Abstract
Background
Current research in behavioral cardiology reveals a significant association between posttraumatic stress disorder (PTSD) and increased risk for cardiovascular disease and mortality; however, the underlying mechanisms remain poorly understood. We hypothesized that patients with PTSD would exhibit endothelial dysfunction, a potential mechanism involved in the development and progression of cardiovascular disease.
Methods and Results
A total of 214 outpatients treated at the San Francisco Veterans Affairs Medical Center underwent tests of endothelial function and evaluation for PTSD. Flow‐mediated vasodilation of the brachial artery was performed to assess endothelial function, and current PTSD status was defined by the PTSD Checklist, based on the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition), with a score ≥40. Multivariable linear regression models were used to estimate the association between PTSD status and endothelial function. Patients with PTSD (n=67) were more likely to be male (99% versus 91%, P=0.04) and to have depression (58% versus 8%, P<0.0001) and were less likely to be on an angiotensin‐converting enzyme inhibitor (17% versus 36%, P=0.007) or β‐blocker treatment (25% versus 41%, P=0.03). Univariate analysis demonstrated that patients with PTSD had significantly lower flow‐mediated vasodilation (5.8±3.4% versus 7.5±3.7%; P=0.003); furthermore, lower flow‐mediated vasodilation was associated with increasing age (P=0.008), decreasing estimated glomerular filtration rate (P=0.003), hypertension (P=0.002), aspirin (P=0.03), and β‐blocker treatments (P=0.01). In multivariable analysis, PTSD remained independently associated with lower flow‐mediated vasodilation (P=0.0005).
Conclusions
After adjusting for demographic, comorbidity, and treatment characteristics, PTSD remained associated with worse endothelial function in an outpatient population. Whether poor endothelial function contributes to the higher risk of cardiovascular disease in patients with PTSD deserves further study.
Keywords: endothelial dysfunction, endothelial function, mental disorder, posttraumatic stress disorder
Subject Categories: Endothelium/Vascular Type/Nitric Oxide, Mental Health, Cardiovascular Disease
Introduction
Allostasis is the process by which living organisms maintain biological homeostasis during a diverse array of challenges, including those of an environmental or physiological nature.1, 2 It is thought that cumulative effects of stress (repetitive or continuous), referred to as allostatic load, affect several physiological systems including the hypothalamic–pituitary–adrenal axis; the autonomic nervous system; and the cardiovascular, metabolic, and immune systems.1, 2 Because ≈7.7 million US persons suffer from posttraumatic stress disorder (PTSD) in a given year3 and the expected lifetime prevalence of PTSD is 8% in the general population,4 better understanding of the impact of PTSD on cardiovascular health has become critical.
In a large internationally representative epidemiologic research study,5 the effects of psychosocial factors on myocardial infarction were even larger than well‐established medical risk factors such as diabetes, hypertension, and obesity. Multiple prospective cohort studies have estimated the association of PTSD with incident cardiovascular events and/or cardiovascular death with hazard ratios ranging from 1.46 to 3.28 compared with those of patients without PTSD (reviewed by Edmondson and Cohen6). Despite the disproportionate association of PTSD and cardiovascular disease (CVD), the underlying mechanisms remain poorly understood. A possible explanation is that patients with PTSD have higher rates of traditional CVD risk factors, including hypertension, dyslipidemia, diabetes, obesity, and tobacco use,7 but neither these risk factors nor depression fully account for the increased rate of CVD in the PTSD population.6
Nitric oxide is known to be a key mediator of endothelial function,8 and a recent study reports that biomarkers of nitric oxide synthetic capacity are significantly impaired in veterans with PTSD compared with those without PTSD.9 Acute stress has been shown to increase circulating markers of endothelial dysfunction and to impair vasoreactivity, as assessed by endothelium‐dependent flow‐mediated vasodilation (FMD).10, 11, 12 We hypothesized that chronic stress, as experienced by veterans with PTSD, leads to less vasoreactivity (ie, worse endothelial function) than that of an age‐matched control veteran population without PTSD.
Methods
Study Participants
Between June 2011 and August 2015, 214 patients were recruited from the San Francisco Veterans Affairs Medical Center (VAMC) for evaluation of their vascular function and for the presence of PTSD (cross‐sectional design), including 136 veterans who were referred to the surgery clinic and 78 veterans who were receiving care in the medical clinic. The investigator‐initiated protocol was approved by the University of California, San Francisco (UCSF), Committee on Human Research, and all patients gave informed consent.
PTSD and Comorbid Psychiatric Diagnosis
The research team administered the PTSD Checklist (PCL), a self‐report measure composed of 17 five‐point Likert scales that provide a total score for PTSD (range 17–85 points), according to the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition).13 Only patients with completed PCL testing were included. A score of ≥40 was used to define the presence of PTSD, as suggested by the VAMC National Center for PTSD.14 In a sample of male veterans in PTSD treatment, the correlation of the PCL with the Clinician‐Administered PTSD Scale is reported to be 0.79.15 To assess comorbid psychiatric diagnosis, the Patient Health Questionnaire was also used to evaluate depressive symptoms (score of ≥10). This self‐report instrument measures the frequency of depressive symptoms corresponding to the 9‐symptom criteria in the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition).16
Vascular Reactivity of Brachial Arteries
Endothelial function was measured using brachial artery endothelium‐dependent FMD in the Vascular Integrated Physiology and Experimental Therapeutics (VIPERx) Laboratory at UCSF, according to current guidelines and standards previously described by our group.17, 18, 19, 20, 21, 22 Under fasting conditions, including abstention from nicotine and caffeine, participants acclimated in the laboratory for 10 minutes in a supine position in a temperature‐controlled, low‐lit room. A 5‐cm tourniquet blood pressure cuff was placed on the upper arm distal to the insertion of the deltoid. Although there is no consensus on the location of the cuff (upper arm versus lower arm), our laboratory has adopted the upper arm technique, which is supported by current guidelines.23 The patient's blood pressure was recorded in the contralateral arm. The brachial artery was surveyed by B‐mode ultrasound (Philips HD11; Philips) using a broadband linear array transducer with a range of 3 to 12 MHz (Philips L12‐3; Philips) to identify a segment suitable for imaging. Criteria for a suitable segment include a straight arterial segment with adequate visualization of a “double line sign” on the near‐ and far‐wall intima–media lines and an accompanying registration structure such as a crossing vein. The baseline diameter of the vessel was recorded using EKG‐gated image‐capture software (Brachial Imager; Medical Imaging Applications LLC), and baseline Doppler spectral waveform was recorded using an insonation angle of 60°. Mean diameter and velocity at baseline were calculated from measurements collected for 60 seconds.
The blood pressure cuff was then inflated to 250 or 50 mm Hg above the participant's systolic blood pressure, whichever first induced cessation of brachial artery blood flow by sonography, for a period of 5 minutes. Blood flow cessation was confirmed by sonographic monitoring of the brachial artery during cuff inflation. Following cuff deflation, hyperemia‐stimulated brachial artery luminal diameter and Doppler spectral waveforms were again recorded for a total duration of 3 minutes after cuff release. Vasorelaxation determined by this method has been shown to be mediated predominantly by nitric oxide with little influence from other endothelium‐derived relaxing factors.
Postacquisition image analysis and calculation of hemodynamic parameters was performed using continuous edge‐detection software (Brachial Analyzer; Medical Imaging Applications LLC). The lumen diameter was measured from the near‐wall lumen–intima interface to the far‐wall lumen–intima interface. The velocity–time integral was calculated by the peak velocity method of integrating the spectral waveform over the first 4 cardiac cycles after cuff release. Blood flow was calculated as the product of blood vessel cross‐sectional area and velocity–time integral modeling the artery as a circle. Mean shear stress was calculated by the Hagen‐Poiseuille equation, in which Tw is shear stress in dynes/cm2 and mean volumetric flow is Q. The viscosity of blood (μ) is assumed to be 0.0035 poise, and lumen radius (r) is in centimeters:
| (1) |
Corresponding hyperemia parameters were calculated over a predetermined time window of 55 to 65 seconds after cuff release. FMD% was calculated as ([60‐second Hyperemia diameter?mean baseline diameter/mean baseline diameter]×100).
Quality control was assessed at all points in data acquisition. Image quality was evaluated by a second person and graded on a 6‐point scale that included registration structure (landmark), horizontally directed artery, correct longitudinal alignment, clearly visualized near‐ and far‐wall intima–media thickness, and at least 5 mm of clearly visualized artery. The interobserver variability in our laboratory is 0.05±0.16%, and the intraobserver variability is 0±0.15%.
Other Patient Characteristics
Demographic and anthropometric data were collected at the time of FMD testing to better characterize research participants and included age, race, sex, hip and waist circumference, and body mass index. Blood pressure was measured using standard sphygmomanometry. We collected from the participants a history of CVD, such as coronary artery disease, cerebrovascular disease, and previous lower extremity vascular disease, as well as risk factors including hypertension, diabetes mellitus types 1 and 2, hyperlipidemia, and history of current or past tobacco use. History of smoking was defined as current or former smoker. Concurrent medications and pertinent cardiovascular examination findings were also recorded.
Lipid, Metabolic, and Inflammatory Measurements
Blood samples were collected in a fasting state for measurement of albumin, total cholesterol, triglycerides, low‐density lipoprotein, high‐density lipoprotein, high‐sensitivity C‐reactive protein, and hemoglobin A1c by the VAMC clinical laboratory per standard methodology (Beckman Coulter analyzer). The coefficient of variation for high‐sensitivity C‐reactive protein using this procedure was 5.1%. Estimated glomerular filtration rate was calculated using the abbreviated Modification of Diet in Renal Disease formula based on age, sex, race, and serum creatinine level.24
Statistical Analysis
Characteristics of patients with and without PTSD were compared, and statistically significant associations were identified with t tests for continuous variables and with chi‐square tests for categorical variables. We used maximum likelihood regression models to estimate mean (95% CI) values of FMD by levels of patient characteristics, singly (univariate) and together (multivariable), and to test the significance of these associations using likelihood ratio tests. Multivariable adjustment was made for demographic characteristics and traditional cardiovascular risk factors with P<0.05 in univariate models; however, depression status was omitted from the multivariable model of FMD because it is very strongly associated with PTSD status, and our goal was to study association of FMD with PTSD.
In addition to analyzing associations with PTSD status, if PCL scores ≥40 defined the presence of PTSD, we used linear regression models to estimate associations of patient characteristics and mean brachial artery FMD with PCL quartiles and to test for statistically significant associations using 1‐ and 3‐df likelihood ratio statistics, respectively. An illustrative plot of predicted FMD by PCL quartile, against a background of observed FMD values by logarithm base‐10 PCL scores, was created using SAS transreg (SAS Institute). Statistical analyses were performed using Stata/SE 12 (StataCorp) and SAS version 9.4.
Results
Among the 214 participants in this study, the range of PCL scores was 17 to 85, and 64 (31%) were found to have PTSD, as defined by a score ≥40 (Table 1). Patients with PTSD were more likely to be male (99% versus 91%, P=0.04) and to have depression (56% versus 8%, P<0.0001) but were less likely to be on an angiotensin‐converting enzyme inhibitor (17% versus 36%, P=0.007) or β‐blocker treatment (25% versus 41%, P=0.03). There was no difference in the proportion of patients who were active smokers between the 2 groups (PTSD 27% versus non‐PTSD 25%, P=0.81). Quartiles of PCL scores showed that the majority of the sample was at lower risk levels (17–20, 21–30, 31–46, 47–85). When patient characteristics were examined by PCL quartile (Table S1), the trends were similar to those observed by PTSD status.
Table 1.
Characteristics of Patients With and Without PTSDa
| No PTSD (147) | PTSD (n=67) | P Value | |
|---|---|---|---|
| Demographics | |||
| Age, y | 69±9 | 68±6 | 0.39 |
| Male sex | 91% | 99% | 0.04 |
| White | 74% | 81% | 0.31 |
| Comorbidities and risk factors | |||
| History of smoking | 83% | 78% | 0.36 |
| Hypertension | 73% | 84% | 0.11 |
| Hyperlipidemia | 71% | 79% | 0.22 |
| Diabetes mellitus | 27% | 27% | 0.93 |
| Coronary artery disease | 26% | 30% | 0.49 |
| Depression (PHQ‐9 score ≥10) | 8% | 58% | <0.0001 |
| Systolic blood pressure, mm Hg | 138±18 | 143±21 | 0.08 |
| Diastolic blood pressure, mm Hg | 79±10 | 81±11 | 0.26 |
| Medications | |||
| Aspirin | 59% | 71% | 0.15 |
| ACEI | 36% | 17% | 0.007 |
| β‐Blocker | 41% | 25% | 0.03 |
| Statin | 75% | 74% | 0.90 |
| Laboratory studies | |||
| Total cholesterol, mg/dL | 169±40 | 164±44 | 0.44 |
| LDL, mg/dL | 92±34 | 89±35 | 0.59 |
| HDL, mg/dL | 49±14 | 49±13 | 0.91 |
| Triglycerides, mg/dL | 137±87 | 127±72 | 0.40 |
| CRP, mg/L | 4.3±5.1 | 3.7±3.6 | 0.40 |
| eGFR, mL/min | 77±22 | 81±19 | 0.19 |
| HgA1c (%) | 5.9±1.0 | 6.0±1.0 | 0.78 |
ACEI indicates angiotensin‐converting enzyme inhibitor; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; HgA1c, hemoglobin A1c; LDL, low‐density lipoprotein; PHQ‐9, Patient Health Questionnaire; PTSD, posttraumatic stress disorder.
Continuous characteristics are summarized by mean±SD and categorical characteristics as percentage of PTSD level having the characteristic.
Among 209 participants with nonmissing outcomes, the overall brachial artery FMD was 6.9±3.7%. Patients with PTSD had worse endothelial function, as measured by significantly lower brachial artery FMD (5.8±3.4% versus 7.5±3.7%; P=0.003) (Table 2).
Table 2.
Brachial Artery Flow‐Mediated Vasodilation in Patients Without and With PTSD
| Measurea | No PTSD (147) | PTSD (n=67) | P Valueb |
|---|---|---|---|
| Baseline diameter, cm | 0.38±0.07 | 0.41±0.05 | 0.01 |
| Reactive hyperemia diameter, cm | 0.41±0.07 | 0.43±0.05 | — |
| Change from baseline with RH | 0.03±0.01 | 0.02±0.01 | — |
| % Change from baseline (brachial FMD) | 7.5±3.7 | 5.8±3.4 | 0.003 |
| Baseline velocity, m/s | 0.16±0.07 | 0.15±0.06 | 0.54 |
| Reactive hyperemia velocity, m/s | 0.78±0.28 | 0.82±0.25 | — |
| Change from baseline with RH, m/s | 0.62±0.28 | 0.66±0.26 | 0.25 |
| Baseline flow, mL/min | 113±64 | 121±54 | 0.36 |
| Reactive hyperemia flow, mL/min | 651±315 | 726±248 | — |
| Change from baseline with RH, mL/min | 537±287 | 604±241 | 0.10 |
| Baseline shear stress, dynes/cm2 | 12±7 | 11±5 | 0.14 |
| Reactive hyperemia shear stress, dynes/cm2 | 54±21 | 54±19 | — |
| Change from baseline with RH, dynes/cm2 | 41±20 | 43±19 | 0.57 |
FMD indicates flow‐mediated vasodilation; PTSD, posttraumatic stress disorder; RH, reactive hyperemia.
Continuous characteristics are summarized by mean±SD.
Student t test. To avoid inflating the type 1 error rate (false‐positive claims), statistical tests examined 1 measure each of variation at start and at end of vasodilation challenge.
We used a regression model to examine linearity of FMD as a function of logarithm base‐10 PCL scores, stratified by PCL quartile (Figure). This model estimated that FMD declines nonlinearly with logarithm base‐10 PCL scores, with mean differences between quartiles of −1.8%, −1.0%, and −0.45%, respectively (3‐df likelihood ratio test P=0.060) (Figure and Table S2).
Figure 1.
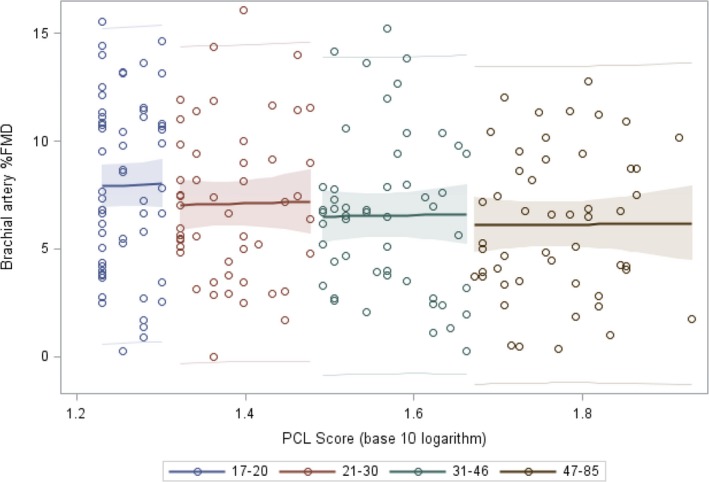
Mean (with 95% CI and prediction limits) brachial artery FMD per sample quartile of PCL score suggests that greater worsening of endothelial function occurs at earlier stages of PTSD. FMD indicates flow‐mediated vasodilation; PCL, PTSD Checklist; PTSD, posttraumatic stress disorder.
Univariate analysis demonstrated that lower FMD also was significantly associated with a diagnosis of PTSD (P=0.003), increasing age (P=0.008), decreasing estimated glomerular filtration rate (P=0.003), hypertension (P=0.002), aspirin use (P=0.03), and β‐blocker treatment (P=0.01). In multivariate analysis (Table 3), PTSD remained independently associated with FMD (P=0.0005). In an adjusted model, this corresponded to an FMD of 4.9% (95% CI 3.7–6.0%) in patients with PTSD compared with 7.3% (95% CI 6.7–8.0%) in those without PTSD.
Table 3.
Univariate and Multivariate Associations With Endothelial Function Measured by Brachial Artery Flow‐Mediated Vasodilation
| Patient Characteristic | Univariate Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| Mean (95% CI) Coefficienta | P Value | Mean (95% CI) Coefficienta | P Value | |
| PTSD | −1.7 (−2.7 to −0.6) | 0.002 | −2.5 (−3.8 to −1.1) | 0.0005 |
| Sex (male) | 0.03 (−1.98 to 2.04) | 0.97 | 2.0 (−1.4 to 5.5) | 0.25 |
| Age | −0.09 (−0.15 to −0.02) | 0.008 | −0.09 (−0.17 to −0.002) | 0.06 |
| Race (white) | 0.02 (−1.16 to 1.21) | 0.97 | 0.57 (−0.92 to 2.03) | 0.46 |
| eGFR | 0.04 (0.01–0.06) | 0.003 | 0.03 (0.001–0.06) | 0.04 |
| Hypertension | −1.8 (−3.0 to −0.68) | 0.002 | −0.5 (−2.2 to 1.3) | 0.59 |
| ASA | −1.3 (−2.5 to −0.15) | 0.03 | −0.6 (−2.2 to 0.65) | 0.35 |
| β‐Blockers | −1.3 (−2.3 to −0.3) | 0.01 | −1.1 (−2.3 to 0.16) | 0.09 |
| ACEI | −0.7 (−1.8 to 0.45) | 0.24 | −0.7 (−1.9 to 0.6) | 0.31 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ASA, acetylsalicylic acid; eGFR, estimated glomerular filtration rate; FMD, flow‐mediated dilation; PTSD, posttraumatic stress disorder.
A regression model coefficient estimates the mean (95% CI) change in FMD per unit change in a patient characteristic; for example, the univariate analysis estimated a mean change of −1.7 as PTSD status changed from 0 to 1 (Table 3), which is consistent with the FMD means of 7.5 and 5.8 for PTSD absence and presence, respectively, reported in Table 2.
FMD was also significantly associated with the presence of depressive symptoms, which are frequently comorbid with PTSD (coefficient −1.3; 95% CI −2.5 to −0.12; P=0.03). After additional adjustment for depression in the full model presented in Table 3, the relationship between FMD and PTSD remained significant (coefficient −1.8; 95% CI −3.4 to −0.2; P=0.03).
Discussion
In a cohort of outpatients treated at the San Francisco VAMC, PTSD was associated with worse endothelial function, as measured by FMD. This association remained significant after adjusting for other potential cofounders. To our knowledge, these data represent the largest cohort in which endothelial function with FMD was measured in patients with PTSD. These data provide evidence of a clear association between PTSD and vascular function among veterans and illustrate the need to empirically determine the optimal multidisciplinary strategies to treat patients with comorbid PTSD and CVD risk.
Patients with PTSD have higher rates of traditional biological and behavioral CVD risk factors, including hypertension, dyslipidemia, diabetes, obesity, and tobacco use.7 Nevertheless, in our work and that of others, the association of PTSD and CVD has not been fully explained by these risk factors or by depression, suggesting that the pathway is not merely related to poor health behaviors or traditional CVD risk factors.6, 25, 26, 27 Rather, a direct relationship between PTSD and vascular function appears to be involved. In fact, our study demonstrated an association between PTSD and endothelial dysfunction measured by brachial artery FMD after adjustment for age, hypertension, and renal function. Our work expands on prior examinations of PTSD and vascular function.10, 11, 12, 28 To date, only 1 study in the literature focused on PTSD and vascular function measured with FMD. This study, conducted in a cohort of 100 police officers, found that higher levels of PTSD symptoms were associated with a nearly 2‐fold decrease in FMD after adjustment for demographics and health behaviors.29 Consequently, our current data represent the largest study reporting the association between PTSD and endothelial dysfunction using brachial artery FMD.
Our study demonstrated a 2.4% adjusted difference in FMD between patients with and without PTSD. This difference has significant predictive potential for the risk of cardiovascular events. In fact, in recent meta‐analysis, it has been estimated that a 1% decrease in FMD is predictive of a 10% absolute increase in future cardiovascular events and mortality.30, 31 It is currently unknown whether treatment of PTSD would improve vascular function or future cardiovascular risk.
Based on current data, damage to the endothelial lining of blood vessels appears to be worsened by mental or psychological stress. This supports the notion that repeated provocation of endothelial stress reactivity, as may occur with persistent PTSD, may have an atherogenic effect on the endothelium over time. Mechanisms may include impairments of nitric oxide pathways via stress‐arousal mediators,9, 32 an increase in inflammation10, 33 (although not present in this study), and a perturbation in major stress systems such as the hypothalamic–pituitary–adrenal axis and the autonomic nervous system.2 Patients with PTSD have lower peripheral cortisol levels,34, 35 which could contribute to a proinflammatory milieu and an inflammatory activation of endothelial cells.36, 37, 38 It is also possible that psychological stress leads to endothelial dysfunction through an increase in oxidative stress39 or through the release of potent vasoconstrictors including endothelin40 and angiotensin II.41
Limitations
Although this work represents the largest study to date on the association between PTSD and endothelial function, this study was observational and used a cross‐sectional design. The patient population studied included predominantly male white veterans from the San Francisco VAMC. It is also important to note that depression has been associated with worse FMD.42 In our study, a percentage of the patients met criteria for both PTSD and depression (based on scores on the Patient Health Questionnaire). Depressive symptoms in some cases can reflect the overlap of the diagnostic criteria with PTSD (anhedonia, cognitive decrements, sleep disturbance, guilt). Furthermore, some argue that unless major depressive disorder was established prior to PTSD, the co‐occurrence may represents a trauma‐related phenomenon that is distinct from major depressive disorder.43 In the current study, the timing of occurrence of diagnosis was not established. Furthermore, in the majority of patients, use of antidepressants or antianxiety drugs was not recorded. This represents another limitation of the study. Although participants were recruited through both the surgery and medical clinics, all vascular testing was conducted by the same laboratory (VIPERx), and PTSD was measured in the same fashion. Last, it should be noted that the baseline diameter was different between the groups and possibly could contribute to the difference seen in FMD. This is however less likely since the FMD equation adjusts for baseline diameter as demonstrated in the methods section.
Conclusions
In summary, our results support the hypothesis that patients with PTSD have worse endothelial function, as demonstrated by lower brachial artery FMD. Prospective studies are needed to establish whether PTSD contributes to the increased risk of cardiovascular events via endothelial dysfunction. Furthermore, future research is needed to understand whether treatment of PTSD could improve measures of endothelial function and decrease the risk of CVD or CVD events.
Sources of Funding
This work was supported by startup funds from the University of California San Francisco and the Northern California Institute for Research and Education (Dr Grenon), by Award Number KL2RR024130 from the National Center for Research Resources, Award Number 1K23HL122446‐01 from the National Institute of Health/NHLBI, and a Society for Vascular Surgery Seed Grant and Career Development Award (Dr Grenon). This project was also supported by K23 HL 094765‐01 from the National Heart Lung and Blood Institute (Dr Cohen), the Department of Defense, the American Heart Association, the Brain and Behavior Research Foundation, the Northern California Institute for Research and Education, the University of California, San Francisco, and the Irene Perstein Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The funding organizations were not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Disclosures
None.
Supporting information
Table S1. Characteristics of Patients by Quartiles of the PTSD Checklist
Table S2. Brachial Artery Flow‐Mediated Vasodilation by Sample Quartiles of the PTSD Checklist Score
(J Am Heart Assoc. 2016;5:e003010 doi: 10.1161/JAHA.115.003010)
Accompanying Tables S1 and S2 are available at http://jaha.ahajournals.org/content/5/3/e003010/suppl/DC1
References
- 1. McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. [DOI] [PubMed] [Google Scholar]
- 2. McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. [DOI] [PubMed] [Google Scholar]
- 3. NIH Fact Sheet for PTSD. Available at: http://report.nih.gov/nihfactsheets/ViewFactSheet.aspx?csid=58. Accessed 12/3/15.
- 4. Manguno‐Mire G, Laurel Franklin C. Post‐traumatic stress disorder In: Carlstedt RA, ed. Handbook of integrative Clinical Psychology, Psychiatry, and Behavioral Medicine: Perspectives, Practices, and Research. New York, NY: Springer; 2009:358–378. [Google Scholar]
- 5. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case‐control study. Lancet. 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 6. Edmondson D, Cohen BE. Posttraumatic stress disorder and cardiovascular disease. Prog Cardiovasc Dis. 2013;55:548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohen BE, Marmar C, Ren L, Bertenthal D, Seal KH. Association of cardiovascular risk factors with mental health diagnoses in Iraq and Afghanistan war veterans using VA health care. JAMA. 2009;302:489–492. [DOI] [PubMed] [Google Scholar]
- 8. Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Luscher TF. Nitric oxide is responsible for flow‐dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–1319. [DOI] [PubMed] [Google Scholar]
- 9. Bersani FS, Wolkowitz OM, Lindqvist D, Yehuda R, Flory J, Bierer LM, Makotine I, Abu‐Amara D, Coy M, Reus VI, Epel ES, Marmar C, Mellon SH. Global arginine bioavailability, a marker of nitric oxide synthetic capacity, is decreased in PTSD and correlated with symptom severity and markers of inflammation. Brain Behav Immun. 2016;52:153–160. [DOI] [PubMed] [Google Scholar]
- 10. Heinz A, Hermann D, Smolka MN, Rieks M, Graf KJ, Pohlau D, Kuhn W, Bauer M. Effects of acute psychological stress on adhesion molecules, interleukins and sex hormones: implications for coronary heart disease. Psychopharmacology. 2003;165:111–117. [DOI] [PubMed] [Google Scholar]
- 11. Spieker LE, Hurlimann D, Ruschitzka F, Corti R, Enseleit F, Shaw S, Hayoz D, Deanfield JE, Luscher TF, Noll G. Mental stress induces prolonged endothelial dysfunction via endothelin‐A receptors. Circulation. 2002;105:2817–2820. [DOI] [PubMed] [Google Scholar]
- 12. Ghiadoni L, Donald AE, Cropley M, Mullen MJ, Oakley G, Taylor M, O'Connor G, Betteridge J, Klein N, Steptoe A, Deanfield JE. Mental stress induces transient endothelial dysfunction in humans. Circulation. 2000;102:2473–2478. [DOI] [PubMed] [Google Scholar]
- 13. Blanchard EB, Jones‐Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD checklist (PCL). Behav Res Ther. 1996;34:669–673. [DOI] [PubMed] [Google Scholar]
- 14. Using the PTSD Checklist for DSM‐IV (PCL). Available at: http://www.ptsd.va.gov/professional/pages/assessments/assessment-pdf/PCL-handout.pdf. Accessed 12/3/15.
- 15. Keen SM, Kutter CJ, Niles BL, Krinsley KE. Psychometric properties of PTSD Checklist in sample of male veterans. J Rehabil Res Dev. 2008;45:465–474. [DOI] [PubMed] [Google Scholar]
- 16. Kroenke K, Spitzer RL, Williams JB. The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow‐mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300:H2–H12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grenon SM, Conte MS, Nosova E, Alley H, Chong K, Harris WS, Vittinghoff E, Owens CD. Association between n‐3 polyunsaturated fatty acid content of red blood cells and inflammatory biomarkers in patients with peripheral artery disease. J Vasc Surg. 2013;58:1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grenon SM, Owens CD, Alley H, Chong K, Yen PK, Harris W, Hughes‐Fulford M, Conte MS. n‐3 Polyunsaturated fatty acids supplementation in peripheral artery disease: the OMEGA‐PAD trial. Vasc Med. 2013;18:263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grenon SM, Chong K, Alley H, Nosova E, Gasper W, Hiramoto J, Boscardin WJ, Owens CD. Walking disability in patients with peripheral artery disease is associated with arterial endothelial function. J Vasc Surg. 2014;59:1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nosova EV, Yen P, Chong KC, Alley HF, Stock EO, Quinn A, Hellmann J, Conte MS, Owens CD, Spite M, Grenon SM. Short‐term physical inactivity impairs vascular function. J Surg Res. 2014;190:672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alley H, Owens CD, Gasper WJ, Grenon SM. Ultrasound assessment of endothelial‐dependent flow‐mediated vasodilation of the brachial artery in clinical research. J Vis Exp. 2014;e52070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard‐Herman M, Herrington D, Vallance P, Vita J, Vogel R; International Brachial Artery Reactivity Task F . Guidelines for the ultrasound assessment of endothelial‐dependent flow‐mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. [DOI] [PubMed] [Google Scholar]
- 24. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 25. Kubzansky LD, Koenen KC. Is posttraumatic stress disorder related to development of heart disease? An update Cleve Clin J Med. 2009;76(suppl 2):S60–S65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vaccarino V, Goldberg J, Rooks C, Shah AJ, Veledar E, Faber TL, Votaw JR, Forsberg CW, Bremner JD. Posttraumatic stress disorder and incidence of coronary heart disease: a Twin Study. J Am Coll Cardiol. 2013;62:970–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ahmadi N, Hajsadeghi F, Mirshkarlo HB, Budoff M, Yehuda R, Ebrahimi R. Post‐traumatic stress disorder, coronary atherosclerosis, and mortality. Am J Cardiol. 2011;108:29–33. [DOI] [PubMed] [Google Scholar]
- 28. von Kanel R, Hepp U, Traber R, Kraemer B, Mica L, Keel M, Mausbach BT, Schnyder U. Measures of endothelial dysfunction in plasma of patients with posttraumatic stress disorder. Psychiatry Res. 2008;158:363–373. [DOI] [PubMed] [Google Scholar]
- 29. Violanti JM, Andrew ME, Burchfiel CM, Dorn J, Hartley T, Miller DB. Posttraumatic stress symptoms and subclinical cardiovascular disease in police officers. Int J Stress Manag. 2006;13:541–554. [Google Scholar]
- 30. Ras RT, Streppel MT, Draijer R, Zock PL. Flow‐mediated dilation and cardiovascular risk prediction: a systematic review with meta‐analysis. Int J Cardiol. 2013;168:344–351. [DOI] [PubMed] [Google Scholar]
- 31. Xu Y, Arora RC, Hiebert BM, Lerner B, Szwajcer A, McDonald K, Rigatto C, Komenda P, Sood MM, Tangri N. Non‐invasive endothelial function testing and the risk of adverse outcomes: a systematic review and meta‐analysis. Eur Heart J Cardiovasc Imaging. 2014;15:736–746. [DOI] [PubMed] [Google Scholar]
- 32. Liu Y, Mladinov D, Pietrusz JL, Usa K, Liang M. Glucocorticoid response elements and 11 beta‐hydroxysteroid dehydrogenases in the regulation of endothelial nitric oxide synthase expression. Cardiovasc Res. 2009;81:140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dugue B, Leppanen E, Grasbeck R. Preanalytical factors (biological variation) and the measurement of serum soluble intercellular adhesion molecule‐1 in humans: influence of the time of day, food intake, and physical and psychological stress. Clin Chem. 1999;45:1543–1547. [PubMed] [Google Scholar]
- 34. Boscarino JA. Posttraumatic stress disorder, exposure to combat, and lower plasma cortisol among Vietnam veterans: findings and clinical implications. J Consult Clin Psychol. 1996;64:191–201. [DOI] [PubMed] [Google Scholar]
- 35. Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress‐related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. [DOI] [PubMed] [Google Scholar]
- 36. Maes M, Lin AH, Delmeire L, Van Gastel A, Kenis G, De Jongh R, Bosmans E. Elevated serum interleukin‐6 (IL‐6) and IL‐6 receptor concentrations in posttraumatic stress disorder following accidental man‐made traumatic events. Biol Psychiatry. 1999;45:833–839. [DOI] [PubMed] [Google Scholar]
- 37. Szotowski B, Antoniak S, Poller W, Schultheiss HP, Rauch U. Procoagulant soluble tissue factor is released from endothelial cells in response to inflammatory cytokines. Circ Res. 2005;96:1233–1239. [DOI] [PubMed] [Google Scholar]
- 38. von Kanel R, Hepp U, Kraemer B, Traber R, Keel M, Mica L, Schnyder U. Evidence for low‐grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res. 2007;41:744–752. [DOI] [PubMed] [Google Scholar]
- 39. Aschbacher K, O'Donovan A, Wolkowitz OM, Dhabhar FS, Su Y, Epel E. Good stress, bad stress and oxidative stress: insights from anticipatory cortisol reactivity. Psychoneuroendocrinology. 2013;38:1698–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Noll G, Wenzel RR, Schneider M, Oesch V, Binggeli C, Shaw S, Weidmann P, Luscher TF. Increased activation of sympathetic nervous system and endothelin by mental stress in normotensive offspring of hypertensive parents. Circulation. 1996;93:866–869. [DOI] [PubMed] [Google Scholar]
- 41. Kosunen KJ. Plasma renin activity, angiotensin II, and aldosterone after mental arithmetic. Scand J Clin Lab Invest. 1977;37:425–429. [PubMed] [Google Scholar]
- 42. Chen H, Zhang L, Zhang M, Song X, Zhang H, Liu Y, Lv S. Relationship of depression, stress and endothelial function in stable angina patients. Physiol Behav. 2013;118:152–158. [DOI] [PubMed] [Google Scholar]
- 43. Flory JD, Yehuda R. Comorbidity between post‐traumatic stress disorder and major depressive disorder: alternative explanations and treatment considerations. Dialogues Clin Neurosci. 2015;17:141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of Patients by Quartiles of the PTSD Checklist
Table S2. Brachial Artery Flow‐Mediated Vasodilation by Sample Quartiles of the PTSD Checklist Score


