Abstract
Cell-based neurotransmitter fluorescent engineered reporters (CNiFERs) provide a new tool for neuroscientists to optically detect the release of neurotransmitters in the brain in vivo. A specific CNiFER is created from a human embryonic kidney cell that stably expresses a specific G protein-coupled receptor, which couples to Gq/11 G proteins, and a FRET-based Ca2+-detector, TN-XXL. Activation of the receptor leads to an increase in the FRET signal. CNiFERs have nM sensitivity and a temporal response of seconds because a CNiFER clone utilizes the native receptor for a particular neurotransmitter, e.g., D2R for dopamine. CNiFERs are directly implanted into the brain, enabling them to sense neurotransmitter release with a spatial resolution of less than one hundred µm, making them ideal to measure volume transmission in vivo. CNiFERs can also be used to screen other drugs for potential cross-reactivity in vivo. We recently expanded the family of CNiFERs to include GPCRs that couple to Gi/o G proteins. CNiFERs are available for detecting acetylcholine (ACh), dopamine (DA) and norepinephrine (NE). Given that any GPCR can be used to create a novel CNiFER and that there are approximately 800 GPCRs in the human genome, we describe here the general procedure to design, realize, and test any type of CNiFER.
Keywords: Neuroscience, Issue 111, Optical imaging, FSCV, dialysis, volume transmission, neurotransmitters, neuropeptides, TPLSM, biosensor, GPCR
Introduction
To fully understand how neurons communicate in the brain, it is necessary to have a method to measure the release of neurotransmitters in vivo. There are several well-established techniques for measuring neurotransmitters in vivo. One commonly used technique is microdialysis, in which a cannula is inserted into the brain and a small volume of cerebrospinal fluid is collected and analyzed using high-performance liquid chromatography and electrochemical detection1. Microdialysis has a spatial resolution on the order of a few diameters of the probe, e.g., ~0.5 mm for a 200 μm diameter microprobe. The temporal resolution of this technique, however, is slow due to sampling intervals that typically last ~5 min or longer1. Moreover, analyses are not made in real-time. Another technique is fast scanning cyclic voltammetry (FSCV), which uses a carbon-fiber probe that is inserted into the brain. FSCV has excellent temporal resolution (subsecond), high sensitivity (nanomolar), and spatial resolution with probe diameters of 5 to 30 μm. However, FSCV is limited to transmitters that produce a characteristic oxidation and reduction profile with voltage on a carbon potentiometric probe2.
A third technique to measure neurotransmitters is directly through genetically-encoded neurotransmitter (NT) biosensors3. With this method, a fusion protein is created that contains a ligand-binding domain for a transmitter coupled to a fluorescence resonance energy transfer (FRET)-based pair of fluorophores4 or a permutated GFP5. Unlike the previous two methods, these biosensors are genetically encoded and expressed on the surface of a host cell, such as a neuron, through the production of transgenic animals or acutely with the use of viral agents to infect cells. To date, genetically-encoded biosensors have been only developed for detecting glutamate and GABA3-5. Limitations with these techniques have been the low sensitivity, in the nM range, and the inability to expand the detection to the large number of transmitters, e.g., classical neurotransmitters, neuropeptides and neuromodulators, which signal through G protein-coupled receptors (GPCRs). In fact, there are nearly 800 GPCRs in the human genome.
To address these shortfalls, we have developed an innovative tool to optically measure release of any neurotransmitter that signals through a GPCR. CNiFERs (cell-based neurotransmitter fluorescent engineered reporters) are clonal HEK293 cells engineered to express a specific GPCR that, when stimulated, triggers an increase in intracellular [Ca2+] that is detected by a genetically encoded FRET-based Ca2+ sensor, TN-XXL. Thus, CNiFERs transform neurotransmitter receptor binding into a change in fluorescence, providing a direct and real-time optical read-out of local neurotransmitter activity. By utilizing the native receptor for a given neurotransmitter, CNiFERs retain the chemical specificity, affinity and temporal dynamics of the endogenously expressed receptors. To date, we have created three types of CNiFERs, one for detecting acetylcholine using the M1 receptor, one for detecting dopamine using the D2 receptor, and one for detecting norepinephrine using the α1a receptor6,7. The CNiFER technology is readily expandable and scalable, making it amenable to any type of GPCR. In this JoVE article, we describe and illustrate the methodology to design, realize, and test in vivo CNiFERs for any application.
Protocol
All animal procedures performed in this study are in accordance with Institutional Animal Care and Use Committee (IACUC) guidelines, and have been approved by the IACUCs at the Icahn School of Medicine at Mount Sinai and the University of California, San Diego.
1. Generate GPCR-expressing Lentivirus for Transforming HEK293 Cells
Obtain the cDNA for a specific GPCR from a commercial source, e.g., cdna.org. Alternatively, amplify the GPCR gene from a cDNA library using PCR. Obtain a lentivirus-expressing vector, such as pCDH-CMV-MCS-EF1-Puro (pCDH). Use this vector to propagate the DNA as well as to generate lentivirus.
Clone the GPCR cDNA into the lentivirus-expressing vector by PCR. See Lorenz8, for details on PCR subcloning.
Expand and purify the GPCR-pCDH DNA using an endotoxin-free 'maxi' prep kit as per the manufacturer instructions. Confirm that the GPCR cDNA subcloned into pCDH is mutation-free by DNA sequencing. Note: Prior to submitting the DNA for virus production, digest an aliquot with appropriate restriction enzyme to confirm size of insert and purity of DNA.
Generate lentivirus using a virus core facility, such as one at The Salk Institute, University of Penn., or University of North Carolina, etc., or generate in-house9. Use approximately 25 μg (>1 μg/μl) of endotoxin-free DNA for transfection of HEK cells in a T75 flask. Ensure that the DNA is of high purity, having an absorbance ratio (A260/A280) of ~1.8. Note: Titers of virus ~1011-1012 GC/ml are optimal for transduction of HEK293 cells.
2. Choosing HEK293/TN-XXL Backbone Cell Type for Culturing In Vitro
Note: Determine the G protein coupling specificity, e.g., Gi/o, Gq/11, or Gs G proteins, of the GPCR, as this dictates whether a G protein chimera is needed for the CNiFER. For Gq-coupled receptors, e.g., M1 muscarinic receptor, choose HEK293/TN-XXL(#3g8) as the backbone HEK293 cell type. For Gi/o-coupled receptors, the chimeric G protein Gqi5 is needed10. For Gs-coupled receptor, the Gqs5 chimera is needed10. In this protocol, the construction of a D2R CNiFER is used as an example. D2R signals through Gi/o G proteins and requires HEK293 cells that stably express the chimeric G protein, Gqi5, e.g., HEK293/TN-XXL/Gqi5_#qi5.6.
Obtain the HEK293/TN-XXL/Gqi5_#qi5.6 clonal cells from a research lab. Note: The following clonal cells, HEK293/TN-XXL(#3g8) for Gq-coupled receptors, HEK293/TN-XXL/Gqi5 (#qi5.6) for Gi/o-coupled receptors, and HEK293/TN-XXL/Gqs5 (#qs5.47) for Gs-coupled receptors, are freely available upon request6,7.
Grow and expand HEK293/TN-XXL/Gqi5_#qi5.6 to ~90% confluency in a T25 flask with 5 ml of HEK293 growth media (Table 1). Grow the cells in a humidified incubator at 37 °C with 5% (v/v) CO2. Note: All work with culturing HEK293 cells should be carried out using standard sterile tissue culture techniques.
Harvest the HEK293 cells by first aspirating media from T25 flask. Wash the cells gently by adding 5 ml of PBS and rocking flask.
Remove the PBS and add 1 ml of 0.05% (w/v) trypsin/EDTA (Table 2). Incubate for 1 to 2 min at 37°C with 5% (v/v) CO2.
Collect cells and transfer to sterile 15 ml conical tube. Centrifuge for 5 min at 1,000 x g in a cell culture centrifuge. Aspirate the supernatant.
Resuspend the cell pellet in 5 ml of HEK293 growth media. Count the cells in a hemocytometer using trypan blue. Calculate the cell density and proceed to step 3.
3. Lentiviral Transduction of HEK293/TN-XXL/Gqi5 Cells
Seed a T25 flask with 0.7 x 106 HEK293/TN-XXL/Gqi5 cells. Grow the cells in a humidified incubator at 37 °C with 5% (v/v) CO2 until ~50% confluent, after approximately 1 day. Freeze remaining cells (steps 8.2-8.3). These cells will serve as the CNiFER control, i.e., a CNiFER that lacks the GPCR.
On the day of the infection, dilute the GPCR expressing lentivirus (step 1.4) to a final concentration of ~109 GC/ml in a total volume of 2 ml of HEK293 growth media (Table 1). For example, add 20 μl of 1011 GC/ml virus to 2 ml media in a T25 flask. Note: The virus titer information should be provided by the virus core facility. Combine lentivirus and media in a centrifuge tube and triturate gently. High titers of lentivirus are biosafety level 2 (BSL-2).
Aspirate the media from the T25 flask. Add the 2 ml of virus/media mixture from step 3.2. Incubate the T25 flask O/N at 37 °C with 5% (v/v) CO2.
After one day of infection, aspirate the virus/media mixture and replace it with HEK293 growth media containing puromycin (2 μg/ml; Table 1). Puromycin selects for the transduced cells. Incubate the flask at 37 °C with 5% (v/v) CO2 until about 90% confluent, after ~1-2 days.
Prepare a 96-well plate (black with clear bottom) coated with fibronectin, for generating a 10-point agonist/activation curve in step 4.1. In a sterile hood, add 50 μl fibronectin (5 μg/ml) per well in Rows A and B of the 96-well plate. Incubate the plate at room temp for 1 hr. Rinse twice for 5 min per rinse with PBS. Add 50 μl of HEK293 growth media and incubate O/N at 37 °C with 5% (v/v) CO2. Note: Fibronectin-treated plates are commercially available.
Harvest the cells in the T25 flask as described in steps 2.3-2.6 (Table 2).
Resuspend the cell pellet in 5 ml of HEK293 growth media. Seed a T25 flask with 1.5 ml of cells for FACS analysis. Also, seed a T75 flask with 1 ml of cells for freezing and storage (see steps 8.2-8.3)
For the 10-point agonist curve, seed the first two rows (A and B) of a fibronectin-coated 96-well plate (from step 3.5) with 100 μl of the cell suspension per well.
Incubate HEK293 cells growing in a T25 flask, a T75 flask and a 96-well plate until about ~90% confluent at 37 °C with 5% (v/v) CO2, after ~1-2 days.
4. FACS and Isolation of Single CNiFER Clones
- Use the 96-well plate for generating a 10-point agonist activation curve. Note: Before starting fluorescence activated cell sorting (FACS) analysis, it is important to confirm the expression of the GPCR by testing transduced cells for an agonist response (10-point agonist curve). This test is carried out using a fluorometric plate reader.
- Prepare a drug plate for the 10-point agonist activation curve. Choose 10 different agonist concentrations that bracket the predicted EC50, which can be determined from the literature. Note: The drug plate contains 3-fold of each concentration (in duplicate) to adjust for the 1:3 dilution in the CNiFER plate. For example, the drug plate for testing a D2 CNiFER contains 10 different concentrations of dopamine at 3-fold concentrations; 0.2, 0.5, 1, 3, 5, 10, 20, 30, 50, and 1,000 nM. In the CNiFER plate, therefore, the final concentrations of dopamine are 0.067, 0.167, 0.333, 1.00, 1.67, 3.33, 6.67, 10.0, 16.7, and 333 nM.
- Prepare the agonist solutions using artificial cerebral spinal fluid (ACSF) (Table 1). Use two wells for 'ACSF', e.g., A1 and A2, and two wells for 'no cells', e.g., B1 and B2. Note: Prepare different concentrations of drugs using a serial dilution method. Create a template to keep track of CNiFER clones and the drug concentrations (Figure 3).
- Use the software to program the 96-well fluorometric plate reader for measuring FRET and performing solution transfers.
- Set the plate reader temperature to 37 °C.
- For measuring FRET with TN-XXL, set the excitation wavelength to 436 ± 4.5 nm (center ± HWHM). Set the emission filters to 485 ± 7.5 nm for eCFP and to 527 ± 7.5 nm for Citrine. Set the cutoff filter to 475 and 515 nm for eCFP and Citrine, respectively.
- Program the plate reader to measure emission at 485 nm and 527 nm every 4 sec for a total of 180 sec. Choose the option to deliver 50 µl from the 3-fold drug plate to the 100 μl in the CNiFER plate, after collecting 30 sec of baseline fluorescence.
- Aspirate the media from Rows A and B and add 100 µl of ACSF to the 96-well CNiFER plate that is ~90% confluent (step 3.9).
- Load the 96-well CNiFER plate and the '3-fold' drug plate into the plate reader. Allow ~30 min to equilibrate the plates at 37 °C. Then, start the program.
- To analyze plate reader data, export the fluorescence values to a spreadsheet. Create a formula to subtract the background measurements (taken for each signal from wells without cells) from wells with CNiFERs. Normalize fluorescence intensities to pre-stimulus baselines, FCitrine(t)/FCitrine(baseline), and calculate the FRET ratio (ΔR/R; Eqn. 1) using the peak responses at 527 nm and 485 nm emissions (see step 11). Note: If there is a significant change in ΔR/R with the agonist, then this indicates expression of the GPCR and one can proceed to the FACS analysis (step 4.2). If there is no FRET response with agonist, troubleshoot by using a Ca2+ ionophore, e.g., A21387, to test the Ca2+ response and confirm that FRET-based sensor is working. If the ionophore works, then the receptor was not likely expressed.
On the day before FACS, prepare four 96-well plates coated with fibronectin (see step 3.5) for collecting the sorted cells. Add 50 μl of HEK293 growth media and incubate O/N at 37 °C with 5% (v/v) CO2.
Prepare 5% (w/v) BSA in PBS (5 g/100 ml) and filter (0.2 μm) into a sterile bottle.
Harvest the cells grown in the T25 flask (see steps 2.3-2.5, Table 2). Resuspend the cell pellet in 4 ml of 5% (w/v) BSA in PBS. Centrifuge the cells at 1,000 x g for 5 min.
Aspirate the media and resuspend the cell pellet in ~5 ml of 5% (w/v) BSA in PBS to give a final concentration of ~5 x 106 cells/ml. Note: Check with the FACS core facility for specific requirements on cell density and sorting conditions.
Filter the resuspended cells with a 40 μm cell strainer to remove the clumps. Transfer the cells to a 5 ml polypropylene round bottom tube. Place the tube on ice for transport to the FACS facility.
Sort the transduced HEK293 cells at a FACS facility. Program the parameters on a FACS flow cytometer as follows: set 4 °C for the sample holder, 100 μm for the nozzle and 20 p.s.i.. Based on the pre-sort analysis, select cells, i.e., choose a 'gate', that have a large eCFP fluorescence (eCFP excitation, eCFP emission) and large FRET (eCFP excitation, Citrine emission) fluorescence (see Results below, Figure 2).
Deposit individual, sorted cells into a 96-well plate prepared in step 4.2, with one clone per well containing 50 μl of HEK293 growth media with puromycin. Add 50 μl of HEK293 growth media with puromycin (Table 1) for a total of 100 μl per well. Maintain cells O/N at 37 °C with 5 % (v/v) CO2. Note: HEK293 growth media contains puromycin for selection of transduced cells.
5. Culturing and Expansion of Sorted, Clonal CNiFERs
Maintain the CNiFERs in the 96-well plates by removing 50 μl of old media from each well and replacing with 50 μl of fresh HEK293 growth media containing puromycin (Table 1). Repeat this every 5 to 7 days until ~90% confluent, after 2-3 weeks.
Harvest CNiFER cells by gently aspirating the media and rinsing once gently with PBS. Remove the PBS and add 20 μl of 0.05% (w/v) trypsin/EDTA. Incubate for 1 to 2 min at 37° C with 5% (v/v) CO2.
Add 100 μl of HEK293 growth media to trypsin-treated cells and resuspend the cells. Transfer contents to a 24-well plate containing 400 μl fresh HEK293 growth media with puromycin. In the 24-well plate, maintain cells by replacing 250 μl of HEK293 growth media every 5-7 days until wells are ~90% confluent.
6. Identify Candidate CNiFERs Based on FRET Response Using Fluorometric Plate Reader
Note: With four 96-well plates following FACS, there should be >100 testable clones that survive and expand to the 24-well plate stage, since many of the original clones fail to grow. To identify potential candidate CNiFERs, use a 3-point analysis for the FRET response with cognate agonist, e.g., dopamine for D2R.
When the cells are ~90% confluent in the 24-well plate, gently aspirate the media. Add ~100 μl of 0.05 % (w/v) trypsin/EDTA and incubate for 1-2 min at 37 °C with 5% (v/v) CO2. Add 400 μl of HEK293 growth media to trypsin-treated cells and mix the cells by gentle trituration.
Set up the 3-point agonist curve for the initial screening of the clones. For each CNiFER clone, i.e., one of the wells from the 24-well plate, aliquot 100 μl of the cell suspension (~4 x 103 cells/well) into each of three wells, e.g., A1, A2, A3, of a fibronectin-coated 96-well plate (black with clear bottom) (see step 3.5).
Transfer the remaining ~200 μl of the cell suspension to a 12-well plate containing 1,000 μl of HEK293 growth media (1.2 ml final volume). Incubate both plates at 37 °C with 5% (v/v) CO2, until ~90% confluent. The 96-well plate is for the fluorometric assay and the 12-well plate is for growing and expanding the clones.
For the 3-point analysis, determine three different concentrations of agonist that are 0.1-, 1.0-, and 10-times the EC50 for the specific GPCR. Prepare agonist concentrations in a drug plate as described in steps 4.1.1-4.1.2. Perform a fluorometric plate reader assay as described in steps 4.1.3-4.1.5.
Calculate the FRET ratio (ΔR/R) as described in step 4.1.6. Choose CNiFERs that have appropriate sensitivity and largest FRET response for expansion, freezing back, and more comprehensive analyses (step 7).
For the clones that were selected in step 6.5 and are growing in the 12-well plate, remove and replace 600 μl of the HEK293 growth media every 5 to 7 days, until ~90% confluent.
Gradually expand the clones from a 12-well plate to a 6-well plate, and then to a T25 flask (steps 2.3 - 2.6 and Table 2). When the T25 flask is ~90% confluent, harvest cells as described (steps 2.3-2.5). Resuspend the cell pellet in 5 ml of HEK293 growth media.
Add 1 ml of cell suspension to a T75 flask with 9 ml of HEK293 growth media. Use the remaining 4 ml of the cell suspension for cryoprotection and storage in liquid N2 (steps 8.2-8.3). Prepare eight 1.5 ml cryotubes and place on ice.
In the T75 flask, maintain cells by replacing the media with fresh HEK293 growth media, e.g., 10 ml every 3-5 days until 70-80% confluent, after ~1-2 weeks.
7. Final Selection of CNiFER Clones Using Fluorometric Plate Reader
- On the day before the plate reader assay:
- Harvest cells from a ~90% confluent T75 flask (see steps 2.3-2.6, Table 2). Seed a 96-well fibronectin-coated plate (black with clear bottom) at 5 x 104/well with ~100 μl of cell suspension. Note: One clone is distributed into a single 96-well plate.
- Prepare a drug plate for the comprehensive screening of CNiFER clones, distinguishing specific agonist responses from non-specific CNiFER responses.
- To generate a full dose-response curve, choose 10 different agonist concentrations around the predicted EC50. Use two wells for 'no cells' and two wells for 'ACSF'.
- For determining non-specific responses, choose three concentrations of 12 different neurotransmitters or modulators (72 wells) (Figure 3). Like the agonist, the drug plate contains 3-fold concentrations in duplicate. For example, 100 μl of three different concentrations of acetylcholine, glutamate, orexin, VIP, adenosine, serotonin, norepinephrine, GABA, Substance P, melatonin, somatostatin and histamine, each at a 3-fold concentration of 50, 1,000, and 3,000 nM are loaded into the 96-well drug plate.
Set the parameters on the fluorometric plate reader for measuring FRET and performing solution transfers as described in steps 4.1.3-4.1.5.
For analyzing the full dose response curve, calculate the peak FRET ratio (ΔR/R) (step 4.1.6), plot as a function of log agonist concentration and fit with the Hill equation (step 11.4). Determine the EC50, Hill coefficient, and maximal FRET ratio. For the 12 other neurotransmitters/modulators, plot peak ΔR/R as a function of drug concentration.
Choose ~10 CNiFER clones that have a large FRET ratio, an appropriate EC50 for the cognate agonist, and little or no background responses to other neurotransmitter agonists (non-specific response).
8. Freeze-back Selected CNiFER Clones
Use a ~90% confluent T75 flask of an individual CNiFER clone. Harvest cells as described (steps 2.3-2.5, Table 2). For freezing cells, resuspend the cell pellet in 5 ml of HEK293 growth media. Label ten 1.5 ml cryotubes and set on ice.
For cryoprotection, mix cells 1:1 with 20% (v/v) DMSO in HEK293 growth media, e.g., 5 ml of the 20% (v/v) DMSO/media mixture is gently mixed with 5 ml of cell suspension (final concentration of DMSO is 10%).
Aliquot 1 ml into each of the cryotubes. Freeze tubes with cells in a -80 °C freezer O/N, in a foam-insulated box (see Materials). Transfer cryotubes to liquid nitrogen for long-term storage.
9. CNiFER Implantation into Mouse Cortex
Sterilize all surgical tools in an autoclave before surgery. Prepare a semi-sterile field for surgery by wiping with 70% ethanol and laying down a clean lab diaper.
Prepare the CNiFER injection pipet by pulling a glass capillary (i.d. of 0.53 mm) on a vertical electrode puller. Use a pair of no. 5 fine-tip forceps to break the tip of the electrode to a diameter of ~40 μm. Note: This is best accomplished under a stereo zoom microscope with a graticule.
Anesthetize an adult (postnatal day 60-90) C57BL/6 mouse with isoflurane: 4% (v/v) for induction and 1.5 to 2.0% (v/v) for maintenance. Use tail or toe pinch to make sure that the mouse is fully anesthetized. Note: Re-pinch periodically and assess whisker twitching throughout surgery to reassess depth of anesthesia.
Cover the eyes with ophthalmic ointment to prevent drying. Mount the mouse in a stereotaxic frame with ear bars. Maintain the mouse body temperature at 37 °C using a heat pad regulated by a rectal probe.
Shave an area approximately 5 mm by 12 mm with an animal electric shaver. Apply Betadine followed by 70% (v/v) isopropanol. Use a scalpel blade to cut and remove the skin over the skull surface. Use a scalpel blade to remove the periosteum from the surface of the skull. Expose and clean the surface of the skull, as described for stereotaxic surgery11.
Lower an empty glass pipet to bregma and record the antero-posterior (A/P) and medio-lateral (M/L) coordinates. Referring to the mouse brain atlas, calculate the position of the injection site. Shift the pipet to the target site and mark the skull for subsequent window formation. See Cetin et al. for details on stereotaxic injections with rodents11. Note: The site of injection and window depend on the region to be studied and the distribution of neurotransmitter or peptide releasing projections in the cortex. For instance, in a recent publication7, the stereotaxic coordinates +1.0 to +2.0 mm A/P and +1.0 to +2.0 mm M/L were used to inject CNiFER cells into frontal cortex for in vivo imaging of dopamine release during classical conditioning.
Form a 2 mm x 3 mm thinned-skull window as previously described12,13. Note: The bone in the window should be 15-20 μm thick. The small white spots in the bone should not be visible when the skull surface is moistened, if the bone is sufficiently thinned12,13.
Place an ACSF soaked sponge on the window to keep it moist while preparing cells to inject.
Harvest the CNiFER clone that was grown in a T75 flask to ~80% confluency. Aspirate the media and wash the cells with sterile PBS. Note: Trypsin is omitted for these steps.
Remove PBS and use 10 ml of ACSF to dislodge cells from the bottom of the flask. Triturate cells to dissociate cell clumps. Centrifuge and resuspend the pellet in 100 μl of ACSF. Centrifuge for 30 sec at 1,400 x g and remove the supernatant, leaving a pellet covered in ACSF. This step leaves a clump of cells in suspension.
Backfill the injection pipet prepared in step 9.2 with mineral oil, load the pipet onto a nanoinjector, and advance the plunger to eject a small bead of oil. Put 5 μl of CNiFER cell suspension onto a strip of plastic paraffin film near the mouse preparation. Draw up either the CNiFERs or control CNiFER cells into the pulled pipette.
Move the pipette to the target X and Y coordinates, i.e., A/P and M/L noted in step 9.5. Lower the pipette, piercing the thinned skull, and continue to ~200-400 μm below the skull surface, to deposit CNiFER cells in layers 2/3 of cortex.
Inject ~4.6 nl of CNiFER cells at the deepest site with the nanoinjector, note movement in the oil and cell interface and then wait for 5 min for the cells to dispense. Withdraw the pipette ~100 μm and inject another ~4.6 nl of CNiFER cells, wait 5 min. Then withdraw the pipette slowly and gently to prevent backflow of the CNiFERs. Repeat injection at one or more adjacent sites.
Repeat injection steps 9.8-9.12 with control HEK293 cells (i.e., HEK293/TN-XXL/Gqi5 clone lacking GPCR). Separate the CNiFER and control cell injection sites by ~200 μm.
After completing cell implantations, rinse the thinned-skull window with ACSF and wait for the skull to dry. Apply a drop of cyanoacrylate glue (see Materials) over the window and quickly place a pre-cut sterile cover glass on top of the glue. Gently push the cover glass against the skull for a few seconds. Let the glue dry for 2 min12,13.
Seal the edges of the cover glass with dental cement and form a well around the window to hold water for the dipping objective.
For immobilizing the mouse's head during imaging, attach a custom-built head-bar with a small drop of cyanoacrylate glue behind the window (see14 for details on dimension and materials). Let the glue dry thoroughly and then add additional dental cement to further reinforce the custom-built head-bar.
Cover the rest of the skull surface, except for the window, with a layer of dental cement. Make sure the edges of the skin are covered by cement and let it dry for 20 min.
Following the surgery, stop isoflurane administration and leave the mouse on a heating pad in a cage until it fully recovers from anesthesia. Inject 5% (w/v) glucose in saline (s.c.) for rehydration and 0.05 to 0.1 mg/kg buprenorphine (i.p., instant release) for post-operative analgesia. Note: To minimize potential immunological reaction to the human CNiFERs, inject the mouse daily with 20 μl/100 g cyclosporine (i.p.) starting the day before injection of CNiFERs.
Return the mouse to its home cage for food and water.
10. In Vivo Imaging of CNiFER Clones
Note: Live imaging is conducted with mice using a two-photon microscope and a head-fixed apparatus. No anesthesia is needed during the imaging sessions. When imaging animals in the awake state, limit head restraint to only a few hours at a time to reduce stress levels. Return the animal to it home cage between imaging sessions for food and water. Potential stress is minimized by darkening the room lights and surrounding part of the mouse in an enclosure.
On the day after surgery, mount the mouse on an imaging platform by screwing the metal head-bar implanted on the skull to the head-fixation frame. Note: When imaging awake mice, the imaging session should not exceed a few hours due to the potential stress induced by the head-restraint device.
Place the imaging platform with the head-restrained mouse in a two-photon imaging microscope equipped with a 10X (0.30 NA) and 40X (0.80 NA) water immersion objectives.
Insert filter cube for FRET imaging (eCFP and Citrine) that has a dichroic mirror at 505 nm and band-pass filters that span 460 nm to 500 nm for measuring eCFP and 520 nm to 560 nm for measuring Citrine.
Add ACSF to the well containing thinned-skull window and lower the water immersion objective into the ACSF. Use the eyepiece in conjunction with mercury lamp and GFP filter cube to locate the surface of the cortex and vasculature below the window. Note: The pattern of the vasculature helps locate and image the same region over repeated days of imaging.Switch to the 40X water immersion objective to locate CNiFERs by manually focusing on surface of the cortex over the cells using the GFP filter cube and a mercury lamp.
Set up for two-photon imaging. Select the appropriate light path for two-photon imaging. For a typical commercial system, use the software to switch to two-photon imaging mode and redirect light to the photomultiplier tubes (PMTs) in the non-descanned detectors. Turn on the near infrared femtosecond pulsed laser, select a wavelength of 820 nm and a power setting of 5-15%. Note: 5% power typically provides ~25 mW at the specimen.
Set the PMT1 & PMT2 voltage close to the maximum value, typically 700-1,000 V depending on the PMT. Set the gain to 1 for each channeland zero the z position for the objective.
Lower the objective ~100 to 200 μm from the cortical surface and start the x-y scan. Adjust the laser power, gain, and PMT voltage for each channel, i.e., eCFP and Citrine, to optimize the signal-to-noise ratio of the CNiFER fluorescence.
Use the zoom feature in the software to restrict the image to a region that contains the CNiFER cells as well as a background region. Use a scan rate no slower than one frame every 2 sec (0.5 Hz) at 4 μs per pixel. Adjust the line averaging for suitable signal-to-noise ratio, e.g., Kalman 2 line averaging.
Draw a region-of-interest (ROI) around CNiFER cells, surrounding about 3 to 4 cells per plane. Set up real-time analysis of ROI average intensities. Start acquisition to monitor CNiFER fluorescence over time.
Collect fluorescence from CNiFERs before and during experimental manipulations, e.g., electrical stimulation, ChR2 stimulation, behavior, as determined by the user.
When the imaging experiment is completed, return the mouse to its home cage. Repeat the imaging across days, as desired. When re-imaging the cells refer to the previously acquired low-magnification vasculature image to orient back to the same imaging field (step 10.4). Note: Implanted CNiFERs can be imaged for at least 7 days.
11. Data Analysis
Open imaging file and select ROI's for CNiFERs and one ROI for background. Select 'series analysis' for both channels of each ROI. Export average fluorescence intensity for each ROI as a tab-delimited file.
Use a mathematical software (see Materials) program to analyze fluorescence values. Low-pass filter (0.3 Hz) each signal and then subtract the background fluorescence in each ROI from eCFP and Citrine fluorescence intensities.
Calculate average fluorescence for baseline and calculate ratios as described in Equation 1 to measure the FRET ratio ΔR(t)/R.
To determine the sensitivity of CNiFERs, plot the FRET ratio as a function of log agonist concentration. Fit with the Hill equation to determine the EC50 and Hill coefficient (n), using scientific statistical software and the Hill Equation (Equation 2).

Representative Results
A CNiFER is derived from a human embryonic kidney (HEK293) cell that is engineered to stably express at least two proteins: a specific G-protein coupled receptor (GPCR) and a genetically encoded [Ca2+] sensor, TN-XXL. TN-XXL undergoes fluorescence resonance energy transfer (FRET) between cyan and yellow fluorescent proteins, eCFP and Citrine, respectively, in response to Ca2+ ions6,15. Activation of GPCRs that couple to endogenous Gq G proteins trigger an increase in cytosolic [Ca2+] through the PLC/IP3 pathway, leading to an increase in FRET from the TNXXL Ca2+ detector (Figure 1).
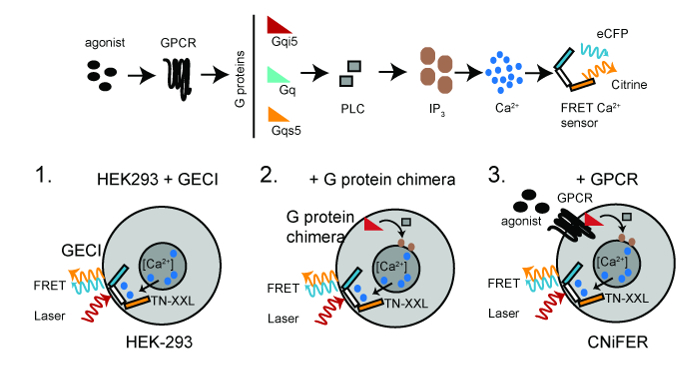 Figure 1: Scheme for Developing CNiFERs. Top, GPCR-Ca2+ signaling pathway required for creating a CNiFER cell. Bottom, the basic steps for constructing CNiFERs using HEK293 cells. Step 1. Transduce with genetically encoded FRET-based Ca2+-detector (TN-XXL). Step 2. Transduce Gα G-protein chimera, i.e., Gqs5, Gqi5, if necessary. Step 3. Transduce unique GPCR to create CNiFER. Two-photon excitation light (red) excites eCFP, which undergoes FRET, producing both an eCFP emission (cyan) and Citrine emission (yellow). Please click here to view a larger version of this figure.
Figure 1: Scheme for Developing CNiFERs. Top, GPCR-Ca2+ signaling pathway required for creating a CNiFER cell. Bottom, the basic steps for constructing CNiFERs using HEK293 cells. Step 1. Transduce with genetically encoded FRET-based Ca2+-detector (TN-XXL). Step 2. Transduce Gα G-protein chimera, i.e., Gqs5, Gqi5, if necessary. Step 3. Transduce unique GPCR to create CNiFER. Two-photon excitation light (red) excites eCFP, which undergoes FRET, producing both an eCFP emission (cyan) and Citrine emission (yellow). Please click here to view a larger version of this figure.
The increase in FRET provides a rapid optical read-out of the change in neurotransmitter levels. To develop a CNiFER for a particular type of neurotransmitter, first determine the type of G protein that couples to the GPCR. For Gq-coupled GPCRs, the GPCR uses Gq proteins endogenously expressed in HEK293 cells. For Gi/o-coupled GPCRs, a clonal HEK293 line is first created that expresses a chimeric G protein that redirects the GPCR to the Gq-PLC/IP3 pathway. This is accomplished with a chimeric G protein, Gqi5, which contains primarily Gαq sequence and five amino acids of the carboxyl terminus of Gi. These five amino acids are sufficient for Gqi5 to communicate with Gi/o-coupled GPCRs, but signal through the Gq pathway. For Gs-coupled GPCRs, a Gqs5 chimera is used10. The general strategy for producing a CNiFER is to: 1) create a clonal HEK293 cell that is stably expressing an optical Ca2+ detector, i.e., TN-XXL, using a lentivirus transduction of HEK cells, 2) stably express a G protein chimera, if necessary, in the HEK293 cell clone expressing TN-XXL, and 3) create a stably expressing GPCR clone in the HEK293 cell clone expressing TN-XXL and the chimeric G protein. The clonal HEK293 line that lacks the GPCR but has the TN-XXL and chimeric G protein serves as the 'control CNiFER'. The control CNiFER is needed to confirm that the CNiFER response is due specifically to activation of the engineered receptors, i.e., D2R, and not to activation of other receptors endogenously expressed in HEK293 cells.
To generate lentivirus, a lentivector expression system is used, e.g., pCDH-CMV-MCS-EF1-Puro, which contains the genetic elements responsible for packaging, transduction, stable integration of the viral expression construct into genomic DNA, and expression of the target gene sequence. To produce a high titer of viral particles, expression and packaging vectors are transiently co-transfected into producer mammalian cells and virus is collected. There are several viral core facilities in the US that can generate high titer lentivirus. Following infection of HEK293 cells, the Puro gene provides antibiotic resistance for identifying transduced HEK293 cells.
In order to identify specific clonal lines, transduced HEK293 cells are sorted using a fluorescence activated cell sorting (FACS) system. The objective is to isolate a clone that contains a high expression level of FRET-based Ca2+ detector and the capability of undergoing FRET. In this example of FACS analysis, the fluorescence of the eCFP emission is plotted against the FRET signal (eCFP excitation and Citrine emission). The boxes mark regions (P2 and P3) that will be subsequently selected ("gated") for sorting into 96-well plates (Figure 2). Generally, about four 96-well plates are sufficient to screen for successful creation of CNiFERs. From these 4 plates, approximately 100 clones are suitable for fluorometric plate reader analysis.
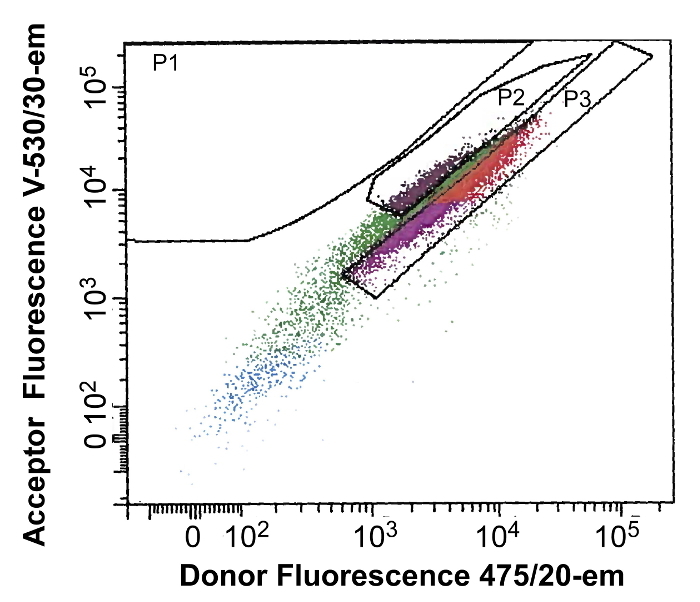 Figure 2: Example of FACS Analysis. A sample of the output following a FACS analysis. The graph plots eCFP emission ("475/20-A") as a function of Citrine emission ("FRET V-530/30-A"), using eCFP excitation for each cell. Regions P2 and P3 show areas selected, i.e., gated, for sorting into individual cells. Colors are arbitrary. Please click here to view a larger version of this figure.
Figure 2: Example of FACS Analysis. A sample of the output following a FACS analysis. The graph plots eCFP emission ("475/20-A") as a function of Citrine emission ("FRET V-530/30-A"), using eCFP excitation for each cell. Regions P2 and P3 show areas selected, i.e., gated, for sorting into individual cells. Colors are arbitrary. Please click here to view a larger version of this figure.
Once the sorted cells have grown to sufficient density, the FRET response following agonist activation is determined using a 96-well fluorometric plate reader system equipped with solution handling. To narrow down the number of clones to study, a "3-point" agonist curve is used to screen ~100 clones and select CNiFERs with the best responses. Approximately 10 clones are then analyzed further with determination of the complete dose-response with the cognate agonist, and the non-specific responses, probed with 12 other neurotransmitters or modulators. A 96-well drug plate is prepared as three-fold concentration (final concentration is diluted 1:3 in plate) of drugs (e.g., agonists, antagonists, etc.) in ACSF. In this example, a drug plate is set up for testing a D2 CNiFER with its cognate agonist, dopamine, and potential non-specific responses with a variety of other neurotransmitter and peptide agonists (Figure 3). The backbone CNiFER, which lacks the GPCR, serves as an important control for the newly created CNiFER.
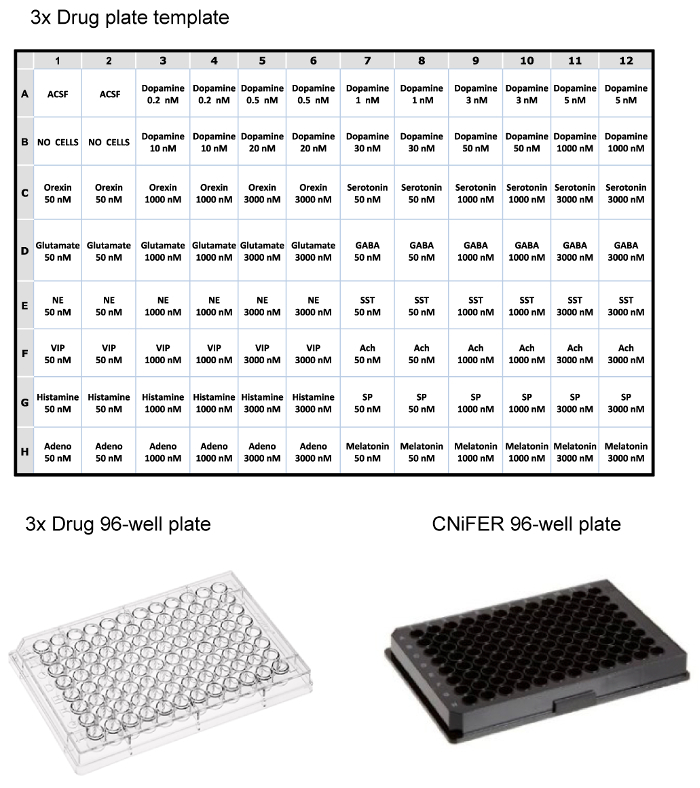 Figure 3: Examples of Layout for 96-well Plates. Top, table of the layout for loading a 3x drug plate for fluorometric plate reader, using three-fold concentrations of various neurotransmitters and peptides. Bottom, examples of clear plastic 96-well drug plate and black 96-well plate for seeding CNiFERs and measuring in plate reader. Please click here to view a larger version of this figure.
Figure 3: Examples of Layout for 96-well Plates. Top, table of the layout for loading a 3x drug plate for fluorometric plate reader, using three-fold concentrations of various neurotransmitters and peptides. Bottom, examples of clear plastic 96-well drug plate and black 96-well plate for seeding CNiFERs and measuring in plate reader. Please click here to view a larger version of this figure.
Stimulation of the GPCR is expected to increase the FRET response, as a consequence of an elevation of intracellular [Ca2+] and detection by TN-XXL. Under these conditions, FRET is produced by eCFP and Citrine moving closer, so that excitation of eCFP produces a smaller eCFP emission and larger Citrine emission. In this example, excitation is set to 436 nm and emission filters are set to 485 ± 7.5 nm for eCFP and 527 ± 7.5 nm for Citrine (Figure 4). Thirty sec of baseline fluorescence is measured and then 50 µl from the "three-fold" agonist in ACSF plate is delivered to each well containing 100 μl ACSF (1:3 dilution). eCFP and Citrine emission fluorescence are measured every 3.8 seconds for 180 sec. Background measurements are taken from wells without cells and subtracted, if necessary. Fluorescence intensities are normalized to pre-stimulus baselines (F(t)/F(baseline)), and peak responses are measured to calculate the FRET ratio (ΔR/R) using the F(t)/F(baseline) of the 527 nm and 485 nm channels (Equation 1). A dose response curve is then constructed by plotting the FRET ratio as a function of different agonist concentrations and fit with the Hill equation to determine the EC50 and Hill coefficient (Figure 5) (Equation 2). An optimal CNiFER exhibits a large FRET ratio and an appropriate EC50 for the cognate agonist, and exhibits little or no background responses to other neurotransmitter agonists. By contrast, the control CNiFER should show little response to the cognate agonist.
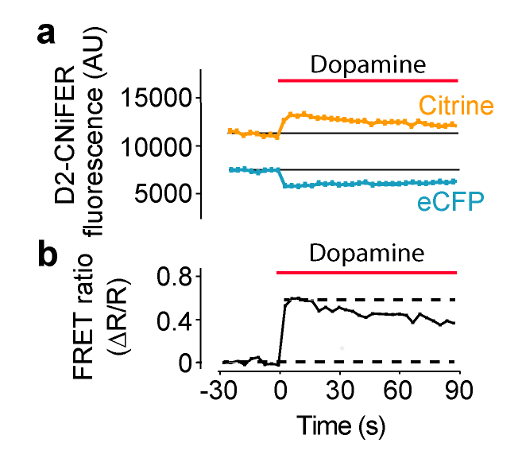 Figure 4: Example of Agonist-induced FRET Response. D2R CNiFER FRET response measured on a plate reader with a solution delivery system. (A) A plot of the FRET response, i.e., eCFP excitation with eCFP and Citrine emissions, during application of dopamine (red bar) to D2 CNiFERs. Note that eCFP emission decreases while Citrine emission increases with agonist (dopamine). (B)A plot of the FRET ratio (Equation 1) for the response in (A) Figure modified from Muller et al., 20147. Please click here to view a larger version of this figure.
Figure 4: Example of Agonist-induced FRET Response. D2R CNiFER FRET response measured on a plate reader with a solution delivery system. (A) A plot of the FRET response, i.e., eCFP excitation with eCFP and Citrine emissions, during application of dopamine (red bar) to D2 CNiFERs. Note that eCFP emission decreases while Citrine emission increases with agonist (dopamine). (B)A plot of the FRET ratio (Equation 1) for the response in (A) Figure modified from Muller et al., 20147. Please click here to view a larger version of this figure.
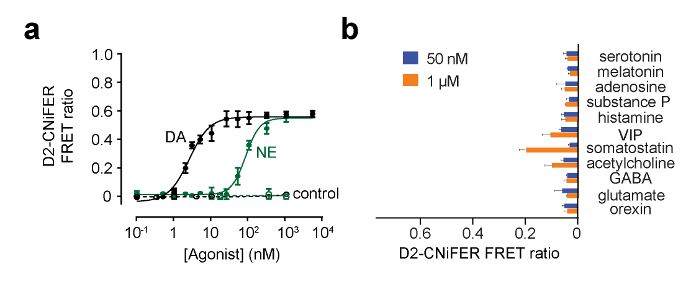 Figure 5: Examples of Dose Response Curves for D2 CNiFER. (A) Dose response curves for response of D2 CNiFERs to dopamine (DA, black) and for norepinephrine (NE, green). In addition, the response of "control" CNiFERs lacking the D2R is shown. (B) The bar graph shows the FRET ratio response for other neurotransmitters and modulators at 50 nM and 1 μM. Values are mean ± SEM. Figure modified from Muller et al., 20147. Please click here to view a larger version of this figure.
Figure 5: Examples of Dose Response Curves for D2 CNiFER. (A) Dose response curves for response of D2 CNiFERs to dopamine (DA, black) and for norepinephrine (NE, green). In addition, the response of "control" CNiFERs lacking the D2R is shown. (B) The bar graph shows the FRET ratio response for other neurotransmitters and modulators at 50 nM and 1 μM. Values are mean ± SEM. Figure modified from Muller et al., 20147. Please click here to view a larger version of this figure.
CNiFER clones can be assessed further for possible receptor-dependent desensitization and for their temporal resolution, discriminating the presentation of two different agonist pulses (for details, see Muller et al., 20147). Having constructed a CNiFER clone, the next step is to test its function in vivo. To monitor the fluorescence in vivo, it is necessary to use a two-photon microscope. After preparing a thinned-skull window, CNiFERs are loaded into a glass pipette and injected into layers 2/3 of cortex. The mouse is then prepared for in vivo imaging by attaching a glass cover slip to the thinned skull, and implanting a head bar for fixing the head during imaging (Figure 6).
To determine that the implanted CNiFERs are viable in vivo, known concentrations of agonist can be injected near the site of implantation and the FRET ratio determined7. To further validate the activity of implanted CNiFERs, stimulating the input neurons should be examined. For example, with the D2 CNiFER, the effect of electrical stimulation of the midbrain dopamine neurons that project to the cortex was examined. A 0.1 MΩ tungsten bipolar stimulating electrode with a tip separation of 500 μm was implanted into the substantia nigra (−3.2 mm A/P, −1.3 mm M/L, −4.4 mm D/V). Figure 6 shows an example of electrically stimulating the substantia nigra at different intensities and observing an increase in the FRET ratio for D2 CNiFERs7. Note that systemic intra-peritoneal (i.p.) injection of a D2 receptor antagonist, eticlopride (1 mg/kg), blocks the D2 CNiFER response. On the other hand, injection of cocaine (15 mg/kg), which blocks reuptake of dopamine, enhances the electrically evoked D2 CNiFER response7.
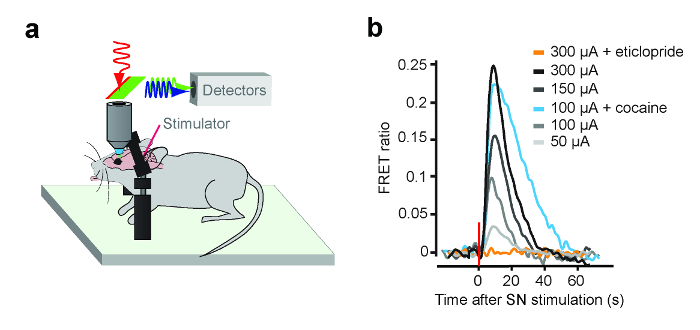 Figure 6: Example of D2 CNiFER Response In Vivo Following Electrical Stimulation of Substantia Nigra. (A) A cartoon shows a head-fixed mouse prepared for in vivo two-photon imaging and electrical stimulation. Two-photon light (red, 820 nm) for excitation and 475 nm emission for eCFP (blue) and 530 nm emission for Citrine (green). (B) The line plot shows the FRET ratio for D2 CNiFER injected into cortex following electrical stimulation of substantia nigra, i.e., 200 μsec pulses of 50 to 300 μA at 50 Hz for 500 msec, and following electrical stimulation in the presence of a D2R antagonist (eticlopride) or cocaine. Figure modified from Muller et al., 20147. Please click here to view a larger version of this figure.
Figure 6: Example of D2 CNiFER Response In Vivo Following Electrical Stimulation of Substantia Nigra. (A) A cartoon shows a head-fixed mouse prepared for in vivo two-photon imaging and electrical stimulation. Two-photon light (red, 820 nm) for excitation and 475 nm emission for eCFP (blue) and 530 nm emission for Citrine (green). (B) The line plot shows the FRET ratio for D2 CNiFER injected into cortex following electrical stimulation of substantia nigra, i.e., 200 μsec pulses of 50 to 300 μA at 50 Hz for 500 msec, and following electrical stimulation in the presence of a D2R antagonist (eticlopride) or cocaine. Figure modified from Muller et al., 20147. Please click here to view a larger version of this figure.
 Table 1: List of chemicals and reagents for making HEK293 growth medium and ACSF.
Table 1: List of chemicals and reagents for making HEK293 growth medium and ACSF.
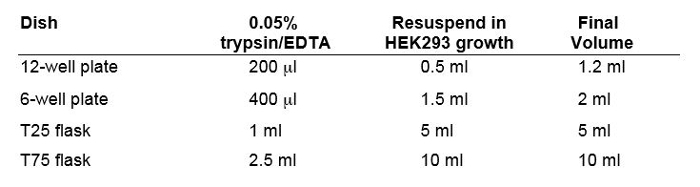 Table 2: Volumes for Harvesting Cells From Different Size Culture Plates or Flasks.
Table 2: Volumes for Harvesting Cells From Different Size Culture Plates or Flasks.
Discussion
The creation of CNiFERs provides an innovative and unique strategy for optically measuring release of neurotransmitters in the brain in vivo. CNiFERs are ideally suited for measuring extrasynaptic release, i.e., volume conduction, for neurotransmitters. Importantly, each CNiFER possesses the properties of the native GPCR, providing a physiological optical measurement of the changes in levels of neurotransmitters in the brain. To date, CNiFERs have been created for detecting acetylcholine (M1-CNiFER)6, dopamine (D2-CNiFER)7 and norepinephrine (α1a-CNiFER)7.
In principal, a CNiFER can be created for any neurotransmitter that signals through a GPCR. For the case where the GPCR signals through Gq G proteins, no further modification is needed to the HEK293 cell. GPCRs that signal through Gi/o, however, require coexpression of a Gqi5 chimeric G protein to couple the GPCR to the Gq/PLC pathway7,10. Similarly, GPCRs that signal through Gs will require coexpression of a chimeric Gqs5 G protein10. Once completed, each CNiFER clone is screened and only those CNiFER clones that have an affinity comparable to the native receptor, exhibit little or no desensitization and provide a signal-to-noise ratio that is adequate for measuring with in vivo two-photon microscopy, are selected for in vivo studies.
For in vivo studies, it is highly recommended to treat the mice with cyclosporine to minimize any potential immunological response. There is a possibility of rejection or an immunological response with implanting human CNiFER cells into the rodent brain. This was investigated previously by examining expression of GFAP and MAC17, following CNiFER implantation. CNiFERs did not appear to produce glial scars or generate any significant MAC1 staining7.
Two major issues to consider in constructing CNiFERs are sensitivity and desensitization. If the EC50 is too high, i.e., low affinity, relative to the native receptor, then the CNiFER may not have sufficient sensitivity to detect release of neurotransmitter in vivo. One solution is to rescreen clones and choose a different CNiFER clone that has higher affinity. An alternative strategy would be to test other types of genetically encoded fluorescent Ca2+-detectors that may have a higher Ca2+ sensitivity, which can shift the EC50 for GPCR activation. Because the CNiFER design is modular, it is easily adapted to other types of genetically encoded Ca2+-detectors, such as GCaMP16. Isolating CNiFER clones with the same receptor but different EC50s could be advantageous for extending the dynamic range of detecting release of endogenous neurotransmitters in vivo.
Desensitization of the CNiFER will also limit its use in vivo. If the peak response gradually decreases with each pulse of agonist, then the receptor may be desensitizing. In this case, examine other clones and determine if they respond the same way. Modifications to the receptor amino acid sequence, or use of another subtype of receptor may be necessary to address the agonist-dependent desensitization. If there are known sites of phosphorylation or amino acids identified that associate with G protein receptor kinases (GRKs), it would be advisable to construct a non-desensitizing variant of the GPCR by mutating one or more sites. The mechanism of desensitization must be determined for each receptor on a case-by-case basis.
Thus far, CNiFERs have been only implanted into superficial layers of cortex6,7, due to spectroscopic limitations with imaging fluorophores with two-photon microscopy17,18. In the future, it may be possible to adapt CNiFER technology with fiber-based measurements of fluorescence19 so that CNiFERs can be implanted in subcortical brain regions.
Disclosures
The authors have nothing to disclose.
Acknowledgments
We thank B. Conklin (University of California, San Francisco) for providing the Gqi5 and Gqs5 cDNAs, A. Schweitzer for assistance with the electronics, N. Taylor for assistance with screening of clones, Ian Glaaser and Robert Rifkin for proof reading, and Olivier Griesbeck for TN-XXL. This work was supported by research grants through the US National Institute on Drug Abuse (NIDA) (DA029706; DA037170), the National Institute of Biomedical Imaging and Bioengineering (NIBIB) (EB003832), Hoffman-La Roche (88610A) and the "Neuroscience Related to Drugs of Abuse" training grant through NIDA (DA007315).
References
- Day JC, Kornecook TJ, Quirion R. Application of in vivo. microdialysis to the study of cholinergic systems. Methods. 2001;23:21–39. doi: 10.1006/meth.2000.1103. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Venton BJ, Heien ML, Wightman RM. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clin Chem. 2003;49:1763–1773. doi: 10.1373/49.10.1763. [DOI] [PubMed] [Google Scholar]
- Liang R, Broussard GJ, Tian L. Imaging Chemical Neurotransmission with Genetically Encoded Fluorescent Sensors. ACS Chem Neurosci. 2015. [DOI] [PubMed]
- Okubo Y, et al. Imaging extrasynaptic glutamate dynamics in the brain. Proc. Natl. Acad. Sci. USA. 2010;107:6526–6531. doi: 10.1073/pnas.0913154107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin JS, et al. An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat Methods. 2013;10:162–170. doi: 10.1038/nmeth.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen QT, et al. An in vivo biosensor for neurotransmitter release and in situ receptor activity. Nat Neurosci. 2010;13:127–132. doi: 10.1038/nn.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A, Joseph V, Slesinger PA, Kleinfeld D. Cell-based reporters reveal in vivo dynamics of dopamine and norepinephrine release in murine cortex. Nat Methods. 2014;11:1245–1252. doi: 10.1038/nmeth.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz TC. Polymerase chain reaction: basic protocol plus troubleshooting and optimization strategies. J Vis Exp. 2012. p. e3998. [DOI] [PMC free article] [PubMed]
- Wang X, McManus M. Lentivirus production. J Vis Exp. 2009. [DOI] [PMC free article] [PubMed]
- Conklin BR, Farfel Z, Lustig KD, Julius D, Bourne HR. Substitution of three amino acids switches receptor specificity of Gqα to that of Gjα. Nature. 1993;363:274–276. doi: 10.1038/363274a0. [DOI] [PubMed] [Google Scholar]
- Cetin A, Komai S, Eliava M, Seeburg PH, Osten P. Stereotaxic gene delivery in the rodent brain. Nat Protoc. 2006;1:3166–3173. doi: 10.1038/nprot.2006.450. [DOI] [PubMed] [Google Scholar]
- Shih AY, Mateo C, Drew PJ, Tsai PS, Kleinfeld D. A polished and reinforced thinned-skull window for long-term imaging of the mouse brain. J Vis Exp. 2012. [DOI] [PMC free article] [PubMed]
- Drew PJ, et al. Chronic optical access through a polished and reinforced thinned skull. Nat Methods. 2010;7:981–984. doi: 10.1038/nmeth.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih AY, et al. Two-photon microscopy as a tool to study blood flow and neurovascular coupling in the rodent brain. J Cereb Blood Flow Metab. 2012;32:1277–1309. doi: 10.1038/jcbfm.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi JG, et al. Characterizing ligand-gated ion channel receptors with genetically encoded Ca2+ sensors. PLoS One. 2011;6:e16519. doi: 10.1371/journal.pone.0016519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerboom J, et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J. Neurosci. 2012;32:13819–13840. doi: 10.1523/JNEUROSCI.2601-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmchen F, Denk W. Deep tissue two-photon microscopy. Nat Methods. 2005;2:932–940. doi: 10.1038/nmeth818. [DOI] [PubMed] [Google Scholar]
- Svoboda K, Yasuda R. Principles of two-photon excitation microscopy and its applications to neuroscience. Neuron. 2006;50:823–839. doi: 10.1016/j.neuron.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Cui G, et al. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature. 2013;494:238–242. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]


