Abstract
Growing by an alarming rate in the Western world, obesity has become a condition associated with a multitude of diseases such as diabetes, metabolic syndrome and various cancers. Generally viewed as an abnormal accumulation of hypertrophied adipocytes, obesity is also a poor prognostic factor for recurrence and chemoresistance in cancer patients. With more than two-thirds of the adult population in the United States considered clinically overweight or obese, it is critical that the relationship between obesity and cancer is further emphasized and elucidated. Adipocytes are highly metabolically active cells, which, through release of adipokines and cytokines and activation of endocrine and paracrine pathways, affect processes in neighboring and distant cells, altering their normal homeostasis. This work will examine specifically how adipocyte-derived factors regulate the cellular metabolism of malignant cells within the tumor niche. Briefly, tumor cells undergo metabolic pressure towards a more glycolytic and hypoxic state through a variety of metabolic regulators and signaling pathways, i.e., phosphoinositol-3 kinase (PI3K), hypoxia-inducible factor-1 alpha (HIF-1α), and c-MYC signaling. Enhanced glycolysis and high lactate production are hallmarks of tumor progression largely because of a process known as the Warburg effect. Herein, we review the latest literature pertaining to the body of work on the interactions between adipose and tumor cells, and underlining the changes in cancer cell metabolism that have been targeted by the currently available treatments.
Keywords: adipose tissue, cancer, glycolysis, metabolic pathologies, obesity, tumor metabolism
Introduction: fifty shades of fat
Adipose tissue is a versatile organ, crucial for maintaining homeostasis by storing and dispersing energy, producing and releasing adipokines and cytokines, with the ability to influence other cells of the body in autocrine, paracrine and endocrine fashion [1]. This highly metabolically active tissue is distributed throughout the body in discrete depots, and its development, expansion and energy balance are regulated by an integrated network of genetic, environmental, epigenetic and pharmacological factors [1, 2]. When unbalanced, or when caloric intake exceeds energy expenditure, adipose tissue becomes problematic and can detrimentally affect physiological processes.
Different types of adipose tissue: brown vs. white fat
Long-thought to have homogenous characteristics throughout the entire body, adipose tissue actually exhibits depot-specific differences in metabolic profiles, and these variations appear to correlate with susceptibility to obesity and specific metabolic disorders [1]. In addition to its localization-based classification, adipose tissue is also commonly categorized based on its coloration, and is divided into brown, white and beige tissues with distinct functional, metabolic and endocrine differences [1].
The main role of brown adipose tissue (BAT) is to provide non-shivering thermogenesis by the means of energy expenditure. Mitochondria and cytochrome content are abundant in BAT, a characteristic that attributes, in part, to the color and name of BAT [3]. Brown adipocytes are multilocular, meaning they contain multiple fat droplets. Uniquely, they express an uncoupling protein-1 (UCP1), the function of which is to uncouple respiratory chain proteins in the abundant cellular mitochondria. The uncoupling of respiratory chain proteins results in the metabolic substrates being oxidized purely for the purpose of heat energy dissipation [3]. The progenitors of BAT can be traced through the expression of myogenic factor 5 (Myf5), which is also expressed in skeletal myocytes [4]. In humans, BAT develops during the fetal stage and is the most abundant throughout the body at infancy and throughout the first decade of life. It eventually declines in its abundance and retires predominately to areas surrounding vital organs such as suprarenal and para-aortic [3, 5] and the supraclavicular area [6]. Cold temperature [6–8] and β-adrenergic stimulation [9, 10] can trigger the expression of UCP1, induction of substrate oxidation and activation of BAT. Increased expression of UCP1 in rodents caused by over-feeding sprouted a theory of the relevance of BAT in evading obesity. The fact that activity of BAT declines in overweight individuals [7, 11] supports the evidence of inverse correlation between propensity to obesity and abundance of BAT [12, 13].
In contrast to BAT, the white adipose tissue (WAT) development begins in utero and continues to evolve throughout life [14, 15]. WAT serves as primary energy storage and based on its location in the body, it is often referred to as subcutaneous or visceral (intra-abdominal) fat that includes mesenteric, epididymal and perirenal depots. In humans, subcutaneous fat develops prior to visceral [16] and can be distinctively different in its pathophysiological processes [17]. Importantly, the morphology of white adipocytes differs from brown as they are unilocular cells containing less mitochondria and do not express UCP1 [18].
“Browning” is a phenomenon described when adipocytes located in the typical WAT sites switch from anabolic to catabolic mechanism, producing “beige” adipocytes [19]. They do so by developing multilocular morphology with increased numbers of mitochondria and expression of UCP1. This occurrence has been well-described in rodents and is triggered by cold exposure and β3-adrenergic stimulation [20–22]. Although closely resembling the brown fat morphology, BAT residing in the WAT depots does not express the same lineage marker, Myf5, as a “classical BAT” [23]. There has been a growing interest in understanding the capacity of brown and beige adipocytes to counteract obesity, diabetes and other metabolic diseases [19]. Strategies are being developed to selectively enhance respiratory uncoupling in adipose tissue to induce weight loss and reverse obesity-driven pathological processes.
Bone marrow fat and its roles in physiological processes and disease
Bone marrow fat, known as yellow adipose tissue (YAT), represents a depot dispersed throughout the bone marrow with primary localization to trabecular cavities [24, 25], and often viewed as having mixed characteristics of both WAT and BAT [25–27]. No longer considered just a “space-filler”, YAT is recognized as a highly active organ, functions of which extend far beyond the storage of triglycerides and lipid metabolism, and they include systemic energy regulation and management of insulin sensitivity [28, 29]. Importantly, the systemic changes related to adipose tissue homeostasis critically affect glucose and energy balance [30] and reciprocally influence bone health. If the stability in signaling pathways that integrate bone remodeling and energy metabolism gets perturbed by metabolic events related to age and obesity, the physiological processes in the bone, like osteogenesis and hematopoiesis, are critically affected [25, 28]. The latter results in susceptibility to pro-inflammatory events and dysregulated bone remodeling [24].
It is well-established, that during the normal aging process, healthy, hematopoietically active red marrow of the bone is progressively replaced by the fatty yellow marrow [24–26]. There is also increasing evidence that obesity and associated metabolic pathologies can have detrimental effects on bone health that go beyond age-driven changes in skeletal homeostasis [24, 31, 32]. Until recently, obesity was thought to have a protective effect on bone metabolism due to the positive impact of body weight on bone formation [31, 33]. Current evidence suggests that percent body fat, waist circumference, and waist-to-hip ratio correlate with the risk of osteoporotic fractures, especially in men, who have larger amounts of marrow fat than age-matched women [34–36]. These epidemiological data are mirrored by the results of animal studies, where marrow adiposity has been shown to result in decreases in trabecular bone volume and overall reduced bone mineral density (BMD) [31, 37, 38]. Despite these findings, the relationship between adiposity and bone turnover remains controversial and additional, controlled studies are needed to truly understand the effects of obesity on bone health in humans.
There is growing clinical and epidemiological evidence that metabolic syndrome (MetS), a cluster of metabolic abnormalities that include abdominal obesity, hypertriglyceridemia, low high-density lipoprotein (HDL) cholesterol, high blood pressure, and glucose intolerance [39–41] is a strong contributor to marrow adiposity. This condition is highly prevalent in the United States as demonstrated by its presence in approximately 25–30% of adults over the age of 18 years [42, 43], and is a strong risk factor for cardiovascular disease, diabetes and stroke [44, 45]. A study of metabolic syndrome in normal-weight individuals with only regional accumulation of fat (visceral/abdominal and inter-muscular) was associated with fasting hyperinsulinemia, a risk factor for type 2 diabetes mellitus (T2DM) [46, 47]. Importantly, general obesity was shown to correlate with accumulation of marrow fat in both control and diabetic individuals [48]; however, only in diabetic patients was marrow adiposity correlated with visceral adipose tissue (VAT) [48]. This finding pinpoints the potential importance of visceral fat depot in bone health, and its implications for development of diabetes. This also underlines the importance of distinguishing VAT from other adipose tissues in studies investigating the impact of obesity and metabolic disorders on skeletal health, because using the central obesity measures continues to lead to inconsistent results [49].
One specific metabolic consequence of excess adiposity is diabetes, a condition highly linked with marrow adiposity [48, 50] and profound effects on bone health [51, 52]. Numerous reports suggest that the following are potential biological links between obesity and diabetes: changes in insulin levels, altered calcium metabolism, reduced renal function, vitamin D de-regulation, higher concentrations of inflammatory molecules and collagen glycation products, polypeptides, such as osteocalcin and osteopontin, and certain adipokines [27–29, 53, 54]. Increases in circulating levels of bone resorption markers such as tartrate resistant acid phosphatase (TRAP 5b) and cathepsin K (CTSK) have been reported in diabetic patients [55] and animal experimental models of diabetes [56, 57]. Serum levels of osteocalcin, an osteoblast-specific polypeptide, were reported to be inversely correlated with adiposity and measures of insulin resistance [58, 59]. In contrast, a positive association with insulin sensitivity and HDL cholesterol was demonstrated for osteoprotegerin, a known inhibitor of bone resorption, further evidence of clear association between the metabolic features and bone degradation [60]. It is noteworthy, similar to other fat depots, that adipogenesis in the bone marrow is under the regulation of PPARγ [28]. This has led to serious concerns in terms of treatment with anti-diabetic thiazolidinedione drugs, which were shown to induce bone marrow adiposity [61], likely even further exacerbating the environment already altered by diabetes itself. It is also important to keep in mind that marrow adiposity associated with diabetes appears to be characterized by low unsaturation and high saturation levels of fats [48, 62]. This suggests that perhaps apart from an overall increase in adiposity, the composition of marrow fat might be a more important factor in bone health, and potentially other physiological processes, a phenomenon that warrants further investigations.
Adipocyte artillery
Contrary to the previous view of adipocytes being metabolically inert, growing evidence from the last decades of research has revealed that they are in fact metabolically active cells highly involved in the uptake, production, and secretion of many different factors with systemic implications [63]. Through the production of lipids and secretion of hormones, cytokines or adipokines, adipocytes have the ability to influence neighboring cells within their microenvironment and throughout the body as a whole, working as a functional paracrine and endocrine tissue [1, 2, 63].
Hormones
The two most commonly studied hormones secreted by white adipocytes are adiponectin and leptin. Adiponectin is a protein hormone responsible for regulating multiple metabolic processes [64]. This hormone is secreted primarily by adipocytes and is released into the bloodstream where it binds to adiponectin receptor 1 (AdipoR1), adiponectin receptor 2 (AdipoR2), and has the potential to bind a membrane receptor, T-cadherin [65, 66]. Adiponectin-receptor binding results in activation of AMP-activated protein kinase (AMPK) and subsequent signaling through peroxisome proliferator-activated receptor (PPAR)-α transcription factor [67–69]. Adiponectin levels have been associated with many different metabolic diseases. Interestingly, adiponectin is shown to be down-regulated in patients with obesity and/or diabetes and is upregulated upon treatment with insulin-sensitizers [70, 71]. It was recently discovered that bone marrow adipose tissue (MAT), in response to caloric restriction and chemotherapy, secretes adiponectin at a much larger scale as compared to the levels secreted by WAT, suggesting that MAT-derived adiponectin is circulated throughout the body, exhibiting endocrine and metabolic effects on cells [72]. Circulating adiponectin is shown to be decreased in patients with T2DM, cardiovascular disease, liver disease, and hypertension [73–75]. In addition, adiponectin binding to AdipoR1 and AdipoR2 has been shown to have anti-diabetic effects, which further underlines positive effects of this hormone on metabolic homeostasis [76].
Leptin, also known as the “satiety hormone”, is the other most commonly studied factor produced and secreted by white adipocytes [77, 78]. Canonical leptin signaling occurs through the leptin receptor which, upon the binding of its ligand, dimerizes and induces phosphorylation and activation of Janus tyrosine kinase-2 (JAK2) [79]. This leads to STAT3 phosphorylation and downstream transcription of leptin target genes. Mutations in the gene encoding leptin or its receptors in the hypothalamus result in disturbed leptin signaling and consequently promote hyperphagic obesity, diabetes mellitus, and neuroendocrine dysfunctions [80]. Interestingly, although leptin is secreted by adipocytes to inhibit hunger, this adipokine is produced and secreted at high rates in obese individuals [81]. Enhanced leptin signaling has been implicated in many different cancers [82]. Leptin binding to its receptor on mammary cancer cells has been shown to play a role in maintaining cancer stem cell phenotype and promoting stem cell-like properties of triple negative breast cancers [83].
Inflammatory cytokines
Obesity is characterized as a state of chronic inflammation. It has been speculated that expansion of adipose tissue occurring in obesity results in oxygen deprivation of adipocytes which are most distant from the capillary network [84]. This hypoxia triggers the activation of hypoxia-inducible factor 1-alpha (HIF-1α), which in turn, signals for the macrophage infiltration and induction of inflammation [85]. There is growing evidence that multiple pro- and anti-inflammatory cytokines in obese adipose tissue form a functional circuitry that regulates local and systemic glucose tolerance and insulin sensitivity [86]. These cytokines are secreted by adipocytes, macrophages and other cell types residing in the inflamed tissue [86, 87].
TNF-α was one of the first identified WAT-derived pro-inflammatory cytokines, thought to be primarily secreted by myeloid cells via activation of MAPK and NFκB signaling pathways and stimulating the release of other inflammatory cytokines, such as IL-1β and IL-6 [88]. It has since been determined that adipocytes themselves are a significant source of TNF-α, whose induction in fat cells occurs in response to free fatty acids (FFA), and activation of JNK signaling pathway [89]. TNF-α, in turn, via activation of ERK signaling pathway stimulates lipolysis, a process resulting in a positive feedback mechanism that further contributes to the chronic state of obesity-induced inflammation [90]. The abundant secretion of this TNF-α has been directly linked to obesity-associated insulin resistance [89, 91] and tumorigenesis [92].
IL-6 is a pleiotropic cytokine, that is released in response to hypoxic stimulation of white adipocytes [93] and its secretion is associated with insulin resistance [94], immune responses and host defense mechanisms [88], as well as tumorigenesis and metastatic potential [95]. Approximately 30% of circulating IL-6 levels are thought to originate from WAT, categorizing it as an adipokine [88]. Circulating levels of IL-6 appear to correlate with increased body mass, waist circumference and FFA concentrations; however, its functions in obesity and insulin resistance in regards to tissue and metabolic rate remain controversial [88].
IL-1β is another important regulator of inflammatory responses whose levels are elevated in obesity and associated metabolic disorders [86]. A blockade of IL-1β activity in animal models and human subjects with neutralizing antibodies to this cytokine or its receptor improve insulin sensitivity and help to treat T2DM [86, 96, 97]. However, IL-1β-deficient animals have reported glucose intolerance, while IL-1RA-null mice are resistant to diet-induced obesity, findings revealing the need for further investigations of pro-inflammatory axes in obesity and metabolic disorders.
Lipolysis
Key components released by all types of adipocytes that can influence metabolic processes in neighboring cells are glycerol and FFA. In times of excess energy, fatty acids are stored as triglycerides, forming lipid droplets that are housed in the specialized domains within the endoplasmic reticulum [98, 99]. Fat cells are constantly both synthesizing triglycerides, and breaking them down to glycerol and fatty acids during a catabolic process known as lipolysis [100]. This process is driven by activation of the rate-limiting enzyme, adipose triglyceride lipase (ATGL), phosphorylation and activation of hormone-sensitive lipase (HSL), and monoacylglycerol hydrolysis by monoglyceride lipase (MGL). Lipolysis and its rates are regulated by hormonal and biochemical signaling. The process of lipid breakdown is stimulated through the binding of catecholamines, epinephrine and norepinephrine, to β-adrenergic receptors 1 and 2 and the α-adrenergic receptor [101]. A key process for lipolysis and lipase regulation occurs through activation or suppression of protein kinase A (PKA) [102, 103]. PKA has the ability to both activate HSL and also facilitate the trafficking of proteins involved in lipolysis [104]. ATGL, conversely, is not a direct target of PKA and has high affinity for triacylglycerides and no activity against either diacylglycerides or monoacylglycerides [105]. HSL-null mice exhibit severely impaired glycerol release and large accumulation of DAG in several tissues, confirming that this lipase is a rate-limiting enzyme in DAG hydrolysis [106]. In contrast to HSL, ATGL deficiency leads to severe lipid-associated phenotype with high lipid accumulation, poor lipid mobilization, reduced biochemically-induced lipolysis, and myopathy [107–112], indicating its essential function in lipolysis. Absence of ATGL reduces fatty acid release from adipose tissue by 75% and a mutation in the ATGL gene in humans causes lipid storage dysfunction called neutral lipid storage disease with myopathy (NLSDM) [111, 113]. Because lipolysis is such a fundamental and crucial process for energy homeostasis and metabolism, dysfunction in this process has been suggested as a hallmark to the onset or maintenance of obesity [114].
Obesity-cancer link: the concerning problem
Currently, obesity is a global epidemic characterized by excess adipocyte size and numbers. Recent reports indicate that more than two-thirds of Americans are overweight or obese and this number has been increasing for decades [115, 116]. Obesity is a serious health concern and a major risk for the development and onset of a multitude of different cancers [117–119]. Studies have demonstrated that the fraction of patients that have cancer caused by excess weight has reached about 20% of all cancers [119]. The Million Women Study reported that around 50% of cancers in postmenopausal women are linked to obesity [120]. For the high-risk obese patients in general, the most common malignancies appear to be esophageal adenocarcinoma, colorectal, postmenopausal breast, prostate, and renal cancers [121, 122]. Malignant melanoma, thyroid cancers, leukemias, non-Hodgkin’s lymphomas, and multiple myelomas have been associated with obesity but to a lesser extent [123, 124].
Role of circulating adipokines in tumorigenesis and tumor progression
As experimental and epidemiological evidence linking obesity with cancer risk or recurrence increases, the mechanisms behind this association are still largely unknown. It is becoming increasingly accepted that dysregulation of adipocyte function and obesity-driven chronic inflammation are the main culprits in adiposity-induced tumorigenesis [117, 125]. This is particularly evident in cancers that grow in adipocyte-rich environments like breast carcinomas, or cancers that have propensity to metastasize to fat-rich sites, such as ovarian or gastric malignancies [126]. In addition to acting as local paracrine signaling molecules, adipokines also exert systemic effects and allow for communication with distant sites. The increased levels of adipose tissue-derived factors, such as TNF-α, IL-6, IL-8, macrophage chemoattractant protein (MCP-1), and leptin and their role in tumor progression have been well-documented [82, 126].
Levels of circulating leptin are enhanced in obese individuals, and elevated leptin is a poor prognostic factor for breast cancer patients, underlining the role of this adipokine in tumor progression [127]. Leptin expression is higher in patients that have prostate cancer compared to benign prostate hyperplasia and higher in patients with advanced, metastatic disease compared to patients with localized, early stage prostate cancer, implicating leptin expression as a biomarker for prostate cancer staging and prognosis [128, 129]. Notably, a polymorphism associated with an overexpression of the mutated leptin in some patients has been suggested as a risk factor for prostate cancer [130]. Furthermore, increased levels of leptin receptor were reported in breast cancer tissue as compared to normal tissue and suggested to correlate with immune response, angiogenesis, reproduction, growth factor signaling and lipid metabolism pathways [131–134]. In gastric cancer, leptin has been shown to increase tumor invasiveness by activating Rho/ROCK signaling pathways [135] while inhibitory effects of this adipokine on mitochondrial respiration have been linked with colon cancer progression [136].
In contrast to leptin, adiponectin, an adipokine with insulin-sensitizing effects, has been suggested to have anti-tumor effects [126, 137]. Low levels of adiponectin, as observed in obese individuals, have been correlated with an increased risk of prostate cancer [138]. Treatment with recombinant adiponectin has resulted in anti-tumor effects in some cancer types such as fibrosarcoma, myelomonocytic leukemia, and breast carcinoma [139–142]. Similarly, inhibitory effects of adiponectin on survival and proliferation of prostate cancer cells was reported, with anti-tumor effects linked to the high molecular form (HMW) of this adipokine, which is known to be responsible for its biological activity [143, 144]. These results were shown both in androgen-dependent LNCaP-FGC cells and androgen-independent DU145 cells, indicating a global effect on prostate cancer cells regardless of androgen receptor status.
Bone marrow adipocytes and skeletal metastases
Although numerous studies have identified obesity as a risk factor for various cancers [124, 145–147], it is only recently that accumulation of bone marrow fat has emerged as a risk factor for the development and progression of skeletal metastases, particularly from prostate cancer [24, 148]. Specifically, we and others have shown that marrow adipocytes mediate translocation of the lipids to the metastatic cancer cells [149, 150]. These adipocyte-supplied lipids serve as an energy source for cancer cells, and consequently induce tumor cell proliferation, motility and invasion [148, 151]. Moreover, fatty acid binding protein 4 (FABP4), a lipid transporter expressed predominantly in adipocytes, macrophages, and endothelial cells [152], and originally identified as a key mediator of adipocyte-tumor interactions in ovarian cancer [126, 153], is highly upregulated in metastatic prostate cancer cells interacting with adipocytes [148]. We have shown that through its interplay with PPARγ and IL-1β, FABP4 is involved in driving the aggressiveness of prostate tumors in bone [148]. Our studies have also demonstrated that additional pro-inflammatory factors such as cyclooxygenase-2 (COX-2) and MCP-1 are highly induced in metastatic tumor cells under conditions of high marrow adiposity [24]. This underlines the interaction between the lipid-driven and the inflammatory pathways in bone and offers new avenues for investigation of mechanisms behind development and progression of skeletal metastases.
Feeding the enemy
Warburg Effect
The role of adipocytes in regulating tumor metabolism is largely understudied and not well-understood. The growth-, proliferation-, and survival-promoting effects of fat cells on the tumor cells have been clearly demonstrated in breast, prostate, gastric, colon and ovarian cancers [126]; however, little is known about the contribution of altered tumor metabolism to these effects. A recent publication by Nieman et al. showed that ovarian cancer cells utilize white adipocytes to gain energy for rapid division by inducing fat cell-driven lipolysis and increasing availability of lipids for uptake by the tumor cells [153]. Subsequent to lipid uptake, there is an overexpression of fatty acid transporter, FABP4, and significant elevation of β-oxidation, which can be blocked by the treatment with inhibitor of carnitine-palmitoyltransferase 1 (CPT-1), etomoxir [153]. Notably, β-oxidation has also been shown to be a main source of energy in prostate cancer cells [154], further suggesting that metabolic reprogramming may be playing an important role in tumorigenesis.
For most of the normal cells in the human body, glucose is an essential energy source. In the presence of oxygen glucose is broken down to pyruvate, which enters the mitochondria and is further oxidized to carbon dioxide with the release of energy in the form of ATP [155]. In the absence of oxygen, normal cells will produce high rates of lactate and undergo a metabolic shift to a more glycolytic phenotype. It has been well-documented that unlike normal cells, tumor cells show high rates of glycolysis and lactate production, regardless of the presence or absence of oxygen [156]. This metabolic switch to aerobic glycolysis, also known as the Warburg effect, provides energy and essential carbon sources for lipogenesis and nutrient production for the rapidly dividing cancer cells [157, 158]. This enhanced glycolytic phenotype was originally postulated to be a direct effect of mitochondrial dysfunction within cancer cells [157]. Under normal physiological conditions ATP is generated through oxidative phosphorylation in the mitochondria in which acetyl-CoA is oxidized to CO2, releasing energy in the form ATP [159]. It has been shown that oncogenic transformation leads to an increase in glycolytic genes, while tumor suppressor proteins induce expression of oxidative phosphorylation (OxPhos) genes, showing the implications of glycolysis in carcinogenesis [160, 161].
Many of the enzymes responsible for glucose metabolism in both normal and cancer cells have significant functions that are non-glycolytic and tumor promoting [162]. Specifically, it was revealed that hexokinase II (HKII) has an anti-apoptotic effect on the mitochondria by binding to the mitochondrial membrane, antagonizing interaction with pro-apoptotic factors Bad and Bax [163–165]. Along the same lines, pyruvate kinase M2 (PKM2) was shown to have non-glycolytic functions in facilitating tumor survival [166, 167]. PKM2 appears to be activated through epidermal growth factor receptor (EGFR) signaling and obese patients have higher levels of serum heparin-binding epidermal-like growth factor, which is able to activate EGFR [168, 169]. Other functions of PKM2 include the phosphorylation of histone H3 and releasing histone deacytelase 3, which leads to induction of many cell cycle genes including Cyclin D and metabolic regulator c-MYC [170]. PKM2 is also known to act as a transcriptional regulator through its interactions with Oct4, a transcription factor that drives the expression of many genes in tumorigenesis and nuclear signaling [171, 172].
Glycolysis is much less energy-efficient compared to the OxPhos pathway as it generates two net ATP molecules, vs. 36 molecules of ATP produced by the OxPhos pathway. Consequently, cancer cells must undergo very high rates of glycolysis in order to generate a large amount of ATP quickly. Interestingly, cells utilizing aerobic glycolysis have high ratios of ATP/ADP and NADH/NAD+ even when proliferating at high rates [173]. The aerobic glycolytic phenotype is important for tumor progression through the following postulates: 1) high rates of lactate production and secretion can break down and degrade the surrounding extracellular matrix and aid tumor expansion and metastasis; 2) enhanced glycolysis supplies an abundance of ATP to the cancer cells; and 3) associated mitochondrial dysregulation inhibits or reduces apoptosis [174–176]. It has also been shown that aerobic glycolysis creates byproducts that increase the ability of the cells to produce precursors for biosynthesis of multiple different macromolecules essential for rapid division such as lipids, nucleic acids, and proteins [177]. Also, the generated lactate can create a toxic environment for immune cells, contributing to decreased immunosurveillance and thus the ability of the tumor to hide from an immune response within its microenvironment and prevent detection [178]. Excess lactate production has also been implicated in the stimulation of endothelial cells surrounding the tumor to allow vascularization of the tumor and to provide nutrients through the circulation [179, 180].
It is known that the transcription factor c-MYC acts as a master regulator of cellular metabolism by actively transcribing genes associated with glycolysis. Specifically, it has been shown that c-MYC upregulates lactate dehydrogenase alpha (LDH-α), an enzyme crucial for the conversion of pyruvate to lactate during the Warburg effect [181, 182]. The c-MYC transcription factor is also known to regulate key proteins involved in both nucleic acid synthesis and fatty acid synthesis, processes utilized by tumor cells to meet the demands of rapid cellular division, which underlines its role as a crucial regulator of cellular metabolism [183, 184]. Furthermore, overexpression of c-MYC has been correlated with upregulation of pyruvate kinase M2, the splice variant most commonly seen in tumor cells during aerobic glycolysis [185–187]. The M2 isoform of pyruvate kinase is overexpressed in cancer cells through c-MYC-regulated overexpression of heterogeneous nuclear ribonucleoprotein 1 and 2 (hnRNPA1 and hnRNPA2). These ribonucleoproteins preferentially splice the M2 isoform over the M1 isoform, which is critical for aerobic glycolysis [185, 188]. There is a proposed positive feedback in which PKM2 is upregulated by c-MYC and, in turn, PKM2 is involved in the upregulation of c-MYC [189]. Notably, c-MYC has been shown to be overexpressed in an estimated 50% of all human cancers [190, 191].
Along with an enhanced glycolytic phenotype, tumorigenesis is often associated with mitochondrial dysfunction [192]. Mitochondria become dysfunctional when the mitochondrial DNA (mtDNA) is reduced or mutated and obese individuals have been shown to have a reduction in mitochondrial DNA in white adipocytes [193]. Dysregulation in mitochondrial activity has also been shown to play a role in the inhibition of tumor suppressor protein p53 in epithelial cells, leading to aberrant proliferation checkpoints and tumorigenesis [194]. There is also emerging evidence demonstrating that obesity-induced adipokines promote mitochondrial defects and promote glycolytic phenotype in normal tissue, thereby driving tumorigenesis [195].
HIF-1α signaling pathways in cancer
Anaerobic respiration, a hallmark of tumorigenesis, drives a hypoxic phenotype in cancer cells even in the presence of oxygen [196]. Hypoxic signaling occurs when transcription factor HIF-1α becomes stabilized and translocates to the nucleus where it binds to and activates hypoxic response elements (HRE) [197]. In normoxia, prolyl hydroxylase domain (PHD) proteins hydrolyze HIF-1α, which is then ubiquitinated by Von Hippel-Lindau (VHL) and targeted to the proteasome. However, under hypoxic conditions, PHD is inhibited and HIF-1α is stabilized and translocated to the nucleus where it dimerizes with HIF-1β to activate hypoxia-responsive genes [198]. In addition to the hypoxic effect seen in low oxygenated tissue, there is also an oxygen-independent HIF-1α signaling in which HIF-1α becomes stabilized and activates target genes in the presence or absence of oxygen [199].
The role of HIF-mediated signaling pathway in tumorigenesis and clinical response to treatments is well-established [200]. Many tumors have areas of low oxygenation or intratumoral hypoxia. Patients with poorly oxygenated primary tumors have a higher risk of both metastases and mortality owing to a more aggressive cancer phenotype [201]. Elevated HIF-1α expression has been correlated to increased mortality risk in a plethora of different cancers including solid tumors of the bladder, brain, breast, colon, esophageal, head and neck, oropharynx, liver, lung, pancreas, skin, stomach, and uterus as well as acute lymphocytic and myeloid leukemias [202].
HIF-1α acts as a transcriptional activator and regulates the expression of many different glycolytic enzymes involved in metabolic reprogramming. Specifically, LDH-α, the enzyme responsible for the high conversion of pyruvate to lactate in cancer cells, is trans-activated only through HIF-1α transcriptional regulation [203]. Along with HIF-1α, the HIF-2α isoform signals in a similar way, but trans-activates different target genes. A study in renal cell carcinoma cells showed that HIF-1α and HIF-2α signaling converge at genes involved in glucose transport [i.e., glucose transporter 1 (Glut1)], lipid metabolism [i.e., adipose differentiation-related protein (ADRP)], pH homeostasis [i.e., carbonic anhydrase IX (CAIX)], interleukin responses, (i.e., IL-6), and angiogenesis [i.e., vascular endothelial growth factor (VEGF)] [204]. Interestingly, however, there were many significant differences in gene regulation between HIF-1α and HIF-2α. This study also revealed that HIF-1α but not HIF-2α is highly involved in a glycolytic response, functioning as a trans-activating factor for the following enzymes involved in glycolysis: hexokinase1 (HK1), hexokinase2 (HK2), phosphofructokinase (PFK), aldolase A (ALDA), phosphoglycerate kinase 1 (PGK1), and LDH-α [204]. There has also been a proposed feed-forward mechanism in which the Warburg Effect-associated enzyme PKM2 can act as a coactivator for HIF-1α target gene transcription [205]. It was recently demonstrated that Jumonji c domain-containing dioxygenase (JMJD5), is upregulated through HIF-1α signaling and that JMJD5 interacts with PKM2, enhancing its translocation to the nucleus and is recruited to the LDH-α promoter [206, 207]. The inhibition of JMJD5 causes a decrease in glucose metabolism and lactate secretion associated with Warburg effect [206, 207].
HIFs do not only regulate glycolytic genes to promote a more glycolytic phenotype, but they are also involved in mitochondrial effects and decreased OxPhos activity. Among HIF target genes are microRNAs, particularly miR-210, which has been reported to be overexpressed in hypoxia in a number of cancer cells [208]. MicroRNAs bind to sequences in messenger RNA and either inhibit their translation or, in some cases, initiate their degradation [209]. MiR-210 targets the iron-sulfur cluster assembly enzyme (ISCU) gene, which is required for the activity of complex I in the mitochondrial electron transport chain during oxidative metabolism, the constituents of cytochrome c oxidase assembly protein (COX10), NADH-dehydrogenase 1a subcomplex 4 (NDUFA4), and subunit D of succinate dehydrogenase complex (SDHD) [210–214]. Accordingly, miR-210 has been labeled as a biomarker of tumor hypoxia, and its high levels have been implicated in poor patient prognosis for several cancers [215]. Because many of the targets of miR-210 affect mitochondrial activity, it is evident that this molecule plays a central role in cellular metabolism and homeostasis. Notably, it was also reported that there is a positive feedback loop between miR-210 and HIF-1α in human lung cancer cell lines, where miR-210 stabilizes HIF-1α [213]. This leads to increased transcription of HIF-1α target genes and establishment of hypoxic tumor microenvironment. In addition, lung cancer cells that overexpress miR-210 have been shown to have decreased mitochondrial activity and increased glycolytic phenotype, and exhibit elevated resistance to radiotherapy [213]. This decrease in β-oxidation in parallel with increased HIF activity is important for tumor cell survival because it prevents malignant cells from developing high levels of reactive oxygen species (ROS) and allows them to survive under hypoxic stress.
Another important effect of hypoxia on tumor cells is the initiation of angiogenesis, a process of formation of new blood vessels from pre-existing vasculature. Tumor growth and metastatic progression depend heavily on angiogenesis for the continuing supply of nutrients [216]. Accordingly, studies have shown that tumors with functional angiogenesis grow much larger than those without proper vascularization and blood supply, and that reduced blood supply often results in necrosis or apoptosis [217, 218]. The most well-known pro-angiogenic factor regulated by HIF-1α is vascular endothelial growth factor (VEGF) [219]. It has been shown that VEGF transcript and protein levels are upregulated in response to hypoxia and that targeting HIF-1α with small-interfering RNA (siRNA) significantly reduces VEGF gene and protein expression in epithelial cells [220]. Interestingly, we have previously demonstrated that treatment of tumor cells with media conditioned by bone marrow adipocytes in vitro, as well as in vivo establishment of skeletal tumors under conditions of high marrow adiposity result in significant upregulation of oxidative stress markers and VEGF, suggesting potential activation of HIF-1α [148]. Particularly important to the obesity-cancer link might be the evidence that obese patients have higher levels of HIF-1α activity in white adipocytes due to rapid proliferation and expansion of the fat cells and an increase in VEGF expression [221–225]. VEGF alone has been implicated in tumorigenesis through the stimulation of proliferative signaling pathways through the vascular endothelial growth factor receptor (VEGFR) and through cancer stem cell maintenance [226, 227]. Further studies are required to elucidate the role of adipose tissue hypoxia and VEGF secretion in obesity on tumor initiation and maintenance.
ER stress and tumor metabolism
It is well-established that high levels of endoplasmic reticulum (ER) activity occur in cancer cells because high rates of cellular division generate a need for a surplus of protein synthesis and proper folding [228]. Elevated levels of fatty acids caused by conditions such as obesity can also contribute to ER stress and mitochondrial damage in neighboring cells, including cancer cells [229]. Consequently, misfolded proteins accumulate in the ER lumen triggering a series of intracellular signaling pathways called the unfolded protein response (UPR). UPR can either trigger apoptosis or, oppositely, stimulate the cells to overcome stress, contributing to tumorigenesis and survival.
ER stress has been viewed as one of the mechanisms of cell survival and tumor progression [230–232]. Hypoxia was demonstrated to activate UPR via PERK/eIF2α signaling in tumor cells as a mechanism to promote metastases [233]. ER stress allows malignant cells to thrive in hostile hypoxic microenvironments where nutrients are limited and pH is generally lower than physiological range [234–236]. In fact, there is emerging data that links hypoxia, ER stress response, and metabolic adaptation by the tumor cells. Specifically, a recent study by Chen et al. reported that ER stress in triple negative breast cancer cells leads to the activation of X-box binding protein-1 (XBP1) and that XBP1 complexes with HIF-1α to enhance a hypoxic phenotype [237]. They further demonstrated that XBP1/HIF-1α complex can bind to and regulate pyruvate dehydrogenase kinase-1 (PDK1), a gene associated with the Warburg Effect. It was also shown that activating transcription factor 4 (ATF4), an ER stress response protein, has the ability to bind to HREs and HIF-1α target genes such as VEGF, potentiating HIF-1α activity under stress conditions [238, 239]. Enhanced hypoxia in white adipocytes of obese mice was shown to correlate with increased production of unfolded proteins, and overexpression of ER stress factor CHOP [221]. In turn, CHOP activation attenuated the expression of adiponectin by interfering with the activity of its promoter, revealing potential links between ER stress and low circulating adiponectin levels seen in obesity [221]. Cumulatively, above-described results indicate that the crosstalk between ER stress, HIF signaling and tumor metabolism might be critical driver of tumor cell survival and chemoresistance (Figure 1).
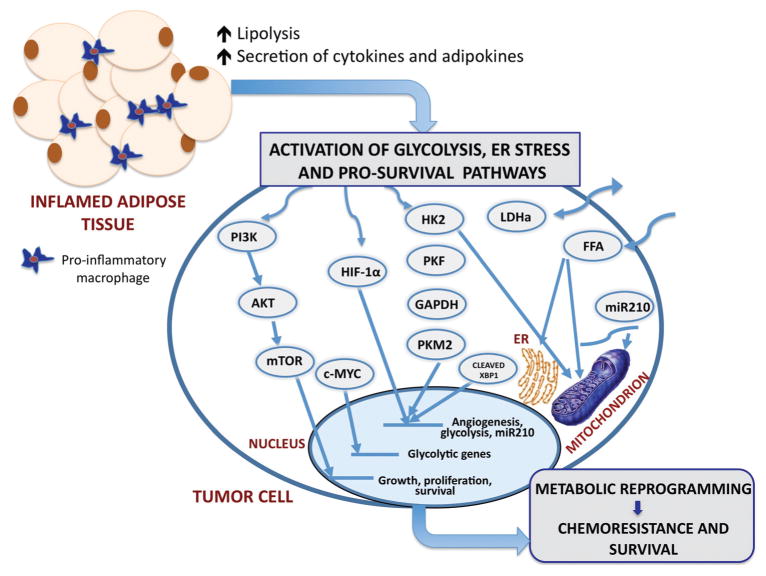
Figure 1.
The proposed schematic of possible mechanisms of metabolic regulation of tumor cells by dysfunctional adipocytes in obesity The major consequence of obesity is adipose tissue inflammation and associated increases in circulating levels of lipolysis-generated lipids and pro-inflammatory cytokines and adipokines. Through paracrine, autocrine, and endocrine effects, adipocyte-derived factors activate metabolic pathways in tumor cells and facilitating growth and survival. Pathways of interest include the following: phosphoinositol 3-kinase (PI3-K) signaling cascade, hypoxia-inducible factor 1α (HIF-1α), increased glucose uptake and enhanced glycolysis, and the potentially oncogenic endoplasmic reticulum (ER stress) pathway. The stimulation of PI3-K pathway leads to downstream activation of Akt and mTOR, enhancing the transcription of genes involved in growth, proliferation and survival. PI3-K signaling can also activate c-MYC and lead to the induction of glycolytic genes. A potential crosstalk between the glycolysis pathway and the HIF-1α signaling axis potentiates HIF activity, and exacerbates the glycolytic and hypoxic phenotypes. HIF-1α signaling leads to the expression of miRNA-210, which disrupts mitochondrial integrity, affecting cellular metabolism. Additionally, FFA have the ability to disrupt mitochondrial and ER membrane integrity and cause mitochondrial dysfunction and ER stress. The interactions of the ER stress response protein, XBP-1, with HIF-1α drive the expression of HIF- and glycolysis-targeted genes. This adipocyte-driven dynamic network of events results in metabolic adaptation of tumor cells, implicating adiposity in tumor aggressiveness and chemoresistance to therapy. PI3K, phosphoinositide 3-kinase; AKT, protein kinase b; mTOR, mammalian target of rapamycin; c-MYC, myc proto-oncogene; HIF-1α, hypoxia-inducible factor 1; HK2, hexokinase 2; PKF, phosphofructokinase; GAPDH, glyceralaldehyde-3-phosphate dehydrogenase; PKM2, pyruvate kinase isoform; LDHα, lactate dehydrogenase; FFA, free fatty acids; miR-210, micro RNA 210.
Adipocyte artillery and their effects on metabolism
Few studies have demonstrated the effects of adipocyte-derived factors on tumor metabolism. The majority of reported studies focus on TNF-α, leptin, and lipids or lipolysis products [240, 241]. A study utilizing genetically obese ob/ob mice showed an association between TNF-α secretion and OxPhos dysregulation [242]. A marked decrease in mitochondrial respiratory chain activity in liver cells of ob/ob mice was reported. Also reported were elevated levels of TNF-α, inducible nitric oxide synthase (iNOS), and tyrosine nitrated proteins correlated with increased adiposity. It was determined that ob/ob mice not only have diminished activity of OxPhos system, but also a reduction in the assembly of the OxPhos subunits in the mitochondria to about 50–60% [243]. Most of the decreases were seen in subunits transcribed by mitochondrial DNA, which was reduced by approximately 60% relative to control mice.
Along with TNF-α, white adipocyte-secreted factors such as leptin and Wnt peptides can also cause mitochondrial impairment [244, 245]. The observed effects of leptin appear to not only result from canonical leptin signaling, but also from its non-canonical signals associated with crosstalk with both the phosphatidylinositol 3-kinase (PI3-K) pathway and the Ras-dependent pathways [246]. These pathways are both commonly deregulated in cancers and affect cellular survival, growth, and metabolism [247, 248]. PI3-K activation leads to subsequent activation of the downstream target protein Akt, and PI3-K/Akt signaling has been shown to directly regulate cellular metabolism [248]. Induction of this pathway leads to the expression of glucose, amino acid, lipoprotein, and iron transporters at the cellular surface [156]. Additional effects include stimulation of glycolytic enzymes hexokinase and phosphofructokinase, increased transcription of glycolytic genes, and relative increases in protein synthesis essential for rapid cellular division [249, 250].
It has been recently demonstrated, that Wnt signaling, which, when elevated, is commonly associated with tumorigenesis and tumor survival, suppresses mitochondrial respiration and cytochrome C oxidase activity [251]. An enhanced Wnt signaling through the β-catenin pathway leads to the inhibition of cytochrome C oxidase subunits COXVIc, COXVIIa, and COXVIIc, and this inhibition of mitochondrial activity results in an enhanced glycolytic phenotype. It has been postulated that with higher white adipocyte content, levels of Wnt ligands are increased, leading to enhanced positive correlation with Wnt signaling in neighboring cells [252, 253].
Little is known to date on how adipocyte-derived lipids directly influence tumor metabolism; however, there is increasing evidence that lipids generated by the tumor cells during lipogenesis modulate metabolic pathways in cancer cells and stimulate the Warburg Effect. One consequence of the Warburg Effect is an increase in lipid biosynthesis, and de novo lipogenesis is, in fact, performed at high rates in cancer cells [243]. An example of a bioactive lipid with implications for prostate tumorigenesis is sphingosine-1/2 phosphate (S-1/2P) [254, 255]. S1P is activated by the phosphorylation of sphingosine by sphingosine kinase 1 or 2 (SK1 or SK2). S1P has been shown to induce cell growth, survival, and migration and to play a role in variety of cancers [256]. Similarly, upregulation of SK1 has been associated with glioblastomas, lung, thyroid, and breast cancers [257–261]. Notably, S1P has also been shown to highly present in obese patients compared to lean patients [262] and its potential involvement in obesity-driven tumorigenesis calls for further investigations.
Current therapeutic options in targeting tumor metabolism
Tools to regulate glycolysis
Because tumor metabolism is deregulated in almost all cancers, targeting glycolytic intermediates has become a hot topic in therapeutic research. One of the first inhibitors developed to target glycolysis was 2-deoxyglucose (2-DG), a glucose analog that downregulates glucose metabolism through competitive inhibition [263]. 2-DG is transported into the cell and phosphorylated by hexokinase to 2-deoxyglucose-phosphate (2-DG-P). 2-DG-P cannot be further metabolized and accumulates in the cells, leading to competitive inhibition of hexokinase during glycolysis [264]. In vitro studies have shown that this effect causes a decrease in cellular ATP production, and leads to the blockage of cell cycle progression and subsequent cell death [265]. A recent study has demonstrated that a combination of 2-DG treatment with photodynamic therapy induces tumor cell death in a synergistic manner [266]. Decreased cellular proliferation and increased apoptosis of cancer cells was also demonstrated upon 2-DG treatment in the N-diethyl-nitrosamine-induced rat hepatocarcinoma model [267]. Along with a decrease in glycolysis, there was an observable decrease in the tricarboxylic acid (TCA) cycle activity, fatty acid and cholesterol biosynthesis, and ATP production, all pathways associated with tumor progression and metabolism. Other studies have shown that inhibiting glyoxylase 1, an enzyme responsible for the conversion of the glycolysis byproduct methylyglyoxyl to D-lactate, in a highly metabolically active tumor cells leads to an increase in apoptosis and a decrease in cellular proliferation [268, 269].
Targeting glycolysis in order to reverse the Warburg Effect has sparked interest as a potential anti-cancer therapy and has led to recent breakthroughs in therapeutics. One particularly intriguing target gaining a significant amount of attention from the pharmaceutical industry is LDH-α, an enzyme converting pyruvate to lactate, and a biomarker of advanced disease, poor prognosis, and resistance to therapy in many different cancers [270–272]. LDH-α inhibitors have a high specificity for cancer cells because of the high demand for lactate production in cancer cells during aerobic glycolysis [273]. It was recently reported that inhibition of LDH-α reduced ATP levels and led to an accumulation of ROS in lymphoma cells [274]. This accumulation of ROS resulted in increased incidence of apoptosis, suggesting that LDH-α is critical for tumor maintenance and cancer cell metabolism [274, 275]. These results have been recapitulated in a variety of diverse cancer types including lung cancer, renal cancer, breast cancer, hepatocellular carcinoma, nasopharyngeal carcinoma, and pancreatic cancer [274, 276–282].
There has been clinical success with drugs designed to target other enzymes in the glycolysis pathway, such as hexokinase II (HKII), phosphofructokinase (PFK), glyceraldehyde-3 phosphate dehydrogenase (GAPDH), and pyruvate kinase M2 (PKM2). Lonidamine, a selective HKII inhibitor, reached phase III trials in the 1990s as a therapeutic option for patients with lung cancer and was mildly successful but had toxic side effects [283, 284]. A novel approach by Wang et al. used the natural compound curcumin as a potential drug that could target HKII specifically and was shown to have anti-cancer effects in vitro [285]. The authors reported that colorectal cancer cells treated with curcumin in vitro have decreased mitochondria-associated anti-apoptotic HKII, leading to enhanced cell death. 3-bromopyruvate (3-BrPA), a selective inhibitor of another glycolytic enzyme, glyceralaldehyde-3-phosphate dehydrogenase (GAPDH), has been shown to downregulate PI3K/Akt signaling axis, leading to induced apoptosis in breast cancer cells [286, 287]. This finding suggests potential benefits of dual targeting of glycolysis enzymes and PI3K/Akt-mediated cellular metabolism. 3-BrPA has been FDA approved for phase I clinical trials as a selective glycolysis inhibitor [288–290] and was shown to induce ER stress, inhibit global protein synthesis and thereby induce tumor cell death [291]. Attempts have also been made to target phosphofructokinase, particularly PFKB3, the isoform commonly upregulated in cancers. Specifically, the use of selective PFKB3 inhibitors, such as 3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one (3PO) shown that inhibition of PFKB3 leads to autophagy and could be an effective anti-tumor therapy [292]. 3PO is currently being tested in clinical trials [293].
Metabolic transporters as targets for therapy
Monocarboxylate transporters (MCTs) are proteins over-expressed in most cancers and responsible for the import (MCT1) and export (MCT4) of lactate [294]. MCTs are strong contributors to high production and secretion of lactate resulting from the Warburg effect, which makes them attractive targets for anti-cancer therapies. MCT-targeting agents showed significant anti-cancer effects, and a novel inhibitor developed by AstraZeneca, AZD3965, is currently being developed in a phase I clinical trial in patients with advanced cancer [295]. Significant efforts have recently been made to selectively target the glucose transporters, the proteins responsible for the uptake of glucose to be utilized in glycolysis [296, 297]. Promising results with WZB117, a small molecule inhibitor of glucose transporter one (GLUT1) showed in vitro and in vivo inhibition of glucose uptake by WZB117 [298]. Treatment with WZB117 caused down-regulation of glycolysis, reduced cellular ATP, reduced levels of cell cycle genes, and a more than 70% reduction in tumor size in mice injected with human lung cancer cells [298].
Modulating tumor metabolism with nanoparticle-based therapies
A newly developing field in cancer therapeutics, nanomedicine, revolves around the use of synthesized targeting molecules that can “carry” an encapsulated or bound drug for specific delivery to its target site without toxic effects on non-target tissues. The use of nanomolecules to target glycolysis specifically in tumor cells has been a growing area of research. Ryland et al. used C6-ceramide nanoliposomes to target the Warburg Effect in chronic lymphocytic leukemia (CLL) [299]. Their results demonstrated that treatment of CLL cells in vitro and in vivo with the C6-ceramide nanoliposomes leads to a marked decrease gene and protein expression of GAPDH, a reduction in cellular ATP, and a consequent tumor regression [299].
New approaches to anti-cancer therapies have revolved around finding novel ways to repurpose traditional compounds. Of interest, shikonin, a natural napthoquinone, along with its traditional role as an anti-inflammatory medicine, has been shown to alter cellular metabolism as an anti-cancer therapeutic [300]. Shikonin-loaded antibodies-armed nanoparticles have been developed specifically as a targeted therapy for ovarian cancer [301]. Shikonin was shown to target PKM2 and to have a necroptotic effect on cancer cells [302, 303]. Furthermore, a study by Matthaiou et al., reported that shikonin-loaded antibody-armed nanoparticles exhibit selective cytotoxicity against ovarian tumor cells in vitro [301]. Shikonin was also shown to induce autophagy in human pancreatic cancer cells in a PI3-K-dependent manner [303]. Cancer cells treated with shikonin had increased expression of autophagy-associated genes LC3-II/LC3-I and a marked reduction in phosphorylation and activation of the phosphoinositol 3-kinase (PI3-K)/Akt signaling pathway [303].
Targeting PI3-K in tumor metabolism
Since the discovery of PI3-K in the 1980s and the demonstration of its clinical importance for tumor survival, significant research efforts have been aimed at inhibiting this cancer-favoring signaling pathway. One of the original inhibitors to target PI3-K was wortmannin, a compound shown to inactivate this kinase by covalent interactions with Lys-802, leading to the inability to transfer a phosphate group [304]. The rationale is that by inhibiting PI3-K/Akt signaling – which, when active, induces glycolytic genes – there would be an observed decrease in glycolysis and production of ATP and lactate, with a subsequent increase in cell death and chemoresistance. Indeed, studies with glycolytic glioma cells have shown that wortmannin treatment leads to suppression in glycolytic migration and an increase in glycogenic phenotype [305]. Additionally, the use of PX-866, a selective PI3-K inhibitor, has shown promising in vitro and in vivo anti-cancer effects and has had success in initial phase I studies in the clinic [306–311]. A treatment with PI3-K inhibitor, LY294002, resulted in a decrease in levels of glycolytic enzymes in human colorectal cancer cells and an increase in apoptosis, suggesting that PI3-K inhibitors have the potential to negatively regulate cancer cell metabolism and to be therapeutically beneficial. Unfortunately, although wortmannin and LY294002 have been effective in vitro, the associated toxicities kept these compounds from being translated to the clinic [312, 313]. However, there have been 15 different PI3-K inhibitors that have successfully entered clinical trials and idelalisib, a selective PI3-K-delta inhibitor was recently approved by the FDA [314]. Idelalisib, used in combination with CD20 antibody rituximab has shown promising results in patients with CLL, which is known to be associated with high rates of glycolysis [315–317].
c-MYC as a potential metabolic target
The transcription factor, c-MYC is regulated by mammalian target of rapamycin (mTOR), which is activated downstream of PI3-K/Akt and has well-established effects on cellular metabolism [318–320]. Studies utilizing mouse embryonic fibroblasts have demonstrated that mTOR signaling leads to induction of genes involved in glycolysis, the pentose phosphate signaling pathway, and lipogenesis, all pathways upregulated during aerobic glycolysis [321–323]. Treatment with rapamycin, the mTOR inhibitor, revealed significant decrease in the glycolytic capacity of cancer cells [324, 325]. The glycolytic inhibitor 2-DG has shown efficacy in downregulating HIF-1α and c-MYC in non-Hodgkin lymphoma cells, enhancing tumor cell sensitivity to methylprednisolone, resulting in cell cycle arrest and apoptosis, and showing a positive feedback between glycolysis and c-MYC induction [326]. Interestingly, a study using, diclofenac, a nonsteroidal anti-inflammatory drug, showed suppression of c-MYC. This down-regulation of c-MYC that resulted in significantly reduced levels of GLUT1, LDH-α, MCT1 and coincident decrease in glucose import and lactate secretion by human melanoma cells in vitro, validating the importance of targeting this transcription factor for anti-cancer therapy [327–329]. The most current, ongoing clinical trials targeting various aspects of metabolism in cancer cells are listed in Table 1.
Table 1.
Current, on-going clinical trials targeting different aspects of cellular metabolism in different cancer types.
| Target | Drug | Clinical trial cancer | Clinical trial reference ID |
|---|---|---|---|
| Phosphofructokinase Isoform 3 (PFK3B) | ACT-PFK-158 | Advanced solid malignancies [330] | NCT02044861 |
| Myc | Lenalidomide | B-cell lymphoma [331] | NCT02213913 |
| Multiple myeloma [332] | NCT01380106 | ||
| Hodgkin’s lymphoma [333] | NCT01460940 | ||
| Pyruvate dehydrogenase kinase 1 (PDK1) | Dichloroacetate | Head and neck cancer [334] | NCT01163487 |
| mTOR | Rad001 | Prostate cancer [335] | NCT00657982 |
| Triple negative breast cancer [336] | NCT01939418 | ||
| Glioma [337] | NCT00823459 | ||
| Gastric cancer [338] | NCT01514110 | ||
| Hexokinase II (HK2) | Curcumin | Breast cancer [339] | NCT01975363 |
| Prostate cancer [340] | NCT01917890 | ||
| Leukemia/lymphoma [341] | NCT02100423 | ||
| Phosphoinositol 3-kinase | GDC-0980/GDC-0941 | Breast cancer [342] | NCT01437566 |
| Advanced solid tumors [343] | NCT01540253 | ||
| BKM120 | Head and neck cancer [344] | NCT01816984 | |
| Wnt | LGK974 | Multiple cancers [345] | NCT01351103 |
| CWP232291 | Acute myeloid leukemia [346] | NCT01398462 | |
| PRI-724 | Advanced myeloid malignancies [347] | NCT01606579 | |
| TNF-α | L19TNFα | Advanced solid tumors [348] | NCT02076620 |
| HIF-1α | Digoxin | Breast cancer [349] | NCT01763931 |
| Ganetespib | Multiple cancers [350] | NCT02192541 | |
| CRLX101 | Ovarian/tubal/peritoneal cancer [351] | NCT01652079 | |
| Phenelzine | Prostate cancer [352] | NCT01253642 |
Conclusions
With obesity toll spreading to pandemic levels, it is critical that the underlying mechanisms linking obesity to metabolic pathologies and tumorigenesis are elucidated. It is clear that factors and lipolysis products derived from any type of adipocyte have the capacity to alter cellular homeostasis in neighboring cells. Abnormal adiposity and chronic inflammation in obesity can lead to the secretion of a multitude of factors, all of which can influence tumor metabolism. Our understanding of tumor metabolism over the last century has revealed a complex, integrated network of enzymes and metabolites cooperating together to facilitate tumor cell growth and survival. The dynamic functions of metabolic proteins make tumor metabolism an intricate and attractive field of research. Further understanding of the interactions between these metabolites and their oncogenic nature will provide insight into elucidating targetable mechanisms and development of novel therapies. Many leaps have been made in cancer therapies in a context of tumor metabolism. It remains crucial to advance our understanding of adipose tissue and disease in order to determine the molecular mechanisms behind adiposity and pathologies and, specifically, cancer.
Acknowledgments
Grant support: National Institutes of Health (NIH)/NCI 1 R01 CA181189-01, DOD W81XWH-14-1-0036.
Footnotes
The authors disclose no potential conflicts of interest.
Contributor Information
Jonathan Diedrich, Department of Pharmacology and Karmanos Cancer Institute, Wayne State University School of Medicine, Detroit, MI 48201, USA.
Halina Chkourko Gusky, Department of Pharmacology and Karmanos Cancer Institute, Wayne State University School of Medicine, Detroit, MI 48201, USA.
Izabela Podgorski, Department of Pharmacology and Karmanos Cancer Institute, Wayne State University School of Medicine, 540 E. Canfield, Rm 6304, Detroit, MI 48201, USA.
References
- 1.Sethi JK, Vidal-Puig AJ. Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007;48:1253–62. doi: 10.1194/jlr.R700005-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frayn KN, Karpe F, Fielding BA, Macdonald IA, Coppack SW. Integrative physiology of human adipose tissue. Int J Obes Relat Metab Disord. 2003;27:875–88. doi: 10.1038/sj.ijo.0802326. [DOI] [PubMed] [Google Scholar]
- 3.Lean ME. Brown adipose tissue in humans. Proc Nutr Soc. 1989;48:243–56. doi: 10.1079/pns19890036. [DOI] [PubMed] [Google Scholar]
- 4.Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N, Hamilton DL, Gimeno RE, Wahlestedt C, Baar K, Nedergaard J, Cannon B. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci USA. 2007;104:4401–6. doi: 10.1073/pnas.0610615104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heaton JM. The distribution of brown adipose tissue in the human. J Anat. 1972;112(Pt 1):35–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–17. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–8. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 8.Ouellet V, Labbe SM, Blondin DP, Phoenix S, Guerin B, Haman F, Turcotte EE, Richard D, Carpentier AC. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest. 2012;122:545–52. doi: 10.1172/JCI60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melicow MM. Hibernating fat and pheochromocytoma. AMA Arch Pathol. 1957;63:367–72. [PubMed] [Google Scholar]
- 10.Soderlund V, Larsson SA, Jacobsson H. Reduction of FDG uptake in brown adipose tissue in clinical patients by a single dose of propranolol. Eur J Nucl Med Mol Imaging. 2007;34:1018–22. doi: 10.1007/s00259-006-0318-9. [DOI] [PubMed] [Google Scholar]
- 11.Lee P, Swarbrick MM, Ho KK. Brown adipose tissue in adult humans: a metabolic renaissance. Endocrine Rev. 2013;34:413–38. doi: 10.1210/er.2012-1081. [DOI] [PubMed] [Google Scholar]
- 12.Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest. 1998;102:412–20. doi: 10.1172/JCI3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vijgen GH, Bouvy ND, Teule GJ, Brans B, Hoeks J, Schrauwen P, van Marken Lichtenbelt WD. Increase in brown adipose tissue activity after weight loss in morbidly obese subjects. J Clin Endocrinol Metab. 2012;97:1229–33. doi: 10.1210/jc.2012-1289. [DOI] [PubMed] [Google Scholar]
- 14.Giralt M, Villarroya F. White, brown, beige/brite: different adipose cells for different functions? Endocrinology. 2013;154:2992–3000. doi: 10.1210/en.2013-1403. [DOI] [PubMed] [Google Scholar]
- 15.Lee YH, Mottillo EP, Granneman JG. Adipose tissue plasticity from WAT to BAT and in between. Biochim Biophys Acta. 2014;1842:358–69. doi: 10.1016/j.bbadis.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrington TA, Thomas EL, Frost G, Modi N, Bell JD. Distribution of adipose tissue in the newborn. Pediatr Res. 2004;55:437–41. doi: 10.1203/01.PDR.0000111202.29433.2D. [DOI] [PubMed] [Google Scholar]
- 17.Hamdy O, Porramatikul S, Al-Ozairi E. Metabolic obesity: the paradox between visceral and subcutaneous fat. Curr Diabetes Rev. 2006;2:367–73. doi: 10.2174/1573399810602040367. [DOI] [PubMed] [Google Scholar]
- 18.Nishino N, Tamori Y, Tateya S, Kawaguchi T, Shibakusa T, Mizunoya W, Inoue K, Kitazawa R, Kitazawa S, Matsuki Y, Hiramatsu R, Masubuchi S, Omachi A, Kimura K, Saito M, Amo T, Ohta S, Yamaguchi T, Osumi T, Cheng J, Fujimoto T, Nakao H, Nakao K, Aiba A, Okamura H, Fushiki T, Kasuga M. FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. J Clin Invest. 2008;118:2808–21. doi: 10.1172/JCI34090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–63. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 20.Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Penicaud L, Casteilla L. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci. 1992;103(Pt 4):931–42. doi: 10.1242/jcs.103.4.931. [DOI] [PubMed] [Google Scholar]
- 21.Granneman JG, Li P, Zhu Z, Lu Y. Metabolic and cellular plasticity in white adipose tissue I: effects of beta3-adrenergic receptor activation. Am J Physiol. 2005;289:E608–16. doi: 10.1152/ajpendo.00009.2005. [DOI] [PubMed] [Google Scholar]
- 22.Himms-Hagen J, Cui J, Danforth E, Jr, Taatjes DJ, Lang SS, Waters BL, Claus TH. Effect of CL-316,243, a thermogenic beta 3-agonist, on energy balance and brown and white adipose tissues in rats. Am J Physiol. 1994;266:R1371–82. doi: 10.1152/ajpregu.1994.266.4.R1371. [DOI] [PubMed] [Google Scholar]
- 23.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–7. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardaway AL, Herroon MK, Rajagurubandara E, Podgorski I. Bone marrow fat: linking adipocyte-induced inflammation with skeletal metastases. Cancer Metastasis Rev. 2014;33:527–43. doi: 10.1007/s10555-013-9484-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lecka-Czernik B. Marrow fat metabolism is linked to the systemic energy metabolism. Bone. 2011;50:534–9. doi: 10.1016/j.bone.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lecka-Czernik B, Rosen CJ, Kawai M. Skeletal aging and the adipocyte program: New nsights from an “old” molecule. Cell Cycle. 2010;9:3648–54. doi: 10.4161/cc.9.18.13046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr. 2009;19:109–24. doi: 10.1615/critreveukargeneexpr.v19.i2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lecka-Czernik B. PPARs in bone: the role in bone cell differentiation and regulation of energy metabolism. Curr Osteoporos Rep. 2010;8:84–90. doi: 10.1007/s11914-010-0016-1. [DOI] [PubMed] [Google Scholar]
- 29.Paula FJ, Rosen CJ. Obesity, diabetes mellitus and last but not least, osteoporosis. Arq Bras Endocrinol Metabol. 2010;54:150–7. doi: 10.1590/s0004-27302010000200010. [DOI] [PubMed] [Google Scholar]
- 30.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–69. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao JJ. Effects of obesity on bone metabolism. J Orthop Surg Res. 2011;6:30. doi: 10.1186/1749-799X-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duque G. Bone and fat connection in aging bone. Curr Opin Rheumatol. 2008;20:429–34. doi: 10.1097/BOR.0b013e3283025e9c. [DOI] [PubMed] [Google Scholar]
- 33.Villareal DT, Apovian CM, Kushner RF, Klein S American Society for N, Naaso TOS. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr. 2005;82:923–34. doi: 10.1093/ajcn/82.5.923. [DOI] [PubMed] [Google Scholar]
- 34.Kugel H, Jung C, Schulte O, Heindel W. Age- and sex-specific differences in the 1H-spectrum of vertebral bone marrow. J Magn Reson Imaging. 2001;13:263–8. doi: 10.1002/1522-2586(200102)13:2<263::aid-jmri1038>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 35.Nielson CM, Srikanth P, Orwoll ES. Obesity and fracture in men and women: an epidemiologic perspective. J Bone Miner Res. 2012;27:1–10. doi: 10.1002/jbmr.1486. [DOI] [PubMed] [Google Scholar]
- 36.Owusu W, Willett W, Ascherio A, Spiegelman D, Rimm E, Feskanich D, Colditz G. Body anthropometry and the risk of hip and wrist fractures in men: results from a prospective study. Obesity Res. 1998;6:12–9. doi: 10.1002/j.1550-8528.1998.tb00309.x. [DOI] [PubMed] [Google Scholar]
- 37.Cao JJ, Sun L, Gao H. Diet-induced obesity alters bone remodeling leading to decreased femoral trabecular bone mass in mice. Ann N Y Acad Sci. 2010;1192:292–7. doi: 10.1111/j.1749-6632.2009.05252.x. [DOI] [PubMed] [Google Scholar]
- 38.Halade GV, Rahman MM, Williams PJ, Fernandes G. High fat diet-induced animal model of age-associated obesity and osteoporosis. J Nutr Biochem. 2010;21:1162–9. doi: 10.1016/j.jnutbio.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 40.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary Cardiol Rev. 2005;13:322–7. [PubMed] [Google Scholar]
- 41.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44:720–32. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Ford ES, Abbasi F, Reaven GM. Prevalence of insulin resistance and the metabolic syndrome with alternative definitions of impaired fasting glucose. Atherosclerosis. 2005;181:143–8. doi: 10.1016/j.atherosclerosis.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. J Am Med Assoc. 2002;287:356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 44.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 45.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 46.Goodpaster BH, Krishnaswami S, Resnick H, Kelley DE, Haggerty C, Harris TB, Schwartz AV, Kritchevsky S, Newman AB. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26:372–9. doi: 10.2337/diacare.26.2.372. [DOI] [PubMed] [Google Scholar]
- 47.Mori Y, Hoshino K, Yokota K, Itoh Y, Tajima N. Differences in the pathology of the metabolic syndrome with or without visceral fat accumulation: a study in pre-diabetic Japanese middle-aged men. Endocrine. 2006;29:149–53. doi: 10.1385/endo:29:1:149. [DOI] [PubMed] [Google Scholar]
- 48.Baum T, Yap SP, Karampinos DC, Nardo L, Kuo D, Burghardt AJ, Masharani UB, Schwartz AV, Li X, Link TM. Does vertebral bone marrow fat content correlate with abdominal adipose tissue, lumbar spine bone mineral density, and blood biomarkers in women with type 2 diabetes mellitus? J Magn Reson Imaging. 2012;35:117–24. doi: 10.1002/jmri.22757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheu Y, Cauley JA. The role of bone marrow and visceral fat on bone metabolism. Curr Osteoporos Rep. 2011;9:67–75. doi: 10.1007/s11914-011-0051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Botolin S, McCabe LR. Bone loss and increased bone adiposity in spontaneous and pharmacologically induced diabetic mice. Endocrinology. 2007;148:198–205. doi: 10.1210/en.2006-1006. [DOI] [PubMed] [Google Scholar]
- 51.Schwartz AV, Sellmeyer DE. Diabetes, fracture, and bone fragility. Curr Osteoporos Rep. 2007;5:105–11. doi: 10.1007/s11914-007-0025-x. [DOI] [PubMed] [Google Scholar]
- 52.Strotmeyer ES, Cauley JA. Diabetes mellitus, bone mineral density, and fracture risk. Curr Opin Endocrinol Diabetes Obes. 2007;14:429–35. doi: 10.1097/MED.0b013e3282f1cba3. [DOI] [PubMed] [Google Scholar]
- 53.Isidro ML, Ruano B. Bone disease in diabetes. Curr Diabetes Rev. 2010;6:144–55. doi: 10.2174/157339910791162970. [DOI] [PubMed] [Google Scholar]
- 54.Lee P, van der Wall H, Seibel MJ. Looking beyond low bone mineral density: multiple insufficiency fractures in a woman with post-menopausal osteoporosis on alendronate therapy. J Endocrinol Invest. 2007;30:590–7. doi: 10.1007/BF03346353. [DOI] [PubMed] [Google Scholar]
- 55.Takizawa M, Suzuki K, Matsubayashi T, Kikuyama M, Suzuki H, Takahashi K, Katsuta H, Mitsuhashi J, Nishida S, Yamaguchi S, Yoshimoto K, Itagaki E, Ishida H. Increased bone resorption may play a crucial role in the occurrence of osteopenia in patients with type 2 diabetes: Possible involvement of accelerated polyol pathway in its pathogenesis. Diabetes Res Clin Pract. 2008;82:119–26. doi: 10.1016/j.diabres.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 56.Duarte VM, Ramos AM, Rezende LA, Macedo UB, Brandao-Neto J, Almeida MG, Rezende AA. Osteopenia: a bone disorder associated with diabetes mellitus. J Bone Miner Metab. 2005;23:58–68. doi: 10.1007/s00774-004-0542-y. [DOI] [PubMed] [Google Scholar]
- 57.Hie M, Shimono M, Fujii K, Tsukamoto I. Increased cathepsin K and tartrate-resistant acid phosphatase expression in bone of streptozotocin-induced diabetic rats. Bone. 2007;41:1045–50. doi: 10.1016/j.bone.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 58.Pittas AG, Harris SS, Eliades M, Stark P, Dawson-Hughes B. Association between serum osteocalcin and markers of metabolic phenotype. J Clin Endocrinol Metab. 2009;94:827–32. doi: 10.1210/jc.2008-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saleem U, Mosley TH, Jr, Kullo IJ. Serum osteocalcin is associated with measures of insulin resistance, adipokine levels, and the presence of metabolic syndrome. Arterioscler Thromb Vasc Biol. 2010;30:1474–8. doi: 10.1161/ATVBAHA.110.204859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gannage-Yared MH, Fares F, Semaan M, Khalife S, Jambart S. Circulating osteoprotegerin is correlated with lipid profile, insulin sensitivity, adiponectin and sex steroids in an ageing male population. Clin Endocrinol. 2006;64:652–8. doi: 10.1111/j.1365-2265.2006.02522.x. [DOI] [PubMed] [Google Scholar]
- 61.Lazarenko OP, Rzonca SO, Hogue WR, Swain FL, Suva LJ, Lecka-Czernik B. Rosiglitazone induces decreases in bone mass and strength that are reminiscent of aged bone. Endocrinology. 2007;148:2669–80. doi: 10.1210/en.2006-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patsch JM, Li X, Baum T, Yap SP, Karampinos DC, Schwartz AV, Link TM. Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. J Bone Miner Res. 2013;28:1721–8. doi: 10.1002/jbmr.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–56. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 64.Diez JJ, Iglesias P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol. 2003;148:293–300. doi: 10.1530/eje.0.1480293. [DOI] [PubMed] [Google Scholar]
- 65.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocrine Rev. 2005;26:439–51. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 66.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci USA. 2004;101:10308–13. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tomas E, Tsao T-S, Saha AK, Murrey HE, Zhang Cc, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA. 2002;99:16309–13. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 69.Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J, Takata M, Eto K, Terauchi Y, Komeda K, Tsunoda M, Murakami K, Ohnishi Y, Naitoh T, Yamamura K, Ueyama Y, Froguel P, Kimura S, Nagai R, Kadowaki T. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278:2461–8. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- 70.Liu M, Liu F. Up- and down-regulation of adiponectin expression and multimerization: mechanisms and therapeutic implication. Biochimie. 2012;94:2126–30. doi: 10.1016/j.biochi.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ryan AS, Berman DM, Nicklas BJ, Sinha M, Gingerich RL, Meneilly GS, Egan JM, Elahi D. Plasma adiponectin and leptin levels, body composition, and glucose utilization in adult women with wide ranges of age and obesity. Diabetes Care. 2003;26:2383–8. doi: 10.2337/diacare.26.8.2383. [DOI] [PubMed] [Google Scholar]
- 72.Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, Ning X, Bree AJ, Schell B, Broome DT, Soliman SS, DelProposto JL, Lumeng CN, Mitra A, Pandit SV, Gallagher KA, Miller JD, Krishnan V, Hui SK, Bredella MA, Fazeli PK, Klibanski A, Horowitz MC, Rosen CJ, MacDougald OA. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014;20:368–75. doi: 10.1016/j.cmet.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–9. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 74.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–5. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 75.Spranger J, Kroke A, Möhlig M, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361:226–8. doi: 10.1016/S0140-6736(03)12255-6. [DOI] [PubMed] [Google Scholar]
- 76.Iwashima Y, Katsuya T, Ishikawa K, Ouchi N, Ohishi M, Sugimoto K, Fu Y, Motone M, Yamamoto K, Matsuo A, Ohashi K, Kihara S, Funahashi T, Rakugi H, Matsuzawa Y, Ogihara T. Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension. 2004;43:1318–23. doi: 10.1161/01.HYP.0000129281.03801.4b. [DOI] [PubMed] [Google Scholar]
- 77.Williams KW, Scott MM, Elmquist JK. From observation to experimentation: leptin action in the mediobasal hypothalamus. Am J Clin Nutr. 2009;89:985S–90S. doi: 10.3945/ajcn.2008.26788D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schwartz MW, Woods SC, Porte D, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 79.Myers MG. Leptin receptor signaling and the regulation of mammalian physiology. Recent Prog Horm Res. 2004;59:287–304. doi: 10.1210/rp.59.1.287. [DOI] [PubMed] [Google Scholar]
- 80.Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM, Basdevant A, Bougneres P, Lebouc Y, Froguel P, Guy-Grand B. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 81.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. New Engl J Med. 1996;334:292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 82.Garofalo C, Surmacz E. Leptin and cancer. J Cell Physiol. 2006;207:12–22. doi: 10.1002/jcp.20472. [DOI] [PubMed] [Google Scholar]
- 83.Zheng Q, Banaszak L, Fracci S, Basali D, Dunlap SM, Hursting SD, Rich JN, Hjlemeland AB, Vasanji A, Berger NA, Lathia JD, Reizes O. Leptin receptor maintains cancer stem-like properties in triple negative breast cancer cells. Endocr Relat Cancer. 2013;20:797–808. doi: 10.1530/ERC-13-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Neels JG, Olefsky JM. Inflamed fat: what starts the fire? J Clin Invest. 2006;116:33–5. doi: 10.1172/JCI27280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, Bouloumie A, Barbatelli G, Cinti S, Svensson PA, Barsh GS, Zucker JD, Basdevant A, Langin D, Clement K. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–86. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 86.Sun S, Ji Y, Kersten S, Qi L. Mechanisms of inflammatory responses in obese adipose tissue. Annu Rev Nutr. 2012;32:261–86. doi: 10.1146/annurev-nutr-071811-150623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity- related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. 2013:139239. doi: 10.1155/2013/139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nguyen MT, Satoh H, Favelyukis S, Babendure JL, Imamura T, Sbodio JI, Zalevsky J, Dahiyat BI, Chi NW, Olefsky JM. JNK and tumor necrosis factor-alpha mediate free fatty acid-induced insulin resistance in 3T3-L1 adipocytes. J Biol Chem. 2005;280:35361–71. doi: 10.1074/jbc.M504611200. [DOI] [PubMed] [Google Scholar]
- 90.Souza SC, Palmer HJ, Kang YH, Yamamoto MT, Muliro KV, Paulson KE, Greenberg AS. TNF-alpha induction of lipolysis is mediated through activation of the extracellular signal related kinase pathway in 3T3-L1 adipocytes. J Cell Biochem. 2003;89:1077–86. doi: 10.1002/jcb.10565. [DOI] [PubMed] [Google Scholar]
- 91.Hivert MF, Sullivan LM, Fox CS, Nathan DM, D’Agostino RB, Sr, Wilson PW, Meigs JB. Associations of adiponectin, resistin, and tumor necrosis factor-alpha with insulin resistance. J Clin Endocrinol Metab. 2008;93:3165–72. doi: 10.1210/jc.2008-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang B, Wood IS, Trayhurn P. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Archiv. 2007;455:479–92. doi: 10.1007/s00424-007-0301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol. 2001;280:E745–51. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 95.Yadav A, Kumar B, Datta J, Teknos TN, Kumar P. IL-6 promotes head and neck tumor metastasis by inducing epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling pathway. Mol Cancer Res. 2011;9:1658–67. doi: 10.1158/1541-7786.MCR-11-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lagathu C, Yvan-Charvet L, Bastard JP, Maachi M, Quignard-Boulange A, Capeau J, Caron M. Long-term treatment with interleukin-1beta induces insulin resistance in murine and human adipocytes. Diabetologia. 2006;49:2162–73. doi: 10.1007/s00125-006-0335-z. [DOI] [PubMed] [Google Scholar]
- 97.Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–26. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 98.Martin S, Parton RG. Lipid droplets: a unified view of a dynamic organelle. Nat Rev Mol Cell Biol. 2006;7:373–8. doi: 10.1038/nrm1912. [DOI] [PubMed] [Google Scholar]
- 99.Rajala MW, Scherer PE. Minireview: The adipocyte–at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765–73. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- 100.Langin D. Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacol Res. 2006;53:482–91. doi: 10.1016/j.phrs.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 101.Lafontan M, Berlan M. Characterization of physiological agonist selectivity of human fat cell alpha 2-adrenoceptors: adrenaline is the major stimulant of the alpha 2-adrenoceptors. Eur J Pharmacol. 1982;82:107–11. doi: 10.1016/0014-2999(82)90562-3. [DOI] [PubMed] [Google Scholar]
- 102.Honnor RC, Dhillon GS, Londos C. cAMP-dependent protein kinase and lipolysis in rat adipocytes. I. Cell preparation, manipulation, and predictability in behavior. J Biol Chem. 1985;260:15122–9. [PubMed] [Google Scholar]
- 103.Honnor RC, Dhillon GS, Londos C. cAMP-dependent protein kinase and lipolysis in rat adipocytes. II. Definition of steady-state relationship with lipolytic and antilipolytic modulators. J Biol Chem. 1985;260:15130–8. [PubMed] [Google Scholar]
- 104.Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem. 1991;266:11341–6. [PubMed] [Google Scholar]
- 105.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–6. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 106.Haemmerle G, Zimmermann R, Hayn M, Theussl C, Waeg G, Wagner E, Sattler W, Magin TM, Wagner EF, Zechner R. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J Biol Chem. 2002;277:4806–15. doi: 10.1074/jbc.M110355200. [DOI] [PubMed] [Google Scholar]
- 107.Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S, Kratky D, Wagner EF, Klingenspor M, Hoefler G, Zechner R. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–7. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 108.Kershaw EE, Hamm JK, Verhagen LA, Peroni O, Katic M, Flier JS. Adipose triglyceride lipase: function, regulation by insulin, and comparison with adiponutrin. Diabetes. 2006;55:148–57. [PMC free article] [PubMed] [Google Scholar]
- 109.Miyoshi H, Perfield JW, Souza SC, Shen W-J, Zhang H-H, Stancheva ZS, Kraemer FB, Obin MS, Greenberg AS. Control of adipose triglyceride lipase action by serine 517 of perilipin A globally regulates protein kinase A-stimulated lipolysis in adipocytes. J Biol Chem. 2007;282:996–1002. doi: 10.1074/jbc.M605770200. [DOI] [PubMed] [Google Scholar]
- 110.Akiyama M, Sakai K, Ogawa M, McMillan JR, Sawamura D, Shimizu H. Novel duplication mutation in the patatin domain of adipose triglyceride lipase (PNPLA2) in neutral lipid storage disease with severe myopathy. Muscle Nerve. 2007;36:856–9. doi: 10.1002/mus.20869. [DOI] [PubMed] [Google Scholar]
- 111.Fischer J, Lefèvre C, Morava E, Mussini J-M, Laforêt P, Negre-Salvayre A, Lathrop M, Salvayre R. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat Genet. 2007;39:28–30. doi: 10.1038/ng1951. [DOI] [PubMed] [Google Scholar]
- 112.Kobayashi K, Inoguchi T, Maeda Y, Nakashima N, Kuwano A, Eto E, Ueno N, Sasaki S, Sawada F, Fujii M, Matoba Y, Sumiyoshi S, Kawate H, Takayanagi R. The lack of the C-terminal domain of adipose triglyceride lipase causes neutral lipid storage disease through impaired interactions with lipid droplets. J Clin Endocrinol Metab. 2008;93:2877–84. doi: 10.1210/jc.2007-2247. [DOI] [PubMed] [Google Scholar]
- 113.Zechner R, Kienesberger PC, Haemmerle G, Zimmermann R, Lass A. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J Lipid Res. 2009;50:3–21. doi: 10.1194/jlr.R800031-JLR200. [DOI] [PubMed] [Google Scholar]
- 114.Schiffelers SL, Saris WH, Boomsma F, van Baak MA. beta(1)- and beta(2)-Adrenoceptor-mediated thermogenesis and lipid utilization in obese and lean men. J Clin Endocrinol Metab. 2001;86:2191–9. doi: 10.1210/jcem.86.5.7506. [DOI] [PubMed] [Google Scholar]
- 115.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. J Am Med Assoc. 2014;311:806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJ, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang X, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon SJ, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJ, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 2014. The Lancet. 2014;384:766–81. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Calle E, Thun M. Obesity and cancer. Oncogene. 2004;23:6365–78. doi: 10.1038/sj.onc.1207751. [DOI] [PubMed] [Google Scholar]
- 118.Gong Z, Agalliu I, Lin DW, Stanford JL, Kristal AR. Obesity is associated with increased risks of prostate cancer metastasis and death after initial cancer diagnosis in middle-aged men. Cancer. 2007;109:1192–202. doi: 10.1002/cncr.22534. [DOI] [PubMed] [Google Scholar]
- 119.Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist. 2010;15:556–65. doi: 10.1634/theoncologist.2009-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D Million Women Study C. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. Br Med J. 2007;335:1314. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gibson TM, Park Y, Robien K, Shiels MS, Black A, Sampson JN, Purdue MP, Beane Freeman LE, Andreotti G, Weinstein SJ, Albanes D, Fraumeni JF, Jr, Curtis RE, Berrington de Gonzalez A, Morton LM. Body mass index and risk of second obesity-associated cancers after colorectal cancer: a pooled analysis of prospective cohort studies. J Clin Oncol. 2014;32:4004–11. doi: 10.1200/JCO.2014.56.8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kaidar-Person O, Bar-Sela G, Person B. The two major epidemics of the twenty-first century: obesity and cancer. Obes Surg. 2011;21:1792–7. doi: 10.1007/s11695-011-0490-2. [DOI] [PubMed] [Google Scholar]
- 123.Kitahara CM, Platz EA, Freeman LE, Hsing AW, Linet MS, Park Y, Schairer C, Schatzkin A, Shikany JM, Berrington de González A. Obesity and thyroid cancer risk among U.S. men and women: a pooled analysis of five prospective studies. Cancer Epidemiol Biomarkers Prev. 2011;20:464–72. doi: 10.1158/1055-9965.EPI-10-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lichtman MA. Obesity and the risk for a hematological malignancy: leukemia, lymphoma, or myeloma. Oncologist. 2010;15:1083–101. doi: 10.1634/theoncologist.2010-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–5S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 126.Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta. 2013;1831:1533–41. doi: 10.1016/j.bbalip.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vona-Davis L, Rose DP. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocr Relat Cancer. 2007;14:189–206. doi: 10.1677/ERC-06-0068. [DOI] [PubMed] [Google Scholar]
- 128.Hoon Kim J, Lee SY, Myung SC, Kim YS, Kim T-H, Kim MK. Clinical significance of the leptin and leptin receptor expressions in prostate tissues. Asian J Androl. 2008;10:923–8. doi: 10.1111/j.1745-7262.2008.00438.x. [DOI] [PubMed] [Google Scholar]
- 129.Tewari R, Rajender S, Natu SM, Goel A, Dalela D, Goel MM, Tondon P. Significance of obesity markers and adipocytokines in high grade and high stage prostate cancer in North Indian men – a cross-sectional study. Cytokine. 2013;63:130–4. doi: 10.1016/j.cyto.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 130.Ribeiro R, Vasconcelos A, Costa S, Pinto D, Morais A, Oliveira J, Lobo F, Lopes C, Medeiros R. Overexpressing leptin genetic polymorphism (?.2548 G/A) is associated with susceptibility to prostate cancer and risk of advanced disease. Prostate. 2004;59:268–74. doi: 10.1002/pros.20004. [DOI] [PubMed] [Google Scholar]
- 131.Margetic S, Gazzola C, Pegg GG, Hill RA. Leptin: a review of its peripheral actions and interactions. Int J Obes Relat Metab Disord. 2002;26:1407–33. doi: 10.1038/sj.ijo.0802142. [DOI] [PubMed] [Google Scholar]
- 132.Muoio DM, Lynis Dohm G. Peripheral metabolic actions of leptin. Best Pract Res Clin Endocrinol Metab. 2002;16:653–66. doi: 10.1053/beem.2002.0223. [DOI] [PubMed] [Google Scholar]
- 133.Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga-Koda L, Golaszewska J, Russo A, Sulkowski S, Surmacz E. Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin Cancer Res. 2006;12:1447–53. doi: 10.1158/1078-0432.CCR-05-1913. [DOI] [PubMed] [Google Scholar]
- 134.Ishikawa M, Kitayama J, Nagawa H. Enhanced expression of leptin and leptin receptor (OB-R) in human breast cancer. Clin Cancer Res. 2004;10:4325–31. doi: 10.1158/1078-0432.CCR-03-0749. [DOI] [PubMed] [Google Scholar]
- 135.Dong Z, Fu S, Xu X, Yang Y, Du L, Li W, Kan S, Li Z, Zhang X, Wang L, Li J, Liu H, Qu X, Wang C. Leptin-mediated regulation of ICAM-1 is Rho/ROCK dependent and enhances gastric cancer cell migration. Br J Cancer. 2014;110:1801–10. doi: 10.1038/bjc.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yehuda-Shnaidman E, Nimri L, Tarnovscki T, Kirshtein B, Rudich A, Schwartz B. Secreted human adipose leptin decreases mitochondrial respiration in HCT116 colon cancer cells. PLoS ONE. 2013;8:e74843. doi: 10.1371/journal.pone.0074843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: a review of current evidence. Endocrine Rev. 2012;33:547–94. doi: 10.1210/er.2011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Goktas S, Yilmaz MI, Caglar K, Sonmez A, Kilic S, Bedir S. Prostate cancer and adiponectin. Urology. 2005;65:1168–72. doi: 10.1016/j.urology.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 139.Brakenhielm E, Veitonmaki N, Cao R, Kihara S, Matsuzawa Y, Zhivotovsky B, Funahashi T, Cao Y. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci USA. 2004;101:2476–81. doi: 10.1073/pnas.0308671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, Kihara S, Funahashi T, Tenner AJ, Tomiyama Y, Matsuzawa Y. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–32. [PubMed] [Google Scholar]
- 141.Kang JH, Lee YY, Yu BY, Yang B-S, Cho K-H, Yoon DK, Roh YK. Adiponectin induces growth arrest and apoptosis of MDA-MB-231 breast cancer cell. Arch Pharm Res. 2005;28:1263–9. doi: 10.1007/BF02978210. [DOI] [PubMed] [Google Scholar]
- 142.Dieudonne M-N, Bussiere M, Dos Santos E, Leneveu M-C, Giudicelli Y, Pecquery R. Adiponectin mediates antiproliferative and apoptotic responses in human MCF7 breast cancer cells. Biochem Biophys Res Commun. 2006;345:271–9. doi: 10.1016/j.bbrc.2006.04.076. [DOI] [PubMed] [Google Scholar]
- 143.Bub JD, Miyazaki T, Iwamoto Y. Adiponectin as a growth inhibitor in prostate cancer cells. Biochem Biophys Res Commun. 2006;340:1158–66. doi: 10.1016/j.bbrc.2005.12.103. [DOI] [PubMed] [Google Scholar]
- 144.Suzuki S, Wilson-Kubalek EM, Wert D, Tsao TS, Lee DH. The oligomeric structure of high molecular weight adiponectin. FEBS Lett. 2007;581:809–14. doi: 10.1016/j.febslet.2007.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 146.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 147.Vucenik I, Stains JP. Obesity and cancer risk: evidence, mechanisms, and recommendations. Ann N Y Acad Sci. 2012;1271:37–43. doi: 10.1111/j.1749-6632.2012.06750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Herroon MK, Rajagurubandara E, Hardaway AL, Powell K, Turchick A, Feldmann D, Podgorski I. Bone marrow adipocytes promote tumor growth in bone via FABP4-dependent mechanisms. Oncotarget. 2013;4:2108–23. doi: 10.18632/oncotarget.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gazi E, Gardner P, Lockyer NP, Hart CA, Brown MD, Clarke NW. Direct evidence of lipid translocation between adipocytes and prostate cancer cells with imaging FTIR microspectroscopy. J Lipid Res. 2007;48:1846–56. doi: 10.1194/jlr.M700131-JLR200. [DOI] [PubMed] [Google Scholar]
- 150.Tokuda Y, Satoh Y, Fujiyama C, Toda S, Sugihara H, Masaki Z. Prostate cancer cell growth is modulated by adipocyte-cancer cell interaction. BJU Int. 2003;91:716–20. doi: 10.1046/j.1464-410x.2003.04218.x. [DOI] [PubMed] [Google Scholar]
- 151.Brown MD, Hart CA, Gazi E, Bagley S, Clarke NW. Promotion of prostatic metastatic migration towards human bone marrow stoma by Omega 6 and its inhibition by Omega 3 PUFAs. Br J Cancer. 2006;94:842–53. doi: 10.1038/sj.bjc.6603030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hardaway AL, Podgorski I. IL-1beta, RAGE and FABP4: targeting the dynamic trio in metabolic inflammation and related pathologies. Future Med Chem. 2013;5:1089–108. doi: 10.4155/fmc.13.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB, Hotamisligil GS, Yamada SD, Peter ME, Gwin K, Lengyel E. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu Y. Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer Prostatic Dis. 2006;9:230–4. doi: 10.1038/sj.pcan.4500879. [DOI] [PubMed] [Google Scholar]
- 155.Akram M. Mini-review on glycolysis and cancer. J Cancer Educ. 2013;28:454–7. doi: 10.1007/s13187-013-0486-9. [DOI] [PubMed] [Google Scholar]
- 156.Jones RG, Thompson CB. Tumor suppressors and cell metabolism: a recipe for cancer growth. Genes Dev. 2009;23:537–48. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 158.Baron A, Migita T, Tang D, Loda M. Fatty acid synthase: a metabolic oncogene in prostate cancer? J Cell Biochem. 2004;91:47–53. doi: 10.1002/jcb.10708. [DOI] [PubMed] [Google Scholar]
- 159.Brown GC. Control of respiration and ATP synthesis in mammalian mitochondria and cells. Biochem J. 1992;284:1–13. doi: 10.1042/bj2840001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 161.Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010;330:1340–4. doi: 10.1126/science.1193494. [DOI] [PubMed] [Google Scholar]
- 162.Kim J-W, Dang CV. Multifaceted roles of glycolytic enzymes. Trends Biochem Sci. 2005;30:142–50. doi: 10.1016/j.tibs.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 163.Majewski N, Nogueira V, Bhaskar P, Coy PE, Skeen JE, Gottlob K, Chandel NS, Thompson CB, Robey RB, Hay N. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol Cell. 2004;16:819–30. doi: 10.1016/j.molcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 164.Pastorino JG, Shulga N, Hoek JB. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J Biol Chem. 2002;277:7610–8. doi: 10.1074/jbc.M109950200. [DOI] [PubMed] [Google Scholar]
- 165.Sun L, Shukair S, Naik TJ, Moazed F, Ardehali H. Glucose phosphorylation and mitochondrial binding are required for the protective effects of hexokinases I and II. Mol Cell Biol. 2008;28:1007–17. doi: 10.1128/MCB.00224-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tamada M, Suematsu M, Saya H. Pyruvate kinase M2: multiple faces for conferring benefits on cancer cells. Clin Cancer Res. 2012;18:5554–61. doi: 10.1158/1078-0432.CCR-12-0859. [DOI] [PubMed] [Google Scholar]
- 167.Lu Z. Nonmetabolic functions of pyruvate kinase isoform M2 in controlling cell cycle progression and tumorigenesis. Chin J Cancer. 2012;31:5–7. doi: 10.5732/cjc.011.10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Matsumoto S, Kishida K, Shimomura I, Maeda N, Nagaretani H, Matsuda M, Nishizawa H, Kihara S, Funahashi T, Matsuzawa Y, Yamada A, Yamashita S, Tamura S, Kawata S. Increased plasma HB-EGF associated with obesity and coronary artery disease. Biochem Biophys Res Commun. 2002;292:781–6. doi: 10.1006/bbrc.2002.6720. [DOI] [PubMed] [Google Scholar]
- 169.Henriksen L, Grandal MV, Knudsen SLJ, van Deurs B, Grøvdal LM. Internalization mechanisms of the epidermal growth factor receptor after activation with different ligands. PLoS One. 2013;8:e58148. doi: 10.1371/journal.pone.0058148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yang W, Xia Y, Hawke D, Li X, Liang J, Xing D, Aldape K, Hunter T, Alfred Yung WK, Lu Z. PKM2 phosphorylates histone H3 and promotes gene transcription and tumorigenesis. Cell. 2012;150:685–96. doi: 10.1016/j.cell.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lee J, Kim HK, Han Y-M, Kim J. Pyruvate kinase isozyme type M2 (PKM2) interacts and cooperates with Oct-4 in regulating transcription. Int J Biochem Cell Biol. 2008;40:1043–54. doi: 10.1016/j.biocel.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 172.Morfouace M, Lalier L, Oliver L, Cheray M, Pecqueur C, Cartron PF, Vallette FM. Control of lioma cell death and differentiation by PKM2-Oct4 interaction. Cell Death Dis. 2014;5:e1036. doi: 10.1038/cddis.2013.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 174.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–9. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 175.Cairns RA, Bennewith KL, Graves EE, Giaccia AJ, Chang DT, Denko NC. Pharmacologically increased tumor hypoxia can be measured by 18F-Fluoroazomycin arabinoside positron emission tomography and enhances tumor response to hypoxic cytotoxin PR-104. Clin Cancer Res. 2009;15:7170–4. doi: 10.1158/1078-0432.CCR-09-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Michelakis ED, Sutendra G, Dromparis P, Webster L, Haromy A, Niven E, Maguire C, Gammer TL, Mackey JR, Fulton D, Abdulkarim B, McMurtry MS, Petruk KC. Metabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med. 2010;2:31ra34. doi: 10.1126/scitranslmed.3000677. [DOI] [PubMed] [Google Scholar]
- 177.Soga T. Cancer metabolism: key players in metabolic reprogramming. Cancer Sci. 2013;104:275–81. doi: 10.1111/cas.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Husain Z, Huang Y, Seth P, Sukhatme VP. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J Immunol. 2013;191:1486–95. doi: 10.4049/jimmunol.1202702. [DOI] [PubMed] [Google Scholar]
- 179.Beckert S, Farrahi F, Aslam RS, Scheuenstuhl H, Konigsrainer A, Hussain MZ, Hunt TK. Lactate stimulates endothelial cell migration. Wound Repair Regen. 2006;14:321–4. doi: 10.1111/j.1743-6109.2006.00127.x. [DOI] [PubMed] [Google Scholar]
- 180.Polet F, Feron O. Endothelial cell metabolism and tumour angiogenesis: glucose and glutamine as essential fuels and lactate as the driving force. J Intern Med. 2013;273:156–65. doi: 10.1111/joim.12016. [DOI] [PubMed] [Google Scholar]
- 181.Shim H, Dolde C, Lewis BC, Wu CS, Dang G, Jungmann RA, Dalla-Favera R, Dang CV. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc Natl Acad Sci USA. 1997;94:6658–63. doi: 10.1073/pnas.94.13.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kim Jw, Zeller KI, Wang Y, Jegga AG, Aronow BJ, O’Donnell KA, Dang CV. Evaluation of Myc E-Box phylogenetic footprints in glycolytic genes by chromatin immunoprecipitation assays. Mol Cell Biol. 2004;24:5923–36. doi: 10.1128/MCB.24.13.5923-5936.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mannava S, Grachtchouk V, Wheeler LJ, Im M, Zhuang D, Slavina EG, Mathews CK, Shewach DS, Nikiforov MA. Direct role of nucleotide metabolism in C-MYC-dependent proliferation of melanoma cells. Cell Cycle. 2008;7:2392–400. doi: 10.4161/cc.6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liu Y-C, Li F, Handler J, Huang CRL, Xiang Y, Neretti N, Sedivy JM, Zeller KI, Dang CV. Global regulation of nucleotide biosynthetic genes by c-Myc. PLoS One. 2008;3:e2722. doi: 10.1371/journal.pone.0002722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.David CJ, Chen M, Assanah M, Canoll P, Manley JL. HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature. 2010;463:364–8. doi: 10.1038/nature08697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975–2011. Bethesda, MD: National Cancer Institute; Apr, 2014. http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission, posted to the SEER web site. [Google Scholar]
- 187.Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–3. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 188.Clower CV, Chatterjee D, Wang Z, Cantley LC, Vander Heiden MG, Krainer AR. The alternative splicing repressors hnRNP A1/A2 and PTB influence pyruvate kinase isoform expression and cell metabolism. Proc Natl Acad Sci USA. 2010;107:1894–9. doi: 10.1073/pnas.0914845107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wong N, De Melo J, Tang D. PKM2, a central point of regulation in cancer metabolism. Int J Cell Biol. 2013;2013:1–11. doi: 10.1155/2013/242513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vita M, Henriksson M. The Myc oncoprotein as a therapeutic target for human cancer. Semin Cancer Biol. 2006;16:318–30. doi: 10.1016/j.semcancer.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 191.Liu H, Radisky DC, Yang D, Xu R, Radisky ES, Bissell MJ, Bishop JM. MYC suppresses cancer metastasis by direct transcriptional silencing of alphav and beta3 integrin subunits. Nat Cell Biol. 2012;14:567–74. doi: 10.1038/ncb2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Boland ML, Chourasia AH, Macleod KF. Mitochondrial dysfunction in cancer. Front Oncol. 2013;3:292. doi: 10.3389/fonc.2013.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kaaman M, Sparks LM, van Harmelen V, Smith SR, Sjolin E, Dahlman I, Arner P. Strong association between mitochondrial DNA copy number and lipogenesis in human white adipose tissue. Diabetologia. 2007;50:2526–33. doi: 10.1007/s00125-007-0818-6. [DOI] [PubMed] [Google Scholar]
- 194.Compton S, Kim C, Griner NB, Potluri P, Scheffler IE, Sen S, Jerry DJ, Schneider S, Yadava N. Mitochondrial dysfunction impairs tumor suppressor p53 expression/function. J Biol Chem. 2011;286:20297–312. doi: 10.1074/jbc.M110.163063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bournat JC, Brown CW. Mitochondrial dysfunction in obesity. Curr Opin Endocrinol Diabetes Obes. 2010;17:446–52. doi: 10.1097/MED.0b013e32833c3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Robey IF, Lien AD, Welsh SJ, Baggett BK, Gillies RJ. Hypoxia-inducible factor-1alpha and the glycolytic phenotype in tumors. Neoplasia. 2005;7:324–30. doi: 10.1593/neo.04430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Benizri E, Ginouves A, Berra E. The magic of the hypoxia-signaling cascade. Cell Mol Life Sci. 2008;65:1133–49. doi: 10.1007/s00018-008-7472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bruick RK. Oxygen sensing in the hypoxic response pathway: regulation of the hypoxia-inducible transcription factor. Genes Dev. 2003;17:2614–23. doi: 10.1101/gad.1145503. [DOI] [PubMed] [Google Scholar]
- 199.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 200.Ogawa K, Chiba I, Morioka T, Shimoji H, Tamaki W, Takamatsu R, Nishimaki T, Yoshimi N, Murayama S. Clinical significance of HIF-1alpha expression in patients with esophageal cancer treated with concurrent chemoradiotherapy. Anticancer Res. 2011;31:2351–9. [PubMed] [Google Scholar]
- 201.Vaupel P, Mayer A, Höckel M. Tumor hypoxia and malignant progression. Meth Enzymol. 2004;381:335–54. doi: 10.1016/S0076-6879(04)81023-1. [DOI] [PubMed] [Google Scholar]
- 202.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and herapeutics. Oncogene. 2010;29:625–34. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Semenza GL. HIF-1 mediates metabolic responses to intra-tumoral hypoxia and oncogenic mutations. J Clin Invest. 2013;123:3664–71. doi: 10.1172/JCI67230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361–74. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Luo W, Hu H, Chang R, Zhong J, Knabel M, O’Meally R, Cole Robert N, Pandey A, Semenza GL. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732–44. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wang H-J, Hsieh Y-J, Cheng W-C, Lin C-P, Lin Y-s, Yang S-F, Chen C-C, Izumiya Y, Yu J-S, Kung H-J, Wang W-C. JMJD5 regulates PKM2 nuclear translocation and reprograms HIF-1α-mediated glucose metabolism. Proc Natl Acad Sci USA. 2014;111:279–84. doi: 10.1073/pnas.1311249111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hsia DA, Tepper CG, Pochampalli MR, Hsia EYC, Izumiya C, Huerta SB, Wright ME, Chen H-W, Kung H-J, Izumiya Y. KDM8, a H3K36me2 histone demethylase that acts in the cyclin A1 coding region to regulate cancer cell proliferation. Proc Natl Acad Sci USA. 2010;107:9671–6. doi: 10.1073/pnas.1000401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ivan M, Harris AL, Martelli F, Kulshreshtha R. Hypoxia response and microRNAs: no longer two separate worlds. J Cell Mol Med. 2008;12:1426–31. doi: 10.1111/j.1582-4934.2008.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Macfarlane LA, Murphy PR. MicroRNA: biogenesis, function and role in cancer. Curr Genom. 2010;11:537–61. doi: 10.2174/138920210793175895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Devlin C, Greco S, Martelli F, Ivan M. miR-210: More than a silent player in hypoxia. IUBMB Life. 2011;63:94–100. doi: 10.1002/iub.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chan SY, Zhang Y-Y, Hemann C, Mahoney CE, Zweier JL, Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab. 2009;10:273–84. doi: 10.1016/j.cmet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chen Z, Li Y, Zhang H, Huang P, Luthra R. Hypoxia-regulated microRNA-210 modulates mitochondrial function and decreases ISCU and COX10 expression. Oncogene. 2010;29:4362–8. doi: 10.1038/onc.2010.193. [DOI] [PubMed] [Google Scholar]
- 213.Grosso S, Doyen J, Parks SK, Bertero T, Paye A, Cardinaud B, Gounon P, Lacas-Gervais S, Noël A, Pouysségur J, Barbry P, Mazure NM, Mari B. MiR-210 promotes a hypoxic phenotype and increases radioresistance in human lung cancer cell lines. Cell Death Dis. 2013;4:e544. doi: 10.1038/cddis.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Puisségur MP, Mazure NM, Bertero T, Pradelli L, Grosso S, Robbe-Sermesant K, Maurin T, Lebrigand K, Cardinaud B, Hofman V, Fourre S, Magnone V, Ricci JE, Pouysségur J, Gounon P, Hofman P, Barbry P, Mari B. miR-210 is overexpressed in late stages of lung cancer and mediates mitochondrial alterations associated with modulation of HIF-1 activity. Cell Death Differ. 2011;18:465–78. doi: 10.1038/cdd.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Camps C, Buffa FM, Colella S, Moore J, Sotiriou C, Sheldon H, Harris AL, Gleadle JM, Ragoussis J. hsa-miR-210 is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin Cancer Res. 2008;14:1340–8. doi: 10.1158/1078-0432.CCR-07-1755. [DOI] [PubMed] [Google Scholar]
- 216.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 217.Holmgren L, O’Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1:149–53. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- 218.Parangi S, O’Reilly M, Christofori G, Holmgren L, Grosfeld J, Folkman J, Hanahan D. Antiangiogenic therapy of transgenic mice impairs de novo tumor growth. Proc Natl Acad Sci USA. 1996;93:2002–7. doi: 10.1073/pnas.93.5.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–13. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhang P, Wang Y, Hui Y, Hu D, Wang H, Zhou J, Du H. Inhibition of VEGF expression by targeting HIF-1 alpha with small interference RNA in human RPE cells. Ophthalmologica. 2007;221:411–7. doi: 10.1159/000107502. [DOI] [PubMed] [Google Scholar]
- 221.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–11. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 222.Yin J, Gao Z, He Q, Zhou D, Guo Z, Ye J. Role of hypoxia in obesity-induced disorders of glucose and lipid metabolism in adipose tissue. Am J Physiol. 2009;296:E333–42. doi: 10.1152/ajpendo.90760.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58:718–25. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes. 2008;32:451–63. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- 225.Elias I, Franckhauser S, Ferre T, Vila L, Tafuro S, Munoz S, Roca C, Ramos D, Pujol A, Riu E, Ruberte J, Bosch F. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes. 2012;61:1801–13. doi: 10.2337/db11-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Liang Y, Brekken RA, Hyder SM. Vascular endothelial growth factor induces proliferation of breast cancer cells and inhibits the anti-proliferative activity of anti-hormones. Endocr Relat Cancer. 2006;13:905–19. doi: 10.1677/erc.1.01221. [DOI] [PubMed] [Google Scholar]
- 227.Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13:871–82. doi: 10.1038/nrc3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–9. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 229.Yuzefovych LV, Musiyenko SI, Wilson GL, Rachek LI. Mitochondrial DNA damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat diet-induced insulin resistance mice. PLoS One. 2013;8:e54059. doi: 10.1371/journal.pone.0054059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bi M, Naczki C, Koritzinsky M, Fels D, Blais J, Hu N, Harding H, Novoa I, Varia M, Raleigh J, Scheuner D, Kaufman RJ, Bell J, Ron D, Wouters BG, Koumenis C. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J. 2005;24:3470–81. doi: 10.1038/sj.emboj.7600777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Romero-Ramirez L, Cao H, Nelson D, Hammond E, Lee AH, Yoshida H, Mori K, Glimcher LH, Denko NC, Giaccia AJ, Le QT, Koong AC. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res. 2004;64:5943–7. doi: 10.1158/0008-5472.CAN-04-1606. [DOI] [PubMed] [Google Scholar]
- 232.Rouschop KM, van den Beucken T, Dubois L, Niessen H, Bussink J, Savelkouls K, Keulers T, Mujcic H, Landuyt W, Voncken JW, Lambin P, van der Kogel AJ, Koritzinsky M, Wouters BG. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J Clin Invest. 2010;120:127–41. doi: 10.1172/JCI40027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Mujcic H, Nagelkerke A, Rouschop KM, Chung S, Chaudary N, Span PN, Clarke B, Milosevic M, Sykes J, Hill RP, Koritzinsky M, Wouters BG. Hypoxic activation of the PERK/eIF2alpha arm of the unfolded protein response promotes metastasis through induction of LAMP3. Clin Cancer Res. 2013;19:6126–37. doi: 10.1158/1078-0432.CCR-13-0526. [DOI] [PubMed] [Google Scholar]
- 234.Martinon F. Targeting endoplasmic reticulum signaling pathways in cancer. Acta Oncol. 2012;51:822–30. doi: 10.3109/0284186X.2012.689113. [DOI] [PubMed] [Google Scholar]
- 235.Healy SJ, Gorman AM, Mousavi-Shafaei P, Gupta S, Samali A. Targeting the endoplasmic reticulum-stress response as an anticancer strategy. Eur J Pharmacol. 2009;625:234–46. doi: 10.1016/j.ejphar.2009.06.064. [DOI] [PubMed] [Google Scholar]
- 236.Ma Y, Hendershot LM. The role of the unfolded protein response in tumour development: friend or foe? Nat Rev Cancer. 2004;4:966–77. doi: 10.1038/nrc1505. [DOI] [PubMed] [Google Scholar]
- 237.Chen X, Iliopoulos D, Zhang Q, Tang Q, Greenblatt MB, Hatziapostolou M, Lim E, Tam WL, Ni M, Chen Y, Mai J, Shen H, Hu DZ, Adoro S, Hu B, Song M, Tan C, Landis MD, Ferrari M, Shin SJ, Brown M, Chang JC, Liu XS, Glimcher LH. XBP1 promotes triple-negative breast cancer by controlling the HIF1α pathway. Nature. 2014;508:103–7. doi: 10.1038/nature13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Pereira ER, Frudd K, Awad W, Hendershot LM. Endoplasmic reticulum (ER) stress and hypoxia response pathways interact to potentiate hypoxia-inducible factor 1 (HIF-1) transcriptional activity on targets like vascular endothelial growth factor (VEGF) J Biol Chem. 2014;289:3352–64. doi: 10.1074/jbc.M113.507194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Koumenis C. ER stress, hypoxia tolerance and tumor progression. Curr Mol Med. 2006;6:55–69. doi: 10.2174/156652406775574604. [DOI] [PubMed] [Google Scholar]
- 240.Balistreri CR, Caruso C, Candore G. The role of adipose tissue and adipokines in obesity-related inflammatory diseases. Media inflam. 2010;2010:802078. doi: 10.1155/2010/802078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Khan S, Shukla S, Sinha S, Meeran SM. Role of adipokines and cytokines in obesity-associated breast cancer: therapeutic targets. Cytokine Growth Factor Rev. 2013;24:503–13. doi: 10.1016/j.cytogfr.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 242.García-Ruiz I, Rodríguez-Juan C, Díaz-Sanjuan T, del Hoyo P, Colina F, Mulñoz-Yagüe T, Solís-Herruzo JA. Uric acid and anti-TNF antibody improve mitochondrial dysfunction in ob/ob mice. Hepatology. 2006;44:581–91. doi: 10.1002/hep.21313. [DOI] [PubMed] [Google Scholar]
- 243.García-Ruiz I, Solís-Muñoz P, Fernández-Moreira D, Grau M, Colina F, Muñoz-Yagüe T, Solís-Herruzo JA. High-fat diet decreases activity of the oxidative phosphorylation complexes and causes nonalcoholic steatohepatitis in mice. Dis Model Mech. 2014;7:1287–96. doi: 10.1242/dmm.016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Silva-Alvarez C, Arrazola MS, Godoy JA, Ordenes D, Inestrosa NC. Canonical Wnt signaling protects hippocampal neurons from Abeta oligomers: role of non-canonical Wnt-5a/Ca(2+) in mitochondrial dynamics. Front Cell Neurosci. 2013;7:97. doi: 10.3389/fncel.2013.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Yoon JC, Ng A, Kim BH, Bianco A, Xavier RJ, Elledge SJ. Wnt signaling regulates mitochondrial physiology and insulin sensitivity. Genes Dev. 2010;24:1507–18. doi: 10.1101/gad.1924910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Donato J, Frazão R, Elias CF. The PI3K signaling pathway mediates the biological effects of leptin. Arq Bras Endocrinol Metabol. 2010;54:591–602. doi: 10.1590/s0004-27302010000700002. [DOI] [PubMed] [Google Scholar]
- 247.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13:140–56. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Braccini L, Ciraolo E, Martini M, Pirali T, Germena G, Rolfo K, Hirsch E. PI3K keeps the balance between metabolism and cancer. Adv Biol Reg. 2012;52:389–405. doi: 10.1016/j.jbior.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 249.Edinger AL. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol Biol Cell. 2002;13:2276–88. doi: 10.1091/mbc.01-12-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, Zhang J, Signoretti S, Loda M, Roberts TM, Zhao JJ. Essential roles of PI(3)K–p110β in cell growth, metabolism and tumorigenesis. Nature. 2008;454:776–9. doi: 10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Lee SY, Jeon HM, Ju MK, Kim CH, Yoon G, Han SI, Park HG, Kang HS. Wnt/Snail signaling regulates cytochrome c oxidase and glucose metabolism. Cancer Res. 2012;72:3607–17. doi: 10.1158/0008-5472.CAN-12-0006. [DOI] [PubMed] [Google Scholar]
- 252.Mori H, Prestwich TC, Reid MA, Longo KA, Gerin I, Cawthorn WP, Susulic VS, Krishnan V, Greenfield A, Macdougald OA. Secreted frizzled-related protein 5 suppresses adipocyte mitochondrial metabolism through WNT inhibition. J Clin Invest. 2012;122:2405–16. doi: 10.1172/JCI63604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Tan X, Wang X, Chu H, Liu H, Yi X, Xiao Y. SFRP5 correlates with obesity and metabolic syndrome and increases after weight loss in children. Clin Endocrinol. 2014;81:363–9. doi: 10.1111/cen.12361. [DOI] [PubMed] [Google Scholar]
- 254.Sekine Y, Suzuki K, Remaley AT. HDL and sphingosine-1-phosphate activate stat3 in prostate cancer DU145 cells via ERK1/2 and S1P receptors, and promote cell migration and invasion. Prostate. 2011;71:690–9. doi: 10.1002/pros.21285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Beckham TH, Lu P, Cheng JC, Zhao D, Turner LS, Zhang X, Hoffman S, Armeson KE, Liu A, Marrison T, Hannun YA, Liu X. Acid ceramidase-mediated production of sphingosine 1- phosphate promotes prostate cancer invasion through upregulation of cathepsin B. Int J Cancer. 2012;131:2034–43. doi: 10.1002/ijc.27480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- 257.Johnson KR, Johnson KY, Crellin HG, Ogretmen B, Boylan AM, Harley RA, Obeid LM. Immunohistochemical distribution of sphingosine kinase 1 in normal and tumor lung tissue. J Histochem Cytochem. 2005;53:1159–66. doi: 10.1369/jhc.4A6606.2005. [DOI] [PubMed] [Google Scholar]
- 258.Van Brocklyn JR, Jackson CA, Pearl DK, Kotur MS, Snyder PJ, Prior TW. Sphingosine kinase-1 expression correlates with poor survival of patients with glioblastoma multiforme: roles of sphingosine kinase isoforms in growth of glioblastoma cell lines. J Neuropathol Exp Neurol. 2005;64:695–705. doi: 10.1097/01.jnen.0000175329.59092.2c. [DOI] [PubMed] [Google Scholar]
- 259.Guan H, Liu L, Cai J, Liu J, Ye C, Li M, Li Y. Sphingosine kinase 1 is overexpressed and promotes proliferation in human thyroid cancer. Mol Endocrinol. 2011;25:1858–66. doi: 10.1210/me.2011-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Watson C, Long JS, Orange C, Tannahill CL, Mallon E, McGlynn LM, Pyne S, Pyne NJ, Edwards J. High expression of sphingosine 1-phosphate receptors, S1P1 and S1P3, sphingosine kinase 1, and extracellular signal-regulated kinase-1/2 is associated with development of tamoxifen resistance in estrogen receptor-positive breast cancer patients. Am J Pathol. 2010;177:2205–15. doi: 10.2353/ajpath.2010.100220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Ohotski J, Edwards J, Elsberger B, Watson C, Orange C, Mallon E, Pyne S, Pyne NJ. Identification of novel functional and spatial associations between sphingosine kinase 1, sphingosine 1-phosphate receptors and other signaling proteins that affect prognostic outcome in estrogen receptor-positive breast cancer. Int J Cancer. 2013;132:605–16. doi: 10.1002/ijc.27692. [DOI] [PubMed] [Google Scholar]
- 262.Błachnio-Zabielska AU, Pułka M, Baranowski M, Nikołajuk A, Zabielski P, Górska M, Górski J. Ceramide metabolism is affected by obesity and diabetes in human adipose tissue. J Cell Physiol. 2012;227:550–7. doi: 10.1002/jcp.22745. [DOI] [PubMed] [Google Scholar]
- 263.Brown J. Effects of 2-deoxyglucose on carbohydrate metablism: review of the literature and studies in the rat. Metab Clin Exp. 1962;11:1098–112. [PubMed] [Google Scholar]
- 264.Weindruch R, Keenan KP, Carney JM, Fernandes G, Feuers RJ, Floyd RA, Halter JB, Ramsey JJ, Richardson A, Roth GS, Spindler SR. Caloric restriction mimetics: metabolic interventions. J Gerontol A Biol Sci Med Sci. 2001;56:20–33. doi: 10.1093/gerona/56.suppl_1.20. [DOI] [PubMed] [Google Scholar]
- 265.Maher JC, Krishan A, Lampidis TJ. Greater cell cycle inhibition and cytotoxicity induced by 2-deoxy-D-glucose in tumor cells treated under hypoxic vs aerobic conditions. Cancer Chemother Pharmacol. 2004;53:116–22. doi: 10.1007/s00280-003-0724-7. [DOI] [PubMed] [Google Scholar]
- 266.Feng X, Zhang Y, Wang P, Liu Q, Wang X. Energy metabolism targeted drugs synergize with photodynamic therapy to potentiate breast cancer cell death. Photochem Photobiol Sci. 2014;13:1793–803. doi: 10.1039/c4pp00288a. [DOI] [PubMed] [Google Scholar]
- 267.Wang Z, Zhang L, Zhang D, Sun R, Wang Q, Liu X. Glycolysis inhibitor 2-deoxy-D-glucose suppresses carcinogen-induced rat hepatocarcinogenesis by restricting cancer cell metabolism. Mol Med Rep. 2014;11:1917–24. doi: 10.3892/mmr.2014.2945. [DOI] [PubMed] [Google Scholar]
- 268.Creighton DJ, Zheng ZB, Holewinski R, Hamilton DS, Eiseman JL. Glyoxalase I inhibitors in cancer chemotherapy. Biochem Society Trans. 2003;(31 Pt):1378–82. doi: 10.1042/bst0311378. [DOI] [PubMed] [Google Scholar]
- 269.Antognelli C, Palumbo I, Aristei C, Talesa VN. Glyoxalase I inhibition induces apoptosis in irradiated MCF-7 cells via a novel mechanism involving Hsp27, p53 and NF-kappaB. Br J Cancer. 2014;111:395–406. doi: 10.1038/bjc.2014.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Girgis H, Masui O, White NM, Scorilas A, Rotondo F, Seivwright A, Gabril M, Filter ER, Girgis AH, Bjarnason GA, Jewett MA, Evans A, Al-Haddad S, Siu KM, Yousef GM. Lactate dehydrogenase A is a potential prognostic marker in clear cell renal cell carcinoma. Mol Cancer. 2014;13:101. doi: 10.1186/1476-4598-13-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Koukourakis MI, Giatromanolaki A, Sivridis E, Bougioukas G, Didilis V, Gatter KC, Harris AL Tumour, Angiogenesis Research G. Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. Br J Cancer. 2003;89:877–85. doi: 10.1038/sj.bjc.6601205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Miao P, Sheng S, Sun X, Liu J, Huang G. Lactate dehydrogenase A in cancer: a promising target for diagnosis and therapy. IUBMB Life. 2013;65:904–10. doi: 10.1002/iub.1216. [DOI] [PubMed] [Google Scholar]
- 273.Fiume L, Manerba M, Vettraino M, Di Stefano G. Inhibition of lactate dehydrogenase activity as an approach to cancer therapy. Future Med Chem. 2014;6:429–45. doi: 10.4155/fmc.13.206. [DOI] [PubMed] [Google Scholar]
- 274.Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL, Dang CV. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci USA. 2010;107:2037–42. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Xie H, Hanai J, Ren JG, Kats L, Burgess K, Bhargava P, Signoretti S, Billiard J, Duffy KJ, Grant A, Wang X, Lorkiewicz PK, Schatzman S, Bousamra M, 2nd, Lane AN, Higashi RM, Fan TW, Pandolfi PP, Sukhatme VP, Seth P. Targeting lactate dehydrogenase – a inhibits tumorigenesis and tumor progression in mouse models of lung cancer and impacts tumor-initiating cells. Cell Metab. 2014;19:795–809. doi: 10.1016/j.cmet.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Yang Y, Su D, Zhao L, Zhang D, Xu J, Wan J, Fan S, Chen M. Different effects of LDH-A inhibition by oxamate in non-small cell lung cancer cells. Oncotarget. 2014;5:11886–96. doi: 10.18632/oncotarget.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Xie H, Valera VA, Merino MJ, Amato AM, Signoretti S, Linehan WM, Sukhatme VP, Seth P. LDH-A inhibition, a therapeutic strategy for treatment of hereditary leiomyomatosis and renal cell cancer. Mol Cancer Ther. 2009;8:626–35. doi: 10.1158/1535-7163.MCT-08-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Wang ZY, Loo TY, Shen JG, Wang N, Wang DM, Yang DP, Mo SL, Guan XY, Chen JP. LDH-A silencing suppresses breast cancer tumorigenicity through induction of oxidative stress mediated mitochondrial pathway apoptosis. Breast Cancer Res Treat. 2012;131:791–800. doi: 10.1007/s10549-011-1466-6. [DOI] [PubMed] [Google Scholar]
- 279.Fiume L, Manerba M, Vettraino M, Di Stefano G. Impairment of aerobic glycolysis by inhibitors of lactic dehydrogenase hinders the growth of human hepatocellular carcinoma cell lines. Pharmacology. 2010;86:157–62. doi: 10.1159/000317519. [DOI] [PubMed] [Google Scholar]
- 280.Sheng SL, Liu JJ, Dai YH, Sun XG, Xiong XP, Huang G. Knockdown of lactate dehydrogenase A suppresses tumor growth and metastasis of human hepatocellular carcinoma. FEBS J. 2012;279:3898–910. doi: 10.1111/j.1742-4658.2012.08748.x. [DOI] [PubMed] [Google Scholar]
- 281.Zhai X, Yang Y, Wan J, Zhu R, Wu Y. Inhibition of LDH-A by oxamate induces G2/M arrest, apoptosis and increases radiosensitivity in nasopharyngeal carcinoma cells. Oncol Rep. 2013;30:2983–91. doi: 10.3892/or.2013.2735. [DOI] [PubMed] [Google Scholar]
- 282.Maftouh M, Avan A, Sciarrillo R, Granchi C, Leon LG, Rani R, Funel N, Smid K, Honeywell R, Boggi U, Minutolo F, Peters GJ, Giovannetti E. Synergistic interaction of novel lactate dehydrogenase inhibitors with gemcitabine against pancreatic cancer cells in hypoxia. Br J Cancer. 2014;110:172–82. doi: 10.1038/bjc.2013.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Price GS, Page RL, Riviere JE, Cline JM, Thrall DE. Pharmacokinetics and toxicity of oral and intravenous lonidamine in dogs. Cancer Chemother Pharmacol. 1996;38:129–35. doi: 10.1007/s002800050460. [DOI] [PubMed] [Google Scholar]
- 284.Gatzemeier U, Cavalli F, Häussinger K, Kaukel E, Koschel G, Martinelli G, Neuhauss R, von Pawel J. Phase III trial with and without lonidamine in non-small cell lung cancer. Semin Oncol. 1991;18(2 Suppl):42–8. [PubMed] [Google Scholar]
- 285.Wang K, Fan H, Chen Q, Ma G, Zhu M, Zhang X, Zhang Y, Yu J. Curcumin inhibits aerobic glycolysis and induces mitochondrial- mediated apoptosis through hexokinase II in human colorectal cancer cells in vitro. Anticancer Drugs. 2015;26:15–24. doi: 10.1097/CAD.0000000000000132. [DOI] [PubMed] [Google Scholar]
- 286.Liu Z, Zhang Y-Y, Zhang Q-W, Zhao S-R, Wu C-Z, Cheng X, Jiang C-C, Jiang Z-W, Liu H. 3-Bromopyruvate induces apoptosis in breast cancer cells by downregulating Mcl-1 through the PI3K/Akt signaling pathway. Anticancer Drugs. 2014;25:447–55. doi: 10.1097/CAD.0000000000000081. [DOI] [PubMed] [Google Scholar]
- 287.Ganapathy-Kanniappan S, Geschwind J-F, Kunjithapatham R, Buijs M, Vossen JA, Tchernyshyov I, Cole RN, Syed LH, Rao PP, Ota S, Vali M. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is pyruvylated during 3-bromopyruvate mediated cancer cell death. Anticancer Res. 2009;29:4909–18. [PMC free article] [PubMed] [Google Scholar]
- 288.Ihrlund LS, Hernlund E, Khan O, Shoshan MC. 3-Bromopyruvate as inhibitor of tumour cell energy metabolism and chemopotentiator of platinum drugs. Mol Oncol. 2008;2:94–101. doi: 10.1016/j.molonc.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Zhao Y, Butler EB, Tan M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis. 2013;4:e532. doi: 10.1038/cddis.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Chapiro J, Sur S, Savic LJ, Ganapathy-Kanniappan S, Reyes J, Duran R, Thiruganasambandam SC, Moats CR, Lin M, Luo W, Tran PT, Herman JM, Semenza GL, Ewald AJ, Vogelstein B, Geschwind JF. Systemic delivery of microencapsulated 3-bromopyruvate for the therapy of pancreatic cancer. Clin Cancer Res. 2014;20:6406–17. doi: 10.1158/1078-0432.CCR-14-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Ganapathy-Kanniappan S, Vali M, Kunjithapatham R, Buijs M, Syed LH, Rao PP, Ota S, Kwak BK, Loffroy R, Geschwind JF. 3-Bromopyruvate: a new targeted antiglycolytic agent and a promise for cancer therapy. Curr Pharm Biotechnol. 2010;11:510–7. doi: 10.2174/138920110791591427. [DOI] [PubMed] [Google Scholar]
- 292.Klarer AC, O’Neal J, Imbert-Fernandez Y, Clem A, Ellis SR, Clark J, Clem B, Chesney J, Telang S. Inhibition of 6-phospho-fructo-2-kinase (PFKFB3) induces autophagy as a survival mechanism. Cancer Metab. 2014;1:2. doi: 10.1186/2049-3002-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Clem BF, O’Neal J, Tapolsky G, Clem AL, Imbert-Fernandez Y, Kerr DA, 2nd, Klarer AC, Redman R, Miller DM, Trent JO, Telang S, Chesney J. Targeting 6-phosphofructo-2-kinase (PFKFB3) as a therapeutic strategy against cancer. Mol Cancer Ther. 2013;12:1461–70. doi: 10.1158/1535-7163.MCT-13-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Pinheiro C, Longatto-Filho A, Azevedo-Silva J, Casal M, Schmitt FC, Baltazar F. Role of monocarboxylate transporters in human cancers: state of the art. J Bioenerg Biomembr. 2012;44:127–39. doi: 10.1007/s10863-012-9428-1. [DOI] [PubMed] [Google Scholar]
- 295.Cancer Research UK. A Phase I Trial of AZD3965 in Patients With Advanced Cancer. Bethesda (MD): National Library of Medicine (US); 2013. ClinicalTrials.gov [Internet] [cited 2014 Dec 20]. Available from: https://clinicaltrials.gov/ct2/show/NCT01791595. NLM identifier: NCT01791595. [Google Scholar]
- 296.Doherty JR, Yang C, Scott KE, Cameron MD, Fallahi M, Li W, Hall MA, Amelio AL, Mishra JK, Li F, Tortosa M, Genau HM, Rounbehler RJ, Lu Y, Dang CV, Kumar KG, Butler AA, Bannister TD, Hooper AT, Unsal-Kacmaz K, Roush WR, Cleveland JL. Blocking lactate export by inhibiting the Myc target MCT1 Disables glycolysis and glutathione synthesis. Cancer Res. 2014;74:908–20. doi: 10.1158/0008-5472.CAN-13-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Curry JM, Tuluc M, Whitaker-Menezes D, Ames JA, Anantharaman A, Butera A, Leiby B, Cognetti DM, Sotgia F, Lisanti MP, Martinez-Outschoorn UE. Cancer metabolism, stemness and tumor recurrence: MCT1 and MCT4 are functional biomarkers of metabolic symbiosis in head and neck cancer. Cell Cycle. 2013;12:1371–84. doi: 10.4161/cc.24092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Liu Y, Cao Y, Zhang W, Bergmeier S, Qian Y, Akbar H, Colvin R, Ding J, Tong L, Wu S, Hines J, Chen X. A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol Cancer Ther. 2012;11:1672–82. doi: 10.1158/1535-7163.MCT-12-0131. [DOI] [PubMed] [Google Scholar]
- 299.Ryland LK, Doshi UA, Shanmugavelandy SS, Fox TE, Aliaga C, Broeg K, Baab KT, Young M, Khan O, Haakenson JK, Jarbadan NR, Liao J, Wang H-G, Feith DJ, Loughran TP, Jr, Liu X, Kester M. C6-ceramide nanoliposomes target the Warburg effect in chronic lymphocytic leukemia. PLoS One. 2013;8:e84648. doi: 10.1371/journal.pone.0084648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Wiench B, Eichhorn T, Paulsen M, Efferth T. Shikonin directly targets mitochondria and causes mitochondrial dysfunction in cancer cells. eCAM. 2012;2012:726025. doi: 10.1155/2012/726025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Omidi Y, Matthaiou E, Barar J, Coukos G, Sandaltzopoulos R, Li C. Shikonin-loaded antibody-armed nanoparticles for targeted therapy of ovarian cancer. Int J Nanomedicine. 2014;15:1855–70. doi: 10.2147/IJN.S51880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Chen J, Xie J, Jiang Z, Wang B, Wang Y, Hu X. Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase-M2. Oncogene. 2011;30:4297–306. doi: 10.1038/onc.2011.137. [DOI] [PubMed] [Google Scholar]
- 303.Shi S, Cao H. Shikonin promotes autophagy in BXPC-3 human pancreatic cancer cells through the PI3K/Akt signaling pathway. Oncology Lett. 2014;8:1087–9. doi: 10.3892/ol.2014.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Wymann MP, Bulgarelli-Leva G, Zvelebil MJ, Pirola L, Vanhaesebroeck B, Waterfield MD, Panayotou G. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol Cell Biol. 1996;16:1722–33. doi: 10.1128/mcb.16.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Beckner ME, Gobbel GT, Abounader R, Burovic F, Agostino NR, Laterra J, Pollack IF. Glycolytic glioma cells with active glycogen synthase are sensitive to PTEN and inhibitors of PI3K and gluconeogenesis. Lab Invest. 2005;85:1457–70. doi: 10.1038/labinvest.3700355. [DOI] [PubMed] [Google Scholar]
- 306.Levy B, Spira A, Becker D, Evans T, Schnadig I, Camidge DR, Bauman JE, Hausman D, Walker L, Nemunaitis J, Rudin CM, Halmos B, Bowles DW. A randomized, phase 2 trial of Docetaxel with or without PX-866, an irreversible oral phosphatidylinositol 3-kinase inhibitor, in patients with relapsed or metastatic non-small-cell lung cancer. J Thorac Oncol. 2014;9:1031–5. doi: 10.1097/JTO.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 307.Ihle NT, Paine-Murrieta G, Berggren MI, Baker A, Tate WR, Wipf P, Abraham RT, Kirkpatrick DL, Powis G. The phosphatidylinositol-3-kinase inhibitor PX-866 overcomes resistance to the epidermal growth factor receptor inhibitor gefitinib in A-549 human non-small cell lung cancer xenografts. Mol Cancer Ther. 2005;4:1349–57. doi: 10.1158/1535-7163.MCT-05-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Koul D, Shen R, Kim YW, Kondo Y, Lu Y, Bankson J, Ronen SM, Kirkpatrick DL, Powis G, Yung WK. Cellular and in vivo activity of a novel PI3K inhibitor, PX-866, against human glioblastoma. Neuro Oncol. 2010;12:559–69. doi: 10.1093/neuonc/nop058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Bowles DW, Ma WW, Senzer N, Brahmer JR, Adjei AA, Davies M, Lazar AJ, Vo A, Peterson S, Walker L, Hausman D, Rudin CM, Jimeno A. A multicenter phase 1 study of PX-866 in combination with docetaxel in patients with advanced solid tumours. Br J Cancer. 2013;109:1085–92. doi: 10.1038/bjc.2013.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Hong DS, Bowles DW, Falchook GS, Messersmith WA, George GC, O’Bryant CL, Vo AC, Klucher K, Herbst RS, Eckhardt SG, Peterson S, Hausman DF, Kurzrock R, Jimeno A. A multicenter phase I trial of PX-866, an oral irreversible phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2012;18:4173–82. doi: 10.1158/1078-0432.CCR-12-0714. [DOI] [PubMed] [Google Scholar]
- 311.Bowles DW, Senzer N, Hausman D, Peterson S, Vo A, Walker L, Cohen RB, Jimeno A. A multicenter phase 1 study of PX-866 and cetuximab in patients with metastatic colorectal carcinoma or recurrent/metastatic squamous cell carcinoma of the head and neck. Invest New Drugs. 2014;32:1197–203. doi: 10.1007/s10637-014-0124-3. [DOI] [PubMed] [Google Scholar]
- 312.Marone R, Cmiljanovic V, Giese B, Wymann MP. Targeting phosphoinositide 3-kinase: moving towards therapy. Biochim Biophys Acta. 2008;1784:159–85. doi: 10.1016/j.bbapap.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 313.Knight ZA, Shokat KM. Chemically targeting the PI3K family. Biochem Soc Trans. 2007;35:245–9. doi: 10.1042/BST0350245. [DOI] [PubMed] [Google Scholar]
- 314.FDA approves first PI3K inhibitor. Nat Rev Drug Discov. 2014;13:644–5. Available at: http://www.nature.com/nrd/journal/v13/n9/full/nrd4425.html. [Google Scholar]
- 315.Tili E, Michaille J-J, Luo Z, Volinia S, Rassenti LZ, Kipps TJ, Croce CM. The down-regulation of miR-125b in chronic lymphocytic leukemias leads to metabolic adaptation of cells to a transformed state. Blood. 2012;120:2631–8. doi: 10.1182/blood-2012-03-415737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Martinez Marignac VL, Smith S, Toban N, Bazile M, Aloyz R. Resistance to Dasatinib in primary chronic lymphocytic leukemia lymphocytes involves AMPK-mediated energetic re- programming. Oncotarget. 2013;4:2550–66. doi: 10.18632/oncotarget.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, Barrientos JC, Zelenetz AD, Kipps TJ, Flinn I, Ghia P, Eradat H, Ervin T, Lamanna N, Coiffier B, Pettitt AR, Ma S, Stilgenbauer S, Cramer P, Aiello M, Johnson DM, Miller LL, Li D, Jahn TM, Dansey RD, Hallek M, O’Brien SM. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. New Engl J Med. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Dang CV. MYC, Metabolism, cell growth, and tumorigenesis. Cold Spring Harbor Persp Med. 2013;3:a014217-a. doi: 10.1101/cshperspect.a014217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–30. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 320.Gera JF. AKT activity determines sensitivity to mammalian target of rapamycin (mTOR) inhibitors by regulating cyclin D1 and c-myc expression. J Biol Chem. 2003;279:2737–46. doi: 10.1074/jbc.M309999200. [DOI] [PubMed] [Google Scholar]
- 321.Shimobayashi M, Hall MN. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol. 2014;15:155–62. doi: 10.1038/nrm3757. [DOI] [PubMed] [Google Scholar]
- 322.Düvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, Vander Heiden MG, MacKeigan JP, Finan PM, Clish CB, Murphy LO, Manning BD. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39:171–83. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Ben-Sahra I, Howell JJ, Asara JM, Manning BD. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science. 2013;339:1323–8. doi: 10.1126/science.1228792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Edinger AL, Linardic CM, Chiang GG, Thompson CB, Abraham RT. Differential effects of rapamycin on mammalian target of rapamycin signaling functions in mammalian cells. Cancer Res. 2003;63:8451–60. [PubMed] [Google Scholar]
- 325.Peng T, Golub TR, Sabatini DM. The immunosuppressant rapamycin mimics a starvation-like signal distinct from amino acid and glucose deprivation. Mol Cell Biol. 2002;22:5575–84. doi: 10.1128/MCB.22.15.5575-5584.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Pang Y-Y, Wang T, Chen F-Y, Wu Y-L, Shao X, Xiao F, Huang H-H, Zhong H, Zhong J-H. Glycolytic inhibitor 2-deoxy-d-glucose suppresses cell proliferation and enhances methylprednisolone sensitivity in non-Hodgkin lymphoma cells through down-regulation of HIF-1α and c-MYC. Leuk Lymphoma. 2014:1–10. doi: 10.3109/10428194.2014.963575. [DOI] [PubMed] [Google Scholar]
- 327.Gottfried E, Lang SA, Renner K, Bosserhoff A, Gronwald W, Rehli M, Einhell S, Gedig I, Singer K, Seilbeck A, Mackensen A, Grauer O, Hau P, Dettmer K, Andreesen R, Oefner PJ, Kreutz M. New Aspects of an old drug – diclofenac targets MYC and glucose metabolism in tumor cells. PLoS One. 2013;8:e66987. doi: 10.1371/journal.pone.0066987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Hoferová Z, Fedorocko P, Hofmanová J, Hofer M, Znojil V, Minksová K, Soucek K, Egyed A, Kozubík A. The effect of non-steroidal antiinflammatory drugs ibuprofen, flurbiprofen, and diclofenac on in vitro and in vivo growth of mouse fibrosarcoma. Cancer Invest. 2002;20:490–8. doi: 10.1081/cnv-120002149. [DOI] [PubMed] [Google Scholar]
- 329.Mayorek N, Naftali-Shani N, Grunewald M. Diclofenac inhibits tumor growth in a murine model of pancreatic cancer by modulation of VEGF levels and arginase activity. PLoS One. 2010;5:e12715. doi: 10.1371/journal.pone.0012715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Advanced Cancer Therapeutics. Phase 1 Safety Study of ACT-PFK-158, 2HCL in Patients with Advanced Solid Malignancies. Bethesda (MD): National Library of Medicine (US); 2014. ClinicalTrials.gov [Internet] [cited 2014 Dec 20]. Available from: https://clinicaltrials.gov/ct2/show/NCT02044861. NLM identifier: NCT02044861. [Google Scholar]
- 331.University of Chicago. Lenalidomide and Combination Chemotherapy (DA-EPOCH-R) in Treating Patients With MYC-Associated B-Cell Lymphomas. Bethesda (MD): National Library of Medicine (US); 2014. ClinicalTrials.gov [Internet] [cited 2014 Dec 20]. Available from: https://clinicaltrials.gov/ct2/show/NCT02213913. NLM identifier: NCT02213913. [Google Scholar]
- 332.Boston VA Research Institute. Study to Evaluate Two Lenalidomide Dose Regimens With Low Dose Dexamethasone for the Treatment Relapsed Multiple Myeloma. Bethesda (MD): National Library of Medicine (US); 2013. ClinicalTrials.gov [Internet] [cited 2014 Dec 20]. Available from: https://clinicaltrials.gov/ct2/show/NCT01380106. NLM identifier: NCT01380106. [Google Scholar]
- 333.Blum Kristie. A Phase II Trial of Panobinostat and Lenalidomide in Patients With Relapsed or Refractory Hodgkin’s Lymphoma. Bethesda (MD): National Library of Medicine (US); 2013. ClinicalTrials.gov [Internet] [cited 2013 Dec 20]. Available from: https://clinicaltrials.gov/ct2/show/NCT01460940. NLM identifier: NCT01460940. [Google Scholar]
- 334.Chang DT Stanford University. Phase I Trial of Metabolic Reprogramming Therapy for Treatment of Recurrent Head and Neck Cancers. Bethesda (MD): National Library of Medicine (US); 2014. ClinicalTrials.gov [Internet] [cited 2014 Dec 20]. Available from: https://clinicaltrials.gov/ct2/show/NCT01163487. NLM identifier: NCT01163487. [Google Scholar]
- 335.Sheba Medical Center. Phase II Study of RAD001 in a Neoadjuvant Setting in Men With Intermediate or High Risk Prostate Cancer. Bethesda (MD): National Library of Medicine (US); 2009. ClinicalTrials.gov [Internet] [cited 2014 Dec 20]. Available from: https://clinicaltrials.gov/ct2/show/NCT00657982. NLM identifier: NCT00657982. [Google Scholar]
- 336.National Cancer Center, Korea. Phase Ib/ II Trials of RAD001 in Triple Negative Metastatic Breast Cancer. Bethesda (MD): National Library of Medicine (US); 2014. ClinicalTrials.gov [Internet] [cited 2014 Dec 20]. Available from: https://clinicaltrials.gov/ct2/show/NCT01939418. NLM identifier: NCT01939418. [Google Scholar]
- 337.University of California, San Francisco. Phase II Trial of RAD001 in Patients With Recurrent Low Grade Glioma. Bethesda (MD): National Library of Medicine (US); 2014. ClinicalTrials.gov [Internet] [cited 2014 Dec 20]. Available from: https://clinicaltrials.gov/ct2/show/NCT00823459. NLM identifier: NCT00823459. [Google Scholar]
- 338.Chinese University of Hong Kong. Gastric Cancer RAD001 Study. Bethesda (MD): National Library of Medicine (US); 2014. ClinicalTrials.gov [Internet] [cited 2014 Dec 20]. Available from: https://clinicaltrials.gov/ct2/show/NCT01514110. NLM identifier: NCT01514110. [Google Scholar]
- 339.Ohio State University Comprehensive Cancer Center. Pilot Study of Curcumin for Women With Obesity and High Risk for Breast Cancer. Bethesda (MD): National Library of Medicine (US); 2014. Clinical- Trials.gov [Internet] [cited 2014 Dec 20]. Available from: https://clinicaltrials.gov/ct2/show/NCT01975363. NLM identifier: NCT01975363. [Google Scholar]
- 340.Shahid Beheshtu Medical University. Radiosensitizing and Radioprotectve Effects of Curcumin in Prostate Cancer. Bethesda (MD): National Library of Medicine (US); 2013. ClinicalTrials.gov [Internet] [cited 2014 Dec 20]. Available from: https://clinicaltrials.gov/ct2/show/NCT01917890. NLM identifier: NCT01917890. [Google Scholar]
- 341.Case Comprehensive Cancer Center. Curcumin and Cholecalciferol in Treating Patients With Previously Untreated Stage 0-II Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma. Bethesda (MD): National Library of Medicine (US); 2014. ClinicalTrials.gov [Internet] [cited 2014 Dec 20]. Available from: https://clinicaltrials.gov/ct2/show/NCT02100423. NLM identifier: NCT02100423. [Google Scholar]
- 342.Genentech, Inc. Study of GDC-0941 or GDC-0980 With Fulvestrant Versus Fulvestrant in Advanced or Metastatic Breast Cancer in Patients Resistant to Aromatase Inhibitor Therapy. Bethesda (MD): National Library of Medicine (US); 2014. ClinicalTrials.gov [Internet] [cited 2014 Dec 20]. Available from: https://clinicaltrials.gov/ct2/show/NCT01437566. NLM identifier: NCT01437566. [Google Scholar]
- 343.Roswell Park Cancer Institute. PI3K Inhibitor BKM120 and Docetaxel in Treating Patients With Advanced Solid Tumor That is Locally Advanced, Cannot Be Removed By Surgery, or Metastatic. Bethesda (MD): National Library of Medicine (US); 2014. ClinicalTrials.gov [Internet] [cited 2014 Dec 20]. Available from: https://clinicaltrials.gov/ct2/show/NCT01540253. NLM indetifier: NCT01540253. [Google Scholar]
- 344.University of Chicago. PI3K Inhibitor BKM120 and Cetuximab in Treating Patients With Recurrent or Metastatic Head and Neck Cancer. Bethesda (MD): National Library of Medicine (US); 2014. ClinicalTrials.gov [Internet] [cited 2014 Dec 20]. Available from: https://clinicaltrials.gov/ct2/show/NCT01816984. NLM identifier: NCT01816984. [Google Scholar]
- 345.Novartus Pharmaceuticals. A Study of Oral LGK974 in Patients With Malignancies Dependent on Wnt Ligands. Bethesda (MD): National Library of Medicine (US); 2014. ClinicalTrials.gov [Internet] [cited 2014 Dec 20]. Available from: https://clinicaltrials.gov/ct2/show/NCT01351103. NLM identifier: NCT01351103. [Google Scholar]
- 346.JW Pharmaceutical. Phase I Clinical Study of CWP232291 in Acute Myeloid Leukemia Patients. Bethesda (MD): National Library of Medicine (US); 2014. ClinicalTrials.gov [Internet] [cited 2014 Dec 20]. Available from: https://clinicaltrials.gov/ct2/show/NCT01398462. NLM identifier: NCT01398462. [Google Scholar]
- 347.Prism Pharma Co, Ltd. Safety and Efficacy Study of PRI-724 in Subjects With Advanced Myeloid Malignancies. Bethesda (MD): National Library of Medicine (US); 2014. ClinicalTrials.gov [Internet] [cited 2014 Dec 20]. Available from: https://clinicaltrials.gov/ct2/show/NCT01606579. NLM identifier: NCT01606579. [Google Scholar]
- 348.Philogen SpA. L19TNFα in Combination With Doxorubicin in Patients With Advanced Solid Tumours. Bethesda (MD): National Library of Medicine (US); 2014. ClinicalTrials.gov [Internet] [cited 2014 Dec 20]. Available from: https://clinicaltrials.gov/ct2/show/NCT02076620. NLM identifier: NCT02076620. [Google Scholar]
- 349.Sidney Kimmel Comprehensive Cancer Center. DIG-HIF1 Pharmacodynamic Trial in Newly Diagnosed Operable Breast Cancer. Bethesda (MD): National Library of Medicine (US); 2014. ClinicalTrials.gov [Internet] [cited 2014 Dec 20]. Available from: https://clinicaltrials.gov/ct2/show/NCT01763931. NLM identifier: NCT01763931. [Google Scholar]
- 350.National Cancer Institute (NCI) Ganetespib and Ziv-Aflibercept in Refractory Gastrointestinal Carcinomas, Non-Squamous Non-Small Cell Lung Carcinomas, Urothelial Carcinomas, and Sarcomas. Bethesda (MD): National Library of Medicine (US); 2014. ClinicalTrials.gov [Internet] [cited 2014 Dec 20]. Available from: https://clinicaltrials.gov/ct2/show/NCT02192541. NLM identifier: NCT02192541. [Google Scholar]
- 351.Massachusetts General Hospital. CRLX101 in Combination With Bevacizumab for Recurrent Ovarian/Tubal/ Peritoneal Cancer. Bethesda (MD): National Library of Medicine (US); 2014. ClinicalTrials.gov [Internet] [cited 2014 Dec 20]. Available from: https://clinicaltrials.gov/ct2/show/NCT01652079. NLM identifier: NCT01652079. [Google Scholar]
- 352.OHSU Knight Cancer Institute. Phenelzine Sulfate and Docetaxel in Treating Patients With Prostate Cancer With Progressive Disease After First-Line Therapy With Docetaxel. Bethesda (MD): National Library of Medicine (US); 2014. ClinicalTrials.gov [Internet] [cited 2014 Dec 20]. Available from: https://clinicaltrials.gov/ct2/show/NCT01253642. NLM identifier: NCT01253642. [Google Scholar]