Abstract
Background
Endometriosis is a prevalent gynecologic disease associated with systemic chronic inflammation, heightened oxidative stress and atherogenic lipid profile that may increase women's risk for Coronary heart disease (CHD).
Methods and Results
We examined the prospective association between laparoscopically-confirmed endometriosis and subsequent CHD among 116,430 women in the Nurses’ Health Study II (1989-2009). Participants with a history of heart disease and stroke were excluded. Compared to women without endometriosis, women with laparoscopically-confirmed endometriosis had a higher risk of myocardial infarction (relative risk, 1.52; 95% confidence interval, 1.17-1.98), angiographically-confirmed angina (1.91; 1.59-2.29), coronary artery bypass graft surgery/coronary angioplasty procedure/stent (1.35; 1.08-1.69), or any of these CHD endpoints combined (1.62; 1.39-1.89), independent of potential demographic, anthropometric, family history, reproductive, and lifestyle confounders. Relative risk for the combined CHD endpoint was highest among women age ≤40 (3.08; 2.02-4.70), and decreased as age increased (40<age ≤50, 1.65, 1.35-2.02; 50<age ≤55, 1.44, 1.07-1.94; age >55, 0.98, 0.56-1.72; p-value, test for heterogeneity=0.001). Having had a hysterectomy/oophorectomy was associated with higher risk of combined CHD compared to not having had a hysterectomy/oophorectomy (1.51, 1.34-1.71). 42% of the association between endometriosis and CHD could be explained by greater frequency of hysterectomy/oophorectomy and earlier age at surgery following endometriosis diagnosis.
Conclusions
In this large, prospective cohort, laparoscopically-confirmed endometriosis was associated with increased risk of CHD. The association was strongest among young women. Hysterectomy/oophorectomy was associated with higher risk of CHD and could partially explain the association between endometriosis and CHD.
Keywords: endometriosis, coronary artery disease, epidemiology
Introduction
Endometriosis is a chronic and estrogen-dependent gynecologic disorder that affects ~10% of women of reproductive age in the United States.(1) It is defined as the presence of endometrium-like tissue thriving outside the uterus, primarily on the pelvic peritoneum and ovaries.(2) Signs and symptoms include chronic pelvic pain, dysmenorrhea, dyspareunia, and reduced fertility.(3)
Endometriosis has been linked to systemic chronic inflammation, heightened oxidative stress and an atherogenic lipid profile. Various inflammatory factors, e.g. interleukin-1, interleukin-6, tumor necrosis factor-α, have been observed to be elevated both in peritoneal fluid (4-6) and the peripheral blood (4, 5, 7) of women with endometriosis compared to controls. Studies also have suggested an increase of markers of oxidative stress but a decrease of antioxidants in the peritoneal fluid (8, 9) and peripheral blood (10, 11) among women with endometriosis. Moreover, women with endometriosis have been suggested to have higher serum levels of low-density lipoprotien (10-12) but lower high-density lipoprotein. (10, 11)
Inflammation, oxidative stress and an atherogenic lipid profile play key roles in the pathogenesis of atherosclerotic CHD.(13-15) Chronic systemic inflammation contributes to vascular insult and atheromatous plaque progression.(16) Increasing evidence supports that reactive oxygen species contributes to the process of atherogenesis, as important mediators of signaling pathways that lead to vascular inflammation.(15) Elevated concentration of low-density lipoprotein enhances its retention under the arterial wall(17); retention and oxidation of low-density lipoprotein are fundamental events in atherogenesis.(18) In contrast, high-density lipoprotein is antiatherogenic, removing cholesterol from cells in the arterial intima.(19) Therefore, the presence of endometriosis may promote coronary artery atherosclerosis formation and progression, increasing the risk of CHD.
In addition, the treatments of endometriosis, such as hysterectomy or oophorectomy (20-24) and analgesics (25) may confer increased risk of CHD to women with endometriosis. Hysterectomy or oophorectomy is a surgical treatment for endometriosis, thus women with endometriosis are much more likely than the general population to undergo hysterectomy or oophorectomy and also receive the surgery at a much younger age. The surgically-induced menopause prior to a natural menopause may increase risk of CHD among women, and this elevated risk may be most evident at a younger age (i.e., before all women reach natural menopause). (20-24)
In sum, women with endometriosis may have higher risk of CHD compared to women without endometriosis,(26) and this association may differ by age group. To test these hypotheses, we examined the prospective associations between endometriosis and myocardial infarction, angiographically-confirmed angina, and coronary artery bypass graft surgery/coronary angioplasty procedure/stent within the Nurses’ Health Study II, a large prospective cohort study.
Methods
Study Population
Nurses’ Health Study II is a prospective cohort study with 116,430 registered female nurses who were 25 to 42 and resided in 14 of the United States at enrollment in 1989. At baseline, participants completed a detailed questionnaire regarding demographic, medical, lifestyle, reproductive and other information and have continued to do so biennially. This research was approved by the Institutional Review Boards of Brigham and Women's Hospital and Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Assessment of endometriosis
In 1993, women were first asked if they had “ever had physician-diagnosed endometriosis.” If “yes,” they were asked during which two year follow-up period the diagnosis had occurred and if it had been confirmed by laparoscopy–the gold standard for diagnosing endometriosis.(27, 28) They were asked again in each subsequent biennial questionnaire.
As previously detailed, (1) the surgical report validation for self-reported laparoscopically-confirmed endometriosis cases within this cohort is 96% (n=101/105). Among women without laparoscopic confirmation, evidence of clinical diagnosis or symptoms was found in only 54% (n=14/26) of the records. Thus, self-reported physician-diagnosed endometriosis without laparoscopic confirmation may be substantially misclassified, and therefore, endometriosis exposure was restricted to women who reported laparoscopic confirmation. Those who reported endometriosis diagnosis but never laparoscopic confirmation were censored at the report of clinical diagnosis. In addition, this validation study determined that the average diagnostic delay (from symptom onset to surgical diagnosis) was 4 years in this cohort, while a study in 10 countries observed an average delay of 6.7 years.(29) A shorter diagnostic delay within Nurses’ Health Study II compared to the general population may be attributed to the medical knowledge and greater access to care of this cohort of registered nurses.
Assessment of CHD
We assessed incident myocardial infarction cases (fatal and non-fatal), angiographically-confirmed angina and coronary artery bypass graft surgery/angioplasty/stent cases that occurred between enrollment and the 2007 questionnaire cycle (which ended in May 2009). Clinicians blinded to the questionnaire information reviewed medical records from self-reported non-fatal myocardial infarction events. Non-fatal myocardial infarctions were classed as “confirmed” if they met the criteria of the World Health Organization: symptoms and either diagnostic electrocardiographic changes or raised cardiac enzymes,(30) as “probable” if hospital records were not obtained but they were corroborated in writing or a telephone interview. Fatal myocardial infarction was confirmed by hospital records, the National Death Index, or autopsy. Physician-diagnosed angina confirmed by angiography, coronary artery bypass graft surgery/angioplasty/stent and time of diagnosis were self-reported. When myocardial infarction, angiography-confirmed angina and coronary artery bypass graft surgery/angioplasty/stent cases were combined as the outcome (the combined CHD), we used the time of the first CHD event among those three to define the first age of occurrence.
Statistical Analysis
Those who experienced myocardial infarction, stroke, angiographically-confirmed angina or coronary artery bypass graft surgery/angioplasty/stent prior to enrollment into Nurses’ Health Study II in 1989 were excluded from this study. Person-months at risk were calculated from age at enrollment to age at death, CHD incidence, and end of follow-up for the present aims, whichever occurred first.
Association between endometriosis and CHD
As the main analysis, we quantified the association between endometriosis and CHD using multivariable Cox proportional hazards models. To evaluate potential confounding, we adjusted for age at the beginning of each questionnaire cycle and calendar time and then additionally adjusted for demographic, anthropometric, family history, reproductive, and lifestyle potential confounders including known risk factors for CHD to calculate crude and adjusted relative risks and 95% confidence intervals. All time-varying covariates were updated prospectively. Given that most questions were asked in every questionnaire across the nearly 20 years of follow-up, we observed little missing data. For example, there were less than 1% missing data for age at menarche (asked at baseline) and smoking history (asked in each questionnaire) among 2,019,360 person-years. The highest percentage of missing values observed was for birth weight (5%), body mass index (5%) and postmenopausal hormone use (6%). Missing covariate values were handled by the missing indicator method.(31) Multiple imputation to handle the missing data also was performed as a sensitivity analysis. Standard multivariable Cox models may be biased when there exist time-dependent confounders that are also affected by previous exposure.(32) For example, diet and physical activity change over time and can be influenced by previous endometriosis diagnosis. Therefore, we also applied marginal structural models with inverse probability weighting to adjust for potential time-dependent confounding.
Age as a modifier on the association between endometriosis and CHD
Secondly, we examined if and how age modifies the association between endometriosis and CHD. To do so, we stratified the association between endometriosis and combined CHD by age groups (<40, 40-50, 50-55, ≥55), and tested the statistical significance of the interaction between age and endometriosis with likelihood ratio tests.(33) We plotted the absolute incidence rate of combined CHD against age for women with and without laparoscopically-confirmed endometriosis, calculated by multivariable adjusted pooled logistic regression. This incidence rate was calculated at the mean or mode of all covariates.
Relationship between endometriosis, hysterectomy/oophorectomy and combined CHD
Thirdly, we investigated the relationship between endometriosis, hysterectomy/oophorectomy and combined CHD. As the first step, we assessed the association between hysterectomy/oophorectomy and combined CHD. In addition to the covariates adjusted in the main analysis, to further account for risk factors of hysterectomy, we added adjustment for household income, husband's education and geographic region of residence. We also further adjusted for potential indication for hysterectomy including infertility history and analgesics use (in addition to oral contraceptive) as indicators of endometriosis clinical severity.
As the second step, we calculated if proportions of the association between endometriosis and combined CHD could be statistically accounted for by the surgical treatments hysterectomy/oophorectomy. To do so, we added adjustment for hysterectomy/oophorectomy to the base multivariate model (i.e. now a surgery-adjusted model). Then, we calculated the proportion of the association between endometriosis and combined CHD that was statistically accounted for by hysterectomy/oophorectomy by dividing the log relative risk for combined CHD in relation to endometriosis estimated from the surgery-adjusted model by the log relative risk for combined CHD in relation to endometriosis from the base model. Confidence intervals for these estimated proportions account are calculated as per Lin et al. (34). We repeated this method to calculate the proportions of association between endometriosis and combined CHD that were statistically accounted for by postmenopausal hormone use (estrogen, progesterone/progestin, or estrogen and progesterone/progestin combined) and duration of use, and analgesic use.
Results
Association between endometriosis and CHD
At baseline in 1989, 116,430 women were enrolled in this cohort. The response rate across the 20 years since enrollment has been consistently over 90% for each questionnaire cycle and did not vary by endometriosis status. During the 20 years follow up among 1,971,574 person-years, there were 1,438 incident combined CHD cases. At baseline (1989), women who had laparoscopically-confirmed endometriosis history (n = 5,296) were slightly older, had earlier age at menarche, were more likely to be nulliparous, had lower parity, were more likely to use oral contraceptives and more likely to have a family history of myocardial infarction <age 60 years old, compared to women who had not been diagnosed with endometriosis at baseline (Table 1). Women with endometriosis were also much more likely to have had a hysterectomy and/or oophorectomy and to have had these at an earlier age, be surgically postmenopausal, and use postmenopausal hormones and analgesics.
Table 1.
Age-standardized baseline characteristics of women in the Nurses' Health Study II in 1989 by laparoscopically-confirmed endometriosis history
| Laparoscopically-Confirmed
Endometriosis (yes/no |
||
|---|---|---|
| Yes (n=5,296) | No (n=109,161) | |
| Age at baseline (years)*, mean(SD) | 36.0(4.2) | 34.7(4.7) |
| White race, % | 94 | 92 |
| Body mass index at baseline (kg/m2) | ||
| - <18.5 (underweight), % | 4 | 3 |
| - 18.5-22.4, % | 45 | 44 |
| - 22.5-24.9, % | 23 | 22 |
| - 25.0-29.9 (overweight), % | 19 | 19 |
| - 30.0+ (obese), % | 9 | 12 |
| Age at menarche | ||
| - ≤ 11 years old, % | 29 | 24 |
| - 12-13 years old, % | 56 | 58 |
| - ≥ 14 years old, % | 15 | 18 |
| Parity | ||
| - Nulliparous, % | 42 | 30 |
| - 1 pregnancy >6 months, % | 23 | 19 |
| - 2 pregnancy >6 months, % | 26 | 33 |
| - 3+ pregnancy >6 months, % | 9 | 18 |
| Oral contraceptive use, ever, % | 89 | 83 |
| Cigarette smoking history, never, % | 64 | 65 |
| Alcohol intake (grams), mean(SD) | 2.9(5.8) | 3.1(6.1) |
| Alternate healthy eating index 2010†, mean(SD) | 48.0(11.0) | 48.6(11.0) |
| Physical activity, (metabolic equivalents (METS)/week)‡, mean(SD) | 24.6(35.0) | 24.9(36.9) |
| Multivitamin use, % | 47 | 44 |
| Parental myocardial infarction< age 60 years, % | 20 | 18 |
| Health care usage, % | 98 | 95 |
| Physician-diagnosed hypertension, % | 7 | 5 |
| Physician-diagnosed hypercholesterolemia, % | 14 | 11 |
| Physician-diagnosed type 2 diabetes, % | 1 | 1 |
| Menopausal status | ||
| - premenopausal, % | 86 | 98 |
| - postmenopausal, % | 14 | 2 |
| Ever use postmenopausal hormones, % | 32 | 10 |
| Hysterectomy, % | 21 | 4 |
| Oophorectomy | ||
| - Unilateral, % | 4 | 1 |
| - Bilateral, % | 13 | 1 |
| Analgesic use§ (≥2days/week), % | 51 | 41 |
Values are means (SD) or percentages and are standardized to the age distribution of the study population.
Values of categorical variables may not sum to 100% due to rounding
Value is not age adjusted
Alternate healthy eating index 2010 (35) is a score that measures adherence to a diet pattern based on foods and nutrients most predictive of disease risk in the literature (minimum score=0, maximum score=110)
Metabolic equivalents from recreational and leisure-time activities
Analgesic included acetaminophen, aspirin, ibuprofen, indometacin, naproxen, nabumetone, ketoprofen, celecoxib, rofecoxib and valdecoxib.
In age and calendar year adjusted analyses, the relative risks - comparing women with laparoscopically-confirmed endometriosis (n = 11,903 by end of follow-up) to women without - were 1.63 (95% confidence interval, 1.27 to 2.11) for myocardial infarction, 2.07 (1.73 to 2.47) for angiographically-confirmed angina, 1.49 (1.19 to 1.86) for coronary artery bypass graft surgery/angioplasty/stent and 1.73 (1.49 to 2.00) for combined CHD (Table 2). Results remained statistically significant after adjustment for potential confounders (myocardial infarction, 1.52, 1.17 to 1.98; angina, 1.91, 1.59 to 2.29; coronary artery bypass graft surgery/angioplasty/stent, 1.35, 1.08 to 1.69; combined CHD, 1.62, 1.39 to 1.89) (Table 2). Results were unchanged when using marginal structural models. Results were also unchanged when multiple imputation was applied instead of the missing indicator method to address missing data in any covariate. The significant association between endometriosis and combined CHD remained after adjustment for each treatment factor.
Table 2.
Relative risks and 95% confidence intervals of incident myocardial infarction, angiographically-confirmed angina, coronary artery bypass graft surgery/coronary angioplasty procedure/stent and combined coronary heart disease events according to laparoscopically-confirmed endometriosis history.
| Myocardial Infarction | Angina | Coronary Bypass/Angioplasty/Stent | Combined Coronary Heart Disease | |||||
|---|---|---|---|---|---|---|---|---|
| Endometriosis confirmed by
laparoscopy (No/Yes) |
||||||||
| No | Yes | No | Yes | No | Yes | No | Yes | |
| Number of CHD cases | 429 | 69 | 742 | 149 | 599 | 91 | 1231 | 207 |
| Person-years | 1,822,783 | 154,696 | 1,820,499 | 153,892 | 1,821,888 | 154,555 | 1,818,018 | 153,556 |
| Age and calendar year adjusted | 1.00 | 1.63 (1.27 to 2.11) | 1.00 | 2.07 (1.73 to 2.47) | 1.00 | 1.49 (1.19 to 1.86) | 1.00 | 1.73 (1.49 to 2.00) |
| Multivariable-adjusted* | 1.00 | 1.52 (1.17 to 1.98) | 1.00 | 1.91 (1.59 to 2.29) | 1.00 | 1.35 (1.08 to 1.69) | 1.00 | 1.62 (1.39 to 1.89) |
Relative risks and 95% confidence intervals stratified by age in months and questionnaire cycle and adjusted for race (white, black, asian and other), age at menarche (≤11; 12-13; ≥14 years old), birth weight (not full term; <5.5; 5.5-6.9; 7-8.3; ≥8.4 lb), maternal or paternal history of myocardial infarction <60 years old (yes/no), body mass index at age 18 (<18.5; 18.5-22.4; 22.5-24.9; 25-29.9; ≥30 kg/m2), and time-varying risk factors: current body mass index (<18.5; 18.5-22.4; 22.5-24.9; 25-29.9; 30-34.9; 35-39.9; ≥40 kg/m2), parity (nulliparous; 1; 2; 3; ≥4 pregnancies that were longer than 6 months), total months of breast feeding (no breast feeding or < 1 month; <6; 6-11; 12-23; 24 months), oral contraceptive use (never; past; current), smoking history (never; past; current), alcohol consumption (none; <5; 5-10; >10 gram/day), alternate healthy eating index quintile 2010 (A score that measures adherence to a diet pattern based on foods and nutrients most predictive of disease risk in the literature), physical activity (<3; 3-8; 9-17; 18-26; 27-41; ≥42 metabolic equivalent, metabolic equivalents - hour/week), multivitamin use (yes/no), and health care utilization rate (a cumulative score was calculated based on the answers to several questions that asked if the nurse has had a physical exam, pap smear, pelvic exam, or a breast exam by a clinician).
Age as a modifier on the association between endometriosis and CHD
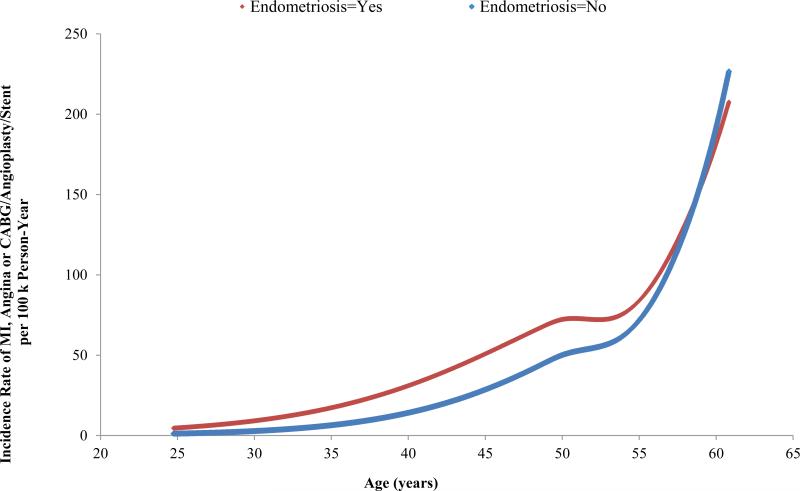
We observed a statistically significant interaction between endometriosis and age for the combined CHD endpoint. The relative risk was highest among women age≤40 (3.08, 2.02 to 4.70) and decreased as age increased (40<age ≤50, 1.65, 1.35 to 2.02; 50<age ≤55, 1.44, 1.07 to 1.94; age >55, 0.98, 0.56 to 1.72; p-value, test for heterogeneity=0.001). Figure 1 demonstrates that women with endometriosis had a higher absolute incidence rate of combined CHD compared to women without endometriosis across ages before late the 50s; the two absolute incidence rates crossed at ages in the late 50s - after which the two groups had similar incidence rates (Figure 1). The incidence rate difference between women with endometriosis and women without endometriosis increased slowly as age increased until around age 50.
Figure 1.
Incidence Rate of Myocardial Infaction, Angina and Coronary Bypass/Angioplasty/Stent Across Age in Years (covariates set to mean or mode value).
Relationship between endometriosis, hysterectomy/oophorectomy and combined CHD
We observed that women who had a hysterectomy/oophorectomy had higher age-standardized incidence rates of CHD. Having had a hysterectomy/oophorectomy was associated with higher age-standardized absolute incidence rates of CHD compared to those who had not: 139 vs. 60 per 100,000 person years; having had a hysterectomy/oophorectomy was associated with higher risk of combined CHD compared to not having had a hysterectomy/oophorectomy (1.51, 1.34 to 1.71). Adjustment for potential risk factors and indication for hysterectomy/oophorectomy did not attenuate this association.
We observed that 42% of the association between endometriosis and CHD was statistically significantly accounted for by greater frequency of hysterectomy/oophorectomy and earlier age at these surgeries among women with endometriosis (Table 3). While 31% of the association could be statistically accounted for by greater frequency and longer duration of postmenopausal hormone use, this was primarily driven by greater frequency and earlier age at hysterectomy/oophorectomy.
Table 3.
Proportions and 95% confidence intervals (CI)* of the association between endometriosis and combined coronary heart disease event (myocardial infarction, angiographically-confirmed angina and/or coronary artery bypass graft surgery/coronary angioplasty procedure/stent) mediated by treatment factors.
| Treatment factors | Proportion (95% CI)* |
|---|---|
| Hysterectomy/oophorectomy and age at surgery | 42 (25-60) |
| Postmenopausal hormone use (yes/no) + postmenopausal hormone use duration (continuous years) | 31 (l7-44) |
| Analgesic use‡ (≥2 times/week vs. <2 times/week) | 5 (3-8) |
| All treatment factors combined | 55 (33-77) |
Proportions and CIs were calculated following Lin et al.(34), comparing the full model that includes the exposure, treatment factor(s) and any covariates with a partial model without the treatment factor (i.e. 1-log(relative risk of model 1)/log(relative risk of model 2)). The multivariable-adjusted model 2 (Table 2) represents the average effect of endometriosis given the distribution of age and treatment in the Nurses' Health Study. The mediation proportion estimated from the model presented here can be interpreted as the mediation proportion to be observed in this study population or any other study population with a similar proportion of treatment and a similar age distribution.
† Categorical variable, defined as: no hysterectomy/oophorectomy, hysterectomy/oophorectomy at age≤45, age 45-50, age 50-55 and age>55.
Analgesic included acetaminophen, aspirin, ibuprofen, indometacin, naproxen, nabumetone, ketoprofen, celecoxib, rofecoxib and valdecoxib.
Discussion
Compared to women without endometriosis, laparoscopically-confirmed endometriosis was associated with a significantly increased risk of myocardial infarction, angiographically-confirmed angina, and coronary artery bypass graft surgery/angioplasty/stent. Adjustment for potential confounders did not attenuate this association. Hysterectomy/oophorectomy was associated with a higher risk of CHD and explained a portion of the association between endometriosis and CHD.
To our knowledge, this is the first prospective cohort study investigating the association between endometriosis and CHD. One case-control study found null results when investigating whether women with endometriosis (n=66) had greater subclinical atherosclerosis than controls (n=66), measured by intima-media thickness on the common carotid artery.(36) As noted by the author, the mean age of the study population was only 41 years. Given the small sample size, the study had insufficient statistical power to detect increased atherosclerosis at such early age. Also, the control group was defined as women with uterine myomas, ovarian cysts or pelvic pain, who may have greater risk of subclinical atherosclerosis than the general population. Another case control study also investigated the association between carotid intima-media thickness and endometriosis and found null results.(37) However, this study similarly had a very young age group (mean age 33 in cases and 35 in controls) and very small sample size (37 cases and 31 controls).
Systemic chronic inflammation, heightened oxidative stress and atherogenic lipid profile associated with endometriosis and the synergistic effect of the three may underpin the biologic mechanism for the association between endometriosis and CHD. Additionally, endometriosis and CHD may share common genetic susceptibilities. Multiple independent loci in the CDKN2BAS1 (also known as ANRIL) region on chromosome 9p21 have been robustly associated with endometriosis in genome-wide association studies in Japanese and European ancestry populations,(38, 39) and has also been robustly associated with CHD outcomes.(40-42) Chr9p21 encodes the long non-coding RNA(ncRNA) ANRIL, which has been demonstrated to regulate cell proliferation, adhesion and apoptosis,(43, 44) - central mechanisms of atherogenesis (13, 43) and endometriosis. (2, 3)
Apart from these biologic mechanisms, it is possible that the crude association between endometriosis and CHD can be explained by CHD risk factors that predate and cause endometriosis (for example, diet). However, the small attenuation of relative risk from the crude models to the comprehensive multivariable models suggests that strong confounding is unlikely. Moreover, because the results of multivariable Cox models and marginal structural models were almost identical, the potential bias that may remain within multivariable Cox models for adjustment of time-varying confounding (32) was negligible.
We observed that the increased risk of CHD in women with endometriosis was greatest among younger women, and there was no increased risk from endometriosis after women reached the late 50s. This association may have important clinical and public health implications among women in their early 40s to early 50s. It is possible that the observed increased risk associated with surgical menopause from hysterectomy/oophorectomy among women with endometriosis started to diminish with age partially because nearly every woman had reached menopause by age 55.
We observed higher risk of CHD among women who had a hysterectomy/oophorectomy compared to women who did not have a hysterectomy/oophorectomy. These associations were not altered when potential confounding factors, e.g., age, parity, BMI, race/ethnicity (45-47), were adjusted for in the multivariable analyses. Another important determinant of the decisions regarding hysterectomy/oophorectomy is education (45, 47), which is very homogenous in this population of nurses, and so we additionally adjusted for husband's education level and household income, again with no evidence of confounding. Confounding by indication of this hysterectomy association could be due to severity of endometriosis disease. Therefore, we further adjusted for infertility history and analgesics use (in addition to oral contraceptive use) as proxies of endometriosis disease severity as the main presentations of endometriosis are infertility and pain (1, 3). Adjustment did not change the association between hysterectomy/oophorectomy and CHD. These results were supported by the literature: bilateral oophorectomy at ages younger than 50 has been associated with an increased incidence and mortality of cardiovascular diseases, although results have been inconclusive for bilateral oophorectomy at ages older than 50.(20-22) A nationwide study from Sweden showed that hysterectomy without oophorectomy was associated with an increased risk of cardiovascular diseases in women age<50 but not in women age ≥50.(23, 24) Loss of ovarian function and subsequent deficiency of endogenous estrogens may be the biologic mechanism for association between bilateral oophorectomy and cardiovascular diseases, while simple hysterectomy can interfere with ovarian blood flow and may result in premature ovarian failure.(23)
We observed that approximately 40% of the observed significant association between endometriosis and CHD may be statistically accounted for by hysterectomy/oophorectomy and age at surgery. This may be because 1) women with endometriosis were subsequently much more likely to have a hysterectomy/oophorectomy and had the surgery at a younger age compared to women without endometriosis; and 2) hysterectomy/oophorectomy is associated with higher risk of CHD. The mediation proportion estimated from this model can be interpreted as the mediation proportion to be observed in this study population or any other study population with a similar proportion of treatment and a similar age distribution. The remaining ~50% of the significant association between endometriosis and CHD was not explained by endometriosis treatments.
These data raise concerns regarding treating endometriosis with hysterectomy/oophorectomy. Physicians need to consider the potential long term impact that the surgeries may cause and weigh the risks and benefits of the treatment in dialogue with patients, particularly with respect to endometriosis where pain recurrence risk remains.(48) Although oophorectomy confers obvious prevention for ovarian cancer, with which endometriosis has been associated (49), CHD is the leading cause of mortality and morbidity in women in the US and UK. CHD incidence and mortality are orders of magnitude greater than ovarian cancer, with incidence 30 times greater and mortality 26 times greater in the US in 2010.(50) However, we acknowledge that endometriosis management decisions are not random, and there are many individual issues that contribute to treatment decisions between a physician and patient.
Strengths and limitations of the study
Regarding limitations, our unexposed group (i.e. never clinically or surgically diagnosed with endometriosis) may include asymptomatic endometriosis or symptomatic without confirmatory diagnosis. If so, our results would be biased toward the null. However, in sensitivity analyses expanded definition of endometriosis cases by attributing person time from endometriosis without laparoscopic confirmation to the exposed group did not alter the results. More importantly, as Zondervan (51) et al. quantified, the likely prevalence of undiagnosed endometriosis should not exceed 2% of the unexposed population, and therefore too low to impact on the results, particularly in our large study population among whom undiagnosed endometriosis become diluted among tens of thousands of those with no endometriosis. We did not have information on other hormonal treatments for endometriosis, such as danazol (a synthetic androgen) and Leuprolide (lupron, gonadotropin-releasing hormone analog) to assess to what extent the association between endometriosis and CHD could have been explained by those treatments. We also did not have information on the extent of excision of endometriosis lesions during laparoscopy to evaluate whether the excision could alter the elevated CHD risk associated with endometriosis, although no current cohort of sufficient size has these data. Regarding generalizability, these medical professionals have greater knowledge of and access to medical care, minimizing misclassification of the primary exposure (endometriosis) or the outcomes and thus maximizing precision of hazard ratio estimation, it is unlikely that the underlying biologic relation observed between endometriosis and CHD, if true, would differ between these women and women in the general population. Finally, the timing of exposure was defined by time of surgical diagnosis, not disease onset. However, this is true for all studies of endometriosis regardless of design; furthermore, sensitivity analyses predating diagnosis time of endometriosis for 2, 4, 6 or 8 years didn't change our results.
Our study has many strengths. The longitudinal study design, large sample size and 20 years of follow-up allowed us to document the prospective association between surgically diagnosed endometriosis and subsequent risk of CHD. The wealth of time-varying data allowed for detailed adjustment for known and suspected potential confounders and risk factors for CHD. Minimal confounding was seen. We were able to assess proportions of the association statistically accounted for by various treatment factors. Lastly, the results were robust across multiple definitions of CHD.
Conclusions
In this large prospective cohort, laparoscopically-confirmed endometriosis was associated with higher risk of CHD. The association was stronger among young women. Hysterectomy/oophorectomy was associated with higher risk of CHD and could explain a portion of the association between endometriosis and CHD. These data have implications for clinical management of endometriosis patients, suggesting women with endometriosis may represent a high risk group for CHD -- particularly at a young age, indicating the need for risk awareness and subsequent screening for CHD and healthy lifestyle promotion among primary care and public health specialists.
What is Known
Endometriosis has been associated with systemic chronic inflammation, heightened oxidative stress and atherogenic lipid profile that may increase women's risk for coronary heart disease.
However, to our knowledge, no previous studies have explored the association between endometriosis and risk of coronary heart disease.
What the Study Adds
This is the first study investigating the association between endometriosis and risk of coronary heart disease, and our data show that endometriosis is associated with higher risk of coronary heart disease independent of known and hypothesized confounders and coronary heart disease risk factors, especially among younger women.
Moreover, we observed that a portion of this association could be explained by treatment factors associated with endometriosis diagnosis, particularly hysterectomy/oophorectomy.
Acknowledgments
We gratefully acknowledge the Nurses’ Health Study II participants for their continuing contributions.
Funding Sources: This study was supported by research grants HD52473 and HD57210 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The Nurses’ Health Study II is supported by the Public Health Service grant CA50385 from the National Cancer Institute, NIH, U.S. Department of Health and Human Services. The study sponsors were not involved in the study design nor collection, analysis, or interpretation of data, or the writing of the manuscript or the decision to submit it for publication. The authors were wholly independent from study sponsors.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160:784–796. doi: 10.1093/aje/kwh275. [DOI] [PubMed] [Google Scholar]
- 2.Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 3.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 4.Agic A, Xu H, Finas D, Banz C, Diedrich K, Hornung D. Is endometriosis associated with systemic subclinical inflammation? Gynecol Obstet Invest. 2006;62:139–47. doi: 10.1159/000093121. [DOI] [PubMed] [Google Scholar]
- 5.Bedaiwy MA, Falcone T, Sharma RK, Goldberg JM, Attaran M, Nelson DR, Agarwal A. Prediction of endometriosis with serum and peritoneal fluid markers: a prospective controlled trial. Hum Reprod. 2002;17:426–431. doi: 10.1093/humrep/17.2.426. [DOI] [PubMed] [Google Scholar]
- 6.Akoum A, Al-Akoum M, Lemay A, Maheux R, Leboeuf M. Imbalance in the peritoneal levels of interleukin 1 and its decoy inhibitory receptor type II in endometriosis women with infertility and pelvic pain. Fertil Steril. 2008;89:1618–1624. doi: 10.1016/j.fertnstert.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 7.Koumantakis E, Matalliotakis I, Neonaki M, Froudarakis G, Georgoulias V. Soluble serum interleukin-2 receptor, interleukin-6 and interleukin-1a in patients with endometriosis and in controls. Arch Gynecol Obstet. 1994;255:107–112. doi: 10.1007/BF02390936. [DOI] [PubMed] [Google Scholar]
- 8.Gupta S, Agarwal A, Krajcir N, Alvarez JG. Role of oxidative stress in endometriosis. Reprod Biomed Online. 2006;13:126–34. doi: 10.1016/s1472-6483(10)62026-3. [DOI] [PubMed] [Google Scholar]
- 9.Van Langendonckt A, Casanas-Roux F, Donnez J. Oxidative stress and peritoneal endometriosis. Fertil Steril. 2002;77:861–870. doi: 10.1016/s0015-0282(02)02959-x. [DOI] [PubMed] [Google Scholar]
- 10.Verit FF, Erel O, Celik N. Serum paraoxonase-1 activity in women with endometriosis and its relationship with the stage of the disease. Hum Reprod. 2008;23:100–104. doi: 10.1093/humrep/dem340. [DOI] [PubMed] [Google Scholar]
- 11.Turgut A, Ozler A, Goruk NY, Tunc SY, Evliyaoglu O, Gul T. Copper, ceruloplasmin and oxidative stress in patients with advanced-stage endometriosis. Eur Rev Med Pharmacol Sci. 2013;17:1472–1478. [PubMed] [Google Scholar]
- 12.Melo AS, Rosa-e-Silva JC, Rosa-e-Silva AC, Poli-Neto OB, Ferriani RA, Vieira CS. Unfavorable lipid profile in women with endometriosis. Fertil Steril. 2010;93:2433–2436. doi: 10.1016/j.fertnstert.2009.08.043. [DOI] [PubMed] [Google Scholar]
- 13.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 14.Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, Gallimore JR, Pepys MB. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonomini F, Tengattini S, Fabiano A, Bianchi R, Rezzani R. Atherosclerosis and oxidative stress. Histol Histopathol. 2008;23:381–390. doi: 10.14670/HH-23.381. [DOI] [PubMed] [Google Scholar]
- 16.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen LB. Transfer of low density lipoprotein into the arterial wall and risk of atherosclerosis. Atherosclerosis. 1996;123:1–15. doi: 10.1016/0021-9150(96)05802-9. [DOI] [PubMed] [Google Scholar]
- 18.Skalen K, Gustafsson M, Rydberg EK, Hulten LM, Wiklund O, Innerarity TL, Boren J. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature. 2002;417:750–754. doi: 10.1038/nature00804. [DOI] [PubMed] [Google Scholar]
- 19.Barter PJ, Rye KA. High density lipoproteins and coronary heart disease. Atherosclerosis. 1996;121:1–12. doi: 10.1016/0021-9150(95)05675-0. [DOI] [PubMed] [Google Scholar]
- 20.Rivera CM, Grossardt BR, Rhodes DJ, Brown RD, Jr., Roger VL, Melton LJ, 3rd, Rocca WA. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16:15–23. doi: 10.1097/gme.0b013e31818888f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atsma F, Bartelink ML, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause. 2006;13:265–279. doi: 10.1097/01.gme.0000218683.97338.ea. [DOI] [PubMed] [Google Scholar]
- 22.Parker WH, Feskanich D, Broder MS, Chang E, Shoupe D, Farquhar CM, Berek JS, Manson JE. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses’ health study. Obstet Gynecol. 2013;121:709–716. doi: 10.1097/AOG.0b013e3182864350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingelsson E, Lundholm C, Johansson AL, Altman D. Hysterectomy and risk of cardiovascular disease: a population-based cohort study. Eur Heart J. 2011;32:745–750. doi: 10.1093/eurheartj/ehq477. [DOI] [PubMed] [Google Scholar]
- 24.Howard BV, Kuller L, Langer R, Manson JE, Allen C, Assaf A, Cochrane BB, Larson JC, Lasser N, Rainford M, Van Horn L, Stefanick ML, Trevisan M. Risk of cardiovascular disease by hysterectomy status, with and without oophorectomy: the Women's Health Initiative Observational Study. Circulation. 2005;111:1462–1470. doi: 10.1161/01.CIR.0000159344.21672.FD. [DOI] [PubMed] [Google Scholar]
- 25.Garcia Rodriguez LAG-PA, Bueno H, Hwa J. NSAID use selectively increases the risk of non-fatal myocardial infarction: a systematic review of randomised trials and observational studies. PLoS One. 2011:6. doi: 10.1371/journal.pone.0016780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Missmer SA. Commentary: Endometriosis--epidemiologic considerations for a potentially ‘high-risk’ population. Int J Epidemiol. 2009;38:1154–1155. doi: 10.1093/ije/dyp249. [DOI] [PubMed] [Google Scholar]
- 27.Duleba AJ. Diagnosis of endometriosis. Obstet Gynecol Clin North Am. 1997;24:331–346. doi: 10.1016/s0889-8545(05)70307-7. [DOI] [PubMed] [Google Scholar]
- 28.Pardanani SBR. The gold standard for the surgical diagnosis of endometriosis: visual findings or biopsy results? J Gynecol Techn. 1998;4:121–124. [Google Scholar]
- 29.Nnoaham KE, Hummelshoj L, Webster P, d'Hooghe T, de Cicco Nardone F, de Cicco Nardone C, Jenkinson C, Kennedy SH, Zondervan KT. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96:366–373 e8. doi: 10.1016/j.fertnstert.2011.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rose GABH. Cardiovascular survey methods. 2nd ed. World Health Organization; Geneva: 1982. (WHO monograph series No 58.) [PubMed] [Google Scholar]
- 31.Miettinen OS. Theoretical epidemiology: principles of occurrence research. John Wiley & Sons; New York (NY): 1985. [Google Scholar]
- 32.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Greenland S. Tests for interaction in epidemiologic studies: a review and a study of power. Stat Med. 1983;2:243–251. doi: 10.1002/sim.4780020219. [DOI] [PubMed] [Google Scholar]
- 34.Lin DY, Fleming TR, De Gruttola V. Estimating the proportion of treatment effect explained by a surrogate marker. Stat Med. 1997;16:1515–1627. doi: 10.1002/(sici)1097-0258(19970715)16:13<1515::aid-sim572>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 35.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–1018. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pretta S, Remorgida V, Abbamonte LH, Anserini P, Ragni N, Del Sette M, Gandolfo C, Ferrero S. Atherosclerosis in women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2007;132:226–231. doi: 10.1016/j.ejogrb.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 37.Santoro L, D'Onofrio F, Campo S, Ferraro PM, Tondi P, Campo V, Flex A, Gasbarrini A, Santoliquido A. Endothelial dysfunction but not increased carotid intima-media thickness in young European women with endometriosis. Hum Reprod. 2012;27:1320–1326. doi: 10.1093/humrep/des062. [DOI] [PubMed] [Google Scholar]
- 38.Rahmioglu N, Nyholt DR, Morris AP, Missmer SA, Montgomery GW, Zondervan KT. Genetic variants underlying risk of endometriosis: insights from meta-analysis of eight genome-wide association and replication datasets. Hum Reprod Update. 2014;20:702–716. doi: 10.1093/humupd/dmu015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uno S, Zembutsu H, Hirasawa A, Takahashi A, Kubo M, Akahane T, Aoki D, Kamatani N, Hirata K, Nakamura Y. A genome-wide association study identifies genetic variants in the CDKN2BAS locus associated with endometriosis in Japanese. Nat Genet. 2010;42:707–10. doi: 10.1038/ng.612. [DOI] [PubMed] [Google Scholar]
- 40.Samani NJ, Erdmann J, Hall AS, Hengstenberg C, Mangino M, Mayer B, Dixon RJ, Meitinger T, Braund P, Wichmann HE, Barrett JH, Konig IR, Stevens SE, Szymczak S, Tregouet DA, Iles MM, Pahlke F, Pollard H, Lieb W, Cambien F, Fischer M, Ouwehand W, Blankenberg S, Balmforth AJ, Baessler A, Ball SG, Strom TM, Braenne I, Gieger C, Deloukas P, Tobin MD, Ziegler A, Thompson JR, Schunkert H. Genomewide association analysis of coronary artery disease. N Engl J Med. 2007;357:443–53. doi: 10.1056/NEJMoa072366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Helgadottir A, Thorleifsson G, Manolescu A, Gretarsdottir S, Blondal T, Jonasdottir A, Sigurdsson A, Baker A, Palsson A, Masson G, Gudbjartsson DF, Magnusson KP, Andersen K, Levey AI, Backman VM, Matthiasdottir S, Jonsdottir T, Palsson S, Einarsdottir H, Gunnarsdottir S, Gylfason A, Vaccarino V, Hooper WC, Reilly MP, Granger CB, Austin H, Rader DJ, Shah SH, Quyyumi AA, Gulcher JR, Thorgeirsson G, Thorsteinsdottir U, Kong A, Stefansson K. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316:1491–1493. doi: 10.1126/science.1142842. [DOI] [PubMed] [Google Scholar]
- 42.McPherson R, Pertsemlidis A, Kavaslar N, Stewart A, Roberts R, Cox DR, Hinds DA, Pennacchio LA, Tybjaerg-Hansen A, Folsom AR, Boerwinkle E, Hobbs HH, Cohen JC. A common allele on chromosome 9 associated with coronary heart disease. Science. 2007;316:1488–1491. doi: 10.1126/science.1142447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holdt LM, Hoffmann S, Sass K, Langenberger D, Scholz M, Krohn K, Finstermeier K, Stahringer A, Wilfert W, Beutner F, Gielen S, Schuler G, Gabel G, Bergert H, Bechmann I, Stadler PF, Thiery J, Teupser D. Alu elements in ANRIL non-coding RNA at chromosome 9p21 modulate atherogenic cell functions through trans-regulation of gene networks. PLoS Genet. 2013;9:e1003588. doi: 10.1371/journal.pgen.1003588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Motterle A, Pu X, Wood H, Xiao Q, Gor S, Ng FL, Chan K, Cross F, Shohreh B, Poston RN, Tucker AT, Caulfield MJ, Ye S. Functional analyses of coronary artery disease associated variation on chromosome 9p21 in vascular smooth muscle cells. Hum Mol Genet. 2012;21:4021–4029. doi: 10.1093/hmg/dds224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Determinants of hysterectomy and oophorectomy in women attending menopause clinics in Italy. Maturitas. 2000;36:19–25. doi: 10.1016/s0378-5122(00)00135-3. [DOI] [PubMed] [Google Scholar]
- 46.Plusquin C, Fastrez M, Vandromme J, Rozenberg S. Factors affecting gynaecologists’ decision to perform prophylactic oophorectomy concomitantly with hysterectomy: a Belgian survey. Maturitas. 2011;70:391–394. doi: 10.1016/j.maturitas.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 47.Sievert LL, Murphy L, Morrison LA, Reza AM, Brown DE. Age at menopause and determinants of hysterectomy and menopause in a multi-ethnic community: the Hilo Women's Health Study. Maturitas. 2013;76:334–341. doi: 10.1016/j.maturitas.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shakiba K, Bena JF, McGill KM, Minger J, Falcone T. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstet Gynecol. 2008;111:1285–1292. doi: 10.1097/AOG.0b013e3181758ec6. [DOI] [PubMed] [Google Scholar]
- 49.Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, Nagle CM, Doherty JA, Cushing-Haugen KL, Wicklund KG, Chang-Claude J, Hein R, Lurie G, Wilkens LR, Carney ME, Goodman MT, Moysich K, Kjaer SK, Hogdall E, Jensen A, Goode EL, Fridley BL, Larson MC, Schildkraut JM, Palmieri RT, Cramer DW, Terry KL, Vitonis AF, Titus LJ, Ziogas A, Brewster W, Anton-Culver H, Gentry-Maharaj A, Ramus SJ, Anderson AR, Brueggmann D, Fasching PA, Gayther SA, Huntsman DG, Menon U, Ness RB, Pike MC, Risch H, Wu AH, Berchuck A. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol. 2012;13:385–94. doi: 10.1016/S1470-2045(11)70404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zondervan KT, Cardon LR, Kennedy SH. What makes a good case-control study? Design issues for complex traits such as endometriosis. Hum Reprod. 2002;17:1415–1423. doi: 10.1093/humrep/17.6.1415. [DOI] [PubMed] [Google Scholar]