Abstract
Allosteric regulation of protein function is recognized to be widespread throughout biology; however, knowledge of allosteric mechanisms, the molecular changes within a protein that couple one binding site to another, is limited. Although mutagenesis is often used to probe allosteric mechanisms, we consider herein what the outcome of a mutagenesis study truly reveals about an allosteric mechanism. Arguably, the best way to evaluate the effects of a mutation on allostery is to monitor the allosteric coupling constant (Qax), a ratio of the substrate binding constants in the absence versus presence of an allosteric effector. A range of substitutions at a given residue position in a protein can reveal when a particular substitution causes gain-of-function, which addresses a key challenge in interpreting mutation-dependent changes in the magnitude of Qax. Thus, whole-protein mutagenesis studies offer an acceptable means of identifying residues that contribute to an allosteric mechanism. With this focus on monitoring Qax, and keeping in mind the equilibrium nature of allostery, we consider alternative possibilities for what an allosteric mechanism might be. We conclude that different possible mechanisms (rotation-of-solid-domains, movement of secondary structure, side-chain repacking, changes in dynamics, etc.) will result in different findings in whole-protein mutagenesis studies.
Introduction
Due to the seemingly ubiquitous role that allosteric regulation has in controlling metabolic and signal transduction pathways, it has been called “the second secret of life” (1, 2). Despite its central role in living systems, there is a general lack of understanding of the details that contribute to the molecular/atomic level mechanism of allosteric regulation for individual proteins. In particular, little is known about how regions between two communicating binding sites on a protein contribute to allostery. In a large protein, this “great-in-between” can span distances of tens to hundreds of Ångstroms. Studying areas between the two relevant ligand binding sites is the current focus of not only our research, but also of others interested in characterizing allosteric systems. These systems can be of two types: V-type allostery, in which effector binding alters the rate of catalysis, and K-type allostery, in which effector binding alters substrate affinity. It is this second K-type allostery that is the focus of this article.
Whole-protein, site-directed mutagenesis is the approach that we consider herein as a potential tool to evaluate the role of the “great-in-between” in allosteric function. Regarding data analyses in such a study, we explore possible outcomes of the mutagenic probing of allosteric proteins and how mutational studies might best be designed and interpreted to gain insights into allosteric mechanisms. The concepts of induced-fit and conformational selection, which sometimes influence interpretations of mutational analyses of allostery, are also discussed, even though they are not directly relevant to all definitions of allostery; however, rather than compare and contrast allostery definitions (the focus of Fenton (1) and Fenton et al. (3)), we will, instead, relate them to the energy-cycle definition of allostery.
As an example of the strategy of using mutagenesis to probe which residues contribute to determining the magnitude of allosteric coupling (Qax, defined below), we recently published a mutagenesis probing study of the Fru-1,6-BP (fructose-1,6-bisphosphate) allosteric binding site of human liver pyruvate kinase (hL-PYK) (4). L-PYK is allosterically activated by Fru-1,6-BP and allosterically inhibited by alanine. The outcome of both of these allosteric responses is a modification in the apparent affinity of the hL-PYK for its substrate phosphoenolpyruvate. An advantage to working directly within an allosteric binding site, as in our study above (4), is that we can start with the assumption that some part of the local region in that site must of necessity contribute to allosteric function. Although we will not have that same advantage when considering the expanded area between binding sites, functional outcomes from the previous study will be useful empirical examples for this discussion. Because the reference mutagenesis data set derives from the study of a PYK isozyme, we will use the study of allostery in isozymes from the PYK family of proteins as a convenient reference throughout this article. Also, due to the enzymatic nature of PYK, we will refer to the two relevant ligands as substrate and effector; however, all discussion points included herein are applicable to allosterically regulated binding proteins that lack enzymatic functions.
Materials and Methods
Qax is the coupling constant of an equilibrium allosteric mechanism
Defining allostery as an equilibrium mechanism
To focus on the function side of the structure/function relationship, we define allosteric function in a heterotropic K-type system (the primary focus herein) as how an enzyme binds a substrate differently in the presence, versus in the absence, of an allosteric effector (1). This definition also includes the assumption that the effector binds at a site distinct from the active site, and requires a consideration of the four enzyme forms (the free enzyme and three enzyme complexes) that constitute the four corners of a thermodynamic energy cycle (Fig. 1). In this allosteric energy cycle, an enzyme/protein/macromolecule (E) can bind one substrate (A) and one allosteric effector (X). Kia is the binding of the substrate to the enzyme in the absence of effector. Kia/x is the binding of the substrate to the enzyme in the presence of saturating concentrations of effector. Kix is the binding of effector to the enzyme when substrate is absent. Kix/a is the binding of effector to the enzyme in the presence of saturating concentrations of substrate. Allosteric coupling is defined as (1, 5, 6, 7):
| (1) |
Note that according to our definition of allostery, when Qax ≠ 1, there is allosteric coupling between the binding of A to a protein and the binding of X to the same protein; Qax = 1 indicates that the system is not allosteric. Because Qax is a ratio of binding constants, the magnitude of this allosteric specific parameter is not dictated by the magnitude of any one ligand binding constant. This fact allows for the possibility that the enzyme residues that contribute the most to ligand binding might be different from those that contribute to allostery (4, 8, 9, 10). The ability to quantify the magnitude of the allosteric response offers an opportunity to probe which residues and regions of the enzyme are important in determining that magnitude. However, without incorporating a probing approach, a single measurement of the magnitude of the allosteric coupling constant (Qax) of the wild-type enzyme does not provide insights into the mechanism that gives rise to allostery in that enzyme. For this reason, there is an appeal to the idea of using mutations to probe individual residue positions and asking if the modification results in a change in the magnitude of Qax.
Figure 1.
Reaction scheme for an allosteric energy cycle in which an enzyme (E) can bind one substrate (A) and one allosteric effector (X).
Word use
With this brief definition of what allostery is (and before highlighting what it is not), we will carefully define words that are used to transition between structure and function concepts. The words structure, conformation, ensemble, dynamics, and conformational changes are used in very different contexts throughout the literature. Therefore, it is necessary to clearly state how we will use these words here. Because changes in the structural ensemble as detected by solution techniques are commonly interpreted as conformational changes, we use conformation to refer to the average of the various unique three-dimensional folds/structures that constitute an ensemble under any given condition (e.g., the presence of a given ligand, an enzyme complex). Unique three-dimensional protein folds/structures determined by high-resolution x-ray crystallography will be called “structures”. Therefore, multiple structures for a given protein as determined by x-ray crystallography may be represented as different fractions of the ensemble of structures that are present under a given condition (e.g., the presence of a given ligand, an enzyme complex). Again, the average of those represented structures in the ensemble is the conformation. The term “conformational change” will be reserved for cases when the average of the ensemble shifts.
Due to the equilibrium nature of allostery, the dynamics that are relevant to allosteric regulation are those motions present at equilibrium, which are different among the four equilibrated enzyme forms (E, EA, XE, and XEA in Fig. 1). Therefore, dynamics are the fluctuations that a single protein molecule will sample around the conformational average given sufficient time, and are equivalent to the sum of all of the unique structures that are present in the ensemble of molecules at a single snapshot in time. For considerations of allostery, changes in dynamics among the four allosterically relevant enzyme forms include changes in motion amplitudes. Based on this discussion, we define the allosteric mechanism as those changes in the conformation and/or dynamics that contribute to the modified ligand binding affinity as quantified in Qax.
In contrast to the equilibrium-related dynamics, we will use the term “conetics” (conformational kinetics) to refer to the structural changes that occur in transitioning from one conformation to another. Our hope is that this term will avoid the confusion and assumptions often resulting from the widespread use of the term “kinetics” to describe a breadth of behaviors and concepts. Conetics is applicable to both conformational-selection and induced-fit, and is independent of whether the structural changes are causative (i.e., effector binding causes change A; change A causes change B; change B causes change C, etc.) or temporal (each of the changes, although associated with the original binding event, may be independent of other changes).
What allostery is not
Our definition of allostery (Eq. 1) designates this phenomenon as an equilibrium process. Despite the equilibrium nature of allostery, proposed mechanistic models of allostery are often envisioned as being initiated by an association event between the effector and protein, followed by a sequential series of changes (i.e., causative in nature with effector binding causing change A; change A causing change B; change B causing change C, etc.) in a pathway of residues that results in modification of the active site (11, 12, 13, 14). Unfortunately, such models incorrectly imply that it is the conetics of the transitions among conformational forms of an enzyme that are important to an allosteric mechanism (3). Because conetics (e.g., induced-fit or conformational-selection; see below) are not state functions, the conetics immediately after association of the protein with a ligand are not required to be the reverse of the conetics that occur when a protein-ligand complex dissociates (15). Furthermore, each protein molecule in a sample could experience a unique conetic pathway as it transitions among its various liganded states (see Fig. 1). There is currently considerable attention devoted to identifying structural/dynamic changes, evaluating the timing/rates of changes, and determining the causative/temporal relationships of changes that contribute to conetics. Although such studies can certainly illuminate important details about protein structure and/or function, an understanding of these conetics-associated topics is not necessary to evaluate equilibrium processes, including allostery.
We admit that it is challenging to identify words that are consistent with the equilibrium nature of allostery and do not imply a conetic mechanism (e.g., effects are propagated away from an effector binding site; an effector elicits a response; binding energy is transduced through the protein; etc.). Therefore, we find it especially important to emphasize the equilibrium nature of allosteric regulation. As a result of the equilibrium nature of allostery, we are not seeking a sequential chain of events when we characterize an allosteric mechanism; instead, we are looking for differences among the equilibrated conformations of the enzyme forms included in Fig. 1.
V-type allostery is also equilibrium in nature
Although considerations in this article primarily focus on K-type systems, V-type allosteric systems have been studied for some time (16, 17, 18, 19). The potential role of dynamics in allosteric mechanisms has received attention in proteins that display modified Vmax activity in response to allosteric effectors (20, 21). A V-type change implies that when an effector is bound to a protein there is a change in the rate-determining rate constant that contributes to the catalytic mechanism or that some other step becomes rate-determining. However, the modified protein dynamics that might contribute to this V-type response are still those that are present in a protein complex that is equilibrated with the effector bound. We should not entertain the notion that V-type allostery is a result of the effector/protein association event initiating an energy wave that alters the probability of formation of the catalytic transition state and that the allosteric effect only occurs during that very short time in which the energy wave passes through the active site. In contrast to that traveling energy wave (i.e., conetics) idea, in an equilibrated complex in which an allosteric effector is present at saturating conditions, the effector is not cycling on and off the protein at a rate comparable to the rate of catalysis. Instead, the bound effector likely alters the equilibrated protein dynamics to increase (for an activator) the likelihood of the transition state being formed (22, 23, 24).
One interesting possibility for a V-type allosteric system is if product release is the rate-limiting step. In this case, the influence of an allosteric effector on the protein’s affinity for product could give rise to modified Vmax activity via a mechanism very similar to that of a K-type system (25, 26).
Is Qax the only useful parameter in a mutational study of allostery?
By detailing what an allosteric mechanism is and is not, we hope to emphasize the equilibrium nature of an allosteric mechanism. This emphasis also focuses attention on the importance of Qax as a parameter specific for allosteric function (3). To appreciate the full implications of Qax, let us consider a simple schematic of a protein in which the active site and the allosteric site communicate using an (oversimplified) linear network of residues (Fig. 2). Please note that we used the term “pathway” to denote a series of causative changes in which effector binding causes change A; change A causes change B; change B causes change C, etc. In contrast, we use the term “network” to denote the relative locations of residues that contribute to allosteric function, with no implication for a series of changes. If we make a mutation in this network (Fig. 2, point A), we anticipate that Qax will be modified, unless there is compensation (see below). Will Kia, Kix, or both, also be modified?
Figure 2.

Oversimplified protein with one active site and one allosteric site and a linear network of residues that contribute to the allosteric mechanism (arrow). Point A marks a residue position that contributes to the native allosteric mechanism in the wild-type protein. A mutation at point A is expected to modify Qax, but it might also alter Kia, Kix, or both. Point B marks a residue position that does not contribute to the native allosteric mechanism. Nonetheless, a mutation at point B has the potential of causing structural changes that alter the allosteric mechanism. To see this figure in color, go online.
For demonstration, let us consider introducing a mutation that is directly in the binding site for the allosteric effector. Mutations in this effector binding site, by necessity, either play a role in the allosteric mechanism or are very close to residues that do so. It is likely that a mutation in the effector binding site could be identified that modifies substrate affinity by mimicking some allosterically relevant change associated with effector binding to the wild-type protein. (The S531E mimic of Fru-1,6-BP activation of hL-PYK is as an example of this (4).) Therefore, a mutation in the effector site might modify Kia for binding of the substrate in the active site. The influence of a mutation on Qax could result from the fact that the mutation already caused the allosterically relevant change (compared to wild-type), thus binding of the effector does not elicit the change in the mutant enzyme that it does in the wild-type protein. In this example, both Qax and Kia would be modified due to the effector site mutation.
We can also envision a scenario in which Qax is sensitive to a point mutation, but Kia is not. In this scenario, the effector site mutation might remove a key allosterically relevant side chain, such that effector binding fails to elicit an allosteric response. (A loop deletion mutation in the 527–533 loop of hL-PYK is an example of this possibility (4).) In this case, there would be no allosteric response upon effector binding to the mutant protein (Qax = 1), and the substrate affinity of the mutant protein in the absence of effector would be unperturbed.
The two scenarios just introduced can be replicated for mutations made in the active site while monitoring Qax and effector binding (Kix). The result of the two effector-site mutation scenarios and the two active-site mutation scenarios is that we can envision that Kia and Kix values may be modified when an allosterically relevant residue is mutated. Without additional considerations, an evaluation of the influence of mutations on Kia and Kix seems important for a study of allostery. However, before attempting to interpret mutational effects on Kia or Kix, consider that even proteins that bind only a single ligand (i.e., are not allosteric) will have modified ligand affinity if the correct mutation is introduced. Furthermore, areas of a simple binding protein that are far from the binding site can play a role in binding. (Energetic coupling between a single binding event and a protein residue that is distant from the binding site has been referred to as allosteric, although it does not fit our definition of allostery (1).) Because binding constants can be modified by mutations distant from the binding site in proteins that are not allosteric, we can extrapolate to allosteric systems and state that the influence of a mutation on the affinity of the protein for one ligand (Kia or Kix) in the absence of a change in Qax does not necessarily indicate that the mutation has perturbed the native allosteric mechanism. Therefore, we reaffirm that by our definition of allostery, Qax is the relevant parameter to be evaluated in a mutational study of allostery, and Kia and Kix should only be considered while also evaluating Qax.
Results and Discussion
Potential allosteric mechanisms and predicted outcomes of mutations
Given the above reminder that allostery is an equilibrium process and that we can monitor changes in Qax as an allosteric specific readout, we can next consider potential allosteric mechanisms and what a mutational study of each mechanism might reveal.
Rotation of solid domains in allostery
Structural comparisons of various enzyme forms of a given enzyme often report rotation-of-solid-domains. Although not confirmed, the suggestion that the allosteric mechanism of PYK functions by rotation-of-solid-domains serves as an example (27, 28, 29). In this section, we will consider this rotation-of-solid-domains mechanism and use the illustrations to conclude that a whole-protein mutagenesis study of a protein that uses this allosteric mechanism might only identify a few residues involved in the relevant domain contacts as having roles in allostery.
To appreciate this rotation-of-solid-domains mechanism, let us simplify a protein with three domains into three solid geometric shapes connected to a solid surface (Fig. 3 A). We will represent potential interactions between domains as being complementary in shape. Furthermore, we will restrict motion of the two outside domains (A and C) and allow only the middle B domain to move. In the absence of ligands, the protein is an ensemble of many conformations. In Fig. 3, we include three unique structures to represent the ensemble; in contrast to this oversimplified illustration, we anticipate a very large number of structures to be represented in a real protein ensemble.
Figure 3.
The allosteric energy cycle included in Fig. 1, expanded to allow each enzyme form to be an ensemble of structures. (A) An introduction to the three structures present in each ensemble. The hypothetical protein has two stationary domains (A and C) and a domain that is attached by a flexible tether (B). Domain-domain interactions and protein-ligand interactions in this simplified model are restricted to shape complementation. (B) For each of the four enzyme forms, the presence of ligands competes with the docking of domain B to the other two domains. This influence of ligands on the population of structures present in each ensemble is presented as bar graphs above each structure. To see this figure in color, go online.
Despite the potential of the B-domain to move in the absence of ligands (the free enzyme in Fig. 3 B), stabilizing interactions with other domains will result in the population of protein molecules that have the B domain docked to either the A or the C domains being higher than the population of molecules with the B domain at intermediate positions between A and C. Based on the equilibrium concepts just discussed, we can envision that bound inhibitor simply blocks the potential of forming the stabilizing interactions between the B and C domains (compare free enzyme and EX forms of the enzyme in Fig. 3 B). Once inhibitor is bound and due to the flexibility of the B domain tether, this B domain will diffuse within a constrained space until new stabilizing bonds are formed (between the A and B domains in our example) and a new equilibrated ensemble is reached. Due to the bonding potential between A and B, the ensemble will now include more protein molecules in which the B domain is docked to the A domain, as opposed to the B-domain being bound to the now effector-blocked C domain or at various intermediate positions in the space between domains A and C. A parallel discussion can focus on the influence of protein-bound substrate on the B domain (compare free enzyme and EA forms of the enzyme in Fig. 3 B).
Upon formation of either of the two binary complexes (EA and XE), one of two possible docking sites for the B domain is removed. This loss of one docking site for the B domain increases the effective concentration of the unbound B domain. Therefore, binding substrate or binding inhibitor alone increases the effective concentration of the unbound B domain. That increase in effective concentration of B domain acts as a competitive inhibitor: when substrate is added to the XE complex, the increased effective B domain concentration competes with substrate for binding to the A domain. If we return to our original definition for allostery, substrate can bind easier to the free enzyme than it can to the XE complex. The outcome of this example would be allosteric inhibition. In our illustration, at increased concentrations of substrate and inhibitor, a condition under which simultaneous binding of both ligands occurs, the B domain will not likely be docked to either the A domain or the C domain (see the fractional population of the XEA complex in Fig. 3 B). Overall, each enzyme form will have a unique conformation. Based on these considerations, we find it feasible that rotation-of-solid-domains could then give rise to Qax ≠ 1 as defined in Eq. 1.
A primary reason for illustrating a potential allosteric mechanism is that it helps one to predict potential outcomes of a mutational study of a protein using that mechanism. In a potential rotation-of-solid-domains mechanism, a very limited number of interactions on the surfaces of the rotating domains may be sufficient to give rise to an allosteric mechanism. In turn, if a rotating domain allosteric mechanism is present in a protein, a whole-protein mutagenesis study might identify only a few residues involved in the relevant domain contacts as having roles in allostery.
Although rotation-of-solid-domains can give rise to allosterism, it should be noted that the rotation-of-solid-domains in response to the binding of one ligand (we will continue to call this an effector for consistency) does not necessarily elicit an allosteric response on a second (e.g., substrate) binding event. A possible example of such a rotation in the absence of allosterism was observed with PYK. Following earlier scattering applications (30), we revisited the potential of a rotation-of-solid-domain allosteric mechanism in PYK using small-angle x-ray scattering. We observed that even effector analogs that do not elicit an allosteric response cause a large conformational change as detected by scattering (31). Assuming that the conformational changes detected by scattering studies reflect the rotation-of-solid-domains observed by comparisons of x-ray crystallography structures, we conclude that although the rotation-of-solid-domains may be associated with effector binding, it is not sufficient for allostery. Our small-angle x-ray scattering study did not discriminate whether the allosteric mechanism is a result of changes that are in addition to the binding-associated rotation-of-solid-domains or if the allosteric relevant changes are completely independent of the rotation-of-solid-domains; however, our findings with PYK introduce the concept that large structural changes can be associated with effector affinity even when Qax = 1.
Fig. 4 illustrates how domains can be changed after the effector (again, this term is used for nomenclature consistency) binding, but with no influence on substrate affinity. Fig. 4 is similar to Fig. 3, with the exception that the active site has been moved to domain C. As described in Fig. 4, the changes in domain positions that occur due to effector binding can occur in this model; however, the substrate binding site on the far right side of the C domain is not influenced by the B domain rotations. This new scenario would not satisfy our definition of allostery, because binding of the effector would not influence substrate binding (i.e., Qax = 1). This potential for large conformational changes associated with a single ligand binding event is a primary reason why conformational changes alone should not be used to define allostery (1).
Figure 4.
A protein that binds two ligands in a nonallosteric manner. In contrast to the example in Fig. 3, the active site has been moved to the outside of domain C. In this example, the nonsubstrate ligand is labeled as an effector to be consistent with Fig. 3. When this effector is bound to the protein, it modifies domain contacts, resulting in a solid domain rotation. Those domain rotations do not, however, influence substrate binding. To see this figure in color, go online.
Shifting secondary structure and side-chain repacking
A question that has yet to be addressed in the study of allostery is the resolution at which allostery occurs (e.g., subunit changes, domain changes, secondary structure changes, residue changes, combinations of the above, etc.). The schematic in Fig. 3 serves as a general discussion for all of these levels of resolution. For example, assume that the three geometric shapes included in any one structure in Fig. 3 (A–C) might represent three aligned helices within a domain. This new higher-resolution model would imply that shifts in secondary structure could give rise to allostery, in which case, a whole-protein mutagenesis study would be expected to identify allosteric roles for residues at the interface of secondary structures.
We could further increase the resolution of Fig. 3 by considering each of the three geometric shapes in a given structure to represent three amino acids. At this resolution, the schematic in Fig. 3 would represent side-chain repacking due to ligand binding. If allosteric mechanisms function through altered side-chain packing, a whole-protein mutagenesis study would be expected to identify a network of residues that connect the active site and the effector site.
Importantly, there is no reason to dismiss the notion that multiple levels of resolution might simultaneously contribute to an allosteric mechanism. As one example, a domain rotation might stabilize side-chain repacking in a neighboring domain. Therefore, a whole-protein mutagenesis study might identify only residues on the surface of one domain, but a network of residues in the second domain.
Allostery by changes in protein dynamics
Potential allosteric mechanisms are not limited to conformational changes, and can include changes in protein dynamics. In many discussions of the contributions of dynamics to allostery, however, the fact that allosteric regulation is an equilibrium process is absent. Qax can be expressed in free energy (1, 5, 6, 7, 32):
| (2) |
where ΔGax is the free energy for the following disproportionation equilibrium:
| (3) |
As with the ΔG for any other reaction, ΔGax includes contributions from both ΔHax and ΔSax. Any changes in the dynamic properties of a protein that are associated with allostery are expected to contribute to the ΔSax. Despite the fact that energy cycles, with associated Qax, ΔGax, and ΔSax parameters, have been used to consider allosteric systems since the 1970s (7), the much more recent ability to monitor dynamics in proteins using nuclear magnetic resonance has made it fashionable to emphasize how protein dynamics might contribute to allosteric mechanisms.
As noted early in this article, it is the differences in the dynamics of the four equilibrated enzyme forms (E, EA, EX, and EAX) included in the allosteric energy cycle that are of relevance to allosteric regulation. Amplitudes of motions are included under this heading of dynamics. An increase in the amplitudes of motions would translate into an increase in the number of structures included in the ensemble and a broadening of the ensemble distribution. An increase in the amplitudes of dynamic motions can be accommodated, however, with no change in the average conformation. This concept is the basis of the idea that allosteric regulation can occur without conformational change (33); however, in studies of allostery, the absence of evidence for a conformational change (e.g., no difference in crystal structures) should not be interpreted as evidence for the absence of that change.
The dynamics contributing to allostery can occur at multiple levels of resolution (e.g., change in amplitudes of domain rotations, secondary structure shifts, side-chain repacking, etc.) (34). Dynamics of each level of resolution occur on different timescales, and therefore, detecting the dynamics of each level of resolution requires different techniques (35, 36). The two methods that have become popular for simultaneously measuring dynamics of a large fraction of a protein’s structure are nuclear magnetic-resonance relaxation methods (37, 38, 39, 40, 41, 42) and hydrogen/deuterium exchange as detected by mass spectrometry (43, 44, 45, 46, 47).
One study of protein dynamics in an allosteric protein reports that methyl-containing side chains show dynamic motions in a range of times until the two energetically coupled ligands are simultaneously bound (37). Upon formation of that ternary complex, residues that form a network between the two ligand binding sites experience enhancement of conformational motions on the millisecond timescale. Although this methyl labeling-based technique monitors only methyl-containing side chains without following other motions in the protein, a whole-protein mutational study would likely identify a network of residues connecting the active site and the effector site, given that a network of fluctuating side chains has been observed in that protein.
Conformational-selection and induced-fit association models
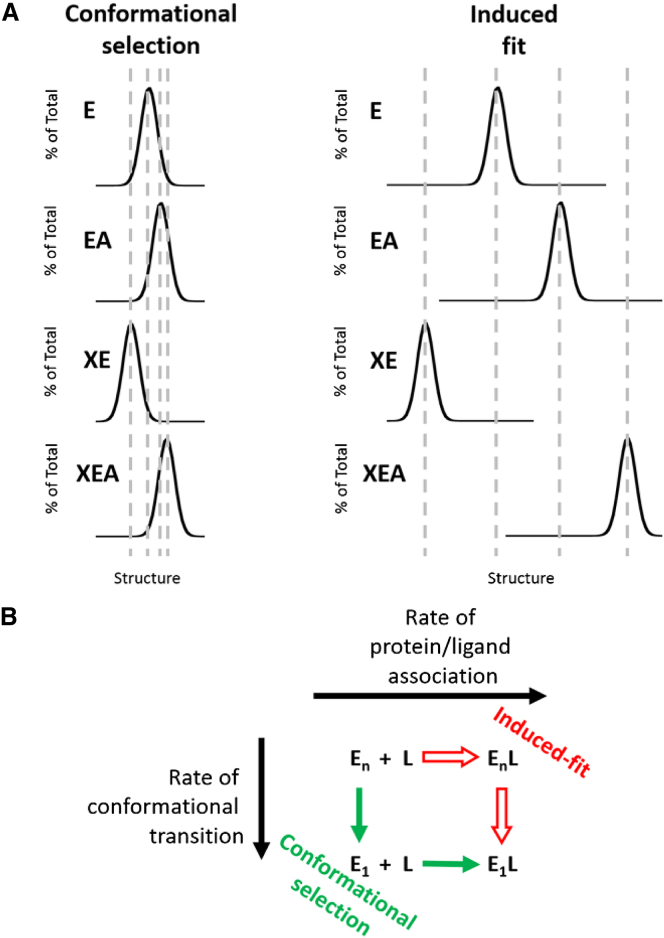
How does our treatment of events associated with ligand binding take into account these two conceptually different models, both of which relate equilibrated protein conformations with the conetics associated with ligand-protein binding? In Fig. 3, we include only three structures to represent the free enzyme ensemble, and for simplicity, include the same three structures in each structural ensemble of each enzyme form with bound ligand(s). As a result of this simplification, this figure is unintentionally more consistent with a conformational-selection model. In contrast, the induced-fit model would show the full set of ensemble structures to be present for a particular enzyme form only when ligand was bound. Despite the oversimplification of the illustration in Fig. 3, all discussion points included in this article accommodate the equilibrium components of either conformational-selection or induced-fit association models equally well.
These two models are compared in Fig. 5, where it can be observed that the key difference between the equilibrium components of both is the overlap of the structures represented in the enzyme form ensembles (Fig. 5 A). A fair question to ask is whether in an allosteric energy cycle, conformational selection could play a role in transitioning between some enzyme forms, while induced-fit could play a role in the transition between other enzyme forms. At the conetic/kinetic level (Fig. 5 B), conformational-selection and induced-fit models can be considered as mirroring kinetic differences, in that conformational change occurs either faster than protein/ligand association in the former or slower in the latter (48, 49, 50). The treatment of conformational-selection and induced-fit models as mutually exclusive is likely not justified, as both could simultaneously contribute to an association event (51). Most importantly, binding is an equilibrium process, and distinguishing between these two models for protein-ligand association events is not necessary to evaluate the equilibrium binding constant for any one binding event. As an extension of the simpler single ligand binding, we do not need to understand conetic processes to evaluate allostery as defined herein.
Figure 5.
Conformational-selection versus induced-fit. (A) Ensemble distribution consideration of induced-fit versus conformational-selection in the ligand association events. Rather than limiting the structural ensemble to three structures as presented in Fig. 3, a more realistic ensemble distribution would include many structures that would constitute a bell-shaped distribution. A conformational-selection mechanism for ligand association events would indicate that the structures present in each protein form are also present at various abundances in other protein forms; therefore, ligand binding stabilizes one conformation and causes a redistribution of the ensemble. In contrast, an induced-fit mechanism for ligand association would indicate that those structures present in a given ligand-bound protein form are not present in other protein forms. (B) The differences between conformational-selection and induced-fit mechanisms for ligand association are a result of whether the rate of transitions between other structures in the ensemble (En) to the ligand binding competent structure (E1) occurs faster or slower than the association rate of the ligand to enzyme. As concluded in the text, we do not need to understand conetic processes associated with ligand association to evaluate allostery, which is the comparison of multiple binding parameters. To see this figure in color, go online.
Challenges of interpreting changes in Qax in terms of molecular mechanisms of allostery
The purpose of this section is to highlight challenges associated with interpreting changes in Qax to facilitate insights into molecular mechanisms, and when possible, provide experimental designs that can be used to address these challenges.
Mutation of residues not involved in the native allosteric mechanism can modify Qax
We highlight above that Qax is the primary parameter that should be evaluated in mutational studies aimed at elucidating residues that contribute to an allosteric mechanism; however, when a mutated position does result in Qax being modified, this does not necessarily mean that position is important in the native allosteric mechanism present in the wild-type enzyme. A change in Qax resulting from a mutation could be the result of either (1) the removal of the original side chain that contributed to function in the wild-type protein (loss-of-function), or (2) the introduction of a new function due to the physicochemistry associated with introducing the replacement side chain (gain-of-function). In Fig. 2, we can envision a residue of the enzyme for which the side chain does not contribute to ligand binding or to the native allosteric mechanism (Fig. 2, point B); we will consider that residue to be valine for the point of this discussion. If this valine residue is replaced by an isoleucine residue, it is possible that the protein can rearrange to accommodate the introduced greater bulk of the side chain, but the overall rearrangement could modify the allosteric mechanism. This type of idea is often expressed in terms of whether a mutation has a global effect (the type of overall protein rearrangement just described) versus having only a local effect (direct interactions with the side chain of interest). Now recall that we do not know the protein resolution at which allostery functions. Therefore, we have no way of knowing how extensive a global change might need to be before it could influence the allosteric mechanism. Although alanine is a reasonable first choice to substitute as an alternative side chain, even that does not completely escape the potential for a gain-of-function outcome because of its own nonneutral properties, such as increasing helix propensity (52).
For those modifications that alter the value of Qax, the challenge then becomes determining whether the residue in question plays a direct role in the native allosteric mechanism present in the wild-type enzyme, or instead reflects a gain-of-function mutation at a site that does not have a role in allostery in the wild-type protein. Without a sufficient number of examples, we have no estimate of the likelihood of observing the latter outcome. As with mutational studies of any other protein function, a common approach to resolve this question is to make a series of substitutions to determine which side-chain properties do and do not modify function (i.e., Ka, Kix, and Qax in this study). This strategy was used at each residue position in hL-PYK to investigate its allosteric effector binding sites (4).
Qax is a composite term
Any single substitution at a given residue position may cause multiple perturbations in the protein, and these various perturbations can potentially compensate for each other in a given protein function (1). “Compensation” is often associated with a compensation between ΔH and TΔS, and contributions from these two factors may fully or partially cancel each other in the overall ΔGax associated with allosteric coupling. The extreme case of complete compensation results in the substrate binding differently at the structural/ΔH levels in the presence of the effector, but with the same ΔGa observed in the absence of the effector (31, 53, 54, 55). As a second type of compensation, consider two ligand binding sites coupled through two different (and potentially independent) networks. The ΔGax contributions from these two networks may fully, or partially, compensate in the overall ΔGax (56). With these two types of compensation in mind, it follows that a mutation might modify both ΔH and ΔS and/or two different communication networks contributing to Qax/ΔGax. In fact, these compensating mechanisms may be one reason that chemically dissimilar side chains substituted at a given position can result in similar protein function (57). It follows, then, that deciphering at the molecular level how a single mutation alters different parameters contributing to the measured Qax, is another challenge in interpreting a change in the value of Qax. Thus, even though any change in Qax that results from a point mutation can be assigned as resulting from the introduced modification, neither that modification, nor the change in Qax on its own, provides insights into the molecular details of the pertinent changes throughout the protein (conformational or dynamic) that give rise to that observed change in Qax.
In the case of complete compensation, residues that participate in an allosteric mechanism may fail to be identified in a mutagenesis study. For a screening approach to identify such residues, the introduction of multiple substitutions at a given position greatly increases the likelihood of identifying substitutions that do not introduce complete compensation, as a perturbation in Qax will likely be observed for at least some residue substitutions. As noted above, this strategy of introducing a series of substitutions at given protein positions was used to investigate the allosteric effector binding sites of hL-PYK (4).
Changes in homotropic cooperativity influence the observed magnitude of heterotropic allostery
In addition to the compensation mechanism just described, the extent of homotropic cooperativity among multiple substrate binding events and/or homotropic cooperativity among multiple effector binding events can influence the magnitude of the heterotropic coupling measured in an allosteric system (58). This is an important consideration given that many allosteric proteins also display homotropic cooperativity in ligand binding (indicated by the Hill coefficient, nH). However, Qax is only influenced if the extent of homotropic cooperativity of binding of one type of ligand (e.g., substrate) is modified when the second ligand type (e.g., effector) is prebound to the protein (5, 6, 7, 32). Unfortunately, due to the scope of a potential whole-protein mutagenesis study, determining nH values associated with effector binding for all mutant proteins is likely not feasible. Nonetheless, we can include mutation-induced changes in homotropic cooperativity as one potential cause of the perturbations that result in a modification in the apparent Qax detected for individual mutant proteins. The influence of mutations on cooperative ligand binding can then be evaluated in follow-up studies.
Mutations can modify the energetic reference state
The energy cycle and respective binding constants represented in Fig. 1 can be evaluated as an energy diagram (7, 34, 59, 60). On an energy diagram (Fig. 6), binding of both the substrate (A) and the effector (X) is favorable; thus, the respective EA and XE complexes are lower on the energy diagram than the fully folded free enzyme (E). The energy difference between the enzyme forms (the vertical separation between enzyme forms in Fig. 6) represents the ΔG associated with the respective binding event. Likewise the separation between the free enzyme and the ternary XEA complex represents the Gibbs free energy difference between these enzyme forms, and ΔGax (i.e., ΔGa+x – [ΔGix + ΔGia] (7)) is the free energy associated with Qax. This energy diagram highlights the fact that the energies of all enzyme forms in the energy cycle are related to that of the free enzyme, E.
Figure 6.
Energy diagram of the allosteric energy cycle in Fig. 1, with the added energy state of the denatured protein. To see this figure in color, go online.
Using the energy diagram, we can again ask what is the outcome resulting from the creation of a mutation in the protein. The mutation might change the energy state of the EA complex, i.e., change ΔGa and thus the measured Kia, or the mutation might change the energy state of the XE complex, thus modifying ΔGx and Kix. However, the mutation might also change the energy state of the XEA complex, or it might even change the energy state of E, the reference state. Likely, all four enzyme forms in the allosteric energy cycle will have modified energy as a result of introducing a mutation. Of course, when binding and allosteric functions are measured, we are witnessing the effect of differences in energy states and not detecting the actual energy states themselves.
When functions of mutated proteins are compared to those of the wild-type protein, an intrinsic assumption is usually made that the energy of the reference state has not been altered. In other words, the free-enzyme form of the wild-type protein and the free-enzyme form of the mutated protein are assumed equivalent. Unfortunately, we have no way to definitively confirm this assumed equivalence. Many studies report similarities in measurable properties of folded protein for mutant and wild-type proteins, such as equivalent circular-dichroism spectra or similar structures determined by x-ray crystallography. However, because we do not understand the resolution at which allostery functions or the potential contribution of dynamics, even these structure-related quality controls may not indicate an energetic equivalence of unliganded wild-type and unliganded mutant proteins. One might be tempted to suggest that the denatured wild-type and mutant proteins could provide a common reference state (Fig. 6). It has been argued, however, that the denatured protein is not a true random coil; thus, mutants could modify the potential residual structure that remains in this denatured state (61, 62). Consequently, the denatured state also does not necessarily offer a reference state that is equivalent in wild-type and mutant variants.
Without a common reference state, the energy cycle for the mutant protein can never truly be related to that of the wild-type protein. For all other challenges noted in this section, we offer either an experimental design or an interpretation that is useful to address the challenge; however, for the potential influence of a mutation on the reference state, we are left with the qualitative comparison of Qax. Thus, one must realize that the ratio of binding constants that define Qax reflects differences in energy states of enzyme forms in which the free-energy reference states for wild-type and mutant proteins may not be equivalent.
Alanine-scanning mutagenesis, while monitoring Qax
We conclude that the use of a range of substitutions at each position in a protein would be the ideal approach when using mutagenesis to screen for positions that contribute to allosteric function. Although we used this strategy to investigate the allosteric effector binding sites of hL-PYK (4), many allosteric proteins are so large that using a multiple-substitution-at-each-position approach would be excessively laborious. Deep mutational scanning (63) could be useful, but would require the development of genotype-phenotype coupled assays that are sensitive to allosteric functions. To address the drawback of working with excessive numbers of mutant proteins, we noted in our previous study that several of the overall conclusions could also be drawn after considering the subset of mutant proteins in which only alanine was used as a substitution (4). Therefore, we propose using whole-protein alanine-scanning mutagenesis (64, 65) as a first step to identify residues that might contribute to allosteric mechanisms.
In essence, the influence of an alanine-scan on the magnitude of Qax can be used as a first-pass assessment if the mutated position does contribute to allosteric function. The obvious benefit of considering an alanine-scanning approach as a first-pass screen is the more manageable number of mutant proteins to be characterized (e.g., there are fewer than 500 non-alanine/non-glycine residues in hL-PYK). As discussed above, we anticipate that such a first-pass screen would fail to identify residue positions at which alanine can fulfill the same allosterically relevant role as the native residue (i.e., full compensation with no or minimal change in Qax). This cautionary note parallels that offered for the use of alanine-scanning mutagenesis to evaluate hot spots in protein-protein interactions (66). This possibility would result in false-negative data for the role of residues that contribute to the allosteric function, and unfortunately, those false-negative positions would not likely be further investigated. In contrast, if positions in the protein identified for allosteric roles from the alanine-scanning approach are subsequently subjected to mutations using a range of substitutions, false-positives due to gain-of-function mutations would likely be identified. Therefore, a reasonable overall strategy would be to use whole-protein alanine-scanning mutagenesis to identify residues that potentially contribute to allosteric mechanisms, with follow-up studies using a range of substitutions at each position identified.
Conclusions
Creating mutations has been a longstanding tool to probe complex protein functions. The use of mutations becomes more challenging as extended regions of a protein (the “great-in-between”) contribute to the function being studied and the number of positions to be probed increases. Fortunately, improved high-throughput techniques have facilitated mutagenesis studies involving large sets of mutant proteins, including mutational evaluations of allosteric mechanisms (4, 17, 57, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78). A whole-protein mutagenesis design (possibly initiated with an alanine-scan) seems like a logical next step to identify residues/positions that contribute to allosteric mechanisms, and of course, a range of residues should be substituted at each position of interest. It might be noted that the logic in such a mutagenesis approach is opposite to that used in the more common structural comparisons, given that one first attempts to identify which protein residues contribute to the allosteric mechanism, but reserves hypothesizing on the mechanism until after the actual allosterically relevant residues have been identified. Also, a whole-protein mutagenesis study is not a self-contained technique that can be expected to provide all the information needed to understand an allosteric mechanism. Mutations with a modified Qax can be characterized using other biochemical and biophysical tools. The only unique attribute to such follow-up characterizations is that each mutated protein must be characterized in all four of the enzyme forms included in Fig. 1 (as opposed to characterizing only the free enzyme in protein stability studies or two enzyme forms in ligand binding studies).
Author Contributions
A.W.F. and G.M.C. both contributed to writing this article.
Acknowledgments
Development of ideas included here was initiated by a discussion with Dr. C. Nick Pace (Texas A&M). We are grateful for Dr. Pace’s input.
The authors’ research was supported by National Institutes of Health grants No. DK78076 (to A.W.F.) and No. DK32953 (to G.M.C.).
Editor: Brian Salzberg.
References
- 1.Fenton A.W. Allostery: an illustrated definition for the ‘second secret of life’. Trends Biochem. Sci. 2008;33:420–425. doi: 10.1016/j.tibs.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monod J. Penguin Books; London, UK: 1977. Chance and Necessity: Essay on the Natural Philosophy of Modern Biology. [Google Scholar]
- 3.Fenton A.W., Johnson T.A., Holyoak T. The pyruvate kinase model system, a cautionary tale for the use of osmolyte perturbations to support conformational equilibria in allostery. Protein Sci. 2010;19:1796–1800. doi: 10.1002/pro.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishwar A., Tang Q., Fenton A.W. Distinguishing the interactions in the fructose 1,6-bisphosphate binding site of human liver pyruvate kinase that contribute to allostery. Biochemistry. 2015;54:1516–1524. doi: 10.1021/bi501426w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reinhart G.D. Quantitative analysis and interpretation of allosteric behavior. Methods Enzymol. 2004;380:187–203. doi: 10.1016/S0076-6879(04)80009-0. [DOI] [PubMed] [Google Scholar]
- 6.Reinhart G.D. Linked-function origins of cooperativity in a symmetrical dimer. Biophys. Chem. 1988;30:159–172. doi: 10.1016/0301-4622(88)85013-0. [DOI] [PubMed] [Google Scholar]
- 7.Weber G. Ligand binding and internal equilibria in proteins. Biochemistry. 1972;11:864–878. doi: 10.1021/bi00755a028. [DOI] [PubMed] [Google Scholar]
- 8.Alontaga A.Y., Fenton A.W. Effector analogues detect varied allosteric roles for conserved protein-effector interactions in pyruvate kinase isozymes. Biochemistry. 2011;50:1934–1939. doi: 10.1021/bi200052e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urness J.M., Clapp K.M., Fenton A.W. Distinguishing the chemical moiety of phosphoenolpyruvate that contributes to allostery in muscle pyruvate kinase. Biochemistry. 2013;52:1–3. doi: 10.1021/bi301628k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams R., Holyoak T., Fenton A.W. Differentiating a ligand’s chemical requirements for allosteric interactions from those for protein binding. Phenylalanine inhibition of pyruvate kinase. Biochemistry. 2006;45:5421–5429. doi: 10.1021/bi0524262. [DOI] [PubMed] [Google Scholar]
- 11.Li G., Magana D., Dyer R.B. Anisotropic energy flow and allosteric ligand binding in albumin. Nat. Commun. 2014;5:3100. doi: 10.1038/ncomms4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDonald L.R., Boyer J.A., Lee A.L. Segmental motions, not a two-state concerted switch, underlie allostery in CheY. Structure. 2012;20:1363–1373. doi: 10.1016/j.str.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ribeiro A.A., Ortiz V. Energy propagation and network energetic coupling in proteins. J. Phys. Chem. B. 2015;119:1835–1846. doi: 10.1021/jp509906m. [DOI] [PubMed] [Google Scholar]
- 14.Lockless S.W., Ranganathan R. Evolutionarily conserved pathways of energetic connectivity in protein families. Science. 1999;286:295–299. doi: 10.1126/science.286.5438.295. [DOI] [PubMed] [Google Scholar]
- 15.Miksovská J., Day J.H., Larsen R.W. Volume and enthalpy profiles of CO rebinding to horse heart myoglobin. J. Biol. Inorg. Chem. 2003;8:621–625. doi: 10.1007/s00775-003-0457-4. [DOI] [PubMed] [Google Scholar]
- 16.Pawlyk A.C., Pettigrew D.W. Transplanting allosteric control of enzyme activity by protein-protein interactions: coupling a regulatory site to the conserved catalytic core. Proc. Natl. Acad. Sci. USA. 2002;99:11115–11120. doi: 10.1073/pnas.132393599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu P., Lasagna M., Pettigrew D.W. IIAGlc inhibition of glycerol kinase: a communications network tunes protein motions at the allosteric site. Biochemistry. 2007;46:12355–12365. doi: 10.1021/bi7010948. [DOI] [PubMed] [Google Scholar]
- 18.Yu P., Pettigrew D.W. Linkage between fructose 1,6-bisphosphate binding and the dimer-tetramer equilibrium of Escherichia coli glycerol kinase: critical behavior arising from change of ligand stoichiometry. Biochemistry. 2003;42:4243–4252. doi: 10.1021/bi027142l. [DOI] [PubMed] [Google Scholar]
- 19.Grant G.A. Contrasting catalytic and allosteric mechanisms for phosphoglycerate dehydrogenases. Arch. Biochem. Biophys. 2012;519:175–185. doi: 10.1016/j.abb.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boehr D.D., McElheny D., Wright P.E. The dynamic energy landscape of dihydrofolate reductase catalysis. Science. 2006;313:1638–1642. doi: 10.1126/science.1130258. [DOI] [PubMed] [Google Scholar]
- 21.Goodey N.M., Benkovic S.J. Allosteric regulation and catalysis emerge via a common route. Nat. Chem. Biol. 2008;4:474–482. doi: 10.1038/nchembio.98. [DOI] [PubMed] [Google Scholar]
- 22.Schramm V.L. Transition states and transition state analogue interactions with enzymes. Acc. Chem. Res. 2015;48:1032–1039. doi: 10.1021/acs.accounts.5b00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zoi I., Motley M.W., Schwartz S.D. Enzyme homologues have distinct reaction paths through their transition states. J. Phys. Chem. B. 2015;119:3662–3668. doi: 10.1021/jp511983h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nashine V.C., Hammes-Schiffer S., Benkovic S.J. Coupled motions in enzyme catalysis. Curr. Opin. Chem. Biol. 2010;14:644–651. doi: 10.1016/j.cbpa.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casey A.K., Schwalm E.L., Frantom P.A. V-type allosteric inhibition is described by a shift in the rate-determining step for α-isopropylmalate synthase from Mycobacterium tuberculosis. Biochemistry. 2013;52:6737–6739. doi: 10.1021/bi401186v. [DOI] [PubMed] [Google Scholar]
- 26.Kumar G., Frantom P.A. Evolutionarily distinct versions of the multidomain enzyme α-isopropylmalate synthase share discrete mechanisms of V-type allosteric regulation. Biochemistry. 2014;53:4847–4856. doi: 10.1021/bi500702u. [DOI] [PubMed] [Google Scholar]
- 27.Valentini G., Chiarelli L.R., Mattevi A. Structure and function of human erythrocyte pyruvate kinase. Molecular basis of nonspherocytic hemolytic anemia. J. Biol. Chem. 2002;277:23807–23814. doi: 10.1074/jbc.M202107200. [DOI] [PubMed] [Google Scholar]
- 28.Jurica M.S., Mesecar A., Stoddard B.L. The allosteric regulation of pyruvate kinase by fructose-1,6-bisphosphate. Structure. 1998;6:195–210. doi: 10.1016/s0969-2126(98)00021-5. [DOI] [PubMed] [Google Scholar]
- 29.Rigden D.J., Phillips S.E., Fothergill-Gilmore L.A. The structure of pyruvate kinase from Leishmania mexicana reveals details of the allosteric transition and unusual effector specificity. J. Mol. Biol. 1999;291:615–635. doi: 10.1006/jmbi.1999.2918. [DOI] [PubMed] [Google Scholar]
- 30.Consler T.G., Uberbacher E.C., Lee J.C. Domain interaction in rabbit muscle pyruvate kinase. II. Small angle neutron scattering and computer simulation. J. Biol. Chem. 1988;263:2794–2801. [PubMed] [Google Scholar]
- 31.Fenton A.W., Williams R., Trewhella J. Changes in small-angle x-ray scattering parameters observed upon binding of ligand to rabbit muscle pyruvate kinase are not correlated with allosteric transitions. Biochemistry. 2010;49:7202–7209. doi: 10.1021/bi100147w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reinhart G.D. The determination of thermodynamic allosteric parameters of an enzyme undergoing steady-state turnover. Arch. Biochem. Biophys. 1983;224:389–401. doi: 10.1016/0003-9861(83)90225-4. [DOI] [PubMed] [Google Scholar]
- 33.Cooper A., Dryden D.T. Allostery without conformational change. A plausible model. Eur. Biophys. J. 1984;11:103–109. doi: 10.1007/BF00276625. [DOI] [PubMed] [Google Scholar]
- 34.Motlagh H.N., Wrabl J.O., Hilser V.J. The ensemble nature of allostery. Nature. 2014;508:331–339. doi: 10.1038/nature13001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henzler-Wildman K., Kern D. Dynamic personalities of proteins. Nature. 2007;450:964–972. doi: 10.1038/nature06522. [DOI] [PubMed] [Google Scholar]
- 36.Lewandowski J.R. Advances in solid-state relaxation methodology for probing site-specific protein dynamics. Acc. Chem. Res. 2013;46:2018–2027. doi: 10.1021/ar300334g. [DOI] [PubMed] [Google Scholar]
- 37.Lipchock J.M., Loria J.P. Nanometer propagation of millisecond motions in V-type allostery. Structure. 2010;18:1596–1607. doi: 10.1016/j.str.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDonald L.R., Whitley M.J., Lee A.L. Colocalization of fast and slow timescale dynamics in the allosteric signaling protein CheY. J. Mol. Biol. 2013;425:2372–2381. doi: 10.1016/j.jmb.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L., Bouguet-Bonnet S., Buck M. Combining NMR and molecular dynamics studies for insights into the allostery of small GTPase-protein interactions. Methods Mol. Biol. 2012;796:235–259. doi: 10.1007/978-1-61779-334-9_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zerbetto M., Anderson R., Buck M. Analysis of 15N-1H NMR relaxation in proteins by a combined experimental and molecular dynamics simulation approach: picosecond-nanosecond dynamics of the Rho GTPase binding domain of plexin-B1 in the dimeric state indicates allosteric pathways. J. Phys. Chem. B. 2013;117:174–184. doi: 10.1021/jp310142f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brüschweiler S., Konrat R., Tollinger M. Allosteric communication in the KIX domain proceeds through dynamic repacking of the hydrophobic core. ACS Chem. Biol. 2013;8:1600–1610. doi: 10.1021/cb4002188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi L., Kay L.E. Tracing an allosteric pathway regulating the activity of the HslV protease. Proc. Natl. Acad. Sci. USA. 2014;111:2140–2145. doi: 10.1073/pnas.1318476111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prasannan C.B., Villar M.T., Fenton A.W. Identification of regions of rabbit muscle pyruvate kinase important for allosteric regulation by phenylalanine, detected by H/D exchange mass spectrometry. Biochemistry. 2013;52:1998–2006. doi: 10.1021/bi400117q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frantom P.A., Zhang H.M., Blanchard J.S. Mapping of the allosteric network in the regulation of α-isopropylmalate synthase from Mycobacterium tuberculosis by the feedback inhibitor L-leucine: solution-phase H/D exchange monitored by FT-ICR mass spectrometry. Biochemistry. 2009;48:7457–7464. doi: 10.1021/bi900851q. [DOI] [PubMed] [Google Scholar]
- 45.Beckett D. Hydrogen-deuterium exchange study of an allosteric energy cycle. Methods Mol. Biol. 2012;796:261–278. doi: 10.1007/978-1-61779-334-9_14. [DOI] [PubMed] [Google Scholar]
- 46.Laine O., Streaker E.D., Beckett D. Allosteric signaling in the biotin repressor occurs via local folding coupled to global dampening of protein dynamics. J. Mol. Biol. 2008;381:89–101. doi: 10.1016/j.jmb.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Underbakke E.S., Iavarone A.T., Marletta M.A. Nitric oxide-induced conformational changes in soluble guanylate cyclase. Structure. 2014;22:602–611. doi: 10.1016/j.str.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vogt A.D., Pozzi N., Di Cera E. Essential role of conformational selection in ligand binding. Biophys. Chem. 2014;186:13–21. doi: 10.1016/j.bpc.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vogt A.D., Di Cera E. Conformational selection is a dominant mechanism of ligand binding. Biochemistry. 2013;52:5723–5729. doi: 10.1021/bi400929b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vogt A.D., Di Cera E. Conformational selection or induced fit? A critical appraisal of the kinetic mechanism. Biochemistry. 2012;51:5894–5902. doi: 10.1021/bi3006913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daniels K.G., Tonthat N.K., Oas T.G. Ligand concentration regulates the pathways of coupled protein folding and binding. J. Am. Chem. Soc. 2014;136:822–825. doi: 10.1021/ja4086726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chakrabartty A., Kortemme T., Baldwin R.L. Helix propensities of the amino acids measured in alanine-based peptides without helix-stabilizing side-chain interactions. Protein Sci. 1994;3:843–852. doi: 10.1002/pro.5560030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Braxton B.L., Tlapak-Simmons V.L., Reinhart G.D. Temperature-induced inversion of allosteric phenomena. J. Biol. Chem. 1994;269:47–50. [PubMed] [Google Scholar]
- 54.Tlapak-Simmons V.L., Reinhart G.D. Obfuscation of allosteric structure-function relationships by enthalpy-entropy compensation. Biophys. J. 1998;75:1010–1015. doi: 10.1016/S0006-3495(98)77589-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fisher H.F. Detecting “silent” allosteric coupling. Methods Mol. Biol. 2012;796:71–96. doi: 10.1007/978-1-61779-334-9_5. [DOI] [PubMed] [Google Scholar]
- 56.Fenton A.W., Reinhart G.D. Disentangling the web of allosteric communication in a homotetramer: heterotropic inhibition in phosphofructokinase from Escherichia coli. Biochemistry. 2009;48:12323–12328. doi: 10.1021/bi901456p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meinhardt S., Manley M.W., Jr., Swint-Kruse L. Rheostats and toggle switches for modulating protein function. PLoS One. 2013;8:e83502. doi: 10.1371/journal.pone.0083502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fenton A.W., Reinhart G.D. Isolation of a single activating allosteric interaction in phosphofructokinase from Escherichia coli. Biochemistry. 2002;41:13410–13416. doi: 10.1021/bi026450g. [DOI] [PubMed] [Google Scholar]
- 59.Hilser V.J. Biochemistry. An ensemble view of allostery. Science. 2010;327:653–654. doi: 10.1126/science.1186121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hilser V.J. Modeling the native state ensemble. Methods Mol. Biol. 2001;168:93–116. doi: 10.1385/1-59259-193-0:093. [DOI] [PubMed] [Google Scholar]
- 61.Bond C.J., Wong K.B., Daggett V. Characterization of residual structure in the thermally denatured state of barnase by simulation and experiment: description of the folding pathway. Proc. Natl. Acad. Sci. USA. 1997;94:13409–13413. doi: 10.1073/pnas.94.25.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Griko Y.V. Denaturation versus unfolding: energetic aspects of residual structure in denatured α-lactalbumin. J. Protein Chem. 1999;18:361–369. doi: 10.1023/a:1021099714901. [DOI] [PubMed] [Google Scholar]
- 63.Fowler D.M., Fields S. Deep mutational scanning: a new style of protein science. Nat. Methods. 2014;11:801–807. doi: 10.1038/nmeth.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clackson T., Wells J.A. A hot spot of binding energy in a hormone-receptor interface. Science. 1995;267:383–386. doi: 10.1126/science.7529940. [DOI] [PubMed] [Google Scholar]
- 65.Cunningham B.C., Wells J.A. High-resolution epitope mapping of hGH-receptor interactions by alanine-scanning mutagenesis. Science. 1989;244:1081–1085. doi: 10.1126/science.2471267. [DOI] [PubMed] [Google Scholar]
- 66.DeLano W.L. Unraveling hot spots in binding interfaces: progress and challenges. Curr. Opin. Struct. Biol. 2002;12:14–20. doi: 10.1016/s0959-440x(02)00283-x. [DOI] [PubMed] [Google Scholar]
- 67.Cade C., Swartz P., Clark A.C. Modifying caspase-3 activity by altering allosteric networks. Biochemistry. 2014;53:7582–7595. doi: 10.1021/bi500874k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zayner J.P., Antoniou C., Sosnick T.R. Investigating models of protein function and allostery with a widespread mutational analysis of a light-activated protein. Biophys. J. 2013;105:1027–1036. doi: 10.1016/j.bpj.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fenton A.W., Paricharttanakul N.M., Reinhart G.D. Identification of substrate contact residues important for the allosteric regulation of phosphofructokinase from Escherichia coli. Biochemistry. 2003;42:6453–6459. doi: 10.1021/bi034273t. [DOI] [PubMed] [Google Scholar]
- 70.Li J., Lee J.C. Modulation of allosteric behavior through adjustment of the differential stability of the two interacting domains in E. coli cAMP receptor protein. Biophys. Chem. 2011;159:210–216. doi: 10.1016/j.bpc.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhan H., Camargo M., Matthews K.S. Positions 94–98 of the lactose repressor N-subdomain monomer-monomer interface are critical for allosteric communication. Biochemistry. 2010;49:8636–8645. doi: 10.1021/bi101106x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhan H., Sun Z., Matthews K.S. Functional impact of polar and acidic substitutions in the lactose repressor hydrophobic monomer-monomer interface with a buried lysine. Biochemistry. 2009;48:1305–1314. doi: 10.1021/bi801357f. [DOI] [PubMed] [Google Scholar]
- 73.Walters J., Schipper J.L., Clark A.C. Allosteric modulation of caspase 3 through mutagenesis. Biosci. Rep. 2012;32:401–411. doi: 10.1042/BSR20120037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eginton C., Naganathan S., Beckett D. Sequence-function relationships in folding upon binding. Protein Sci. 2015;24:200–211. doi: 10.1002/pro.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pettigrew D.W., Romeo P.H., Ackers G.K. Probing the energetics of proteins through structural perturbation: sites of regulatory energy in human hemoglobin. Proc. Natl. Acad. Sci. USA. 1982;79:1849–1853. doi: 10.1073/pnas.79.6.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ackers G.K., Smith F.R. Effects of site-specific amino acid modification on protein interactions and biological function. Annu. Rev. Biochem. 1985;54:597–629. doi: 10.1146/annurev.bi.54.070185.003121. [DOI] [PubMed] [Google Scholar]
- 77.LiCata V.J., Ackers G.K. Long-range, small magnitude nonadditivity of mutational effects in proteins. Biochemistry. 1995;34:3133–3139. doi: 10.1021/bi00010a001. [DOI] [PubMed] [Google Scholar]
- 78.Murtaugh M.L., Fanning S.W., Horn J.R. A combinatorial histidine scanning library approach to engineer highly pH-dependent protein switches. Protein Sci. 2011;20:1619–1631. doi: 10.1002/pro.696. [DOI] [PMC free article] [PubMed] [Google Scholar]