Abstract
Various types of weapon traits found in insect order Coleoptera are known as outstanding examples of sexually selected exaggerated characters. It is known that the sex determination gene doublesex (dsx) plays a significant role in sex-specific expression of weapon traits in various beetles belonging to the superfamily Scarabaeoidea. Although sex-specific weapon traits have evolved independently in various Coleopteran groups, developmental mechanisms of sex-specific expression have not been studied outside of the Scarabaeoidea. In order to test the hypothesis that dsx-dependent sex-specific expression of weapon traits is a general mechanism among the Coleoptera, we have characterized the dsx in the sexually dimorphic broad-horned beetle Gnatocerus cornutus (Tenebrionidea, Tenebirionidae). By using molecular cloning, we identified five splicing variants of Gnatocerus cornutus dsx (Gcdsx), which are predicted to code four different isoforms. We found one male-specific variant (GcDsx-M), two female-specific variants (GcDsx-FL and GcDsx-FS) and two non-sex-specific variants (correspond to a single isoform, GcDsx-C). Knockdown of all Dsx isoforms resulted in intersex phenotype both in male and female. Also, knockdown of all female-specific isoforms transformed females to intersex phenotype, while did not affect male phenotype. Our results clearly illustrate the important function of Gcdsx in determining sex-specific trait expression in both sexes.
Sexually dimorphic weapons in beetles
Sexually selected weapon traits are among the most prominent morphological characteristics in animals1. Various types of weapon traits in Coleoptera are known as some of the outstanding examples of sexually-selected exaggerated characters2,3. Weapon characters usually express in a sex-specific (mostly male-specific) manner and function in individuals combat over limited resources1,2,3,4. Although the developmental mechanisms of the sex-specific expression of weapon traits have long remained elusive, recent progress using molecular tools in several beetle species revealed that the sex-determination gene doublesex plays an important role in sex-specific expression of weapon traits3,5,6,7.
The sex-determination gene doublesex
doublesex (dsx) is known as a key downstream gene in the sex-determination cascade8. In holometabolous insects studied to date, dsx has at least two isoforms which are expressed in a sex-specific manner. That is, one isoform expresses only in males and functions in male differentiation, and another expresses only in females and functions in female differentiation. Dsx regulates development of both primary sexual traits (e.g. gonads and genitals) and secondary sexual traits (e.g. sex combs in male Drosophila melanogaster)8,9,10. In weaponed beetles, the function of dsx has been examined in four species: Onthophagus taurus and O. sagitarius (Scarabaeoidea, Scarabaeidae5), Trypoxylus dichotomus (Scarabaeoidea, Scarabaeidae6), and Cyclommatus metallifer (Scarabaeoidea, Lucanidae7). Although it is unlikely that the weapons of these beetles share a common evolutionary origin11, all have their sex-specific expression organized by Dsx (reviewed in3,12). However, all of these beetles are also closely related, belonging to the superfamily Scarabaeoidea, and functional studies of dsx in weapon trait expression outside of the Scarabaeoidea have been limited.
Gnatocerus cornutus: sexually dimorphic weapon traits and strength as an experimental model
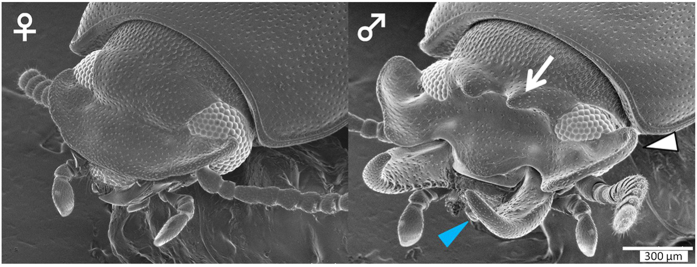
The broad-horned beetle Gnatocerus cornutus is a sexually dimorphic, weaponed beetle belonging to the superfamily Tenebrionidea and family Tenebrionidae. In this species, males have well-developed mandibles which are used for combat13. In addition to mandibles, males also possess other male-specific traits such as well-developed genae and a pair of short head horns, although their function in combat is unclear13. In females, mandibles are never elongated like males and other traits such as genae and horns are not expressed13,14 (Fig. 1). It is a unique characteristic of this beetle that both novel (horns) and size-modified traits (mandibles and genae) are expressed in a sexually dimorphic manner.
Figure 1. Adult female and male of Gnatocerus cornutus.
(Left) Scanning electron microscope (SEM) image of adult female G. cornutus. (Right) SEM image of adult male G. cornutus. A pair of elongated mandibles, enlarged genae, and a pair of small head horns in the male are indicated by a light blue arrowhead, white arrowhead and white arrow respectively.
Compared to other previously studied weaponed scarab beetles, this species is easy to rear and breed in the laboratory. It has a shorter generation time (approximately 2 months at 25 °C incubation). Also, G. cornutus is phylogenetically close to the model beetle species Tribolium castaneum15, whose genome information is available16. Both of species belong to same family Tenebrionidae, so that the Tribolium genome can be used as an ideal reference genome for G. cornutus in potential next generation sequencing analyses. On the other hand, Gnatocerus cornutus is distantly related to previously studied scarab beetles with weapon characters. Estimated divergence time between Tenebrionidea and Scarabaeoidea is approximately 240 million years ago17. Considering these characteristics and phylogenetic position (distant from other weaponed scarab beetles and close to T. castaneum), G. cornutus can be an important new model system for investigating molecular mechanisms of the sex-specific expression of weapon traits.
Here, we identify and perform expression and functional analyses of dsx in order to test the hypothesis that dsx-dependent sex-specific expression of weapon traits is a general mechanism among different groups in the Coleoptera. Our results clearly demonstrate the significant function of dsx in G. cornutus weapon development.
Materials and Methods
Insects
The strain of the broad horned beetle Gnatocerus cornutus used in a previous study13 was also used in this study. We kept them in the laboratory according to Okada et al.13. Briefly, larvae were kept together in plastic containers at 25 °C and at a humidity higher than 60%. We used flour (Okura-bussann, Chiba, Japan) enriched by dried yeast (Asahi food and healthcare, Tokyo, Japan) in a 9:1 ratio as food. Larvae were transferred to 24-well plates (Becton Dickinson Labware, NJ, USA) to induce pupation.
Identification of sex-specific genome sequences in G. cornutus via RAPD-PCR
The sex of G. cornutus is indistinguishable by external morphology during larval and prepupal periods, so we developed PCR-dependent sexing methods before the molecular analyses of dsx. To develop PCR-based sexing method in this species we performed RAPD-PCR, using RAPD 10mer Kits (Operon Biotechnology, Tokyo, Japan). Genomic DNA (gDNA) was extracted from whole bodies of unmated adult male and female G. cornutus by dissecting a single leg, washing it in water, homogenizing it in 50 μl of TES (0.1 M Tris-HCL (pH 9.0), 0.1 M EDTA, 1% SDS) and incubating it at 70 °C for 30 min. Then 7 μl of 8 M K-Acetate was added and the samples left on ice for 30 min. After centrifugation, the gDNA was ethanol precipitated. The gDNA was used in as a template in PCR performed with AmpliTaq Gold 360 Master Mix (Applied Biosystems, Foster City, CA, USA) according to manufactures’ protocol. The PCR program was: 95 °C for 9 min, and 45 cycles of [94 °C for 1 min, 35 °C for 1 min and 72 °Cfor 2 min]. PCR products were amplified using from male (but not female) samples using the A-09 primer (5′-GGGTAACGCC-3′). This male-specific PCR product was isolated by electrophoresis on a 2% agarose gel (MetaPhor Agarose, FMC BioProducts, Rockland, ME, USA) and purified using the MagExtractor PCR & Gel-Clean up kit (TOYOBO, Osaka, Japan). The purified product was subcloned using a TOPO TA Cloning Kit (Invitrogen, Carlsbad, CA, USA), and sequenced using the BigDye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems). From the obtained male-specific genome sequence, we designed a primer-pair for PCR dependent sexing:
Gc-Y-05; 5′-AGTGTTGACGCAAACCTATC-3′
Gc-Y-06; 5′-AGTTCTGCAGCCATATCAGT-3′
For sexing PCR, DNA was prepared as follows: whole bodies (for larvae and prepupae) or dissected legs (for adults) were washed and homogenized in 100 μl of 50 mM NaOH and incubated at 95 °C for 15 min. Then, 100 μl of 200 mM Tris-HCl (pH 8.0) was added and the samples were centrifuged at 15,000 rpm for 10 min). The supernatant was then used directly as a template for PCR. PCR was performed with AmpliTaq Gold 360 Master Mix (Applied Biosystems), under the following cycling parameters: 95 °C for 7 min, and 35 cycles of [94 °C for 1 min, 60 °C for 30 sec and 72 °C for 30 sec]. For positive control of gDNA PCR, amplification of a section of the 28S rRNA gene sequence was used as above, except with an annealing temperature of 50 °C. Primer sequences used for PCR amplification of the 28S fragment were according to Kim et al.18 as below:
28S-F: 5′- GAC TAC CCC CTG AAT TTA AGC AT -3′
28S-R: 5′- GAC TCC TTG GTC CGT GTT TCA AG -3′.
Molecular cloning of a doublesex gene fragment from Gnatocerus cornutus
The molecular cloning a doublesex gene fragment was performed via PCR with degenerate primers. The template cDNA was prepared from single male or female adults. Total RNA was extracted using TRIZOL (Invitrogen, Carlsbad, CA, USA). Then, reverse transcription was performed with SuperScript II RNase H Reverse Transcriptase (Invitrogen) using 1000 ng of total RNA. Degenerate primers were designed from the doublesex DM and OD2 domains conserved across insects. We also cloned the rp49 gene from G. cornutus for use as an internal control in PCR. Degenerate primer sequences are listed in Table 1. PCR and subsequent subcloning were performed according to Ito et al.6 with minor modifications. Briefly, amplified PCR products were separated by electrophoresis on 1% agarose gels and purified using MagExtractor gel cleaner kit (Toyobo). Purified PCR products were subcloned using the pBlueScript KS+ vector (Stratagene, La Jolla, CA, USA) and XL1-Blue competent cells. Subcloned inserts were sequenced using an automatic DNA sequencer (DNA sequencer 3130 genetic analyser; Applied Biosystems). Database searches for identified sequence homology were performed using BlastX at the NCBI server (http://blast.ncbi.nlm.nih.gov/Blast.cgi). ClustalX was used to construct a phylogenic NJ tree of a conserved DM domain of 47 amino acids from Dsx from the following coleopteran species: Tribolium castaneum19, Onthophagus taurus5, Trypoxylus dichotomus6 and Cyclommatus metallifer7. The DNA Data Bank of Japan (DDBJ)/European Molecular Biology Laboratory (EMBL)/GenBank accession number for Gcrp49 is LC107876.
Table 1. Degenerate and RACE-PCR primers.
| Gcdsx-degenerate-F | 5′-AAYTGYGCIMGITGYMGIAAYCA-3′ |
| Gcdsx-degenerate-R | 5′-TACATIARIGGCATCATYTCCCA-3′ |
| Gcrp49-F | 5′-ACIAARMAITTYATIMGICA-3′ |
| Gcrp49-R | 5′-TGIGCIATYTCISCRCARTA-3′ |
| 5′-RACE-GSP | 5′-GACTCTTCTGCAGGACATGCGGGTCTAT-3′ |
| 5′-RACE-NGSP | 5′-ACTTGCAGTACCTCTTGTGGCCCTTGA-3′ |
| 3′-RACE-GSP | 5′-CTCAAGGGCCACAAGAGGTACTGCAAG-3′ |
| 3′-RACE-NGSP | 5′-GTCCTGCAGAAGAGTCCTTCGCCGATAC-3′ |
RACE-PCR amplification of full-length Gcdsx transcript variants
For amplification of full-length of Gcdsx we performed RACE-PCR. Using total RNA isolated as above, we synthesized cDNA for RACE-PCR using the SMART RACE kit (Clontech, Mountain View, CA, USA) according to the manufacture’s protocol. We designed four gene-specific primers (primers for initial and nested PCR for both 3′ and 5′-RACE) from the sequence of the Gcdsx fragment described above (Table 1). PCR was performed with Advantage2 polymeerase (Clontech, Mountain View, CA USA) using the following PCR: five cycles of [94 °C for 5 sec and 72 °C for 3 min], five cycles of [94 °C for 5 sec, 70 °C for 10 sec and 72 °C for 3 min], and 25 cycles of [94 °C for 5 sec, 68 °C for 10 sec and 72 °C for 3 min]. Using 0.5 μl of PCR product from the initial PCR reaction, nested PCR was carried out under the following parameters: 40 cycles of [94 °C for 5 sec, 70 °C for 10 sec and 72 °C for 3 min]. Amplified DNA bands were then subcloned and sequenced as described previously. The accession numbers for Gcdsx-M, Gcdsx-FS, Gcdsx-FL, Gcdsx-C1, Gcdsx-C2 are LC105647-LC105651.
Expression analyses of Gcdsx variants by PCR
In order to investigate expression pattern of each Gcdsx variant, we performed expression analyses via PCR. We dissected heads of last instar, early and late prepupal period, and early and late pupal period G. cornutus and then preserved them at −80 °C until use. Prepupal and pupal stages were judged based on external appearance and pigmentation level, respectively. Using the rest of whole body, we extracted genomice DNA and determined sex for each individual using the PCR-dependent method described before. For each sex, five heads were used for RNA extraction. Total RNA was extracted with the RNeasy Mini Kit (QIAGEN, Hilden, Germany) by the auto-RNA extractor QIAcube (QIAGEN, Hilden, Germany) according to the manufacturers’ protocol. 431.2 ng of RNA was reverse transcribed as described procedure previously. Using this synthesized cDNA as a template, PCR was performed using AmpliTaq Gold 360 Master Mix (Applied Biosystems) at 95 °C for 9 min followed by 35 cycles of [94 °C for 1 min, 45 °C for 30 sec and 72 °C for 1 min].
Functional analyses of Gcdsx variants via RNAi
For investigating function of Gcdsx, we performed analyses via RNAi. Using primer pairs designed for each region (Table 2), partial sequences of Gcdsx were amplified by PCR and subcloned into TOPO vector (pCR4-TOPO) with the TOPO TA cloning Kit (Invitrogen). Then, insert regions were amplified with the universal primer pair with the T7 sequence (5′- TAATACGACTCACTATAGGGAGACCACGTCCTGCAGGTTTAAACG-3′ and 5′- TAATACGACTCACTATAGGGAGACCACCGAATTGAATTTAGCGGC-3′). Amplified PCR products were then purified as previously described. dsRNAs were synthesized using the MEGAscript T7 kit (Ambion, Austin, Tx, USA). dsRNA of the DsRed sequence negative control sequence was synthesized by the same method. Injection of dsRNA into larvae was performed using a microinjector (FemtoJet, Eppendolf, Hamburg, Germany) with a glass needle (Natsume-Kogaku, Nagano, Japan). The concentration of the dsRNA solution was 10 μg/μl and approximately 0.10 to 0.68 μl was injected into late instar larvae. Injected larvae were reared separately to induce pupation. Eclosed adult phenotypes were observed by binocular microscope and photographed with a VHX-900 digital microscope (Keyence, Osaka, Japan).
Table 2. Primers for dsRNA synthesis.
| Gcdsx-exon1,2-F | 5′-AAYTGYGCIMGITGYMGIAAYCA-3′ |
| Gcdsx-exon1,2-R | 5′-TACATIARIGGCATCATYTCCCA-3′ |
| Gcdsx-exon4-F | 5′-CCAAGAAAGGAGAATCAACGG-3′ |
| Gcdsx-exon4-R | 5′-CAACAAAGTGACGTCGCCGCTGGG-3′ |
| Gcdsx-exon5-F | 5′-TGCACAAGAACTCAACAAGAAG-3′ |
| Gcdsx-exon5-R | 5′-TTGGGACAAACGCTCCAGT-3′ |
| Gcdsx-exon8-F | 5′-TTCCAAACCGTGAATCACAA-3′ |
| Gcdsx-exon8-R | 5′-CTTGGAGCCCACTCTGAATC-3′ |
Gcdsx-exon1,2-F and R are identical to Gcdsx-denegenerate-F and R.
Scanning electron microscopy (SEM)
Magnified images of G. cornutus heads were captured by SEM (VE-9800, Keyence) without any pretreatment.
Results and Discussion
Development of PCR-based sexing method in G. cornutus
The sex of G. cornutus is indistinguishable during the larval periods, and it is also indistinguishable in individuals that have been phenotypically disrupted by dsx RNAi treatment. Thus, we first developed PCR-based sexing methods. We performed PCR with twenty different 10-mer primers provided with the a RAPD kit (A-01 to A-20) using gDNA of male or female G. cornutus. We thus obtained a 402 bp male-specific amplicon using the A-09 primer (Fig. 2). This sequence did not show significant homology with any previously identified sequence by Blastn using the NCBI database (http://www.ncbi.nlm.nih.gov/). We then designed a pair of sexing primers (Gc-Y-05 and 06) based on this male-specific sequence, which amplified a 203 bp PCR product only when male-gDNA was used as the template (See Supplementary Fig. 1S). Considering that this species has XY sex determination system20, this male-specific sequence might be on Y chromosome. Thus, we used this PCR-based sexing method for identifying the original genetic sexes in phenotypically disrupted dsx RNAi individuals in later experiments (data not shown).
Figure 2. A male-specific RAPD marker of G. cornutus.
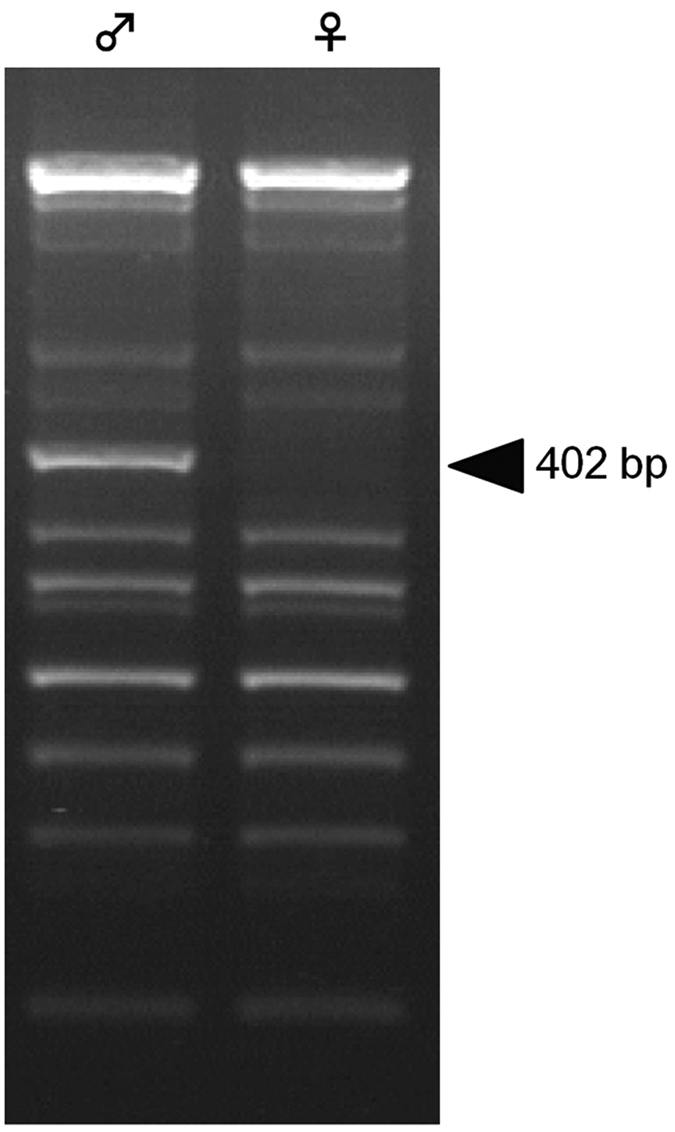
Amplification pattern from male and female genomic DNA of G. cornutus using A-09 primer. Arrowhead indicates a male-specific PCR product.
In the expression and functional analyses of sex-determination genes, it is important to know the sex of sample individuals in advance. However, some insects do not show morphological dimorphism, especially in larval and prepupal periods, which are critical periods in investigating development of sexually-selected exaggerated traits in some beetles2. Furthermore, when trying to identify the initial sex-determination molecular signal, it is necessary to know the sex of early embryos21, which are often difficult to determine morphologically. Our new male-specific primer pair enabled us to distinguish the sample sex in any developmental stage.
This PCR marker is also allows us to determine the original sex of dsx RNAi-treated individuals in the present study and will be used in future investigations of sex-determination mechanisms in this species.
Identification of dsx in G. cornutus
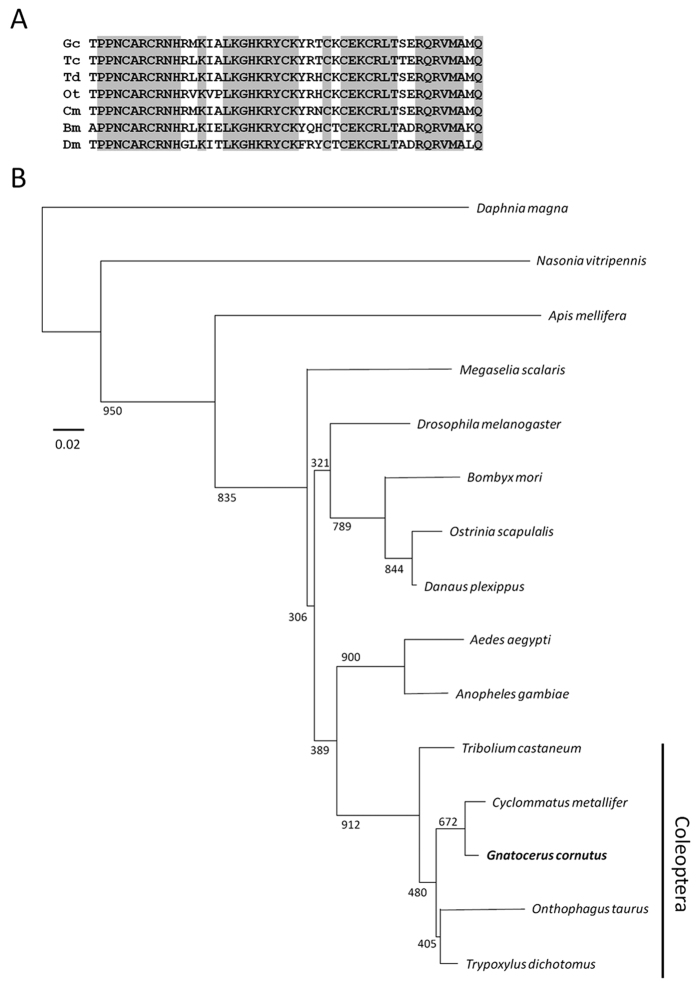
First, by using degenerate PCR, we identified a partial sequence of a putative G. cornutus dsx homolog. We obtained the full-length clone of this gene by subsequent RACE-PCR. The putative protein coded by the identified gene sequence contains amino acids of conserved DNA binding domain (DM domain/OD1 domain) (Fig. 3A) and conserved dimerization domain (OD2 domain). The DM domain is found in all members of the Dmrt gene family, including Dsx, while the OD2 domain is specific to Dsx22. Phylogenetic analysis using this conserved DM domain sequence indicated that the identified sequence from G. cornutus was grouped with other coleopteran Dsx sequences (Fig. 3B). These results strongly suggest that the identified gene was the homolog of dsx from G. cornutus. Consequently we named this gene Gcdsx.
Figure 3. Alignment and phylogeny of GcDsx with other insect Dsx proteins.
(A) Alignment of the conserved 47 amino acid DM domain of Dsx proteins of five coleopteran species (Gc, Gnatocerus cornutus; Tc, Tribolium castaneum; Td, Trypoxylus dichotomus; Ot, Onthophagus taurus; Cm, Cyclommatus metallifer) and other holometabolous insects (Bm, Bombyx mori; Dm, Drosophila melanogaster). Amino acids conserved among all seven species are highlighted in gray. (B) Phylogenetic tree of the 47 amino acid conserved DM domain of Dsx constructed using the neighbor-joining method with bootstrap support values (1000 iterations) indicated next to branch nodes.
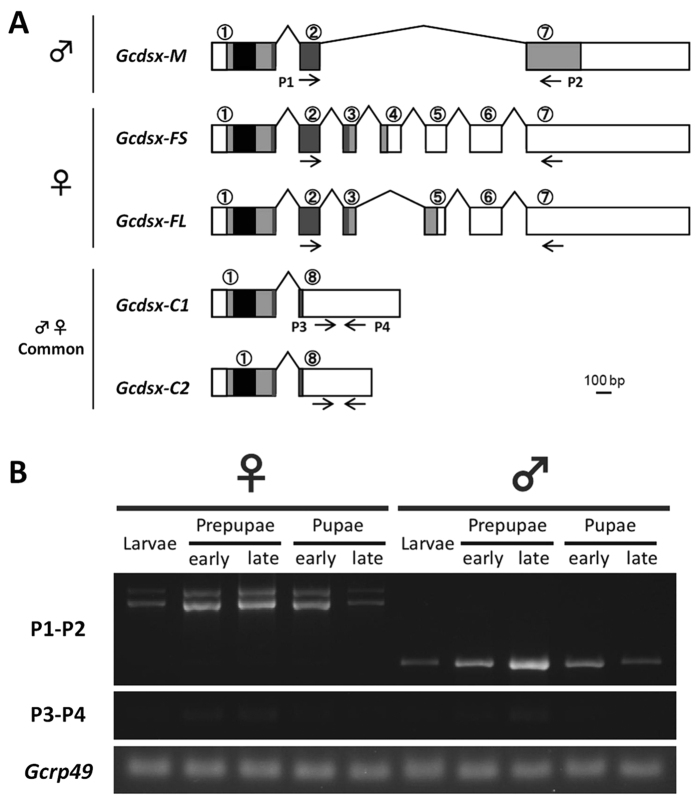
We identified five different splicing variants of Gcdsx via RACE-PCR. One variant was isolated only from male, two variants were only from females and two variants were from both sexes, thus we named those variants Gcdsx-M, Gcdsx-FS, Gcdsx-FL, Gcdsx-C1 and Gcdsx-C2 (Fig. 4A). All of the variants have the same 5′ exon (exon1) encoding the DM domain, so all of the variants differed in the 3′ region (Fig. 4A). Exons 3, 5 and 6 were specific to Gcdsx-FS and Gcdsx-FL (Fig. 4A). Gcdsx-FS and Gcdsx-FL were distinguished by Gcdsx-FS specific exon 4 (Fig. 4A). We could not identify any Gcdsx-M specific exons. Exon 8 appeared in the non-sex-specific variants Gcdsx-C1 and Gcdsx-C2, which differed in some non-coding regions, but had identical putative ORFs. The lengths of the putative ORFs (in amino acids) for the Gcdsx variants were: 319 (GcDsx-M), 224 (GcDsx-FS), 249 (GcDsx-FL) and 149 (GcDsx-C1 and GcDsx-C2).
Figure 4. Predicted gene model and expression patterns of Gcdsx splicing variants.
(A) Schematic gene model of identified Gcdsx splicing variants. Boxes indicate exons. White boxes indicate UTRs. Black and dark grey boxes indicate DM and OD2 domains, respectively. Light grey boxes indicate other translated portions of the proteins. Arrows indicate the primer positions used in expression analyses via RT-PCR. (B) Expression pattern of each splicing variant of Gcdsx during postembryonic development in both sexes. P1-P2 pair can amplify Gcdsx-M, Gcdsx-FS and Gcdsx-FL. P3-P4 pair can amplify Gcdsx-C1 and Gcdsx-C2. Gcrp49 was used as internal control.
Next, to examine the expression of these isoforms, we performed expression analysis between sexes by RT-PCR using the primers in listed in Fig. 4A. These results indicated that the sex-specificity of those variants were unchanged during all stages of postembryonic development (Fig. 4B). On the other hand, the expression level of sex-specific Gcdsx variants seemed to be greater during the prepupal period than in the late larval or late pupal periods in both sexes (Fig. 4B).
Comparison of GcDsx isoforms with other coleopteran Dsx proteins
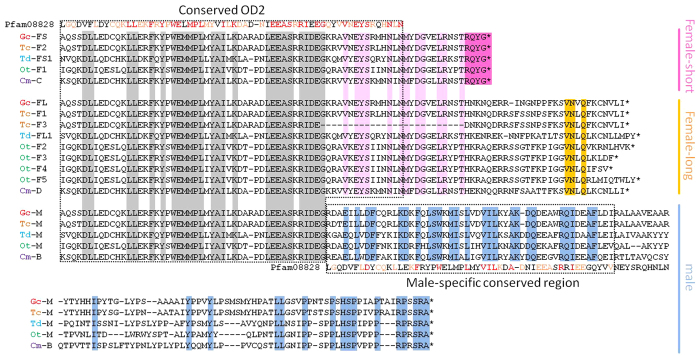
Alignment of GcDsx isoforms with other coleopteran Dsx proteins indicated that three classes of Dsx isoforms are likely to be shared within coleopteran species (Fig. 5). Considering the expression patterns of those isoforms in this species and other species5,6,7,19, the three classes can be categorized as female-specific short (Dsx-FS), female-specific long (Dsx-FL) and male-specific (Dsx-M) (Fig. 5). The Dsx-FS class can be distinguished from Dsx-FL by protein size and the presence of four highly conserved residues (RQYG) in the C-terminal end (Fig. 5), while Dsx-FL has longer C-terminal ends (Fig. 5). Conservation of amino acid sequence of Dsx-FL among species is relatively lower than the other classes, and variation in isoforms within single species can be recognized (e.g. O. taurus has four Dsx-FL isoforms with different C-terminal residues) (Fig. 5). Dsx-M has the longest protein sequence in all of the five coleopteran species and is easily distinguishable from Dsx-FL or Dsx-FS by lacking 14 residues in the C-terminus of the OD2 domain (Fig. 5). All Dsx-M sequences have similar protein length and share other structural characteristics, such as a well conserved RP(S/R)SRA sequence at the protein’s C-terminus, and especially possession of a conserved region just after the carboxy terminus of the OD2 domain (Fig. 5). This male-specific conserved domain shows weak similarity to the OD2 sequence. For example, the OD2 domain has two highly conserved sequences (WEMMPL) and (LEEAS(R/K)RIDEG), which are partially found in the male-specific domain. Gcdsx-C1 and C2 encode the same protein, GcDsx-C, which possesses a conserved DM (OD1) domain, but lack the OD2 domain. This isoform is truncated by the insertion of a stop codon at 5′ end of exon 8. T. dichotomus has a DsxC1 homolog with a similar size (144 aa) and characteristics (i.e. complete lack of an OD2 domain) as GcDsx-C.
Figure 5. Comparison of Dsx isoforms among coleopteran species.
Alignments of Dsx isoforms among five coleopteran species (Gc, Gnatocerus cornutus; Tc, Tribolium castaneum; Td, Trypoxylus dichotomus; Ot, Onthophagus taurus; Cm, Cyclommatus metallifer). Highlighted residues in grey, light pink, dark pink, orange and light blue indicate conserved residues among five species in OD2 region, female-specific region, female-short isoforms, female-long isoforms and male isoforms, respectively. Conserved OD2 domain (Pfam08828) sequences are aligned with Dsx sequences. Colored characters of Pfam08828 sequence indicated the residues conserved among all of five species (red) or conserved in at least two species (orange). Accession numbers of amino acid sequences of coleopteran Dsx proteins used for alignments are Gc-FS(LC105648), Tc-F2(AFQ62107), Td-FS1(BAM93344), Ot-F1(AEX92939), Cm-C(BAO23810), Gc-FL(LC105649), Tc-F1(AFQ62106), Tc-F3(AFQ62108), Td-FL1(BAM93341), Ot-F2(AEX92940), Ot-F3(AEX92941),Ot-F4(AEX92942), Ot-F5(AEX92943), Cm-D(BAO23811), Gc-M(LC105647), Tc-M(XP_001807448), Td-M1(BAM93339), OT-M(AEX92938) and Cm-B(BAO23809).
In general, insect Dsx proteins have wide structural diversity on their C-terminal sides, so that it is difficult to align many regions of Dsx proteins from different insect orders. But within the coleopteran lineage, Dsx isoforms are well conserved both in structure and expression patterns5,6,7,19. Here, we propose a shared set of Dsx isoforms which may indicate common ancestry within the Coleoptera. That is, males express a single sex-specific isoform (Dsx-M) and females express two different sex-specific isoforms, one long one short (Dsx-FL and Dsx-FS, respectively) (Fig. 5).
Functional analyses of Gcdsx
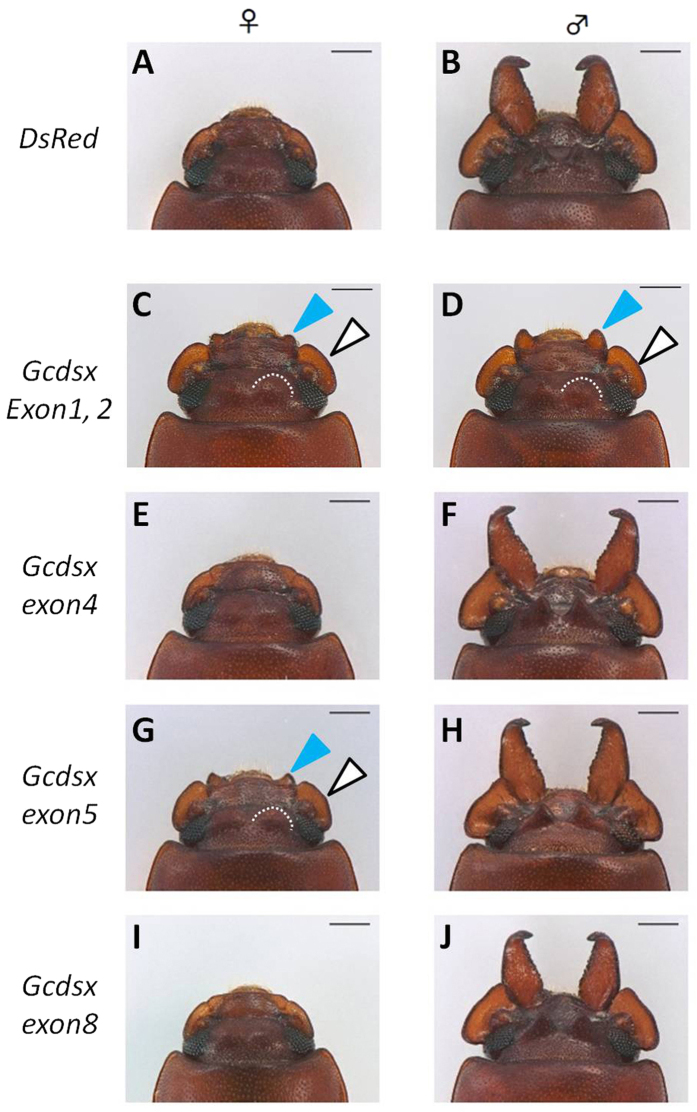
In order to reveal the function of Gcdsx, we performed RNAi knockdowns of Gcdsx isoforms. By knocking down the function of these isoforms, we clearly demonstrated their critical function in sex-specific trait development (Table 3, Fig. 6). In individuals injected with DsRed dsRNA as a control, none of the sexually dimorphic structures were affected in comparison with wild-type (non-injected) individuals of either sex (Fig. 6A,B). However, when we injected dsRNA against Gcdsx exon 1 and 2, which are shared with all of the Gcdsx variants, injected individuals had phenotypically disrupted morphology in both sexes. In Gcdsx exon1,2 RNAi females, mandibles became slightly longer and genae became wider than DsRed injected control individuals. Additionally a small pair of bumps was formed between the eyes, where a pair of horns normally forms in males (Fig. 6C). On the other hand, in Gcdsx exon1, 2 RNAi males had much smaller sexually dimorphic structures (Fig. 6D). That is, mandibles became much shorter and genae became narrower. A pair of small horns became a pair of faint bumps rather than obvious horns (Fig. 6D). In conclusion, Gcdsx exon1,2 knocked-down males and females showed a similar intersexual phenotype, which is likely to be a developmental default state of this species (Fig. 6C,D). These results indicate critical function of GcDsx in weapon expression in G. cornutus as same as in other previously studied weaponed beetles. Thus, it is suggested that dsx gene has been repeatedly co-opted as a developmental regulator of sexually dimorphic weapon trait formation in the various beetle lineages.
Table 3. Summary of Gcdsx RNAi experiments.
| dsRNA | Sex | Injected number | Lethal stage | Adult head morphology | |||
|---|---|---|---|---|---|---|---|
| larva | pupa | female | intersex | male | |||
| exon1,2 | female | 18 | 4 | 1 | 0 | 13 | 0 |
| male | 16 | 8 | 1 | 0 | 6 | 1 | |
| exon4 | female | 8 | 1 | 0 | 7 | 0 | 0 |
| male | 28 | 7 | 0 | 0 | 0 | 21 | |
| exon5 | female | 4 | 1 | 0 | 0 | 3 | 0 |
| male | 8 | 2 | 0 | 0 | 0 | 6 | |
| exon8 | female | 6 | 1 | 0 | 5 | 0 | 0 |
| male | 19 | 11 | 0 | 0 | 0 | 8 | |
| DsRed | female | 19 | 3 | 0 | 16 | 0 | 0 |
| male | 17 | 1 | 1 | 0 | 0 | 15 | |
Figure 6. Anterior morphological phenotypes of Gcdsx RNAi individuals.
(A) Negative control DsRed RNAi female. No male weapon traits (large mandibles, genae and a pair of small horn) are expressed. (B) Negative control DsRed RNAi male whose weapon traits (large mandibles, genae and a pair of small horns) were normally expressed the same as wild type. (C) Gcdsx exon1 and exon2 knockdown female. The mandibles became slightly larger (light blue arrowhead) and a pair of genae show significant growth (white arrowhead) compared to the control. Also, a pair of small horns was apparent between the eyes (white dashed line). (D) Gcdsx exon1 and exon2 knockdown male. Compared to control DsRed RNAi males, mandibles became smaller (light blue arrowhead) and the size of genae (white arrowhead) and head horns (white dashed line) was decreased. Both sexes treated with Gcdsx (exon1, 2) RNAi showed a similar intersexual phenotype. (E,F) Gcdsx exon4 RNAi (i.e. GcDsx-FS specific knockdown) females and males did not show any altered morphologies. (G,H) Gcdsx exon5 RNAi (i.e. both GcDsx-FS and GcDsx-FL specific knockdown) female and male. Female morphology changed to an intersexual phenotype that is characterized by slightly enlarged mandibles (light blue arrowhead), enlarged genae (white arrowhead) and a pair of horn bumps (white dashed line), while males were not affected. (I,J) Gcdsx exon8 RNAi (i.e. GcDsx-C specific knockdown) female and male. No changes in morphologies. Scale bars indicate 250 μm.
Next, we performed RNAi experiments using dsRNA against isoform-specific regions of Gcdsx. We designed dsRNA corresponding to exon 4 (i.e. Gcdsx-FS specific knockdown), exon 5 (i.e. knockdown of both female-specific Gcdsx-FS and Gcdsx-FL) and exon 8 (i.e. knockdown of both non-sex-specific Gcdsx-C1 and Gcdsx-C2). Knockdown of Gcdsx-FS via injection of dsRNA of exon 4 and Gcdsx-C1/C2 via injection of dsRNA of exon 8 did not affect any morphological traits in either sex (Fig. 6E,F,I,J). In contrast, knockdown of both of female-specific Gcdsx (Gcdsx-FS and Gcdsx-FL) via injection of dsRNA of exon 5 caused an intersexual phenotype in females (Fig. 6G), the same as with the knockdown of all Gcdsx isoforms (Fig. 6C), but did not affect phenotype in males (Fig. 6H). Considering that intersexual phenotypes (short mandibles, narrow genae and a pair of faint bumps) is likely to be a developmental default state, GcDsx has a critical function on sex-specific trait expression in males, and inhibition of male sex-specific traits in females. It is known that insect Dsx protein functions as transcription factor, so that GcDsx-M and GcDsx-FL appear to play a central role in the regulation of downstream genes controlling male differentiation (longer mandibles, wider genae and a pair of horn) and female differentiation (tiny mandibles, absence of genae and horns), respectively.
This and previous studies have demonstrated by isoform specific RNAi that Dsx-M and Dsx-F are essential for expression or inhibition of sex-specific weapon characters in males and females, respectively6,23. Structural differences in the C-terminal region of Dsx proteins are critical for sex-differentiation or more specifically, for sex-specific transcriptional regulation of downstream genes.
On the other hand, we could not find evidence for a function of Dsx-FS alone in sex-specific trait development, i.e. Dsx-FS isoform-specific RNAi did not affect sexually dimorphic weapon characters. There are two non-mutually exclusive interpretations of these results. First, Dsx-FS may have functions in other sexual traits such as gonad development or in different developmental stages. To date, all functional studies of dsx in weaponed coleopteran species have focused on postembryonic development, especially on the prepupal period when sexually dimorphic adult structures develop. Thus, although Dsx-FS type isoforms seems to be non-functional for sex-specific weapon traits via knock-down during prepupal period, it is necessary to investigate Dsx-FS function in gonad development in prepupal periods and early sex-determination including early germ cell differentiation during the embryonic stage. The second possibility is that Dsx-FL can compensate for Dsx-FS function, so that the effects of Dsx-FS knockdown were masked by the Dsx-FL isoform. In Tribolium castaneum, dsxFS RNAi affected ovarian development19. This result can be explained by either of those two possibilities. Further studies are necessary to reveal the functions of the two structurally different female isoforms of Dsx in coleopteran insects.
Additional Information
How to cite this article: Gotoh, H. et al. Molecular cloning and functional characterization of the sex-determination gene doublesex in the sexually dimorphic broad-horned beetle Gnatocerus cornutus (Coleoptera, Tenebrionidae). Sci. Rep. 6, 29337; doi: 10.1038/srep29337 (2016).
Supplementary Material
Acknowledgments
We thank Drs. L. Lavine and M. Lavine for their comments and English corrections on the manuscript, and thank Drs. M. Kobayashi and M. Ikeda for helpful discussions of this work, and Ms. H. Kawaguchi for the maintenance of beetle laboratory stocks. This work was supported in part by MEXT KAKENHI Grant Numbers 25128706 and 16H01452, JSPS KAKENHI Grant Number 25660265 and H. Gotoh was supported by JSPS fellow.
Footnotes
Author Contributions T.N. designed the study. M.I., H.N. and S.M. performed the experiments. T.N., M.I., H.N., S.M., H.G. and T.Y. analyzed the data. K.O. and T.M. supplied the materials. H.G., T.N. and S.M. wrote and all authors reviewed the manuscript.
References
- Emlen D. J. Animal Weapons: The Evolution of Battle. (Henry Holt and Company, 2014). [Google Scholar]
- Warren I. A., Gotoh H., Dworkin I. M., Emlen D. J. & Lavine L. C. A general mechanism for conditional expression of exaggerated sexually‐selected traits. Bioessays 35, 889–899 (2013). [DOI] [PubMed] [Google Scholar]
- Lavine L., Gotoh H., Brent C. S., Dworkin I. & Emlen D. J. Exaggerated trait growth in insects. Ann. Rev. Entomol. 60, 453–472 (2015). [DOI] [PubMed] [Google Scholar]
- Emlen D. J., Warren I. A., Johns A., Dworkin I. & Lavine L. C. A mechanism of extreme growth and reliable signaling in sexually selected ornaments and weapons. Science 337, 860–864 (2012). [DOI] [PubMed] [Google Scholar]
- Kijimoto T., Moczek A. P. & Andrews J. Diversification of doublesex function underlies morph-, sex-, and species-specific development of beetle horns. Proc. Natl. Acad. Sci. USA 109, 20526–20531 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y. et al. The role of doublesex in the evolution of exaggerated horns in the Japanese rhinoceros beetle. EMBO Rep. 14, 561–567 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh H. et al. Developmental link between sex and nutrition; doublesex regulates sex-specific mandible growth via juvenile hormone signaling in stag beetles. PLoS Genet. 10, e1004098 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gempe T. & Beye M. Function and evolution of sex determination mechanisms, genes and pathways in insects. Bioessays 33, 52–60 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtis K. C. & Baker B. S. Drosophila doublesex gene controls somatic sexual differentiation by producing alternatively spliced mRNAs encoding related sex-specific polypeptides. Cell 56, 997–1010 (1989). [DOI] [PubMed] [Google Scholar]
- Robinett C. C., Vaughan A. G., Knapp J. M. & Baker B. S. Sex and the single cell. II. There is a time and place for sex. PLoS Biol. 8, e1000365 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emlen D. J., Szafran Q., Corley L. S. & Dworkin I. Insulin signaling and limb-patterning: candidate pathways for the origin and evolutionary diversification of beetle ‘horns’. Heredity 97, 179–191 (2006). [DOI] [PubMed] [Google Scholar]
- Moczek A. P. & Kijimoto T. Development and evolution of insect polyphenisms: novel insights through the study of sex determination mechanisms. Curr. Opi. Insect Sci. 1, 52–58 (2014). [DOI] [PubMed] [Google Scholar]
- Okada K., Miyanoshita A. & Miyatake T. Intra-sexual dimorphism in male mandibles and male aggressive behavior in the broad-horned flour beetle Gnatocerus cornutus (Coleoptera: Tenebrionidae). J. Insect Behav. 19, 457–467 (2006). [Google Scholar]
- Okada K. & Miyatake T. Genetic correlations between weapons, body shape and fighting behaviour in the horned beetle Gnatocerus cornutus. Animal Behav. 77, 1057–1065 (2009). [Google Scholar]
- Angelini D. R. & Jockusch E. L. Relationships among pest flour beetles of the genus Tribolium (Tenebrionidae) inferred from multiple molecular markers. Mol. Phylogent. Evol. 46, 127–141 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S. et al. The genome of the model beetle and pest Tribolium castaneum. Nature 452, 949–955 (2008). [DOI] [PubMed] [Google Scholar]
- Hunt T. et al. A comprehensive phylogeny of beetles reveals the evolutionary origins of a superradiation. Science 318, 1913–1916 (2007). [DOI] [PubMed] [Google Scholar]
- Kim C. G. et al. Pattern of morphological diversification in the Leptocarabus ground beetles (Coleoptera: Carabidae) as deduced from mitochondrial ND5 gene and nuclear 28S rDNA sequences. Mol. Biol. Evol. 17, 137–145 (2000). [DOI] [PubMed] [Google Scholar]
- Shukla J. N. & Palli S. R. Doublesex target genes in the red flour beetle, Tribolium castaneum. Sci. Rep. 2, 978 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan C. & Petitpierre E. Chromosome numbers and sex-determining systems in Tenebrionidae (Coleoptera). In Advances in Coleopterology (ed. Zunino H. M., Belles X. & Blas M.) 167–176 (European Association of Coleopterology, 1991). [Google Scholar]
- Gotoh H., Nishikawa H., Sahara K., Yaginuma T. & Niimi T. A new molecular technique for determining the sex of Harmonia axyridis. J. Insect Biotech. Sericol. 84, 9–15 (2015). [Google Scholar]
- Price D. C., Egizi A. & Fonseca D. M. The ubiquity and ancestry of insect doublesex. Sci. Rep. 5, 13068 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh H. et al. Identification and functional analyses of sex determination genes in the sexually dimorphic stag beetle Cyclommatus metallifer. BMC Genomics 17, 250 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.