Abstract
Acute kidney injury (AKI) is increasingly recognized as a common problem in children undergoing cardiac surgery, with well documented increases in morbidity and mortality in both the short and the long term. Traditional approaches to the identification of AKI such as changes in serum creatinine have revealed a large incidence in this population with significant negative impact on clinical outcomes. However, the traditional diagnostic approaches to AKI diagnosis have inherent limitations that may lead to under-diagnosis of this pathologic process. There is a dearth of randomized controlled trials for the prevention and treatment of AKI associated with cardiac surgery, at least in part due to the paucity of early predictive biomarkers. Novel non-invasive biomarkers have ushered in a new era that allows for earlier detection of AKI. With these new diagnostic tools, a more consistent approach can be employed across centers that may facilitate a more accurate representation of the actual prevalence of AKI and more importantly, clinical investigation that may minimize the occurrence of AKI following pediatric cardiac surgery. A thoughtful management approach is necessary to mitigate the effects of AKI after cardiac surgery, which is best accomplished in close collaboration with pediatric nephrologists. Long-term surveillance for improvement in kidney function and potential development of chronic kidney disease should also be a part of the comprehensive management strategy.
Keywords: Acute kidney injury, Acute renal failure, Cardiac surgery, Congenital heart surgery, Biomarkers, Children
1. Introduction
Acute kidney injury (AKI) is common following pediatric cardiac surgery and occurs in ~40–50% of cases [1]. This is likely an underestimate and offers an important target for improved clinical care. The occurrence of AKI has been consistently reported as a risk factor for morbidity and mortality in this vulnerable population and is related to prolonged length of stay, post-operative ventilator duration, fluid disturbances, electrolyte derangements, and abnormal drug metabolism [1–3]. AKI in adults undergoing cardiac surgery has been more extensively studied and is associated with increased hospital cost and in-hospital mortality, increased need for hemodialysis after discharge, increased incidence of chronic kidney disease (CKD), and decreased survival [4–7]. However, many patients regardless of age fail to be diagnosed with AKI. There are many potential reasons that AKI is underappreciated, including a lack of awareness by clinicians and limitations in the current diagnostic approach to AKI. Herein, we report the current definitions, epidemiology, mechanisms, clinical approach, and the development of novel diagnostic strategies for AKI after pediatric cardiac surgery.
2. Definition of AKI
Prior to the development of the RIFLE (Risk, Injury, Failure, Loss, and End-Stage) criteria in 2004, the reported incidence of AKI varied widely and resulted in many patients with AKI not being recognized [8]. The RIFLE criteria consists of three graded levels of injury (Risk, Injury, and Failure) based upon either the fold increase in serum creatinine (1.5-, 2-, or 3-fold respectively) or reduction in urine output. A modification termed as the Acute Kidney Injury Network (AKIN) criteria was developed which defined AKI as a ≥0.3 mg/dl increase in serum creatinine within a restrictive 48-hour period. However, the AKIN criteria have not been adequately validated for use in children, and the restricted diagnostic timeframe of 48 h for a rise in serum creatinine may limit its utility. Recently, the criteria for diagnosis of AKI have been further refined by the Kidney Disease Improving Global Outcomes (KDIGO) group and are now widely accepted for children and adults (Table 1) [9]. These criteria still rely heavily on a rise in serum creatinine (SCr) and changes in urine output. The degree of SCr increase is also still used to define AKI severity using a 3-level staging system with stage 1 being the least severe and stage 3 being most severe. However, the use of SCr in the diagnosis and staging of AKI has many inherent limitations. Even normal SCr can vary widely with age, gender, diet, muscle mass, nutrition status, medications, and hydration status. An increase in SCr does not differentiate the nature, type and timing of the renal insult. Dialysis readily clears serum creatinine, rendering this marker useless in the assessment of renal function once dialysis has begun. In the acute setting, it is estimated that more than 50% of kidney function must be lost before SCr even begins to rise. Changes in SCr often lag behind changes in renal function due to an acute insult until a steady state has been reached, which can take several days. This leads to delayed diagnosis of AKI and greatly limits opportunity for timely clinical intervention. Even with these recognized limitations, changes in either serum creatinine or urine output do offer powerful diagnostic and prognostic capacity. If both worsening serum creatinine and decreased urine output occur, the severity of disease is worse with associated higher mortality. The duration of AKI also offers important prognostic information and depending on AKI stage, can alter outcome. Kellum et al. recently evaluated a cohort of over 23,000 patients that developed AKI as classified by the KDIGO criteria [10]. In patients meeting stage 3 AKI diagnosis by one criteria, hospital mortality and need for renal replacement therapy (RRT) were < 18% and < 3.5%, respectively. There was a dramatic change in these outcomes when stage 3 AKI was diagnosed by both criteria, with need for RRT increasing to 55% and mortality increasing to 51%. They also documented that the duration of AKI was a strong predictor of death or end stage renal disease regardless of AKI stage. Additionally, data from this report suggest that changes in SCr for those with higher baseline values may be more important clinically than a change of similar magnitude in patients with low baseline SCr values.
Table 1.
The Kidney Disease Improving Global Outcomes (KDIGO) AKI criteria. Adapted from reference [9].
| Stage | Serum creatinine | Urine output |
|---|---|---|
| 1 | 1.5–1.9 times baseline, OR ≥0.3 mg/dl (≥26.5 µmol/l) increase |
<0.5 ml/kg/h for 6–12 h |
| 2 | 1.0–2.9 times baseline | <0.5 ml/kg/h for ≥12 h |
| 3 | 3.0 times baseline, OR SCr ≥4.0 mg/dl (≥353.6 µmol/l), OR Initiation of renal replacement therapy, OR eGFR <35 ml/min per 1.73 m2 (<18 years) |
<0.3 ml/kg/h for ≥24 h, OR Anuria for ≥12 h |
SCr, serum creatinine; eGFR, estimated glomerular filtration rate.
3. Incidence and Etiology of Cardiac Surgery-associated Acute Kidney Injury
In recent years, improvements in surgical techniques have expanded the complexity of congenital heart lesions that are correctible, but with an associated increase in the incidence of cardiac surgery-associated AKI (CS-AKI) [11]. Recent reports of AKI using current definitions in large pediatric populations undergoing congenital heart disease surgery have revealed an incidence of 40–50% [1,3,12–14], with an even higher incidence of 64% among neonates [15]. More importantly, pediatric CS-AKI has been consistently associated with a number of complications including increased morbidity and mortality in this population. In the short term, after adjustment for covariates, pediatric CS-AKI is independently associated with longer duration of ventilation, longer intensive care unit and hospital stay, increased inotropic support, increased occurrence of low cardiac output syndrome, and increased mortality [1,3,12–19]. In the long term, severe pediatric CS-AKI is independently associated with increased mortality [3,15], lower z-score for height [15], as well as evidence for chronic kidney disease [20]. The short- and long-term implications of AKI in this setting clearly underscore the importance of a better understanding of the pathogenesis and risk factors, the significance of making an early diagnosis in the post-operative period, and the need for long-term follow up.
Historically, it had been thought that AKI in the setting of cardiac disease was secondary to low cardiac output and/or impaired arterial perfusion of the kidneys. Current data would suggest that the more pertinent contributory mechanisms include venous congestion along with an upregulation of inflammatory cytokines [21,22]. The pathogenesis of CS-AKI is complex, incompletely understood, and based largely on animal models. Primary mechanisms include ischemia, ischemia–reperfusion injury, mechanical blood trauma, oxidative stress, nephrotoxins, and activation of inflammatory cascades [11,23,24]. Reduced mean arterial pressure and non-pulsatile renal blood flow during cardiopulmonary bypass (CPB) leads to renal ischemia and hypoxia, with resultant activation of apoptotic and necrotic pathways of tubule and endothelial cell death [25,26]. Direct and convincing evidence for low renal oxygenation during pediatric CPB has been obtained using near-infrared spectroscopy [27–29]. Not surprisingly, the duration of CPB has been the single most consistently identified risk factor for pediatric CS-AKI [1]. It has been directly demonstrated that human kidneys can safely tolerate 30–60 min of controlled clamp ischemia with only mild structural changes and no acute functional loss [30]. Reperfusion following release of aortic cross-clamps results in activation of oxidative pathways that further exacerbate tubular and microvascular injury [25,26]. Mechanical trauma to red blood cells in the CPB circuit causes hemolysis and the release of free hemoglobin and iron, which exacerbate oxidant-mediated injury. Injured tubule and endothelial cells initiate a local inflammatory response. In addition, a systemic inflammatory response is induced by CPB, triggered by contact between blood and the artificial surfaces of the CPB circuit. Activation of neutrophils, platelets, and other pro-inflammatory cascades further exacerbates the damage to renal tubule and endothelial cells. While inflammation is prominent in animal models of AKI, its role in humans undergoing CPB is not fully known. During the post-operative period, the use of nephrotoxic agents such as non-steroidal anti-inflammatory agents, antibiotics, and contrast agents can worsen the kidney injury. These multiple mechanisms of injury are likely to be activated at different time points with varying intensities and may act synergistically.
Numerous risk factors have been identified for the development of pediatric CS-AKI, in the pre-operative, intra-operative, and post-operative phases [31,32]. The most consistently identified independent risk factors for pediatric CS-AKI include younger age, longer duration of CPB, higher surgical complexity (as reflected by the risk-adjusted classification for congenital heart surgery [RACHS-1] score), preoperative ventilator support, and lower preoperative serum creatinine [1,12,15]. Many pre-operative risk factors such as lower age and genetic predisposition are not modifiable but should be considered in decision making if known. Other potentially modifiable pre-operative risk factors include diabetes mellitus, lower preoperative serum creatinine, preoperative ventilator support, history of congestive heart failure, and hypertension. Although these may not be fully correctible, optimal management should be targeted. In addition, screening to assess for other preoperative risk factors such as renal dysfunction or reduced left ventricular ejection fraction (LVEF) should be completed. Appropriate assessment for causes of these conditions and subsequent treatment could result in an improvement in kidney and/or systolic function. There are also recognized intra-operative risk factors, including low blood pressure and low hemoglobin concentrations, both of which can be optimized to improve oxygen delivery during CPB. Some intra-operative risks, such as use of deep hypothermic circulatory arrest and longer duration of bypass, may be modified but often will be dependent on the surgical approach in particular centers [32]. However, some risks are unavoidable such as need for emergent surgical intervention or need for thoracic aortic surgery. Post-operative factors that increase the risk of AKI include volume status, use of renal replacement therapy, kidney perfusion pressure, kidney function immediately following surgery, prior existing chronic kidney disease, and use of mechanical circulatory support [32,33]. Appropriate control of blood pressure and volume status in the post-operative period offers clinicians potential strategies to mitigate or avoid AKI.
4. Novel Diagnostic Strategies in AKI
There are inherent limitations to relying on changes in SCr and urine output as diagnostic vehicles for AKI. To assist clinicians in the diagnosis of AKI, novel biomarkers in both the serum and urine have been developed that may offer additive benefit to the traditional diagnostic approach in AKI. Specifically in the field of pediatric CS-AKI, the use of unbiased functional genomic and proteomic technologies has uncovered a number of highly promising non-invasive biomarkers that are currently being validated [34–37]. There has been a significant expansion in the investigational resources in this space and these biomarkers are rapidly becoming part of the framework for research investigation and clinical management [38,39]. Many of these have been developed to detect direct damage to the kidney and offer additional information to commonly used biomarkers such as SCr and urine output. Novel biomarkers leverage the possibility of detecting damage before obvious functional changes such as decrease in urine output or rise in SCr develop. These biomarkers can be divided into three categories based on their characteristics and underlying physiologic actions and include: 1)markers of glomerular filtration rate (GFR), 2) markers of tubular injury, 3) markers of cell cycle arrest, and 4) markers of inflammation. Biomarkers of AKI are a highly targeted area of clinical investigation with over 30 markers being reported in the recent literature [39]. The following sections will focus on the current status of the most promising of these biomarkers for the prediction of AKI and its adverse outcomes, specifically in the setting of pediatric cardiac surgery.
4.1. Markers of GFR
Traditional assessment of glomerular filtration uses SCr which is an imprecise and delayed marker of changes in GFR, limiting overall utility as a biomarker [40]. Cystatin C has been reported as an alternative to serum creatinine and is a superior biomarker in the estimation of GFR [41]. Unlike SCr, cystatin C concentrations are unaffected by gender or muscle mass. Measurement of cystatin C, which is widely available in most hospitals and laboratories, also has better diagnostic capability in chronic kidney disease [42,43]. The use of cystatin C as a diagnostic tool for AKI has recently been explored. Two meta-analyses have revealed that serum cystatin C outperforms SCr as a predictor of AKI and a marker of AKI severity [44,45]. A multicenter prospective study of children undergoing cardiac surgery showed that early measurements of cystatin C (within 6 h of initiating CPB) strongly and independently predicted the development of SCr-based AKI with an adjusted odds ratio of 17.2 and adjusted area under the receiver operating characteristic curve (AUC) of 0.89 [46]. The postoperative cystatin C level also independently predicted longer duration of ventilation and intensive care unit stay [46]. Other recent reports have provided confirmatory results, albeit with a slightly reduced AUC of 0.71–0.81 [47–49]. A recent multicenter prospective cohort study assessed the change in tubular injury biomarkers of AKI with changes in cystatin C in children undergoing cardiac surgery with CPB [50]. This report found that compared with a serum creatinine-based definition of AKI, a cystatin C-based definition of AKI was more strongly associated with changes in tubular injury biomarkers. Finally, in a small prospective study of infants undergoing CPB, postoperative serum cystatin C outperformed SCr as a biomarker to predict AKI defined by the tubular injury biomarker NGAL, with an AUC of 0.87 for predicting NGAL-positive AKI [51]. Collectively, the data to date on 913 children undergoing CPB and 313 AKI events provide preliminary evidence for the utility of early plasma cystatin C measurements as a promising biomarker for AKI and its adverse outcomes in pediatric CS-AKI (Table 2), with an average AUC of 0.79. Prior to advocating for its routine clinical use, further confirmation in larger multi-center prospective studies is required.
Table 2.
Plasma cystatin C for the prediction of acute kidney injury after pediatric cardiac surgery.
| Reference | Sample size | AKI events | AKI definition | Timing (hours) | AUC (95% CI) | Cut-off (mg/l) |
|---|---|---|---|---|---|---|
| Zappitelli [46] | 288 | 121 | ≥50% increase in SCr | <6 h | 0.89 (0.81–0.97) | 1.16 |
| Krawczeski [47] | 374 | 119 | ≥50% increase in SCr | 12 h | 0.81 (0.74–0.88) | 1.16 |
| Peco-Antic [48] | 112 | 18 | ≥25% decrease in eCCl | 6 h | 0.73 (NR) | NR |
| Seitz [49] | 139 | 55 | ≥50% increase in SCr | 6 h | 0.71 (NR) | 0.945 |
| Total | 913 | 313 | 0.79 |
SCr, serum creatinine; eCCl, estimated creatinine clearance; NR, not reported; timing refers to hours since starting cardiopulmonary bypass when the plasma sample was obtained.
4.2. Markers of Tubular Injury
Non-invasive biomarkers of tubular injury in the urine and blood have been widely investigated in diagnosing AKI in children undergoing cardiac surgery. Some of the most frequently reported include neutrophil gelatinase-associated protein (NGAL), interleukin-18 (IL-18), liver-type fatty acid-binding protein (L-FABP), and kidney injury molecule-1 (KIM-1).
The most extensively studied biomarker in pediatric CS-AKI is NGAL. Unbiased preclinical transcriptome profiling studies have consistently identified Ngal (also known as lipocalin 2 or lcn2) to be one of the most upregulated genes in the kidney very early after acute injury in animal models [34,52,53]. Downstream proteomic analyses also revealed NGAL to be one of the most highly induced proteins in the kidney after ischemic or nephrotoxic AKI in animal models [54–56]. Studies utilizing the NGAL reporter mouse model have unequivocally demonstrated that NGAL derives specifically from the kidney, where it is rapidly induced in the injured distal nephron segments in response to intrinsic AKI (and not in prerenal AKI induced by volume depletion) [57]. The resultant secretion of NGAL into the urine comprises the major fraction of urinary NGAL protein. Plasma NGAL also derives largely from the injured kidney, with additional systemic contributions from activated neutrophils and macrophages. The consistent finding that NGAL protein is easily detected in the urine and plasma soon after AKI in animal studies has inspired a large number of translational studies to evaluate NGAL as a non-invasive biomarker in human AKI. Several investigators have focused on establishing the reference ranges for NGAL in normal healthy children as well as mature and premature infants [58–62]. These studies have highlighted small but significant age and gender differences in normal infants and children that need to be accounted for when interpreting NGAL values.
Well over 300 publications have now reported on NGAL in human AKI, to the point that several systematic reviews and meta-analyses of its diagnostic utility have now appeared [63–65]. The diagnostic accuracy of NGAL for the prediction of AKI has remained high. This is particularly applicable to the pediatric cardiac surgery setting, and the pertinent studies are reviewed herein. In several prospective single center as well as multicenter studies of infants and children who underwent elective cardiac surgery with CPB, AKI (usually defined as a 50% increase in SCr) occurred 1–3 days after surgery. In contrast, serial NGAL measurements revealed a 10-fold or greater increase in the urine and plasma, within 2–6 h of initiating CPB, in children who subsequently developed AKI [66–77]. The diagnostic performance of urine NGAL for the prediction of pediatric CS-AKI is summarized in Table 3, and that of plasma NGAL in Table 4. Collectively, the data to date on nearly 3000 children undergoing CPB and nearly 900 AKI events provide strong evidence for the utility of early NGAL measurements to predict AKI after pediatric cardiac surgery, with an average AUC of 0.83 for urine NGAL and 0.86 for plasma NGAL. The addition of NGAL significantly improved AKI risk prediction over clinical models alone, as measured by net reclassification improvement and integrated discrimination improvement, thereby clearly providing added value to the clinician [69,70]. In addition, early NGAL measurements in the setting of pediatric CPB are strongly correlated with and predictive of graded AKI severity as well as AKI duration [67–70,76]. In children who develop CS-AKI based on an increase in SCr, urinary NGAL reliably discriminates between transient azotemia and true intrinsic AKI with structural damage with 100% specificity and 100% positive predictive value [78]. Early NGAL measurements also provide graduated relationships with, and moderate to strong prediction of, adverse outcomes in pediatric CS-AKI, including length of hospital stay, duration of mechanical ventilation, dialysis requirement, and mortality [67–74,76,79,80]. A recent economic impact analysis confirmed the cost-effectiveness of urinary NGAL in the early diagnosis of AKI after cardiac surgery [81]. Because of its strong predictive properties for CS-AKI, NGAL is also emerging as an early biomarker for monitoring interventional trials. In a recent prospective randomized double-blind controlled trial of infants undergoing cardiac surgery with CPB, the group that received intra-operative fenoldopam demonstrated a renoprotective effect by a dramatic reduction in post-operative urine NGAL levels compared to the placebo group [82]. The fenoldopam group also showed a significant reduction in the need for diuretics and vasodilators.
Table 3.
Urine NGAL for the prediction of acute kidney injury after pediatric cardiac surgery.
| Reference | Sample size | AKI events | AKI definition | Timing (hours) | AUC | Cut-off (ng/ml) |
|---|---|---|---|---|---|---|
| Mishra [66] | 71 | 20 | ≥50% increase in SCr | 2 h | 0.99 | 50 |
| Bennett [67] | 196 | 99 | ≥50% increase in SCr | 2 h | 0.95 | 100 |
| Krawczeski [68] | 374 | 112 | ≥50% increase in SCr | 2 h | 0.92 | 50 |
| Krawczeski [69] | 220 | 60 | ≥50% increase in SCr | 2 h | 0.90 | 185 |
| Parikh [70] | 311 | 53 | ≥100% increase in SCr | <6 h | 0.71 | 70 |
| Peco-Antic [48] | 112 | 18 | ≥25% decrease in eCCl | 6 h | 0.70 | NR |
| Zheng [71] | 58 | 29 | ≥50% increase in SCr | 4 h | 0.86 | 54 |
| Cantinotti [72] | 135 | 52 | ≥50% increase in SCr | 2 h | 0.85 | 50 |
| Hazle [73] | 49 | 42 | ≥50% increase in SCr | 2 h | 0.79 | 76 |
| Bojan [74] | 200 | 27 | Severe AKI (KDIGO stage 3) | 3–6 h | 0.8 | NR |
| Seitz [49] | 139 | 55 | ≥50% increase in SCr | 6 h | 0.60 | 27.6 |
| Alcaraz [75] | 106 | 36 | ≥50% increase in SCr | 3 h | 0.86 | 75 |
| Total | 1971 | 603 | 0.83 |
SCr, serum creatinine; eCCl, estimated creatinine clearance; NR, not reported; timing refers to hours since starting cardiopulmonary bypass when the urine sample was obtained.
Table 4.
Plasma NGAL for the prediction of acute kidney injury after pediatric cardiac surgery.
| Reference | Sample size | AKI events | AKI definition | Timing (hours) | AUC | Cut-off (ng/ml) |
|---|---|---|---|---|---|---|
| Mishra [66] | 71 | 20 | ≥50% increase in SCr | 2 h | 0.90 | 50 |
| Dent [76] | 120 | 45 | ≥50% increase in SCr | 2 h | 0.96 | 150 |
| Krawczeski [47] | 374 | 112 | ≥50% increase in SCr | 2 h | 0.94 | 45 |
| Parikh [70] | 311 | 53 | ≥100% increase in SCr | <6 h | 0.56 | 260 |
| Fadel [77] | 40 | 32 | ≥50% increase in SCr | 2 h | 0.95 | 100 |
| Total | 916 | 262 | 0.86 |
SCr, serum creatinine; timing refers to hours since starting cardiopulmonary bypass when the plasma sample was obtained.
The widespread availability of standardized clinical analytical platforms (outside USA) has contributed to the explosion of NGAL studies and the emergence of NGAL as an excellent stand-alone troponin-like structural kidney damage biomarker in the urine and plasma globally [64,83–85]. This has resulted in the concept of “sub-clinical AKI” in patients who are “NGAL-positive but creatinine-negative”. Two large studies enrolled about 4000 adult and pediatric cardiac surgical and critically ill subjects and grouped them according to their NGAL and SCr status [86,87]. Both studies confirmed the hypothesis that NGAL levels complemented the information obtained from SCr measurements in predicting AKI and its prognosis. A substantial proportion of subjects (about 20%) had increased NGAL concentrations even in the absence of a rise in SCr concentration. This previously undetectable condition of damage biomarker-positive but SCr-negative “subclinical AKI” was associated with a 2–3 fold increased risk of death or dialysis requirement compared to patients with normal NGAL concentrations. In patients with loss of kidney function, measurement of NGAL still added prognostic value, since patients who were both NGAL- and SCr-positive displayed by far the worst prognosis.
However, in anticipation of the widespread use of NGAL in routine practice, the clinician should be familiar with its potential limitations. First, the majority of pediatric studies reported to date have been single center studies that have excluded patients with chronic kidney disease, which is a well-known contributor to the development of AKI [88]. The available data suggest that patients with stable chronic kidney disease display low concentrations of NGAL similar to normal controls, but those with progressive chronic kidney disease may exhibit higher concentrations that may approach those in milder forms of AKI. Indeed, three meta-analyses have shown that the performance of NGAL in older adults is inferior to that in children, likely related to the comorbidities (such as chronic kidney disease, chronic heart failure, atherosclerosis, diabetes, medications) that can hinder NGAL's predictive ability [63–65]. Second, there are no uniformly accepted cut-offs, although the literature would suggest that an NGAL cut-off value of < 100 ng/ml (measured on a standardized clinical platform) would rule out AKI in those with normal baseline function, and a cut-off of > 150 ng/ml can be diagnostic for AKI, leaving a “grey zone” where NGAL concentrations may be indeterminate [63–65,89]. Cut-offs may be dependent on age, gender, underlying clinical setting, and the specific assay employed. Third, the definitions of AKI employed in published NGAL studies have been variable, but always based on substantial elevations in SCr concentration, which raises the challenge of using a flawed outcome variable to analyze the performance of a novel biomarker. For example, in patients with true tubular injury but no significant change in SCr, as is often encountered in critically ill subjects, [86,87] an appropriately elevated NGAL concentration would be deemed as a false positive. Similarly, patients with absence of tubular injury but with elevation in SCr due to pre-renal causes will display appropriately normal NGAL levels, which would be labeled as a false negative. Additional prospective multicenter studies that examine the ability of NGAL to predict pertinent short- and long-term clinical end points will further verify its diagnostic and prognostic value. Finally, given the multifactorial mechanisms of AKI, it is likely that a combination of tubular injury biomarkers in addition to NGAL might improve the diagnostic and prognostic accuracy, a concept that is further discussed in Section 4.4. However, at the present time, a standardized clinical platform for measuring other tubular injury biomarkers such as IL-18, L-FABP, and KIM-1 is not available, and all of these markers are subject to the same limitations as mentioned above for NGAL. These additional biomarkers have only been examined in a limited number of pediatric CPB studies, as outlined below.
Interleukin-18 (IL-18) is a pro-inflammatory cytokine that is induced and cleaved in the proximal tubule, and subsequently easily detected in the urine following ischemic AKI in animal models [90]. Urinary IL-18 was shown to represent an early, predictive biomarker in children undergoing cardiac surgery in a small single center study. In patients who developed an increase in SCr 1–3 days after CPB, urinary IL-18 levels increased at 6 h and peaked at 12 h post-CPB, with an AUC of 0.75 for predicting AKI [91]. Subsequent pediatric single center [69,71, 73] and multicenter [70] studies have confirmed the ability of urine IL-18 levels obtained during the 6–12 hour post-CPB time window to moderately predict AKI, with AUCs in the 0.72–0.82 range (Table 5). The addition of urinary IL-18 improved AKI risk prediction over clinical models alone, and correlated with adverse outcomes including length of hospital stay and duration of mechanical ventilation [70].
Table 5.
Urine IL-18 for the prediction of acute kidney injury after pediatric cardiac surgery.
| Reference | Sample size | AKI events | AKI definition | Timing (hours) | AUC | Cut-off (pg/ml) |
|---|---|---|---|---|---|---|
| Parikh [91] | 71 | 20 | ≥50% increase in SCr | 12 h | 0.75 | 70 |
| Krawczeski [69] | 220 | 60 | ≥50% increase in SCr | 12 h | 0.82 | NR |
| Parikh [70] | 311 | 53 | ≥100% increase in SCr | 6 h | 0.72 | 364 |
| Zheng [71] | 58 | 29 | ≥50% increase in SCr | 6 h | 0.77 | 49 |
| Hazle [73] | 49 | 42 | ≥50% increase in SCr | 6 h | 0.76 | 800 |
| Total | 709 | 204 | 0.76 |
SCr, serum creatinine; NR, not reported; timing refers to hours since starting cardiopulmonary bypass when the urine sample was obtained.
Liver-type fatty acid binding protein (L-FABP) is another protein that is induced in the proximal tubule early after AKI in animal models, and is thought to be nephroprotective due to its antioxidant properties [92]. Urinary L-FABP represented an early, predictive biomarker in children undergoing cardiac surgery in a small single center study. In patients who developed an increase in SCr 2–3 days after CPB, urinary L-FABP levels increased at 4 h, with a predictive AUC of 0.81 for AKI [93]. Subsequent pediatric single center studies [48,69] have indicated the utility of urine L-FABP levels obtained 6 h post-CPB to moderately predict AKI, with AUCs in the 0.75–0.78 range.
Kidney injury molecule-1 (KIM-1) was first identified by subtractive hybridization screening as a gene that is markedly upregulated in the proximal tubule cells of ischemic rat kidneys [94], a finding that has been confirmed in additional transcriptome profiling studies. KIM-1 is one of the most highly induced proteins in the kidney after experimental AKI, where it mediates phagocytosis of damaged cells and thereby limits injury [95]. A proteolytically processed extra-cellular domain of KIM-1 can be detected in the urine of patients with AKI, using experimental laboratory assays. In a small case–control study of 40 children undergoing CPB, urinary KIM-1 levels were markedly increased in the 20 subjects that developed AKI, with an AUC of 0.83 at 12 h post-CPB for predicting AKI [96]. Subsequent pediatric single center studies have yielded conflicting results, with one indicating moderate accuracy for predicting AKI at 12 h (AUC 0.70) [69] and others indicating a poorer predictive performance [48,73].
4.3. Markers of Cell Cycle Arrest
Tissue inhibitor of metalloproteinases-2 (TIMP-2) and insulin-like growth factor binding protein 7 (IGFBP7), both markers of cell cycle arrest, are upregulated after kidney injury in order to limit proliferation of damaged tubule cells. The product of these markers is easily measured in the urine using a point-of-care kit that has been approved by the FDA [97,98]. In a preliminary case–control study of 51 children undergoing CPB, the product of urinary TIMP-1 and IGFBP7 levels was increased in the 12 subjects that developed AKI, with an AUC of 0.85 (CI: 0.72–0.94) at 4 h post-CPB for predicting AKI [99]. The corresponding AUC for urinary NGAL was reported to be comparably good at 0.87 (CI: 0.74–0.95). Additional studies are required in the pediatric CPB population to further extend the potential role for these cell cycle markers as predictors of AKI.
4.4. Markers of Inflammation
Animal models of AKI are characterized by inflammation, with elevation in serum interleukin (IL)-6 representing the major pro-inflammatory cytokine response [100]. In children undergoing CPB, contact between blood and the artificial surfaces of the CPB circuit activates inflammatory cells to release pro-inflammatory cytokines such as IL-6, which further exacerbate the damage to renal tubule and endothelial cells. In a small case–control study of 39 children, serum IL-6 levels at 2 h post-CPB were increased in the 18 subjects that developed AKI, with an AUC of 0.76 for predicting AKI and an AUC of 0.95 to predict prolonged mechanical ventilation [101]. This was confirmed in a subsequent study of 47 children, in which serum IL-6 levels at 2 h post-CPB moderately predicted AKI in 19 children, with a lower AUC of 0.68 [102]. However, two subsequent larger studies did not document an association between early IL-6 concentrations and subsequent development of AKI after pediatric CPB [103,104]. Further studies are required to evaluate serum IL-6, a central pro-inflammatory cytokine, as an early biomarker of AKI after pediatric CPB.
4.5. Biomarker Combinations
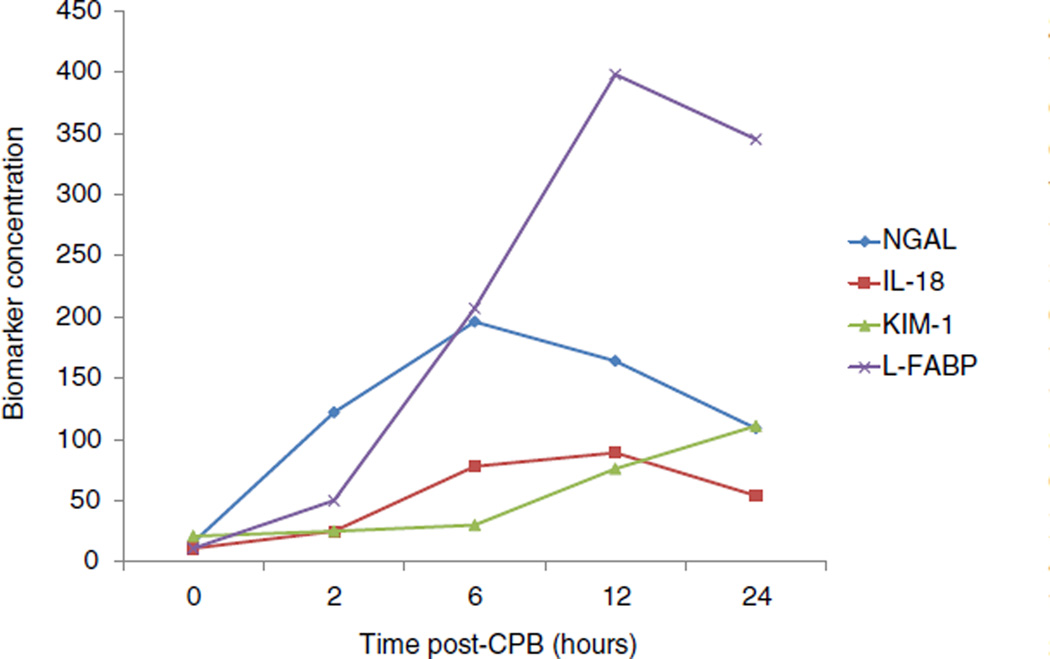
The utility of combining functional markers (cystatin C) and tubular damage markers (NGAL) for improving prediction of AKI severity and subtype (transient prerenal versus sustained intrinsic renal) has recently been confirmed in a cohort of children undergoing cardiac surgery [78]. Furthermore, a combined analysis of the tubular injury biomarkers NGAL, IL-18, L-FABP and KIM-1 in a cohort of 220 children undergoing CPB showed a temporal sequence of elevation in the 60 subjects that developed a 50% increase in SCr 1–3 days post-CPB (Fig. 1) [69]. At the 2 hour post-CPB time point, only urine NGAL was significantly elevated, and improved AKI prediction above a clinical model from an AUC of 0.74 to 0.85. At 6 h, NGAL, IL-18, and L-FABP were all elevated, and provided improvements in the AUC for AKI prediction from0.72 to 0.91, 0.84, and 0.77 respectively. A significant increase in urinary KIM-1 was first detected at 12 h post-CPB. At the 6 hour time point, the combination of NGAL + IL-18 provided the best results in terms of AUC improvement over any single biomarker, net reclassification improvement, and integrated discrimination improvement. At the 12 h time point, the combination of NGAL + IL-18 + L-FABP resulted in the best AUC improvement over single biomarkers. Thus, a combination of tubular injury biomarkers could allow clinicians to pinpoint the timing of kidney insult, and thereby drive the appropriate therapeutic intervention [69].
Fig. 1.
Profiles of tubular injury biomarker concentrations at various time points after cardiopulmonary bypass (CPB) initiation. Data are from Krawczeski et al. [69]. Urinary biomarker concentrations are shown for NGAL (ng/ml), IL-18 (pg/ml), L-FABP (ng/ml), and KIM-1 (pg/0.1 ml).
5. Therapeutic Strategies
The primary target for treatment in AKI is prevention. This necessitates identification of high risk patients. Recognized risk factors for the development of AKI have been published and include comorbidities such as existing chronic kidney disease or sepsis [105]. Based on knowledge of predisposing factors for the development of AKI, some institutions are leveraging the electronic medical record (EMR) data to identify at-risk individuals and notify providers. This offers clinicians a pre-emptive opportunity to be mindful of careful surveillance for AKI and potentially avoid situations or nephrotoxic therapies that may increase the likelihood of kidney injury. Current efforts at the development of AKI risk scores may limit the incidence of AKI in the future. Therapeutic strategies are quite limited in those patients that develop AKI. Volume resuscitation may be used in appropriate situations and careful dosing or avoidance of nephrotoxic drugs should be observed. As AKI is a commonly reported complication of cardiac surgery, therapies have been considered but with little clinical impact. Past reports have suggested that the heart and the kidneys can be protected against significant ischemia–reperfusion during cardiac interventions injury by instituting preceding, brief periods of ischemia–reperfusion to remote organs, which has been called remote ischemic preconditioning [106, 107]. Recently, the impact of remote ischemic preconditioning was studied in 1612 adult patients undergoing cardiac surgery requiring cardiopulmonary bypass on combined cardiovascular endpoints as well as acute kidney injury [108]. The use of remote ischemic preconditioning did not confer protection against AKI as assessed by rise in serum creatinine or changes in plasma NGAL levels.
No effective clinical treatments have been developed specifically for AKI but this will likely be on the horizon. There is increasing interest in the identification of genetic risk factors for AKI, using genome-wide association studies. The identification of patients that may be genetically predisposed to AKI prior to surgery would offer additional steps to be taken regarding monitoring as well as avoidance of things such as hypovolemia. But, perhaps more importantly, a broader understanding of genetics in AKI may help elucidate biological pathways that could be targets for other diagnostic tests and potential therapies.
6. Conclusion
Acute kidney injury in pediatric patients following cardiac surgery is an increasingly recognized clinical concern with important implications with regard to prognosis and management. However, many children experience AKI in the post-operative setting that are not diagnosed. Furthermore, many that are diagnosed may not be managed optimally to mitigate AKI. Often, this management does not include consultation with a pediatric nephrologist. Although many pediatric patients experiencing AKI following cardiac surgery recover kidney function, long-term surveillance for development of CKD is not performed. A greater emphasis on early detection of AKI with appropriate and thoughtful interventions is needed avoid long term sequelae. Although current diagnostic strategies for AKI are widely available, they fail to diagnose injury in a timely manner which may result in delayed intervention. Newer diagnostic tools are becoming more available internationally that will facilitate earlier recognition of AKI in a more reliable manner. The combination of functional biomarkers along with markers of tubular injury offers a greater diagnostic opportunity in pediatric patients following cardiac surgery. The data collected from the increased use of these markers will offer clinicians a greater opportunity to consider AKI in ongoing post-operative care and develop overarching strategies to mitigate untoward long-term effects. Clinicians managing pediatric patients that have undergone cardiac surgery should be aware of the long-term implications of AKI. If AKI is diagnosed during the post-operative period, referral to a pediatric nephrologist is strongly advocated for ongoing monitoring and institution of medical therapy when indicated. Given the expanding focus on AKI and the cardio-renal syndrome in cardiac surgery, we are obliged to make this an area of increased collaboration and clinical investigation to improve outcomes for children with congenital heart disease across the globe.
Footnotes
Conflict of interest
PD is a co-inventor on submitted patents on the use of NGAL as a biomarker of kidney injury.
References
- 1.Li S, Krawczeski CD, Zappitelli M, et al. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: a prospective multicenter study. Crit Care Med. 2011;39:1493–1499. doi: 10.1097/CCM.0b013e31821201d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devarajan P. Pediatric acute kidney injury: different from acute renal failure but how and why. Curr Pediatr Rep. 2013;1(1):34–40. doi: 10.1007/s40124-012-0003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watkins SC, Williamson K, Davidson M, Donahue BS. Long-term mortality associated with acute kidney injury in children following congenital cardiac surgery. Paediatr Anaesth. 2014;24:919–926. doi: 10.1111/pan.12419. [DOI] [PubMed] [Google Scholar]
- 4.Hobson C, Ozrazgat-Baslanti T, Kuxhausen A, et al. Cost and mortality associated with postoperative acute kidney injury. Ann Surg. 2015;261:1207–1214. doi: 10.1097/SLA.0000000000000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wald R, Quinn RR, Luo J, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302:1179–1185. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 6.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442–448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hobson CE, Yavas S, Segal MS, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119:2444–2453. doi: 10.1161/CIRCULATIONAHA.108.800011. [DOI] [PubMed] [Google Scholar]
- 8.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute Dialysis Quality Initiative w. Acute renal failure — definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 10.Kellum JA, Sileanu FE, Murugan R, Lucko N, Shaw AD, Clermont G. Classifying AKI by urine output versus serum creatinine level. J Am Soc Nephrol. 2015;26:2231–2238. doi: 10.1681/ASN.2014070724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pederson K. Acute kidney injury in children undergoing surgery for congenital heart disease. Eur J Pediatr Surg. 2012;22:426–433. doi: 10.1055/s-0032-1322540. [DOI] [PubMed] [Google Scholar]
- 12.Aydin SI, Seiden HS, Blaufox AD, et al. Acute kidney injury after surgery for congenital heart disease. Ann Thorac Surg. 2012;94:1589–1595. doi: 10.1016/j.athoracsur.2012.06.050. [DOI] [PubMed] [Google Scholar]
- 13.Blinder JJ, Goldstein SL, Lee VV, et al. Congenital heart surgery in infants: effects of acute kidney injury on outcomes. J Thorac Cardiovasc Surg. 2012;143:368–374. doi: 10.1016/j.jtcvs.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 14.dos Santos El Halal MG, Carvalho PR. Acute kidney injury according to pediatric RIFLE criteria is associated with negative outcomes after heart surgery in children. Pediatr Nephrol. 2013;28:1307–1314. doi: 10.1007/s00467-013-2495-7. [DOI] [PubMed] [Google Scholar]
- 15.Morgan CJ, Zappitelli M, Robertson CMT, et al. Risk factors for and outcomes of acute kidney injury in neonates undergoing complex cardiac surgery. J Pediatr. 2013;162:120–127. doi: 10.1016/j.jpeds.2012.06.054. [DOI] [PubMed] [Google Scholar]
- 16.Zappitelli M, Bernier P-L, Saczkowski RS, et al. A small post-operative rise in serum creatinine predicts acute kidney injury in children undergoing cardiac surgery. Kidney Int. 2009;76:885–892. doi: 10.1038/ki.2009.270. [DOI] [PubMed] [Google Scholar]
- 17.Toth R, Breuer T, Cserep Z, et al. Acute kidney injury is associated with higher morbidity and resource utilization in pediatric patients undergoing heart surgery. Ann Thorac Surg. 2012;93:1984–1991. doi: 10.1016/j.athoracsur.2011.10.046. [DOI] [PubMed] [Google Scholar]
- 18.Gil-Ruiz Gil-Esparza MA, Alcaraz Romero AJ, Romero Otero A, et al. Prognostic relevance of early AKI according to pRIFLE criteria in children undergoing cardiac surgery. Pediatr Nephrol. 2014;29:1265–1272. doi: 10.1007/s00467-014-2757-z. [DOI] [PubMed] [Google Scholar]
- 19.Piggott KD, Soni M, Decampli WM, et al. Acute kidney injury and fluid overload in neonates following surgery for congenital heart disease. World J Pediatr Congenit Heart Surg. 2015;6:401–406. doi: 10.1177/2150135115586814. [DOI] [PubMed] [Google Scholar]
- 20.Cooper DS, Claes D, Goldstein SL, et al. Follow-up renal assessment of injury long-term after acute kidney injury (FRAIL-AKI) Clin J Am Soc Nephrol. 2016 doi: 10.2215/CJN.04240415. [Epub ahead of publication] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dupont M, Mullens W, Tang WH. Impact of systemic venous congestion in heart failure. Curr Heart Fail Rep. 2011;8:233–241. doi: 10.1007/s11897-011-0071-7. [DOI] [PubMed] [Google Scholar]
- 22.Holdsworth SR, PY Gan. Cytokines: names and numbers you should care about. Clin J Am Soc Nephrol. 2015 doi: 10.2215/CJN.07590714. [Epub ahead of publication] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Axelrod DM, Sutherland SM. Acute kidney injury in the pediatric cardiac patient. Pediatr Anesth. 2014;24:899–901. doi: 10.1111/pan.12448. [DOI] [PubMed] [Google Scholar]
- 24.Thiele RH, Isbell JM, Rosner MH. AKI associated with cardiac surgery. Clin J Am Soc Nephrol. 2015;10:500–514. doi: 10.2215/CJN.07830814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006;17:1503–1520. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- 26.Devarajan P. Cellular and molecular derangements in acute tubular necrosis. Curr Opin Pediatr. 2005;17:193–199. doi: 10.1097/01.mop.0000152620.59425.eb. [DOI] [PubMed] [Google Scholar]
- 27.Owens GE, King K, Gurney JG, Charpie JR. Low renal oximetry correlates with acute kidney injury after infant cardiac surgery. Pediatr Cardiol. 2011;32:183–188. doi: 10.1007/s00246-010-9839-x. [DOI] [PubMed] [Google Scholar]
- 28.Hazle MA, Gajarski RJ, Aiyagari R, et al. Urinary biomarkers and renal near-infrared spectroscopy predict intensive care unit outcomes after cardiac surgery in infants younger than six months of age. J Thorac Cardiovasc Surg. 2013;146:861–867. doi: 10.1016/j.jtcvs.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruf B, Bonelli V, Balling G, et al. Intraoperative renal near-infrared spectroscopy indicates developing acute kidney injury in infants undergoing cardiac surgery with cardiopulmonary bypass: a case–control study. doi: 10.1186/s13054-015-0760-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parekh DJ, Weinberg JM, Ercole B, et al. Tolerance of the human kidney to isolated controlled ischemia. J Am Soc Nephrol. 2013;24:506–517. doi: 10.1681/ASN.2012080786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hobson C, Singhania G, Bihorac A. Acute kidney injury in the surgical patient. Crit Care Clin. 2015;31:705–723. doi: 10.1016/j.ccc.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parolari A, Pesce LL, Pacini D, et al. Risk factors for perioperative acute kidney injury after adult cardiac surgery: role of perioperative management. Ann Thorac Surg. 2012;93:584–591. doi: 10.1016/j.athoracsur.2011.09.073. [DOI] [PubMed] [Google Scholar]
- 33.Raimundo M, Crichton S, Syed Y, et al. Low systemic oxygen delivery and BP and risk of progression of early AKI. CJASN. 2015;10:1340–1349. doi: 10.2215/CJN.02780314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devarajan P. Genomic and proteomic characterization of acute kidney injury. Nephron. 2015;131:85–91. doi: 10.1159/000437237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuh MP, Nehus E, Ma Q, et al. Long-term stability of urinary biomarkers of acute kidney injury in children. Am J Kidney Dis. 2015 May 29; doi: 10.1053/j.ajkd.2015.04.040. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devarajan P, Krawczeski CD, Nguyen MT, et al. Proteomic identification of early biomarkers of acute kidney injury after cardiac surgery in children. Am J Kidney Dis. 2010;56:632–642. doi: 10.1053/j.ajkd.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beger RD, Holland RD, Sun J, et al. Metabonomics of acute kidney injury in children after cardiac surgery. Pediatr Nephrol. 2008;23:977–984. doi: 10.1007/s00467-008-0756-7. [DOI] [PubMed] [Google Scholar]
- 38.McCullough PA, Shaw AD, Haase M, et al. Diagnosis of acute kidney injury using functional and injury biomarkers: workgroup statements from the tenth Acute Dialysis Quality Initiative Consensus Conference. Contrib Nephrol. 2013;182:13–29. doi: 10.1159/000349963. [DOI] [PubMed] [Google Scholar]
- 39.Koyner JL, Parikh CR. Clinical utility of biomarkers of AKI in cardiac surgery and critical illness. Clin J Am Soc Nephrol. 2013;8:1034–1042. doi: 10.2215/CJN.05150512. [DOI] [PubMed] [Google Scholar]
- 40.Cirillo M. Evaluation of glomerular filtration rate and of albuminuria/proteinuria. J Nephrol. 2010;23:125–132. [PubMed] [Google Scholar]
- 41.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shlipak MG, Matsushita K, Arnlov J, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369:932–943. doi: 10.1056/NEJMoa1214234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz GJ, Schneider MF, Maier PS, et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int. 2012;82:445–453. doi: 10.1038/ki.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng Y, Zhang Y, Li G, Wang L. Relationship of cystatin-C change and the prevalence of death or dialysis need after acute kidney injury: a meta-analysis. Nephrology (Carlton) 2014;19:679–684. doi: 10.1111/nep.12312. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Z, Lu B, Sheng X, Jin N. Cystatin C in prediction of acute kidney injury: a systemic review and meta-analysis. Am J Kidney Dis. 2011;58(3):356–365. doi: 10.1053/j.ajkd.2011.02.389. [DOI] [PubMed] [Google Scholar]
- 46.Zappitelli M, Krawczeski CD, Devarajan P, et al. Early postoperative serum cystatin C predicts severe acute kidney injury following pediatric cardiac surgery. Kidney Int. 2011;80:655–662. doi: 10.1038/ki.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krawczeski CD, Vandevoorde RG, Kathman T, et al. Serum cystatin C is an early predictive biomarker of acute kidney injury after pediatric cardiopulmonary bypass. Clin J Am Soc Nephrol. 2010;5:1552–1557. doi: 10.2215/CJN.02040310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peco-Antic A, Ivanisevic I, Vulicevic I, et al. Biomarkers of acute kidney injury in pediatric cardiac surgery. Clin Biochem. 2013;46:1244–1251. doi: 10.1016/j.clinbiochem.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 49.Seitz S, Rauh M, Gloeckler M, et al. Cystatin C and neutrophil gelatinase-associated lipocalin: biomarkers for acute kidney injury after congenital heart surgery. Swiss Med Wkly. 2013;143:w13744. doi: 10.4414/smw.2013.13744. [DOI] [PubMed] [Google Scholar]
- 50.Zappitelli M, Greenberg JH, Coca SG, et al. Association of definition of acute kidney injury by cystatin C rise with biomarkers and clinical outcomes in children undergoing cardiac surgery. JAMA Pediatr. 2015;169:583–591. doi: 10.1001/jamapediatrics.2015.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herbert C, Patel M, Nugent A, et al. Serum cystatin C as an early marker of neutrophil gelatinase-associated lipocalin-positive acute kidney injury resulting from cardiopulmonary bypass in infants with congenital heart disease. Congenit Heart Dis. 2015;10:E180–E188. doi: 10.1111/chd.12253. [DOI] [PubMed] [Google Scholar]
- 52.Supavekin S, Zhang W, Kucherlapati R, et al. Differential gene expression following early renal ischemia–reperfusion. Kidney Int. 2003;63:1714–1724. doi: 10.1046/j.1523-1755.2003.00928.x. [DOI] [PubMed] [Google Scholar]
- 53.Devarajan P, Mishra J, Supavekin S, Patterson LT, Potter SS. Gene expression in early ischemic renal injury: clues towards pathogenesis, biomarker discovery, and novel therapeutics. Mol Genet Metab. 2003;80:365–376. doi: 10.1016/j.ymgme.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 54.Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel urinary biomarker for ischemic injury. J Am Soc Nephrol. 2003;4:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 55.Mishra J, Mori K, Ma Q, et al. Neutrophil gelatinase-associated lipocalin (NGAL): a novel urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol. 2004;24:307–315. doi: 10.1159/000078452. [DOI] [PubMed] [Google Scholar]
- 56.Mori K, Lee HT, Rapoport D, et al. Endocytic delivery of lipocalin–siderophore–iron complex rescues the kidney from ischemia–reperfusion injury. J Clin Invest. 2005;115:610–621. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paragas N, Qiu A, Zhang Q, et al. The NGAL reporter mouse detects the response of the kidney to injury in real time. Nat Med. 2011;17:216–222. doi: 10.1038/nm.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bennett MR, Nehus E, Haffner C, Ma Q, Devarajan P. Pediatric reference ranges for acute kidney injury biomarkers. Pediatr Nephrol. 2015;30:677–685. doi: 10.1007/s00467-014-2989-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McWilliam SJ, Antoine DJ, Sabbisetti V, et al. Reference intervals for urinary renal injury biomarkers KIM-1 and NGAL in healthy children. Biomark Med. 2014;8:1189–1197. doi: 10.2217/bmm.14.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lavery AP, Meinzen-Derr JK, Anderson JK, et al. Urinary NGAL in premature infants. Pediatr Res. 2008;64:423–428. doi: 10.1203/PDR.0b013e318181b3b2. [DOI] [PubMed] [Google Scholar]
- 61.Askenazi DJ, Koralkar R, Levitan EB, et al. Baseline values of candidate urine acute kidney injury biomarkers vary by gestational age in premature infants. Pediatr Res. 2011;70:302–306. doi: 10.1203/PDR.0b013e3182275164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saeidi B, Koralkar R, Griffin RL, Halloran B, Ambalavanan N, Askenazi DJ. Impact of gestational age, sex, and postnatal age on urine biomarkers in premature neonates. Pediatr Nephrol. 2015;30:2037–2044. doi: 10.1007/s00467-015-3129-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–1024. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 64.Haase-Fielitz A, Haase M, Devarajan P. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury: a critical evaluation of current status. Ann Clin Biochem. 2014;51:335–351. doi: 10.1177/0004563214521795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou F, Luo Q, Wang L, Han L. Diagnotic value of neutrophil gelatinase-associated lipocalin for early diagnosis of cardiac surgery-associated acute kidney injury: a meta-analysis. Eur J Cardiothorac Surg. 2015 doi: 10.1093/ejcts/ezv199. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 66.Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 67.Bennett M, Dent CL, Ma Q, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3:665–673. doi: 10.2215/CJN.04010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krawczeski CD, Woo JG, Wang Y, Bennett MR, Ma Q, Devarajan P. Neutrophil gelatinase-associated lipocalin concentrations predict development of acute kidney injury in neonates and children after cardiopulmonary bypass. J Pediatr. 2011;158:1009–1015. doi: 10.1016/j.jpeds.2010.12.057. [DOI] [PubMed] [Google Scholar]
- 69.Krawczeski CD, Goldstein SL, Woo JG, et al. Temporal relationship and predictive value of urinary acute kidney injury biomarkers after pediatric cardiopulmonary bypass. J Am Coll Cardiol. 2011;58:2301–2309. doi: 10.1016/j.jacc.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parikh CR, Devarajan P, Zappitelli M, et al. TRIBE-AKI consortium. postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J Am Soc Nephrol. 2011;22:1737–1747. doi: 10.1681/ASN.2010111163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng J, Xiao Y, Yao Y, et al. Comparison of urinary biomarkers for early detection of acute kidney injury after cardiopulmonary bypass surgery in infants and young children. Pediatr Cardiol. 2013;34:880–886. doi: 10.1007/s00246-012-0563-6. [DOI] [PubMed] [Google Scholar]
- 72.Cantinotti M, Storti S, Lorenzoni V, et al. The combined use of neutrophil gelatinase-associated lipocalin and brain natriuretic peptide improves risk stratification in pediatric cardiac surgery. Clin Chem Lab Med. 2012;50:2009–2017. doi: 10.1515/cclm-2012-0125. [DOI] [PubMed] [Google Scholar]
- 73.Hazle MA, Gajarski RJ, Aiyagari R, et al. Urinary biomarkers and renal near-infrared spectroscopy predict intensive care unit outcomes after cardiac surgery in infants younger than 6 months of age. J Thorac Cardiovasc Surg. 2013;146:861–867. doi: 10.1016/j.jtcvs.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bojan M, Vicca S, Lopez-Lopez V, et al. Predictive performance of urine neutrophil gelatinase-associated lipocalin for dialysis requirement and death following cardiac surgery in neonates and infants. Clin J Am Soc Nephrol. 2014;9:285–294. doi: 10.2215/CJN.04730513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alcaraz AJ, Gil-Ruiz A, Castillo A, et al. Postoperative neutrophil gelatinase-associated lipocalin predicts acute kidney injury after pediatric cardiac surgery. Pediatr Crit Care Med. 2014;15:121–130. doi: 10.1097/PCC.0000000000000034. [DOI] [PubMed] [Google Scholar]
- 76.Dent CL, Ma Q, Dastrala S, et al. Plasma neutrophil gelatinase-associated lipocalin predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: a prospective uncontrolled cohort study. Crit Care. 2007;11:R127. doi: 10.1186/cc6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fadel FI, Abdel Rahman AMO, Mohamed MF, et al. Plasma neutrophil gelatinase-associated lipocalin as an early biomarker for prediction of acute kidney injury after cardio-pulmonary bypass in pediatric cardiac surgery. Arch Med Sci. 2012;8:250–255. doi: 10.5114/aoms.2012.28552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Basu RK, Wong HR, Krawczeski CD, et al. Combining functional and tubular damage biomarkers improves diagnostic precision for acute kidney injury after cardiac surgery. J Am Coll Cardiol. 2014;64:2753–2762. doi: 10.1016/j.jacc.2014.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Z, Ma S, Zappitelli M, Parikh C, Wang CY, Devarajan P. Penalized count data regression with application to hospital stay after pediatric cardiac surgery. Stat Methods Med Res. 2014 doi: 10.1177/0962280214530608. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Z, Ma S, Wang CY, Zappitelli M, Devarajan P, Parikh C. EM for regularized zero-inflated regression models with applications to postoperative morbidity after cardiac surgery in children. Stat Med. 2014;33:5192–5208. doi: 10.1002/sim.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shaw AD, Chalfin DB, Kleintjens J. The economic impact and cost-effectiveness of urinary neutrophil gelatinase-associated lipocalin after cardiac surgery. Clin Ther. 2011;33:1713–1725. doi: 10.1016/j.clinthera.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 82.Ricci Z, Luciano R, Favia I, et al. High-dose fenoldopam reduces postoperative neutrophil gelatinase-associated lipocalin and cystatin C levels in pediatric cardiac surgery. Crit Care. 2011;15:R160. doi: 10.1186/cc10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Devarajan P. Review: neutrophil gelatinase-associated lipocalin: a troponin-like biomarker for human acute kidney injury. Nephrology (Carlton) 2010;15:419–428. doi: 10.1111/j.1440-1797.2010.01317.x. [DOI] [PubMed] [Google Scholar]
- 84.Devarajan P. Biomarkers for the early detection of acute kidney injury. Curr Opin Pediatr. 2011;23:194–200. doi: 10.1097/MOP.0b013e328343f4dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Devarajan P. Neutrophil gelatinase-associated lipocalin: a promising biomarker for human acute kidney injury. Biomark Med. 2010;4:265–280. doi: 10.2217/bmm.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nickolas TL, Schmidt-Ott KM, Canetta P, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J Am Coll Cardiol. 2012;59:246–255. doi: 10.1016/j.jacc.2011.10.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Haase M, Devarajan P, Haase-Fielitz A, et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57:1752–1761. doi: 10.1016/j.jacc.2010.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney diseases as interconnected syndromes. N Engl J Med. 2014;371:58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Soto K, Papoila AL, Coelho S, et al. Plasma NGAL for the diagnosis of AKI in patients admitted from the emergency department setting. Clin J Am Soc Nephrol. 2013;8:2053–2063. doi: 10.2215/CJN.12181212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Melnikov VY, Ecder T, Fantuzzi G, et al. Impaired IL-18 processing protects caspase-1 deficient mice from ischemic acute renal failure. J Clin Invest. 2001;107:1145–1152. doi: 10.1172/JCI12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Parikh CR, Mishra J, Thiessen-Philbrook H, et al. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006;70:199–203. doi: 10.1038/sj.ki.5001527. [DOI] [PubMed] [Google Scholar]
- 92.Kamijo-Ikemori A, Sugaya T, Obama A, et al. Liver-type fatty acid-binding protein attenuates renal injury induced by unilateral ureteral obstruction. Am J Pathol. 2006;169:1107–1117. doi: 10.2353/ajpath.2006.060131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Portilla D, Cent C, Sugaya T, et al. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2008;73:465–472. doi: 10.1038/sj.ki.5002721. [DOI] [PubMed] [Google Scholar]
- 94.Ichimura T, Bonventre JC, Bailly V, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 95.Yang L, Brooks CR, Xiao S, et al. KIM-1-mediated phagocytosis reduces acute injury to the kidney. J Clin Invest. 2015;125:1620–1636. doi: 10.1172/JCI75417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Han WK, Waikar SS, Johnson A, et al. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2009;76:348–349. doi: 10.1038/sj.ki.5002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Meersch M, Schmidt C, Van Aken H, et al. Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS One. 2014;9:e93460. doi: 10.1371/journal.pone.0093460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Meersch M, Schmidt C, Van Aken H, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury after pediatric cardiac surgery. PLoS One. 2014;9:e110865. doi: 10.1371/journal.pone.0110865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hoke TS, Douglas IS, Klein CL, et al. Acute renal failure after bilateral nephrectomy is associated with cytokine-mediated pulmonary injury. J Am Soc Nephrol. 2007;18:155–164. doi: 10.1681/ASN.2006050494. [DOI] [PubMed] [Google Scholar]
- 101.Liu KD, Altmann C, Smits G, et al. Serum interleukin-6 and interleukin-8 are early biomarkers of acute kidney injury and predict prolonged mechanical ventilation in children undergoing cardiac surgery: a case–control study. Crit Care. 2009;13:R104. doi: 10.1186/cc7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miklaszewska M, Korohoda P, Zachwieja K, et al. Serum interleukin 6 levels as an early marker of acute kidney injury in children after cardiac surgery. Adv Clin Exp Med. 2013;22:377–386. [PubMed] [Google Scholar]
- 103.Greenberg JH, Whitlock R, Zhang WR, et al. Interleukin-6 and interleukin-10 as acute kidney injury biomarkers in pediatric cardiac surgery. Pediatr Nephrol. 2015;30:1519–1527. doi: 10.1007/s00467-015-3088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Morgan CJ, Gill PJ, Lam S, Joffe AR. Peri-operative interventions, but not inflammatory mediators, increase risk of acute kidney injury after cardiac surgery: a prospective cohort study. Intensive Care Med. 2013;39:934–941. doi: 10.1007/s00134-013-2849-4. [DOI] [PubMed] [Google Scholar]
- 105.Finlay S, Bray B, Lewington AJ, et al. Identification of risk factors associated with acute kidney injury in patients admitted to acute medical units. Clin Med. 2013;13:233–238. doi: 10.7861/clinmedicine.13-3-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heusch G, Botker HE, Przyklenk K, Redington A, Yellon D. Remote ischemic conditioning. J Am Coll Cardiol. 2015;65:177–195. doi: 10.1016/j.jacc.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Deftereos S, Giannopoulos G, Tzalamouras V, et al. Renoprotective effect of remote ischemic post-conditioning by intermittent balloon inflations in patients undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2013;61:1949–1955. doi: 10.1016/j.jacc.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 108.Hausenloy DJ, Candilio L, Evans R, et al. Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med. 2015;373:1408–1417. doi: 10.1056/NEJMoa1413534. [DOI] [PubMed] [Google Scholar]