Abstract
Background
Transdermal nicotine, with behavioral counseling, is among the most popular approaches used to quit smoking. Yet, 6-month cessation rates rarely exceed 20–25%. Identifying factors associated with cessation success may help researchers and clinicians develop enhanced interventions that can improve quit rates. This study examined longitudinal changes in withdrawal, craving, depression and anxiety symptoms, and alternative reinforcers, from a baseline assessment to a 6-month outcome, as predictors of 6-month smoking cessation outcomes following 8 weeks of nicotine patch treatment and counseling.
Methods
A sample of 180 smokers, who completed an effectiveness trial that provided counseling and 8 weeks of 21mg nicotine patches, was analyzed. Generalized estimating equations evaluated changes in withdrawal and craving, depression and anxiety symptoms, and alternative reinforcers over time, between participants who were smoking at 6-months and participants who were abstinent (confirmed with carbon monoxide) at 6-months. Multiple logistic regression assessed changes in these variables as predictors of relapse.
Results
Controlling for covariates associated with cessation (i.e., nicotine dependence, patch adherence, and rate of nicotine metabolism), participants who were abstinent at 6 months showed significantly lower craving and withdrawal and significantly higher substitute reinforcers from baseline to 6 months, vs. those who were smoking at 6 months (p < .001). An increase in craving predicted relapse to smoking (p < .05).
Conclusions
These results support continued efforts to strengthen interventions that reduce withdrawal and craving and the development of interventions to address alternative reinforcers in order to promote long-term smoking abstinence following nicotine patch treatment.
Keywords: smoking, behavioral economics, alternative reinforcers, withdrawal, craving
1. INTRODUCTION
Owing to its availability as an over-the-counter medication, relative affordability, and favorable side effect profile, transdermal nicotine patches remain as one of the most popular methods that smokers use to make a quit attempt. Among smokers making a quit attempt, transdermal nicotine is the most popular form of nicotine replacement therapy and the second most popular form of pharmacotherapy, next to varenicline (Kasza et al., 2015). While popular, long-term cessation rates among users of the nicotine patch (with counseling) rarely exceed 20–25% (Fiore et al., 2008). Identifying variables that enhance the effectiveness of transdermal nicotine by characterizing smokers who are successful in their attempt, vs. those who are unable to quit, may highlight novel treatment targets to boost treatment responsiveness.
Abstinence symptoms, emotional responses to quitting, and sources of alternative reinforcement are three such targets. Studies have documented the role that withdrawal symptoms and abstinence-induced craving have on relapse early in a quit attempt (al’Absi et al., 2007; Piasecki et al., 2000; Swan et al., 1996) and medications used to treat nicotine dependence are, in part, designed to reduce withdrawal and craving (Henningfield et al., 2009). However, even while abstinent, withdrawal and craving are highly variable and the impact of such variation across time on smoking abstinence is under-studied (Javitz et al., 2012). Likewise, elevated levels of depression and anxiety symptoms pre-cessation and early in the quit attempt have been linked to poorer smoking cessation outcomes at the end of treatment (Niaura et al., 2001; Leventhal et al., 2008; 2014; Kenford et al., 2002; Zvolensky et al., 2009). Yet, few studies have examined the prospective relationship between emotional responses to quitting and smoking cessation outcomes. Lastly, more recent research has identified novel predictors of quit attempt outcomes, including complementary and alternative reinforcers. Rooted in Behavioral Economic Theory (Green and Freed, 1993), increased engagement in substitute reinforcers, which are healthier alternative activities to smoking (e.g., physical activity), and decreased engagement in complementary reinforcers, which are activities that increase the reinforcing value of smoking (e.g., consuming alcohol), have been associated with greater probability of successful cessation. Results from a small lab study (Stoops et al., 2011) and a clinical trial of short-term abstinence (Goelz et al., 2014) indicate the need to examine the predictive significance of alternative reinforcers on long-term abstinence. Such analyses could help support the development and evaluation of novel behavioral interventions based on Behavioral Economic Theory to promote tobacco cessation. Thus, emerging from these studies is the picture that smokers attempting to quit may have a greater probability for success if they are able to better manage craving and withdrawal, negative emotional responses, and substitute and complementary reinforcers.
To date, however, few studies have prospectively examined long-term changes in these factors following treatment with transdermal nicotine as predictors of successful long-term cessation. Thus, this study, using data from a large clinical trial, examined changes in withdrawal and craving, emotional responses, and complementary and substitute reinforcers among successful abstainers 6 months after treatment initiation vs. those who returned to smoking.
2. METHODS
2.1 Participants
Participants in this study completed an effectiveness clinical trial that evaluated 8, 24, and 52 weeks of transdermal nicotine (Schnoll et al., 2015; ClinicalTrials.gov Identifier: NCT01047527). For the current study, only participants randomized to 8 weeks of treatment were included in order to standardize nicotine patch treatment duration and to follow up on our prior study of predictors of short-term cessation (Goelz et al., 2014). To be eligible for the clinical trial, participants had to be 18 years of age or older, report smoking 10 cigarettes per day or more, and indicate an interest in quitting smoking. Exclusion criteria were limited to minimizing potential subject risk (see Schnoll et al., 2015) and, as a result, the present sample was relatively unique (e.g., included smokers with past or current psychiatric disorders).
2.2 Study Procedures
Methods were approved by the University of Pennsylvania and Northwestern University Institutional Review Boards. Participants, recruited through advertisements, completed an in-person intake session to confirm eligibility. Eligible participants were randomized to 8, 24, or 52 weeks of transdermal nicotine and 12 behavioral counseling sessions. Participants received open-label 21mg transdermal nicotine (Nicoderm CQ; GlaxoSmithKline, Research Triangle Park, NC) for 8 weeks and 8 behavioral counseling sessions. Counseling was brief, based on established treatment guidelines (Fiore et al., 2008), and focused on preparing for cessation, managing urges and triggers to smoking, and developing strategies to avoid relapse. Data for this study were taken from assessments conducted at baseline (week −2), week 8, and 6 months. At 6 months, self-reported smoking cessation was confirmed using breath carbon monoxide (CO) assessed using the Vitalograph BreathCO monitor.
2.3 Measures
2.3.1. Covariates
At the baseline assessment, participants were asked to provide information about demographic (e.g., age, sex, race) and smoking-related (e.g., cigarettes per day, the Fagerström Test for Nicotine Dependence [FTND; Heatherton et al., 1991]) characteristics. In addition, as described previously (Kaufmann et al., 2015), saliva samples (5ml) were collected at baseline to determine participant rate of nicotine metabolism based on the nicotine metabolite ratio (NMR).
2.3.2. Withdrawal and Craving
The Minnesota Nicotine Withdrawal Scale (MNWS) measured withdrawal symptoms associated with quitting smoking (Hughes and Hatsukami, 1986). The MNWS assesses 7 American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) dimensions of nicotine withdrawal (i.e., anger, anxiety, depression, difficulty concentrating, increased appetite, insomnia, restlessness, impatience, and craving); a summary score was calculated. The 10-item brief Questionnaire of Smoking Urges (QSU; Cox et al., 2001) was used to assess craving for cigarettes; a total summary score was used.
2.3.3. Inventory of Depressive Symptomatology
The 30-item Inventory of Depressive Symptomatology (IDS; Rush et al., 1986; 1996) assessed the severity and frequency of depressive symptoms. The IDS includes symptoms of major depressive disorder as defined by the DSM-IV. Each item is scaled from 0 (not present) to 3 (very severe), yielding a total score. Depression severity can be classified as normal (≤15), mild (16–24), moderate (25–32), moderate to severe (33–40) and severe (≥41).
2.3.4. Beck Anxiety Inventory
We used the 21-item Beck Anxiety Inventory (BAI) to determine participant symptoms of anxiety (Beck et al., 1988). Each item was rated on a 4-point scale ranging from 0 to 3 and items were summed to obtain a total score. Total scores from 0–7 are considered to reflect a minimal level of anxiety; scores of 8–15 indicate mild anxiety; scores of 16–25 reflect moderate anxiety; and scores of 26–63 indicate severe anxiety.
2.3.5. Pleasant Events Schedule
The Pleasant Events Schedule (PES; MacPhillamy and Lewinsohn, 1982) is a self-report measure of the frequency and enjoyability of common rewarding activities and events engaged in by an individual over the past 30 days. We used the 45-item short-form version of the PES as in past studies (Audrain-McGovern et al., 2009, 2011). First, the cross product of the frequency score (0 = has not happened to 2 = happened often) and enjoyability score (0 = not pleasurable to 2 = very pleasurable) for each item provided a measure of an individual’s reinforcement from that activity. Second, participants were asked whether they associated each activity with smoking or the urge to smoke; activities associated with smoking were defined as complementary reinforcers whereas activities not associated with smoking were defined as alternative reinforcers. The cross products of the substitute reinforcers were summed to provide an individual’s overall index of substitute reinforcers and the cross products of the complementary reinforcers were summed to provide an individual’s overall index of complementary reinforcers.
2.3.6. Transdermal Nicotine (Patch) Adherence
Daily patch use from week 0 (target quit day) to week 8 was assessed at each session using a timeline follow-back measure (Brown et al., 1998), a method used in previous trials (Schnoll et al., 2009). The average number of days per week that a patch was used was calculated to represent to degree to which participants were adherent.
2.3.7. Smoking Behavior
We used the timeline follow-back measure to assess smoking from baseline to 6 months (Brown et al., 1998). At 6 months, participants who self-reported that they had not smoked for the previous 7 days were asked to provide a breath sample for biochemical verification of smoking status (within 3 days of the self-report). Level of breath CO was measured in parts per million (ppm). Consistent with established guidelines (SRNT Subcommittee on Biochemical Verification, 2002), participants were considered abstinent at 6 months if they self-reported abstinence for 7 days prior to the assessment and had a breath sample with CO ≤ 10ppm. Participants who withdrew from the study, failed to provide a CO sample, or had a CO greater than 10ppm were considered smokers. The same procedures were used to determine smoking cessation at 8 weeks.
2.4 Statistical Analysis
This intent-to-treat analysis included all participants randomized to the 8-week treatment arm who completed the first counseling session (baseline; n = 180). Sample characteristics, including demographic data and smoking history, were examined using descriptive statistics (e.g., mean, standard deviation, proportions). We assessed differences between participants who were abstinent at 6 months or smoking at 6 months using chi-square tests (for categorical variables) or ANOVA (for continuous variables). Variables associated with 6 month abstinence (p ≤ .10) were included in a multiple logistic model to identify potential covariates for the primary analyses.
Next, we used Generalized Estimating Equations (GEE) to determine whether changes in withdrawal and craving, depression and anxiety symptoms, and substitute and complementary reinforcers, from baseline to 6 months were related to smoking abstinence at 6 months. Main and interaction effects for the repeated measures independent variable (i.e., time: baseline, week 8, 6 months) and the between-group independent variable (i.e., 6 month smoking status: smoking or abstinent) were assessed. Separate models were conducted for each construct and covariates were included.
Lastly, multivariate logistic regression was used to assess whether changes in withdrawal and craving, depression and anxiety symptoms, and substitute and complementary reinforcers from baseline to week 8 were associated with relapse to smoking between week 8 and 6 months. For these analyses, we selected only participants who were confirmed abstinent at week 8 and examined difference scores for our predictors of relapse to smoking between week 8 and 6 months (with relapse defined as 7 consecutive days of smoking). Models included covariates. All analyses were completed using SPSS (Version 20.0, IBM Corp., Armonk, NY) or STATA (Version 13.1, StataCorp, College Station, Texas).
3. RESULTS
3.1. Sample Characteristics and Covariates1
Table 1 shows the characteristics of the sample. The quit rate at 6 months was 21.7% (39/180). Characteristics associated with abstinence at 6 months were level of education, level of nicotine dependence, baseline rate of smoking, rate of nicotine metabolism, and current or past psychiatric disorder. Compared to participants who were smoking at 6 months, abstainers reported a significantly higher level of education (χ2 [1] = 4.85, p = .05), lower level of nicotine dependence (F[1,177] = 4.86, p = .05), lower rate of nicotine metabolism (F[1,169] = 3.84, p = .05), and higher rate of weekly use of the nicotine patch (F[1,178] = 10.84, p = .001), and were significantly less likely to report a current or past psychiatric disorder (χ2[1] = 8.01, p < .03). These variables were included in a logistic regression model predicting 6 month abstinence. When included together in the model, predictors of 6 month abstinence included: nicotine dependence (β = .83, 95% CI: 0.67–1.03, p = .10), nicotine metabolism rate (β = .14, 95% CI: 0.02–1.18, p = .07), and patch adherence (β = 1.44, 95% CI: 1.06–1.95, p = .02). These variables were included as covariates in the models below.
Table 1.
Participant Characteristics (N = 180)
| Characteristic | 6 month Smokers (n = 141) | 6 month Abstainers (n = 39) | Total (n = 180) |
|---|---|---|---|
| Sex (% male) | 48.9 | 53.8 | 50.0 |
| Age (mean, SD) | 45.6 (12.6) | 47.1 (11.1) | 45.9 (12.3) |
| Race (% Caucasian) | 52.6 | 47.2 | 51.5 |
| Marital status (% married) | 31.9 | 30.8 | 31.7 |
| Sexual orientation (% heterosexual) | 93.1 | 84.4 | 91.4 |
| Education (% ≤ GED)* | 32.6 | 15.4 | 28.9 |
| Income (% ≤ 50,000, annually) | 74.3 | 66.7 | 72.6 |
| Nicotine Dependence (mean, SD)* | 5.5 (1.9) | 4.7 (1.8) | 5.3 (1.9) |
| Cigarettes per day (mean, SD) | 17.3 (8.8) | 15.2 (6.6) | 16.9 (8.4) |
| Age of smoking initiation (mean, SD) | 15.8 (5.4) | 16.9 (4.3) | 16.0 (5.2) |
| Years of smoking (mean, SD) | 28.4 (13.3) | 29.4 (11.2) | 28.6 (12.9) |
| Nicotine Metabolite Ratio (mean, SD)* | 0.36 (0.24) | 0.28 (0.15) | 0.35 (0.22) |
| Nicotine Patch Adherence (mean, SD)* | 4.4 (2.1) | 5.5 (1.2) | 4.6 (2.0) |
| Past or Current Psychiatric Disorder (% with)* | 11.1 | 0 | 8.6 |
Note. Differences between 6 month Smokers and Abstainers:
p’s < .10.
3.2. Withdrawal and Craving
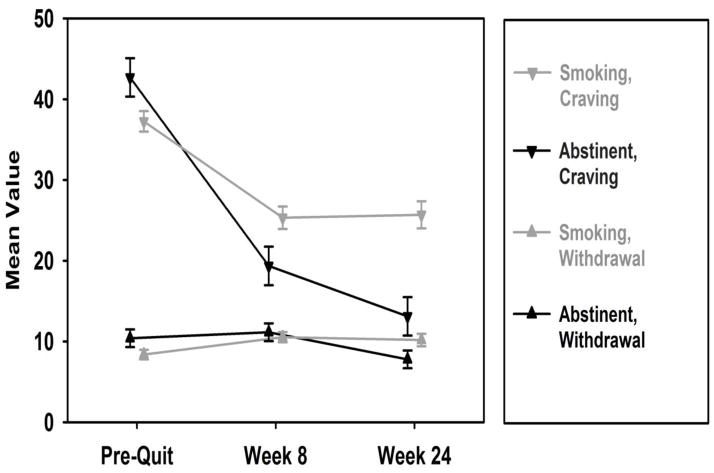
Controlling for covariates, the GEE analyses yielded a significant time x smoking status interaction for symptoms of withdrawal (χ2 [2] = 9.25, p < .001). As shown in Table 2, the main effects for time, for both baseline to week 8 withdrawal and baseline to 6 months withdrawal, were significant (β = 2.15, 95% CI: 0.76 to 3.54, p = .002; β = 1.81, 95% CI: 0.19 to 3.43, p = .03). While the main effect of smoking status (β = 2.03, 95% CI: −0.46 to 4.51, p = .11) and the interaction effect for change in withdrawal from baseline to week 8 and smoking status at 6 months (β = −1.42, 95% CI: −4.19 to 1.34, p = .31) were not significant, the interaction effect for baseline to 6 months was significant (β = −4.43, 95% CI: −7.32 to −1.54, p = .003). As shown in Figure 1, withdrawal symptoms increased initially for both 6 month smokers and abstainers; however, while withdrawal symptoms remained elevated for 6 month smokers between week 8 and 6 months, they decreased significantly for 6 month abstainers during this time-period.
Table 2.
Separate GEE Analyses of Withdrawal, Craving, Depressive Symptoms, Anxiety Symptoms, and Substitute and Complementary Reinforcers by 6 month Smoking Status over Time
| Dependent Variable: Withdrawal | β | 95% CI | p |
|---|---|---|---|
| Constant | 5.93 | 2.51 to 9.35 | .001 |
| Nicotine Dependence | 0.68 | 0.25 to 1.12 | .002 |
| Patch Adherence | −0.53 | −0.98 to −0.07 | .02 |
| Nicotine Metabolite Ratio | 3.95 | 0.37 to 7.63 | .03 |
| Time (Baseline vs. Week 8) | 2.15 | 0.76 to 3.54 | .002 |
| Time (Baseline vs. 6 Months)* | 1.81 | 0.19 to 3.43 | .03 |
| 6 Months Smoking Status | 2.03 | −0.46 to 4.51 | .11 |
| Time (Baseline vs. Week 8) x 6 Months Smoking Status* | −1.42 | −4.19 to 1.34 | .31 |
| Time (Baseline vs. 6 months) x 6 Months Smoking Status* | −4.43 | −7.32 to −1.54 | .003 |
| Dependent Variable: Craving | β | 95% CI | p |
| Constant | 23.92 | 16.65 to 31.18 | < .001 |
| Nicotine Dependence | 2.63 | 1.72 to 3.55 | < .001 |
| Patch Adherence | −0.39 | −1.36 to 0.58 | .43 |
| Nicotine Metabolite Ratio | 3.15 | −4.40 to 10.71 | .41 |
| Time (Baseline vs. Week 8)* | −11.93 | −15.06 to −8.79 | <.001 |
| Time (Baseline vs. 6 Months)* | −11.57 | −15.20 to −7.93 | <.001 |
| 6 Months Smoking Status | 5.45 | 0.08 to 10.83 | .05 |
| Time (Baseline vs. Week 8) x 6 Months Smoking Status* | −11.42 | −17.67 to −5.18 | <.001 |
| Time (Baseline vs. 6 months) x 6 Months Smoking Status* | −18.03 | −24.54 to −11.52 | <.001 |
| Dependent Variable: Depressive Symptoms | β | 95% CI | p |
| Constant | 5.78 | 1.55 to 10.00 | .007 |
| Nicotine Dependence | 0.85 | 0.31 to 1.39 | .002 |
| Patch Adherence | −0.47 | −1.03 to 0.96 | .10 |
| Nicotine Metabolite Ratio | 7.85 | 3.40 to 12.29 | .001 |
| Time (Baseline vs. Week 8)* | −1.74 | −3.41 to −0.09 | .04 |
| Time (Baseline vs. 6 Months)* | −2.84 | −4.78 to −0.90 | .004 |
| 6 Months Smoking Status | 1.86 | −1.17 to 4.90 | .23 |
| Time (Baseline vs. Week 8) x 6 Months Smoking Status* | −1.00 | −4.31 to 2.30 | .55 |
| Time (Baseline vs. 6 months) x 6 Months Smoking Status* | −2.81 | −6.26 to 0.64 | .11 |
| Dependent Variable: Anxiety Symptoms | β | 95% CI | p |
| Constant | −1.02 | −5.18 to 3.14 | .63 |
| Nicotine Dependence | 0.87 | 0.34 to 13.97 | .001 |
| Patch adherence | −0.44 | −0.99 to 0.12 | .12 |
| Nicotine Metabolite Ratio | 6.89 | 2.53 to 11.25 | .001 |
| Time (Baseline vs. Week 8)* | 1.14 | −0.55 to 2.83 | .19 |
| Time (Baseline vs. 6 Months) * | 1.63 | −0.34 to 3.60 | .11 |
| 6 Months Smoking Status | 2.18 | −0.84 to 5.20 | .16 |
| Time (Baseline vs. Week 8) x 6 Months Smoking Status* | 2.74 | −0.63 to 6.11 | .11 |
| Time (Baseline vs. 6 months) x 6 Months Smoking Status* | 0.10 | −3.42 to 3.62 | .96 |
| Dependent Variable: Substitute Reinforcers | β | 95% CI | p |
|---|---|---|---|
| Constant | 39.02 | 27.29 to 50.76 | < .001 |
| Nicotine Dependence | −3.03 | −4.53 to −1.54 | < .001 |
| Patch adherence | 1.62 | 0.06 to 3.18 | .04 |
| Nicotine Metabolite Ratio | −8.09 | −20.46 to 4.29 | .20 |
| Time (Baseline vs. Week 8)* | 17.81 | 13.27 to 22.34 | < .001 |
| Time (Baseline vs. 6 Months)* | 11.38 | 6.10 to 16.66 | < .001 |
| 6 Months Smoking Status | −7.62 | −16.02 to 0.78 | .08 |
| Time (Baseline vs. Week 8) x 6 Months Smoking Status* | 8.32 | −0.68 to 17.33 | .07 |
| Time (Baseline vs. 6 months) x 6 Months Smoking Status* | 18.29 | 8.89 to 27.97 | < .001 |
| Dependent Variable: Complementary Reinforcers | β | 95% CI | p |
| Constant | 37.50 | 25.42 to 49.58 | < .001 |
| Nicotine Dependence | 1.44 | −0.12 to 3.00 | .07 |
| Patch adherence | −2.12 | −3.71 to −0.54 | .009 |
| Nicotine Metabolite Ratio | 1.48 | −11.52 to 14.47 | .80 |
| Time (Baseline vs. Week 8)* | −22.34 | −26.07 to −18.61 | <.001 |
| Time (Baseline vs. 6 Months)* | −22.00 | −26.27 to −17.52 | <.001 |
| 6 Months Smoking Status | −0.31 | −8.53 to 7.91 | .94 |
| Time (Baseline vs. Week 8) x 6 Months Smoking Status* | −3.41 | −10.78 to 3.96 | .36 |
| Time (Baseline vs. 6 months) x 6 Months Smoking Status* | −7.70 | −15.19 to 0.17 | .05 |
Note. The constant coefficient provides a reference value, and coefficients reflect changes relative to that constant;
denotes time-varying constructs in the model.
Figure 1.
Changes in Withdrawal and Craving among 6 Month Smokers and Abstainers
Note. FTND score, NMR, and patch adherence were controlled for in GEE analyses.
Likewise, controlling for covariates, the time x smoking status interaction for craving was significant (χ2 [2] = 30.8, p < .0001). As shown in Table 2, the main effects for time, for both baseline to week 8 craving and baseline to 6 months craving, were significant (β = −11.93, 95% CI: −15.06 to −8.79, p < .001; β = −11.57, 95% CI: −15.20 to −7.93, p < .001), as was the main effect of smoking status at 6 months (β = 5.45, 95% CI: 0.08 to 10.83, p = .05). Further, the time x smoking status interactions at week 8 and 6 months were significant (β = −11.42, 95% CI: −17.67 to −5.18, p < .001; β = −18.03, 95% CI: −24.54 to −11.52, p < .001). As shown in Figure 1, the reduction in craving over time was significantly greater for 6 month abstainers than for 6 month smokers, and the reduction in craving continued for abstinent participants from week 8 to 6 months but plateaued for smokers during this time.
3.3. Depressive and Anxiety Symptoms
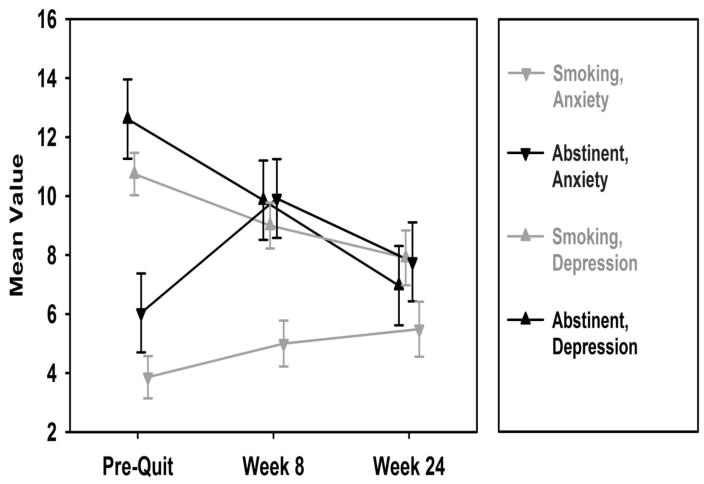
As shown in Table 2, depressive symptoms decreased significantly from baseline to week 8 (β = −1.74, 95% CI: −3.41 to −0.09, p = .04) and from baseline to 6 months (β = −2.84, 95% CI: −4.78 to −0.9, p = .004). However, the main effect for smoking status was not significant, the overall time x smoking status interaction was not significant (χ2 [2] = 2.58, p = .28), and the interaction effects for baseline to week 8 and baseline to 6 months were not significant (Table 2). As shown in Figure 2, depressive symptoms decreased over time similarly for 6 month smokers and abstainers. With regard to anxiety symptoms, there were no significant main effects (Table 2), the overall interaction effect (χ2 [2] = 3.16, p = .21) was not significant, and there were no individual significant interaction effects (Table 2).
Figure 2.
Changes in Depression and Anxiety Symptoms among 6 Month Smokers and Abstainers
Note. FTND score, NMR, and patch adherence were controlled for in GEE analyses.
3.4. Alternative Reinforcers
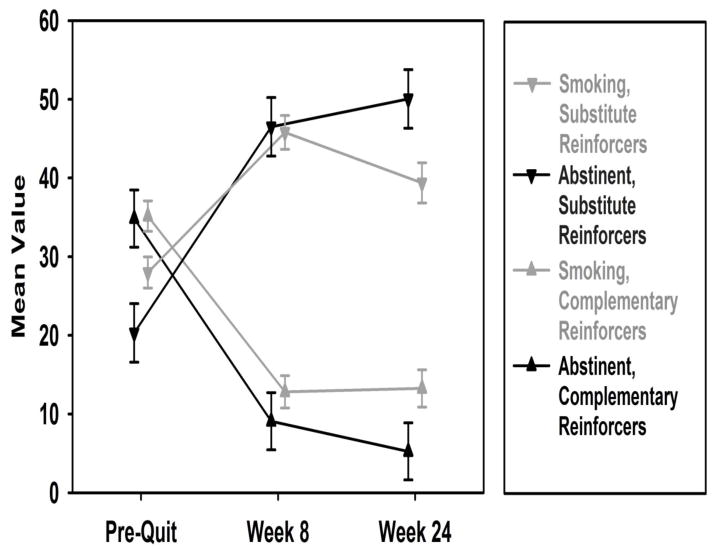
As shown in Table 2, for substitute reinforcers, there were significant main effects for time, for both baseline to week 8 (β = −17.81, 95% CI: 13.27 to 22.34, p < .001) and baseline to 6 months (β = 11.38, 95% CI: 6.10 to 16.66, p < .001). The main effect for smoking status at 6 months was not significant (β = −7.62, 95% CI: −16.02 to 0.78, p = .08). Assessment of the interaction effects showed that the level of substitute reinforcers from baseline to 6 months increased across time more for those demonstrating 6 month abstinence, vs. those who were smoking at 6 months (χ2[2] = 14.54, p < .0001; Figure 3). This effect was evident after week 8 (β = 18.29, 95% CI: 8.89 to 27.97, p < .001).
Figure 3.
Changes in Substitute and Complementary Reinforcers among 6 Month Smokers and Abstainers
Note. FTND score, NMR, and patch adherence were controlled for in GEE analyses.
As shown in Table 2, for complementary reinforcers, there were significant main effects for time, for both baseline to week 8 (β = −22.34, 95% CI: −26.07 to −18.61, p < .001) and baseline to 6 months (β = −22.00, 95% CI: −26.27 to −17.52, p < .001). The main effect for smoking status at 6 months was not significant (β = −0.31, 95% CI: −8.53 to 7.91, p = .94). Assessment of the interaction effects showed that the level of complementary reinforcers from baseline to 6 months decreased across time more for those demonstrating 6 month abstinence, vs. those who were smoking at 6 months, however the overall interaction was not significant (χ2[2] = 3.82, p = .15; Figure 3). As shown in Table 2, this was due to the non-significant interaction effect for baseline to week 8 (β = −3.41, 95% CI: −10.78 to 3.96, p = .36), compared to the interaction effect for baseline to 6 months (β = −7.70, 95% CI: −15.19 to 0.17, p = .05).
3.5. Models of Relapse
The regression models predicting relapse between week 8 and 6 months, controlling for covariates, showed that participants who showed a greater reduction in craving from baseline to week 8 had a significantly lower likelihood of relapsing to smoking between week 8 and 6 months (OR = 0.96, 95% CI: 0.93–0.99), p = .02). Changes in depressive and anxiety symptoms, withdrawal, and complementary and alternative reinforcers from baseline to week 8 were not predictive of relapse from week 8 to 6 months (p’s > .05).
4. DISCUSSION
Withdrawal, craving, and emotional distress are common barriers to successful smoking cessation. Yet, there are relatively few studies that have documented such relationships prospectively and with long-term cessation following treatment with transdermal nicotine. In addition, few studies have examined substitute and complementary reinforcers as predictors of success, especially long-term success. Thus, this study was designed to evaluate these factors as predictors of 6-month cessation outcomes following treatment with nicotine patch and behavioral counseling. Below, we discuss the main findings from this study.
Our first observation is that individuals who are successful at quitting smoking after 6-months from an initial quit attempt using the nicotine patch report greater reductions in withdrawal and craving, compared to individuals who were unsuccessful; a greater reduction in craving, in particular, during the first 8 weeks, significantly reduced the risk for subsequent relapse. These results conform to previous studies (al’Absi et al., 2007; Piasecki et al., 2000; Swan et al., 1996) and converge with our understanding of an important mechanism of effect for the nicotine patch (Henningfield et al., 2009). Further, these results extend our understanding of the important role played by the ability to manage craving and withdrawal by documenting these relationships out to six months following an initial target quit date. With few exceptions (e.g., Javitz et al., 2012), there are limited data illustrating the long-term prospective significance of managing craving and withdrawal symptoms for long-term abstinence following nicotine patch treatment. The present data show that, for abstainers, symptoms of withdrawal and craving substantially decline after 8 weeks, whereas, for smokers, these symptoms remain at a steady plateau.
Second, with regard to depression symptoms, we did not find that smokers and abstainers differed over time; both groups showed a significant reduction in symptoms from baseline to 6 months. This finding is inconsistent with previous analyses of this sample which showed that depressive symptoms were related to cessation outcome 8 weeks of treatment (Goelz et al., 2014). Perhaps depressive symptoms play a more prominent role during the initial weeks of a quit attempt, but are less predictive of long-term cessation outcomes. Further, while this finding is inconsistent with other past findings (e.g., Berlin and Covey, 2006; Catley et al., 2003), it does converge with more recent analyses showing that depressive symptoms do not always predict cessation outcomes in nicotine patch trials (Weinberger et al., 2013) and that anhedonia, rather than global depression symptomatology, may be a more consistent predictor of smoking cessation outcomes (Cook et al., 2015; Leventhal et al., 2014)2. Assessment of the link between depression and abstinence should consider more fine-grained definitions of depressive symptoms as outlined in recent theoretical models (e.g., Ameringer and Leventhal, 2010). Our findings concerning anxiety were not convergent with studies indicating the significance of such symptoms in determining smoking cessation outcomes (McDermott et al., 2013; Piper et al., 2011). The lack of effect may be related to the measure used since alternative approaches, which have focused less on anxiety symptomatology and more on anxiety vulnerability measures such as anxiety sensitivity, have been more consistently associated with smoking cessation outcomes (e.g., Zvolensky et al., 2009). As with depressive symptoms, recent conceptual frameworks of affective predictors of smoking behavior incorporate such vulnerability processes rather than current symptomatology, which may better account for these linkages (see Leventhal and Zvolensky, 2015).
Lastly, our results show the potential importance of substitute and complementary reinforcers as predictors of abstinence following nicotine patch treatment. Our earlier study showed that a reduction in complementary reinforcers and an increase in substitute reinforcers predicted short-term (8-week) abstinence (Goelz et al., 2014). Here, we extend that initial finding by showing that substitute environmental reinforcers are associated with long-term abstinence as well and provide support for the continuing investigation of constructs central to behavioral economics as predictors of smoking cessation outcomes. The application of behavioral economic theory to smoking cessation treatment has mostly involved the use of financial incentives in the workplace (e.g., Halpern et al., 2015). This approach may be limited by focusing on substitute reinforcement (i.e., money for abstinence) without adequate attention to therapeutic elements designed to reduce complementary reinforcers as well. Behavioral activation therapy and community reinforcement models (e.g., Higgins et al., 2003; Abbott, 2009; MacPherson et al., 2010) may represent more suitable approaches for addressing nicotine dependence.
The results of this study should be considered in the context of its limitations. First, while the prospective nature of the data is a strength, the results should not be interpreted with causal inferences. Variables associated with cessation were controlled for in the models, however the lack of randomization means that not all potential confounding factors were controlled. Second, the present findings are generalizable only to smokers seeking treatment within a formal program. These results may not be applicable to the general population of smokers who may be less motivated to quit smoking. Third, these results are limited to the context of smoking cessation treatment using the nicotine patch and behavioral counseling. It is unclear if the relationships found here would be replicated if other forms of treatment for nicotine dependence were used. Lastly, while the use of CO and the cut-off of 10ppm have been recommended to verify self-reported smoking abstinence, more recent studies have suggested using lower cut-offs (Cropsey et al., 2014). Similarly, breath CO may be effected by the device used to assess CO (Karelitz et al., in press) and by the speed of emptying the lungs (Raiff et al., 2010). Additional potential limitations include use of the time-line follow-back procedure (vs. contemporaneous assessment) and the coarse temporal sampling to assess change.
In summary, this study supports the use of existing or novel interventions that address nicotine withdrawal and craving. One clinical recommendation is dual use of nicotine replacement therapy, in particular the use of the nicotine lozenge or gum, with the patch, which can improve the suppression of craving, versus the nicotine patch alone (Bolt et al., 2012; Cahill et al., 2013). Likewise, these results lend support to the evaluation of clinical interventions directed towards enhancing engagement in substitute reinforcers in addition to reducing engagement in complimentary reinforcers. A preliminary study of behavioral activation therapy, which focused on reinforcers showed promise for smoking cessation (MacPherson et al., 2010) but larger trials are needed to evaluate this approach relative to standard behavioral interventions. Augmenting approaches to helping people quit smoking by using ancillary interventions that better suppress withdrawal and craving and help smokers address the need for alternative environmental reinforcers may improve the effectiveness of standard forms of nicotine dependence treatment such as the nicotine patch and counseling.
Highlights.
Lower craving and withdrawal and greater substitute reinforcers increases cessation
The results support the dual use of nicotine replacement therapy
The results support the testing of interventions to address alternative reinforcers
Acknowledgments
Role of Funding Source
This research was supported by grants from the National Institute on Drug Abuse (R01 DA025078 and R01 DA033681) and from the National Cancer Institute (R01 CA165001 and P50 CA143187).
Footnotes
Please see Schnoll et al. (2015) for the CONSORT diagram for this trial, which indicates that 69 participants were lost-to-follow-up.
To explore this issue with the present data, we used only the pleasure items from the PES and four items from the IDS (i.e., response of your mood to good events, general interest, capacity for pleasure, and interest in sex) as indicators of anhedonia in the GEE analyses. The results for the PES anhedonia items converged with the results for substitute reinforcers (i.e., a significant increase for abstainers) but the results for the IDS items were not statistically significant, as was found for the full IDS. Such analyses should be done with measures specifically designed to assess anhedonia.
Conflict of Interest
Dr. Schnoll and Dr. Hitsman receive medication and placebo free of charge from Pfizer and have provided consultation to Pfizer. Dr. Schnoll has also consulted with GlaxoSmithKline. These companies had no involvement in this study.
Contributors
Dr. Schnoll conceived of the project, ascertained the study funding, and led the University of Pennsylvania site. Dr. Hitsman assisted with preparing the grant application and led the Northwestern University site. Sonja Blazekovic oversaw the collection of data at the University of Pennsylvania and Anna Veluz-Wilkens oversaw the collection of data at Northwestern University. Frank Leone oversaw participant eligibility and side effect monitoring and E. Paul Wileyto oversaw data analysis. Janet Audrain-McGovern advised on the inclusion of additional study measures and helped prepare the manuscript. All authors reviewed the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott PJ. A review of the community reinforcement approach in the treatment of opioid dependence. J Psychoactive Drugs. 2009;41:379–385. doi: 10.1080/02791072.2009.10399776. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Carr SB, Bongard S. Anger and psychobiological changes during smoking abstinence and in response to acute stress: prediction of smoking relapse. Int J Psychophysiol. 2007;66:109–115. doi: 10.1016/j.ijpsycho.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameringer KJ, Leventhal AM. Applying the tripartite model of anxiety and depression to cigarette smoking: an integrative review. Nicotine Tob Res. 2010;12:1183–1194. doi: 10.1093/ntr/ntq174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Epstein LH, Rodgers K, Cuevas J, Wileyto EP. Young adult smoking: what factors differentiate ex-smokers, smoking cessation treatment seekers and nontreatment seekers? Addict Behav. 2009;34:1036–1041. doi: 10.1016/j.addbeh.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Rodgers K, Cuevas J. Declining alternative reinforcers link depression to young adult smoking. Addiction. 2011;106:178–187. doi: 10.1111/j.1360-0443.2010.03113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolt DM, Piper ME, Theobald WE, Baker TB. Why two smoking cessation agents work better than one: role of craving. J Consult Clin Psychol. 2012;80:54–65. doi: 10.1037/a0026366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R, Burgess E, Sales S, Whiteley J. Reliability and validity of a smoking timeline follow-back interview. Psychol Addict Behav. 1988;12:101–112. [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Berlin I, Covey LS. Pre-cessation depressive mood predicts failure to quit smoking: the role of coping and personality traits. Addiction. 2006;101:1814–1821. doi: 10.1111/j.1360-0443.2006.01616.x. [DOI] [PubMed] [Google Scholar]
- Cahill K, Moher M, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;2:CD003440. doi: 10.1002/14651858.CD009329.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catley D, Ahluwalia JS, Resnicow K, Nazir N. Depressive symptoms and smoking cessation among inner-city African Americans using the nicotine patch. Nicotine Tob Res. 2003;5:61–68. [PubMed] [Google Scholar]
- Cook JW, Piper ME, Leventhal AM, Schlam TR, Fiore MC, Baker TB. Anhedonia as a component of the tobacco withdrawal syndrome. J Abnorm Psychol. 2015;124:215–225. doi: 10.1037/abn0000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Cropsey KL, Trent LR, Clark CB, Stevens EN, Lahti AC, Hendricks PS. How low should you go? Determining the optimal cutoff for exhaled carbon monoxide to confirm smoking abstinence when using cotinine as reference. Nicotine Tob Res. 2014;16:1348–1355. doi: 10.1093/ntr/ntu085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Dorfman SF, Froelicher ES, Goldstein MG, Healton CG, Henderson PN, et al. PHS Guideline Update Liaisons, 2008 PHS Guideline Update Staff, 2008. Treating tobacco use and dependence: 2008 update US Public Health Service Clinical Practice Guideline executive summary. Respir Care. 2008;53:1217–1222. [PubMed] [Google Scholar]
- Green L, Freed DE. The substitutability of reinforcers. J Exp Anal Behav. 1993;60:141–158. doi: 10.1901/jeab.1993.60-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goelz PM, Audrain-McGovern JE, Hitsman B, Leone FT, Veluz-Wilkins A, Jepson C, Wileyto EP, D’Avanzo PA, Rivera J, Schnoll RA. The association between changes in alternative reinforcers and short-term smoking cessation. Drug Alcohol Depend. 2014;138:67–74. doi: 10.1016/j.drugalcdep.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, Shiffman S, Ferguson SG, Gritz ER. Tobacco dependence and withdrawal: science base, challenges and opportunities for pharmacotherapy. Pharmacol Ther. 2009;123:1–16. doi: 10.1016/j.pharmthera.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence - a Revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Higgins S, Sigmon S, Wong C, Heil SH, Badger GJ, Donham R, Dantona RL, Anthony S. Community reinforcement therapy for cocaine-dependent outpatients. Arch Gen Psychiatry. 2003;60:1043–1052. doi: 10.1001/archpsyc.60.9.1043. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Halpern SD, French B, Small DS, Saulsgiver K, Harhay MO, Audrain-McGovern J, Loewenstein G, Brennan TA, Asch DA, Volpp KG. Randomized trial of four financial-incentive programs for smoking cessation. N Engl J Med. 2015;372:2108–2117. doi: 10.1056/NEJMoa1414293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitz HS, Lerman C, Swan GE. Comparative dynamics of four smoking withdrawal symptom scales. Addiction. 2012;107:1501–1511. doi: 10.1111/j.1360-0443.2012.03838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karelitz JL, Michael VC, Perkins KA. Analysis of agreement between expired-air carbon monoxide monitors. J Smok, Cessat. doi: 10.1017/jsc.2015.18. in press. available on CJO2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasza KA, Cummings KM, Carpenter MJ, Cornelius ME, Hyland AJ, Fong GT. Use of stop-smoking medications in the United States before and after the introduction of varenicline. Addiction. 2015;110:346–355. doi: 10.1111/add.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann A, Hitsman B, Goelz PM, Veluz-Wilkins A, Blazekovic S, Powers L, Leone FT, Gariti P, Tyndale RF, Schnoll RA. Rate of nicotine metabolism and smoking cessation outcomes in a community-based sample of treatment-seeking smokers. Addict Behav. 2015;51:93–99. doi: 10.1016/j.addbeh.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenford SL, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Predicting relapse back to smoking: contrasting affective and physical models of dependence. J Consult Clin Psychol. 2002;70:216–227. [PubMed] [Google Scholar]
- Leventhal AM, Ramsey SE, Brown RA, LaChance HR, Kahler CW. Dimensions of depressive symptoms and smoking cessation. Nicotine Tob Res. 2008;10:507–517. doi: 10.1080/14622200801901971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Trujillo M, Ameringer KJ, Tidey JW, Sussman S, Kahler CW. Anhedonia and the relative reward value of drug and nondrug reinforcers in cigarette smokers. J Abnorm Psychol. 2014;123:375–386. doi: 10.1037/a0036384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Zvolensky MJ. Anxiety, depression, and cigarette smoking: a transdiagnostic vulnerability framework to understanding emotion-smoking comorbidity. Psychol Bull. 2015;141:176–212. doi: 10.1037/bul0000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson L, Tull MT, Matusiewicz AK, Rodman S, Strong DR, Kahler CW, Hopko DR, Zvolensky MJ, Brown RA, Lejeuz CW. Randomized controlled trial of behavioral activation smoking cessation treatment for smokers with elevated depressive symptoms. J Consult Clin Psychol. 2010;78:55–61. doi: 10.1037/a0017939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPhillamy DJ, Lewinsohn PM. The Pleasant Events Schedule - studies on reliability, validity, and scale intercorrelation. J Consult Clin Psychol. 1982;50:363–380. [Google Scholar]
- McDermott MS, Marteau TM, Hollands GJ, Hankins M, Aveyard P. Change in anxiety following successful and unsuccessful attempts at smoking cessation: cohort study. Br J Psychiatry. 2013;202:62–67. doi: 10.1192/bjp.bp.112.114389. [DOI] [PubMed] [Google Scholar]
- Niaura R, Britt DM, Shadel WG, Goldstein M, Abrams D, Brown R. Symptoms of depression and survival experience among three samples of smokers trying to quit. Psychol Addict Behav. 2001;15:13–17. doi: 10.1037/0893-164x.15.1.13. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Niaura R, Shadel WG, Abrams D, Goldstein M, Fiore MC, Baker TB. Smoking withdrawal dynamics in unaided quitters. J Abnorm Psychol. 2000;109:74–86. doi: 10.1037//0021-843x.109.1.74. [DOI] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Baker TB. Anxiety diagnoses in smokers seeking cessation treatment: relations with tobacco dependence, withdrawal, outcome and response to treatment. Addiction. 2011;106:418–427. doi: 10.1111/j.1360-0443.2010.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiff BR, Faix C, Turturici M, Dallery J. Breath carbon monoxide output is affected by speed of emptying the lungs: implications for laboratory and smoking cessation research. Nicotine Tob Res. 2010;12:834–838. doi: 10.1093/ntr/ntq090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Giles DE, Schlesser MA, Fulton CL, Weissenburger J, Burns C. The Inventory of Depressive Symptomatology (IDS) - preliminary findings. Psychopharmacol Bull. 1986;22:985–990. doi: 10.1016/0165-1781(86)90060-0. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Schnoll RA, Goelz PM, Veluz-Wilkins A, Blazekovic S, Powers L, Leone FT, Gariti P, Wileyto EP, Hitsman B. A randomized clinical trial of long-term nicotine replacement therapy in a community-based sample of smokers. JAMA Intern Med. 2015;175:504–511. doi: 10.1001/jamainternmed.2014.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Patterson F, Wileyto EP, Tyndale RF, Benowitz N, Lerman C. Nicotine metabolic rate predicts successful smoking cessation with transdermal nicotine: a validation study. Pharmacol Biochem Behav. 2009;92:6–11. doi: 10.1016/j.pbb.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Poole MM, Vansickel AR, Rush CR. Influence of escalating alternative reinforcer values on cigarette choice. Behav Proc. 2011;87:302–305. doi: 10.1016/j.beproc.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, Ward MM, Jack LM. Abstinence effects as predictors of 28-day relapse in smokers. Addict Behav. 1996;21:481–490. doi: 10.1016/0306-4603(95)00070-4. [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Mazure CM, Morlett A, McKee SA. Two decades of smoking cessation treatment research on smokers with depression: 1990–2010. Nicotine Tob Res. 2013;15:1014–1031. doi: 10.1093/ntr/nts213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Stewart SH, Vujanovic AA, Gavric D, Steeves D. Anxiety sensitivity and anxiety and depressive symptoms in the prediction of early smoking lapse and relapse during smoking cessation treatment. Nicotine Tob Res. 2009;11:323–331. doi: 10.1093/ntr/ntn037. [DOI] [PMC free article] [PubMed] [Google Scholar]