Abstract
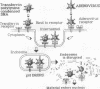
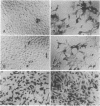
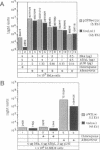
One limit to successful receptor-mediated gene delivery is the exit of the endocytosed material from the endosome. We demonstrate here the delivery of marker genes to tissue culture cells using a modification of the receptor-mediated gene delivery technique that exploits the endosomolytic activity of defective adenovirus particles. In particular, greater than 90% of the transfected-cell population is found to express a beta-galactosidase gene, and, most importantly, this high level of expression can be obtained with psoralen-inactivated virus particles. Furthermore, because the delivered gene is not carried within the genome of the adenovirus particle, the size constraints are relieved, and we can, therefore, show the delivery of a 48-kilobase cosmid DNA molecule.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkner K. L. Development of adenovirus vectors for the expression of heterologous genes. Biotechniques. 1988 Jul-Aug;6(7):616–629. [PubMed] [Google Scholar]
- Chardonnet Y., Dales S. Early events in the interaction of adenoviruses with HeLa cells. I. Penetration of type 5 and intracellular release of the DNA genome. Virology. 1970 Mar;40(3):462–477. doi: 10.1016/0042-6822(70)90189-3. [DOI] [PubMed] [Google Scholar]
- Collis P., Antoniou M., Grosveld F. Definition of the minimal requirements within the human beta-globin gene and the dominant control region for high level expression. EMBO J. 1990 Jan;9(1):233–240. doi: 10.1002/j.1460-2075.1990.tb08100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotten M., Längle-Rouault F., Kirlappos H., Wagner E., Mechtler K., Zenke M., Beug H., Birnstiel M. L. Transferrin-polycation-mediated introduction of DNA into human leukemic cells: stimulation by agents that affect the survival of transfected DNA or modulate transferrin receptor levels. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4033–4037. doi: 10.1073/pnas.87.11.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel D. T., Agarwal S., Wagner E., Cotten M. Adenovirus enhancement of transferrin-polylysine-mediated gene delivery. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8850–8854. doi: 10.1073/pnas.88.19.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel D. T., Wagner E., Cotten M., Birnstiel M. L., Agarwal S., Li C. M., Loechel S., Hu P. C. High-efficiency gene transfer mediated by adenovirus coupled to DNA-polylysine complexes. Hum Gene Ther. 1992 Apr;3(2):147–154. doi: 10.1089/hum.1992.3.2-147. [DOI] [PubMed] [Google Scholar]
- Donti E., Longo L., Tonini G. P., Verdona G., Melodia A., Lanino E., Cornaglia-Ferraris P. Cytogenetic and molecular study of two human neuroblastoma cell lines. Cancer Genet Cytogenet. 1988 Feb;30(2):225–231. doi: 10.1016/0165-4608(88)90188-4. [DOI] [PubMed] [Google Scholar]
- Fernández-Puentes C., Carrasco L. Viral infection permeabilizes mammalian cells to protein toxins. Cell. 1980 Jul;20(3):769–775. doi: 10.1016/0092-8674(80)90323-2. [DOI] [PubMed] [Google Scholar]
- Hearst J. E., Thiry L. The photoinactivation of an RNA animal virus, vesicular stomatitis virus, with the aid of newly synthesized psoralen derivatives. Nucleic Acids Res. 1977;4(5):1339–1347. doi: 10.1093/nar/4.5.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Shenk T. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanne D., Straub K., Rapoport H., Hearst J. E. Psoralen-deoxyribonucleic acid photoreaction. Characterization of the monoaddition products from 8-methoxypsoralen and 4,5'8-trimethylpsoralen. Biochemistry. 1982 Mar 2;21(5):861–871. doi: 10.1021/bi00534a008. [DOI] [PubMed] [Google Scholar]
- Keller G., Paige C., Gilboa E., Wagner E. F. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985 Nov 14;318(6042):149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Characterization of DNA condensates induced by poly(ethylene oxide) and polylysine. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4288–4292. doi: 10.1073/pnas.72.11.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemay P., Boudin M. L., Milleville M., Boulanger P. Human adenovirus type 2 protein IIIa. I. Purification and characterization. Virology. 1980 Feb;101(1):131–143. doi: 10.1016/0042-6822(80)90490-0. [DOI] [PubMed] [Google Scholar]
- Lim K., Chae C. B. A simple assay for DNA transfection by incubation of the cells in culture dishes with substrates for beta-galactosidase. Biotechniques. 1989 Jun;7(6):576–579. [PubMed] [Google Scholar]
- Lin L., Wiesehahn G. P., Morel P. A., Corash L. Use of 8-methoxypsoralen and long-wavelength ultraviolet radiation for decontamination of platelet concentrates. Blood. 1989 Jul;74(1):517–525. [PubMed] [Google Scholar]
- MacGregor G. R., Caskey C. T. Construction of plasmids that express E. coli beta-galactosidase in mammalian cells. Nucleic Acids Res. 1989 Mar 25;17(6):2365–2365. doi: 10.1093/nar/17.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olins D. E., Olins A. L. Model nucleohistones: the interaction of F1 and F2al histones with native T7 DNA. J Mol Biol. 1971 May 14;57(3):437–455. doi: 10.1016/0022-2836(71)90102-1. [DOI] [PubMed] [Google Scholar]
- Otero M. J., Carrasco L. Proteins are cointernalized with virion particles during early infection. Virology. 1987 Sep;160(1):75–80. doi: 10.1016/0042-6822(87)90046-8. [DOI] [PubMed] [Google Scholar]
- Pearse B. M., Crowther R. A. Structure and assembly of coated vesicles. Annu Rev Biophys Biophys Chem. 1987;16:49–68. doi: 10.1146/annurev.bb.16.060187.000405. [DOI] [PubMed] [Google Scholar]
- Quantin B., Perricaudet L. D., Tajbakhsh S., Mandel J. L. Adenovirus as an expression vector in muscle cells in vivo. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2581–2584. doi: 10.1073/pnas.89.7.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M. A., Yoshimura K., Trapnell B. C., Yoneyama K., Rosenthal E. R., Dalemans W., Fukayama M., Bargon J., Stier L. E., Stratford-Perricaudet L. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992 Jan 10;68(1):143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- Samulski R. J., Chang L. S., Shenk T. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. J Virol. 1989 Sep;63(9):3822–3828. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P., Fitzgerald D. J., Willingham M. C., Pastan I. Role of a low-pH environment in adenovirus enhancement of the toxicity of a Pseudomonas exotoxin-epidermal growth factor conjugate. J Virol. 1984 Sep;51(3):650–655. doi: 10.1128/jvi.51.3.650-655.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P., Fitzgerald D., Ginsberg H., Willingham M., Pastan I. Evidence that the penton base of adenovirus is involved in potentiation of toxicity of Pseudomonas exotoxin conjugated to epidermal growth factor. Mol Cell Biol. 1984 Aug;4(8):1528–1533. doi: 10.1128/mcb.4.8.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J. T., Leng M., Felsenfeld G. Deoxyribonucleic acid-polylysine complexes. Structure and nucleotide specificity. Biochemistry. 1969 Aug;8(8):3219–3232. doi: 10.1021/bi00836a014. [DOI] [PubMed] [Google Scholar]
- Stratford-Perricaudet L. D., Levrero M., Chasse J. F., Perricaudet M., Briand P. Evaluation of the transfer and expression in mice of an enzyme-encoding gene using a human adenovirus vector. Hum Gene Ther. 1990 Fall;1(3):241–256. doi: 10.1089/hum.1990.1.3-241. [DOI] [PubMed] [Google Scholar]
- Tratschin J. D., West M. H., Sandbank T., Carter B. J. A human parvovirus, adeno-associated virus, as a eucaryotic vector: transient expression and encapsidation of the procaryotic gene for chloramphenicol acetyltransferase. Mol Cell Biol. 1984 Oct;4(10):2072–2081. doi: 10.1128/mcb.4.10.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E., Cotten M., Foisner R., Birnstiel M. L. Transferrin-polycation-DNA complexes: the effect of polycations on the structure of the complex and DNA delivery to cells. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4255–4259. doi: 10.1073/pnas.88.10.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E., Cotten M., Mechtler K., Kirlappos H., Birnstiel M. L. DNA-binding transferrin conjugates as functional gene-delivery agents: synthesis by linkage of polylysine or ethidium homodimer to the transferrin carbohydrate moiety. Bioconjug Chem. 1991 Jul-Aug;2(4):226–231. doi: 10.1021/bc00010a006. [DOI] [PubMed] [Google Scholar]
- Wagner E., Zatloukal K., Cotten M., Kirlappos H., Mechtler K., Curiel D. T., Birnstiel M. L. Coupling of adenovirus to transferrin-polylysine/DNA complexes greatly enhances receptor-mediated gene delivery and expression of transfected genes. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6099–6103. doi: 10.1073/pnas.89.13.6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E., Zenke M., Cotten M., Beug H., Birnstiel M. L. Transferrin-polycation conjugates as carriers for DNA uptake into cells. Proc Natl Acad Sci U S A. 1990 May;87(9):3410–3414. doi: 10.1073/pnas.87.9.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. L., Hsu M. T. Psoralen-cross-linking study of the organization of intracellular adenovirus nucleoprotein complexes. J Virol. 1988 Apr;62(4):1227–1234. doi: 10.1128/jvi.62.4.1227-1234.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. Y., Wilson J. M., Shalaby F., Grossman M., Shafritz D. A., Wu C. H. Receptor-mediated gene delivery in vivo. Partial correction of genetic analbuminemia in Nagase rats. J Biol Chem. 1991 Aug 5;266(22):14338–14342. [PubMed] [Google Scholar]
- Wu G. Y., Wu C. H. Receptor-mediated in vitro gene transformation by a soluble DNA carrier system. J Biol Chem. 1987 Apr 5;262(10):4429–4432. [PubMed] [Google Scholar]