Significance
Testosterone stimulation during the pubertal period is necessary for the full expression of male-type social behaviors. However, it is not known whether estrogen receptor (ER) α may be involved in pubertal organizational action of testosterone on the regulation of male-type social behaviors. In this study, we showed that the prepubertal knockdown of ERα in the medial amygdala (MeA) of gonadally intact male mice reduced both sexual and aggressive behaviors as well as the number of MeA neurons later in adulthood. These results indicate that not only aromatization but also ERα expression in the MeA during the pubertal period is required for organizational action of testosterone to fully masculinize neural circuitry responsible for the expression of male-type social behaviors.
Keywords: estrogen receptor α, pubertal period, testosterone, medial amygdala, social behavioral network
Abstract
Testosterone plays a central role in the facilitation of male-type social behaviors, such as sexual and aggressive behaviors, and the development of their neural bases in male mice. The action of testosterone via estrogen receptor (ER) α, after being aromatized to estradiol, has been suggested to be crucial for the full expression of these behaviors. We previously reported that silencing of ERα in adult male mice with the use of a virally mediated RNAi method in the medial preoptic area (MPOA) greatly reduced sexual behaviors without affecting aggressive behaviors whereas that in the medial amygdala (MeA) had no effect on either behavior. It is well accepted that testosterone stimulation during the pubertal period is necessary for the full expression of male-type social behaviors. However, it is still not known whether, and in which brain region, ERα is involved in this developmental effect of testosterone. In this study, we knocked down ERα in the MeA or MPOA in gonadally intact male mice at the age of 21 d and examined its effects on the sexual and aggressive behaviors later in adulthood. We found that the prepubertal knockdown of ERα in the MeA reduced both sexual and aggressive behaviors whereas that in the MPOA reduced only sexual, but not aggressive, behavior. Furthermore, the number of MeA neurons was reduced by prepubertal knockdown of ERα. These results indicate that ERα activation in the MeA during the pubertal period is crucial for male mice to fully express their male-type social behaviors in adulthood.
Testosterone plays a central role in the regulation of male-type social behaviors in many mammalian species. It is known that testosterone facilitates the expression of sexual and aggressive behaviors through two kinds of actions. During the developmental period, testosterone exerts its “organizational action” to irreversibly masculinize the sexually undifferentiated brain and build the male-type neural network. In adulthood, testosterone exerts “activational action” to regulate the function of the fully masculinized neural network. As well as acting through androgen receptors (ARs), testosterone also acts through estrogen receptor (ER) α or ERβ, after being aromatized to estradiol (E2). Because the expression of sexual and aggressive behaviors is greatly reduced in aromatase knockout (ArKO) and ERα knockout (αERKO) male mice, aromatization of testosterone and its action via ERα have been suggested to be crucial for the facilitation of male-type social behaviors in mice (1–8). However, the exact timing and brain site(s) of ERα activation crucial for the expression of sexual and aggressive behaviors remain undetermined.
In our previous study, we demonstrated site-specific involvement of ERα in the activational action of testosterone by using a virally mediated RNAi method. In this study, knocking down of ERα in the medial preoptic area (MPOA) of gonadally intact adult male mice suppressed sexual behavior while in the ventromedial nucleus (VMN) of hypothalamus reduced both sexual and aggressive behaviors (9). On the other hand, ERα silencing in the medial amygdala (MeA) did not affect either behavior (9), contrary to our predictions. Numerous studies have shown that neurons in the MeA express a high level of ERα (10–12) and play a role in the facilitation of both male sexual and aggressive behaviors (13–18). However, consistent with our findings, Paisley et al. (19) reported that the site-specific suppression of ERα with antisense in the MeA did not affect the expression of sexual behavior in gonadally intact male rats. Considering these findings, we hypothesized that ERα in the MeA may be involved in the organizational action of testosterone.
In addition to the classically known perinatal critical period, it is now well-documented that testosterone may also exert its organizational action during the pubertal period. Studies using male Syrian hamsters have reported that deprivation of testosterone during this period leads to an irreversible alteration in male-type social behaviors, tested in adults with testosterone replacement (20–23). Moreover, it is known that structural sexual dimorphism in the MeA reported in adult rats and hamsters is due to irreversible changes induced by testosterone during the pubertal period (24, 25). However, it is not known whether ERα may be involved in the pubertal organizational action of testosterone on the regulation of male-type social behaviors and/or formation of sexually dimorphic brain structure.
In the present study, we performed site-specific ERα knockdown before the onset of puberty in male mice with the use of an AAV-mediated RNAi method and examined the effects on the expression of male sexual and aggressive behaviors in adults (experiment 1). Among a number of brain areas expressing high levels of ERα, we focused on the MeA, in which ERα silencing only in adulthood had no effects on both behaviors, and on the MPOA, where it disrupted sexual, but not aggressive, behaviors. In addition, we investigated the effects of prepubertal ERα knockdown on sexually dimorphic brain morphology of the MeA (experiment 2).
Results
Experiment 1: Effects of Site-Specific Knockdown of ERα During the Prepubertal Period on the Expression of Male-Type Social Behaviors.
MeA.
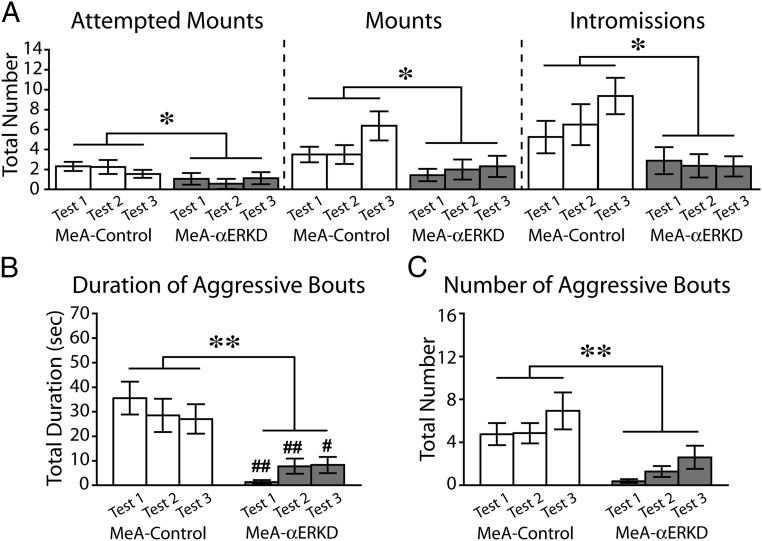
Prepubertal knockdown of ERα in the MeA caused a great reduction of sexual behavior in adult male mice (Fig. 1A). Repeated measurements ANOVA revealed that the MeA-αERKD group showed a significantly lower number of attempted mounts [treatment, F1,30 = 4.227, P < 0.05; treatment × test number, F2,60 = 0.880, not significant (N.S.)], mounts (treatment, F1,30 = 5.488, P < 0.05; treatment × test number, F2,60 = 1.405, N.S.), and intromissions (treatment, F1,30 = 6.753, P < 0.05; treatment × test number, F2,60 = 2.123, N.S.) compared with the MeA-control group.
Fig. 1.
Effects of prepubertal ERα knockdown in the MeA on male sexual and aggressive behaviors. (A) Number of attempted mounts (Left), mounts (Middle), and intromissions (Right) were significantly reduced in the MeA-αERKD compared with the MeA-control group. (B and C) Both the duration and number of aggressive bouts were significantly reduced in the MeA-αERKD compared with the MeA-control group. **P < 0.01, *P < 0.05, as indicated in the figures. ##P < 0.01, #P < 0.05 vs. MeA-control group in the respective test. Data are presented as mean ± SEM.
Prepubertal ERα silencing in the MeA also caused a great reduction of the aggressive behaviors tested in adult (Fig. 1 B and C). Repeated measurements ANOVA revealed that the total duration (treatment, F1,30 = 17.093, P < 0.01; treatment × test number, F2,60 = 3.394, P < 0.05) (Fig. 1B) and the number (treatment, F1,30 = 12.618, P < 0.01; treatment × test number, F2,60 = 0.175, N.S.) (Fig. 1C) of aggressive bouts were significantly reduced in the MeA-αERKD group compared with the MeA-control group. We found that the control group consistently showed longer total duration of aggressive bouts than the αERKD group in all three tests (by Bonferroni post hoc test: tests 1 and 2, P < 0.01; test 3, P < 0.05) even though there was a significant interaction between treatment and test number.
MPOA.
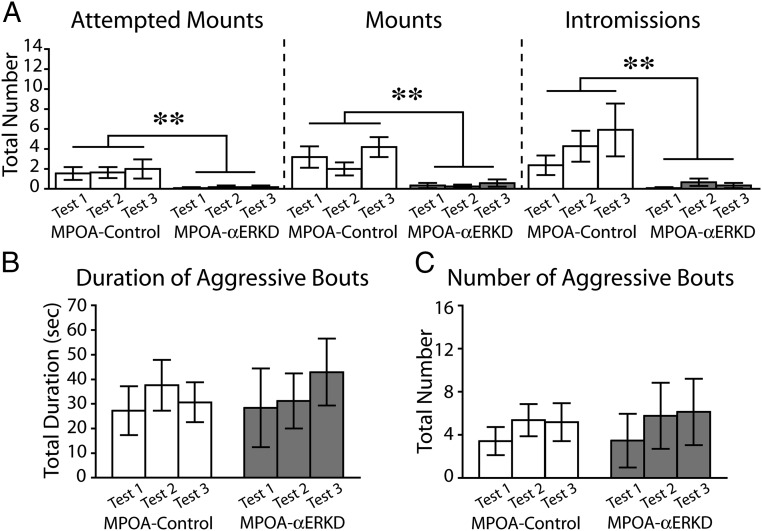
Knocking down of ERα in the MPOA caused a great reduction of sexual behaviors (Fig. 2A) whereas it did not affect the levels of aggressive behaviors (Fig. 2 B and C). MPOA-αERKD mice rarely showed any components of sexual behaviors in all three tests. Repeated measurements ANOVA revealed that the number of attempted mounts (treatment, F1,21 = 11.376, P < 0.01; treatment × test number, F2,42 = 0.101, N.S.), mounts (treatment, F1,21 = 14.826, P < 0.01; treatment × test number, F2,42 = 1.615, N.S.), and intromissions (treatment, F1,21 = 9.338, P < 0.01; treatment × test number, F2,42 = 1.103, N.S.) were significantly reduced in MPOA-αERKD compared with the MPOA-control group (Fig. 2A).
Fig. 2.
Effects of prepubertal ERα knockdown in the MPOA on male sexual and aggressive behaviors. (A) Number of attempted mounts (Left), mounts (Middle), and intromissions (Right) were significantly reduced in the MPOA-αERKD compared with the MPOA-control group. (B and C) There was no difference between the MPOA-control and MPOA-αERKD groups in either (B) duration or (C) number of aggressive bouts. **P < 0.01. Data are presented as mean ± SEM.
Unlike sexual behavior, MPOA-αERKD mice showed equivalent levels of aggressive behavior toward male intruder stimuli as mice in the MPOA-control group (Fig. 2 B and C). There were no statistically significant differences between MPOA-αERKD and MPOA-control groups in the total duration (treatment, F1,21 = 0.021, N.S.; treatment × test number, F2,42 = 0.163, N.S.) (Fig. 2B) or the number (treatment, F1,21 = 0.024, N.S.; treatment × test number, F2,42 = 1.008, N.S.) (Fig. 2C) of aggressive bouts.
Immunohistochemical evaluation of ERα knockdown.
Successful uptake of AAV vectors by the cells in the targeted areas was verified by the presence of GFP-immunopositive cells. In both control and αERKD mice, we observed GFP-positive cells throughout the rostral-caudal extent of the MeA (Fig. S1 A and B) and MPOA (Fig. S1 D and E), as shown in the representative photomicrographs. Double immunohistochemical staining for ERα and GFP revealed that a substantial number of GFP positive cells expressed ERα in the control mice but none of them expressed ERα in αERKD mice in both MeA (Fig. S2A) and MPOA (Fig. S2B) groups.
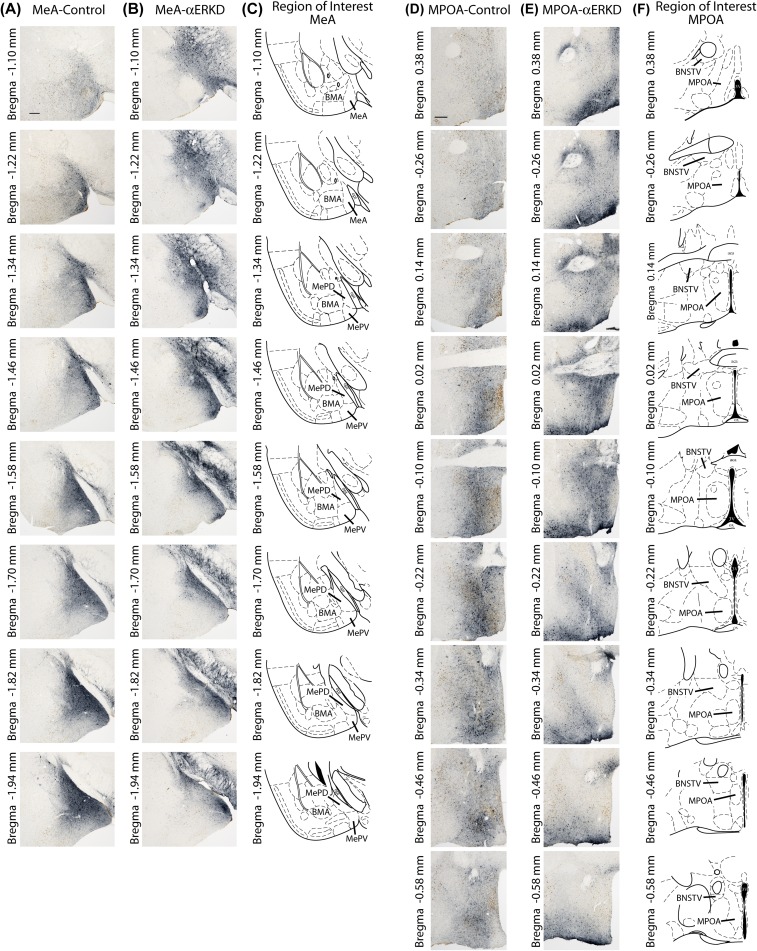
Fig. S1.
GFP distribution patterns throughout the rostral-caudal extent of the target regions. (A and B) Representative photomicrographs of ERα-GFP double-labeled sections of MeA-control and MeA-αERKD mice, and (C) histological diagrams of the MeA (defined as the area combined MePD and MePV) throughout the rostral-caudal extent (Bregma −1.10 to −1.94 with 120-µm intervals). (D and E) Representative photomicrographs of ERα-GFP double-labeled sections of MPOA-control and MPOA-αERKD mice, and (F) histological diagrams of the MPOA throughout the rostral-caudal extent (Bregma 0.38 to −0.58 with 120-µm intervals). (Scale bars: 200 µm.) BMA, basomedial amygdala; BNSTV, bed nucleus of the stria terminalis, ventral; MePD, medial amygdala, posterodorsal; MePV, medial amygdala, posteroventral; MPOA, medial preoptic area.
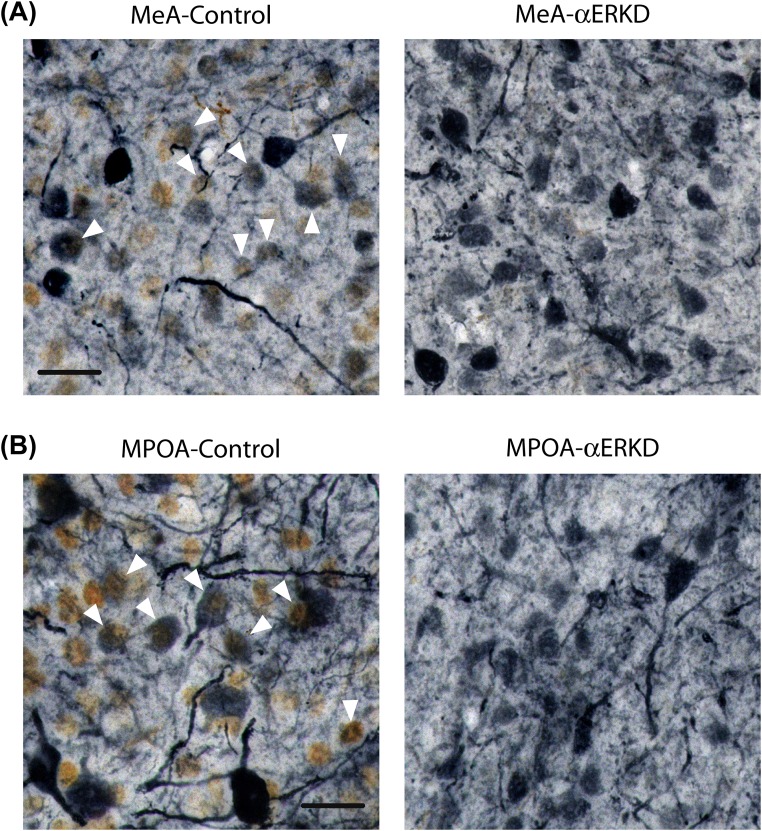
Fig. S2.
Double-label immunohistochemistry for ERα (brown) and GFP (blue-gray). (A) Representative photomicrographs of double-labeled MeA sections (Bregma −1.67) of MeA-control and MeA-αERKD. (B) Representative photomicrographs of double-labeled MPOA sections (Bregma −0.07) of MPOA-control and MPOA-αERKD. The absence of ERα-GFP double-labeled cells in the MeA-αERKD or MPOA-αERKD sections indicates that AAV-shERα used in the present study successfully knocked down ERα expression in the transfected cells. (Scale bars: 20 µm.) The white arrowheads indicate ERα-GFP double-labeled cells.
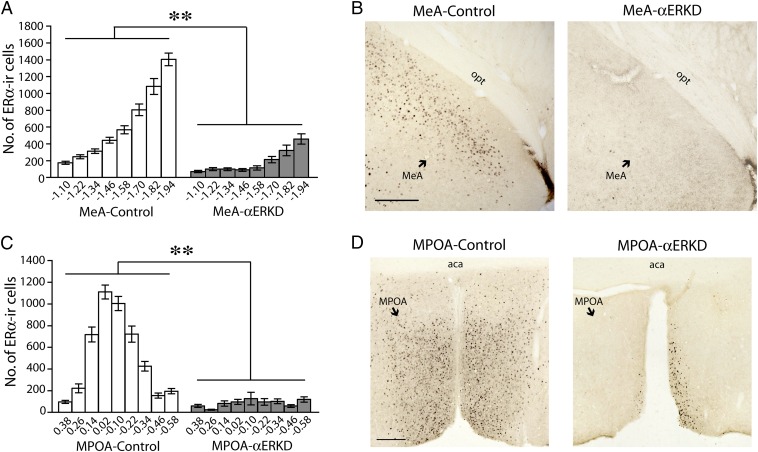
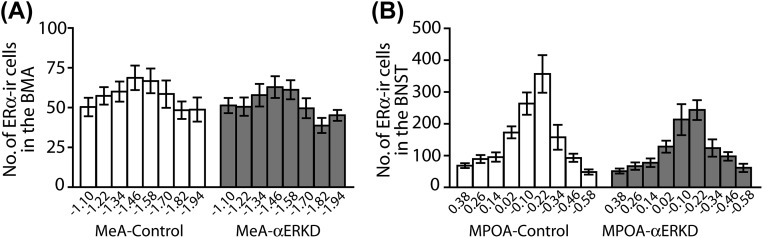
Quantification of ERα-immunopositive cells along the rostral-caudal axis further confirmed that the site-specific injection of AAV-shERα caused a great reduction of ERα expression in the target areas without affecting the expression in adjacent areas. In the MeA injected mice, an ∼70% reduction of ERα-positive cells in the MeA region (see Fig. S1C for definition) was observed in the MeA-αERKD compared with the MeA-control group (treatment, F1,30 = 111.149, P < 0.01) (Fig. 3A) as shown in the representative photomicrographs (Fig. 3B). On the other hand, the number of ERα-positive cells in the basomedial amygdala (BMA), the area adjacent to the MeA (see Fig. S1C for definition), was not affected (F1,30 = 0.587, N.S.) (Fig. S3A). In the MPOA injected mice, an ∼84% reduction of ERα-positive cells in the MPOA region (see Fig. S1F for definition) was observed in the MPOA-αERKD group compared with the MPOA-control group (treatment, F1,21 = 342.083, P < 0.01) (Fig. 3C), as shown in the representative photomicrographs (Fig. 3D). In the ventral portion of the bed nucleus of the stria terminalis (BNST), the area adjacent to the MPOA (see Fig. S1F for definition), there was no treatment group difference in the number of ERα-positive cells (F1,21 = 1.075, N.S.) (Fig. S3B).
Fig. 3.
Immunohistochemical evaluations of ERα knockdown in the MeA and MPOA. (A and C) The number of ERα-immunoreactive cells in the target regions was significantly reduced in the αERKD groups compared with their respective control groups throughout the rostrocaudal axis (A) in the MeA (Bregma −1.10 to −1.94; defined as the area combined MePD and MePV) (Fig. S1C) and (C) in the MPOA (Bregma 0.38 to −0.58) (Fig. S1F). **P < 0.01. Data are presented as mean ± SEM. (B and D) Representative photomicrographs of ERα-immunoreactive cells in brain sections from (B) the MeA-control and MeA-αERKD groups and (D) the MPOA-control and MPOA-αERKD groups. (Scale bars: 200 µm.)
Fig. S3.
Confirmation of site specificity of ERα knockdown. (A) Number of ERα-immunoreactive cells in the BMA, the lateral adjacent area to the MeA, was not different between the MeA-αERKD and MeA-control groups throughout the rostrocaudal axis (Bregma −1.10 to −1.94) (see Fig. S1C for the definition of the BMA). (B) Number of ERα-immunoreactive cells in the ventral portion of the BNST, the dorsal adjacent area to the MPOA, was not different between the MPOA-αERKD and MPOA-control groups throughout the rostrocaudal axis (Bregma 0.38 to −0.58) (see Fig. S1F for the definition of the ventral portion of the BNST). All data are presented as mean ± SEM.
Experiment 2: Effects of the Site-Specific Knockdown of ERα During the Prepubertal Period on the Morphological Development of the MeA.
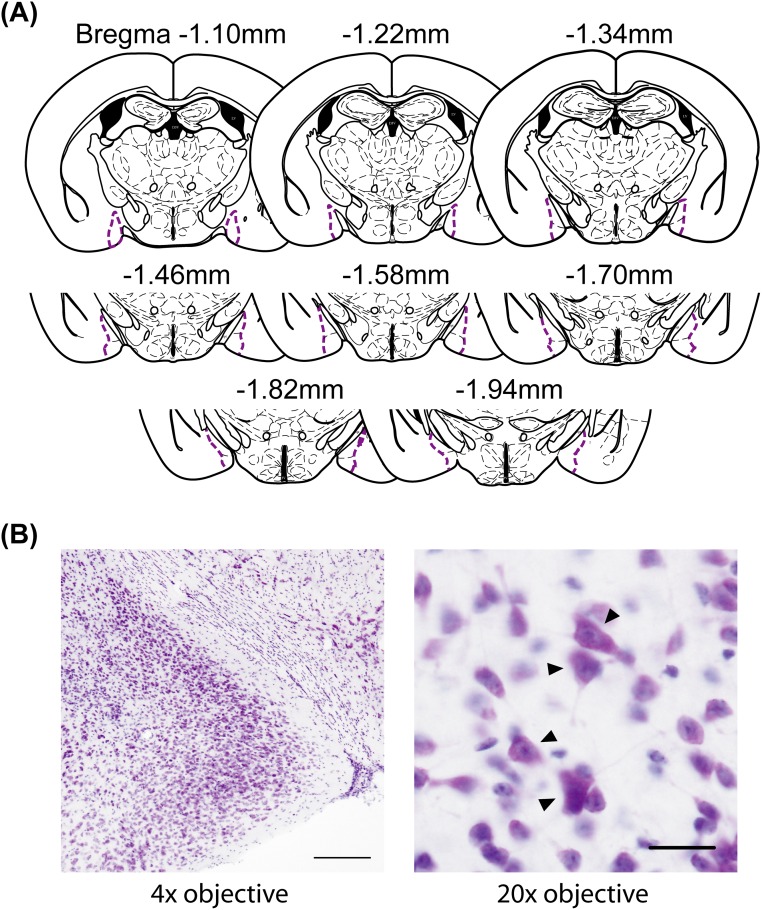
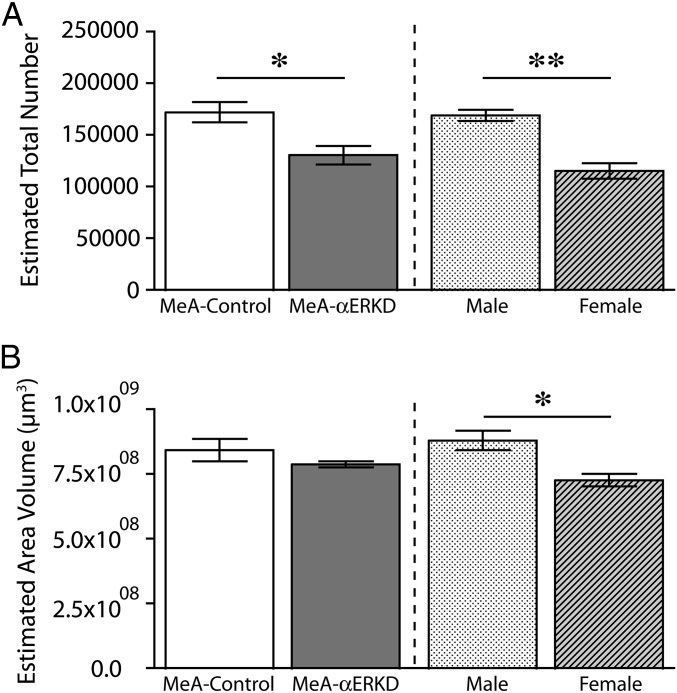
Prepubertal knockdown of ERα in the MeA caused a significant reduction in the number of neuronal cells within the region examined (see Fig. S4 A and B) in adulthood [t(9) = 2.986, P < 0.05] (Fig. 4A). In contrast to the neuronal cell number, there was no difference in the volume of the MeA between MeA-αERKD mice and MeA-control mice [t(9) = 1.591, N.S.] (Fig. 4B). In the uninjected reference groups used for examination of sex differences of the MeA morphology, both number of neurons [t(6) = 5.794, P < 0.01] (Fig. 4A) and the regional volume [t(6) = 3.446, P < 0.05] (Fig. 4B) were significantly greater in gonadally intact males compared with gonadally intact females.
Fig. S4.
(A) Histological diagrams of the MeA included in the morphological evaluation. (B) Representative photomicrographs of a Nissl-stained MeA section viewed under 4× objective (Left) and the same section viewed under 20× objective (Right). (Scale bars: Left, 200 µm; Right, 20 µm.) The black arrowheads indicate neuronal cells.
Fig. 4.
Effects of prepubertal ERα knockdown on the morphology of the MeA examined in adults. (A) The number of neuronal cells in the MeA was significantly reduced in the MeA-αERKD compared with the MeA-control (Left). In uninjected reference groups, gonadally intact males had a significantly greater number of neuronal cells in the MeA compared with gonadally intact females (Right). (B) There was no difference between the MeA-control and MeA-αERKD groups in the regional volume of the MeA (Left) whereas gonadally intact males had greater regional volume compared with gonadally intact females (Right). **P < 0.01, *P < 0.05, as indicated. Data are presented as mean ± SEM.
Discussion
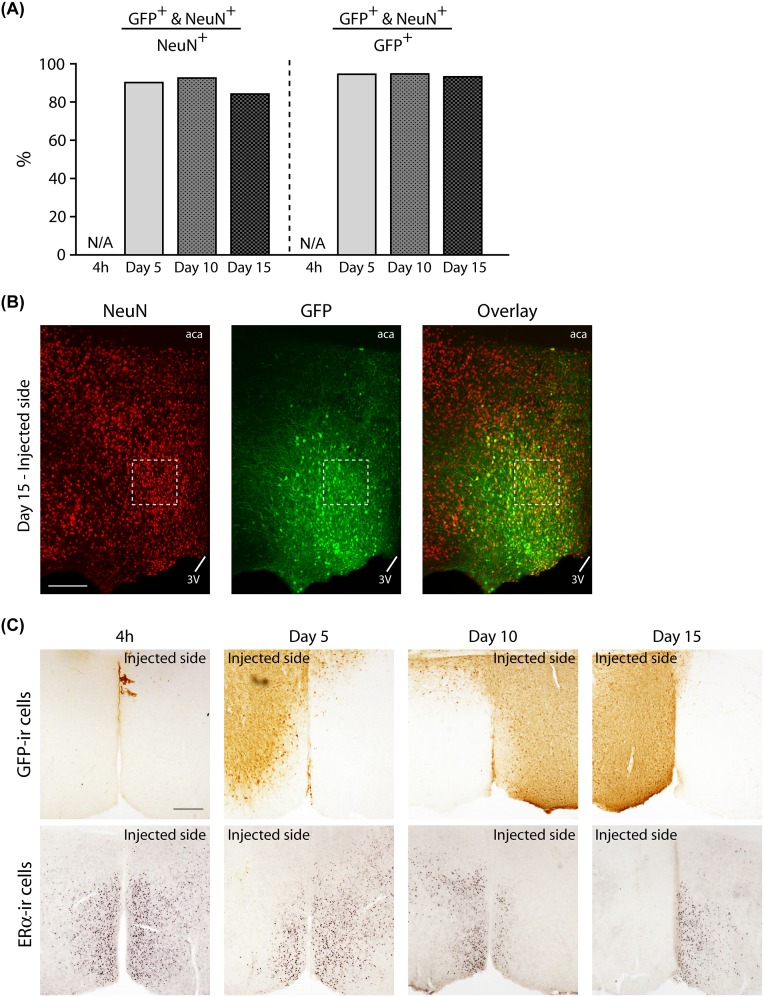
This study aimed to investigate the involvement of ERα and its site specificity in the pubertal organizational action of gonadal steroids on the regulation of male-type social behaviors. The onset of puberty in rodents is known to be around postnatal day (PND) 28. In male mice, testosterone production in testis begins to rise around PND 28, and plasma concentration also starts to rise around PND 30 (26, 27). In our pilot experiment, we observed the uptake of vectors by ∼90% of neuronal cells at as early as the fifth day after injection (Fig. S5 A and B). We also confirmed that a 5-d interval was sufficient for the AAV-shERα to almost completely silence the expression of ERα in the target area after AAV injection on PND 21 (Fig. S5C). Therefore, it is assumed that ERα must have been absent in the targeted area well before the mice used in the present study reached the age of puberty onset.
Fig. S5.
Results of a supplemental experiment performed to confirm successful vector uptake and ERα knockdown before the onset of puberty in mice unilaterally injected with AAV-shERα vectors on PND 21. (A) Percentage of NeuN and GFP double-labeled (NeuN+ and GFP+) cells in the total number of NeuN+ (Left) or GFP+ (Right) cells. About 90% of NeuN+ cells in the MPOA also expressed GFP at the time point of 5, 10, or 15 d after AAV vector injection. All data are presented as mean of two mice. N/A, not applicable due to absence of GFP expression. (B) Representative photomicrographs of a NeuN-GFP double-labeled section in the injected side of the MPOA at the time point of 15 d after vector injection. (Scale bar: 200 µm.) (C) Representative photomicrographs demonstrating immunohistochemical labeling for GFP in the MPOA sections (Top) and that for ERα in the adjacent sections (Bottom) at four different time points (4 h and on 5, 10, or 15 d) after unilateral injection of AAV-shERα. At 4 h, GFP expression was not detected in the injected side (left hemisphere), and the expression of ERα was not affected. On 5, 10, and 15 d after injection, GFP-immunopositive cells were detected, and expression of ERα were greatly reduced in the injected side. These results suggest that AAV-shERα used in the present study successfully knocked down ERα expression in the targeted regions by the fifth day after injection: i.e., PND 26, before the onset of puberty. (Scale bar: 200 µm.)
In experiment 1, we found that prepubertal ERα knockdown in the MeA greatly reduced both sexual and aggressive behavior in male mice tested in adulthood. It should be noted that AAV-shERα used in this study irreversibly knocked down ERα expression in transfected cells. However, we have previously shown that site-specific injection of AAV-shERα in the MeA at 16 wk of age (well after the end of the pubertal period) did not affect the expression of sexual or aggressive behaviors in male mice (9). Thus, behavioral alterations found in the present study must be due to the lack of ERα activation by testosterone in the MeA during the pubertal period. It should be noted that all mice were tested as gonadally intact in the present study. Therefore, it is expected that endogenous testosterone action via ARs was intact. In addition, testosterone action via ERα and ERβ, after being aromatized to estradiol, was not disrupted except that through ERα in the target region. Nevertheless, prepubertal, but not postpubertal, ERα knockdown in the MeA greatly altered both sexual and aggressive behaviors in adults. These facts indicate that the ERα in the MeA is crucial for the pubertal organizational action of testosterone on male-type social behaviors.
It may be argued that the behavioral alteration may simply be due to reduced levels of testosterone at the time of testing, potentially caused by prepubertal ERα knockdown. This possibility cannot be completely ruled out because we did not measure circulating levels of gonadal steroids at the time of behavioral tests in the present study. However, this possibility seems less likely because previous studies have reported that adult αERKO male mice have rather elevated levels of testosterone (28, 29). Nevertheless, it is necessary to investigate whether similar behavioral effects of prepubertal ERα knockdown may be observed in mice gonadectomized and treated with a fixed amount of testosterone before behavioral testing in adulthood. More importantly, prepubertal knockdown of ERα in the MeA may have affected onset of puberty itself, including timing and/or magnitude of pubertal testosterone rise, and may have caused behavioral alterations found in the present study. This possibility, which further suggests the importance of ERα in the MeA for the regulation of puberty onset, needs to be addressed in future studies.
In contrast to the findings in the MeA, the effect of ERα silencing in the MPOA before the onset of puberty did not differ from that induced by AAV-shERα injection in adulthood. In our previous study, knocking down of ERα in the MPOA at 16 wk of age greatly reduced the expression of male sexual, but not aggressive, behaviors (9). These results suggest that ERα activation in MPOA neuronal cells at the time of testing in adults is necessary for induction of sexual behaviors. However, we could not determine whether ERα stimulation during the pubertal period may also be necessary for the full masculinization of male sexual behavior because prepubertal injection of AAV-shERα permanently blocked ERα gene expression in the MPOA. Thus, this possibility still needs to be probed using different techniques in a future study. On the other hand, as for aggressive behavior, we found that lack of ERα in the MPOA even before the onset of puberty did not affect the expression of this behavior in adult. Therefore, it is concluded that ERα gene expression in the MPOA is not involved in the regulation of male aggressive behaviors by aromatized testosterone in terms of both pubertal organizational action and activational action in adult. However, it remains to be determined in future studies whether and how ERα in the MPOA may be responsible for perinatal organizational action of testosterone.
In our previous study investigating the effects of site-specific ERα knockdown in adults, we found that activation of ERα in the VMN at the time of testing is necessary for the induction of both sexual and aggressive behaviors in male mice (9). Lee et al. (30) have also shown that ERα-expressing neurons in the ventrolateral subdivision of the VMN regulate the expression of various social behaviors, including both sexual and aggressive behavior in the activity level-dependent manner in adult male mice. These reports together clearly suggest the significant contribution of ERα in the VNM for the facilitation of male social behaviors. However, whether ERα stimulation during the pubertal period may also be necessary for the full masculinization of male sexual and aggressive behaviors remain unknown.
Differential effects of prepubertal ERα knockdown in the MeA and MPOA suggest that the role of ERα for masculinization of neural circuitry for male social behaviors through organizational action of gonadal steroids is site-specific. To further characterize the effects of prepubertal ERα knockdown in the MeA at neuroanatomical levels, we performed thorough analysis of MeA cells (experiment 2). We found that the number of neuronal cells was reduced in the MeA-αERKD group compared with the MeA-control group. A similar difference was also observed between males and females analyzed as a reference. It is known that MeA undergoes structural changes during the pubertal period in a sex-specific manner that leads to sexually dimorphic adult phenotypes in various species (24, 25, 31, 32). In gonadally intact rats, it is reported that a greater number of neuronal cells were proliferated in the MeA during the pubertal period in males compared with females and that these sexual differences were eliminated by prepubertal gonadectomy (24). In contrast, gonadectomy in adulthood did not affect the number of neuronal cells in the MeA of male rats (32). Thus, the number of neuronal cells in the MeA undergoes irreversible change during the pubertal period in a gonadal steroid-dependent manner. Our findings showing a reduced number of MeA neurons in the MeA-αERKD group suggest that the presence of ERα is required for testosterone to masculinize the neuronal number in the MeA during the pubertal period. The importance of the MeA in the regulation of male-type social behaviors has been demonstrated in numerous studies (13–18). Particularly, the MeA plays a significant role in chemosensory information processing (15, 16, 33, 34) as part of the social behavior network in the CNS (35). It relays socially meaningful signals to activate or inhibit various downstream brain sites, such as the MPOA, anterior hypothalamic area (AHA), and VMN, that are more directly involved in the execution of male sexual and aggressive behaviors (14, 36–40). Thus, incomplete masculinization of the MeA may cause an improper functioning of the neural circuitry for the expression of male-type social behaviors. Testosterone and/or estradiol are involved in development of sexually dimorphic brain morphology by promoting cell proliferation, differentiation, or survival, or by inducing apoptosis in a sex-specific manner in certain brain regions (41–45). However, the exact mechanisms of ERα-mediated action of testosterone for full masculinization of the MeA structure and function during the pubertal period still remain unknown and need to be elucidated in future studies. In addition, it is our great interest to identify the profile of the lost neuronal population(s) that might be responsible for the disruption of sexual and aggressive behaviors by prepubertal knockdown of ERα. Optogenetic stimulation of the GABAergic neuronal population in the posterodorsal region of the MeA has been shown to promote sexual and aggressive behaviors in a scalable manner in male mice (17). Another study with pharmacogenomical manipulations has reported that aromatase-expressing neurons in the sexually dimorphic posterodorsal region of the MeA are necessary for both male and female mice to express their sex-specific forms of aggression whereas they do not seem to be crucial for the expression of sexual behaviors (18). Further studies determining the identity of the lost neuronal population(s) in MeA-αERKD mice will provide further insight into the mechanisms of how exactly the function of the MeA is organized in a sex-specific manner.
Taken together, our results clearly demonstrated that the absence of ERα only in the MeA (while all of the other physiological conditions are kept intact) dramatically alters brain development and expression of male social behaviors in adults. To our knowledge, this study is the first study indicating that not only aromatization of testosterone but also ERα expression in the MeA during the pubertal period is absolutely necessary for testosterone to fully organize/masculinize neural circuitry governing male-type social behaviors. Furthermore, considering that the amygdala is a major region responsible for sex differences in socio-emotional states that become more prominent after the onset of puberty, our findings offer insights for better understating of the neuroendocrine basis of gender-specific neurobehavioral development in human adolescence.
Materials and Methods
Subjects.
A total of 70 male and 4 female ICR/Jcl mice were used. All procedures were approved by the University of Tsukuba Institutional Animal Care and Use Committee and the University of Tsukuba Institutional Recombinant DNA Use Committee and were conducted strictly in accordance with National Institutes of Health guidelines. All efforts were made to minimize the number of animals and their suffering.
Design of shRNA for ERα Silencing.
Adeno-associated virus (AAV) vectors expressing a small hairpin RNA (shRNA) against either the sequence specific for the ERα gene or the sequence specific for luciferase (LUC) as control were used. These vectors also expressed enhanced green fluorescent protein (GFP) as a reporter to visually detect transduced neurons. Details of shRNAs used in this study are described in Musatov et al. (46) and also in SI Materials and Methods.
Stereotaxic Surgery.
Stereotaxic surgery for all experiments was performed on PND 21. After being weaned from their mothers, gonadally intact male mice were bilaterally injected with either AAV-shERα or AAV-shLUC in one of two brain regions: the medial amygdala (MeA) or medial preoptic area (MPOA). Each group was designated as MeA-αERKD, MeA-control, MPOA-αERKD, and MPOA-control. Detailed procedures of stereotaxic surgery are described in SI Materials and Methods.
Experimental Design.
Experiment 1: Effects of the site-specific knockdown of ERα during the prepubertal period on the expression of male-type social behaviors.
A total of 55 male mice received bilateral injection of AAV-shERα or AAV-shLUC in the MeA or MPOA (MeA-αERKD, n = 16; MeA-control, n = 16; MPOA-αERKD, n = 12; MPOA-control, n = 11) on PND 21. At 11 wk of age, all mice were individually housed in plastic cages (29 × 19 × 12 cm). Starting 1 wk later, they were tested for sexual and aggressive behaviors biweekly in an alternating manner. Behavioral tests were performed during the dark phase (starting 2 h after lights off) of the light–dark cycle under red light illumination. All tests were video-recorded and scored off-line by an observer not aware of the treatment of the mouse with the use of a digital event recorder program (Recordia 1.0b; O’Hara & Co., Ltd). After the completion of behavioral testing, all mice were transcardially perfused, and brain tissues were processed for immunohistochemistry of either ERα single or ERα-GFP double labeling. Eight MeA sections (Bregma −1.10 to −1.94) and nine MPOA sections (Bregma 0.38 to −0.58) were analyzed for the number and distribution of ERα-immunopositive cells.
Experiment 2: Effects of site-specific knockdown of ERα during the prepubertal period on the morphological development of the MeA.
A total of 11 male mice were bilaterally injected with either AAV-shERα or AAV-shLUC in the MeA (MeA-αERKD, n = 7; MeA-control, n = 4) on PND 21. At the age of 12 wk old, all mice were transcardially perfused, and brain tissues were processed for immunohistochemical labeling of GFP for confirmation of vector uptake in the targeted area, and for histological staining of Nissl substance with 0.2% thionin blue solution for stereological analysis of MeA neurons. Sections were also collected from uninjected gonadally intact 12-wk-old male (n = 4) and female (n = 4) ICR/Jcl mice. They were used for stereological analysis to examine sex differences in the MeA neuronal morphology as a reference for evaluation of demasculinization caused by prepubertal ERα silencing.
Statistics.
Behavioral data and the number of ERα-immunopositive cells were analyzed by two-way ANOVAs for repeated measurements. ANOVAs were followed by a Bonferroni post hoc test when appropriate. The number of neuronal cells and the regional volume across treatment were compared using independent t tests. Statistically significant differences were considered when P < 0.05 (two-tailed). All data are presented as mean ± SEM. All data were analyzed using the SPSS 19.0 (SPSS Inc.) statistical package.
The detailed procedures of sexual and aggressive behavioral tests, brain tissue preparation, immunohistochemistry, ERα-immunopositive cell count, and stereological analysis are described in SI Materials and Methods.
SI Materials and Methods
Subjects.
Adult ICR/Jcl male and female mice were purchased from a commercial breeder (CLEA) and mated. When females became visibly pregnant, they were housed individually in plastic cages (29 × 19 × 12 cm) with cotton nesting material. They were then monitored for parturition once a day at the end of the light phase. The day of parturition was defined as postnatal day (PND) 0. On PND 21, male offspring were weaned and used for the experiments. All mice were housed at constant temperature (23 ± 2 °C) with a 12:12-h light/dark cycle (lights off at 1200). Food and water were provided ad libitum.
Design of shRNA for ERα Silencing.
Adeno-associated virus (AAV) vectors expressing a small hairpin RNA (shRNA) against either the sequence specific for the ERα gene.
(AAV-shERα: 5′-GATCCCCGGCATGGAGCATCTCTACACTTCCTGTCA TGTAGAGATGCTCCATGCCTTTTTTGGAT-3′ and 5′-CTAGATTCCAAAAAA GGCATGGAGCATCTCTACA TGACAGGAAGTGTAGAGATGCTCCATGCCGGG-3′) or the sequence specific for luciferase (LUC) as control (AAV-shLUC: 5′-GATCCCCCCCGTGGAGAGCAACTGCAT CTTCCTGTCA ATGCAGTTGCTCTCCAGCGGTTTTTGGAA-3′ and 5′-CTAGTTCCAAAAACCGCTGGAGAGCAACTGCAT TGACAGGAAGATGCAGTTGCTCTCCAGCGGGG-3′) were used. The nucleotides specific for ERα or luciferase are underlined. These vectors also expressed enhanced green fluorescent protein (GFP) as a reporter to visually detect transduced neurons. The detailed information of shRNAs used in this study is described in Musatov et al. (46).
Stereotaxic Surgery.
Mice were anesthetized with sodium pentobarbital (60 mg/kg) and then placed in a stereotaxic frame. A 5-μL Hamilton syringe was aimed at either the MeA [anteroposterior (AP) −1.25, mediolateral (ML) ± 2.2, dorsoventral (DV) −5.15] or MPOA (AP +0.02, ML ±0.5, DV −5.2). These coordinates were determined based on The Mouse Brain Stereotaxic Coordinates (47) with an adjustment for brain size on PND 21. Each mouse was given bilateral injection of 1 µL of either AAV-shERα or AAV-shLUC (1012 packaged genomic particles, 0.5 μL per hemisphere) using a micropump injector (World Precision Instruments Inc.). The injection lasted 5 min, and the needle was left in place for an additional 5 min after the end of infusion. After surgery, all mice were group housed with their littermates (∼4–5 mice per cage) until further use.
Sexual Behavior Test.
Each male mouse was tested in its home cage for sexual behavior against a group-housed, ovariectomized ICR/Jcl female mouse biweekly for a total of three trials. The duration of each trial was 30 min. All female stimuli were hormonally primed with s.c. injections of estradiol benzoate dissolved in sesame oil (10 μg/0.1 mL) at 48 and 24 h before testing and progesterone dissolved in sesame oil (500 μg/0.1 mL) 4–6 h before testing to ensure high sexual receptivity. Each male encountered a different receptive female mouse for each trial. The number of attempted mounts, mounts, intromissions, and ejaculations was scored for each mouse. Because no male mice exhibited ejaculation in any group, no further statistical analysis was performed on ejaculatory behavior.
Aggressive Behavior Test.
Aggressive behavior of experimental mice toward an intruder male mouse was assessed biweekly in a resident–intruder paradigm. Each week, mice were tested on three consecutive days for a total of nine trials. Each mouse was tested in its home cage against a gonadally intact, group-housed (four mice per cage), olfactory bulbectomized (OBX) ICR/Jcl male intruder mouse. The expression of aggressive behaviors in mice is highly dependent on olfactory cues, and OBX male mice rarely show aggression. Thus, testing experimental mice against OBX intruder mice allowed us to observe offensive aggressive responses from the resident mouse that were not influenced by any experience of defeat. It should be noted that our focus in the present study was on observing offensive aggressive responses although aggressive behavior has been known to consist of offensive and defensive response components (48). The duration of each trial was 15 min. Each mouse encountered a different intruder mouse in each of the nine trials. Duration and number of aggressive bouts were scored for each experimental mouse. Data from three trials performed each week were averaged for each mouse and used for statistical analysis. An aggressive behavior bout was defined as a set of behavioral interactions including at least one of the following behavioral acts: chasing, boxing, wrestling, tail rattling, biting, and offensive lateral attack. If the interval between two aggressive bouts did not exceed 3 s, the two bouts were considered continuous and scored as one bout.
Brain Tissue Preparation for Immunohistochemistry.
All mice were deeply anesthetized with a solution of 1:1 mixture of sodium pentobarbital (60 mg/kg) and heparin (1,000 units/kg) and transcardially perfused with 0.1 M PBS, pH 7.2, followed by 4% paraformaldehyde (PFA) in 0.1 M phosphate-buffer (PB), pH 7.2. Brains were removed and postfixed overnight at 4 °C in 4% PFA in 0.1 M PB. They were then rinsed with 0.1 M PB and cryoprotected in 0.1 M PB containing 30% sucrose. After the completion of tissue processing, free-floating coronal sections of brain (30-μm thickness) split into four series were prepared on a freezing microtome.
ERα Immunohistochemistry.
Sections were incubated in a rinse buffer, TBS-X (0.05 M Tris-buffered saline, pH 7.2, and 0.2% Triton X-100), containing 1% hydrogen peroxide for 20 min to inhibit endogenous peroxidase activity, and then blocked in an incubation buffer (3% skim milk and 3% BSA in TBS-X) for 2 h at room temperature. Sections were incubated in a rabbit polyclonal anti-ERα antiserum (C1355, 1:25,000; 48 h; Upstate) dissolved in the incubation buffer for 48 h at 4 °C. Sections were then treated with a 1:250 dilution of biotinylated goat anti-rabbit secondary antibody (Vector Laboratories) in the incubation buffer for 2 h at room temperature, followed by 1-h incubation with avidin–biotin complex (Vectastain ABC Elite kit; Vector Laboratories) in TBS to amplify the staining intensity. After the completion of antiserum reaction procedures, sections were visualized with 0.02% diaminobenzidine, 0.15% nickel ammonium sulfate, and 0.003% hydrogen peroxide in TBS.
ERα and GFP Double-Label Immunohistochemistry.
Sections were incubated in a rinse buffer, PBS-X (0.05 M Tris-buffered saline, pH 7.4, and 0.1% Triton X-100), containing 0.6% hydrogen peroxide for 30 min to inhibit endogenous peroxidase activity, and then blocked in an incubation buffer (5% normal goat serum in PBS-X) for 1 h at room temperature. Sections were incubated in buffer containing either a rabbit polyclonal anti-ERα antiserum (C1355, 1:10,000; 48 h; Upstate) at 4 °C. Sections were then treated with a goat anti-rabbit secondary antibody (K4003; DAKO) for 30 min at room temperature. After the completion of antiserum reaction procedures, sections were visualized with DAB (Liquid DAB+ Substrate Chromogen System, K3468; DAKO). After the visualization of ERα, antigens were retrieved from sections using 0.1 M glycine-HCl buffer solution (pH 2.2). Sections were then incubated in PBS-X containing 0.6% hydrogen peroxide for 30 min to inhibit endogenous peroxidase activity. Sections were incubated in buffer containing either a rabbit polyclonal anti-GFP antiserum [ab290, 1:30,000; overnight (o/n); Abcam] at 4 °C. Sections were then treated with a goat anti-rabbit secondary antibody (K4003; DAKO) for 30 min at room temperature. After the completion of antiserum reaction procedures, sections were visualized with a VECTOR SG Peroxidase (HRP) Substrate Kit (SK-4700; Vector).
All sections were mounted on gelatin-coated slides, air-dried, and dehydrated through an ascending alcohol series, which were cleared with xylene, and coverslipped with Permount (Fisher Scientific).
To verify the specificity of immunohistochemical procedures, we included negative controls that the primary antiserums (ERα or GFP) were omitted from the staining procedure. In these conditions, neither cells nor fibers were stained. In all experiments, only the mice who showed a spread of GFP-immunopositive cells in the targeted area were used for statistical analysis of anatomical and/or behavioral data.
ERα-Immunopositive Cell Count.
Eight MeA sections (Bregma −1.10 to −1.94) and nine MPOA sections (Bregma 0.38 to −0.58) were analyzed for number and distribution of ERα-immunopositive cells. Each brain area was photographed at 40× magnification with a digital camera mounted on an Olympus microscope (BX61; Olympus), and ERα-immunopositive cells were bilaterally counted using the Adobe Photoshop Creative Suite software (Adobe Systems Inc.). To confirm that ERα knockdown was limited within the targeted area, ERα-immunopositive cells in adjacent regions, such as the basomedial amygdala (BMA) for the MeA groups and the ventral portion of the bed nucleus of the stria terminalis (BNST) for the MPOA groups were also counted bilaterally.
Stereological Analysis of the MeA.
The MeA was observed on a PC monitor with the aid of a CCD camera connected to a light microscope (DM5000B; Leica Microsystems), and the total number of neuronal cells in the MeA and the regional volume of the MeA was estimated bilaterally with the aid of Stereo Investigator software (MBF Bioscience, Inc.). Eight sections (30-µm thickness, 90-µm intervals) containing the MeA (Fig. S4A) (Bregma −1.10 to −1.94 according to The Mouse Brain Stereotaxic Coordinates (47) were analyzed in each mouse. To measure the MeA neuronal cell number and volume, the optical fractionator method in accordance with the system workflow of the Stereo Investigator (MBF Bioscience, Inc.) was used. The outlines of the MeA were determined on digital images to define the region for cell counts and calculate the estimated volume of the MeA. The counting frame size (40 × 40 µm), the grid size for arrangement of the counting frame size (120 × 120 µm), the highest optical dissector setting (10–12 µm), and the highest top guard zone setting (2 µm) were set first. Neuronal cells within the counting frames were then counted manually, by an experimenter not aware of the treatment of the sample, to estimate the total numbers of neuronal cells in the MeA. Neuronal cells were defined as those containing blue or purple colored rough endoplasmic reticulum (Nissl bodies) in the cytoplasm and having an oval or spherical nucleus with a blue or purple colored nucleolus (Fig. S4B).
Additional Procedure for Fig. S5.
A total of eight mice were unilaterally injected with AAV-shERα in the right or left hemisphere of the MPOA on PND 21. At the time point of 4 h (PND 21, n = 2), 5 d (PND 26, n = 2), 10 d (PND 31, n = 2), or 15 d (PND 36, n = 2) after the injection, they were transcardially perfused, and brain tissues were processed for NeuN and GFP double-label fluorescent immunohistochemistry or single-label immunohistochemistry for GFP or ERα.
NeuN and GFP Double-Label Fluorescent Immunohistochemistry.
Sections were incubated in a rinse buffer, TBS-X (0.05 M Tris-buffered saline, pH 7.2, and 0.2% Triton X-100), containing 3% hydrogen peroxide for 20 min to inhibit endogenous peroxidase activity, and then blocked in an incubation buffer (3% skim milk and 3% BSA in TBS-X) for 2 h at room temperature. Sections were incubated in buffer containing a rabbit polyclonal anti-NeuN antiserum (ab104224, 1:5,000; 48 h; Abcam) at 4 °C. Sections were then treated with a 1:400 dilution of donkey anti-rabbit IgG Alexa 594 (Invitrogen) in the incubation buffer for 4 h at room temperature. After the visualization of NeuN, sections were incubated in buffer containing a goat polyclonal anti-GFP antiserum (ab6673, 1:3,000; o/n; Abcam) at 4 °C. Sections were then treated with a 1:400 dilution of donkey anti-goat IgG Alexa 488 (Invitrogen) in the incubation buffer for 4 h at room temperature. To verify the specificity of immunohistochemical procedures, we included negative controls that the primary antiserums (GFP or NeuN) were omitted from the staining procedure.
GFP Single-Label Immunohistochemistry.
Sections were incubated in a rinse buffer, TBS-X (0.05 M Tris-buffered saline, pH 7.2, and 0.2% Triton X-100), containing 1% hydrogen peroxide for 20 min to inhibit endogenous peroxidase activity, and then blocked in an incubation buffer (3% skim milk and 3% BSA in TBS-X) for 2 h at room temperature. Sections were incubated in buffer containing a goat polyclonal anti-GFP antiserum (ab6673, 1:5,000; o/n; Abcam) at 4 °C. Sections were then treated with a 1:250 dilution of biotinylated goat anti-rabbit secondary antibody (Vector Laboratories) in the incubation buffer for 2 h at room temperature, followed by a 1-h incubation with avidin–biotin complex (Vectastain ABC Elite kit; Vector Laboratories) in TBS to amplify the staining intensity. After the completion of antiserum reaction procedures, sections were visualized with 0.02% diaminobenzidine, 0.15% nickel ammonium sulfate, and 0.003% hydrogen peroxide in TBS. To verify the specificity of immunohistochemical procedures, we included negative controls that the primary antiserums (GFP) were omitted from the staining procedure.
Acknowledgments
We thank Dr. M. C. Tsuda for technical advice and Dr. C. Pavlides for reviewing the manuscript. This study was supported by Grants-in-Aid for Scientific Research 23240057 and 15H05724 (to S.O.) and University of Tsukuba Research Project Grants (to K.S. and S.O.). K.S. and M.N. were recipients of the Japan Society for Promotion of Science Research Fellowship for Young Scientists.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1524907113/-/DCSupplemental.
References
- 1.Ogawa S, Lubahn DB, Korach KS, Pfaff DW. Behavioral effects of estrogen receptor gene disruption in male mice. Proc Natl Acad Sci USA. 1997;94(4):1476–1481. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogawa S, et al. Modifications of testosterone-dependent behaviors by estrogen receptor-α gene disruption in male mice. Endocrinology. 1998;139(12):5058–5069. doi: 10.1210/endo.139.12.6358. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa S, et al. Abolition of male sexual behaviors in mice lacking estrogen receptors α and β (α β ERKO) Proc Natl Acad Sci USA. 2000;97(26):14737–14741. doi: 10.1073/pnas.250473597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toda K, Saibara T, Okada T, Onishi S, Shizuta Y. A loss of aggressive behaviour and its reinstatement by oestrogen in mice lacking the aromatase gene (Cyp19) J Endocrinol. 2001;168(2):217–220. doi: 10.1677/joe.0.1680217. [DOI] [PubMed] [Google Scholar]
- 5.Toda K, et al. Oestrogen at the neonatal stage is critical for the reproductive ability of male mice as revealed by supplementation with 17β-oestradiol to aromatase gene (Cyp19) knockout mice. J Endocrinol. 2001;168(3):455–463. doi: 10.1677/joe.0.1680455. [DOI] [PubMed] [Google Scholar]
- 6.Scordalakes EM, Rissman EF. Aggression in male mice lacking functional estrogen receptor α. Behav Neurosci. 2003;117(1):38–45. [PubMed] [Google Scholar]
- 7.Bakker J, Honda S, Harada N, Balthazart J. Restoration of male sexual behavior by adult exogenous estrogens in male aromatase knockout mice. Horm Behav. 2004;46(1):1–10. doi: 10.1016/j.yhbeh.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Honda S, Wakatsuki T, Harada N. Behavioral analysis of genetically modified mice indicates essential roles of neurosteroidal estrogen. Front Endocrinol (Lausanne) 2011;2:40. doi: 10.3389/fendo.2011.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sano K, Tsuda MC, Musatov S, Sakamoto T, Ogawa S. Differential effects of site-specific knockdown of estrogen receptor α in the medial amygdala, medial pre-optic area, and ventromedial nucleus of the hypothalamus on sexual and aggressive behavior of male mice. Eur J Neurosci. 2013;37(8):1308–1319. doi: 10.1111/ejn.12131. [DOI] [PubMed] [Google Scholar]
- 10.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol. 1997;388(4):507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Mitra SW, et al. Immunolocalization of estrogen receptor β in the mouse brain: comparison with estrogen receptor α. Endocrinology. 2003;144(5):2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- 12.Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor α and β in the mouse central nervous system: In vivo autoradiographic and immunocytochemical analyses. J Comp Neurol. 2004;473(2):270–291. doi: 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- 13.Kondo Y. Lesions of the medial amygdala produce severe impairment of copulatory behavior in sexually inexperienced male rats. Physiol Behav. 1992;51(5):939–943. doi: 10.1016/0031-9384(92)90074-c. [DOI] [PubMed] [Google Scholar]
- 14.Kondo Y, Arai Y. Functional association between the medial amygdala and the medial preoptic area in regulation of mating behavior in the male rat. Physiol Behav. 1995;57(1):69–73. doi: 10.1016/0031-9384(94)00205-j. [DOI] [PubMed] [Google Scholar]
- 15.Maras PM, Petrulis A. Lesions that functionally disconnect the anterior and posterodorsal sub-regions of the medial amygdala eliminate opposite-sex odor preference in male Syrian hamsters (Mesocricetus auratus) Neuroscience. 2010;165(4):1052–1062. doi: 10.1016/j.neuroscience.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maras PM, Petrulis A. The anterior medial amygdala transmits sexual odor information to the posterior medial amygdala and related forebrain nuclei. Eur J Neurosci. 2010;32(3):469–482. doi: 10.1111/j.1460-9568.2010.07289.x. [DOI] [PubMed] [Google Scholar]
- 17.Hong W, Kim D-W, Anderson DJ. Antagonistic control of social versus repetitive self-grooming behaviors by separable amygdala neuronal subsets. Cell. 2014;158(6):1348–1361. doi: 10.1016/j.cell.2014.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Unger EK, et al. Medial amygdalar aromatase neurons regulate aggression in both sexes. Cell Reports. 2015;10(4):453–462. doi: 10.1016/j.celrep.2014.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paisley JC, et al. Sexual responses of the male rat medial preoptic area and medial amygdala to estrogen. I. Site specific suppression of estrogen receptor alpha. Horm Behav. 2012;62(1):50–57. doi: 10.1016/j.yhbeh.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 20.Romeo RD, Richardson HN, Sisk CL. Puberty and the maturation of the male brain and sexual behavior: recasting a behavioral potential. Neurosci Biobehav Rev. 2002;26(3):381–391. doi: 10.1016/s0149-7634(02)00009-x. [DOI] [PubMed] [Google Scholar]
- 21.Schulz KM, et al. Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Horm Behav. 2004;45(4):242–249. doi: 10.1016/j.yhbeh.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Horm Behav. 2009;55(5):597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulz KM, Sisk CL. Pubertal hormones, the adolescent brain, and the maturation of social behaviors: Lessons from the Syrian hamster. Mol Cell Endocrinol. 2006;254-255:120–126. doi: 10.1016/j.mce.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed EI, et al. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008;11(9):995–997. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Lorme KC, Schulz KM, Salas-Ramirez KY, Sisk CL. Pubertal testosterone organizes regional volume and neuronal number within the medial amygdala of adult male Syrian hamsters. Brain Res. 2012;1460:33–40. doi: 10.1016/j.brainres.2012.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Shaughnessy PJ, Baker PJ, Heikkilä M, Vainio S, McMahon AP. Localization of 17β-hydroxysteroid dehydrogenase/17-ketosteroid reductase isoform expression in the developing mouse testis--androstenedione is the major androgen secreted by fetal/neonatal leydig cells. Endocrinology. 2000;141(7):2631–2637. doi: 10.1210/endo.141.7.7545. [DOI] [PubMed] [Google Scholar]
- 27.Wu X, Arumugam R, Zhang N, Lee MM. Androgen profiles during pubertal Leydig cell development in mice. Reproduction. 2010;140(1):113–121. doi: 10.1530/REP-09-0349. [DOI] [PubMed] [Google Scholar]
- 28.Eddy EM, et al. Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology. 1996;137(11):4796–4805. doi: 10.1210/endo.137.11.8895349. [DOI] [PubMed] [Google Scholar]
- 29.Akingbemi BT, et al. Estrogen receptor-α gene deficiency enhances androgen biosynthesis in the mouse Leydig cell. Endocrinology. 2003;144(1):84–93. doi: 10.1210/en.2002-220292. [DOI] [PubMed] [Google Scholar]
- 30.Lee H, et al. Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature. 2014;509(7502):627–632. doi: 10.1038/nature13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernier PJ, Bédard A, Vinet J, Lévesque M, Parent A. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc Natl Acad Sci USA. 2002;99(17):11464–11469. doi: 10.1073/pnas.172403999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris JA, Jordan CL, Breedlove SM. Sexual dimorphism in neuronal number of the posterodorsal medial amygdala is independent of circulating androgens and regional volume in adult rats. J Comp Neurol. 2008;506(5):851–859. doi: 10.1002/cne.21536. [DOI] [PubMed] [Google Scholar]
- 33.Meredith M, Westberry JM. Distinctive responses in the medial amygdala to same-species and different-species pheromones. J Neurosci. 2004;24(25):5719–5725. doi: 10.1523/JNEUROSCI.1139-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samuelsen CL, Meredith M. Categorization of biologically relevant chemical signals in the medial amygdala. Brain Res. 2009;1263:33–42. doi: 10.1016/j.brainres.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newman SW. The medial extended amygdala in male reproductive behavior: A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- 36.Dominguez J, Riolo JV, Xu Z, Hull EM. Regulation by the medial amygdala of copulation and medial preoptic dopamine release. J Neurosci. 2001;21(1):349–355. doi: 10.1523/JNEUROSCI.21-01-00349.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dominguez JM, Hull EM. Stimulation of the medial amygdala enhances medial preoptic dopamine release: Implications for male rat sexual behavior. Brain Res. 2001;917(2):225–229. doi: 10.1016/s0006-8993(01)03031-1. [DOI] [PubMed] [Google Scholar]
- 38.Canteras NS. The medial hypothalamic defensive system: Hodological organization and functional implications. Pharmacol Biochem Behav. 2002;71(3):481–491. doi: 10.1016/s0091-3057(01)00685-2. [DOI] [PubMed] [Google Scholar]
- 39.Choi GB, et al. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46(4):647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, He Z, Zhao C, Li L. Medial amygdala lesions modify aggressive behavior and immediate early gene expression in oxytocin and vasopressin neurons during intermale exposure. Behav Brain Res. 2013;245:42–49. doi: 10.1016/j.bbr.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7(10):1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- 42.Fowler CD, Johnson F, Wang Z. Estrogen regulation of cell proliferation and distribution of estrogen receptor-α in the brains of adult female prairie and meadow voles. J Comp Neurol. 2005;489(2):166–179. doi: 10.1002/cne.20638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsukahara S. Sex differences and the roles of sex steroids in apoptosis of sexually dimorphic nuclei of the preoptic area in postnatal rats. J Neuroendocrinol. 2009;21(4):370–376. doi: 10.1111/j.1365-2826.2009.01855.x. [DOI] [PubMed] [Google Scholar]
- 44.Tsukahara S, et al. Effects of aromatase or estrogen receptor gene deletion on masculinization of the principal nucleus of the bed nucleus of the stria terminalis of mice. Neuroendocrinology. 2011;94(2):137–147. doi: 10.1159/000327541. [DOI] [PubMed] [Google Scholar]
- 45.Kanaya M, et al. Regional difference in sex steroid action on formation of morphological sex differences in the anteroventral periventricular nucleus and principal nucleus of the bed nucleus of the stria terminalis. PLoS One. 2014;9(11):e112616. doi: 10.1371/journal.pone.0112616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. RNAi-mediated silencing of estrogen receptor α in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proc Natl Acad Sci USA. 2006;103(27):10456–10460. doi: 10.1073/pnas.0603045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2nd Ed Academic; San Diego: 2001. [Google Scholar]
- 48.Blanchard RJ, Wall PM, Blanchard DC. Problems in the study of rodent aggression. Horm Behav. 2003;44(3):161–170. doi: 10.1016/s0018-506x(03)00127-2. [DOI] [PubMed] [Google Scholar]