Abstract
Background
Helicobacter pylori (H. pylori) is a well-recognized gastroduodenal pathogen and class I carcinogen. Dual oxidase-2 (DUOX2), a member of NADPH oxidase family, has several critical physiological functions, including thyroid hormone biosynthesis and host mucosal defense.
Aim
To investigate the effect of H. pylori infection on DUOX2 gene expression in human stomach.
Materials and Methods
The biopsies were obtained from patients who underwent endoscopic diagnosis. The patient serum was assayed for two virulence factors of H. pylori, CagA IgG and VacA. The inflammation in gastric mucosa was analyzed with histology. Real-time quantitative PCR was used to detect the expression of three members of NADPH oxidase, NOX1, NOX2, and DUOX2, as well as lactoperoxidase (LPO) in the gastric mucosa. NOX2, DUOX2, and myeloperoxidase (MPO) protein levels were quantified by Western blots or immunohistochemistry.
Results
The H. pylori-infected gastric mucosa had more severe inflammation than uninfected samples. However, the expression of DUOX2 mRNA and protein was lower in gastric mucosa of patients with H. pylori infection compared to the uninfected. Among the H. pylori-infected patients, those having CagA IgG or VacA in the serum had lower DUOX2 expression levels than those infected with H. pylori without either virulence factor. The NOX2 and MPO levels were higher in those patients infected with H. pylori irrespective of the virulence factors than those uninfected patients. NOX1 and LPO mRNA were undetectable in the gastric mucosa.
Conclusion
CagA+ or VacA+ H. pylori in the stomach of patients may suppress DUOX2 expression to promote its own survival. Increased NOX2 could not eliminate H. pylori infection.
Electronic supplementary material
The online version of this article (doi:10.1007/s10620-016-4144-z) contains supplementary material, which is available to authorized users.
Keywords: Dual oxidase-2 (DUOX2), Gastric mucosa, Helicobacter pylori (H. pylori), Lactoperoxidase (LPO), NOX2
Introduction
Helicobacter pylori (H. pylori) is a Gram-negative, microaerophilic bacterium that colonizes the luminal surface of the gastric epithelium [1]. Approximately half of the world’s population is infected with H. pylori, and 15–20 % of the infected individuals develop clinical diseases [2]. Helicobacter pylori is a well-recognized gastroduodenal pathogen and a group I carcinogen. Helicobacter pylori can cause duodenal and gastric ulcers, non-ulcer dyspepsia, gastric carcinoma, and gastric mucosa-associated lymphoid tissue (MALT) lymphoma [3, 4]. However, recent evidence suggests that H. pylori protects against esophageal reflux, Barrett’s esophagus [5, 6], allergy, asthma [7], and even inflammatory bowel diseases [8]. Most microorganisms cannot survive the gastric acidity. However, H. pylori is able to secrete urease, which hydrolyzes urea to produce alkaline ammonia, thus promotes its own survival in the stomach [4]. Furthermore, H. pylori is resistant to the immune responses that it activates in gastric mucosa by using a highly sophisticated mechanism contributed by pathogenicity-related factors [9–11].
Several virulence factors of H. pylori, including urease, cytotoxin-associated gene A (CagA), and vacuolating cytotoxin A (VacA), are used clinically for strain diagnosis and virulence judgment [12, 13]. CagA, one of the most abundant H. pylori proteins, is translocated into epithelial cells by type IV secretion system [14]. The CagA gene is located in the 47-kb Cag pathogenicity island (Cag-PAI), which consists of approximately 27 genes. Cag-PAI-containing H. pylori is more virulent, partially due to the fact that Cag-PAI genes also encode type IV secretion system [15, 16]. CagA facilitates H. pylori colonization in gastric mucosa by disrupting the gap junctions, cell polarity, and modulation of signal pathways to stimulate proliferation [14, 17, 18]. VacA is a secreted protoxin, which is internalized through interference with membrane trafficking of gastric epithelial cells. Once getting inside of the cells, VacA causes cytochrome C release and cell apoptosis by destabilizing mitochondria [19].
Helicobacter pylori infection will elicit host immune responses with varied intensities depending on the bacterial strain and virulence. Pro-inflammatory cytokines, enzymes, and reactive oxygen species (ROS) produced by gastric epithelial cells and inflammatory cells (i.e., neutrophils and monocytes) all contribute to inflammation [20]. There are seven members in the family of ROS-generating nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, including NOX1–5 and two dual oxidases (DUOX1 and DUOX2). NADPH oxidases are transmembrane proteins that accept electrons from cytosolic NADPH, transport them through flavin adenine dinucleotide (FAD), membrane-imbedded hemes, and then donate a single electron to reduce oxygen to superoxide [21]. NOX1 is highly expressed in the normal colon epithelium and plays a role in intestinal inflammation [22–24]. Guinea pig gastric NOX1 is highly responsive to lipopolysaccharide from H. pylori to generate peroxide anion [25].
NOX2 is the prototype NADPH oxidase and is referred to as the phagocyte NADPH oxidase because it was first described in neutrophils and macrophages [26]. The distribution of NOX2 is not limited to phagocytes; other type of cells, such as lymphocytes, neurons, and endothelium, also express NOX2 [26, 27]. NOX2 is a major ROS-producing enzyme with bactericidal activity [22]. Here, we investigated the levels of NOX2 and myeloperoxidase (MPO), the most abundant neutrophil granule proteins, as inflammation markers in the gastric mucosa.
DUOXs have an intrinsic Ca2+-NADPH-dependent H2O2-generating activity [28]. DUOX2, but not DUOX1, was found in the epithelium of the digestive tract [29]. The physiological functions of DUOX2 include thyroid hormone biosynthesis and host mucosal defense [21]. A putative mechanism of action is via DUOX–LPO–SCN system, in which the DUOX-produced H2O2 and the ubiquitous thiocyanate (SCN−) are catalyzed by LPO to form bactericidal isothiocyanate (OSCN−) in the airway, salivary gland, and intestinal mucosal epithelial cells [30–33]. The DUOX2 and LPO are highly expressed in the lower GI tract, especially in the rectum [22, 34]. Increased DUOX2 expression was found in colonic epithelial cell in patients with inflammatory bowel disease [35–37]. Moreover, DUOX2 is regulated by NOD2 upon ligand binding in the intestine [36]. In animal studies, DUOX2 gene expression is elevated in the H. pylori-infected stomach of rhesus macaques in a Cag-PAI-dependent manner [38] and in the Salmonella typhimurium-infected mouse colon [39]. DUOX2 prevented H. felis infection and consequently reduced inflammation in the mouse stomach [40]. Whether DUOX2 gene expression is also activated by H. pylori colonization in human gastric mucosa is unclear.
In this study, we investigated NADPH oxidase, NOX1, NOX2, DUOX2, LPO, and MPO expressions in the gastric biopsies of patients with and without H. pylori infection. Higher inflammation scores, NOX2 and MPO expression levels were detected in the samples from H. pylori-infected patients compared to those uninfected. Unexpectedly, the DUOX2 protein and mRNA levels were lower in patients infected with CagA+ or VacA+ H. pylori than patients infected with H. pylori without virulence factors and uninfected patients. The latter two groups had the same DUOX2 mRNA levels. We speculate that the virulence factors of H. pylori may inhibit DUOX2 gene expression in human gastric mucosa.
Materials and Methods
Tissue Procurement
Gastric biopsies were obtained from patients with alimentary tract symptoms who underwent endoscopic diagnosis at the First Affiliated Hospital of Henan University of Science and Technology. To ensure accurate histopathological diagnosis, minimally four biopsies of gastric mucosa were collected during endoscopy at 2.5-cm-circle area from the pylorus, as well as one from gastric incisura angularis for the study. More biopsies were taken from the abnormal areas for pathological diagnosis. One biopsy was used for rapid urease test (RUT). One biopsy was fixed in 10 % formalin, embedded in paraffin, and then sectioned. The sections were stained with hematoxylin and eosin (H&E) for pathological scoring and for IHC staining. The remaining samples were stored at −80 °C for RNA extraction and Western blotting. RNAlater (Qiagen, Hilden, Germany, Cat. No. 76106) was used to prevent RNA degradation. Patient serum was collected during endoscopy and was tested for CagA IgG and VacA using enzyme-linked immunosorbent assays (ELISA). The clinical data collected from each patient included age, gender, medical history, medication usage, and endoscopy diagnosis (Table 1). Patient exclusion criteria were as follows: (1) undergoing proton pump inhibitor and antibiotic treatment, such as amoxicillin and clarithromycin, within 1 month of biopsy; (2) having esophageal or gastric cancers; (3) upper gastrointestinal bleeding; (4) severe disease in the liver, kidney, cardiovascular or cerebrovascular system; and (5) pregnant or breast-feeding. Informed consent was obtained from all patients, and the study was approved by the Clinical Research Ethics Committee of the Hospital.
Table 1.
Clinical features of gastric biopsies
| Hp+/total | Hp+/gender | Age (year)a | ||
|---|---|---|---|---|
| Male | Female | Male/female | ||
| Superficial gastritis | 68/132 | 39/71 | 29/61 | 54/53 |
| Atrophic gastritis | 1/5 | 0/2 | 1/3 | 66/58 |
| Gastric ulcer | 27/43 | 19/25 | 8/18 | 52/53 |
| Duodenal ulcer | 15/20 | 11/15 | 4/5 | 43/46 |
| Total | 111/200 | 69/113 | 42/87 | |
aThe patient’s age ranges from 18 to 70 years
Diagnosis of H. pylori Infection and Diseases
Both RUT and C13 or C14 urea breath test (UBT) were used to determine H. pylori infection without performing colony-forming assay. When either one was positive, the patient was considered colonized with H. pylori. The diagnosis of chronic superficial gastritis and atrophic gastritis was based on endoscopy and pathology [41, 42]. Intestinal metaplasia (IM) was diagnosed by the presence of goblet cells. The gastric and duodenal ulcers were diagnosed by endoscopy.
Histological Analysis
The criteria for inflammation scores in gastric mucosa were based on the Updated Sydney System [41]. The pathologic features included (1) neutrophil infiltration, (2) monocyte infiltration, (3) atrophy, and (4) IM. The score for each feature is the following: normal to very mild as 0, mild as 1, moderate as 2, and marked to severe as 3. The total scores for normal tissue are below 4. The visual analogue scale was applied to microscopic examination results [41, 43].
ELISA of CagA IgG and VacA of H. Pylori
The serum CagA IgG and VacA protein levels were measured using a CagA IgG, TSZ ELISA kit (Biotang Inc., Lexington, MA, USA, Catalog no. HU9659) and a human VacA ELISA kit (Catalog no. HU8333) following manufacturer’s instructions. Briefly, CagA IgG and VacA were detected with biotinylated monoclonal antibodies. After incubation with streptavidin–horseradish peroxidase (HRP) for 30 min, the peroxidase activity was analyzed with the addition of chromogenic substrate 3,3-,5,5-tetramethyl benzidine and hydrogen peroxide. After 15 min at room temperature, the reaction was stopped with H2SO4 and the absorbance was measured at 450 nm. According to the manufacturer’s specifications, above 1.2 ng/mL CagA IgG or 142 pg/mL VacA is considered positive (+).
IHC
Four-micron sections of paraffin-embedded samples were mounted on poly-l-lysine-coated slides. IHC was performed using a modified biotin–peroxidase complex method as described [44]. Briefly, tissue sections were dewaxed in xylene, rehydrated through graded alcohol, and the antigen retrieval was done by microwave-boiling the slides for 10 min in 0.1 N sodium citrate, pH 6.0. The endogenous peroxidase was blocked by incubating in 3 % H2O2 for 10 min, and non-specific binding was blocked by 5 % bovine serum albumin (Sigma, USA) for 20 min. Sections were incubated overnight at 4 °C with a rabbit anti-DUOX2 Ab (raised against a KLH-conjugated 501–600 amino acids of human DUOX2; Bioss, Beijing, China, Cat. No. bs-11432R). A rabbit anti-MPO Ab (1:200 dilution, Abcam, UK, Cat. No ab9535) was used to detect MPO. The DUOX2-Ab and MPO-Ab complexes were detected with biotinylated goat anti-rabbit Ab (Boster Biological Technology Co. Ltd, Wuhan, China, Cat. No SA1020) and streptavidin–HRP, and visualized with 3,3-diaminobenzidine substrate. A goat anti-NOX2 Ab (1:100 dilution, Santa Cruz, CA, USA, Cat. No sc-5826) and a biotinylated rabbit anti-goat Ab (1:300 dilution) (Boster Biological Technology Co. Ltd, Wuhan, China, Cat. No SA1023) were used as to detect NOX2. The sections were counterstained with hematoxylin. The positive control for anti-DUOX2 Ab was thyroid gland tissues, and the negative controls were either omitting the primary Ab or using an unrelated rabbit Ab.
The protein levels were evaluated blindly in 10 fields under 400× magnification from each slide. One hundred cells per field were categorized as follows: “−,” 0 %, no staining; “+,” >25 % cells were stained; “++,” 26–50 % cells were stained; and “+++,” >50 % cells stained.
Real-Time qPCR
Total RNA was extracted using TRIzol Reagent (Invitrogen, USA). Two μg of total RNA was used for cDNA synthesis using PrimeScript™ RT Master Mix (Takara, Japan) in a 40-μl reaction mixture, following these steps: 37 °C for 15 min,85 °C for 5 s, and 4 °C for 10 min. The primer sequences for DUOX2, NOX1, NOX2, LPO, MPO, and β-actin (Table 2) were designed by using Primer3.0 software [45] and synthesized by Sangon Biotechnology (Zhengzhou, China). Real-time qPCR was done with a CFX96™ Real-Time PCR system (Bio-Rad Labs, USA). In 25-µl reaction mixture contained 2 μl of cDNA, 12.5 μl of 2 × SYBR Premix Ex Taq II (Takara, Japan), 8.5 μl of H2O, and 2 ul of 0.4 µM primers. A two-step method was used for DUOX2, NOX1, LPO, and β-actin cDNAs detection because of the 60 °C annealing temperature; the reaction had an initial step of 95 °C for 30 s and 40 cycles of 95 °C for 5 s plus 60 °C for 30 s. A three-step method (40 cycles of 95 °C for 5 s, 57 °C for 30 s, and 72 °C for 30 s) was used for NOX2 cDNA, which had 57 °C annealing temperature. Each sample was assayed in triplicates. The efficiency of PCR amplification was 97–105 %. A melting curve was performed to evaluate product specificity. RNA levels were quantified using the Ct (2−ΔΔCt) method and normalized to β-actin. The gene is considered as not expressed when the Ct value is higher than 35.
Table 2.
Primer sequence for quantitative real-time PCR
| mRNA | Gene | Primer sequence | Amplicon (bp) | |
|---|---|---|---|---|
| NM-014080 | DUOX2 | Forward | 5′-CCTCAGGACCACCATGCTAT | 133 |
| Reserve | 5′-CTGCAGGGAGTTGAAGAAGG | |||
| NM-007052 | NOX1 | Forward | 5′-TTTGTCGGCCTTCTCATATT | 159 |
| Reserve | 5′-GAATCTTCCCTGTTGCCTAGAA | |||
| NM-000397 | NOX2 | Forward | 5′-AATCCCTGCTCCCACTAACA | 108 |
| Reserve | 5′-TTTCAAGATGCGTGGAAACTAC | |||
| NM-006151 | LPO | Forward | 5′-CAGAGCTCATGGCGGTCTTC | 122 |
| Reserve | 5′-TACCACAAGAGCGCAGACTAC | |||
| NM-001101 | β-actin | Forward | 5′-CTCTTCCAGCCTTCCTTCCT | 116 |
| Reserve | 5′-AGCACTGTGTTGGCGTACAG |
Western Blotting Analysis
Protein lysates were prepared from tissues by homogenization in RIPA lysis buffer (Soliarbio Co., Beijing, China) on ice with a grinder. The supernatant was collected after centrifugation for 15 min at 12,000 rpm and determined for protein concentrations with bicinchoninic acid (BCA, Solarbio Company, Beijing). Thirty µg of protein was resolved by 10 % SDS-PAGE and then transferred onto polyvinylidene difluoride membranes (Millipore; USA). A rabbit anti-DUOX2 Ab (200×, Abcam, UK; Cat. No. ab65813; raised against 400–500 a.a. of human DUOX2) and rabbit anti-GAPDH r Ab (10,000×, Abcam; UK, Cat. No. ab37168) were used as primary Ab. After hybridization with the HRP-conjugated anti-rabbit IgG (300X, Boster Biological Technology Co. Ltd, Wuhan, China, Cat. No. BA1054), the Ag/Ab complex was detected by an enhanced chemiluminescence reagent (Pierce; Minneapolis, MN, USA). The image was captured by ChemiDoc XRS (Bio-Rad, USA), and the intensity was quantified with ImageJ 1.48v program (NIH, USA).
Statistical Analysis
Student’s t test and Mann–Whitney U test were used to assess the difference in each pair. One-way ANOVA and Kruskal–Wallis tests were used to compare the data among at least three groups. Significant difference was defined as P < 0.05. Data were reported as mean ± standard error of the mean (SEM). All statistical analysis was performed by using the SPSS 19.0 statistics package (SPSS Inc., Chicago, IL, USA).
Results
Helicobacter pylori Infection Rate and Distribution of CagA+ IgG and VacA+ in Patients with Gastritis
Among the 200 patients recruited in the study, 113 patients were male and 87 were female (Table 1). Besides the higher disease incidence in males than females, there was no gender difference in disease characteristics. The average ages of male and female were 53.5 and 52.7 years. Most patients (66 %, 132/200) had chronic superficial gastritis; other patients had atrophic gastritis (2.5 %, 5/200), gastric ulcer (21.5 %, 43/200), and duodenal ulcer (10 %, 20/200). While only one of the five atrophic gastritis patients was positive for H. pylori infection, 52 % (68/132), 63 % (27/43), and 75 % (15/20) of patients with superficial gastritis, gastric ulcer, and duodenal ulcer, respectively, were positive for H. pylori infection. The total H. pylori infection rate was 55.5 % (111/200) (Table 1). This distribution pattern of H. pylori infection is similar to other studies in China [46].
We evaluated H. pylori virulence with CagA IgG and VacA ELISA on 82 of 111 patient sera. We found that 58.5 % of patients (n = 48) were negative for both CagA and VacA (CagA−/VacA−), 9.7 % (n = 8) were CagA−/VacA+, 13.4 % (n = 11) were CagA+/VacA−, and 18.3 % (n = 15) were double positive (CagA+/VacA+). The CagA IgG+ rate in our study was also similar to other studies conducted in China [47].
Helicobacter pylori+ Patients Had Higher Inflammation in Gastric Mucosa than Uninfected Patients
We analyzed the inflammation scores and compared the scores between the H. pylori-negative (Hp−) and H. pylori-positive (Hp+) groups (Table 3). The pathology scores were based on inflammation, atrophy, and intestinal metaplasia (IM). The inflammation, but not atrophy or IM, was significantly higher in the Hp+ group.
Table 3.
Inflammation in gastric mucosa of patients
| Hp | Normal/very mild | Mild | Medium | Severe | ||||
|---|---|---|---|---|---|---|---|---|
| – | + | – | + | – | + | – | + | |
| Inflammation | 36 | 10 | 37 | 32 | 11 | 34a | 5a | 35b |
| Atrophy | 74 | 83 | 14 | 25 | 1 | 2 | 0 | 1 |
| IM | 80 | 89 | 8 | 17 | 1 | 3 | 0 | 2 |
IM intestinal metaplasia
aMore Hp+ patient with medium inflammation compared to Hp− patients (P = 0.002)
bMore Hp+ patients with severe inflammation compared to Hp− subjects (P < 0.0001)
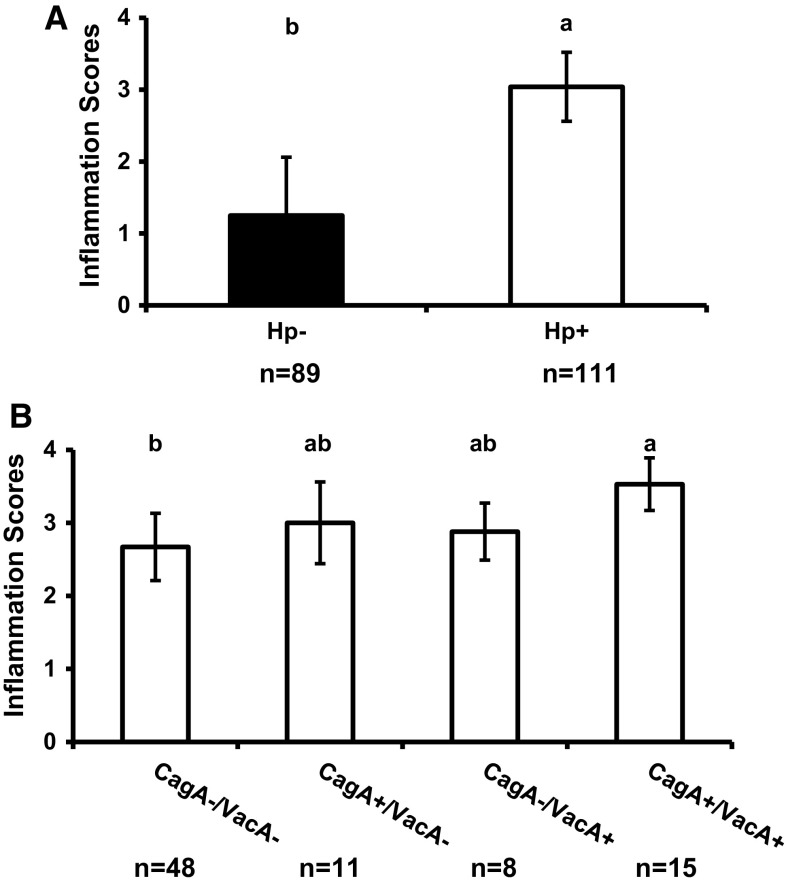
We also compared the inflammation scores between the Hp+ and Hp− groups as well as among the subgroups of Hp+ patients based on CagA IgG and VacA in their serum (Fig. 1a, b). The inflammation scores in gastric mucosa of Hp+ patients were significantly higher than Hp- group. Also, the inflammation scores of patients of CagA+/VacA+ group are significantly higher than CagA−/VacA− group (Fig. 1b). However, the inflammation scores of patients positive for CagA IgG or VacA+ H. pylori infection were not different from any other infected groups.
Fig. 1.
Comparison of gastric inflammation scores in Hp− and Hp+ patients. a The comparison between Hp+ and Hp− group, and b the comparison of Hp+ patients categorized by CagA and VacA expressions. The error bar shows SEM. Different letters above the columns indicate that the means are different, where a > b (P < 0.05). The columns that share a letter are not different, i.e., ab is not different from either a or b
Detection of Pro-Oxidant Gene Expression by qPCR
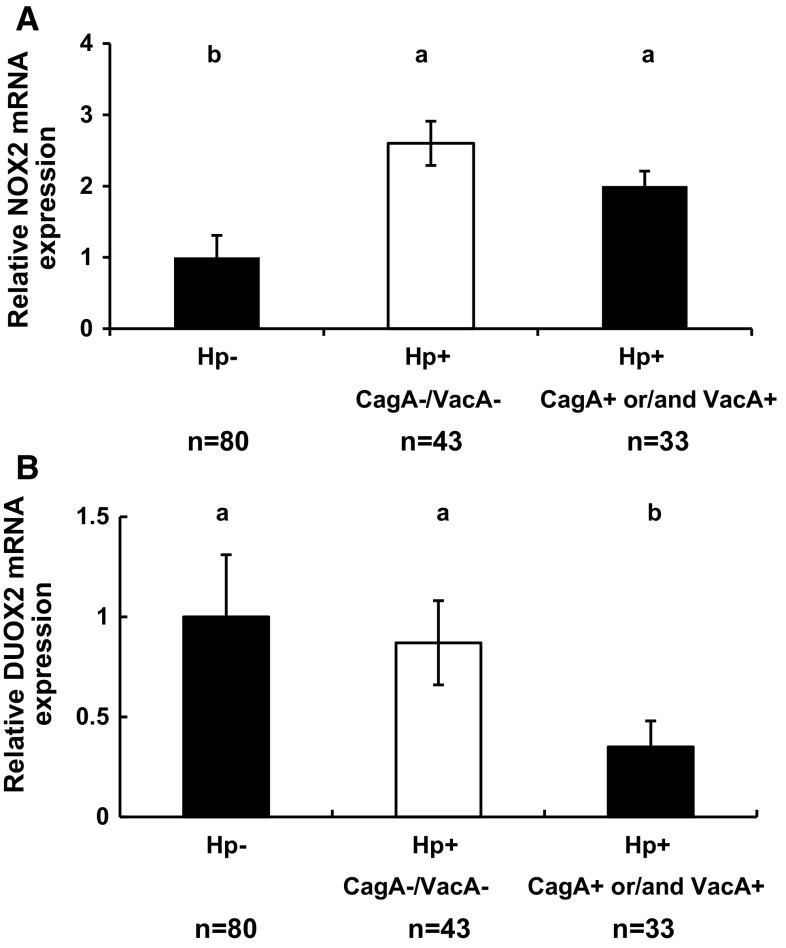
NOX2 protein is highly expressed in the neutrophils and macrophages, which are recruited into the inflammatory sites by H. pylori infection [48]. The NOX2 mRNA level is twofold higher in the gastric mucosa of Hp+ patients than in Hp− patients, and no difference is found among the infected groups stratified with CagA and VacA status (Fig. 2a and Supplementary Figure 1A).
Fig. 2.
Comparison of NOX2 and DUOX2 mRNA levels in the gastric mucosa of Hp− and Hp+ patients. a Comparison of NOX2 mRNA levels between Hp− patients, Hp+ CagA−VacA−, and patients infected with Hp+ secreted one of CagA and VacA or both. b Comparison of DUOX2 mRNA levels in the same groups as NOX2. The message levels are normalized with beta-actin mRNA levels. The error bars are SEM. Different letters above the columns indicate that the means are different, where a > b (P < 0.05)
Because DUOX2 protected against Helicobacterfelis infection in mouse stomach [40], we analyzed DUOX2 mRNA levels in our patients. Unexpectedly, the DUOX2 RNA level is decreased in the gastric mucosa of patients who are infected with the H. pylori expressing either of the virulence factors compared to Hp + patients without either virulence factors and in Hp- patients (Fig. 2b and Supplementary Figure 1A). The DUOX2 mRNA level of the CagA−/VacA− group is similar to that in the Hp- group.
Because DUOX2 and LPO have been postulated to form a defense mechanism against bacterial infection and LPO is expressed in the intestine [22, 34], we also analyzed LPO gene expression in the gastric mucosa. No LPO mRNA can be detected in both Hp+ and Hp− patients (n = 20 for each group; data not shown). Furthermore, because NOX1 plays a role in wound-healing [49] and promotes inflammation in a mouse model of ileocolitis [24], we also analyzed NOX1 mRNA levels in the gastric mucosa. Similar to LPO, no NOX1 mRNA was detected in the gastric mucosa (n = 20 each for Hp+ and Hp− groups, as well as two IM samples (data not shown).
Detection of DUOX, NOX2, and MPO Protein Expression
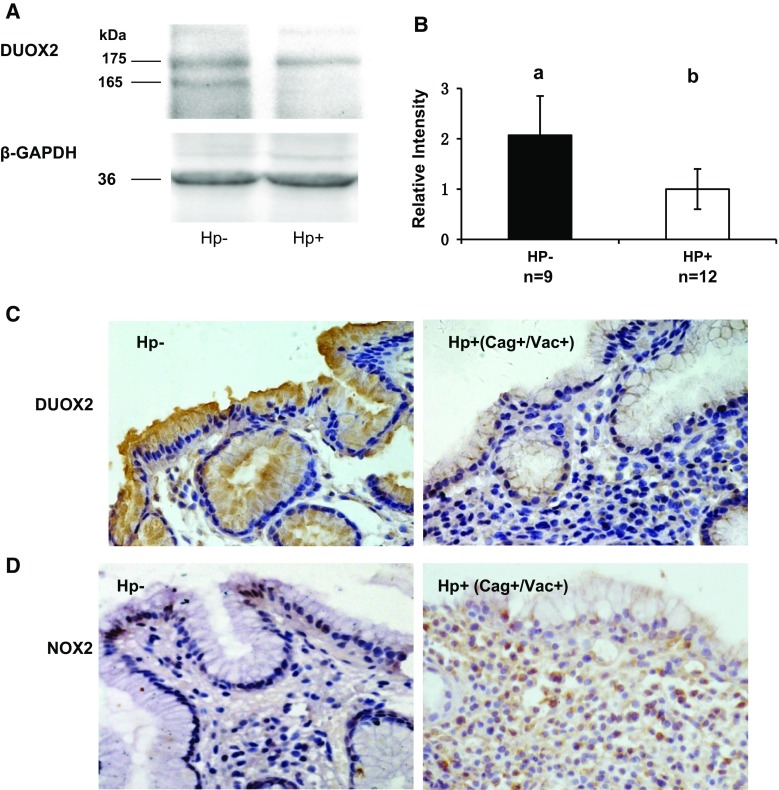
We quantified DUOX protein levels with Western blotting (Fig. 3a, b). Consistent with DUOX2 mRNA levels, twofold higher DUOX protein level was present in Hp− gastric mucosa than Hp+ samples. After confirming the Ab specificity by IHC of thyroid tissues (data not shown), this antibody was used to compare DUOX2 protein levels in the gastric mucosa among the Hp+ subgroups and Hp− group by IHC. A stronger DUOX staining was detected in Hp− patients especially at the apical surface, although it is also present in the cytoplasm of the epithelial cells (Fig. 3c). Consistent with DUOX2 mRNA levels, mucosa of CagA+ or VacA+ group had weaker DUOX2 staining than the CagA−/VacA− and Hp- groups (Table 4). Also, the CagA−/VacA− group had similar DUOX2 staining intensity as the Hp− group.
Fig. 3.
Comparison of DUOX protein expression in gastric mucosa of Hp− and Hp+ patients. a Western blot of DUOX and GAPDH from a representative Hp− and Hp+ patients. The expected m.w. of DUOX is 175 kDa, and the Hp− has an additional 165-kDa band recognized by anti-DUOX2 Ab (Abcam, which cannot distinguish DUOX2 from DUOX1). b Bar graph of DUOX protein quantified from the Western blots analyzed from 9 Hp− and 12 Hp+ patients. The error bar is SEM; the different letters above the columns indicate that the means are different, where a > b (P < 0.05). c IHC stained gastric mucosa from Hp− and Hp+ (CagA+/VacA) patients. Hp+ gastric mucosa has more infiltrating cells and weaker DUOX staining (brown color; the original magnification is ×400). d That there is higher NOX2 staining in inflammatory cells in the Hp+ (CagA+/VacA) gastric mucosa than that in Hp− (brown color; the original magnification is ×400)
Table 4.
DUOX2 immunohistochemical staining
| Intensity | Hp−a (n = 51) | CagA−/VacA−b (n = 15) | CagA+ or VacA+ (n = 33) |
|---|---|---|---|
| + | 9 | 2 | 15 |
| ++ | 27 | 4 | 16 |
| +++ | 15 | 9 | 2 |
a P < 0.01 compared between Hp− group and patients with at least one virulence factor, CagA+ or VacA+
b P < 0.05 compared between patients without virulence factors (CagA−/VacA−) and with at least one virulence factor, CagA+ or VacA+
NOX2 IHC was performed on the gastric mucosa of Hp+ and Hp− groups of patients (n = 20 for each group, Fig. 3d). In the Hp+ groups of patients, a stronger NOX2 staining was detected in the infiltrating inflammatory cells compared to that in Hp− gastric mucosa (IHC scores not shown). Similarly to NOX2 IHC pattern, a higher level of MPO+ inflammatory cells is detected in the Hp+ groups than in the Hp− patients (n = 15 for each group, data not shown).
Discussion
In this study, we found that the gastric mucosa of patients infected with H. pylori expressing either CagA+ or VacA+ had a lower level of DUOX2 expression than patients infected with CagA−/VacA− H. pylori and uninfected patients. This result suggests that the virulence factors in H. pylori are able to suppress DUOX2 expression. Helicobacter pylori can survive in the host by defying the host innate and adaptive immune systems that it triggers [12, 50]. CagA and VacA promote mucosal inflammation through their multifactorial functions, such as induction of pro-inflammatory cytokines, disruption of cell conjunction regulation of host immunity, activation of proliferation and apoptosis [12]. These two virulence factors can manipulate and inhibit human T cells [11]. CagA mediates H. pylori colonization on the apical surface of gastric epithelium by manipulating host’s immune response to allow its persistence in the host [20]. The intracellular CagA forms a complex with the oncogenic c-Met to stimulate cell proliferation [18]. Since DUOX2 is expressed in the mature epithelium [29], and an in vitro study has shown that H. pylori infection induces oxidative stress-associated cell death leading to loss of DUOX2-expressing gastric epithelial cells [51], these may explain why the epithelial cells from CagA+ or VagA+ patients have lower DUOX2 expression. Alternatively, the decrease in DUOX2 expression in the gastric mucosa of Hp + patients may be a part of the strategy of H. pylori to evade host’s innate immune response [30, 31]. The exact mechanism for H. pylori to suppress DUOX2 expression needs to be further investigated.
Clearly, our finding is different from the animal studies. Grasberger et al. [40] reported that DUOX2 protected mouse stomach from H. felis infection and colonization, and Hornsby et al. found that H. pylori infection induced DUOX2 mRNA expression in the stomach of rhesus macaques [41]. Although H. felis can infect human stomach, it does not cause gastric pathology [52]. It is possible that H. felis induces a different response in mice compared to human’s response to H. pylori. After the rhesus macaques infected with Cag-PAI-positive H. pylori, the DUOX2 mRNA levels were elevated at weeks 4 and 8 and decreased at week 13. This result suggests that H. pylori induce DUOX2 expression temporally; after the initial induction, the H. pylori-infected cells may progress to adapting the infection. It is possible that the long-term infected patients had a different response from that in the newly infected animals.
H. pylori-infected human gastric epithelial cells recruited inflammatory cells, including neutrophils and macrophages, which induced bactericidal ROS, most notably from NOX2 [53–56]. Helicobacter pylori encodes an array of antioxidant enzymes, including catalase, superoxide dismutase, and a unique bacterial peroxiredoxin, to reduce ROS and escape from ROS-mediated injury [57, 58]. Other studies suggest that H. pylori infection activates NOX2 activity to launch inflammatory responses but fails to eradicate bacterial colonization [59, 60]. Although the virulence factors affected the severity of inflammation, in which CagA+/VagA+-infected patients had more severe inflammation than other subgroups, these factors did not further enhance NOX2 gene expression. Our result supports the view that H. pylori infection activates NOX2 gene expression. The NOX2 gene induction may occur in macrophages rather than neutrophils. The mature neutrophils have few mRNA, and NOX2 protein is synthesized and stored in the granules. Upon stimulation, the mature neutrophils are recruited to the inflammatory site without the need for de novo synthesis of proteins [61, 62]. MPO is a marker for neutrophils and monocytes [24]. A similar pattern of NOX2 and MPO protein expression suggests that either NOX2 or MPO is a good marker for infiltrating inflammatory cells.
DUOX2 has been proposed to defend against bacterial infection by teaming up with LPO and SCN. Although we did not detect LPO mRNA in gastric mucosa, because LPO is highly expressed in the salivary gland, it is possible that LPO can be ingested into the stomach to form a DUOX–LPO–SCN antimicrobial defense network as in the airway and the lower intestine [22, 30]. NOX1 is highly expressed in normal colon but not detectable in normal stomach [26, 27, 55]. A few studies showed that NOX1 was present in gastric cancer [63], and its transcript was detected in H. pylori-associated gastric diseases [64]. In our hands, NOX1 mRNA is not detectable in the gastric epithelium regardless of H. pylori infection status and existing intestinal metaplasia.
In this study, we demonstrated the presence of DUOX2 in the human gastric mucosa. Unexpectedly, the levels of DUOX2 expressed in the gastric mucosa of patients infected with VacA+ or CagA+ H. pylori were lower than patients infected with VacA−/CagA− H. pylori and uninfected patients. This result suggests that VacA or CagA of H. pylori can modulate DUOX2 gene expression in gastric epithelial cells to promote its own survival. How VacA and CagA can affect DUOX2 gene expression is worthy of further studies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Dr. Ran Qi, Yonggan Fan, Xianli Liu, and Wenzhao Zhao for their help to this study. This study was supported by a Grant (No. 81370487 to Q.Gao) from National Natural Science Foundation of China.
Compliance with ethical standards
Conflict of interest
All authors declare no conflict of interest.
Footnotes
Hongqian Li and Yunfeng Zhou have contributed equally to this study.
Contributor Information
Hongqian Li, Email: 1550613332@qq.com.
Yunfeng Zhou, Email: zhouyf1980@szu.edu.cn.
Yufeng Zheng, Email: 15896573698@163.com.
Hong Guo, Email: guohongguohong@126.com.
Lei Gao, Email: a_ha_2001@163.com.
Pan Chen, Email: chpp24016@163.com.
Dandan Feng, Email: 305950651@qq.com.
Lijuan Wu, Email: wlj6968@163.com.
Moli Yang, Email: 475949639@qq.com.
Yanli Qi, Email: qyly1@126.com.
Hao Guo, Email: xinxianggh@126.com.
Yongchao Chang, Email: hkdyfyjyk@sina.com.
Fong-Fong Chu, Email: fchu@coh.org.
Qiang Gao, Phone: +86-010-56981364, Email: gaoq@mail.haust.edu.cn.
References
- 1.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. doi: 10.1016/S0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 2.Everhart JE. Recent developments in the epidemiology of Helicobacter pylori. Gastroenterol Clin North Am. 2000;29:559–578. doi: 10.1016/S0889-8553(05)70130-8. [DOI] [PubMed] [Google Scholar]
- 3.Malnick SD, Melzer E, Attali M, Duek G, Yahav J. Friend or foe? World J Gastroenterol. 2014;20:8979–8985. doi: 10.3748/wjg.v20.i27.8979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362:1597–1604. doi: 10.1056/NEJMcp1001110. [DOI] [PubMed] [Google Scholar]
- 5.Sonnenberg A, Lash RH, Genta RM. A national study of Helicobactor pylori infection in gastric biopsy specimens. Gastroenterology. 2010;139:1894–1901. doi: 10.1053/j.gastro.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Corley DA, Kubo A, Levin TR, Block G, Habel L, Rumore G, et al. Helicobacter pylori and gastroesophageal reflux disease: a case-control study. Helicobacter. 2008;13:352–360. doi: 10.1111/j.1523-5378.2008.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med. 2007;167:821–827. doi: 10.1001/archinte.167.8.821. [DOI] [PubMed] [Google Scholar]
- 8.Luther J, Dave M, Higgins PD, Kao JY. Association between Helicobacter pylori infection and inflammatory bowel disease: a meta-analysis and systematic review of the literature. Inflamm Bowel Dis. 2010;16:1077–1084. doi: 10.1002/ibd.21116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gebert B, Fischer W, Haas R. The Helicobacter pylori vacuolating cytotoxin: from cellular vacuolation to immunosuppressive activities. Rev Physiol Biochem Pharmacol. 2004;152:205–220. doi: 10.1007/s10254-004-0027-3. [DOI] [PubMed] [Google Scholar]
- 10.Yuan J, Li P, Tao J, Shi X, Hu B, Chen H, et al. H. pylori escape host immunoreaction through inhibiting ILK expression by VacA. Cell Mol Immunol. 2009;6:191–197. doi: 10.1038/cmi.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salama NR, Hartung ML, Muller A. Life in the human stomach: persistence strategies of the bacterial pathogen Helicobacter pylori. Nat Rev Microbiol. 2013;11:385–399. doi: 10.1038/nrmicro3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Posselt G, Backert S, Wessler S. The functional interplay of Helicobacter pylori factors with gastric epithelial cells induces a multi-step process in pathogenesis. Cell Commun Signal. 2013;11:77. doi: 10.1186/1478-811X-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bridge DR, Merrell DS. Polymorphism in the Helicobacter pylori CagA and VacA toxins and disease. Gut Microbes. 2013;4:101–117. doi: 10.4161/gmic.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones KR, Whitmire JM, Merrell DS. A Tale of Two Toxins: Helicobacter Pylori CagA and VacA modulate host pathways that impact disease. Front Microbiol. 2010;1:115. doi: 10.3389/fmicb.2010.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Censini S, Lange C, Xiang Z, Crabtree JE, Ghiara P, Borodovsky M, et al. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Odenbreit S, Puls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497–1500. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]
- 17.Backert S, Tegtmeyer N, Selbach M. The versatility of Helicobacter pylori CagA effector protein functions: the master key hypothesis. Helicobacter. 2010;15:163–176. doi: 10.1111/j.1523-5378.2010.00759.x. [DOI] [PubMed] [Google Scholar]
- 18.McCracken KW, Cata EM, Crawford CM, Sinagoga KL, Schumacher M, Rockich BE, et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature. 2014;516:5. doi: 10.1038/nature13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rassow J. Helicobacter pylori vacuolating toxin A and apoptosis. Cell Commun Signal. 2011;9:26. doi: 10.1186/1478-811X-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease. J Clin Invest. 2004;113:321–333. doi: 10.1172/JCI20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katsuyama M, Matsuno K, Yabe-Nishimura C. Physiological roles of NOX/NADPH oxidase, the superoxide-generating enzyme. J Clin Biochem Nutr. 2012;50:9–22. doi: 10.3164/jcbn.11-06SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rada B, Leto TL. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib Microbiol. 2008;15:164–187. doi: 10.1159/000136357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szanto I, Rubbia-Brandt L, Kiss P, Steger K, Banfi B, Kovari E, et al. Expression of NOX1, a superoxide-generating NADPH oxidase, in colon cancer and inflammatory bowel disease. J Pathol. 2005;207:164–176. doi: 10.1002/path.1824. [DOI] [PubMed] [Google Scholar]
- 24.Esworthy RS, Kim BW, Chow J, Shen B, Doroshow JH, Chu FF. Nox1 causes ileocolitis in mice deficient in glutathione peroxidase-1 and -2. Free Radic Biol Med. 2014;68:315–325. doi: 10.1016/j.freeradbiomed.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teshima S, Rokutan K, Nikawa T, Kishi K. Guinea pig gastric mucosal cells produce abundant superoxide anion through an NADPH oxidase-like system. Gastroenterology. 1998;115:1186–1196. doi: 10.1016/S0016-5085(98)70090-3. [DOI] [PubMed] [Google Scholar]
- 26.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 27.Salles N, Szanto I, Herrmann F, Armenian B, Stumm M, Stauffer E, et al. Expression of mRNA for ROS-generating NADPH oxidases in the aging stomach. Exp Gerontol. 2005;40:353–357. doi: 10.1016/j.exger.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Ameziane-El-Hassani R, Morand S, Boucher JL, Frapart YM, Apostolou D, Agnandji D, et al. Dual oxidase-2 has an intrinsic Ca2 + -dependent H2O2-generating activity. J Biol Chem. 2005;280:30046–30054. doi: 10.1074/jbc.M500516200. [DOI] [PubMed] [Google Scholar]
- 29.El Hassani RA, Benfares N, Caillou B, Talbot M, Sabourin JC, Belotte V, et al. Dual oxidase2 is expressed all along the digestive tract. Am J Physiol Gastrointest Liver Physiol. 2005;288:G933–G942. doi: 10.1152/ajpgi.00198.2004. [DOI] [PubMed] [Google Scholar]
- 30.Bae YS, Choi MK, Lee WJ. Dual oxidase in mucosal immunity and host-microbe homeostasis. Trends Immunol. 2010;31:278–287. doi: 10.1016/j.it.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Allaoui A, Botteaux A, Dumont JE, Hoste C, De Deken X. Dual oxidases and hydrogen peroxide in a complex dialogue between host mucosae and bacteria. Trends Mol Med. 2009;15:571–579. doi: 10.1016/j.molmed.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Moskwa P, Lorentzen D, Excoffon KJ, Zabner J, McCray PB, Jr, Nauseef WM, et al. A novel host defense system of airways is defective in cystic fibrosis. Am J Respir Crit Care Med. 2007;175:174–183. doi: 10.1164/rccm.200607-1029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geiszt M, Witta J, Baffi J, Lekstrom K, Leto TL. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 2003;17:1502–1504. doi: 10.1096/fj.02-1104fje. [DOI] [PubMed] [Google Scholar]
- 34.Kim BW, Esworthy RS, Hahn MA, Pfeifer GP, Chu FF. Expression of lactoperoxidase in differentiated mouse colon epithelial cells. Free Radic Biol Med. 2012;52:1569–1576. doi: 10.1016/j.freeradbiomed.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacFie TS, Poulsom R, Parker A, Warnes G, Boitsova T, Nijhuis A, et al. DUOX2 and DUOXA2 form the predominant enzyme system capable of producing the reactive oxygen species H2O2 in active ulcerative colitis and are modulated by 5-aminosalicylic acid. Inflamm Bowel Dis. 2014;20:514–524. doi: 10.1097/01.MIB.0000442012.45038.0e. [DOI] [PubMed] [Google Scholar]
- 36.Lipinski S, Till A, Sina C, Arlt A, Grasberger H, Schreiber S, et al. DUOX2-derived reactive oxygen species are effectors of NOD2-mediated antibacterial responses. J Cell Sci. 2009;122:3522–3530. doi: 10.1242/jcs.050690. [DOI] [PubMed] [Google Scholar]
- 37.Csillag C, Nielsen OH, Vainer B, Olsen J, Dieckgraefe BK, Hendel J, et al. Expression of the genes dual oxidase 2, lipocalin 2 and regenerating islet-derived 1 alpha in Crohn’s disease. Scand J Gastroenterol. 2007;42:454–463. doi: 10.1080/00365520600976266. [DOI] [PubMed] [Google Scholar]
- 38.Hornsby MJ, Huff JL, Kays RJ, Canfield DR, Bevins CL, Solnick JV. Helicobacter pylori induces an antimicrobial response in rhesus macaques in a cag pathogenicity island-dependent manner. Gastroenterology. 2008;134:1049–1057. doi: 10.1053/j.gastro.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esworthy RS, Kim BW, Wang Y, Gao Q, Doroshow JH, Leto TL, et al. The Gdac1 locus modifies spontaneous and Salmonella-induced colitis in mice deficient in either Gpx2 or Gpx1 gene. Free Radic Biol Med. 2013;65:1273–1283. doi: 10.1016/j.freeradbiomed.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grasberger H, El-Zaatari M, Dang DT, Merchant JL. Dual oxidases control release of hydrogen peroxide by the gastric epithelium to prevent Helicobacter felis infection and inflammation in mice. Gastroenterology. 2013;145:1045–1054. doi: 10.1053/j.gastro.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney system. International workshop on the histopathology of gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Fang JYLW, Li ZS, Du YQ, Ji XL, Ge ZZ, Li YQ, et al. Consensus on chronic gastritis in China-third national consensus meeting on chronic gastritis, (2012 Shanghai, China) Chin J Dig Dis. 2013;2013:11. [Google Scholar]
- 43.Koh H, Noh TW, Baek SY, Chung KS. Nodular gastritis and pathologic findings in children and young adults with Helicobacter pylori infection. Yonsei Med J. 2007;48:240–246. doi: 10.3349/ymj.2007.48.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao Q, Meijer MJ, Kubben FJ, Sier CF, Kruidenier L, van Duijn W, et al. Expression of matrix metalloproteinases-2 and -9 in intestinal tissue of patients with inflammatory bowel diseases. Dig Liver Dis. 2005;37:584–592. doi: 10.1016/j.dld.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 45.Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, et al. Primer3–new capabilities and interfaces. Nucleic Acids Res. 2012;40:e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh K, Ghoshal UC. Causal role of Helicobacter pylori infection in gastric cancer: an Asian enigma. World J Gastroenterol. 2006;12:1346–1351. doi: 10.3748/wjg.v12.i9.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Limburg P, Qiao Y, Mark S, Wang G, Perez-Perez G, Blaser M, et al. Helicobacter pylori seropositivity and subsite-specific gastric cancer risks in Linxian, China. J Natl Cancer Inst. 2001;93:226–233. doi: 10.1093/jnci/93.3.226. [DOI] [PubMed] [Google Scholar]
- 48.Cadamuro AC, Rossi AF, Maniezzo NM, Silva AE. Helicobacter pylori infection: host immune response, implications on gene expression and microRNAs. World J Gastroenterol. 2014;20:1424–1437. doi: 10.3748/wjg.v20.i6.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leoni G, Alam A, Neumann PA, Lambeth JD, Cheng G, McCoy J, et al. Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J Clin Invest. 2013;123:443–454. doi: 10.1172/JCI65831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson KT, Crabtree JE. Immunology of Helicobacter pylori: insights into the failure of the immune response and perspectives on vaccine studies. Gastroenterology. 2007;133:288–308. doi: 10.1053/j.gastro.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 51.Ding SZ, Minohara Y, Fan XJ, Wang J, Reyes VE, Patel J, et al. Helicobacter pylori infection induces oxidative stress and programmed cell death in human gastric epithelial cells. Infect Immun. 2007;75:4030–4039. doi: 10.1128/IAI.00172-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fritz EL, Slavik T, Delport W, Olivier B, van der Merwe SW. Incidence of Helicobacter felis and the effect of coinfection with Helicobacter pylori on the gastric mucosa in the African population. J Clin Microbiol. 2006;44:1692–1696. doi: 10.1128/JCM.44.5.1692-1696.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Q, Dawodu JB, Etolhi G, Husain A, Gemmell CG, Russell RI. Relationship between the mucosal production of reactive oxygen radicals and density of Helicobacter pylori in patients with duodenal ulcer. Eur J Gastroenterol Hepatol. 1997;9:261–265. doi: 10.1097/00042737-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 54.Davies GR, Simmonds NJ, Stevens TR, Sheaff MT, Banatvala N, Laurenson IF, et al. Helicobacter pylori stimulates antral mucosal reactive oxygen metabolite production in vivo. Gut. 1994;35:179–185. doi: 10.1136/gut.35.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rokutan K, Kawahara T, Kuwano Y, Tominaga K, Nishida K, Teshima-Kondo S. Nox enzymes and oxidative stress in the immunopathology of the gastrointestinal tract. Semin Immunopathol. 2008;30:315–327. doi: 10.1007/s00281-008-0124-5. [DOI] [PubMed] [Google Scholar]
- 56.Keenan JI, Peterson RA, 2nd, Hampton MB. NADPH oxidase involvement in the pathology of Helicobacter pylori infection. Free Radic Biol Med. 2005;38:1188–1196. doi: 10.1016/j.freeradbiomed.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 57.Wang G, Alamuri P, Maier RJ. The diverse antioxidant systems of Helicobacter pylori. Mol Microbiol. 2006;61:847–860. doi: 10.1111/j.1365-2958.2006.05302.x. [DOI] [PubMed] [Google Scholar]
- 58.Bryk R, Griffin P, Nathan C. Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature. 2000;407:211–215. doi: 10.1038/35025109. [DOI] [PubMed] [Google Scholar]
- 59.Allen LA, Beecher BR, Lynch JT, Rohner OV, Wittine LM. Helicobacter pylori disrupts NADPH oxidase targeting in human neutrophils to induce extracellular superoxide release. J Immunol. 2005;174:3658–3667. doi: 10.4049/jimmunol.174.6.3658. [DOI] [PubMed] [Google Scholar]
- 60.Satin B, Del Giudice G, Della Bianca V, Dusi S, Laudanna C, Tonello F, et al. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a protective antigen and a major virulence factor. J Exp Med. 2000;191:1467–1476. doi: 10.1084/jem.191.9.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sheshachalam A, Srivastava N, Mitchell T, Lacy P, Eitzen G. Granule protein processing and regulated secretion in neutrophils. Front Immunol. 2014;5:448. doi: 10.3389/fimmu.2014.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kruger P, Saffarzadeh M, Weber AN, Rieber N, Radsak M, von Bernuth H, et al. Neutrophils: between host defence, immune modulation, and tissue injury. PLoS Pathog. 2015;11:e1004651. doi: 10.1371/journal.ppat.1004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tominaga K, Kawahara T, Sano T, Toida K, Kuwano Y, Sasaki H, et al. Evidence for cancer-associated expression of NADPH oxidase 1 (Nox1)-based oxidase system in the human stomach. Free Radic Biol Med. 2007;43:1627–1638. doi: 10.1016/j.freeradbiomed.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 64.Augusto AC, Miguel F, Mendonca S, Pedrazzoli J, Jr, Gurgueira SA. Oxidative stress expression status associated to Helicobacter pylori virulence in gastric diseases. Clin Biochem. 2007;40:615–622. doi: 10.1016/j.clinbiochem.2007.03.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.