Abstract
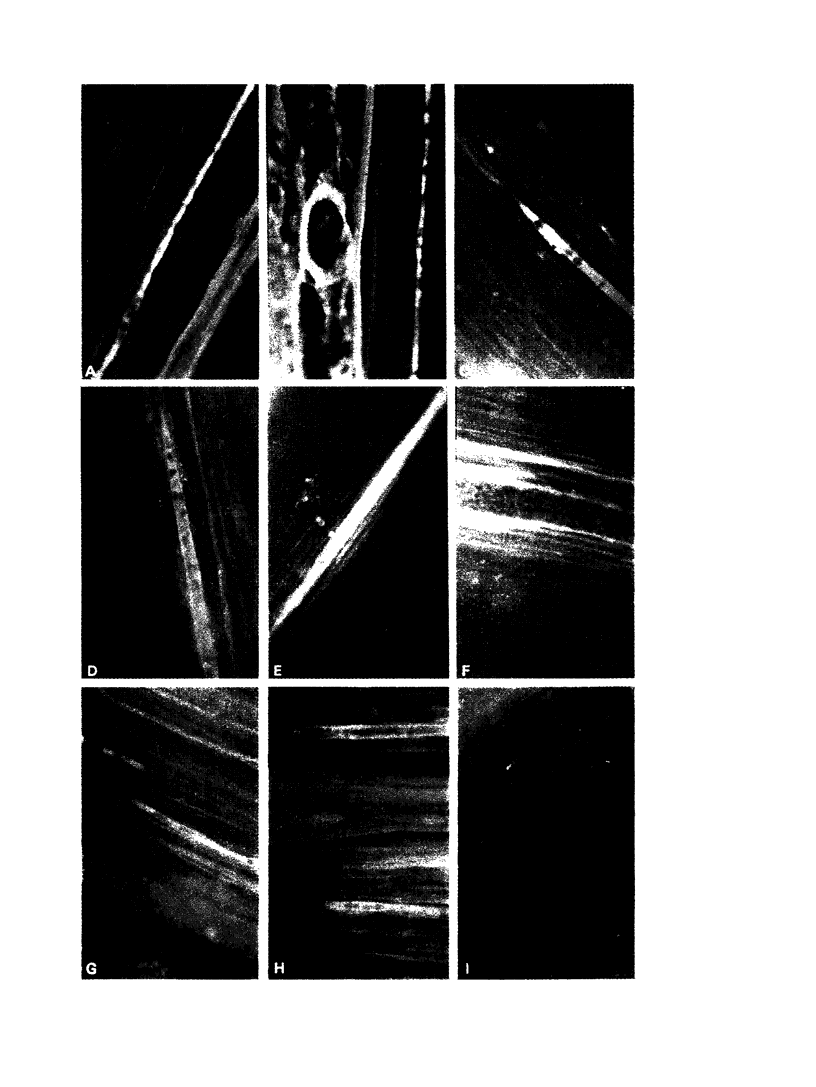
An albumin-Evans blue conjugate has been used as a fluorescent tracer to demonstrate the increased permeability of endoneurial capillaries and perineurial sheath of the sciatic nerve of the alloxan-diabetic rat. The significance of the extravasation of protein into the endoneurial space is discussed in relation to the altered dynamics of the endoneurial microcirculation. It is suggested that tissue hypoxia produced in this way may be a cause of the segmental demyelination which occurs in these nerves.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AAGENAES O., MOE H. Light- and electron-microscopic study of skin capillaries of diabetics. Diabetes. 1961 Jul-Aug;10:253–259. doi: 10.2337/diab.10.4.253. [DOI] [PubMed] [Google Scholar]
- Adams W. E. The blood supply of nerves: I. Historical review. J Anat. 1942 Jul;76(Pt 4):323–341. [PMC free article] [PubMed] [Google Scholar]
- BERGSTRAND A., BUCHT H. The glomerular lesions of diabetes mellitus and their electron-microscope appearances. J Pathol Bacteriol. 1959 Jan;77(1):231–242. doi: 10.1002/path.1700770122. [DOI] [PubMed] [Google Scholar]
- BURSTEIN R., SOULE S. D., BLUMENTHAL H. T. Histogenesis of pathological processes in placentas of metabolic disease in pregnancy. II. The diabetic state. Am J Obstet Gynecol. 1957 Jul;74(1):96–104. doi: 10.1016/s0002-9378(16)37007-7. [DOI] [PubMed] [Google Scholar]
- BUSINCO L. Connective tissue, histiocapillary unity and rheumatism. Int Arch Allergy Appl Immunol. 1959;14(3-4):205–216. doi: 10.1159/000228513. [DOI] [PubMed] [Google Scholar]
- Bischoff A. Diabetic neuropathy. Morbid anatomy, patho-physiology and pathogenesis based on electron-microscopic findings. Ger Med Mon. 1968 May;13(5):214–passim. [PubMed] [Google Scholar]
- Bloodworth J. M., Jr, Engerman R. L., Powers K. L. Experimental diabetic microangiopathy. I. Basement membrane statistics in the dog. Diabetes. 1969 Jul;18(7):455–458. doi: 10.2337/diab.18.7.455. [DOI] [PubMed] [Google Scholar]
- Bloodworth J. M., Jr, Molitor D. L. Ultrastructural aspects of human and canine diabetic retinopathy. Invest Ophthalmol. 1965 Dec;4(6):1037–1048. [PubMed] [Google Scholar]
- Burkel W. E. The histological fine structure of perineurium. Anat Rec. 1967 Jun;158(2):177–189. doi: 10.1002/ar.1091580207. [DOI] [PubMed] [Google Scholar]
- CHURG J., MAUTNER W., GRISHMAN E., EISNER G. M. Structure of glomerular capillaries in proteinuria. Arch Intern Med. 1962 Jan;109:97–115. doi: 10.1001/archinte.1962.03620130099011. [DOI] [PubMed] [Google Scholar]
- CRESCITELLI F. Nerve sheath as a barrier to the action of certain substances. Am J Physiol. 1951 Aug;166(2):229–240. doi: 10.1152/ajplegacy.1951.166.2.229. [DOI] [PubMed] [Google Scholar]
- Camerini-Davalos R. A., Oppermann W., Mittl R., Ehrenreich T. Studies of vascular and other lesions in KK mice. Diabetologia. 1970 Jun;6(3):324–329. doi: 10.1007/BF01212246. [DOI] [PubMed] [Google Scholar]
- Cravioto H. The perineurium as a diffusion barrier. Ultrastructural correlates. Bull Los Angeles Neurol Soc. 1966 Oct;31(4):196–208. [PubMed] [Google Scholar]
- Ditzel J. The in vivo reactions of the small blood vessels to diabetes mellitus. Acta Med Scand Suppl. 1967;476:123–134. doi: 10.1111/j.0954-6820.1967.tb12691.x. [DOI] [PubMed] [Google Scholar]
- Dolman C. L. The pathology and pathogenesis of diabetic neuropathy. Bull N Y Acad Med. 1967 Sep;43(9):773–783. [PMC free article] [PubMed] [Google Scholar]
- EMIROGLU F. The permeability of the peripheral nerve sheath in frogs. Arch Int Physiol Biochim. 1955 Jun;63(2):161–180. doi: 10.3109/13813455509150404. [DOI] [PubMed] [Google Scholar]
- Federman J. L., Gerritsen G. C. The retinal vasculature of the Chinese hamster: a preliminary study. Diabetologia. 1970 Jun;6(3):186–191. doi: 10.1007/BF01212228. [DOI] [PubMed] [Google Scholar]
- Garner A. Pathology of diabetic retinopathy. Br Med Bull. 1970 May;26(2):137–142. doi: 10.1093/oxfordjournals.bmb.a070765. [DOI] [PubMed] [Google Scholar]
- Hildebrand J., Joffroy A., Graff G., Coërs C. Neuromuscular changes with alloxan hyperglycemia. Electrophysiological, biochemical, and histological study in rats. Arch Neurol. 1968 Jun;18(6):633–641. doi: 10.1001/archneur.1968.00470360055005. [DOI] [PubMed] [Google Scholar]
- JAIN S. N., SHARMA A. P. R OLE OF COMPRESSION IN THE AETIOLOGY OF BELL'S PALSY. AN EXPERIMENTAL STUDY. J Laryngol Otol. 1964 Mar;78:266–272. doi: 10.1017/s002221510006206x. [DOI] [PubMed] [Google Scholar]
- KLEBANOFF S. J., GREENBAUM A. L. The effect of pH on the diabetogenic action of alloxan. J Endocrinol. 1954 Nov;11(4):314–322. doi: 10.1677/joe.0.0110314. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC K. Some observations on perfused frog sciatic nerves. J Physiol. 1954 Feb 26;123(2):338–356. doi: 10.1113/jphysiol.1954.sp005055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohner E. M., Dollery C. T., Paterson J. W., Oakley N. W. Arterial fluorescein studies in diabetic retinopathy. Diabetes. 1967 Jan;16(1):1–10. doi: 10.2337/diab.16.1.1. [DOI] [PubMed] [Google Scholar]
- Kristensson K. Transport of fluorescent protein tracer in peripheral nerves. Acta Neuropathol. 1970;16(4):293–300. doi: 10.1007/BF00686894. [DOI] [PubMed] [Google Scholar]
- LEVENE R., LAZZARINI-ROBERTSON A., Jr, FOGLIA V. G., SINGER J. THE RETINA IN EXPERIMENTAL DIABETIC RATS. Arch Ophthalmol. 1963 Aug;70:253–255. doi: 10.1001/archopht.1963.00960050255019. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A. Electrotonus in frog spinal roots and sciatic trunk. Acta Physiol Scand. 1951 Aug 25;23(2-3):234–262. doi: 10.1111/j.1748-1716.1951.tb00813.x. [DOI] [PubMed] [Google Scholar]
- Lampert P., Garro F., Pentschew A. Tellurium neuropathy. Acta Neuropathol. 1970;15(4):308–317. doi: 10.1007/BF00684729. [DOI] [PubMed] [Google Scholar]
- Lieberman A. R. The connective tissue elements of the mammalian nodose ganglion. An electron microscope study. Z Zellforsch Mikrosk Anat. 1968;89(1):95–111. doi: 10.1007/BF00332655. [DOI] [PubMed] [Google Scholar]
- MARTIN K. H. UNTERSUCHUNGEN UEBER DIE PERINEURALE DIFFUSIONSBARRIERE AN GEFRIERGETROCKNETEN NERVEN. Z Zellforsch Mikrosk Anat. 1964 Oct 23;64:404–428. [PubMed] [Google Scholar]
- MAYER R. F., DENNY-BROWN D. CONDUCTION VELOCITY IN PERIPHERAL NERVE DURING EXPERIMENTAL DEMYELINATION IN THE CAT. Neurology. 1964 Aug;14:714–726. doi: 10.1212/wnl.14.8_part_1.714. [DOI] [PubMed] [Google Scholar]
- Mellick R. S., Cavanagh J. B. Changes in blood vessel permeability during degeneration and regeneration in peripheral nerves. Brain. 1968 Mar;91(1):141–160. doi: 10.1093/brain/91.1.141. [DOI] [PubMed] [Google Scholar]
- Olsson Y., Reese T. S. Permeability of vasa nervorum and perineurium in mouse sciatic nerve studied by fluorescence and electron microscopy. J Neuropathol Exp Neurol. 1971 Jan;30(1):105–119. doi: 10.1097/00005072-197101000-00011. [DOI] [PubMed] [Google Scholar]
- Olsson Y. Studies on vascular permeability in peripheral nerves. I. Distribution of circulating fluorescent serum albumin in normal, crushed and sectioned rat sciatic nerve. Acta Neuropathol. 1966 Sep 1;7(1):1–15. doi: 10.1007/BF00686605. [DOI] [PubMed] [Google Scholar]
- Olsson Y. Topographical differences in the vascular permeability of the peripheral nervous system. Acta Neuropathol. 1968 Jan 2;10(1):26–33. doi: 10.1007/BF00690507. [DOI] [PubMed] [Google Scholar]
- Orci L., Stauffacher W., Amherdt M., Pictet R., Renold A. E., Rouiller C. The kidney of spiny mice (Acomys cahirinus): electron microscopy of glomerular changes associated with ageing and tubular glycogen accumulation during hyperglycemia. Diabetologia. 1970 Jun;6(3):343–355. doi: 10.1007/BF01212248. [DOI] [PubMed] [Google Scholar]
- PETTE E., MANNWEILER K., PALACIOS O., MUETZE B. PHENOMENA OF THE CELL MEMBRANE AND THEIR POSSIBLE SIGNIFICANCE FOR THE PATHOGENESIS OF SO-CALLED AUTOIMMUNE DISEASES OF THE NERVOUS SYSTEM. Ann N Y Acad Sci. 1965 Mar 31;122:417–428. doi: 10.1111/j.1749-6632.1965.tb20224.x. [DOI] [PubMed] [Google Scholar]
- Preston G. M. Peripheral neuropathy in the alloxan-diabetic rat. J Physiol. 1967 Apr;189(2):49P–50P. [PMC free article] [PubMed] [Google Scholar]
- RATNOFF O. D., LEPOW I. H. COMPLEMENT AS A MEDIATOR OF INFLAMMATION. ENHANCEMENT OF VASCULAR PERMEABILITY BY PURIFIED HUMAN C'1 ESTERASE. J Exp Med. 1963 Nov 1;118:681–698. doi: 10.1084/jem.118.5.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHANES A. M. Effects of sheath removal on the sciatic of the toad, Bufo marinus. J Cell Physiol. 1954 Feb;43(1):87–98. doi: 10.1002/jcp.1030430107. [DOI] [PubMed] [Google Scholar]
- SHANTHAVEERAPPA T. R., BOURNE G. H. The 'perineural epithelium', a metabolically active, continuous, protoplasmic cell barrier surrounding peripheral nerve fasciculi. J Anat. 1962 Oct;96:527–537. [PMC free article] [PubMed] [Google Scholar]
- Seneviratne K. N., Peiris O. A. The effect of ischaemia on the excitability of sensory nerves in diabetes mellitus. J Neurol Neurosurg Psychiatry. 1968 Aug;31(4):348–353. doi: 10.1136/jnnp.31.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneviratne K. N., Peiris O. A. The effects of hypoxia on the excitability of the isolated peripheral nerves of alloxan-diabetic rats. J Neurol Neurosurg Psychiatry. 1969 Oct;32(5):462–469. doi: 10.1136/jnnp.32.5.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneviratne K. N., Peiris O. A. The role of diffusion barriers in determining the excitability of peripheral nerve. J Neurol Neurosurg Psychiatry. 1970 Jun;33(3):310–318. doi: 10.1136/jnnp.33.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinwall O., Klatzo I. Selective vulnerability of the blood-brain barrier in chemically induced lesions. J Neuropathol Exp Neurol. 1966 Oct;25(4):542–559. doi: 10.1097/00005072-196610000-00004. [DOI] [PubMed] [Google Scholar]
- THOMAS P. K. The connective tissue of peripheral nerve: an electron microscope study. J Anat. 1963 Jan;97:35–44. [PMC free article] [PubMed] [Google Scholar]
- TOUSSAINT D., DUSTIN P. Electron microscopy of normal and diabetic retinal capillaries. Arch Ophthalmol. 1963 Jul;70:96–108. doi: 10.1001/archopht.1963.00960050098015. [DOI] [PubMed] [Google Scholar]
- Trap-Jensen J. Increased capillary permeability to 131-iodide and [51Cr]EDTA in the exercising forearm of long-term diabetics. Clin Sci. 1970 Jul;39(1):39–49. doi: 10.1042/cs0390039. [DOI] [PubMed] [Google Scholar]
- WAKSMAN B. H. Experimental study of diphtheritic polyneuritis in the rabbit and guinea pig. III. The bloodnerve barrier in the rabbit. J Neuropathol Exp Neurol. 1961 Jan;20:35–77. doi: 10.1097/00005072-196101000-00003. [DOI] [PubMed] [Google Scholar]
- WEISS M., ROHLICH P. Significance of the interstice of the peripheral nerve. Acta Morphol Acad Sci Hung. 1954;4(3):309–318. [PubMed] [Google Scholar]



