drug-inducible transgenes: differentiation and transplantion into the mouse brain. 2009 doi: 10.3171/jns.2005.103.1.0115. at < http://thejns.org/doi/abs/10.3171/jns.2005.103.1.0115>. [DOI] [PubMed]
- 198.Rathjen J, et al. Directed differentiation of pluripotent cells to neural lineages: homogeneous formation and differentiation of a neurectoderm population. Development. 2002;129:2649–61. doi: 10.1242/dev.129.11.2649. [DOI] [PubMed] [Google Scholar]
- 199.Hancock CR, Wetherington JP, Lambert NA, Condie BG. Neuronal differentiation of cryopreserved neural progenitor cells derived from mouse embryonic stem cells. Biochem Biophys Res Commun. 2000;271:418–21. doi: 10.1006/bbrc.2000.2631. [DOI] [PubMed] [Google Scholar]
- 200.Scheffler B, et al. Functional network integration of embryonic stem cell-derived astrocytes in hippocampal slice cultures. Development. 2003;130:5533–41. doi: 10.1242/dev.00714. [DOI] [PubMed] [Google Scholar]
- 201.Misumi S, et al. Differentiation of Oligodendrocytes from Mouse Induced Pluripotent Stem Cells Without Serum. Transl Stroke Res. 2013;4:149–157. doi: 10.1007/s12975-012-0250-1. [DOI] [PubMed] [Google Scholar]
- 202.Kitazawa A, Shimizu N. Differentiation of mouse induced pluripotent stem cells into neurons using conditioned medium of dorsal root ganglia. N Biotechnol. 2011;28:326–33. doi: 10.1016/j.nbt.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 203.Mohamad O, et al. Efficient neuronal differentiation of mouse ES and iPS cells using a rotary cell culture protocol. Differentiation. 2013;86:149–158. doi: 10.1016/j.diff.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 204.Chen X, et al. Directed neuronal differentiation of mouse embryonic and induced pluripotent stem cells and their gene expression profiles. Int J Mol Med. 2013;32:25–34. doi: 10.3892/ijmm.2013.1372. [DOI] [PubMed] [Google Scholar]
- 205.Najm FJ, et al. Transcription factor-mediated reprogramming of fibroblasts to expandable, myelinogenic oligodendrocyte progenitor cells. Nat Biotechnol. 2013;31:426–33. doi: 10.1038/nbt.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zujovic V, Taupin V. Use of cocultured cell systems to elucidate chemokine-dependent neuronal/microglial interactions: control of microglial activation. Methods. 2003;29:345–50. doi: 10.1016/s1046-2023(02)00358-4. [DOI] [PubMed] [Google Scholar]
- 207.Williams EC, et al. Mutant astrocytes differentiated from Rett syndrome patients-specific iPSCs have adverse effects on wild-type neurons. Hum Mol Genet. 2014;23:2968–80. doi: 10.1093/hmg/ddu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ferraiuolo L, et al. Dysregulation of astrocyte-motoneuron cross-talk in mutant superoxide dismutase 1-related amyotrophic lateral sclerosis. Brain. 2011;134:2627–41. doi: 10.1093/brain/awr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wilhelm I, Krizbai IA. In vitro models of the blood-brain barrier for the study of drug delivery to the brain. Mol Pharm. 2014;11:1949–63. doi: 10.1021/mp500046f. [DOI] [PubMed] [Google Scholar]
- 210.Park JW, Vahidi B, Taylor AM, Rhee SW, Jeon NL. Microfluidic culture platform for neuroscience research. Nat Protoc. 2006;1:2128–36. doi: 10.1038/nprot.2006.316. [DOI] [PubMed] [Google Scholar]
- 211.Park J, Koito H, Li J, Han A. A multi-compartment CNS neuron-glia Co-culture microfluidic platform. J Vis Exp. 2009 doi: 10.3791/1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hosmane S, Yang IH, Ruffin A, Thakor N, Venkatesan A. Circular compartmentalized microfluidic platform: Study of axon-glia interactions. Lab Chip. 2010;10:741–7. doi: 10.1039/b918640a. [DOI] [PubMed] [Google Scholar]
- 213.Majumdar D, Gao Y, Li D, Webb DJ. Co-culture of neurons and glia in a novel microfluidic platform. J Neurosci Methods. 2011;196:38–44. doi: 10.1016/j.jneumeth.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Floden AM, Combs CK. Microglia repetitively isolated from in vitro mixed glial cultures retain their initial phenotype. J Neurosci Methods. 2007;164:218–24. doi: 10.1016/j.jneumeth.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Liao B, Zhao W, Beers DR, Henkel JS, Appel SH. Transformation from a neuroprotective to a neurotoxic microglial phenotype in a mouse model of ALS. Exp Neurol. 2012;237:147–52. doi: 10.1016/j.expneurol.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Derventzi A, et al. An in vitro model for investigating human autologous neuronal-astrocyte and immune cell interactions underlying neurodegenerative and immunosuppressive processes in neuropathy. Brain Res. 2014;1587:1–14. doi: 10.1016/j.brainres.2014.08.058. [DOI] [PubMed] [Google Scholar]