Abstract
Case series
Patient: Male, 87 • Male, 62
Final Diagnosis: Spinal subdural abscess
Symptoms: Fever • pain • weakness
Medication: —
Clinical Procedure: Laminectomy • durotomy • drainage • debridement
Specialty: Neurosurgery
Objective:
Rare disease
Background:
Spinal subdural abscesses, also known as empyemas, are rare infectious lesions, the exact incidence of which is unknown. Presentation is typically dramatic, with back pain, fever, motor, and sensory deficits. Rapid identification and surgical intervention with laminectomy, durotomy, and washout provides the best outcomes. While hematogenous spread of an extra-spinal infection is the most common cause of this condition, a significant number of cases result from iatrogenic mechanisms, including lumbar punctures, epidural injections, and surgery.
Case Report:
Here we present 2 cases: 1) an 87-year-old man with type 2 diabetes, schizophrenia, mild cognitive impairment, and symptomatic lumbar spinal stenosis and 2) a 62-year-old man with a prior L3–4 spinal fusion with symptomatic lumbar spinal stenosis. In both cases, patients underwent laminectomy for spinal stenosis and developed epidural abscess. Following successful drainage of the epidural abscess, they continued to be symptomatic, and repeat imaging revealed the presence of a subdural abscess that was subsequently evacuated. Case 1 had significant improvement with residual lower-extremity weakness, while Case 2 made a complete neurological recovery.
Conclusions:
These cases illustrate patients at increased risk for developing this rare spinal infection, and demonstrate that rapid recognition and surgical treatment is key to cure and recovery. Review of the literature highlights pertinent risk factors and demonstrates nearly one-third of reported cases have an iatrogenic etiology. The cases presented here demonstrate that a subdural process should be suspected in any patient with intractable pain following treatment of an epidural abscess.
MeSH Keywords: Empyema, Subdural; Epidural Abscess; Staphylococcal Infections
Background
Subdural abscesses are suppurative infections of the space between the dura and arachnoid. More common intracranially, such an infection in the spinal cord is rare [1]. Less than 70 cases have been reported in adults [2–48], with an additional 73 reported in pediatric populations [49]. Here, we report 2 additional cases and provide a comprehensive review of all adult cases reported to date. In the first case, a laminectomy for spinal stenosis was complicated by a spinal abscess initially treated with drainage and IV antibiotics. Upon completing the antibiotic course, the infection recrudesced as a subdural abscess requiring emergent surgical intervention. Case 2 is, to the best of our knowledge, only the second case reported in the literature of a dural tear serving as a precursor to subdural infection.
Case Report
Case 1
History and clinical presentation
An 87-year-old man with type 2 diabetes, schizophrenia, mild cognitive impairment, and symptomatic lumbar spinal stenosis underwent uncomplicated L3-4 laminectomies with partial laminotomies of L2 and L5. Three months after the initial operation, he presented to an outside hospital with lethargy, weakness, and groin pain, and was found to have methicillin-resistant Staph. aureus (MRSA) bacteremia and MRSA spinal abscess for which he underwent incision and drainage, followed by a 6-week course of vancomycin at a skilled nursing facility. One week after completion of antibiotics and discharge to home, the patient began having back, buttock, and groin pain, and again presented to an outside hospital, where he was noted to have a white blood cell count of 23.1 × 109/L and blood cultures positive for MRSA.
On arrival, his affect was altered, his speech was tangential, and he appeared profoundly encephalopathic. He was unable to accurately localize his pain, but his abdomen, groin, and thoracic spine were tender to palpation. He had a low-grade fever of 38.2°C. His basic metabolic panel (BMP) was remarkable for sodium of 128 mEQ/L and a blood glucose of 265 mg/dL. The physical exam was limited by patient cooperation; however, there appeared to be lower-extremity weakness bilaterally; sensory function appeared grossly intact. Review of outside hospital spinal MRI revealed extensive thoracic and lumbar spinal abscess compressing the spinal cord, as well as vertebral osteomyelitis (Figure 1). Radiology officially read this exam as epidural abscess. At this time, he underwent emergent surgery.
Figure 1.
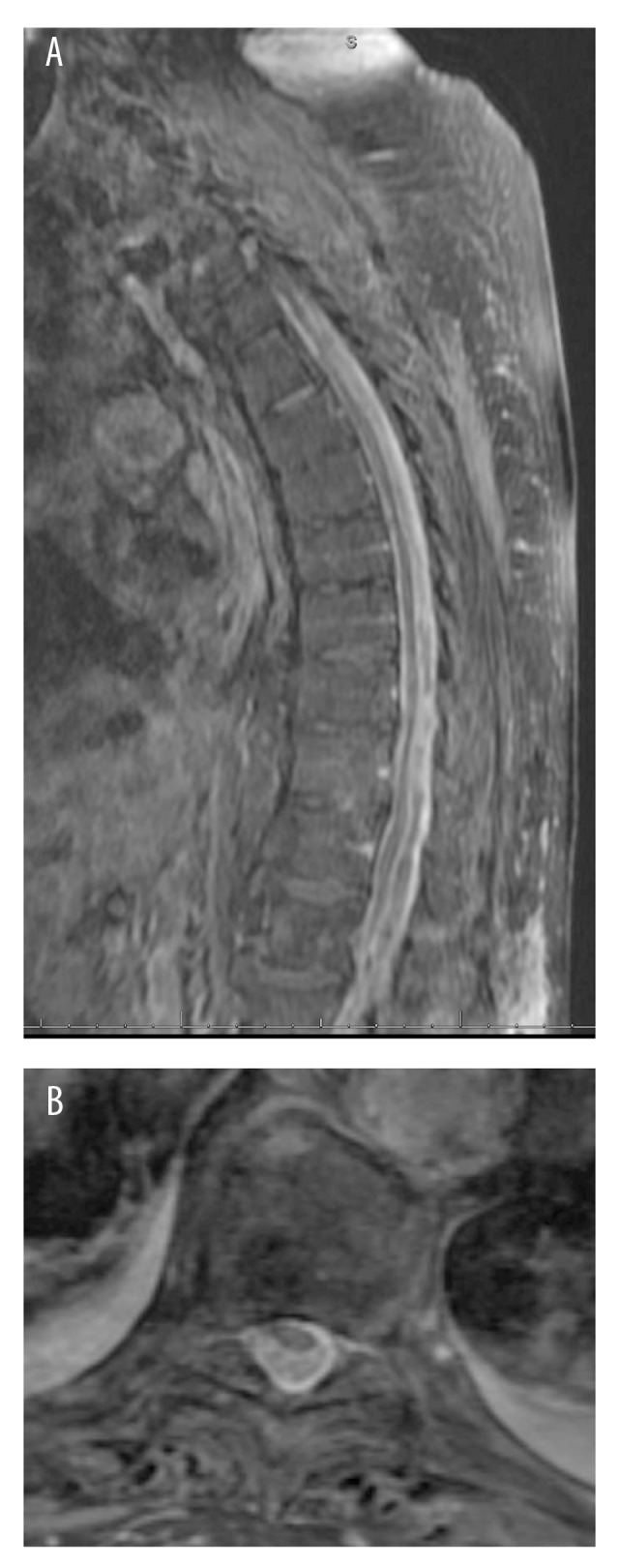
(A) Sagittal T1-weighted with contrast, and (B) Axial T1-weighted with contrast MRI that arrived with the patient from an outside hospital, showing extensive abscess with cord compression.
Operative procedure
Dissection down to the spine revealed no purulent material. An L2 laminectomy was performed, which uncovered an inflamed and thickened dura, but no compression or abscess, as was expected by preoperative imaging. Right-sided hemilaminectomies were performed also at T10 and T12, again revealing no epidural abscess. Due to the concern for subdural abscess, the dura was incised at L2, returning frank pus that was cultured and later grew MRSA. Based on MRI imaging showing extensive thoracic involvement and now confirmed subdural abscess, the T10 laminectomy was completed and a new T5 laminectomy was created. Incision of the dura at T10 again expressed frank pus and this was copiously irrigated; the T5 dural incision returned small amounts of pus and hemosiderin-stained material. Using a combination of red rubber catheters, vancomycin-containing irrigation was instilled at all 3 dural openings, flushing out pus and adherent phlegmon. This process was continued until clear fluid returned. The dural openings were sutured and covered with Tisseel, and the remaining wound was washed-out with vancomycin solution.
Post-operative course
The patient was continued on vancomycin, 1 gram every 12 h, with vancomycin troughs showing therapeutic levels. Despite this treatment, he had a persistent leukocytosis. Repeat MRI of the spine on post-operative day 6 demonstrated improvement of the subdural empyema but residual subdural abscess at T12–L2, epidural abscess at T11–12, and a new lumbar paravertebral abscess. MRI of the brain at this time revealed new scattered bilateral hemispheric foci of restricted diffusion and FLAIR hyperintensity, concerning for cardiogenic septic emboli, although a trans-esophageal echocardiogram revealed no valvular lesions and his blood cultures remained negative.
The paravertebral abscess was drained by interventional radiology; cultures grew MRSA. In consultation with the infectious disease service, he was placed on combination IV vancomycin (trough goal 15–20 mg/L) and oral rifampin (450 mg twice daily), a standard regimen for epidural abscess [50]. The patient remained afebrile, and inflammatory markers began to fall. He continued to slowly improve, but it took many weeks for his encephalopathy to improve. He was ultimately discharged on post-operative day 54. On follow-up 4 months later, he was alert and interactive without any complaints of pain. Motor strength was 4/5 in bilateral lower extremities. Inflammatory markers had normalized, and repeat imaging revealed improvement of osteomyelitis and gross resolution of the subdural abscess.
Case 2
History and clinical presentation
A 62-year-old man with a lumbar 3–4 (L3–4) spinal fusion and laminectomy 6 years prior presented with progressive back pain, thigh numbness, intermittent urinary incontinence, and radicular right leg pain. Magnetic resonance imaging (MRI) without contrast revealed severe stenosis at L2–3 and he underwent an L2 laminectomy and revision of his L3 laminectomy. A dural tear complicated this revision surgery, though the arachnoid appeared intact and no spinal fluid leak was noted. At that time the tear was covered with Tisseel fibrin sealant (Baxter Healthcare Corp.; Deerfield, IL, USA) and the patient was kept supine 24 h postoperatively. Spinal instrumentation significantly increases perioperative infection risk [50]; with this in mind, all standard precautions against infection were strictly adhered to, including double-gloving, copious surgical site prepping, use perioperative antibiotics, and careful attention to hemostasis. No break in sterility was noted during the operation. The patient recovered well from this procedure, with improving pain and no signs of infection until 2 weeks later, when he noticed purulent drainage from the wound and acutely increased back pain. He presented to his local emergency department, where he received 1 dose of vancomycin and ampicillin/sulbactam before urgent transfer to our facility. He denied any fever or chills, but noted increased lower-extremity weakness, which he attributed to intense pain with movement.
Upon arrival he had a 1-cm wound dehiscence draining dark yellow fluid. His exam revealed bilateral thigh numbness, stable from his baseline. Strength testing of the hamstrings and quadriceps was limited by pain; strength was otherwise intact bilaterally. MRI revealed an epidural abscess and he was taken to the OR for incision and drainage. Cultures grew methicillin-sensitive Staph. aureus (MSSA) and he was started on rifampin and cefazolin in the post-operative period. After failure of medical management of continued severe pain for 5 days, a repeat MRI was ordered to evaluate any other possible sources. This MRI demonstrated a complex, multi-loculated, enhancing fluid collection in the lumbar spine read by neuroradiology as epidural abscess (Figure 2), and he was taken to the OR for repeat drainage.
Figure 2.
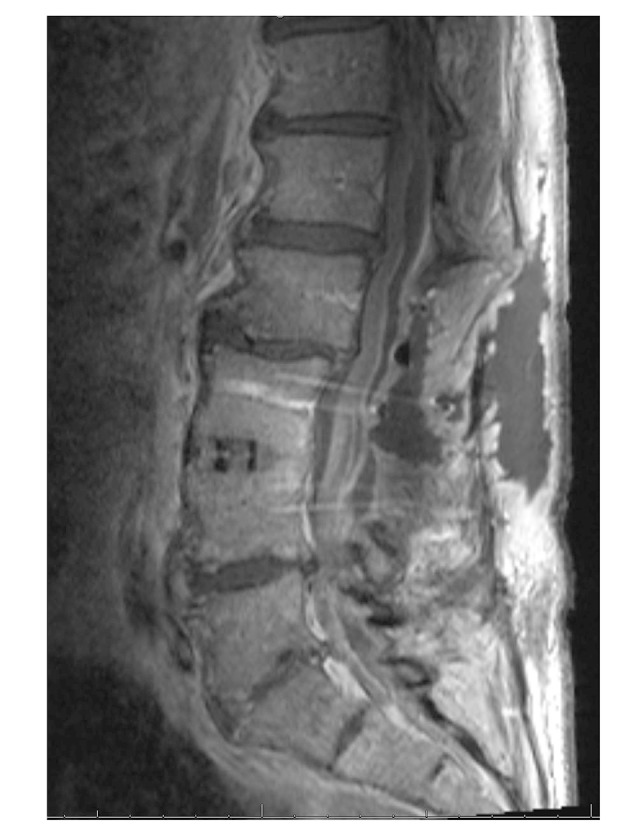
T1-weighted image with contrast taken 5 days after epidural drainage, demonstrating complex fluid collection identified at surgery as a subdural abscess.
Operative procedure
On dissection down to the fascia, some seroma was encountered but no frank pus. The fascia was opened along its prior lines, and again no pus was encountered. The dura was inflamed and thickened without purulence. The previously noted dural tear was identified, with intact but opaque arachnoid and no sign of cerebrospinal fluid (CSF) egress. The old hardware at L4–5 was removed, and a midline incision in the dura was made. There was a thick phlegmon tightly adherent to the nerve roots and dura. After careful debridement, the subdural space was irrigated with a vancomycin solution using a red rubber catheter. When irrigating cranially, only clear fluid returned. Caudally, there appeared to be more phlegmon, so an L5 laminectomy was performed. Incision of the dura at this level expressed frank pus, and there was again thickly adherent phlegmon. After debridement, the subdural space was again irrigated with vancomycin solution and the dura was closed. The previous dural tear could not be approximated, and it was covered with a muscle patch. Tisseel was placed over the suture line; no CSF leak was noted with Valsalva maneuver.
Postoperative course
The patient made a quick recovery and was mobile within 2 days after his operation. His exam demonstrated 5/5 strength throughout his bilateral lower extremities. In consultation with infectious disease service, he was discharged home on postop day 8 with an infusion pump to complete a 6-week course of intravenous vancomycin (750 mg every 8 h) and cefepime (2 g every 12 h), the standard regimen used at our institution for Staph. bacteremia. On 3-month follow-up, inflammatory markers had normalized. He denied any fever, back pain, weakness, numbness, or problems with bowel/bladder control, and is pleased with his surgical outcome.
Discussion and Review of Literature
Spinal subdural abscesses are rare, with the first case reported in 1927 [51]. A comprehensive review of the English-language literature found 66 cases in adults (including the 2 in the present study), with an additional 73 cases reported in pediatric populations [49]. Below, we provide a review of adult patient populations, risk factors, history and clinical presentation, treatment, and etiology.
Incidence and risk factors
The exact incidence of spinal subdural abscess is unknown; however, multiple longitudinal single-institution studies suggest it is quite rare [17,52]. One recent study found just 4 cases over a 10-year period, compared to 68 incidences of epidural spinal abscess [52].
Our literature review demonstrated that spinal subdural abscess is equally common in men and women, with a male: female ratio of 0.9. It is most commonly seen in men between the ages of 40 and 70, with peak incidence in the 6th decade of life. In women it appears slightly later, between the ages of 50 and 70, with peak incidence in the 7th decade. Subdural abscess is most commonly seen in the lumbar spine, with a significant number having extension into the thoracic spine, as was seen in Case 1. The infection has been described at every level of the spine, including cases with evidence for intracranial involvement and spread throughout the entire spinal axis [36,53].
There were a significant number of cases in which the patient had risk factors for infection (Table 1). Many of these risk factors have been identified as factors contributing to surgical site infection during spinal surgery [50]. Diabetes was very commonly seen (15% of cases). Other immune-modulating conditions encountered included HIV/AIDS and rheumatoid arthritis. Intravenous drug use provides a mechanism for bacteremia and direct hematogenous seeding of the spinal cord [26,38,41]. Complications of pregnancy and delivery, including septic abortion [51] and endometritis [11], provide a source for spinal infection, as does use of epidural anesthesia [9]. Dermoid cyst is a more common source of infection in pediatric populations, but there is 1 case of an adult presenting with an infected cyst [40]. Finally, a large group of patients (12.1% of cases), including the patients presented here, had pre-existing spinal disease. These included symptomatic spinal stenosis [48], herniated disc [23,24], degenerative disc disease [28], and ruptured disc [17]. All of these patients had surgery or injections performed, which was likely the initial source of infection; however, it is also possible that spinal disease itself is a predisposing factor for infection, secondary to chronic inflammatory changes.
Table 1.
Predisposing factors. Table of predisposing factors associated with formation of spinal subdural abscess. Only patients with identified risk factors are counted.
| Factor | Number of cases (%) |
|---|---|
| Diabetes | 10 (15.2) |
| Spinal disease* | 8 (12.1) |
| Intravenous drug abuse | 5 (7.5) |
| Pregnancy | 4 (6.1) |
| Dermoid cyst | 1 (1.5) |
| HIV | 1 (1.5) |
| Multiple sclerosis | 1 (1.5) |
| Rheumatoid arthritis | 1 (1.5) |
Spinal stenosis, degenerative disc disease, ruptured disc, herniated disc.
History and clinical presentation
As early as 1948, Heusner suggested the clinical progression of spinal epidural abscess: 1) spinal pain, 2) root pain, 3) weakness, sensory loss, and/or loss of bowel/bladder control, and finally 4) paralysis [54]. This progression is similar in subdural abscess [4,45], with fever and pain the most common initial or presenting symptoms, and motor, sensory, and loss of sphincter control happening as a late event in the course (Figure 3). The most common symptom is back pain, which is often accompanied by fever. This pain can be localized or radicular in quality. In Case 1, the presenting complaint was lower-extremity and groin pain with weakness; in Case 2, the patient presented with fever and intractable back pain and eventually developed weakness before intervention.
Figure 3.
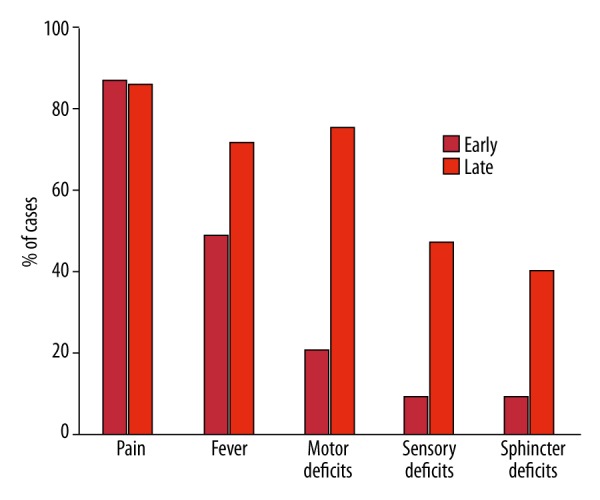
Early and late symptoms of spinal subdural abscess. Early symptoms are the symptoms at presentation or the initial symptoms in the clinical course. Late symptoms are any symptoms present immediately before intervention.
Definitive diagnosis can be made with MRI with contrast, and it is the preferred modality for detection of subdural spinal abscess [24,26,34]. In the vast majority of cases in which MRI was used, abscess was detected. The differentiation of epidural and subdural abscess can sometimes be made based on MRI imaging alone, with a typical finding of preservation of the epidural fat [24]. In both of our cases, the preoperative radiological diagnosis was epidural abscess and subdural abscess was discovered at surgery. Practically, a mass-occupying abscess requires urgent surgical drainage; therefore, pre-operative localization to the epidural vs. subdural space is not necessarily required.
Other diagnostic tests that may be useful are inflammatory markers, including erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), as well as leukocyte count, which are nonspecific but sensitive for infection. Notably, only 68% of cases in which white count was reported demonstrated a leukocytosis, while nearly all had elevated ESR (94.1%) and CRP (100%). Both of the patients reported here had an elevated white count and markedly elevated CRP.
Treatment and outcome
The definitive treatment is drainage and antibiotics; 57 reported cases received this treatment. Complete recovery was seen in 42% of surviving patients, while 14% of patients died. Partial recovery was seen in the remaining 44% of cases and ranges from residual numbness and weakness to complete paralysis. Of the 5 patients that received no treatment or treatment only with antibiotics, all died [2,14,19,44,53].
The cases presented here are an interesting study in the efficacy of antibiotics alone vs. antibiotics and drainage. Case 1 had a previous spinal abscess that was drained; after a full 6-week course of antibiotics, his pain and weakness returned and he presented with subdural abscess and encephalopathy. It is not clear if his subdural abscess was present at the time of the initial operation or if it developed later, but the fact that the spinal infection recrudesced rapidly upon antibiotic cessation suggests antibiotic therapy alone would never have been curative. It is possible that Case 2 had concomitant epidural and subdural abscesses; after drainage of the epidural abscess, his back pain persisted and in fact worsened, even with antibiotic treatment. His rapid recovery after subdural irrigation demonstrates the necessity of surgical intervention.
Surgical drainage in our 87-year-old patient was accomplished with a conservative approach; the abscess extending through the thoracolumbar subdural space was irrigated after performing 3 laminectomies at L2, T10, and T5. Red rubber catheters were used to ensure irrigation of the entire space. While most surgeries for subdural spinal abscess involve multiple consecutive laminectomies with durotomy, these cases and others [24,38] demonstrate that good outcomes can be obtained with limited skip laminectomies for the satisfactory drainage of a subdural abscess spanning multiple levels.
Etiology
In both of the cases reported here and in the majority of cases reported to date, Staphylococcus aureus was the most frequently implicated causative organism (56% of cases). Other reported organisms include anaerobes [16,28,40,44], M. tuberculosis [31,34], E. coli [4,32], and various Streptococcus species (Table 2). The predominance of Staph. suggests spread from soft-tissue infections or nosocomial infections, both of which are common sources in these patients. Staph. bacteremia can result in multiple abscesses in the epidural and subdural space as well as in adjacent soft tissue [29], as seen in our first case.
Table 2.
Causative organisms. Table of causative organisms of subdural spinal abscess.
| Organism | Number of cases (%) |
|---|---|
| S. aureus | 37 (56.1) |
| Anaerobes | 4 (6.0) |
| E. coli | 2 (3.0) |
| Beta hemolytic Strep. | 2 (3.0) |
| S. viridans | 2 (3.0) |
| S. milleri | 2 (3.0) |
| M. tuberculosis | 2 (3.0) |
| S. agalactae | 1 (1.5) |
| S. intermedius | 1 (1.5) |
| Microaerophilic Staph. | 1 (1.5) |
| D. pneumoniae | 1 (1.5) |
| Pseudomonas | 1 (1.5) |
| M. hominis | 1 (1.5) |
| Unknown | 10 (13.6) |
The pathophysiology of spinal subdural abscess formation remains unclear, but it is thought that 3 major mechanisms can account for seeding of the infection: hematogenous spread from a distant site, direct spread from adjacent infection, and iatrogenic sources. Hematogenous spread is the most common route; the most common source of infection is soft-tissue abscess, furunculosis, and cellulitis (Table 3). The most common sources of direct spread are infection secondary to congenital deformities or dysraphism (especially in pediatric populations) [49], decubitus ulcers [44,53], and retropharyngeal abscess [8,26].
Table 3.
Etiology of spinal subdural abscess. Table showing the mechanism of infection for spinal subdural abscess, with presumed source. Percent of total cases in parenthesis.
| Hematogenous spread | 24 (36.4) |
| Soft tissue infection/abscess | 11 (16.7) |
| Bacteremia unknown source | 6 (9.1) |
| Urinary tract infection | 2 (3.0) |
| Respiratory infection | 2 (3.0) |
| Endometritis | 2 (3.0) |
| Endocarditis | 1 (1.5) |
| Direct spread | 8 (12.1) |
| Meningitis | 3 (4.5) |
| Decubitus ulcer | 2 (3.0) |
| Retropharyngeal abscess | 2 (3.0) |
| Dermoid cyst | 1(1.5) |
| Iatrogenic | 22 (33.3) |
| Epidural Injection | 6 (9.1) |
| Lumbar Puncture | 6 (9.1) |
| Spinal Surgery | 6 (9.1) |
| Other Injection | 4 (6.1) |
| Lumbar Drain | 1 (1.5) |
| Unknown | 12 (18.2) |
There were a striking number of cases with iatrogenic etiology (33% of reported cases) – nearly as many as those caused by hematogenous spread. Lumbar puncture in the setting of meningitis or bacteremia is thought to provide a direct route for seeding [15,16,33]. Similarly, epidural injections [9,17,20,24,27,46] allow a direct route for inoculation. Therapeutic injections into and around the spine have caused subdural abscess in 4 cases; this includes 1 case of acupuncture for neck pain [7] and 1 complication from contrast injection for discography [28]. In an unusual case, a man with a history of uncomplicated injections for thoracic back pain developed a spinal subdural abscess acutely after an acromial bursal injection [10].
In addition to Case 2 presented here, there is only 1 other incidence of a dural tear acting as the presumed site of entry into the subdural space [48]. In the other case, a dural tear complicated a laminectomy for spinal stenosis; no CSF egress was noted. The patient’s postoperative course was complicated by S. aureus pneumonia and bacteremia, and she went on to develop an S. aureus subdural abscess 10 days later. In our case, the inadvertent dural tear from the earlier operation likely facilitated spread to the subdural space from an epidural abscess. By contrast, our first case illustrates spread of an epidural abscess into the subdural space without a known existing dural defect. Of note, this patient also developed a psoas abscess and had evidence of septic emboli on brain MRI. It is therefore possible that septic emboli could have seeded the epidural and subdural spaces independently.
Conclusions
While rare, spinal subdural abscess can cause permanent disability or death if not quickly recognized and treated. The 2 cases presented here demonstrate a typical clinical course and treatment with limited but effective laminectomy and durotomy followed by copious irrigation. Our review of the literature highlights pertinent risk factors, including previous spine disease, and demonstrates nearly one-third of cases in the adult literature have an iatrogenic source. This report demonstrates that epidural spinal abscesses can precede subdural spinal abscesses, and a subdural process should be suspected in any patient with intractable symptoms following successful epidural abscess drainage.
References:
- 1.De Bonis P, Anile C, Pompucci A, et al. Cranial and spinal subdural empyema. Br J Neurosurg. 2009;23(3):335–40. doi: 10.1080/02688690902939902. [DOI] [PubMed] [Google Scholar]
- 2.Abbott KH. Acute pyogenic spinal subdural abscess; A review of the literature and report of a case. Bull Los Angel Neuro Soc. 1940;18(2):91–102. [PubMed] [Google Scholar]
- 3.Abramovitz JN, Batson RA, Yablon JS. Vertebral osteomyelitis. The surgical management of neurologic complications. Spine. 1986;11(5):418–20. [PubMed] [Google Scholar]
- 4.Bartels RH, de Jong TR, Grotenhuis JA. Spinal subdural abscess. Case report. J Neurosurg. 1992;76(2):307–11. doi: 10.3171/jns.1992.76.2.0307. [DOI] [PubMed] [Google Scholar]
- 5.Bennett AE, Keegan JJ. Circumscribed suppurations of the spinal cord and meninges. Report of a case of subdural abscess with functional recovery following operation. Arch Neurol Psychiatry. 1928;19:329–33. [Google Scholar]
- 6.Butler EG, Dohrmann PJ, Stark RJ. Spinal subdural abscess. Clin Exp Neurol. 1988;25:67–70. [PubMed] [Google Scholar]
- 7.Chen M-H, Chen M-H, Huang J-S. Cervical subdural empyema following acupuncture. J Clin Neurosci. 2004;11(8):909–11. doi: 10.1016/j.jocn.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Chern S-H, Wei C-P, Hsieh R-L, Wang J-L. Methicillin-resistant Staphylococcus aureus retropharyngeal abscess complicated by a cervical spinal subdural empyema. J Clin Neurosci. 2009;16(1):144–46. doi: 10.1016/j.jocn.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Collis RE, Harries SE. A subdural abscess and infected blood patch complicating regional analgesia for labour. Int J Obstet Anesth. 2005;14(3):246–51. doi: 10.1016/j.ijoa.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Coumans J-VCE, Walcott BP. Rapidly progressive lumbar subdural empyema following acromial bursal injection. J Clin Neurosci. 2011;18(11):1562–63. doi: 10.1016/j.jocn.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Dacey RG, Winn HR, Jane JA, Butler AB. Spinal subdural empyema: Report of two cases. Neurosurgery. 1978;3(3):400–3. doi: 10.1227/00006123-197811000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Dus V. Spinal peripachymeningitis (epidural abscess). Report of 8 cases. J Neurosurg. 1960;17(6):972–83. doi: 10.3171/jns.1960.17.6.0972. [DOI] [PubMed] [Google Scholar]
- 13.Fraser RA, Ratzan K, Wolpert SM, Weinstein L. Spinal subdural empyema. Arch Neurol. 1973;28(4):235–38. doi: 10.1001/archneur.1973.00490220043005. [DOI] [PubMed] [Google Scholar]
- 14.Freedman H, Alpers BJ. Spinal subdural abscess. Arch Neurol Psychiatry. 1948;60(1):49–60. doi: 10.1001/archneurpsyc.1948.02310010055004. [DOI] [PubMed] [Google Scholar]
- 15.Gelfand MS, Bakhtian BJ, Simmons BP. Spinal sepsis due to Streptococcus milleri: Two cases and review. Rev Infect Dis. 1991;13(4):559–63. doi: 10.1093/clinids/13.4.559. [DOI] [PubMed] [Google Scholar]
- 16.Harries-Jones R, Hernandez-Bronchud M, Anslow P, Davies CJ. Meningitis and spinal subdural empyema as a complication of sinusitis. J Neurol Neurosurg Psychiatr. 1990;53(5):441. doi: 10.1136/jnnp.53.5.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris LF, Haws FP, Triplett JN, Maccubbin DA. Subdural empyema and epidural abscess: Recent experience in a community hospital. South Med J. 1987;80(10):1254–58. doi: 10.1097/00007611-198710000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Heindel CC, Ferguson JP, Kumarasamy T. Spinal subdural empyema complicating pregnancy. Case report. J Neurosurg. 1974;40(5):654–56. doi: 10.3171/jns.1974.40.5.0654. [DOI] [PubMed] [Google Scholar]
- 19.Hirson C. Spinal subdural abscess. Lancet. 1965;2(7424):1215–17. doi: 10.1016/s0140-6736(65)90637-9. [DOI] [PubMed] [Google Scholar]
- 20.Hos NJ, Bauer C, Liebig T, et al. Autoinfection as a cause of postpartum subdural empyema due to Mycoplasma hominis. Infection. 2015;43(2):241–44. doi: 10.1007/s15010-014-0713-2. [DOI] [PubMed] [Google Scholar]
- 21.Khalil JG, Nassr A, Diehn FE, et al. Thoracolumbosacral spinal subdural abscess: Magnetic resonance imaging appearance and limited surgical management. Spine. 2013;38(13):E844–47. doi: 10.1097/BRS.0b013e31828d5f30. [DOI] [PubMed] [Google Scholar]
- 22.Knudsen LL, Voldby B, Stagaard M. Computed tomographic myelography in spinal subdural empyema. Neuroradiology. 1987;29(1):99. doi: 10.1007/BF00341052. [DOI] [PubMed] [Google Scholar]
- 23.Ko MW, Osborne B, Jung S, et al. Papilledema as a manifestation of a spinal subdural abscess. J Neurol Sci. 2007;260(1–2):288–92. doi: 10.1016/j.jns.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 24.Kraeutler MJ, Bozzay JD, Walker MP, John K. Spinal subdural abscess following epidural steroid injection. J Neurosurg Spine. 2015;22(1):90–93. doi: 10.3171/2014.9.SPINE14159. [DOI] [PubMed] [Google Scholar]
- 25.Kurokawa Y, Hashi K, Fujishige M, et al. [Spinal subdural empyema diagnosed by MRI and recovered by conservative treatment] No To Shinkei. 1989;41(5):513–17. [in Japenese] [PubMed] [Google Scholar]
- 26.Levy ML, Wieder BH, Schneider J, et al. Subdural empyema of the cervical spine: Clinicopathological correlates and magnetic resonance imaging. Report of three cases. J Neurosurg. 1993;79(6):929–35. doi: 10.3171/jns.1993.79.6.0929. [DOI] [PubMed] [Google Scholar]
- 27.Lim H-Y, Choi H-J, Kim S, Kuh S-U. Chronic spinal subdural abscess mimicking an intradural-extramedullary tumor. Eur Spine J. 2013;22(Suppl. 3):S497–500. doi: 10.1007/s00586-013-2700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lownie SP, Ferguson GG. Spinal subdural empyema complicating cervical discography. Spine. 1989;14(12):1415–17. doi: 10.1097/00007632-198912000-00023. [DOI] [PubMed] [Google Scholar]
- 29.McCabe JJ, Murphy RP. Spinal subdural abscess. JAMA Neurol. 2013;70(2):266–67. doi: 10.1001/jamaneurol.2013.588. [DOI] [PubMed] [Google Scholar]
- 30.McCall TD, Fults DW, Schmidt RH. Use of resorbable collagen dural substitutes in the presence of cranial and spinal infections-report of 3 cases. Surg Neurol. 2008;70(1):92–66. doi: 10.1016/j.surneu.2007.04.007. discussion 96–97. [DOI] [PubMed] [Google Scholar]
- 31.Mikić D, Roganović Z, Culafić S, et al. Subdural tuberculous abscess of the lumbar spine in a patient with chronic low back pain. Vojnosanit Pregl. 2012;69(12):1109–13. [PubMed] [Google Scholar]
- 32.Nadkarni T, Shah A, Kansal R, Goel A. An intradural-extramedullary gas-forming spinal abscess in a patient with diabetes mellitus. J Clin Neurosci. 2010;17(2):263–65. doi: 10.1016/j.jocn.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Negrin J, Clark RA. Pyogenic subdural abscess of the spinal meninges; Report of two cases. J Neurosurg. 1952;9(1):95–100. doi: 10.3171/jns.1952.9.1.0095. [DOI] [PubMed] [Google Scholar]
- 34.Ozates M, Ozkan U, Kemaloglu S, et al. Spinal subdural tuberculous abscess. Spinal Cord. 2000;38(1):56–58. doi: 10.1038/sj.sc.3100949. [DOI] [PubMed] [Google Scholar]
- 35.Patronas NJ, Marx WJ, Duda EE. Radiographic presentation of spinal abscess in the subdural space. Am J Roentgenol. 1979;132(1):138–39. doi: 10.2214/ajr.132.1.138. [DOI] [PubMed] [Google Scholar]
- 36.Pompucci A, De Bonis P, Sabatino G, et al. Cranio-spinal subdural empyema due to S. intermedius: A case report. J Neuroimaging. 2007;17(4):358–60. doi: 10.1111/j.1552-6569.2007.00084.x. [DOI] [PubMed] [Google Scholar]
- 37.Raskind R, Weiss SR. Subdural and extradural abscess with unusual complication and complete recovery. Int Surg. 1969;52(6):440–43. [PubMed] [Google Scholar]
- 38.Sathi S, Schwartz M, Cortez S, Rossitch E. Spinal subdural abscess: Successful treatment with limited drainage and antibiotics in a patient with AIDS. Surg Neurol. 1994;42(5):424–27. doi: 10.1016/0090-3019(94)90351-4. [DOI] [PubMed] [Google Scholar]
- 39.Schiller F, Shadle OW. Extrathecal and intrathecal suppuration. Report of two cases and discussion of the spinal subdural space. Arch Neurol. 1962;7:33–36. doi: 10.1001/archneur.1962.04210010039003. [DOI] [PubMed] [Google Scholar]
- 40.Schnegg JF, Glauser M, de Tribolet N. Infection of a lumbar dermoid cyst by an anaerobic peptococcus. Acta Neurochir (Wien) 1981;58(1–2):127–29. doi: 10.1007/BF01401691. [DOI] [PubMed] [Google Scholar]
- 41.Scully RE, Mark EJ, McNeely BU. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 47–1984. A 65-year-old abuser of intravenous drugs with fever and neck pain. N Engl J Med. 1984;311(21):1365–70. doi: 10.1056/NEJM198411223112108. [DOI] [PubMed] [Google Scholar]
- 42.Sorar M, Er U, Seçkin H, et al. Spinal subdural abscess: A rare cause of low back pain. J Clin Neurosci. 2008;15(3):292–94. doi: 10.1016/j.jocn.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 43.Theodotou B, Woosley RE, Whaley RA. Spinal subdural empyema: Diagnosis by spinal computed tomography. Surg Neurol. 1984;21(6):610–12. doi: 10.1016/0090-3019(84)90278-7. [DOI] [PubMed] [Google Scholar]
- 44.Usoltseva N, Medina-Flores R, Rehman A, et al. Spinal subdural abscess: A rare complication of decubitus ulcer. Clin Med Res. 2014;12(1–2):68–72. doi: 10.3121/cmr.2013.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Velissaris D, Aretha D, Fligou F, Filos KS. Spinal Subdural Staphylococcus aureus Abscess: Case report and review of the literature. World J Emerg Surg. 2009;4(1):31. doi: 10.1186/1749-7922-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Volk T, Hebecker R, Ruecker G, et al. Subdural empyema combined with paraspinal abscess after epidural catheter insertion. Anesth Analg. 2005;100(4):1222–23. doi: 10.1213/01.ANE.0000149040.54969.B4. [DOI] [PubMed] [Google Scholar]
- 47.Vural M, Arslantaş A, Adapinar B, et al. Spinal subdural Staphylococcus aureus abscess: Case report and review of the literature. Acta Neurol Scand. 2005;112(5):343–46. doi: 10.1111/j.1600-0404.2005.00496.x. [DOI] [PubMed] [Google Scholar]
- 48.Wu AS, Griebel RW, Meguro K, Fourney DR. Spinal subdural empyema after a dural tear. Case report. Neurosurg Focus. 2004;17(6):E10. doi: 10.3171/foc.2004.17.6.10. [DOI] [PubMed] [Google Scholar]
- 49.Sandler AL, Thompson D, Goodrich JT, et al. Infections of the spinal subdural space in children: a series of 11 contemporary cases and review of all published reports. A multinational collaborative effort. Childs Nerv Syst. 2013;29(1):105–17. doi: 10.1007/s00381-012-1916-4. [DOI] [PubMed] [Google Scholar]
- 50.Lazennec JY, Fourniols E, Lenoir T, et al. Infections in the operated spine: update on risk management and therapeutic strategies. Orthop Traumatol Surg Res. 2011;97(6 Suppl.):S107–16. doi: 10.1016/j.otsr.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 51.Sittig O. Metastatischer Riickenmarksabscess bei septischem Abortus. Z Neurol Pschiatr. 1927;(107):146–51. [in German] [Google Scholar]
- 52.Angsuwat M, Kavar B, Lowe AJ. Early detection of spinal sepsis. J Clin Neurosci. 2010;17(1):59–63. doi: 10.1016/j.jocn.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 53.Baker RP, Brown EM, Coakham HB. Overwhelming cranial and spinal subdural empyema secondary infected sacral decubitus ulcers. Br J Neurosurg. 2003;17(6):572–73. doi: 10.1080/02688690310001626886. [DOI] [PubMed] [Google Scholar]
- 54.Heusner AP. Nontuberculous spinal epidural infections. N Engl J Med. 1948;239(23):845–54. doi: 10.1056/NEJM194812022392301. [DOI] [PubMed] [Google Scholar]


