Abstract
Mercury (Hg) bioaccumulation in fish poses well-known health risks to wildlife and humans through fish consumption. Yet fish Hg concentrations are highly variable, and key factors driving this variability remain unclear. One little studied source of variation is the influence of habitat-specific feeding on Hg accumulation in lake fish. However, this is likely important because most lake fish feed in multiple habitats during their lives, and the Hg and caloric content of prey from different habitats can differ. This study used a three-pronged approach to investigate the extent to which habitat-specific prey determine differences in Hg bioaccumulation in fish. This study first compared Hg concentrations in common nearshore benthic invertebrates and pelagic zooplankton across five lakes and over the summer season in one lake, and found that pelagic zooplankton generally had higher Hg concentrations than most benthic taxa across lakes, and over a season in one lake. Second, using a bioenergetics model, the effects of prey caloric content from habitat-specific diets on fish growth and Hg accumulation were calculated. This model predicted that the consumption of benthic prey results in lower fish Hg concentrations due to higher prey caloric content and growth dilution (high weight gain relative to Hg from food), in addition to lower prey Hg levels. Third, using data from the literature, links between fish Hg content and the degree of benthivory, were examined, and showed that benthivory was associated with reduced Hg concentrations in lake fish. Taken together, these findings support the hypothesis that higher Hg content and lower caloric content make pelagic zooplankton prey greater sources of Hg for fish than nearshore benthic prey in lakes. Hence, habitat-specific foraging is likely to be a strong driver of variation in Hg levels within and between fish species.
Keywords: Biomagnification, Growth dilution, Bioenergetics, Trophic transfer, Aquatic food webs, Contaminants
Graphical abstract
1. Introduction
In order to minimize mercury (Hg) exposure risks to humans and fish-consuming wildlife, there is a need to continue to refine and improve predictions of Hg concentrations in fish. Human consumption of fish is increasing worldwide (Fisheries and Agriculture Organization of the United Nations (FAO), 2014), and continues to be a primary source of Hg exposure to wildlife and humans (UNEP, 2003). Mercury exposure and its concomitant health risks are difficult to manage because fish Hg concentrations are highly variable, even within a species (Karimi et al., 2012; Sunderland, 2007). Fish obtain Hg from their diet (Hall et al., 1997), with Hg concentrations in fish prey, as well as bioenergetic factors such as fish growth and metabolism, known to influence fish Hg concentrations (Essington and Houser, 2003; Schindler et al., 1995; Trudel and Rasmussen, 2006; Ward et al., 2010).
To refine our understanding and improve predictions of fish Hg concentrations within and across systems, it is necessary to continue to identify and examine unexplored sources of Hg to fish. One potential source of variation that is not well understood is the effect of fish foraging in different habitats, where prey Hg and fish bioenergetic rates can differ widely. Many lake fish species consume invertebrate prey from both pelagic and nearshore benthic (shallow sediments and macrophytes along the shoreline) habitats for at least part of their lives (Schindler and Scheuerell, 2002; VanderZanden and Vadeboncoeur, 2002). For example, young-of-year largemouth bass (Hodgson et al., 1993; Post, 2003) and yellow perch of 1–3 years (Prout et al., 1990; Wu and Culver, 1992) eat both benthic and pelagic invertebrates (zooplankton) before switching to piscivory (Pelham et al., 2001; Post, 2003; Schindler et al., 1997a). Similarly, bluegill sunfish consume zooplankton as young-of-year, switch to benthic invertebrates as juveniles, then consume zooplankton as adults (Mittelbach and Osenberg, 1993; Osenberg et al., 1992; Werner and Hall, 1988). These cross-habitat diet shifts can occur on relatively short timescales, such as intermittently throughout the summer (Mittelbach, 1981) or on a diel basis (Baumann and Kitchell, 1974; Keast and Welsh, 1968). Further, piscivorous fish indirectly consume both benthic and pelagic prey by consuming forage fish. Evidence suggests that comparable amounts of benthic and pelagic-derived carbon are transferred to top predators. For instance, Vadeboncoeur et al. (2002) showed that an average of 65% of the diet of numerous forage and piscivorous fish species comes from benthic sources due to direct and indirect consumption. Clearly, cross-habitat prey consumption is neither rare nor insignificant. Therefore, the relative importance of benthic and pelagic sources of Hg to fish must be quantified to understand and predict patterns of Hg accumulation in lake fish.
Studies have begun to measure concentrations of Hg in nearshore benthic and pelagic prey as sources to fish in freshwater (Back et al., 2003; Chetelat et al., 2011; Gorski et al., 2003) and marine (Chen et al., 2014; Chen et al., 2009) ecosystems. These studies provide crucial information to understand Hg patterns in lower trophic level organisms that are key sources of Hg into the food web (Cleckner et al., 1999; Folt et al., 2002). However, existing evidence regarding the relative importance of freshwater zooplankton and benthic invertebrates as sources of Hg to lake fish is sparse and contradictory. Two studies suggest that while some benthic invertebrates have lower Hg concentrations than zooplankton (Back et al., 2003; Gorski et al., 2003), predacious water scorpions and notonectids have the highest Hg concentrations among all invertebrates, consistent with higher Hg in predators due to biomagnification. Among zooplankton, cladocerans have significantly higher Hg concentrations than copepods (Back and Watras, 1995; Pickhardt et al., 2002; Pickhardt et al., 2005; Watras et al., 1998).
Patterns of Hg concentrations among fish provide indirect evidence that in general, pelagic prey may be more important sources of Hg to fish than benthic prey. Gorski et al. (2003) found higher Hg concentrations in fish (adult pike and large adult yellow perch) in a lake with a pelagic-based food web compared to a lake with a more benthic-based food web, even when fish trophic level, size and age were similar between lakes. Similarly, studies show that benthivorous lake fish have lower Hg concentrations than pelagic-feeding lake fish (Becker and Bigham, 1995; Kidd et al., 2003; Power et al., 2002; Willacker et al., 2013). Studies of estuarine and marine systems also found greater biomagnification of Hg (indicated by a higher slope of the Hg-δ15N relationship) in pelagic compared to benthic food chains (Lavoie et al., 2010), and that a higher degree of pelagic feeding (determined from δ13C) is associated with higher Hg concentrations in fish and shellfish (Chen et al., 2014; Chen et al., 2009; Karimi et al., 2013). Overall, these findings are consistent with higher Hg concentrations in zooplankton and other pelagic prey. Yet, there are numerous exceptions to this pattern. For example, one study found similar methylmercury (MeHg) concentrations among zooplankton and benthic invertebrates (Wyn et al., 2009). Also, benthic prey can be relatively more important Hg sources in contaminated (Eagles-Smith et al., 2008a; Eagles-Smith et al., 2008b) or shallow (Chumchal et al., 2008) lakes. Differences in relative Hg concentrations between benthic and pelagic invertebrates among studies likely depend on the taxa collected, and the sources of Hg to habitat-specific prey (e.g., concentrations of bioavailable Hg from sediments, consumption of detrital material versus periphyton or algae). Mercury concentrations for a given taxon can be highly variable (Tremblay et al., 1996), further complicating our ability to make general comparisons of Hg concentrations in lower trophic level taxa across ecosystems. Currently, there are insufficient data to assess the generality of these patterns across lake ecosystems, and compare the overall importance of benthic and pelagic invertebrates as sources of Hg to fish.
Past Hg studies also have not examined the potential influence of differences in benthic and pelagic prey quality on fish growth and subsequent somatic growth dilution of Hg (Karimi et al., 2007; Ward et al., 2010). Past research showed that rapid, efficient growth can reduce Hg concentrations in the body by decreasing the amount of Hg obtained from food relative to weight gain (Karimi et al., 2007; Karimi et al., 2010). This process of somatic growth dilution is hypothesized to be particularly important for MeHg, the organic, dominant form found in fish (Watras and Bloom, 1992), and other contaminants with low rates of efflux (excretion) that tend to persist in the body and biomagnify through the food chain (Karimi et al., 2010; Reinfelder et al., 1998). Among the factors that influence fish growth, those that most strongly increase growth efficiency (weight gain relative to consumption, and Hg intake) are more likely to result in somatic growth dilution (Karimi et al., 2007; Karimi et al., 2010; Trudel and Rasmussen, 2006). Such factors include prey quality (caloric content, digestibility), and fish activity level (including energy expended to capture and consume prey), with higher prey quality and lower fish activity level hypothesized to increase somatic growth dilution (Trudel and Rasmussen, 2006). Despite the growing recognition of the influence of growth dilution on Hg bioaccumulation, the relative importance of differences in fish growth from habitat-specific diets on fish Hg content is unknown.
The overarching goal of this study was to compare nearshore benthic versus pelagic prey as sources of Hg to fish based on differences in prey Hg content and caloric content using three different approaches. The first approach was to conduct a field study to compare Hg concentrations in common nearshore benthic and pelagic invertebrates across five New England lakes, and over a summer season in one lake. We hypothesized that Hg concentrations would be lower in benthic invertebrates than pelagic zooplankton in the lakes included in this study. Second, to gain a better mechanistic understanding of fish Hg accumulation from different habitats, the second approach examined the influence of habitat-specific diet composition (nearshore benthic, pelagic or mixed-habitat) and prey quality on Hg accumulation in fish using a bioenergetics-Hg mass balance model parameterized with invertebrate and zooplankton Hg concentrations from the field studies and invertebrate and zooplankton energy densities or caloric content (cal g−1) from published studies. Third, the relationship between the degree of benthivory (proportion of nearshore benthic prey in the diet) and fish Hg content among fish species using data from the literature was examined.
2. Materials and methods
2.1. Field sampling approach and study sites
To compare Hg content in zooplankton and nearshore benthic invertebrates, zooplankton and nearshore benthic invertebrates from multiple lakes (multi-lake study), and over a summer season in one lake (seasonal study) were collected. These organisms were collected in 2003 and 2004 for a study examining overall trace metal composition and variability. We include them in this study to focus on Hg patterns. For the multi-lake study, total Hg concentrations were measured for common nearshore benthic invertebrate taxa in 5 lakes in New Hampshire and Vermont United States in July and August 2003. We chose lakes that ranged from oligotrophic to eutrophic (Gregg Lake (NH), Post Pond (NH), Canobie Lake (NH), Weatherhead Hollow Pond (VT) and Horseshoe Pond (NH)), to include variation in Hg concentrations across lake types. Detailed lake characteristics are provided elsewhere (Chen et al., 2000; Ward et al., 2012). Briefly, all sites have no known point sources of Hg pollution and had no major land use changes between sampling periods. For the multi-lake study, we used Hg concentrations in zooplankton collected from 3 of the 5 lakes (Gregg Lake, Post Pond and Horseshoe Pond) in 2001–2002 from a previous study (Ward et al., 2012). We examined the potential for, and found no evidence of, interannual differences in prey Hg content (see 2.4 Statistical analysis of field data). In the multi-lake study, samples were collected within one month to minimize seasonal variation. For the seasonal study conducted in mesotrophic Post Pond, Hg concentrations of a subset of nearshore benthic invertebrate taxa were compared to zooplankton composite samples each month from June through October 2004. Mercury concentration data from the multi-lake and seasonal studies were analyzed separately.
2.2. Invertebrate collection
Nearshore benthic invertebrates from shallow sediments and shoreline macrophytes, where many lake fish species commonly feed, were collected using a stratified random design. Three to five macrophyte stands were identified at each lake. Organisms were collected from three random subsections within each stand. Collection and sorting procedures used trace metal clean techniques (Back et al., 2003; Chen et al., 2000). A 250 μm mesh d-frame net was used to collect benthic organisms from macrophytes by sweeping, and by dragging across the top 5 cm of sediments. For each lake, net contents were sieved through 250 μm mesh and pooled across stands and subsections into polyethylene buckets with filtered lake water for transport. Bulk zooplankton samples were collected by vertical tows using a 202 μm plankton net at maximum lake depth.
Common benthic invertebrate taxa, representing different functional groups, were collected from each lake in the multi-lake study. These taxa included amphipods (grazers), libellulids (dragonfly naiads, predators), coenagrionids (damselfly naiads, predators), chironomids (grazers, detritivores, predators), snails (grazers) and unionid bivalves (filter feeders). In the seasonal study, macrozooplankton (>202 μm) and a subset of soft-bodied benthic invertebrate taxa collected in the multi-lake study (amphipods and coenagrionids), were collected. The taxonomic resolution of the samples varied, and included subphylum (zooplankton; crustacea), order (amphipod; amphipoda) and family (chironomids, chironomidae; damselflies, coenagrionidae; dragonflies, libellulidae; snails, planorbidae and viviparidae; bivalves, unionidae). Bulk zooplankton samples were collected using trace metal clean techniques. In the multi-lake study, zooplankton were collected as in Chen et al. (2000) and Ward et al. (2012). Briefly, zooplankton were collected from 1 to 5 vertical tows in the deepest part of the lake using a >202 μm plankton net. Zooplankton from multiple tows were split equally among 3 replicate samples for Hg analysis, and 3 replicate samples for paired biomass measurements. For Hg analysis, zooplankton were filtered onto Teflon® filters and placed into Teflon® vials in the field. Sample blanks consisting of Teflon filters filtered with deionized water in the field were taken to account for background Hg concentrations. For biomass measurements used to calculate mass-specific Hg concentrations for the multi-lake study zooplankton were filtered using preflushed glass fiber filters in the field. In the seasonal study, 3 replicate zooplankton samples per sampling date were taken for both Hg and biomass measurements, and were filtered onto preweighed Teflon® filters and placed into Teflon® vials in the field. For the seasonal study, duplicate zooplankton samples were preserved as a reference for taxonomy and abundance of different zooplankton taxa at each sampling point. Taxonomy samples were anaesthetized in carbonated water and preserved in buffered formalin sucrose solution (Stemberger and Lazorchek, 1994). Abundance and biomass of individual plankton taxa were estimated from 3 replicate 1 ml subsamples as in Stemberger and Lazorchek (1994).
Benthic organisms were sorted live in the lab, by taxa, into Teflon® sample vials within 48 h of collection. Organisms were triple-rinsed and placed in nanopure water for 1–2 h to allow stomach contents to depurate, although complete depuration is unlikely. Snails were rinsed, frozen, thawed, and removed from their shells before Hg analysis. Sample blanks (nanopure rinse water in Teflon vials) were collected throughout the sorting period to account for background Hg levels. For each taxon, three replicates were taken per lake (multi-lake study), or per month (seasonal study). Samples consisted of single individuals for larger organisms (libellulids). For smaller organisms, samples consisted of composites (~20 chironomids or amphipods), to ensure detectable Hg levels. We preserved representative individuals of each benthic taxon when possible to serve as body size and taxonomy references in an effort to keep these factors consistent between sampling points.
2.3. Mercury analysis
Invertebrate samples were analyzed for total Hg (the sum total of organic and inorganic Hg species) with a high resolution inductively-coupled plasma mass spectrometer (HR-ICP-MS, Finnigan MAT, Bremen, Germany) at the Trace Element Analysis Core Facility at Dartmouth College. Hg analysis was conducted within five months of sample collection. Total Hg includes both inorganic and organic forms of Hg. The organic form, MeHg, is the dominant form of Hg found in fish (Watras and Bloom, 1992), and tends to biomagnify in aquatic food chains (Watras et al., 1998). However, total Hg was measured because of the lower cost of sample analysis, greater ability to measure accurate concentrations in small biomass samples, and studies have found strong relationships between total Hg content in fish and their invertebrate prey (Ward et al., 2010). Benthic samples were frozen in Teflon® vials, lyophilized, weighed, acidified and homogenized with trace metal grade 70% HNO3 (Seastar®) and H2SO4. Sulfuric acid was added to digest total P for a separate study (Karimi and Folt, 2006). Benthic invertebrate samples were microwave digested and diluted with nanopure water before analysis. Zooplankton samples were digested with trace metal grade 70% HNO3 (Seastar®) and H2SO4 on a 100 °C hot plate overnight in a trace metal-clean hood. Quality control procedures reported elsewhere (Chen et al., 2000; Karimi and Folt, 2006) included digesting and analyzing standard reference materials (DORM-2, NRC-CNRC Canada, Prawn CRM, China, and a standard aqueous reference material), and recovery rates ranged from 95 to 105%. We subtracted lab blank concentrations from samples of the same sorting period only when the mean blank concentrations were significantly above zero. The detection limit was 2 ng g−1. Sample Hg concentrations below detection (N = 9 for the study) were assumed to be at a concentration of one-half the detection limit (Clarke, 1998).
2.4. Statistical analysis of field data
To evaluate differences between nearshore benthic and pelagic prey across lakes in the multi-lake study, we compared Hg concentrations in benthic invertebrates (collected in 2003) to Hg concentrations in macrozooplankton (>202 μm) collected in August 2001 (Gregg Lake), and 2002 (Horseshoe Pond and Post Pond) (Ward et al., 2012). To minimize effects of seasonal differences in the multi-lake study, we analyzed zooplankton sampled on particular dates in August (2001 in August (2002) that most closely matched dates for benthic invertebrate sampling (August 2003). The potential for significant inter-annual variation in invertebrate Hg concentrations was examined by comparing zooplankton Hg concentrations in Post Pond between 2001–2002 samples and 2004 by a one-way ANOVA using JMP 5.01 (SAS Inst., Cary, NC). There were no significant differences in zooplankton Hg concentrations among sampling years in July (P 0.55), August (P = 0.56), and September (P = 0.13). Earlier analyses also show that between-lake differences in metal concentrations of aquatic biota significantly exceed between-year variation within lakes (Chen et al., 2000). Benthic invertebrate and zooplankton Hg concentrations were compared in Gregg Lake, Post Pond and Horseshoe Pond each by a one-way ANOVA using JMP 5.01. Two outliers out of 60 total samples from the multi-lake study were removed for each quantitative analysis. Both outliers were amphipod samples from two different lakes that had element concentrations that were orders of magnitude higher than the remaining replicates from the same lakes, indicating sample contamination. Removing both outliers did not change the qualitative results. Tukey’s HSD was used to make multiple comparisons among taxa. Significant differences were assessed with a Bonferroni correction (α = 0.05).
For the seasonal study, Hg concentrations among invertebrates in Post Pond over the summer season were compared with a two-way MANOVA-Repeated Measures (MANOVAR) on log10−transformed values, analyzing the effects of taxonomic identity, sampling month and their interaction on Hg concentrations in benthic invertebrates and zooplankton using JMP 5.01.
2.5. Modeling fish mercury accumulation
To predict the effects of prey quality (caloric content) on fish Hg level, fish Hg accumulation under four habitat-specific diet scenarios were modeled using Fish Bioenergetics 3.0 (Hanson et al., 1997). This model uses empirically derived physiological rates for a variety of fish species to estimate growth, metabolism, waste losses and contaminant accumulation, given user-defined parameters such as those describing diet and prey Hg concentrations. Bluegill sunfish (Lepomis macrochirus) was selected as a model species because, among fish with known physiological rates in the Fish Bioenergetics 3.0 model, their foraging behaviors across lake habitats are well-studied (Mittelbach and Osenberg, 1993; Osenberg et al., 1992; Werner and Hall, 1988). Hg concentrations in typical, individual forage fish were estimated by parameterizing the model with invertebrate prey Hg concentrations from the multi-lake field study (Tables 1, 2) and prey energy densities (caloric content, cal g−1) from the literature (Table 2). Values for Hg assimilation efficiency and depuration were based on studies of MeHg, because it is the form of Hg that is primarily trophically transferred and biomagnified through the food web (Watras et al., 1998). The percent MeHg of total Hg can vary widely for both benthic invertebrates (e.g., 17–93% for dytiscids, 51–84% for odonates, Tremblay et al., 1996) and for zooplankton (e.g., 30–70% (Back et al., 2003), or 11–83% (Watras et al., 1998) for >153 μm size fraction). Therefore, we assumed that percent MeHg of total Hg concentrations in the prey was 50% for all invertebrates, and that fish assimilated MeHg with the same efficiency (0.8) from all prey types (Wang and Wong, 2003). We also assumed that Hg depuration was a function of temperature and fish weight, given previously described relationships for MeHg (Trudel and Rasmussen, 1997). Temperature (20 °C), prey energy densities, MeHg assimilation efficiency and prey Hg concentrations were held constant.
Table 1.
Mercury concentrations in benthic invertebrates and zooplankton from 5 NH and VT lakes (means based on dry weights ± SD). N = 3 for each taxon and lake, except N = 2 amphipods in both Horseshoe and Post Pond. Means across lakes are presented in bold for each taxon.
| Taxon | Site | Mean Hg (ng g−1) ± SD |
|---|---|---|
| Amphipoda | Canobie Lake | 131 ± 3 |
| Gregg Lake | 155 ± 40 | |
| Horseshoe Pond | 32 ± 41 | |
| Post Pond | 106 ± 27 | |
| Weatherhead Hollow Pond | 1 ± 0 | |
| MEAN | 88 ± 67 | |
| Chironomidae | Canobie Lake | 151 ± 25 |
| Gregg Lake | 75 ± 68 | |
| Horseshoe Pond | 54 ± 13 | |
| Post Pond | 147 ± 34 | |
| Weatherhead Hollow Pond | 148 ± 18 | |
| MEAN | 115 ± 53 | |
| Crayfish | Post Pond | 74 ± 12 |
| Weatherhead Hollow Pond | 129 ± 23 | |
| MEAN | 102 ± 34 | |
| Odonata (Gomphidae) | Post Pond | 70 ± 31 |
| Odonata (Libellulidae) | Canobie Lake | 264 ± 222 |
| Gregg Lake | 101 ± 14 | |
| Horseshoe Pond | 34 ± 38 | |
| Post Pond | 176 ± 22 | |
| Weatherhead Hollow Pond | 94 ± 26 | |
| MEAN | 134 ± 119 | |
| Snail | Gregg Lake | 137 ± 10 |
| Horseshoe Pond | 127 ± 55 | |
| Post Pond | 515 ± 240 | |
| Weatherhead Hollow Pond | 17 ± 28 | |
| MEAN | 205 ± 234 | |
| Unionidae | Post Pond | 144 ± 44 |
| Zooplankton (>202 μm) | Gregg Lake | 351 ± 75 |
| Horseshoe Pond | 199 ± 37 | |
| Post Pond | 249 ± 204 | |
| MEAN | 266 ± 129 | |
| Odonata (Coenagrionidae) | Canobie Lake | 212 ± 103 |
| Gregg Lake | 288 ± 27 | |
| Horseshoe Pond | 52 ± 3 | |
| Post Pond | 378 ± 158 | |
| Weatherhead Hollow Pond | 13 ± 19 | |
| MEAN | 188 ± 160 |
Table 2.
Summary of habitat-specific diet scenario conditions and the predicted effects on fish Hg accumulation.
| Diet variables
|
Fish responses
|
||||||
|---|---|---|---|---|---|---|---|
| Diet composition % | Prey energy density (J g−1 w.w.)a | Prey Hg ± SE(ng g−1)b | Fish Hg (mg kg−1 w.w.)c | Hg uptake (mg)c | Specific consumption rate (J g−1 d−1)c | Specific growth rate (J g−1 d−1)c | Specific respiration rate (%)(J g−1 d−1)c |
| Benthic diet | |||||||
| Libellulids (48) | 4284 | 134 ± 31 | |||||
| Gastropods (22) | 1807 | 205 ± 70 | |||||
| Chironomids (19) | 2756 | 115 ± 14 | |||||
| Coenagrionids (7) | 3365 | 188 ± 41 | |||||
| Amphipods (4) | 3924 | 88 ± 19 | |||||
| MEAN (Min Hg, Max Hg) | 3370 | 148 (111, 184) | 0.14 (0.11, 0.17) | 0.02 (0.01, 0.03) | 202 | 63 | 62 |
| Cladoceran-dominated pelagic diet | |||||||
| Cladocerans (75) | 2202 | 380 ± 61 | |||||
| Copepods (25) | 2310 | 152 ± 25 | |||||
| MEAN (Min Hg, Max Hg) | 2229 | 323 (271, 375) | 0.40 (0.33, 0.46) | 0.05 (0.04, 0.06) | 134 | 21 | 61 |
| Copepod-dominated pelagic diet | |||||||
| Cladocerans (25) | 2202 | 380 ± 61 | |||||
| Copepods (75) | 2310 | 152 ± 25 | |||||
| MEAN (Min Hg, Max Hg) | 2283 | 209 (175, 243) | 0.25 (0.21, 0.29) | 0.03 (0.03, 0.04) | 137 | 23 | 61 |
| Mixed diet | |||||||
| Benthic Prey (50) | |||||||
| Libellulids (24) | 4284 | 134 ± 31 | |||||
| Gastropods (11) | 1807 | 205 ± 70 | |||||
| Chironomids (9.5) | 2756 | 115 ± 14 | |||||
| Coenagrionids (3.5) | 3365 | 188 ± 41 | |||||
| Amphipods (2) | 3924 | 88 ± 19 | |||||
| Pelagic Prey (50) | |||||||
| Cladocerans (37.5) | 2202 | 380 ± 61 | |||||
| Copepods (12.5) | 2310 | 152 ± 25 | |||||
| MEAN (Min Hg, Max Hg) | 2800 | 235 (191, 280) | 0.25 (0.22, 0.28) | 0.04 (0.03, 0.04) | 168 | 42 | 61 |
Prey energy density values from (Cummins and Wuycheck, 1971) and converted using dry weight: wet mass ratios from (Dumont et al., 1975) (Dumont et al., 1975) for zooplankton, (McPeek et al., 2001) (McPeek et al., 2001) for coenagrionids and assumed to be 0.2 for libellulids (Cummins and Wuycheck, 1971) (Cummins and Wuycheck, 1971).
Prey Hg values are from Table 1. See Methods for calculations of cladoceran and copepod values.
At 3.3 g wet weight.
The effects of four habitat-specific diet scenarios on fish MeHg content were tested. Scenario parameter values are summarized in Table 2. To test for effects of previously observed differences in Hg concentrations between cladocerans and copepods on fish Hg accumulation, diet scenarios included 2 pelagic diets, a cladoceran-dominated diet (75% cladoceran, 25% copepod) and a copepod-dominated pelagic diet (75% copepod, 25% cladoceran). Cladoceran and copepod Hg concentrations were estimated from the mean bulk zooplankton Hg concentration from multiple lakes (Table 1). We assumed that cladocerans and copepods each comprised an equal proportion (50%) of the bulk zooplankton samples, and that the cladoceran concentration was 2.5 times higher than copepods (Pickhardt et al., 2005). To calculate a standard error for each of the cladoceran and copepod Hg values, we assumed that both cladoceran and copepod Hg concentrations had the same coefficient of variation and sample size (9) as the bulk zooplankton samples.
A mixed diet (50% cladoceran-dominated pelagic diet, 50% nearshore benthic diet) and a nearshore benthic diet scenario (Table 2) were also included. Diet composition for bluegill, in lakes of this region, are not well described. Therefore, the nearshore benthic diet was based on gut content data for pumpkinseed (Lepomis gibbosus) from Post Pond (Dionne, 1991; Dionne and Folt, 1991). Also, pumpkinseed fish are a congener of bluegill, thus are more likely to have similar physiological rates to bluegill, than other shoreline-feeding fish species. However, pumpkinseed consume snails, (Mittelbach et al., 1992; Osenberg et al., 1992) while bluegill and other common lake fish that feed on nearshore benthos do not. Therefore, we noted the general implications of including snails in the benthic diet component of the model scenarios, and how diets of forage fish that do not specialize on snails would qualitatively compare.
For each diet, simulations were run for mean, minimum, and maximum prey Hg concentrations based on mean ± SE of Hg content for each prey taxa in the diet. Simulations were based on physiological rates for small, juvenile bluegill sunfish (L. macrochirus), with a starting size of 1 g, and run for 2 years with a daily time step. Simulations were run assuming a daily ration of 6% fish wet mass per day, similar to that observed for bluegill in the field (Keast and Welsh, 1968). Small juvenile fish were used as a model to capture differences in Hg bioaccumulation before ontogenetic diet shifts occur. Fish MeHg concentrations were compared once fish reached 3.3 g wet weight. Beyond this size, both pumpkinseed and bluegill are known to shift their diets (Osenberg et al., 1988).
Finally, sensitivity of the model to differences in %MeHg of total Hg in each diet scenario were examined. For each diet scenario, fish Hg accumulation was modeled in response to a minimum and maximum MeHg content in the diet based on the widest range of %MeHg values reported from the literature, and the minimum and maximum total Hg values for the diet. Specifically, for a given scenario, the minimum MeHg concentration was calculated as the minimum %MeHg ∗ minimum total Hg, and the maximum MeHg concentration was calculated as the maximum %MeHg ∗ maximum total Hg, for that diet. We assumed the %MeHg for nearshore benthic diet ranged from 17 to 93% (Tremblay et al., 1996), and 11–83% for the pelagic diet (Watras et al., 1998). We assumed the %MeHg for the mixed diet contained the widest range of 11–93%, reflecting the combined range of benthic and pelagic prey. The range of predicted fish MeHg content resulting from the range of MeHg content in prey was compared across diet scenarios at the benchmark fish size of 3.3 g.
2.6. Analysis of literature data
Finally, the relationship between fish Hg content and the degree of benthivory (proportion of fish diet from nearshore benthic prey) across lake fish species was examined using data from the literature. Specifically, principal component analysis was conducted to compare the Hgbenthivory relationship relative to other diet-related factors (fish trophic position (a continuous measure of position in the food web) and body size) that are known to influence fish Hg levels (Cabana et al., 1994; Karimi et al., 2013). Mean Hg fillet concentrations and mean length for common fish species were obtained from a study summarizing these values across lakes from the northeastern United States (Kamman et al., 2005). Estimates of the degree of benthivory (benthivory index) were used from one study, based on the proportion of benthic prey in the diet (VanderZanden and Vadeboncoeur, 2002). Trophic position values were obtained for the same fish species from studies that based estimates on fish diet, or gut content data (percent prey item in diet by volume) and trophic position of prey (VanderZanden et al., 1997; VanderZanden and Rasmussen, 1996). From the study by VanderZanden and Rasmussen (1996), values for Class 2 Lakes that contained forage fish and lacked the invertebrate predator Mysis relicta, and values for warm-water lake trout, were used. In addition, trophic position was estimated for 6 fish species that did not have estimates from these studies, based on general knowledge of feeding habits for these fish. Specifically, we assumed that these species (longnose sucker, white sucker, bluegill, brown bullhead) are forage fish with relatively lower trophic positions compared to piscivorous fish in the dataset (Lake et al., 2001; Power et al., 2002; VanderZanden et al., 1997; Werner and Hall, 1988). The final, merged dataset included 14 common lake fish species (Supplementary Table A1). Thus, the degree of benthivory, Hg content, body size, and trophic position values in this dataset were obtained from different sources and lake systems. Therefore, in combining these datasets, we assume values for these factors are similar across lakes for a given species.
3. Results
3.1. Multi-lake comparisons
Taxonomic differences in Hg concentrations for invertebrates varied across lakes. However, some consistent taxonomic patterns emerged. Pelagic zooplankton generally had higher Hg concentrations than most soft-bodied nearshore benthic invertebrates in the same lakes (Fig. 1). Snails and damselfly larvae also had similar, high Hg concentrations compared to zooplankton in two of the three lakes. In each lake, there were significant differences in invertebrate Hg concentrations among taxa (F4,10 = 17.05, P = 0.0002, Gregg Lake; F4,9 = 16.83, P = 0.0003, Horseshoe Pond; F4,9 = 2.04, P = 0.1715, Post Pond). Zooplankton had significantly higher Hg concentrations than benthic invertebrates except damselfly naiads in Gregg Lake (Tukey’s HSD, Fig. 1). Zooplankton Hg concentrations were significantly higher than those of all benthic invertebrates except snails in Horseshoe Pond. However, zooplankton and benthic invertebrates shared similar Hg concentrations in Post Pond. These same patterns were consistent when comparing with other size fractions of zooplankton (45–100, 100–202 μm Ward et al., 2012). Among the benthic taxa, relative Hg concentrations were variable across lakes. For example, snails had among the highest Hg concentrations in Post Pond, but not in the other two lakes. Across lakes, odonates had consistently low (libellulid dragonfly larvae) or variable (damselfly larvae) Hg levels, even though odonates prey on other invertebrates, and are higher in the benthic food chain. Also, among benthic taxa, chironomids had consistently low Hg content across lakes.
Fig. 1.
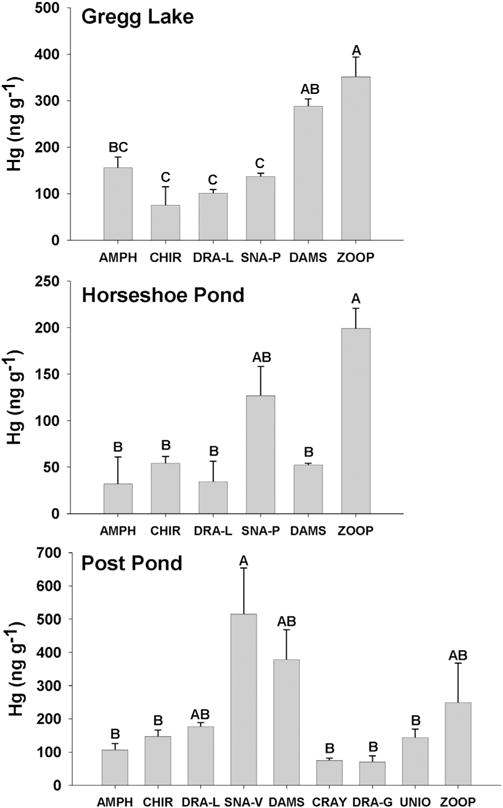
Mean Hg concentrations (±SE) in zooplankton (2001) and benthic invertebrates (2003) in three lakes (n = 3 per taxon per lake; n = 2 for snails in Gregg Lake, and amphipods in Horseshoe and Post Pond). Groups with the same letter indicate nonsignificant differences. Taxa include amphipods (AMPH), chironomids (CHIR), dragonflies (libellulidae, DRA-L and gomphidae, DRA-G), snails (planorbidae, SNA-P and viviparidae, SNA-V), damselflies (DAMS), crayfish (CRA), unionids (UNIO) and zooplankton (ZOOP).
3.2. Seasonal comparisons
While the quantitative differences in Hg concentrations among taxa varied over the season (F8,8 = 4.23, P = 0.03), zooplankton clearly and consistently had higher Hg concentrations than both amphipods and damselfly larvae throughout a season (June–Oct) in Post Pond (Fig. 2). Seasonal differences in overall invertebrate Hg concentrations were marginally significant (F4,3 = 9.28, P = 0.05). Seasonal dynamics were notably more dramatic in zooplankton Hg content than in the benthic invertebrates. Zooplankton Hg concentrations were particularly high in September, during destratification, at which time Daphnia comprised a much larger portion (82%) of the macrozooplankton assemblage compared to other months (Fig. 2).
Fig. 2.
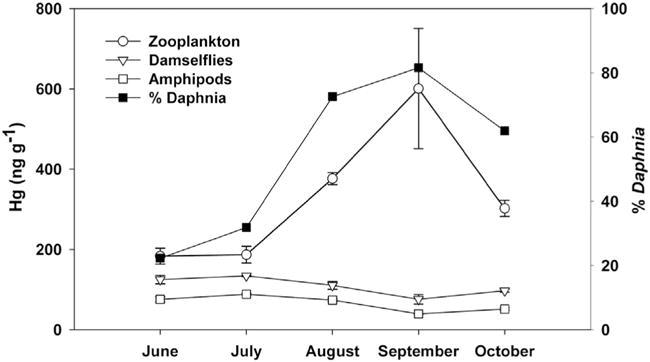
Seasonal Hg concentrations (ng g−1 dry weight) in zooplankton, coenagrionid odonates (damselflies) and amphipods from Post Pond, 2004. Means ± SE are shown for each month, n = 3 for each taxon per month. Filled squares show the proportion of Daphnia comprising the zooplankton assemblage at each time point (based on μg dry weight L−1).
3.3. Model results
The bioenergetic model estimated that Hg concentrations are greatest in fish consuming a cladoceran-dominated, pelagic diet due to higher cladoceran Hg concentrations as well as lower fish growth rates. Fish Hg concentrations were highest from the cladoceran-dominated pelagic diet, lowest from the nearshore benthic diet, and intermediate from the copepod-dominated pelagic diet and mixed near-shore benthic-pelagic diet (Fig. 3, Table 2). This pattern was robust to fish size, and thus would be qualitatively the same if the analysis started with a larger sized juvenile fish. At the benchmark size of 3.3 g wet weight, the benthic diet resulted in a mean Hg concentration 65% lower than the cladoceran-dominated pelagic diet (Table 2). Even though daily ration was fixed for all diet scenarios, specific growth rate was highest in fish consuming the nearshore benthic diet due to higher mean prey quality (prey energy density, Table 2). Due to the faster growth rate, specific consumption rate, which scales with body size, was also highest in fish consuming the nearshore benthic diet. When prey Hg concentrations were held constant (equal to the mean for the mixed diet), the range in prey quality resulted in fish consuming the highest quality prey to have 25% lower Hg concentrations than fish consuming the lowest quality prey (Fig. 4).
Fig. 3.
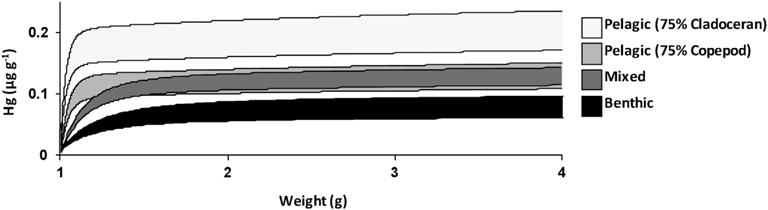
The predicted range of Hg concentrations for given fish sizes based on four habitat-specific diet scenarios.
Fig. 4.
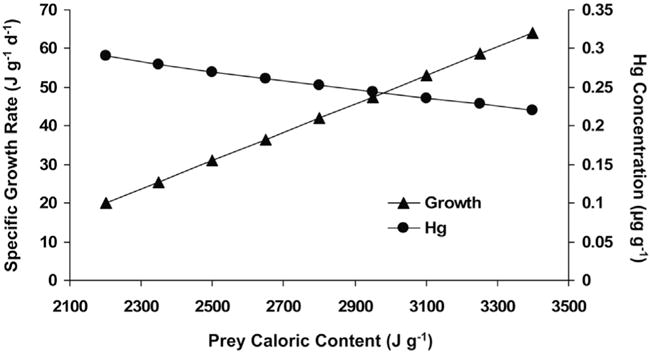
The effect of invertebrate prey caloric content on fish growth rate and fish Hg concentration.
The analysis of the sensitivity of habitat-specific fish Hg bioaccumulation to variability in %MeHg in the diet shows overlap in fish MeHg content from these diets at low fish MeHg levels when prey MeHg concentrations are low (i.e., when %MeHg or total Hg in prey is low). However, fish MeHg concentrations can reach higher maximum values from the pelagic, cladoceran-dominated-diet. This higher maximum MeHg value for pelagic fish occurs even though the %MeHg reaches a lower maximum in pelagic zooplankton (83%) compared to benthic invertebrates (93%). Thus, the higher maximum values for fish MeHg from cladoceran-dominated diet was due to the combination of higher maximum total Hg, and lower caloric content of pelagic prey relative to the other diets.
3.4. Relationship between fish Hg and benthivory from the literature
Principal component analysis showed a clear, negative relationship between fish Hg and the degree of benthivory, and positive relationships between fish Hg, trophic position, and fish length (Fig. 5). These associations were reflected in Component 1, for which fish Hg, trophic position, and length loaded positively, and benthivory loaded negatively (Table 3). Fish length also loaded positively on Component 2 (Table 3). Together, the first two components explained 85% of the variation among observations in the dataset.
Fig. 5.
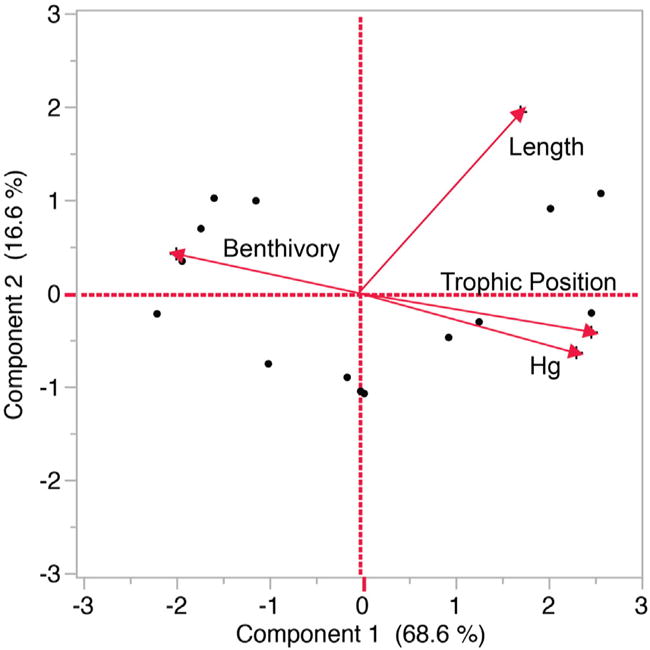
PCA biplot on mean values across fish species. Hg content is negatively related to benthivory, and positively related to fish length and trophic position.
Table 3.
Principal component eigenvalues, percent variance explained, and variable loadings (loadings with an absolute value >0.4 in bold).
| Component 1 | Component 2 | |
|---|---|---|
| Eigenvalue | 2.74 | 0.66 |
| % Variance | 68.58 | 16.62 |
| Cumulative % | 68.58 | 85.20 |
| Benthivory | −0.75 | 0.16 |
| Trophic position | 0.96 | −0.16 |
| Hg | 0.90 | −0.25 |
| Length | 0.67 | 0.74 |
4. Discussion
Results from this study support the hypothesis that the consumption of nearshore benthic prey reduces Hg bioaccumulation in fish due to generally lower Hg concentrations in benthic invertebrates, and higher prey quality compared to pelagic zooplankton. Understanding processes that influence these Hg patterns in lower trophic level organisms, particularly as they relate to food quality and growth, is important, because these organisms are key sources of Hg and other contaminants to the food web.
Findings from this study show that zooplankton generally have higher Hg concentrations than most nearshore benthic invertebrate taxa measured in this study. Concentrations of many other metals (As, Se, Zn) from the same field samples also were higher in zooplankton than nearshore benthic invertebrates (Karimi and Folt, 2006). There are notable exceptions to this general pattern. For example, in Post Pond, Hg concentrations in zooplankton and benthic invertebrates were similar in the multi-lake study (Fig. 1), but significantly higher in zooplankton in the seasonal study (Fig. 2), possibly due to differences in the life stage and ages of the benthic invertebrates sampled between field studies, or due to introduced variability from measuring biomass in paired samples, separate from the Hg samples, in the multi-lake study. Second, snails had the highest Hg concentrations in Post Pond, but not in Gregg Lake and Horseshoe Pond, possibly because snails in Post Pond (pulmonate snails) were physiologically and taxonomically distinct from snails in the other lakes (prosobranch, or gill-breathing, snails). Third, damselfly larvae also had relatively high Hg content compared to other taxa in two lakes, consistent with previous studies that found other predacious benthic invertebrate taxa to have high Hg content (Back et al., 2003; Gorski et al., 2003). Finally, evidence from the literature suggests that while total Hg concentrations are generally higher in zooplankton, MeHg content is highly variable in both zooplankton and benthic invertebrates (Back et al., 2003; Kainz et al., 2002; Tremblay et al., 1996; Watras et al., 1998). Thus, contrasting concentrations of organic Hg species in lower trophic level organisms from different lake habitats (e.g., Chetelat et al., 2011) merits further study. Overall, results from this and previous studies (Back et al., 2003; Gorski et al., 2003), suggest that while many common benthic invertebrate taxa have lower Hg content than pelagic zooplankton, exceptions to this pattern (e.g., in shallow (Chumchal et al., 2008) or contaminated lakes (Eagles-Smith et al., 2008a; Eagles-Smith et al., 2008b)) occur and are not well understood. Future work should identify the major factors that drive Hg accumulation in invertebrates and other lower trophic level organisms in benthic and pelagic lake habitats. Differences in MeHg at the base of the food web (periphyton, phytoplankton), body size, lifestage, and bioenergetic factors are likely to influence Hg patterns in benthic and pelagic invertebrates.
In addition to Hg content in prey, findings from this study also show that prey quality can strongly influence Hg bioaccumulation in fish through somatic growth dilution. Specifically, the model results show that consuming a nearshore benthic diet reduces Hg content in fish due to higher caloric content, and lower Hg content in the benthic invertebrates included in this study (Table 2). As a result, benthivorous fish obtained more energy per gram prey consumed, grew faster than planktivores (Table 2), and increased their net biomass gain relative to the amount of Hg ingested from their prey, thus decreasing their weight-based Hg concentration. In the benthic and mixed diet scenarios, snails were included in the benthic diet component. On average, gastropods have lower caloric content and higher Hg content than other benthic taxa included in these scenarios (Table 2). Therefore, excluding snails from the diet would result in even lower Hg accumulation in benthivorous fish other than pumpkinseed that do not consume snails.
Studies have shown somatic growth dilution of Hg in fish populations in the field (Harris and Bodaly, 1998; Ward et al., 2010). However, less is known about growth dilution of Hg in lower trophic level organisms (e.g., Karimi et al., 2007; Karimi et al., 2010). In general, prey quality, respiration, and other factors that influence growth rate and efficiency should be examined relative to Hg bioaccumulation in fish and lower trophic level organisms to gain a broader understanding of Hg trophic transfer in lake and other aquatic food webs. Other prey characteristics (e.g. digestibility, handling time, nutrient content), as well as systematic differences in fish bioenergetic rates between habitats not tested in this study, may also influence fish growth. Little is known about the overall, relative quality of benthic invertebrates and zooplankton for fish growth. Zooplankton, particularly Daphnia, are generally higher in phosphorus content (Elser et al., 1996) than benthic invertebrates (Frost et al., 2003), and may be of higher quality for fish that are phosphorus-limited. Similarly, differences in prey fatty acid content may influence relative prey quality and fish growth (Copeman et al., 2002; Kainz et al., 2006). Alternatively, when fish are energy-limited (Schindler and Eby, 1997b), benthic invertebrates may be of higher quality due to their higher caloric content. In this study, differences in prey Hg concentrations had a much stronger influence (65% difference) on fish Hg accumulation than prey quality (25% difference). However, one laboratory study found strong effects of algal nutrient quality on Hg accumulation in Daphnia (Karimi et al., 2007) via the process of growth dilution. Thus, the overall importance of growth dilution of Hg driven by habitat associated differences in prey quality, or other factors, may vary widely.
Consistent differences in bioenergetic factors and food sources at the base of the food web among invertebrate taxa may also explain higher Hg concentrations in zooplankton, as well as the exceptions to this general pattern. Zooplankton, particularly large-bodied cladocerans, have exceptionally high filtering rates (Haney, 1973), thus are likely to ingest food-borne Hg at a greater rate than benthic invertebrates, and may explain higher Hg content in cladocerans among zooplankton (Back and Watras, 1995; Pickhardt et al., 2002; Pickhardt et al., 2005; Watras et al., 1998). Zooplankton also have higher metabolic rates (Glazier, 2005) than benthic invertebrates (Hamburger and Dall, 1990). In particular, while metabolic costs decrease with increasing body size in benthic invertebrates, cladocerans and other pelagic organisms maintain a constant metabolic cost, possibly due to the larger demands required to maintain buoyancy and avoid predators in the pelagic zone compared to the sedentary behaviors typical of benthic organisms (Glazier, 2005; Glazier, 2006). As a result, zooplankton may increase their weight-based Hg concentrations by respiring a greater proportion of biomass per unit Hg ingested. Thus, increased metabolic demands in zooplankton and other pelagic organisms may make pelagic zones important conduit habitats of Hg and other food-borne-contaminants, in freshwater as well as marine systems. Bioenergetic processes may also explain differences in Hg and other metals among closely related benthic taxa. For example, pulmonate snails, including viviparidae in Post Pond, have higher respiration rates than prosobranch snails (Berg and Ockelmann, 1959), such as planorbidae in Horseshoe Pond and Gregg Lake. These higher respiration rates could result in higher weight-based Hg concentrations (Essington and Houser, 2003; Trudel and Rasmussen, 2006) as observed in Post Pond snails (Fig. 1). Finally, differences in Hg content among food sources at the base of the food web likely influence differences in Hg among benthic and pelagic invertebrates. Bioconcentration of Hg from water to phytoplankton is extremely high (Driscoll et al., 2007), and is considered the basis for Hg in the pelagic food web. In contrast, benthic feeders may derive some of their food intake from organic carbon in sediments that in some cases can have much lower MeHg concentrations than particulates (Balcom et al., 2015).
Results from this study show that zooplankton are particularly important sources of Hg to fish, due to high Hg concentrations in zooplankton, particularly in late summer, and lower caloric content. Seasonal shifts in zooplankton Hg concentrations coincided with Daphnia abundance, and were highest in late summer when Daphnia comprised a much larger percentage of the macrozooplankton assemblage (Fig. 2). This observation is consistent with studies that have shown cladocerans to have significantly higher Hg concentrations than copepods (Back and Watras, 1995; Pickhardt et al., 2002; Pickhardt et al., 2005; Watras et al., 1998). Seasonal variation in Hg also can result from abiotic factors such as destratification (Herrin et al., 1998), or biotic factors, such as periods of high phytoplankton or zooplankton abundance that can cause bloom dilution, or trophic dilution of Hg, respectively (Chen and Folt, 2005a; Chen et al., 2005b; Pickhardt et al., 2002; Walters et al., 2015). We did not observe dramatic seasonal variation in nearshore benthic invertebrate Hg concentrations, although this has been found for other metals in highly contaminated systems (Hare and Campbell, 1992). Seasonal variation in benthic invertebrate Hg concentrations may be lower because benthic invertebrates are longer lived, and therefore integrate Hg intake over a longer time compared to zooplankton, or due to lower variability in the Hg content in benthic food sources. Nevertheless, these results confirm findings from other studies that cladocerans, such as Daphnia, have higher Hg concentrations than other freshwater zooplankton taxa.
These findings, that zooplankton are conduits of Hg to fish due to relatively high Hg concentrations and lower prey quality, are an important step toward a thorough understanding of Hg trophic transfer. This study exemplifies how the trophic transfer of Hg, even in one step of the food chain from zooplankton to fish, is highly complex and involves many factors that should be more fully examined. First, differences in Hg content among benthic and pelagic prey are sensitive to the taxa examined. For example, snails and damselfly larvae each have variable Hg concentrations across lakes resulting in variable, relative differences in Hg content among taxa within each lake. Such variability challenges our ability to generalize both absolute and relative Hg concentrations for broad taxonomic groups. Second, the potential influence of seasonal and inter-annual variability in MeHg production and bioavailability on MeHg content among lower trophic level organisms warrants further study, in order to determine whether habitat-specific differences in prey MeHg content are consistent over time. Third, the shifts in Hg bioaccumulation and growth dilution that may occur across ontogenetic life stages of fish are not adequately known. The model analysis examines Hg bioaccumulation in the juvenile stage, and shows that differences in habitat-specific Hg content in fish are maintained as body size increases (Fig. 3). There is a need to build on this information to understand how habitat-specific patterns of Hg bioaccumulation change from juvenile to adult phases of growth, as ontogenetic diet shifts occur, influencing the relative importance of different prey items as sources of Hg.
In addition, studies that directly measure MeHg concentrations in small organisms rather than, or in addition to total Hg, would provide precision to estimate the transfer of MeHg, the more toxic, biomagnifying form of Hg, through the food web. Results from this study suggest that fish MeHg concentrations can reach higher maximum values from diets containing zooplankton, particularly cladoceran-dominated diets, even when %MeHg in the diet is highly variable. However, the wide range of %MeHg and total Hg values in both benthic and pelagic invertebrates may result in cases where pelagic feeding fish have similar, or lower MeHg concentrations than benthic feeding fish, even though prey quality and growth dilution of Hg from the pelagic diet is low. Currently, MeHg measurements on individual taxa are rare due to the difficulty detecting MeHg concentrations in organisms with low biomass. Differences in total Hg content can be similar to differences in MeHg content among invertebrate taxa (Cremona et al., 2008). Also, total Hg content in invertebrates is strongly related to total Hg content in fish (Ward et al., 2010), of which MeHg is the dominant form (Watras and Bloom, 1992). Nevertheless, progress toward directly measuring MeHg in lower trophic level organisms would help determine habitat, taxonomic, and seasonal differences in MeHg content in lower trophic levels, and increase our ability to predict fish Hg content.
The major implication of these findings is that, all else being equal, nearshore benthivory can reduce fish Hg concentrations. Both the model results, and analysis of fish diet and Hg content data from the literature (Fig. 5) support this hypothesis. This finding is consistent with observations from other studies that found benthivores, such as bullhead and juvenile perch, often have lower Hg content than planktivores, such as shiners, cisco, and alewives (Becker and Bigham, 1995; Power et al., 2002). This pattern is somewhat counterintuitive, as many assume that bottom-feeding fish are prone to exposure from sediments that are thought to be repositories of MeHg (Morel et al., 1998), in addition to being of generally poor quality for human consumption due to relatively low fatty acid content (Institute of Medicine, 2006; Mozzafarian and Rimm, 2006). Lower Hg concentrations in nearshore benthic organisms are also somewhat surprising given that mercury methylation occurs primarily in sediments (Morel et al., 1998), periphyton (Cleckner et al., 1999) and macrophyte roots (Mauro et al., 2002) of benthic habitats. Nevertheless, given the importance of benthic prey in lake food webs, it is likely that the ameliorating effects of benthivory on Hg accumulation extend from forage fish to piscivores.
5. Conclusions
In summary, this study demonstrated the value of examining physiological processes together with Hg patterns in field populations in order to understand habitat-specific differences in Hg trophic transfer. Differences in the abundance of different prey types within each habitat (e.g., Daphnia), temperature, activity levels, and other factors that influence bioenergetic rates are also likely to influence differences in Hg bioaccumulation across habitats. Characteristic differences in bioenergetic rates among organisms in different habitats may strongly influence differences in Hg content among fish as well as lower trophic level organisms. Such differences in bioenergetics may explain the growing evidence that pelagic feeding is associated with higher Hg bioaccumulation in fish in freshwater as well as marine systems. Therefore, while differences in Hg concentrations among prey items may distinguish trophic transfer from habitat-specific food chains, understanding bioenergetic-based mechanisms will ultimately allow us to better predict the accumulation of Hg in fish and, ultimately, humans. Finally, differences in prey Hg content, prey quality, and somatic growth dilution are key determinants of Hg content in fish in lakes and other aquatic ecosystems, and as such should be considered in the management and mitigation of human risks due to fish consumption in different lakes.
Supplementary Material
HIGHLIGHTS.
We examined habitat-specific feeding and Hg accumulation in lake fish.
Zooplankton had higher Hg content than many benthic prey.
Literature analysis shows lower Hg content in fish associated with benthivory.
Model shows role of lower Hg, higher calories, growth dilution from benthivory.
Growth dilution is important to understand habitat-specific Hg accumulation.
Acknowledgments
We thank Kathy Cottingham, Matt Ayres and Bob Sterner for helpful comments on earlier drafts. We thank Stefan Stürup for element analyses and Brandon Mayes and Stephen ‘Chip’ Glaholt for field and lab assistance. This research was supported by NIH grant number P42 ESO7373-7 to C.L. Folt and C.Y. Chen from the National Institute of Environmental Health Sciences and by NSF/MRI-0215913 to X. Feng and the Gelfond Fund for Mercury Research and Outreach to R. Karimi. These funding sources had no role in the study design, the collection, analysis, or interpretation of data, or in the writing and publication of the study.
References
- Back RC, Gorski PR, Cleckner LB, Hurley JP. Mercury content and speciation in the plankton and benthos of Lake Superior. Sci Total Environ. 2003;304:349–354. doi: 10.1016/S0048-9697(02)00580-6. [DOI] [PubMed] [Google Scholar]
- Back RC, Watras CJ. Mercury in zooplankton of Northern Wisconsin lakes - taxonomic and site-specific trends. Water Air Soil Pollut. 1995;80:931–938. [Google Scholar]
- Balcom PH, Schartup AT, Mason RP, Chen CY. Sources of water column methylmercury across multiple estuaries in the Northeast U.S. Mar Chem. 2015;177:721–730. doi: 10.1016/j.marchem.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann PC, Kitchell JF. Diel patterns of distribution and feeding of bluegill (Lepomis macrochirus) in Lake Wingra, Wisconsin. Trans Am Fish Soc. 1974;103:255–260. [Google Scholar]
- Becker DS, Bigham GN. Distribution of mercury in the aquatic food web of Onondaga Lake, New York. Water Air Soil Pollut. 1995;80:563–571. [Google Scholar]
- Berg K, Ockelmann KW. The respiration of freshwater snails. J Exp Biol. 1959;36:690–708. [Google Scholar]
- Cabana G, Tremblay A, Kalff J, Rasmussen JB. Pelagic food chain structure in Ontario lakes-a determinant of mercury levels in lake trout (Salvelinus namaycush) Can J Fish Aquat Sci. 1994;51:381–389. [Google Scholar]
- Chen CY, Borsuk ME, Bugge DM, Hollweg T, Balcom PH, Ward DM, et al. Benthic and pelagic pathways of methylmercury bioaccumulation in estuarine food webs of the northeast United States. PLoS One. 2014;9 doi: 10.1371/journal.pone.0089305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Dionne M, Mayes BM, Ward DM, Sturup S, Jackson BP. Mercury bioavailability and bioaccumulation in estuarine food webs in the Gulf of Maine. Environ Sci Technol. 2009;43:1804–1810. doi: 10.1021/es8017122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Folt CL. High plankton densities reduce mercury biomagnification. Environ Sci Technol. 2005a;39:115–121. [PubMed] [Google Scholar]
- Chen CY, Stemberger RS, Kamman NC, Mayes BM, Folt CL. Patterns of Hg bioaccumulation and transfer in aquatic food webs across multi-lake studies in the northeast US. Ecotoxicology. 2005b;14:135–147. doi: 10.1007/s10646-004-6265-y. [DOI] [PubMed] [Google Scholar]
- Chen CY, Stemberger RS, Klaue B, Blum JD, Pickhardt PC, Folt CL. Accumulation of heavy metals in food web components across a gradient of lakes. Limnol Oceanogr. 2000;45:1525–1536. [Google Scholar]
- Chetelat J, Amyot M, Garcia E. Habitat-specific bioaccumulation of methylmercury in invertebrates of small mid-latitude lakes in North America. Environ Pollut. 2011;159:10–17. doi: 10.1016/j.envpol.2010.09.034. [DOI] [PubMed] [Google Scholar]
- Chumchal MM, Drenner RW, Fry B, Hambright KD, Newland LW. Habitat-specific differences in mercury concentration in a top predator from a shallow lake. Trans Am Fish Soc. 2008;137:195–208. [Google Scholar]
- Clarke JU. Evaluation of censored data methods to allow statistical comparisons among very small samples with below detection limit observations. Environ Sci Technol. 1998;32:177–183. [Google Scholar]
- Cleckner LB, Gilmour CC, Hurley JP, Krabbenhoft DP. Mercury methylation in periphyton of the Florida Everglades. Limnol Oceanogr. 1999;44:1815–1825. [Google Scholar]
- Copeman LA, Parrish CC, Brown JA, Harel M. Effects of docosahexaenoic, eicosapentaenoic, and arachidonic acids on the early growth, survival, lipid composition and pigmentation of yellowtail flounder (Limanda ferruginea): a live food enrichment experiment. Aquaculture. 2002;210:285–304. [Google Scholar]
- Cremona F, Planas D, Lucotte M. Assessing the importance of macroinvertebrate trophic dead ends in the lower transfer of methylmercury in littoral food webs. Can J Fish Aquat Sci. 2008;65:2043–2052. [Google Scholar]
- Cummins KW, Wuycheck JC. Caloric equivalents for investigations in ecological energetics. Mitt Internat Verein Limnol. 1971;18:1–151. [Google Scholar]
- Dionne M. Biology. Dartmouth College; Hanover: 1991. How littoral macrophyte growth form influences foraging ecology of pumpkinseed fish; p. 141. [Google Scholar]
- Dionne M, Folt CL. An experimental analysis of macrophyte growth forms as fish foraging habitat. Can J Fish Aquat Sci. 1991;48:123–131. [Google Scholar]
- Driscoll CT, Han YJ, Chen CY, Evers DC, Lambert KF, Holsen TM, et al. Mercury contamination in forest and freshwater ecosystems in the Northeastern United States. Bioscience. 2007;57:17–28. [Google Scholar]
- Dumont HJ, Vandevelde I, Dumont S. Dry weight estimate of biomass in a selection of cladocera, copepoda and rotifera from plankton, periphyton and benthos of continental waters. Oecologia. 1975;19:75–97. doi: 10.1007/BF00377592. [DOI] [PubMed] [Google Scholar]
- Eagles-Smith CA, Suchanek TH, Colwell AE, Anderson NL. Mercury trophic transfer in a eutrophic lake: the importance of habitat-specific foraging. Ecol Appl. 2008a;18:A196–A212. doi: 10.1890/06-1476.1. [DOI] [PubMed] [Google Scholar]
- Eagles-Smith CA, Suchanek TH, Colwell AE, Anderson NL, Moyle PB. Changes in fish diets and food web mercury bioaccumulation induced by an invasive planktivorous fish. Ecol Appl. 2008b;18:A213–A226. doi: 10.1890/06-1415.1. [DOI] [PubMed] [Google Scholar]
- Elser JJ, Dobberfuhl DR, MacKay NA, Schampel JH. Organism size, life history, and N:P stoichiometry. Bioscience. 1996;46:674–684. [Google Scholar]
- Essington TE, Houser JN. The effect of whole-lake nutrient enrichment on mercury concentration in age-1 yellow perch. Trans Am Fish Soc. 2003;132:57–68. [Google Scholar]
- Fisheries and Agriculture Organization of the United Nations (FAO) The State of the World Fisheries and Aquaculture. United Nations; Rome: 2014. [Google Scholar]
- Folt CL, Chen CY, Pickhardt PC. Using plankton food web variables as indicators for the accumulation of toxic metals in fish. In: Was, Wilson SH, editors. Biomarkers of Environmentally Associated Disease: Technologies, Concepts, and Perspectives. CRC Press/Lewis Publishers; 2002. [Google Scholar]
- Frost PC, Tank SE, Turner MA, Elser JJ. Elemental composition of littoral invertebrates from oligotrophic and eutrophic Canadian lakes. J N Am Benthol Soc. 2003;22:51–62. [Google Scholar]
- Glazier DS. Beyond the ‘3/4-power law’: variation in the intra-and interspecific scaling of metabolic rate in animals. Biol Rev. 2005;80:611–662. doi: 10.1017/S1464793105006834. [DOI] [PubMed] [Google Scholar]
- Glazier DS. The 3/4-power law is not universal: evolution of isometric, ontogenetic metabolic scaling in pelagic animals. Bioscience. 2006;56:325–332. [Google Scholar]
- Gorski PR, Cleckner LB, Hurley JP, Sierszen ME, Armstrong DE. Factors affecting enhanced mercury bioaccumulation in inland lakes of Isle Royale National Park, USA. Sci Total Environ. 2003;304:327–348. doi: 10.1016/S0048-9697(02)00579-X. [DOI] [PubMed] [Google Scholar]
- Hall BD, Bodaly RA, Fudge RJP, Rudd JWM, Rosenberg DM. Food as the dominant pathway of methylmercury uptake by fish. Water Air Soil Pollut. 1997;100:13–24. [Google Scholar]
- Hamburger K, Dall PC. The respiration of common benthic invertebrate species from the shallow littoral zone of Lake Esrom, Denmark. Hydrobiologia. 1990;199:117–130. [Google Scholar]
- Haney JF. In situ examination of grazing activities of natural zooplankton communities. Archiv Fur Hydrobiologie. 1973;72:87–132. [Google Scholar]
- Hanson PC, Johnson TB, Schindler DE, Kitchell JF. Fish Bioenergetics 3.0. University of Wisconsin Sea Grant Institute; Madison: 1997. Technical Report WISCU-T-9-7-001. [Google Scholar]
- Hare L, Campbell PGC. Temporal variations of trace metals in aquatic insects. Freshw Biol. 1992;27:13–27. [Google Scholar]
- Harris RC, Bodaly RA. Temperature, growth and dietary effects on fish mercury dynamics in two Ontario lakes. Biogeochemistry. 1998;40:175–187. [Google Scholar]
- Herrin RT, Lathrop RC, Gorski PR, Andren AW. Hypolimnetic methylmercury and its uptake by plankton during fall destratification: a key entry point of mercury into lake food chains? Limnol Oceanogr. 1998;43:1476–1486. [Google Scholar]
- Hodgson JR, Xi H, Kitchell JF, Kitchell JF. The fish populations. In: Carpenter SR, editor. The trophic cascade in lakes. Cambridge University Press; Cambridge, UK: 1993. pp. 43–68. [Google Scholar]
- Nesheim MC, Yaktine AL, editors. Institute of Medicine. Seafood Choices: Balancing Benefits and Risks. Institute of Medicine of the National Academies; Washington D.C.: 2006. p. 608. [Google Scholar]
- Kainz M, Lucotte M, Parrish CC. Methyl mercury in zooplankton - the role of size, habitat, and food quality. Can J Fish Aquat Sci. 2002;59:1606–1615. [Google Scholar]
- Kainz M, Telmer K, Mazumder A. Bioaccumulation patterns of methyl mercury and essential fatty acids in lacustrine planktonic food webs and fish. Sci Total Environ. 2006;368:271–282. doi: 10.1016/j.scitotenv.2005.09.035. [DOI] [PubMed] [Google Scholar]
- Kamman NC, Burgess NM, Driscoll CT, Simonin HA, Goodale W, Linehan J, et al. Mercury in freshwater fish of northeast North America - a geographic perspective based on fish tissue monitoring databases. Ecotoxicology. 2005;14:163–180. doi: 10.1007/s10646-004-6267-9. [DOI] [PubMed] [Google Scholar]
- Karimi R, Chen CY, Pickhardt PC, Fisher NS, Folt CL. Stoichiometric controls of mercury dilution by growth. Proc Natl Acad Sci U S A. 2007;104:7477–7482. doi: 10.1073/pnas.0611261104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi R, Fisher NS, Folt CL. Multielement stoichiometry in aquatic invertebrates: when growth dilution matters. Am Nat. 2010;176:699–709. doi: 10.1086/657046. [DOI] [PubMed] [Google Scholar]
- Karimi R, Fitzgerald TP, Fisher NS. A quantitative synthesis of mercury in commercial seafood and implications for exposure in the United States. Environ Health Perspect. 2012;120:1512–1519. doi: 10.1289/ehp.1205122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi R, Folt CL. Beyond macronutrients: element variability and multi-element stoichiometry in freshwater invertebrates. Ecol Lett. 2006;9:1–11. doi: 10.1111/j.1461-0248.2006.00979.x. [DOI] [PubMed] [Google Scholar]
- Karimi R, Frisk M, Fisher NS. Contrasting food web factor and body size relationships with Hg and Se concentrations in marine biota. PLoS One. 2013;9:e74695. doi: 10.1371/journal.pone.0074695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keast A, Welsh L. Daily feeding periodicities food uptake rates and dietary changes with hour of day in some lake fishes. J Fish Res Board Can. 1968;25:1133–1144. [Google Scholar]
- Kidd KA, Bootsma HA, Hesslein RH, Lockhart WL, Hecky RE. Mercury concentrations in the food web of Lake Malawi, East Africa. J Great Lakes Res. 2003;29:258–266. [Google Scholar]
- Lake JL, McKinney RA, Osterman FA, Pruell RJ, Kiddon J, Ryba SA, et al. Stable nitrogen isotopes as indicators of anthropogenic activities in small freshwater systems. Can J Fish Aquat Sci. 2001;58:870–878. [Google Scholar]
- Lavoie RA, Hebert CE, Rail JF, Braune BM, Yumvihoze E, Hill LG, et al. Trophic structure and mercury distribution in a Gulf of St. Lawrence (Canada) food web using stable isotope analysis. Sci Total Environ. 2010;408:5529–5539. doi: 10.1016/j.scitotenv.2010.07.053. [DOI] [PubMed] [Google Scholar]
- Mauro JBN, Guimaraes JRD, Hintelmann H, Watras CJ, Haack EA, Coelho-Souza SA. Mercury methylation in macrophytes, periphyton, and water-comparative studies with stable and radio-mercury additions. Anal Bioanal Chem. 2002;374:983–989. doi: 10.1007/s00216-002-1534-1. [DOI] [PubMed] [Google Scholar]
- McPeek MA, Grace M, Richardson JML. Physiological and behavioral responses to predators shape the growth/predation risk trade-off in damselflies. Ecology. 2001;82:1535–1545. [Google Scholar]
- Mittelbach GG. Foraging efficiency and body size-a study of optimal diet and habitat use by bluegills. Ecology. 1981;62:1370–1386. [Google Scholar]
- Mittelbach GG, Osenberg CW. Stage-structured interactions in bluegill-consequences of adult resource variation. Ecology. 1993;74:2381–2394. [Google Scholar]
- Mittelbach GG, Osenberg CW, Wainwright PC. Variation in resource abundance affects diet and feeding morphology in the pumpkinseed sunfish (Lepomis gibbosus) Oecologia. 1992;90:8–13. doi: 10.1007/BF00317802. [DOI] [PubMed] [Google Scholar]
- Morel FMM, Kraepiel AML, Amyot M. The chemical cycle and bioaccumulation of mercury. Annu Rev Ecol Syst. 1998;29:543–566. [Google Scholar]
- Mozzafarian D, Rimm EB. Fish intake, contaminants, and human health evaluating the risks and the benefits. J Am Med Assoc. 2006;296:1885–1899. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- Osenberg CW, Mittelbach GG, Wainwright PC. 2-stage life histories in fish - the interaction between juvenile competition and adult performance. Ecology. 1992;73:255–267. [Google Scholar]
- Osenberg CW, Werner EE, Mittelbach GC, Hall DJ. Growth patterns in bluegill (Lepomis macrochirus) and pumpkinseed (L. gibbosus) sunfish: environmental variation and the importance of ontogenetic niche shifts. Can J Fish Aquat Sci. 1988;45:17–26. [Google Scholar]
- Pelham ME, Pierce CL, Larscheid JG. Diet dynamics of the juvenile piscivorous fish community in Spirit Lake, Iowa, USA, 1997–1998. Ecol Freshw Fish. 2001;10:198–211. [Google Scholar]
- Pickhardt PC, Folt CL, Chen CY, Klaue B, Blum JD. Algal blooms reduce the uptake of toxic methylmercury in freshwater food webs. Proc Natl Acad Sci U S A. 2002;99:4419–4423. doi: 10.1073/pnas.072531099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickhardt PC, Folt CL, Chen CY, Klaue B, Blum JD. Impacts of zooplankton composition and algal enrichment on the accumulation of mercury in an experimental freshwater food web. Sci Total Environ. 2005;339:89–101. doi: 10.1016/j.scitotenv.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Post DM. Individual variation in the timing of ontogenetic niche shifts in largemouth bass. Ecology. 2003;84:1298–1310. [Google Scholar]
- Power M, Klein GM, Guiguer K, Kwan MKH. Mercury accumulation in the fish community of a sub-Arctic lake in relation to trophic position and carbon sources. J Appl Ecol. 2002;39:819–830. [Google Scholar]
- Prout MW, Mills EL, Forney JL. Diet, growth, and potential competitive interactions between age-0 white perch and yellow perch in Oneida Lake, New York. Trans Am Fish Soc. 1990;119:966–975. [Google Scholar]
- Reinfelder JR, Fisher NS, Luoma SN, Nichols JW, Wang WX. Trace element trophic transfer in aquatic organisms: a critique of the kinetic model approach. Sci Total Environ. 1998;219:117–135. doi: 10.1016/s0048-9697(98)00225-3. [DOI] [PubMed] [Google Scholar]
- Schindler DE, Eby LA. Stoichiometry of fishes and their prey: implications for nutrient recycling. Ecology. 1997b;78:1816–1831. [Google Scholar]
- Schindler DE, Hodgson JR, Kitchell JF. Density-dependent changes in individual foraging specialization of largemouth bass. Oecologia. 1997a;110:592–600. doi: 10.1007/s004420050200. [DOI] [PubMed] [Google Scholar]
- Schindler DE, Scheuerell MD. Habitat coupling in lake ecosystems. Oikos. 2002;98:177–189. [Google Scholar]
- Schindler DW, Kidd KA, Muir DCG, Lockhart WL. The effects of ecosystem characteristics on contaminant distribution in northern fresh water lakes. Sci Total Environ. 1995;161:1–17. [Google Scholar]
- Stemberger RS, Lazorchek JM. Zooplankton assemblage responses to disturbance gradients. Can J Fish Aquat Sci. 1994;51:2435–2447. [Google Scholar]
- Sunderland EM. Mercury exposure from domestic and imported estuarine and marine fish in the US seafood market. Environ Health Perspect. 2007;115:235–242. doi: 10.1289/ehp.9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay A, Lucotte M, Meili M, Cloutier L, Pichet P. Total mercury and methylmercury contents of insects from boreal lakes: ecological, spatial, and temporal patterns. Water Qual Res J Can. 1996;31:851–873. [Google Scholar]
- Trudel M, Rasmussen JB. Modeling the elimination of mercury by fish. Environ Sci Technol. 1997;31:1716–1722. [Google Scholar]
- Trudel M, Rasmussen JB. Bioenergetics and mercury dynamics in fish: a modelling perspective. Can J Fish Aquat Sci. 2006;63:1890–1902. [Google Scholar]
- UNEP. Global mercury assessment. United Nations Environment Programme; NY, New York: 2003. [Google Scholar]
- Vadeboncoeur Y, Vander Zanden MJ, Lodge DM. Putting the lake back together: reintegrating benthic pathways into lake food web models. Bioscience. 2002;52:44–54. [Google Scholar]
- VanderZanden MJ, Cabana G, Rasmussen JB. Comparing trophic position of freshwater fish calculated using stable nitrogen isotope ratios (delta N-15) and literature dietary data. Can J Fish Aquat Sci. 1997;54:1142–1158. [Google Scholar]
- VanderZanden MJ, Rasmussen JB. A trophic position model of pelagic food webs: impact on contaminant bioaccumulation in lake trout. Ecol Monogr. 1996;66:451–477. [Google Scholar]
- VanderZanden MJ, Vadeboncoeur Y. Fishes as integrators of benthic and pelagic food webs in lakes. Ecology. 2002;83:2152–2161. [Google Scholar]
- Walters DM, Raikow DF, Hammerschmidt CR, Mehling MG, Kovach A, Oris JT. Methylmercury bioaccumulation in stream food webs declines with increasing primary production. Environ Sci Technol. 2015;49:7762–7769. doi: 10.1021/acs.est.5b00911. [DOI] [PubMed] [Google Scholar]
- Wang WX, Wong RSK. Bioaccumulation kinetics and exposure pathways of inorganic mercury and methylmercury in a marine fish, the sweetlips Plectorhinchus gibbosus. Mar Ecol Prog Ser. 2003;261:257–268. [Google Scholar]
- Ward DM, Mayes B, Sturup S, Folt CL, Chen CY. Assessing element-specific patterns of bioaccumulation across New England lakes. Sci Total Environ. 2012;421:230–237. doi: 10.1016/j.scitotenv.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward DM, Nislow KH, Chen CY, Folt CL. Rapid, efficient growth reduces mercury concentrations in stream-dwelling Atlantic salmon. Trans Am Fish Soc. 2010;139:1–10. doi: 10.1577/T09-032.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watras CJ, Back RC, Halvorsen S, Hudson RJM, Morrison KA, Wente SP. Bioaccumulation of mercury in pelagic freshwater food webs. Sci Total Environ. 1998;219:183–208. doi: 10.1016/s0048-9697(98)00228-9. [DOI] [PubMed] [Google Scholar]
- Watras CJ, Bloom NS. Mercury and methylmercury in individual zooplankton-implications for bioaccumulation. Limnol Oceanogr. 1992;37:1313–1318. [Google Scholar]
- Werner EE, Hall DJ. Ontogenetic habitat shifts in bluegill-the foraging rate predation risk trade-off. Ecology. 1988;69:1352–1366. [Google Scholar]
- Willacker JJ, von Hippel FA, Ackerly KL, O’Hara TM. Habitat-specific foraging and sex determine mercury concentrations in sympatric benthic and limnetic ecotypes of threespine stickleback. Environ Toxicol Chem. 2013;32:1623–1630. doi: 10.1002/etc.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Culver DA. Ontogenic diet shift in Lake Erie age-0 yellow perch (Perca flavescens) - a size-related response to zooplankton density. Can J Fish Aquat Sci. 1992;49:1932–1937. [Google Scholar]
- Wyn B, Kidd KA, Burgess NM, Curry RA. Mercury biomagnification in the food webs of acidic lakes in Kejimkujik National Park and National Historic Site, Nova Scotia. Can J Fish Aquat Sci. 2009;66:1532–1545. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


