Abstract
Chlorine (Cl2) is utilized worldwide for a diverse range of industrial applications, including pulp bleaching, sanitation, and pharmaceutical development. Though Cl2 has widespread use, little is known regarding the mechanisms of toxicity associated with Cl2 exposure, which occurs during industrial accidents or acts of terrorism. Previous instances of Cl2 exposure have led to reported episodes of respiratory distress that result in high morbidity and mortality. Furthermore, studies suggest that acute Cl2 exposure also results in systemic vascular injury and subsequent myocardial contractile dysfunction. Here we review both lung and cardiac pathology associated with acute Cl2 inhalation and discuss recently published data that suggests that mitochondrial dysfunction underlies the pathogenesis of Cl2-induced toxicity. Lastly, we discuss our findings that suggest that upregulation of autophagy protects against Cl2-induced lung inflammation and can be a potential therapeutic target for ameliorating the toxic effects of Cl2 exposure.
Keywords: chlorine, mitochondrial dysfunction, autophagy, lung injury, cardiac injury
Chlorine as an occupational and public health hazard
Chlorine (Cl2), a common toxic inhalant, is an essential chemical widely used in numerous industrial applications, including plastics manufacturing, waste sanitation, water treatment, and pharmaceutical development. Cl2 is a water-soluble yellow-green gas that commonly irritates the eyes, integumentary, and respiratory systems following exposure.1 Like any toxic inhalant, extent of injury is limited primarily by duration of exposure and intensity of dose. Most injuries are localized to sites in direct contact with the gas.2 Skin exposure to Cl2 can result in chemical burns and cell death resulting in the generation of dermal lesions.3 The Cl2 Institute in Arlington, Virginia, estimates that approximately 65 million tons of Cl2 are manufactured yearly worldwide, with the United States alone producing 13.8 million tons. With such widespread production and use, Cl2 exposure has the potential to be a significant global public health threat. An estimated 9000 calls occur annually to U.S. poison control centers for Cl2-related exposures.4
Wide-scale Cl2 exposures can occur in a number of scenarios, from accidental spills and occupational exposures to chemical warfare and acts of terrorism. Cl2 accidents have resulted in thousands of individuals being exposed to the toxic gas. Over the last 20 years, there have been 30 reported cases of large-scale accidental Cl2 release into urban centers. On January 6, 2005, a train derailment and subsequent 54,422-kg (120,000-lb) chlorine gas spill occurred in the cotton mill town of Graniteville, South Carolina.5–9 Several hundred people became immediately sick and thousands more were exposed.10–12 This is the largest civilian U.S. population ever exposed to chlorine gas.13 Data has been aggregated from 1979 to 2006 for over 8000 Graniteville millworkers who have at least 3 years of pre-event spirometry and related health and covariate assessment.14 Individual chlorine exposures from the event have been estimated using validated plume models.13,15,16 Concentrations of Cl2 upon the initial release of the gas reached up to 500 ppm, a dose above the lethal concentration of Cl2 in many animal studies.1,17 In addition, adverse environmental damages from the accident were extensive, as animal deaths and bleaching of vegetation occurred up to 1 km from the site of the accident.17–19
Other notable Cl2 exposure incidents include an industrial accident at a hazardous waste facility in Apex, North Carolina in 2006 and a Cl2 delivery-system malfunction in a Sacramento, California waterpark in 2011, both of which led to numerous hospitalizations for severe respiratory complaints.20 Even more recently, a British military nurse developed respiratory distress and reactive airway disease following Cl2 gas inhalation during routine procedures for disinfecting an Ebola treatment facility in Sierra Leone.6 These events highlight the hazards that Cl2 poses as both an accidental and occupational exposure risk.
More troublingly, Cl2 facilities are also implicated as potential terrorist targets. The U.S. Department of Homeland Security considers Cl2 production and storage facilities as potential high-risk targets that, according to the projections, could result in thousands of fatalities and up to 100,000 hospitalizations (The Homeland Security Council. Planning Scenarios: Executive Summaries. 004; 8–1). The use of Cl2 as a weapon is not a novel concept. During World War I, German forces would wait for favorable meteorological conditions before opening cylinders of Cl2 to surprise unsuspecting forces caught downwind of the suffocating greenish-yellow cloud.1 In 2007, insurgents in the Iraqi Conflict adopted this chemical warfare agent to terrorize regions of conflict by detonating explosives containing Cl2 gas in large urban centers.21
There is no specific antidote for individuals exposed to Cl2. Standards for treatment following Cl2 exposure are principally symptomatic, focusing on ameliorating conditions that potentially develop, such as pulmonary edema and upper and lower airway obstruction.1,2 Postexposure therapeutic management includes oxygen administration and treatment of bronchospasms with beta agonists.22 In cases of severe respiratory distress, intubation may be required. Corticosteroids have been shown to ameliorate acute respiratory distress following Cl2 exposure in animal studies, but their efficacy in improving respiratory function following human exposures has yet to be established.23
Lung injury after Cl2 exposure
In the year of the Graniteville accident, a significant reduction in mean forced expiratory volume in 1 second (FEV1) (−4.2% predicted, P = 0.019) was recorded when compared to the year before the incident. In the second year, partial recovery in the mean forced vital capacity (FVC) percent predicted level was seen, but the cohorts' average FEV1/FVC percent predicted level continued to decrease over time. Severe annual FEV1 decline was most prevalent in the year of the accident and independent of millworker smoking status.11
The toxic effects of Cl2 are most related to its oxidant potential. Upon inhalation, Cl2 first reacts with antioxidants in lung epithelial lining fluid (ELF).24 Once antioxidant stores are depleted, soluble Cl2 rapidly undergoes hydrolysis to generate hypochlorous (HOCl) and hydrochloric acids (HCl).1 These Cl2 products then react with protein side chains, DNA, and lipids of the cells that line the airway epithelium to generate toxic reactants.24–26 Some of these reactants formed are long lasting, contributing to injury long after the duration of the initial exposure event. Chloramines generated by Cl2 reactions with proteins activate inflammatory cascades through the mitogen-activated protein kinase (MAP kinase)27 and the nuclear factor κ light chain enhancer of activated B cells (NK-κB) pathways,28 resulting in inflammatory cell infiltration into the alveolar space. Furthermore, chloramines may predispose individuals to pulmonary edema by damaging epithelial sodium channel activity and disrupting fluid clearance in the lung.29 In addition, chlorinated lipids (sterols, fatty acids, and phospholipid chlorohydrines generated from reactions with Cl2 or HOCl) are considered proinflammatory.30
Along the respiratory tract, Cl2 distribution is primarily influenced by the concentration of the gas. It has been noted that, at low concentrations, Cl2 injury is predominantly limited to nasal and tracheal injury.31, 32 On the basis of diffusion modeling, at low Cl2 concentrations, up to 90% of Cl2 is absorbed within the nasal cavity and nasopharynx, with less than 10% reaching the hypopharynx or beyond.31 However, at higher concentrations, Cl2 not only damages the upper airways, but also penetrates deeper and damages the lower respiratory system.31,33
The dose of Cl2 also determines physiologic symptoms, responses, and consequences. Mild nasal irritation and throat irritation can occur at levels as low as 0.014–0.04 ppm, and ocular irritation, dyspnea, and headaches can occur at levels of 1 ppm.2 Acute exposure to doses as low as 1ppm causes sensory irritation,34 while chronic exposure to even lower doses (0.1–0.4ppm) can evoke ocular irritation and airway epithelial degeneration.32,35 These irritating effects were shown to be primarily mediated by the activation of transient receptor potential ankyrin 1 (TRPA1) ion channels, which interact with airway neurons to mediate neurological and airway responses to inhaled irritants, such as the cough reflex.36,37 Other clinical effects at low concentrations include lung inflammation, reversible bronchospasm, bradypnea, and dyspnea.38–41
At concentrations greater than 50 ppm, chemical pneumonitis develops.1 Animal studies have shed light into the pathologic sequence of injury following Cl2 exposure at higher concentrations: acute exposure to high doses of Cl2 induces bronchial epithelial sloughing, followed by a period of interstitial edema and infiltration of immunocompetent cells into the airways.42 These pathological events result in the development of pneumonitis, necrosis, and pulmonary edema.24,32 Uncorrected injury can potentially progress to respiratory failure and death.43 Mortality can occur at exposure to concentrations of 430 ppm for 30 min or more or concentrations greater than 1000 ppm for a few minutes.1,17
Another common sequel of Cl2 exposures in humans is reactive airway disease syndrome (RADS), a disorder characterized by wheezing, airflow obstruction, air trapping, and other asthma-like symptoms that persist beyond the typical recovery period for an inhaled toxic irritant. Not all individuals that are exposed to Cl2 develop RADS, and the phenotypic and genotypic risk factors that predispose individuals to its pathogenesis are still poorly understood.44 Although initially postulated to be secondary to only high-level inhalation exposures,45 later case reports demonstrated that low levels of Cl2 can also induce long-term reactive changes in the airway.44 These findings of reactive airway disease syndrome were recapitulated in animal studies: animals that survive the initial Cl2 insult often develop severe airway hyperreactivity, elevated airway resistance to methacholine challenge, and mucous metaplasia following exposure.38, 46
Evidence also suggests that Cl2 exposure increases susceptibilities to pulmonary infections. Following the Graniteville derailment, the South Carolina Department of Health and Environmental Control sent out a health alert message to all healthcare providers within the South Carolina health alert network within 24 h of the Graniteville disaster. This message alerted medical providers to the risks of secondary pneumonia infection within patients treated for chlorine injury and recommended that all hospitalized patients receive a pneumococcal vaccine before hospital discharge.
Following Cl2 exposure, mucous metaplasia results in an increase in mucous-producing goblet cells and a subsequent decrease in secretory serous cells in the conducting airways. Overproduction of mucous impairs respiratory clearance of pathogens and leads to airway obstruction and atelectasis. In addition, lower numbers of serous cells results in decreased production of their antimicrobial granules, lactoferrin and lysozyme, which predisposes to infection.42,47 Furthermore, HOCl reacts with amino acids in carbohydrate-recognition domains of surfactant protein (SP)-A, thereby decreasing its ability to bind and kill pathogens.48 HOCl also disrupts disulfide crosslinking of SP-D, inhibiting its pathogen-aggregating activity following infectious challenge.49 Finally, studies have demonstrated that Cl2 impairs antimicrobial superoxide generation and inflammatory production of IL-17A and IL-22 (both critical cytokines in inducing the epithelial antimicrobial response) of myeloid cells in the lung, thereby increasing susceptibility to certain pulmonary fungal infections by 500 fold.50
Cardiovascular effects of Cl2 toxicity
There have been some isolated reports in humans of systemic injury following gas exposure targeting the brain, liver, and heart. Specifically, white matter hemorrhages and liver transaminitis and cardiomegaly have been observed in cases of high-dose Cl2 exposure.17,53,54 In animal models, Cl2 exposure has also been shown to injure systemic vasculature and impair vascular function. Rats exposed to Cl2 (250–400 ppm) for 30 min had significantly decreased endothelial nitric oxide synthase (eNOS) protein expression. These animals also displayed marked attenuation in eNOS-dependent vasodilation (stimulated by acetycholine) at 24–48 h after exposure, suggesting that NO dysregulation may underlie the pathophysiology of Cl2 inhalation–induced systemic endothelial dysfunction.55 However, results also demonstrated that during the initial stages of Cl2 induced injury, inflammatory, cell-derived, inducible NOS (iNOS) may compensate for the loss of eNOS and prevent spike in the mean blood pressure. However, when iNOS was inhibited, significantly higher systemic blood pressures were recorded.55
Cardiotoxicity is another important risk factor for more severe immediate outcomes from Cl2 injury. In rats, inhalation of 500 ppm Cl2 for 30 min increases lactate in the coronary sinus, suggesting an increase in anaerobic metabolism by the heart. Cl2 inhalation also attenuates myocardial contractile force, reduces systolic and diastolic blood pressures, and causes biventricular failure and death.56 These results can be reproduced by sole exposure to chloramine (a potential circulating Cl2 reactant product), suggesting an independent and distinctive role of Cl2 (and its reactants) in inducing cardiac toxicity.26
Interestingly, cardiomegaly was observed on autopsy in eight of nine immediate victims of the acute chlorine release from the Graniteville, South Carolina train derailment in January 2005.19 Upon further examination, the one victim who died in the hospital was found to have cardiomegaly by chest X-ray in the hospital, later confirmed by autopsy. Similarly, four of the 71 victims hospitalized were found to have had cardiomegaly by chest X-ray. Furthermore, of those hospitalized, 64% presented with tachycardia and 52% with hypertension in the emergency department. Before the Graniteville chlorine disaster, there was little recognition of any potential cardiovascular effects from chlorine exposure in humans. There was some evidence that exposure to high levels of chlorine gas may result in vascular injury and the depression of cardiac function in humans and rats,56,57 which could exacerbate existing conditions leading to stroke.56–58 But there had not been any evidence of such long-term cardiovascular effects in a human cohort study.
The Graniteville cohort,59 which now has 305 patients enrolled, is the only systematic long-term longitudinal cohort study of a population exposed to a single high-concentration chlorine gas event with pre–post individual exposure and health comparisons and prospective longitudinal lung function assessment. In the cohort, it was observed that the immediate lung function loss between 2004 and 200514 had not fully recovered at 7–9 years postevent for some individuals. Furthermore, surprisingly, 12% of the participants were medically ineligible for spirometry testing owing to their high blood pressure even after repeated attempts to medically control it, and the modeled chlorine dose was significantly associated with their increased blood pressure (P < 0.05) 7–9 years later. When studying the rates of hospitalizations for cardiovascular diseases within the larger Graniteville population, hospitalizations for high blood pressure doubled from 110 per 10,000 residents in 2005 to 220 hospitalizations per 10,000 residents in 2012. This data indicates that the cardiovascular effects of chlorine exposure may last for many years.
Mitochondrial bioenergetic dysfunction in Cl2-induced injury
Although Cl2 exposure results in local and systemic injury with both acute and chronic consequences, effective therapeutics to ameliorate Cl2 toxicity are lacking. Further dissection of the pathophysiologic mechanisms of Cl2-induced injury can direct the generation of novel therapeutics. Mitochondria play a significant role in determining cell survival and apoptosis following a stress response.60 Mitochondria are also an important source of reactive oxygen species that may be elevated after cell injury. Interestingly, these mitochondrial oxidants can further propagate cell injury and disrupt other mitochondria that were not affected by the initial injury, resulting in an indirect perpetuation of ongoing inflammation and cell death.61–63 This results in both an acute and delayed deleterious effect from Cl2, even with sublethal Cl2 exposures.
In our recent study, we found that mitochondrial function was impaired after Cl2 exposure.64 We exposed H441 (human airway Clara cell–like cells) cells to 100 ppm Cl2 for 15 min, a dose and duration similar to those found in documented Cl2 disasters. Cl2 exposure decreased the maximal mitochondrial oxygen consumption rate (OCR) and the bioenergetic reserve capacity, an index of cellular energy for repair and resistance to oxidative damage.65 Cl2 also increased non-mitochondrial OCR, which represents an increased capacity to generate reactive oxygen species.
Interestingly, Cl2 exposure increased superoxide production in the mitochondria of Clara cells64 and primary cultures of alveolar type II cells.66 Further, we also demonstrated that mitochondrial oxidative stress played a significant role in the inhibition of cellular bioenergetics.64 Treatment of Cl2-exposed H441 cells with MitoQ, a mitochondrial-targeted antioxidant, partially prevented the Cl2-dependent decrease in maximal OCR. Exposure to Cl2 also decreased the mitochondrial membrane potential, which was prevented by MitoQ pretreatment.64 Surprisingly, exposure to Cl2 also resulted in a significant decrease in the maximal extracellular acidification rate (ECAR), an indicator of glycolysis,67 suggesting that glycolysis may also be impaired in these cells. Again, treatment with MitoQ attenuated this decrease in maximal ECAR to near normal levels. Our study also found that Cl2 specifically inhibits complex I and II of the mitochondrial electron transport chain.64
The mitochondrial injury also seems to be an important mediator of Cl2-induced cardiac toxicity. In rats, (500 ppm for 30 min) inhalation decreased ATP content in heart tissue and primary cardiomyocytes, resulting in increased anaerobic metabolism, as evidenced by the accumulation of lactate in the coronary sinus.68 Ahmad et al. attributed this impairment of the mitochondrial function to the formation of circulating Cl2 reactants and chlorination and inactivation of cardiac sarcoendoplasmic Ca2+ ATPase (SERCA) and subsequent increase in cytosolic Ca2+ overload. Excessive cytosolic Ca2+ causes increased production of reactive oxygen species by the mitochondria.69,70 In addition, the mitochondrial reactive species can itself perturb the cytosolic Ca2+ homeostasis and cause cardiac dysfunction.71,72 SERCA regulates cardiac intracellular Ca2+ homeostasis by transporting cytosolic Ca2+ into the sarco/endoplasmic reticulum at diastole and thus lowering intracellular Ca2+ levels.68,73 SERCA can be readily oxidized and its activity impaired by reactive species like hypochlorous acid (HOCl), a product of chlorine and water.68, 73 Interestingly, the administration of the SERCA stabilizer ranolazine or the SERCA activator istaroxime prevented chlorine-induced cardiomyocyte death and preserved mitochondrial membrane potential and ATP after chlorine exposure.68
The role of autophagy in Cl2-induced injury
Autophagy is generally an inducible adaptive response to lung injury. During autophagy, damaged organelles or denatured proteins are disposed through the lysosomal degradation pathway.74 Autophagic degradation of dysfunctional or damaged mitochondria is termed mitophagy.74 Mitochondria that are damaged by reactive oxygen species are typically removed by the lysosomal–mitophagy systems in order to maintain adequate mitochondrial function and control.60,75,76 During a minimal stress response, the lysosomal–autophagy system is capable of allowing mitophagy of dysfunctional mitochondria; however, if a more significant insult occurs, this system cannot appropriately maintain the large amount of mitochondria damaged by the reactive species.
The ubiquitin-like conjugation protein microtubule-associated protein 1, light chain 3B (LC3B) is a principal mediator of autophagy. In mammals, the conversion of LC3BI (unconjugated cytosolic form) to LC3BII (autophagosomal membrane–associated phosphatidylethanolamine-conjugated form) is a hallmark of autophagosome formation.50, 51 In addition, the nucleoporin p62 protein is involved in recognizing toxic cellular waste and targeting it for autophagy.77 Decreased levels of p62 are associated with induction of autophagy.78 Interestingly, in our studies we found that LC3BII levels were increased, while the levels of p62 protein were decreased, 6 h after Cl2 exposure in H441 cells. These results suggested that Cl2 exposure leads to an increase in autophagy, presumably to clear damaged mitochondria.
In addition, we explored whether the modulation of autophagy would affect mitochondrial bioenergetics following Cl2 inhalation. For this, we pretreated human club cells with either trehalose, an activator of autophagy, or 3-MA (3-methyladenine), an inhibitor of autophagy, before exposure to Cl2. Preincubation of cells with trehalose resulted in an increase in LC3BII in both air- and Cl2-exposed cells, associated with elevated levels of autophagy. More interestingly, trehalose attenuated the decrease in maximal OCR of cells following Cl2 exposure, suggesting an improvement of mitochondrial respiration function. Conversely, 3-MA pretreatment resulted in further bioenergetic dysfunction of Cl2-exposed cells.64
We also studied the effects of autophagy upregulation on lung inflammatory responses in vivo after Cl2-inhalation injury. Acute pretreatment of mice with trehalose via aerosolization increased lung LC3BII levels after Cl2 (400 ppm, 30 min) exposure, which correlated with decreased lung permeability and airway protein leakage into bronchoalveolar lavage fluid. Chronic treatment of mice with trehalose 6 weeks before Cl2 exposure resulted in a decrease in lung inflammatory cells and improved tissue healing.64
Conclusions
In conclusion, given the widespread use and easy attainability of Cl2, exposure to the toxic gas poses a major health threat that currently has few available therapeutic treatments. Exposure to Cl2 gas results in both local and systemic responses, resulting in high morbidity and mortality secondary to both cardiac and pulmonary dysfunction. Our studies show that an increase in mitochondrial ROS and subsequent dysfunction in mitochondrial bioenergetics are hallmarks of Cl2-inhalation injury. Furthermore, preventing oxidative damage of SERCA or stabilizing the SERCA activity can prevent Ca2+ overload, mitochondrial dysfunction, and cardiac damage. In addition, upregulating mitochondrial autophagy pathways in damaged cells can likely ameliorate mitochondrial bioenergetics dysfunction and subsequent pulmonary damage resulting from acute Cl2 exposure (Fig. 1). Trehalose administration represents a novel treatment method that improves autophagy of dysfunctional mitochondria and can possibly decrease the deleterious effects of Cl2-inhalational injury. It is important to note that immediate treatment after Cl2 exposure may play significant role in mitigating long-term cardiopulmonary pathologies associated with chronic Cl2 toxicity. However, further studies are required to assess the long-term beneficial effects of mitigating mitochondrial dysfunction after Cl2 exposure.
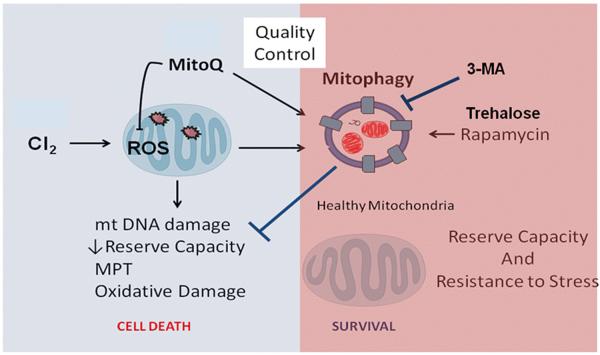
Figure 1.
Role of autophagy on chlorine (Cl2)-induced mitochondrial dysfunction. Mitochondria are the key regulators of cell survival in response to Cl2-induced cellular stress. Healthy mitochondria produce reactive oxygen species (ROS) that serve a cell signaling function; however, damaged mitochondria produce excessive amounts of ROS and become a major contributor of cellular and organ injury leading to mitochondrial DNA damage, formation of mitochondrial permeability transition (MPT) pores, and cell death. Mitochondria modified by ROS are targeted for removal by mitophagy, a critical step in maintaining mitochondrial quality control and limit cellular and organ injury. Upregulation of mitophagy by rapamycin or trehalose prevents mitochondrial oxidative damage and is beneficial in ameliorating Cl2 toxicity. Alternatively, decreasing mitophagy with 3-methyladenine (3-MA) enhances Cl2-induced injury.
Acknowledgments
Supported by the CounterACT Program, National Institutes of Health Office of the Director (NIH OD), the National Institute of Neurological Disorders and Stroke (NINDS), and the National Institute of Environmental Health Science. Grant Numbers: (S.M.) (5R21 ES024027 02, 1R21ES025423 01, and 1U01ES026458-01A1) and (E.S.) 5R01ES15532 05.
Footnotes
Conflicts of interest The authors declare no conflicts of interest.
References
- 1.Winder C. The toxicology of chlorine. Environ Res. 2001;85:105–114. doi: 10.1006/enrs.2000.4110. [DOI] [PubMed] [Google Scholar]
- 2.Evans RB. Chlorine: state of the art. Lung. 2005;183:151–167. doi: 10.1007/s00408-004-2530-3. [DOI] [PubMed] [Google Scholar]
- 3.Snider TH, et al. A dynamic system for delivering controlled bromine and chlorine vapor exposures to weanling swine skin. Cutan Ocul Toxicol. 2014;33:161–167. doi: 10.3109/15569527.2013.806524. [DOI] [PubMed] [Google Scholar]
- 4.Becker M, Forrester M. Pattern of chlorine gas exposures reported to Texas poison control centers, 2000 through 2005. Tex Med. 2008;104:52–57. 51. [PubMed] [Google Scholar]
- 5.Plant JF. Rail safety: Targeting oversight and assessing results. Public Administration Review. 2008;68:137–140. [Google Scholar]
- 6.Dunning AE, Oswalt JL. Train wreck and chlorine spill in Graniteville, South Carolina. Transportation Research Record. 2007:130–135. [Google Scholar]
- 7.Ball LJ, Dworak J. Disaster in Graniteville. S C Nurse. 2005;12:1. [PubMed] [Google Scholar]
- 8.B NTS. Collision of Norfolk Southern Freight Train 192 With Standing Norfolk Southern Local Train P22 With Subsequent Hazardous Materials Release at Graniteville, South Carolina, January 6, 2005. Washington, DC: 2005. [Google Scholar]
- 9.O'Connor J, Leach L, Hinshaw D. The State. 2005. `Y'all got to get off this floor!'. [Google Scholar]
- 10.Clark KA, et al. Respiratory symptoms and lung function 8–10 months after community exposure to chlorine gas: a public health intervention and cross-sectional analysis. BMC Public Health. 2013;13:945. doi: 10.1186/1471-2458-13-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ginsberg JP, et al. Posttraumatic stress and tendency to panic in the aftermath of the chlorine gas disaster in Graniteville, South Carolina. Soc Psychiatry Psychiatr Epidemiol. 2012;47:1441–1448. doi: 10.1007/s00127-011-0449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svendsen ER, et al. GRACE: public health recovery methods following an environmental disaster. Arch Environ Occup Health. 2010;65:77–85. doi: 10.1080/19338240903390222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Svendsen ER, et al. Epidemiologic methods lessons learned from environmental public health disasters: Chernobyl, the World Trade Center, Bhopal, and Graniteville, South Carolina. Int J Environ Res Public Health. 2012;9:2894–2909. doi: 10.3390/ijerph9082894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark KA, et al. Lung Function before and after a Large Chlorine Gas Release in Graniteville, South Carolina, USA. Ann Am Thorac Soc. 2015;13(3):356–63. doi: 10.1513/AnnalsATS.201508-525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reed D, Feigley C, Svendsen E. Exposure Estimation Technology for Large Environmental Chemical Releases: Feasibility of an Atmospheric Plume Dispersion Model Without Microenvironmental Weather Data. Epidemiology. 2011;22:S105–S106. [Google Scholar]
- 16.Jani DD, et al. Modeling an irritant gas plume for epidemiologic study. Int J Environ Health Res. 2016;26:58–74. doi: 10.1080/09603123.2015.1020414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buckley RL, et al. A case study of chlorine transport and fate following a large accidental release. Atmospheric Environment. 2012;62:184–198. [Google Scholar]
- 18.Mackie E, et al. Management of chlorine gas-related injuries from the Graniteville, South Carolina, train derailment. Disaster Med Public Health Prep. 2014;8:411–416. doi: 10.1017/dmp.2014.81. [DOI] [PubMed] [Google Scholar]
- 19.Van Sickle D, et al. Acute health effects after exposure to chlorine gas released after a train derailment. Am J Emerg Med. 2009;27:1–7. doi: 10.1016/j.ajem.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holusha J. NY TImes. 2006. Blaze in North Carolina Prompts Huge Evacuation. [Google Scholar]
- 21.Semple K. [Accessed April 15, 2016];Attacks Kill 2 Iraqis and Expose Hundreds to Chlorine Gas. NY Times. 2007 http://www.nytimes.com/2007/03/17/world/middleeast/17cnd-iraq.html?_r=0.
- 22.Sexton JD, Pronchik DJ. Chlorine inhalation: the big picture. J Toxicol Clin Toxicol. 1998;36:87–93. doi: 10.3109/15563659809162593. [DOI] [PubMed] [Google Scholar]
- 23.Bosse GM. Nebulized sodium bicarbonate in the treatment of chlorine gas inhalation. J Toxicol Clin Toxicol. 1994;32:233–241. doi: 10.3109/15563659409017956. [DOI] [PubMed] [Google Scholar]
- 24.Squadrito GL, Postlethwait EM, Matalon S. Elucidating mechanisms of chlorine toxicity: reaction kinetics, thermodynamics, and physiological implications. Am J Physiol Lung Cell Mol Physiol. 2010;299:L289–300. doi: 10.1152/ajplung.00077.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hawkins CL, Pattison DI, Davies MJ. Hypochlorite-induced oxidation of amino acids, peptides and proteins. Amino Acids. 2003;25:259–274. doi: 10.1007/s00726-003-0016-x. [DOI] [PubMed] [Google Scholar]
- 26.den Hartog GJ, et al. Efficacy of HOCl scavenging by sulfur-containing compounds: antioxidant activity of glutathione disulfide? Biol Chem. 2002;383:709–713. doi: 10.1515/BC.2002.073. [DOI] [PubMed] [Google Scholar]
- 27.Midwinter RG, Vissers MC, Winterbourn CC. Hypochlorous acid stimulation of the mitogen-activated protein kinase pathway enhances cell survival. Arch Biochem Biophys. 2001;394:13–20. doi: 10.1006/abbi.2001.2530. [DOI] [PubMed] [Google Scholar]
- 28.Midwinter RG, et al. IkappaB is a sensitive target for oxidation by cell-permeable chloramines: inhibition of NF-kappaB activity by glycine chloramine through methionine oxidation. Biochem J. 2006;396:71–78. doi: 10.1042/BJ20052026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song W, et al. Inhibition of lung fluid clearance and epithelial Na+ channels by chlorine, hypochlorous acid, and chloramines. J Biol Chem. 2010;285:9716–9728. doi: 10.1074/jbc.M109.073981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spickett CM. Chlorinated lipids and fatty acids: an emerging role in pathology. Pharmacol Ther. 2007;115:400–409. doi: 10.1016/j.pharmthera.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Nodelman V, Ultman JS. Longitudinal distribution of chlorine absorption in human airways: a comparison to ozone absorption. J Appl Physiol (1985) 1999;87:2073–2080. doi: 10.1152/jappl.1999.87.6.2073. [DOI] [PubMed] [Google Scholar]
- 32.Wolf DC, et al. Two-year inhalation exposure of female and male B6C3F1 mice and F344 rats to chlorine gas induces lesions confined to the nose. Fundam Appl Toxicol. 1995;24:111–131. doi: 10.1006/faat.1995.1013. [DOI] [PubMed] [Google Scholar]
- 33.Buckley LA, et al. Respiratory tract lesions induced by sensory irritants at the RD50 concentration. Toxicol Appl Pharmacol. 1984;74:417–429. doi: 10.1016/0041-008x(84)90295-3. [DOI] [PubMed] [Google Scholar]
- 34.Barrow CS, et al. Comparison of the sensory irritation response in mice to chlorine and hydrogen chloride. Arch Environ Health. 1977;32:68–76. doi: 10.1080/00039896.1977.10667258. [DOI] [PubMed] [Google Scholar]
- 35.Klonne DR, et al. One-year inhalation toxicity study of chlorine in rhesus monkeys (Macaca mulatta) Fundam Appl Toxicol. 1987;9:557–572. doi: 10.1016/0272-0590(87)90037-6. [DOI] [PubMed] [Google Scholar]
- 36.Bessac BF, Jordt SE. Sensory detection and responses to toxic gases: mechanisms, health effects, and countermeasures. Proc Am Thorac Soc. 2010;7:269–277. doi: 10.1513/pats.201001-004SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bessac BF, et al. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest. 2008;118:1899–1910. doi: 10.1172/JCI34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fanucchi MV, et al. Post-exposure antioxidant treatment in rats decreases airway hyperplasia and hyperreactivity due to chlorine inhalation. Am J Respir Cell Mol Biol. 2012;46:599–606. doi: 10.1165/rcmb.2011-0196OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D'Alessandro A, et al. Exaggerated responses to chlorine inhalation among persons with nonspecific airway hyperreactivity. Chest. 1996;109:331–337. doi: 10.1378/chest.109.2.331. [DOI] [PubMed] [Google Scholar]
- 40.Bonetto G, et al. Longitudinal monitoring of lung injury in children after acute chlorine exposure in a swimming pool. Am J Respir Crit Care Med. 2006;174:545–549. doi: 10.1164/rccm.200509-1392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kennedy SM, et al. Lung health consequences of reported accidental chlorine gas exposures among pulpmill workers. Am Rev Respir Dis. 1991;143:74–79. doi: 10.1164/ajrccm/143.1.74. [DOI] [PubMed] [Google Scholar]
- 42.Das R, Blanc PD. Chlorine gas exposure and the lung: a review. Toxicol Ind Health. 1993;9:439–455. doi: 10.1177/074823379300900304. [DOI] [PubMed] [Google Scholar]
- 43.Demnati R, et al. Time-course of functional and pathological changes after a single high acute inhalation of chlorine in rats. Eur Respir J. 1998;11:922–928. doi: 10.1183/09031936.98.11040922. [DOI] [PubMed] [Google Scholar]
- 44.Hickmann MA, et al. Are high-dose toxic exposures always associated with reactive airways dysfunction syndrome (RADS)? Arch Environ Health. 2001;56:439–442. doi: 10.1080/00039890109604479. [DOI] [PubMed] [Google Scholar]
- 45.Alberts WM, do Pico GA. Reactive airways dysfunction syndrome. Chest. 1996;109:1618–1626. doi: 10.1378/chest.109.6.1618. [DOI] [PubMed] [Google Scholar]
- 46.Gorguner M, et al. Reactive airways dysfunction syndrome in housewives due to a bleach-hydrochloric acid mixture. Inhal Toxicol. 2004;16:87–91. doi: 10.1080/08958370490265004. [DOI] [PubMed] [Google Scholar]
- 47.Menaouar A, et al. Chlorine gas induced acute lung injury in isolated rabbit lung. Eur Respir J. 1997;10:1100–1107. doi: 10.1183/09031936.97.10051100. [DOI] [PubMed] [Google Scholar]
- 48.Bowes D, Clark AE, Corrin B. Ultrastructural localisation of lactoferrin and glycoprotein in human bronchial glands. Thorax. 1981;36:108–115. doi: 10.1136/thx.36.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crouch EC, et al. Myeloperoxidase-dependent inactivation of surfactant protein D in vitro and in vivo. J Biol Chem. 2010;285:16757–16770. doi: 10.1074/jbc.M109.097048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gessner MA, et al. Chlorine gas exposure increases susceptibility to invasive lung fungal infection. Am J Physiol Lung Cell Mol Physiol. 2013;304:L765–773. doi: 10.1152/ajplung.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li C, et al. Chlorine induces the unfolded protein response in murine lungs and skin. Am J Respir Cell Mol Biol. 2013;49:197–203. doi: 10.1165/rcmb.2012-0488RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.White CW, Martin JG. Chlorine gas inhalation: human clinical evidence of toxicity and experience in animal models. Proc Am Thorac Soc. 2010;7:257–263. doi: 10.1513/pats.201001-008SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baader EW. Anhydrous chlorine poisoning; catastrophe of Walsum. Med Deporte Trab. 1952;17:5252–5259. [PubMed] [Google Scholar]
- 54.Leube G, Kreiter H. Acute chlorine poisoning. Case reports of 90 patients with acute poisoning. Med Klin. 1971;66:354–357. [PubMed] [Google Scholar]
- 55.Honavar J, et al. Chlorine gas exposure causes systemic endothelial dysfunction by inhibiting endothelial nitric oxide synthase-dependent signaling. Am J Respir Cell Mol Biol. 2011;45:419–425. doi: 10.1165/rcmb.2010-0151OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zaky A, et al. Chlorine inhalation-induced myocardial depression and failure. Physiol Rep. 2015;3 doi: 10.14814/phy2.12439. doi: 10.14814/phy2.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suzuki S, et al. Fatal pulmonary arterial thrombosis associated with chlorine gas poisoning. Clin Appl Thromb Hemost. 2001;7:356–358. doi: 10.1177/107602960100700420. [DOI] [PubMed] [Google Scholar]
- 58.Kose A, et al. Myocardial infarction, acute ischemic stroke, and hyperglycemia triggered by acute chlorine gas inhalation. Am J Emerg Med. 2009;27:1022, e1021–1024. doi: 10.1016/j.ajem.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 59.Balte P, et al. Decline in Lung Functions after Exposure to Chlorine: a Pre and Post Exposure Analysis. Presented at Environ Health Perspect; Seattle, Washington. 2014. [Google Scholar]
- 60.Lee J, Giordano S, Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J. 2012;441:523–540. doi: 10.1042/BJ20111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hill BG, et al. Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem J. 2009;424:99–107. doi: 10.1042/BJ20090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klamt F, Shacter E. Taurine chloramine, an oxidant derived from neutrophils, induces apoptosis in human B lymphoma cells through mitochondrial damage. J Biol Chem. 2005;280:21346–21352. doi: 10.1074/jbc.M501170200. [DOI] [PubMed] [Google Scholar]
- 64.Jurkuvenaite A, et al. Upregulation of autophagy decreases chlorine-induced mitochondrial injury and lung inflammation. Free Radic Biol Med. 2015;85:83–94. doi: 10.1016/j.freeradbiomed.2015.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hill BG, et al. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol Chem. 2012;393:1485–1512. doi: 10.1515/hsz-2012-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lazrak A, et al. Regulation of alveolar epithelial Na+ channels by ERK1/2 in chlorine-breathing mice. Am J Respir Cell Mol Biol. 2012;46:342–354. doi: 10.1165/rcmb.2011-0309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dranka BP, et al. Assessing bioenergetic function in response to oxidative stress by metabolic profiling. Free Radic Biol Med. 2011;51:1621–1635. doi: 10.1016/j.freeradbiomed.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ahmad S, et al. Sarcoendoplasmic reticulum Ca(2+) ATPase. A critical target in chlorine inhalation-induced cardiotoxicity. Am J Respir Cell Mol Biol. 2015;52:492–502. doi: 10.1165/rcmb.2014-0005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sedlic F, et al. Mitochondrial depolarization underlies delay in permeability transition by preconditioning with isoflurane: roles of ROS and Ca2+ Am J Physiol Cell Physiol. 2010;299:C506–515. doi: 10.1152/ajpcell.00006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dedkova EN, Seidlmayer LK, Blatter LA. Mitochondria-mediated cardioprotection by trimetazidine in rabbit heart failure. J Mol Cell Cardiol. 2013;59:41–54. doi: 10.1016/j.yjmcc.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yun Y, Hou L, Sang N. SO(2) inhalation modulates the expression of pro-inflammatory and pro-apoptotic genes in rat heart and lung. J Hazard Mater. 2011;185:482–488. doi: 10.1016/j.jhazmat.2010.09.057. [DOI] [PubMed] [Google Scholar]
- 72.Yancey DM, et al. Cardiomyocyte mitochondrial oxidative stress and cytoskeletal breakdown in the heart with a primary volume overload. Am J Physiol Heart Circ Physiol. 2015;308:H651–663. doi: 10.1152/ajpheart.00638.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cook NL, et al. Myeloperoxidase-derived oxidants inhibit sarco/endoplasmic reticulum Ca2+-ATPase activity and perturb Ca2+ homeostasis in human coronary artery endothelial cells. Free Radic Biol Med. 2012;52:951–961. doi: 10.1016/j.freeradbiomed.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ryter SW, et al. Autophagy in pulmonary diseases. Annu Rev Physiol. 2012;74:377–401. doi: 10.1146/annurev-physiol-020911-153348. [DOI] [PubMed] [Google Scholar]
- 76.Goldman SJ, et al. Autophagy and the degradation of mitochondria. Mitochondrion. 2010;10:309–315. doi: 10.1016/j.mito.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rusten TE, Stenmark H. p62, an autophagy hero or culprit? Nat Cell Biol. 2010;12:207–209. doi: 10.1038/ncb0310-207. [DOI] [PubMed] [Google Scholar]
- 78.Araya J, et al. Insufficient autophagy in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2013;304:L56–69. doi: 10.1152/ajplung.00213.2012. [DOI] [PubMed] [Google Scholar]