Abstract
Purpose of review
Atherosclerosis is chronic disease, whose progression is orchestrated by the balance between proinflammatory and anti-inflammatory mechanisms. Various myeloid cells, including monocytes, macrophages, dendritic cells and neutrophils can be found in normal and atherosclerotic aortas, in which they regulate inflammation and progression of atherosclerosis. The lineage relationship between blood monocyte subsets and the various phenotypes and functions of myeloid cells in diseased aortas is under active investigation.
Recent findings
Various subsets of myeloid cells play diverse roles in atherosclerosis. This review discusses new findings in phenotypic and functional characterization of different subsets of macrophages, in part determined by the transcription factors IRF5 and Trib1, and dendritic cells, characterized by the transcription factor Zbtb46, in atherosclerosis.
Summary
Improved understanding proinflammatory and anti-inflammatory mechanisms of macrophages and dendritic cell functions is needed for better preventive and therapeutic measures in atherosclerosis.
Keywords: atherosclerosis, cytokines, inflammation, myeloid cells, transcription factors
INTRODUCTION
Macrophages and dendritic cells (DC) are principal components of the immune system. Dendritic cells are required for host defense against pathogens, for self-tolerance and prevention of autoimmunity [1]. Macrophages and dendritic cells play prominent roles in many pathological conditions, such as cancer and autoimmune disorders, which, like atherosclerosis, have an inflammatory component [2–5]. Dendritic cells and macrophages are major regulators of inflammation and atherosclerosis progression [6,7], but are also present in normal arteries [8,9], in which their homeostatic functions are unknown. Dendritic cell and macrophage populations are significantly expanded during atherosclerosis development [9]. Neutrophils are also found early in atherosclerotic lesions and play a role in atherosclerosis [10]. The role of myeloid cells in atherosclerosis has been covered in many excellent reviews [11–20] underlining their predominant important roles in promoting vascular inflammation and disease progression as well as in suppression of inflammation and atherosclerosis [21,22,23▪▪]. In this review, we focus on new findings to characterize functional roles of different subsets of myeloid cells present in healthy and atherosclerotic aortas.
MACROPHAGES OR DENDRITIC CELLS?
Historically, the differentiation between macrophages and dendritic cells was based on microscopic studies of their shape and functional characteristics: macrophages are active phagocytes [24] and dendritic cells are specialized for antigen presentation [1]. Surface marker expression is widely used to distinguish dendritic cells from macrophages [25]. Although CD11c is used as a ‘bona fide’ dendritic cell marker [1], recent studies have demonstrated that its not dendritic cell-specific [25,26]. Monocytes in hypercholesterolemia and mature macrophages in atherosclerotic lesions can express CD11c [27,28▪]. In addition, most macrophages and some dendritic cells express CD11b, whereas CD68 and F4/80 expression is mostly restricted to macrophages [3]. Thus, macrophages and dendritic cells express many of the same surface markers, making unequivocal distinction between them difficult [3,29]. CD64 (FcγR1) and MERTK (Mer tyrosine kinase receptor) have recently been proposed as macrophage-specific markers [25], but their use for vascular macrophage characterization is not yet established.
KEY POINTS.
Macrophages and dendritic cells could perform different functions even though they express similar surface markers.
The type of macrophage activation (M1/M2) is determined by environmental cues including cytokines.
The transcription factors IRF5 and Trib1 regulate M1/M2 macrophage differentiation.
Zbtb46 is a master regulator transcription factor for cDC.
Desmosterol accumulation in foam cells decreases their inflammatory properties.
Atherosclerotic lesions contain CD11b+ CD11c−, CD11b+CD11c+ and CD11b−CD11c+ myeloid cells [28▪]. About half of CD11b+CD11c− and CD11b+CD11c+ cells express F4/80 and therefore can be classified as macrophages, according to the current nomenclature, whereas F4/80−CD11b+CD11c+cells could be conventional (c)DC. Indeed, in lymph nodes, CD11b+CD11c+ cDC were functionally defined as potent antigen presenting cells [30]. Another type of CD11b+CD11c+ DC called ‘inflammatory DC’ or ‘TNF and iNOS producing’ (TIP)-DC appears in highly inflammatory environments, like during bacterial infection [31]. They derive from Gr1/Ly6Chi inflammatory monocytes [31], which also can give rise to inflammatory macrophages with the same CD11b+CD11c+ surface phenotype [32]. Whether CD11b+CD11c+ inflammatory DC and inflammatory macrophages is a mixture of distinct dendritic cells and macrophages, or whether they are the same cell population is unclear.
Both macrophages and dendritic cells can present antigens to CD4, CD8 and NK T cells in the context of MHC-II, MHC-I and CD1d, respectively. It is generally assumed that only dendritic cells are able to initiate activation of naive T cells, whereas macrophages typically can only restimulate previously activated T cells. The antigen-presenting function of dendritic cells during atherosclerosis development recently received considerable attention [33]. In an imaging study, antigen-presenting cells (APCs) marked by CD11c driven YFP expression were shown to interact with activated CD4 T cells in the arterial wall, resulting in production of T cell-derived inflammatory cytokines [28▪].
MACROPHAGES IN ATHEROSCLEROSIS: PRO-INFLAMMATORY AND ANTI-INFLAMMATORY FUNCTIONS
Atherosclerosis is a disease with metabolic, inflammatory and autoimmune elements. Immune cells accumulate in the wall of arteries and in plaque (Fig. 1a–c). Myeloid cells are key players in regulating inflammation and plaque growth in the vessel wall [5,14]. Similar to other tissues, healthy artery walls contain tissue-resident macrophages, which as in other tissues appear during embryonic development from yolk sac cells [34▪▪]. Most tissue macrophages persist in the body independently of hematopoietic stem cell progenitors (HSC) and develop independently of the transcription factor Myb [34▪▪,35▪].
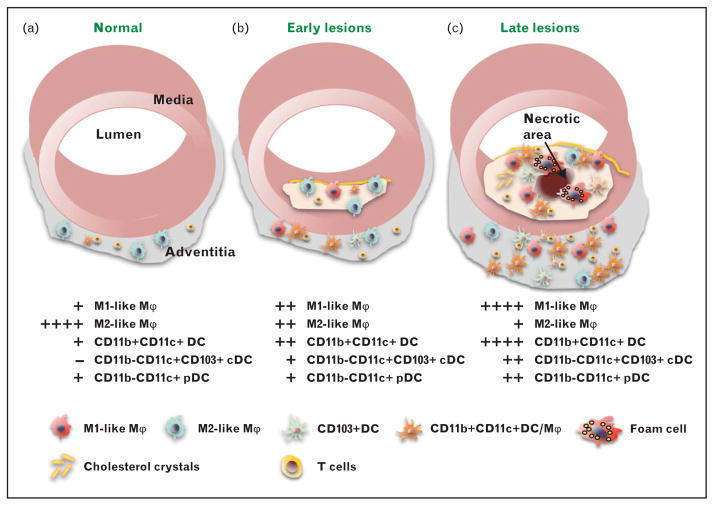
FIGURE 1.
Myeloid cells in atherosclerosis development. (a) The normal aorta contains some T lymphocytes and a small number of myeloid cells that express M2 markers and may be resident vascular macrophages. (b) Early atherosclerotic lesions are characterized by accumulation of myeloid cells in the subendothelial space. The number of cells in the adventitia is significantly increased. The macrophage population now represents a mixture of ‘M1-like’ and ‘M2-like’ macrophages. Various types of dendritic cells (DC) start to accumulate in both adventitia and plaque. (c) Late atherosclerotic lesions are characterized by accumulation of cholesterol crystals and various types of immune cells including foam cells, whose death leads to formation of the necrotic core, which is related to plaque instability and rupture. The macrophage population is mainly ‘M1-like’. High numbers of dendritic cell with potential pro-inflammatory and anti-inflammatory functions are detected in the adventitia and atherosclerotic plaque. High numbers of interactions between antigen-specific T cells and antigen-presenting cells (dendritic cell and macrophages) are observed in plaque and adventitia. pDC, plasmacytoid dendritic cell, cDC, conventional dendritic cell, Mϕ, macrophage.
Under normal conditions, immune cells migrate to the vessel wall (where they can be detected under steady state conditions) and leave in a few days, returning to the circulation [9]. In the early stages of atherosclerosis, high concentrations of lipoproteins in the plasma, and high blood pressure lead to activation of endothelium, upregulation of adhesion molecules, and influx of bone marrow-derived monocytes and other immune cells into the arterial wall [36]. These cells become activated and produce inflammatory mediators and further enhance the recruitment of inflammatory cells, which contributes to plaque growth [18,37].
Monocytes
Monocytes originate from monocyte-dendritic cell precursors (MDP) [38] in the bone marrow and circulate in the blood. In mice, two subsets of monocytes with different functions were distinguished based on the surface expression of CD11b, CD115, and variable levels of Ly6C, CX3CR1 and CCR2: Ly6Chi ‘inflammatory’ and Ly6Clo ‘patrolling’ monocytes [39]. Ly6Chi monocytes express high levels of CCR2 [17] and are functionally similar to CD14+CD16− cells, the most abundant monocyte population in humans. Ly6Clo ‘patrolling’ monocytes do not express CCR2 and are similar to CD14dimCD16+ ‘patrolling’ monocytes in humans in their ability to scan the vessel walls under normal conditions [40,41]. Patrolling monocytes sense nucleic acids and viruses via Toll-like receptor (TLR)7 and TLR8 [42], and thus serve an unique ‘housekeeping’ function: they control neutrophil recruitment and focal necrosis of endothelial cells, thereby convey anti-inflammatory and endothelium-protective functions [41]. The lack of patrolling monocytes in Nur77-deficient Nr4a1−/− mice might underlie the observed acceleration of atherosclerosis [43,44]. Ly6clo monocytes do not need to extravasate to fulfill their endothelium and athero-protective functions [41].
Under hypercholesterolemic conditions, the expression of CD11c is upregulated on Ly6Clo patrolling monocytes [27,45,46]. Whether Ly6Chi monocytes also upregulate CD11c expression remains to be investigated.
The Ly6Chi subpopulation of monocytes is the first population recruited to the arterial wall upon atherosclerosis initiation [47,48]. These cells give rise to CD11b+CD11c+ inflammatory macrophages and dendritic cells in situ [49] [26], which produce proinflammatory cytokines such as TNF, IL-12, IL-6 and also iNOS. Almost all CD11b+CD11c+cells express high levels of MHC-II, CD80 and CD86, suggesting a strong antigen-presenting capacity. CD11b+CD11c+ cells promote inflammation via cytokine production and activation of T-helper responses [28▪]. It is possible that environmental cues characteristic for atherosclerosis, such as hypercholesterolemia and necrotic cell death, regulate the appearance of these cells in the tissue and their ability to differentiate into either macrophages or dendritic cells under inflammatory conditions.
At the late stages of development, atherosclerosis is characterized by nonresolving inflammation (Fig. 1c), fueled by various factors, including high plasma levels of LDLs and elevated blood pressure [18,50,51]. Within the atherosclerotic aortas, macrophages, which represent the largest population of CD45+ immune cells, ingest excessive lipids and become foam cells [52]. Lipid accumulation in macrophages, coupled with their inability to leave the plaque, leads to in situ macrophage necrosis. The ‘necrotic core’ destabilizes plaque and promotes plaque rupture, which leads to adverse cardiovascular events (Fig. 2) [6].
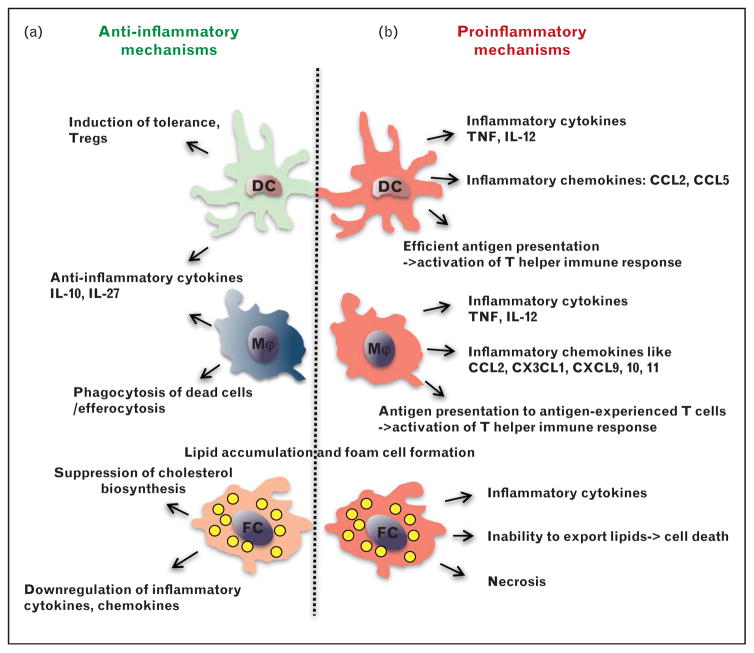
FIGURE 2.
Proinflammatory and anti-inflammatory functions of macrophages and dendritic cells (DC) in atherosclerosis. (a) Atheroprotective mechanisms at early stages of atherosclerosis are mediated by anti-inflammatory functions of macrophages and DC, which includes induction of regulatory T cells (Treg) and anti-inflammatory cytokine production. The phagocytic function of macrophages contributes to clearance of dead cells and extracellular lipids. (b) During the later stages of atherosclerosis the proinflammatory mechanisms dominate. Both macrophages and dendritic cell produce proinflammatory cytokines and chemokines, which promote local inflammation. Antigen presentation is a signature of dendritic cells, however macrophages are able to present antigens to antigen-experienced T cells. Both macrophages and dendritic cells could become foam cells. Filled with lipids, foam cells can die forming the necrotic core inside the plaque, resulting in plaque instability and potential rupture.
Pattern-recognition receptors (such as TLRs) together with scavenger receptors regulate lipid accumulation and macrophage activation [53,54]. Activated plaque macrophages in situ have been reported to produce elevated levels of inflammatory cytokines and chemokines [55], but it is not clear whether this is a cell autonomous effect [23▪▪].
Macrophages: the M1/M2 hypothesis
In the presence of the growth factors M-CSF or GM-CSF, macrophages can be grown in vitro [56,57]. Upon stimulation with proinflammatory or anti-inflammatory cytokines, macrophages possess a large degree of plasticity and can acquire different gene transcription profiles and functional characteristics [2,58]. The presence of the Th-1 cytokine IFNγ, especially together with bacterial lipopolysaccharide (LPS), leads to the appearance of ‘classically’ activated, macrophages (M1-like). M1 macrophages are characterized by a proinflammatory expression profile, including the production of TNF, iNOS and IL-12, each of them with known proatherogenic functions [59–64], as well as elevated expression of MHC-II and co-stimulatory molecules important for the induction of T-cell responses [65]. On the contrary, Th-2 cytokines, such as IL-4 and IL-13, drive ‘alternative’ or ‘M2-like’ macrophages activation [66]. ‘M2-like’ macrophages express high levels of immunosuppressive arginase I and anti-inflammatory IL-10 as well as low levels of MHC-II and co-stimulatory molecules, suggesting their potential physiological role in resolving of inflammation, tissue repair and wound healing. In vitro, phenotypically different M2 macrophages can be observed when naive macrophages are activated by IL-4 and IL-13 (M2a subtype), IL-10 (M2b) or glucocorticoids or immune complexes (M2c) [66]. The chemokine CXCL4 induces an additional M4 macrophage phenotype with potential implications for atherosclerosis [67]. Oxidized lipids induce yet another phenotype of macrophages that has been termed ‘Mox’ [68].
Certain markers have been proposed to be indicative of macrophage polarization. However, M1 and M2 markers are not the same for mouse and human macrophages and M4 have only been studied in humans and Mox only in mice. In atherosclerosis, macrophages with both M1 and M2 markers were found in the same lesions side-by-side, and mixed phenotypes were also seen [68]. In a laser capture microdissection study, CD68+ cells from regressing plaque were enriched for expression of genes associated with an M2 macrophage phenotype [69], resulting in hypothesis that M2 macrophage polarization may be anti-atherogenic and M1 polarization drives atherosclerosis [19]. However, M1 and M2 phenotypes were defined in vitro and probably do not exist in the same form in vivo. Mouse studies suggest that both M1-like and M2-like macrophages can be derived from Ly-6Chi monocytes [46].
Likely, macrophage heterogeneity in vivo is explained by their plasticity rather than by their ability to form terminally differentiated subsets [49]. Thus, macrophage populations in atherosclerosis represent a wide spectrum of different activation states, which depend on in-situ environmental cues and the timing of exposure to these cues [70▪,71]. In-vivo inflammatory macrophages (similar to ‘M1’ macrophages in vitro) could switch gene expression toward an anti-inflammatory profile in response to IL-4 [70▪]. Early atherosclerotic lesions are populated with ‘M2-like’ macrophages, whose original functions may be homeostatic phagocytosis and repair of tissue injury [72]. Upon atherosclerosis development, these ‘M2-like’ macrophages are gradually substituted by ‘M1-like’ inflammatory macrophages [71]. Recent studies suggest that not only newly recruited Ly6Chi inflammatory monocytes give rise to ‘M1’ macrophages [47], which then overpopulate the aorta, but resident ‘M2-like’ macrophages actually convert into ‘M1’ in situ [71]. Likely, the proinflammatory environment of the vessel wall regulates changes in macrophage gene expression, leading to a switch of their gene expression program from anti-inflammatory to proinflammatory. In addition, macrophage phenotype is influenced by inflammatory cytokines from the cells of the adaptive immune system during plaque formation [28▪].
Toll-like and scavenger receptor ligands
Macrophages in atherosclerotic lesions express TLRs and scavenger receptors [12]. TLR ligands (such as LPS for TLR4) induce an inflammatory gene expression program, which later is succeeded by ‘LPS tolerance’ and production of various negative feedback regulatory molecules [73,74]. Therefore, the ability of macrophages to produce proinflammatory or anti-inflammatory mediators in situ may be explained by the timing of their recent exposure to TLR ligands. Possibly, high fat high cholesterol diet driving atherosclerosis development can induce changes in the intestinal microbiome and increase translocation of LPS into the circulation [75]. There is good evidence for the functional involvement of TLR and NLR ligands in atherosclerosis progression [53,54,76]. Oxidized LDL (oxLDL), a ligand for scavenger receptor CD36, can trigger a switch in the macrophage gene expression profile from ‘M2-like’ to ‘M1-like’ in vitro [77]. Furthermore, obesity progression results in a switch from ‘M2-like’ to ‘M1-like’ cells, with the NLRP3 inflammasome serving as a sensor of obesity–associated danger signals [78]. A proinflammatory role of cholesterol crystals, which activate inflammasomes [79,80], suggests that similar mechanisms of macrophage activation could operate in atherosclerosis.
The developmental origin of different subsets of macrophages and dendritic cells in the arterial wall is not known. Recently, lineage-defining transcription factors that control macrophage terminal differentiation in vivo have been described. IRF5 has been proposed to regulate ‘M1-like’ macrophage differentiation in humans. High IRF5 expression correlated with induction of IL12p35, IL12p40 and IL23p19, but downregulation of IL-10 in macrophages [81]. Trib1 was shown to regulate ‘M2-like’ differentiation [82▪▪]. Mice lacking Trib1 display decreased adipose tissue mass and increased lipolysis when fed a normal chow diet, whereas high-fat diet drives insulin resistance, hypertriglyceridemia and inflammatory cytokine production [82▪▪]. The transcription factor IRF4 is also implicated in M2 differentiation, however its role in vivo is still unclear [83,84].
Mice lacking the orphan transcription factor Nur77 (Nr4a1) show an almost complete absence of Ly6Clo (’patrolling’) monocytes, thereby establishing Nur77 as a critical regulator of the Ly6clo subset differentiation [85]. Interestingly, in the absence of Nur77, the remaining macrophages exhibited enhanced inflammatory properties resulting in aggravated inflammation and atherosclerosis [43,44].
DENDRITIC CELLS IN ATHEROSCLEROSIS: ORIGIN, PROINFLAMMATORY AND ANTI-INFLAMMATORY FUNCTION
The adaptive immune system exerts both pro-inflammatory and anti-atherogenic effects (reviewed in [37]). Since the immune response is largely shaped by dendritic cells, their role in atherosclerosis was proposed, but not directly assessed until recently. Mice deficient in chemokine receptors involved in dendritic cell migration, including CX3CR1, CCR2 and CCR5, demonstrated significant reduction of atherosclerosis correlating with decreased dendritic cell accumulation [86–88]. However, these same chemokine receptors are also expressed on monocytes and regulate monocyte recruitment. Moreover, CX3CR1 deficiency also affects survival of Ly-6Clo blood monocytes, which also could explain the decrease in macrophage and dendritic cell content in the atherosclerotic aortas [89].
Despite extensive progress made recently, the origin of dendritic cells in normal and atherosclerotic aortas in vivo is still unclear. The current paradigm suggests that both monocytes and dendritic cells derive from a common monocyte dendritic cell precursor (MDP) cell. A common dendritic cell precursor (CDP) has been identified that no longer produces monocytes and differentiates to cDC via a precDC stage [90], in which Flt3L serves as a main regulator of cDC development [91]. The lineage of cDC has been well defined in peripheral lymphoid organs [26,92]. The search for master regulators of specific dendritic cell subsets yielded several transcription factors, which are essential to control the development of various types of dendritic cells. Batf3 was shown to regulate the development of CD8α+CD11c+ cDC [93], while E2–2 is a crucial regulator of pDC development [94]. Moreover, Batf3, IRF8 and Id2 were shown to regulate CD8α+ CD11b-CD11c+ cells in lymphoid tissue and their functional analogs in nonlymphoid tissue CD103+CD11b-CD11c+ cells [29,95,96]. The transcription factor Ztbt46 was recently found to be highly upregulated in cDC [97▪▪,98] and plays a crucial role in their maintenance [99▪▪]. Therefore, Ztbt46 may be a master regulator of cDC differentiation and can serve as a definitive marker of cDC lineage. It remains to be investigated whether Zbtb46 levels are increased in atherosclerotic aortas and whether Zbtb46-driven dendritic cells are found there and fulfill distinct functions.
Early studies described the presence of dendritic cells in healthy nonatherosclerotic aortas [8], but these cells were not phenotyped beyond morphologic description. Healthy vessels contain some CD11b+CD11c+ cells and a small population of CD11b-CD11c+ dendritic cells [28▪]. In atherosclerosis, several populations of dendritic cells were described in the arterial wall [21,28▪,100], with rapid accumulation of CD11b+CD11c+ cells. As discussed above, these CD11b+CD11c+ cells likely originate from monocytes and represent a heterogeneous population comprising both inflammatory macrophages and dendritic cells. The number of CD11b-CD11c+ cells is also increased in aortas and in the plaques during atherosclerosis. Several populations of classical dendritic cells including CD11b-CD103+CD11c+ [21], CD11b-CD8α+CD11c+ + (E. K. and K.L. unpublished) and plasmacytoid dendritic cells (pDC) [101,102] were also found.
Functionally, dendritic cells serve as a bridge to connect innate and adaptive immune responses in atherosclerosis. Previous reports demonstrated that deletion of molecules involved in antigen presentation, such as co-stimulatory molecules B7.1-B7.2 and CD74, required for dendritic cell migration, decreases atherosclerosis [33,103]. Recently, we demonstrated that APC expressing YFP under the CD11c promoter can present antigens to T cells and promote IFN-γ production [28▪]. However, not all dendritic cell subsets function uniformly in atherosclerosis (Fig. 2). CD103+CD11c+ dendritic cells were shown to play an immunosuppressive role by sustaining Treg homeostasis [21], very similar to their already known role in the gut [104,105]. The differentiation of CD103+CD11c+ dendritic cells is driven by Flt3L, and therefore mice deficient in FLT3L lack (among other dendritic cell subsets) CD103+CD11c+ cells, which aggravated atherosclerosis [21]. As other subsets of cDCs including CD8α+CD11c+ cells are also FLT3L dependent [106], it is possible that more than one subset of immunosuppressive dendritic cells is absent or deregulated in the absence of FLT3L. Signaling through the adapter molecule MyD88 (involved in TLR and IL-1 receptor signaling) in CD11c+ dendritic cells is crucial for Treg priming and monocyte accumulation in atherosclerosis [22]. Further studies will illuminate the mechanisms by which dendritic cells curb monocyte recruitment to inflamed aortas during atherosclerosis.
Several studies tried to address the role of dendritic cells in atherosclerosis by their depletion in vivo using diphtheria toxin receptor (DTR) expressed under the CD11c promoter (CD11cDTR mice) [107]. Transient (24 h) depletion of CD11c+ cells resulted in hypercholesterolemia, suggesting a possible role of dendritic cells in regulation of cholesterol homeostasis; however the study did not report atherosclerotic lesion sizes [108]. In another study, a single injection of diphtheria toxin (DT) resulted in significant reduction of CD11c+ cells (likely both dendritic cells and CD11c+ monocyte-derived cells), with their numbers returning to 75% of baseline by day 21 after depletion. However no changes in atherosclerotic lesion size were observed [100]. When CD11c+ cells were depleted for 2 weeks by multiple DT injection in mice after bone marrow transplantation, a very modest decrease of overall lesion size was found. This was accompanied by a significant decrease of CD11b-CD11c+ cell numbers (with highest expression of CD11c) and a rather modest depletion of CD11b+CD11c+ cells (a mixed cell population perhaps with different levels of CD11c expression) [28▪]. Since CD11c is not a definitive and specific dendritic cell marker, the interpretation of results obtained with CD11cDTR mice where some but not other CD11c+ cells are depleted, is complicated. Perhaps, specific depletion of cDC using zDCDTR mice [99▪▪] can serve as a better model for determination of the role of cDC in atherosclerosis.
Accumulation of lipids and foam cell formation
Accumulation of foam cells inside the atherosclerotic plaque is a hallmark of atherogenesis [11,109]. The origin of foam cells is not completely understood. Historically, macrophages were thought to accumulate lipid droplets and thus become foam cells. However, foam cells generated from human blood monocytes in vitro show gene expression patterns similar to dendritic cells [110]. Gene expression profiling of CD68+ cells harvested from atherosclerotic lesions by laser capture microdis-section were not analyzed for macrophage and dendritic cell markers [69], so the cellular identity of foam cells remains unclear. Existence of various macrophage subpopulations, which differ by gene expression profile, suggests that recruited monocyte-derived cells may preferentially take up lipids to become foam cells. It is not clear whether bona fide dendritic cell can become foam cells and if so, which dendritic cell subsets are responsible for lipid accumulation. CD68+CD11c+ cells in the subendothelial space of the aorta were reported to accumulate lipids and potentially become foam cells, contributing to plaque growth [100]. While the expression of CD11b, F4/80 or other macrophage markers like CD64 was not analyzed, expression of 33D1 and the presence of dendrites suggest that these cells could be dendritic cells. However, the lineage and origin of this cell population remains to be defined. It is also possible that foam cells can derive from CD11b+CD11c+ cells of monocyte origin [28▪].
Foam cells are characterized by the presence of large amounts of cholesterol and cholesterol esters in lipid droplets in the cytoplasm. Inability to export lipids from the cell eventually leads to cell death [111]. Dead foam cells are normally taken up by other macrophages, but impaired efferocytosis in atherosclerosis promotes the growth of the necrotic area in the plaque, leading to plaque rupture and clinically relevant adverse events [112]. A large necrotic area is known to contribute to increased inflammation, since stress proteins are released that trigger innate immunity [112].
On the contrary, foam cells generated in the peritoneal cavity of Ldlr−/− mice on atherogenic diet show reduced expression of many inflammatory genes [23▪▪]. The cholesterol metabolite desmosterol accumulates in peritoneal macrophage-derived foam cells and downregulates (by acting as an LXR ligand) several clusters of genes, including inflammatory genes and genes responsible for lipid metabolism [23▪▪]. Therefore, it seems that foam cells have an intrinsic lipid-dependent mechanism to decrease their inflammatory properties [23▪▪]. These findings seem at odds with the observation that foam cells appear to be proinflammatory in the context of atherosclerosis (Fig. 2).
Previously, foam cells in the plaque were implicated as a direct source of cytokines and inflammatory signals [113] (Fig. 2). However, their ability to die by necrosis may be even more important to activate neighboring freshly recruited myeloid cells, which have not yet been exposed to inflammatory stimuli and have not yet accumulated enough lipids and therefore would demonstrate more pronounced inflammatory response [111]. Foam cells produce less inflammatory molecules than LPS-activated macrophages, which are not overloaded with lipids [23▪▪]. However, when constantly challenged, foam cells may still produce more inflammatory mediators than unchallenged naive macrophages in normal nonatherosclerotic aortas. Another possibility is that cytokines produced by T cells might set up the proinflammatory environment formed in atherosclerotic plaques. Indeed, T cells isolated from mice with already established atherosclerosis produce much more IFNγ than naive T cells when exposed to APCs in the context of atherosclerotic aorta [28▪].
CONCLUSION
Despite the progress in describing various subsets of myeloid cells in atherosclerosis, they are understudied. Our ability to harness endogenous immune mechanisms in atherosclerosis depends on a better understanding of cytokines, cholesterol metabolism in macrophages, macrophage and dendritic cell phenotypes, and their interactions with adaptive immune system. This requires integration of knowledge from at least four disparate fields: cholesterol metabolism, macrophage biology, monocyte lineage and development, and dendritic cell and T-cell immunology. This emerging area of vascular immunology holds great promise for future preventive and therapeutic approaches in atherosclerosis.
Acknowledgments
This work was supported by AHA SDG 13SDG14490059 to E.K, NIH RO1 115232 to K.L. and NIH P01 055798 to C.C.H.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 444).
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–166. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koltsova EK, Ley K. How dendritic cells shape atherosclerosis. Trends Immunol. 2011;32:540–547. doi: 10.1016/j.it.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bobryshev YV, Babaev VR, Lord RS, Watanabe T. Ultrastructural identification of cells with dendritic cell appearance in atherosclerotic aorta of apolipoprotein E deficient mice. J Submicrosc Cytol Pathol. 1999;31:527–531. [PubMed] [Google Scholar]
- 9.Galkina E, Kadl A, Sanders J, et al. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med. 2006;203:1273–1282. doi: 10.1084/jem.20052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soehnlein O. Multiple roles for neutrophils in atherosclerosis. Circ Res. 2012;110:875–888. doi: 10.1161/CIRCRESAHA.111.257535. [DOI] [PubMed] [Google Scholar]
- 11.Glass CK, Witztum JL. Atherosclerosis the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 12.Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogenesis. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:1506–1516. doi: 10.1161/ATVBAHA.110.221127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soehnlein O, Weber C. Myeloid cells in atherosclerosis: initiators and decision shapers. Semin Immunopathol. 2009;31:35–47. doi: 10.1007/s00281-009-0141-z. [DOI] [PubMed] [Google Scholar]
- 15.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802–815. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 16.Randolph GJ. Emigration of monocyte-derived cells to lymph nodes during resolution of inflammation and its failure in atherosclerosis. Curr Opin Lipidol. 2008;19:462–468. doi: 10.1097/MOL.0b013e32830d5f09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gautier EL, Jakubzick C, Randolph GJ. Regulation of the migration and survival of monocyte subsets by chemokine receptors and its relevance to atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1412–1418. doi: 10.1161/ATVBAHA.108.180505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 19.Williams HJ, Fisher EA, Greaves DR. Macrophage differentiation and function in atherosclerosis: opportunities for therapeutic intervention? J innate Immun. 2012;4:498–508. doi: 10.1159/000336618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi JH, Cheong C, Dandamudi DB, et al. Flt3 signaling-dependent dendritic cells protect against atherosclerosis. Immunity. 2011;35:819–831. doi: 10.1016/j.immuni.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Subramanian M, Thorp E, Hansson GK, Tabas I. Treg-mediated suppression of atherosclerosis requires MYD88 signaling in dendritic cells. J Clin Invest. 2013;123:179–188. doi: 10.1172/JCI64617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23▪▪.Spann NJ, Garmire LX, McDonald JG, et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–152. doi: 10.1016/j.cell.2012.06.054. This article suggests that high-fat diet promotes foam cell formation but reduces the expression of many inflammatory genes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hume DA. The mononuclear phagocyte system. Curr Opin Immunol. 2006;18:49–53. doi: 10.1016/j.coi.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 25.Gautier EL, Shay T, Miller J, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118–1128. doi: 10.1038/ni.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merad M, Sathe P, Helft J, et al. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu H, Gower RM, Wang H, et al. Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation. 2009;119:2708–2717. doi: 10.1161/CIRCULATIONAHA.108.823740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28▪.Koltsova EK, Garcia Z, Chodaczek G, et al. Dynamic T cell-APC interactions sustain chronic inflammation in atherosclerosis. J Clin Invest. 2012;122:3114–3126. doi: 10.1172/JCI61758. This article demonstrates interactions between antigen-experienced T cells and antigen-presenting cells in the wall of the mouse aorta. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Satpathy AT, Wu X, Albring JC, Murphy KM. Re(de)fining the dendritic cell lineage. Nat Immunol. 2012;13:1145–1154. doi: 10.1038/ni.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis KL, Reizis B. Dendritic cells: arbiters of immunity and immunological tolerance. Cold Spring Harb Perspect Biol. 2012;4:a007401. doi: 10.1101/cshperspect.a007401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serbina NV, Salazar-Mather TP, Biron CA, et al. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 32.Dunay IR, Damatta RA, Fux B, et al. Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity. 2008;29:306–317. doi: 10.1016/j.immuni.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buono C, Pang H, Uchida Y, et al. B7-1/B7-2 costimulation regulates plaque antigen-specific T-cell responses and atherogenesis in low-density lipoprotein receptor-deficient mice. Circulation. 2004;109:2009–2015. doi: 10.1161/01.CIR.0000127121.16815.F1. [DOI] [PubMed] [Google Scholar]
- 34▪▪.Schulz C, Gomez Perdiguero E, Chorro L, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. This work provides evidence for local development of tissue-resident macrophages. [DOI] [PubMed] [Google Scholar]
- 35▪.Hashimoto D, Chow A, Noizar C, et al. Tissue-resident macrophages self maintain locally throught adult life with minimal contribution from circulating monocytes. Immunity. 2013:792–804. doi: 10.1016/j.immuni.2013.04.004. This work provides evidence for local development of tissue-resident macrophages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 37.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 38.Fogg DK, Sibon C, Miled C, et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 39.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 40.Auffray C, Fogg DK, Narni-Mancinelli E, et al. CX3CR1+ CD115+ CD135+ common macrophage/dendritic cell precursors and the role of CX3CR1 in their response to inflammation. J Exp Med. 2009;206:595–606. doi: 10.1084/jem.20081385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carlin LM, Stamatiades EG, Auffray C, et al. Nr4a1-dependent Ly6Clow monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013;153:362–375. doi: 10.1016/j.cell.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cros J, Cagnard N, Woollard K, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hanna RN, Shaked I, Hubbeling HG, et al. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ Res. 2012;110:416–427. doi: 10.1161/CIRCRESAHA.111.253377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamers AA, Vos M, Rassam F, et al. Bone marrow-specific deficiency of nuclear receptor Nur77 enhances atherosclerosis. Circ Res. 2012;110:428–438. doi: 10.1161/CIRCRESAHA.111.260760. [DOI] [PubMed] [Google Scholar]
- 45.Gower RM, Wu H, Foster GA, et al. CD11c/CD18 expression is upregulated on blood monocytes during hypertriglyceridemia and enhances adhesion to vascular cell adhesion molecule-1. Arterioscler Thromb Vasc Biol. 2011;31:160–166. doi: 10.1161/ATVBAHA.110.215434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tacke F, Alvarez D, Kaplan TJ, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swirski FK, Libby P, Aikawa E, et al. Ly-6Chi monocytes dominate hyperch-olesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 49.Geissmann F, Manz MG, Jung S, et al. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blake GJ, Ridker PM. C-reactive protein and other inflammatory risk markers in acute coronary syndromes. J Am Coll Cardiol. 2003;41:37S–42S. doi: 10.1016/s0735-1097(02)02953-4. [DOI] [PubMed] [Google Scholar]
- 51.Hansson GK, Robertson AK, Soderberg-Naucler C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- 52.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis. Annu Rev Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stewart CR, Stuart LM, Wilkinson K, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choi SH, Harkewicz R, Lee JH, et al. Lipoprotein accumulation in macrophages via toll-like receptor-4-dependent fluid phase uptake. Circ Res. 2009;104:1355–1363. doi: 10.1161/CIRCRESAHA.108.192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller YI, Choi SH, Wiesner P, et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108:235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang X, Goncalves R, Mosser DM. In: The isolation and characterization of murine macrophages. Current protocols in immunology. Unit 14. Coligan John E, et al., editors. Chapter 14. Hoboken, NJ: John Wiley & Sons; 2008. p. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vogt G, Nathan C. In vitro differentiation of human macrophages with enhanced antimycobacterial activity. J Clin Investig. 2011;121:3889–3901. doi: 10.1172/JCI57235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mantovani A, Garlanda C, Locati M. Macrophage diversity and polarization in atherosclerosis: a question of balance. Arterioscler Thromb Vasc Biol. 2009;29:1419–1423. doi: 10.1161/ATVBAHA.108.180497. [DOI] [PubMed] [Google Scholar]
- 59.Branen L, Hovgaard L, Nitulescu M, et al. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:2137–2142. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- 60.Detmers PA, Hernandez M, Mudgett J, et al. Deficiency in inducible nitric oxide synthase results in reduced atherosclerosis in apolipoprotein E-deficient mice. J Immunol. 2000;165:3430–3435. doi: 10.4049/jimmunol.165.6.3430. [DOI] [PubMed] [Google Scholar]
- 61.Zhang X, Niessner A, Nakajima T, et al. Interleukin 12 induces T-cell recruitment into the atherosclerotic plaque. Circ Res. 2006;98:524–531. doi: 10.1161/01.RES.0000204452.46568.57. [DOI] [PubMed] [Google Scholar]
- 62.Lee TS, Yen HC, Pan CC, Chau LY. The role of interleukin 12 in the development of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:734–742. doi: 10.1161/01.atv.19.3.734. [DOI] [PubMed] [Google Scholar]
- 63.Davenport P, Tipping PG. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol. 2003;163:1117–1125. doi: 10.1016/S0002-9440(10)63471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hauer AD, Uyttenhove C, de Vos P, et al. Blockade of interleukin-12 function by protein vaccination attenuates atherosclerosis. Circulation. 2005;112:1054–1062. doi: 10.1161/CIRCULATIONAHA.104.533463. [DOI] [PubMed] [Google Scholar]
- 65.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 66.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 67.Gleissner CA, Shaked I, Little KM, Ley K. CXC chemokine ligand 4 induces a unique transcriptome in monocyte-derived macrophages. J Immunol. 2010;184:4810–4818. doi: 10.4049/jimmunol.0901368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kadl A, Meher AK, Sharma PR, et al. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res. 2010;107:737–746. doi: 10.1161/CIRCRESAHA.109.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feig JE, Vengrenyuk Y, Reiser V, et al. Regression of atherosclerosis is characterized by broad changes in the plaque macrophage transcriptome. PLoS One. 2012;7:e39790. doi: 10.1371/journal.pone.0039790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70▪.Egawa M, Mukai K, Yoshikawa S, et al. Inflammatory Monocytes Recruited to Allergic Skin Acquire an Anti-inflammatory M2 Phenotype via Basophil-Derived Interleukin-4. Immunity. 2013;38:570–580. doi: 10.1016/j.immuni.2012.11.014. This work demonstrates the relationship between mouse monocyte substes and tissue macrophages and elucidates mechanisms of M2 differentiation in vivo. [DOI] [PubMed] [Google Scholar]
- 71.Khallou-Laschet J, Varthaman A, Fornasa G, et al. Macrophage plasticity in experimental atherosclerosis. PLoS One. 2010;5:e8852. doi: 10.1371/journal.pone.0008852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mantovani A, Biswas SK, Galdiero MR, et al. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 73.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 74.Hargreaves DC, Horng T, Medzhitov R. Control of inducible gene expression by signal-dependent transcriptional elongation. Cell. 2009;138:129–145. doi: 10.1016/j.cell.2009.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wiesner P, Choi SH, Almazan F, et al. Low doses of lipopolysaccharide and minimally oxidized low-density lipoprotein cooperatively activate macrophages via nuclear factor kappa B and activator protein-1: possible mechanism for acceleration of atherosclerosis by subclinical endotoxemia. Circ Res. 2010;107:56–65. doi: 10.1161/CIRCRESAHA.110.218420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mullick AE, Soldau K, Kiosses WB, et al. Increased endothelial expression of Toll-like receptor 2 at sites of disturbed blood flow exacerbates early atherogenic events. J Exp Med. 2008;205:373–383. doi: 10.1084/jem.20071096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Tits LJ, Stienstra R, van Lent PL, et al. Oxidized LDL enhances pro-inflammatory responses of alternatively activated M2 macrophages: a crucial role for Kruppel-like factor 2. Atherosclerosis. 2011;214:345–349. doi: 10.1016/j.atherosclerosis.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 78.Vandanmagsar B, Youm YH, Ravussin A, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grebe A, Latz E. Cholesterol crystals and inflammation. Curr Rheumatol Rep. 2013;15:313. doi: 10.1007/s11926-012-0313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Krausgruber T, Blazek K, Smallie T, et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12:231–238. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- 82▪▪.Satoh T, Kidoya H, Naito H, et al. Critical role of Trib1 in differentiation of tissue-resident M2-like macrophages. Nature. 2013;495:524–528. doi: 10.1038/nature11930. Authors demonstrate that M2 differentiation is regulated by Trib-1 transcription factor in vivo. [DOI] [PubMed] [Google Scholar]
- 83.Satoh T, Takeuchi O, Vandenbon A, et al. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol. 2010;11:936–944. doi: 10.1038/ni.1920. [DOI] [PubMed] [Google Scholar]
- 84.El Chartouni C, Schwarzfischer L, Rehli M. Interleukin-4 induced interferon regulatory factor (Irf) 4 participates in the regulation of alternative macrophage priming. Immunobiology. 2010;215:821–825. doi: 10.1016/j.imbio.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 85.Hanna RN, Carlin LM, Hubbeling HG, et al. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C-monocytes. Nat Immunol. 2011;12:778–785. doi: 10.1038/ni.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Combadiere C, Potteaux S, Gao JL, et al. Decreased atherosclerotic lesion formation in CX3CR1/apolipoprotein E double knockout mice. Circulation. 2003;107:1009–1016. doi: 10.1161/01.cir.0000057548.68243.42. [DOI] [PubMed] [Google Scholar]
- 87.Combadiere C, Potteaux S, Rodero M, et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 88.Liu P, Yu YR, Spencer JA, et al. CX3CR1 deficiency impairs dendritic cell accumulation in arterial intima and reduces atherosclerotic burden. Arterioscler Thromb Vasc Biol. 2008;28:243–250. doi: 10.1161/ATVBAHA.107.158675. [DOI] [PubMed] [Google Scholar]
- 89.Landsman L, Bar-On L, Zernecke A, et al. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood. 2009;113:963–972. doi: 10.1182/blood-2008-07-170787. [DOI] [PubMed] [Google Scholar]
- 90.Liu K, Victora GD, Schwickert TA, et al. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schmid MA, Kingston D, Boddupalli S, Manz MG. Instructive cytokine signals in dendritic cell lineage commitment. Immunol Rev. 2010;234:32–44. doi: 10.1111/j.0105-2896.2009.00877.x. [DOI] [PubMed] [Google Scholar]
- 92.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 93.Hildner K, Edelson BT, Purtha WE, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cisse B, Caton ML, Lehner M, et al. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell. 2008;135:37–48. doi: 10.1016/j.cell.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schiavoni G, Mattei F, Sestili P, et al. ICSBP is essential for the development of mouse type I interferon-producing cells and for the generation and activation of CD8alpha(+) dendritic cells. J Exp Med. 2002;196:1415–1425. doi: 10.1084/jem.20021263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hacker C, Kirsch RD, Ju XS, et al. Transcriptional profiling identifies Id2 function in dendritic cell development. Nat Immunol. 2003;4:380–386. doi: 10.1038/ni903. [DOI] [PubMed] [Google Scholar]
- 97▪▪.Satpathy AT, Kc W, Albring JC, et al. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J Exp Med. 2012;209:1135–1152. doi: 10.1084/jem.20120030. This work describes the transcription factor Zbtb46 that specifically regulates conventional dendritic cell development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Meredith MM, Liu K, Kamphorst AO, et al. Zinc finger transcription factor zdendritic cell is a negative regulator required to prevent activation of classical dendritic cells in the steady state. J Exp Med. 2012;209:1583–1593. doi: 10.1084/jem.20121003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99▪▪.Meredith MM, Liu K, Darrasse-Jeze G, et al. Expression of the zinc finger transcription factor zdendritic cell (Zbtb46, Btbd4) defines the classical dendritic cell lineage. J Exp Med. 2012;209:1153–1165. doi: 10.1084/jem.20112675. This work describes the transcription factor Zbtb46 that specifically regulates conventional dendritic cell development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Paulson KE, Zhu SN, Chen M, et al. Resident intimal dendritic cells accumulate lipid and contribute to the initiation of atherosclerosis. Circ Res. 2010;106:383–390. doi: 10.1161/CIRCRESAHA.109.210781. [DOI] [PubMed] [Google Scholar]
- 101.Daissormont IT, Christ A, Temmerman L, et al. Plasmacytoid dendritic cells protect against atherosclerosis by tuning T-cell proliferation and activity. Circ Res. 2011;109:1387–1395. doi: 10.1161/CIRCRESAHA.111.256529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Macritchie N, Grassia G, Sabir SR, et al. Plasmacytoid dendritic cells play a key role in promoting atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2012;32:2569–2579. doi: 10.1161/ATVBAHA.112.251314. [DOI] [PubMed] [Google Scholar]
- 103.Sun J, Hartvigsen K, Chou MY, et al. Deficiency of antigen-presenting cell invariant chain reduces atherosclerosis in mice. Circulation. 2010;122:808–820. doi: 10.1161/CIRCULATIONAHA.109.891887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sun CM, Hall JA, Blank RB, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, et al. A functionally specialized population of mucosal CD103+ dendritic cells induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity. 2011;35:323–335. doi: 10.1016/j.immuni.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jung S, Unutmaz D, Wong P, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gautier EL, Huby T, Saint-Charles F, et al. Conventional dendritic cells at the crossroads between immunity and cholesterol homeostasis in atherosclerosis. Circulation. 2009;119:2367–2375. doi: 10.1161/CIRCULATIONAHA.108.807537. [DOI] [PubMed] [Google Scholar]
- 109.Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399–409. doi: 10.1038/nrcardio.2009.55. [DOI] [PubMed] [Google Scholar]
- 110.Cho HJ, Shashkin P, Gleissner CA, et al. Induction of dendritic cell-like phenotype in macrophages during foam cell formation. Physiol Genom. 2007;29:149–160. doi: 10.1152/physiolgenomics.00051.2006. [DOI] [PubMed] [Google Scholar]
- 111.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thorp E, Tabas I. Mechanisms and consequences of efferocytosis in advanced atherosclerosis. J Leuk Biol. 2009;86:1089–1095. doi: 10.1189/jlb.0209115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Choudhury RP, Lee JM, Greaves DR. Mechanisms of disease: macrophage-derived foam cells emerging as therapeutic targets in atherosclerosis. Nat Clin Pract Cardiovasc Med. 2005;2:309–315. doi: 10.1038/ncpcardio0195. [DOI] [PubMed] [Google Scholar]