ABSTRACT
The formation of SCFA is the result of a complex interplay between diet and the gut microbiota within the gut lumen environment. The discovery of receptors, across a range of cell and tissue types for which short chain fatty acids SCFA appear to be the natural ligands, has led to increased interest in SCFA as signaling molecules between the gut microbiota and the host. SCFA represent the major carbon flux from the diet through the gut microbiota to the host and evidence is emerging for a regulatory role of SCFA in local, intermediary and peripheral metabolism. However, a lack of well-designed and controlled human studies has hampered our understanding of the significance of SCFA in human metabolic health. This review aims to pull together recent findings on the role of SCFA in human metabolism to highlight the multi-faceted role of SCFA on different metabolic systems.
KEYWORDS: gut microbiota, glucose homeostasis, host metabolic health, inflammation, short chain fatty acids
Introduction
Short chain fatty acids (SCFA) are the primary end-products of fermentation of non-digestible carbohydrates (NDC) that become available to the gut microbiota. They represent the major flow of carbon from the diet, through the microbiome to the host. The discovery that SCFA appear to be the natural ligands for free fatty acid receptor 2 and 3 (FFAR 2/3), found on a wide range of cell types, including enteroendocrine and immune cells, has led to renewed interest in the role of SCFA in human health.1-3 The link between dietary intake, gut microbiota diversity and function and their significance to human health is an active area of research at the present time. This reflects: 1) long-standing epidemiological evidence linking long-term high-fiber diets to improved health outcomes, 2) more recent observations from metagenomics studies on variations in gut microbiome diversity in metabolic disease and 3) improvements in our understanding of the intricate molecular signaling (referred to as “cross-talk”) between the gut microbiome and the host. Together, these represent a new frontier in our understanding of the determinants of major diseases in Western Societies. This is in part driven by the potential to intervene over the life-course with relatively simple and cost-effective interventions. However, at the present time, there is insufficient evidence to inform appropriate, evidence-based clinical or public health interventions with clearly defined outcomes using SCFA formulations. This review aims to examine the recent evidence around the role of SCFA as key signaling molecules between the gut microbiome and host health and bring together an integrated view of the role SCFA in human metabolic health.
SCFA production by the gut microbiota
SCFA are produced mainly through saccharolytic fermentation of carbohydrates that escape digestion and absorption in the small intestine and the pathways of SCFA production are relatively well understood4 and recently described in detail.5 The major products are formate, acetate, propionate and butyrate. Lactate is also a major organic acid produced from the fermentation of selected, often rapidly fermentable NDCs.6 Relatively minor amounts of branched chain fatty acids are also produced, mainly through fermentation of protein-derived branched chain amino acids.7 Amino acid fermentation may also contribute to SCFA, mainly via acetate and propionate production. Relatively little is known about the role of formate in the gut. It has been linked to methanogenesis and appears to be elevated in inflammatory conditions.8,9 Lactate can also be further metabolised to acetate, propionate and butyrate by a number of cross-feeding organisms.10-12
Metagenomic approaches have facilitated characterization of bacteria responsible for SCFA production. Acetate production pathways are widely distributed among bacterial groups whereas pathways for propionate, butyrate and lactate production appear more highly conserved and substrate specific. For example, propionate production although distributed across a number of phyla is dominated by relatively few bacterial genera.13 Species such as Akkermansia municiphilla have been identified as key propionate producing mucin degrading organisms.14 On the other hand, deoxy-sugars such as fucose and rhamnose are particularly propiogenic because of metabolic pathways present to reduce the carbon skeleton, via the intermediate 1,2-propanediol, in select organisms.13 Fermentation of resistant starch is thought to contribute significantly to butyrate production in the colon and is dominated by Ruminococcus bromii, such that absence of the organism significantly reduces resistant starch fermentation.15 A surprisingly small number of organisms, dominated by Faecalibacterium prausnitzii, Eubacterium rectale, Eubacterium hallii and R. bromii, appear to be responsible for the major fraction of butyrate production.16 The link between diet, microbiome composition and SCFA production, although relatively well characterized is rather more difficult to predict and has often relied on in vitro fermentation data and animal models. In silico modeling of the complex dynamic relationships between dietary substrate, microbiota composition and substrate production holds promise enabling predictions of SCFA production from diet-gut microbiome interactions.17 However, high-level evidence from controlled human trials supporting SCFA as key regulation factors in human metabolism is largely lacking and there is significant reliance on associative studies, rather than interventional studies.
The field has been hampered by a lack of methodology to measure SCFA production directly in human studies, although recent work suggests that stable isotope techniques may hold promise.18 Observations in humans have largely relied on the measurement of stool SCFA output, although it is unclear whether stool SCFA output is a suitable proxy for luminal SCFA production.19 However, there is emerging evidence that diet-driven changes in microbiota diversity lead to variations in SCFA. In a recent diet-switch study, where African Americans were fed a high-fiber, low-fat African-style diet and rural Africans a high-fat, low-fiber western-style diet, the investigators observed profound shifts in gut microbiota composition, and SCFA and bile acids in the faecal water.20 A shift toward the butyrate producing organisms Roseburia intestinalis, Eubacterium rectale and Clostridium symbiosum along with increased butyrogenesis was observed on low-fat, high fiber feeding. Increases in CD3+ intra-epithelial lymphocytes and CD68+ lamina propria macrophages were also observed on high fat, low fiber diets suggesting increased inflammation in the absence of saccharolytic breakdown of fiber. Whether these changes translate into long-term impacts on host metabolism require intervention studies of longer duration. Changes in the microbiota of patients with inflammatory bowel disease (IBD) have been linked with decreased bacterial diversity and a loss of butyrate producing organisms such as F. prausnitzii.21,22 A similar loss of diversity has been observed in other auto-immune pathologies, such as psioaritic arthritis, suggesting a role for the microbiota and its metabolites in immune regulation.23 Insight into the links between diversity and function are also observed in interventions that have profound impacts on the diet - gut microbiome axis. Roux-en Y (RYGB) gastric bypass surgery led to enrichment of Bacteroidetes, Verrucomicrobia, and Proteobacteria at the phylum level and relatively greater propionate and lower acetate production suggesting that the gut microbiota contribute to reduced host weight and adiposity after RYGB surgery.24 These studies highlight the interdependence between diet (substrate), the gut microbiota and host metabolism and that, changes in SCFA and the microbiota are at least associated with profound effects on host metabolism.
Site of SCFA production and biological gradient from gut lumen to the periphery
It is important to consider the site of SCFA production and the biological gradient across the various down-stream tissues to fully understand the biological effects of SCFA in humans. This is particularly pertinent for the translation of findings from animal studies which often utilize oral SCFA supplementation or high dietary fiber supplementation to induce changes in SCFA production. Oral SCFA are rapidly absorbed and oxidised, best exemplified by the use of sodium 13C-acetate as a tracer for liquid phase of gastric emptying.25,26 High circulating concentrations of SCFA (>1mmol/L; normal range, 0–5 μmol/L for propionate and butyrate), other than acetate, are observed in acidaemic disease in humans and have profound impacts on metabolism because of the toxicity of these organic acids at high concentrations.27 Whether oral SCFA feeding studies that lead to high circulating SCFA concentration in animals, particularly propionate, represent an appropriate model for human SCFA physiology requires further validation. Animal studies which use dietary fiber supplementation to manipulate colonic SCFA tend to use relatively high fiber supplementation; 5 – 20% w/w NDC in animal dry matter intake (DMI). Using the UK National Diet and Nutrition Survey (NDNS), estimates of human daily DMI (sum of protein, total fat, total carbohydrate, micronutrients and vitamins) can be obtained.28 Mean daily DMI from the NDNS survey in the UK for men and women (aged 19–64) is calculated at 418.3 g/d and 326.3 g/d respectively. Thus translating the fiber supplementation rates from animal experiments, a daily fiber supplementation in the range 20.9 – 83.7 g/d for men and 16.3 – 65.3 g/d of dietary fiber would be broadly comparable with the 5 – 20% w/w diet loadings used in animal studies. Also from UK NDNS data, mean dietary fiber intake in the UK diet has been measured at 14.7 g/d for men (aged 19–64) and 12.8 g/d for women (aged 19–64) respectively (measured as non-starch polysaccharide). Even the lowest supplementation levels used in animal studies represent a comparable substantial increase above habitual dietary fiber intake in humans.
There is a strong biological gradient for each SCFA from the gut lumen to the periphery which leads to differing cell and tissue SCFA exposure (Fig. 1). The seminal work in sudden-death victims was first to highlight the significant reduction in butyrate, relative to acetate and propionate across the gut epithelium and also the significant reduction in propionate relative to acetate across the liver in humans.29 This has also been observed more recently with stable isotope flux studies in man where hepatic capacity to utilize SCFA balances gut SCFA production, leading to non-significant splanchnic propionate and butyrate output.30,31 These observations suggest that the roles of SCFA should be considered in each cell or tissue type within this biological gradient. The interplay between epithelial utilization and integrity, splanchnic utilization and peripheral availability requires delineation to determine whether increased production of all SCFA, or selective increases in individual SCFA at specific tissues, determines some of the observed metabolic effects.
Figure 1.
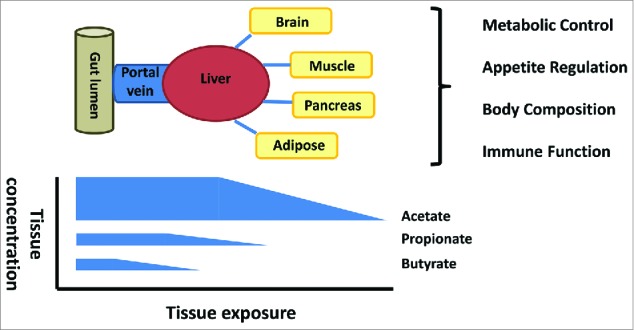
The gut lumen is the major site of production but the concentration gradient falls from the lumen to the periphery with selective uptake of butyrate at the epithelium, propionate at the liver and acetate in the periphery. The significance for host physiology of this biological gradient is poorly understood.
SCFA and gut integrity
It is well established that SCFA, and butyrate in particular, are important substrates for maintaining the colonic epithelium. Butyrate is the preferred fuel utilised by coloncytes and the primary site of butyrate sequestration in the body is the gut epithelium.31,32 Butyrate appears to have a dual role, sometimes referred to as the “butyrate paradox” whereby it induces proliferation in healthy colonocytes but terminal differentiation and apoptosis in transformed cells.33,34 However, in a mouse model of colorectal cancer, butyrate appears to fuel hyper-proliferation in MSH2 deficient colon epithelial cells leading to enhanced tumor formation.35 SCFA also appear to play an important role in regulating the integrity of the epithelial barrier through co-ordinated regulation of tight junction proteins (TJP) which themselves regulate the intracellular molecular highway between the lumen and hepatic portal system. Increased permeability is associated with translocation of bacteria and/or their cell wall components which trigger an inflammatory cascade that has been associated with obesity and insulin resistance.36 Increased bacterial lipopolysaccharide (LPS) triggers a toll-like receptor 4 (TLR4) mediated pro-inflammatory cascade in immune cells (monocytes, macrophages and Kupffer cells), leading to the activation of downstream signaling pathways, such as nuclear factor kappa β (NF-kΒ) and mitogen-activated protein kinase (MAPK), which can lead to inflammation driven by cytokines such as TNF-a and IL-6.37 Of the SCFA produced in the colon, butyrate appears to be the most important regulator of TJP and has been shown to enhance intestinal barrier function through increased expression of claudin-1 and Zonula Occludens-1 (ZO-1) and occludin redistribution; proteins which are critical components of the tight junction assembly.38 Butyrate has been shown to reverse the aberrant expression of ZO-1 and decrease LPS translocation leading to inhibition of macrophage activation, pro-inflammatory cytokine production and neutrophil infiltration resulting in reduced hepatic liver injury in rats.39 Further work is urgently needed in human models to determine whether SCFA play an important role in mucosal maintenance and integrity.
SCFA and glucose homeostasis
A potential regulatory role for SCFA in glucose homeostasis, mediated through FFAR 2/3, has led to significant interest in pharmacological interventions targeting this receptor mediated pathway in metabolic disease.40 In the liver, propionate is gluconeogenic while acetate and butyrate are lipogenic.41 From stoichiometric equations of daily SCFA production from dietary fiber intake,42 daily propionate production, estimated to be 29.5 mg/kg/day for an average 85 kg human, is likely to make only a relatively small contribution to endogenous glucose production (2.2 mg/kg/min43) of which approximately 50% may be attributable to gluconeogenesis.43 The potential role of SCFA as signaling molecules regulating hepatic glucose homeostasis however has not been fully elucidated in humans. Acetate, propionate and butyrate appear to regulate hepatic lipid and glucose homeostasis in an adenosine monophosphate-activated protein kinase dependent manner involving peroxisome proliferator-activated receptor-γ regulated effects on gluconeogenesis and lipogenesis.44 Recently, evidence has also emerged for a homeostatic signal in the hepatic portal system derived from increased intestinal gluconeogenesis from propionate which involves induction of gluconeogenic genes by butyrate.45,46 Given the concomitant exposure of the epithelium to butyrate and propionate, this mechanism suggests an elegant homeostatic nutrient sensor. In addition, increased propionate flux through the liver has been shown to reduce intrahepatic triglyceride which is also likely to elicit an improvement in hepatic and whole-body glucose homeostasis.47 Furthermore, acetate has been linked to suppression of adipocyte lipolysis, thus reducing free fatty acid (FFA) flux to the liver mitigating against fatty liver induced deterioration in glucose homeostasis.48,49 Finally, elevated plasma acetate has been shown to be inversely related to plasma insulin levels.50 A mechanism to explain this effect involves improved insulin response in pancreatic β cells, mediated by FFAR2 which induces improved glucose control.51,52 Taken together, the evidence suggests that SCFA elicit effects on multiple tissues in a concerted action to improve intestinal, hepatic and whole-body glucose homeostasis.
In addition to the direct SCFA derived signal from the gut there are concomitant signals, generated by primary SCFA production in the gut lumen. Gut hormones produced by the enteroendocrine cells in the colonic epithelium also exert beneficial effects on glucose homeostasis. The mechanisms of gut hormone driven effects on glucose homeostasis have been comprehensively reviewed elsewhere53,54 and are beyond the scope of this review. However, the production of SCFA by the microbiota in the gut lumen is an important initiating event for the gut-hormone derived signal.55,56
SCFA effects on lipid metabolism
SCFA elicit effects on lipid metabolism and adipose tissue at several levels. In the liver, the fate of acetate is de novo lipogenesis (DNL) and cholesterogenesis, both of which appear to be inhibited by propionate.57,58 Thus the ratio propionate : acetate may be an important determinant of the contribution of colonic acetate to lipid stores. Recent work has also demonstrated that propionate alone is able to reduce visceral fat and liver fat.47 Increased circulating SCFA are associated with reduced adipocyte lipolysis and adipogenesis.59 SCFA also inhibit insulin stimulated lipid accumulation in adipocytes via FFAR 2 signaling, resulting in small more responsive adipocytes which is associated with reduced adipose inflammatory infiltrate.60,61 Acetate also appears to stimulate leptin secretion in adipocytes.62 Leptin is an important adipose derived homeostatic signal which regulates energy balance and appetite.63 The inhibition of adipose tissue lipolysis leads to reduced free FFA from the adipose tissue to the liver. In fatty liver disease, adipose derived FFA have been shown to contribute 60% of fatty acids to newly synthesized triglyceride in the liver while DNL contributes 26%.64 Rectal infusion of acetate and propionate has demonstrated a 40% reduction in serum FFA.49 The contribution of exogenous (gut microbiota derived) acetate production to whole-body acetate flux has been estimated to be approximately 44%65 but how this proportional contribution is affected by different NDCs and microbiome activity is largely unknown. Increasing peripheral SCFA availability from NDC fermentation may be a novel strategy to inducing regulation of FFA flux in the obese phenotype. However, controversy still exists regarding the role of SCFA in obesity. A number of studies have advanced the “energy harvesting” hypothesis, whereby SCFA are thought to contribute additional calories through fermentation in the obese as an explanation for additional weight gain.66 However, this is not supported by the observational evidence in humans where high-fiber diets, which would be expected to increase SCFA production, protect against weight gain.67-69 Further well controlled studies in humans are urgently needed to dissect the role of SCFA in lipid turnover and energy homeostasis.
SCFA and appetite regulation
The role of SCFA in appetite regulation and energy intake has recently been reviewed in detail elsewhere.70,71 In addition to endocrine mediated effects, SCFAs can modulate neuronal activity and visceral reflexes directly via receptors expressed on neurons of the peripheral, autonomic and somatic nervous systems providing an additional mechanism of SCFA action.72 Whether these observations are driven by a single SCFA or a synergistic combination of SCFAs remains to be elucidated. In human intervention studies, only a small number of studies have demonstrated that fermentable fiber is associated with improved appetite regulation.73-77 Supplementation of habitual fiber intake in the range 16 – 35 g/d (approximately 5 – 10 % DMI) is necessary to induce these effects and may reflect the more consistent findings in animals at these equivalent fiber loadings and above. Whether these high fiber intakes elicit their appetite-regulating effects via SCFAs and FFAR 2/3 signaling pathways awaits a method to quantify SCFA production in vivo. Tantalising evidence of the role of SCFA in appetite regulation has recently appeared in a study using selective modulation of colonic propionate in humans which demonstrated that propionate appears to induce short-term appetite regulation though PYY and GLP-1 mediated mechanisms.47 Further work is needed to fully elucidate the role of each SCFA however.
SCFA and immune function
An exciting area of recent investigation has arisen from the discovery that SCFA play a role in regulating the immune system and inflammatory response. Early work at the turn of this century had demonstrated the potential role of butyrate in immune regulation when it was shown that butyrate inhibits nuclear factor kappa β (NF-κΒ) activation in macrophages and also inhibits histone deacetylation (HDAc) in acute myeloid leukemia.78,79 NF-κΒ is a eukaryotic transcription factor that is involved in the control of a plethora of normal cellular processes, including immune and inflammatory responses. HDAc inhibition plays a role in specific inflammatory signaling pathways as well as epigenetic mechanisms.80 Recently, 2 studies have highlighted a potential role for propionate and butyrate in regulatory T cell production and function at the whole-animal level through inhibition of HDAc.81,82 The extra-thymic conditioning of regulatory T cell response by SCFA suggests that these molecules are an important link in the cross-talk between the microbiome and the immune system. Whether SCFA act as a signal to induce tolerance to the host-associated microbiome or directly reduce inflammatory responses remains to be fully elucidated. SCFA do appear able to reduce the responsiveness of lamina propria macrophages to commensal bacteria, via nitric oxide, IL-6, and IL-12 independent of FFAR signaling, to induce tolerance.83 SCFA, in particular propionate and butyrate, have also been shown to inhibit the expression of lipopolysaccharide (LPS)-induced cytokines, IL-6 and IL-12p40 in human mature dendritic cells.84 Of the few clinical studies that have used SCFA therapeutically in inflammatory disease in a controlled trial setting, improvements in clinical and histological indices of IBD and therapeutic efficacy in acute radiation proctitis have been observed supporting a direct anti-inflammatory role of butyrate at sites of inflammation.85,86 Decreases in butyrate-producing organisms have been observed in metabolic aberrations such as type-2 diabetes,87 which is a disease characterized by low-grade inflammation. Thus, an evolving body of evidence appears to support a crucial role for SCFA in shaping the local and peripheral immune system which impacts on host metabolism via inflammatory pathways.
The liver hosts a range of cell types that interact via small molecules and secondary immune cytokine signaling. Gut barrier permeability is thought to be a key factor in determining the pro-inflammatory load reaching the liver.36 Abrogation of hepatocyte triglyceride accumulation and fatty acid esterification, and decreasing fatty acid oxidation and insulin responsiveness has been observed in a murine Kupffer cell depletion model, largely mediated by TNF-α.88 Recent data also suggests butyrate suppresses TNF-α, IL-6, and myeloperoxidase activity by preventing NF-κΒ activation in Kupffer cells.89 There is a paucity of data regarding acetate and propionate, which would be present at higher flux through the liver. Although further evidence is required to establish the role of SCFA in regulating liver inflammation, either directly or indirectly, these studies demonstrate the importance of the gut-liver axis in inflammatory and metabolic systems and that SCFA may play important roles in both.
The role of SCFA also extends to peripheral immune function. A recent study has demonstrated that acetate mediates joint inflammation in a murine gout model through inflammasome assembly and IL-1β production that is partially FFAR2 dependent.90 A similar protective effect has been recently observed for butyrate in a peripheral blood mononuclear cell gout model, although high concentrations of butyrate were required to moderate production of the pro-inflammatory cytokines IL-1β, IL-6, IL-8 and IL-1β.91 Consideration of the biological gradient for SCFA exposure for different immune cell types may be critical to defining physiologically relevant outcomes in immune-mediated and inflammatory disease.
Conclusions and future perspectives
It is tempting to overlook the potential for small ubiquitous microbiota derived molecules like SCFA to act as important molecular signals between the microbiota and host or as metabolic substrates regulating host cellular metabolism. A continually emerging body of evidence supports the role of SCFA as key mediators of cell function in a range of local, intermediary and peripheral tissues (summarised in Fig. 2) raising the question as to whether SCFA represent the key molecular link between diet, the microbiome and health? The renewed interest in SCFA, coupled with the revolution in the tools available to dissect the complex molecular biology associated with cell signaling and metabolism, is beginning to provide evidence of the central role of SCFA in the diet-gut microbiome-host metabolism axis. There are however some key questions remaining that require further investigation. 1) Is the microbiome a passive substrate-degrading system or are signaling molecules actively involved in microbiome-host “crosstalk”? Undoubtedly the answer to this quandary is yes, the molecules produced through microbiota activity provide important regulatory signals to the host and in the “-omics” revolution, metabolomics is a key enabling technology which will enable the identification of the repertoire of signals between microbiome and host. 2) Can the microbiota and its function be manipulated in a predictable way to have a clinically relevant impact on disease risk? The answer to this is highly likely to be yes, but probiotic studies designed to change microbiota diversity have been far from convincing in terms of outcome measures in metabolic health92 and weight management.93 Prebiotics have had varying degrees of success and the lesson from animal feeding studies may be that high doses of NDC are needed in Western populations to drive physiologically relevant changes in SCFA in order to induce a physiologically relevant effect. This presents a major challenge to translating effective treatments into clinical practice or into strategies that improve population health. New targeted approaches may be needed. 3) In Western societies and developing countries, what role do changes in diet and microbiome function play in future risk of disease? There is an increasing focus on the prevention of diseases that cluster around obesity, inactivity and a Western-type diet because of the present and predicted future economic health burden. The multifaceted roles of SCFA suggest that they may play an important role over the life-course in protecting the body against deteriorating metabolic control and inflammatory status associated with Western lifestyles. Whether manipulating the diet-gut microbiome-host metabolism axis represents a panacea for prevention of these leading causes of morbidity and mortality remains to be seen but it is a tantalising prospect because of the wide ranging benefits that could be brought about through relatively simple and cost-effective interventions if they could be targeted appropriately.
Figure 2.
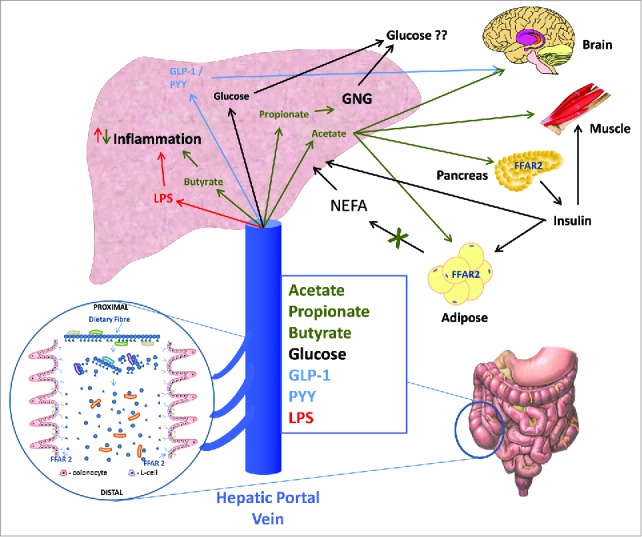
SCFA along with other metabolites entering the hepatic portal system are rapidly transported to the liver. The role of molecular signaling on different liver cell types is poorly characterized. SCFA can act on resident macrophages and hepatocytes although there may be functional selectivity for each SCFA. Incretion hormones may also act on hepatocytes and peripheral tissues. The overall impact of this dual signaling system appears to be maintenance of a healthy liver through regulation of hepatic metabolism and inflammation and control of adipose derived FFA flux. The peripheral effects of SCFA appear tissue specific. SCFA can regulate insulin in the pancreas, FFA flux from adipocytes, appetite centers in the brain and provide a fuel for the muscle. This multi-faceted role however, requires further investigation with well-designed and controlled studies in humans.
Abbreviations
- SCFA
short chain fatty acids
- NDC
non-digestible carbohydrates
- FFAR
free fatty acid receptor
- IBD
inflammatory bowel disease
- DMI
dry matter intake
- UK NDNS
United Kingdom national diet and nutrition survey
- TJP
tight junction proteins
- LPS
lipopolysaccharide
- TLR
toll-like receptor
- NF-kΒ
nuclear factor kappa β
- MAPK
mitogen-activated protein kinase
- TNF-a
tissue necrosis factor-α
- IL
interleukin
- ZO-1
Zonula Occludens-1
- FFA
free fatty acid
- DNL
de novo lipogenesis
- HDAc
histone deacetylation
Disclosure of potential conflicts of interest
The authors have no conflicts of interest to declare.
Funding
The Stable Isotope Biochemistry Laboratory at SUERC is supported in part by grants from BBSRC (BB/L004259/1,BB/H004815/1,BB/H532091/1,BB/L025418/1) and Scottish Government (RESAS) Strategic Partnership.
References
- [1].Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van Damme J, et al.. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem 2003; 278:25481-9; PMID:12711604; http://dx.doi.org/ 10.1074/jbc.M301403200 [DOI] [PubMed] [Google Scholar]
- [2].Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, et al.. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 2003; 278:11312-9; PMID:12496283; http://dx.doi.org/ 10.1074/jbc.M211609200 [DOI] [PubMed] [Google Scholar]
- [3].Nilsson NE, Kotarsky K, Owman C, Olde B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem Biophys Res Commun 2003; 303:1047-52; PMID:12684041; http://dx.doi.org/ 10.1016/S0006-291X(03)00488-1 [DOI] [PubMed] [Google Scholar]
- [4].Miller TL, Wolin MJ. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl Environ Microbiol 1996; 62:1589-92; PMID:8633856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Flint HJ, Duncan SH, Scott KP, Louis P. Links between diet, gut microbiota composition and gut metabolism. Proc Nutr Soc 2015; 74:13-22; PMID:25268552; http://dx.doi.org/ 10.1017/S0029665114001463 [DOI] [PubMed] [Google Scholar]
- [6].Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc 2003; 62:67-72; PMID:12740060; http://dx.doi.org/ 10.1079/PNS2002207 [DOI] [PubMed] [Google Scholar]
- [7].Russell WR, Gratz SW, Duncan SH, Holtrop G, Ince J, Scobbie L, Duncan G, Johnstone AM, Lobley GE, Wallace RJ, et al.. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am J Clin Nutr 2011; 93:1062-72; PMID:21389180; http://dx.doi.org/ 10.3945/ajcn.110.002188 [DOI] [PubMed] [Google Scholar]
- [8].Vanderhaeghen S, Lacroix C, Schwab C. Methanogen communities in stools of humans of different age and health status and co-occurrence with bacteria. FEMS Microbiol Lett 2015; 362:fnv092; PMID:26025070 [DOI] [PubMed] [Google Scholar]
- [9].Bereswill S, Fischer A, Plickert R, Haag LM, Otto B, Kuhl AA, Dasti JI, Zautner AE, Muñoz M, Loddenkemper C, et al.. Novel murine infection models provide deep insights into the “menage a trois” of Campylobacter jejuni, microbiota and host innate immunity. PLoS One 2011; 6:e20953; PMID:21698299; http://dx.doi.org/ 10.1371/journal.pone.0020953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bourriaud C, Robins RJ, Martin L, Kozlowski F, Tenailleau E, Cherbut C, Michel C. Lactate is mainly fermented to butyrate by human intestinal microfloras but inter-individual variation is evident. J Appl Microbiol 2005; 99:201-12; PMID:15960680; http://dx.doi.org/ 10.1111/j.1365-2672.2005.02605.x [DOI] [PubMed] [Google Scholar]
- [11].Belenguer A, Duncan SH, Calder AG, Holtrop G, Louis P, Lobley GE, Flint HJ. Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl Environ Microbiol 2006; 72:3593-9; PMID:16672507; http://dx.doi.org/ 10.1128/AEM.72.5.3593-3599.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Morrison DJ, Mackay WG, Edwards CA, Preston T, Dodson B, Weaver LT. Butyrate production from oligofructose fermentation by the human faecal flora: what is the contribution of extracellular acetate and lactate? Br J Nutr 2006; 96:570-7; PMID:16925864 [PubMed] [Google Scholar]
- [13].Reichardt N, Duncan SH, Young P, Belenguer A, McWilliam Leitch C, Scott KP, Flint HJ, Louis P. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J 2014; 8:1323-35; PMID:24553467; http://dx.doi.org/ 10.1038/ismej.2014.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol 2004; 54:1469-76; PMID:15388697; http://dx.doi.org/ 10.1099/ijs.0.02873-0 [DOI] [PubMed] [Google Scholar]
- [15].Ze X, Duncan SH, Louis P, Flint HJ. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J 2012; 6:1535-43; PMID:22343308; http://dx.doi.org/ 10.1038/ismej.2012.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Louis P, Young P, Holtrop G, Flint HJ. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ Microbiol 2010; 12:304-14; PMID:19807780; http://dx.doi.org/ 10.1111/j.1462-2920.2009.02066.x [DOI] [PubMed] [Google Scholar]
- [17].Munoz-Tamayo R, Laroche B, Walter E, Dore J, Duncan SH, Flint HJ, Leclerc M. Kinetic modelling of lactate utilization and butyrate production by key human colonic bacterial species. FEMS Microbiol Ecol 2011; 76:615-24; PMID:21388423; http://dx.doi.org/ 10.1111/j.1574-6941.2011.01085.x [DOI] [PubMed] [Google Scholar]
- [18].Boets E, Deroover L, Houben E, Vermeulen K, Gomand SV, Delcour JA, Verbeke K. Quantification of in Vivo Colonic Short Chain Fatty Acid Production from Inulin. Nutrients 2015; 7:8916-29; PMID:26516911; http://dx.doi.org/ 10.3390/nu7115440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vogt JA, Wolever TM. Fecal acetate is inversely related to acetate absorption from the human rectum and distal colon. J Nutr 2003; 133:3145-8; PMID:14519799 [DOI] [PubMed] [Google Scholar]
- [20].Holmes E, Li JV, Marchesi JR, Nicholson JK. Gut microbiota composition and activity in relation to host metabolic phenotype and disease risk. Cell Metab 2012; 16:559-64; PMID:23140640; http://dx.doi.org/ 10.1016/j.cmet.2012.10.007 [DOI] [PubMed] [Google Scholar]
- [21].Wang W, Chen L, Zhou R, Wang X, Song L, Huang S, Wang G, Xia B. Increased proportions of Bifidobacterium and the Lactobacillus group and loss of butyrate-producing bacteria in inflammatory bowel disease. J Clin Microbiol 2014; 52:398-406; PMID:24478468; http://dx.doi.org/ 10.1128/JCM.01500-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, Ballet V, Claes K, Van Immerseel F, Verbeke K, et al.. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014; 63:1275-83; PMID:24021287; http://dx.doi.org/ 10.1136/gutjnl-2013-304833 [DOI] [PubMed] [Google Scholar]
- [23].Scher JU, Ubeda C, Artacho A, Attur M, Isaac S, Reddy SM, Marmon S, Neimann A, Brusca S, Patel T, et al.. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol 2015; 67:128-39; PMID:25319745; http://dx.doi.org/ 10.1002/art.38892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Liou AP, Paziuk M, Luevano JM Jr., Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med 2013; 5:178ra41; PMID:23536013; http://dx.doi.org/ 10.1126/scitranslmed.3005687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Braden B, Adams S, Duan LP, Orth KH, Maul FD, Lembcke B, Hör G, Caspary WF. The [13C]acetate breath test accurately reflects gastric emptying of liquids in both liquid and semisolid test meals. Gastroenterology 1995; 108:1048-55; PMID:7698571 [DOI] [PubMed] [Google Scholar]
- [26].Hendry PO, van Dam RM, Bukkems SF, McKeown DW, Parks RW, Preston T, Dejong CH, Garden OJ, Fearon KC, et al.. Randomized clinical trial of laxatives and oral nutritional supplements within an enhanced recovery after surgery protocol following liver resection. Br J Surg 2010; 97:1198-206; PMID:20602497; http://dx.doi.org/ 10.1002/bjs.7120 [DOI] [PubMed] [Google Scholar]
- [27].Carrillo-Carrasco N, Venditti C. Propionic Acidemia In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, et al., eds. GeneReviews(R). Seattle (WA): University of Washington, SeattleUniversity of Washington, Seattle; All rights reserved., 1993 [Google Scholar]
- [28]. 2015 National Diet and Nutrition Survey: results from Years 1 to 4 (combined) of the rolling programme for 2008 and 2009 to 2011 and 2012 - Publications - GOV.UK. [Google Scholar]
- [29].Macfarlane GT, Gibson GR, Cummings JH. Comparison of fermentation reactions in different regions of the human colon. J Appl Bacteriol 1992; 72:57-64; PMID:1541601 [DOI] [PubMed] [Google Scholar]
- [30].Bloemen JG, Venema K, van de Poll MC, Olde Damink SW, Buurman WA, Dejong CH. Short chain fatty acids exchange across the gut and liver in humans measured at surgery. Clin Nutr 2009; 28:657-61; PMID:19523724; http://dx.doi.org/ 10.1016/j.clnu.2009.05.011 [DOI] [PubMed] [Google Scholar]
- [31].van der Beek CM, Bloemen JG, van den Broek MA, Lenaerts K, Venema K, Buurman WA, Dejong CH. Hepatic Uptake of Rectally Administered Butyrate Prevents an Increase in Systemic Butyrate Concentrations in Humans. J Nutr 2015; 145(9):2019-24 [DOI] [PubMed] [Google Scholar]
- [32].Clausen MR, Mortensen PB. Kinetic studies on colonocyte metabolism of short chain fatty acids and glucose in ulcerative colitis. Gut 1995; 37:684-9; PMID:8549946; http://dx.doi.org/ 10.1136/gut.37.5.684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lupton JR. Microbial degradation products influence colon cancer risk: the butyrate controversy. J Nutr 2004; 134:479-82; PMID:14747692 [DOI] [PubMed] [Google Scholar]
- [34].Donohoe DR, Collins LB, Wali A, Bigler R, Sun W, Bultman SJ. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol Cell 2012; 48:612-26; PMID:23063526; http://dx.doi.org/ 10.1016/j.molcel.2012.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Belcheva A, Irrazabal T, Robertson SJ, Streutker C, Maughan H, Rubino S, Moriyama EH, Copeland JK, Kumar S, Green B, et al.. Gut microbial metabolism drives transformation of MSH2-deficient colon epithelial cells. Cell 2014; 158:288-99; PMID:25036629; http://dx.doi.org/ 10.1016/j.cell.2014.04.051 [DOI] [PubMed] [Google Scholar]
- [36].Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008; 57:1470-81; PMID:18305141; http://dx.doi.org/ 10.2337/db07-1403 [DOI] [PubMed] [Google Scholar]
- [37].Manco M, Putignani L, Bottazzo GF. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev 2010; 31:817-44; PMID:20592272; http://dx.doi.org/ 10.1210/er.2009-0030 [DOI] [PubMed] [Google Scholar]
- [38].Wang HB, Wang PY, Wang X, Wan YL, Liu YC. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig Dis Sci 2012; 57:3126-35; PMID:22684624; http://dx.doi.org/ 10.1007/s10620-012-2259-4 [DOI] [PubMed] [Google Scholar]
- [39].Liu B, Qian J, Wang Q, Wang F, Ma Z, Qiao Y. Butyrate protects rat liver against total hepatic ischemia reperfusion injury with bowel congestion. PLoS One 2014; 9:e106184; PMID:25171217; http://dx.doi.org/ 10.1371/journal.pone.0106184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ulven T. Short-chain free fatty acid receptors FFA2/GPR43 and FFA3/GPR41 as new potential therapeutic targets. Front Endocrinol (Lausanne) 2012; 3:111; PMID:23060857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].den Besten G, Lange K, Havinga R, van Dijk TH, Gerding A, van Eunen K, Müller M, Groen AK, Hooiveld GJ, Bakker BM, et al.. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am J Physiol Gastrointest Liver Physiol 2013; 305:G900-10; PMID:24136789; http://dx.doi.org/ 10.1152/ajpgi.00265.2013 [DOI] [PubMed] [Google Scholar]
- [42].Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev 2001; 81:1031-64; PMID:11427691 [DOI] [PubMed] [Google Scholar]
- [43].Rajpal A, Dube S, Carvalho F, Simoes AR, Figueiredo A, Basu A, Jones J, Basu R. Effects of transaldolase exchange on estimates of gluconeogenesis in type 2 diabetes. Am J Physiol Endocrinol Metab 2013; 305:E465-74; PMID:23736541; http://dx.doi.org/ 10.1152/ajpendo.00245.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].den Besten G, Bleeker A, Gerding A, van Eunen K, Havinga R, van Dijk TH, Oosterveer MH, Jonker JW, Groen AK, Reijngoud DJ, et al.. Short-chain fatty acids protect against high-fat diet-induced obesity via a PPARgamma-dependent switch from lipogenesis to fat oxidation. Diabetes 2015; 64:2398-408; PMID:25695945; http://dx.doi.org/ 10.2337/db14-1213 [DOI] [PubMed] [Google Scholar]
- [45].De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Bäckhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014; 156:84-96; PMID:24412651; http://dx.doi.org/ 10.1016/j.cell.2013.12.016 [DOI] [PubMed] [Google Scholar]
- [46].Soty M, Penhoat A, Amigo-Correig M, Vinera J, Sardella A, Vullin-Bouilloux F, Zitoun C, Houberdon I, Mithieux G. A gut-brain neural circuit controlled by intestinal gluconeogenesis is crucial in metabolic health. Mol Metab 2015; 4:106-17; PMID:25685698; http://dx.doi.org/ 10.1016/j.molmet.2014.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Chambers ES, Viardot A, Psichas A, Morrison DJ, Murphy KG, Zac-Varghese SEK, MacDougall K, Preston T, Tedford C, Finlayson GS, et al.. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 2014; 64(11):1744-54; PMID:25500202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Crouse JR, Gerson CD, DeCarli LM, Lieber CS. Role of acetate in the reduction of plasma free fatty acids produced by ethanol in man. J Lipid Res 1968; 9:509-12; PMID:5725882 [PubMed] [Google Scholar]
- [49].Wolever TM, Brighenti F, Royall D, Jenkins AL, Jenkins DJ. Effect of rectal infusion of short chain fatty acids in human subjects. Am J Gastroenterol 1989; 84:1027-33; PMID:2773895 [PubMed] [Google Scholar]
- [50].Layden BT, Yalamanchi SK, Wolever TM, Dunaif A, Lowe WL Jr. Negative association of acetate with visceral adipose tissue and insulin levels. Diabetes Metab Syndr Obes 2012; 5:49-55; PMID:22419881; http://dx.doi.org/ 10.2147/DMSO.S29244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tang C, Ahmed K, Gille A, Lu S, Grone HJ, Tunaru S, Offermanns S. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nat Med 2015; 21:173-7; PMID:25581519; http://dx.doi.org/ 10.1038/nm.3779 [DOI] [PubMed] [Google Scholar]
- [52].McNelis JC, Lee YS, Mayoral R, van der Kant R, Johnson AM, Wollam J, Olefsky JM. GPR43 Potentiates β-Cell Function in Obesity. Diabetes 2015; 64:3203-17; PMID:26023106; http://dx.doi.org/ 10.2337/db14-1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Todd JF, Bloom SR. Incretins and other peptides in the treatment of diabetes. Diabet Med 2007; 24:223-32; PMID:17263764; http://dx.doi.org/ 10.1111/j.1464-5491.2006.02071.x [DOI] [PubMed] [Google Scholar]
- [54].Psichas A, Reimann F, Gribble FM. Gut chemosensing mechanisms. J Clin Invest 2015; 125:908-17; PMID:25664852; http://dx.doi.org/ 10.1172/JCI76309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012; 61:364-71; PMID:22190648; http://dx.doi.org/ 10.2337/db11-1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Psichas A, Sleeth ML, Murphy KG, Brooks L, Bewick GA, Hanyaloglu AC, Ghatei MA, Bloom SR, Frost G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes (Lond) 2015; 39:424-9; PMID:25109781; http://dx.doi.org/ 10.1038/ijo.2014.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Nishina PM, Freedland RA. Effects of propionate on lipid biosynthesis in isolated rat hepatocytes. J Nutr 1990; 120:668-73; PMID:2366102 [DOI] [PubMed] [Google Scholar]
- [58].Demigne C, Morand C, Levrat MA, Besson C, Moundras C, Remesy C. Effect of propionate on fatty acid and cholesterol synthesis and on acetate metabolism in isolated rat hepatocytes. Br J Nutr 1995; 74:209-19; PMID:7547838; http://dx.doi.org/ 10.1079/BJN19950124 [DOI] [PubMed] [Google Scholar]
- [59].Hong YH, Nishimura Y, Hishikawa D, Tsuzuki H, Miyahara H, Gotoh C, Choi KC, Feng DD, Chen C, Lee HG, et al.. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology 2005; 146:5092-9; PMID:16123168; http://dx.doi.org/ 10.1210/en.2005-0545 [DOI] [PubMed] [Google Scholar]
- [60].Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani T, et al.. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun 2013; 4:1829; PMID:23652017; http://dx.doi.org/ 10.1038/ncomms2852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest 2011; 121:2094-101; PMID:21633177; http://dx.doi.org/ 10.1172/JCI45887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Zaibi MS, Stocker CJ, O'Dowd J, Davies A, Bellahcene M, Cawthorne MA, Brown AJ, Smith DM, Arch JR. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett 2010; 584:2381-6; PMID:20399779; http://dx.doi.org/ 10.1016/j.febslet.2010.04.027 [DOI] [PubMed] [Google Scholar]
- [63].Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 1995; 269:543-6; PMID:7624777; http://dx.doi.org/ 10.1126/science.7624777 [DOI] [PubMed] [Google Scholar]
- [64].Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005; 115:1343-51; PMID:15864352; http://dx.doi.org/ 10.1172/JCI23621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Fernandes J, Vogt J, Wolever TM. Kinetic model of acetate metabolism in healthy and hyperinsulinaemic humans. Eur J Clin Nutr 2014; 68:1067-71; PMID:25052228; http://dx.doi.org/ 10.1038/ejcn.2014.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006; 444:1027-31; PMID:17183312; http://dx.doi.org/ 10.1038/nature05414 [DOI] [PubMed] [Google Scholar]
- [67].Ludwig DS, Pereira MA, Kroenke CH, Hilner JE, Van Horn L, Slattery ML, Jacobs DR Jr. Dietary fiber, weight gain, and cardiovascular disease risk factors in young adults. Jama 1999; 282:1539-46; PMID:10546693; http://dx.doi.org/ 10.1001/jama.282.16.1539 [DOI] [PubMed] [Google Scholar]
- [68].Maskarinec G, Takata Y, Pagano I, Carlin L, Goodman MT, Le Marchand L, Nomura AM, Wilkens LR, Kolonel LN. Trends and dietary determinants of overweight and obesity in a multiethnic population. Obesity (Silver Spring) 2006; 14:717-26; PMID:16741275; http://dx.doi.org/ 10.1038/oby.2006.82 [DOI] [PubMed] [Google Scholar]
- [69].Du H, van der AD, Boshuizen HC, Forouhi NG, Wareham NJ, Halkjaer J, Tjønneland A, Overvad K, Jakobsen MU, Boeing H, et al.. Dietary fiber and subsequent changes in body weight and waist circumference in European men and women. Am J Clin Nutr 2010; 91:329-36; PMID:20016015; http://dx.doi.org/ 10.3945/ajcn.2009.28191 [DOI] [PubMed] [Google Scholar]
- [70].Byrne CS, Chambers ES, Morrison DJ, Frost G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int J Obes (Lond), 2015; 39(9):1331-8; PMID:25971927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Chambers ES, Morrison DJ, Frost G. Control of appetite and energy intake by SCFA: what are the potential underlying mechanisms? Proc Nutr Soc 2015; 74:328-36; PMID:25497601; http://dx.doi.org/ 10.1017/S0029665114001657 [DOI] [PubMed] [Google Scholar]
- [72].Nohr MK, Egerod KL, Christiansen SH, Gille A, Offermanns S, Schwartz TW, Møller M. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience 2015; 290:126-37; PMID:25637492; http://dx.doi.org/ 10.1016/j.neuroscience.2015.01.040 [DOI] [PubMed] [Google Scholar]
- [73].Archer BJ, Johnson SK, Devereux HM, Baxter AL. Effect of fat replacement by inulin or lupin-kernel fibre on sausage patty acceptability, post-meal perceptions of satiety and food intake in men. Br J Nutr 2004; 91:591-9; PMID:15035686; http://dx.doi.org/ 10.1079/BJN20031088 [DOI] [PubMed] [Google Scholar]
- [74].Daud NM, Ismail NA, Thomas EL, Fitzpatrick JA, Bell JD, Swann JR, Costabile A, Childs CE, Pedersen C, Goldstone AP, et al.. The impact of oligofructose on stimulation of gut hormones, appetite regulation and adiposity. Obesity (Silver Spring) 2014; 22:1430-8; PMID:24715424; http://dx.doi.org/ 10.1002/oby.20754 [DOI] [PubMed] [Google Scholar]
- [75].Pedersen C, Lefevre S, Peters V, Patterson M, Ghatei MA, Morgan LM, Frost GS. Gut hormone release and appetite regulation in healthy non-obese participants following oligofructose intake. A dose-escalation study. Appetite 2013; 66:44-53; PMID:23474087; http://dx.doi.org/ 10.1016/j.appet.2013.02.017 [DOI] [PubMed] [Google Scholar]
- [76].Cani PD, Joly E, Horsmans Y, Delzenne NM. Oligofructose promotes satiety in healthy human: a pilot study. Eur J Clin Nutr 2006; 60:567-72; PMID:16340949; http://dx.doi.org/ 10.1038/sj.ejcn.1602350 [DOI] [PubMed] [Google Scholar]
- [77].Parnell JA, Reimer RA. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am J Clin Nutr 2009; 89:1751-9; PMID:19386741; http://dx.doi.org/ 10.3945/ajcn.2009.27465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Luhrs H, Gerke T, Muller JG, Melcher R, Schauber J, Boxberge F, Scheppach W, Menzel T. Butyrate inhibits NF-kappaB activation in lamina propria macrophages of patients with ulcerative colitis. Scand J Gastroenterol 2002; 37:458-66; PMID:11989838; http://dx.doi.org/ 10.1080/003655202317316105 [DOI] [PubMed] [Google Scholar]
- [79].Maeda T, Towatari M, Kosugi H, Saito H. Up-regulation of costimulatory/adhesion molecules by histone deacetylase inhibitors in acute myeloid leukemia cells. Blood 2000; 96:3847-56; PMID:11090069 [PubMed] [Google Scholar]
- [80].Akimova T, Beier UH, Liu Y, Wang L, Hancock WW. Histone/protein deacetylases and T-cell immune responses. Blood 2012; 119:2443-51; PMID:22246031; http://dx.doi.org/ 10.1182/blood-2011-10-292003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Arpaia N, Campbell C, Fan XY, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, et al.. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013; 504:451-5; PMID:24226773; http://dx.doi.org/ 10.1038/nature12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, et al.. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013; 504:446-50; PMID:24226770; http://dx.doi.org/ 10.1038/nature12721 [DOI] [PubMed] [Google Scholar]
- [83].Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A 2014; 111:2247-52; PMID:24390544; http://dx.doi.org/ 10.1073/pnas.1322269111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Nastasi C, Candela M, Bonefeld CM, Geisler C, Hansen M, Krejsgaard T, Biagi E, Andersen MH, Brigidi P, Ødum N, et al.. The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Sci Rep 2015; 5:16148; PMID:26541096; http://dx.doi.org/ 10.1038/srep16148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Vernia P, Fracasso PL, Casale V, Villotti G, Marcheggiano A, Stigliano V, Pinnaro P, Bagnardi V, Caprilli R. Topical butyrate for acute radiation proctitis: randomised, crossover trial. Lancet 2000; 356:1232-5; PMID:11072942; http://dx.doi.org/ 10.1016/S0140-6736(00)02787-2 [DOI] [PubMed] [Google Scholar]
- [86].Vernia P, Annese V, Bresci G, d'Albasio G, D'Inca R, Giaccari S, Ingrosso M, Mansi C, Riegler G, Valpiani D, et al.. Topical butyrate improves efficacy of 5-ASA in refractory distal ulcerative colitis: results of a multicentre trial. Eur J Clin Invest 2003; 33:244-8; PMID:12641543; http://dx.doi.org/ 10.1046/j.1365-2362.2003.01130.x [DOI] [PubMed] [Google Scholar]
- [87].Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, et al.. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012; 490:55-60; PMID:23023125; http://dx.doi.org/ 10.1038/nature11450 [DOI] [PubMed] [Google Scholar]
- [88].Huang W, Metlakunta A, Dedousis N, Zhang P, Sipula I, Dube JJ, Scott DK, O'Doherty RM. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes 2010; 59:347-57; PMID:19934001; http://dx.doi.org/ 10.2337/db09-0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Qiao YL, Qian JM, Wang FR, Ma ZY, Wang QW. Butyrate protects liver against ischemia reperfusion injury by inhibiting nuclear factor kappa B activation in Kupffer cells. J Surg Res 2014; 187:653-9; PMID:24445056; http://dx.doi.org/ 10.1016/j.jss.2013.08.028 [DOI] [PubMed] [Google Scholar]
- [90].Vieira AT, Macia L, Galvao I, Martins FS, Canesso MC, Amaral FA, Garcia CC, Maslowski KM, De Leon E, Shim D, et al.. A Role for Gut Microbiota and the Metabolite-Sensing Receptor GPR43 in a Murine Model of Gout. Arthritis Rheumatol 2015; 67:1646-56; PMID:25914377; http://dx.doi.org/ 10.1002/art.39107 [DOI] [PubMed] [Google Scholar]
- [91].Cleophas MC, Crisan TO, Lemmers H, Toenhake-Dijkstra H, Fossati G, Jansen TL, Dinarello CA, Netea MG, Joosten LA. Suppression of monosodium urate crystal-induced cytokine production by butyrate is mediated by the inhibition of class I histone deacetylases. Ann Rheum Dis 2015. http://dx.doi.org/ 10.1136/annrheumdis-2014-206258 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [92].Razmpoosh E, Javadi M, Ejtahed HS, Mirmiran P. Probiotics as beneficial agents in the management of diabetes mellitus: a systematic review. Diabetes Metab Res Rev 2015; PMID:25963407; http://dx.doi.org/ 10.1002/dmrr.2665 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [93].Park S, Bae JH. Probiotics for weight loss: a systematic review and meta-analysis. Nutr Res 2015; 35:566-75; PMID:26032481; http://dx.doi.org/ 10.1016/j.nutres.2015.05.008 [DOI] [PubMed] [Google Scholar]


