Abstract
Soybean seed coat exists in a range of colors from yellow, green, brown, black, to bicolor. Classical genetic analysis suggested that soybean seed color was a moderately complex trait controlled by multi-loci. However, only a couple of loci could be detected using a single biparental segregating population. In this study, a combination of association mapping and bulk segregation analysis was employed to identify genes/loci governing this trait in soybean. A total of 14 loci, including nine novel and five previously reported ones, were identified using 176,065 coding SNPs selected from entire SNP dataset among 56 soybean accessions. Four of these loci were confirmed and further mapped using a biparental population developed from the cross between ZP95-5383 (yellow seed color) and NY279 (brown seed color), in which different seed coat colors were further dissected into simple trait pairs (green/yellow, green/black, green/brown, yellow/black, yellow/brown, and black/brown) by continuously developing residual heterozygous lines. By genotyping entire F2 population using flanking markers located in fine-mapping regions, the genetic basis of seed coat color was fully dissected and these four loci could explain all variations of seed colors in this population. These findings will be useful for map-based cloning of genes as well as marker-assisted breeding in soybean. This work also provides an alternative strategy for systematically isolating genes controlling relative complex trait by association analysis followed by biparental mapping.
Introduction
Soybean [Glycine max (L.) Merr.] is the most widely grown grain legumes in the world, which is widely used as the major sources of vegetable oils and plant proteins [1]. Soybean seed contains eight essential amino acids which could not be produced by human body [2]. Seed coat color is an important attribute determining outward appearance of soybean seed, which exists in a range of colors from yellow, green, brown, black, to bicolor. It is usually considered as a useful phenotypic marker in breeding due to convenience for observation [3, 4]. Compared with yellow seeds of most grown soybean varieties, black/brown seeds usually accumulate flavonoids and anthocyanins within the epidermal layer of the seed coat, which are currently attracting great interest in their antioxidant properties and flavors [5]. Seed coat color is also an evolutionary trait within the soja subgenus and it was changed from black in wild soybean to various colors in cultivated soybeans during domestication [6, 7]. In addition, several studies have also concerned partial pigmentation of seed coat as a result of chilling stress or viral diseases, indicating crosstalk between regulation of seed coat pigmentation and stress responses [8–13].
Soybean seed color has moderately complex inheritance which is controlled by multi-loci. At least five genetic loci (I, R, T, W1, and O) were identified by classical genetics, most of which were involved in flavonoid-based pigmentation pathway [13, 14]. Among them, three (I, R, and T) are involved in the biosynthesis of the pigments while O and W1 only influence the pigmentation under the background of recessive alleles of i r or i t, respectively [14]. There are four alleles (known as I, ii, ik, and i) at I locus controlling the presence/absence and spatial distribution of anthocyanin and proanthocyanidin via posttranscriptional gene silencing. Soybeans possessing dominant I allele exhibit complete colorless of seed coat while soybeans with i allele give rise to colored seed coat [12]. The other two alleles (ii and ik) restrict pigments to the hilum and saddle regions of the seed coat [14]. R and T loci control the type and abundance of pigments in seed coat, resulting in specific colors including black (i,R,T), imperfect black (i,R,t), brown (i,r,T), or buff (i,r,t) [15, 16]. W1 locus only affects seed color under iRt background and W1 and w1 alleles give imperfect black and buff colors, respectively. O locus affects color of brown seed and soybeans with the recessive o allele under irT background exhibit red-brown seed coat [14]. In addition, mutants with different combinations (single, double or triple mutants) of G, d1 and d2 loci give rise to green seed color and segregation of G1, G2, and G3 for green color has also been studied previously [17–19].
Molecular cloning of these loci suggested that many of them were structural or regulatory genes involving in anthocyanin biosynthesis pathway. I locus was mapped to a region harboring a cluster of chalcone synthase (CHS) genes on chromosome 8 of soybean genome [20–22]. The recessive i allele had a deletion of CHS4 or CHS1 promoter sequences, resulting in an increased accumulation of chalcone synthase (CHS) transcripts in the seed coat due to the abolishment of posttranscriptional RNA silencing [23, 24]. Cloning of genomic and cDNA sequences of flavonoid 3’-hydroxylase (F3’H) gene suggested that this gene cosegregated with T locus [25, 26]. Chromatographic experiments and genetic analysis also revealed that W1 might encode a flavonoid 3’ 5’ hydroxylase (F3’5’H) as a 65-bp insertion in this gene cosegregated with the mutant phenotype [15, 27]. R locus was initially mapped to LG K (chromosome 9) [28] and then restricted to a region between molecular markers A668_1 and K387_1 [29]. Candidate gene analysis suggested that loss function of a seed coat-expressed R2R3-MYB gene was responsible for recessive phenotype of R locus [30, 31]. Furthermore, O locus has been found to correspond to an anthocyanidin reductase (ANR) gene, which needs to be further confirmed [13]. Recently, cloning and characterization of D1 and D2 revealed that they were homologs of the STAY-GREEN (SGR) genes from other plant species and were duplicated as a result of the most recent whole genome duplication in soybean [32, 33].
Both biparental and association mapping are two main approaches for genetic dissection of important traits in plants [34]. Traditionally, biparental mapping served as a powerful tool to identify genes for QTLs in model plants Arabidopsis and rice [35–41]. In the subsequent processes of positional cloning, the most effective way for characterization of individual locus is the use of near isogenic lines (NILs) which differ only at a single QTL region. However, it still has limitations in isolation of genes for QTLs in plants with complex genome such as soybean, which is mainly due to limited allelic diversity existing in two parental lines and low recombination events incurring during population development. Especially, development of NILs through repeated backcrossing is still a time-consuming and laborious process for soybean. Therefore, only a few reports have been published in successful isolation of genes responsible for QTLs in soybean [42, 43]. Alternatively, association mapping using natural population has also proven to be an effective strategy to identify marker-trait associations in animals and plants [44]. Association mapping enables the study of many genotypes at once and generates more precise QTL positions if a sufficient number of molecular markers are used. Therefore, this mapping method has been shown to have potential in dissecting the genetic basis of various traits in Arabidopsis, rice, and maize [45–47]. However, no correction for multiple testing possibly led to false positive associations [48]. The development of high-throughput sequencing technologies provides the opportunity to combine these two approaches together, which mitigates each other's limitations [49–51].
Classical genetic analysis demonstrated that multi-loci controlled seed coat color in soybean, accessions possessing the same color possibly having different genotypes at these loci. In this study, association mapping coupled with biparental mapping were employed to systematically dissect genes/loci controlling seed coat color of soybean. SNPs in coding regions among 56 soybean accessions were selected for association mapping and a total of 14 genomic regions were identified to be associated with seed coat color. A segregating population derived from two accessions with different colors was used to confirm association mapping results. The inheritance of seed color in this biparental population was dissected into simple color pairs by development of residual heterozygous lines (RHLs). All four loci governing this trait were systematically identified by bulk segregation analysis (BSA) and fine mapping. All these results suggested that association mapping combined with BSA in biparental population acted as a useful strategy for dissecting relative complex traits in soybean, thus providing a valuable tool for marker-assisted breeding.
Materials and Methods
Plant materials
For association mapping, a panel of 56 accessions including G. soja and G. max were used, which were resequenced in the previous reports [7, 52]. Among them, 21 wild soybeans and three landraces have black seed coat color while four wild soybeans and four landraces have brown. Seed coats of the other five landraces and all 20 breeding lines are yellow (S1 Table). The segregating population consisting of 171 lines was derived from the cross between ZP95-5383 (yellow seed coat) and NY279 (brown seed coat). RHLs were developed by phenotypic selection and self-fertility of specific lines for several generations.
Genotypic data analysis
SNP data of all 56 accessions were downloaded from NCBI web site (http://www.ncbi.nlm.nih.gov/ SNP/snp_viewTable.cgi?handle = NFCRI_MOA_CAAS). Three sets of SNPs (Set A, B, and C) were selected from entire data set. These sets include SNPs appeared in coding regions (Set A), coding SNPs removal of synonymous ones (Set B) and non-synonymous coding SNPs (Set C). The number of alleles and the polymorphism information content (PIC) per locus were calculated using POWERMAKER 3.25 software [53]. The population structure was assessed by using STRUCTURE software version 2.2 [54]. To determine the number of genetic clusters (K), ten independent runs were carried out for each value of K (from 1 to 10) with 500,000 iterations, followed by a burn-in period of 500,000 iterations. The likely number of sub-populations present was estimated following Evanno et al.[55], in which the number of sub-groups (∆k) was maximized. The Q matrix that lists the estimated membership coefficients of individuals in each cluster was utilized for subsequent association mapping.
Association mapping
TASSEL 3.0 software package was used to conduct association mapping and identify associated SNPs with MLM model (Q+K) [48, 56]. Population structure (Q) and the kinship matrix (K) were based on the results of population structure analysis. All SNP-trait pairs with P-value < 0.001 were considered significant, which was determined according to the result of QQ-Plot analysis. QQ plots and manhattan plots for association mapping were drawn using the qqman R package [57]. The genotypes of most significant associated SNPs in different soybean accessions were examined using GGT software [58].
DNA isolation
Genomic DNA was isolated from fresh young leaves of soybeans using the sodium dodecyl sulphate (SDS) method [59, 60]. The extracted DNA was quantified using Quawell Q5000 spectrophotometer (Quawell Technology, Inc. USA) and all DNA samples were normalized to 50ng/μL for PCR amplification.
Molecular marker analysis
Polymorphic SSR markers in specific mapping regions were developed using parental lines of the segregating population and the progeny were genotyped as previously described [61]. Primer sequences of SSR markers were obtained from SoyBase (http://soybase.org/resources/ssr.php) and Song et al. [62]. PCR was performed in a 20μL reaction system using 1μL of DNA sample in each reaction and conducted in a PTC-200 thermocycler (Bio-Rad, USA).
Bulk segregation analysis and fine mapping
Residual heterozygous lines with separation of different seed color pairs were used for rough mapping. DNA samples isolated from 20 plants with dominant trait and 20 plants with recessive traits from each RHL population were pooled together to construct two bulks for BSA, respectively. DNA of parental lines and all bulks was screened with SSR markers near loci identified by association mapping. The physical positions of all markers were according to soybean reference genome assembly v1.1 [63]. Once an associated locus was confirmed in a RHL population, the progeny of this RHL were genotyped with additional polymorphic markers from this genomic region. Based on the exchanges between genotypes of markers and specific locus, the recombinants were identified and used for fine mapping.
Genetic analysis of different loci
SSR markers closely linked to qSC1;5;7 loci and dCAPs marker of qSC2/T locus [64] were used for genotyping entire F2 population. dCAPS marker was developed by artificial introduction of a restriction enzyme recognition site at the end of the forward primer for GmF3’H gene [64]. PCR products were digested with restriction enzyme EcoNI at 37°C for more than 1h, and separated on 2% agarose gels stained with EB followed by photography. The relationship of genotype and phenotype were applied for genetic analysis of different loci.
Results
SNP marker selection and distribution analysis
After filtering from more than 5.1 million high quality SNPs identified by combining resequencing data of 31 and 25 soybean accessions [7, 52], three sets of SNPs located in coding regions were selected. There are 176, 065 SNPs in Set A, which appear in coding regions of predicted genes and represent coding SNPs. Set B (98,244 SNPs) represents SNPs removal of synonymous coding SNPs from Set A, including non-synonymous, nonsense and read through coding SNPs. Set C contains 94,261 SNPs and represents only non-synonymous coding SNPs among all 56 accessions (Table 1).
Table 1. Distribution of coding SNPs selected from resequencing data.
| Chr. | Length (Mb) | No. of SNPs | SNPs/kb | No. of predicted genes | Set A (Coding SNPs)a | Set B (Non-synonymous, nonsense and read through coding SNPs)b | Set C (Non-synonymous coding SNPs) c | |||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of SNPs | SNPs/Gene | No. of SNPs | SNPs/Gene | No. of SNPs | SNPs/Gene | |||||
| 1 | 55.92 | 268,724 | 4.8 | 2,428 | 7681 | 3.2 | 4374 | 1.8 | 4186 | 1.7 |
| 2 | 51.66 | 234,553 | 4.5 | 3,158 | 8495 | 2.7 | 4644 | 1.5 | 4463 | 1.4 |
| 3 | 47.78 | 282,502 | 5.9 | 2,641 | 9145 | 3.5 | 5115 | 1.9 | 4913 | 1.9 |
| 4 | 49.24 | 240,026 | 4.9 | 2,575 | 7258 | 2.8 | 3984 | 1.5 | 3842 | 1.5 |
| 5 | 41.94 | 184,474 | 4.4 | 2,636 | 7139 | 2.7 | 3872 | 1.5 | 3741 | 1.4 |
| 6 | 50.72 | 294,307 | 5.8 | 3,296 | 10797 | 3.3 | 6112 | 1.9 | 5898 | 1.8 |
| 7 | 44.68 | 235,011 | 5.3 | 2,801 | 8916 | 3.2 | 4980 | 1.8 | 4807 | 1.7 |
| 8 | 47.00 | 266,154 | 5.7 | 3,867 | 11465 | 3.0 | 6278 | 1.6 | 6052 | 1.6 |
| 9 | 46.84 | 257,758 | 5.5 | 2,729 | 8583 | 3.1 | 4732 | 1.7 | 4574 | 1.7 |
| 10 | 50.97 | 247,193 | 4.8 | 2,986 | 8449 | 2.8 | 4595 | 1.5 | 4428 | 1.5 |
| 11 | 39.17 | 179,976 | 4.6 | 2,866 | 7020 | 2.4 | 3852 | 1.3 | 3717 | 1.3 |
| 12 | 40.11 | 192,646 | 4.8 | 2,484 | 7061 | 2.8 | 3885 | 1.6 | 3753 | 1.5 |
| 13 | 44.41 | 277,854 | 6.3 | 3,786 | 10917 | 2.9 | 6002 | 1.6 | 5817 | 1.5 |
| 14 | 49.71 | 238,506 | 4.8 | 2,201 | 7553 | 3.4 | 4315 | 2.0 | 4161 | 1.9 |
| 15 | 50.94 | 320,440 | 6.3 | 2,715 | 9510 | 3.5 | 5606 | 2.1 | 5390 | 2.0 |
| 16 | 37.40 | 251,100 | 6.7 | 2,138 | 10235 | 4.8 | 5927 | 2.8 | 5694 | 2.7 |
| 17 | 41.91 | 223,989 | 5.3 | 2,685 | 7497 | 2.8 | 3980 | 1.5 | 3831 | 1.4 |
| 18 | 62.31 | 412,564 | 6.6 | 2,580 | 12887 | 5.0 | 7336 | 2.8 | 7045 | 2.7 |
| 19 | 50.59 | 254,340 | 5.0 | 2,641 | 7709 | 2.9 | 4240 | 1.6 | 4078 | 1.5 |
| 20 | 46.77 | 240,127 | 5.1 | 2,369 | 7748 | 3.3 | 4415 | 1.9 | 4231 | 1.8 |
| Scaffold | 23.51 | - | - | 205 | - | - | - | - | - | - |
| Total | 973.58 | 5,102,244 | 5.2 | 55,787 | 176065 | 3.2 | 98244 | 1.8 | 94621 | 1.7 |
aSet A represented coding SNPs in which all SNPs appeared in coding regions of predicted genes.
bSet B represented SNPs removal of synonymous coding SNPs from Set A, including non-synonymous, nonsense and read through coding SNPs.
cSet C represented only non-synonymous coding SNPs.
The distribution of selected SNPs was fairly uniform across all soybean chromosomes (Table 1). The largest number of coding SNPs was observed on chromosome 18, followed by chromosome 8, and the lowest number of SNPs was found on chromosomes 11 and 12. On average, about 3.2 coding SNPs/gene were selected from 5.2 SNPs/kb for the entire genome. For each chromosome, the distribution of coding SNPs varied from 2.4 SNPs/gene on Chromosome 11 to 5.0 SNPs/gene on chromosome 18 (Table 1).
Population structure analysis
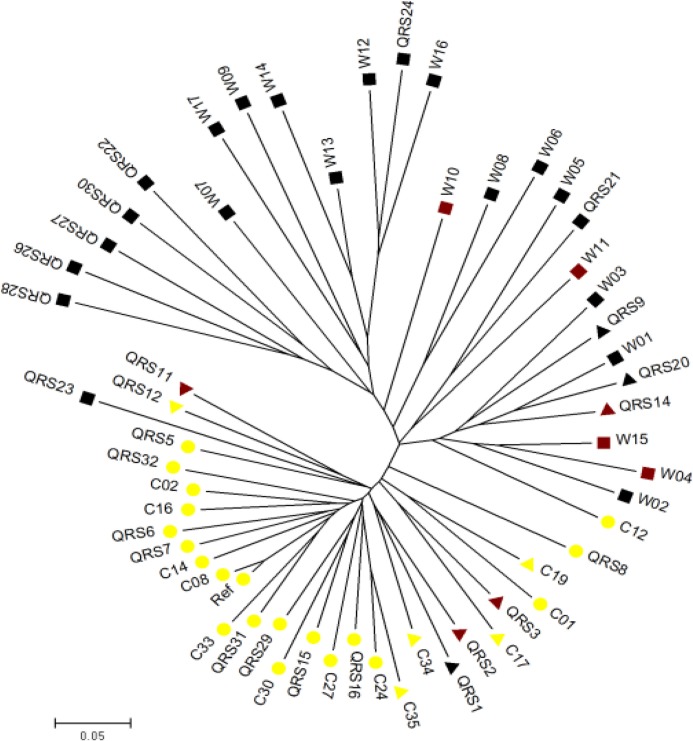
To study the relationship of these 56 soybean accessions, a neighbor-joining tree based on genetic distances was constructed by Powermarker using coding SNPs. The results showed that all these accessions could be classified into two major groups (Fig 1). Majority accessions of G. max or G. soja separated completely with only three exceptions (QRS23 in subgroup I mainly containing G. max and QRS14 and QRS20 in subgroup II mainly containing G. soja). Meanwhile, population structure was also assessed to estimate the most likely number (K) of subgroups among these accessions. The value of LnP(D) increased continuously for K values ranging from 1 to 10 and only one significant change of ΔK was observed at K = 2 (S1 Fig A), suggesting that this natural population could be clustered into two major subgroups (S1 Fig B). Subgroup I included mainly G. max while subgroup II contained mainly G. soja, which was in accordance with the neighbor-joining tree (Fig 1).
Fig 1. Phylogenetic tree of 56 soybean accessions.
The phylogenetic tree was constructed by Powermarker using the coding SNPs. Different shapes indicated different types of accessions (square, wild soybean; triangle, landrace; circle, breeding line) and color of the shape (yellow, brown, and black) indicated seed coat color.
Identification of loci associated with seed coat color by association mapping
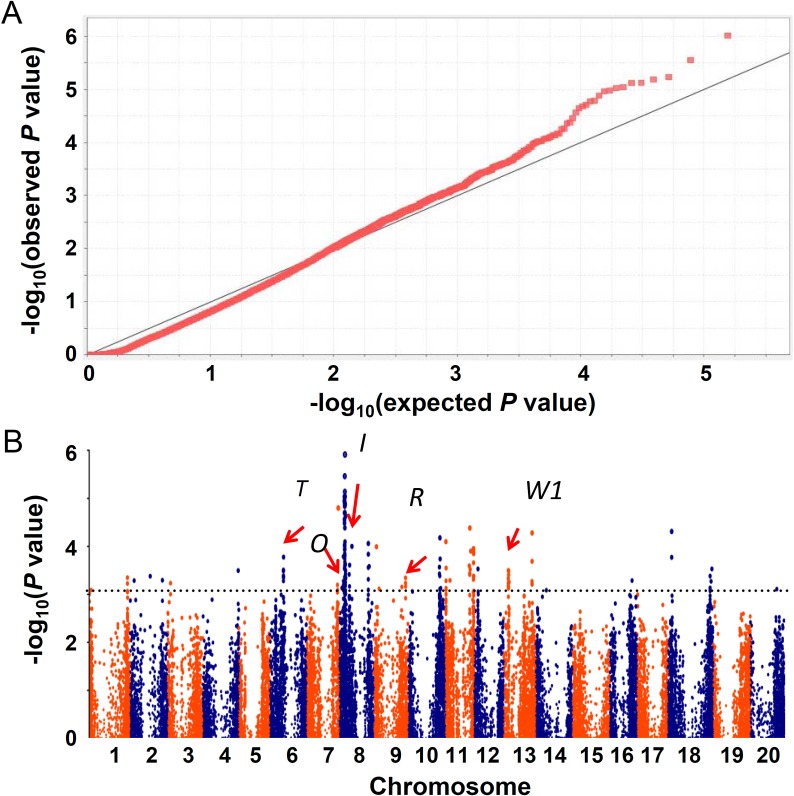
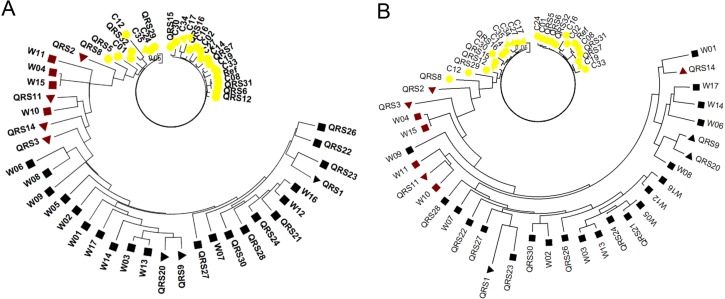
Association mapping was performed with MLM using the phenotypic data and three sets of SNPs. To reduce both false positive and false negative risks caused by population structure, only SNPs detected by K = 2 were taken into account. The QQ-Plot analysis showed that expected -log (P) matched observed -log (P) best using SNPs from Set A (Fig 2A). Association mapping revealed that 146 SNPs located in 14 genomic regions on 10 chromosomes (designated as qSC1-qSC14, Fig 2B, Table 2) were significantly associated with seed coat color. Nearly all of 14 regions contained more than five significant associated SNPs except qSC11 on chromosome 12. The physical distances of these associated regions ranged from 53 kb to 5,142 kb (Table 2). Moreover, similar results were also obtained by using the other two sets (Set B and C) of SNPs (S2 Fig and S2 Table). Interestingly, all five loci identified by classic genetics were detected in our result of association mapping (Fig 2B and Table 2), suggesting the representative of soybean accessions used in this study and the accuracy of mapping result. Furthermore, associated SNPs located in all 14 loci could separate soybeans with different seed colors properly no matter they were wild soybeans, landraces or breeding lines while only SNPs in five previous reported loci could not separate them completely (Fig 3). Even more, the combination of most significant associated SNP in each locus could also identify different seed coat colors of all these accessions (S3 Fig).
Fig 2. Association mapping of seed coat color in soybean.
(A) Expect -log (P) matched observed -log (P) best from the QQ-Plot. (B) Manhattan plots showed -log (P) from a genome-wide scan were plotted against positions of SNPs across 20 chromosomes of soybean. The horizontal line represented threshold of significant association and red arrows indicated the positions of five classical genetic loci.
Table 2. Details of loci associated with seed coat color identified via association mapping.
| Locus | Chr. | Position of most significant SNPs | P-value | R2 | No. of coding SNPs | Significant region | Classic loci | ||
|---|---|---|---|---|---|---|---|---|---|
| Start | End | Range(kb) | |||||||
| qSC1 | 1 | 52,438,308 | 3.96E-04 | 0.2685304 | 5 | 51,388,129 | 52,467,583 | 1079 | - |
| qSC2 | 6 | 19,047,336 | 1.45E-04 | 0.3022027 | 7 | 18,878,314 | 19,047,336 | 169 | T |
| qSC3 | 7 | 43,082,019 | 1.32E-05 | 0.4644675 | 6 | 41,940,392 | 43,082,019 | 1142 | - |
| qSC4 | 8 | 5,501,159 | 1.42E-04 | 0.318538 | 6 | 3,417,779 | 5,512,389 | 2095 | O |
| qSC5 | 8 | 7,589,623 | 9.56E-07 | 0.6492089 | 51 | 6,783,439 | 8,648,879 | 1865 | I |
| qSC6 | 8 | 39,553,339 | 7.40E-05 | 0.3314216 | 8 | 39,473,028 | 40,585,746 | 1113 | - |
| qSC7 | 9 | 43,418,250 | 3.55E-04 | 0.2882047 | 5 | 38,277,760 | 43,419,283 | 5142 | R |
| qSC8 | 10 | 43,409,021 | 5.61E-05 | 0.3567043 | 15 | 42,771,760 | 43,820,987 | 1049 | - |
| qSC9 | 11 | 681,423 | 6.77E-05 | 0.3912334 | 9 | 681,423 | 1,055,084 | 374 | - |
| qSC10 | 11 | 38,426,187 | 9.55E-05 | 0.3558796 | 12 | 38,370,581 | 38,636,896 | 266 | - |
| qSC11 | 12 | 5,404,396 | 2.57E-04 | 0.2872759 | 3 | 5,404,396 | 5,649,464 | 245 | - |
| qSC12 | 13 | 7,117,537 | 2.95E-04 | 0.271697 | 9 | 6,661,165 | 7,743,106 | 1082 | W1 |
| qSC13 | 13 | 39,050,259 | 4.40E-05 | 0.4384943 | 5 | 39,045,030 | 39,157,835 | 113 | - |
| qSC14 | 18 | 57,962,686 | 3.67E-04 | 0.2844445 | 5 | 57,910,138 | 57,962,686 | 53 | - |
Fig 3.
Phylogenetic tree constructed by Powermarker using associated SNPs (A) and SNPs in five previous reported loci (B). (A) SNPs located in all 14 associated loci were used for constructing phylogenetic tree and accessions with different seed colors could be separated properly. (B) SNPs in five previous reported loci were used for constructing phylogenetic tree and soybeans with different seed colors can not be separated completely. Different shapes indicated different types of accessions (square, wild soybean; triangle, landrace; circle, breeding line) and color of the shape (yellow, brown, and black) indicated seed coat color.
Validation of loci governing seed coat color using bi-parental population
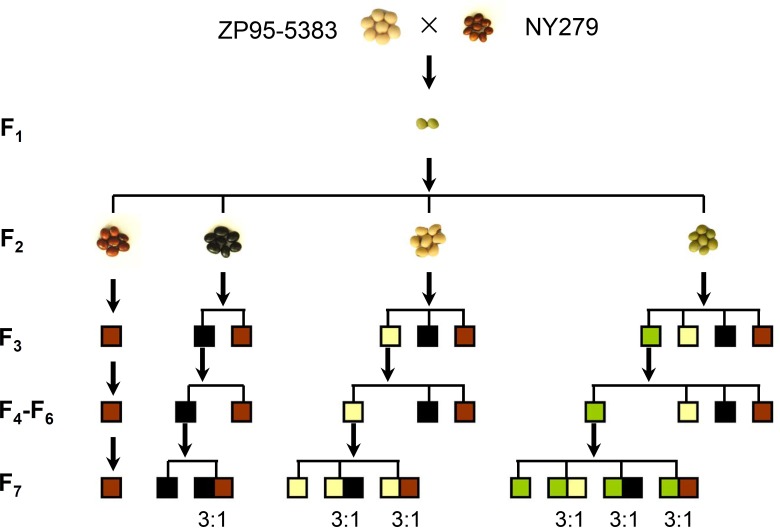
To confirm the candidate loci identified in association mapping, a biparental population derived from the cross between ZP95-5383 (yellow seed coat) and NY279 (brown seed coat) was used. Seed coat color of F1 plant was green and four different colors were observed in F2 generation (30, 109, 17, and 15 individuals showed yellow, green, brown and black seed coat separately). Genetic analysis of different generations from F2 to F7 revealed that brown seed coat did not segregate at all while individuals with black seed only generated progeny with black or brown seed. Some individuals possessing yellow seed coat could generate soybeans with yellow, black, and brown colors and the segregation of green seed coat was just like that in F1 generation (Fig 4).
Fig 4. The inheritance of seed coat color in a segregating population derived from the cross between ZP95-5383 and NY279.
Squares with different colors (green, yellow, black, and brown) represented soybeans with corresponding seed colors. Similar pattern of inheritance from soybeans with black, yellow, green seed in F3-F7 generation were not shown completely in this diagram.
Bulk segregation analysis was carried out using 33 polymorphic SSR markers (S3 Table) near fourteen associated loci. DNA bulks from F2 individuals with green, yellow, black, and brown seed were first screened with polymorphic markers. The results suggested that only two loci cosegregated with specific colors of bulks. Four markers located in qSC1 region co-segregated with yellow seed coat and three markers in qSC5 region co-segregated with black and brown.
To further dissect other loci controlling seed coat color in this segregating population, several RHLs were developed for different color pairs including green/yellow, green/black, green/brown, yellow/black, yellow/brown, black/brown after phenotypic selection and self-fertility for several generations (Fig 4). DNA bulks of different color pairs from these RHL populations were also identified with polymorphic markers. Similar to the results from F2 individuals, markers in qSC1 region co-segregated with color pair of green/yellow and markers in qSC5 co-segregated with green/black, green/brown, yellow/black, yellow/brown. However, three markers located in qSC2 region and three markers in qSC7 were all identified to co-segregate with color pair of black/brown in two different RHL populations, which was not detected from bulks of F2 individuals.
Fine mapping of loci identified by combining association mapping and bulk segregation analysis
To further map these four loci of qSC1;2;5;7, individuals consisting of DNA pools were all genotyped with the polymorphic markers for each locus. The results revealed that all markers at every locus clearly co-segregated with the phenotype of different seed coat colors. Among them, qSC1 was a novel one controlling green/yellow while the other three loci located at similar regions of T, I, and R loci. Using different RHL populations, qSC1, qSC2, qSC5, and qSC7 were successfully mapped between markers BARCSOYSSR_1_1503 and 1_1546, 6_942 and 6_998, 8_459 and 8_480, and Sat_352 and Satt196, respectively (Table 3).
Table 3. Details of loci governing seed coat color identified via BSA in soybean.
| Locus | Chr. | Associated phenotype | Genetic region | Physical position (assembly v1.1) | Physical position (assembly v2.0) | Physical distance (kb) |
|---|---|---|---|---|---|---|
| qSC1 | 1 | Green/Yellow | 1_1503~1_1546 | 51,910,240~52,633,016 | 52,797,517~53,517,890 | 723 |
| qSC2/T | 6 | Black/Brown | 6_942~6_998 | 17,443,860~18,713,575 | 17,498,347~18,918,726 | 1,270 |
| qSC5/I | 8 | Green/Black, Green/Brown, Yellow/Black, Yellow/Brown | 8_459~8_480 | 8,321,840~8,745,942 | 8,326,164 ~8,775,965 | 424 |
| qSC7/R | 9 | Black/Brown | Sat_352~Satt196 | 41,890,948~43,310,941 | 45,087,036~46,515,708 | 1,420 |
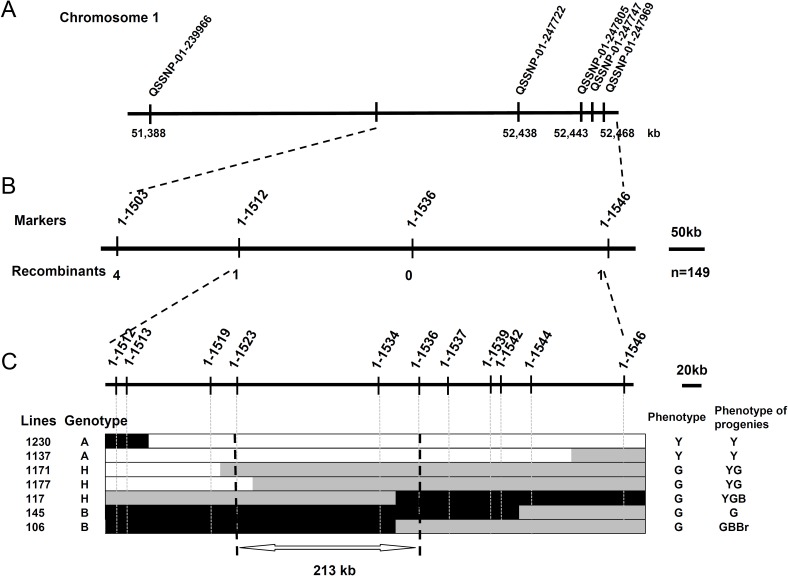
Since genes corresponding to I, T and R loci have been identified in the previously reports [23–26, 30], selected RHL populations were used for fine mapping of qSC1 locus. Eleven polymorphic markers between BARCSOYSSR_1_1503 and 1_1546 were developed and subsequent marker-phenotype analysis enabled us to refine qSC1 region into a 213-kb interval (23 candidate genes) between markers BARCSOYSSR_1_1523 and 1_1536 (Fig 5).
Fig 5. Fine mapping of qSC1 locus.
(A) Chromosomal location of qSC1 identified by association mapping on chromosome 1. The significant associated SNPs were indicated above the line. (B) Roughly mapping of qSC1 by using RHL populations. Vertical lines represented polymorphism markers. The names of markers and the number of recombinants between qSC1 and each marker were shown above and below the line separately. (C) Fine mapping of qSC1 locus by detailed marker-phenotype analysis of recombinants. The genotype of each recombinant was confirmed based on the phenotypes of its progeny. The black/gray/white colors indicated homozygousity/heterozygousity/homozygousity of markers based on genotypes of parental lines and the delimited region for the qSC1 locus is indicated by bold arrow. Y (Yellow), G (Green), B (Black), and Br (Brown) represented different seed colors of recombinants and their progeny. All the physical positions of markers were according to assembly v1.1 of soybean genome.
Molecular marker development and the interaction of different loci
Combinations of different loci can be used to infer genetic effect of each locus for specific trait. Three SSR markers closely linked to qSC1, qSC5/I, qSC7/R loci (BARCSOYSSR_1_1528, 8_466, and 9_1491) and a dCAPS marker of GmF3’H gene for qSC2/T locus were used for genotyping entire F2 population. The results revealed that all individuals possessing dominant qSC5/I allele showed green or yellow seed coats while soybeans with recessive qsc5/i allele showed black or brown coats. In the qSC5/I background, seed colors of all individuals possessing dominant qSC1 allele were green while soybeans with recessive qsc1 allele showed yellow seed coats. Furthermore, seed colors of individuals with recessive qsc2/t locus in the qsc5/i background were brown. However, when individuals possessed dominant allele of qSC2/T and recessive allele of qsc5/i, qSC7/R locus could be used for distinguishing black and brown seed coat (Table 4). From these results the interaction of different loci can be concluded, in which qSC5/I locus controlled pigmentation of seed coat to dark colors and qSC1 governed further pigmentation of relative light color on the basis of qSC5/I locus. In addition, qSC2/T and qSC7/R loci were responsible for pigmentation of different degrees of dark colors and qSC2/T locus might function upstream of qSC7/R in this network.
Table 4. The relationship of genotypes and seed coat colors in F2 segregating population.
| Genotype | No. of individuals | ||||||
|---|---|---|---|---|---|---|---|
| Green | Yellow | Black | Brown | ||||
| qSC5/Ia | qSC1 | qSC2/T | qSC7/R | 57 | 0 | 0 | 0 |
| qSC5/I | qSC1 | qSC2/T | qsc7/r | 16 | 0 | 0 | 0 |
| qSC5/I | qSC1 | qsc2/t | qSC7/R | 26 | 0 | 0 | 0 |
| qSC5/I | qSC1 | qsc2/t | qsc7/r | 10 | 0 | 0 | 0 |
| qSC5/I | qsc1 | qSC2/T | qSC7/R | 0 | 15 | 0 | 0 |
| qSC5/I | qsc1 | qSC2/T | qsc7/r | 0 | 4 | 0 | 0 |
| qSC5/I | qsc1 | qsc2/t | qSC7/R | 0 | 8 | 0 | 0 |
| qSC5/I | qsc1 | qsc2/t | qsc7/r | 0 | 3 | 0 | 0 |
| qsc5/ib | qSC1 | qSC2/T | qSC7/R | 0 | 0 | 12 | 0 |
| qsc5/i | qsc1 | qSC2/T | qSC7/R | 0 | 0 | 3 | 0 |
| qsc5/i | qSC1 | qSC2/T | qsc7/r | 0 | 0 | 0 | 2 |
| qsc5/i | qsc1 | qSC2/T | qsc7/r | 0 | 0 | 0 | 0 |
| qsc5/i | qSC1 | qsc2/t | qSC7/R | 0 | 0 | 0 | 8 |
| qsc5/i | qsc1 | qsc2/t | qSC7/R | 0 | 0 | 0 | 1 |
| qsc5/i | qSC1 | qsc2/t | qsc7/r | 0 | 0 | 0 | 3 |
| qsc5/i | qsc1 | qsc2/t | qsc7/r | 0 | 0 | 0 | 3 |
aThe uppercase letter of locus symbol indicated dominant or heterozygous alleles.
bThe lowercase letter of locus symbol indicated recessive allele.
Discussion
Combination of association mapping and biparental mapping enhance the mapping resolution
Association mapping has been proven to be a powerful tool to identify loci associated with important traits even at single gene resolution in Arabidopsis, rice and maize [45–47]. In soybean, only hundreds of SSR markers or few thousands of SNPs have been used in association analysis at the early stage [65–69]. However, the marker density was too low to detect QTLs powerfully, resulting in difficult isolation of genes. A couple of recent reports have increased markers to several thousands or tens of thousands with GBS (genotyping by sequencing) or SNP chips, but the resolution is still not very high because of the long-range LD (linkage disequilibrium) [51, 70–72]. In addition, it is likely that contributions of coding SNPs to phenotypic variations would be higher than SNPs in non-coding regions [73]. Therefore, association analysis with SNPs in coding regions may get more specific results compared to SNPs in non-coding regions. Moreover, our results also indicated that non-synonymous and synonymous coding SNPs have similar effects on association mapping.
Association and biparental mapping have complementary advantages and disadvantages and their limitations could be mitigated by using both analysis [34]. The combination of these two approaches has been employed in model plants and successful isolation of gene for QTL has proven the usefulness of this strategy [74, 75]. A locus having an effect in multiple accessions could be detected in association mapping while only loci harboring major effects can be mapped in a biparental population [76]. Therefore, once a trait is correlated with the structure of a natural population, the power of association analysis is reduced, whereas biparental mapping can be used to detect QTLs in a population derived from accessions belonging to different subgroups [50, 77]. Therefore, identification of QTLs using both biparental and association mapping in the same study will provide more robust understanding of genetic architecture than any single method. In this study, although a total of 14 loci were identified in association mapping, only four of them were confirmed by BSA in the biparental population used. The other loci may be validated using other segregating populations.
Comparison of identified loci with previously reported QTLs
Seed coat color is not only related to biochemical functions of secondary metabolism, antioxidant activity, and disease resistance but also a morphological trait for classification of germplasm and evolutionary analysis [3–5]. Apart from five genetic loci controlling flavonoid-based pigmentation [13, 14], eight QTLs on five chromosomes have also been identified through QTL mapping but mapping regions were always too large due to limited number of markers used [78–80]. Among them, two QTLs (seed coat color2-1 and 3–1) were all close to I locus, but it was difficult to confirm whether they were the same QTL due to the large genomic regions in their studies. Moreover, some reports also revealed that combination of gene-based markers of T and W1 loci or two SNP outliers could partially increase selection efficiency for seed colors [64, 81]. Therefore, soybean seed color has a relative complex genetic basis and accessions with same color possibly having different genotypes at these loci.
All five loci identified previously were detected in our results of association mapping, suggesting the representative of soybean accessions used. Meanwhile, all associated SNPs at 14 loci could separate soybeans with different colors more properly than only using five previous reported loci (Fig 3), further indicating the accuracy of our association analysis. Moreover, four loci including a novel one were confirmed by biparental mapping, indicating that we identified common or major loci in both natural and biparental populations. Eight of the rest ten loci could be further confirmed using segregating populations developed from other accessions because our biparental population even did not detect W1 and O loci. In addition, previous studies also revealed that G locus linked with D1 was mapped to LG D1a (chromosome 1) of soybean but no detailed information of physical position [82, 83]. Cloning and characterization of D1 supported that Glyma01g42390 is D1 controlling stay-green in soybean [32, 33]. Therefore, qSC1 on chromosome 1 may be considered as G locus and the fine mapping region of 213kb will be useful for map-based cloning of G gene.
Systematic dissection of complex trait as a powerful tool for discovering genes
Even though a high quality and well annotated genome sequence has been available [63, 84], isolation of genes for QTLs is still somewhat difficult in soybean. Majority of QTL mapping studies in soybean using hundreds of molecular markers with population size of a few hundred always identified dozens or even hundreds of QTLs (http://www.soybase.org). However, few of these QTLs are common to all mapping efforts. Moreover, the difficult of developing NILs in soybean further restrict the usage of this approach for fine mapping of QTLs. Development of RHLs is another choice for evaluating QTLs in soybean since some relative complex traits could be divided into several simple trait pairs in RHL populations [42, 43, 85]. In this study, four kinds of seed colors in segregating population were dissected into six simple color pairs by continuous self-fertility and selection of progeny. Finally all four QTLs were identified and validated by using these RHL populations and markers located in associated regions.
When BSA method was used to confirm loci identified in association analyses, only two major loci (qSC1 and qSC5/I) were confirmed from bulks of F2 individuals. After continuously developing RHLs, another two loci (qSC2/T and qSC7/R) were further identified. These four loci explained all genetic variations of seed coat colors in this segregating population, indicating that the strategy of systematically dissecting relative complex trait to simple trait pairs could serve as a powerful approach for discovering multiple genes which may have little effect.
The interaction of different loci controlling seed coat color
Previous reports indicated that I locus had major effect on controlling pigmentation of seed coat [14, 21, 22]. Our results from association mapping, BSA of F2 individuals and RHL populations all supported this conclusion as qSC5/I locus could be used for distinguishing dark and light colors of seed coat. Since the seed colors of wild soybeans and modern cultivars are mainly black and yellow respectively, qSC5/I locus may undergo selection during soybean domestication. Previous report on resequencing of wild and cultivated soybeans also indentified three genes in qSC5/I region with strong selection signals [7]. qSC1 locus was proven to co-segregate with green color and dominant qSC1 allele could pigment light green color in qSC5/I allele background. Up to now, few reports illustrated the genetic basis of green seed color in soybean, partially because the segregation of this kind of individuals is more complex than others. There is also a possibility that the green color is fading out at maturity of soybean seed and becoming yellow under the control of v1 or g1 locus [17, 86]. Since qSC5/I was proven to regulate the expression of CHS genes which had function in early step in flavonoids and anthocyanins biosynthesis [23, 24, 87, 88], we postulated that qSC1 might affect the coloration of seed coat in an independent pathway.
Furthermore, previous studies also revealed that T and R loci were associated with black and brown seed coats [14–16, 31]. Characterization of R locus suggested that functional R gene acted to promote transcription of structural genes encoding U3FGT and ANS which were located in downstream of flavonoid 3’-hydroxylase (encoded by GmF3’H gene) in anthocyanin pathway [30]. In this study, qSC7/R locus could be used for distinguish black and brown seed coats only under the background of dominant qSC2/T locus, also indicating that qSC7/R locus was involved in the downstream of qSC2/T. Therefore, further fine mapping and cloning of qSC1 will contribute to construct regulatory network of seed coat pigmentation in soybean.
Conclusions
A total of 14 loci distributed across ten chromosomes were identified to be associated with soybean seed coat colors using coding SNPs among a natural population. These loci could distinguish all tested soybean accessions with different colors more properly than five previous reported loci. Four of them including one novel locus were confirmed using several RHLs derived from a biparental population. The moderately complex trait of seed coat color was divided into simple color pairs and all four QTLs controlled this trait were systematically dissected by bulk segregation analysis and fine mapping (Fig 6). Even more, the regulation mechanism of these four loci was illustrated by genotyping entire F2 population using flanking markers of them. The results exhibiting in the manuscript could provide in-depth understanding of the inheritance of seed coat color and domestication analysis of different loci in soybean. The genetic information of these loci was useful for map-based cloning as well as marker-assisted selection in breeding program. Moreover, this work also provide an alternative strategy for systematically discovering genes by association analysis with high-throughput sequence data in natural population following bulk segregation analysis among dissected segregating populations.
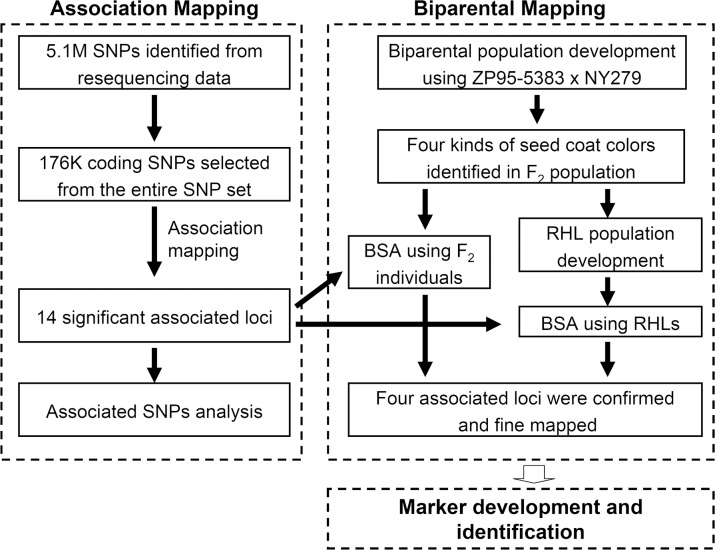
Fig 6. Flowchart of the approach to combine association and biparental mapping.
Results of association mapping and bulk segregation analysis were summarized side by side to clearly describe the entire study.
Supporting Information
(A) Estimated ln (probability of the data) calculated for K ranging from 2 to 9. (B) Population structure of soybean accessions, each accession was represented by a single vertical line and every color represented one cluster. The red color indicated Subgroup I and the green color indicated subgroup II.
(PDF)
Association mapping of seed coat color in soybean with SNPs in Sets B and C. (A) Expect -log (P) matched observed -log (P) best from the QQ-Plot using SNPs from Set B. (B) Manhattan plots showed -log(P) from a genome-wide scan were plotted against positions of SNPs on 20 chromosomes using SNPs from Set B. (C) Expect -log (P) matched observed -log (P) best from the QQ-Plot using SNPs from Set C. (D) Manhattan plots showed -log(P) from a genome-wide scan were plotted against positions of SNPs on 20 chromosomes using SNPs from Set C.
(PDF)
Red represented allele of each locus present in the reference genome (Williams 82) and blue represented the alternate allele. In addition, green represented the heterozygous alleles and grey represented missing data.
(PDF)
(PDF)
Comparative analysis of the association mapping results using SNPs from Sets A, B, and C.
(PDF)
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Agricultural Science and Technology Innovation Program (ASTIP) of Chinese Academy of Agricultural Sciences, recipient: LJQ; the State High-tech Research and Development Program, grant no. 2013AA102602, recipient: YG; National Natural Science Foundation of China, grant no. 31271753; Accurate Identification and Display of Soybean Germplasm, grant no. NB2010-2130315-25-05; and National Crop Germplasm Platform, grant no. 2012-004; 2014-004. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hartman GL, West ED, Herman TK. Crops that feed the world 2. Soybean-worldwide production, use, and constraints caused by pathogens and pests. Food Security. 2011;3:5–17. [Google Scholar]
- 2.Carpenter J, Felsot A, Goode T, Hamming M, Onstad D, Sankula S. Comparative environmental impacts of biotechnology-derived and traditional soybean, corn, and cotton crops Ames, IA: Council for Agricultural Science and Technology; 2002; p. 15–50. [Google Scholar]
- 3.Holton TA, Cornish EC. Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell. 1995;7(7):1071–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koes R, Verweij W, Quattrocchio F. Flavonoids: a colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005;10(5):236–242. [DOI] [PubMed] [Google Scholar]
- 5.Dixon RA, Sumner LW. Legume natural products: understanding and manipulating complex pathways for human and animal health. Plant Physiol. 2003;131(3):878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hymowitz T, Newell C. Taxonomy, speciation, domestication, dissemination, germplasm resources, and variation in the genus Glycine In: Summerfield RJ BA, editor. Advances in Legume Science. Kew, Richmond, Surrey: Royal Botanical Gardens; 1980. p. 251–264. [Google Scholar]
- 7.Li YH, Zhao SC, Ma JX, Li D, Yan L, Li J, et al. Molecular footprints of domestication and improvement in soybean revealed by whole genome re-sequencing. BMC Genomics. 2013;14:579 10.1186/1471-2164-14-579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gore MA, Hayes AJ, Jeong SC, Yue YG, Buss GR, Maroof S. Mapping tightly linked genes controlling potyvirus infection at the Rsv1 and Rpv1 region in soybean. Genome. 2002;45(3):592–599. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi R. Association of soybean genes I and T with low-temperature induced seed coat deterioration. Crop Sci. 1997;37:1755–1759. [Google Scholar]
- 10.Takahashi R, Asanuma S. Association of T gene with chilling tolerance in soybean. Crop Sci. 1996;36:559–562. [Google Scholar]
- 11.Benitez ER H. F, Kaneko Y, Matsuzawa Y, Bang SW, Takahashi R. Soybean maturity gene effects on seed coat pigmentation and cracking in response to low temperatures. Crop Sci. 2004;44:2038–2042. [Google Scholar]
- 12.Senda M, Masuta C, Ohnishi S, Goto K, Kasai A, Sano T, et al. Patterning of virus-infected Glycine max seed coat is associated with suppression of endogenous silencing of chalcone synthase genes. Plant Cell. 2004;16(4):807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang K, Jeong N, Moon JK, Lee YH, Lee SH, Kim HM, et al. Genetic analysis of genes controlling natural variation of seed coat and flower colors in soybean. J Hered. 2010;101(6):757–768. 10.1093/jhered/esq078 [DOI] [PubMed] [Google Scholar]
- 14.Palmer RG, Pfeiffer TW, Buss GR, Kilen TC. Qualitative genetics Soybeans: improvement, production, and uses 3rd ed. Madison (WI): ASA, CSSA, and SSSA; 2004. p. 137–214. [Google Scholar]
- 15.Buzzetl RI, Buttery BR, MacTavish DC. Biochemical genetics of black pigmentation of soybean seed. J Hered. 1987;78:53–54. [Google Scholar]
- 16.Todd JJ, Vodkin LO. Pigmented soybean (Glycine max) seed coats accumulate proanthocyanidins during development. Plant Physiol. 1993;102(2):663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodworth CM. Inheritance of cotyledon, seed-coat, hilum, and pubescence colors in soybeans. Genetics. 1921;6(6):487–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guiamet JJ, Giannibelli MC. Nuclear and cytoplasmic ''stay-green'' mutations of soybean alter the loss of leaf soluble proteins during senescence. Physiol Plantarum. 1996;96(4):655–661. [Google Scholar]
- 19.Reese PFJ, Boerma HR. Additional genes for green seed coat in soybean. J Hered. 1989;80(1):86–88. [Google Scholar]
- 20.Clough SJ, Tuteja JH, Li M, Marek LF, Shoemaker RC, Vodkin LO. Features of a 103-kb gene-rich region in soybean include an inverted perfect repeat cluster of CHS genes comprising the I locus. Genome. 2004;47(5):819–831. [DOI] [PubMed] [Google Scholar]
- 21.Todd JJ, Vodkin LO. Duplications that suppress and deletions that restore expression from a chalcone synthase multigene family. Plant Cell. 1996;8(4):687–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tuteja JH, Clough SJ, Chan WC, Vodkin LO. Tissue-specific gene silencing mediated by a naturally occurring chalcone synthase gene cluster in Glycine max. Plant Cell. 2004;16(4):819–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Senda M, Kurauchi T, Kasai A, Ohnishi S. Suppressive mechanism of seed coat pigmentation in yellow soybean. Breeding Sci. 2012;61(5):523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuteja JH, Zabala G, Varala K, Hudson M, Vodkin LO. Endogenous, tissue-specific short interfering RNAs silence the chalcone synthase gene family in Glycine max seed coats. Plant Cell. 2009;21(10):3063–3077. 10.1105/tpc.109.069856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toda K, Yang D, Yamanaka N, Watanabe S, Harada K, Takahashi R. A single-base deletion in soybean flavonoid 3'-hydroxylase gene is associated with gray pubescence color. Plant Mol Biol. 2002;50(2):187–196. [DOI] [PubMed] [Google Scholar]
- 26.Zabala G, Vodkin L. Cloning of the pleiotropic T locus in soybean and two recessive alleles that differentially affect structure and expression of the encoded flavonoid 3' hydroxylase. Genetics. 2003;163(1):295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zabala G, Vodkin LO. A rearrangement resulting in small tandem repeats in the F3'5'H gene of white flower genotypes is associated with the soybean locus. Crop Sci. 2007;47:S113–124. [Google Scholar]
- 28.Lark KG, Weisemann JM, Matthews BF, Palmer R, Chase K, Macalma T. A genetic map of soybean (Glycine max L.) using an intraspecific cross of two cultivars: 'Minosy' and 'Noir 1'. Theor Appl Genet. 1993;86(8):901–906. 10.1007/BF00211039 [DOI] [PubMed] [Google Scholar]
- 29.Song QJ, Marek LF, Shoemaker RC, Lark KG, Concibido VC, Delannay X, et al. A new integrated genetic linkage map of the soybean. Theor Appl Genet. 2004;109(1):122–128. [DOI] [PubMed] [Google Scholar]
- 30.Gillman JD, Tetlow A, Lee JD, Shannon JG, Bilyeu K. Loss-of-function mutations affecting a specific Glycine max R2R3 MYB transcription factor result in brown hilum and brown seed coats. BMC Plant Biol. 2011;11:155 10.1186/1471-2229-11-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zabala G, Vodkin LO. Methylation affects transposition and splicing of a large CACTA transposon from a MYB transcription factor regulating anthocyanin synthase genes in soybean seed coats. PLoS One. 2014;9(11):e111959 10.1371/journal.pone.0111959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang C, Li CC, Li WY, Wang Z, Zhou ZK, Shen YT, et al. Concerted evolution of D1 and D2 to regulate chlorophyll degradation in soybean. Plant J. 2014;77(5):700–712. 10.1111/tpj.12419 [DOI] [PubMed] [Google Scholar]
- 33.Nakano M, Yamada T, Masuda Y, Sato Y, Kobayashi H, Ueda H, et al. A green-cotyledon/stay-green mutant exemplifies the ancient whole-genome duplications in soybean. Plant Cell Physiol. 2014;55(10):1763–1771. 10.1093/pcp/pcu107 [DOI] [PubMed] [Google Scholar]
- 34.Mitchell-Olds T. Complex-trait analysis in plants. Genome Biol. 2010;11(4):113 10.1186/gb-2010-11-4-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henry IM, Dilkes BP, Tyagi A, Gao J, Christensen B, Comai L. The BOY NAMED SUE quantitative trait locus confers increased meiotic stability to an adapted natural allopolyploid of Arabidopsis. Plant Cell. 2014;26(1):181–194. 10.1105/tpc.113.120626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Fan C, Xing Y, Jiang Y, Luo L, Sun L, et al. Natural variation in GS5 plays an important role in regulating grain size and yield in rice. Nat Genet. 2011;43(12):1266–1269. 10.1038/ng.977 [DOI] [PubMed] [Google Scholar]
- 37.Ren Z, Zheng Z, Chinnusamy V, Zhu J, Cui X, Iida K, et al. RAS1, a quantitative trait locus for salt tolerance and ABA sensitivity in Arabidopsis. Proc Natl Acad Sci U S A. 2010;107(12):5669–5674. 10.1073/pnas.0910798107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song XJ, Huang W, Shi M, Zhu MZ, Lin HX. A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nat Genet. 2007;39(5):623–630. [DOI] [PubMed] [Google Scholar]
- 39.Xue W, Xing Y, Weng X, Zhao Y, Tang W, Wang L, et al. Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet. 2008;40(6):761–767. 10.1038/ng.143 [DOI] [PubMed] [Google Scholar]
- 40.Yano M, Katayose Y, Ashikari M, Yamanouchi U, Monna L, Fuse T, et al. Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell. 2000;12(12):2473–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Z, Ober JA, Kliebenstein DJ. The gene controlling the quantitative trait locus EPITHIOSPECIFIER MODIFIER1 alters glucosinolate hydrolysis and insect resistance in Arabidopsis. Plant Cell. 2006;18(6):1524–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watanabe S, Hideshima R, Xia Z, Tsubokura Y, Sato S, Nakamoto Y, et al. Map-based cloning of the gene associated with the soybean maturity locus E3. Genetics. 2009;182(4):1251–1262. 10.1534/genetics.108.098772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watanabe S, Xia Z, Hideshima R, Tsubokura Y, Sato S, Yamanaka N, et al. A map-based cloning strategy employing a residual heterozygous line reveals that the GIGANTEA gene is involved in soybean maturity and flowering. Genetics. 2011;188(2):395–407. 10.1534/genetics.110.125062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Appels R, Barrero R, Bellgard M. Advances in biotechnology and informatics to link variation in the genome to phenotypes in plants and animals. Funct Integr Genomics. 2013;13(1):1–9. 10.1007/s10142-013-0319-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atwell S, Huang YS, Vilhjalmsson BJ, Willems G, Horton M, Li Y, et al. Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature. 2010;465(7298):627–631. 10.1038/nature08800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang X, Zhao Y, Wei X, Li C, Wang A, Zhao Q, et al. Genome-wide association study of flowering time and grain yield traits in a worldwide collection of rice germplasm. Nat Genet. 2012;44(1):32–39. [DOI] [PubMed] [Google Scholar]
- 47.Li H, Peng Z, Yang X, Wang W, Fu J, Wang J, et al. Genome-wide association study dissects the genetic architecture of oil biosynthesis in maize kernels. Nat Genet. 2013;45(1):43–50. 10.1038/ng.2484 [DOI] [PubMed] [Google Scholar]
- 48.Yu J, Pressoir G, Briggs WH, Vroh Bi I, Yamasaki M, Doebley JF, et al. A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat Genet. 2006;38(2):203–208. [DOI] [PubMed] [Google Scholar]
- 49.Brachi B, Faure N, Horton M, Flahauw E, Vazquez A, Nordborg M, et al. Linkage and association mapping of Arabidopsis thaliana flowering time in nature. PLoS Genet. 2010;6(5):e1000940 10.1371/journal.pgen.1000940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Famoso AN, Zhao K, Clark RT, Tung CW, Wright MH, Bustamante C, et al. Genetic architecture of aluminum tolerance in rice (Oryza sativa) determined through genome-wide association analysis and QTL mapping. PLoS Genet. 2011;7(8):e1002221 10.1371/journal.pgen.1002221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sonah H, O'Donoughue L, Cober E, Rajcan I, Belzile F. Identification of loci governing eight agronomic traits using a GBS-GWAS approach and validation by QTL mapping in soya bean. Plant Biotechnol J. 2015;13(2):211–221. 10.1111/pbi.12249 [DOI] [PubMed] [Google Scholar]
- 52.Lam HM, Xu X, Liu X, Chen W, Yang G, Wong FL, et al. Resequencing of 31 wild and cultivated soybean genomes identifies patterns of genetic diversity and selection. Nat Genet. 2010;42(12):1053–1059. 10.1038/ng.715 [DOI] [PubMed] [Google Scholar]
- 53.Liu K, Muse SV. PowerMarker: an integrated analysis environment for genetic marker analysis. Bioinformatics. 2005;21(9):2128–2129. [DOI] [PubMed] [Google Scholar]
- 54.Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: dominant markers and null alleles. Mol Ecol Notes. 2007;7:574–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol. 2005;14(8):2611–2620. [DOI] [PubMed] [Google Scholar]
- 56.Bradbury PJ, Zhang Z, Kroon DE, Casstevens TM, Ramdoss Y, Buckler ES. TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics. 2007;23(19):2633–2635. [DOI] [PubMed] [Google Scholar]
- 57.Turner S D. qqman: an R package for visualizing GWAS results using QQ and manhattan plots. bioRxiv, 2014, 005165.
- 58.van Berloo R. GGT 2.0: Versatile software for visualization and analysis of genetic data. J Hered. 2008;99(2):232–236. 10.1093/jhered/esm109 [DOI] [PubMed] [Google Scholar]
- 59.Dellaporta SL, Wood J, Hicks JB. A plant DNA minipreparation: Version II. Plant Mol Biol Rep. 1983;1:19–21. [Google Scholar]
- 60.Murray MG, Thompson WF. Rapid isolation of high molecular-weight plant DNA. Nucleic Acids Res. 1980;8:4321–4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cregan PB, Jarvik T, Bush AL, Shoemaker RC, Lark KG, Kahler AL, et al. An integrated genetic linkage map of the soybean genome. Crop Sci. 1999;39(5):1464–1490. [Google Scholar]
- 62.Song QJ, Jia GF, Zhu YL, Grant D, Nelson RT, Hwang EY, et al. Abundance of SSR motifs and development of candidate polymorphic SSR markers (BARCSOYSSR_1.0) in soybean. Crop Sci. 2010;50(5):1950–1960. [Google Scholar]
- 63.Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, Nelson W, et al. Genome sequence of the palaeopolyploid soybean. Nature. 2010;463(7278):178–183. 10.1038/nature08670 [DOI] [PubMed] [Google Scholar]
- 64.Guo Y, Qiu LJ. Allele-specific marker development and selection efficiencies for both flavonoid 3'-hydroxylase and flavonoid 3',5'-hydroxylase genes in soybean subgenus soja. Theor Appl Genet. 2013;126(6):1445–1455. 10.1007/s00122-013-2063-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hao D, Cheng H, Yin Z, Cui S, Zhang D, Wang H, et al. Identification of single nucleotide polymorphisms and haplotypes associated with yield and yield components in soybean (Glycine max) landraces across multiple environments. Theor Appl Genet. 2012;124(3):447–458. 10.1007/s00122-011-1719-0 [DOI] [PubMed] [Google Scholar]
- 66.Korir PC, Zhang J, Wu K, Zhao T, Gai J. Association mapping combined with linkage analysis for aluminum tolerance among soybean cultivars released in Yellow and Changjiang river valleys in China. Theor Appl Genet. 2013;126(6):1659–1675. 10.1007/s00122-013-2082-0 [DOI] [PubMed] [Google Scholar]
- 67.Li YH, Smulders MJ, Chang RZ, Qiu LJ. Genetic diversity and association mapping in a collection of selected Chinese soybean accessions based on SSR marker analysis. Conserv Genet. 2011;12(5):1145–1157. [Google Scholar]
- 68.Mamidi S, Chikara S, Goos RJ, Hyten DL, Annam D, Moghaddam SM, et al. Genome-wide association analysis identifies candidate genes associated with iron deficiency chlorosis in soybean. Plant Genome. 2011;4(3):154–164. [Google Scholar]
- 69.Niu Y, Xu Y, Liu XF, Yang SX, Wei SP, Xie FT, et al. Association mapping for seed size and shape traits in soybean cultivars. Mol Breeding. 2013;31(4):785–794. [Google Scholar]
- 70.Bastien M, Sonah H, Belzile F. Genome wide association mapping of Sclerotinia sclerotiorum resistance in soybean with a Genotyping-by-Sequencing approach. Plant Genome. 2014; 7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mamidi S, Lee RK, Goos JR, McClean PE. Genome-wide association studies identifies seven major regions responsible for iron deficiency chlorosis in soybean (Glycine max). PLoS One. 2014;9(9):e107469 10.1371/journal.pone.0107469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vuong TD, Sonah H, Meinhardt CG, Deshmukh R, Kadam S, Nelson RL, et al. Genetic architecture of cyst nematode resistance revealed by genome-wide association study in soybean. BMC Genomics. 2015;16:593 10.1186/s12864-015-1811-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tardivel A, Sonah H, Belzile F, O'Donoughue LS. Rapid identification of alleles at the soybean maturity gene E3 using genotyping by sequencing and a haplotype-based approach. Plant Genome. 2014;7(2). [Google Scholar]
- 74.Motte H, Vercauteren A, Depuydt S, Landschoot S, Geelen D, Werbrouck S, et al. Combining linkage and association mapping identifies RECEPTOR-LIKE PROTEIN KINASE1 as an essential Arabidopsis shoot regeneration gene. Proc Natl Acad Sci U S A. 2014;111(22):8305–8310. 10.1073/pnas.1404978111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sterken R, Kiekens R, Boruc J, Zhang F, Vercauteren A, Vercauteren I, et al. Combined linkage and association mapping reveals CYCD5;1 as a quantitative trait gene for endoreduplication in Arabidopsis. Proc Natl Acad Sci U S A. 2012;109(12):4678–4683. 10.1073/pnas.1120811109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang J, McClean PE, Lee R, Goos RJ, Helms T. Association mapping of iron deficiency chlorosis loci in soybean (Glycine max L. Merr.) advanced breeding lines. Theor Appl Genet. 2008;116(6):777–787. 10.1007/s00122-008-0710-x [DOI] [PubMed] [Google Scholar]
- 77.Kadam S, Vuong TD, Qiu D, Meinhardt CG, Song L, Deshmukh R, et al. Genomic-assisted phylogenetic analysis and marker development for next generation soybean cyst nematode resistance breeding. Plant Sci. 2016;242:342–350. 10.1016/j.plantsci.2015.08.015 [DOI] [PubMed] [Google Scholar]
- 78.Githiri SM, Yang D, Khan NA, Xu D, Komatsuda T, Takahashi R. QTL analysis of low temperature induced browning in soybean seed coats. J Hered. 2007;98(4):360–366. [DOI] [PubMed] [Google Scholar]
- 79.Ohnishi S, Funatsuki H, Kasai A, Kurauchi T, Yamaguchi N, Takeuchi T, et al. Variation of GmIRCHS (Glycine max inverted-repeat CHS pseudogene) is related to tolerance of low temperature-induced seed coat discoloration in yellow soybean. Theor Appl Genet. 2011;122(3):633–642. 10.1007/s00122-010-1475-6 [DOI] [PubMed] [Google Scholar]
- 80.Oyoo M, Benitez E, Kurosaki H, Ohnishi S, Miyoshi T, Kiribuchi-Otobe C, et al. QTL analysis of soybean seed coat discoloration associated with II TT Genotype. Crop Sci. 2010;51(2):464–469. [Google Scholar]
- 81.Li YH, Reif JC, Jackson SA, Ma YS, Chang RZ, Qiu LJ. Detecting SNPs underlying domestication-related traits in soybean. BMC Plant Biol. 2014;14(1):251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lohnes DG, Specht JE, Cregan PB. Evidence for homoeologous linkage groups in the soybean. Crop Sci. 1997;37(1):254–257. [Google Scholar]
- 83.Weiss MG. Genetic linkage in soybeans: Linkage group II and III. Crop Sci. 1970;10:300–303. [Google Scholar]
- 84.Li YH, Zhou G, Ma J, Jiang W, Jin LG, Zhang Z, et al. De novo assembly of soybean wild relatives for pan-genome analysis of diversity and agronomic traits. Nat Biotechnol. 2014;32(10):1045–1052. 10.1038/nbt.2979 [DOI] [PubMed] [Google Scholar]
- 85.Guan R, Qu Y, Guo Y, Yu L, Liu Y, Jiang J, et al. Salinity tolerance in soybean is modulated by natural variation in GmSALT3. Plant J. 2014;80(6):937–950. 10.1111/tpj.12695 [DOI] [PubMed] [Google Scholar]
- 86.Owen V. Inheritance studies in soybeans III. Seed-coat color and summary of all other mendelian characters thus far reported. Genetics. 1928;13(1):50–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cho YB, Jones SI, Vodkin L. The transition from primary siRNAs to amplified secondary siRNAs that regulate chalcone synthase during development of Glycine max seed coats. PLoS One. 2013;8(10):e76954 10.1371/journal.pone.0076954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Senda M, Nishimura S, Kasai A, Yumoto S, Takada Y, Tanaka Y, et al. Comparative analysis of the inverted repeat of a chalcone synthase pseudogene between yellow soybean and seed coat pigmented mutants. Breeding Sci. 2013;63(4):384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Estimated ln (probability of the data) calculated for K ranging from 2 to 9. (B) Population structure of soybean accessions, each accession was represented by a single vertical line and every color represented one cluster. The red color indicated Subgroup I and the green color indicated subgroup II.
(PDF)
Association mapping of seed coat color in soybean with SNPs in Sets B and C. (A) Expect -log (P) matched observed -log (P) best from the QQ-Plot using SNPs from Set B. (B) Manhattan plots showed -log(P) from a genome-wide scan were plotted against positions of SNPs on 20 chromosomes using SNPs from Set B. (C) Expect -log (P) matched observed -log (P) best from the QQ-Plot using SNPs from Set C. (D) Manhattan plots showed -log(P) from a genome-wide scan were plotted against positions of SNPs on 20 chromosomes using SNPs from Set C.
(PDF)
Red represented allele of each locus present in the reference genome (Williams 82) and blue represented the alternate allele. In addition, green represented the heterozygous alleles and grey represented missing data.
(PDF)
(PDF)
Comparative analysis of the association mapping results using SNPs from Sets A, B, and C.
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.